5
Environmental Impacts
The workshop’s final session featured five panelists providing different perspectives of how advances in catalysis can have an impact on the environmental issues associated with greenhouse gas emissions. The five panelists were Carl Mesters, managing researcher and chief scientist at Shell; David Allen, the Gertz Regents Professor of Chemical Engineering and director of the Center for Energy and Environmental Resources at The University of Texas at Austin; Richard Helling, director of sustainable chemistry for The Dow Chemical Company; Bala Subramaniam, the Dan F. Servey Distinguished Professor of Chemical Engineering at the University of Kansas; and Klaus Harth, vice president for environmental catalysis research at Badische Anilin und Soda Fabrik (BASF).
In his introductory remarks, Monty Alger, director of the Pennsylvania State University Institute for Natural Gas Research and professor of chemical engineering noted that chemicals are a subset of the energy system, which can be a city, a campus, a company, a nation, or the world. Thinking of the energy system as a whole, and considering the majority of activities related to the energy using today’s existing technologies, it is feasible to get to a zero-carbon state. The only obstacle would be the cost of investment. The real challenge is not just to become sustainable but to do so at the right level of cost-effectiveness and productivity cost; given that today there is no economic value proposition of moving to a zero-carbon world and creating the incentive to invest in a new infrastructure to support such a transition.
Another challenge, said Alger, is to create policy that incentivizes
using the plentiful carbon resources this workshop has been considering in a way that generates the positive economic and environmental benefits that many of the technologies described and discussed at this workshop could enable. He cited several examples of policies and associated regulations—the Clean Air Act, the Clean Water Act, and policies banning the use of ozone-destroying chlorofluorocarbons—that triggered investment and transformation of systems using new and available technologies. Alger noted, though, that sustainability has to be built considering the entire value chain. So while one company might produce substantial carbon dioxide emissions, its products might enable other companies or industries to drastically reduce their emissions. Looking across the value chain can be challenging, because companies do not think horizontally to measure sustainability, but having said that, Alger noted that there is the capability and technology to measure what goes into the atmosphere in order to produce a total system measurement.
As a final comment, Alger said that the energy business in general and the chemical industry in particular are capital intensive. The best new technologies, then, will be ones that not only offer a benefit in terms of environmental impacts and operating costs, but also make use of existing infrastructure or reduce the cost of transitioning to a new infrastructure.
ENVIRONMENTAL IMPACTS ON ENERGY-MOBILITY CHEMICALS
The shale gas revolution, said Mesters, has already enabled the United States to reduce its carbon emission by replacing coal with methane, since coal burning emits nearly twice the amount of carbon dioxide per unit of energy produced compared with burning natural gas to carbon dioxide and water (see Table 5-1). However, stated Mesters, the most efficient way to get energy from methane would be to convert methane to carbon and water, which would produce more energy per pound of methane but also eliminate carbon dioxide as byproduct. The challenges, then, are to develop the appropriate technology and to find a use for the carbon currently produced that would offset the cost of the carbon tax that would incentivize changing the way energy is produced from natural gas.
Another way in which natural gas could reduce harmful emissions, said Mesters, is by converting them to a liquid fuel that could substitute for diesel fuel made from oil. Shell, for example, is producing what it calls GTL (gas to liquid) Gasoil, a product that when burned in a properly tuned diesel engine produces significantly lower emissions of nitrogen oxides, particulate matter, hydrocarbons, and carbon monoxide.
With regard to converting methane into chemicals, Mesters said there are three basic routes to forming carbon–carbon bonds, all of which cur-
| Fuel Source | CO2 Emission (lb/mm BTU) |
|---|---|
| Coal (anthracite) | 228.6 |
| Coal (bituminous) | 205.7 |
| Coal (lignite) | 215.4 |
| Coal (subbituminous) | 214.3 |
| Diesel fuel and heating oil | 161.3 |
| Gasoline | 157.2 |
| Propane | 139.0 |
| Natural gas | 117.0 |
SOURCE: http://www.eia.gov/tools/faqs/faq.cfm?id=73&t=11 (accessed March 7, 2016).
rently have drawbacks that integrated efforts in catalysis, process design, and separations science may be able to address. The direct pyrolysis of methane to olefins is energy intensive, produces a great deal of coke, and requires complex separations. Oxidative coupling suffers from competing kinetics and low methane conversion, generates a tremendous amount of heat, and can require complex separations. The indirect route via syngas or using alternative oxidants that first produce methyl-X compounds requires multiple chemical reactions in series, which drives up capital costs.
CHANGING THE SYSTEM OF CHEMICAL MANUFACTURING PROCESSES
Chemical manufacturing is a systems-based operation, said Allen, and as the industry and policy makers begin to assess how the industry’s environmental footprint will change with the transformations this workshop has discussed, it is important to do so from a systems perspective. For example, changing raw materials from petroleum to natural gas-based feedstocks changes the manufacturing system and creates new bottleneck processes, said Allen. In the case of butadiene, there are large effects on price and acetaldehyde becomes a bottleneck intermediate (DeRosa and Allen, 2015). Similarly, an analysis of methane-to-aromatics technologies identifies key cost points and maps cascading effects through the xylene and toluene supply chains.
He summarized the points he wanted the workshop to consider by saying that changing feedstocks, process chemistries, and process tech-
nologies changes the system of chemical manufacturing processes. As a result, the indirect impacts of changes in energy consumption, materials consumption, water use, and other measures of environmental impact can be larger than the direct impacts. Often, the net effect can be counterintuitive, he said.
LIFECYCLE ASSESSMENT
Lifecycle analysis, said Helling, helps make good decisions and is a complementary tool to economic analysis when considering whether to deploy a new process technology. It can be a particularly powerful tool to use when looking at environmental impacts because it can account for the follow-on benefits that can result when a new chemical material enables changes outside of the chemical industry that have a positive environmental impact. For example, a new lightweight but strong material could have no net effect on chemical industry emissions, but it could make vehicles more energy efficient and reduce overall emissions significantly. However, calculating those benefits requires understanding how to determine the positive and negative environmental impacts of the new processes used to make that material.
Lifecycle analysis starts, then, with feedstocks and the first few steps of the reaction path to a new material, explained Helling, and proceeds through the entire lifecycle of a material to when it is disposed of or recycled. He also explained that thinking about the lifecycle of a product or material and using that information to influence purchases or investments, that is called “lifecyle thinking.” Making that more quantitative, he said, is referred to as “lifecycle assessment” and it takes “a few orders of magnitude more work than lifecycle thinking,” said Helling. At Dow, lifecycle thinking is used far more often than lifecycle assessment when making decisions.
One common metric used in lifecycle assessment is cumulative energy demand, which includes the energy content of a material, its fuel value, and the energy that goes into its manufacture. He said the simple rule of thumb is that the fuel value and energy for manufacturing are about equal, plus or minus 30 percent, though the uncertainty can get down to plus or minus 5 percent. “A priori, there is no way to dismiss one or the other fact as they both can be very important,” said Helling.
Energetics alone, however, does not do justice to lifecycle assessment because it is fundamentally a multi-attribute assessment technique, and as Helling explained, it is rare that one option is better than an alternative in every way it can be examined. “There are almost always tradeoffs, and lifecycle assessment helps you understand quantitatively what those tradeoffs are,” he said. As an example, a process might reduce greenhouse
gas emissions but require more fresh water to do so, which might be a big problem in Texas but not in Michigan. As a result, while the calculations that go into a lifecycle assessment can be robust, Helling said, “it takes the values part of it to know what we do with the information and how we compare those things and come to a decision.” Acknowledging that Dow does not do a full lifecycle assessment for every project, Helling concluded his remarks by promoting what he calls a 1-day lifecycle assessment. “That involves making enough assumptions that you can come to a directionally correct analysis as rapidly as possible,” he explained.
QUANTITATIVE SUSTAINABILITY-GUIDED PROCESS DESIGN
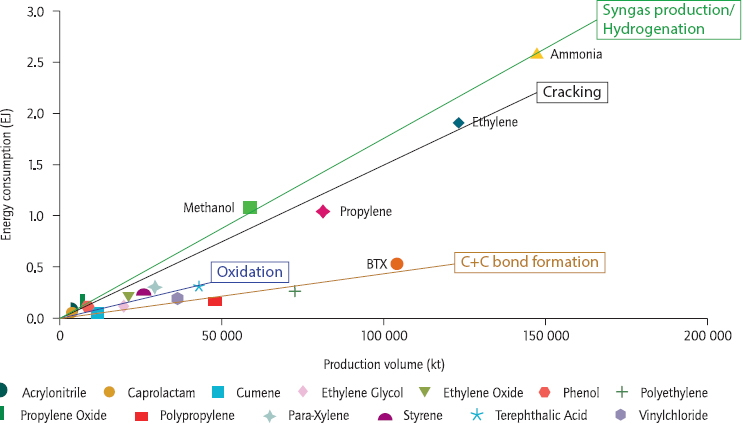
The U.S. chemical industry uses approximately 5 billion British thermal units (BTUs) per year, accounting for 5.9 percent of the nation’s energy use, and production of the top 18 commodity chemicals consume 80 percent of that energy and account for 75 percent of the industry’s greenhouse gas emissions, said Subramaniam. He noted that hydrogenations are the most energy-intensive processes, followed by cracking, oxidation, and carbon–carbon bond formation (see Figure 5-1). In his opinion,
SOURCE: U.S. International Energy Agency, International Council of Chemical Associations, and DECHEMA, 2013.
NOTE: LCA = lifecycle analysis.
SOURCE: Subramaniam, 2016.
catalyst- and process-related improvements can reduce the industry’s energy consumption and environmental impact. The challenge, he said, is to develop novel catalytic technologies that are not only economically viable, but also exhibit high carbon atom economy. Lifecycle analysis can help determine which technologies will meet both of those requirements (see Figure 5-2).
Subramaniam explained that his industry partners want this analysis to start early in process development and want to conduct a process scale simulation to perform the techno-economic analysis. As soon as he and his collaborators receive a process flow diagram, which includes stream and energy flows, they can conduct a lifecycle analysis that can even account for any environmental impacts that might accrue from producing the feedstock for the process. As examples, he discussed two processes, both for making the precursors to polyethylene terephthalate. The first analysis (Ghanta et al., 2013) compared a liquid-phase hydrogen peroxide–based process that eliminates carbon dioxide as a byproduct with a gas-phase oxygen-based silver–catalyzed process for ethylene epoxidation. The key question, he said, was whether the need to use hydrogen peroxide, which requires the use of methane, cancels the zero carbon dioxide benefit. This analysis identified performance metrics that could help yield an economically viable process and could show what parts of the process, including feedstock production, can be changed to reduce its environmental footprint. The analysis revealed that without such changes, the quantitative overall environmental impacts on air quality, water quality, and greenhouse gas emissions would be similar for both processes and lie within the uncertainties of such predictions.
In the second example, he and his collaborators compared terephthalic acid produced in a spray reactor process with the conventional process. This analysis showed there were clear economic and environmental advantages to the spray reactor process. The main economic
advantages were a 50 percent reduction in capital costs arising from eliminating the hydrogenation step in the current process and a 15 percent reduction in operating costs, or approximately $0.07 per pound for a multi-billion-pound compound. The main environmental advantage comes from reducing the amount of solvent burned, which would result in a substantial reduction in greenhouse gas emissions. He noted that his industry partners are now negotiating licenses for this process.
ENVIRONMENTAL CATALYSIS RELATED TO FEEDSTOCK CHANGE
Addressing the challenges raised during the workshop, said Harth, is of high importance to the chemical industry, particularly with regard to yield, energy utilization, capital investment, and sustainability. “We have to look at all of these criteria if we want to come up with the innovation and new processes based on shale gas,” said Harth. He reiterated, though, the message that others had made, which is that the impact of changes that the chemical industry makes will be important, but will nonetheless be small compared with changes required in the energy sector. How can catalysis impact the energy sector? Harth said that having catalysts that can oxidize natural gas, which occurs in auto exhaust, would provide a great environmental benefit.
DISCUSSION
Mark Barteau from the University of Michigan, commenting on Subramaniam’s figure showing the energy intensity of the top 18 commodity chemicals (see Figure 5-2), noted that he draws a different conclusion from that figure. Two-thirds of the carbon footprint of the hydrogenation processes, he said, comes from generating hydrogen, and the cracking processes are endothermic, so discounting those two curves by the things that catalysis cannot change suggests, in his opinion, that the industry has figured out the optimal inefficiency for a wide variety of processes across the chemical industry that is independent of feedstock variations, price fluctuations, inversion of processes, and any other factors. Subramaniam said that where catalysis can change that equilibrium is by maximizing carbon atom efficiency. Helling agreed with Barteau and said that putting a firmer value on carbon emissions will make decisions easier because instead of them being made on the basis of complex value judgments, there will be a true, measurable economic cost. Mark Jones from The Dow Chemical Company said he agreed with both Barteau and Subramaniam and noted that in his opinion, Subramaniam is arguing that
running processes at maximum carbon efficiency will be good regardless of policy.
Helling then noted that the U.S. chemical industry’s switch to shale gas as its major feedstock has already made it more sustainable, but that improvements are required to be among the natural gas producers who are using older technology. He suggested something akin to a “cash for clunkers” program that would encourage producers to use equipment that would greatly reduce the current methane leakage rates. Allen added that the latest data he has seen show the average leakage rate is between 1 and 1.5 percent of the methane extracted from a shale gas well is released into the atmosphere before it is used and that leakage is dominated by what he called “super-emitters.” Two percent of the sites in the Barnett shale formation, he said, accounts for 50 percent of the methane emissions (Zavala-Araiza et al., 2015).
Along the same lines, Pallavi Chitta, from the University of Utah, noted that natural gas flaring is wasting approximately $1 billion of natural gas per year and the energy equivalent of approximately 20 percent of U.S. electricity generation while emitting carbon dioxide equivalent to the emissions of approximately 1 million cars per year during the environmental panel open discussion. He added, and Helling agreed, that flaring was a better option than simply venting methane given that methane is approximately 25 times more potent than carbon dioxide as a greenhouse gas, but the better option still would be to make something from that natural gas. Helling added that naphtha accounts only for approximately 8 percent of U.S. chemical feedstocks, though globally that percentage is still as high as 40 percent.
Wayne Schammel from Siluria Technologies commented that process efficiency is a key aspect of sustainability, but that efficiency has to encompass an entire process developed in conjunction by chemists and chemical engineers. As an example, he cited a process for oxidizing p-xylene to terephthalic acid that achieves 100 percent conversion with 98 percent selectivity, but that generates methyl bromide, a greenhouse gas, and uses a dual water–acetic acid solvent. The most energy-intensive step in this process involves converting 80 percent acetic acid to 95 percent acetic acid for reuse. His point was that if someone developed a different process for oxidizing p-xylene terephthalic acid that eliminated methyl bromide production and operated at room temperature instead of 200°C, it would not be of much use because it would also eliminate the generation of heat that is used in the process’s dehydration tower. Mesters added that efficiency optimization modeling today does not include carbon dioxide emissions and often ignores water, too, which must change going forward.








