2
Medical Isotope Production and Utilization
This chapter provides a primer on the production of molybdenum-99 (Mo-99), technetium-99m (Tc-99m), iodine-131 (I-131), and xenon-133 (Xe-133), and it also describes the Mo-99/Tc-99m supply chain and economics. It is intended for nonexpert readers.
Nuclear medicine is a medical specialty that utilizes radioactive isotopes, referred to as radionuclides, to diagnose and treat disease. These radionuclides are incorporated into radiopharmaceuticals1 and introduced into the body by injection, swallowing, or inhalation. Physiologic/metabolic processes in the body concentrate the tracers in specific tissues and organs; the radioactive emissions from the tracers can be used to noninvasively image these processes (see Sidebar 2.1) or kill cells in regions where radionuclides have concentrated.
Other types of noninvasive diagnostic procedures—for example, computed tomography (CT) and magnetic resonance imaging (MRI)—can detect anatomical changes in tissues and organs as the result of disease. Nuclear medicine procedures can often detect the physiological and metabolic changes associated with disease before any anatomical changes occur. Such procedures can be used to identify disease at early stages and evaluate patients’ early responses to therapeutic interventions.
The radionuclide of primary interest in this report is Tc-99m, the radioactive decay product of Mo-99 (see Section 2.1 in this chapter). Other
___________________
1 Radiopharmaceuticals typically consist of radionuclides bound chemically to organic molecules that are part of biological pathways. The molecules are selected based on their affinities for organs or tissues of concern for a particular medical procedure.
radionuclides of interest are I-131 and Xe-133. Their production and use are described in the following sections.
2.1 Tc-99m
Tc-99m is used in approximately 80 percent of all nuclear medicine procedures performed worldwide each year. Historically, about half of these procedures have been performed in the United States (Verbeek, 2008); that proportion likely remains about the same today.
Tc-99m is a particularly useful imaging radionuclide because it
- Can be chemically incorporated into radiopharmaceuticals that have affinities for different tissue and organ systems.
- Has a sufficiently long half-life (~6 hours) to be usable in nuclear medicine procedures.
- Emits energetic gamma rays (140 kiloelectron volts [keV]) that can be detected efficiently with widely available camera technologies (see Sidebar 2.1).
- Can be supplied efficiently to hospitals and clinics using technetium generators2 (see Section 2.5.4 of this chapter).
- Provides low patient doses for some procedures because of its short half-life and lack of alpha or beta radiations.
___________________
2 The official Food and Drug Administration (FDA) name of the device is technetium Tc-99m generator.
Tc-99m-based radiopharmaceuticals are used to diagnose disease in a large number of tissue and organ systems, including bone, brain, heart, kidneys, liver, and lungs. About 50 percent of Tc-99m utilization in the United States is in nuclear cardiology, predominantly for myocardial perfusion imaging3 which images blood flow through heart muscle. Table 2.1 shows some commonly used Tc-99m-based radiopharmaceuticals. The list is not intended to be exhaustive; rather, it illustrates the wide application range of Tc-99m radiopharmaceuticals.
2.2 I-131 AND Xe-133
I-131 and Xe-133 also have important nuclear medicine applications. Xe-133 is used to image the distribution and rate of exchange of air in the lungs. It decays with a half-life of ~5.2 days and emits 81 keV gamma rays, which can be detected using existing camera technologies (see Sidebar 2.1). Xe-133 is the only approved tracer for this application in the United States. Many other countries use TechnegasTM, a radiopharmaceutical containing a dispersion of Tc-99m-labeled carbon, for lung imaging.
I-131 is used as a therapeutic agent to treat several types of disease. Its ~8-day half-life and emission of high-energy beta particles (mean energy ~190 keV) make it effective for killing cancer cells. The most commonly used I-131 therapeutic agents are
- I-131-labeled sodium iodide, used in the treatment of hyperthyroid disorders and thyroid cancer. Iodine-based therapies are effective for treating these diseases because iodine is naturally taken up by the thyroid (Van Nostrand and Wartofsky, 2007).
- I-131-labeled metaiodobenzylguanidin, used in the treatment of neuroblastomas4 and some cancers of the adrenal glands—for example, pheochromocytoma.
2.3 PRODUCTION OF Mo-99
Almost all of the Tc-99m used in nuclear medicine today is produced by radioactive decay of Mo-99.5 Mo-99 decays with about a 66-hour half-life by emitting a beta particle. About 88 percent of the decays produce Tc-99m via the pathway depicted in Figure 2.1.
___________________
3 Nuclear cardiology is not as highly utilized in other countries. For example, in Europe, nuclear cardiology represents only about 14 percent of the nuclear medicine procedures performed. See Delbeke and Segall (2011).
4 Cancer of the nerve tissue that occurs in children.
5 Tc-99m can also be produced directly using cyclotrons; see Section 2.3.2 in this chapter.
Mo-99 can be produced by a number of processes using research reactors or accelerators (see Sidebar 2.2). The primary production method is fission of uranium-235 (U-235) in research reactors. Other production processes are in small-scale use today, and others are under development. A comprehensive list of potential Mo-99/Tc-99m production processes is shown in Figure 2.2. These processes can be subdivided into two groups:
- Reactor-based production processes
- Accelerator-based production processes
Some key production processes are described in the following sections.
2.3.1 Reactor-Based Production
Mo-99 has been produced for medical use for decades by irradiating targets containing uranium or molybdenum with neutrons produced by research reactors (see Sidebar 2.2). Mo-99 is produced through the following two reactions (see Figure 2.2):
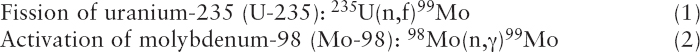
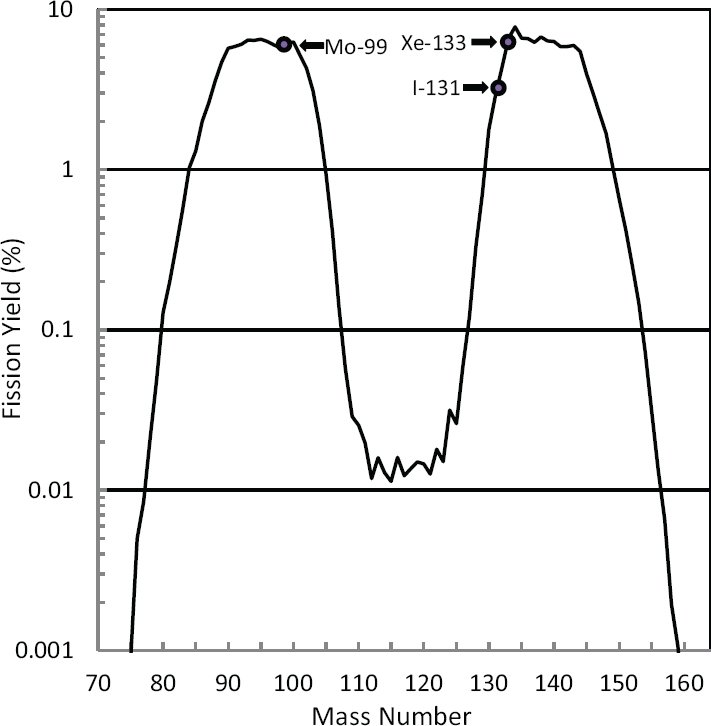
In reaction (1), fission (f) of U-235 by irradiation with neutrons (n) produces the double-hump distribution of fission products shown in Figure 2.3. About 6.1 percent of the fissions result in the production of Mo-99. I-131 and Xe-133 are also uranium fission products; their abundances are also shown in Figure 2.3. Uranium fission is considered to be the “gold standard” process for producing Mo-99 because (1) the production process is highly efficient, especially when highly enriched uranium (HEU; see Sidebar 1.2 in Chapter 1) is used; and (2) the Mo-99 produced has a high specific activity6 (>1,000 curies per gram [Ci/g]), making it suitable for use in conventional technetium generators (see Section 2.5.4 in this chapter).
In reaction (2), Mo-98 captures a neutron (n) and transmutes to Mo-99 after emitting a gamma ray (γ). Neutron capture is a less efficient process for producing Mo-99 than is fission because the neutron capture cross section for Mo-98 is over three orders of magnitude smaller than the fission cross section for U-235.7 Moreover, Mo-99 produced by neutron capture
___________________
6 Specific activity is defined as radioactivity per unit mass, usually expressed as becquerel (Bq) per gram or curies (Ci) per gram. 1 Bq = 3.7 × 1010 Ci.
7 Cross section is a measure of the probability of a neutron reaction, expressed as an area. The fission cross section for U-235 is about 580 × 10–24 cm2; the neutron capture cross section for Mo-98 is 0.13 × 10–24 cm2. Both cross sections are for thermal neutrons (i.e., neutrons in thermal equilibrium at about room temperature).
TABLE 2.1 Commonly Used Tc-99m-Based Radiopharmaceuticals in the United States
| Radiopharmaceutical | Imaging | Manufacturer | Trade Name | FDA Approval Date |
| Tc-99m-Bicisate | Brain Perfusion | Lantheus Medical Imaging (“Lantheus”) | Neurolite® | 1994 |
| Tc-99m-Exametazine | Brain Perfusion | GE Healthcare | Ceretec™ | 1988 |
| Tc-99m-Macroaggregated albumin (MAA) | Pulmonary Perfusion | DraxImage | Technetium Tc-99m Albumin Aggregated Kit | 1987 |
| Tc-99m-Mebrofenin | Hepatobiliary Imaging | Bracco Diagnostics | Choletec® | 1987 |
| Tc-99m-Medronate | Bone Imaging | Jubilant DraxImage | DraxImage MDP-25 | 2004 |
| Pharmalucence | CIS-MDP | 1982 | ||
| Bracco | MDP-Bracco | Approved prior to 1982 | ||
| Tc-99m-Mertiadide | Kidney Imaging | Mallinckrodt Nuclear Medicine LLC (“Mallinckrodt”) | Technescan MAG3™ | 1990 |
| Tc-99m-Oxidronate | Skeletal Imaging | Mallinckrodt | Technescan HDP | 1981 |
| Tc-99m-Pentetate (DTPA) | Brain and Kidney Imaging | DraxImage | DTPA | 1989 |
| Tc-99m-Sodium Pertechnetate | Brain, Thyroid, Salivary Gland, Blood Pool, and Urinary Bladder Imaging | GE Healthcare | Technetium Tc-99m Generator | 2013 |
| Lantheus | Technelite | 1976 | ||
| Mallinckrodt | Ultra-Technekow™ DTE | 1973 | ||
| Tc-99m-Pyrophosphate | Cardiac, Bone, and Blood Pool Imaging | Mallinckrodt | Technescan™ PYP™ | 1974 |
| Pharmalucence | CIS-PYRO | 1987 |
| Tc-99m-Red Blood Cells | Red Blood Cell Imaginga | Mallinckrodt | UltraTag RBC | 1991 |
| Tc-99m-Sestamibi | Cardiac Perfusion | Cardinal Health | Technetium Tc-99m Sestamibi | 2009 |
| DraxImage | Technetium Tc-99m Sestamibi | 2009 | ||
| Lantheus | Cardiolite | 1990 | ||
| Mallinckrodt | Technetium Tc-99m Sestamibi Injection | 2008 | ||
| Pharmalucence | Technetium Tc-99m Sestamibi | 2009 | ||
| Tc-99m-Sulfur Colloid | Liver, Spleen, and Bone Marrow Imaging | Pharmalucence | An-Sulfur Colloid | Approved prior to 1982 |
| Tc-99m-Tetrofosmin | Cardiac Perfusion | GE Healthcare | Myoview™ | 1995 |
| Tc-99m-Tilmanocept | Lymphatic Mapping and Guiding Sentinel Lymph Node Biopsy | Navidea Biopharmaceuticals, Inc. | Lymphoseek® | 2013 |
NOTE: FDA = Food and Drug Administration.
a For example, to detect gastrointestinal bleeding and evaluate left ventricle function.
SOURCE: Adopted from http://www.cardinalhealth.com/content/dam/corp/web/documents/fact-sheet/CardinalHealth-FDAApprovedRadiopharmaceuticalsandApprovedUses.pdf with additional information from some current technetium generator suppliers. Information on FDA approval dates was generated by the committee.
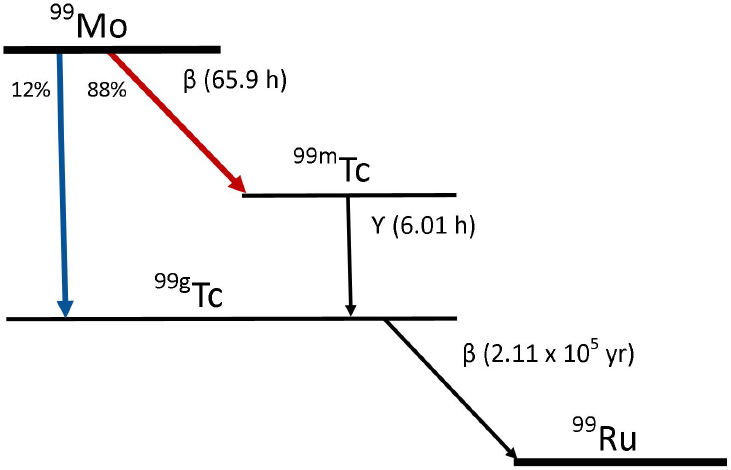
has a lower specific activity (typically 0.1-1 Ci/g), too low for use in conventional technetium generators.
2.3.2 Accelerator-Based Production
Mo-99 and Tc-99m can be produced by irradiating uranium or molybdenum with neutrons, protons, or photons from accelerators (Sidebar 2.2). Several reaction pathways have been suggested and/or investigated, although none are used at present to produce Mo-99 or Tc-99m for medical use. Some key pathways include the following:
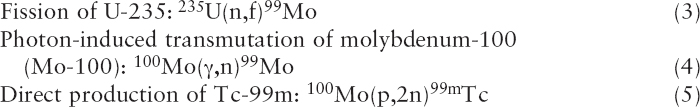
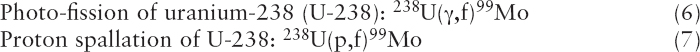
Reaction (3) is identical to reaction (1) except that neutrons are produced by accelerators instead of research reactors. Neutrons are produced by accelerating protons (p) or deuterons (a proton-neutron pair, denoted D) into high-atomic-mass materials (i.e., high-Z material)—for example, tungsten.8 The Mo-99 produced by accelerator fission has a high specific
___________________
8 Bombardment of high-Z materials with protons or deuterons strips neutrons from the nuclei of the target material, a process known as spallation.
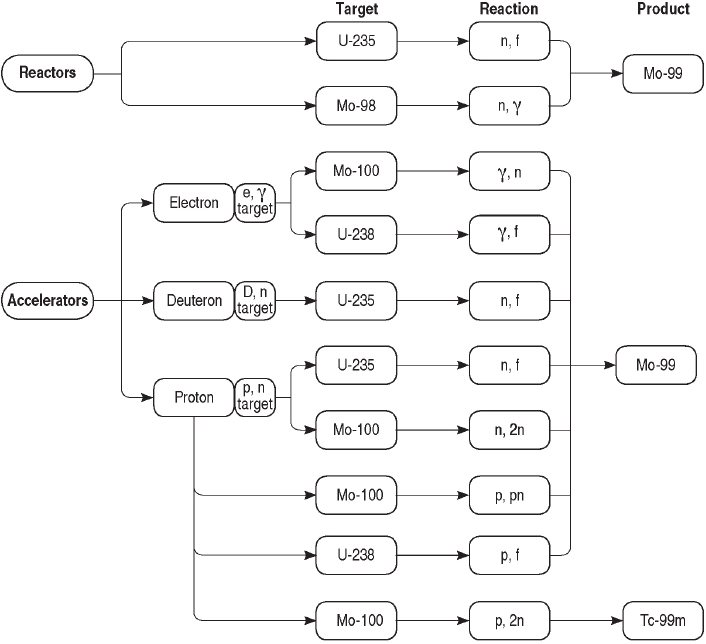
activity (>1,000 Ci/g); however, neutron fluxes and corresponding Mo-99 production rates are typically one or two orders of magnitude lower in accelerators compared to reactors. Production of Mo-99 by accelerator fission is currently under active development in the United States (see Chapter 4).
Reaction (4) produces Mo-99 by spalling neutrons from Mo-100 nuclei with high-energy (14 MeV) photons. These photons are produced by accel-
erating high-intensity (35-50 MeV) electron beams into high-Z materials.9 The Mo-99 produced using this reaction has a low specific activity (1-10 Ci/g), slightly higher than that produced from neutron activation of Mo-98, namely reaction (2).
Reaction (5) produces Tc-99m directly using protons (p) to remove
___________________
9 Photons are produced by the slowing down of electrons in high-Z material; these photons are referred to as Bremsstrahlung radiation.
neutrons (n) from the nuclei of Mo-100 atoms. The Tc-99m produced by this reaction can be used directly in radiopharmaceuticals without further purification or packaging into a generator. The cyclotron10 production of Tc-99m is under active development in Canada (see Chapter 4).
Reactions (6) and (7) are similar to reaction (1) except that high-energy photons (reaction (6)) or protons (reaction (7)) are used to fission U-238 instead of U-235. U-238 is more abundant in natural uranium than is U-235 (see Sidebar 1.2 in Chapter 1); however, the reaction cross sections are orders of magnitude lower than are those for reaction (1).
2.4 PRODUCTION OF I-131 AND Xe-133
I-131 and Xe-133 are products of U-235 fission and are coproduced with Mo-99 when U-235 is irradiated with neutrons (Figure 2.3):

Some current Mo-99 suppliers (see Section 2.5.3 in this chapter) co-recover Mo-99 and Xe-133 from irradiated uranium. There is currently no other production method for Xe-133.
I-131 can also be co-recovered with Mo-99, but no current suppliers of I-131 do so at present. Instead, they make I-131 by irradiating tellurium-130 (Te-130) with neutrons (El Bakkari et al., 2015; IAEA, 2003):
![]()
Irradiation of Te-130 with neutrons produces Te-131 or Te-131m, which have half-lives of 25 minutes and 30 hours, respectively. Te-131 subsequently decays to I-131 by beta (β–) emission.
2.5 OVERVIEW OF Mo-99/Tc-99m SUPPLY CHAIN
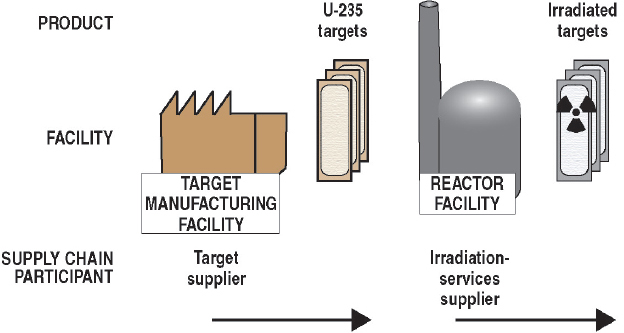
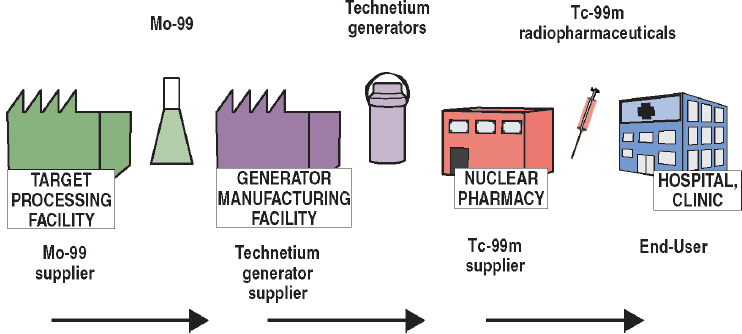
As noted previously, almost all Mo-99 for medical use is produced by irradiating targets containing U-235 in research reactors (reaction (1) in Section 2.3.1). The supply chain for this production process is illustrated graphically in Figure 2.4 and described below. This description is organized around the organizations that participate in the supply chain and the services they provide:
- Target suppliers: Organizations that fabricate U-235 targets for Mo-99 production.
___________________
10 A cyclotron accelerates charged particles such as protons along spiral paths. Cyclotrons having energy ranges between 14 and 24 megaelectron volts (MeV) can be used to produce Tc-99m.
- Irradiation services suppliers: Organizations that irradiate U-235 targets to produce Mo-99.
- Mo-99 suppliers: Organizations that process irradiated targets to recover and purify Mo-99 for commercial sale.
- Technetium generator suppliers: Organizations that manufacture technetium generators for commercial sale.
- Tc-99m suppliers: Organizations that sell Tc-99m sodium pertechnetate (NaTcO4) and/or Tc-99m-labeled radiopharmaceuticals to end users.
- Tc-99m end users: Hospitals and clinics that purchase Tc-99m sodium pertechnetate and/or Tc-99m-labeled radiopharmaceuticals for use in medical procedures.
This supply chain is designed to deliver Mo-99/Tc-99m on a weekly or more frequent basis. Such “just-in-time” delivery is essential to the successful operation of the supply chain because Mo-99 and Tc-99m have short half-lives (~66 and 6 hours, respectively) and therefore cannot be stockpiled. The activity of Mo-99 declines by about 1 percent per hour because of radioactive decay. It must be moved through the supply chain quickly to minimize decay losses. The elapsed time from production of Mo-99 in a reactor to the delivery of a Tc-99m dose to a hospital or clinic can be as short as 4-5 days.
The quantity of Mo-99 in the supply chain is time-dependent because of radioactive decay. The quantity of supply is conventionally measured in 6-day curies,11 that is, the amount of Mo-99 available 6 days after the point of measurement (see Sidebar 2.3). The point of measurement is not fixed in the supply chain but is instead determined by supply chain participants to suit their particular needs. This metric is used throughout this report to specify Mo-99 quantities.
2.5.1 Target Suppliers
A target contains U-235 (HEU or low enriched uranium [LEU]) in a form that is suitable for irradiation in research reactors. Most of the targets
___________________
11 The 6-day curie is not an SI (the International System of Units) unit and therefore does not conform to international standards. The becquerel (Bq) is the SI equivalent to the curie for reporting the activity of a radionuclide. 1 curie equals 37 gigabecquerals (GBq). The Mo-99 supply chain and regulators report exclusively in 6-day curies and for this reason the committee uses this quantity instead of the SI equivalent in this report.
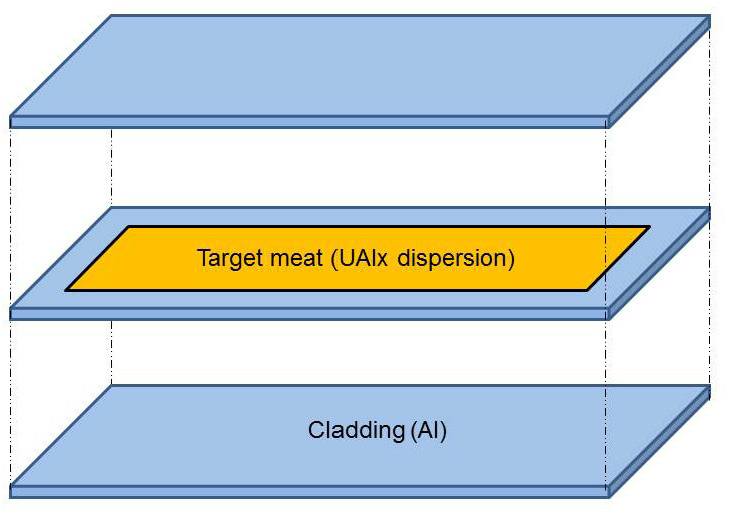
used to produce Mo-99 for medical use today have a sandwich design12 (see Figure 2.5). The meat of the sandwich contains uranium-aluminum alloy particles dispersed in aluminum alloy matrices. The meat is encapsulated in an aluminum alloy cladding that provides a barrier to the release of fission products and transfers heat to the reactor coolant. Targets are typically 3-5 cm in width, 10-15 cm in length, and about 1-2 mm in thickness.
A small number of supplier companies manufacture targets essentially by hand (see Chapter 5). These companies work under contract to Mo-99 suppliers (see Section 2.5.3 in this chapter) to produce targets in accord to those suppliers’ specifications using HEU or LEU purchased by those suppliers from national governments. (At present, most uranium used in target manufacture is purchased from the United States; see Chapter 5.) Targets are not interchangeable among Mo-99 suppliers because of their unique designs. It can take a year or more to obtain the uranium, manufacture it into the appropriate alloy form, and fabricate it into targets. Because of these long lead times, most Mo-99 suppliers stockpile targets at target irradiation facilities (see next section).
___________________
12 One current Mo-99 supplier (Nordion) uses pin (i.e., rod-shaped) targets containing uranium oxide.
2.5.2 Irradiation Services Suppliers
Research reactors are designed to produce large neutron fluxes (typically around 1014 neutrons/cm2-s) in a compact core (see Sidebar 2.2). These fluxes, which are higher than those produced in commercial power reactors, make research reactors suitable for a wide range of scientific, engineering, and industrial missions, including Mo-99 production. These reactors are owned by governments or universities but may be operated by private companies.
Research reactors sell irradiation services to multiple customers on a contract basis. Mo-99 production is an important revenue source for many of these reactors (see Section 2.7 in this chapter). Mo-99 suppliers (see next section) have long-term contracts with research reactors to irradiate and in some cases ship irradiated targets to suppliers’ facilities for processing.
Targets are typically irradiated in reactors for about 5-7 days to allow Mo-99 to build up to between about 70 and 80 percent of saturation concentration13 (see Sidebar 2.3, Figure S2.3). Only about 3 percent of the U-235 in the target is consumed during irradiation. After irradiation, the targets are removed from the reactor and set aside for a day or less to allow for decay of short-half-life fission products. The targets are then
___________________
13 At saturation concentration, the production of Mo-99 in the target is balanced exactly by the loss of Mo-99 from radioactive decay.
packaged and shipped by truck to Mo-99 suppliers’ facilities.14 Shipping time can vary from less than an hour to about a day depending on distance and transport logistics.
2.5.3 Mo-99 Suppliers
Mo-99 suppliers chemically process irradiated targets to recover Mo-99 for commercial sale. Target processing takes place in heavily shielded,
___________________
14 Transportation is constrained to land-based methods because of the size and weight of the shipping containers and transport regulations.
remote-controlled containment compartments, referred to as hot cells,15 which are located in suppliers’ facilities. Most current Mo-99 suppliers use an alkaline dissolution process16 for processing irradiated targets. This process is well suited to target materials that contain aluminum, and it allows for the coproduction of I-131 if desired.
The process is described as alkaline dissolution because the targets are dissolved in sodium hydroxide. The uranium precipitates out as oxides, hydrated oxides, and/or hydroxides; the aluminum and some of the fission products, including Mo-99, remain in solution. The solutions are processed to separate and purify Mo-99 and package it for shipment. Further details about this process are provided in Chapter 5. Target processing typically takes 12-24 hours.
The separated Mo-99, which is contained in solution as molybdate (MoO42–), is shipped to technetium generator suppliers (see next section) in specialized transport containers. These containers can be shipped by road or by air. Shipment can take from a few hours to 1-2 days depending on distance and transport logistics.
2.5.4 Technetium Generator Suppliers
Technetium generators are systems that store Mo-99 and allow its decay product, Tc-99m, to be recovered for use. Most technetium generators are designed to be used with high-specific-activity Mo-99 (>1,000 Ci/g) produced by U-235 fission. The generator consists of an alumina (Al2O3) column having the diameter of a large pencil along with associated filters and tubing for obtaining Tc-99m (see Figure 2.6). This apparatus is installed into radiation-shielded packages for shipment to Tc-99m suppliers (see next section). The generator includes both the package (the plastic container shown in Figure 2.6) and its contained apparatus. Technetium generators contain from 1 to 19 Ci of Mo-99, matched to address the needs and workloads of Tc-99m suppliers (see next section).
It takes 18-24 hours to prepare technetium generators for shipment. Preparation involves loading the molybdate solution onto the columns and sterilizing them; installing the columns, tubing, and filters into the shielded generator package; and packaging the generators for shipment. Tc-99m generators are typically shipped to Tc-99m suppliers within a day of their manufacture. Generators are shipped in regulatory-compliant boxes. The delivery methods can be air, ground, or a combination of both depending on customer location and contracted transportation network.
___________________
15 The term “hot” in hot cell refers to radioactivity, not temperature.
16 One Mo-99 supplier (Nordion) uses an acidic process to produce Mo-99. All of the remaining global producers use an alkaline process.

2.5.5 Tc-99m Suppliers
Technetium generators are delivered primarily to two types of Tc-99m suppliers: regional nuclear pharmacies and hospital nuclear pharmacies. A small number of generators are also delivered directly to hospital nuclear medicine departments for on-site emergency (on-call) situations during afterhours, weekends, and holidays.
Tc-99m is obtained from technetium generators through a process referred to as elution. Tc-99m pertechnetate (TcO4–) is produced on the alumina column as Mo-99 decays. A saline solution (0.9% NaCl) is used to
wash the pertechnetate from the column.17 The recovered sodium pertechnetate (NaTcO4 as noted previously) is mixed with cold kits18 to produce radiopharmaceuticals. A typical elution of a technetium generator takes about 5 minutes.
The Tc-99m eluted from the generator should ideally contain no radionuclide impurities.19 However, Mo-99 is sometimes co-eluted from the generator. This process is referred to as Mo-99 breakthrough. The contamination of Tc-99m with Mo-99 can interfere with radiopharmaceutical production, reduce image quality (Mo-99 emits high-energy [740 and 780 keV] gamma rays), and expose patients to unnecessary radiation.
The accepted activity limit of Mo-99 in the eluate is 0.15 microcuries (μCi) per millicurie (mCi) of Tc-99m in all generators.20 Package inserts that accompany the generators recommend that customers test each elution for Mo-99 breakthrough. However, the U.S. Nuclear Regulatory Commission only requires the measurement of Mo-99 concentration in the first eluate recovered after receipt of a Mo-99/Tc-99m generator.
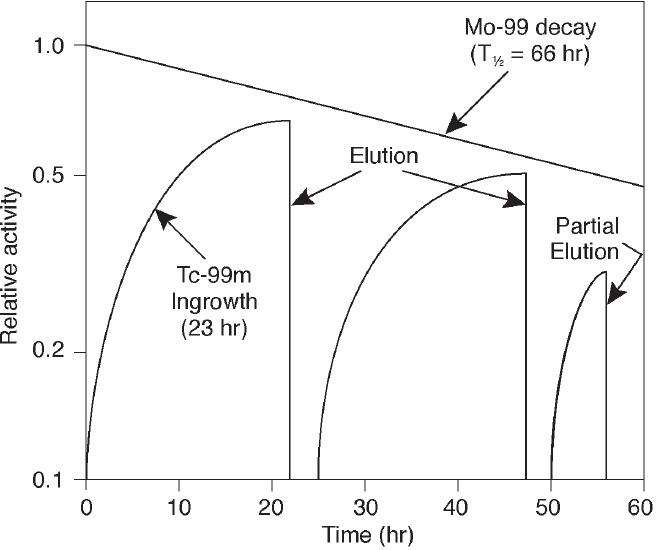
Technetium generators are typically eluted once or twice per day for 1 to 2 weeks. Maximum buildup of Tc-99m occurs approximately 24 hours after each elution (see Figure 2.7). More frequent generator elutions prior to maximum Tc-99m buildup can increase the amount of Tc-99m available for the day. For example, 50 percent of maximum Tc-99m buildup is reached in about 4.5 hours and 75 percent of maximum buildup is reached in about 8 hours.
The amount of Tc-99m in a generator decreases by approximately 20 percent each day. Consequently, Tc-99m suppliers must manage their generators to maximize use of Tc-99m. The most efficient distribution of Tc-99m radiopharmaceuticals happens in centralized nuclear pharmacies because they can most efficiently match the timing of generator delivery to radiopharmaceutical demand. Centralized nuclear pharmacies typically receive a number of generator deliveries staggered throughout the week, and they have the staff needed to produce and distribute radiopharmaceuticals either in multi-dose vials or as single doses to multiple imaging centers and hospitals.
___________________
17 The pertechnetate ion is less tightly bound to the alumina because it has only a single negative charge, versus the double negative charge for molybdate.
18 These kits provide prepackaged chemicals to simplify incorporation of Tc-99m into molecules and preparation of Tc-99m radiopharmaceuticals for specific imaging tests. These kits are characterized as “cold” because they do not contain radioactivity.
19 Possible impurities include Mo-99, I-131, ruthenium-103, strontium-89, and strontium-90.
20 Title 10 of the Code of Federal Regulations, Part 35, Section 35.204 (10 CFR 35.204), “Permissible molybdenum-99, strontium-82, and strontium-85 concentrations.”
2.5.6 Tc-99m End Users
Tc-99m is supplied to hospitals and clinics for use in medical isotope procedures. Tc-99m may be supplied as bulk sodium pertechnetate or as single doses of Tc-99m-labeled radiopharmaceuticals for administration to specific patients. End users can receive Tc-99m one or more times per day depending on their patient loads.
2.6 WASTE MANAGEMENT
The production of Mo-99 from irradiated uranium targets produces four waste streams:
- Solids containing uranium.
- Processing off-gases, primarily the noble gases xenon (Xe-131m, Xe-133, Xe-133m, and Xe-135) and krypton (Kr-85).
- Process liquids from target dissolution.
- Other solid wastes produced during target processing: for example, radioactively contaminated processing equipment.
All of these waste streams are generated in Mo-99 supplier facilities. The management of these wastes is described in Chapter 5.
Xenon plays an important role in monitoring international compliance with the Comprehensive Nuclear-Test-Ban Treaty (CTBT), which prohibits nuclear weapons testing (see, for example, Matthews et al., 2010). This noble gas is mostly nonreactive and has a high yield from uranium fission (see Figure 2.3). Several short-lived isotopes of xenon are produced when a nuclear weapon is detonated, notably Xe-133 (~5.2-day half-life) and Xe-135 (~9.1-hour half-life). A global network of sensitive monitors has been established to measure atmospheric levels of these isotopes. This network can be used to detect illicit nuclear weapons tests, even when they occur underground.21
Radioxenon isotopes are also produced when uranium targets are irradiated to make Mo-99. Xenon and other radioactive off-gases are captured during target processing, temporarily stored to allow for radioactive decay, and subsequently released to the atmosphere. Mo-99 suppliers release sufficient Xe-133 from their target processing facilities to be detected by the CTBT monitoring network. The background radioxenon signals from these facilities can potentially interfere with CTBT compliance monitoring (Matthews et al., 2010).
Technetium generator suppliers also have to manage smaller amounts of Mo-99/Tc-99m waste produced during the manufacture of generators. This waste is typically stored for decay (about 60 hours for Tc-99m and 1 month for Mo-99) and then disposed of as regular trash. Spent technetium generators are usually returned to the generator suppliers for dismantlement (IAEA, 1998). The shielding is reused but the internal apparatus is replaced.
2.7 SUPPLY CHAIN ECONOMICS
The supply chain for Tc-99m and associated medical isotopes is a public-private partnership involving national and state governments, government-owned entities, and private companies. These partners have diverse and sometimes conflicting interests, but their collective success requires mutual cooperation and coordination.
___________________
21 Gaseous xenon can escape to the atmosphere by migrating through pore spaces and fractures created by an underground detonation.
Mo-99 intended for medical use is produced mostly in multipurpose research reactors constructed with government funding. These reactors were built primarily for research and materials testing; target irradiation for Mo-99 production was a secondary activity. However, this activity has become a progressively larger part of reactors’ workloads as demand for Mo-99 has increased, beginning in the late 1970s. Some reactors now obtain most of their revenues from irradiation of targets for Mo-99 production.
Historically, reactor facilities have charged suppliers of Mo-99 only for the marginal operating costs associated with target irradiation. Suppliers were not charged for the life-cycle costs of operating the reactor facilities, which include depreciation of capital costs for facility construction, general operations, maintenance, and decommissioning (OECD-NEA, 2010). These costs were absorbed by the governments that own the reactors.
Reactor facilities—and by extension, their government owners—have also absorbed the costs for maintaining extra irradiation capacity beyond that needed for the routine production of Mo-99. This extra capacity, referred to as outage reserve capacity, allows the reactor to rapidly scale up Mo-99 production to meet demand when other irradiation facilities shut down for scheduled or unscheduled maintenance. The facilities that process irradiated targets also have reserve processing capacity.
Outage reserve capacity helps to ensure that adequate supplies of Mo-99 are available during planned and unplanned outages of target irradiation and/or processing facilities. Mo-99 suppliers traditionally paid for this reserve capacity only when they used it. This created an incentive for irradiation services suppliers to use this reserve capacity for other customers to gain revenue, further driving down prices for irradiation services (OECD-NEA, 2010). In recent years, Mo-99 suppliers have started to pay for the costs for maintaining the outage reserve capacity. However, there is no direct way of measuring the amount of paid outage reserve capacity that is maintained throughout the supply chain (OECD-NEA, 2016).
Governments also subsidize—to various extents—the purchase of Tc-99m for medical procedures. Many governments, including the U.S. government, provide health care to all or a portion of their citizens22 and set reimbursement rates for medical procedures, including those that utilize Tc-99m. Many private insurance companies use government reimbursement rates as guides for setting their own reimbursement rates. Costs for Tc-99m are frequently bundled with the medical procedure rather than being reimbursed separately.
The other supply chain participants—Mo-99 suppliers, technetium
___________________
22 For example, the U.S. government provides health care to individuals who are over 65 years of age and disabled (through Medicare) and to families and individuals with low income and limited resources (through Medicaid).
generator suppliers, and Tc-99m suppliers—are mostly private companies and can set their own prices for the products they sell. These entities have limited pricing power for their products, however, because
- The products they sell (i.e., Mo-99, technetium generators, and Tc-99m) are commodities that are available from several companies;
- These commodities are usually sold through multiyear contracts that limit sellers’ abilities to raise prices; and
- Governments and private insurance companies set reimbursement rates for the nuclear medicine procedures.
Some of the cold kits used to produce Tc-99m radiopharmaceuticals are proprietary items and may be available from only one technetium generator supplier. These companies can make a profit by bundling these kits and a technetium generator in purchase agreements, even if they only break even or incur a small loss for selling generators. This profit does not flow back up the supply chain to Mo-99 suppliers (OECD-NEA, 2010).
2.7.1 Full-Cost Recovery
The High-level Group on the Security of Supply of Medical Radioisotopes (HLG-MR) was established in 2009 by the Economic Co-operation and Development’s Nuclear Energy Agency (OECD-NEA) to examine the underlying causes of global Mo-99/Tc-99m shortages and recommend actions to ensure adequate supplies in the future. The HLG-MR is comprised of approximately 40 experts representing the governments of 17 countries as well as from the European Commission and the International Atomic Energy Agency. The HLG-MR has been working to achieve several goals, including reducing government subsidies for reactors that produce Mo-99.
The HLG-MR has developed six principles to promote full-cost recovery of medical isotope production (see Sidebar 2.4) and the availability of outage reserve capacity to be paid for by higher prices in the supply chain rather than through continued government subsidies. The governments of the HLG-MR member countries agreed to implement the policy approach within 3 years of its adoption (by 2014). This deadline was not met, and progress to implement full-cost recovery is slower than desired (OECD-NEA, 2014a).
The HLG-MR recognizes that existing long-term contracts between irradiation services suppliers and Mo-99 suppliers and/or ongoing government support prevents implementation of full-cost recovery. In addition, some supply chain participants are resisting the price increases necessary for full-cost recovery to occur.
Moreover, it is unclear whether full-cost recovery can ever be implemented across the supply chain in a free market. Some Mo-99 suppliers are in a strong bargaining position relative to the irradiation services suppliers. Two of the five current global Mo-99 suppliers (IRE and Mallinckrodt; see Chapter 3) use multiple irradiation suppliers.23 This gives these Mo-99
___________________
23 All of the other global producers are tied to single reactors: ANSTO (OPAL), Nordion (NRU), and NTP (SAFARI-1).
suppliers bargaining power over irradiation costs. If these Mo-99 suppliers offer to purchase irradiation services at prices above marginal costs but below full-cost recovery, it is better for the irradiation services suppliers to accept those prices to generate revenues to cover reactor operating costs, even if those revenues are insufficient to cover general operating costs and depreciation. These prices may not be sustainable in the long run, but irradiation services suppliers would lose less in the short run by accepting lower prices.
Additional discussion of full-cost recovery is provided in Section 4.5 in Chapter 4.


























