CHAPTER TWO
Remembering Yesterday
Atmospheric chemistry research has provided some spectacular successes, including in improving air quality, reducing acid deposition, and addressing upper atmosphere ozone depletion. These advances have saved lives, protected ecosystems, and improved the social welfare of people in the United States and around the world. There is a similar theme to each of these stories. Each involves atmospheric chemistry research focused on rapid change in the environment that led to improved understanding of the drivers of a problem with real-world implications. Further research improved predictive capability to inform policy choices and responses to the problem, with research continuing to inform the status of the problem today. A brief history of these examples and the central role played by atmospheric chemistry research and researchers, including those whose contributions were recognized with the Nobel Prize and the U.S. National Medal of Science, follows.
2.1 PAST SUCCESSES OF ATMOSPHERIC CHEMISTRY IN A CHANGING WORLD
Improving Air Quality
Air pollution had been recognized as a problem in cities for hundreds of years (Brimblecombe, 1978; Gaffney and Marley, 2003), but by the 19th century it had become much more visible and pervasive. The industrial revolution led to dramatically increased emissions of gases and particles into the atmosphere from fossil fuel combustion. Early episodes of air pollution were typically driven by high emissions of particles and sulfur dioxide, which is converted to sulfuric acid in a process that is accelerated in fog and clouds. This situation led to the description of air pollution as “smog,” a combination of the words “smoke” (from particles) and “fog.”The adverse health effects of smog have been documented for hundreds of years (Brimblecombe, 1978). In the United States, 20 deaths were attributed to extreme smog conditions in Donora, PA, in 1948. The “poster child” for severe air pollution was an episode in London in December 1952 that led to an estimated 4,500 excess deaths during the episode, and a total of 13,500 through the following March (Bell et al., 2004; Snyder, 1994).
In the late 1940s, a new type of damage to agricultural crops was observed in Southern California and traced to interactions with atmospheric constituents (Middleton, 1956; Middleton et al., 1950). Shortly thereafter, chemist Arie Jan Haagen-Smit (see
Box 2.1) demonstrated in a series of laboratory studies that photolysis in air of a mixture of hydrocarbons and nitrogen oxides (NOx) led to the formation of ozone (O3) and a number of associated pollutants whose effects were consistent with the observed plant damage. Despite recalcitrance on the part of industry to acknowledge the contributions from vehicle exhaust emissions (Hahn, 1967), this explanation is now well established: The increasing emissions of volatile organic compounds (VOCs) and NOx that came in large part from automobile exhaust were major contributors to this new kind of air pollution, known as “photochemical” smog (e.g., Haagen-Smit, 1950). In his classic book Photochemistry of Air Pollution, Philip Leighton described the fundamental reaction mechanisms involved (Leighton, 1961). Although it would be almost a decade before the importance of hydroxyl radicals in air was recognized, this book presented the likely importance of free radical chemistry and recycling in the formation of photochemical air pollution.
Levels of fine particles and O3 in the atmosphere are key determinants of air quality (e.g., Jerrett et al., 2009; Rao, 2015; Samet et al., 2000) because they are associated with adverse effects on human health and welfare, agricultural damage, and reduced ecosystem productivity (EPA, 2014a,b; UNEP and WMO, 2011). Understanding the processes driving photochemical smog formation has developed over many decades. Ground-level O3 is known to be formed from reactions involving NOx, VOCs, and sunlight (Finlayson-Pitts and Pitts, 2000; Haagen-Smit and Fox, 1954; Leighton, 1961; NRC, 1991; Seinfeld and Pandis, 2006). Particles can be directly emitted or formed by reactions of gaseous precursors in air. Particulate matter ranges in size from particles smaller than 10 nanometers in diameter to particles as large as 10 micrometers. A measure of fine particles widely used by regulatory agencies is PM2.5, which refers to particulate matter smaller than 2.5 micrometers in diameter. The majority of urban PM2.5 forms in the atmosphere from reactions of NOx, sulfur dioxide (SO2), ammonia, amines, and VOCs. There is increasing concern about ultrafine particles (particulate matter smaller than 100 nanometers in diameter) from outdoor and indoor pollution sources, and their toxicity. Understanding the emissions and chemical transformations of precursor gases to PM and O3 has been instrumental in driving regulations to improve air quality.
A number of regulatory policies to address air pollution have been put into place at both the federal and state levels in the United States. These include the Air Pollution Control Act (P.L. 84-159, 69 Stat. 322, 1955) and the Clean Air Act (P.L. 88-206, 77 Stat. 392, 1963), which with subsequent amendments have significantly improved air quality and reduced adverse effects over the last 50 years. For example, regulatory policies emphasizing NOx control have led to significant decreases in O3 in polluted areas such as Southern California, along with declines in CO, PM2.5, and related pollutants over
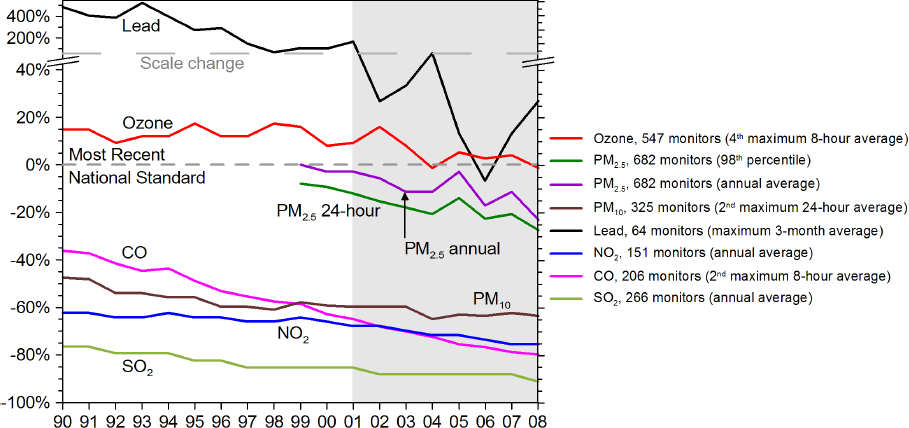
recent decades (Hidy and Blanchard, 2015; NRC, 1991). Figure 2.1 shows the trend in air pollutants falling from unsafe levels to values below National Ambient Air Quality Standards over the United States.
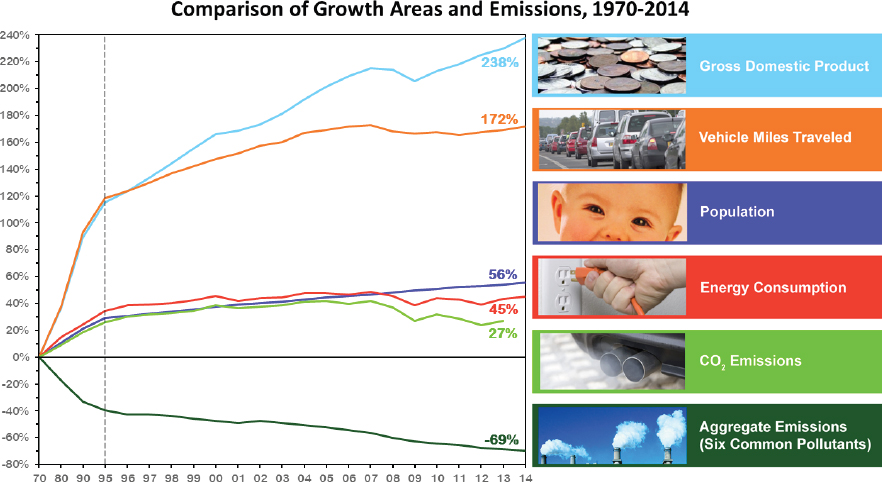
Reasons for this decrease in pollutant emissions include progress in energy efficiency, shifts to renewable technologies, and the role of regulations in raising the cost of business for polluting firms that then increases the competitive advantage of companies using clean technologies (EIA, 2015; IEA, 2014). As a result, California air quality improvements, for example, have been associated with increased productivity, life expectancy, and medical cost savings (CAPCOA, 2015). On a national scale, economic growth has occurred in concert with increased energy consumption and reduced pollutant emissions (see Figure 2.2). The atmospheric chemistry research community has made major contributions to the development of such demonstrably effective control strategies.
The lessons learned from reducing air pollutant levels in the United States provide a scientific foundation for other areas in the world where emissions have increased
dramatically in recent years. Poor air quality remains an issue in many parts of the developing world. It has been estimated that outdoor air pollution is responsible for 3.3 million premature deaths per year globally, with more than two-thirds of the deaths occurring in Asia (Lelieveld et al., 2015). Because long-range transport can carry pollutants to downwind regions or countries, international strategies may be a part of future air quality management. In addressing these challenges, atmospheric chemistry will continue to play a vital role in improving the understanding of the impact of air pollution on human health (see Chapter 4.2).
Reducing Acid Deposition
Deposition of acids from the air onto surfaces is a direct consequence of fossil fuel burning. Combustion emits NOx and SO2, which are converted in the atmosphere into nitric and sulfuric acids, respectively (NRC, 1983, 1986; Oppenheimer, 1983). When
these compounds are returned to Earth, acidification of ecosystems and surface waterways occurs, inducing a cascade of deleterious effects. The writer John Evelyn first described the negative effects of city air on ancient marble in 17th century England (Evelyn, 1661). However, the topic of acid pollution coming from the atmosphere was not widely reported until the Industrial Revolution dramatically increased emissions of NOx and SO2. For example, in 1852, chemist Robert Smith coined the term “acid rain” when he noted higher acidity rainfall in industrial regions relative to coastal areas in England and indicated it was a result of sulfuric acid from coal combustion (Smith, 1852).
More than a century after Smith’s finding, Odén (1968) showed that much of the acidity in Scandinavian rains resulted from long-range atmospheric transport following emissions of sulfur from England and central Europe. Also in the 1960s, multidisciplinary research was initiated to understand the consequences of acid rain falling in the eastern United States and Canada. Biologists and limnologists conducted large-scale manipulative experiments on farms and lakes to probe the links between reduced agricultural crop production, “dead” lakes, damaged forests, whole-ecosystem effects, and acid deposition (Beamish and Harvey, 1972; Lee et al., 1981). Through their studies of the regional distribution of acid rain and its importance for aquatic and terrestrial ecosystems at the Hubbard Brook experimental forest in New Hampshire beginning in the early 1960s, Gene Likens (see Box 2.1) and colleagues were instrumental in bringing the problem of acid rain to public attention in the United States (Likens et al., 1972). Coupled with research to better understand long-range transport of atmospheric pollutants and consistent with Odén’s work in Sweden, chemists proposed that power plant emissions from midwestern states could arrive in the northeastern United States and damage ecosystems through the deposition of acid rain (Nelson et al., 1984).
Throughout the 1970s and 1980s, researchers discovered that acid deposition reduces biodiversity and can substantially alter ecosystems with smaller changes in acidity than previously believed (Schindler, 1988). Furthermore, the negative impacts of direct acid deposition are compounded because acidity causes toxic species, such as trace metals, to remobilize in the environment (Exley et al., 1991). In addition to the ecosystem effects of acid deposition, the formation of highly concentrated inhalable droplets of acidic fog was proposed to likely have substantial effects on human and ecosystem health (Hoffman, 1984; Jacob et al., 1985).
Elucidating the causes and effects of acid deposition has been a broad effort involving researchers from a variety of fields. Fundamental atmospheric chemistry research played a vital role in understanding the mechanism behind the conversion of SO2 and
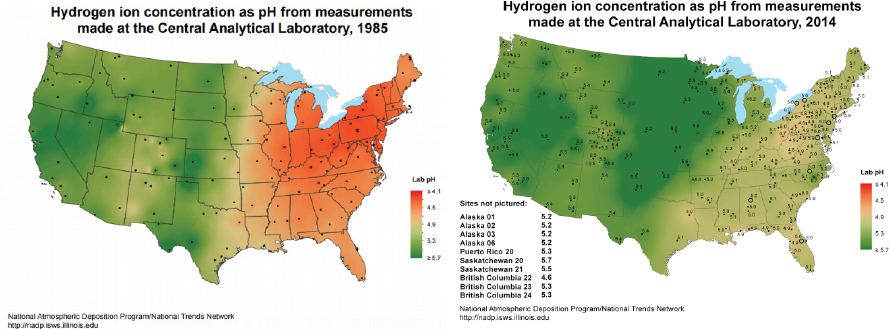
NOx into acids and with this knowledge, researchers were able to predict that decreases in emissions of SO2 and NOx would lead to lower acid rain levels. The recognition that pollution can cause effects well beyond local environments began reaching the public in the 1970s, leading to widespread pressure for political and legal action. This realization resulted in the 1980 Acid Deposition Act, which was passed to comprehensively support a program addressing the sources of acid rain and dry deposition and their long-term effects over a broad geographic area. Research was also funded to examine potential pollution control measures, which resulted in Clean Air Act amendments and supporting legislation such as the Cross-State Air Pollution Rule1 to ameliorate effects of pollutant transport between states. Thus, informed public support for policy change ultimately led to the development of strategies to dramatically reduce emissions, and hence acid deposition in the northeastern United States (Driscoll et al., 2001). Figure 2.3 illustrates the dramatic success of this legislation in leading to significant decreases in the acidity of precipitation over the eastern United States.
The success in reducing these emissions was largely due to improvements in environmental technology that emerged in response to the Clean Air Act and its amendments. As one example, between the late 1880s and the late 1960s the number of patents filed for flue gas desulfurization systems to limit emissions of SO2 from coal-fired power plants, did not exceed four per year; after passage of the Clean Air Act amendment of 1970 the number of patents filed annually was substantially greater (Taylor et al., 2003). Furthermore, since 1970 capital costs for new systems and operating costs decreased significantly (Taylor et al., 2003), highlighting the potential for environmental regulation to drive technological innovation.
Addressing Upper Atmosphere Ozone Depletion
Absorption of solar radiation by O3 in the stratosphere (the region of the atmosphere 10–50 km above the Earth’s surface) makes life on Earth possible because it reduces the flux of harmful ultraviolet radiation to the surface (NRC, 1979, 1982, 1984b). Decreases in this O3 ultraviolet filter lead to increases in skin cancer, as well as ecosystem and climate effects such as reduced productivity, altered reproduction and development, and increased mutations (Hader et al., 2007; van Dijk et al., 2013; WMO, 2014). Sydney Chapman described the natural cycle in the upper atmosphere that involves oxygen molecules, oxygen atoms, and ozone. Known as the “Chapman Cycle,” this reaction sequence generates a steady-state concentration of O3 that provides the filter for
___________________
1 Cross-State Air Pollution Rule: https://www.epa.gov/airmarkets/interstate-air-pollution-transport.
ultraviolet radiation from the sun. The recognition that anthropogenic emissions could alter the balance of this cycle came in the early 1970s when Paul Crutzen (see Box 2.2) predicted that oxides of nitrogen could alter the Chapman Cycle (Crutzen, 1970, 1971) via photolysis of nitrous oxide that had been proposed to be transported from the troposphere (Bates and Hays, 1967). In 1971, Harold Johnston (see Box 2.1) predicted that direct emissions of NO into the stratosphere by a proposed fleet of supersonic transports would also lead to significant ozone loss (Johnston, 1971).
Use of chlorofluorocarbons (CFCs) expanded greatly after World War II in a number of applications such as propellants in spray cans and as refrigerants and blowing agents. Because they do not have carbon-hydrogen bonds with which the hydroxyl radical (OH) can react, they are inert in the lower atmosphere and do not contribute to photochemical air pollution. However, their long lifetimes in the lower atmosphere mean that they can reach the stratosphere where they absorb short-wavelength ultraviolet radiation, generating highly reactive chlorine atoms. Stolarski and Cicerone (1974) proposed that if there were a source of chlorine atoms in the stratosphere, chain destruction of ozone could occur in a manner qualitatively similar to that by oxides of nitrogen, but at the time there was no obvious source of chlorine to the upper atmosphere. Inspired by the detection and measurement of a global distribution of CFCs in the troposphere by James Lovelock and coworkers (Lovelock et al., 1973), Mario Molina and Sherwood Rowland probed the potential atmospheric fates of CFCs and showed that they could indeed be a major source of stratospheric chlorine, perturbing the Chapman Cycle and leading to stratospheric O3 loss (see Box 2.2).
Measurement of CFCs also enabled new developments in predictive modeling tools, as Lovelock recognized with his seminal 1971 paper (Lovelock, 1971). Tropospheric measurements of semi-inert tracers like CFCl3 and radiogenic gases such as 222Rn and 85Kr, provided fundamental tests for the development of 3-D chemistry transport models (Jacob et al., 1987; Prather et al., 1987) and became a community standard for testing chemistry-transport modeling (Jacob et al., 1997; Pyle and Prather, 1996).
While initial doubt existed regarding the predictions of the atmospheric effects of CFCs, the loss of ozone in the Antarctic “ozone hole” discovered in the mid-1980s was clear and much more dramatic than expected based on known chemistry at the time. Confirmation of stratospheric ozone depletion by as much as 10–35 percent was first noted by Shigeru Chubachi, Joseph Farman, Brian Gardiner, and Jonathan Shanklin (Chubachi, 1985; Farman et al., 1985), and corroborated by satellite measurements (Krueger et al., 1987; Stolarski et al., 1986). These findings motivated Antarctic expeditions by a burgeoning community of scientists charged with discovering the causes and mechanisms of ozone hole formation. The fact that the ozone loss was severe over Antarctica and localized in the lower stratosphere near the ozone concentration maximum was an unforeseen phenomenon. Given what was known at the time, it was expected that CFC-induced ozone loss would be greatest at high altitudes in the tropical and mid-latitude stratosphere. Researchers used a combination of laboratory and theory studies, field observations, and predictive modeling to show that reactions of gases on polar stratospheric cloud particles activated temporary reservoirs for chlorine, which had originated in the stratospheric breakdown of CFCs (Anderson et al., 1991; Crutzen and Arnold, 1986; McElroy et al., 1986; Solomon et al., 1986; see Box 2.2). The integration of laboratory studies, theory, models, and field observations built a detailed understanding of the phenomena responsible for stratospheric ozone depletion, including the unique chemical processes that led to formation of the Antarctic ozone hole (Cicerone et al., 1983; Molina and Molina, 1987; Prather et al., 1984). This defining decade of atmospheric chemistry research demonstrated unequivocally that CFCs were the cause of ozone depletion.
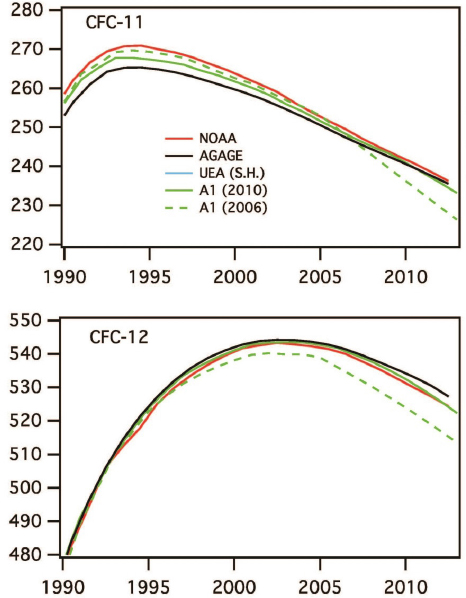
The overwhelming evidence for the role of CFCs as the source of chlorine that caused stratospheric ozone depletion led to enhanced public awareness and demand for action. In 1987, an international agreement resulted in the Montreal Protocol2 treaty and its amendments, which phased out the production of CFCs and other ozone depleting substances. Atmospheric chemists played a key role in providing information to help develop the initial Montreal Protocol, and the information leading to its amendments that broadened the suite of ozone depleting substances included and accelerated the timeline for their phase-out. Figure 2.4 shows that the previously increasing trends in atmospheric concentrations of CFCs reversed in the period 1994–2002 in response to these international controls. By decreasing atmospheric CFC concentrations, this science-based treaty is projected to have averted a number of adverse effects, including millions of skin cancer deaths (van Dijk et al., 2013) and the Antarctic ozone hole has been shown to be healing (Solomon et al., 2016). Emission controls have also
___________________
2 The Montreal Protocol on Substances that Deplete the Ozone Layer: http://ozone.unep.org/en/treaties-and-decisions/montreal-protocol-substances-deplete-ozone-layer.
curbed the contribution of CFCs to global climate change, since they are also greenhouse gases (Hansen et al., 1989; IPCC, 2014).
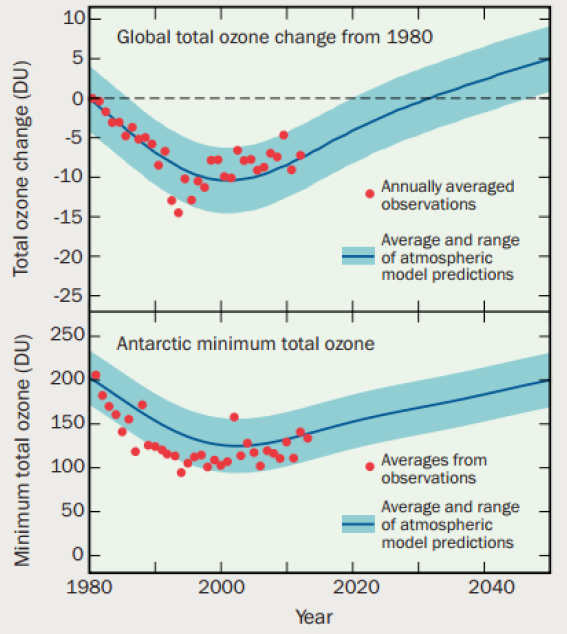
However, even though it is on a positive path toward healing, the Antarctic ozone hole is expected to continue to have an impact on regional climate (e.g., on temperature, winds, and precipitation) in the Southern Hemisphere for some time (WMO, 2014). Atmospheric chemists will continue to play an important role in understanding stratospheric chemistry and how stratospheric ozone is changing in response to changes in emissions as well as changes in atmospheric temperature and structure (see Figure 2.5), in particular as the climate continues to change. This ongoing work is needed to monitor these changes and predict future changes with implications for human health and welfare.
2.2 CONCLUSION
As discussed in the three case studies presented in this chapter, the results of atmospheric chemistry research have historically provided a strong foundation of scientific understanding of issues that affect human and global welfare. This understanding has enabled the identification of the root causes to major problems and the development of predictive capabilities that informed scientifically based control strategies. Research continues to inform ongoing assessment of environmental issues as they affect the United States, as well as in developing countries where emission rates of many pollutants are increasing rapidly.
As will be discussed in the chapters that follow, as the atmosphere undergoes changes at an unprecedented rate due to human activities, emerging problems have the potential to significantly affect the health of humans and ecosystems, as well as climate and weather on many scales. These changes will undoubtedly include those that are unforeseen and/or happen abruptly (NRC, 2013), similar to the emergence of the Antarctic ozone hole. The ability to stay ahead of rapidly evolving environmental issues will require having a range of the most advanced tools possible in order to be ready to respond to the next event, be it a volcanic eruption, an oil spill, or emissions of methane from destabilizing permafrost.
Some of the problems that have been successfully addressed in the United States are being repeated in other countries around the world as economic growth outstrips the implementation of regulations. Given the importance of long-range transport and of global consequences of emissions, what happens in those countries can have substantial impacts in other regions around the world. It is therefore in our national interest to advance the understanding of atmospheric chemistry to provide a sound basis for the global development and implementation of rational, scientifically based strategies to minimize the impacts of these challenges.
This page intentionally left blank.














