CHAPTER FOUR
Anticipating Tomorrow: Societal Challenges
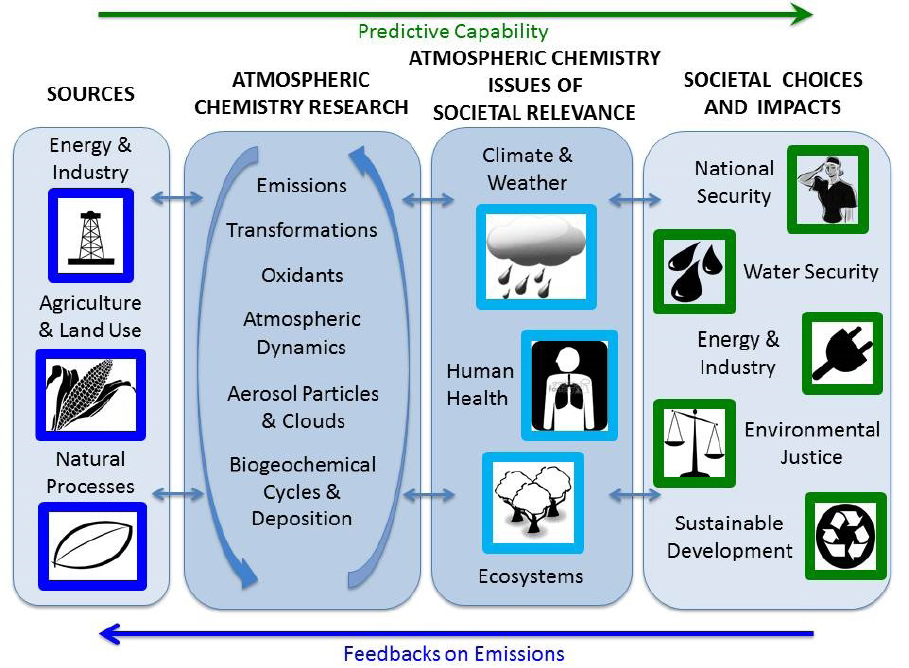
As described in Chapter 2, atmospheric chemists have contributed to the response to many societal problems with use-inspired basic research. The first three sections (see Chapters 4.1–4.3) in this chapter focus on key areas where atmospheric chemistry research is currently contributing to the understanding of societally relevant issues and where the Committee believes there should be increased attention in the coming decade. These include the relationships of atmospheric chemistry to the weather and climate system, human health, and ecosystems, areas where rapid changes are occurring and where there are opportunities for important progress in the coming decade to better inform societal choices. The last section (Chapter 4.4) examines a number of areas where atmospheric chemistry affects society and where societal choices influence atmospheric composition, ultimately affecting people in different ways. The relationship among these areas is shown schematically in Figure 4.1.
4.1 ATMOSPHERIC GASES AND PARTICLES AFFECT CLIMATE AND WEATHER
The increase in the atmospheric abundance of greenhouse gases and particles due to human activity has altered climate with profound implications for society. Global mean temperature has increased by 0.8°C since the pre-industrial era, and 15 of the 16 warmest years since 1880 have occurred since 2000.1 Despite the importance of this trend, the change in global mean temperature is just one indicator of the changing climate system. Other impacts of a changing climate include rising sea levels, increasing frequency of heavy precipitation, and significant seasonal ice loss in the Arctic. Some types of extreme events, which can incur large costs on the economy, are known to be exacerbated by climate change (NASEM, 2016). For the period 1980–2011, it was estimated that droughts and heat waves cost the U.S. economy a total of $210 billion and floods cost $85 billion (Smith and Katz, 2013). These extreme climate events also have implications for global food security. The drought in the United States in 2012 resulted in a 21 percent reduction in the yield of maize, relative to the previous five nondrought years, which led to a 54 percent increase in the price of maize (Boyer et al.,
___________________
1 NOAA global analysis (2015): https://www.ncdc.noaa.gov/sotc/global/201513.
2013; Gilbert, 2016). This alteration highlights the fact that regional climate impacts have global implications in the interconnected global economy.
These changes to the climate are driven by increases in greenhouse gases, including carbon dioxide, methane, nitrous oxide, and ozone. Greenhouse gases absorb infrared radiation, and the increase in their atmospheric abundance due to human activity has produced a radiation imbalance in the atmosphere, which is referred to as radiative forcing of the climate system. Aerosol particles scatter and absorb solar radiation, producing a direct effect on climate. Aerosol particles also have an indirect effect on climate through their influence on clouds, which can lead to either warming or cooling of the climate system, although the overall global impact is cooling. See Figure 4.2
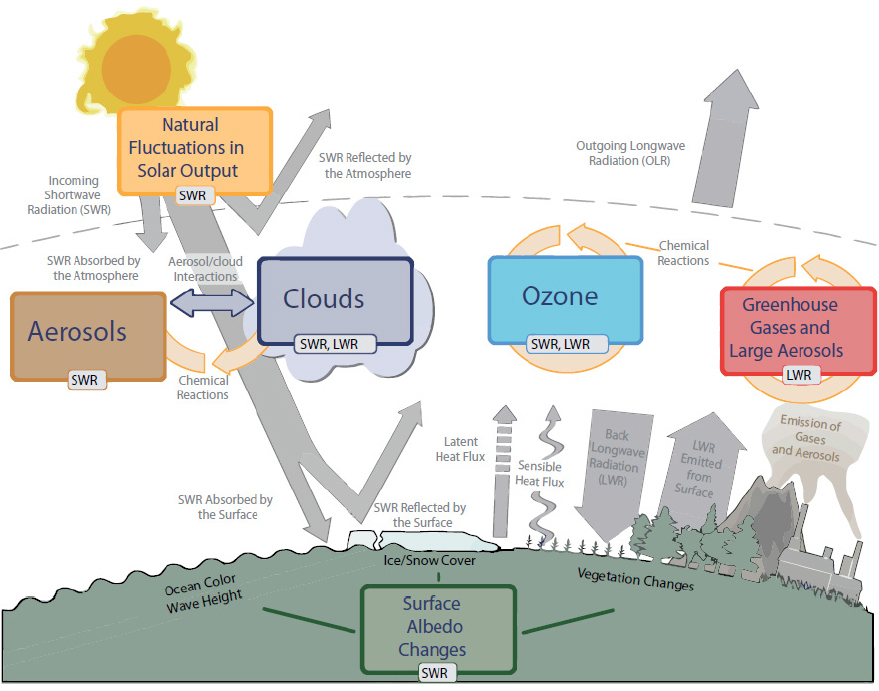
for an overview of the role of atmospheric gases, aerosol particles, and clouds in the climate system.
Strong feedbacks exist between climate change and atmospheric composition. Changes in climate can impact ecosystems, resulting in changes in emission of gases and particles to the atmosphere (Bonan, 2008; Cramer et al., 2001; Hoegh-Guldberg and Bruno, 2010). A prime example is in the Arctic, which has experienced the greatest regional warming since the pre-industrial era. Continued warming in the Arctic could result in increased emissions of methane from the permafrost, which will feed back on the climate system directly, as methane is a greenhouse gas. Increases in atmospheric methane will increase tropospheric ozone and stratospheric water vapor, which are
also greenhouse gases and play an important role in the chemistry of the troposphere and stratosphere, respectively (Akimoto et al., 2015; Isaksen et al., 2014; West et al., 2012). Many sources, such as wildfire and dust, respond to changes in climate and land use that may increase emissions of some gases and aerosol particles (Ginoux et al., 2012; Kloster et al., 2012; Knorr et al., 2016). For example, during the last glacial maximum, atmospheric dust concentrations were two to five times higher than interglacial periods (Maher et al., 2010). In addition, dust, black carbon (soot), and organic carbon deposited on snow and ice sheets reduce albedo, resulting in surface warming and faster melting (Yasunari et al., 2015). Obtaining an improved understanding of this coupling between atmospheric chemistry and climate is essential for reliably predicting future changes in both atmospheric composition and climate.
The radiative forcing of climate on a global scale by well-mixed greenhouse gases is relatively well understood, and according to the IPCC (2013) the level of confidence in estimates of the radiative forcing is high for methane and halocarbons, and very high for carbon dioxide (CO2) and nitrous oxide. However, projecting the regional response in climate to radiative forcing is challenging, and the coupling between the regional response and atmospheric composition and chemistry is a particular source of uncertainty. Climate-related changes in storm tracks will impact weather patterns as well as the transport of pollution, which has implications for air quality. Changes in the structure and intensity of convection influence the transport of pollution from the surface to the upper troposphere and lower stratosphere. These changes also impact the frequency of lightning, which is an important source of NOx, a key precursor of tropospheric ozone (Schumann and Huntrieser, 2007; Seinfeld and Pandis, 2006). Changes in the lightning source of NOx will have a dominant influence on ozone abundance in the upper troposphere, where ozone has the greatest capacity to affect climate.
There is less confidence in the radiative forcing associated with changes in the abundance of short-lived gases. There is also significant regional and seasonal variability in radiative forcing from these more chemically active gases (e.g., Bowman and Henze, 2012; Shindell et al., 2006). It has been suggested that controlling the abundance of short-lived climate pollutants, those with an atmospheric lifetime of less than 20 years, could provide a means of mitigating some of the expected changes in climate. For example, Shindell et al. (2012) showed that controlling methane and black carbon could reduce global mean warming by about 0.5°C by 2050. The initial focus for these pollutants has been on methane, tropospheric ozone, and black carbon, which are all warming agents. In addition, methane is a precursor of troposphere ozone, and ozone and black carbon are air pollutants. Consequently, reducing the atmospheric abundance of these short-lived pollutants would have the co-benefit of improving air quality (see
Box 4.1). How radiatively-important short-lived pollutants have changed since preindustrial times is not well understood.
The impact of aerosol particles on the climate system is less well understood than the influence of well-mixed greenhouse gases, particularly their influence on clouds. Modeling, satellite remote sensing, laboratory, and field measurement studies over
the last two decades have focused on understanding the impacts of aerosol particles on clouds and global climate. During this time, some scientific gaps have been closed, and additional processes have been identified that still elude quantification. As with many complex systems in intermediate stages of understanding, this progress has not yet reduced the overall magnitude of uncertainty, leaving major deficiencies in the ability to project future climate (Seinfeld et al., 2016). The root of this uncertainty is a lack of understanding of the contribution of natural and anthropogenic sources of global aerosol particles (Carslaw et al., 2013), their synergies and chemical transformations that affect how particles absorb and scatter light, as well as how particles take up water and their ability to serve as cloud and ice nuclei (e.g., Farmer et al., 2015; Fierce et al., 2013; Hoose and Mohler, 2012; McFiggans et al., 2006). The multiscale and multiphase coupling of aerosol particles with clouds and radiation further complicates its robust representation in models (Stevens and Feingold, 2009). Although particle radiative forcing (both direct and indirect) is still recognized as a leading source of uncertainty in current estimates of climate sensitivity, anthropogenic aerosol particle precursor emissions and the resulting radiative forcing are not expected to increase considerably compared to current levels. This expectation, together with the ever-increasing levels of greenhouse gases (and associated warming), has spawned one view in the climate community that continuing efforts on aerosol and aerosol–cloud interactions in climate studies may be of secondary importance.
However, this projection neglects the dominant contribution of natural aerosol particles, and how a changing climate may alter their abundance and impacts on the Earth’s energy balance. Furthermore, describing the impacts of the changing aerosol abundances over previous decades is essential to the accurate estimation of climate sensitivity and the predictive capability of climate models. In addition, many studies point to the pivotal role that reactive gases, aerosol particles and their interactions with radiation and clouds can have on atmospheric phenomena that span the range from the weather to climate scale.
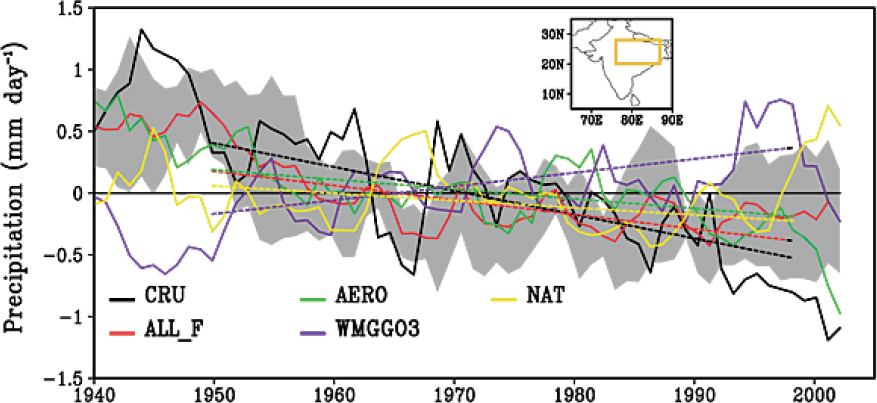
Aerosol particles impact radiative forcing on regional signals that are not reflected on the global scale (Shindell and Faluvegi, 2009), such as impacts on the Asian summer monsoon, rainfall patterns in Southeast Asia, North Atlantic variability, the evolution of tropical cyclones, and the invigoration of precipitation and intensification of precipitation. The changing abundance of atmospheric particles over Europe and North America has been linked to changes in climate over these regions (Mickley et al., 2012; Philipona et al., 2009). Leibensperger et al. (2012) estimated that between 1970 and 1990, atmospheric particles resulted in an annual mean net cooling of the central and eastern United States by 0.5–1.0°C, offsetting some of the warming due to greenhouse
gases, and an annual mean reduction in precipitation of 0.2 mm/day along the U.S. east coast. Atmospheric particles have also been linked to changes in storm tracks over the North Pacific and North Atlantic (Booth et al., 2012; Dunstone et al., 2013; Wang et al., 2014; Zhang et al., 2007) and to changes in Hadley circulation in the tropics (e.g., Allen, 2015; Hwang et al., 2013; Ming and Ramaswamy, 2011). In addition, Bell et al. (2008) argued that satellite observations of rainfall over the Unites States show weekly variation in cloud top heights and precipitation, with a midweek maximum in afternoon rainfall and storm intensity over the southeastern United States that are correlated with particle loading in the atmosphere, suggesting a strong connection between weather and anthropogenic pollution.
Many studies have concluded that changing anthropogenic aerosol particles can shift the tropical patterns of rainfall, contributing to the Sahel drought (Held et al., 2005) and threatening the Amazon rainforest (Cox et al., 2008). Large anthropogenic aerosol particle abundances over Asia appear to delay the onset of summer monsoons and are spatially shifting rainfall patterns in SE Asia (Gautam et al., 2011; Kuhlmann and Quaas, 2010; Lee et al., 2014). Aerosol particle–cloud interactions have also been suggested to modulate North Atlantic variability (Booth et al., 2012; Dunstone et al., 2013) as well as the Pacific Decadal Oscillation (Allen et al., 2014). Saharan dust outbreaks may influence the organization and evolution of tropical cyclones (Reale et al., 2014).
The indirect effect of aerosol particles on climate and weather depends on the microphysical properties of the particles. An increase in cloud condensation nuclei (CCN) results in a greater concentration of smaller cloud droplets, which increases the reflection of solar radiation and reduces surface heating. The particles also alter the precipitation efficiency of clouds by reducing the rate at which cloud drops coalesce into raindrops, resulting in less warm rain production and a longer cloud lifetime (Albrecht, 1989). Although some modeling studies have shown that atmospheric particles can suppress downwind precipitation (Changnon, 1981; Schmid and Niyogi, 2013), other studies have seen little impact from the particles and have ascribed this lack of impact to changes in meteorology (Carrió et al., 2010). Still other studies have shown that biomass burning particles invigorate convection and enhance tornado formation (Saide et al., 2015). Dust and bioparticles can change cloud microphysics by promoting the formation of ice, which in turn may alter the precipitation efficiency of clouds (Creamean et al., 2013). The range of cloud changes from modeling studies highlights the poor understanding of the influence of the composition and size of particles on the interactions between atmospheric chemistry, climate, and weather. This weak understanding translates into high uncertainty in the consequences of geoengineering through deliberate introduction of particles into marine clouds.
Changes in climate since the pre-industrial era have been linked to changes in the frequency of extreme weather events, which have substantial societal costs. Robust statistical formulations to describe accurately attributable risk due to human perturbation of the climate system for a single observed extreme weather event are difficult, even when the studied event is unprecedented in the historical record (Hansen et al., 2014). However, it is possible to estimate the increased likelihood of the occurrence of extreme events, such as hurricanes (Webster et al., 2005), heat waves (Stott et al., 2004), heavy rainfall (Lau and Kim, 2006; Lau et al., 2006), tropical and landfall typhoons (Wang et al., 2015b), floods (Milly et al., 2002), and drought (Pongracz et al., 2014), due to the changing composition of the atmosphere (Stott, 2015). Probabilities for heat waves in western North America and central Asia, cold outbreaks in eastern North America, and droughts in central North America, Europe, and central Asia are predicted to increase in the future (Screen and Simmonds, 2014), and the societal costs for some of these events can be quite high. It was estimated that there were more than 70,000 additional deaths in Europe in 2003, compared to a reference period of the 5 years prior, due to the severe heat wave that year (Robine et al., 2008). The changing atmosphere likely played a role in the severe heat wave in the southwestern United States in 2013 that caused hospitalizations, cancellation of flights, and deadly wildfires (Shiogama et al., 2014).
4.2 ATMOSPHERIC CHEMISTRY AFFECTS HUMAN HEALTH
Many trace gases and particles emitted to or produced in the atmosphere impact human health. Air pollution has been reported to negatively impact human physical and mental health for at least 800 years (Evelyn, 1661; Finlayson-Pitts and Pitts, 2000; Goodhill, 1971), with one of the most dramatic episodes being an estimated 13,500 excess deaths in the London smog of 1952 (see Figure 4.3). As indicated in Chapter 1, one out of eight deaths on a global basis is currently attributed to outdoor and indoor air pollution combined (WHO, 2014), and a recent study estimates 3.3 million premature deaths annually due to ambient air pollution worldwide (Lelieveld et al., 2015). The total cost due to health effects of some air pollutants in the United States is estimated to be between $71–$277 billion each year, the majority of that resulting from detrimental health impacts and premature deaths (Muller and Mendelsohn, 2007). Children are particularly susceptible to the effects of air pollution due to increased dosage through more rapid breathing and spending more time outdoors; they also have more susceptible metabolic, immune, and lung systems (EPA, 2013; Office of Environmental Health Hazard Assessment, 2015; WHO, 2005).

The U.S. Environmental Protection Agency has designated a list of hazardous air pollutants found indoors and outdoors that are known to be carcinogenic or cause other serious health effects ranging from eye and skin irritation to impacts on reproductive, neurological, and respiratory systems (see Figure 4.4).2 Three gaseous criteria pollutants3—carbon monoxide, nitrogen oxides, and sulfur dioxide—which are directly emitted to the atmosphere (particularly from fossil fuel combustion), cause a range of detrimental health impacts. These include asthma symptoms, cough, cardiovascular, cardiac, and respiratory stress and mortality (Anderson et al., 2007; WHO, 2013). Ozone, which is created through the reactions of nitrogen oxides and volatile
___________________
2 EPA Health Effects Notebook for Hazardous Air Pollutants: http://www3.epa.gov/ttn/atw/hlthef/hapindex.html.
3 U.S. EPA has designated six common pollutants as “criteria” pollutants for which national air quality standards are set to protect people and the environment: ground-level ozone, particle pollution, lead, sulfur dioxide, carbon monoxide, and nitrogen dioxide.
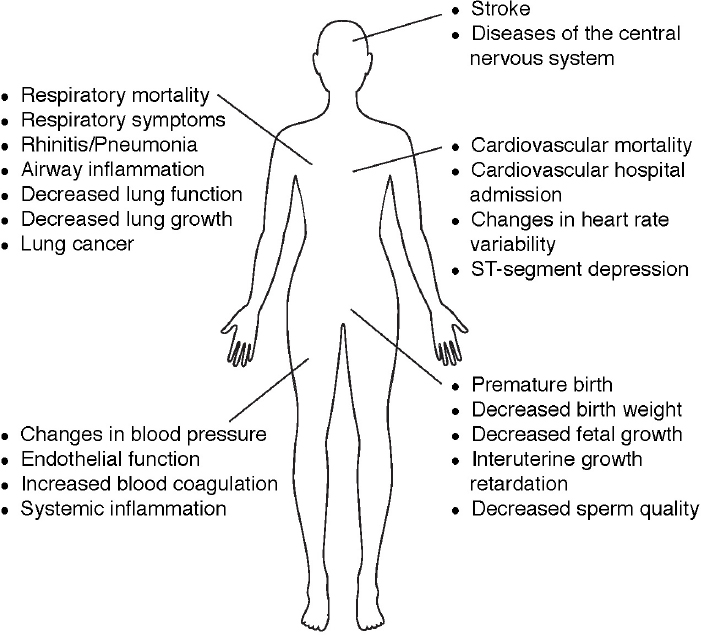
organic compounds in the presence of sunlight (see Chapter 2), also has well-known adverse health impacts (EPA, 2013), including premature cardiac and respiratory mortality (e.g., Jerrett et al., 2009; Lipfert et al., 2006), increased hospital visits, asthma incidence and severity, decreased lung development in children (Gauderman, 2006; Office of Environmental Health Hazard Assessment, 2015; WHO, 2005, 2013), and possible impacts on reproductive (Olsson et al., 2013) and cognitive health (Chen and Schwartz, 2009; EPA, 2009).
Airborne particulate matter (PM) is also a criteria pollutant. Particles have a wide range of sizes and chemical composition depending on sources and removal processes from the atmosphere. Particulate matter has been linked to a range of health impacts, including asthma, cardiopulmonary disease, lung cancer, reproductive health problems (WHO, 2005), and increased mortality (Pope and Dockery, 2006; Pope et al., 2013; see Box 4.2). Recent studies suggest an association of atmospheric particles with neuro--
degenerative diseases such as Alzheimer’s and Parkinson’s, strokes, autism, and anxiety (Brauer, 2015; Calderon-Garciduenas and Torres-Jardon, 2015; Power et al., 2015; von Ehrenstein et al., 2014). It has been suggested that ultrafine particles (<100 nm in diameter) may have disproportionate impacts on health (Araujo, 2011a,b). Particles can have particularly adverse effects on children, including deficits in lung function development (Gauderman, 2006). Aggravation and increased incidence of asthma and airway inflammation and an increased risk of bronchitis and wheezing in children have been documented (Chen et al., 2015; WHO, 2005), and effects on neural development in children have also been suggested (Calderon-Garciduenas et al., 2014). Bioparticles also play a major role in the transport of disease and have been linked with indoor health effects (Douwes et al., 2003; Fernstrom and Goldblatt, 2013). Recent studies have shown an association between atmospheric wind patterns and Kawasaki disease in three countries (Rodo et al., 2011). Thus, understanding the chemistry of atmospheric particles is central to understanding health effects.
A number of mechanisms have been proposed to link exposure to the health effects of particles. Reactive oxygen species (ROS) are thought to play a key role in inflammation, pulmonary oxidative stress, vascular dysfunction, atherosclerosis and lung cancer (Araujo, 2011a; Hamra et al., 2014). ROS include free radicals such as OH, HO2, superoxide anion, peroxynitrites, H2O2, and organic peroxides and can appear in both the gas and aerosol phases. The toxicity and health effects of particles thus also depend to a large and under-appreciated extent on atmospheric chemical transformations that depend strongly on aging processes in the atmosphere.
Exposures to higher levels of air pollutants can occur indoors, where people typically spend approximately 90 percent of their time (Nazaroff and Goldstein, 2015). A particular problem in the developing world is the emissions of gases and particles from biomass burning for cooking and heating (Lelieveld et al., 2015; Smith et al., 2014).
It is difficult to separate the effects of specific gases and particles on human health, and there are likely synergistic effects between gases and particles; in addition, some compounds are found in and move between the two phases. Further complexity arises from the fact that many of the gases and particles that cause significant health effects are not directly emitted, but rather are formed via atmospheric chemical reactions (Finlayson-Pitts and Pitts, 2000; Pandis and Seinfeld, 2006; Pöschl, 2005). Without knowledge of the nature and chemistry of gases and particles as well as how they are emitted, formed, and altered in the atmosphere from the atmospheric chemistry research community, reliable guidance for societal choices to minimize impacts on human health is not possible. These mechanisms place atmospheric chemistry at the fulcrum of connecting exposure to health effects and highlight the potential contributions of the atmospheric chemistry community to human health research.
4.3 ATMOSPHERIC CHEMISTRY INTERACTS WITH NATURAL AND MANAGED ECOSYSTEMS
Terrestrial and marine ecosystems support society through the production of food and energy, and play an important socio-cultural role in how we view our world. Ecosystems are unique, dynamic components of the Earth system that continually respond to changing conditions, including those in the atmosphere. Ecosystems rely on the atmosphere for uptake of life-sustaining elements such as carbon, oxygen, nitrogen, and trace elements through deposition processes and in turn contribute to the atmosphere and impact climate through the emission of gases and particles (Behrenfeld et al., 2006; Maher et al., 2010; Swap et al., 1992). As a result, changes in atmospheric chemistry affect key ecosystem services, including the health of forests, agricultural lands, and oceans. The interaction with atmospheric chemistry can influence the value of ecosystem services (de Groot et al., 2012) through the reliance on ecosystems for food production, the maintenance of wildlife and their habitats for biodiversity and sustainability, and the aesthetic effects of particulate matter on visibility in public lands (NRC, 1993).
Ecosystems are often described as natural or managed by human activity. Natural ecosystems support all life on Earth. Managed ecosystems such as croplands are manipulated by humans to increase and modify food and energy production, and they provide food, energy, and other essential and beneficial services (Millennium Ecosystem Assessment, 2005). Many gas and particle phase atmospheric compounds deposit from the atmosphere to the Earth’s surface through wet or dry deposition. Ecosystem damage, for example through deposition of acidic compounds formed via fossil fuel combustion (e.g., Section 3.5) or the clearing of land for agriculture, can trigger a cascade of ecological effects through particle and trace gas exchange and atmospheric chemical reactions. Because natural and managed ecosystems are so closely tied to the atmosphere, atmospheric chemical reactions and chemical composition play a central role in understanding how ecosystems respond to global change and affect climate change. Therefore, there is a crucial need to understand how terrestrial and marine ecosystems contribute to, and are controlled by, the chemistry of the atmosphere.
While ecosystem productivity is dominantly a function of climate conditions and CO2 concentrations, chemical reactions in the atmosphere create compounds that also influence ecosystem productivity. The exchange of these compounds (e.g., reactive nitrogen, organic compounds, and other inorganic compounds) between ecosystems and the atmosphere can potentially harm or benefit ecosystems, depending on the chemical compound. For example, oxidants such as ground-level ozone damage plant tissue and slow growth and other functions (Felzer et al., 2007); see Figure 4.5.

Ozone can be taken up by plants, reducing the productivity of natural (Chappelka and Samuelson, 1998) and managed ecosystems such as agriculture (Booker et al., 2009; Fiscus et al., 2005). This can have significant impacts on crop production: for example, one study estimated that global cereal crop losses due to exposure to ozone in 2000 ranged from $11–$18 billion (Avnery et al., 2011).
Nutrients such as nitrogen and phosphorus deposited to the surface can benefit ecosystems, unless anthropogenic pollution drives an excess that may be harmful (e.g., acid rain deposition, eutrophication of aquatic systems). Nitrogen deposition can also alter ecosystem productivity by affecting biological diversity (Bobbink et al., 1998), nutrient cycling, and the rate at which plants grow (Vitousek et al., 1997), all of which have substantial associated costs (Jones et al., 2014). A significant fraction of the global anthropogenic emissions of atmospheric nitrogen deposits on the ocean and
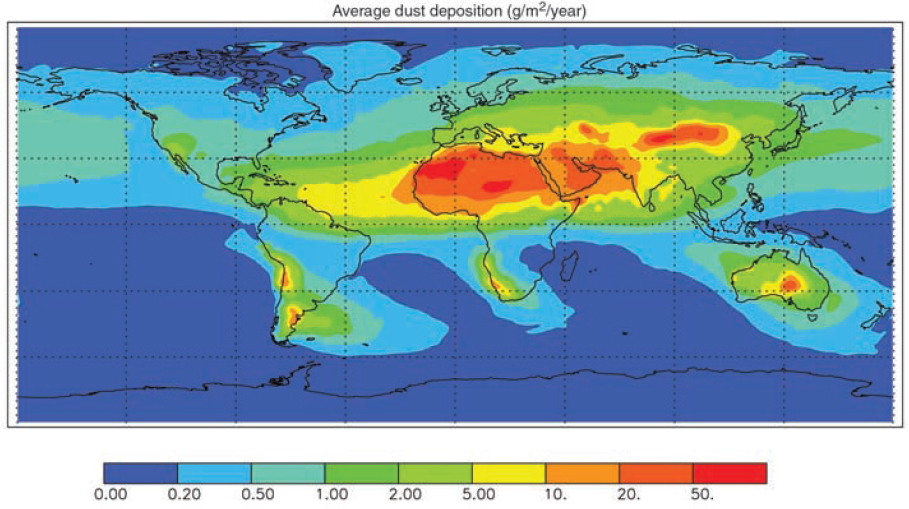
alters new marine biological growth (Duce et al., 2008; Krishnamurthy et al., 2007), and the atmospheric deposition of mineral dust is an important source of the nutrients iron and phosphorus in many open ocean regions (Jickells et al., 2005; Mahowald et al., 2005, 2008; see Figure 4.6). Toxic compounds, e.g., mercury, are known to deposit to terrestrial and marine ecosystems (Landis and Keeler, 2002). While much of the deposition of heavy metals such as mercury, lead, and cadmium does not directly affect productivity, it may influence the quality of food produced. Long-range transport of dust also plays a role in adversely affecting the health of coral reefs due to the deposition of soil-associated fungal spores (Shinn et al., 2000).
To add complexity, many ecosystems play an active role in atmospheric chemical processes through their emissions of gases and particles, which can impact both tropospheric (e.g., volatile organic compounds [VOCs]) and stratospheric (e.g., nitrous oxide) composition and chemistry. Understanding this complex two-way exchange of constituents between the atmospheric reservoir and ecosystems is necessary to determine how atmospheric chemistry affects, and is affected by, ecosystems. As an example of this complexity, organic forms of carbon and nitrogen that are oxidized,
reactive, and thermally labile are thought to be important in understanding the fluxes of carbon and nitrogen at the biosphere (soil, plant, ocean)/atmosphere interface. Measurement of these species is often difficult, but necessary to close the reactive carbon and nitrogen budgets.
In many regions of the world, atmospheric composition and chemistry are driven by terrestrial and marine ecosystems. Terrestrial vegetation emits a significant amount of VOCs (Guenther et al., 1995). Biologically produced VOCs are key contributors to tropospheric ozone formation and, depending on the oxidation pathway, VOC oxidation products form or grow secondary organic aerosol particles. Ecosystems also produce many types of bioparticles, ranging from plant debris, pollen, fungi, and bacteria that can oxide (NO) and these emissions comprise 15–25 percent of the industrial era NOx in the global atmosphere (Holland et al., 1999). Terrestrial and marine ecosystems also emit nitrous oxide, an important greenhouse gas. Concentrations of nitrous oxide have been increasing in the industrial era due to fertilizer applications in managed ecosystems, and food production is estimated to account for 80 percent of the increase in atmospheric nitrous oxide (Ciais et al., 2013). The burning of terrestrial vegetation, either occurring naturally or ignited by humans, can inject large quantities of NOx, carbon monoxide, and aerosol particles to the atmosphere, and these also play an important role in climate and air quality.
Similar to terrestrial ecosystems, the ocean is an important natural source of many trace gases and particles that can influence atmospheric chemistry. Marine emissions include greenhouse gases (e.g., nitrous oxide), halocarbons, VOCs, sea spray, bioparticles, primary organic carbon aerosol particles, and sulfur species such as dimethyl sulfide and organosulfates (Quinn et al., 2015). The impacts of ocean acidification on the processes that control the production of these species are potentially important but still uncertain (Gruber, 2011). It is generally believed that marine ecosystem emissions are less than terrestrial emissions, however far fewer measurements of reactive organic species have been made over the oceans so the uncertainties in marine emissions remain quite large (Carpenter and Nightingale, 2015; Shaw et al., 2010). Because these emissions occur in remote regions that might not be strongly influenced by terrestrial emissions, they can have an important impact on gas-phase chemistry and aerosol formation in these regions.
Ecosystems are increasingly affected by numerous perturbations and stressors, including land use changes, water pollution, and climate change. Habitat destruction, competition from invasive species, unsustainable exploitation of species for economic gain, and climate change have already increased extinction rates and affected species ranges and migratory patterns (Barnosky et al., 2012; Lenton, 2011; NRC, 2013).
Because the impact of air pollution and deposition of nutrients and contaminants can be substantial, improved understanding of the interactions of atmospheric chemistry on natural and managed ecosystems is urgently needed.
4.4 SOCIETAL CHOICES AND IMPACTS
Societal needs involving health and welfare, economic and intellectual development, and many other aspects of lifestyle (beyond just food, clothing, and shelter) benefit from basic science, including atmospheric chemistry. Actions taken to achieve certain benefits have repercussions on the environment and often result in changes in atmospheric composition. Atmospheric chemistry research can benefit societal development by attributing changes to specific aspects of development. Optimally, this basic scientific knowledge can point the way to alternatives and less damaging technologies. Following are several areas where the relationship between atmospheric chemistry and societal choices and impacts are particularly important
National Security
Threats to a nation’s security that are potentially destabilizing can impact the U.S. military or diplomatic missions and thus present a concern for U.S. national security. The U.S. governmental entities interested in national security include the military, intelligence, international development, and diplomatic corps. Their concerns are global and include, inter alia, food and water shortages, pandemic disease, refugees, clashes over resources, and devastation by natural disasters. Most of these crises involve humanitarian emergencies with a strong health and welfare component. They may be exacerbated by slow environmental degradation from poor air or water quality, or they may involve destruction of societal function and governance by natural disasters such as earthquakes, tsunamis, volcanoes, floods, or hurricanes. A changing atmosphere not only directly affects human health, agricultural productivity, and the state of the environment, but is also a substantial contributor to climate change. Climate change may be shifting water resources as well as the frequency and strength of extreme weather such as hurricanes, storm surges, and heat waves. The rapid changes in the Arctic are an example where climate change presents a challenge to U.S. national security (see Box 4.3). All of these changes can have important effects on national and global stability and security. As discussed above (see Chapter 4.1), research in atmospheric chemistry can improve the fidelity of climate models, which can improve the ability to anticipate these changes.
The science connecting national security and climate change is a growing research area (e.g., Brown, 1989; Busby, 2007, 2008; Smith, 2007). For example, a university-based program in Climate Change and African Political Stability was initiated in 2009 with a Department of Defense grant. The Center for Naval Analyses’ Military Advisory Board of retired generals and admirals continues to point out the increasing risks for national security (CNA MAB, 2014). The Center for Climate and Security (also including ex-military and government leaders) publishes reports like The Arab Spring and Climate Change (Werrell and Femia, 2013), including essays such as “Chinese Drought, Wheat, and the Egyptian Uprising: How a Localized Hazard Became Globalized.”
The U.S. government’s recent interest in the significance of future climate change on national security began with the setting of new priorities for the intelligence community that specifically included climate change. To highlight this, a recent National Intelligence Assessment relied on the Intergovernmental Panel on Climate Change 4th Assessment Report as its primary source for climate science, which it then linked to the security aspects (Fingar, 2008). The importance of climate change for national security is treated in a number of recent reports and assessments, including a White House summary, “The National Security Implications of a Changing Climate” (White House, 2015). Similarly, the National Intelligence Council (NIC, 2015) acts as a source of reports on water availability and climate. The Secretary of Defense recently released a Climate Change Adaptation Roadmap (DoD, 2014). There are a number of efforts to address these concerns. For example, the State Department initiated the Climate and Clean Air Coalition to Reduce Short-Lived Climate Pollutants4 as an effort to support international development goals as well as to gain international partners, and the EPA recently announced the Clean Power Plan, which establishes final emission guidelines for states to follow in developing plans to reduce CO2 emissions from existing fossil fuel-fired electric generating units. (However, this plan does not address other greenhouse gases such as nitrous oxide and methane.) All of these reports highlight the growing interest and concern of the U.S. government about the related issues of climate change and national security.
In addition to the climate-national security connection, atmospheric chemistry research is important for understanding the fates of chemical or radioactive contaminants on populations. For example, the dispersion of the Chernobyl fallout required accurate chemistry-transport modeling, and the chemical properties of warfare agents will determine their degradation and dispersion. It is important that the best current knowledge of atmospheric science is included in assessments of complex issues of
___________________
national security, and as new research is identified that is vital to the understanding of national security, it is included as a central part of the atmospheric chemistry research portfolio.
Water Security
The availability of fresh water is central to human health and welfare, including food and energy production. In many regions of the world insufficient rainfall exists, which means irrigation must be used to water crops. Currently, 70 percent of global fresh water consumption is used for agriculture, and a 20 percent increase in water consumption for agriculture is expected by mid-century. Increased demand for fresh water for agriculture and livestock, together with other uses such as energy production, will add substantially to the current stress on nonrenewable groundwater sources. The water cycle is already changing due to changes in Earth’s climate driven by increasing greenhouse gas concentrations. For example, the amount of rain that falls during the most intense 1 percent of storms has increased by almost 20 percent over the past 50 years (USGCRP, 2009). Some regions of the world are in the midst of historic droughts. In 2015 alone, 10 weather and climate disaster events occurred with losses exceeding $1 billion each across the United States, including drought and flooding events.5
Atmospheric chemistry plays a key role in impacting the water cycle largely through serving as the conduit for the formation and transport of aerosol particles which act as cloud condensation and ice nuclei (CCN and IN) (see Figure 4.7). The dynamics of atmospheric transport, cloud formation, weather patterns, and precipitation processes, all play a role in controlling the delivery of fresh water to the Earth’s surface.
Through their ability to heat or cool the atmosphere and seed clouds, atmospheric aerosol particles have been shown to play a critical role in affecting the amount, type, and location where precipitation occurs (see Chapter 3.5). It has been suggested that in regions with high levels of pollution, the amount of precipitation can be reduced due to the presence of too many cloud nuclei that form drops small enough to inhibit rain formation (Rosenfeld et al., 2008). In contrast, certain aerosol particles can form ice in mixed phase clouds which can lead to increased amounts of precipitation (Ault et al., 2011; Muhlbauer and Lohmann, 2009). Increases in particles that act as ice nuclei may have a proportionally larger effect on precipitation than corresponding changes in cloud condensation nuclei (Rosenfeld et al., 2008), although this largely depends on the thermodynamic and dynamic state of the clouds in question. Only a small portion
___________________
5 Billion-Dollar Weather and Climate Disasters: http://www.ncdc.noaa.gov/billions/events.
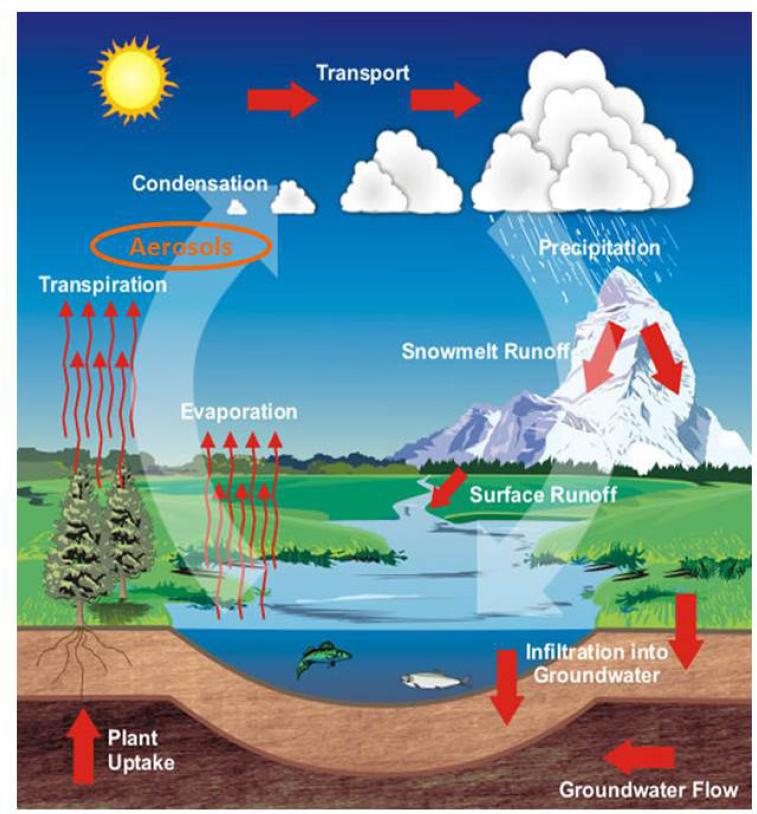
of the available moisture in clouds is transformed into precipitation that reaches the surface (Bruintjes, 1999). Thus, depending on their sources and composition, aerosol particles can lead to a re-distribution of water resources with more extreme events such as flooding and drought. This shift translates into less water in regions where reservoirs can capture the water with more runoff in other regions which lead to poor water quality. The overall effects of aerosol particles on precipitation formation and redistribution are highly uncertain (Huntington, 2006), highlighting the need for improved understanding of the sources and properties of aerosol particles that impact precipitation (see Figure 4.8).
Energy and Industry
Energy and industry are a core part of societal growth and development. The world’s energy demands have continually evolved with expanding population and advancing technology. Energy sources, from biofuel to fossil fuels to alternative energies, have broadened as the global energy demand has expanded. The exploration and development of energy sources usually creates waste, both in the atmosphere and in water, that degrades the human and natural environment. Further, the generation of energy, particularly from fossil fuels, has been notoriously polluting, thus inspiring development of systems that are designed to result in clean air and clean water (e.g., U.S. Clean Air Act of 1963 [P.L. 88-206, 77 Stat. 392]; U.S. Clean Water Act of 1972 [P.L. 92-500, 86 Stat. 816]). Since the Los Angeles experience with photochemical smog (Haagen-Smit, 1952; see Chapter 2), society has come to realize that the products of combustion, as
well as solvents and other chemicals released by industry, can rapidly pollute the air, making it unhealthy, degrading visibility, and in general reducing urban and even rural amenities.
Fortunately, an understanding of energy and industrial emissions combined with research on atmospheric chemical reactions and removal processes have enabled society to clean up the air in many urban areas (see Chapter 2). Increased future demands for energy and the associated industrial activities will result in increased requirements for the control of emissions of gases and particles from these processes.
The buildup of CO2 and methane from fossil fuels is a major contributor to anthropogenic climate change (IPCC, 2014). For example, Paulik et al. (2015) have shown increased emissions of methane and other gases such as ethane (see Figure 3.1) as well as increased levels of atmospheric polycyclic aromatic hydrocarbons in the vicinity of hydraulic fracturing (“fracking”) activities. As discussed in Chapter 3.1 research in atmospheric chemistry can improve the understanding of trends in emissions.
The chemical industry has developed elegant technologies like the synthetic chlorofluorocarbons that appeared at first benign but were later tied to stratospheric ozone loss with associated increases in skin cancer, and more recently, climate change (IPCC, 2014; Seinfeld and Pandis, 2006). Research in atmospheric chemistry must keep pace with the magnitude of development and new technologies in the energy sector and other industrial sectors in order to best protect environmental services.
Environmental Justice
Environmental justice is defined by the U.S. Environmental Protection Agency as the “fair treatment and meaningful involvement of all people regardless of race, color, national origin, or income with respect to the development, implementation, and enforcement of environmental laws, regulations, and policies.”6 Systematic integration of perspectives from all communities will enable management of the Earth system in a way that is beneficial for all its inhabitants. A closer linkage of atmospheric chemistry research to those using the information and directly affected by the pollution will strengthen the research program and make it more useful to policy makers and communities. Some important principles follow.
The issue of fair treatment is based on the fact that societal impacts of changing atmospheric composition like climate change are not distributed equally. Studies of
___________________
6 Environmental Justice: https://www3.epa.gov/environmentaljustice.
these distributional effects find that negative environmental impacts tend to disproportionately fall on low-income populations (Brown, 1995; Jerrett et al., 2001; Morello-Frosch and Jesdale, 2006). Environmental justice studies examine the physical, social, economic, and equity implications of impact distribution, rather than just average effects across an entire population. Atmospheric chemistry research can contribute to a greater understanding of environmental justice by employing low-cost, widespread monitoring and high-resolution modeling to characterize the spatial distribution of pollution and its impacts, and by integrating this analysis with socioeconomic characterization. The unequal distribution of poor air quality should be a key part of assessments of future development and climate change.
The issue of meaningful involvement is based on a growing recognition that involvement and integration of local knowledge and perspectives is a key component of sustainable development (e.g., Cash et al., 2003). Some approaches to broadening perspectives are termed community-based participatory research (Minkler et al., 2010) or working with indigenous knowledge (Grenier, 1998). These approaches seek to identify and integrate principles and practices embedded in local context and culture, rather than those defined more globally by the research community. For example, communities may be aware of impacts of degraded air quality on populations and ecosystems, or how local actions alter emissions or exposure, in ways not apparent to the research community (Gonzalez et al., 2011; Nasir et al., 2014). Community engagement may provide key knowledge about indoor air pollution, which is affected by individual or community practices of smoking, cooking, and related activities. Indigenous knowledge can also describe the conditions and practices that govern tropical deforestation and biomass burning. Integration of scientific and community knowledge can provide improvements in both communities’ quality of life and in scientific understanding of atmospheric chemistry.
To understand community-scale pollution, impact, and response, and hence facilitate advances, key information is found in nonacademic knowledge systems. An open question is—how can such collaborations be fostered? While collaborative research is often motivated by knowledge generation and scientific reputation, such benefits may not be attractive to participants outside the traditional research community. Guidance for ensuring collaborations are beneficial to both sides is needed. Furthermore, innovation and advances may be found in disciplines such as psychology, sociology, and ethics, which are further removed from atmospheric chemistry than typical partnerships such as Earth system modeling.
Sustainable Development
Development almost always comes with industrialization, transportation, and the expansion of agriculture that pollute air and water, bringing ill health and poor living conditions. An understanding of the chemistry of this pollution has proven essential to designing healthy and sustainable communities in a changing world. Sustainable development thus involves atmospheric chemistry.
In September 2015, the United Nations released a list of 17 Sustainable Development Goals (SDGs), each supported by quantitative and qualitative targets to be met by 2030 (United Nations, 2015a). The primary obstacles to attaining the SDGs are implementation, funding, and commitment rather than a lack of research. Nevertheless, many of the goals were developed from intricate geoscience knowledge in general, and atmospheric chemistry in particular. SDGs in which atmospheric chemistry plays a role are summarized in Box 4.4. The specific contributions of atmospheric chemistry research to each goal are discussed in the text below, where the targets relevant to both atmospheric chemistry and the overarching Sustainable Development Goals are identified.
Atmospheric chemistry research has an important role to play in sustainable development through minimizing environmental risks from exposure, preparing for extreme events, demonstrating lower-impact societal pathways and through international cooperation.
- Minimizing environmental risks from exposures: Understanding the sources, fate, and transport of chemicals in the atmosphere can lead to better identification of hazards and management of releases (Goal 12) and a reduction in deaths and illnesses from hazardous chemicals and air pollution (Goal 3). Indoor air pollution is a major cause of preventable deaths of children under 5 years old (Goal 3), a hazard that will be reduced by providing modern energy (Goal 7).
- Preparing for extreme events: The SDGs call for reducing the exposure of vulnerable populations to extreme events, which is often related to poverty (Goal 1), developing food production systems resilient under climate change (Goal 2), and strengthening resilience to climate-related hazards (Goal 13). They also promote managing national and global health risks with early-warning systems (Goal 3). Atmospheric chemistry, as part of Earth system models, can provide predictive understanding of how energy consumption and agricultural activity affect the pollution of the atmosphere, land, coastal and blue waters.
- Demonstrating lower-impact societal pathways: The SDGs recommend several pathways that will affect human environments and the Earth system. These include better air quality and safer public transport in cities (Goal 11), where concentrated pollutants are now generated. They also include greater emphasis on renewable energy and energy efficiency (Goal 7), and industry retrofits to achieve efficiency (Goal 9). These trends can be included in future scenarios of air pollutants and greenhouse gases to demonstrate environmental costs and benefits of each course of action. Scientific characterization of the Earth system is part of ensuring “that people everywhere have the relevant information and awareness for sustainable development and lifestyles in harmony with nature” (Goal 12).
- International cooperation: In order for scientific research to proceed in tandem with and be useful for sustainable development, scientific partnerships must cross national boundaries, and scientists, practitioners, and policy makers must integrate their disparate forms of knowledge. Participatory decision making (Goal 16), enhanced knowledge sharing, and capacity building (Goal 17) are all part of cooperative international initiatives such as Future Earth.
The ambitious goals charted in “Transforming Our World—The 2030 Agenda for Sustainable Development” require social, economic, and political support. Progress and cooperation in science—including atmospheric chemistry—are also integral to success.
4.5 CONCLUSION
Human choices are affecting the chemistry of the atmosphere now and are certain to do so in the future. As a scientific community, we are beginning to understand the impacts that changes in atmospheric chemistry have on pressing societal issues such as climate and weather, human health, and natural and managed ecosystems. These impacts have resulted not only in environmental degradation, but also the loss of billions of dollars to the global economy. As society makes choices that will affect the atmospheric environment, preparing for future quality of life needs to include a predictive framework that is sufficiently robust to generate predictions about the environmental impacts of those choices—whether local, regional, or global. In turn, those predictions need to be based on fundamental knowledge of atmospheric chemistry. Refining such an effective predictive capability for tomorrow is an ultimate goal of atmospheric chemistry research.
This page intentionally left blank.




























