How do the microbiomes of the different indoor environments in which humans spend time for working, living, learning, and playing impact human health and well-being? What building conditions support microbial communities that benefit or harm human health and well-being? If most microorganisms do not infect humans, do those that thrive in indoor environments influence human health for good or bad, and if so, by what mechanisms? These questions are among those that motivate the study of microbiomes of the built environment. This chapter begins by laying the groundwork for understanding how microorganisms found in buildings may influence health. The chapter then addresses, in turn, infection transmission in indoor environments, noninfectious health outcomes associated with indoor microorganisms, and potential benefits of microbial exposures. The chapter concludes with summary observations and a discussion of knowledge gaps in the area of health impacts of built environment microbial exposures. Chapters 3–5 examine how building characteristics and occupants shape the indoor microbiome (see Chapter 3), tools that can be used in research on microbiomes of the built environment (see Chapter 4), and potential interventions that can alter these microbiomes (see Chapter 5).
INFLUENCE OF BUILDING MICROBIOMES ON HUMAN HEALTH: ECOLOGIC AND BIOLOGIC PLAUSIBILITY
Several considerations support the plausibility of the influence of building microbiomes on human health. First, in developed areas of the world, indoor environments are the primary ecosystems inhabited by people. Second, the environments people inhabit may influence the human microbiome, which may in turn impact human health. For example, microorganisms present in the environment may proliferate in niche-specific ecosystems of the human host—such as in airways, the gut, and on skin. Third, a wide array of microbial components and characteristics are known to impact
human health. Finally, a number of sources of microorganisms within indoor environments impact human health.
Implications of Building Microbiomes for the Diversity of the Human Microbiome
In developed areas of the world, humans are born and spend the vast majority of their lives indoors, which may limit the diversity of microorganisms to which they are exposed. A building’s envelope (foundation, walls, windows, and roofs) separates the indoor and outdoor environments, thus reducing exposure to microorganisms that thrive outdoors and potentially increasing exposure to organisms that thrive indoors.
The diversity of the microbiomes of the built environments in which humans live may impact the microbiomes of their bodies. Studies have shown that humans who spend significant time outdoors or live in dwellings with more open building envelope designs that result in high levels of unfiltered or minimally filtered air exchange with the outdoors have more diverse microbiomes relative to those who live in dwellings with less open designs (Clemente et al., 2015; Hanski et al., 2012).
The extent to which the indoor microbiome contributes to this diversity or lack thereof is not well understood, however. It has also been suggested that exposure to reduced microbial (especially bacterial) diversity may be less a function of the microbial content of buildings than a side effect of the modern human diet, which is less diverse than that of our ancestors: it varies little with the seasons; may be affected by the use of antibiotics; and may select for a limited number of human microbial taxa, particularly in the gut (Yatsunenko et al., 2012). This diversity may benefit human health as the microbiomes to which humans are exposed may be important for immune development and the processing of nutrients in the gut, which may not function as well when gut microbial communities change. People in economically disadvantaged and less developed societies who spend more time outdoors can have higher infectious disease risk and higher infant mortality (Clemente et al., 2015; Hanski et al., 2012). However, this may be due more to their health when exposed to infectious agents than to the diversity of the microorganisms to which they are exposed; as noted, some evidence suggests beneficial immune system effects from exposure to diverse microbes (see the section on “Beneficial Effects of Microbes”). Thus, the relative lack of microbial diversity may have positive or adverse effects on human physiologic and immune responses and health and may in turn influence risk of chronic noninfectious symptoms and diseases. By separating themselves from the outdoors, humans may have eroded the diversity of their own, as well as their environmental, microbiomes.
Environmental Influences on the Human Microbiome
Mounting evidence indicates that the human microbiome is influenced by the environment and that it is integral to human development. One of the most studied influences is the transmission of particular infectious microorganisms. For example, fomites are surfaces or objects on which microorganisms can deposit and that allow for transmission to a host (Julian, 2010). Fomites are well documented in the spread of infectious disease, and there is research associated with human exposure to indoor pathogens (Dick et al., 1987; Wong et al., 2010).
Another known influence of the environmental microbiome on the human microbiome is the process of birth. Each individual’s microbiome is acquired both in utero and from the environment at birth. Babies delivered vaginally and by Cesarean delivery show differences in their microbiome composition (Bokulich et al., 2016; Hill et al., 2017; Rutayisire et al., 2016). Yet the human microbiome does not fully stabilize to adult patterns until 2–3 years of age (Dethlefsen et al., 2006). New technologies and bioinformatics techniques for genomic analysis of microbial DNA extracted from environmental samples are providing insights in this area not previously possible (see Box 2-1). One topic of interest is the nature of nonpathogenic interactions between indoor and human microbiomes.
Neonatal studies provide evidence that the microbes from the environment that are of human origin may influence the human microbiome. Other studies also have provided evidence that dogs and humans have bacteria in common (Song et al., 2013). Yet there is no concrete evidence that the human microbiome can be colonized by bacteria that originate from a building. For example, an in-depth study of the indoor and human microbiomes in which seven families were followed over 6 weeks indicated that the majority of the building microbiome measurable on home surfaces originated from the occupants. This study also found that the building microbiome did not appear to influence the occupants’ skin microbial structure or composition (Lax et al., 2014). Further research will be needed to understand the reproducibility and generalizability of the findings of this study, and how temperature, humidity, building materials, and the integrity of the building structure impact the interchange between the indoor and human bacterial microbiomes.
Microbial Components Associated with Human Health Effects
Microorganisms can impact human health through a variety of mechanisms. The dominant microbial components linked to human health include pathogen-associated molecular patterns (PAMPs). PAMPs are molecules such as endotoxin and lipopolysaccharide (LPS) (a component of bacterial cell
membranes), flagellin (from bacteria), and (1–3)-β-D glucans (also referred to as triple helical glucan, from fungi wall membranes). These molecules are associated with groups of microbes (bacteria or fungi) that may influence human innate immune system responses, interact with airway epithelial cell or irritant receptors (Lambrecht and Hammad, 2013, 2014), or have toxic effects. For example, many indoor fungi produce metabolites that can induce respiratory or systemic toxicity upon exposure (Kuhn and Ghannoum, 2003).
Mycotoxins, products of fungal metabolism (Robbins et al., 2000), can also provoke physiological responses. These fungal metabolites have been shown in mechanistic, toxicological studies to be relevant to human health, and more than 300 mycotoxins are potentially harmful with respect to food contamination (Alshannaq and Yu, 2017). This situation is much less clear for inhalation exposure to indoor-relevant mycotoxins. Only a handful of studies support a role of these compounds in inflammatory processes when combined with exposure to other microbial components (endotoxin, glucans) (Korkalainen et al., 2017). The potential airway epithelial toxicity of microbial components may be increased by the presence of tobacco smoke or other factors that disturb the epithelial barriers, tight junctions, antimicrobial production, or mucocilliary ability to clear bacteria (Lambrecht and Hammad, 2013, 2014).
A large respiratory and allergy literature that has evolved over two decades suggests complex positive, as well as negative, associations of various PAMPs with allergy and respiratory outcomes. In the observational epidemiologic literature, the directionality (whether the risk or protective factors) and the magnitude of associations with microbially produced molecules such as LPS/endotoxin appear to be dependent on dose, human body compartment (e.g., airway, gut), host, and stage of life (Perzanowski et al., 2006; Sordillo et al., 2010). Recent experimental literature also provides supporting evidence that PAMPs and other fungal (e.g., chitin) (Mohapatra et al., 2016; O’Dea et al., 2014) and bacterial components may “train” innate immune responses, with downstream effects on the body’s ability to deal with either infection or allergic responses. While experimental models find direct effects of bacterial or fungal components (e.g., endotoxin or glucans), more recent observational birth cohort studies suggest that endotoxin and other PAMPs may be markers for complex communities of environmental bacteria and fungi, many of which have previously not been associated with disease (Manor et al., 2014).
Sources of Indoor Microbiomes That Are Relevant to Human Health
Recent observations suggest that occupants and outdoor microbes entering buildings through ventilation and tracked in through dust are the dominant origin of indoor environmental bacteria, particularly those that can be airborne (Adams et al., 2015; Prussin et al., 2015). Occupants contributing to the indoor microbiome include humans and nonhuman occupants, such as rodents and cockroaches, as well as pets, which are sources of bacteria and therefore can be direct sources of bacterial PAMPs (Thorne, 2015). In farm studies, likely sources for indoor PAMPs have included animal feed and farm animals. In urban environments, LPS/endotoxin sources include not only pets but also associations with moisture, such as that due to concrete basements, humidifiers (Park et al., 2001), and water damage in situations less extreme than flood conditions. Endotoxin on dirt and decaying plant material can be tracked into a house by its inhabitants. On the other hand, the relative contribution of endotoxin- or microbe-containing outdoor airborne particles to the suite of indoor microbial components (Hanson et al., 2016; Manzano-León, 2013) is not well understood. Although not causal or definitive, there is evidence that LPS/endotoxin exposures may be protective against the development of allergies in rural (Thorne, 2015) and U.S. urban environments (Park et al., 2001).
Sources of fungi indoors vary. Typically, in non-water-damaged buildings, fungi enter a building through leakage in the building envelope and through ventilation systems, are carried indoors by occupants, or may be brought indoors in association with building materials. The growth of fungi
is generally moisture dependent. In cases of extreme water damage—as in flooding in New Orleans, Louisiana (Mitchell et al., 2012); in Cedar Rapids, Iowa (Hoppe et al., 2012); or in Boulder, Colorado (Emerson et al., 2015)—high levels of fungi not originating from building occupants have been measured on building surfaces and in air. Even in non-water-damaged buildings, fungi can grow in or on building materials when sufficient moisture is present (Adan and Samson, 2011; Macher et al., 2017), and their growth is affected by such factors as the chemical composition of building materials.
Less is currently understood about the origins of viruses in the built environment (beyond transmission of specific pathogens). Evidence suggests, however, either enhanced sources of bacteria relative to viruses indoors or preferential removal of viruses as air penetrates indoors (Prussin et al., 2015). See Chapter 3 for greater detail.
TRANSMISSION OF INFECTION IN INDOOR ENVIRONMENTS
Much prior work investigating the impact of environmental conditions on the survival of microorganisms and their transmission to and between humans has focused on infectious organisms. As noted earlier, fomites (Julian, 2010) are well documented in the spread of infectious disease, and there is research documenting aerosol transmission of pathogens in different nonresidential indoor environments (Dick et al., 1987; Wong et al., 2010). This section examines the modes of transmission and complex, mixed exposures or coinfections for selected pathogens.
Modes of Transmission
Some viruses, bacteria, fungi, protozoa, and algae in the indoor environment have long been known to be pathogens, with the potential to cause infectious disease or allergic illness (Burge, 1980). Potentially infectious organisms can vary in terms of their transmissibility (ease of spread), their mode of spread indoors, and their virulence (a quantitative measure of pathogenicity or potential to cause disease). Subspecies within a bacterial species also may have highly divergent health effects, highlighting the need for detailed microbial information (Ponnusamy et al., 2016). Transmissibility, virulence, and mode of spread all influence the mode and effectiveness of infection control, a topic generally beyond the scope of this report. Recent interventions to reduce transmission of tuberculosis1 indoors, for example, have shown the benefit of advancing understand-
___________________
1 Tuberculosis is caused by infection with the bacterium Mycobacterium tuberculosis.
ing of transmissibility and drug resistance patterns (Barrera et al., 2015; Dharmadhikari et al., 2014; Mphaphlele et al., 2015; Nardell, 2016).
Table 2-1 summarizes modes of transmission for selected pathogens that have been associated with infection due to exposure in the indoor environment. The organisms listed in this table vary widely in their transmissibility and virulence. Influenza A, for example, can be challenging to contain, as person-to-person transmission can begin on contact with asymptomatic individuals or 24 hours before symptoms are present. Severe acute respiratory syndrome (SARS), a virus more virulent than influenza A, may be somewhat easier to contain, in that symptoms are likely to be present when the virus is transmissible. Viral pathogens such as influenza also may have multiple modes of transmission—for example, by droplet inhalation or by fomite contact—that may vary in their relative contribution to transmission according to such building conditions as indoor temperature and humidity (Koep et al., 2013). Although some of the organisms listed in Table 2-1 have infectious potential, some may be present in buildings but are not highly virulent and rarely cause infection in people with healthy lungs and healthy immune systems. For example, many immunocompetent people breathe in a variety of Aspergillus species (including Aspergillus fumigatis) indoors on a regular basis without becoming infected.
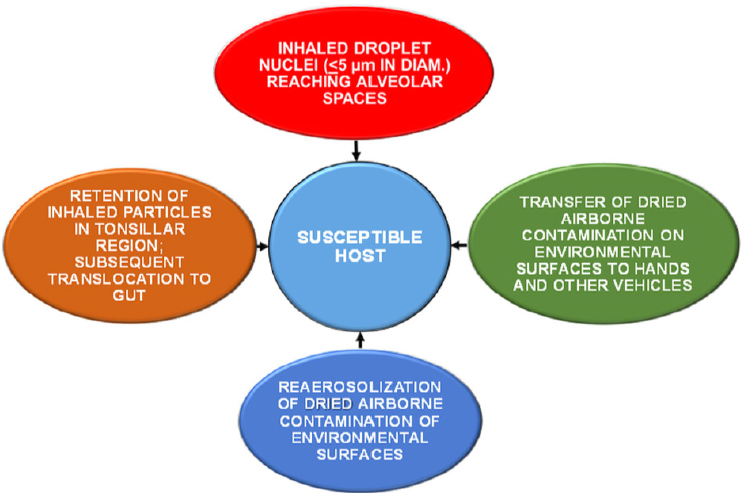
Figure 2-1 illustrates various routes of transmission of infectious agents in the indoor environment. Chapter 3 explores indoor sources and reservoirs of microorganisms in greater detail, but one important pathway for human exposure is inhalation of microorganisms carried or resuspended in room air, as well as microorganisms found in building water systems that become aerosolized. Relevant exposure routes include both aerosolization of fine particles that can travel into the deep lung and inhalation of coarse particles that may be deposited in the upper respiratory system (Hatch, 1961) or be translocated to the gastrointestinal tract via the mucociliary escalator (Harada and Repine, 1985).
Another important route of transmission is via fomites, in which the microorganism is transferred from a surface. The initial deposition of microorganisms onto a fomite can occur by deposition of aerosols or dusts or by transference via contact from an individual. A susceptible host can become infected by touching a microbially laden fomite and subsequently touching the mouth, eye, nose, or other body surface. Many microorganisms can be transmitted in this manner, especially viruses, including rhinovirus, influenza virus, coronavirus, norovirus, rotavirus, hepatitis A virus, adenovirus, and astrovirus (Boone and Gerba, 2007). Multiple modes of transmission often occur for a particular microorganism (Nicas and Jones, 2009). For example, influenza may be transmitted by aerosolization or by contact with a virus-laden fomite, and the predominant mode of transmission may be related in part to the absolute humidity inside the building
| Super Kingdom | Mode of Transmission | Examples |
|---|---|---|
| Bacteria | Inhalation | Bacillus anthracis |
| Coxiella burnetii | ||
| Chlamydia psittaci | ||
| Legionella | ||
| Mycobacterium tuberculosis | ||
| Atypical mycobacteria | ||
| Fomites | Clostridium difficile | |
| Staphylococcus aureus | ||
| Enterococcus | ||
| Fungi | Inhalation | Cryptococcus neoformans |
| Histoplasma capsulatum | ||
| Aspergillus fumigatus | ||
| Fomites | Trichophyton mentagrophytes | |
| Trichophyton rubrum | ||
| Protozoa | Inhalation | Acanthamoeba spp. |
| Viruses | Inhalation | Variola (smallpox) |
| Rubella | ||
| Norovirus | ||
| Rotavirus | ||
| Adenovirus | ||
| Coxsackie virus | ||
| Influenza | ||
| Rhinovirus | ||
|
Coronaviruses (Middle East respiratory syndrome [MERS], severe acute respiratory syndrome [SARS]) |
||
| Fomites | Variola (smallpox) | |
| Rubella | ||
| Norovirus | ||
| Rotavirus | ||
| Adenovirus | ||
| Coxsackie virus | ||
| Influenza | ||
| Rhinovirus | ||
| Coronaviruses (MERS, SARS) |
SOURCES: Table created using data from Burrell (1991), Couch (1981), and Yu et al. (2004).
SOURCE: Sattar, 2016.
(McDevitt et al., 2010; Tellier, 2009; Yang and Marr, 2011). Thus, transmission of microorganisms within the built environment is a complex process that is contingent not only on the class of microorganism itself but also the state of the building (e.g., its humidity or ventilation) and the number and behavior of building occupants.
A recently recognized class of pathogens, termed saprozoic (Ashbolt, 2015), is capable of amplifying on wetted surfaces such as those that may be found in built environments. In some cases, amplification is facilitated by growth of amoebae in biofilms that may harbor pathogens. A recent report suggests that fungi may also facilitate growth of saprozoites (Alum and Isaacs, 2016). Organisms of this class, which have been shown to be transmitted by indoor aerosolization or from fomites, include nontuberculosis Mycobacterium spp. Legionella pneumophila can similarly grow in association with biofilms, in this case in water systems. L. pneumophila can cause legionellosis and Pontiac fever, a nonfatal flu-like respiratory disease with a short incubation period and for which recovery usually occurs without medical intervention (OSHA, 2017; Principe et al., 2017). Locales in which amplification may occur along with subsequent aerosolization and infection include hot tubs and whirlpools (Falkinham, 2003); indoor fountains
and architectural features (Haupt et al., 2012; O’Loughlin et al., 2007); shower heads and hoses (Feazel et al., 2009; Schoen and Ashbolt, 2011); heating, ventilation, and air conditioning (HVAC) systems and humidifiers (Stetzenbach, 2007); and toilets (Azuma et al., 2012).
Biofilms can also exist in piping systems and provide habitats for pathogen amplification (Wang et al., 2012). Corroded pipes may provide a more favorable environment for this microbial amplification, and this factor has been associated with a spike in legionellosis cases in Flint, Michigan (Schwake et al., 2016) (see Box 2-2).
Building design and operational characteristics can affect the relative importance of different modes of transmission (Li et al., 2007), as can cleaning and handwashing practices (Sandora et al., 2008) and the use of masks (Wei and Li, 2016). For example, in multiunit residential (and likely commercial) buildings, cross-transmission of aerosols via the ventilation system may serve as a conduit for disease transmission (Mao and Gao, 2015; Nardell et al., 1991).
Complex, Mixed Exposures
The potential exists in any environmental exposure for multiple agents to affect a single host concomitantly. Outbreaks of legionellosis have provided evidence that coinfection can adversely impact patient outcomes, although it is not clear whether the coinfections occurred following the initial L. pneumophila exposure (e.g., in a hospital) (Fernandez et al., 2002). Animal studies have shown that coinfections can modulate immune system response to one of the challenges (Redford et al., 2014). However, reports of multiple pathogen impacts in the context of indoor exposures in the built environment are currently unavailable.
Somewhat more information is available on the interaction between exposure to infectious agents and concomitant respiratory exposure to adverse chemical or physical agents. Most of this information is derived from animal models. Controlled rodent experiments showed that susceptibility to inhaled Klebsiella was increased by prior exposure to nitrogen dioxide (NO2), as well as to aerosols of cadmium and nickel (as chloride) (Gardner, 1982). Intratracheal administration of combustion particles to mice was found to increase the lethality of inhaled Streptococcus (Hatch et al., 1985). Using a similar assay, Arany and colleagues (1986) showed that inhalation of a variety of volatile organic compounds either enhanced lethality or reduced the mice’s ability to fight off the inhaled Klebsiella infection. It can be anticipated that many human exposures in the built environment will be complex, and future studies will need to explore in more detail the potentially interacting or modulating effects of combined chemical, physical particulate matter, and microbial exposures on health.
In summary, the indoor environment can be a venue for exposure to a variety of infectious agents, including bacteria, protozoa, fungi, and viruses. These exposures may occur via inhalation (including of aerosols from premise plumbing) or contact with fomites. For bacteria, in particular, wetted surfaces can serve as a habitat for biofilms, which can amplify certain species, including several pathogens. These exposures are likely to
be from a combination of multiple microorganisms, which can affect how the microorganisms impact the human host in a variety of ways, many of which have not yet been studied.
DAMP INDOOR ENVIRONMENTS, INDOOR MICROBIAL EXPOSURES, AND RESPIRATORY OR ALLERGIC DISEASE OUTCOMES
Another area in which there has been much previous work is the impacts of damp indoor environments on human health. This research has typically focused on a number of noninfectious2 health effects from exposures to indoor microorganisms, with the greatest number of studies focusing on dampness, observations of microbial growth or detection of mold odors, and respiratory and allergic symptoms.
Damp building conditions promote the growth of mold, bacteria, and other microbial agents. Damp buildings may also contain other living organisms, such as dust mites and cockroaches (along with their associated microbial communities), which can potentially contribute to exacerbation of respiratory issues. Occupants in damp buildings can be exposed to pollutants in the air not only from biological contaminants but also from the deterioration of building materials, which can be accelerated by the presence of dampness inside the building.
Table 2-2 summarizes evidence from three review publications that damp buildings influence health (IOM, 2004; Mendell et al., 2011; WHO, 2009). There is a larger body of literature on upper and lower respiratory outcomes, with more limited attention to nonrespiratory outcomes. Over the years since the 2004 Institute of Medicine (IOM) report Damp Indoor Spaces and Health was issued, the evidence has become stronger for an association between damp buildings and the exacerbation of asthma and, importantly, the development of asthma. Furthermore, the 2011 review by Mendell and colleagues evaluates a number of health outcomes not considered by the IOM committee in 2004 (Mendell et al., 2011). Not all health effects that have been suggested as having possible associations with damp indoor environments have been the subject of sufficient published literature to enable evaluation. The 2004 IOM report lists a number of health outcomes as having inadequate or insufficient evidence with which to determine whether an association exists, including airflow obstruction (in otherwise healthy persons), skin symptoms, mucous membrane irritation
___________________
2 The term “noninfectious” is used here to represent potential health associations that are not known to be due to specific infection, although conventional clinical testing through pathogen culture methods may not fully consider the breadth of diversity and community structure of indoor microbiomes that has been increasingly characterized.
TABLE 2-2 Associations Between Health Outcomes and Exposure to Damp Indoor Environments
| Health Outcome | Strength of Association | ||
|---|---|---|---|
| IOM, 2004 | WHO, 2009 | Mendell et al., 2011 | |
| Upper respiratory (nasal and throat) tract symptoms | Sufficient Evidence | Sufficient Evidence | Sufficient Evidence |
| Wheeze | Sufficient Evidence | Sufficient Evidence | Sufficient Evidence |
| Cough | Sufficient Evidence | Sufficient Evidence | Sufficient Evidence |
| Shortness of breath | Limited or Suggestive Evidence | Sufficient Evidence | Sufficient Evidence |
| Exacerbation of existing asthma | Sufficient Evidence | Sufficient Evidence | Sufficient* Evidence |
| Development of asthma | Limited or Suggestive Evidence | Sufficient Evidence | Sufficient Evidence |
| Current asthma | Not Evaluated | Sufficient Evidence | Sufficient Evidence |
| Ever-diagnosed asthma | Not Evaluated | Not Evaluated | Sufficient Evidence |
| Bronchitis | Not Evaluated | Limited or Suggestive Evidence | Sufficient Evidence |
| Respiratory infections | Not Evaluated | Sufficient Evidence | Sufficient Evidence |
| Allergic rhinitis | Not Evaluated | Limited or Suggestive Evidence | Sufficient Evidence |
| Eczema | Not Evaluated | Not Evaluated | Sufficient Evidence |
| Common cold | Not Evaluated | Not Evaluated | Limited or Suggestive Evidence |
| Allergy/atopy | Not Evaluated | Inadequate/ Insufficient Evidence | Limited or Suggestive Evidence |
| Hypersensitivity pneumonitis | Clinical Evidence | Clinical Evidence | Clinical Evidence |
*Evidence judged to be strongly suggestive of causation.
syndrome, gastrointestinal tract problems, chronic obstructive pulmonary disease, fatigue, inhalation fevers (nonoccupational exposures), neuropsychiatric symptoms, lower respiratory illness in otherwise healthy adults, cancer, acute idiopathic pulmonary hemorrhage in infants, reproductive effects, and rheumatologic and other immune diseases. In their 2011 review, Mendell and colleagues identify altered lung function as having inadequate evidence available.
These reviews indicate stronger evidence for adverse health effects due to signs of dampness relative to those due to measures of indoor environmental microorganisms, a point reiterated in a recent review on the associations of health effects with observational assessment of dampness and mold (Mendell and Kumagai, 2017). The potential benefits of reducing moisture problems in buildings have been discussed in the literature (Mendell, 2007; Mendell and Kumagai, 2017; Mendell et al., 2008; Mudarri and Fisk, 2007; WHO, 2009).
The rest of this section selectively discusses studies published in the past decade concerning indoor dampness and respiratory or allergic health outcomes, and it addresses the question “Can specific indoor microbial exposures account in part for the adverse respiratory effects of building dampness?” In summary, results have been inconsistent from study to study, and it is currently not understood which specific contaminants or combinations thereof in damp indoor environments cause the various health effects under what circumstances.
A review by Quansah and colleagues (2012) on dampness and mold in homes and asthma development found that associations with the presence of visible mold and with mold odor were evidence for mold-related causal agents for asthma (Quansah et al., 2012). In a recent review Sharpe and colleagues (2015) conclude that there is some evidence that in indoor environments Penicillium, Aspergillus, Cladosporium, and Alternaria species are associated with asthma development and with worsening of asthma symptoms, but that more work is needed on the role of fungal diversity. Another recent review on indoor exposures associated with the exacerbation of asthma found that many indoor exposures exacerbated asthma, including indoor dampness or dampness-related agents such as endotoxin, culturable Penicillium, and total fungi (Kanchongkittiphon et al., 2015). On the other hand, in an earlier paper, Hägerhed-Engman and colleagues (2009) report no association between concentrations of measured mold species and asthma in the Swedish Dampness in Buildings and Health study (Hägerhed-Engman et al., 2009). Similarly, a recent study of Danish schools found that high classroom dampness was associated with lower lung function and wheezing, but microbial components were not consistently associated with health outcomes (Holst et al., 2016).
Data from large cross-sectional studies of approximately 46,000 8- to 12-year-old children in 20 countries during phase two of the International Study of Asthma and Allergies in Childhood (ISAAC) found significant and consistent associations of current exposure to dampness or visible mold in homes with respiratory and allergic symptoms (Weinmayr et al., 2013). Associations with current exposure included wheezing, coughing up phlegm without a cold, rhinitis, rhinoconjunctivitis, and eczema. Children
were similarly affected by dampness regardless of whether they had allergic sensitization or parents with allergy.
Studies of individual episodes of water-damaged buildings using detailed exposure measures and precise case definitions have subsequently produced stronger evidence that a variety of definable microbial groups may be partly responsible for associations between damp environments and respiratory health. In a water-damaged 20-story office building in the Northeast United States, linear associations were found between respiratory illnesses (particularly physician-diagnosed asthma) and hydrophilic fungi and ergosterol (a molecule in fungal cell membranes) (Park et al., 2008). In another study of a water-damaged U.S. building, associations were found between adverse respiratory functioning and thermophilic actinomycetes (a phylum of bacteria) and nontuberculosis mycobacteria (Park et al., 2017). While these cases are instructive, the range of microbial ecosystems in the built environment that may have long-term adverse effects on respiratory health is not well defined.
Higher fungal counts, specific fungal or bacterial species, and higher endotoxin/LPS levels have been associated with building dampness (Park et al., 2008). The absence of a gold standard for measuring mold (Chew et al., 2016) means that complementary measurement methods often yield a better perspective on which mold or mold component exposures linked to dampness may be relevant to health. As discussed by Cox-Ganser and colleagues (2011), mycotoxins have been posited as contributors to these symptoms, but their effects in the context of damp and poorly maintained buildings are not well understood.
Outright flooding, building-related water damage, indoor point sources of dampness and water, suboptimally maintained cooling towers, or poor maintenance of buildings leading to indoor moisture problems may lead to proliferation of microbial communities that can cause noninfectious adverse allergic and nonallergic respiratory responses. This is the case particularly in susceptible populations such as children and people with preexisting asthma.
Association with Asthma Development and Worsening of Asthma Control
Living, working, or attending school in damp indoor environments has been associated with onset or worsening and exacerbation of asthsma in children and adults. A meta-analysis of 33 studies estimates that exposure to dampness and mold in the home raises the risk for asthma development, history of asthma, and current asthma by about 30–50 percent (Fisk et al., 2007). It has been estimated that 21 percent of current asthma cases in the United States can be attributed to dampness and mold, which translates to 4.6 million of the 21.8 million U.S. asthma cases at the time of the esti-
mate (Mudarri and Fisk, 2007). This study also estimates the annual cost of asthma attributable to exposure to dampness and mold at $3.5 billion.
Recent evidence suggests that both allergic and nonallergic asthma are more frequent in damp indoor environments. This evidence is important because it suggests that not all of the effects of dampness result from allergic responses to allergens from microbial and nonmicrobial sources (e.g., allergens on dust mites and cockroaches) that proliferate in damp conditions. As mentioned previously, microorganisms include components other than allergens. Fungal and bacterial components such as PAMPs, for example, can cause irritant or inflammatory symptoms through nonallergic biologic pathways.
While inheritance is presumed to play a role in how children or adults respond to exposures to indoor microorganisms such as fungi and bacteria, the genetics that might modulate either adverse or protective microbial responses resulting in the development or worsening of allergic or nonallergic respiratory disease is not well understood. Fungal components (e.g., chitin [Da Silva et al., 2008]) or bacterial components (e.g., LPS [Simpson and Martinez, 2010]) are known to stimulate innate pathways that are under genetic regulation, but the implications of this knowledge for characterization of susceptibility to disease are not well defined. Gene–environment interactions are likely to be complex and may even be sex-specific, since investigators of genes related to immununoglobulin E (IgE) or asthma have identified sex-specific polymorphisms of genes that regulate asthma or allergy responses, such as interleukin-17 receptor B (IL17RB) and thymic stromal lymphopoietin (TSLP) (Hunninghake et al., 2008, 2011). To complicate matters, a genetic polymorphism may be a risk factor for asthma, but it may increase the protective effects (on allergic asthma) of an environmental exposure such as the fungal and bacterial microbes on a farm (Loss et al., 2016).
A 9-year prospective follow-up study on onset of asthma was conducted on 7,104 young adults from 13 countries who had participated in the European Community Respiratory Health Survey (ECRHS) I and II and had not reported baseline respiratory symptoms or asthma (Norback et al., 2013). The findings strongly support the connection between indoor dampness and/or visible mold and new onset of asthma or bronchial hyperresponsiveness.
Various articles have reviewed the accumulating literature strengthening the links among dampness, dampness-related agents, and asthma exacerbation occurring in nonallergic as well as allergic children (Kanchongkittiphon et al., 2015). For example, a 2010 study of inner-city children indicated that indoor fungi originating from both indoor and outdoor sources could worsen asthma (Pongracic et al., 2010).
Prior work by investigators from the U.S. National Institute for Occupational Safety and Health showed that in occupants of a historically
water-damaged office building, asthma was associated with concentrations of hydrophilic fungi in floor dust (Park et al., 2008) and that there was a synergistic effect of fungal and endotoxin exposure in respiratory health effects (Park et al., 2006). Other analyses of this office building population found evidence for exacerbation of building-related asthma. The onset of posthire asthma was associated with a lower prevalence of positive skin-prick reactions to common allergens, including indoor and outdoor mold mixes (Cox-Ganser et al., 2005). In summary, this study showed that building occupants who had developed asthma after working in the building were less allergic to common allergens, so the asthma must have developed directly in concert with something present in the building.
Fungi are a source of many different components that may have health effects. Allergens and other antigens on indoor fungi are one set of microbial components known to worsen respiratory symptoms in people who have established asthma and are allergic to the specific fungal allergen they inhale. Paradoxically, in early life, higher exposure to some nonmicrobial allergens (e.g., peanut allergen) may actually promote tolerance and protection from allergic responses (Du Toit et al., 2015). However, it is not known whether tolerance can occur with early-childhood exposure to fungal allergens. Overall, early-life fungal exposures worsen the health of young children, but many of the negative effects in early life may not be due to the allergens. Annex Table 2-1 at the end of this chapter summarizes epidemiologic studies that explore interventions aimed at reducing exposures and improving asthma outcomes.
Association with Rhinitis
Jaakkola and colleagues (2013) conducted a meta-analysis of the literature on the association between exposure to fungi in damp buildings and rhinitis (nasal inflammation). They concluded that there is evidence that dampness and mold exposures at home can cause or exacerbate rhinitis and its subcategories of allergic rhinitis and rhinoconjunctivitis (which produces nose and eye symptoms such as sneezing and itching). The strongest associations occurred with the presence of mold odor, which suggests that microbial agents were involved in some fashion in the development or exacerbation of this health effect.
The Role of Microbial Metabolic Compounds
The largest body of research on potential health associations of exposures to microbial metabolites focuses on fungi. Work by Miller and colleagues (2010) and Rand and colleagues (2011, 2013) on effects of fungal secondary compounds (mycotoxins) indicates that these compounds, in
concentrations that may be found in indoor environments, can cause inflammation on a cellular level that points toward nonallergic asthma. With their models, they have shown that exposure to very low concentrations of these compounds precipitates such lung changes as secretion and modification of mucoids on lung surfaces, as well as changes in the respiratory cell composition obtained from sampling. This has been found with many pure compounds from a range of xerophilic, mesophilic, and hydrophilic fungi, as well as triple helical glucan. Moreover, it has been demonstrated that these compounds act on well-understood receptors (e.g., dectin-1 and NfkB). In addition, these compounds significantly modulate downstream gene transduction, transcription, and cytokine expression patterns associated with a variety of immune system pathways related to inflammatory and/or asthma provocation (including TH1, TH2, and TH3) in compound-, dose-, and time-dependent ways.
Association with Eczema
In a recent analysis of data on children in the ISAAC study, residential exposure to dampness and mold was found to be significantly associated with eczema, a type of skin inflammation, in the previous year. Dampness and mold in the first year of life also was associated with higher odds of the child’s ever having had eczema, by parental report. Allergic sensitization of the child did not modify the association, suggesting that dampness or mold might increase the risk of eczema through mechanisms other than IgEmediated allergy, although parental allergic disease did increase the odds of eczema with exposure (Tsakok et al., 2015). Occupational studies support the possibility of other mechanisms through which dampness-associated microbes of the built environment could increase the incidence of eczema, including contact dermatitis. However, little is known about mechanisms in the context of the indoor environments in which the children lived.
Association with Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis, also known as allergic alveolitis, is a condition in which inhaled dust, fungi, or chemicals lead to inflammation in the lung. This condition has been recognized in relation to nonindustrial indoor environments for decades. Early publications on outbreaks usually implicated microbial dissemination from humidification and ventilation systems, although the specific organism(s) were rarely identified. Ventilation- and humidifier-related hypersensitivity pneumonitis may have decreased with recognition of the need for design changes and maintenance of cleanliness in these systems. More recent publications have implicated damp indoor environments, along with other exposures (e.g., chemicals),
in causing hypersensitivity pneumonitis. Buildings with long-standing water damage from roof leaks, other building envelope water incursion, plumbing leaks, and below-grade moisture problems have all had reported clusters of hypersensitivity pneumonitis (Borchers et al., 2017).
NONAIRWAY AND NONALLERGY EFFECTS
The impact of bacterial or fungal communities in buildings on health outcomes other than those related to respiratory conditions or allergy is less well established. Epidemiologic and toxicologic data suggest that effects of environmental microbial exposures on human health are likely to be dependent on dose, stage of life, physiologic compartment (e.g., gut, nose, lung, skin), and/or host (including sex).
Endocrine Disruption and Child Development
Viable indoor microbes likely metabolize chemicals present in the built environment. A number of chemicals found in common household products or furnishings, such as bisphenol A, perfluorooctane sulfonate (PFOS), and perfluorooctanoate (PFOA), have been shown when ingested to influence endocrine function (e.g., glucose metabolism or diabetes risk) and child growth, including the risk of becoming overweight or obese (Heindel et al., 2017). Laboratory experiments show that environmental microbes can metabolize these and other chemicals with endocrine disruptive properties, sometimes creating more bioactive or bioavailable chemicals and sometimes reducing their toxicity (Blavier et al., 2016; Bradley et al., 2016; Gramec and Mašič, 2016; Janicki et al., 2016; Koestel et al., 2017; Vejdovszky et al., 2017). However, the chemical metabolites of indoor microbes have not been well characterized. Moreover, little is known about the ingestion, inhalation, transdermal, or other exposures of small children to chemical by-products of microbial metabolism and whether they influence endocrine function or growth. Fungi themselves can also produce mycotoxins that affect the production of estrogen, but the relevance of this finding to health and whether these fungi are found in buildings are similarly unknown (Vejdovszky et al., 2017).
In addition to producing active metabolites or metabolizing chemicals in the indoor environment, the indoor environmental microbiome itself may influence the human microbiome in ways that lead to health effects. The skin and its commensal microbial communities are instrumental in protection against the environment. These communities, which vary in composition by location on the body, are determined largely by environmental and physiologic conditions. The microbial communities on the skin modulate the health status of the body through immune responses that
maintain health or, in specific situations, may promote disease (Barnard and Li, 2017). While knowledge has grown about the diversity and characteristics of skin microbial communities, relatively little is understood about the strains responsible for the function of these communities in maintaining protection and health or in promoting disease (Barnard and Li, 2017; Belkaid and Segre, 2014). Personal care products and other household chemicals that can act as endocrine disruptors have been shown to affect the skin and its microbiome (Bouslimani et al., 2015, 2016). However, there are no data to suggest that the indoor environmental microbiome influences the composition or structure of the skin microbiome (Lax et al., 2014).
Toxicologic and epidemiologic studies provide supporting evidence that specific groups of chemicals in plastics, furnishings, and personal care products may have endocrine-disrupting properties that increase the risk of hormone-related conditions such as diabetes or obesity, particularly in children exposed in early life. While it is known that microbes metabolize chemicals, and limited data show that building materials influence microbial degradation of those materials, it is not known whether metabolism of building materials, furnishings, or household chemicals by microbes results in active metabolites relevant to human health, specifically to endocrine disruption. These chemicals can also impact the skin microbiome and its ability to protect the body against disease, but modulation of the skin microbiome and its direct impact on human health is also not yet well characterized. While degradation or metabolism of building materials by microbes may be partly related to moisture, additional factors play into the health and safety aspects of these materials and the likelihood of their degradation by microbes. Further research in this area may inform the choice of “healthy” building materials and furnishings.
Future studies could focus on testing whether environmental microorganisms or by-products of household chemicals with endocrine-disrupting properties interact at major sites when encountering the human body. Research could elucidate whether these encounters lead to systematic changes in how the body functions and develops, with influences on endocrine or immune function or child growth. Such potential sites of interaction include the skin; airway; and gastrointestinal tract, including the oropharyngeal sites. Concurrently with the present study, a study by another committee of the National Academies of Sciences, Engineering, and Medicine (Advancing Understanding of the Implications of Environment–Chemical Interactions with Human Microbiomes) is under way, focused on developing a research agenda to guide the chemical risk assessment community in understanding how chemical exposures may modulate the human microbiome and how the human microbiome may modulate the effects of chemical exposures (via metabolism of chemicals) on human health outcomes. With that study’s focus on the human microbiome and
risk assessment for health outcomes related to indoor and other environmental chemical exposures, the proposed research agenda is likely to be of interest to the built environment community as it will likely suggest gaps in research needed to better understand the concerns outlined in this section.
Brain Health and Neurologic Outcomes
Studies in the United States and internationally have suggested that adult or child brain health may be influenced by aspects of homes or public buildings such as offices, schools, and hospitals. These studies have evaluated outcomes ranging from central nervous system (CNS) symptoms such as headaches, to mood (e.g., depression) and sleep disorders, to changes in neurocognitive or behavioral function. The findings of these studies have prompted investigators to posit that indoor microbial communities may mediate a portion of the observed associations of brain health outcomes with building characteristics or with other potential microbial sources within or proximal to buildings.
There are several potential pathways by which such effects could occur. Airborne indoor microbial components or metabolites may enter the brain directly via the olfactory bulb (Block et al., 2012). Alternatively, they may have indirect neurologic effects through airway autonomic stimulation or by causing airway or systemic inflammation. Microorganisms and their metabolites are also found in building water systems. Under certain circumstances, microbial deterioration of building or indoor plumbing materials may result in release of toxic chemicals into the indoor water system, and the absorption of these chemicals may lead to negative brain effects. To date, with specific exceptions, evidence to support these hypotheses is scant, in part because of methodologic challenges in exposure and outcome measurement and because of the potential complexity of the biologic response to exposures.
Another possible mechanism for the influence of indoor environmental microorganisms on brain development is through their potential interactions with the human gut microbiome. However, apart from the increasingly well-documented association of indoor dogs (Fujimura et al., 2014) or farm animals with the enrichment of certain beneficial microbes in the human gut and the diversification of home microbial communities, data to support a relationship between the indoor microbiome and the human microbiome are scant. As noted previously, the human microbiome can be influenced by the environment. For example, the ElderMet study found that subjects in long-term care facilities had different and less diverse gut microbiomes relative to those living in the community, although such factors as diet and health status appear to play important roles in explaining this variability (Claesson et al., 2012).
The question then arises of whether the environmental microbiome can influence the gut or respiratory microbiome. From a compartmental and stage-of-life point of view, infants and toddlers are more likely than adults to ingest environmental microbes, which may in turn influence the composition and function of the gut microbiome. A preliminary investigation using data on 20 infants from the Canadian Healthy Infant Longitudinal Development (CHILD) study, for example, found associations between house dust and fecal samples for several classes of bacteria (Konya et al., 2014). Other potential mechanisms by which environmental microbes might influence brain health might not require the ingestion or proliferation of microbes, and they could include responses to airway or skin encounters with microbial components or metabolites. Accumulating evidence suggests an association between microbiota present in the gut and brain function (Burokas et al., 2015), as the existence of bidirectional neural and immune interactions between the intestine and the brain has been proven (Keunen et al., 2015). While the exact mechanisms by which the microbiome can influence the development of the CNS are not completely understood, proposed communication between these systems is termed the microbiome–gut–brain axis. There are numerous complex interactions between the microbes that reside in the gastrointestinal tract and immune, endocrine, and neurologic systems. The vagus nerve directly connects the gastrointestinal nervous system to the brain. The immune system monitors the presence of microbes and reacts to changes in their structure and composition, transmitting this information to the CNS. Furthermore, human commensal organisms release metabolites that are precursors of important neurotransmitters such as gamma amino-butyric acid (GABA) and serotonin precursors (e.g., 5-hydroxytryptophan [5-HT]) or might induce the production of 5-HT by enteroendocrine cells, which in turn influences the nervous system and the brain (O’Mahony et al., 2015).
Altered microbial community structure, or dysbiosis, in the setting of stress and disease has been associated with alterations in behavior, cognition, emotion, and levels of inflammatory cells (Cryan and Dinan, 2012). Germ-free mice demonstrate altered risk-taking behavior, memory, and anxiety (Al-Asmakh et al., 2012; Cryan and Dinan, 2012; Neufeld et al., 2011). Diaz Heijtz and colleagues (2011) used an elegant experiment to demonstrate the role of the microbiome in brain and CNS development: germ-free mice had an altered neurologic response when subjected to stress tests, and this response was reversed only when bacteria were transplanted into the mouse caecum during infancy, rather than during adulthood.
Perturbing initial colonization and microbiome development has been shown to affect brain development and to pose a risk of developing neurologic disorders later in life (Borre et al., 2014; Diaz Heijtz et al., 2011). Therefore, environmental exposure to the built environment microbiome
early in life could have much more significant effects on neurologic development than those that occur later in life.
Studies supporting the influence of gut microbial composition and function on a complex and bidirectional gut–brain axis were recently reviewed by Jasarevic and colleagues (2016). Microbial metabolites, such as short chain fatty acids and chemotactic peptides, may influence the brain directly or may bind to intestinal epithelial cell receptors to enable the secretion of peptide neurotransmitters. Microbes can also interact with gut immune cells, and resulting cytokines may influence brain function. Centrally activated neural circuits that may be activated by various stressors may also influence gut microbial composition or function. In addition to the gut, the nasal or lower airway epithelial layer, including its mucus interface, is a barrier, interactive site, or portal through which microbes or their components may influence immune function (and perhaps brain function) through multiple mechanisms. These mechanisms may include disruption of the mucous barrier, stimulation of toll-like receptors (TLRs) or other immune receptors (Davies, 2014), or direct translocation from the upper respiratory tract to the brain.
Moisture-Damaged Buildings, Poor-Quality Housing, and Brain Health
Brain health outcomes have also been studied for associations with moisture-damaged buildings, poor housing conditions in disadvantaged communities, outdoor traffic proximal to schools and homes, pets in homes, and the construction of what is colloquially termed “green” housing (see the section on “Beneficial Effects of Microbes”). It is important to note that the use of the term “green” encompasses a variety of design and building approaches, potential interventions, and actual success at achieving more healthful or energy-efficient buildings.
In addition to the large literature linking damp buildings, buildings with water damage, and housing in poor repair with respiratory symptoms, some studies also link these conditions to reduction in brain health, with symptoms of headache, nausea, mood disorders, difficulty concentrating, or sleep difficulties (Ansarin et al., 2013; Casas et al., 2013; Chambers et al., 2016; Cox-Ganser et al., 2010, 2011; Faber et al., 2015; Francisco et al., 2016; Jacobs et al., 2015; Oudin et al., 2016; Park et al., 2008, 2017; Schiffman et al., 2005, Shiue, 2015; Singh and Kenney, 2013; Tiesler et al., 2015). In circumstances such as the post–Hurricane Katrina experience, victims living in trailers suffer from stress and mood disorders, but it has been difficult to disentangle the trauma of the experience from responses to the physical environment, including molds and their products, as well as other airborne exposures. Mold odors and visible mold have been linked to sleep difficulties (Ansarin et al., 2013; Chambers et al., 2016; Faber et al., 2015;
Jacobs et al., 2015; Oudin et al., 2016; Shiue, 2015; Singh and Kenney, 2013; Tiesler et al., 2015), and it is unknown whether proinflammatory upper airway influences combine with the direct brain effects from mold to contribute to such difficulties, including sleep-disordered breathing.
The physical exposures linked to poverty, and thereby connected to lower socioeconomic status, are compounded by many other disparities, including reduced access to an adequate diet, an enriching environment, health care, and education, as well as the presence of environmental toxins. Children in poverty have a significantly increased risk for developmental delay, poor school performance, and behavioral problems (Blay et al., 2015; Chambers et al., 2016; Evans and Schamberg, 2009; Hanson et al., 2013; Saigal and Doyle, 2008). Poverty has even been linked to changes in brain structure (Hanson et al., 2013; Jednorog et al., 2012). Many of the aspects associated with poverty (e.g., poor diet and health, depression, and anxiety) are associated with alteration of the human microbiome (Myles, 2014; Rook et al., 2014). Even less obvious factors, such as the number of caregivers and the indoor and outdoor home environment (e.g., presence of animals, access to outdoor green spaces), can significantly impact the human microbiome (Lax et al., 2015). Providing clean, dry, well-maintained housing has recently been shown to improve respiratory health (Colton et al., 2014), but it is not known whether this could occur in part through changing microbial exposures.
Epidemiologic and toxicologic studies, including studies using rodent models, provide growing evidence that outdoor particulate and gaseous pollutants influence brain health. The Outdoor Air Pollution and Brain Health Workshop (Block et al., 2012) was followed by additional studies providing evidence that outdoor pollutants have effects on the brain, including children’s neurodevelopment and neurocognitive and behavioral function in school (Basagana et al., 2016; Clifford et al., 2016; Dadvand et al., 2015; Harris et al., 2015; Kicinski et al., 2015; Sunyer et al., 2015), as well as cognitive function and decline in elders. A small but growing body of literature demonstrates the presence of microbes or microbial components on outdoor particles that may penetrate indoors and on indoor particles that may have both outdoor and indoor sources, which can be of local, regional, or even transoceanic origin (Frankel et al., 2012). Their contribution to effects on brain health is poorly understood. Although an in-depth discussion of outdoor environmental pollution is beyond the scope of this study, air, water, and other materials from the outdoor environment come indoors into built environments. The integration of disparate areas of knowledge that will underpin a clearer understanding of how indoor microbial exposures can lead to health outcomes and how this understanding could lead to practical application will include efforts to clarify the ties between the outdoors and the indoors.
While building conditions have been associated with adverse neurocognitive, behavioral, and other brain health outcomes, the specific contribution of the indoor microbiome to these adverse effects is unknown. The availability of evolving microbiologic, genomic, bioinformatics, and statistical technologies may facilitate a fuller assessment of whether and how brain health is influenced by indoor environmental microbes. An increased mechanistic understanding of how indoor microbes can act on neurologic outcomes would inform future environmental interventions designed to protect brain health. In addition, multiple adverse environmental exposures that are greater in disadvantaged populations and neighborhoods may add to or modify effects of indoor microbial exposures. Thus, it is important to conduct studies of indoor microbial effects on neurocognitive outcomes in built environments reflecting a range of socioeconomic circumstances and resources and to incorporate prospective longitudinal studies in future assessments.
BENEFICIAL EFFECTS OF MICROBES
Over the past two decades, interest in the potentially protective influences of indoor environmental microbes has been stimulated in part by farm community studies (see Box 2-3 and Annex Table 2-2 at the end of this chapter) that reproducibly demonstrate a reduced risk of allergic asthma with certain microbial exposures at home in early life. These exposures are estimated through measurement of bacteria components, microbial culture, or first-generation molecular biologic tools. Additionally, new metagenomics tools have opened a window into better understanding of the vast number of microbes that inhabit the human body and the microbial communities that are in the built environment.
Indoor microbes and their components and metabolites may have beneficial health effects in some circumstances and detrimental health effects in others. Characteristics of the built environment, the microbial community, and human behaviors within that environment may modulate the dose of the microorganism or the compartment exposed, and they may in turn influence whether the microorganism has a beneficial, adverse, or null effect on health. The same community of indoor microorganisms and their cell wall components may benefit human health in some circumstances and be detrimental in others depending on such circumstances as building characteristics, life stage of the person being exposed, exposure route, coexposures, dose, and genetic sensitivity. For example, a baby who ingests microorganisms while crawling on the floor may respond differently from an adult with asthma who inhales the same microorganisms. Potentially beneficial microbes include primarily microorganisms that train or modulate the human immune system (Kelly et al., 2005), produce small molecules
that mediate human health (Neish, 2009), or enable other functions that improve well-being in a human host (Reber et al., 2016; Rook and Lowry, 2008).
This knowledge has led to the identification of biomarkers that are associated with health benefit and an extensive body of research aimed at understanding the mechanisms of how microbial exposures could benefit human health (Heederik and von Mutius, 2012; Torow and Hornef, 2017; von Mutius, 2016; von Mutius and Vercelli, 2010; Wlasiuk and Vercelli,
2012). Annex Table 2-2 at the end of this chapter summarizes selected studies that have examined potential beneficial microbial exposures in the indoor environment.
This field of research needs many more targeted, longitudinal observational studies and intervention studies in order to pinpoint where beneficial tips into adverse, as well as the reverse, and to build the knowledge base needed to modulate built environments so as to positively impact human health.
Association of Microbial Exposures with Protection from Asthma and Respiratory Symptoms
While the adverse effects of microorganisms, their components, and their products have well-documented influence on the development, progression, or exacerbation of asthma and allergies (Eggleston et al., 1998; Lai et al., 2015; Quansah et al., 2012), there is also a substantive literature addressing protection from the development of asthma and allergy conditions through microbial exposures (Behbod et al., 2015; Celedón et al., 2007; Sordillo et al., 2010). Recent evidence suggests that the bacterial communities in dust in homes near farms may be reducing the incidence of asthma in certain populations (Stein et al., 2016) (see Box 2-3).
Dog-associated dust and the bacterial communities therein also have been shown to reduce atopy symptoms in mice (Fujimura et al., 2014), a finding suggesting that young children who live in homes with dogs may be less likely to develop asthma (Fall et al., 2015). The mechanism is hypothesized to be attributable to features of the microbial communities associated with animals. These microbes may act by shaping immune responses on skin, on airway mucosal surfaces, and in the gut (von Mutius, 2016). Differences in gut microbiota, including increased concentration of Veillonella spp., Lachnospira spp., and Faecalibacterium spp. from the phylum Firmicutes (Arrieta et al., 2015), in the first 3 months of life appear to play a role in asthma protection. Along with these taxa and Bacteroidetes (Lynch et al., 2014), increasing evidence suggests associations between exposure to high bacterial and fungal diversity in early life and protection from asthma and wheeze (Dannemiller et al., 2014; Ege et al., 2011; Tischer et al., 2016).
Exposure to insect and mammal stool within the first year of life has been shown to reduce the risk of development of preasthmatic wheeze (Dami and Bracken, 2016), while reduced exposure to certain species of Firmicutes and Bacteroidetes associated with house dust has been associated with an increase in atopy (Lynch et al., 2014). As these bacterial phyla are often associated with the mammalian gut, their absence suggests a reduction in stool in the environment. The observation that reducing mouse and cockroach stool can increase the probability of wheezing and asthma may
be seen as paradoxical, but it accords with current hypotheses that immune system challenges can reduce atopy.
In addition to the effects of exposures to bacteria discussed above, a small but growing literature indicates selected beneficial effects of early-life exposures to fungi in relation to the development of allergy and respiratory disease (Behbod et al., 2015; Tischer et al., 2016). However, especially in the case of fungi (but also to some extent in the case of bacteria), researchers still know very little about what products specific microbes are making or dispersing that may benefit human health if encountered in early life. More longitudinal studies of early-life microbial effects on subsequent child health are needed that define specific taxa and microbial community structure and function. Observations in these studies will need to be further validated in animal models to elucidate the mode of transfer from the environment to human compartments and biologic mechanisms of immune, physiologic, and/or other health effects. These studies may not result in recommendations that suggest reproducing the lifestyle or building structure that is associated with protection (e.g., most people will not live in a house with cows inhabiting a barn below), but they may help define the components of microbial exposures that are of potential therapeutic benefit for some people. As has been shown with endotoxin, it is likely that various microbial components may be good for some people and bad for others, depending on dose, compartment (whether inhaled or ingested), stage of life at which they are exposed (early life or adulthood), and/or susceptibility factors (heredity and additional environmental factors covarying with poverty). This concept is not easily applied to building design, where the goal is to provide healthier buildings for all. Nonetheless, longitudinal studies that include microbial measurements can define conditions and specific exposures that are adverse or protective for specific groups of people.
Potential Beneficial Effects Associated with Green Buildings and Green Spaces
Green Building Design
“Green building” design aims to promote environmental and energy sustainability, and the concept has become adopted more widely within the architectural and design community: the U.S. Green Building Council’s Leadership in Energy and Environmental Design (LEED) standard is one example. Green building designs reflect a mix of efforts focused on energy, water, and indoor environmental quality, but they offer no guarantees of meeting specific requirements for energy savings or healthful design. Green building design and what it entails is discussed in more detail in Chapter 3.
Green building design could potentially impact occupant health by improving air quality. Yet there have been no randomized trials of residents moving from conventional to green housing and how this change is associated with health outcomes. Findings of a few recent observational studies, some of them quite small, suggest that reducing home dampness through weatherization and by using green building approaches may improve asthma control.3 These findings are based on the assumption that appropriate standards and guidelines on outdoor air ventilation rates and selection of building materials should be followed. To the committee’s knowledge, there have been no published studies investigating ties among design, indoor microbiology, and health outcomes.
Researchers also have attempted to link green building design to neurocognitive outcomes. In one recent study, moving from poorly maintained or extremely aging housing to newly renovated or constructed green housing was found to result in fewer self-reported lost schooldays or workdays; less disturbed sleep, sadness, nervousness, and restlessness; and improved child behavior (Jacobs et al., 2015). Yet these improvements may have resulted from living in newer and better maintained housing, irrespective of green design features. Furthermore, it is unknown whether environmental microbial exposures contributed to the reported improvements.
While green buildings have repeatedly been cited as an approach to improving health (Allen et al., 2015, 2016; NRC, 2006), the specific attributes of green buildings that may contribute to improved health need to be broken down in order to understand how or why certain approaches to improved construction and operation under specific ecologic conditions may make such contributions. It is important to understand as well that many features of green buildings may make no contributions to improved indoor environmental quality or to the health of occupants.
Green Space
Outdoor green space surrounding buildings has been associated with improved patient outcomes (see Center for Health Care Design, 1995; IOM, 2007; Ulrich et al., 2004) and overall health status (Gong et al., 2016; Nieuwenhuijsen, 2016). Scientists have posited that exposure to green space may contribute to health benefits through exposure to plant-associated environmental microbiota. Studies in Finland, for example, have shown that living close to green space and agriculture rather than close to a town increases the biodiversity of the skin microbiota and correlates with reduced allergic sensitization (Hanski et al., 2012). For a review of potential
___________________
3 See http://www.enterprisecommunity.org/solutions-and-innovation/green-communities (accessed April 25, 2017).
links among green space, the microbiome, and immune system function, see Rook (2013). Rook and Knight (2015) recently called for city planning and architectural designs that optimize the biodiversity of microbial exposure in urban settings, with an emphasis on green spaces.
SUMMARY OBSERVATIONS AND KNOWLEDGE GAPS
Summary Observations
The ability of microorganisms within the built environment to affect human health is supported by data for many types of infectious bacteria, viruses, fungi, and protozoa. Selected examples of microorganisms that can be encountered by humans in the built environment and can result in infections from inhalation or from contacts with fomites are provided in Table 2-1 presented earlier in this chapter.
A variety of health effects that are not infectious in nature have also been reported. Extensive research demonstrates that exposure to damp, water-damaged buildings and “sick buildings” results in negative respiratory health effects for building occupants. These respiratory effects often are not directly related to allergy and may also be caused by irritant or proinflammatory components of microbes. Connections between the built environment and a number of nonrespiratory health outcomes have been suggested—including effects on child development, brain health, and mental health—although less is known about whether these effects are due to exposures to indoor environmental microbes and through which physiologic mechanisms they occur.
Beneficial effects on health from exposure to microorganisms in built environments have also been reported, particularly for exposures that occur in early life. Evidence for mechanisms by which microbial exposures can have positive effects is starting to accumulate and can be built upon to better understand what constitutes microbial communities that have such effects and the potential mechanisms of action involved. In particular, a small but growing literature shows that selected early-life microbial exposures are associated with positive benefits in relation to the development of allergy and respiratory disease.
Research connecting microbial exposures in the built environment with health impacts draws on a number of study approaches, including epidemiologic observational studies, such as longitudinal cohort studies, as well as dose-response studies. For example, results of observational studies that suggest connections between built environment microbiomes and human health can benefit from further validation in animal models to elucidate the microbial communities or components responsible for protective or adverse health responses, the modes of transfer from the environment to humans,
and the types and mechanisms of physiologic responses. There also may be opportunities to leverage data from existing health studies. These study designs are discussed in more detail in Chapter 4.
Knowledge Gaps
On the basis of the above summary observations and the information developed in this chapter connecting indoor microbial exposures to human health effects, the committee identified the following goals for research to address knowledge gaps and advance the field:
- Improve understanding of the transmission and impacts of infectious microorganisms within the built environment. Continued elucidation of the transmission of infectious microorganisms in a variety of built environments would be useful, including studies on modes of transmission for emerging respiratory pathogens; for pathogens with evolving patterns of hosts (animal as well as human); and for pathogens with problematic characteristics, such as drug resistance (e.g., Mycobacterium tuberculosis, Clostridium difficile [Peng et al., 2017]). Improved understanding of the relationship between microbial transmission and the timing of symptom onset also would be useful in informing future strategies for minimizing exposures.
- Clarify the relationships between microbial communities that thrive in damp buildings and negative allergic, respiratory, neurocognitive, and other health outcomes. A number of studies link human exposure to damp and water-damaged buildings with allergic and other respiratory health impacts. But further research is needed to identify how building conditions and maintenance result in dampness that leads to the proliferation of communities of microbes that can adversely affect respiratory health; to distinguish among the microbial and nonmicrobial effects of dampness; to understand the relationships among microbes, building materials, and chemicals within damp buildings; and to assess how human health is impacted when dampness is reduced.
- Elucidate the immunologic, physiologic, or other biologic mechanisms through which microbial exposures in built environments may influence human health. A number of possible health impacts from microbial exposures have been suggested (beyond infectious disease and the association between dampness and respiratory health), including developmental and neurocognitive effects. Much remains unknown about how the composition of the microbial communities, stage of life, route of exposure, and other factors affect human biologic responses and potential health outcomes. For example, a
-
growing body of literature suggests that the human microbiome, particularly the microbial communities in the gut, can influence health. But questions remain about the extent to which indoor microbiomes influence the composition and function of the human microbiome (on the skin and in the gut, oral, or airway compartments) and what that may mean for health outcomes.
- Gain further understanding of the beneficial impacts of exposures to microbial communities on human health. Several studies have documented associations between early-life microbial exposures and exposure to diverse microorganisms associated with animals and later protective health effects. Further longitudinal studies of the effects of early-life microbial exposures on subsequent child and adult health will be needed to understand these connections more fully. Also useful would be additional data with which to further explore the beneficial impacts of exposures to specific microbial communities and clarify such factors as the extent to which these impacts vary with the characteristics of a building’s occupants, stage of life, and the routes through which the occupants are exposed.
- Develop an improved understanding of complex, mixed exposures in the built environment. Responses to the complex and compound exposures that occur routinely in built environments, such as exposures to multiple microorganisms and to combinations of microorganisms and chemicals, have not been thoroughly elucidated to date.
- Design studies to test health-related hypotheses, drawing on the integrated expertise of health professionals, microbiologists, chemists, building scientists, and engineers. Many of the studies investigating how human microbial exposures relate to health outcomes have been conducted in ways that make them difficult to reproduce in other buildings and make it difficult to understand how specific building attributes affect both the microbial exposures and the health outcomes. A variety of further studies will need to be developed and implemented to ensure that the experiments are reproducible and produce results that can be translated into actionable outcomes.
REFERENCES
Aagaard, K., K. Riehle, J. Ma, N. Segata, T. A. Mistretta, C. Coarfa, S. Raza, S. Rosenbaum, I. Van den Veyver, A. Milosavljevic, D. Gevers, C. Huttenhower, J. Petrosino, and J. Versalovic. 2012. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLOS ONE 7(6):e36466.
Adams, R. I., A. C. Bateman, H. M. Bik, and J. F. Meadow. 2015. Microbiota of the indoor environment: A meta-analysis. Microbiome 3:49. doi:10.1186/s40168-015-0108-3.
Adan, O. C. G., and R. A. Samson. 2011. Fundamentals of mold growth in indoor environments and strategies for healthy living. Wageningen, The Netherlands: Wageningen Academic Publishers.
Al-Asmakh, M., F. Anuar, F. Zadjali, J. Rafter, and S. Pettersson. 2012. Gut microbial communities modulating brain development and function. Gut Microbes 3(4):366-373.
Allen, J. G., P. MacNaughton, J. G. Laurent, S. S. Flanigan, E. S. Eitland, and J. D. Spengler. 2015. Green buildings and health. Current Environmental Health Reports 2(3):250-258.
Allen, J. G., P. MacNaughton, U. Satish, S. Santanam, J. Vallarino, and J. D. Spengler. 2016. Associations of cognitive function scores with carbon dioxide, ventilation, and volatile organic compound exposures in office workers: A controlled exposure study of green and conventional office environments. Environmental Health Perspectives 124(6):805-812.
Alshannaq, A., and J. H. Yu. 2017. Occurrence, toxicity, and analysis of major mycotoxins in food. International Journal of Environmental Research and Public Health 14(6). doi:10.3390/ijerph14060632.
Alum, A., and G. Z. Isaacs. 2016. Aerobiology of the built environment: Synergy between Legionella and fungi. American Journal of Infection Control 44 (9 Suppl.):S138-S143.
Ansarin, K., L. Sahebi, and S. Sabur. 2013. Obstructive sleep apnea syndrome: Complaints and housing characteristics in a population in the United States. Sao Paulo Medical Journal 131(4):220-227.
Arany, C., J. A. Shea, W. J. O’Graham, and F. J. Miller. 1986. The effects of inhalation of organic chemical air contaminants on murine lung host defenses. Fundamental and Applied Toxicology 6(4):713-720.
Arrieta, M.-C., L. T. Stiemsma, P. A. Dimitriu, L. Thorson, S. Russell, S. Yurist-Doutsch, B. Kuzeljevic, M. J. Gold, H. M. Britton, D. L. Lefebvre, P. Subbarao, P. Mandhane, A. Becker, K. M. McNagny, M. R. Sears, T. Kollmann, W. W. Mohn, S. E. Turvey, and B. Brett Finlay. 2015. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science Translational Medicine 7(307):307ra152.
Ashbolt, N. J. 2015. Environmental (saprozoic) pathogens of engineered water systems: Understanding their ecology for risk assessment and management. Pathogens 4(2):390-405.
Azuma, K., I. Uchiyama, and J. Okumura. 2012. Assessing the risk of Legionnaires’ disease: The inhalation exposure model and the estimated risk in residential bathrooms. Regulatory Toxicology and Pharmacology 65(1):1-6.
Banda, C. 2016. Offering women safer options than “vaginal seeding” for infants born by caesarean section. British Medical Journal 352:i1734.
Barnard, E., and H. Li. 2017. Shaping of cutaneous function by encounters with commensals. The Journal of Physiology 595(2):437-450.
Barrera, E., V. Livchits, and E. Nardell. 2015. F-A-S-T: A refocused, intensified, administrative tuberculosis transmission control strategy. International Journal of Tuberculosis and Lung Disease 19(4):381-384.
Basagana, X., M. Esnaola, I. Rivas, F. Amato, M. Alvarez-Pedrerol, J. Forns, M. Lopez-Vicente, J. Pujol, M. Nieuwenhuijsen, X. Querol, and J. Sunyer. 2016. Neurodevelopmental deceleration by urban fine particles from different emission sources: A longitudinal observational study. Environmental Health Perspectives 124(10):1630-1636.
Behbod, B., J. E. Sordillo, E. B. Hoffman, S. Datta, T. E. Webb, D. L. Kwan, J. A. Kamel, M. L. Muilenberg, J. A. Scott, G. L. Chew, T. A. E. Platts-Mills, J. Schwartz, B. Coull, H. Burge, and D. R. Gold. 2015. Asthma and allergy development: Contrasting influences of yeasts and other fungal exposures. Clinical & Experimental Allergy 45(1):154-163.
Belkaid, Y., and J. A. Segre. 2014. Dialogue between skin microbiota and immunity. Science 346(6212):954-959.
Birzele, L. T., M. Depner, M. J. Ege, M. Engel, S. Kublik, C. Bernau, G. J. Loss, J. Genuneit, E. Horak, M. Schloter, C. Braun-Fahrländer, H. Danielewicz, D. Heederik, E. von Mutius, and A. Legatzki. 2016. Environmental and mucosal microbiota and their role in childhood asthma. Allergy 72(1):109-119.
Blavier, J., G. Songulashvili, C. Simon, M. Penninckx, S. Flahaut, M. L. Scippo, and F. Debaste. 2016. Assessment of methods of detection of water estrogenicity for their use as monitoring tools in a process of estrogenicity removal. Environmental Technology 37(24):3104-3119.
Blay, S. L., A. J. Schulz, and G. Mentz. 2015. The relationship of built environment to health-related behaviors and health outcomes in elderly community residents in a middle income country. Journal of Public Health Research 4(2):548.
Block, M. L., A. Elder, R. L. Auten, S. D. Bilbo, H. Chen, J. C. Chen, D. A. Cory-Slechta, D. Costa, D. Diaz-Sanchez, D. C. Dorman, D. R. Gold, K. Gray, H. A. Jeng, J. D. Kaufman, M. T. Kleinman, A. Kirshner, C. Lawler, D. S. Miller, S. S. Nadadur, B. Ritz, E. O. Semmens, L. H. Tonelli, B. Veronesi, R. O. Wright, and R. J. Wright. 2012. The outdoor air pollution and brain health workshop. Neurotoxicology 33(5):972-984.
Bokulich, N. A., D. A. Mills, and M. A. Underwood. 2013. Surface microbes in the neonatal intensive care unit: Changes with routine cleaning and over time. Journal of Clinical Microbiology 51(8):2617-2624.
Bokulich, N. A., J. Chung, T. Battaglia, N. Henderson, M. Jay, H. Li, D. Lieber A, F. Wu, G. I. Perez-Perez, Y. Chen, W. Schweizer, X. Zheng, M. Contreras, M. G. Dominguez-Bello, and M. J. Blaser. 2016. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Science Translational Medicine 8(343):343-382.
Boone, S. A., and C. P. Gerba. 2007. Significance of fomites in the spread of respiratory and enteric viral disease. Applied and Environmental Microbiology 7(6):1687-1696.
Borchers, A.T., C. Chang, and E.M. Gershwin. 2017. Mold and human health: A reality check. Clinical Reviews in Allergy and Immunology 52(3):305-322.
Borre, Y. E., R. D. Moloney, G. Clarke, T. G. Dinan, and J. F. Cryan. 2014. The impact of microbiota on brain and behavior: Mechanisms & therapeutic potential. Advances in Experimental Medicine and Biology 817:373-403.
Bouslimani, A., C. Porto, C. M. Rath, M. Wang, Y. Guo, A. Gonzalez, D. Berg-Lyon, G. Ackermann, G. J. Moeller Christensen, T. Nakatsuji, L. Zhang, A. W. Borkowski, M. J. Meehan, K. Dorrestein, R. L. Gallo, N. Bandeira, R. Knight, T. Alexandrov, and P. C. Dorrestein. 2015. Molecular cartography of the human skin surface in 3D. Proceedings of the National Academy of Sciences of the United States of America 112(17):E2120-E2129.
Bouslimani, A., A. V. Melnik, Z. Xu, A. Amir, R. R. da Silva, M. Wang, N. Bandeira, T. Alexandrov, R. Knight, and P. C. Dorrestein. 2016. Lifestyle chemistries from phones for individual profiling. Proceedings of the National Academy of Sciences of the United States of America 113(48):E7645-E7654.
Bradley, P. M., W. A. Battaglin, L. R. Iwanowicz, J. M. Clark, and C. A. Journey. 2016. Aerobic biodegradation potential of endocrine-disrupting chemicals in surface-water sediment at Rocky Mountain National Park, USA. Environmental Toxicology and Chemistry 35(5):1087-1096.
Breysse, J., D. E. Jacobs, W. Weber, S. Dixon, C. Kawecki, S. Aceti, and J. Lopez. 2011. Health outcomes and green renovation of affordable housing. Public Health Reports 126(Suppl 1):64-75.
Breysse, J., S. Dixon, J. Gregory, M. Philby, D. E. Jacobs, and J. Krieger. 2014. Effect of weatherization combined with community health worker in-home education on asthma control. American Journal of Public Health 104:e57-e64. doi:10.2105/AJPH.2013.301402.
Burge, H. 1980. “Bioaerosols” prevalence and health effects in the indoor environment. Journal of Allergy and Clinical Immunology 86(5):687-701.
Burokas, A., R. D. Moloney, T. G. Dinan, and J. F. Cryan. 2015. Microbiota regulation of the Mammalian gut-brain axis. Advances in Applied Microbiology 91:1-62.
Burrell, R. 1991. Microbiological agents as health risks in indoor air. Environmental Health Perspectives 95:29-34.
Casas, L., M. Torrent, J. P. Zock, G. Doekes, J. Forns, M. Guxens, M. Täubel, J. Heinrich, and J. Sunyer. 2013. Early life exposures to home dampness, pet ownership and farm animal contact and neuropsychological development in 4 year old children: A prospective birth cohort study. International Journal of Hygiene and Environmental Health 216(6):690-697.
Cavaleiro Rufo, J., J. Madureira, I. Paciencia, L. Aguiar, C. Pereira, D. Silva, P. Padrão, P. Moreira, L. Delgado, I. Annesi-Maesano, E. Oliveira Fernandes, J. P. Teixeira, and A. Moreira. 2017. Indoor fungal diversity in primary schools may differently influence allergic sensitization and asthma in children. Pediatric Allergy and Immunology 28(4):332-339.
Celedón, J. C., D. K. Milton, C. D. Ramsey, A. A. Litonjua, L. Ryan, T. A. Platts-Mills, and D. R. Gold. 2007. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. Journal of Allergy and Clinical Immunology 120(1):144-149.
Center for Health Care Design. 1995. Gardens in healthcare facilities: Uses, therapeutic benefits, and design recommendations. https://www.healthdesign.org/sites/default/files/Gardens%20in%20HC%20Facility%20Visits.pdf (accessed July 13, 2017).
Chambers, E. C., M. S. Pichardo, and E. Rosenbaum. 2016. Sleep and the housing and neighborhood environment of urban Latino adults living in low-income housing: The AHOME Study. Behavioral Sleep Medicine 14(2):169-184.
Chew, G. L., J. Wilson, F. A. Rabito, F. Grimsley, S. Iqbal, T. Reponen, M. L. Muilenberg, P. S. Thorne, D. G. Dearborn, and R. L. Morley. 2006. Mold and endotoxin levels in the aftermath of Hurricane Katrina: A pilot project of homes in New Orleans undergoing renovation. Environmental Health Perspectives 114:1883-1889.
Chew, G. L., W. E. Horner, K. Kennedy, C. Grimes, C. S. Barnes, W. Phipatanakul, D. Larenas-Linnemann, J. D. Miller, J. Portnoy, E. Levetin, P. B. Williams, S. Baxi, and J. Scott. 2016. Procedures to assist health care providers to determine when home assessments for potential mold exposure are warranted. The Journal of Allergy and Clinical Immunology: In Practice 4(3):417-422.
Claesson, M. J., I. B. Jeffery, S. Conde, S. E. Power, E. M. O’Connor, S. Cusack, H. M. Harris, M. Coakley, B. Lakshminarayanan, O. O’Sullivan, G. F. Fitzgerald, J. Deane, M. O’Connor, N. Harnedy, K. O’Connor, D. O’Mahony, D. van Sinderen, M. Wallace, L. Brennan, C. Stanton, J. R. Marchesi, A. P. Fitzgerald, F. Shanahan, C. Hill, R. P. Ross, and P. W. O’Toole. 2012. Gut microbiota composition correlates with diet and health in the elderly. Nature 488(7410):178-184.
Clemente, J. C., E. C. Pehrsson, M. J. Blaser, K. Sandhu, Z. Gao, and B. Wang. 2015. The microbiome of uncontacted Amerindians. Science Advances 1(3):e1500183. doi:10.1126/ sciadv.1500183.
Clifford, A., L. Lang, R. Chen, K. J. Anstey, and A. Seaton. 2016. Exposure to air pollution and cognitive functioning across the life course—A systematic literature review. Environmental Research 147:383-398.
Colton, M. D., P. MacNaughton, J. Vallarino, J. Kane, M. Bennett-Fripp, J. D. Spengler, and G. Adamkiewicz. 2014. Indoor air quality in green vs. conventional multifamily low-income housing. Environmental Science & Technology 48(14):7833-7841.
Colton, M. D., J. G. Laurent, P. MacNaughton, J. Kane, M. Bennett-Fripp, J. Spengler, and G. Adamkiewicz. 2015. Health benefits of green public housing: Associations with asthma morbidity and building-related symptoms. American Journal of Public Health 105:2482-2489.
Couch, R. B. 1981. Viruses and indoor air pollution. Bulletin of the New York Academy of Medicine 57(10):907-921.
Cox-Ganser, J. M., S. K. White, R. Jones, K. Hilsbos, E. Storey, P. L. Enright, C. Y. Rao, and K. Kreiss. 2005. Respiratory morbidity in office workers in a water-damaged building. Environmental Health Perspectives 113(4):485-490.
Cox-Ganser, J. M., J.-H. Park, and K. Kreiss. 2010. Office workers and teachers. In Occupational and environmental lung diseases, edited by P. Cullinan, S. Tarlo, and B. Nemery. Chichester, UK: John Wiley & Sons, Ltd.
Cox-Ganser, J. M., J.-H. Park, and R. Kanwal. 2011. Epidemiology and health effects in moisture-damaged damp buildings. In Sick building syndrome and related illness: Prevention and remediation of mold contamination, edited by W. Goldstein. Boca Raton, FL: CRC Press, Taylor & Francis Group. Pp. 11-22.
Cryan, J. F., and T. G. Dinan. 2012. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience 13(10):701-712.
Cunnington, A. J., K. Sim, A. Deierl, J. S. Kroll, E. Brannigan, and J. Darby. 2016. “Vaginal seeding” of infants born by caesarean section. British Medical Journal 352:i227.
Da Silva, C. A., D. Hartl, W. Liu, C. G. Lee, and J. A. Elias. 2008. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. Journal of Immunology 181(6):4279-4286.
Dadvand, P., M. J. Nieuwenhuijsen, M. Esnaola, J. Forns, X. Basagana, M. Alvarez-Pedrerol, I. Rivas, M. Lopez-Vicente, M. De Castro Pascual, J. Su, M. Jerrett, X. Querol, and J. Sunyer. 2015. Green spaces and cognitive development in primary schoolchildren. Proceedings of the National Academy of Sciences of the United States of America 112(26):7937-7942.
Dami, A. J., and S. J. Bracken. 2016. Breathing better through bugs: Asthma and the microbiome. The Yale Journal of Biology and Medicine 89(3):309-324.
Dannemiller, K. C., M. J. Mendell, J. M. Macher, K. Kumagai, A. Bradman, and N. Holland. 2014. Sequencing reveals that low fungal diversity in house dust is associated with childhood asthma development. Indoor Air 24(3):236-247.
Davies, D. E. 2014. Epithelial barrier function and immunity in asthma. Annals of the American Thoracic Society 11(Suppl. 5):S244-S251.
Dethlefsen, L., P. B. Eckburg, E. M. Bik, and D. A. Relman. 2006. Assembly of the human intestinal microbiota. Trends in Ecology & Evolution 21(9):517-523.
Dharmadhikari, A. S., M. Mphahlele, K. Venter, A. Stoltz, R. Mathebula, T. Masotla, M. van der Walt, M. Pagano, P. Jensen, and E. Nardell. 2014. Rapid impact of effective treatment on transmission of multidrug-resistant tuberculosis. International Journal of Tuberculosis and Lung Disease 18(9):1019-1025.
Diaz Heijtz, R., S. Wang, F. Anuar, Y. Qian, B. Bjorkholm, A. Samuelsson, M. L. Hibberd, H. Forssberg, and S. Pettersson. 2011. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America 108(7):3047-3052.
Dick, E. C., L. C. Jennings, K. A. Mink, C. D. Wartgow, and S. L. Inborn. 1987. Aerosol transmission of rhinovirus colds. The Journal of Infectious Diseases 156(3):442-448.
Du Toit, G., G. Roberts, P. H. Sayre, H. T. Bahnson, S. Radulovic, A. F. Santos, H. A. Brough, D. Phippard, M. Basting, M. Feeney, V. Turcanu, M. L. Sever, M. Gomez Lorenzo, M. Plaut, and G. Lack. 2015. Randomized trial of peanut consumption in infants at risk for peanut allergy. New England Journal of Medicine 372:808-813.
Ege, M. J., M. Mayer, A. C. Normand, J. Genuneit, W. O. Cookson, C. Braun-Fahrlander, D. Heederik, R. Piarroux, and E. von Mutius. 2011. Exposure to environmental microorganisms and childhood asthma. New England Journal of Medicine 364(8):701-709.
Eggleston, P., D. Rosenstreich, H. Lynn, P. Gergen, D. Baker, M. Kattan, K. M. Mortimer, H. Mitchell, D. Ownby, R. Slavin, and F. Malveaux. 1998. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. Clinical Immunology 102(4 Pt. 1):563-570.
Emerson, J. B, P. B. Keady, T. E. Brewer, N. Clements, E. E. Morgan, J. Awerbuch, S. L. Miller, and N. Fierer. 2015. Impacts of flood damage on airborne bacteria and fungi in homes after the 2013 Colorado Front Range flood. Environmental Science & Technology 49(5):2675-2684.
Evans, G. W., and M. A. Schamberg. 2009. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences of the United States of America 106(16):6545-6549.
Faber, S., G. M. Zinn, A. Boggess, T. Fahrenholz, J. C. Kern, II, and H. M. Kingston. 2015. A cleanroom sleeping environment’s impact on markers of oxidative stress, immune dysregulation, and behavior in children with autism spectrum disorders. BMC Complementary and Alternative Medicine 15:71.
Falkinham, J. O. 2003. Mycobacterial aerosols and respiratory disease. Emerging Infectious Diseases 9(7):763-767.
Fall, T., C. Lundholm, K. Fall, F. Fang, A. Hedhammar, O. Kämpe, E. Ingelsson, and C. Almqvist. 2015. Early exposure to dogs and farm animals and the risk of childhood asthma. JAMA Pediatrics 169(11):e153219.
Feazel, L. M., L. K. Baumgartner, K. L. Peterson, D. N. Frank, J. K. Harris, and N. R. Pace. 2009. Opportunistic pathogens enriched in showerhead biofilms. Proceedings of the National Academy of Sciences of the United States of America 106(38):16393-16399.
Fernandez, J. A., P. Lopez, D. S. Orozco, and J. Merino. 2002. Clinical study of an outbreak of Legionnaire’s disease in Alcoy, Southeastern Spain. European Journal of Clinical Microbiology and Infectious Diseases 21(10):729-735.
Fisk, W. J., Q. Lei-Gomez, and M. J. Mendell. 2007. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air 17:284-296.
Forno, E., A. B. Onderdonk, J. McCracken, A. A. Litonjua, D. Laskey, M. L. Delaney, A. M. Dubois, D. R. Gold, L. M. Ryan, S. T. Weiss, and J. C. Celedón. 2008. Diversity of the gut microbiota and eczema in early life. Clinical and Molecular Allergy 6:11.
Francisco, P. W., D. E. Jacobs, L. Targos, S. L. Dixon, J. Breysse, W. Rose, and S. Cali. 2016. Ventilation, indoor air quality, and health in homes undergoing weatherization. Indoor Air 27(2):463-477.
Frankel, M., G. Beko, M. Timm, S. Gustavsen, E. W. Hansen, and A. M. Madsen. 2012. Seasonal variations of indoor microbial exposures and their relation to temperature, relative humidity, and air exchange rate. Applied and Environmental Microbiology 78(23):8289-8297.
Fujimura, K. E., T. Demoor, M. Rauch, A. A. Faruqi, S. Jang, and C. C. Johnson. 2014. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proceedings of the National Academy of Sciences of the United States of America 111(2):805-810.
Gardner, D. E. 1982. Use of experimental airborne infections for monitoring altered host defenses. Environmental Health Perspectives 43:99-107.
Gold, D. R., G. Adamkiewicz, S. Hasan Arshad, J. C. Celedón, M. D. Chapman, G. L. Chew, D. N. Cook, A. Custovic, U. Gehring, J. E. Gern, C. C. Johnson, S. Kennedy, P. Koutrakis, B. Leaderer, H. Mitchell, A. A. Litonjua, G. A. Mueller, G. T. O’Connor, D. Ownby, W. Phipatanakul, V. Persky, M. S. Perzanowski, C.D. Ramsey, P. M. Salo, J. M. Schwaninger, J. E. Sordillo, A. Spira, S. F. Suglia, A. Togias, D. C. Zeldin, and E. C. Matsui. 2017. NIAID, NIEHS, NHLBI, and MCAN Workshop Report: The indoor
environment and childhood asthma—implications for home environmental intervention in asthma prevention and management. Journal of Allergy and Clinical Immunology [Epub ahead of print].
Gong, Y., S. Palmer, J. Gallacher, T. Marsden, and D. Fone. 2016. A systematic review of the relationship between objective measurements of the urban environment and psychological distress. Environment International 96:48-57.
Gozdz, J., C. Ober, and D. Vercelli. 2016. Innate immunity and asthma risk. New England Journal of Medicine 375:1897-1899.
Gramec, S. D., and L. P. Mašič. 2016. Bisphenol A and its analogs: Do their metabolites have endocrine activity? Environmental Toxicology and Pharmacology 47:182-199.
Groer, M. W., K. E. Gregory, A. Louis-Jacques, S. Thibeau, and W. A. Walker. 2015. The very low birth weight infant microbiome and childhood health. Birth Defects Research Part C: Embryo Today 105(4):252-264.
Hägerhed-Engman, L., C.-G. Bornehag, and J. Sundell. 2009. Building characteristics associated with moisture related problems in 8,918 Swedish dwellings. International Journal of Environmental Health Research 19(4):251-265.
Hanski, I., L. von Hertzen, N. Fyhrquist, K. Koskinen, K. Torppa, T. Laatikainen, P. Karisola, P. Auvinen, L. Paulin, M. J. Mäkelä, E. Vartiainen, T. U. Kosunen, H. Alenius, and T. Haahtela. 2012. Environmental biodiversity, human microbiota, and allergy are interrelated. Proceedings of the National Academy of Sciences of the United States of America 109(21):8334-8339.
Hanson, B., Y. Zhou, E. Bautista, B. Urch, M. Speck, F. Silverman, M. Muilenberg, W. Phipatanakul, E. Sodergren, D. R. Gold, and J. E. Sordillo. 2016. Characterization of the bacterial and fungal microbiome in indoor dust and outdoor air samples: A pilot study. Environmental Science: Processes & Impacts 18(6):713-724.
Hanson, J. L., N. Hair, D. G. Shen, F. Shi, J. H. Gilmore, B. L. Wolfe, and S. D. Pollak. 2013. Family poverty affects the rate of human infant brain growth. PLOS ONE 8(12):e80954.
Harada, R. N., and J. E. Repine. 1985. Pulmonary host defense mechanisms. CHEST Journal 87(2):247-252.
Harris, M. H., D. R. Gold, S. L. Rifas-Shiman, S. J. Melly, A. Zanobetti, B. A. Coull, J. D. Schwartz, A. Gryparis, I. Kloog, P. Koutrakis, D. C. Bellinger, R. F. White, S. K. Sagiv, and E. Oken. 2015. Prenatal and childhood traffic-related pollution exposure and childhood cognition in the project viva cohort (Massachusetts, USA). Environmental Health Perspectives 123(10):1072-1078.
Hartz, L. E., W. Bradshaw, and D. H. Brandon. 2015. Potential NICU environmental influences on the neonate’s microbiome: A systematic review. Advances in Neonatal Care 15(5):324-335.
Hatch, G. E., E. Boykin, J. A. Graham, J. Lewtas, F. Pott, K. Loud, and J. L. Mumford. 1985. Inhalable particles and pulmonary host defense: In vivo and in vitro effects of ambient air and combustion particles. Environmental Research 36(1):67-80.
Hatch, T. F. 1961. Distribution and deposition of inhaled particles in respiratory tract. Bacteriological Reviews 25(3):237-240.
Haupt, T. E., R. T. Heffernan, J. J. Kazmierczak, H. Nehls-Lowe, B. Rheineck, C. Powell, K. K. Leonhardt, A. S. Chitnis, and J. P. Davis. 2012. An outbreak of Legionnaires disease associated with a decorative water wall fountain in a hospital. Infection Control and Hospital Epidemiology 33(2):185-191.
Heederik, D., and E. von Mutius. 2012. Does diversity of environmental microbial exposure matter for the occurrence of allergy and asthma? Journal of Allergy and Clinical Immunology 130(1):44-50.
Heindel, J. J., L. A. Skalla, B. R. Joubert, C. H. Dilworth, and K. A. Gray. 2017. Review of developmental origins of health and disease publications in environmental epidemiology. Reproductive Toxicology 68:34-48.
Hill, C. J., D. B. Lynch, K. Murphy, M. Ulaszewska, I. B. Jeffery, C. A. O’Shea, C. Watkins, E. Dempsey, F. Mattivi, K. Tuohy, R. P. Ross, C. A. Ryan, P. W. O’Toole, and C. Stanton. 2017. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 5(1):4.
Holst, G. J., A. Høst, G. Doekes, H. W. Meyer, A. M. Madsen, K. B. Plesner, and T. Sigsgaard. 2016. Allergy and respiratory health effects of dampness and dampness related agents in schools and homes: A cross-sectional study in Danish pupils. Indoor Air 26:880-891.
Hoppe, K. A., N. Metwali, S. S. Perry, T. Hart, P. A. Kostle, and P. S. Thorne. 2012. Assessment of airborne exposures and health in flooded homes undergoing renovation. Indoor Air 22(6):446-456.
Hunninghake, G. M., J. Lasky-Su, M. E. Soto-Quirós, L. Avila, C. Liang, S. L. Lake, T. J. Hudson, M. Spesny, E. Fournier, J. S. Sylvia, N. B. Freimer, B. J. Klanderman, B. A. Raby, and J. C. Celedón. 2008. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. American Journal of Respiratory and Critical Care Medicine 177(8):830-836.
Hunninghake, G. M., J. H. Chu, S. S. Sharma, M. H. Cho, B. E. Himes, A. J. Rogers, A. Murphy, V. J. Carey, and B. A. Raby. 2011. The CD4+ T-cell transcriptome and serum IgE in asthma: IL17RB and the role of sex. BMC Pulmonary Medicine 11:17.
Illi, S., M. Depner, J. Genuneit, E. Horak, G. Loss, C. Strunz-Lehner, G. Buchele, A. Boznanski, H. Danielewicz, P. Cullinan, D. Heederik, C. Braun-Fahrlander, and E. von Mutius. 2012. Protection from childhood asthma and allergy in Alpine farm environments: The GABRIEL Advanced Studies. Journal of Allergy and Clinical Immunology 129(6):1470-1477.
IOM (Institute of Medicine). 2004. Damp indoor spaces and health. Washington, DC: The National Academies Press.
IOM. 2007. Green healthcare institutions: Health, environment, and economics: Workshop summary. Washington, DC: The National Academies Press.
Jaakkola, M. S., R. Quansah, T. T. Hugg, S. A. Heikkinen, and J. J. Jaakkola. 2013. Association of indoor dampness and molds with rhinitis risk: A systematic review and meta-analysis. Journal of Allergy and Clinical Immunology 132(5):1099-1110.
Jacobs, D. E., E. Ahonen, S. L. Dixon, S. Dorevitch, J. Breysse, J. Smith, A. Evens, D. Dobrez, M. Isaacson, C. Murphy, L. Conroy, and P. Levavi. 2015. Moving into green healthy housing. Journal of Public Health Management and Practice 21(4):345-354.
Janicki, T., M. Krupiński, and J. Długoński. 2016. Degradation and toxicity reduction of the endocrine disruptors nonylphenol, 4-tert-octylphenol and 4-cumylphenol by the nonligninolytic fungus Umbelopsis isabellina. Bioresource Technology 200:223-229.
Jasarevic, E., K. E. Morrison, and T. L. Bale. 2016. Sex differences in the gut microbiome-brain axis across the lifespan. Philosophical Transactions of the Royal Society B: Biological Sciences 371(1688):20150122. doi:10.1098/rstb.2015.0122.
Jednorog, K., I. Altarelli, K. Monzalvo, J. Fluss, J. Dubois, C. Billard, G. Dehaene-Lambertz, and F. Ramus. 2012. The influence of socioeconomic status on children’s brain structure. PLOS ONE 7(8):e42486.
Julian, T. R. 2010. Fomites in infectious disease transmission: A modeling, laboratory, and field study on microbial transfer between skin and surfaces. Ph.D. dissertation. Stanford, CA: Stanford University.
Kanchongkittiphon, W., M. J. Mendell, J. M. Gaffin, G. Wang, and W. Phipatanakul. 2015. Indoor environmental exposures and exacerbation of asthma: An update to the review by the Institute of Medicine. Environmental Health Perspectives 123(1):6-20.
Kelly, D., S. Conway, and R. Aminov. 2005. Commensal gut bacteria: Mechanisms of immune modulation. Trends in Immunology 26(6):326-333.
Kercsmar, C. M., D. G. Dearborn, M. Schluchter, L. Xue, H. L. Kirchner, J. Sobolewski, S. J. Greenberg, S. J. Vesper, and T. Allan. 2006. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environmental Health Perspectives 114:1574-1580.
Keunen, K., R. M. van Elburg, F. van Bel, and M. J. Benders. 2015. Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatric Research 77(1-2):148-155.
Kicinski, M., G. Vermeir, N. Van Larebeke, E. Den Hond, G. Schoeters, L. Bruckers, I. Sioen, E. Bijnens, H. A. Roels, W. Baeyens, M. K. Viaene, and T. S. Nawrot. 2015. Neurobehavioral performance in adolescents is inversely associated with traffic exposure. Environment International 75:136-143.
Kitsios, G. D., and A. Morris. 2016. Mode of delivery to the brave new (microbial) world: A defining moment for the respiratory microbiome? EBioMedicine 9:25-26.
Koep, T. H., F. T. Enders, C. Pierret, S. C. Ekker, D. Krageschmidt, K. L. Neff, M. Lipsitch, J. Shaman, and W. C. Huskins. 2013. Predictors of indoor absolute humidity and estimated effects on influenza virus survival in grade schools. BMC Infectious Diseases 13:71.
Koestel, Z. L., R. C. Backus, K. Tsuruta, W. G. Spollen, S. A. Johnson, A. B. Javurek, M. R. Ellersieck, C. E. Wiedmeyer, K. Kannan, J. Xue, N. J. Bivens, S. A. Givan, and C. S. Rosenfeld. 2017. Bisphenol A (BPA) in the serum of pet dogs following short-term consumption of canned dog food and potential health consequences of exposure to BPA. Science of the Total Environment 579:1804-1814.
Konya, T., B. Koster, H. Maughan, M. Escobar, M. B. Azad, D. S. Guttman, M. R. Sears, A. B. Becker, J. R. Brook, T. K. Takaro, A. L. Kozyrskyj, J. A. Scott, and CHILD Study Investigators. 2014. Associations between bacterial communities of house dust and infant gut. Environmental Research 131:25-30.
Korkalainen, M., M. Täubel, J. Naarala, P. Kirjavainen, A. Koistinen, A. Hyvärinen, H. Komulainen, and M. Viluksela. 2017. Synergistic proinflammatory interactions of microbial toxins and structural components characteristic to moisture-damaged buildings. Indoor Air 27(1):13-23.
Krieger, J. W., T. K. Takaro, L. Song, and M. Weaver. 2005. The Seattle-King County Healthy Homes Project: A randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. American Journal of Public Health 95:652-659.
Kuhn, D. M., and M. A. Ghannoum. 2003. Indoor mold, toxigenic fungi, and stachybotrys chartarum: Infectious disease perspective. Clinical Microbiology Reviews 16(1):144-172.
Lai, P. S., W. J. Sheehan, J. M. Gaffin, C. R. Petty, B. A. Coull, D R. Gold, and W. Phipatanakul. 2015. School endotoxin exposure and asthma morbidity in inner-city children. Chest 148(5):1251-1258.
Lambrecht, B. N., and H. Hammad. 2013. Asthma: The importance of dysregulated barrier 782 immunity. European Journal of Immunology 43(12):3125-3137.
Lambrecht, B. N., and H. Hammad. 2014. Allergens and the airway epithelium response: 780 gateway to allergic sensitization. Journal of Allergy and Clinical Immunology 134(3):499-507.
Lax, S., and J. A. Gilbert. 2015. Hospital-associated microbiota and implications for nosocomial infections. Trends in Molecular Medicine 21(7):427-432.
Lax, S., D. P. Smith, J. Hampton-Marcell, S. M. Owens, K. M. Handley, N. M. Scott, S. M. Gibbons, P. Larsen, B. D. Shogan, S. Weiss, J. L. Metcalf, L. K. Ursell, Y. Vazquez-Baeza, W. Van Treuren, N. A. Hasan, M. K. Gibson, R. Colwell, G. Dantas, R. Knight, and J. A. Gilbert. 2014. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345(6200):1048-1052.
Lax, S., C. R. Nagler, and J. A. Gilbert. 2015. Our interface with the built environment: Immunity and the indoor microbiota. Trends in Immunology 36(3):121-123.
Li, Y., G. M. Leung, J. W. Tang, X. Yang, C. Y. Chao, J. Z. Lin, J. W. Lu, P. V. Nielsen, J. Niu, H. Qian, A. C. Sleigh, H. J. Su, J. Sundell, T. W. Wong, and P. L. Yuen. 2007. Role of ventilation in airborne transmission of infectious agents in the built environment—a multidisciplinary systematic review. Indoor Air 17(1):2-18.
Lokugamage, A. 2016. Study provides evidence that “vaginal seeding” of infants born by caesarean partially restores microbiota. British Medical Journal 352:i1737.
Loss, G. J., M. Depner, A. J. Hose, J. Genuneit, A. M. Karvonen, A. Hyvärinen, C. Roduit, M. Kabesch, R. Lauener, P. I. Pfefferle, J. Pekkanen, J. C. Dalphin, J. Riedler, C. Braun-Fahrländer, E. von Mutius, and M. J. Ege. 2016. The early development of wheeze: Environmental determinants and genetic susceptibility at 17q21. American Journal of Respiratory and Critical Care Medicine 193(8):889-897.
Ly, N. P., B. Ruiz-Perez, A. B. Onderdonk, A. O. Tzianabos, A. A. Litonjua, C. Liang, D. Laskey, M. L. Delaney, A. M. DuBois, H. Levy, D. R. Gold, L. M. Ryan, S. T. Weiss, and J. C. Celedón. 2006. Mode of delivery and cord blood cytokines: A birth cohort study. Clinical and Molecular Allergy 4:13.
Lynch, S. V., R. A. Wood, H. Boushey, L. B. Bacharier, G. R. Bloomberg, M. Kattan, G. T. O’Connor, M. T. Sandel, A. Calatroni, E. Matsui, C. C. Johnson, H. Lynn, C. M. Visness, K. F. Jaffee, P. J. Gergen, D. R. Gold, R. J. Wright, K. Fujimura, M. Rauch, W. W. Busse, and J. E. Gern. 2014. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. Journal of Allergy and Clinical Immunology 134(3):593-601.
Macher, J. M., M. J. Mendell, W. Chen, and K. Kumagai. 2017. Development of a method to relate the moisture content of a building material to its water activity. Indoor Air 27(3):599-608.
Manor, O., R. Levy, and E. Borenstein. 2014. Mapping the inner workings of the microbiome: Genomic- and metagenomic-based study of metabolism and metabolic interactions in the human microbiome. Cell Metabolism 20(5):742-752.
Manzano-León, N. 2013. Variation in the composition and in vitro proinflammatory effect of urban particulate matter from different sites. Journal of Biochemical and Molecular Toxicology 27(1):87-97.
Mao, J., and N. Gao. 2015. The airborne transmission of infection between flats in high-rise residential buildings: A review. Building and Environment 94(Part 2):516-531.
Martin, R., H. Makino, A. Cetinyurek Yavuz, K. Ben-Amor, M. Roelofs, E. Ishikawa, H. Kubota, S. Swinkels, T. Sakai, K. Oishi, A. Kushiro, and J. Knol. 2016. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLOS ONE 11(6):e0158498.
McDevitt, J., S. Rudnick, M. First, and J. Spengler. 2010. Role of absolute humidity in the inactivation of influenza viruses on stainless steel surfaces at elevated temperatures. Applied and Environmental Microbiology 76(12):3943-3947.
MDHHS (Michigan Department of Human and Health Services). 2016. MDHHS issues update to 2015 Legionnaires’ disease report for Genesee County. http://www.michigan.gov/mdhhs/0,5885,7-339--379334--,00%20.html (accessed January 30, 2017).
MDHHS and GCHD (Genesee County Health Department). 2015. Legionellosis outbreak-Genesee County, May 2015–November 2015 summary analysis. https://www.michigan.gov/documents/mdhhs/Updated_5-15_to_11-15_Legionellosis_Analysis_Summary_511707_7.pdf (accessed January 30, 2017).
MDHHS and GCHD. 2016. Legionellosis outbreak-Genesee County, June 2014–March 2015 full analysis. https://www.michigan.gov/documents/mdhhs/6-14_to_3-15_Legionellosis_Report_Full_Analysis_Results_511708_7.pdf (accessed January 30, 2017).
Mendell, M. J. 2007. Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children: A review. Indoor Air 17(4):259-277.
Mendell, M. J., and K. Kumagai. 2017. Observation-based metrics for residential dampness and mold with dose-response relationships to health: A review. Indoor Air 27(3):506-517.
Mendell, M. J., Q. Lei-Gomez, A. G. Mirer, O. Seppänen, and G. Brunner. 2008. Risk factors in heating, ventilating, and air-conditioning systems for occupant symptoms in U.S. office buildings: The U.S. EPA BASE study. Indoor Air 18(4):301-316.
Mendell, M. J., A. G. Mirer, K. Cheung, M. Tong, and J. Douwes. 2011. Respiratory and allergic health effects of dampness, mold, and dampness related agents: A review of the epidemiologic evidence. Environmental Health Perspectives 119:748-756.
Miller, J. D., M. Sun, A. Gilyan, J. Roy, and T. G. Rand. 2010. Inflammation-associated gene transcription and expression in mouse lungs induced by low molecular weight compounds from fungi from the built environment. Chemico-Biological Interactions 183(1):113-124.
Mitchell, H., R. D. Cohn, J. Wildfire, E. Thornton, S. Kennedy, J. M. El-Dahr, P. C. Chulada, M. M. Mvula, L. F. Grimsley, M. Y. Lichtveld, L. E. White, Y. M. Sterling, K. U. Stephens, and W. J. Martin. 2012. Implementation of evidence-based asthma interventions in post-Katrina New Orleans: The Head-off Environmental Asthma in Louisiana (HEAL) study. Environmental Health Perspectives 120(11):1607-1612.
Mohapatra, A., S. J. Van Dyken, C. Schneider, J. C. Nussbaum, H. E. Liang, and R. M. Locksley. 2016. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunology 9(1):275-286.
Mphaphlele, M., A. S. Dharmadhikari, P. A. Jensen, S. N. Rudnick, T. H. van Reenen, M. A. Pagano, W. Leuschner, T. A. Sears, S. P. Milonova, M. van der Walt, A. C. Stoltz, K. Weyer, and E. A. Nardell. 2015. Institutional tuberculosis transmission. Controlled trial of upper room ultraviolet air disinfection: A basis for new dosing guidelines. American Journal of Respiratory and Critical Care Medicine 192(4):477-484.
Mudarri, D., and W. J. Fisk. 2007. Public health and economic impact of dampness and mold. Indoor Air 17(3):226-235.
Myles, I. A. 2014. Fast food fever: Reviewing the impacts of the Western diet on immunity. Nutrition Journal 13:61.
Nardell, E. A. 2016. Indoor environmental control of tuberculosis and other airborne infections. Indoor Air 26(1):79-87.
Nardell, E. A., J. Keegan, S. A. Cheney, and S. C. Etkind. 1991. Airborne infection: Theoretical limits of protection achievable by building ventilation. American Review of Respiratory Diseases 144(2):302-306.
Neish, A. S. 2009. Microbes in gastrointestinal health and disease. Gastroenterology 136(1):65-80.
Neu, J. 2016. The microbiome during pregnancy and early postnatal life. Seminars in Fetal and Neonatal Medicine 21(6):373-379.
Neufeld, K. M., N. Kang, J. Bienenstock, and J. A. Foster. 2011. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology & Motility 23(3):255-264.
Nicas, M., and R. M. Jones. 2009. Relative contributions of four exposure pathways to influenza infection risk. Risk Analysis 29(9):1292-1303.
Nieuwenhuijsen, M. J. 2016. Urban and transport planning, environmental exposures and health: New concepts, methods and tools to improve health in cities. Environmental Health 15(Suppl. 1):38.
Norback, D., J.-P Zock, E. Plana, J. Heinrich, C. Svanes, J. Sunyer, N. Kunzli, S. Villani, M. Olivieri, and A. Soon. 2013. Mould and dampness in dwelling places, and onset of asthma: The population-based cohort ECRHS. Occupational and Environmental Medicine 70(5):325-331.
NRC (National Research Council). 2006. Green schools: Attributes for health and learning. Washington, DC: The National Academies Press.
O’Dea, E. M., N. Amarsaikhan, H. Li, J. Downey, E. Steele, S. J. Van Dyken, R. M. Locksley, and S. P. Templeton. 2014. Eosinophils are recruited in response to chitin exposure and enhance Th2-mediated immune pathology in Aspergillus fumigatus infection. Infection and Immunity 82(8):3199-3205.
O’Loughlin, R. E., L. Kightlinger, M. C. Werpy, E. Brown, V. Stevens, C. Hepper, T. Keane, R. F. Benson, B. S. Fields, and M. R. Moore. 2007. Restaurant outbreak of Legionnaires’ disease associated with a decorative fountain: An environmental and case-control study. BMC Infectious Diseases 7:93.
O’Mahony, S. M., G. Clarke, Y. E. Borre, T. G. Dinan, and J. F. Cryan. 2015. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Research 277:32-48.
OSHA (Occupational Safety and Health Administration). 2017. OSHA technical manual; Section III, Chapter 7; Legionnaires’ disease. https://www.osha.gov/dts/osta/otm/otm_iii/otm_iii_7.html (accessed March 5, 2017).
Oudin, A., J. C. Richter, T. Taj, L. Al-Nahar, and K. Jakobsson. 2016. Poor housing conditions in association with child health in a disadvantaged immigrant population: A cross-sectional study in Rosengård, Malmö, Sweden. BMJ Open 6(1):e007979.
Park, J.-H., D. L. Spiegelman, D. R. Gold, H. A. Burge, and D. K. Milton. 2001. Predictors of airborne endotoxin in the home. Environmental Health Perspectives 109(8):859-864.
Park, J.-H., J. Cox-Ganser, C. Rao, and K. Kreiss. 2006. Fungal and endotoxin measurements in dust associated with respiratory symptoms in a water-damaged office building. Indoor Air 16(3):192-203.
Park, J.-H., J. M. Cox-Ganser, K. Kreiss, S. K. White, and C. Y. Rao. 2008. Hydrophilic fungi and ergosterol associated with respiratory illness in a water-damaged building. Environmental Health Perspectives 116(1):45-50.
Park, J.-H., J. M. Cox-Ganser, S. K. White, A. S. Laney, S. M. Caulfield, W. A. Turner, A. D. Sumner, and K. Kreiss. 2017. Bacteria in a water-damaged building: Associations of actinomycetes and nontuberculous mycobacteria with respiratory health in occupants. Indoor Air 27(1):24-33.
Peng, Z., D. Jin, H. B. Kim, C. W. Stratton, B. Wu, W. Tang, and Z. Sun. 2017. An update on antimicrobial resistance in Clostridium difficile: Resistance mechanisms and antimicrobial susceptibility testing. Journal of Clinical Microbiology 55:7. doi:10.1128/ JCM.02250-16.
Perzanowski, M. S., R. L. Miller, P. S. Thorne, R. G. Barr, A. Divjan, B. J. Sheares, R. S. Garfinkel, F. P. Perera, I. F. Goldstein, and G. L. Chew. 2006. Endotoxin in inner-city homes: Associations with wheeze and eczema in early childhood. Journal of Allergy and Clinical Immunology 117(5):1082-1089.
Pistiner, M., D. R. Gold, H. Abdulkerim, E. Hoffman, and J. C. Celedón. 2008. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. Journal of Allergy and Clinical Immunology 122(2):274-279.
Pongracic, J. A., G. T. O’Connor, M. L. Muilenberg, B. Vaughn, D. R. Gold, M. Kattan, W. J. Morgan, R. S. Gruchalla, E. Smartt, and H. E. Mitchell. 2010. Journal of Allergy and Clinical Immunology 125(3):593-599.
Ponnusamy D., E. V. Kozlova, J. Sha, T. E. Erova, S. R. Azar, E. C. Fitts, M. L. Kirtley, B. L. Tiner, J. A. Andersson, C. J. Grim, R. P. Isom, N. A. Hasan, R. R. Colwell, and A. K. Chopra. 2016. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proceedings of the National Academy of Sciences of the United States of America 113(3):722-727.
Prince, A. L., K. M. Antony, D. M. Chu, and K. M. Aagaard. 2014. The microbiome, parturition, and timing of birth: More questions than answers. Journal of Reproductive Immunology 104-105:12-19.
Prince, A. L., D. M. Chu, M. D. Seferovic, K. M. Antony, J. Ma, and K. M. Aagaard. 2015. The perinatal microbiome and pregnancy: Moving beyond the vaginal microbiome. Cold Spring Harbor Perspectives in Medicine 5(6):a023051. doi:10.1101/cshperspect. a023051.
Principe, L., P. Tomao, and P. Visca. 2017. Legionellosis in the occupational setting. Environmental Research 152:485-495.
Prussin, A. J., E. B. Garcia, and L. C. Marr. 2015. Total virus and bacteria concentrations in indoor and outdoor air. Environmental Science & Technology Letters 2(4):84-88.
Quansah, R., M. S. Jaakkola, T. T. Hugg, S. A. M. Heikkinen, and J. J. K. Jaakkola. 2012. Residential dampness and molds and the risk of developing asthma: Review and meta-analysis. PLOS ONE 7(11):e47526.
Rand, T. G., J. Dipenta, C. Robbins, and J. D. Miller. 2011. Effects of low molecular weight fungal compounds on inflammatory gene transcription and expression in mouse alveolar macrophages. Chemico-Biological Interactions 190(2-3):139-147.
Rand, T. G., C. Robbins, D. Rajaraman, M. Sun, and J. D. Miller. 2013. Induction of Dectin-1 and asthma-associated signal transduction pathways in RAW 264.7 cells by a triple-helical (1, 3)-β-D glucan, curdlan. Archives of Toxicology 87(10):1841-1850.
Reber, S. O., P. H. Siebler, N. C. Donner, J. T. Morton, D. G. Smith, J. M. Kopelman, K. R. Lowe, K. J. Wheeler, J. H. Fox, J. E. Hassell, B. N. Greenwood, C. Jansch, A. Lechner, D. Schmidt, N. Uschold-Schmidt, A. M. Füchsl, D. Langgartner, F. R. Walker, M. W. Hale, G. Lopez Perez, W. Van Treuren, A. González, A. L. Halweg-Edwards, M. Fleshner, C. L. Raison, G. A. Rook, S. D. Peddada, R. Knight, and C. A. Lowry. 2016. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proceedings of the National Academy of Sciences of the United States of America 11(22):E3130-E3139.
Redford, P. S., K. D. Mayer-Barber, E. F. W. McNab, E. Stavropoulos, A. Wack, A. Sher, and A. O’Garra. 2014. Influenza A virus impairs control of Mycobacterium tuberculosis coinfection through a type I interferon receptor-dependent pathway Influenza A virus impairs control of Mycobacterium tuberculosis coinfection through a type I interferon receptor-dependent pathway. Journal of Infectious Diseases 209(2):270-274.
Rhoads, W. J., P. Ji, A. Pruden, and M. A. Edwards. 2015. Water heater temperature set point and water use patterns influence Legionella pneumophila and associated microorganisms at the tap. Microbiome 3(1):1-13.
Robbins, C. A., L. J. Swenson, M. L. Nealley, R. E. Gots, and B. J. Kelman. 2000. Health effects of mycotoxins in indoor air: A critical review. Applied Occupational and Environmental Hygiene 15(10):773-784.
Rook, G. A. 2013. Regulation of the immune system by biodiversity from the natural environment: An ecosystem service essential to health. Proceedings of the National Academy of Sciences of the United States of America 110(46):18360-18367.
Rook, G. A. W., and R. Knight. 2015. Environmental microbial diversity and noncommunicable diseases. In Connecting global priorities: Biodiversity and human heath. A state of knowledge review (Ch. 8). http://www.grahamrook.net/resources/Rook-and-Knight_SOK-biodiversity_15.pdf (accessed July 13, 2017).
Rook, G. A. W., and C. A. Lowry. 2008. The hygiene hypothesis and psychiatric disorders. Trends in Immunology 29(4):150-158.
Rook, G. A. W., C. L. Raison, and C. A. Lowry. 2014. Microbial “old friends,” immunoregulation and socioeconomic status. Clinical & Experimental Immunology 17(1):1-12.
Rutayisire, E., K. Huang, Y. Liu, and F. Tao. 2016. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterology 16(1):86.
Saigal, S., and L. W. Doyle. 2008. An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet 371(9608):261-269.
Sandora, T. J., M. C. Shih, and D. A. Goldmann. 2008. Reducing absenteeism from gastrointestinal and respiratory illness in elementary school students: A randomized, controlled trial of an infection-control intervention. Pediatrics 121(6):e1555-e1562.
Sattar, S. A. 2016. Indoor air as a vehicle for human pathogens: Introduction, objectives, and expectation of outcome. American Journal of Infection Control 44(9 Suppl.):S95-S101.
Schiffman, S. S., C. E. Studwell, L. R. Landerman, K. Berman, and J. S. Sundy. 2005. Symptomatic effects of exposure to diluted air sampled from a swine confinement atmosphere on healthy human subjects. Environmental Health Perspectives 113(5):567-576.
Schoen, M. E., and N. J. Ashbolt. 2011. An in-premise model for Legionella exposure during showering events. Water Research 45(18):5826-5836.
Schwake, D. O., E. Garner, O. R. Strom, A. Pruden, and M. A. Edwards. 2016. Legionella DNA markers in tap water coincident with a spike in Legionnaires’ disease in Flint, MI. Environmental Science & Technology Letters 3(9):311-315.
Sharpe, R. A., N. Bearman, C. R. Thornton, K. Husk, and N. J. Osborne. 2015. Indoor fungal diversity and asthma: A meta-analysis and systematic review of risk factors. Journal of Allergy and Clinical Immunology 135(1):110-122.
Shiue, I. 2015. Indoor mildew odour in old housing was associated with adult allergic symptoms, asthma, chronic bronchitis, vision, sleep and self-rated health: USA NHANES, 2005–2006. Environmental Science and Pollution Research 22(18):14234-14240.
Shogan, B. D., D. P. Smith, A. I. Packman, S. T. Kelley, E. M. Landon, S. Bhangar, G. J. Vora, R. M. Jones, K. Keegan, B. Stephens, T. Ramos, B. C. Kirkup, Jr., H. Levin, M. Rosenthal, B. Foxman, E. B. Chang, J. Siegel, S. Cobey, G. An, J. C. Alverdy, P. J. Olsiewski, M. O. Martin, R. Marrs, M. Hernandez, S. Christley, M. Morowitz, S. Weber, and J. Gilbert. 2013. The Hospital Microbiome Project: Meeting report for the 2nd Hospital Microbiome Project, Chicago, USA, January 15(th), 2013. Standards in Genomic Sciences 8(3):571-579.
Simpson, A., and F. D. Martinez. 2010. The role of lipopolysaccharide in the development of atopy in humans. Clinical and Experimental Allergy 40(2):209-223.
Singh, G. K., and M. K. Kenney. 2013. Rising prevalence and neighborhood, social, and behavioral determinants of sleep problems in U.S. children and adolescents, 2003–2012. Sleep Disorders 2013:394320. doi:10.1155/2013/394320.
Song, S. J., C. Lauber, E. K. Costello, C. A. Lozupone, G. Humphrey, D. Berg-Lyons, J. G. Caporaso, D. Knights, J. C. Clemente, S. Nakielny, J. I. Gordon, N. Fierer, and R. Knight. 2013. Cohabiting family members share microbiota with one another and with their dogs. Elife 2:e00458. doi:10.7554/eLife.00458.
Sordillo, J. E., E. B. Hoffman, J. C. Celedón, A. A. Litonjua, D. K. Milton, and D. R. Gold. 2010. Multiple microbial exposures in the home may protect against asthma or allergy in childhood. Clinical & Experimental Allergy 40:902-910.
Stein, M. M., C. L. Hrusch, J. Gozdz, C. Igartua, V. Pivniouk, S. E. Murray, J. G. Ledford, M. M. dos Santos, R. Anderson, N. Metwali, J. Neilson, R. Maier, J. Gilbert, M. Holbreich, P. Thorne, F. Martinez, E. von Mutius, D. Vercelli, C. Ober, and A. I. Sperling. 2016. Innate immunity and asthma risk in Amish and Hutterite farm children. New England Journal of Medicine 375(5):411-421.
Stetzenbach, L. D. 2007. Airborne bacteria in indoor environments. Sampling and Analysis of Indoor Microorganisms 123-132.
Stokholm, J., J. Thorsen, B. L. Chawes, S. Schjorring, K. A. Krogfelt, K. Bonnelykke, and H. Bisgaard. 2016. Cesarean section changes neonatal gut colonization. Journal of Allergy and Clinical Immunology 138(3):881-889.
Sunyer, J., M. Esnaola, M. Alvarez-Pedrerol, J. Forns, I. Rivas, M. Lopez-Vicente, E. SuadesGonzalez, M. Foraster, R. Garcia-Esteban, X. Basagana, M. Viana, M. Cirach, T. Moreno, A. Alastuey, N. Sebastian-Galles, M. Nieuwenhuijsen, and X. Querol. 2015. Association between traffic-related air pollution in schools and cognitive development in primary school children: A prospective cohort study. PLOS Medicine 12(3):e1001792.
Tellier, R. 2009. Aerosol transmission of influenza A virus: A review of new studies. Journal of the Royal Society Interface 6(Suppl. 6):S783-S790.
Thorne, P. S. 2015. Endotoxin exposure: Predictors and prevalence of associated asthma outcomes in the United States. American Journal of Respiratory and Critical Care Medicine 192(11):1287-1297.
Tiesler, C. M., E. Thiering, C. Tischer, I. Lehmann, B. Schaaf, A. von Berg, and J. Heinrich. 2015. Exposure to visible mould or dampness at home and sleep problems in children: Results from the LISAplus study. Environmental Research 137:357-363.
Tischer, C., F. Weikl, A. J. Probst, M. Standl, J. Heinrich, and K. Pritsch. 2016. Urban dust microbiome: Impact on later atopy and wheezing. Environmental Health Perspectives 124(12):1919-1923.
Torow, N., and M. W. Hornef. 2017. The neonatal window of opportunity: Setting the stage for life-long host-microbial interaction and immune homeostasis. The Journal of Immunology 198(2):557-563.
Torrazza, R. M., M. Ukhanova, X. Wang, R. Sharma, M. L. Hudak, J. Neu, and V. Mai. 2013. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PLOS ONE 8(12):e83304.
Tsakok, T., G. Weinmayr, A. Jaensch, D. P. Strachan, H. C. Williams, and C. Flohr. 2015. Eczema and indoor environment lessons from the International Study of Asthma and Allergies in Childhood ISAAC Phase 2. The Lancet 385:S99.
Ulrich, R. P., X. Quan, C. P. Zimring, A. Joseph, and R. Choudhary. 2004. The role of the physical environment in the hospital of the 21st century: A once-in-a-lifetime opportunity. https://www.healthdesign.org/chd/knowledge-repository/role-physical-environment-hospital-21st-century-once-lifetime-opportunity-0 (accessed July 13, 2017).
Vejdovszky, K., K. Hahn, D. Braun, B. Warth, and D. Marko. 2017. Synergistic estrogenic effects of Fusarium and Alternaria mycotoxins in vitro. Archives of Toxicology 91(3):1447-1460.
von Mutius, E. 2016. The microbial environment and its influence on asthma prevention in early life. Journal of Allergy and Clinical Immunology 137(3):680-689.
von Mutius, E., and D. Vercelli. 2010. Farm living: Effects on childhood asthma and allergy. Nature Reviews Immunology 10(12):861-868.
Wang, H., S. Masters, Y. Hong, J. Stallings, J. O. Falkinham, III, M. A. Edwards, and A. Pruden. 2012. Effect of disinfectant, water age, and pipe material on occurrence and persistence of Legionella, mycobacteria, Pseudomonas aeruginosa, and two amoebas. Environmental Science & Technology 4(21):11566-11574.
Wei, J., and Y. Li. 2016. Airborne spread of infectious agents in the indoor environment. American Journal of Infection Control 44(9 Suppl.):S102-S108.
Weinmayr, G., U. Gehring, J. Genuneit, G. Buchele, A. Kleiner, R. Siebers, K. Wickens, J. Crane, B. Brunekreef, D. P. Strachan, and ISAAC Phase Two Study Group. 2013. Dampness and moulds in relation to respiratory and allergic symptoms in children: Results from Phase Two of the International Study of Asthma and Allergies in Childhood (ISAAC Phase Two). Clinical and Experimental Allergy 43(7):762-774.
WHO (World Health Organization). 2009. WHO guidelines for indoor air quality: Dampness and mold. http://www.euro.who.int/__data/assets/pdf_file/0017/43325/E92645.pdf (accessed April 27, 2017).
Wlasiuk, G., and D. Vercelli. 2012. The farm effect, or: When, what and how a farming environment protects from asthma and allergic disease. Current Opinion in Allergy and Clinical Immunology 12(5):461-466.
Wong, B. C. K., N. Lee, Y. Li, P. K. S. Chan, H. Qiu, and Z. Luo. 2010. Possible role of aerosol transmission in a hospital outbreak of influenza. Clinical Infectious Diseases 51(10):1176-1183.
Yang, W., and L. C. Marr. 2011. Dynamics of airborne influenza A viruses indoors and dependence on humidity. PLOS ONE 6(6):e21481.
Yatsunenko, T., F. E. Rey, M. J. Manary, I. Trehan, M. G. Dominguez-Bello, and M. Contreras. 2012. Human gut microbiome viewed across age and geography. Nature 486(7402):222-227.
Yu, I. T. S., Y. Li, T. W. Wong, W. Tam, A. T. Chan, J. H. W. Lee, D. Y. C. Leung, and T. Ho. 2004. Evidence of airborne transmission of the severe acute respiratory syndrome virus. New England Journal of Medicine 350(17):1731-1739.
| Reference | Population | Study Design | Exposure Focus | Intervention | Exposure Outcome | Asthma Outcome | Comments |
|---|---|---|---|---|---|---|---|
| Krieger et al., 2005 | 274 4- to 12-year-old children with asthma from low-income families (Seattle-King County, Washington) | RCT | Multiple asthma “triggers” | Intervention (n = 138): Multifaceted—5–8 home visits by community health worker over 1 year, including home assessment, education, support for behavior change, and resources to reduce exposures Control (n = 136): 1 visit, limited resources | N/A | Increased parent/caregiver actions to reduce exposures Decreased urgent visits, and increased caregiver QOL No differences in asthma symptoms between groups | Separate effects of each component of intervention unknown Intervention not tailored to child’s sensitivities Exposures not measured Projected 4-year savings: $189–$721 |
| Chew et al., 2006 | Three uninhabited water-damaged homes after a major hurricane (New Orleans, Louisiana) | Pre–post treatment comparison | Mold (spore counts, cultures, PCR analysis, glucan), endotoxin, and PM | Intervention: Removal of drywall, carpet, insulation, and all water-damaged furnishings | Reductions in mold and endotoxin pre–post, but high levels during cleanup | N/A | Pre- and during treatment mold and endotoxin levels orders of magnitudes above those in homes without severe water damage Adequate respirator use recommended during cleanup |
| Reference | Population | Study Design | Exposure Focus | Intervention | Exposure Outcome | Asthma Outcome | Comments |
|---|---|---|---|---|---|---|---|
| Kercsmar et al., 2006 | 62 2- to 17-year-old children with asthma in homes with mold (Cuyahoga County, Illinois) | RCT | Mold scores; allergen levels | Intervention (n = 29) and control (n = 33): asthma action plan, education, individualized problem solving Intervention group only: + household repairs and modifications | At 6 months, but not at 12 months, greater reduction in mold scores in intervention group compared with control | Decreased asthma symptom days and prevalence of exacerbations in intervention group compared with control | Low sample size, limited power frequency of families moving complexity of applying for household repairs and working with landlords |
| Breysse et al., 2011 | 49 adults, 29 children from 31 units in a low-income 3-building, 60-unit apartment complex (Minnesota) | Cross-sectional health survey of pre-/ immediately post renovation health, followed by survey 12–18 months post renovation | Green specifications targeting ventilation, moisture, mold, pests, radon | Intervention: Renovation according to Enterprise Green Communities green specifications, using “healthy housing” features; new mechanical ventilation installed (94) | Reduction in energy use (45 percent) Tightening of building envelope Functional exhaust fans Fresh air at 70 percent of ASHRAE standard Lower radon Annual average indoor CO2 982 ppm | Immediately post renovation: Self-reports of cleaner, more comfortable, safer housing Improvement in overall adult health, in nonasthma respiratory health (adults + children), and in asthma health (adults) | Potential recall bias Nonrandomized, unblinded study design Nonindependence of health reports from residents in same apartment Potential communication problems with non-English-speaking residents Potential selection bias toward healthier residents Some retrofitting required as not all renovations worked Reports of health benefits appear fewer in follow-up |
| Reference | Population | Study Design | Exposure Focus | Intervention | Exposure Outcome | Asthma Outcome | Comments |
|---|---|---|---|---|---|---|---|
| Mitchell et al., 2012 | 182 4- to 12-year-old children with moderate to severe asthma living in post–Hurricane Katrina flooded areas (New Orleans, Louisiana) | Observational, pre–post intervention study | Indoor allergens, moisture and mold | Intervention: Individually tailored multifaceted environmental intervention plus asthma counselor (timing of introduction of counselor varied) | Reduction in bedroom mold spores and in Alternaria in settled dust | Reduction (45 percent) in asthma symptom days Children with asthma counselor had greater symptom decrease | Separate effects of individual interventions unknown Unclear whether mold decrease occurred because of intervention |
| Hoppe et al., 2012 | Families living in 73 flood/water-damaged homes (Cedar Rapids, Iowa) | Cross-sectional assessment of homes and health at two levels of remediation (in progress [n = 24] or complete [n = 49]) | Extensive (e.g., mold, bacteria, endotoxin, PM, allergens) | Intervention: Removal of drywall, carpet, insulation, and all water-damaged furnishings | Levels of mold, bacteria, endotoxin, PM, glucan higher in homes with remediation in progress compared with homes with remediation complete | Compared with before the flood, residents of in-progress homes reported more allergies All residents reported more wheeze and meds for breathing problems | Cross-sectional Stage of in-progress cleanup variable Many in-progress families not moved back full time Potential participation bias |
| Breysse et al., 2014 | 102 low-income households in rental properties with 1 or more children with not-well-controlled asthma (King County, Washington) | Observational, pre–post intervention study with historical comparison group | Intervention (n = 34): Weatherization plus community health worker (CHW) education Historical comparison group (n = 68): CHW education without weatherization | Reduction in evidence of water damage greater with intervention group But no consistent evidence for greater improvement in intervention versus comparison group in other environmental exposures | Increased asthma control Increased caregiver QOL | Separate effects of weatherization and CHW not demonstrated Small study size IPM not used |
| Reference | Population | Study Design | Exposure Focus | Intervention | Exposure Outcome | Asthma Outcome | Comments |
|---|---|---|---|---|---|---|---|
| Colton et al., 2014 | 31 low-income households in rental housing | Observational comparison of exposures and health in green versus conventional housing, including among those who moved between housing types | Intervention (n = 18): Move from conventional to new buildings designed to green standards; smoke-free policies and IPM practices employed Control 1 (n = 6): Move from conventional to conventional housing Control 2 (n = 7): Live in conventional housing (61 visits, including pre and post for 24 who moved) | Green versus conventional housing: Lower PM2.5, NO2, and nicotine Fewer reports of mold, pests, inadequate ventilation, and stuffiness | Fewer sick building syndrome symptoms | Suggested benefits of move to green housing need further assessment Number of controls limit pre–post analysis |
| Colton et al., 2015 | 235 households in 3 Boston public housing 188 residents (80 percent) with 2 visits | Observational comparison of conditions and health in green versus conventional housing. Visits included home inspection and questionnaire | Visits to green units (n = 201) and conventional public housing units (n = 222) | Fewer reports and observations of mold, pests, inadequate ventilation, and secondhand smoke in green compared with conventional housing | Fewer asthma symptoms, hospital visits, school absences for children in green compared with conventional public housing | Suggested benefits of move to green housing Effects observed only for children with asthma; effects on adults not certain |
NOTES: ASHRAE = American Society of Heating, Refrigerating and Air-Conditioning Engineers; IPM = integrated pest management; N/A = not available; NO2 = nitrogen dioxide; PCR = polymerase chain reaction; PM = particulate matter; QOL = quality of life; RCT = randomized controlled trial. References (2000–2016) selected by participants from the National Institute of Allergy and Infectious Diseases (NIAID); National Institute of Environmental Health Sciences (NIEHS); National Heart, Lung, and Blood Institute (NHLBI); and Merck Childhood Asthma Network (MCAN) Workshop on the Indoor Environment and Childhood Asthma as representative and illustrative of asthma management intervention studies in children.
SOURCE: Adapted from Gold et al., 2017.
| Reference | Population | Study Design | Microbiota Exposure Source and Measurement Method | Protective Associations | Adverse Associations | Comments |
|---|---|---|---|---|---|---|
| Ege et al., 2011 | Two European studies comparing children on farms with reference group: PARSIFAL (n = 489); GABRIELA (n = 444) | Cross-sectional | Mattress dust PARSIFAL: screened for bacterial DNA with SSCP* GABRIELA: bacterial and fungal culture | Less asthma associated with: Greater diversity Specific taxa: fungi = eurotium, penicillium; bacteria = Listeria moncytogenecs, bacillus species, coryne-bacterium species and others | N/A | Diversity as outcome has limitations Families of species, but not specific microbes, identified as potentially protective |
| Fujimura et al., 2014 | Two households: one with (D) and one without dog (ND) | Murine model validation of epidemiologic human study | Experiment 1: Mouse dust from D and ND homes Experiment 2: Lactobaccillus johnsonii | Experiment 1: Reduced Th2 (allergy)-related airway responses, cecal enrichment with L. johnsonii Experiment 2: Reduced Th2 (allergic) cytokine expression | N/A | Suggests GI microbiome manipulation may protect individuals against allergic airway disease Dust from two homes Murine model |
| Lynch et al., 2014 | 104 inner-city children (Baltimore, Boston, New York, St. Louis) | Longitudinal birth cohort followed from birth to age 3 | At 3 months of age: Living room dust assessed with 16S rRNA-based phylogenetic microarray | Reduced atopy or atopy/recurrent wheeze with more bacterial richness; protective taxa primarily in Bacteroidetes and Firmicutes phyla (Prevotellaceae, Lachnospiraceae, Ruminococcaceae families) | N/A | Suggests children with highest exposures to multiple bacteria and allergens may get protection from allergy and wheeze Sample of children limited Taxonomic definition limited |
| Behbod et al., 2015 | 408 children (Boston, Massachusetts) | Longitudinal birth cohort followed from birth to age 13 | At 2–3 months of age: Bedroom and outdoor air and bedroom dust; bacterial and fungal culture | Reduced wheeze, fungal sensitization, and asthma by age 13 associated with bedroom floor dust | Increased rhinitis associated with outdoor air Cladosporium, dust Aspergillus at 2–3 months | Suggests responses vary by fungi taxon and mode of exposure Culture misses nonculturable taxa |
| Tischer et al., 2016 | 189 children (Munich, Germany) | Longitudinal birth cohort followed from birth to age 10 | At 3 months of age: Living room floor dust bacterial and fungal diversity assessed with tRFLP** | Reduced aeroallergen sensitization and ever wheezing associated with higher fungal (not bacterial) diversity by age 10 | N/A | Protective associations may attenuate with age Diversity as outcome has limitations |
| Reference | Population | Study Design | Microbiota Exposure Source and Measurement Method | Protective Associations | Adverse Associations | Comments |
|---|---|---|---|---|---|---|
| Stein et al., 2016 | 60 children aged 7–14 from the Amish and Hutterite communities | Cross-sectional | Bedroom and living room dust assessed with 16S rRNA V4-5 amplicon sequencing; bacterial and archaeal diversity and compositional structure; endotoxin levels; animal exposure validation studies | Reduced asthma with increased endotoxin levels and increased microbial diversity Sensitized animals protected from eosinophilia with exposure to dust from Amish homes | N/A | Protective associations may be related only to immune activation in the upper airway Microbial organisms likely associated with bovine animals |
| Birzele et al., 2016 | 86 school-age children | Cross-sectional | Mattress dust and nasal samples assessed with 454 pyrosequencing of 16S rRNA; bacterial diversity and composition based on operational taxonomic units (OTUs) | Reduced asthma with diversity/ richness—stronger for dust than for nasal mucosal samples | N/A | Protective associations may relate not only to upper-airway colonization |
| Cavaleiro Rufo et al., 2017 | 858 8- to 10-year-old children from 20 primary schools (Portugal) | Cross-sectional | Classroom air samples for bacterial and fungal culture and endotoxin measures | Reduced allergic sensitization but not asthma with increased diversity | Higher sensitization with higher air Penicillium spp. and endotoxin | Culture misses nonculturable taxa |
NOTES: GI = gastrointestinal; N/A = not available; SSCP = single-strand conformation polymorphism analysis; tRFLP = terminal restriction fragment length polymorphism. Diversity: small numbers of microbial exposures may be sufficient to stimulate all pattern-recognition receptors.
*Same pattern-recognition receptors on multiple microbial taxa.
**Overgrowth of harmful bacteria might be achieved by a limited number of bacterial species.
This page intentionally left blank.





























































