Multimodal therapeutic approaches have been used successfully to treat some complex neurological diseases such as pain and epilepsy for many years. Indeed, combination therapy is the standard of care for the treatment of PD, said Fiske. For example, levodopa, which is converted to dopamine in the brain and is the first-line treatment for PD, is nearly always given in combination with carbidopa, a levodopa enhancer that enables the use of a lower dose of levodopa and thus reduces the side effects of nausea and vomiting.
These examples not only provide lessons for future multimodal therapy development, but also illuminate the research gaps that need to be addressed in order to move ahead into new disease areas as efficiently and synergistically as possible, said Timothy Strauman. The theoretical considerations of seemingly simple approaches—using two drugs at the same time—are useful and apply to more complicated multimodal therapies, such as those that coadminister drugs with psychosocial intervention or neurostimulation, added Hildebrand.
CO-DELIVERY OF PHARMACOLOGICAL INTERVENTIONS FOR ALZHEIMER’S DISEASE
Currently, more than 35 million people worldwide are estimated to have AD, with these numbers expected to triple by 2050 unless an effective treatment is found to slow or prevent the disease (Alzheimer’s Association, 2014; Prince et al., 2013). Pharmaceutical drug development across all disease areas is risky, but drug development for AD has been particularly disappointing with only about 0.5 percent of compounds successfully proceeding from the preclinical phase to an approved drug (Calcoen et al., 2015), said Hendrix.
Emerging interest in using combination therapy to treat AD derives not only from the urgency to find new treatments, but also from experience in other disease areas such as cancer and HIV/AIDS, where cocktails of drugs have proven to be required for successful treatment (Hendrix et al., 2016). Moreover, the complex neuropathology of AD (Holtzman et al., 2011) suggests that it may be necessary to attack multiple pathways in order to slow the disease, said Hendrix. Indeed, treatments currently in the pipeline are being tested in combination with cholinesterase inhibitors, which are one of only two classes of drugs currently approved for the symptomatic treatment of AD. For example, one of these drugs, AVP923, is being tested in a Phase III trial for the treat-
ment of agitation in AD. AVP923 combines dextromethorphan and quinidine, and is a possible example of super synergy. By itself quinidine has no effect, but it changes the way dextromethorphan is metabolized so it can reach the brain, said Hendrix. The product, marketed under the name Nuedexta, has already been approved for the treatment of pseudobulbar affect in amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS).
Drugs being considered for possible combination therapy in AD span a wide variety of mechanisms, said Hendrix, including those that target amyloid β and tau, the proteins that aggregate as plaques and tangles in the AD brain, and those that are potentially neuroprotective (see Figure 3-1). For each of these targets, there may be multiple intervention possibilities, for example, stopping production or improving clearance of amyloid β or preventing the accumulation of tau. So a combination therapy could potentially attack one pathology in two different ways, attack two different pathologies, or attack one pathology in combination with a neuroprotective agent.
Of course, combining drugs introduces many other challenges related to pharmacokinetics, pharmacodynamics, dose finding, drug interactions, and other adverse events, said Hendrix. The routes of administration and dosing regimens could further complicate a trial, for example, if one drug is administered by infusion every third week while another is taken orally
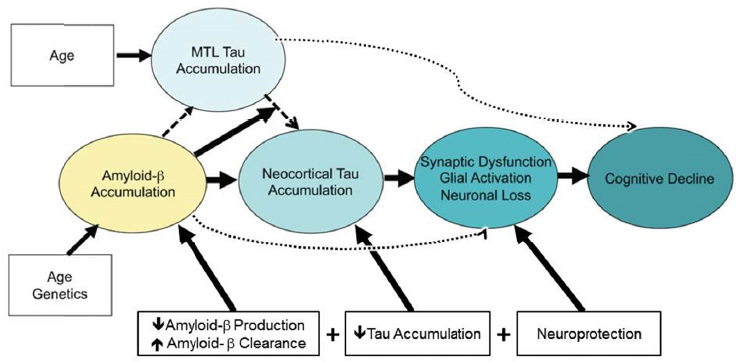
NOTE: MTL = medial temporal lobe.
SOURCES: Presentation by Hendrix, June 14, 2016. Adapted from Sperling et al., 2014.
each day. Finally, regulatory and business issues should be considered, Hendrix said, adding that possibly the biggest barrier is that few companies are large enough and have the capacity and breadth of experience in AD to take on a combination trial. This is where an organization such as the Alzheimer’s Association comes in, building collaborative partnerships with multiple stakeholders. It was with this in mind that they convened a workgroup of regulators, academicians, and industry representatives to map out a path forward for AD combination therapy (Hendrix et al., 2016). The workgroup recommended proceeding with a 2 × 2 factorial four-arm design combining an anti-amyloid and anti-tau drug, where one arm would test the anti-amyloid drug alone, one arm would test the anti-tau drug alone, one arm would combine the two drugs, and one arm would be the placebo.
To advance combination therapy more generally, the workgroup also recommended using transgenic animal models and human neuronal cell culture models to test combinations and using adaptive trials. Ideally, a biomarker that provides a single readout of treatment effect would enable these studies to move forward efficiently, said Hendrix.
CONCOMITANT DELIVERY OF TWO INTERVENTIONS WITH DIFFERENT MODALITIES
In addition to co-delivery of pharmaceuticals, multimodal therapies using two different modalities, such as administration of a drug and neurostimulation from a device or psychosocial therapy, can be effective treatment approaches for neurological and psychiatric conditions such as epilepsy and bipolar disorder in adolescents, said several workshop participants.
Drug−Device Combinations for Epilepsy
Epilepsy is a common but diverse group of neurologic conditions characterized by recurrent seizures and many comorbidities, including depression and anxiety, said Martha Morrell. Many effective anti-epileptic medications are available, each with different features and different mechanisms of action, yet still about 30 percent of patients with partial onset seizures continue to have seizures, and only about 15 percent are candidates for a surgical procedure where a portion of the brain is removed or destroyed to control the seizures.
Neurostimulation is a safe and effective alternative for some of these patients, despite the fact that the mechanism of action is not well understood, said Morrell. The first neuromodulation device to be used in the treatment of epilepsy was the vagus nerve stimulator (VNS), which provides scheduled stimulation to the vagus nerve and has been used to treat partial seizures since 1997. Another approach is deep-brain stimulation (DBS), in which an electrode implanted in the area of brain from which the seizure emanates delivers electrical impulses that stop or shorten the seizure activity (Morrell and Halpern, 2016). While not approved for the treatment of epilepsy in the United States, DBS is available in more than 30 countries worldwide, said Morrell. Brian Fiske commented that DBS is also commonly used in combination with pharmacotherapy to treat PD.
Other neuromodulation devices are used not for treatment per se, but for sensing when a patient is going to have, or has had, a seizure. Some of these devices are widely available from retail outlets, including those that measure galvanic skin response or sense bed shaking and other atypical movements. Morrell described a device she has been working with that combines sensing with responsive neurostimulation, which was approved by the FDA for treatment of uncontrolled partial-onset seizures after 12 years of clinical trials. Such devices offer the potential to move from waiting for an event such as a seizure to occur and having a patient suffer the consequences, to intervening before the event occurs, said Morrell. The Responsive Neurostimulator System (RNS) combines a neurostimulator attached to the skull with electrodes placed according to the patient’s seizure focus, and continuously monitors electrical activity in the brain. A programming device enables the physician to set the device to deliver stimulation when appropriate.
The data captured by the system has proved to be “unexpectedly powerful,” said Morrell. Each patient has a signature that is the same seizure after seizure. The temporal patterns detected by the device are biomarkers that allow physicians to individualize treatment and develop multimodal treatment synergies. For example, if a patient has problems in the morning, the physician can increase medication at that time; or if a female patient has problems associated with the menstrual cycle—what are called catamenial seizures—the physician may choose to prescribe continuous birth control.
Morrell cited a number of potential opportunities for multimodal approaches that combine both multiple therapies (drugs and neurostimulation) as well as stimulation that is combined with a device for sensing brain activity. For example, targeted spatial delivery of a drug with an
implanted device could allow delivery of a pharmacologic agent to the seizure focus when seizure activity is detected. It may also be possible to select drugs that facilitate other drug effects. Finally, she said, these detection and treatment methods can be used in combination with other types of multidisciplinary care, for example, psychotherapy or cognitive-behavioral therapy to deal with suicidal thoughts.
Combining Drugs with Psychosocial Interventions in Adolescents with Bipolar Disorder
Bipolar disorder (BD) is another common and disabling brain disease. According to Kiki Chang, professor of psychiatry and behavioral sciences at Stanford University Medical Center, as much as 2 to 4 percent of the population experiences BD, with onset typically in childhood (Perlis et al., 2004). He plans to use two modalities—pharmacotherapy plus psychotherapy—to treat children with a type of subthreshold BD called “not otherwise specified” or BD-NOS that does not meet the full criteria for mania or BD, as well as children with depression or a family history of BD. All of these children are thought to be at higher risk for later meeting the clinical criteria for BD. Treating them early may prevent progression, said Chang.
Chang’s approach targets circuitry in the brain that is implicated in mood dysregulation, specifically prefrontal-subcortical circuits (Chang et al., 2004). The pharmacotherapy component of his study used the seizure medication divalproex, which has been shown to produce symptomatic improvement as well as a change in brain structure in individuals with BD in an open-label study (Chang et al., 2003), but not in placebo study (Findling et al., 2007), except in those who had a family history of BD. The psychotherapy component of the multimodal therapy used a technique called family-focused therapy, which has been shown to activate dorsal and prefrontal circuits and is accompanied by improvement in symptoms (Garrett et al., 2015). Chang said he hopes the combination of targeted pharmacologic plus psychotherapy at a critical time in brain development will restore healthy neuronal connectivity and function in these at-risk children. Long-term follow-up will be needed to determine if this approach actually prevents the development of BD.
Lisanby suggested an alternative approach: combining two modalities selected because of previous knowledge regarding the mechanism of action of the device, such as inducing plasticity in focal regions of the brain, coupled with a medication that enhances that mechanism. For example,
one approach might combine TMS with a pharmacological agent that selectively promotes neuroplasticity.
SIMULTANEOUS USE OF TWO MODALITIES IN A SINGLE PROCEDURE
The use of different treatment modalities administered at the same time allows researchers and clinicians to leverage the action of one therapy to enhance the effects of the second. Examples of these emerging approaches include the use of noninvasive stimulation with concurrent behavioral training to improve cognitive performance, or combined with psychotherapy to increase mood regulation in depression. The use of feedback derived from electroencephalogram (EEG) recordings to enhance meditation effects in real-time is another example of concurrent use of two different modalities.
Combining Devices with Cognitive Enhancement
TMS is a noninvasive neurostimulation approach to the study of brain function that has both acute and long-lasting effects on the brain, including modulating network activity and neuroplasticity, according to Bruce Luber, staff scientist in the Experimental Therapeutics and Pathophysiology Branch of NIMH. It is approved by the FDA for treatment-resistant depression and has also been shown to enhance cognitive performance (Luber and Lisanby, 2014).
Luber discussed a more active approach for cognitive enhancement, in which direct modulation with TMS is paired with another form of stimulation such as cognitive activity. This approach, called Cognitive Paired Associate Stimulation (C-PAS), is based on the original PAS technique, which combined peripheral stimulation of the median nerve in the hand with TMS to increase excitability changes in the motor cortex and thus potentially increase the therapeutic potential (Ridding and Taylor, 2001). But in C-PAS, instead of stimulating the median nerve peripherally, neural circuits are activated by giving people a visual memory task to do. According to Luber, stimulating a given network with TMS at the same time the network is activated by the cognitive task strengthens the connectivity within the circuit. For example, he has shown that the technique is able to improve cognitive performance (Luber et al., 2007) and eliminate the effects of sleep deprivation on a
targeted cognitive task (Luber et al., 2008). Subsequent studies also showed that the combination of TMS with working memory training may prevent cognitive deficits in people who are sleep deprived (Luber et al., 2013), suggesting that the technique may be able to help people with cognitive deficits or even alleviate memory problems associated with normal aging, which is presently being tested with NIH funding.
Luber acknowledged that there are many challenges to address in further developing the approach. First, the mechanisms underlying TMS-induced cognitive enhancement are unknown, and there is also much to learn about timing, spacing, and intensity parameters involved in TMS dosing. Finally, state dependence, that is, the baseline level of activity in the circuit, is probably the biggest source of variability, he said.
Combining Devices with Psychosocial Intervention
Another application of the concept of tuning different modalities to target the same dysfunctional neurocircuitry was described by Strauman, who combines TMS with a form of psychotherapy called self-system therapy (SST) to treat depression. The rationale, he said, is that TMS will increase plasticity in a network, which should have a synergistic effect on learning behaviors and skills for regulating mood that engage the same network. The approach involves, first, an individualized assessment to identify regions in the left prefrontal cortex that are most responsive to whatever the person finds most rewarding, then targeting that predetermined region/circuit with TMS while engaging the person in SST.
Strauman described preliminary clinical data obtained from five people during early trials, using both clinician report and patient self-report. These early results, which require further validation, suggest that patients who receive the combination treatment showed improvements in depressive symptoms in a shorter period of time than would be seen in response to typical monotherapy approaches, said Strauman. Using pretreatment and posttreatment neuroimaging data, preliminary results also indicate that in response to motivational cues, patients show significantly increased activity in areas of the brain that are relevant for approach and avoidance cues.
Strauman emphasized that confirmation of the mechanism of action and validation of the targeting strategy will be needed before taking this approach to a larger scale clinical trial. In addition, he cited regulatory challenges given that most psychosocial therapies have no regulatory framework to follow.
Integrating Complementary Approaches with Conventional Care
Emmeline Edwards, director of the division of extramural research at the National Center for Complementary and Integrative Health (NCCIH), described another approach that incorporates mindfulness meditation with real-time feedback EEG. Meditation has been shown to increase activity in the posterior cingulate cortex (PCC). Using the EEG feedback, patients can learn to both increase their PCC activity and enhance the effect of meditation, said Edwards (van Lutterveld and Brewer, 2015). She said they are applying this technique to reduce craving for individuals trying to stop smoking. Another study supported by NCCIH incorporates mindfulness meditation for the treatment of pain and demonstrates that mindfulness meditation does not rely on the endogenous opioid activity to reduce pain, since naloxone administration did not block meditation’s pain-relieving effects—an important consideration for using meditation to treat chronic pain and reduce opioid use, said Edwards.
This page intentionally left blank.










