Water Desalination: History, Advances, and Challenges
MANISH KUMAR, TYLER CULP, AND YUEXIAO SHEN
Pennsylvania State University
Desalination is the removal of salt and contaminants from water. It involves a broad range of technologies that yield access to marginal sources of water such as seawater, brackish ground- and surface water, and wastewater. Given the reduction in access to fresh water in recent decades and the uncertainty in availability effected by climate change, desalination is critical for ensuring the future of humanity.
This paper describes advances toward more sustainable desalination and exciting directions that could make this technology more accessible, energy efficient, and versatile. It reviews the emergence of membranes as the preferred technology for desalination, recent advances, challenges to its sustainable implementation, and needs for further research.
INTRODUCTION
Desalination represents a promise of near unlimited water supply and is an attractive potential solution to the age-old conundrum of seawater abundance and practical inaccessibility for potable use. It now encompasses the removal of both salts and dissolved contaminants from various sources such as seawater, brackish surface and groundwater, and industrial and municipal wastewaters.
The primary descriptor of importance for desalination processes is the amount of dissolved solids (primarily inorganic salts) represented by the total dissolved solids (TDS; the solids left over after water is evaporated from particle-free water). Table 1 lists the typical range of TDS levels in waters subjected to desalination-based water treatment processes (Australian NWC 2008).
In addition to being a measure of usability (such as for consumption), TDS
TABLE 1 Typical Water Sources for Desalination and Their Total Dissolved Solids (TDS) Ranges as Well as the Calculated Minimum Energy for Separation per Unit Volume (specific energy consumption).
| Water Source* | Total Dissolved Solids (mg/L) | Minimum Energy for Separation (kwh/m3)** |
|---|---|---|
| Seawater | 15,000–50,000 | 0.67 |
| Brackish water | 1,500–15,000 | 0.17 |
| River water | 500–3,000 | 0.04 |
| Pure water | < 500 | < 0.01 |
| Wastewater (untreated domestic) | 250–1,000 | 0.01 |
| Wastewater (treated domestic) | 500–700 | 0.01 |
* Data from Australian NWC (2008).
** Calculated based on average TDS of the range.
levels determine the bounds for the minimum energy needed to remove these solutes from water (or to move water away from these solutes). Just as energy is released when a solute is dissolved in a compatible solvent, energy is needed to separate the solute from the solvent and is dependent on the concentration of the solute. Table 1 shows that higher-salinity water (such as seawater) requires larger amounts of energy for desalination, whereas water from low-salinity streams (such as those from wastewater reuse) could be much lower.
The growing pressure on limited freshwater sources has focused the world’s attention on seawater and the recovery of water from marginal sources such as brackish ground- and surface water as well as recycled wastewater. It has also raised awareness and catalyzed the implementation of wastewater reuse, where wastewater is treated to a high quality and in some cases used for direct or indirect potable reuse. Desalination is thus a critical technology for humanity to allow for sustainable development.
BACKGROUND AND HISTORY
Desalination has a long history in both mythology and practice. An early and illustrative reference appears in the Bible (Exodus 15:22–26) and is widely considered to be about desalination.
When they came to Marah, they could not drink the water of Marah because it was bitter; therefore it was named Marah. And the people grumbled against Moses, saying, “What shall we drink?” And he cried to the LORD, and the LORD showed him a log, and he threw it into the water, and the water became sweet.
Distillation-Based Technologies
Early scientific descriptions of desalination centered around the application of distillation. In his Meteorologica, Aristotle wrote that “Salt water when it turns into vapour becomes sweet and the vapour does not form salt water again when it condenses” (Forbes 1948, p. 383). This is the definition of distillation, a process used to create fresh water from seawater at larger scales starting in the 1930s (NRC 2008). Distillation-based technologies remained a major approach to water desalination until the development of membranes.
The most common distillation-based desalination methods are thermally driven technologies, including multistage flash distillation, multiple-effect distillation, and mechanical vapor compression processes. In these processes water is evaporated by the addition of heat and in many cases assisted by the use of vacuum. The evaporated water is then condensed to recover desalinated water. Several large plants, primarily in the Middle East, have used thermal distillation since the 1930s (NRC 2008).
But thermal desalination has very high energy consumption and is increasingly being replaced by the use of membranes, specifically reverse osmosis (RO) membranes. Figure 1 shows the energy consumption per unit volume of water
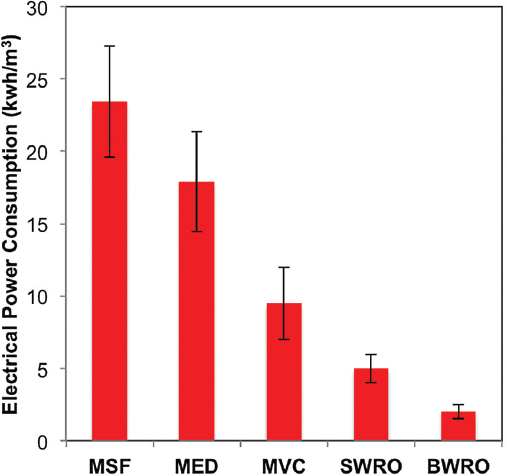
for several commonly used water desalination techniques (Al-Karaghouli and Kazmerski 2013). As is evident from this figure, RO is a substantially more energy-efficient technology for water desalination.
Emergence of Membrane Technology
Membrane technologies arose as a result of a breakthrough in the use of polymer films for separating salt from water in the late 1950s/early 1960s. A brief history of the development of RO membranes is shown in Figure 2, based on Baker (2004).
Reid and Breton (1959) first demonstrated the possibility of desalination using polymeric cellulose films, and thus the first polymeric RO membranes were created. Loeb and Sourirajan (1963) then showed that an asymmetric cellulose acetate membrane can be used for desalination. The permeabilities of these early membranes were low, and RO membranes were considered a novelty separation technique rather than a solution to desalination.
An innovation in the packaging of large membrane areas into small volumes was the development of the spiral wound module (Figure 3) by General Atomics in 1963. The spiral wound configuration is now common in RO applications (Cadotte 1981; Westmoreland 1968). In this module, “leaves” of membranes,
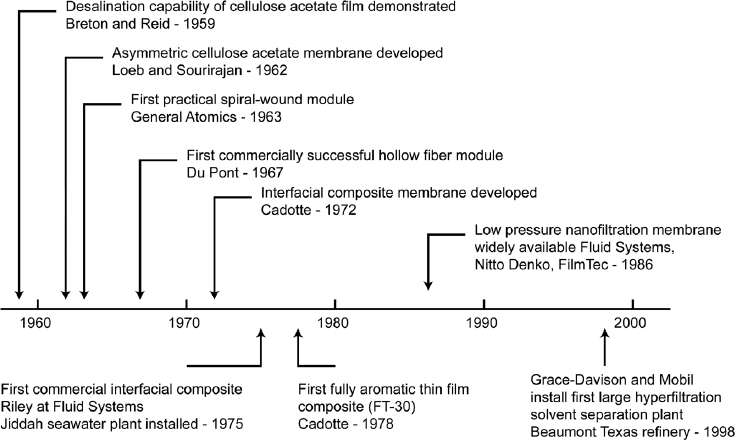
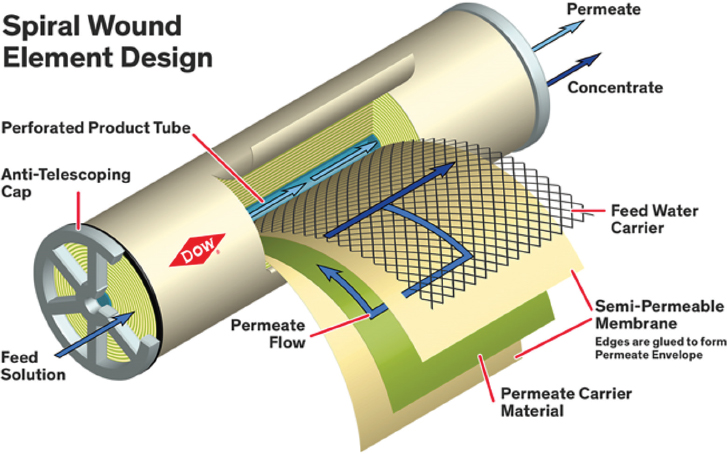
with feed and permeate spacers, are connected to a perforated permeate tube and rolled up in a “jelly roll” configuration. Hollow-fiber modules containing thin fibers were developed a few years later by DuPont, but this configuration is less commonly used for RO.
A major advance in membrane chemistry that has made possible the application of RO membranes is the development of the thin film composite (TFC) architecture. Previously, membranes were either several-micron-thick polymer layers with a uniform architecture or similar-size polymer layers with an “asymmetric” structure with a nonporous salt-rejecting top surface opening up to a more porous support.
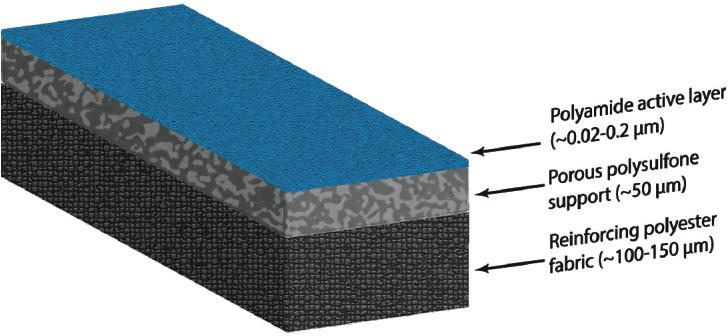
Cadotte (1981) patented the design for the three-layer TFC membrane that is now the industry standard. It provides high permeability while maintaining selectivity for water (vs. salt or other solutes). His major innovation was to make the crosslinked “active layer” of the membrane of nanoscale thickness and support it on a microporous membrane (Figure 4). A 20–200 nm thin crosslinked polyamide layer is supported on (or indeed grown from) a microporous polysulfone layer that is in turn supported on a polyester fabric.
The most common chemistry for modern RO membranes is interfacial polymerization, another major advance in RO membrane manufacturing. The procedure, described in Figure 5, has been the standard for making RO membranes for the past 5 decades.
The energy consumption of RO technology has dramatically declined
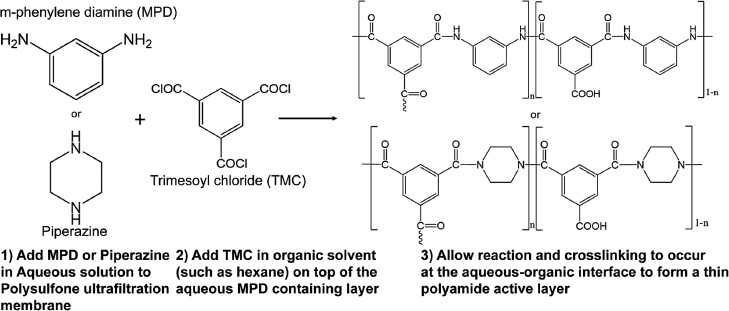
(Figure 6, based on data from Gude 2011 and Elimelech and Phillip 2011) thanks to improvements in formulation, manufacturing procedures, and processes, such as energy recovery from pressurized brine. These advances rapidly enhanced sustainability and exponentially increased the implementation of these membranes for seawater and brackish water desalination as well as wastewater reuse.
For some cases, such as seawater reverse osmosis, it is argued that current membranes have reached very close to the thermodynamic limit of ~1 kWh/m3 and that further improvement in materials may not yield additional energy sustainability (Elimelech and Phillip 2011). On the other hand, advances in permeability and selectivity can still yield major gains in brackish water treatment and wastewater reuse.
Ultrapermeable membranes with very high salt rejection appropriate for reverse osmosis may substantially reduce the necessary energy (~45 percent) or plant infrastructure (pressure vessels, up to 65 percent) in low-salinity sources (Cohen-Tanugi et al. 2014) such as brackish water desalination and water reuse. The energy advantage is significantly lower for high-salinity seawater applications (15 percent less energy) but the plant size can be reduced by 44 percent (Cohen-Tanugi et al. 2014).
A focus on increasing selectivity rather than simply increasing membrane permeability has been proposed in recent work as a sustainable approach to improve membrane materials (Werber et al. 2016a).
ADVANCES IN RO DESALINATION
Recent advances in desalination membranes promise a path to higher sustainability. Some of these advances are described below.
Channel-Based Membranes as an Alternative to Solution-Diffusion RO Membranes
RO membranes currently rely on the solution-diffusion mechanism to separate solutes from water, a transport method in which components of the solution first dissolve into the membrane matrix and then diffuse across the membrane by “jumping” between transiently connected pores. In contrast, biological membranes conduct efficient and selective channel-based transport, in which water or selected solutes are transported “straight through” protein channels (membrane proteins, MPs). MP channels are approximately 4 nm in length in comparison to the tortuous unconnected pores in the 20–200 nm thick RO membrane active layers.
Attention has recently been focused on water channel proteins called aquaporins (AQPs) and their synthetic analogs, carbon nanotubes (CNTs). AQPs selectively transport water across cell membranes in many forms of life (including in humans) (Agre 2004).
Both AQPs and CNTs efficiently transport water at the rate of several billions of molecules per second. They consist of narrow pores lined with hydrophobic surfaces, resulting in single-file water transport (de Groot and Grubmuller 2001; Hinds 2007). While CNTs cannot be made at dimensions that are substantially less than 10 Å in diameter and thus cannot reject salt (hydrated sodium and chloride ions are about 7.2 and 6.6 Å in diameter respectively; Israelachvili 2011), AQPs are highly water selective due to their small pore size (~3 Å) and the presence of amino acid residues that reject charged ions (Agre et al. 2002). The exceptional permeability and selectivity of AQPs has led to research on their incorporation in water purification membranes (Shen et al. 2014), and AQP-based biomimetic membranes were proposed in the mid- to late 2000s in several patents and papers.
There have been many advances since, including methods to incorporate AQPs in stable lipids and lipid-like block copolymers, their packing at high density into membranes, the integration of such layers into various membrane architectures, and finally the development of a scalable membrane where AQPs are inserted into the active layer of RO membranes (Zhao et al. 2012). The latter has resulted in commercially available membranes at small scale, but they face significant challenges to scaleup because of concerns about stability and cost.
Another advance inspired by biological channels and arguably more scalable is the development of artificial water channels and proposals to develop membranes around them (Barboiu 2012). These bioinspired channels are made synthetically using organic synthesis but have until recently been a less studied
area with only a few architectures reported (Shen et al. 2014). We recently demonstrated for the first time that such channels can approach the permeabilities of AQPs and CNTs while providing several advantages (Figure 7) (Licsandru et al. 2016; Shen et al. 2015). The channels tested were peptide-appended pillar[5]arene channels and imidazole-quartet artificial proton channels.
Artificial channels provide distinct advantages for scaleup when compared to CNTs and AQPs because of their compatibility with organic solvents and chemical and biological stability. They could thus be suitable for incorporation in selective high-permeability membranes.
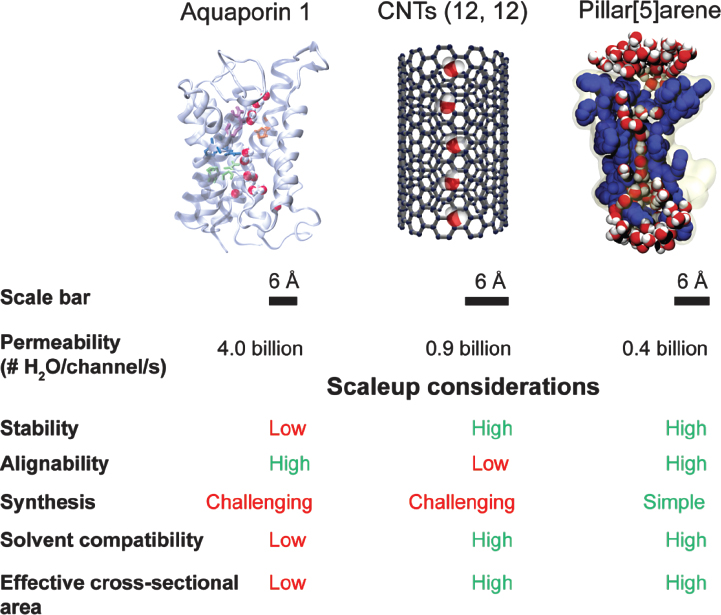
Graphene-based membranes can also be considered as an example of channel-based membranes and may be promising as next-generation RO membranes (Cohen-Tanugi and Grossman 2012; Mi 2014; Werber et al. 2016b). Graphene is a single thin layer of sp2 hybridized carbon that has unusual mechanical, thermal, and electrical properties and may lend itself to a variety of applications.
Pores drilled into graphene may be an option for filtration membranes but currently the pores cannot be made small enough to reject salt (Wang and Karnik 2012). More practical for desalination is the use of oxidized graphene or graphene oxide sheets stacked together so that the distance between the layers can be small enough to reject solutes (Mi 2014). This work is rapidly progressing and could be a new material for sustainable desalination.
Fouling-Resistant Membranes
A major challenge during operation of RO membranes is the deposition of colloidal materials and organic macromolecules on the membrane surface and the growth of microbes. This deposition leads to cake formation, irreversible adsorption, and growth of persistent biofilms, collectively referred to as fouling.
Fouling can cause a substantial increase in power consumption due to additional resistance to flow. In addition, salt accumulates in fouling cake layers. The cake-enhanced concentration polarization and, for biofilms, biofilm-enhanced osmotic pressure (Herzberg and Elimelech 2007; Hoek and Elimelech 2003) increase the effective osmotic pressure to be overcome, thus decreasing the driving force for membrane filtration and increasing power consumption.
Several membrane modification strategies are under consideration to reduce membrane fouling in RO systems. These include the grafting of superhydrophilic or amphiphilic molecules that can prevent adsorption of macromolecules and biological cells; use of nanoparticles, carbon-based materials such as CNTs, and graphene oxide flakes to impart biocidal properties to the RO membrane surface; and use of electroactive or magnetically actuated surfaces to prevent deposition or cause cell death. Methods that interrupt or manipulate cell-to-cell communication are also being explored for biofouling control.
Desalination Powered by Renewable Energy
Desalination has always been considered incompatible with renewable energy infrastructure because of its energy-intensive processes (Charcosset 2009). But with the rapid improvement in RO membranes and systems and concomitant decrease in energy use, more attention is being paid to the coupling of desalination units to solar (using photovoltaics) or wind energy sources. The applications are so far limited to small plants and used for “off-the-grid” applications.
CRITICAL CHALLENGES IN DESALINATION
Notwithstanding rapid progress in the development and deployment of membrane desalination in recent years, there are still persistent fundamental and practical challenges to its sustainable implementation.
Inscrutability of desalination membranes. Although crosslinked TFC RO membranes have been used for a few decades now, the microstructural details of these membranes remain unknown. This lack of knowledge prevents the establishment of a direct link between modifications in the chemistry and microstructure that drive transport properties. Efforts are ongoing to develop tools to enhance understanding of RO membrane structure.
Concentration polarization. When salt is rejected from the surface of RO membranes it forms a concentrated layer adjacent to the membrane, reducing the driving force for transport across the membrane. The thickness of this concentration polarization layer can be reduced by enhancing the back transport of solutes. Several ideas have been tested at various scales but their implementation in a sustainable manner has been challenging.
Seawater intakes and discharges. A particular challenge to the development of seawater desalination plants (including RO plants) is the impingement and entrainment of marine microorganisms during intake to the plant. Impingement is the collision and trapping of marine organisms that are larger than intake screens; entrainment is the passage of small organisms through these screens and the subsequent destruction of these marine organisms. Also, when dense brine is discharged back to the ocean, it can have detrimental effects on the marine environment if proper mixing does not occur. Efforts are needed to better understand these challenges as well as the effect of intake designs and discharge diffusers on the marine environment (Szeptycki et al. 2016).
Inland desalination brine disposal. Whereas coastal plants can discharge concentrated brine to the ocean, inland RO plants need to find a sustainable avenue to manage their brine, which could be as high as 20 percent of the feed flow. Brine minimization and beneficial reuse of brine components as sustainable alternatives to deep well disposal, disposal for municipal sewers, and use of evaporation ponds need to be evaluated carefully.
Lack of chlorine resistance in polyamide membranes. Sodium hypochlo-rite (i.e., bleach) is ubiquitous in water treatment plants for preventing growth of biofilms on surfaces in contact with water, including types of water treatment membranes. But this is not an option for polyamide membranes commonly used for desalination because of their high susceptibility to damage from chlorine. Development of chlorine-resistant membranes is an important practical need.
Translation of new materials. Many new materials have been developed for RO desalination, but their translation to products and use at larger scales is limited. Efforts are needed to translate innovations in materials and process design to actual products and plants.
High-salinity streams. High-salinity streams emerge from energy operations such as hydraulic fracturing (fracking), proposed underground CO2 storage, unconventional oil development, and flue gas desulfurization applications that frequently have TDS values in excess of 100,000 ppm. These pose unique challenges to RO materials, RO process components, and operating strategies.
OUTLOOK
Membrane desalination technology is growing rapidly and becoming a critical tool for ensuring long-term water sustainability around the world. There is intense scientific interest in improving the sustainability of this technology, and several innovations are looking to further reduce the technique’s power consumption and barriers to widespread use and sustainability. The future of this technology is bright, and it is expected to play a major role in the resource-limited future facing the world.
REFERENCES
Agre P. 2004. Aquaporin water channels. Angewandte Chemie 43:4278–4290.
Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. 2002. Aquaporin water channels: From atomic structure to clinical medicine. Journal of Physiology 542:3–16.
Al-Karaghouli A, Kazmerski LL. 2013. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renewable and Sustainable Energy Reviews 24:343–356.
Australian NWC [National Water Commission]. 2008. Emerging Trends in Desalination: A Review. Waterlines Report Series No. 9. Turner, Australian Capital Territory.
Baker RW. 2004. Reverse osmosis. In: Membrane Technology and Applications, 2nd ed. Chichester, UK: John Wiley and Sons.
Barboiu M. 2012. Artificial water channels. Angewandte Chemie International Edition 51:11674–11676.
Cadotte JE. 1981. Interfacially synthesized reverse osmosis membrane. US Patent 4,277,344 A.
Charcosset C. 2009. A review of membrane processes and renewable energies for desalination. Desalination 245:214–231.
Cohen-Tanugi D, Grossman JC. 2012. Water desalination across nanoporous graphene. Nano Letters 12:3602–3608.
Cohen-Tanugi D, McGovern RK, Dave SH, Lienhard JH, Grossman JC. 2014. Quantifying the potential of ultra-permeable membranes for water desalination. Energy & Environmental Science 7:1134–1141.
de Groot BL, Grubmuller H. 2001. Water permeation across biological membranes: Mechanism and dynamics of aquaporin-1 and GlpF. Science 294:2353–2357.
Elimelech M, Phillip WA. 2011. The future of seawater desalination: Energy, technology, and the environment. Science 333:712–717.
Forbes R. 1948. A Short History of the Art of Distillation: From the Beginnings Up to the Death of Cellier Blumenthal. Leiden: EJ Brill.
Gude VG. 2011. Energy consumption and recovery in reverse osmosis. Desalination and Water Treatment 36:239–260.
Herzberg M, Elimelech M. 2007. Biofouling of reverse osmosis membranes: Role of biofilm-enhanced osmotic pressure. Journal of Membrane Science 295:11–20.
Hinds B. 2007. Molecular dynamics: A blueprint for a nanoscale pump. Nature Nanotechnology 2:673–674.
Hoek EM, Elimelech M. 2003. Cake-enhanced concentration polarization: A new fouling mechanism for salt-rejecting membranes. Environmental Science and Technology 37:5581–5588.
Israelachvili JN. 2011. Intermolecular and Surface Forces, rev 3rd ed. Burlington, MA: Academic Press.
Licsandru E, Kocsis I, Shen Y-X, Murail S, Legrand Y-M, van der Lee A, Tsai D, Baaden M, Kumar M, Barboiu M. 2016. Salt-excluding artificial water channels exhibiting enhanced dipolar water and proton translocation. Journal of the American Chemical Society 138:5403–5409.
Loeb S, Sourirajan S. 1963. Sea water demineralization by means of a semipermeable membrane. University of California Los Angeles, Department of Engineering.
Mi B. 2014. Graphene oxide membranes for ionic and molecular sieving. Science 343:740–742.
NRC [National Research Council]. 2008. Desalination: A National Perspective. Washington, DC: National Academies Press.
Reid C, Breton E. 1959. Water and ion flow across cellulosic membranes. Journal of Applied Polymer Science 1:133–143.
Shen Y-X, Saboe PO, Sines IT, Erbakan M, Kumar M. 2014. Biomimetic membranes: A review. Journal of Membrane Science 454:359–381.
Shen Y-X, Si W, Erbakan M, Decker K, De Zorzi R, Saboe PO, Kang YJ, Majd S, Butler PJ, Walz T, Kumar M. 2015. Highly permeable artificial water channels that can self-assemble into two-dimensional arrays. Proceedings of the National Academy of Sciences 112:9810–9815.
Szeptycki L, Hartge E, Ajami N, Erickson A, Heady WN, LaFeir L, Meister B, Verdone L, Koseff JR. 2016. Marine and Coastal Impacts of Ocean Desalination in California. A report of Water in the West, Center for Ocean Solutions, Monterey Bay Aquarium, and the Nature Conservancy. Available at http://waterinthewest.stanford.edu/sites/default/files/Desal_Whitepaper_FINAL.pdf.
Wang EN, Karnik R. 2012. Water desalination: Graphene cleans up water. Nature Nanotechnology 7:552–554.
Werber JR, Deshmukh A, Elimelech M. 2016a. The critical need for increased selectivity, not increased water permeability, for desalination membranes. Environmental Science and Technology Letters 3:112–120.
Werber JR, Osuji CO, Elimelech M. 2016b. Materials for next-generation desalination and water purification membranes. Nature Reviews Materials 1:16018.
Westmoreland JC. 1968. Spirally wrapped reverse osmosis membrane cell. US Patent 3,367,504 A.
Zhao Y, Qiu C, Li X, Vararattanavech A, Shen W, Torres J, Helix-Nielsen C, Wang R, Hu X, Fane AG. 2012. Synthesis of robust and high-performance aquaporin-based biomimetic membranes by interfacial polymerization-membrane preparation and RO performance characterization. Journal of Membrane Science 423:422–428.
This page intentionally left blank.














