3
Value of Controlled Human Inhalation Exposure Studies
INTRODUCTION
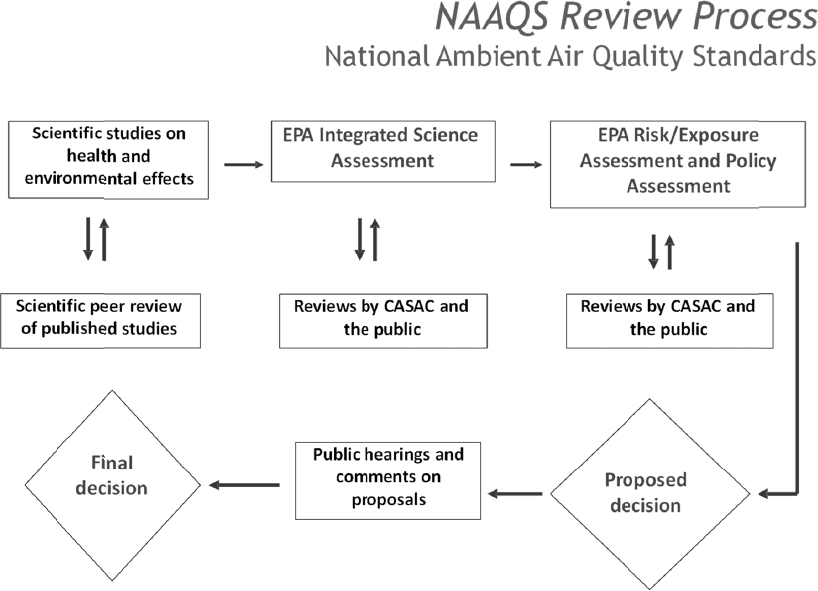
The committee’s assessment of the value of controlled human inhalation exposure (CHIE) studies centered on their contributions to the U.S. Environmental Protection Agency (EPA) regulatory decisionmaking process, especially with respect to promulgating air-quality standards. As discussed in Chapter 2, a key way to understand the value of CHIE studies in this process is by considering EPA’s Integrated Science Assessments (ISAs). The ISAs are extensive reviews of policy-relevant science and consensus documents and are foundational to the process of reviewing the National Ambient Air Quality Standards (NAAQS) for the criteria pollutants.1 (The NAAQS process is illustrated in Figure 3-1.) ISA drafts are reviewed by the Clean Air Scientific Advisory Committee and the public (EPA, 2015a). The use of ISAs is one of the ways the agency provides “access to accurate information sufficient to effectively participate in managing human health and environmental risks” (EPA 2017b). The regulation and control of the six current criteria air pollutants are considered to have broad public health importance because of the pollutants’ anthropogenic origins and widespread distribution to many areas of the country.
Instead of assessing all of the contributions of CHIE studies to the NAAQS decision making for various criteria pollutants, the committee focused on their contributions to the ISAs for the NAAQS for ozone (O3) and airborne particulate matter (PM). However, the committee’s framework for evaluation is relevant to other criteria pollutants as well.
The CHIE studies carried out at EPA’s Human Studies Facility during the past several years have focused mainly on O3 and PM. (See Table C-1 in Appendix C.) They represent a contrast in composition complexity and variability (as discussed in this chapter). O3 is a simple, single molecule, which is used as the indicator pollutant for the complex mixture of photochemical oxidants in ambient air. PM10 refers to particles with an aerodynamic diameter less than or equal to 10 μm. PM2.5 refers to particles with an aerodynamic diameter less than or equal to 2.5 μm. Historically, EPA began by monitoring total suspended particulate matter, and then changed the indicator entity to PM10 in the 1997 PM NAAQS, but over recent decades the agency has focused more on PM2.5 monitoring.
Table 3-1 lists the three NAAQS reviews conducted by EPA for O3 and PM from 1996 to 2015. The most recent evaluation was reported in the Integrated Science Assessment of Ozone and Related Photochemical Oxidants (EPA, 2013). That document informed the review of the O3 NAAQS completed in 2015.
In addition to informing the ISAs, CHIE studies of particles from specific sources (for example, diesel-engine exhaust particles and wood smoke particles) augment the scientific knowledge base for EPA’s decision making concerning regulatory approaches that focus on source emissions (such as EPA’s National Emission Standards for Hazardous Air Pollutants [NESHAP] for Stationary Reciprocating Internal Combustion Engines) (40 CFR Part 63, Subpart ZZZZ).
__________________
1 The term “criteria pollutants” derives from the requirement in the Clean Air Act that EPA establish the scientific criteria for regulation by describing the characteristics and evidence of health and welfare effects of these pollutants.
A FRAMEWORK FOR EVALUATING CHIE STDUIES
The Hill aspects of causality in epidemiology and public health (Hill, 1965) have been used as an approach for assessment of the adequacy of evidence of a causal relationship between exposure to a hazardous agent and a possible health consequence (IOM, 2014). EPA adapted the Hill aspects for consideration of evidence in its ISAs. The considerations used by EPA include specificity of the association between an exposure and an observed response, temporality between the occurrence of an exposure and an observed association, a biologic gradient in the relationship between exposures and responses (such as increasing effects associated with greater exposures), plausibility of a proposed biologic mechanism for the occurrence of an effect, consistency (or reproducibility) of results across independent studies, coherence of observed outcomes across different fields of study or study designs, and experimental results indicating that a change in exposure can cause a change in a response (EPA, 2015a).
The EPA-adapted Hill aspects provided the committee with a framework for assessing the value of CHIE study results to inform EPA’s regulatory decision making and for identifying the kinds of useful information CHIE studies can provide. Here we provide an overview of the values of CHIE studies according to those considerations. Details are provided later in the chapter.
Specificity and Experimental Findings: CHIE studies enable investigators to separate the effects of exposure to individual criteria pollutants, or specific groups of criteria pollutants, from effects associated with exposures to ambient complex mixtures that are observed in epidemiologic studies. The experimental study design of CHIE studies enables formal tests of hypotheses and more unambiguous assessments of short-term exposure–response relationships for specific laboratory-generated pollutants or mixtures. This allows EPA to focus on the causative agents in complex mixtures responsible for the observed health effects.
| Review of Latest Relevant Scientific Informationa | Year Finalized | Year NAAQS Revisions Completed | Indicator | Averaging Time | Level (Concentration) for Primary Standardb | Formc |
|---|---|---|---|---|---|---|
| Air Quality Criteria for Ozone and Related Photochemical Oxidants | 1996 | 1997 | O3 | 8-hour | 0.08 ppm | Annual fourth-highest daily maximum 8-hour concentration, averaged over 3 years |
| Air Quality Criteria for Ozone and Related Photochemical Oxidants | 2006 | 2008 | O3 | 8-houir | 0.075 ppm | Annual fourth-highest daily maximum 8-hour concentration, averaged over 3 years |
| Integrated Science Assessment of Ozone and Related Photochemical Oxidants | 2013 | 2015 | O3 | 8-hour | 0.070 ppm | Annual fourth-highest daily maximum 8-hour concentration, averaged over 3 years |
| Air Quality Criteria for Particulate Matter | 1996 | 1997 | PM2.5 | 24-hour | 65 µg/m3 | 98th percentile, averaged over 3 years |
| Annual | 15.0 µg/m3 | Annual arithmetic mean, averaged over 3 years | ||||
| PM10 | 24-hour | 150 µg/m3 | 99th percentile, averaged over 3 years | |||
| Annual | 50 µg/m3 | Annual arithmetic mean, averaged over 3 years | ||||
| Air Quality Criteria for Particulate Matter | 2004 | 2006 | PM2.5 | 24-hour | 35 µg/m3 | 98th percentile, averaged over 3 years |
| Annual | 15.0 µg/m3 | Annual arithmetic mean, averaged over 3 years | ||||
| PM10 | 24-hour | 150 µg/m3 | Not to be exceeded more than once per year on average over a 3-year period | |||
| Integrated Science Assessment for Particulate Matter | 2009 | 2012 | PM2.5 | 24-hour | 35 µg/m3 | 98th percentile, averaged over 3 years |
| Annual | 12.0 µg/m3 | Annual mean, averaged over 3 years | ||||
| PM10 | 24-hour | 150 µg/m3 | Not to be exceeded more than once per year on average over 3 years |
aIn December 2006 EPA announced a revised process for reviewing and setting NAAQS. The changes included the development of the ISA. Previously, the document reporting on EPA’s periodic reevaluation of newly available scientific information was referred to as the criteria document (EPA 2016c).
bThe primary standard is set for protection of public health.
cThe form defines the air-quality statistic that is to be compared to the level of the standard in determining whether an area attains the NAAQS.
Temporality: CHIE studies have enabled more specific assessment of the timing of responses to short-term exposures to criteria pollutants.
Biologic gradient: Some CHIE studies involving short-term exposures to specific criteria pollutants, particularly those involving ozone (O3) exposures, have contributed to clarification of exposure–response relationships. In addition, CHIE studies allow for the study of specific gaseous or particle pollutant exposure concentrations and durations.
Plausibility, Experimental Findings, Consistency and Coherence: CHIE studies provide evidence to assess plausibility by assessment of multiple biomarker (see below) and physiologic responses to specific exposures, enabling evaluation of potential mechanisms of action of specific criteria pollutants. CHIE study findings might be used to generate new hypotheses or contribute to the strength of evidence regarding biomarker or physiologic responses to pollutants, when the results are consistent across CHIE studies or when they illustrate coherence with results of toxicologic animal studies or observational epidemiologic studies or panel studies.
However humans are not as identical as inbred mice, leading to differences in interpretation of the meaning of consistency and coherence. Particularly with PM CHIE studies, when lack of consistency or coherence/reproducibility occurs, this may be due to factors other than chance or small number of subjects. These factors can include: (1) variability in the composition of the PM; (2) variability in subject susceptibility. With ozone CHIE studies (see below), internal variability in response was an important piece of information about inter-subject susceptibility to the exposure, and that variability in response was reproducible.
New biomarker or physiologic end points related to cognitive function or other noncardiopulmonary outcomes contribute to evidence for plausibility of epidemiologic associations of criteria pollutants or mixtures with other outcomes that have been less well understood or studied.
SENSITIVE GROUPS
Section 109 of the Clean Air Act indicates that the primary NAAQS should allow for an adequate margin of safety to protect public health. The legislative history of Section 109 indicates sensitive subpopulations (or subgroups) are intended to be a specific focus of efforts to provide such protection.2 Broadly speaking, sensitive subpopulations comprise individuals who show stronger biologic responses to increased exposure in terms of concentrations and durations, beginning at lower exposure, relative to the general population (that is, sensitive subpopulations exhibit a shifted exposure–response curve). The sensitivity can be attributable to intrinsic factors (such as asthma) or extrinsic factors (such as tobacco smoking). Therefore, CHIE studies can provide information regarding biologic gradients for sensitive subpopulations.
The committee considers sensitive subpopulations to be an important segment of the general population for several reasons. They are a specific focus of the NAAQS requirements in the Clean Air Act. Developing a scientific understanding of the burden of air pollution on them, without causing harm, is a task that requires continuing synthesis of information, as CHIE study protocols are developed and as research plans are formulated. Because sensitive individuals are likely to be biologically vulnerable, they require special attention from Institutional Review Boards (IRBs) that are asked to approve CHIE study plans.
While CHIE studies can inform NAAQS decision making by contributing to the identification of sensitive subpopulations and assessing sensitivity to exposure, the committee has observed that CHIE studies have not included participants with high baseline risks of serious adverse events (see Chapter 4) and finds that it is not warranted to do so in the future (see Chapter 5). Thus many CHIE studies have
__________________
2 Sometimes sensitive individuals are referred to as susceptible or at-risk individuals.
been limited to involving healthy (and often young) adult subjects, whose biologic responses to controlled exposures would likely differ from those of individuals with established disease. Some CHIE studies, which have been conducted after completion of the PM ISA in 2009, have included subjects with metabolic syndrome or mild asthma, and some whose ages are greater than 65 years old (see Chapter 4). However, even though these studies potentially involved somewhat more sensitive individuals, they were designed to exclude individuals who are most likely to have adverse effects (see Chapter 2). While considering this issue, the committee adhered to the principle that, in CHIE studies, the risk of studying people at high baseline risk of an adverse event outweighs the potential benefit of increased scientific understanding accrued to society. Chapter 5 presents recommendations for improving the definition of inclusion and exclusion criteria for selecting study subjects that need to be considered by EPA and the IRB of record.
CHIE STUDIES IN THE CONTEXT OF TOXICOLOGIC AND EPIDEMIOLOGIC STUDIES, AND THE LARGER RESEARCH AGENDA
The primary value of CHIE studies of air-pollutant exposures is that they generate data on responses to short-term criteria-pollutant or pollutant-mixture exposures for well-defined pollutant concentrations and for specific time periods to inform NAAQS with shorter averaging times (such as 8 or 24 hours) (see Table 3-1). Important secondary values include gaining a better understanding of (1) temporal patterns of short-term responses and recoveries, (2) compartments or specific locations in the human body and kinds of cells affected by air-pollutant exposures, and (3) initial and secondary biologic responses as measured by functional physiologic outcomes and biomarkers.
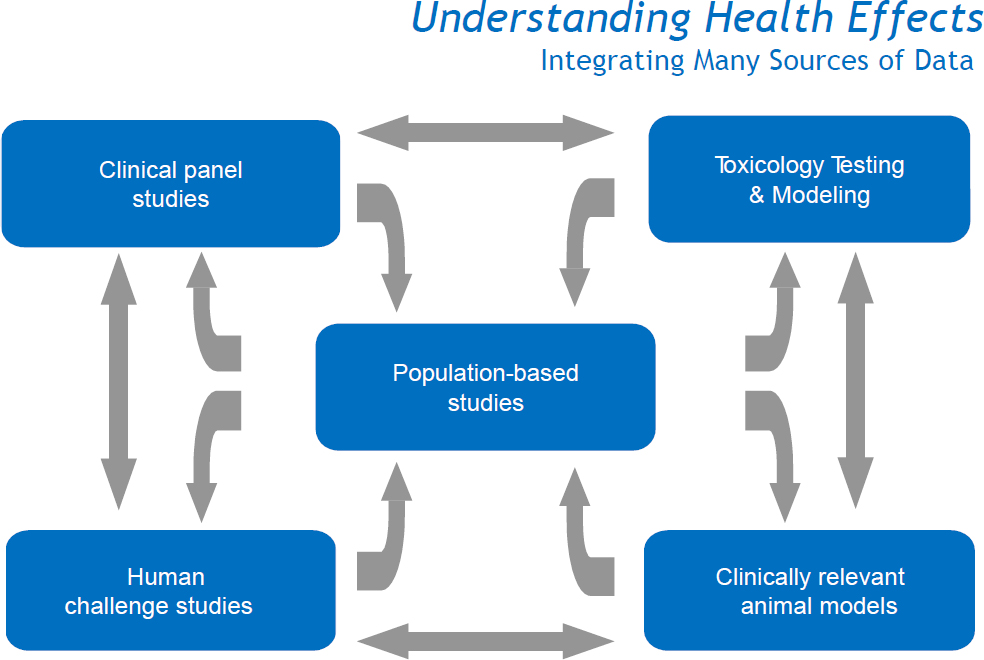
As shown in Figure 3-3, CHIE studies (referred to in the figure as “human challenge studies”) and controlled animal inhalation studies (referred to as clinically relevant animal models) provide information to help in the interpretation of the exposure–response relationships generated by panel studies and larger-scale epidemiologic studies of diverse human populations. CHIE studies can provide unique information that cannot be obtained from animal inhalation studies or from epidemiologic or panel studies of people engaged in their normal daily activities in the real world. EPA considers all three sources of complementary exposure–response information in the challenging task of reviewing, and possibly revising, NAAQS. The role that CHIE studies play in supplementing toxicologic studies and observational epidemiologic or panel studies is discussed extensively in EPA’s ISAs. For example, see EPA (2009, 2013).
USE OF BIOMARKERS IN CHIE STUDIES
The National Research Council report Human Biomonitoring for Environmental Chemicals characterized biomarkers as biologic indicators that generally include biochemical, molecular, genetic, immunologic, or physiologic signals of events in biologic systems (NRC, 2006). There are three broad categories of biomarkers: exposure, response, and susceptibility. As indicated in that report, WHO (2001) defined those categories with respect to environmental chemicals as follows:
Biomarker of exposure. The chemical or its metabolite or the product of an interaction between a chemical and some target molecule or cell that is measured in a compartment in an organism.
Biomarker of effect. A measurable biochemical, physiologic, behavioral, or other alteration in an organism that, depending on the magnitude, can be recognized as associated with an established or possible health impairment or disease.
Biomarker of susceptibility. An indicator of an inherent or acquired ability of an organism to respond to the challenge of exposure to a specific chemical substance.
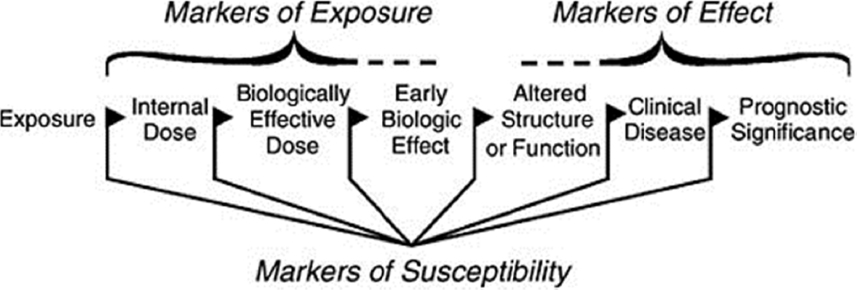
Figure 3-2 shows various classes of biomarkers that can be considered across the steps of an exposure–response sequence. For example, biomarkers of exposure can be used to estimate the concentration of a toxicant in the breathing zone of an individual, and to distinguish it from the dose of the toxicant that is delivered to the airway surface or to a target organ and even to the target receptor for a specific mechanism of action.
In choosing the biomarkers as study end points that focus on perturbations of concern for short-term effects, an important consideration is the short-term effects that might be indicative of the initiation and progression of chronic effects (NRC 2007). The use of a broad array of biomarkers allows scientists in other disciplines, such as exposure scientists, epidemiologists, and toxicologists, to anchor their studies with specific biomarkers.
Biomarkers of short-term responses detected in CHIE studies might be useful in other complementary studies, such as panel studies of human cohorts to assess variations in biologic responses in specific subpopulations, including potentially susceptible subpopulations, to relatively short-term exposures to ambient pollutant mixtures. Biomarkers detected in CHIE studies also might be useful for chronic inhalation exposure studies involving animals, in that seeing similar biomarkers in animals and humans could provide some validation of the animal studies for use in characterizing human risk associated with exposure to air pollutants. However, some biomarkers that are unique to long-term effects might not be identified through CHIE studies. Biomarkers of short-term responses in CHIE studies might also be useful in large-population epidemiologic studies to identify subpopulations at relatively high risk of developing clinically relevant pollutant-induced chronic disease that could benefit from preventive medical intervention.
COMPARING CHIE STUDY EXPOSURES WITH AMBIENT POLLUTANT EXPOSURES
The relevance of the results of controlled inhalation exposures to the potential effects of exposure to similar criteria pollutants in ambient air can vary depending on
- Whether the criteria pollutant represents a variable mixture or has more than one molecular form,
- The complexity and variety of effects of concern, and
- The presence of hazardous air pollutants that co-occur with the criteria pollutant in ambient air.
For carbon monoxide (CO), a criteria pollutant that is in a singular molecular form and whose metabolic products, i.e., carboxyhemoglobin and carboxymyoglobin, are risk factors for a specific adverse health effect, the similarity between controlled exposures and ambient exposures is expected to be very high.
Other current criteria pollutants represent mixtures of ambient air pollutants, including multiple known toxicants in gaseous and particulate forms, such as peroxides as well as O3 for photochemical oxidants, NO and HNO3 as well as NO2 for nitrogen oxides, SO3 and H2SO4 for sulfur oxides, and toxic trace metals and complex polycyclic aromatic hydrocarbons for PM.
The nature of the measurable health-related responses observed in O3 CHIE studies is most similar to those reported in time-series studies of ambient air O3 exposures. It has been established that the magnitude of the pulmonary function responses, per ppb of O3, is greater for ambient air exposures than for exposures in CHIE studies (Spektor et al., 1988). A common interpretation of that finding is that copollutants are also playing a causal role.
PM2.5 is the best example of a criteria pollutant where of the results of controlled laboratory exposures and ambient air exposures tend to be most variable. The epidemiologic evidence demonstrates that there are variable exposure–response relationships, for both acute and chronic responses, between and within cities. Many studies have found that relative toxicity of PM corresponds to the differences in the chemical composition of the PM (see, for example, Thurston et al., 2013, 2016a), or to the sources that the chemical composition represents. However, much is still to be understood about how PM composition influences toxicity. Furthermore, in ambient air, there is always simultaneous exposure of PM2.5, photochemical oxidants, sulfur oxides, and nitrogen oxides in various proportions, and the health effects associated with PM2.5 exposures can be influenced by its copollutants (Lippmann et al., 2013). For CHIE studies of PM2.5 mass concentrations from diesel-engine exhaust (DE), the applicability of the results for to the potential effects of ambient air exposures where there are few sources of DE is more tenuous, because PM2.5 from DE is much richer in organic carbon (OC) and much poorer in transition metals (such as iron and nickel) than is ambient air PM2.5.
CONSIDERATIONS OF CHIE STUDY VALUE FOR EPA DECISION MAKING
The value of CHIE studies for informing the reviews of NAAQS for O3 and PM2.5, and for understanding biologic responses to airborne PM from specific emission sources or of specific compositions, is discussed below.
CHIE studies have been carried out to obtain a detailed understanding of the impact of O3 from two perspectives. One perspective focuses on the considerations of specificity, temporality, and biologic plausibility for providing justification for establishing NAAQS for O3 as an indicator pollutant that is associated with human harm. The second perspective focuses on the consideration of a biologic gradient and connects to the regulator’s task of establishing an averaging time, level (mean concentration over the specified averaging time), and statistical form for a NAAQS, as discussed below.
For airborne PM, scientific investigations and understanding of health impacts occurred through a somewhat different historical route. The epidemiologic evidence of mass-based PM-related health effects (particularly cardiac health effects related to inhalation of PM and entry into the lung) was initially greeted with skepticism and hence CHIE studies have been used to provide evidence for specificity, temporality, and biologic plausibility. There has been less emphasis on consideration of biologic gradient, and investigation of gradient is complicated by the variable complex chemical composition, particle-size distribution, and/or source of a given level of PM.
For O3 and PM, the following discussion provides (1) background on the value of CHIE studies for NAAQS decision making, (2) a summary of CHIE study contributions to the evidence provided in the ISAs (and in select cases, assessments by other expert panel reviews), and (3) a summary of CHIE study contributions to understanding biologic gradients and informing decisions concerning the four basic elements of the NAAQS. The discussion of PM CHIE studies also includes a consideration of the influence of particle size range and chemical composition for NAAQS decision making.
CHIE OZONE STUDIES
Background on the Value of CHIE Studies for the Ozone NAAQS
CHIE study findings have been valuable in informing O3 NAAQS decision making. The strongest evidence for O3-associated health effects is for respiratory effects following short-term exposures. The ISA (EPA, 2013; Table 1-1) concludes that for short-term O3 exposures, evidence supports a causal relationship with respiratory effects and is highly suggestive of a direct or indirect contribution to cardiovascular effects and premature mortality. CHIE studies demonstrated a wide range of respiratory effects, including lung-function decrements and increases in respiratory symptoms, lung inflammation, and airway hyperresponsiveness.
Specificity, Temporality, and Plausibility Considerations
As indicated in the O3 ISA, most CHIE studies investigating the effects of O3 exposure used a randomized, controlled, crossover design in which subjects were exposed, without knowledge of the experimental treatment and in random order to clean filtered air (FA) as the control and, depending on the study, to one or several O3 concentrations, frequencies, and durations. The control exposure provides a direct estimate of the effects of the experimental conditions on the biomarker or physiologic outcomes of interest. Comparison of biologic responses to the FA exposure to those following an O3 exposure allows for estimation of the O3 effects, while controlling for independent effects of the experimental procedures. As individuals may experience small changes in various health end points from exercise, diurnal variation, or other influences, in addition to those of O3 during the course of an exposure, the term “O3induced” is used to designate effects that have been corrected or adjusted for such extraneous responses as measured during FA exposures (EPA 2013).
The initial end points used for the historic CHIE studies were spirometric (lung function) indices obtained via pulmonary function testing. Those indices characterized reproducible physiologic characteristics of individuals, with normal values being dependent on an individual’s age, sex, and height (as an index of lung volume). An extensive body of literature has documented their reproducibility in individual subjects, and they are used not only for human health-effects research related to air pollution exposure, but are used longitudinally for pharmacologic studies as a primary end point by the Food and Drug Administration. Reduced respiratory function is also an independent risk factor for mortality as determined by epidemiologic studies unrelated to those focused on air pollution (Agarwal et al., 2012; Helzebos et al., 2014; Hozawa et al. 2006; Lee et al. 2011; Menezes et al. 2014; Shaaban et al, 2006; Sin et al. 2005) . This measure additionally has been used to stratify the severity of common respiratory diseases including asthma, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis.
Initial CHIE studies looked at responses to O3 breathed in by subjects at rest. Subsequent studies evaluated the impact of O3 inhalation under conditions of moderate exercise and, more recently, under conditions of high ambient temperature. The rationale for evaluating O3 exposure under conditions of exercise is related to the recognition that individuals working outside or engaged in recreational exercise or athletic competition would be expected to breathe at higher than normal minute ventilation and would therefore have a higher internal dose rate. CHIE studies provide an ideal setting to study how exercise modifies the short-term adverse effects of O3 exposure. It should be noted that comparative studies between humans and nonprimates (rodents) showed quite different exposure–response relationships. The differences in breathing pathways and rates, as well as patterns and targets of injury, between rodents and humans resulted in an enhanced appreciation for the value of the CHIE study model.
CHIE studies evaluated the impact of controlled exposure to O3 at progressively lower concentrations and with multihour exposure durations designed to mimic a typical day’s work outside. These studies demonstrated several important findings. The first was an average decline in lung function that became progressively greater with continuing exposure over a 6.6-hour period, with the subjects engaged in intermittent moderate exercise. This finding suggested the need for averaging times of 8 hours duration, whereas the previous NAAQS had a 1-hour averaging time. A second key finding from the CHIE O3 exposure studies was the demonstration of a high degree of interindividual variability in the magnitude of the short-term spirometric decrements with O3 exposure. This finding was unexpected in its magnitude, with interindividual variability of 40% or greater within a predicted average FEV1decrement of 10% for white males aged 18-36 years for 2-hour exposures with intermittent exercise (McDonnell et al., 1997). This result led to the recognition that small average changes in a population masked or obscured variability across the population. This finding stimulated observational population-based epidemiologic investigations of clinical end points, such as respiratory or cardiovascular hospitalizations and exacerbations.
The observation of large interindividual variation in spirometric decrements led to an assessment of whether this was a stable response phenotype. Other studies demonstrated that there was intraindividual stability in the spirometric responses to O3, even while there was persistent large interindividual variability (Folinsbee et al., 1994; Hazucha et al., 2003; McDonnell, 1996; McDonnell et al., 1985).
These studies spurred an avenue of animal toxicologic studies by Kleeberger (1995) evaluating genetic determinants of O3 responsiveness, a productive line of research that continues at this time.
Another important contribution of CHIE studies of O3 was the evaluation of the inflammatory response to controlled exposure to O3. Key papers evaluating bronchoalveolar lavage (BAL) profiles after O3 exposure demonstrated perturbations of the alveolar–capillary interface with influx of inflammatory cells, plasma transudation into the alveolar space, and activation of inflammatory cascades, including the perturbation of the coagulation system. The value of the contributions stem from the simplicity of the exposure, unclouded by concomitant exposures to other toxicants in the ambient air mixture, and the experimental design of CHIE studies. Subsequent epidemiologic studies have developed the knowledge base of the implications of these perturbations in sensitive subpopulations (Alexis et al., 2010; Devlin et al., 1991; Kim et al., 2011; Koren et al., 1989; Lay et al., 2007).
Another line of investigation of O3 exposure examined the relationship between physiologic and inflammatory responses. This provided useful initial information in interpreting time trends in pulmonary
function response to O3. The CHIE studies demonstrated a diminution of the physiologic response with successive daily exposures (“O3 adaptation”). It was unknown whether this was a beneficial response or a manifestation of harm, and CHIE studies allowed an examination of that question. The finding was that the inflammatory response persisted despite a lessening of the lung function decrement. This, in turn, led to the appreciation of dimensions of response to pollutants and cautioned against an overly simplistic interpretation of any single study (Folinsbee et al., 1980).
A subsequent area of investigation in CHIE studies was an exploration of the impact of O3 exposure on the inflammatory response of individuals with preexisting inflammatory airway disease, such as asthma and COPD. Asthma is a syndrome, and great progress is being made in the elucidation of asthma phenotypes, including the role of atopy, obesity, hormonal status (that is, postmenopausal), and viral infection on the inflammatory and physiologic characteristics of asthma. It is important to understand the impact of O3 on these distinct phenotypes, as their pathogenesis is deciphered (Alexis et al., 2000; Hernandez et al., 2010; Peden et al., 1995, 1997).
Similarly, COPD is increasingly understood to be a syndrome with subphenotypes (Kleeberger and Peden, 2005; Speizer and Ware, 2015). One of the potential values of CHIE O3 studies is to understand whether perturbations in the inflammatory profile are similar for these different phenotypes, or distinct. The Clean Air Act has, as a founding principle, the intent to protect sensitive subpopulations with a reasonable margin of safety. CHIE studies with O3 provide an ongoing means to understand and refine the notion of sensitivity. An example of this is more recent findings of association of O3 effects and ambient temperature (Kahle et al., 2015).
Because of epidemiologic observational evidence of strong cardiovascular effects associated with PM rather than with O3, the past decade of CHIE research has focused more on assessment of cardiophysiologic effects of PM2.5, rather than O3. Relatively recent time-series epidemiology studies have reported statistically significant associations not only between PM exposure and daily mortality and/or morbidity due to pulmonary and/or cardiovascular causes, but also between daily ambient O3 concentrations and those outcomes (Basu, 2009; Basu and Malig, 2011; Bell et al., 2004; Ito et al., 2005; Katsouyanni et al., 2009; Rosenthal et al., 2013; Stafoggia et al., 2010; Zanobetti and Schwartz, 2008). Those observations have led EPA investigators to turn to CHIE studies to assess whether there is biologic evidence to support the associations of short-term O3 exposures with clinical outcomes that have been observed through epidemiologic studies. A CHIE study involving sequential 2-hour exposures to clean air and O3, at 22°C and again at 32.5°C, showed an interaction between high temperature and O3 that may activate the fibrinolytic pathway and help to explain the adverse effect of O3 on cardiac mortality and morbidity (Kahle et al., 2015). A CHIE study of young, healthy adults found that O3 can cause an increase in biomarkers of vascular inflammation and changes in markers of fibrinolysis and markers that affect autonomic control of heart rate and repolarization (Devlin et al. 2012).
CHIE studies of subjects at rest have contributed to NAAQS decision making by confirming the reproducibility of physiologic changes associated with O3 exposures. CHIEs studies have contributed to the understanding of O3 effects on lung function: that O3 inhibits the ability to inspire to total lung capacity (Hazucha et al. 1989) thereby reducing FEV1 and FVC. That information helps greatly in understanding the effects on lung function estimated in observational studies. CHIE studies have also demonstrated physiologic effects of O3 exposures under outdoor working conditions, such as elevated ambient temperatures.
Biologic Gradient Considerations Ozone CHIE Study Contributions to the Four Basic Elements of the NAAQS
This section focuses on the use of O3 CHIE studies to establish biologic gradients for O3-associated health effects in order to inform decisions about the primary photochemical oxidant standard. The most recent ISA for O3 was completed in 2013 (EPA, 2013). As indicated in Table 3-1, the components of the current primary NAAQS, set in 2015, include O3 as an indicator, an 8-hour averaging time, a concentration of 70 ppb O3, and a form defined as the annual fourth-highest daily maximum averaged over 3 years. Below we discuss how CHIE studies of O3 contributed to each of these components of the NAAQS.
Indicator: The ISA for O3 noted that O3 and NO2 are the only photochemical oxidants that are routinely monitored and for which a comprehensive ambient air concentration database exists. The findings from CHIE studies, discussed above, provide a sound justification for the selection of O3 as an indicator pollutant. As air pollution oxidant chemistry becomes better understood, opportunities will arise for CHIE studies to address other photochemical oxidants in ambient air, especially peroxides.
Averaging time: CHIE studies provide a basis for evaluating the appropriateness of a primary NAAQS with an 8-hour averaging time, instead of using the shorter exposure duration (1 hour) that was used in earlier O3 NAAQS. The change to an 8-hour averaging time was based on earlier CHIE studies that investigated 6.6- and 8-hour exposures in healthy adults and reported respiratory effects at lower O3 exposure concentrations for 1- and 2-hour exposures with moderate levels of exertion (for example, see McDonnell et al., 1991). O3 causes an inflammatory response in the lungs after a single 1-hour exposure (with exercise) to O3 at a concentration of 300 ppb and that the increased concentrations of some inflammatory cells and mediators persisted for at least 18 hours.
It should be noted that the 6.6-hour exposure study has reported respiratory effects below the lowest effective O3 dose as determined during a 1-hour exposure study in young healthy adults. That suggested that ambient O3 had cumulative daily effects and/or that a longer averaging time than 1 hour is necessary to protect populations from O3’s accumulated effects (McDonnell et al., 1991), thus motivating a longer averaging time for NAAQS for O3 (see below).
Some CHIE studies have been designed to evaluate specific exposure circumstances of interest to regulators. For example, outdoor workers engaged in heavy physical labor were identified as a potentially sensitive subpopulation, and the 6.6-hour experimental protocol was intended to simulate this condition (Folinsbee et al., 1988). The subsequent 8-hour average time for O3 is similar to the exposure periods investigated in this 6.6-hour exposure study.
Ambient O3 concentrations during any 24-hour period vary, with peaks generally occurring in the late morning and/or early afternoon. The timing of the 1-hour maximum concentration could be affected by unusual, sudden increases in the background O3 concentration. The choice of an 8-hour average represents a compromise that takes into account evidence from CHIE studies as well as the necessities of risk management through regulatory implementation.
Level: The current concentration limit for the primary O3 NAAQS was reduced from 0.075 to 0.070 ppm in 2015, based on complementary information from CHIE, epidemiologic, and panel studies. CHIE studies provided essential information on exposure–response relationships for various O3 concentrations for durations up to 8 hours. Available evidence from CHIE 6.6-hour studies show that detectable effects of O3 at constant exposure during the study time on group mean FEV1 (forced expiratory volume in 1 second) were observed at exposure concentrations as low as 60 ppb, but effects were not observed at 40 ppb, in young healthy adults exposed for 6.6 hours while engaged in moderate exercise (EPA, 2013).
Form: The “form” of a NAAQS defines the air-quality statistic (such as the annual fourth-highest daily maximum 8-hour concentration, averaged over 3 years; see Table 3-1) that is to be compared to the level of the standard in determining whether an area attains the NAAQS. EPA indicates the main consideration in selecting a form for current standards is the adequacy of the public health protection provided by the combination of the four elements of the standard (EPA, 2014). The selection of the form of a standard is mainly based on the daily distribution of ambient O3 and risk management target rather than the dose–response relationship obtained from CHIE studies. Also, as mentioned previously, those studies have been complicated by the geographically and temporally variable composition of ambient PM.
Impacts of the Available Results of CHIE Ozone Studies on the Ozone NAAQS
The ISA provides a synthesis and evaluation of the policy-relevant studies as the scientific foundation for the periodic review of the NAAQS required by the Clean Air Act. The primary NAAQS for O3 and related photochemical oxidants is designed to protect against respiratory health effects incurred after short-term exposure to tropospheric (ambient) O3 and related photochemical oxidants.
O3 CHIE studies have been of critical importance for informing NAAQS decision making by providing
- A basis for EPA’s decision to move from a 1-hour to an 8-hour averaging time for O3 concentration. For example, there was the finding of concentration-dependent increases in BAL neutrophils and the inflammatory mediator IL-6 for 6.6-hour exposures to O3 at moderate concentrations (0.080 and 0.10 ppm; Devlin et al., 1991);
- An understanding of the role of risk factors in human physiologic and biologic responses to oxidant pollutant exposures:
- Some individuals in CHIE studies showed no change in lung function while others showed up to a 30% decrease in lung function after a 6-hour O3 exposure. The phenotype of responding to O3 exposure with a decrease in lung function was shown to be reproducible.
- Some individuals in CHIE O3 studies responded with increases in markers of lung inflammation. These were not always the same individuals as those who responded to O3 with a decrease in lung function;
- An understanding of O3 adaptation. Lung function responses to O3 decreased after repeated daily O3 exposures, but the inflammatory response was sustained over repeated exposures (Devlin et al., 1997);
- Identification of decreased lung function, increased airway inflammation, and increased respiratory symptoms in healthy adult subjects after controlled exposure to O3 concentrations less than 75 ppb (EPA, 2014);
- Evidence to support the plausibility of elevated ambient O3 exposures causing increased asthma events observed in sensitive “at-risk” subpopulations;
- Evidence of O3-related health response presented in the 2006 O3 air-quality criteria document (EPA, 2006), providing support for a causal relationship between acute ambient O3 exposures and increased respiratory morbidity outcomes, and the 2013 ISA’s conclusion that it is a causal relationship, providing support for lowering the O3 NAAQS;
- Biologic and physiologic evidence for O3 effects in human health that generated hypotheses for animal studies that looked for risk factors in complementary investigations; and
- An iterative process in which the results of CHIE studies inform the efforts of interdisciplinary teams working to elucidate biologic mechanisms, and those teams identifying new questions to be addressed by CHIE studies.
CHIE PM STUDIES
Background on the Value of CHIE Studies for the PM NAAQS
CHIE study findings have been used to inform decisions about setting the NAAQS for PM2.5 and PM10. As CHIE studies involve short-term exposures and biologic outcomes, they have been specifically relevant to ISA reviews of short-term effects of ambient PM exposure and to the setting of NAAQS related to those effects. In 2009 the most recently completed ISA document (EPA, 2009) cited contributions of CHIE studies in elucidating cardiovascular, respiratory, and other effects (see Chapter 6 and Annex C of the ISA). In 2012, EPA issued a “Provisional Assessment of Recent Studies on Health Effects of Particulate Matter Exposure” (EPA, 2012). An updated version of the PM ISA is in development.
NAAQS have been established for both PM10 and PM2.5 (see Table 3-1). However, as stated in the 2009 ISA and subsequent American Thoracic Society reviews, the specific contribution of the thoracic coarse fraction of PM10 (particles with diameters greater than 2.5 µm and less than or equal to 10 µm [PM10-2.5]) to health outcomes and intermediate physiologic or biomarker outcomes is less well understood than the contribution of PM2.5, and the effects of the coarse fraction of PM10 is an active area of investigation.
The 2009 ISA showed that many CHIE studies provided evidence of biologic plausibility of outcomes observed in time-series epidemiologic studies of short-term responses conducted in the United States and elsewhere by demonstrating perturbations in pathways that are relevant to the development of clinical effects. CHIE study results also showed congruence with outcomes demonstrated in animal toxicity studies. Integrating the complementary data from CHIE studies with observational epidemiologic studies and animal toxicity studies, the 2009 ISA found that the strongest evidence for PM-associated health effects was for associations of short-term exposures to PM2.5 with overall mortality, cardiovascular mortality, and nonfatal events. The ISA also found there was some evidence for respiratory effects associated with short-term PM2.5 exposures. Based on all the evidence, the 2009 ISA concluded there are causal relationships between short-term PM2.5 exposure and cardiovascular effects and mortality, and that the relationship is “likely to be causal” for short-term PM2.5 exposure and respiratory effects (EPA, 2009, Table 2-1).
CHIE studies of O3 involve exposure to a discrete chemical entity. In contrast, CHIE PM mass studies involve exposure to a complex mixture that varies both temporally and spatially in the real world. In its review of the PM NAAQS that was completed in 2012, EPA indicated: “We recognize that important uncertainties remain in this review related to understanding the temporal and spatial variability in PM2.5 concentrations, including PM2.5 components, and associated health impacts across different geographic areas and seasons” (EPA, 2011, pp. 2-25).
However, current PM2.5 and PM10 concentration regulations are based only on particle mass, and such regulations, based on observed reductions in particle mass concentration, have been associated with quantitative improvements in mortality and morbidity in settings with PM of varying chemical and biologic components (Correia et al., 2013; Dockery and Ware, 2015; Gauderman et al. 2015; Hao et al., 2017; Laden et al. 2006; Lepeule et al. 2012; Pope et al., 2009, 2013). Given the available information, EPA had decided to maintain the mass-based PM standards during the PM NAAQS review completed in 2012. Quoting directly from the 2009 ISA:
“Overall, the results … indicate that many constituents of PM can be linked with differing health effects and the evidence is not yet sufficient to allow differentiation of those constituents or sources that are more closely related to specific health outcomes. These findings are consistent with the conclusions of the 2004 PM AQCD (EPA[,] 2004), that a number of source types, including motor vehicle emissions, coal combustion, oil burning, and vegetative burning, are associated with health effects. Although the crustal factor of fine particles was not associated with mortality in the 2004 PM AQCD, recent studies have suggested that PM (both PM2.5 and PM10-2.5) from crustal, soil or road dust sources or PM tracers linked to these sources are associated with cardiovascular effects. In addition, secondary [sulfate] PM2.5 has been associated with both cardiovascular and respiratory effects.”
That conclusion was reaffirmed by EPA’s “Provisional Assessment of Recent Studies on Health Effects of Particulate Matter Exposure” (EPA, 2012).
The impact of PM chemical composition variability on human toxicity is an important issue that is relevant to EPA’s regulatory task (for example, see Bell et al., 2009; Boehm et al., 2015; Cox and Popken, 2015; Dominici et al., 2015; Enstrom, 2005; Greven et al., 2011; Kioumourtzoglou et al., 2015; Young and Xia 2013). If a future ISA concludes that the overall body of research is sufficient to identify regional differences in PM toxicity, then future regulatory approaches that differ by region might be warranted rather than a single, nationwide PM mass-based standard.
In an effort to inform future reviews of the PM NAAQS, it would be impractical to use the CHIE study approach to examine the impact of the full range of PM compositions and dose ranges on biologic perturbations associated with ambient PM exposure. An important research strategic planning task is deciding how to address the range of possible PM compositions for future CHIE studies to increase the understanding of the relative importance of PM components on human toxicity for the purposes of regulation (see Chapter 5).
Specificity, Temporality, and Plausibility Considerations
Because the current PM ISA was completed in 2009 and the next iteration of the document is in preparation, the committee examined additional more-recent publications, including reviews of the state of the art on cardiovascular effects of ambient air pollution, for example, Sun et al. (2010), Crouse et al. (2012), EPA (2012), Hoek et al. (2013), Gold and Mittleman (2013), and Lippmann (2014).
Specificity and temporality: As with the O3 studies cited earlier in the chapter, the majority of CHIE studies investigating the effects of PM exposure involved a randomized, controlled crossover design with random assignment of exposure sequence to clean FA as the control and to one of several possible PM exposures. Comparison of response following an FA exposure to those following a PM exposure allows for estimation of the PM effects on an outcome measure while controlling for independent effects of the experimental procedure, and corrected for small changes due to exercise or other influences. This study design is fundamental to the value of the CHIE study in providing specificity, that is, specifically connecting the exposure of interest with the biologic outcomes, while removing confounding factors. It also provides information on temporality, unequivocally connecting the pollutant exposure to biologic outcomes, excluding the possible influence of diurnal variation through the crossover design with the FA control.
PM CHIE studies have examined a variety of exposures, depending on the location of the study facility, and whether the PM generation method involves the concentration of PM from the ambient air, by resuspension or instillation of source particles that are brought to the study site from different locations, or by onsite generation of PM from a specific source, such as diluted diesel-engine exhaust or wood smoke. The selection of the PM source depends on the goals of the specific protocol. Results of high relevance to the PM NAAQS come from CHIE studies with inertially concentrated airborne particles (CAPs) into a small fraction of the original ambient air volume. CAPs contain elemental carbon (EC), which is a ubiquitous single component of airborne PM, which has frequently been associated with adverse health effects in epidemiologic studies. Another particle fraction is OC, which usually adds more mass to PM2.5 than does EC.
All of the EPA CHIE studies that were cited in the 2009 PM ISA involved exposures to CAPs, as did other studies cited in the ISA that were conducted by other investigators in California and Canada. The cited studies, which involved laboratory-generated EC rather than CAPs, were also performed in U.S. laboratories, with most of them performed by investigators at the University of Rochester. In contrast, nearly all of the cited studies involving controlled human exposures to diluted motor-vehicle engine exhaust were conducted in European countries, which have had different regulations affecting motor vehicle engine exhaust.
Plausibility: The 2009 ISA cited CHIE studies extensively regarding associations between short-term PM exposure and biologic end points. For cardiovascular and systemic effects, CHIE studies were cited in support of the plausibility of these biologic end points:
- Heart rate variability;
- Vasomotor function;
- Systemic inflammation;
- Hemostasis, thrombosis, and coagulation factors; and
- Systemic and cardiovascular oxidative stress.
For respiratory effects, CHIE studies were cited in support of the plausibility of these biologic end points:
- Respiratory symptoms and medication use,
- Pulmonary function,
- Pulmonary inflammation,
- Pulmonary oxidative responses,
- Pulmonary injury, and
- Allergic responses.
The American Heart Association Statistical Update (Mozaffarian et al. 2016) provides recent information on cardiovascular health; a range of major clinical disease conditions (including stroke, congenital heart disease, rhythm disorders, subclinical atherosclerosis, coronary heart disease, heart failure, valvular disease, and peripheral arterial disease).
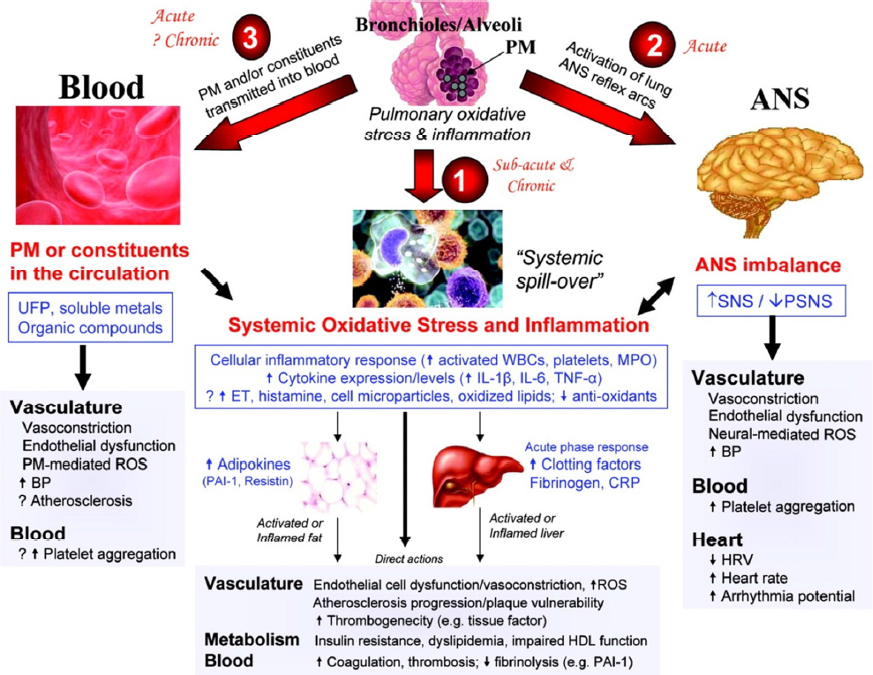
By evaluating cardiophysiologic and biomarker outcomes associated with CAPs exposures, CHIE studies have provided human studies data on the mechanistic plausibility of prior epidemiologic observations suggesting that short-term exposure to elevated concentrations of ambient PM adversely affected cardiac health. Thus CHIEs contributed to understanding how PM inhalation exposure could enter the lung and affect the heart. Figure 3-4 provides a paradigm to frame hypothesis testing, but it does not by any means include all the layers of current understanding of the pathogenesis of various CVD outcomes. While useful, this paradigm is likely to be modified by continuously evolving understanding of CVD etiology.
Specific Cardiovascular Responses
Heart rate variability (HRV): HRV is a measure or marker for cardiovascular autonomic function. Up to a point, more HRV is needed to enable flexibility in response to cardiophysiologic challenges. In longitudinal observational cardiovascular epidemiology studies, reduced HRV (assumed to be chronic) has been a predictor of increased cardiovascular risk. However, it is uncertain in those studies whether HRV is a marker for general ill health or whether it actually influences long- term outcomes directly. CHIE studies have evaluated the acute influence of PM on short-term reversible changes in this physiologic outcome, as part of testing of the plausibility that PM that enters the lung could lead to small reversible perturbations in cardiovascular autonomic function.
There was limited evidence identified in the 2009 ISA to suggest that acute exposure to PM might be associated with HRV changes, suggesting plausibility that particles entering the lung could affect vascular function. With some suggestion of consistency across study designs, many, but not all, animal studies, and human panel studies, as well as CHIE studies, reported altered autonomic function measured by HRV in response to PM. Some studies showed reduced HRV (increased parasympathetic activity relative to sympathetic activity), with other studies showing autonomic responses in the opposite direction. In the CHIE studies PM exposure was most consistently associated with reduced HRV in healthy older adults (Devlin et al., 2003; Gong et al., 2004).
Vasomotor function: In addition to HRV, CHIE studies, as well as observational population-based and panel studies of healthy adults and adults with cardiovascular disease risk factors, have evaluated macrovascular and microvascular subclinical physiologic fine and coarse PM responses with potential relevance to cardiovascular function. Some macrovascular outcomes have included brachial artery diameter, branchial artery flow-mediated dilation (FMD), and blood pressure. The literature was reviewed in the 2009 ISA and in 2010 in an AHA scientific statement (Brook et al. 2010). The AHA statement evaluated the published CHIE studies on PM and physiologic vascular outcomes, including ones in Toronto and Michigan demonstrating associations of concentrated traffic fine particles on brachial artery vasoconstriction or blood pressure in healthy adults (Brook et al. 2002, 2009). While acute subclinical macrovascular responses to PM were not consistently found across all study designs and population, the AHA statement concluded that “even when the few negative studies are considered, the overall evidence supports the concept that ambient PM is capable of impairing vascular function, particularly among higher-risk individuals (for example, those with diabetes [Schneider et al. 2008]) and after traffic-related exposure (Brook et al. 2010, page 2347).”
The 2009 ISA indicated that the cumulative results of several CHIE studies suggest that exposure to diesel exhaust particles (DEPs) is associated with inhibition of endothelium-dependent and endothelium-independent vasodilation (within 2-6 hours), and that the suppression might remain up to 24 hours following exposure (Lund et al., 2009; Mills et al., 2005, 2007; Peretz et al., 2008; Tornqvist et al., 2007). In patients with coronary artery disease, vasodilator function was not affected 6-8 hours after exposure. Shah et al. (2008) suggested that ultrafine particles of EC might produce small changes in systemic vascular function.
Plausibility for systemic inflammatory, prothrombotic, and oxidative stress responses: The ISA and the subsequent American Heart Association (AHA) expert panels reviewed CHIE and other studies on these classes of outcomes.
Systemic inflammation: The ISA reported that CHIE studies of exposures to various PM types have provided limited but inconsistent evidence of a PM-induced increase in markers of systemic inflammation (Barregard et al., 2006; Beckett et al., 2005; Blomberg et al., 2005; Bräuner et al., 2008; Carlsten et al., 2007; Frampton et al., 2006; Gong et al., 2004a,b, 2008; Graff et al., 2009; Mills et al., 2005, 2007, 2008; Peretz et al., 2007; Routledge et al., 2006; Samet et al., 2009; Tornqvist et al., 2007).
Hemostasis, thrombosis, and coagulation: The ISA reported that some CHIE studies provided evidence that short-term exposure to PM2.5 might have small yet statistically significant effects on hemostatic
markers in healthy subjects or patients with coronary artery disease (Barregard et al., 2006; Graff et al., 2009; Lucking et al., 2008; Mills et al., 2005, 2007; Samet et al., 2009).
Systemic and cardiovascular oxidative stress: The ISA reported that CHIE studies of exposure to PM2.5 might increase systemic oxidative and inflammatory responses in human subjects (Barregard et al., 2006; Bräuner et al., 2007; Peretz et al., 2007; Tornqvist et al., 2007).
The ISA reported that a number of CHIE studies suggested that PM2.5 might increase systemic oxidative and inflammatory responses in human subjects. However studies were relatively few with limited consistency in results, as well as in outcomes measured. Subsequently, more published panel studies and CHIE studies have suggested associations of PM2.5 with oxidative stress or inflammatory responses, including some but not all studies reviewed in a 2010 scientific statement from the American Heart Association (Brook et al. 2010). Regarding oxidative stress, statement concluded: “Although not entirely consistent, the available studies demonstrate that acute exposure to PM, perhaps even at ambient levels, may be capable of inducing acute systemic oxidative stress in human subjects under certain circumstances. The assays used to assess the footprint of systemic “oxidative stress” or damage may also play a significant role in the results” (Brook et al. 2010, page 2360).
Plausibility for respiratory responses: Plausibility for respiratory responses was also reviewed by the 2009 ISA and subsequent AHA expert panels.
Symptoms: The ISA reported that CHIE studies found no association between short-term PM2.5 exposure and respiratory symptoms.
Pulmonary function: The majority of CHIE studies cited in the ISA did not provide evidence of PM2.5-induced changes in pulmonary function; however, some investigators observed slight decreases in pulmonary function (Gong et al., 2004b, 2005, 2008; Mudway et al., 2004; Pietropaoli et al., 2004).
Pulmonary inflammation: CHIE studies cited in the ISA provide evidence of PM2.5-induced pulmonary inflammation; however, the response appears to vary substantially depending on the source and composition of the PM. For example, CHIE studies of CAPs from Los Angeles did not show a significant effect on markers of airway inflammation in healthy or health-compromised adults (Gong et al., 2004a, 2004b, 2005, 2008). However, other CHIE studies conducted in Chapel Hill, North Carolina, observed significant indications of pulmonary inflammation among healthy adults following controlled exposures to CAPs (Graff et al., 2009; Samet et al., 2009). Huang et al. (2003) found the increase in BAL fluid neutrophils reported by Ghio et al. (2000) in Chapel Hill to be positively associated with the iron, selenium, and sulfate content of the particles.
Pulmonary oxidative responses: Results of CHIE studies cited in the ISA suggested that short-term exposure to PM2.5 at near-ambient concentrations could produce mild oxidative stress in the lung. For example, Barregard et al. (2008) observed a significant increase in malondialdehyde concentrations in healthy subjects after exposure to wood smoke particles. Limited data suggest that proximal and distal lung regions might be subject to different degrees of oxidative stress during exposures to different pollutant particles (Behndig et al., 2006; Mudway et al., 2004; Schaumann et al., 2004).
Pulmonary injury: One CHIE study cited in the ISA suggests that exposure to wood smoke particles might increase markers of pulmonary injury in healthy adults (Barregard et al., 2008).
Biologic Gradient: PM CHIE Study Contributions to the Four Basic Elements of the NAAQS
PM CHIE studies at EPA have generally focused on questions related to specificity, temporality, and biologic plausibility, rather than biologic gradient. That is to say, PM CHIE studies at EPA have generally used exposures within a narrow range of PM concentrations. This is likely because CHIE study investigators have often not found consistent biomarker or physiologic responses to short-term exposures at lower PM exposure concentrations, even though large epidemiologic studies have found exposure–response associations with health outcomes at lower concentrations than those used in the CHIE studies (EPA, 2009). These differences might be partly due to increased susceptibility of population subgroups in large epidemiologic observational studies, compared to the much smaller number of subjects in CHIE
studies, and partly due to exposure mixture differences. It is possible that exposure to the complex mixtures of PM components and pollutant gases, which are so difficult to disentangle in large epidemiologic studies, has larger effects than exposure to the individual criteria pollutants within them, especially when encountered over longer time periods than the 2-hour durations of CHIE studies.
To facilitate the identification of subgroups at the greatest risk for PM-related health effects, CHIE studies have evaluated factors that contribute to the sensitivity of an individual to criteria pollutant exposures. Such studies aim to evaluate sensitivity to exposure to criteria pollutants on biomarker or physiologic responses. Based on prior knowledge, CHIE studies are designed such that clinical responses are expected to be absent or at least minimal. When studying sensitive groups, CHIE studies involve individuals who might exhibit risk factors to a small degree, but not those who are known through observational epidemiologic studies to be at considerable risk for clinical responses to criteria pollutants.
Indicators: PM2.5 and PM10 are the indicators of the PM NAAQS. EPA monitors the ambient concentrations of those pollutants routinely and extensively and maintains the readings in a publicly available database. However, while pollutant gas concentrations are monitored continuously, most of the PM mass concentrations are based on 24-hour mean concentrations that are monitored only every sixth day. This severely limits their utility for studying transient short-term responses to peak exposures of PM. EPA also maintains a large, but somewhat more selective, database of monitoring values in its Air Quality System database for particle chemical constituents on an every-sixth-day schedule, and they are not used currently as indicator pollutants for NAAQS setting.3
Averaging time: The nation derived substantial calculable public health benefits from the implementation of the pre-2009 annual PM2.5 NAAQS. Reductions in ambient PM2.5 concentration were associated with substantial reductions in annual cardiovascular mortality nationwide, and especially in the northeastern United States (Laden et al., 2006; Pope et al. 2009; Thurston et al., 2013, 2016a).
No CHIE studies were cited in the ISA on health effects of long-term human exposures to PM for setting the annual average 2009 PM NAAQS for PM2.5 and PM10. Given that CHIE study exposure durations are typically a few hours, they are not capable of assessing effects of chronic exposures in humans. Instead, epidemiologic studies are used to investigate chronic effects of importance and offer results that complement the specific insights on acute effects gained through CHIE studies.
Level: The current concentration limit for the primary PM2.5 NAAQS averaged over 24 hours is based primarily on epidemiologic studies with complementary information from CHIE. As noted above, according to the evidence considered during the PM NAAQS review completed in 2012, EPA concluded that reliance on mass concentration limits is warranted, rather than limits on concentrations of the most hazardous particle components. This circumstance reduces EPA’s capacity to focus its air pollution control efforts on the various sources of the most hazardous components of ambient PM10 and PM2.5. This remains an important motivator for future research studies.
Form: In the case of the PM NAAQS, the form of the standard is on a mass basis. The evidence of the adequacy of the form of the current PM NAAQS derives from the evidence of reduced mortality with reduced PM on a mass basis. This evidence accrues from epidemiologic studies, and would not be expected to come from CHIE studies.
Impacts of the Available Results of CHIE PM Studies on the PM NAAQS
As indicated above, CHIE studies have facilitated the identification of subgroups at elevated risk for PM-related health effects. By evaluating biomarker or physiologic responses to PM exposures, CHIE studies have contributed evidence for biologic plausibility of associations of PM with extrapulmonary (e.g., cardiovascular) health effects and to an understanding of factors that determine the sensitivity of an individual to PM pollutant exposures.
__________________
3 See EPA’s Air Quality System, available at https://www.epa.gov/aqs.
Contributions of a Source and Composition-Focused CHIE Study
The results of PM CHIE studies can be influenced by the composition of the PM to which subjects are exposed. The chemical composition of the PM2.5 in diluted DE differs from that of the PM2.5 in the ambient air. DE is greatly enriched in EC and OC, and greatly lowered in terms of toxic metals. Also, the diesel-engine exhaust composition, and the DEPs within the mixture, can vary substantially with engine type and age, duty cycle, and fuel composition, especially in terms of the relatively recent changes in the sulfur content of the fuel.
CHIE studies of DEP in the absence of other components of ambient PM2.5 provide an example of a CHIE study with two types of potential benefits. CHIE studies of DEPs can inform EPA’s decision making concerning initiatives (such as NESHAPs) to reduce PM emissions from a specific source (diesel engines). A second benefit relates to the broader research goal of linking PM composition with biologic perturbations, comparing DEP responses with those from other particle sources. Thus, while it would be anticipated that exposure–response relationships for DEPs to those of ambient-air PM2.5 exposures would differ, the specificity of the response provides value to the PM knowledge base. However, whatever biologic perturbations that are observed in response to controlled DEP exposures could be due, at least in part, to NO2, NO, CO, or OC gases, to condensed OC and toxic trace metals in the exhaust stream, or to their reaction products and interactions. Likewise, in comparing the results of DEP CHIE studies to inhalation exposures near roadways, another source of PM exposure in addition to the diluted tailpipe emissions is road dust resuspension, which adds PM from tire wear, brake and clutch wear, wind-blown soil, and road surface material, all of which add additional toxic trace metals and organics to the inhaled mixture.
CONCLUSIONS
CHIE studies have provided information about specificity, temporality, biologic plausibility for O3 and PM exposure, and relevant end points. Additional information about biologic gradients and susceptible (also referred to as “at-risk”) subpopulations has also been provided. The four elements of the NAAQS (indicator, averaging time, level, and form) have been influenced by CHIE study results to different degrees.
CHIE study findings also help to enrich a scientific understanding of the underlying biologic and physiologic short-term responses to daily inhalation exposures to airborne pollutants, or mixtures thereof.
- Such information is important for future NAAQS reviews and cannot be determined only through observational studies of exposures to complex ambient mixtures among groups of humans whose genetic and other constitutional variables, prior illnesses, occupational exposures, and smoking histories are not as well defined as those for CHIE study subjects.
- For complex mixtures of ambient air pollutants, a further challenge of CHIE studies and epidemiologic studies is the spatial and temporal variability of the chemical compositions of the mixtures, which makes it more difficult to assess exposure–response relationships in terms of considerations of specificity and consistency.
CHIE study results combined with information from observational epidemiologic, panel, and toxicologic studies can facilitate a holistic evaluation of the evidence and thereby provide a well-considered scientific basis for establishing or revising NAAQS.
Developing and refining biomarkers of responses to short-term inhalation exposures to specific pollutants can lead to
- Incorporation of those biomarkers in panel and cohort studies,
- Incorporation of those biomarkers in animal inhalation studies, and
- Mechanistic research to determine the utility of the biomarkers in studies of disease progression.



















