2
Cannabis
HISTORY OF CANNABIS
Cannabis sativa is one of the world’s oldest cultivated plants (Russo, 2007). Although the earliest written records of the human use of cannabis date from the 6th century B.C. (ca. 2,600 cal BP), existing evidence suggests that its use in Europe and East Asia started in the early Holocene (ca. 8,000 cal BP) (Long et al., 2016). Many 19th-century practitioners ascribed medicinal properties to cannabis after the drug found its way to Europe during a period of colonial expansion into Africa and Asia. For example, William B. O’Shaughnessy, an Irish physician working at the Medical College and Hospital in Calcutta, first introduced cannabis (Indian hemp) to Western medicine as a treatment for tetanus and other convulsive diseases (O’Shaughnessy, 1840). At approximately the same time, French physician Jean-Jacques Moreau de Tours experimented with the use of cannabis preparations for the treatment of mental disorders (Moreau de Tours, 1845). Soon after, in 1851, cannabis was included in the 3rd edition of the Pharmacopoeia of the United States (USP). Subsequent revisions of the USP described in detail how to prepare extracts and tinctures of dried cannabis flowers to be used as analgesic, hypnotic, and anticonvulsant (Russo, 2007; U.S. Pharmacopoeial Convention, 1916). Growing concerns about cannabis resulted in the outlawing of cannabis in several states in the early 1900s and federal prohibition of the drug in 1937 with the passage of the Marihuana Tax Act. In response to these concerns, in 1942 the American Medical Association removed cannabis from the 12th edition of U.S. Pharmacopeia (IOM, 1999).
THE CANNABIS PLANT
Cannabis cultivars are considered as part of one genus, Cannabis, family Cannabaceae, order Urticales (Kuddus et al., 2013). Two accepted genera of Cannabaceae are Cannabis and Humulus (hops). There is, however, an ongoing debate concerning the taxonomic differentiation within the Cannabis genus (Laursen, 2015). On the basis of genetic variations, a multitypic genus with at least two putative species, Cannabis sativa and Cannabis indica, has been proposed by some researchers (Clarke and Merlin, 2015; Hillig, 2005). Other researchers have suggested a unique species Cannabis sativa with the genetic differences explained by variations at both the subspecies and the variety level or at a biotype level of putative taxa (Small, 2015).
Chemical Constituents of Cannabis
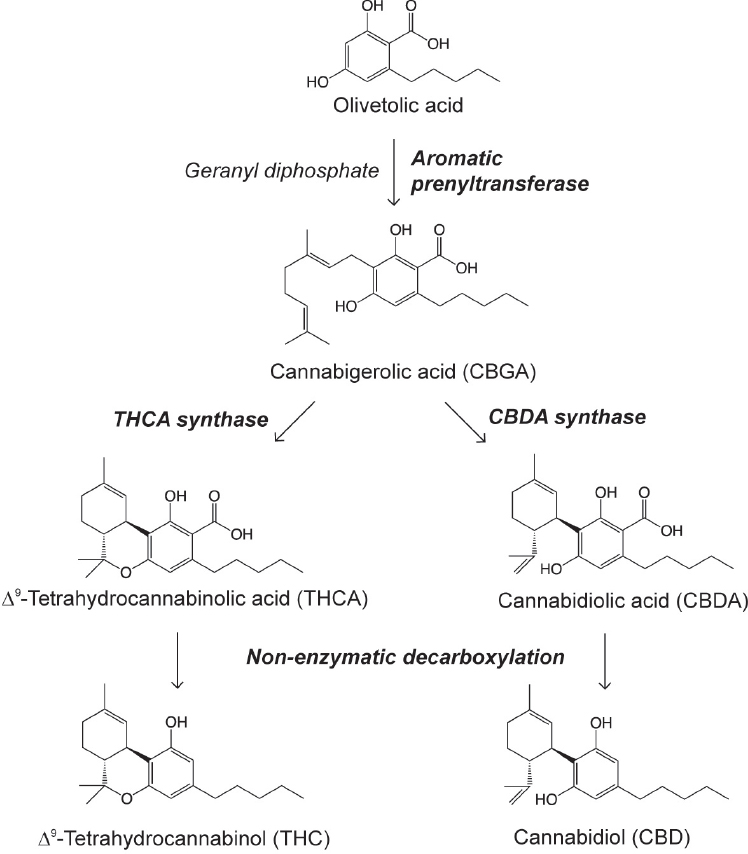
To date, more than 104 different cannabinoids1 have been identified in cannabis (ElSohly and Gul, 2014). Other compounds identified include terpenoids, flavonoids, nitrogenous compounds, and more common plant molecules (American Herbal Pharmacopoeia, 2013). Among these, Δ9-tetrahydrocannabinol (THC) has received the most attention for being responsible for the intoxicated state sought after by recreational cannabis users, owing to its ability to act as a partial agonist2 for type-1 cannabinoid (CB1) receptors. Cannabinoids exist mainly in the plant as their carboxylic precursors (Δ9-tetrahydrocannabinolic acid [THCA] and cannabidiolic acid [CBDA]) and are decarboxylated by light or heat while in storage or when combusted (Grotenhermen, 2003). Δ9-THC is synthesized within the glandular trichomes present in the flowers, leaves, and bracts of the female plant. It shares a common precursor, olivetoic acid, with another quantitatively important constituent of Cannabis sativa, cannabidiol (CBD), which is the most abundant cannabinoid in hemp (see Figure 2-1). For this reason, the genetic profile and relative level of expression of the enzymes responsible for their synthesis (genotype), namely THCA synthase and CBDA synthase, determine the chemical composition of a particular cultivar (chemotype).
Cannabis plants typically exhibit one of the three main different chemotypes based on the absolute and relative concentrations of Δ9-THCA and CBDA (see Table 2-1), which makes it possible to distinguish among the Δ9-THC-type, or drug-type; the intermediate-type; and the CBD-type
___________________
1 Cannabinoids are a group of psychoactive chemical compounds found in the cannabis plant.
2 Partial agonists are ligands that interact with their receptors to produce a level of response that is less than the response to full agonists.
cannabis plants grown for fiber (industrial hemp) or seed oil in which the content of Δ9-THC does not exceed 0.3 percent on a dry-weight basis (Chandra et al., 2013). CBD is pharmacologically active, however, and, therefore, classifying cannabis in terms of drug- and fiber-producing seems inaccurate. Both THC- and CBD-types are considered drug-types, and both cultivars could theoretically be exploited to produce fiber.
TABLE 2-1 Cannabis Phenotypes
NOTE: THCA-predominant strains can yield more than 25 percent Δ9-THC; specifically selected CBDA clones can yield up to 20 percent CBD.
SOURCE: Modified from Galal et al., 2009.
Pharmacological Properties of Δ9-THC
In a series of studies conducted in the late 1930s and early 1940s, Roger Adams and coworkers isolated cannabinol and CBD from hemp oil and then isomerized CBD into a mixture of two tetrahydrocannabinols with “marihuana-like” physiological activity in dogs, proving their structure except for the final placement of one double bond (Adams et al., 1940a,b). Two years later, tetrahydrocannabinol was first isolated from cannabis resin (Wollner et al., 1942). In 1964, thanks to the development of such potent analytical techniques as nuclear magnetic resonance imaging, Gaoni and Mechoulam were able to identify the position of this elusive double bond, thus resolving the final structure of Δ9-THC (Gaoni and Mechoulam, 1964).
In the late 1980s William Devane and Allyn Howlett first postulated the existence of cannabinoid receptors by showing how synthetic molecules designed to mimic the actions of Δ9-THC were able to bind a selective site in brain membranes, thus inhibiting the intracellular synthesis of cyclic adenosine monophosphate (cAMP) through a G protein–mediated mechanism (Devane et al., 1988). The mapping of cannabinoid-binding sites in the rat brain (Herkenham et al., 1990) and the molecular cloning of the first cannabinoid receptor gene (Matsuda et al., 1990) subsequently corroborated this hypothesis. Three years later, a second G protein–coupled cannabinoid receptor was cloned from a promyelocytic cell line and termed CB2 (Munro et al., 1993).
Both CB1 and CB2 signal through the transducing G proteins, Gi and Go, and their activation by Δ9-THC or other agonists causes the inhibition of adenylyl cyclase activity, the closing of voltage-gated calcium channels, the opening of inwardly rectifying potassium channels, and the stimulation of mitogen-activated protein kinases such as extracellular signal–regulated kinases (ERKs) and focal adhesion kinases (FAKs) (Mackie, 2006).
The expression pattern of CB1 receptors in brain structures correlates with the psychoactive effects of cannabis. In mammals, high concen-
trations of CB1 are found in areas that regulate appetite, memory, fear extinction, motor responses, and posture such as the hippocampus, basal ganglia, basolateral amygdala, hypothalamus, and cerebellum (Mackie, 2006). CB1 is also found in a number of nonneural tissues, including the gastrointestinal tract, adipocytes, liver, and skeletal muscle. In addition to CB1, the brain also contains a small number of CB2 receptors, although this subtype is mainly expressed in macrophages and macrophage-derived cells such as microglia, osteoclasts, and osteoblasts (Mackie, 2006).
Pharmacological Properties of Cannabidiol
Cannabidiol was first isolated from hemp oil in 1940 (Adams et al., 1940a) and its structure predicted by chemical methods (Adams et al., 1940b); its fine structure was determined in later studies (Mechoulam and Shvo, 1963). CBD lacks the cannabis-like intoxicating properties of Δ9-THC and, for this reason, has been traditionally considered non-psychoactive. CBD displays very low affinity for CB1 and CB2 cannabinoid receptors (Thomas et al., 2007), but it might be able to negatively modulate CB1 via an allosteric mechanism (Laprairie et al., 2015)3; however, CBD can interfere with the deactivation of the endocannabinoid molecule anandamide, by targeting either its uptake or its enzymatic degradation, catalyzed by fatty-acid amide hydrolase (FAAH), which could indirectly activate CB1 (De Petrocellis et al., 2011; Elmes et al., 2015) (see Box 2-1).
CBD is also a known agonist of serotonin 5-HT1A receptors (Russo et al., 2005) and transient receptor potential vanilloid type 1 (TRPV1) receptors (Bisogno et al., 2001). It can also enhance adenosine receptor signaling by inhibiting adenosine inactivation, suggesting a potential therapeutic role in pain and inflammation (Carrier et al., 2006). The antioxidant and anti-inflammatory properties of this compound may explain its potential neuroprotective actions (Scuderi et al., 2009). Irrespective of the mechanism of action, there is evidence that CBD could potentially be exploited in the treatment and symptom relief of various neurological disorders such as epilepsy and seizures (Hofmann and Frazier, 2013; Jones et al., 2010), psychosis (Leweke et al., 2016), anxiety (Bergamaschi et al., 2011), movement disorders (e.g., Huntington’s disease and amyotrophic lateral sclerosis) (de Lago and Fernandez-Ruiz, 2007; Iuvone et al., 2009), and multiple sclerosis (Lakhan and Rowland, 2009).
___________________
3 Allosteric modulators are ligands that indirectly influence the effects of an agonist or inverse agonist at a target receptor. Allosteric modulators bind to a site distinct from that of the orthosteric agonist binding site.
CANNABIS-DERIVED PRODUCTS
In the United States, cannabis-derived products are consumed for both medical and recreational purposes in a variety of ways. These include smoking or inhaling from cigarettes (joints), pipes (bowls), water pipes (bongs, hookahs), and blunts (cigars filled with cannabis); eating or drinking food products and beverages; or vaporizing the product. These different modes are used to consume different cannabis products, including cannabis “buds” (dried cannabis flowers); cannabis resin (hashish, bubble hash); and cannabis oil (butane honey oil, shatter, wax, crumble). The oil, which may contain up to 75 percent Δ9-THC—versus 5 to 20 percent in the herb or resin (Raber et al., 2015)—is extracted from plant material using organic solvents, such as ethanol, hexane, butane, or supercritical
(or subcritical) CO2, and can be either smoked or vaporized by pressing the extracted oil against the heated surface of an oil rig pipe (dabbing). Cannabinoids can also be absorbed through the skin and mucosal tissues, so topical creams, patches, vaginal sprays, and rectal suppositories are sometimes employed and used as a form of administering Δ9-THC (Brenneisen et al., 1996). A broad selection of cannabis-derived products are also available in the form of food and snack items, beverages, clothing, and health and beauty aid products.
Potency of Cannabis
In the 1990s and early 2000s, the bulk of cannabis consumed in the United States was grown abroad and illicitly imported. The past decade has seen an influx of high-potency cannabis produced within the United States—for example, “sinsemilla”—which is grown from clones rather than from seeds. Data from the U.S. Drug Enforcement Administration (DEA) seizures record a substantial increase in average potency, from 4 percent in 1995 to roughly 12 percent in 2014, both because high-quality U.S.-grown cannabis has taken market share from Mexican imports and because cannabis from both sources has grown in potency (ElSohly et al., 2016; Kilmer, 2014).
Route of Administration
The route of administration of cannabis can affect the onset, intensity, and duration of the psychotropic effects, the effects on organ systems, and the addictive potential and negative consequences associated with its use (Ehrler et al., 2015). The consumption of cannabis causes a particular combination of relaxation and euphoria, commonly referred to as a “high.” When cannabis is smoked, Δ9-THC quickly diffuses to the brain, eliciting a perceived high within seconds to minutes. Blood levels of Δ9-THC reach a maximum after about 30 minutes and then rapidly subside within 1 to 3.5 hours (Fabritius et al., 2013; Huestis et al., 1992). Vaping has an onset, peak, and duration that are similar to those of smoking and produces a similar high (Abrams et al., 2007). “Dabbing,” a term for flash-vaporizing butane hash oil-based concentrates, has been reported to offer a different and stronger intoxicating effect than smoking/vaping (Loflin and Earleywine, 2014). By contrast, eating does not produce effects for 30 minutes to 2 hours, and the perceived high is relatively prolonged, lasting 5 to 8 hours or even longer. The slow action of orally ingested cannabis is due to Δ9-THC being absorbed by the intestine and transported to the liver (hepatic first pass) where it is converted into 11-OH-THC, an equipotent and longer-lasting metabolite (Huestis et al., 1992). Edibles make
it harder to titrate the intoxicating effects due to the delayed and variable onset. Consequently, edibles have been tied to the ingestion of excessive amounts of cannabis under the misperception that the initial dose had not produced the desired effect (Ghosh and Basu, 2015; MacCoun and Mello, 2015). The availability of edibles has also been associated with increased rates of accidental pediatric ingestion of cannabis (Wang et al., 2014).
Trends in Routes of Administration
There are no high-quality nationally representative data on the prevalence of the non-herbal forms of cannabis (e.g., edibles, oils, and other concentrates), but evidence suggests that they are more commonly used by medical cannabis patients in states with recreational or lenient medical cannabis policies (Daniulaityte et al., 2015; Pacula et al., 2016). Forty percent of 12th-grade past-year users reported using cannabis in edible form in medical cannabis states, versus 26 percent in states without medical cannabis laws (NIDA, 2014). In Washington State, an online survey from 2013 found that, among daily and near-daily cannabis users, 27.5 percent had used edibles, 22.8 percent had used hash resin, and 20.4 percent had “dabbed” in the past week (Kilmer et al., 2013).
Data from recreational cannabis sales in Washington and Colorado provide a glimpse of trends that are specific to markets that have legalized cannabis. In Washington State, herbal cannabis remains dominant, having accounted for two-thirds of all sales revenues in June 2016, but it is losing market share as “cannabis extracts for inhalation” become more popular, at 21 percent in June 2016 as compared with 12 percent 1 year prior. The sales of liquid and solid edibles (9 percent) combined account for most of the remaining sales.4 Non-herbal varieties are even more popular on Colorado’s recreational market, where herbal cannabis accounts for a narrow majority (56 percent) and sales of solid concentrates (24 percent) and edibles (13 percent) are on the rise (Castle, 2016).
Partly to provide a guide for the responsible use of non-herbal varieties of cannabis, states that have legalized the recreational cannabis have defined a standard “dose” of THC. Washington State and Colorado have set the standard “dose” of THC as 10 mg, while Oregon chose a lower limit of 5 mg. For perspective, the typical joint size in the United States is 0.66 g (Mariani et al., 2011) and the average potency is 8 percent THC (Fabritius et al., 2013), resulting in an average dose of 8.25 mg THC per joint; higher THC levels ranging from 15–20 percent or higher would yield
___________________
4 Author’s calculations from Washington State Liquor and Cannabis Board’s publicly available August 2016 “traceability” dataset (“biotrackthc_dispensing.csv”). Data requests available at: http://lcb.wa.gov/records/public-records (accessed January 5, 2017).
a THC dose between 9.9–13.2 mg. Occasional users report feeling “high” after consuming only 2–3 mg of THC (Hall and Pacula, 2010); however, users who have developed tolerance to the effects of THC via frequent use may prefer much larger quantities.
CLINICAL FEATURES OF CANNABIS INTOXICATION
During acute cannabis intoxication, the user’s sociability and sensitivity to certain stimuli (e.g., colors, music) may be enhanced, the perception of time is altered, and the appetite for sweet and fatty foods is heightened. Some users report feeling relaxed or experiencing a pleasurable “rush” or “buzz” after smoking cannabis (Agrawal et al., 2014). These subjective effects are often associated with decreased short-term memory, dry mouth, and impaired perception and motor skills. When very high blood levels of Δ9-THC are attained, the person may experience panic attacks, paranoid thoughts, and hallucinations (Li et al., 2014). Furthermore, as legalized medical and recreational cannabis availability increase nationwide, the impairment of driving abilities during acute intoxication has become a public safety issue.
In addition to Δ9-THC dosage, two main factors influence the intensity and duration of acute intoxication: individual differences in the rate of absorption and metabolism of Δ9-THC, and the loss of sensitivity to its pharmacological actions. Prolonged CB1 receptor occupation as a consequence of the sustained use of cannabis can trigger a process of desensitization, rendering subjects tolerant to the central and peripheral effects of Δ9-THC and other cannabinoid agonists (Gonzalez et al., 2005). Animals exposed repeatedly to Δ9-THC display decreased CB1 receptor levels as well as impaired coupling between CB1 and its transducing G-proteins (Gonzalez et al., 2005). Similarly, in humans, imaging studies have shown that chronic cannabis use leads to a down-regulation of CB1 receptors in the cortical regions of the brain and that this effect can be reversed by abstinence (Hirvonen et al., 2012).
CANNABINOID-BASED MEDICATIONS
The U.S. Food and Drug Administration (FDA) has licensed three drugs based on cannabinoids (see Table 2-2). Dronabinol, the generic name for synthetic Δ9-THC, is marketed under the trade name of Marinol® and is clinically indicated to counteract the nausea and vomiting associated with chemotherapy and to stimulate appetite in AIDS patients affected by wasting syndrome. A synthetic analog of Δ9-THC, nabilone (Cesamet®), is prescribed for similar indications. Both dronabinol and nabilone are given orally and have a slow onset of action. In July 2016 the
TABLE 2-2 Cannabinoid-Based Medications
| CANNABINOID-BASED MEDICATIONS | |||
|---|---|---|---|
| Substance | Route of Administration | Description | |
| Natural Product Derived Compounds | Cannabidiol (CBD) | Oral capsule Oromucosal spray |
Cannabinoid extracted from Cannabis plant |
| Cannabis | Multiple |
Multiple active cannabinoids |
|
| Cannador | Oral capsule |
THC and CBD from Cannabis extract |
|
| Epidiolex® (FDA Fast Track) |
Oil |
Concentrated CBD from Cannabis extract |
|
| Nabiximol (Sativex®) (FDA Fast Track) |
Oromucosal spray |
THC and CBD extract from two Cannabis plant varieties |
|
| Tetrahydrocannabinol (THC) | Oral capsule Smoked Oromucosal spray |
Active cannabinoid of Cannabis plant |
|
| THC/CBD | Oral capsule |
Combination of cannabinoids |
|
| Synthetic Compounds | Ajulemic acid (AjA) (FDA PHASE II Active) |
Oral capsule |
Synthetic nonpsychoactive cannabinoid |
| Dronabinol (Marinol®; Syndros®) (FDA approved) | Oral capsule |
Synthetic THC |
|
| Nabilone (Cesamet®) (FDA approved) |
Oral capsule |
Synthetic cannabinoid—THC analogue |
|
FDA approved Syndros®, a liquid formulation of dronabinol, for the treatment of patients experiencing chemotherapy-induced nausea and vomiting who have not responded to conventional antiemetic therapies. The agent is also indicated for treating anorexia associated with weight loss in patients with AIDS. Two additional cannabinoid-based medications have been examined by the FDA. Nabiximols (Sativex®) is an ethanol cannabis extract composed of Δ9-THC and CBD in a one-to-one ratio. Nabiximols is administered as an oromucosal spray and is indicated in the symptomatic relief of multiple sclerosis and as an adjunctive analgesic treatment in cancer patients (Pertwee, 2012). As of September 2016, nabiximols has
been launched in 15 countries, including Canada, Germany, Italy, Spain, the United Kingdom, and has been approved in a further 12, but not in the United States.5 In response to the urgent need expressed by parents of children with intractable epilepsy, in 2013 the FDA allowed investigational new drug studies of Epidiolex®, a concentrated CBD oil (>98 percent CBD), also developed by GW Pharmaceuticals, as an anti-seizure medication for Dravet and Lennox-Gastaut syndromes.
SYNTHETIC CANNABINOIDS AS RECREATIONAL DRUGS
In addition to nabilone, many other synthetic cannabinoids agonists have been described and widely tested on experimental animals to investigate the consequences of cannabinoid receptor activation6 (e.g., CP-55940, WIN-55212-2, JWH-018) (Iversen, 2000; Pertwee, 2012). The therapeutic application of these highly potent molecules is limited by their CB1-mediated psychotropic side effects, which presumably provide the rationale for the illicit use of some of them as an alternative to cannabis (Wells and Ott, 2011). Preclinical and clinical data in support of this claim remain very limited, however. Internet-marketed products such as Spice, K2, and Eclipse are a blend of various types of plant material (typically herbs and spices) that have been sprayed with one of these synthetic cannabinoids (as well as other non-cannabinoid psychoactive drugs). Since 2009 more than 140 different synthetic cannabinoids have been identified in herbal mixtures consumed as recreational drugs. The synthetic cannabinoids used in “herbal mixtures” are chemically heterogeneous, most of them being aminoalkylindole derivatives such as naphthoylindoles (e.g., JWH-018 and JWH-210), cyclopropylindoles (e.g., UR-144, XLR-11), or quinoline esters (e.g., PB-22). They seem to appeal especially to young cannabis and polydrug users because they are relatively inexpensive, easily available through the Internet, and difficult to identify with standard immunoassay drug screenings. In contrast to Δ9-THC, which is a partial agonist of the CB1 receptor, many of the synthetic cannabinoids bind to CB1 receptors with high affinity and efficacy, which may also be associated with higher potential of toxicity (Hermanns-Clausen et al., 2016). According to the National Institute on Drug Abuse (NIDA, 2012, p. 2), people using these various blends have been admitted to Poison Control Centers reporting “rapid heart rate, vomiting, agitation, confusion, and hallucinations.” Synthetic cannabinoids can also raise blood pressure and cause a reduced blood supply to the heart (myocardial ischemia), and in a
___________________
5 For additional information see: http://www.gwpharm.com (accessed January 5, 2017).
6 Due to the determined scope of this report, nontherapeutic synthetic cannabinoids will not be discussed in the forthcoming chapters of the report.
few cases they have been associated with heart attacks. Regular users may experience withdrawal and symptoms of dependence (Tait et al., 2016).
CANNABIS CONTAMINANTS AND ADULTERANTS
The large economic potential and illicit aspect of cannabis has given rise to numerous potentially hazardous natural contaminants or artificial adulterants being reported in crude cannabis and cannabis preparations. Most frequent natural contaminants consist of degradation products, microbial contamination (e.g., fungi, bacteria), and heavy metals. These contaminants are usually introduced during cultivation and storage (McLaren et al., 2008). Growth enhancers and pest control chemicals are the most common risks to both the producer and the consumer. Cannabis can also be contaminated for marketing purposes. This usually entails adding substances (e.g., tiny glass beads, lead) to increase the weight of the cannabis product (Busse et al., 2008; Randerson, 2007) or adding psychotropic substances (e.g., tobacco, calamus) and cholinergic compounds to either enhance the efficacy of low-quality cannabis or to alleviate its side effects (McPartland et al., 2008). Additionally, some extraction and inhalation methods used for certain dosing formulations (tinctures, butane hash oil, “dabs”) can result in substantial pesticide and solvent contamination (Thomas and Pollard, 2016).
REFERENCES
Abrams, D. I., H. P. Vizoso, S. B. Shade, C. Jay, M. E. Kelly, and N. L. Benowitz. 2007. Vaporization as a smokeless cannabis delivery system: A pilot study. Clinical Pharmacology and Therapeutics 82(5):572–578.
Adams, R., M. Hunt, and J. H. Clark. 1940a. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. Journal of the American Chemical Society 62(1):196–200.
Adams, R., D. C. Pease, C. K. Cain, B. R. Baker, J. H. Clark, H. Wolff, and R. B. Wearn. 1940b. Conversion of cannabidiol to a product with marihuana activity. Journal of the American Chemical Society 62(8):2245–2246.
Agrawal, A., P. A. Madden, K. K. Bucholz, A. C. Heath, and M. T. Lynskey. 2014. Initial reactions to tobacco and cannabis smoking: A twin study. Addiction 109(4):663–671.
American Herbal Pharmacopoeia. 2013. Cannabis inflorescence: Cannabis spp.: Standards of identity, analysis, and quality control. Scott’s Valley, CA: American Herbal Pharmacopoeia.
Bergamaschi, M. M., R. H. Queiroz, M. H. Chagas, D. C. de Oliveira, B. S. De Martinis, F. Kapczinski, J. Quevedo, R. Roesler, N. Schröder, A. E. Nardi, R. Martín-Santos, J. E. Hallak, A. W. Zuardi, and J. A. Crippa. 2011. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 36(6):1219–1226.
Bisogno, T., L. Hanus, L. De Petrocellis, S. Tchilibon, D. E. Ponde, I. Brandi, A. S. Moriello, J. B. Davis, R. Mechoulam, and V. Di Marzo. 2001. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. British Journal of Pharmacology 134(4):845–852.
Brenneisen, R., A. Egli, M. A. Elsohly, V. Henn, and Y. Spiess. 1996. The effect of orally and rectally administered delta 9-tetrahydrocannabinol on spasticity: A pilot study with 2 patients. International Journal of Clinical Pharmacology and Therapeutics 34(10):446–452.
Busse, F., L. Omidi, K. Timper, A. Leichtle, M. Windgassen, E. Kluge, and M. Stumvoll. 2008. Lead poisoning due to adulterated marijuana. New England Journal of Medicine 358(15):1641–1642.
Carrier, E. J., J. A. Auchampach, and C. J. Hillard. 2006. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proceedings of the National Academy of Sciences of the United States of America 103(20):7895–7900.
Castle, S. 2016. More growers brings surge in weed supplies, plunge in Boulder County pot prices. Daily Camera, August 26. http://www.dailycamera.com/boulder-business/ci_30295353/bumper-crop-growers-leads-surge-weed-supplies-plunge (accessed November 8, 2016).
Chandra, S., H. Lata, I. A. Khan, M. A. ElSohly. 2013. The role of biotechnology in Cannabis sativa propagation for the production of phytocannabinoid. In S. Chandra, H. Lata, I. A. Khan, and M. A. ElSohly (eds.), Biotechnology for medicinal plants. Berlin: SpringerVerlag. Pp. 123–148.
Clarke, R. C., and M. D. Merlin. 2015. Cannabis: Evolution and ethnobotany. Berkeley: University of California Press.
Daniulaityte, R., R. W. Nahhas, S. Wijeratne, R. G. Carlson, F. R. Lamy, S. S. Martins, E. W. Boyer, G. A. Smith, and A. Sheth. 2015. Time for dabs: Analyzing Twitter data on marijuana concentrates across the U.S. Drug and Alcohol Dependence 155:307–311.
de Lago, E. and J. Fernandez-Ruiz. 2007. Cannabinoids and neuroprotection in motor-related disorders. CNS and Neurological Disorders in Drug Targets 6(6):377–387.
De Petrocellis, L., A. Ligresti, A. S. Moriello, M. Allarà, T. Bisogno, S. Petrosino, C. G. Stott, and V. Di Marzo. 2011. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British Journal of Pharmacology 163(7):1479–1494.
Devane, W. A., F. A. Dysarz, 3rd, M. R. Johnson, L. S. Melvin, and A. C. Howlett. 1988. Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology 34(5):605–613.
Ehrler, M. R., E. C. Deborah, and D. A. Yurgelun-Todd. 2015. Subjective and cognitive effects of cannabinoids in marijuana smokers. In P. Campolongo and L. Fattore (eds.), Cannabinoid modulation of emotion, memory, and motivation. New York: Springer. Pp. 159–181.
Elmes, M. W., M. Kaczocha, W. T. Berger, K. Leung, B. P. Ralph, L. Wang, J. M. Sweeney, J. T. Miyauchi, S. E. Tsirka, I. Ojima, and D. G. Deutsch. 2015. Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Journal of Biological Chemistry 290(14):8711–8721.
ElSohly, M. A., and W. Gul. 2014. Constituents of cannabis sativa. In Handbook of Cannabis. Oxford, UK: Oxford University Press. P. 20.
ElSohly, M. A., Z. Mehmedic, S. Foster, C. Gon, S. Chandra, and J. C. Church. 2016. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biological Psychiatry 79(7):613–619.
Fabritius, M., H. Chtioui, G. Battistella, J. M. Annoni, K. Dao, B. Favrat, E. Fornari, E. Lauer, P. Maeder, and C. Giroud. 2013. Comparison of cannabinoid concentrations in oral fluid and whole blood between occasional and regular cannabis smokers prior to and after smoking a cannabis joint. Analytical and Bioanalytical Chemistry 405(30):9791–9803.
Galal, A. M., D. Slade, W. Gul, A. T. El-Alfy, D. Ferreira, and M. A. Elsohly. 2009. Naturally occurring and related synthetic cannabinoids and their potential therapeutic applications. Recent Patents on CNS Drug Discovery 4:112–136.
Gaoni, Y., and R. Mechoulam. 1964. Isolation, structure, and partial synthesis of an active constituent of hashish. Journal of the American Chemical Society 86(8):1646–1647.
Ghosh, A., and D. Basu 2015. Cannabis and psychopathology: The meandering journey of the last decade. Indian Journal of Psychiatry 57(2):140–149.
Gonzalez, S., M. Cebeira, and J. Fernández-Ruiz. 2005. Cannabinoid tolerance and dependence: A review of studies in laboratory animals. Pharmacology Biochemistry and Behavior 81(2):300–318.
Grotenhermen, F. 2003. Pharmacokinetics and pharmacodynamics of cannabinoids. Clinical Pharmacokinetics 42(4):327–360.
Hall, W. D., and R. L. Pacula. 2010. Cannabis use and dependence: Public health and public policy. (reissue of 2003 first edition). Cambridge, UK: Cambridge University Press.
Herkenham, M., A. B. Lynn, M. D. Little, M. R. Johnson, L. S. Melvin, B. R. de Costa, and K. C. Rice. 1990. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the United States 87(5):1932–1936.
Hermanns-Clausen, M., J. Kithinji, M. Spehl, V. Angerer, F. Franz, F. Eyer, and V. Auwärter. 2016. Adverse effects after the use of JWH-210—A case series from the EU Spice II plus project. Drug Testing and Analysis 8(10):1030–1038.
Hillig, K. W. 2005. Genetic evidence for speciation in Cannabis (Cannabaceae). Genetic Resources and Crop Evolution 52(2):161–180.
Hirvonen, J., R. S. Goodwin, C. T. Li, G. E. Terry, S. S. Zoghbi, C. Morse, V. W. Pike, N. D. Volkow, M. A. Huestis, and R. B. Innis. 2012. Reversible and regionally selective down-regulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Molecular Psychiatry 17(6):642–649.
Hofmann, M. E., and C. J. Frazier. 2013. Marijuana, endocannabinoids, and epilepsy: Potential and challenges for improved therapeutic intervention. Experimental Neurology 244:43–50.
Huestis, M. A., J. E. Henningfield, and E. J. Cone. 1992. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. Journal of Analytical Toxicology 16(5):276–282.
IOM (Institute of Medicine). 1999. Marijuana and medicine: Assessing the science base. Washington, DC: National Academy Press.
Iuvone, T., G. Esposito, D. De Filippis, C. Scuderi, and L. Steardo. 2009. Cannabidiol: A promising drug for neurodegenerative disorders? CNS Neuroscience and Therapeutics 15(1):65–75.
Iversen, L. 2000. The science of marijuana. New York: Oxford University Press.
Jones, N. A., A. J. Hill, I. Smith, S. A. Bevan, C. M. Williams, B. J. Whalley, and G. J. Stephens. 2010. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. Journal of Pharmacology and Experimental Therapeutics 332(2):569–577.
Kilmer, B. 2014. Policy designs for cannabis legalization: Starting with the eight Ps. The American Journal of Drug and Alcohol Abuse 40(4):259–261.
Kilmer, B., J. P. Caulkins, G. Midgette, L. Dahlkemper, R. J. MacCoun, and R. L. Pacula. 2013. Before the grand opening: Measuring Washington State’s marijuana market in the last year before legalized commercial sales. Santa Monica, CA: RAND Corporation.
Kuddus, M., I. A. M. Ginawi, and A. Al-Hazimi. 2013. Cannabis sativa: An ancient wild edible plant of India. Emirates Journal of Food and Agriculture 25(10):736–745.
Lakhan, S. E., and M. Rowland. 2009. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: A systematic review. BMC Neurology 9:59.
Laprairie, R. B., A. M. Bagher, M. E. Kelly, and E. M. Denovan-Wright. 2015. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. British Journal of Pharmacology 172(20):4790–4805.
Laursen, L. 2015. Botany: The cultivation of weed. Nature 525(7570):S4–S5.
Leweke, F. M., J. K. Mueller, B. Lange, and C. Rohleder. 2016. Therapeutic potential of cannabinoids in psychosis. Biological Psychiatry 79(7):604–612.
Li, R. F., G. T. Lu, L. Li, H. Z. Su, G. F. Feng, Y. Chen, Y. Q. He, B. L. Jiang, D. J. Tang, and J. L. Tang. 2014. Identification of a putative cognate sensor kinase for the two-component response regulator HrpG, a key regulator controlling the expression of the hrp genes in Xanthomonas campestris pv. campestris. Environmental Microbiology 16(7):2053–2071.
Loflin, M., and M. Earleywine. 2014. A new method of cannabis ingestion: The dangers of dabs? Addictive Behaviors 39(10):1430–1433.
Long, T., M. Wagner, D. Demske, C. Leipe, and P. E. Tarasov. 2016. Cannabis in Eurasia: Origin of human use and Bronze Age trans-continental connections. Vegetation History and Achaeobotany 25:1–14.
MacCoun, R. J., and M. M. Mello. 2015. Half-baked—The retail promotion of marijuana edibles. New England Journal of Medicine 372(11):989–991.
Mackie, K. 2006. Cannabinoid receptors as therapeutic targets. Annual Review of Pharmacology and Toxicology 46:101–122.
Mariani, J. J., D. Brooks, M. Haney, and F. R. Levin. 2011. Quantification and comparison of marijuana smoking practices: Blunts, joints, and pipes. Drug and Alcohol Dependence 113(2-3):249–251.
Matsuda, L. A., S. J. Lolait, M. J. Brownstein, A. C. Young, and T. I. Bonner. 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346(6284):561–564.
McLaren, J., W. Swift, P. Dillon, and S. Allsop. 2008. Cannabis potency and contamination: A review of the literature. Addiction 103(7):1100–1109.
McPartland, J. M., D. J. Blanchon, and R. E. Musty. 2008. Cannabimimetic effects modulated by cholinergic compounds. Addiction Biology 13(3–4):411–415.
Mechoulam, R., and Y. Shvo. 1963. Hashish. I. The structure of cannabidiol. Tetrahedron 19(12):2073–2078.
Moreau de Tours, J. J. 1845. Du Hachisch et de L’alienation mentale. Paris: Librairie de Fortin, Masson et Ca.
Munro, S., K. L. Thomas, and M. Abu-Shaar. 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365(6441):61–65.
NIDA (National Institute on Drug Abuse). 2012. DrugFacts: Spice (Synthetic Marijuana). Rockville, MD: U.S. Department of Health and Human Services, National Institutes of Health.
NIDA. 2014. Monitoring the Future Survey, Overview of Findings 2014. https://www.drugabuse.gov/related-topics/trends-statistics/monitoring-future/monitoring-futuresurvey-overview-findings-2014 (accessed November 14, 2016).
O’Shaughnessy, W. B. 1840. New remedy for tetanus and other convulsive disorders. The Boston Medical and Surgical Journal 23:153–155.
Pacula, R. L., M. Jacobson, and E. J. Maksabedian. 2016. In the weeds: A baseline view of cannabis use among legalizing states and their neighbours. Addiction 111(6):973–980.
Pertwee, R. G. 2012. Targeting the endocannabinoid system with cannabinoid receptor agonists: Pharmacological strategies and therapeutic possibilities. Philosophical Transactions of the Royal Society of London B: Biological Sciences 367(1607):3353–3363.
Piomelli, D. 2015. Neurobiology of marijuana. In Textbook of Substance Abuse Treatment, M. Galanter, H. D. Kleber, and K. T. Brady, eds. Arlington VA: American Psychiatric Publishing. Pp. 335–350.
Raber, J. C., S. Elzinga, and C. Kaplan. 2015. Understanding dabs: Contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. Journal of Toxicological Science 40(6):797–803.
Randerson, J. 2007. Warning issued over cannabis adulterated with glass beads. The Guardian, January 12. https://www.theguardian.com/society/2007/jan/12/drugsandalcohol.drugs (accessed November 8, 2016).
Russo, E. B. 2007. History of cannabis and its preparations in saga, science, and sobriquet. Chemistry and Biodiversity 4(8):1614–1648.
Russo, E. B., A. Burnett, B. Hall, and K. K. Parker. 2005. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochemical Research 30(8):1037–1043.
Scuderi, C., D. D. Filippis, T. Iuvone, A. Blasio, A. Steardo, and G. Esposito. 2009. Cannabidiol in medicine: A review of its therapeutic potential in CNS disorders. Phytotherapy Research 23(5):597–602.
Small, E. 2015. Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilization. The Botanical Review 81(3):189–294.
Tait, R. J., D. Caldicott, D. Mountain, S. L. Hill, and S. Lenton. 2016. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clinical Toxicology 54(1):1–13.
Thomas, A., G. L. Baillie, A. M. Phillips, R. K. Razdan, R. A. Ross, and R. G. Pertwee. 2007. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. British Journal of Pharmacology 150(5):613–623.
Thomas, B. F., and G. T. Pollard. 2016. Preparation and distribution of cannabis and cannabis-derived dosage formulations for investigational and therapeutic use in the United States. Frontiers in Pharmacology 7:285.
U.S. Pharmacopoeial Convention. 1916. Pharmacopoeia of the United States. Philadelphia, PA: P. Blakiston’s Son & Company.
Wang, G. S., G. Roosevelt, M. C. Le Lait, E. M. Martinez, B. Bucher-Bartelson, A. C. Bronstein, and K. Heard. 2014. Association of unintentional pediatric exposures with decriminalization of marijuana in the United States. Annals of Emerging Medicine 63(6):684–689.
Wells, D. L. and C. A. Ott. 2011. The new marijuana. Annals of Pharmacotherapy 45(3):414–417.
Wollner, H. J., J. R. Matchett, J. Levine, and S. Loewe. 1942. Isolation of a physiologically active tetrahydrocannabinol from Cannabis sativa resin. Journal of the American Chemical Society 64(1):26–29.



















