1
Introduction
Over the last decade, several large-scale US and international programs have been initiated to incorporate advances in molecular and cellular biology, -omics technologies, analytical methods, bioinformatics, and computational tools and methods into the field of toxicology. The overarching goal of the various programs is to move toxicology from a practice that uses whole-animal testing to one that uses primarily modern in vitro assays and computational approaches to predict toxicity on the basis of an understanding of the biological processes that ultimately lead from the initial chemical exposure to adverse effects. Similar efforts are being pursued in the field of exposure science with the goals of obtaining more accurate and complete exposure data on individuals and populations for thousands of chemicals over the lifespan; predicting exposures from use data and chemical-property information; and translating exposures between test systems and humans. It is hoped that the advances in toxicology and exposure science and better integration of the fields will improve risk assessment and thus better support decision-making to improve public and environmental health. With various efforts under way, diverse data are being generated, and their utility for risk assessment investigated. Although the programs and the data being generated are still evolving and will undoubtedly continue to do so, some data could be used now to help to fill gaps and assess chemical risk better. Several federal agencies recognize the potential value of such data in helping them to address their many challenging tasks. Accordingly, the US Environmental Protection Agency (EPA), the US Food and Drug Administration (FDA), the National Center for Advancing Translational Sciences (NCATS), and the National Institute of Environmental Health Sciences (NIEHS), and the asked the National Academies of Sciences, Engineering, and Medicine to consider the integration of modern and emerging scientific approaches and data into risk-based evaluations and to recommend the best ways to do so. As a result of the request, the National Academies convened the Committee on Incorporating 21st Century Science into Risk-Based Evaluations, which prepared this report.
TOXICOLOGY IN THE 21st CENTURY
In the early 2000s, several agencies and organizations began to recognize the potential of various scientific advances in biology and related fields and the possibilities provided by increases in computational power to characterize risks of environmental exposures. Roadmaps were developed to incorporate such advances into their strategic plans for assessing chemicals and other agents (EPA 2003; NTP 2004). In 2007, the National Research Council (NRC) released the report Toxicity Testing in the 21st Century: A Vision and a Strategy,1 which envisioned transforming toxicity testing from a system that relies on animal assays to one that relies primarily on high-throughput in vitro assays and computational methods based on human biology. The primary goals behind the vision were “(1) to provide broad coverage of chemicals, chemical mixtures, outcomes, and life stages, (2) to reduce the cost and time of testing, (3) to use fewer animals and cause minimal suffering in the animals used, and (4) to develop a more robust scientific basis for assessing health effects of environmental agents” (NRC 2007). The committee that prepared the 2007 report emphasized that the transformation would require a focused effort over several decades for full implementation. On release of the report, the NIEHS National Toxicology Program, the EPA National Center for Computational Toxicology, and the Chemical Genomics Center2 of the National Institutes of Health formed a collaboration, known as Tox21, to advance the vision set forth in the 2007 report (Collins et al. 2008). FDA later joined the collaboration.
The goals of the Tox21 collaboration are to identify and characterize specific mechanisms or pathways that lead to adverse effects in humans, to design assays to measure pathway responses, to develop models that can predict toxicity using the assay data, and to set priorities among chemicals for more comprehensive toxicity testing (NCATS 2015a). It is planned that the data generated will ultimately help to inform EPA, FDA, and
___________________
1 Referred to hereafter as the Tox21 report.
2 The Chemical Genomics Center is now part of NCATS.
other agencies on the hazards posed by the chemicals or products that they regulate and will be used by industry to screen for potential toxicity in product development. A phased approach to the research is being taken. Phase I of Tox21 has been completed and involved testing of about 2,800 chemicals in about 50 assays, including ones to assess cytotoxicity, mitochondrial toxicity, cell signaling, DNA damage, immune response, drug metabolism, nuclear-receptor activation, and inhibition of various molecular targets (Tice et al. 2013; NCATS 2015b). Phase II involves testing of over 10,000 chemicals that occupy a diverse chemical and toxicological space and include “industrial chemicals, sunscreen additives, flame retardants, pesticides and selected metabolites, plasticizers, solvents, food additives, natural product components, drinking water disinfection by-products, preservatives, therapeutic agents, and chemical synthesis by-products” (Tice et al. 2013). Phase III will involve identification of physiologically relevant cells, measurement of gene expression in a large number of molecular pathways, and testing of chemical mixtures and extracts (NCATS 2015b).
In 2007, EPA initiated its Toxicity Forecaster (ToxCast) program, which seeks to develop high-throughput screening (HTS) assays for evaluating biological responses that are relevant to prediction of adverse effects of chemical exposures on humans (EPA 2013). A phased approach to research is also being taken in the ToxCast program. Phase I, which has been completed, involved testing of over 300 well-studied chemicals in several hundred HTS assays (Kavlock and Dix 2010). Phase II has also been completed; it involved testing of over 2,000 chemicals—including industrial and consumer products, food additives, and potentially safer chemical alternatives to existing chemicals—in HTS assays for evaluating various cell responses and over 300 signaling pathways (EPA 2013; Silva et al. 2015). ToxCast data are now being evaluated as a means of setting priorities among chemicals for testing in EPA’s Endocrine Disruptor Screening Program and in other programs that require setting priorities for testing.
In addition to US government-led efforts, international efforts are transforming toxicology from an observational to a predictive science. In the European Union, for example, the European Commission and Cosmetics Europe (a trade association for the cosmetics and personal-care industry) have co-funded the research initiative Safety Evaluation Ultimately Replacing Animal Testing (SEURAT 2015). The initiative was started to develop tools to comply with legislation that banned all animal testing for cosmetic ingredients and all marketing of animal-tested cosmetic ingredients and products; a complete ban went into effect in March 2013. Its vision was to eliminate traditional animal testing by adopting a “toxicological mode-of-action framework to describe how any substance may adversely affect human health, and use this knowledge to develop complementary theoretical, computational and experimental (in vitro) models that predict quantitative points of departure needed for safety assessment” (Berggren 2015). The research initiative was a 5-year program (2011–2015) that involved development of in vitro assays that use human pluripotent stem cells, development of a hepatic microfluidic bioreactor, identification and investigation of human biomarkers of chronic toxicity in cellular models, and development of computational tools for predicting chronic toxicity.
Private industry and other organizations are also working to transform the ways in which chemicals are assessed. For example, the pharmaceutical industry has been developing and using in vitro and computational tools as early screens for drug safety for many years (Greene and Song 2011; Bowes et al. 2012). Organizations have developed case studies related to the use of new in vitro assays and computational systems-biology tools for assessment of chemical risk (Daston et al. 2015; Gocht et al. 2015). Cheminformatics research has resulted in the development of rational systems for informing qualitative structure–activity relationship assessments (Wu et al. 2010) and in the development of automated decision trees for identifying toxicity end points, such as developmental and reproductive toxicity (Wu et al. 2013).
Academic institutions are generating a substantial amount of data that could help to inform chemical risk assessment. Academic laboratories tend to focus on end points that are not typically covered in guideline animal studies, such as mammary gland development (Fenton 2006; Soto et al. 2008; Osborne et al. 2015), synaptic morphology and other aspects of nervous system development (Patisaul and Polston 2008), and complex behaviors, including sociality, aggression, cognition, and behavioral hallmarks of psychiatric disorders, such as autism spectrum disorder and attention deficit disorder (Eubig et al. 2010; de Cock et al. 2012; Leon-Olea et al. 2014). Research on genetics, genomics, and epigenetics (including the role of noncoding RNAs) is also abundant and is providing insights on novel biological mechanisms and gene-by-environment interactions (Dolinoy et al. 2007; Rusyn et al. 2010; Tal and Tanguay 2012; Nebert et al. 2013; Yeo et al. 2013). Academic laboratories have been responsible for generating nearly all the data on transgenerational effects (Rissman and Adli 2014); have pioneered the use of nontraditional animal models, including transgenic and population-based models (Churchill et al. 2004; Rusyn et al. 2010; Sullivan et al. 2014); and have conducted most of the epidemiological studies of chemical risk. The enormous volume of data being generated throughout the basic- and clinical-research communities has prompted questions about how the data could best be used for various risk-related activities and decision-making.
EXPOSURE SCIENCE IN THE 21st CENTURY
Exposure science is undergoing a transformation similar to that affecting toxicology with the advances in molecular technologies, computational tools, bioinformatics, sensor systems, and analytical methods. In 2012, the NRC released the report Exposure Science in the 21st Century: A Vision and a Strategy,3 which articulated a long-term vision for exposure science. The primary long-term goal of the vision was to broaden the reach of exposure science from a traditional focus on discrete exposures to an “integrated approach that considers exposures from source to dose, on multiple levels of integration (including time, space, and biological scale), to multiple stressors, and scaled from molecular systems to individuals, populations, and ecosystems” (NRC 2012). The report described scientific and technological progress that has the potential to transform exposure science, including geographic information technologies that can track sources, exposure concentrations, and receptors; monitoring technologies that can collect data on personal exposure of millions of people; highly sensitive analytical technologies that can identify and measure biomarkers that are indicative of internal exposures; and computational tools that can manage the large amounts of data generated. It also highlighted high-priority research, emphasized the need for interagency collaboration and resources, and elaborated the broad concept of the exposome, defined as “the record of all exposures both internal and external that people receive throughout their lifetime” (Rappaport and Smith 2010). Last, it recognized the interdependence of the fields of toxicology, risk assessment, and exposure science and foresaw the need to evolve the risk-assessment paradigm toward one in which exposure science plays a strong role, specifically, a paradigm that is “influenced by and responsive to human and environmental exposure data.” The report described four objectives of exposure science: to set priorities among chemicals for toxicity testing; to provide exposure information to guide toxicity testing; to provide quantitative pharmacokinetic data on absorption, distribution, metabolism, and excretion (ADME) derived from human-exposure studies; and to connect exposure data with biological activity data to identify exposure–response relationships.
In response to the recommendation to improve integration of exposure science throughout the federal government, the Exposure Science in the 21st Century (ES21) Federal Working Group has emerged (EPA 2016a). It consists of representatives of more than 20 federal organizations that have a common interest in exposure-science research and development. The purpose of the working group is to build on the framework recommended in the ES21 report, share information, integrate activities, reduce duplication of efforts among agencies, and promote federal collaboration in the development of exposure science. In addition to the activities of the working group, several research programs are involved in advancing exposure science on paths that are consistent with the vision articulated in the ES21 report. EPA created the Exposure Forecasting (ExpoCast) program, which complements its ToxCast program (EPA 2016b). ExpoCast focuses on developing high-throughput methods for estimating exposure and so far has been used to make exposure predictions related to over 1,900 chemicals. EPA’s goal is to combine the exposure estimates from ExpoCast with bioactivity data from ToxCast to predict human health and environmental risks.
NIEHS is also interested in advancing exposure science and has supported research to develop new sensor systems and to identify biomarkers of response to exposure (NIEHS 2015). It has created the Children’s Health Exposure Analysis Resource (NIEHS 2016), an infrastructure designed to enable and expand incorporation of environmental exposures into studies of children’s health; it includes a data repository, support for statistical analysis, and a network of laboratories to analyze biological samples. The NIEHS strategic plan emphasizes a commitment to supporting research to define and explore the exposome, and the agency has funded the HERCULES center at Emory University to conduct exposome-focused research (NIEHS 2012).
In addition to the efforts in the United States, there are international efforts, such as the Human Early-Life Exposome (HELIX) project and the EXPOsOMICS project. HELIX has the ambitious goal of characterizing early-life exposures and ultimately linking exposures with children’s health outcomes (Vrijheid et al. 2014). The project is studying 32,000 mother–child pairs in six European countries. EXPOsOMICS focuses on the external and internal exposome associated with air pollution and water contamination (Vineis et al. 2013, in press). The project will perform personal-exposure monitoring of air pollutants for hundreds of subjects in Europe, and biological samples from thousands of subjects will be analyzed for internal exposure markers by using -omics technologies (CORDIS 2015).
Like the toxicology initiatives, the exposure programs are generating vast amounts of data, but how the data are best used to inform risk-related tasks and decision-making remains to be determined.
TERMINOLOGY
The recent advances in toxicology and exposure science have given rise to a new vocabulary and a plethora of new terms. Some researchers and practitioners distinguish between terms, but others use the same terms interchangeably and inconsistently. Consequently, there is some confusion as to the specific meanings of various
___________________
3 Referred to hereafter as the ES21 report.
terms. Mode of action, mechanism of action, and adverse outcome pathway are exemplary of the confusion. Each term denotes a progression from some exposure or molecular initiating event to an adverse outcome. Mechanism of action is often distinguished from mode of action by a greater level of biological detail in the understanding and description of the progression from exposure to outcome (EPA 2005; NRC 2007). Mode of action typically describes the progression of key events that result from a chemical exposure whereas adverse outcome pathway conceptually describes the sequential chain of causally linked events at various levels of biological organization starting from a molecular initiating event through to the observable adverse outcome (OECD 2013; Berggren et al. 2015). Although all three terms are used to describe the sequence of steps from an initiating event to an adverse outcome, subtle distinctions between the terms have been made. The subtleties are often lost in practice, and the terms are used interchangeably. In the present report, the committee uses primarily mechanism and defines the term generally to refer to a detailed description of the process by which an agent causes an effect. It uses adverse outcome pathway only in the context of frameworks that have been developed specifically with the phrase. Mechanism is further defined in the context of the new direction of risk assessment in Chapters 5 and 7.
Exposure and dose are two other terms that are often defined and used inconsistently. The NRC (2012) defined exposure broadly as the contact between a stressor and a receptor at any level of biological organization (organism, organ, tissue, or cell). Given that broad definition, the distinction between exposure and dose becomes arbitrary, and dose becomes unnecessary. Exposure is then characterized by the identity of the stressor and the amount, location, and timing of the stressor that comes into contact with the receptor; timing encompasses both duration and the time at which the contact occurs. The committee uses exposure primarily in the present report but acknowledges that it often uses dose in conventional phrases, such as dose–response relationship.
Many terms associated with -omics technologies have been coined in recent years. Box 1-1 provides definitions of various terms used throughout this report. Other terms that are specific to topics discussed in various chapters are defined in those chapters. The committee acknowledges that as the science progresses new terms will be needed, but it urges the scientific community to be judicious in inventing new terms. If needed, new terms should be defined clearly and used consistently.
The committee debated how to refer to all the assays, tools, and methods arising from the “21st century visions” for toxicology and exposure science; some are
no longer “new,” and others are still in development. To simplify the text, the committee often refers to them as Tox21 or ES21 assays, tools, or methods. That notation is meant to be broad and includes all the assays, tools, and methods coming from government, academic, and private laboratories, not only those being developed as part of the Tox21 program previously described.
THE COMMITTEE AND ITS TASK
The committee that was convened as a result of the agencies’ request included experts in toxicology; physiologically based pharmacokinetic modeling; computational methods and bioinformatics; -omics, in vitro models, and alternative methods; epidemiology; exposure assessment; statistics; and risk assessment (see Appendix A for the committee’s biographical information). As noted, the committee was asked to consider and recommend the best uses of the various types of emerging data in risk-based evaluations. The committee’s verbatim statement of task is provided in Box 1-2.
THE COMMITTEE’S APPROACH TO ITS TASK
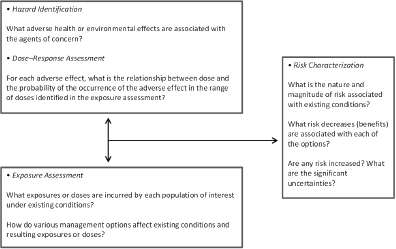
To address its task, the committee held seven meetings, which included three open sessions to hear primarily from various sponsor representatives. Given the potential breadth of its task, the committee devoted substantial time to interpretation of its charge. It used as a basis of its work the risk-assessment framework that was initially proposed in the 1983 report Risk Assessment in the Federal Government: Managing the Process (NRC 1983) and updated most recently in the 2009 report Science and Decisions: Advancing Risk Assessment (NRC 2009) (see Figure 1-1). The committee considered and describes scientific and technological advances in exposure science, toxicology, and epidemiology that could be integrated into and used to improve any of the four elements of risk assessment (hazard identification, dose–response assessment, exposure assessment, and risk characterization). The report, however, is not a catalog of all scientific and technological advances that have been made since publication of the 2007 and 2012 reports (NRC 2007, 2012), but rather a review of the ones most relevant to risk-based evaluations in EPA and FDA.
The committee identified various agency tasks and decision-making contexts (see Box 1-3)—which require different depths of information—and used the tasks and contexts to frame general and specific examples of applications (case studies) for integrating the new science into various components of risk assessment. The examples provide guidance for communicating to various stakeholders how the new science could be used. The committee then considered how data validation, data integration, and uncertainty analysis might need to be adapted to use the new science. The committee recognizes that there will be challenges in using new tools and concepts in fields that are already heavy with practice standards and set protocols.
ORGANIZATION OF THIS REPORT
The committee’s report is organized into seven chapters and five appendixes. Chapters 2, 3, and 4 describe new or emerging methods and tools in exposure science, toxicology, and epidemiology, respectively. Chapter 5 highlights the new direction of risk assessment and describes practical applications for 21st century science.
Chapter 6 discusses issues surrounding model and assay validation and acceptance. Chapter 7 focuses on interpretation and integration of data and evidence. Appendix A provides biographical information on the committee members, and Appendixes B, C, and D provide case studies that demonstrate practical applications of the committee’s recommendations for using new data streams in risk-based evaluations. Appendix E provides a case study in using Bayesian approaches with high-throughput data.
REFERENCES
Berggren, E. 2015. A Path to Validation-SEURAT-1 Case Studies and the Role of ECVAM. Public Forum-Replacing Animal Testing, October 26-27, 2015, London. ToxBank [online]. Available: http://www.toxbank.net/public-forum/ path-validation [accessed January 3, 2017].
Berggren, E., P. Amcoff, R. Benigni, K. Blackburn, E. Carney, M. Cronin, H. Deluyker, F. Gautier, R.S. Judson, G.E. Kass, D. Keller, D. Knight, W. Lilienblum, C. Mahony, I. Rusyn, T. Schultz, M. Schwarz, G. Schüürmann, A. White, J. Burton, A.M. Lostia, S. Munn, and A. Worth. 2015. Chemical safety assessment using read-across: Assessing the use of novel testing methods to strengthen the evidence base for decision making. Environ. Health Perspect. 123(12):1232-1240.
Bowes. J., A.J. Brown, J. Hamon, W. Jarolimek, A. Sridhar, G. Waldron, and S. Whitebread. 2012. Reducing safety-related drug attrition: The use of in vitro pharmacological profiling. Nat. Rev. Drug Discov. 11(12):909-922.
Churchill, G.A, D.C. Airey, H. Allayee, J.M. Angel, A.D. Attie, J. Beatty, W.D. Beavis, J.K. Belknap, B. Bennett, W. Berrettini, A. Bleich, M. Bogue, K.W. Broman, K.J. Buck, E. Buckler, M. Burmeister, E.J. Chesler, J.M. Cheverud, S. Clapcote, M.N. Cook, R.D. Cox, J.C. Crabbe, W.E. Crusio, A. Darvasi, C.F. Deschepper, R.W. Doerge, C.R. Farber,
J. Forejt, D. Gaile, S.J. Garlow, H. Geiger, H. Gershenfeld, T. Gordon, J. Gu, W. Gu, G. de Haan, N.L. Hayes, C. Heller, H. Himmelbauer, R. Hitzemann, K. Hunter, H.C. Hsu, F.A. Iraqi, B. Ivandic, H.J. Jacob, R.C. Jansen, K.J. Jepsen, D.K. Johnson, T.E. Johnson, G. Kempermann, C. Kendziorski, M. Kotb, R.F. Kooy, B. Llamas, F. Lammert, J.M. Lassalle, P.R. Lowenstein, L. Lu, A. Lusis, K.F. Manly, R. Marcucio, D. Matthews, J.F. Medrano, D.R. Miller, G. Mittleman, B.A. Mock, J.S. Mogil, X. Montagutelli, G. Morahan, D.G. Morris, R. Mott, J.H. Nadeau, H. Nagase, R.S. Nowakowski, B.F. O’Hara, A.V. Osadchuk, G.P. Page, B. Paigen, K. Paigen, A.A. Palmer, H.J. Pan, L. Peltonen-Palotie, J. Peirce, D. Pomp, M. Pravenec, D.R. Prows, Z. Qi, R.H. Reeves, J. Roder, G.D. Rosen, E.E. Schadt, L.C. Schalkwyk, Z. Seltzer, K. Shimomura, S. Shou, M.J. Sillanpaa, L.D. Siracusa, H.W. Snoeck, J.L. Spearow, K. Svenson, L.M. Tarantino, D. Threadgill, L.A. Toth, W. Valdar, F.P. de Villena, C. Warden, S. Whatley, R.W. Williams, T. Wiltshire, N. Yi, D. Zhang, M. Zhang, and F. Zou. 2004. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 36(11):1133-1137.
Collins, F.S., G.M. Gray, and J.R. Bucher. 2008. Transforming environmental health protection. Science 319(5865):906-907.
CORDIS (Community Research and Development Information Service). 2015. EXPOSOMICS Result In Brief: Measuring Environmental Exposure. CORDIS, European Commission [online]. Available: http://cordis.europa.eu/result/rcn/151545_en.html [accessed July 13, 2016].
Daston, G., D.J. Knight, M. Schwarz, T. Gocht, R.S. Thomas, C. Mahony, and M. Whelan. 2015. SEURAT: Safety Evaluation Ultimately Replacing Animal Testing–Recommendations for future research in the field of predictive toxicology. Arch. Toxicol. 89(1):15-23.
de Cock, M., Y.G. Maas, and M. van de Bor. 2012. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review Acta Paediatr. 101(8):811-818.
Dolinoy, D.C., J.R. Weidman, and R.L. Jirtle. 2007. Epigenetic gene regulation: Linking early developmental environment to adult disease. Reprod. Toxicol. 23(3):297-307.
EPA (US Environmental Protection Agency). 2003. Framework for Computational Toxicology Research Program in ORD. EPA/600/R-03/065. Office of Research and Development, US Environmental Protection Agency, Washington, DC.
EPA (US Environmental Protection Agency). 2005. Guidelines for Carcinogen Risk Assessment. EPA/630/P-03/001F. Risk Assessment Forum, US Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/sites/production/files/2013-09/documents/cancer_guidelines_final_3-25-05.pdf [accessed July 13, 2016].
EPA (US Environmental Protection Agency). 2013. Toxicity Forecaster (ToxCastTM). Science in Action: Innovative Research for a Sustainable Future. Fact Sheet. Office of Research and Development, US Environmental Protection Agency, Washington, DC [online]. Available: https://www.epa.gov/sites/production/files/2013-12/documents/toxcast-fact-sheet.pdf [accessed July 13, 2016].
EPA (US Environmental Protection Agency). 2016a. The Exposure Science in the 21st Century (ES21) Federal Working Group [online]. Available: https://www.epa.gov/innovation/exposure-science-21st-century-federal-working-group [accessed October 24, 2016].
EPA (US Environmental Protection Agency). 2016b. High-Throughput Exposure Forecasting. Science inAction: Innovative Research for a Sustainable Future. Fact Sheet. Office of Research and Development, US Environmental Protection Agency, Washington, DC. March 2016 [online]. Available: https://www.epa.gov/sites/production/files/2014-12/documents/exposure_forecasting_factsheet.pdf [accessed July 13, 2016].
Eubig, P.A., A. Aguiar, and S.L. Schantz. 2010. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect. 118(12):1654-1667.
Fenton, S.E. 2006. Endocrine-disrupting compounds and mammary gland development: Early exposure and later life consequences. Endocrinology 147(6 Suppl.): S18-S24.
Gocht, T., E. Berggren, H.J. Ahr, I. Cotgreave, M.T. Cronin, G. Daston, B. Hardy, E. Heinzle, J. Hescheler, D.J. Knight, C. Mahony, M. Peschanski, M. Schwarz, R.S. Thomas, C. Verfaillie, A. White, and M. Whelan. 2015. The SEURAT-1 approach towards animal free human safety assessment. ALTEX 32(1):9-24.
Greene, N., and M. Song. 2011. Predicting in vivo safety characteristics using physiochemical properties and in vitro assays. Future Med. Chem. 3(12):1503-1511.
Kavlock, R., and D. Dix. 2010. Computational toxicology as implemented by the US EPA: Providing high throughput decision support tools for screening and assessing chemical exposure, hazard, and risk. J. Toxicol. Environ. Health B Crit. Rev. 13(2-4):197-217.
Leon-Olea, M., C.J. Martyniuk, E.F. Orlando, M.A. Ottinger, C.S. Rosenfeld, J.T. Wolstenholme, and V.L. Trudeau. 2014. Current concepts in neuroendocrine disruption. Gen. Comp. Endocrinol. 203:158-173.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2016. Use of Metabolomics to Advance Research on Environmental Exposures and the Human Exposome: Workshop in Brief. Washington, DC: The National Academies Press.
NCATS (National Center for Advancing Translational Sciences). 2015a. Tox21 Program Goals [online]. Available: http://www.ncats.nih.gov/tox21/about/goals [accessed July 13, 2016].
NCATS (National Center for Advancing Translational Sciences). 2015b. Tox21 Operational Model. Available: http://www.ncats.nih.gov/tox21/about/operations [accessed July 13, 2016].
Nebert, D.W., G. Zhang, and E.S. Vesell. 2013. Genetic risk prediction: Individualized variability in susceptibility to toxicants. Annu. Rev. Pharmacol. Toxicol. 53:355-375.
NIEHS (National Institute of Environmental Health Sciences). 2012. Advancing Science, Improving Health: A Plan for Environmental Health Research. 2012-2017 Strategic Plan [online]. Available: http://www.niehs.nih.gov/about/strategicplan/strategicplan2012_508.pdf [accessed July 13, 2016].
NIEHS (National Institute of Environmental Health Sciences). 2015. Exposure Biology and the Exposome [online]. Available: http://www.niehs.nih.gov/research/supported/dert/programs/exposure/ [accessed July 13, 2016].
NIEHS (National Institute of Environmental Health Sciences). 2016. Children’s Health Exposure Analysis Resource (CHEAR) [online]. Available: http://www.niehs.nih.gov/research/supported/exposure/chear/ [accessed July 13, 2016].
NRC (National Research Council). 1983. Risk Assessment in the Federal Government: Managing the Process. Washington, DC: National Academy Press.
NRC (National Research Council). 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC: The National Academies Press.
NRC (National Research Council). 2009. Science and Decisions: Advancing Risk Assessment. Washington, DC: The National Academies Press.
NRC (National Research Council). 2012. Exposure Science in the 21st Century: A Vision and a Strategy. Washington, DC: The National Academies Press.
NTP (National Toxicology Program). 2004. A National Toxicology Program for the 21st Century: A Roadmap for the Future [online]. Available: https://ntp.niehs.nih.gov/ntp/about_ntp/ntpvision/ntproadmap_508.pdf [accessed July 13, 2016].
OECD (Organisation for Economic Co-operation and Development). 2013. Guidance Document on Developing and Assessing Adverse Outcome Pathways. Series on Testing and Assessment. No. 184. ENV/JM/MONO(2013)6. Paris: OECD [online]. Available: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2013)6&doclanguage=en [accessed July 13, 2016].
Osborne, G., R. Rudel, and M. Schwarzman. 2015. Evaluating chemical effects on mammary gland development: A critical need in disease prevention. Reprod. Toxicol. 54:148-155.
Patisaul, H.B., and E.K. Polston. 2008. Influence of endocrine active compounds on the developing rodent brain. Brain Res. Rev. 57(2):352-362.
Rappaport, S.M., and M.T. Smith. 2010. Environment and disease risks. Science 30(6003):460-461.
Rissman, E.F. and M. Adli. 2014. Transgenerational epigenetic inheritance: Focus on endocrine disrupting compounds. Minireview. Endocrinology 155(8):2770-2780.
Rusyn, I., D.M. Gatti, T. Wiltshire, S.R. Kleeberger, and D.W. Threadgill. 2010. Toxicogenetics: Population-based testing of drug and chemical safety in mouse models. Pharmacogenomics 11(8):1127-1136.
SEURAT (Safety Evaluation Ultimately Replacing Animal Testing). 2015. SEURAT-1 [online]. Available: http://www.seurat-1.eu/ [accessed July 13, 2016].
Silva, M., N. Pham, C. Lewis, S. Iyer, E. Kwok, G. Solomon, and L. Zeise. 2015. A comparison of ToxCast test results with in vivo and other in vitro endpoints for neuro, endocrine, and developmental toxicities: A case study using endosulfan and methidathion. Birth Defects Res. B Dev. Reprod. Toxicol. 104(2):71-89.
Soto, A.M., L.N. Vandenberg, M.V. Maffini, and C. Sonnenschein. 2008. Does breast cancer start in the womb? Basic Clin. Pharmacol. Toxicol. 102(2):125-133.
Sullivan, A.W., E.C. Beach, L.A. Stetzik, A. Perry, A.S. D’Addezio, B.S. Cushing, and H.B. Patisaul. 2014. A novel model for neuroendocrine toxicology: neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster). Endocrinology 155(10):3867-3881.
Tal, T.L., and R.L. Tanguay. 2012. Non-coding RNAs–novel targets in neurotoxicity. Neurotoxicology 33(3):530-544.
Tice, R.R., C.P. Austin, R.J. Kavlock, and J.R. Bucher. 2013. Improving the human hazard characterization of chemicals: A Tox21 Update. Environ. Health Perspect. 121(7):756-765.
Vineis, P., K. van Veldhoven, M. Chadeau-Hyam, and T.J. Athersuch. 2013. Advancing the application of omicsbased biomarkers in environmental epidemiology. Environ. Mol. Mutagen. 54(7):461-467.
Vineis, P., M. Chadeau-Hyam, H. Gmuender, J. Gulliver, Z. Herceg, J. Kleinjan, M. Kogevinas, S. Kyrtopoulos, M. Nieuwenhuijsen, D. Phillips, N. Probst-Hensch, A. Scalbert, R. Vermeulen, and C.P. Wild. In press. The exposome in practice: Design of the EXPOsOMICS project. EXPOsOMICS Consortium. Int. J. Hyg. Environ. Health.
Vrijheid, M., R. Slama, O. Robinson, L. Chatzi, M. Coen, P. van den Hazel, C. Thomsen, J. Wright, T.J. Athersuch, N. Avellana, X. Basagaña, C. Brochot, L. Bucchini, M. Bustamante, A. Carracedo, M. Casas, X. Estivill, L. Fairley, D. van Gent, J.R. Gonzalez, B. Granum, R. Gražulevičienė, K.B. Gutzkow, J. Julvez, H.C. Keun, M. Kogevinas, R.R.C. McEachan, H.M. Meltzer, E. Sabidó, P.E. Schwarze, V. Siroux, J. Sunyer, E.J. Want, F. Zeman, and M.J. Nieuwenhuijsen1. 2014. The human early-life exposome (HELIX): Project rationale and design. Environ. Health Perspect. 122(6):535-544.
Wild, C.P. 2005. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomarkers Prev. 14(8):1847-1850.
Wu, S., K. Blackburn, J. Amburgey, J. Jaworska, and T. Federle. 2010. A framework for using structural, reactivity, metabolic and physicochemical similarity to evaluate the suitability of analogs for SAR-based toxicological assessments. Regul. Toxicol. Pharmacol. 56(1):67-81.
Wu, S., J. Fisher, J. Naciff, M. Laufersweiler, C. Lester, G. Daston, and K. Blackburn. 2013. Framework for identifying chemicals with structural features associated with the potential to act as developmental or reproductive toxicants. Chem. Res. Toxicol. 26(12):1840-1861.
Yeo, M., H. Patisaul, and W. Liedtke. 2013. Decoding the language of epigenetics during neural development is key for understanding development as well as developmental neurotoxicity. Epigenetics 8(11):1128-1132.









