2
Advances in Exposure Science
As described in Chapter 1, the National Research Council (NRC) report Exposure Science in the 21st Century: A Vision and a Strategy articulated a vision for exposure science that was intended to transform, expand, and invigorate the field (NRC 2012). Recent investments in exposome technologies and programs (CHEAR; NIEHS 2016), in new large-scale longitudinal exposure-epidemiology research programs (HELIX; Vrijheid et al. 2014 and EXPOsOMICS; Vineis et al. 2013), and in the rapidly expanding exposure-science programs headed by the National Exposure Research Laboratory and the National Center for Computational Toxicology of the US Environmental Protection Agency (EPA) are examples of the immediate impact of the ES21 report.1 Several research fields have seen substantial advances since the ES21 report was published, and these advances create opportunities for providing guidance to EPA, the US Food and Drug Administration, and others on how best to integrate emerging exposure-science data into risk assessments (Egeghy et al. 2016). Accordingly, this chapter describes the major advances in exposure science since the publication of the ES21 report and applications that would be most relevant and useful for risk-based decision-making. It also presents unaddressed opportunities related to decision-making based on exposure or risk and discusses major obstacles to various applications.
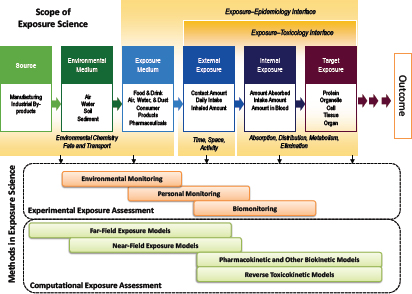
The interrelationship among the fields of exposure science, toxicology, and epidemiology is a central theme of this chapter. Figure 2-1 illustrates the series of events from introduction of a stressor into the environment and its movement through the environment via specific pathways to the receptor and the triggering of a biological response of potential regulatory concern. The figure provides a broad conceptual overview of the scope of exposure science and a general organizational framework as envisaged by the ES21 committee and the present committee. The figure also illustrates the points of integration with toxicology and epidemiology and the fundamental distinctions between fields. Although the continuum is depicted as a linear path, the committee recognizes that multiple interconnecting paths are typically involved in the source-to-outcome continuum. In cases where source identification or mitigation rather than toxicology or risk assessment is the goal, one moves from right to left from measured exposures to sources. Box 2-1 provides some definitions of the key terms used in this chapter related to exposure science.
Organizational frameworks for exposure science, such as the one in Figure 2-1, have been used to describe exposure pathways for contaminated sites and are implicit in all models of environmental or biological fate of chemicals (Wania and Mackay 1999; Koelmans et al. 2001; Schenker et al. 2009). The frameworks have been essential in guiding the acquisition of data, the organization of data, and the use of data in modeling to describe or predict exposure quantitatively. Although some frameworks, such as the Conceptual Site Model (Regens et al. 2002; Mayer et al. 2005), are largely qualitative and conceptual and apply to specific exposure settings or specifically to modeling exercises, others, such as the Aggregate Exposure Pathway framework (Teeguarden et al. 2016), attempt to expand on earlier successes by generalizing the approach to support data acquisition, data organization, conceptualization, and modeling in the broader exposure-science community. As the field of exposure science evolves as a result of advances in the tools and approaches described in this chapter, the use of the frameworks will enable the development of infrastructure to support exposure-data acquisition, collection, organization, and access and to improve the accuracy, completeness, efficiency, and transparency of exposure assessment and modeling.
MAJOR ADVANCES IN EXPOSURE SCIENCE
The committee reviewed advances in the field of exposure science since the publication of the ES21 report with the goal of identifying major advances that have the potential for sustained effects on the important applications described later in this chapter and in the case studies
___________________
1 The present committee refers to Exposure Science in the 21st Century: A Vision and a Strategy (NRC 2012) as the ES21 report and to its committee as the ES21 committee.
described in Appendixes B–D. The advances are summarized in this section.
Remote Sensing and Geospatial Environmental Exposure Assessment
Several substantial advances in exposure science are the result of innovations in remote sensing, global positioning systems (GPSs), and geographic information systems (GISs). Remote sensing is an important tool for enhancing the capacity to assess human and ecological exposures because it provides information on Earth’s surface, water, and atmosphere that cannot be provided by traditional ground-based monitoring systems (Al-Hamdan et al. 2014). Since the ES21 report, remote-sensing data have been used to estimate concentrations of ambient criteria air pollutants (NO2, O3, and PM2.5) on a global scale (Brauer et al. 2015; Geddes et al. 2016; van Donkelaar et al. 2015). Models have estimated the changes in global air pollution and have allowed complete global coverage of key pollutants on a relatively fine spatial scale. The application of remote-sensing technologies with ground-based monitoring will continue to improve human exposure assessment. Several recent key advances include the National Aeronautics and Space Administration (NASA) launch of six Earth-observing missions and the addition of three new instruments to the International Space Station (Seltenrich 2014). NASA and the National Oceanic and Atmospheric Administration provide free access to exposure-relevant data, such as NO2 and PM2.5 concentrations in the troposphere, and environmental data relevant to exposure assessment and interpretation of monitoring data (Seltenrich 2014).
The studies generated with remote sensing data provide even greater insights into human exposures when coupled with GPS and GIS data on populations of interest. GPS data are used to track people in observational exposure and epidemiological studies (Elgethun et al. 2007), and recent advances have allowed more automated coding of GPS data on activities and microenvironments, such as inside and outside at home and at work (Wu et al. 2011; Breen et al. 2014; Nethery et al. 2014; Andra et al. 2015). Data on microenvironments can be used as input for exposure models to refine exposure estimates based on remote sensing data, ground-based ambient air data, and indoor air monitoring data (Breen et al. 2014). Advances in GPS technologies have also been coupled with sensor technologies that measure basic health data, such as heart and respiratory rates and activity level. Information on such measures can be additional inputs for the exposure models and allow further refinement and improvement of exposure classification (Andersen et al. 2015).
Computational Exposure Assessment
For the vast majority of stressors, there are few exposure measurements (Muir and Howard 2006; Egeghy et al. 2012). Various conceptual, empirical, and predictive exposure models are needed to address those data gaps and to enhance the usefulness and application of measured data to exposure and risk assessment. Since the release of the ES21 report, there has been substantial research activity and advancement in the development of computational exposure tools, particularly for calculating near-field chemical exposures of humans, for quantifying relationships between external and internal exposures and between in vivo and in vitro exposures, and for high-throughput exposure estimation that has been used alone and in combination with bioactivity data to set priorities for chemical assessment.
Egeghy et al. (2011) reviewed tools designed to set priorities rapidly for large numbers of chemicals, and Mitchell et al. (2013) conducted an “exposure model prioritization challenge.” A key finding of the challenge was the need to reconcile exposures to chemicals released outdoors (far-field sources) with exposures to chemicals in consumer products applied directly or through indoor-environment exposure pathways (near-field exposures). The recognized absence of tools and exposure information is stimulating research to develop and improve near-field and far-field exposure science. Specifically, the seminal model developed for simulating chemical transport in an indoor environment (Bennett and Furtaw 2004) has been revised to include exposure pathways for which external human exposures (intake fractions) (Shin et al. 2012) and internal exposures (estimates of whole-body concentrations) (Zhang et al. 2014; Webster et al. 2016) can be
estimated. Furthermore, data and models are evolving to improve mechanistic understanding of chemical releases and exposures indoors (Weschler and Nazaroff 2010, 2012; Little et al. 2012). Exposure models for consumer products also are evolving and being evaluated for select chemicals (Young et al. 2012; Gosens et al. 2014; Delmaar et al. 2015; Dudzina et al. 2015). Exposure models and frameworks that combine far-field and near-field pathways for aggregate human exposure assessments are also being developed and applied (Isaacs et al. 2014; Shin et al. 2015; Fantke et al. 2016).
EPA’s ExpoCast project conducts research on and uses computational tools for high-throughput exposure estimation or “forecasting” to set testing or assessment priorities. The ExpoCast project combines various models and data sources to estimate exposures, which can then be compared with high-throughput ToxCast data and other sources of toxicity or bioactivity data. As a part of the ExpoCast exposure estimation, the Systematic Empirical Evaluation of Models (SEEM) framework includes calibration and evaluation of exposure-model estimates against chemical concentrations measured in or estimated from blood and urine samples from a group of nonoccupationally exposed US residents over the age of 6 years (Wambaugh et al. 2013, 2014).2 Exposure-model predictions are compared with available biomonitoring data to estimate the uncertainty in the combined exposure-model calculations (Wambaugh et al. 2013). The Stochastic Human Exposure and Dose Simulation Model for Multimedia, Multipathway chemicals (SHEDS-MM) for exposure-based priority-setting and screening has been revised for high-throughput capacity (SHEDS-HT) (Isaacs et al. 2014) and feeds into the SEEM framework. Other complementary high-throughput aggregate exposure-estimation models that combine existing and emerging tools (see, for example, Shin et al. 2015) can also be incorporated into the SEEM framework, and they are being applied, evaluated, and refined in other contexts.
Improving the amount and quality of the data that are needed to develop parameters for the computational exposure tools is critically important; without such data, the applicability of the tools is limited. Some advances include updated exposure factors (EPA 2011) and the development of the Consumer Product Chemical Profile Database (Goldsmith et al. 2014) and the Chemical/Product Categories Database (Dionisio et al. 2015).3 Numerous quantitative structure–activity relationship (QSAR) models, quantitative structure–property relationship (QSPR) models, and other computational tools for predicting chemical-property information—such as solubilities, partition coefficients, and degradation rates—continue to evolve. The applicability domains of existing tools for calculating chemical-property information require further examination and more explicit definition to ensure that the models are calibrated and applied within the same chemical space. Integrated testing strategies to obtain more high-quality measurements can then be strategically developed to expand the applicability domains of current QSAR models, QSPR models, and other tools used for property estimation.
Because of the extensive measurement-data gaps, the recent advances in computational tools for exposure science are expected to play a crucial role in most aspects of exposure estimation for risk-based assessments, not only high-throughput applications. Higher-tiered models that link exposure databases and spatial information (see, for example, Georgopoulos et al. 2014) and opportunities to combine and integrate measurements and models to characterize and quantify the source-to-receptor relationship more fully (see, for example, McKone et al. 2007) are being developed and applied. Exposure-model uncertainty and sensitivity analyses are useful computational methods that can be used to set priorities for exposure-science research systematically (Arnot et al. 2012; NRC 2012; Arnold and Ramachandran 2014).
Personalized Exposure Assessment
Behavior patterns that determine exposure routes, durations, and conditions combined with the variation in environmental concentrations of stressors over space and time result in unique exposure patterns for individuals and populations. Exposure data that are needed to assess personal exposures can now be generated on various spatial and temporal scales with traditional and emerging methods. New opportunities to measure exposures in and outside the body will help to characterize and quantify personal exposures to an array of stressors. Particularly notable are recent advances in the application of passive sampling techniques to determine internal human concentrations (for example, using silicone implants) (Allan et al. 2013a; Gilbert et al. 2015; O’Connell et al. 2015), external exposure concentrations integrated over time and space (for example, using silicone wristbands) (O’Connell et al. 2014a,b), and chemical concentrations and chemical activities4 in media to which humans are exposed, such as foods (Allan et al. 2013b; Jahnke et al. 2014) and indoor air (Wetzel and Doucette 2015). Por-
___________________
2 Data are from the US National Health and Nutrition Examination Survey.
3 See http://actor.epa.gov/cpcat.
4 Chemical activity is related to the energetic state of a chemical, is a measure of the effective concentration of a chemical in a given exposure medium (Reichenberg and Mayer 2006; Mackay et al. 2011), and is closely related to the freely dissolved concentration. For example, chemical activity is an improved measure of exposure when interaction with media constituents (such as particles in air and organic matter in water) effectively reduces the amount of chemical free to interact with a biological receptor (such as a human), often referred to as the bioavailable fraction.
table sensors for measuring particles and volatile organic chemicals are being refined and are providing valuable information on personal exposures, particularly in vulnerable populations (McGinn et al. 2016). Mobility-based exposure assessment that uses personal devices, such as cell phones, to provide GPS information, can be used to determine time and location of people relative to exposure levels determined from remote sensing information (Adams et al. 2009; de Nazelle et al. 2013; Su et al. 2015). Consumer product and use databases and market research data can provide population and personal exposure information that can help to inform exposure assessment, for example (Goldsmith et al. 2014). All those emerging technologies and data streams will complement existing tools and techniques in the effort to obtain more comprehensive knowledge of the source-to-outcome continuum.
Targeted and Nontargeted Exogenous Chemical Exposure Assessment
Important advances in two complementary approaches for characterizing human exposure—targeted and nontargeted analysis—are improving the accuracy and breadth of human and ecological exposure assessment (Fiehn 2002; Park et al. 2012; O’Connell 2014a,b; Go et al. 2015; Mastrangelo et al. 2015; Sud et al. 2016). Both approaches, whether focused on endogenous or exogenous chemicals, are commonly referred to as metabolomics approaches.5 Targeted analysis focuses on selected chemicals for which standards and methods are available and identifies chemicals on the basis of mass spectrum, elution time, detector signals, or some combination of these measures. Targeted analysis has produced much of the exposure data used in epidemiological studies and risk assessment. The US National Health and Nutrition Examination Survey and the Canadian Health Measures Survey are two extensive biomonitoring programs that use targeted analytical techniques for exposure assessment (Needham et al. 2005; Calafat 2012; Haines and Murray 2012). Although initially limited by throughput and a focus on single chemicals, small groups of chemicals (Casas et al. 2011; Mortensen et al. 2014), or modest-size chemical classes (O’Connell et al. 2014b), targeted methods are emerging for quantitative analysis of hundreds of chemicals (O’Connell et al. 2015). Generally, there is a tradeoff between sensitivity and selectivity that imposes limitations on the number of chemicals that can be analyzed in single runs by using a single instrument or method. Targeted analyses are limited to chemicals for which standards are available. Accepted standards for identification and quantitation have been articulated for most analyte classes (such as metabolites and peptides) (Castle et al. 2006; Fiehn et al. 2006; Goodacre et al. 2007; Sumner et al. 2014), but these standards are inconsistently applied in practice.
Targeted analytical methods for protein and DNA adducts have emerged as an alternative to direct measurement of chemicals in blood. When stable protein or DNA adducts can be easily measured and information on the rates of adduct formation and loss is available, adduct concentrations can be used as proxies for the time-weighted average exposure to the parent chemical. Those approaches are particularly valuable for exposure assessment and exposure reconstruction for short-lived chemicals whose concentrations in blood and other biofluids might be very low and subject to high temporal variability. One example is the use of hemoglobin adducts of acrylamide and its metabolite glycidamide for accurate reconstruction of acrylamide exposure and its concentration in blood over time in humans (Young et al. 2007). Chemical-specific adducts of the carcinogens butadiene, formaldehyde, and acetaldehyde have emerged recently as metrics of exposure to these extremely short-lived chemicals (Swenberg et al. 2007, 2008; Moeller et al. 2013; Yu et al. 2015). The benefits of using stable adducts to measure exposure to short-lived chemicals include the ability to integrate exposure over time (that is, the adducts can serve as integrative measures of exposure because they are more stable) and biological relevance because of the proximity to a target site, such as DNA. Swenberg and co-workers have established highly sensitive methods for specific formaldehyde DNA adducts and pioneered methods for establishing the contribution of endogenous and exogenous formaldehyde to total internal exposure (Edrissi et al. 2013; Moeller et al. 2013; Pottenger et al. 2014; Pontel et al. 2015; Yu et al. 2015). The studies highlight the utility of targeted analysis of adducts for exposure assessment and perhaps a potential for broad assessment of the adductome (Gavina et al. 2014; Pottenger et al. 2014).
Nontargeted analysis has emerged as an approach to provide qualitative information on the large portion of the exposome that is uncharacterized—a portion that includes bioactive endogenous peptides, exogenous chemicals, metabolites, lipids, and other biomolecules. It offers the ability to survey more broadly the presence of all chemicals in the environment and in biofluids regardless of whether standards and methods are available. The nontargeted approach trades selectivity for breadth and produces numerous unidentified analytical features. Comparing unidentified analytical features from large cohorts and correlating them with responses of interest in the cohorts can help to identify analytical features for further investigation (Burgess et al. 2015). Cheminformatics and computational chemistry can be used to identify chemicals with varying levels of confidence; nuclear magnetic resonance spectroscopy can be used to identify chemical structure with high accuracy. Accepted standards for iden-
___________________
5 As defined in Chapter 1 (see Box 1-1), metabolomics is assumed to include exogenous chemicals found in biological systems in their unmetabolized forms.
tification of metabolites (Castle et al. 2006; Fiehn et al. 2006; Sumner et al. 2014) have not been routinely applied to nontargeted approaches, so chemical matches to the analytical features is tentative and association between specific chemicals and disease is uncertain.
Nontargeted approaches are promising, but there are limitations in the use of data produced from nontargeted analyses that should be considered before collecting the data. For example, an unidentified analyte cannot be used to develop a mechanistic argument to support or refute a causal association between the presence of the analyte and a clinical effect, it cannot be quantified in absolute terms, it cannot be subjected to toxicity testing, and it cannot be attributed to sources for purposes of exposure mitigation. Although identifying all analytes is an important objective, reducing the number of analytes—to investigate, for example, on the basis of frequency in samples, membership in an important chemical class, and association with a clinical outcome—will be important until methods for identification of unknown analytes become more efficient.
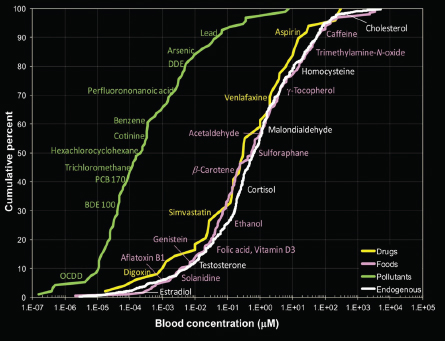
Initial efforts to understand potential contributions of exogenous and endogenous exposure have led to important insights about the role of each and about potential limitations of analytical technologies. Rappaport and co-workers (2014) reported human blood concentrations of many chemicals, their sources, evidence of chronic-disease risks, and numbers of metabolic pathways. Blood concentrations of endogenous chemicals, food chemicals, and drugs were indistinguishable and spanned 11 orders of magnitude; blood concentrations of pollutants were on the average lower by a factor of about 1,000 (see Figure 2-2). Although the findings cannot be generalized to all chemicals or all exposure scenarios, the blood-concentration ranges highlight the importance of using highly sensitive analytical instrumentation to characterize human exposure (Athersuch 2016; Uppal et al. 2016).
Risk assessment and mitigation of sources and risks all depend on knowing absolute quantities of specific chemicals; therefore, targeted analyses will continue to be the primary source of exposure information. Because the results of nontargeted analyses provide only relative or qualitative exposures, they are not readily applicable to conventional risk assessment. However, when unidentified analytical features can be aggregated according to their toxicity or pharmacokinetic behavior, there will be new opportunities to conduct hazard or risk assessments on the basis of similarity to chemicals whose toxicity is known.
Exposure Inference from -Omics Technologies
-Omics technologies that quantify the abundance of biomolecules, such as proteins and transcripts, offer distinct and diverse applications for exposure assessment. In contrast with metabolomic approaches that quantify exposure to specific metabolites of endogenous and exogenous chemicals, proteomic and transcriptomic approaches provide global assessment of biological responses to exposure to multiple stressors. Those -omics approaches can provide biomarkers or biosignatures of response to chemical classes, such as oxidants (Roede et al. 2013; Go and Jones 2014) and potentially genotoxic chemicals (Fenech and Bonassi 2011; Lovreglio et al. 2014; Kalemba-Drozdz 2015; Moro et al. 2015; Tumer et al. 2016). That particular application of -omics technologies, a key element of Wild’s original vision of the exposome (Wild 2005, 2012), is used to infer exposure to one or more chemicals on the basis of a mechanistic understanding of biological response to them. Some biomarkers of exposure can result from changes in the body that are induced by chemical exposure (for example, changes in metabolite or protein profiles), but these types of biomarkers commonly do not provide quantitative exposure information that can be used for risk estimation. The application of -omics technologies to infer exposure to classes of stressors is expected to grow. Although the initial utility will probably be in qualitative exposure inference and in assembling evidence on biological pathways, application should expand to more confident and more quantitative characterization of exposures to chemical classes or groups of stressors that produce the same biological effect, such as oxidation or inflammation.
Novel Exposure Matrices for Exposure Reconstruction
Assessment of occupational and environmental exposures will continue to rely on matrices for which there are established methods of collection, analysis, and interpretation. Those matrices include air, water, soil, food, blood, and urine. The expanding computational exposure-science infrastructure (Arnot et al. 2012; Shin et al. 2012, 2015; Wambaugh et al. 2013, 2014; Isaacs et al. 2014), which uses the traditional data streams to construct population-level exposure assessments, will continue to drive the generation of data on the traditional exposure matrices.
Growing emphasis on near-field exposures (Stapleton et al. 2008; Shin et al. 2012; Wambaugh et al. 2014) and on exposures during development, which is the focus of the Children’s Health Exposure Resource Centers of the National Institute of Environmental Health Sciences, is poised to drive exposure assessment toward new environmental and biological matrices and new approaches. For example, population-level exposure to hundreds of chemicals was recently shown to be dominated by near-field exposures from consumer-product and household use, not by far-field exposures that take place after chemicals are released into the outdoor environment (Shin et al. 2012; Wambaugh et al. 2014). Increased focus on categorizing chemicals in consumer products and on assembling exposure data for use in exposure assessment is one immediate outcome of the recent studies. Continued efforts to measure and estimate concentrations in multimedia sources—such as indoor air, indoor surfaces, dust, and consumer products—are required to address uncertainty in near-field exposures and pathways.
Characterization of exposures during the toxicologically sensitive period of fetal development has historically been limited to drawing inferences about maternal exposure through periodic maternal blood and urine measurements. Responding to the need to improve the characterization of fetal exposures to chemicals, researchers have turned to novel biological matrices, such as teeth, hair, nails, placental tissue, and meconium. The growth properties (the sequential deposition or addition of tissue) and availability of these biospecimens offer the opportunity to extract a record of exposure. For example, laser-ablation inductively coupled mass spectrometry has been used to reconstruct the timing of shifts in primates’ diets that are associated with weaning by measuring calcium:barium ratios in tooth enamel (Austin et al. 2013). The same approach was recently shown to be promising for assessing in utero exposure to manganese. Arora et al. (2012) measured manganese concentrations in tooth dentine specific to the postnatal period and the second and third trimesters and showed a statistically significant relationship between house-dust manganese concentrations and dentine manganese concentrations during the second trimester. Those authors and others (Andra et al. 2015; Palmer et al. 2015) have extended the methods to measure organic chemicals, including phenols and phthalates. Like teeth, hair forms in utero (third trimester), continues to grow, and potentially provides a temporal record of exposure. Initially used widely for forensic analysis of exposure to illicit drugs, hair has emerged as an important matrix for biomonitoring of metals and organic chemicals, such as polybrominated diphenyl ethers (Aleksa et al. 2012; Liu et al. 2015a). Similar methods have been applied to fingernails (Liu et al. 2015a).
Although the new matrices mentioned above have advantages and add valuable information to exposure assessment, they pose challenges in interpretation and application. A common challenge in the use of exposure measures based on the new biological and environmental matrices for quantitative exposure assessment is the need to understand how measured concentrations are related to measures of exposure traditionally used to assess chemical toxicity or risk. Ideally, the new biomonitoring data can be supported by information regarding how measured
concentrations in new matrices are related to conventional measures of internal exposure (serum concentrations, µM) or external exposures (mg/kg-day or mmol/kg-day). New experimental data, such as chemical half-life in the body, and data related to events and processes of exposure, such as time since the exposure, that can inform various relationships and pharmacokinetic models will be useful in interpreting and reconstructing exposures by using the biomonitoring data (see, for example, Lorber and Egeghy 2011; Ritter et al. 2011; Quinn and Wania 2012; Wambaugh et al. 2013; Aylward et al. 2014; Hays et al. 2015). The additional information regarding the exposures provides confidence in using the measured biomonitoring data and supporting the exposure narrative.
Physiologically Based Pharmacokinetic Models and Models for Translating Exposure Between Systems
Physiologically based pharmacokinetic (PBPK) models have made substantial contributions to exposure assessment for more than 30 years. PBPK models have been applied effectively to characterize target-tissue exposure in test animals and humans, to characterize pharmacokinetic variability, and to extrapolate across species, life stages, exposure routes, and, most recently, ecosystem elements (MacLachlan 2010; Weijs et al. 2012; Sonne et al. 2015). PBPK models now provide a common framework similar to environmental fate and transport models for more integrative exposure assessment and are applied more regularly to support aggregate (multiroute) exposure assessment (Esch et al. 2011; Abaci and Shuler 2015), exposure reconstruction from biomonitoring data, and exposure translation between in vitro and in vivo test systems.
The use of PBPK models for exposure reconstruction, known as reverse dosimetry (Liao et al. 2007; Tan et al. 2007; Bartels et al. 2012; Hays et al. 2012; McNally et al. 2012; Yang et al. 2012; Grulke et al. 2013), has led to important advances in the field of biomonitoring. Internal and external exposures can now be related and predicted on the basis of more limited sets of exposure information—for example, urine biomonitoring data (spot samples)—by applying principles of pharmacokinetics. The tools are used to calculate or estimate margins of exposure to chemicals on the basis of blood or urine spot samples and can be used to inform regulatory levels. New methods offer the ability to evaluate the influence of behavior and physiological variability on exposure distributions (Shankaran and Teeguarden 2014).
The use of PBPK models to characterize the influence of biochemical and physiological variability, particularly the role of polymorphisms of metabolizing enzymes in estimates of metabolism and variability (Beaudouin et al. 2010; Bois et al. 2010; Snoeys et al. 2016), has grown substantially and will continue to contribute to exposure assessment and risk assessment. Those advances help to predict pharmacokinetics of potentially sensitive populations, such as preterm infants (Claassen et al. 2015) and children (Yoon et al. 2012). Recently, PBPK models have been applied to disentangle the role of physiological changes related to disease states from the effects of a chemical on disease and to examine the role of reverse causation in published epidemiological studies (Verner et al. 2015; Wu et al. 2015). Accordingly, PBPK models have emerged as new exposure tools capable of supporting inference in epidemiological studies.
One of the major developments concerning PBPK models has been their use for translating exposures between test systems and human-exposure scenarios. In particular, the rapidly expanding use of high-throughput in vitro cell and cell-free systems to characterize the bioactivity of chemicals and materials, such as nanomaterials, has led to a need to translate in vitro exposure data into corresponding in vivo exposures in test systems and humans. Various terms have emerged to describe the applications to do so—for example, in vitro–in vivo extrapolation (IVIVE), reverse toxicokinetics (rTK), and reverse dosimetry. Each describes a kinetics-based and partitioning-based approach to translating exposures from one system of interest (in vitro) to another (in vivo animal or human), and all strive for mass balance. The use of PBPK models and similar biokinetic models of in vitro test systems has produced important methods that can apply PBPK-modeling principles to a broad set of test systems (Rostami-Hodjegan 2012; Yeo et al. 2013; Campbell et al. 2014; Teeguarden et al. 2014; Martin et al. 2015), including microphysiological organ systems or human-on-a-chip systems (Esch et al. 2011; Abaci and Shuler 2015). However, without clear understanding of how exposures in the systems are related to in vivo exposures or human occupational or environmental exposures, their utility will remain limited, as has been the case for standard in vitro cell-culture and cell-free systems.
IVIVE models can be used to calculate human internal exposure concentrations of chemicals from data obtained in high-throughput in vitro systems (Kesisoglou et al. 2015). That approach uses hepatocyte cultures and other biotransformation systems to measure metabolic rate constants that are used to estimate human intrinsic clearance by the liver, a dominant route of metabolic and total clearance in humans. Clearance values can be obtained for different life stages or for populations that are resistant or vulnerable because of polymorphisms of metabolic enzymes. Renal clearance, another major elimination pathway, is often estimated by using data on glomerular filtration rates and measures of protein binding in serum (Rule et al. 2004; Rotroff et al. 2010; Tonnelier et al. 2012; Wetmore et al. 2012). Other aspects of kidney function, such as tubular processing, can also influence clearance rates (Weaver et al. 2016) and various biomark-
er concentrations. Metabolism in other tissues, which can be important, is not evaluated, and this is a limitation of the current state of these systems.6 Combining clearance with computational high-throughput methods for estimating average daily contact and intake rates makes it possible to predict internal concentrations expected in humans. Those concentrations can then be compared with effect levels or no-effect levels from toxicity-testing systems. Addressing some limitations—such as not accounting for metabolism by other tissues, for the potential role of transporters, or for human variability—will be important next steps toward higher confidence in the application of the models. New approaches for better understanding of metabolic and genetic determinants of exposure are detailed in the next section.
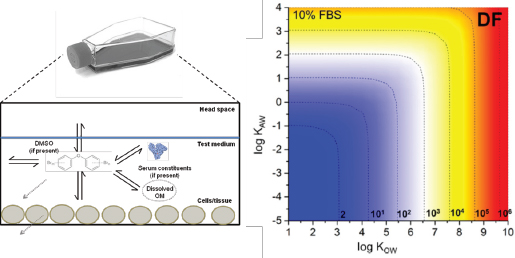
Key challenges in interpreting and applying IVIVE data include the quantification of relevant concentrations that correspond to observed in vitro bioactivity from assumed nominal (administered) concentrations (see Box 2-2 and Figure 2-3). A consistent approach for comparing and extrapolating results could be the use of the free (dissolved aqueous) concentration in the test system because this metric can be applied to cell-based or cell-free systems. The limitations complicate chemical comparisons for potency and toxicity and reduce confidence in the application of in vitro bioassay data that are based only on nominal concentrations in risk-based assessments. Models to calculate in vitro concentrations that cannot be readily measured with traditional sample extraction and analytical techniques need to be developed, evaluated, and applied. Passive dosing and sampling techniques might prove useful in addressing the current analytical challenges and associated uncertainties in quantifying exposures in smaller in vitro test systems (Kramer et al. 2010).
New Approaches for Assessing Biochemical and Physiological Determinants of Internal Exposure
Metabolism, cellular transport, and other processes that control elimination and distribution of chemicals in organisms are essential considerations and important challenges in exposure science, data interpretation, and risk assessment. Metabolism is a key determinant of chemical residence time in the body and can lead to more or less production of toxic chemicals; thus, it plays an important role in the extent of exposure and chemical toxicity (Leung et al. 2012). Reliable measures of metabolic rates are essential for understanding and characterizing differences in metabolism among species and between in vitro and in vivo test systems and for understanding the extent of variability and its effect on susceptibility or resistance. Computational approaches (PBPK, rTK, and IVIVE) can be used to translate in vitro metabolic rates into estimates of chemical clearance (Wilk-Zasadna et al. 2015) and to quantify differences among species and systems for exposure assessment.
High-throughput in vitro assays can be used to investigate metabolism; they now cover many enzymes and isoforms involved in chemical metabolism, including the phase I cytochrome P450 enzymes and a variety of phase II enzymes (admescope; Tolonen and Pelkonen 2015). Direct measures of activity obtained from the assays complement genomic approaches for characterizing the influence of polymorphisms on metabolism. New proteomic tools that use chemical probes can also be used to measure metabolic activity of specific enzymes directly
___________________
6 The committee notes that over-prediction of serum concentrations of parent chemicals and under-prediction of potentially important metabolites is generally a possible outcome of underrepresenting metabolism.
in tissue and cellular preparations (Cravatt et al. 2008; Sadler and Wright 2015). For example, recent publications (Crowell et al. 2013; Sadler et al. 2016) demonstrate that activity-based probes provide better measures of relative enzyme activity for individual enzymes than measures of transcripts or proteins and thus complement conventional metabolism assays. Other in vitro metabolism test systems, such as ones that use hepatocytes and liver spheroids, and computational models to translate metabolic rates and pathways to in vivo clearance continue to evolve (Fitzgerald et al. 2015; Hutzler et al. 2015; Liu et al. 2015b). Higher-throughput systems for measuring and interpreting metabolic rates in hepatocytes have been successful in extending our knowledge from pharmaceuticals to environmental chemicals (Wetmore et al. 2014; Yoon et al. 2014). However, increasing capacity to synthesize chemical standards and test materials will be essential if these approaches are to be successfully applied to the many chemicals in commerce.
As basic hepatic-metabolism data grow, other limitations of the systems to predict chemical kinetics and internal exposures will become important. Extrahepatic metabolism—such as metabolism in the kidney, gastrointestinal tract, and lung—can be important but is not yet addressed in most extrapolations. Similarly, differences in metabolic competence between the cells used in vitro and the in vivo systems can affect the extent of metabolism, the metabolic pathways activated, and the metabolites produced (see, for example, Kolanczyk et al. 2012). Emerging tools that can evaluate potential metabolite production (Tolonen and Pelkonen 2015; Wilk-Zasadna et al. 2015) and the use of multiple in vitro metabolism systems of varied complexity (Zhang et al. 2012) that include more than one tissue or cell type are possible solutions to the challenges. QSAR models that can predict rates of metabolism and clearance in tissues, such as liver and plasma (Berellini et al. 2012; Hsiao et al. 2013), and in the whole body (Obach et al. 2008; Wishart et al. 2008; Arnot et al. 2014) are also promising approaches for obtaining information on metabolism.
Pharmacogenomic profiling has emerged as a valuable approach for characterizing individual and population variabilities in genes that influence absorption, distribution, metabolism, and elimination (ADME) of drugs and environmental chemicals. Variations in ADME processes are important sources of variability in internal exposure. Recent advances in sequencing technologies (De Wit et al. 2015; Heather and Chain 2015; McGinn et al.
2016) now offer unprecedented potential for rapid individual and population-level identification of single-nucleotide polymorphisms that affect metabolic, transport, and clearance processes that together influence individual internal-exposure profiles. Recently, the frequencies of polymorphisms in 1,936 proteins that have documented clinical significance for ADME processes were measured and characterized in a Thai population and compared with findings in other ethnicities (Jittikoon et al. 2016). That and other recent analyses that show greater diversity in polymorphisms in American blacks and other ethnicities (Li et al. 2014; Ortega and Meyers 2014) demonstrate the potential for nearly comprehensive assessment of polymorphisms of ADME-related genes in individuals and populations and for internal-exposure predictions on an individual basis. More comprehensive characterization of ADME-related and other polymorphisms in populations and improved understanding of their function and relevance to exposure and toxicity will be valuable in addressing population variability for risk-based decision-making. The committee notes that compartmental and PBPK models for predicting the resulting effects on population distributions of serum concentrations have been used regularly but for only a few metabolic enzymes (EPA 2010).
Another important process to consider is cellular transport; transport proteins influence both tissue and intracellular concentrations. Pharmaceuticals and environmental chemicals are substrates for transporters (Fardell et al. 2011), and the importance of transporters in affecting internal chemical exposure at target sites is recognized (Wambaugh et al. 2014). QSAR models for predicting chemical interactions with transporters (Sedykh et al. 2013) and a variety of in vitro assays (Xie 2008) have been developed to support incorporation of transporters into determinations of internal exposure.
Continued success in using the new tools described here for measuring and calculating biochemical and physiological determinants of internal exposure will improve exposure assessment and ultimately will support the successful integration of in vitro, computational, and in vivo approaches into risk assessment.
CONFIDENCE LEVELS IN EXPOSURE INFORMATION AND ASSESSMENT
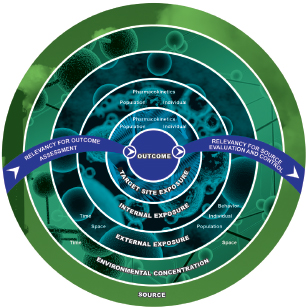
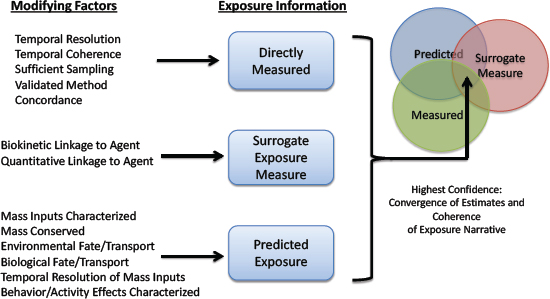
Exposure data from traditional and emerging methods discussed above can be placed in categories spanning the continuum from source to target-site exposure (NRC 2012) (see Figure 2-4). Exposure measures biologically closer to the site of action of the stressor can under some conditions have greater value for linking exposures to effects. For example, the relationship between soil concentrations of a chemical and effects in a population exposed to the soil might be obscured by individual differences in exposure rate, activity patterns, and metabolism. In contrast, individual blood or tissue measures of chemical exposure reflect the combined action of those processes and benefit from being more directly related to the event that initiates adverse effects: interaction of the chemical with a biological receptor (organelle, protein receptor, or DNA). However, soil and air measures of chemicals and biologics can be less confounded sources of information for assessing source contributions to external exposure because fewer processes (absorption, metabolism, and human activity patterns) can obscure relationships between the measured exposure in blood or urine and the source. The committee cautions, however, that internal exposures are not universally better or universally more useful than external exposures for purposes of relating exposures and effects, for example, in epidemiological studies. A long history shows the utility of measures of external exposure for epidemiology. In fact, external exposures might sometimes be superior to internal exposures, for example, when the two are proportional to one another and external measures are easier to acquire. Furthermore, external exposures might be the most biologically relevant when portal-of-entry effects, such as skin sensitization, are the focus. Exposure measures should be carefully selected by considering the strengths and limitations of external and internal measures of exposure and the purpose for which they will be used. Ideally, exposure data are available across the entire spectrum illustrated in Figure 2-4, and approaches for connecting them quantitatively have been developed to enable the use of exposures at any point on the continuum.
There is a spectrum of quality of exposure data from screening-level assessments based on limited information to multiroute, multisource exposure assessments to population-scale longitudinal exposure assessments that use validated exposure biomarkers. Important considerations for the application of exposure data in decision-making are the quality of the data and the context in which the data will be used; data quality can be determined by evaluating accuracy, integrity, suitability, transparency, and concordance of multiple lines of data or evidence (WHO 2016). The degree of confidence that is required for exposure data or exposure assessment is balanced with the cost of data acquisition and determined by the decision context established in problem formulation. In some cases, screening-level exposure data that have greater uncertainty might have sufficient accuracy to support important screening-level decisions made by regulatory agencies and might provide the most cost-effective approach (Wambaugh et al. 2013, 2014; WHO 2016). In those cases, transparency is essential for providing understanding and confidence in decisions that stem from exposure assessment; transparency can be obtained by carefully documenting and reporting data quality, suitability, and integrity (WHO 2016). The use of computationally de-
rived exposure estimates that are based on sparse data is an example of possible applications. That approach might be used to make initial decisions to set priorities among stressors for improved exposure assessment, toxicity assessment, or epidemiological assessment. The same data might also be useful for making initial decisions regarding new applications of a chemical or its inclusion in or removal from new or existing products. In some cases, extensive uncertainty, sensitivity, and variability analyses of exposure-assessment components might indicate that exposures of the magnitude necessary to cause effects fall outside the range of plausibility, in which case such exposure estimates might have sufficient certainty to support decision-making regarding potential risks. As the field moves toward obtaining exposure data on thousands of chemicals in commerce and wider use of cost-effective screening-level analyses, careful reporting of the quality of assessments and associated limitations—for example, through model evaluation and sensitivity analysis—will have high priority. As computational exposure-measurement tools are developed and used, their successful application in risk-based or exposure-based decision-making as described above will involve passing the same quality assessments applied to environmental measures of exposure, for example, by applying EPA or World Health Organization (WHO) guidance to evaluate models (WHO 2005; EPA 2009, 2016a).
Guidance for evaluating exposure data and exposure assessments developed by WHO and EPA and published in the literature focuses more on determining data quality than on establishing confidence in integrating various data streams. For example, integrating emerging data streams (such as computational exposure data) with conventional data (such as those derived from blood and urine biomonitoring and air sampling) is not discussed. Figure 2-5 presents some general considerations for assessing quality of exposure data and for integrating multiple data types. The four attributes for judging the quality of exposure data outlined by WHO—appropriateness, accuracy, integrity and transparency—also apply to Figure 2-5, but there is additional consideration of the strength of agreement between measures and of how each measure is related to the others in the overall exposure narrative. Although computationally derived exposure estimates might be perceived as warranting less confidence than direct measures, consideration of factors related to appropriateness and accuracy might indicate that the computational estimates are of higher quality. For example, direct exposure measures that are made with analytical methods that have not been validated, that are confounded
by sample contamination, that are determined without accounting for external-exposure intake rates and half-lives, or that lack temporal resolution necessary for their application in some decision-making contexts might ultimately be less valuable than indirect or proxy measures that are based on a validated exposure metric. Similarly, computationally derived exposure estimates might be useful for some decision-making contexts, particularly when they are based on extensive experimental data—including pharmacokinetics, total external exposure, and patterns of external exposure—and show mass balance throughout the system. Confidence in any exposure assessment is increased when there is concordance, consistency, or agreement between multiple methods of exposure assessment and is greatest when directly measured exposures, indirect measures of exposure, and computationally derived exposure estimates or simulations agree (McKone et al. 2007; Cowan-Ellsberry et al. 2009; Mackay et al. 2011; Ritter et al. 2011; Teeguarden et al. 2013). Agreement between measured and predicted data streams builds confidence in each method of determination. Convergence between exposure measurements (external and internal) and model simulation results (for example, overlap of concentrations or probability distributions of concentrations) indicate higher confidence in an exposure estimate and in resulting risk-based decisions. Although agreement between exposure measures might be a hallmark of quality and of the ideal, multiple concordant measures of exposure are not required to establish levels of quality required for all decision-making contexts.
Consideration of the level of quality and confidence in exposure assessment in the decision-making context will continue to be important, particularly as new exposure data streams emerge from personal sampling data and from use of new exposure matrices, such as bone, teeth, and hair. The potential for using emerging exposure data streams is high, but without careful evaluation, comparison with other types of exposure-assessment data, and a consistent effort to relate measurements to the appropriate level of biological organization (for example, target site or source), confidence in their use or agreement on their best application might be difficult to obtain.
Guidance has been developed to foster confidence, transparency, and reproducibility in calculated data used for exposure and risk assessment. Specific guidance has been developed for QSAR models for predicting chemical properties and toxicity (OECD 2007), for environmental fate and exposure models (EPA 2009; Buser et al. 2012), and for pharmacokinetic models (McLanahan et al. 2012). As new exposure metrics emerge, it will be important to develop guidance for integrating the various exposure measures and to understand their value and relationships with each other.
APPLICATIONS FOR EXPOSURE SCIENCE
To provide practical guidance on the use of emerging exposure-science data streams for decision-making, the following sections describe applications expected to have near-term and lasting influence on exposure assessment and on risk-based decision-making (see Box 2-3). Each application uses one or more of the advances presented earlier in this chapter to provide a new basis for decision-making, to refine exposure data, or to provide new forms of exposure data.
Aligning Exposures Between Test Systems and Humans
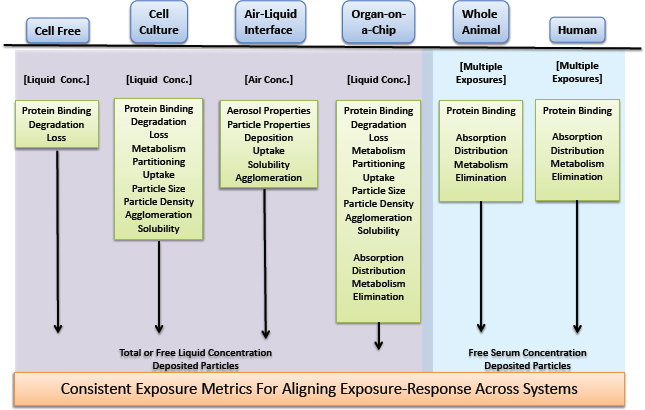
Comparison of biological responses across diverse experimental systems is nearly always an essential step in risk assessment. For example, risk assessors are faced with aligning toxicity data that are based on disparate measures of exposure: nominal liquid concentrations or cell concentrations in in vitro systems and air concentrations, inhaled amounts, or administered doses in rodent studies and human biomonitoring studies. Specificity, sensitivity, and concordance of observed effects across the test systems underlie the value and strength of evidence supporting conclusions about hazard and risk associated with exposure. To compare the responses from different test systems adequately, the exposures (concentrations) need to be expressed in consistent (comparable) units and with due consideration for the matrix in which the chemical is present. For example, a chemical concentration in whole blood that corresponds to an in vivo response can differ from the total concentration in an in vitro test system that corresponds to a related response, although the free (dissolved) concentrations in the aqueous phases in each system might be equal. Thus, the alignment of exposures in the systems is one important step in comparing exposure–response relationships across systems and evaluating concordance and consistency. As in vitro systems, organotypic, or co-culture systems augment or replace traditional animal studies, biological effects are compared over a more diverse array of assay systems and, from an exposure standpoint, over more types of exposure. For example, the most biologically sound comparison of biological effects shown in a cell-free assay, a cell-based assay, and an inhalation-exposure rodent study would involve comparisons of target-site exposures across all three systems: free-liquid concentrations in the cell-free assay, free cell concentrations in the cell-based assay, and free cell concentrations in the target cells of the rodent. As a practical matter, measured free-liquid concentrations in the in vitro assays and serum concentrations in rodent assays or from human studies would typically be considered appropriate measures of exposure-based alignment of the biological effects. However, there are circumstances in which serum concentrations are not good surrogates for tissue dose—for example, when transport proteins facilitate the uptake to and efflux from the tissue (Koch and Brouwer 2012; Wambaugh et al. 2014). The committee emphasizes that for any metric used to align exposure concentrations between systems, one should consider system conditions that might influence the value or interpretation of the data. For example, is the chemical concentration determined under steady-state or dynamic conditions or is the chemical ionic, in which case pH must be considered?
Each experimental system and human exposure situation has a unique set of processes that control or influence the timing, duration, and extent of exposure at the site of action (see Figure 2-6). Many of the processes are biokinetic and measurable with conventional approaches. Characterizing the processes in each test system allows the measurement, calculation, or simulation of chemical exposure at a common site of action. Consistent metrics of exposure, such as free or cell concentration, represent a possible ideal for comparison across systems and do not have the limitations associated with nominal concentrations. The chemical-activity approach has been proposed for ecological risk assessment (Mackay et al. 2011; Gobas et al. 2015) because it can integrate various multimedia exposure data streams (measured and predicted) and tox-
icity data streams (in vitro and in vivo) into a framework with consistent units and might be useful for human health evaluations. Other exposure metrics might be suitable for some decision contexts if they are adequately justified on the basis of pharmacokinetics, physical chemistry, and biology of the end point of interest.
Alignment of exposures between systems can be completed under data-poor and data-rich conditions. High-throughput methods for estimating hepatic and renal clearance can provide data needed for estimating human serum concentrations of chemicals that can be compared with cell-culture concentrations. That approach reflects one extreme—the data-poor case—for which data limitations can be overcome by focused, efficient in vitro and computational methods. Recently, an example of alignment of exposures under data-rich conditions—those with data from in vitro assays, whole-animal studies, and human biomonitoring—was published for systemic effects. Human urine and serum time-course concentration data from multiple studies provided empirical pharmacokinetic data that showed a relationship between serum bisphenol A (BPA) concentrations and urine BPA concentrations (Teeguarden et al. 2011, 2015; Thayer et al. 2015). The empirical relationships were used to calculate the range of human serum concentrations expected in a population of more than 28,000 people on whom there were published biomonitoring urine data. The resulting range of serum concentrations was compared directly with liquid concentrations in low-dose BPA cell-culture and aquatic studies (Teeguarden et al. 2013, 2015). Conclusions concerning the probability of biological effects in humans were drawn by aligning exposures across human biomonitoring and two divergent test systems—vertebrates and cell-culture systems—that used a measure of exposure proximal to target-tissue exposure. Although the role of protein binding was not addressed in that example, the data and tools to do so for BPA and other estrogens have been developed for rodent test systems and humans (Plowchalk and Teeguarden 2002; Teeguarden et al. 2005) and in vitro test systems (Teeguarden and Barton 2004).
A separate set of challenges has prevented widespread alignment of particle and nanoparticle exposures between in vitro and in vivo systems. The deposition of particles in the upper and lower airways of rodents and nonhuman primate toxicity-testing systems and of humans is governed by physical processes (gravity, diffusion, and impaction), breathing patterns, airway structure (size, branching pattern, and geometry), and particle characteristics (size, shape, and density). Similar processes affect gravitational and diffusional transport and eventual particle deposition
on target cells in liquid cell-culture systems and include agglomeration capacity; particle size, shape, density, and agglomeration size and density; media height; and diffusion (Teeguarden et al. 2007; Hinderliter et al. 2010; Cohen et al. 2014; DeLoid et al. 2014). Until recently, toxicity data on particles from in vivo and in vitro systems were compared on different exposure scales—for example, air concentrations and liquid cell concentrations (Sayes et al. 2007)—and this potentially obscured relationships between biological effects in the systems. More recently, direct measurement of target-cell doses has become more common. In addition, with the advent of computational tools that can capture the unique kinetics of particles in solution (Hinderliter et al. 2010) and of supportive experimental methods (Davis et al. 2011; Cohen et al. 2014), computational estimation of cellular doses in in vitro systems is becoming more common. With similar tools for measuring or calculating lung-tissue doses of particles after inhalation exposure (Anjilvel and Asgharian 1995; Asgharian and Anjilvel 1998; Asgharian et al. 1999, 2001, 2006, 2012; Asgharian 2004; Asgharian and Price 2007), approaches that allow comparison of in vitro and in vivo models of experimental particle toxicity have emerged (Teeguarden et al. 2014). The consistency of observed effects between the in vitro and in vivo systems might be revealed by making comparisons with a consistent, biologically relevant measure of exposure. For example, iron oxide nanoparticles were shown to cause expression of the same cytokines in macrophages in vitro and in mouse lungs in vivo when exposures were compared on a particle mass or cell basis.
Research in and development of new methods and more frequent application of existing methods to produce consistent measures of biologically appropriate exposure for toxicity across various test and receptor systems is a potentially high-value application for exposure science.
Improving Exposure Assessment for Epidemiological Studies
Causal inference based on epidemiological evidence can be strengthened when information on health outcomes is combined with clear measures of exposure at the biological site of action or a surrogate for the site of action (such as serum) that is temporally related to the causative biological events. Although that assertion is based on fundamental principles of pharmacology, it is not true that internal exposures are universally better than external exposure for purposes of assessing associations or inferring causation. External-exposure measures have been and will continue to be sufficient, and in some cases superior to internal-exposure measures, for example, where portal-of-entry effects are involved or large population-scale exposure assessments are necessary and internal-exposure assessments are impractical. Reducing or eliminating exposure misclassification and broadening exposure assessment to identify new chemicals that might be causative agents or confounders of existing associations would substantially strengthen the interpretation of epidemiological studies and improve their value for public-health decision-making.
Several advances in the field of exposure science are particularly well suited for improving exposure assessment for epidemiological studies. High-throughput targeted and nontargeted analytical-chemistry tools and new matrices for exposure assessment (such as hair, teeth, and nails) are together expected to offer more temporally relevant exposure assessment of many more chemicals and expand exposure assessment over the full life span. Emerging high-throughput computational-exposure models of external exposure will provide exposure estimates that complement those made through expanded biomonitoring programs. Personal biomonitors and sensor wristbands (O’Connell et al. 2014a,b) offer an unparalleled opportunity to characterize individual exposures and provide temporally and spatially resolved data for understanding patterns of exposure, variability, and the role of behavior and activity levels on exposure. PBPK models could improve exposure assessment by
- Reconstructing exposures from limited biomonitoring samples on the basis of pharmacokinetic understanding (Tan et al. 2006, 2012; Yang et al. 2012).
- Translating external exposures or biomonitoring data into more biologically relevant internal exposures (Teeguarden et al. 2013).
- Reducing the likelihood of reverse causation in epidemiological studies by more clearly delineating the sequences of chemical-induced physiological changes that lead to disease states (Verner et al. 2015; Wu et al. 2015).
- Accounting for population variability that is characterized directly or through the application of pharmacogenomics approaches (Teeguarden et al. 2008; EPA 2010; Ginsberg et al. 2010).
The greater availability of internal-exposure information obtained from direct biomonitoring of human populations or from a combination of computational tools would be of particular value by providing human exposure concentrations at the site of action (tissue or blood). Such information could be compared with measurements in animal and cell-culture studies and might enhance causal inferences derived from epidemiological studies.
Exposure-Based Screening and Priority-Setting
Several exposure-based priority-setting approaches that benefit from the emerging exposure-science tools and data streams have been developed. In an exposure-based
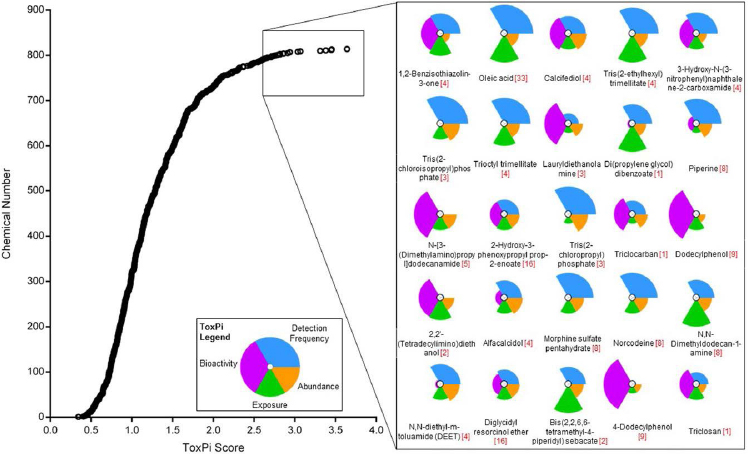
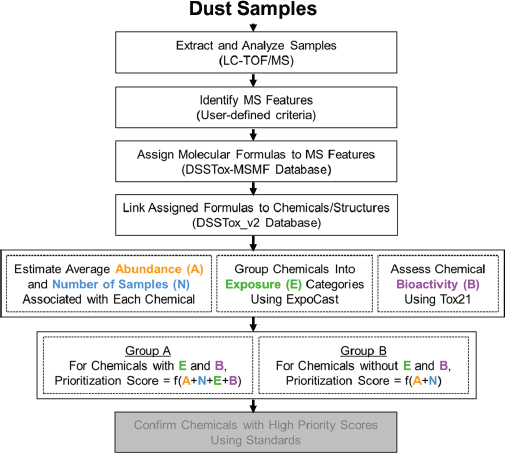
approach, chemicals in the top exposure category are assigned a higher priority for additional tiered toxicological, hazard, or risk assessment than those in the low exposure category; this provides a reproducible, transparent, and knowledge-based framework to inform decisions for testing priorities (Egeghy et al. 2011; Wambaugh et al. 2013, 2014). The European Food Safety Authority and WHO have reviewed the threshold-of-toxicological-concern (TTC) approach as a screening and priority-setting tool that can be used for chemical assessments in cases where hazard data are insufficient and human exposure can be estimated (EFSA 2016). The TTC approach is used principally as a screening tool to assess low-dose chemical exposures and to identify those on which further data are necessary for assessing human health risk.7 In some cases following certain requirements, “exposure-based waiving” for toxicity testing or “exposure-based adaptation of information requirements” approaches can be considered under the European Registration, Evaluation, Authorisation and Restriction of Chemicals legislation (Vermeire et al. 2010; Rowbotham and Gibson 2011). Exposure-based waiving has also been used to propose acceptable exposure levels determined on the basis of generalized chemical-toxicity data and without chemical-specific toxicity data. Such approaches might be useful in making initial decisions about the public-health importance of chemical exposures in lieu of complete exposure and hazard data. Within the bounds of uncertainty and variability of the data, some immediate decisions could be made about the low potential for risk posed by exposures below preselected “critical levels” (Vermeire et al. 2010; Rowbotham and Gibson 2011). Cumulative exposures to chemicals in specific classes might move some chemicals up in priority—an outcome of improved exposure data. Structure-based alerts and TTCs can be applied in such screening contexts to complement the exposure-based decision-making process. EPA recently demonstrated integration of nontargeted and targeted chemical analysis of house-dust samples for exposure-based and bioactivity-based ranking of chemicals for further biomonitoring or toxicity testing as shown in Figure 2-7 (Rager et al. 2016).
___________________
7 The committee notes that TTC approach depends on the set of chemicals used to establish the toxicity distribution that is used to derive the TTC value. The ability of the TTC approach to screen chemicals properly will depend on whether the toxicities of the chemicals of interest are well represented by the toxicities of the chemicals used to establish the distribution.
Biomonitoring data and environmental-monitoring data on most chemicals in commerce are missing or insufficient for exposure-based decision-making. Application of advanced biomonitoring, personal monitoring, and computational exposure-science tools described in this chapter can support high-throughput screening-level exposure assessment and exposure-based priority-setting for later toxicity testing. Exposure models can be applied to screen large numbers of chemicals in commerce and set priorities among specific chemicals or chemical classes on which there are no or few toxicity-testing data (McLachlan et al. 2014). Chemicals that have predicted high concentrations in humans and environmental media can then be used to identify toxicity-data gaps and set priorities for toxicity-testing for risk-based applications. The committee notes that priority-setting based only on exposure might assign a lower priority to chemicals that might be given a higher priority on the basis of toxicity or risk.
Translation of high-throughput data into risk-based rankings is an important application of exposure data for chemical priority-setting. Recent advances in high-throughput toxicity assessment, notably the ToxCast and Tox21 programs (see Chapter 1), and in high-throughput computational exposure assessment (Wambaugh et al. 2013, 2014) have enabled first-tier risk-based rankings of chemicals on the basis of margins of exposure—the ratio of exposures that cause effects (or bioactivity) to measured or estimated human exposures (Wambaugh et al. 2013, 2014; Wetmore et al. 2013, 2014; Shin et al. 2015). Building on work by Wetmore et al. (2012) and Rotroff et al. (2010), Shin et al. (2015) demonstrated a high-throughput method for screening and setting priorities among chemicals on the basis of quantitative comparisons of exposure data with in vitro bioactivity data (bioactivity quotients); this is similar to the margin-of-exposure approach used in risk priority-setting. They used human intake rates estimated with computational exposure models and toxicokinetic models for the in vitro–in vivo extrapolation of ToxCast toxicity data and identified 38 of 180 chemicals for which total estimated exposures equaled or exceeded the estimated oral dose expected to result in blood concentrations that cause a 50% response in an in vitro toxicity-testing system. Population variability due to differences in metabolic capacity was incorporated into the process (Wetmore et al. 2014). Screening-level exposure assessment was used to establish margins of exposure for that group of chemicals for purposes of priority-setting. The committee notes, however, that limitations of such analyses (see section “New Approaches for Assessing Biochemical and Physiological Determinants of Internal Exposure” above) need to be taken into account. Although exposure estimates that exceed in vitro effect estimates might not be conclusive evidence of risk and exposures that fall below in vitro activities might not be conclusive evidence of no risk, the committee sees the potential for the application of computational exposure science to be highly valuable and credible for comparison and priority-setting among chemicals in a risk-based context.
Human-exposure data on a much larger suite of chemicals than is now available would provide important new data for guiding selection of chemicals and exposure concentrations for hazard testing and mechanistic toxicology. The rapid expansion and use of high-throughput in vitro methods for hazard assessment and mechanistic studies presents a growing opportunity to test chemicals for bioactivity at human-exposure levels—levels lower than those typically used in traditional toxicity-testing studies. In vitro test systems—which are less subject to statistical-power limitations, are less expensive, and have fewer ethical considerations than whole-animal studies—might be better suited for testing exposures lower than those in traditional animal studies. Recent animal studies, however, provide useful examples of applying human exposure information to in vivo test systems. For example, recent studies have included exposures at or near those experienced by humans in animal-testing protocols for genistein and synthetic estrogens (NTP 2008; Delclos et al. 2009, 2014; Rebuli et al. 2014; Hicks et al. 2016). For those animal studies, exposures were selected on the basis of measured serum concentrations obtained in pilot animal studies, values estimated with pharmacokinetic models, and measured or estimated serum concentrations in humans. The use of target-tissue exposures or biologically relevant accessible proxies, such as serum, for selecting can in some cases be of greater relevance than the use of external exposure measures. Thus, there is an opportunity to apply many of the new tools described in this chapter—expanded biomonitoring, new biological matrices, and high-throughput computational exposure models—as a guide for the selection of exposures for use in toxicity testing (Gilbert et al. 2015).
Identifying New Chemical Exposures for Toxicity Testing
The totality of exposure that makes up the exposome includes registered chemicals that are used in commerce, their environmental and metabolic degradation products, and endogenously produced chemicals. Traditionally, hazard-testing paradigms focus on satisfying regulatory needs for supporting product registration and contain guidelines for testing commercial chemicals, not their degradation products, metabolites, or similar chemicals produced endogenously. Identification of chemicals that make up the latter groups of untested chemicals has become a key goal of federally funded exposure-science programs, such as the Children’s Health Exposure Analysis Resource. Owing to advances in high-throughput nontargeted analysis (Fiehn 2002; Park et al. 2012; Go et al. 2015; Mastrangelo et al. 2015; Sud et al. 2016), exposure science is in a more
effective position for discovery-based exposure assessment. Combined with environmental-degradation studies to identify novel chemicals, higher-throughput targeted analytical methods also contribute to overall exposure discovery for toxicity testing. For example, researchers in the Oregon State University Superfund Research Program recently discovered novel oxygenated and nitrogenated polycyclic aromatic hydrocarbons produced by conventional remediation methods and have subjected these environmental degradation products to toxicity testing (Knecht et al. 2013; Chibwe et al. 2015; Motorykin et al. 2015). In collaboration with academic scientists, EPA (Rager et al. 2016) recently demonstrated a workflow for nontargeted analysis of house dust with a transition to targeted analysis (measurement of specific target analytes) for ToxCast chemicals and use of frequency of detection information on chemicals as exposure data for priority-setting shown in Figure 2-8. The committee sees the use of nontargeted and targeted analysis as one innovative approach for identifying and setting priorities among chemicals for additional exposure assessment, hazard testing, and risk assessment that complements the current hazard-oriented paradigm.
Predicting Exposure to Support Registration and Use of New Chemicals
About 1,000–2,000 chemicals are introduced into commerce each year (EPA 2004). For newly introduced chemicals, exposure assessment means forecasting likely environmental concentrations or total daily human exposures resulting from expected uses and is not a regular part of the decision-making process. The case of methyl tertiary-butyl ether, a gas additive introduced without fate and transport calculations and later found to be widely distributed in the environment, is a poignant example of the value of predictive exposure modeling (Davis and Farland 2001). A recent NRC report, A Framework to Guide Selection of Chemical Alternatives, found that despite the known importance of exposure, many frameworks for selecting chemical alternatives downplay its importance and focus on inherent hazards posed by chemicals (NRC 2014). The committee that prepared the report recommended an increased emphasis on comparative exposure assessment and stated that inherent hazard should be the focus only in cases where the exposure routes and concentrations of the chemical of concern and its alternatives
are not expected to differ substantially; that is, equivalent exposures should not be automatically assumed. And, it recommended greater reliance on physicochemical data and modeling tools, when high-quality analytical data on exposure are unavailable, to aid in predicting the partitioning of contaminants in the environment and the potential for their persistence, bioaccumulation, and toxicity. Although approaches that are based on both hazard and exposure data are preferred, approaches that are based principally on exposure or hazard data will continue to be valuable depending on the decision context.
Tools to predict chemical properties (environmental or tissue-partitioning properties), stability (degradation and metabolism half-lives), and proposed use scenarios can be used to set parameter values for exposure models that are used to predict concentrations in environmental media and humans, over life spans, and on local and national scales. The estimated concentrations can guide selection of toxicity-testing exposures and can be compared with emerging toxicity data for risk-based assessments. Green-chemistry modeling initiatives can be applied to prescreen candidate chemicals according to the likelihood of biodegradation (Boethling 2011). Candidate chemicals can also be screened by applying more comprehensive methods that consider environmental fate and transport and various chemical use scenarios (release pattern and quantities) (see, for example, Gama et al. 2012). Confidence in the prescreening methods will be greatest when the models and tools cover the applicability domain of the chemicals that are being evaluated and when the tools have already been shown to be effective in predicting fate and transport of chemicals that have similar properties (for example, structural similarity or similar use categories). Hence there is a need to test and evaluate exposure modeling tools and data streams systematically with existing commercial chemicals to foster confidence in applying the same and emerging tools for new premarket chemicals.
Identifying, Evaluating, and Mitigating Sources of Exposure
For chemicals that have multiple relevant exposure pathways, it can be challenging to identify and rank exposure sources for mitigation. Exposure models can be used to reconstruct and identify the sources, behaviors, and pathways that are driving exposures to a particular stressor. Good examples of emerging computational exposure tools that can be used to trace exposures to sources are exposure models for consumer products (Gosens et al. 2014; Delmaar et al. 2015; Dudzina et al. 2015) and exposure models and frameworks that combine far-field and near-field pathways for aggregate human exposure assessments (Isaacs et al. 2014; Shin et al. 2015). For example, Shin et al. (2014) combined exposure models and human-biomonitoring data for nine chemicals to estimate the proportions of total production volumes that are used in selected use categories that correspond to exposure pathways. The models can be used to develop targeted strategies to reduce or virtually eliminate exposures to a particular stressor. For some chemicals, such as those used in pharmaceuticals and personal-care products, the dominant exposure pathways and chemical use rates are relatively obvious, and source mitigation, if necessary, might be relatively straightforward.
The combination of sensor technologies, including personal sensors, with GIS data systems offers new capabilities to identify sources of exposure. Personal sensors—for example, cell-phone–based sulfur oxide and nitrogen oxide sensors—use native GIS systems to collect real-time exposure data, which can be used to identify locations with high exposures and the source locations that contribute to the exposures. Remote sensing can identify high-exposure locations and source locations on a regional or population scale by mapping pollutant concentrations and identifying exposure patterns that might be related to sources.
Some chemicals and materials are poorly degraded and persist in the environment long after production and use are stopped. Some of the highly persistent chemicals also have long residence times in the human body. It can take years or decades for exposures to decline substantially after regulatory action is initiated. Accordingly, highly persistent chemicals that show unacceptable risk should have high priority for mitigation. Models and supporting experimental studies that screen for rates of chemical degradation in environmental media and overall persistence in the environment and in humans can be used to identify persistent chemicals before commercial use and prevent or mitigate potential exposure by finding alternatives.
Emerging exposure-assessment tools can also be used to mitigate sources of exposure to chemicals that cannot be identified confidently. Specifically, nontargeted analysis of environmental samples—air, dust, water, and soil—can be combined with analysis of ecological or human biomonitoring samples to select analytical features that represent internal exposures of potential concern. Geographical mapping of relative concentrations or detection frequency in environmental and human samples can lead to source identification that might in turn help to identify the chemical classes.
Assessing Cumulative Exposure and Exposure to Mixtures
Humans, animals, plants, and other organisms are exposed to numerous stressors that vary in composition and concentration over space and time. For the most part, traditional toxicity testing has been conducted largely on single chemicals, so there are important uncertainties in assessing potential short-term and long-term effects of exposures to a mixture. That issue is a well-recognized
concern for chemical assessment. With advances in exposure data streams and the potential for high-throughput toxicity screening, there are opportunities to address the uncertainty related to potential effects of mixture exposures better. Measurements obtained from human tissue and from environmental media to which humans are exposed can be used directly or indirectly to formulate environmentally relevant concentrations of mixtures for toxicity screening and testing. For example, internal concentrations of persistent organic pollutants from in vivo exposure of humans (silicone implants) were used to determine and test mixture toxicity in in vitro assays (Gilbert et al. 2015). It is also possible to use environmental-monitoring data (sampled water concentrations) to formulate exposure mixtures for toxicity testing (Allan et al. 2012), including approaches that consider population variability in responses to environmentally relevant chemical-mixture concentrations (Abdo et al. 2015). The substantial advances in analytical chemistry noted in this report are producing more complete data on the extent of cumulative exposure to chemicals. Personal sampling devices, such as wristbands and air-sampling devices, provide data on complex cumulative exposures of individuals. -Omics tools appropriate for measuring the aggregate biological response to cumulative exposures to chemical classes that act through similar mechanisms can be combined with measures of real-world cumulative exposures to assess the effects of cumulative exposures more comprehensively. Aggregate-exposure model calculations for individual chemicals could be combined to obtain estimates of cumulative exposures to mixtures, for example, by using models of exposure to consumer products that are supported by databases of chemical concentrations in the product and product-use rates. The exposure-model calculations could be used to address mixture exposures and potential toxicity; this approach would require mixture-toxicity data or mixture-toxicity models for risk-based assessment. For that case, estimating exposure to a mixture of chemical stressors for risk-based assessments is theoretically possible. The reliability of and confidence in the exposure calculations require further evaluation, and methods for including metabolites and nonchemical stressors in cumulative risk-based evaluations are also required.
CHALLENGES AND RECOMMENDATIONS FOR ADVANCING EXPOSURE SCIENCE
A principal objective of improving exposure science is to build confidence in exposure estimates by addressing or reducing uncertainty in the estimates used to support risk-based decision-making. That objective is best met by developing and further integrating monitoring, measurement, and modeling efforts and by harmonizing exposures among test systems, the multimedia environment, and humans. Incrementally increasing the number of chemicals included in monitoring programs can help in evaluating and refining exposure models and in developing new approaches to integrate exposure data and constitutes an initial and pragmatic path. However, increased environmental monitoring alone will not be sufficient to improve exposure science. Interpreting the monitoring data and appropriately applying exposure data in risk-based evaluations will require continued complementary development and evaluation of exposure-assessment tools and information, such as fate and transport models, PBPK models, and data on chemical quantity and use, partitioning properties, reaction rates, and human behavior.
In this section, challenges and recommendations to advance exposure science are discussed further. The points include some guidance initially presented in the ES21 report and some new, more pragmatic points, specifically related to the application of exposure science to risk-based evaluations. The points build on the advances and applications detailed in this chapter, which present key development opportunities for the field recommended by the committee. Generally, the recommendations and challenges cover a continuum: preparation of infrastructure, collection of data, alignment of exposures between systems, integration of exposure data, and use of data for priority-setting. The ES21 Federal Working Group (EPA 2016b) is particularly well-positioned to coordinate and support the recommendations outlined below by further strengthening federal partnerships for the efficient development of exposure-science research and by engaging with other stakeholders to address the challenges that face the development and application of exposure information for risk-based evaluations. The committee notes that several recommendations below call for developing or expanding databases. In all cases, data curation and quality evaluation should be a routine part of database development and maintenance.
Expand and Coordinate Exposure Science Infrastructure to Support Decision-Making
Challenge: A broad spectrum of disciplines and institutions are participating in advancing exposure methods, measurements, and models. Given the many participants in exposure science, most information is fragmented, incompletely organized, and not readily available or accessible in some cases. Thus, the full potential of the existing and emerging information for exposure-based and risk-based evaluations cannot be realized. The committee emphasizes that the rapid growth in exposure science presents unprecedented opportunities for more efficient, complete, and holistic use of exposure information, especially if the information can be well organized into a readily accessible format.
Recommendation: An infrastructure for exposure information should be developed to organize and coordinate better the existing and rapidly evolving components of exposure science and ultimately to improve exposure assessment. The infrastructure should be organized by using conceptual and systems-based frameworks that are commonly used in exposure assessment and should facilitate the generation, acquisition, organization, access, evaluation, integration, and transparent application and communication of exposure information. The infrastructure might best be comprised of an Internet-based network of databases and tools rather than one database and could expand on existing infrastructure and databases. Guidance for generating, evaluating, and applying exposure information (WHO 2005; EPA 2009) should be expanded to enable inclusion of data in the databases.
Recommendation: Coordination and cooperation should be encouraged among the large network of agencies, institutions, and organizations that produce and use exposure information for different but ultimately connected and complementary objectives. Cooperation should increase the efficiency with which the infrastructure described above is developed, and a common ontology of exposure science (Zartarian et al. 2005; Mattingly et al. 2012; EPA 2016b) should continue to evolve to facilitate interdisciplinary communication in the development and application of exposure information.
Identify Chemicals or Other Stressors and Quantify Sources and Exposures
Challenge: Nontargeted analysis in environmental and human media indicates that there are many unknown chemicals in complex uncharacterized mixtures to which humans are exposed. Analytical methods and standards are not available for most chemicals and degradation products, and this hinders the capacity to identify and quantify chemical exposures. Furthermore, uncertainty in source information—product composition, chemical quantity, use, and release rate—is a major obstacle to exposure estimation for most chemicals.
Recommendation: Current efforts to obtain and organize information on chemical quantities in and rates of release from products and materials, particularly consumer products and materials in the indoor environment, should be expanded substantially. Curated databases that contain analytical features that can be used in chemical identification should be expanded, and increasing the availability of analytical standards for chemicals and their degradation products should have high priority. Ultimately, the capacity to conduct targeted and nontargeted analyses to identify and quantify new and existing chemicals and mixtures in environmental media and humans should be increased.
Improve Knowledge of Processes That Determine Chemical Fate in Systems
Challenge: Understanding the influence of processes that control the fate, transport, and ultimately concentration of chemicals in environmental compartments and in animal and cell-based test systems is essential for characterizing and predicting exposures. Information on system properties, processes, and transformation pathways that contribute to chemical exposure is nonexistent, incomplete, and inconsistent, and this limits the capacity for more comprehensive, quantitative exposure-based and risk-based evaluations.
Recommendation: Databases of chemical properties and information on rates and processes that control chemical fate in vitro, in vivo, and in environmental systems should be developed. Information is needed, for example, on partitioning (distribution) coefficients, degradation and transfer rates, and metabolic and environmental transformation pathways. Information might be obtained through experiments or modeling.
Recommendation: Methods for measuring and predicting chemical transformation pathways and rates in environmental media, biological media, and biological test systems should be developed and applied. The methods should be used to quantify human exposures to chemical mixtures (parent chemicals and metabolites) over time and to interpret results from test systems in the context of actual human exposures. In particular, knowledge of environmental, human, and test-system properties and conditions that influence exposures should be improved. Human pharmacokinetic data on metabolism, chemical transporters, and protein binding should be generated for chemicals in consumer products and food-related applications to improve the interpretation of human biomonitoring data from urine, blood, and emerging matrices.
Align Environmental and Test-System Exposures
Challenge: Aligning environmental exposures and information obtained from experimental systems is a critical aspect of risk-based evaluation and is required for improving environmental epidemiology. Various units of quantification, such as administered or unmeasured dose, are often applied, and assumptions, such as steady-state or equilibrium conditions, are made. However, pharmacokinetic and fate processes and other factors often confound the interpretation and translation of exposure information between humans and the environment and experimental systems.
Recommendation: Concentrations in the test-system components should be quantified over time by measurement, which is preferred, or with reliable estimation methods. Methods and models that explicitly translate quantitative information between actual exposures and test-system exposures should be developed and evaluated.
Recommendation: Chemical concentrations that reflect human exposures as derived from biomonitoring measurements or from predictive exposure models should be considered when designing testing protocols for biological assays. Improving knowledge of processes that determine chemical fate in biological and test systems will be necessary to meet this recommendation.
Integrate Exposure Information
Challenge: Integration and appropriate application of exposure data from environmental media, biomonitoring samples, conventional samples (blood and urine), and emerging matrices (hair, nails, teeth, and meconium) is a scientific, engineering, and big-data challenge. The committee emphasizes that integration of measured and modeled data is a key step in developing coherent exposure narratives, in evaluating data concordance, and ultimately in determining confidence in an exposure assessment.
Recommendation: New interdisciplinary projects should be initiated to integrate exposure data and to gain experience that can be used to guide data collection and integration of conventional and emerging data streams. The projects might start as an extension of existing cooperative projects among federal and state agencies, nongovernment organizations, academe, and industry that focus on integrating measurements and models for improved quantitative exposure assessment. High priority should be placed on extending existing (EPA, Centers for Disease Control and Prevention, and WHO) guidance on quality of individual exposure data and assessments to include weighing and evaluating the quality of integrated experimental and modeled information from multiple matrices and data streams.
Determine Exposure-Assessment Priorities
Challenge: All the many uses of exposure data—from selection of chemicals for use in new products to risk-based decision-making to exposure ranking—require exposure data, often for thousands of chemicals, over time and space. Whether or not analytical methods are available for the chemicals, the resources and time that are required for direct measures of exposure are not available, and resource-intensive, high-confidence exposure measurements might not be necessary in some cases. A key challenge for exposure science is how best to focus resources on the highest-priority chemicals, chemical classes, mixtures, and exposure scenarios.
Recommendation: Continued development of computational and experimental tools that maximize the value of existing knowledge for estimating exposure should have high priority. Those approaches might initially focus on selected near-field exposures that are known to be important, on chemical classes that are of high interest because of data on biological effects, or on other objectives, such as exposure ranking of members of a chemical class that are being investigated for use in new products.
Recommendation: Continued development of approaches for exposure-based priority-setting that use uncertainty analysis to establish and communicate levels of confidence to support decision-making should be encouraged. The need to improve models or data that are used for priority-setting should be evaluated on the basis of the level of uncertainty and the tolerance for uncertainty in the decision-making context. Uncertainty and sensitivity analyses should guide selection and priority-setting among data gaps to be filled.
REFERENCES
Abaci, H.E., and M.L. Shuler. 2015. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr. Biol. (Camb) 7(4):383-391.
Abdo, N., B.A. Wetmore, G.A. Chappell, D. Shea, F.A. Wright, and I. Rusyn. 2015. In vitro screening for population variability in toxicity of pesticide-containing mixtures. Environ. Int. 85:147-155.
Adams, C., P. Riggs, and J. Volckens. 2009. Development of a method for personal, spatiotemporal exposure assessment. J.Environ. Monit. 11(7):1331-1339.
Al-Hamdan, M.Z., W.L. Crosson, S.A. Economou, M.G. Estes, Jr., S.M. Estes, S.N. Hemmings, S.T. Kent, M. Puckett, D.A. Quattrochi, D.L. Rickman, G.M. Wade, and L.A. McClure. 2014. Environmental public health applications using remotely sensed data. Geocarto. Int. 29(1):85-98.
Aleksa, K., J. Liesivuori, and G. Koren. 2012. Hair as a biomarker of polybrominated diethyl ethers’ exposure in infants, children and adults. Toxicol. Lett. 210(2):198-202.
Allan, I.J., K. Baek, A. Kringstad, H.E. Roald, and K.V. Thomas. 2013a. Should silicone prostheses be considered for specimen banking? A pilot study into their use for human biomonitoring. Environ. Int. 59:462-468.
Allan, I.J., K. Baek, T.O. Haugen, K.L. Hawley, A.S. Hogfeldt, and A.D. Lillicrap. 2013b. In vivo passive sampling of nonpolar contaminants in brown trout (Salmo trutta). Environ. Sci. Technol. 47(20):11660-11667.
Allan, S.E., B.W. Smith, R.L. Tanguay, and K.A. Anderson. 2012. Bridging environmental mixtures and toxic effects. Environ. Toxicol. Chem. 31(12):2877-2887.
Andersen, Z.J., A. de Nazelle, M.A. Mendez, J. Garcia-Aymerich, O. Hertel, A. Tjonneland, K. Overvad, O. Raaschou-Nielsen, and M.J. Nieuwenhuijsen. 2015. A study of the combined effects of physical activity and air pollution on mortality in elderly urban residents: The Danish Diet, Cancer, and Health Cohort. Environ. Health Perspect. 123(6):557-563.
Andra, S.S., C. Austin, R.O. Wright, and M. Arora. 2015. Reconstructing pre-natal and early childhood exposure to multi-class organic chemicals using teeth: Towards a retrospective temporal exposome. Environ. Int. 83:137-145.
Anjilvel, S., and B. Asgharian. 1995. A multiple-path model of particle deposition in the rat lung. Fundam. Appl. Toxicol. 28(1):41-50.
Armitage, J.M.., F. Wania, and J.A. Arnot. 2014. Application of mass balance models and the chemical activity concept to facilitate the use of in vitro toxicity data for risk assessment. Environ. Sci. Technol. 48(16):9770-9779.
Arnold, S.F., and G. Ramachandran. 2014. Influence of parameter values and variances and algorithm architecture in ConsExpo model on modeled exposures. J. Occup. Environ. Hyg. 11(1):54-66.
Arnot, J.A., T.N. Brown, F. Wania, K. Breivik, and M.S. McLachlan. 2012. Prioritizing chemicals and data requirements for screening-level exposure and risk assessment. Environ. Health Perspect. 120(11):1565-1570.
Arnot, J.A., T.N. Brown, and F. Wania. 2014. Estimating screening-level organic chemical half-lives in humans. Environ. Sci. Technol. 48(1):723-730.
Arora, M., A. Bradman, C. Austin, M. Vedar, N. Holland, B. Eskenazi, and D.R. Smith. 2012. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ. Sci. Technol. 46(9):5118-5125.
Asgharian, B. 2004. A model of deposition of hygroscopic particles in the human lung. Aerosol Sci. Technol.38(9):938-947.
Asgharian, B., and S. Anjilvel. 1998. A multiple-path model of fiber deposition in the rat lung. Toxicol. Sci. 44(1):80-86.
Asgharian, B., and O. T. Price. 2007. Deposition of ultrafine (nano) particles in the human lung. Inhal. Toxicol. 19(13):1045-1054.
Asgharian, B., F.J. Miller, and R. P. Subramaniam. 1999. Dosimetry software to predict particle deposition in humans and rats. CIIT Activities 19(3):1-6.
Asgharian, B., W. Hofman, and R. Bergmann. 2001. Particle deposition in a multiple-path model of the human lung. Aerosol Sci. Technol. 34(4):332-339.
Asgharian, B., O. Price, and G. Oberdorster. 2006. A modeling study of the effect of gravity on airflow distribution and particle deposition in the lung. Inhal. Toxicol. 18(7):473-481.
Asgharian, B., O. Price, G. McClellan, R. Corley, D.R. Einstein, R.E. Jacob, J. Harkema, S.A. Carey, E. Schelegle, D. Hyde, J.S. Kimbell, and F.J. Miller. 2012. Development of a rhesus monkey lung geometry model and application to particle deposition in comparison to humans. Inhal. Toxicol. 24(13):869-899.
Athersuch, T. 2016. Metabolome analyses in exposome studies: Profiling methods for a vast chemical space. Arch. Biochem. Biophys. 589:177-186.
Austin, C., T.M. Smith, A. Bradman, K. Hinde, R. Joannes-Boyau, D. Bishop, D.J. Hare, P. Doble, B. Eskenazi, and M. Arora. 2013. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature 498(7453):216-219.
Aylward, L.L., J.J. Collins, K.M. Bodner, M. Wilken, and C.M. Bodnar. 2014. Intrinsic elimination rate and dietary intake estimates for selected indicator PCBs: Toxicokinetic modeling using serial sampling data in US subjects, 2005-2010. Chemosphere 110:48-52.
Bartels, M., D. Rick, E. Lowe, G. Loizou, P. Price, M. Spen-diff, S. Arnold, J. Cocker, and N. Ball. 2012. Development of PK- and PBPK-based modeling tools for derivation of biomonitoring guidance values. Comput. Methods Programs Biomed. 108(2):773-788.
Beaudouin, R., S. Micallef, and C. Brochot. 2010. A stochastic whole-body physiologically based pharmacokinetic model to assess the impact of inter-individual variability on tissue dosimetry over the human lifespan. Regul. Toxicol. Pharmacol. 57(1):103-116.
Bennett, D.H., and E.J. Furtaw. 2004. Fugacity-based indoor residential pesticide fate model. Environ. Sci. Technol. 38(7):2142-2152.
Berellini, G., N.J. Waters, and F. Lombardo. 2012. In silico prediction of total human plasma clearance. J. Chem. Inf. and Model. 52(8):2069-2078.
Boethling, R.S. 2011. Incorporating environmental attributes into musk design. Green Chem. 13(12):3386-3396.
Bois, F.Y., M. Jamei, and H.J. Clewell. 2010. PBPK modelling of inter-individual variability in the pharmacokinetics of environmental chemicals. Toxicology 278(3):256-267.
Brauer, M., G. Freedman, J. Frostad, A. van Donkelaar, R.V. Martin, F. Dentener, R. Van Dingenen, K. Estep, H. Amini, J.S. Apte, K. Balakrishnan, L. Barregard, D.M. Broday, V. Feigin, S. Ghosh, P.K. Hopke, L.D. Knibbs, Y. Kokubo, Y. Liu, S. Ma, L. Morawska, J.L. Texcalac Sangrador, G. Shaddick, H.R. Anderson, T. Vos, M.H. Forouzanfar, R.T. Burnett, and A. Cohen. 2015. Ambient air pollution exposure estimation for the global burden of disease 2013. Environ. Sci. Technol. 50(1):79-88.
Breen, M.S., T.C. Long, B.D. Schultz, J. Crooks, M. Breen, J.E. Langstaff, K.K. Isaacs, Y.M. Tan, R.W. Williams, Y. Cao, A.M. Geller, R.B. Devlin, S.A. Batterman, and T.J. Buckley. 2014. GPS-based microenvironment tracker (MicroTrac) model to estimate time-location of individuals for air pollution exposure assessments: Model evaluation in central North Carolina. J. Expo. Sci. Environ. Epidemiol. 24(4):412-420.
Burgess, L.G., K. Uppal, D.I. Walker, R.M. Roberson, V. Tran, M.B. Parks, E.A. Wade, A.T. May, A.C. Umfress, K.L. Jarrell, B.O. Stanley, J. Kuchtey, R.W. Kuchtey, D.P. Jones, and M.A. Brantley, Jr. 2015. Metabolome-wide association study of primary open angle glaucoma. Invest. Ophthalmol. Vis. Sci. 56(8):5020-5028.
Buser, A.M., M. MacLeod, M. Scheringer, D. Mackay, M. Bonnell, M.H. Russell, J.V. DePinto, and K. Hungerbühler. 2012. Good modeling practice guidelines for applying multimedia models in chemical assess-ments. Integr. Environ. Assess. Manage. (4):703-708.
Calafat, A.M. 2012. The US National Health and Nutrition Examination Survey and human exposure to environmental chemicals. Int. J. Hyg. Environ. Health 215(2):99-101.
Campbell, J.L., M.E. Andersen, and H.J. Clewell. 2014. A hybrid CFD-PBPK model for naphthalene in rat and human with IVIVE for nasal tissue metabolism and cross-species dosimetry. Inhal. Toxicol. 26(6):333-344.
Casas, L., M.F. Fernandez, S. Llop, M. Guxens, F. Ballester, N. Olea, M.B. Irurzun, L.S. Rodriguez, I. Riano, A. Tar-don, M. Vrijheid, A.M. Calafat, J. Sunyer. and I. Project. 2011. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ. Int. 37(5):858-866.
Castle, A.L., O. Fiehn, R. Kaddurah-Daouk, and J.C. Lindon. 2006. Metabolomics standards workshop and the development of international standards for reporting metabolomics experimental results. Brief Bioinform. 7(2):159-165.
Chibwe, L., M.C. Geier, J. Nakamura, R.L. Tanguay, M.D. Aitken, and S.L. Simonich. 2015. Aerobic bioremediation of PAH contaminated soil results in increased genotoxicity and developmental toxicity. Environ. Sci. Technol. 49(23):13889-13898.
Claassen, K., K. Thelen, K. Coboeken, T. Gaub, J. Lippert, K. Allegaert, and S. Willmann. 2015. Development of a physiologically-based pharmacokinetic model for preterm neonates: Evaluation with in vivo data. Curr. Pharm. Des. 21(39):5688-5698.
Coecke, S., H. Ahr, B.J. Blaauboer, S. Bremer, S. Casati, J. Castell, R. Combes, R. Corvi, C.L. Crespi, M.L. Cunningham, G. Elaut, B. Eletti, A. Freidig, A. Gennari, J.F. Ghersi-Egea, A. Guillouzo, T. Hartung, P. Hoet, M. Ingelman-Sundberg, S. Munn, W. Janssens, B. Ladstetter, D. Leahy, A. Long, A. Meneguz, M. Monshouwer, S. Morath, F. Nagelkerke, O. Pelkonen, J. Ponti, P. Prieto, L. Richert, E. Sabbioni, B. Schaack, W. Steiling, E. Testai, J.A. Vericat, and A. Worth. 2006. Metabolism: A bottleneck in in vitro toxicological test development – The report and recommendations of ECVAM workshop 54. Altern. Lab. Anim. 34(1):49-84.
Cohen, J.M., J.G. Teeguarden, and P. Demokritou. 2014. An integrated approach for the in vitro dosimetry of engineered nanomaterials. Part. Fibre Toxicol. 11:20.
Cowan-Ellsberry, C.E., M.S. McLachlan, J.A. Arnot, M. Macleod, T.E. McKone, and F. Wania. 2009. Modeling exposure to persistent chemicals in hazard and risk assessment. Integr. Environ. Assess. Manag. 5(4):662-679.
Cravatt, B.F., A.T. Wright, and J.W. Kozarich. 2008. Activity-based protein profiling: From enzyme chemistry to proteomic chemistry. Annu. Rev. Biochem. 77:383-414.
Crowell, S.R., A.K. Sharma, S. Amin, J.J. Soelberg, N.C. Sadler, A.T. Wright, W.M. Baird, D.E. Williams, and R.A. Corley. 2013. Impact of pregnancy on the pharmacokinetics of dibenzo[def,p]chrysene in mice. Toxicol. Sci. 135(1):48-62.
Davis, J.A., J.S. Gift, and Q.J. Zhao. 2011. Introduction to benchmark dose methods and US EPA’s benchmark dose software (BMDS) version 2.1.1. Toxicol. Appl. Pharmacol. 254(2):181-191.
Davis, J.M., and W.H. Farland. 2001. The paradoxes of MTBE. Toxicol. Sci. 61(2):211-217.
de Nazelle, A., E. Seto, D. Donaire-Gonzalez, M. Mendez, J. Matamala, M. J. Nieuwenhuijsen, and M. Jerrett. 2013. Improving estimates of air pollution exposure through ubiquitous sensing technologies. Environ. Poll. 176:92-99.
De Wit, P., M.H. Pespeni, and S.R. Palumbi. 2015. SNP genotyping and population genomics from expressed sequences - current advances and future possibilities. Mol. Ecol. 24(10):2310-2323.
Delclos, K.B., C.C. Weis, T.J. Bucci, G. Olson, P. Mellick, N. Sadovova, J.R. Latendresse, B. Thorn, and R.R. New-bold. 2009. Overlapping but distinct effects of genistein and ethinyl estradiol (EE(2)) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod. Toxicol. 27(2):117-132.
Delclos, K.B., L. Camacho, S.M. Lewis, M.M. Vanlandingham, J.R. Latendresse, G.R. Olson, K.J. Davis, R.E. Patton, G. Gamboa da Costa, K.A. Woodling, M.S. Bryant, M. Chidambaram, R. Trbojevich, B.E. Juliar, R.P. Felton, and B.T. Thorn. 2014. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol. Sci. 139(1):174-197.
Delmaar, C., B. Bokkers, W. ter Burg, and G. Schuur. 2015. Validation of an aggregate exposure model for substances in consumer products: A case study of diethyl phthalate in personal care products. J. Expo. Sci. Environ. Epidemiol. 25(3):317-323.
DeLoid, G., J.M. Cohen, T. Darrah, R. Derk, L. Rojanasakul, G. Pyrgiotakis, W. Wohlleben, and P. Demokritou. 2014. Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat. Commun. 5:3514.
Dionisio, K.L., A.M. Frame, M.R. Goldsmith, J.F. Wambaugh, A. Liddell, T. Cathey, D. Smith, J. Vail, A.S. Ernstoff, P. Fantke, O. Jolliet, and R.S. Judson. 2015. Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicol. Rep. 2:228-237.
Dudzina, T., C.J. Delmaar, J.W. Biesterbos, M.I. Bakker, B.G. Bokkers, P.T. Scheepers, J.G. van Engelen, K. Hungerbuehler, and N. von Goetz. 2015. The probabilistic aggregate consumer exposure model (PACEM): Validation and comparison to a lower-tier assessment for the cyclic siloxane D5. Environ. Int. 79:8-16.
Edrissi, B., K. Taghizadeh, B.C. Moeller, D. Kracko, M. Doyle-Eisele, J.A. Swenberg, and P.C. Dedon. 2013. Dosimetry of N(6)-formyllysine adducts following [(1)(3) C(2)H(2)]-formaldehyde exposures in rats. Chem. Res. Toxicol. 26(10):1421-1423.
EFSA (European Food Safety Authority). 2016. Review of the Threshold of Toxicological Concern (TTC) Approach and Development of New TTC Decision Tree [online]. Available: http://www.efsa.europa.eu/en/supporting/pub/1006e [accessed July 15, 2016].
Egeghy, P.P., D.A. Vallero, and E.A. Cohen Hubal. 2011. Exposure-based prioritization of chemicals for risk assessment. Environ. Sci. Pol. 14(8):950-964.
Egeghy, P.P., R. Judson, S. Gangwal, S. Mosher, D. Smith, J. Vail, and E.A. Cohen Hubal. 2012. The exposure data landscape for manufactured chemicals. Sci. Total Environ. 414:159-166.
Egeghy, P.P., L.S. Sheldon, K.K. Isaacs, H. Özkaynak, M.R. Goldsmith, J.F. Wambaugh, R.S. Judson and T.J. Buckley. 2016. Computational exposure science: An emerging discipline to support 21st-century risk assessment. Environ. Health Perspect. 124(6):697-702.
Elgethun, K., M.G. Yost, C.T. Fitzpatrick, T.L. Nyerges, and R.A. Fenske. 2007. Comparison of global positioning system (GPS) tracking and parent-report diaries to characterize children’s time-location patterns. J. Expo. Sci. Environ. Epidemiol. 17(2):196-206.
EPA (US Environmental Protection Agency). 2004. Observational Batteries and Motor Activity. Office of Research and Development, US Environmental Protection Agency, Washington, DC [online]. Available: https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryID=36922 [accessed April 12, 2016].
EPA (US Environmental Protection Agency). 2009. Guidance on the Development, Evaluation and Application of Environmental Models. EPA/100/K-09/003. Office of the Science Advisor, Council for Regulatory Environmental Modeling, US Environmental Protection Agency, Washington, DC [online]. Available: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P1003E4R.PDF [accessed October 24, 2016].
EPA (US Environmental Protection Agency). 2010. Potential for Incorporation of Genetic Polymorphism Data in Human Health Risk Assessment. US Environmental Protection Agency, Washington, DC.
EPA (US Environmental Protection Agency). 2011. Exposure Factors Handbook: 2011 Edition. EPA/600/R-09/052F. National Center for Environmental Assessment, Office of Research and Development, US Environmental Protection Agency, Washington, DC [online]. Available: http://www.nrc.gov/docs/ML1400/ML14007A666.pdf [accessed July 15, 2016].
EPA (US Environmental Protection Agency). 2016a. Modeling EPA Guidance and Publications Developed by Other EPA Organizations [online]. Available: https://www.epa.gov/modeling/modeling-epa-guidance-and-publicationsdeveloped-other-epa-organizations [accessed October 24, 2016].
EPA (US Environmental Protection Agency). 2016b. The Exposure Science in the 21st Century (ES21) Federal Working Group [online]. Available: https://www.epa.gov/innovation/exposure-science-21st-century-federal-working-group [accessed October 24, 2016].
Esch, M.B., T.L. King, and M.L. Shuler. 2011. The role of body-on-a-chip devices in drug and toxicity studies. Annu. Rev. Biomed. Eng. 13:55-72.
Fantke, P., A.S. Ernstoff, L. Huang, S.A. Csiszar, and O. Jolliet. 2016. Coupled near-field and far-field exposure assessment framework for chemicals in consumer products. Environ. Int. 94:508-518.
Fardell, J.E., J. Vardy, I.N. Johnston, and G. Winocur. 2011. Chemotherapy and cognitive impairment: Treatment options. Clin. Pharmacol. Ther. 90(3):366-376.
Fenech, M., and S. Bonassi. 2011. The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis 26(1):43-49.
Fiehn, O. 2002. Metabolomics – The link between genotypes and phenotypes. Plant Mol. Biol. 48(1-2):155-171.
Fiehn, O., B. Kristal, B. van Ommen, L.W. Sumner, S.A. Sansone, C. Taylor, N. Hardy, and R. Kaddurah-Daouk. 2006. Establishing reporting standards for metabolomic and metabonomic studies: A call for participation. OMICS. 10(2):158-163.
Fitzgerald, K.A., M. Malhotra, C.M. Curtin, F.J. Brien, and C.M. O’Driscoll. 2015. Life in 3D is never flat: 3D models to optimise drug delivery. J. Control. Release. 215:39-54.
Gama, S., D. Mackay, and J.A. Arnot. 2012. Selecting and designing chemicals: Application of a mass balance model of chemical fate, exposure and effects in the environment. Green Chem. 14:1094-1102.
Gavina, J.M., C. Yao, and Y.L. Feng. 2014. Recent developments in DNA adduct analysis by mass spectrometry: A tool for exposure biomonitoring and identification of hazard for environmental pollutants. Talanta 130:475-494.
Geddes, J.A., R.V. Martin, B.L. Boys, and A. van Donkelaar. 2016. Long-term trends worldwide in ambient NO concentrations inferred from satellite observations. Environ. Health Perspect. 124(3):281-289.
Georgopoulos, P.G., C.J. Brinkerhoff, S. Isukapalli, M. Dellarco, P.J. Landrigan, and P.J. Lioy. 2014. A tiered framework for risk-relevant characterization and ranking of chemical exposures: Applications to the national children’s study (NCS). Risk Anal. 34(7):1299-1316.
Gilbert, D., P. Mayer, M. Pedersen, and A.M. Vinggaard. 2015. Endocrine activity of persistent organic pollutants accumulated in human silicone implants – Dosing in vitro assays by partitioning from silicone. Environ. Int. 84:107-114.
Ginsberg, G., K. Guyton, D. Johns, J. Schimek, K. Angle, and B. Sonawane. 2010. Genetic polymorphism in metabolism and host defense enzymes: Implications for human health risk assessment. Crit. Rev. Toxicol. 40(7):575-619.
Go, Y.M., and D.P. Jones. 2014. Redox biology: Interface of the exposome with the proteome, epigenome and genome. Redox Biol. 2:358-360.
Go, Y.M., D.I. Walker, Y. Liang, K. Uppal, Q.A. Soltow, V. Tran, F. Strobel, A.A. Quyyumi, T.R. Ziegler, K.D. Pennell, G.W. Miller, and D.P. Jones. 2015. Reference standardization for mass spectrometry and high-resolution metabolomics applications to exposome research. Toxicol. Sci. 148(2):531-543.
Gobas, F.A., S. Xu, G. Kozerski, D.E. Powell, K.B. Wood-burn, D. Mackay, and A. Fairbrother. 2015. Fugacity and activity analysis of the bioaccumulation and environmental risks of decamethylcyclopentasiloxane (D5). Environ. Toxicol. Chem. 34(12):2723-2731.
Goldsmith, M.R., C.M. Grulke, R.D. Brooks, T.R. Tran-sue, Y.M. Tan, A. Frame, P.P. Egeghy, R. Edwards, D.T. Chang, R. Tornero-Velez, K. Isaacs, A. Wang, J. Johnson, K. Holm, M. Reich, J. Mitchell, D.A. Vallero, L. Phillips, M. Phillips, J.F. Wambaugh, R.S. Judson, T.J. Buckley, and C.C. Dary. 2014. Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem. Toxicol. 65(5):269-279.
Goodacre, R., D. Broadhurst, A. Smilde, B. Kristal, J.D. Baker, R. Beger, C. Bessant, S. Connor, G. Capuani, A. Craig, T. Ebbels, D. Kell, C. Manetti, J. Newton, G. Paternostro, R. Somorjai, M. Sjöström, J. Trygg, and F. Wulfert. 2007. Proposed minimum reporting standards for data analysis in metabolomics. Metabolomics 3(3):231-241.
Gosens, I., C.J. Delmaar, W. ter Burg, C. de Heer, and A.G. Schuur. 2014. Aggregate exposure approaches for parabens in personal care products: A case assessment for children between 0 and 3 years old. J. Expo.. Sci. Environ. Epidemiol. 24(2):208-214.
Groothuis, F.A., M.B. Heringa, B. Nicol, J.L. Hermens, B.J. Blaauboer, and N.I. Kramer. 2015. Dose metric considerations in in vitro assays to improve quantitative in vitro-in vivo dose extrapolations. Toxicology 332:30-40.
Grulke, C.M., K. Holm, M.R. Goldsmith, and Y.M. Tan. 2013. PROcEED: Probabilistic reverse dosimetry approaches for estimating exposure distributions. Bioinfor-mation 9(13):707-709.
Gulden, M., and H. Seibert. 2003. In vitro-in vivo extrapolation: estimation of human serum concentrations of chemicals equivalent to cytotoxic concentrations in vitro. Toxicology 189(3):211-222.
Gulden, M., P. Dierickx, and H. Seibert. 2006. Validation of a prediction model for estimating serum concentrations of chemicals which are equivalent to toxic concentrations in vitro. Toxicol In Vitro 20(7):1114-1124.
Haines, D.A., and J. Murray. 2012. Human biomonitoring of environmental chemicals – early results of the 2007-2009 Canadian Health Measures Survey for males and females. Int. J. Hyg. Environ. Health 215(2):133-137.
Hays, S.M., D.W. Pyatt, C.R. Kirman, and L.L. Aylward. 2012. Biomonitoring equivalents for benzene. Regul. Toxicol. Pharmacol. 62(1):62-73.
Hays, S.M., L.L. Aylward, and B.C. Blount. 2015. Variation in urinary flow rates according to demographic characteristics and body mass index in NHANES: Potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ. Health Perspect. 123(4):293-300.
Heather, J.M., and B. Chain. 2015. The sequence of sequencers: The history of sequencing DNA. Genomics 107(1):1-8.
Heringa, M.B., R. Schreurs, F. Busser, P.T. Van Der Saag, B. Van Der Burg, and J.L. Hermens. 2004. Toward more useful in vitro toxicity data with measured free concentrations. Environ. Sci. Technol. 38(23):6263-6270.
Hicks, K.D., A.W. Sullivan, J. Cao, E. Sluzas, M. Rebuli, and H.B. Patisaul. 2016. Interaction of bisphenol A (BPA) and soy phytoestrogens on sexually dimorphic sociosexual behaviors in male and female rats. Horm. Behav. 84:121-126.
Hinderliter, P.M., K.R. Minard, G. Orr, W.B. Chrisler, B.D. Thrall, J.G. Pounds, and J.G. Teeguarden. 2010. ISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part Fibre Toxicol. 7(1):36.
Hsiao, Y.W., U. Fagerholm, and U. Norinder. 2013. In silico categorization of in vivo intrinsic clearance using machine learning. Mol. Pharm. 10(4):1318-1321.
Hutzler, J.M., B.J. Ring, and S.R. Anderson. 2015. Low-turnover drug molecules: A current challenge for drug metabolism scientists. Drug Metab. Dispos. 43(12):1917-1928.
Isaacs, K.K., W.G. Glen, P. Egeghy, M.R. Goldsmith, L. Smith, D. Vallero, R. Brooks, C.M. Grulke, and H. Özkaynak. 2014. SHEDS-HT: An integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources. Environ. Sci. Technol. 48(21):12750-12759.
Jahnke, A., P. Mayer, M.S. McLachlan, H. Wickstrom, D. Gilbert, and M. MacLeod. 2014. Silicone passive equilibrium samplers as ‘chemometers’ in eels and sediments of a Swedish lake. Environ. Sci. Process. Impact. 16(3):464-472.
Jittikoon, J., S. Mahasirimongkol, A. Charoenyingwattana, U. Chaikledkaew, P. Tragulpiankit, S. Mangmool, W. Inunchot, C. Somboonyosdes, N. Wichukchinda, P. Sawanpanyalert, Y. He, H.L. McLeod, and W. Chantratita. 2016. Comparison of genetic variation in drug ADME-related genes in Thais with Caucasian, African and Asian Hap-Map populations. J. Hum. Genet. 61(2):119-127.
Kalemba-Drozdz, M. 2015. The interaction between air pollution and diet does not influence the DNA damage in lymphocytes of pregnant women. Environ. Res. 136:295-299.
Kesisoglou, F., B. Xia, and N.G. Agrawal. 2015. Comparison of deconvolution-based and absorption modeling IVIVC for extended release formulations of a BCS III drug development candidate. AAPS. J. 17(6):1492-1500.
Knecht, A.L., B.C. Goodale, L. Truong, M.T. Simonich, A.J. Swanson, M.M. Matzke, K.A. Anderson, K.M. Waters, and R.L. Tanguay. 2013. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol. Appl. Pharmacol. 271(2):266-275.
Koch, K., and K.L. Brouwer. 2012. A perspective on efflux transport proteins in the liver. Clin. Pharmacol. Ther. 92(5):599-612.
Koelmans, A.A., A. van der Heijde, L.M. Knijff, and R.H. Aalderink. 2001. Integrated modelling of eutrophication and organic contaminant fate & effects in aquatic ecosystems. A review. Water Res. 35(15):3517-3536.
Kolanczyk, R.C., P. Schmieder, W.J. Jones, O.G. Mekenyan, A. Chapkanov, S. Temelkov, S. Kotov, M. Velikova, V. Kamenska, K. Vasilev, and G.D. Veith. 2012. MetaPath: An electronic knowledge base for collating, exchanging and analyzing case studies of xenobiotic metabolism. Regul. Toxicol. Pharmacol. 63(1):84-96.
Kramer, N.I., F.J. Busser, M.T. Oosterwijk, K. Schirmer, B.I. Escher, and J.L. Hermens. 2010. Development of a partition-controlled dosing system for cell assays. Chem. Res. Toxicol. 23(11):1806-1814.
Kramer, N.I., M. Krismartina, A. Rico-Rico, B.J. Blaauboer, and J.L. Hermens. 2012. Quantifying processes determining the free concentration of phenanthrene in basal cytotoxicity assays. Chem. Res. Toxicol. 25(2):436-445.
Leung, L., A.S. Kalgutkar, and R.S. Obach. 2012. Metabolic activation in drug-induced liver injury. Drug Metab. Rev. 44(1):18-33.
Li, J., X. Lao, C. Zhang, L. Tian, D. Lu, and S. Xu. 2014. Increased genetic diversity of ADME genes in African Americans compared with their putative ancestral source populations and implications for pharmacogenomics. BMC Genet 15:52.
Liao, K.H., Y.M. Tan, and H.J. Clewell, III. 2007. Development of a screening approach to interpret human biomonitoring data on volatile organic compounds: Reverse dosimetry on biomonitoring data for trichloroethylene. Risk Anal. 27(5):1223-1236.
Little, J.C., C.J. Weschler, W.W. Nazaroff, Z. Liu, and E.A. Cohen Hubal. 2012. Rapid methods to estimate potential exposure to semivolatile organic compounds in the indoor environment. Environ. Sci. Technol. 46(20):11171-11178.
Liu, L.Y., A. Salamova, K. He, and R.A. Hites. 2015a. Analysis of polybrominated diphenyl ethers and emerging halogenated and organophosphate flame retardants in human hair and nails. J. Chromatogr A. 1406:251-257.
Liu, R., P. Schyman, and A. Wallqvist. 2015b. Critically assessing the predictive power of QSAR models for human liver microsomal stability. J. Chem. Inf. Model. 55(8):1566-1575.
Lorber, M., and P.P. Egeghy. 2011. Simple intake and pharmacokinetic modeling to characterize exposure of Americans to perfluoroctanoic acid, PFOA. Environ. Sci. Technol. 45(19):8006-8014.
Lovreglio, P., F. Maffei, M. Carrieri, M.N. D’Errico, I. Drago, P. Hrelia, G.B. Bartolucci and L. Soleo. 2014. Evaluation of chromosome aberration and micronucleus frequencies in blood lymphocytes of workers exposed to low concentrations of benzene. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 770:55-60.
Mackay, D., J.A. Arnot, F. Wania, and R.E. Bailey. 2011. Chemical activity as an integrating concept in environmental assessment and management of contaminants. Integr. Environ. Assess. Manag. 7(2):248-255.
MacLachlan, D.J. 2010. Physiologically based pharmacokinetic (PBPK) model for residues of lipophilic pesticides in poultry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 27(3):302-314.
Martin, S.A., E.D. McLanahan, P.J. Bushnell, E.S. Hunter, III, and H. El-Masri. 2015. Species extrapolation of life-stage physiologically-based pharmacokinetic (PBPK) models to investigate the developmental toxicology of ethanol using in vitro to in vivo (IVIVE) methods. Toxicol. Sci. 143(2):512-535.
Mastrangelo, A., A. Ferrarini, F. Rey-Stolle, A. Garcia, and C. Barbas. 2015. From sample treatment to biomarker discovery: A tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta 900:21-35.
Mattingly, C.J., T.E. McKone, M.A. Callahan, J.A. Blake, and E.A. Cohen Hubal. 2012. Providing the missing link: The exposure science ontology ExO. Environ. Sci. Technol. 46(6):3046-3053.
Mayer, H.J., M.R. Greenberg, J. Burger, M. Gochfield, C. Powers, D. Kosson, R. Keren, C. Danis, and V. Vyas. 2005. Using integrated geospatial mapping and conceptual site models to guide risk-based environmental cleanup decisions. Risk Anal. 25(2):429-446.
McGinn, S., D. Bauer, T. Brefort, L. Dong, A. El-Sagheer, A. Elsharawy, G. Evans, E. Falk-Sorqvist, M. Forster, S. Fredriksson, P. Freeman, C. Freitag, J. Fritzsche, S. Gibson, M. Gullberg, M. Gut, S. Heath, I. Heath-Brun, A.J. Heron, J. Hohlbein, R. Ke, O. Lancaster, L. Le Reste, G. Maglia, R. Marie, F. Mauger, F. Mertes, M. Mignardi, L. Moens, J. Oostmeijer, R. Out, J.N. Pedersen, F. Persson, V. Picaud, D. Rotem, N. Schracke, J. Sengenes, P.F. Stahler, B. Stade, D. Stoddart, X. Teng, C.D. Veal, N. Zahra, H. Bayley, M. Beier, T. Brown, C. Dekker, B. Ekstrom, H. Flyvbjerg, A. Franke, S. Guenther, A.N. Kapanidis, J. Kaye, A. Kristensen, H. Lehrach, J. Mangion, S. Sauer, E. Schyns, J. Tost, J.M. van Helvoort, P.J. van der Zaag, J.O. Tegenfeldt, A.J. Brookes, K. Mir, M. Nilsson, S. Will-
cocks and, and I.G. Gut. 2016. New technologies for DNA analysis — a review of the READNA Project. N. Biotechnol. 33(3):311-330.
McKone, T.E., R. Castorina, M.E. Harnly, Y. Kuwabara, B. Eskenazi, and A. Bradmanm. 2007. Merging models and biomonitoring data to characterize sources and pathways of human exposure to organophosphorus pesticides in the Salinas Valley of California. Environ. Sci. Technol. 41(9):3233-3240.
McLachlan, M.S., A. Kierkegaard, M. Radke, A. Sobek, A. Malmvärn, T. Alsberg, J.A. Arnot, T.N. Brown, F. Wania, K. Breivik, and S. Xu. 2014. Using model-based screening to help discover unknown environmental contaminants. Environ. Sci. Technol. 48(13):7264-7271.
McLanahan, E.D., H.A. El-Masri, L.M. Sweeney, L.Y. Kopylev, H.J. Clewell, J.F. Wambaugh, and P.M. Schlosser. 2012. Physiologically based pharmacokinetic model use in risk assessment-why being published is not enough. Toxicol. Sci. 126(1):5-15.
McNally, K., R. Cotton, J. Cocker, K. Jones, M. Bartels, D. Rick, P. Price, and G. Loizou. 2012. Reconstruction of exposure to m-xylene from human biomonitoring data using PBPK modelling, Bayesian inference, and Markov Chain Monte Carlo simulation. J. Toxicol. 2012:760281.
Mitchell, J., J.A. Arnot, O. Jolliet, P.G. Georgopoulos, S. Isukapalli, S. Dasgupta, M. Pandian, J. Wambaugh, P. Egeghy, E.A. Cohen Hubal, and D.A. Vallero. 2013. Comparison of modeling approaches to prioritize chemicals based on estimates of exposure and exposure potential. Sci. Total Environ. 458-460:555-567.
Moeller, B.C., L. Recio, A. Green, W. Sun, F.A. Wright, W.M. Bodnar, and J.A. Swenberg. 2013. Biomarkers of exposure and effect in human lymphoblastoid TK6 cells following [13C2]-acetaldehyde exposure. Toxicol. Sci. 133(1):1-12.
Moro, A.M., N. Brucker, M.F. Charao, E. Sauer, F. Freitas, J. Durgante, G. Bubols, S. Campanharo, R. Linden, A.P. Souza, C. Bonorino, R. Moresco, D. Pilger, A. Gioda, S. Farsky, A. Duschl, and S.C. Garcia. 2015. Early hematological and immunological alterations in gasoline station attendants exposed to benzene. Environ. Res. 137:349-356.
Mortensen, M.E., A.M. Calafat, X. Ye, L.Y. Wong, D.J. Wright, J.L. Pirkle, L.S. Merrill, and J. Moye. 2014. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children’s Study. Environ. Res. 129:32-38.
Motorykin, O., J. Schrlau, Y. Jia, B. Harper, S. Harris, A. Harding, D. Stone, M. Kile, D. Sudakin, and S.L. Massey Simonich. 2015. Determination of parent and hydroxy PAHs in personal PM2.5 and urine samples collected during Native American fish smoking activities. Sci. Total Environ. 505:694-703.
Muir, D.C., and P.H. Howard. 2006. Are there other persistent organic pollutants? A challenge for environmental chemists. Environ. Sci. Technol. 40(23):7157-7166.
Needham, L.L., D.B. Barr, and A.M. Calafat. 2005. Characterizing children’s exposures: Beyond NHANES. Neurotoxicology 26(4):547-553.
Nethery, E., G. Mallach, D. Rainham, M. Goldberg, and A. Wheeler. 2014. Using global positioning systems (GPS) and temperature data to generate time-activity classifications for estimating personal exposure in air monitoring studies: an automated method. Environ. Health 13(1):33.
NIEHS (National Institute of Environmental Health Sciences). 2016. Children’s Health Exposure Analysis Resource (CHEAR) [online]. Available: http://www.niehs.nih.gov/research/supported/exposure/chear/ [accessed July 15, 2016].
NRC (National Research Council). 2012. Exposure Science in the 21st Century: A Vision and a Strategy. Washington, DC: The National Academies Press.
NRC (National Research Council). 2014. A Framework to Guide Selection of Chemical Alternatives. Washington, DC: The National Academies Press.
NTP (National Toxicoloy Program). 2008. NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. NIH Publication No. 08-5994 [online]. Available: https://ntp.niehs.nih.gov/ntp/ohat/bisphenol/bisphenol.pdf [accessed July 15, 2016].
Obach, R.S., F. Lombardo, and N.J. Waters. 2008. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab. Dispos. 36(7):1385-1405.
O’Connell, S.G., L.D. Kind, and K.A. Anderson. 2014a. Silicone wristbands as personal passive samplers. Environ. Sci. Technol. 48(6):3327-3335.
O’Connell, S.G., M.A. McCartney, L.B. Paulik, S.E. Allan, L.G. Tidwell, G. Wilson, and K.A. Anderson. 2014b. Improvements in pollutant monitoring: Optimizing silicone for co-deployment with polyethylene passive sampling devices. Environ. Poll. 193:71-78.
O’Connell, S.G., N.I. Kerkvliet, S. Carozza, D. Rohlman, J. Pennington, and K.A. Anderson. 2015. In vivo contaminant partitioning to silicone implants: Implications for use in biomonitoring and body burden. Environ. Int. 85:182-188.
OECD (Organisation for Economic Co-operation and Development). 2007. Guidance Document on the Validation of (Quantitative) Structure-Activity Relationship [(Q)SAR] Models. Series on Testing and Assessment No. 69. Paris: OECD [online]. Available: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2007)2&doclanguage=en [accessed July 15, 2016].
Ortega, V.E., and D.A. Meyers. 2014. Pharmacogenetics: Implications of race and ethnicity on defining genetic profiles for personalized medicine. J. Allergy Clin. Immunol. 133(1):16-26.
Palmer, R.F., L. Heilbrun, D. Camann, A. Yau, S. Schultz, V. Elisco, B. Tapia, N. Garza, and C. Miller. 2015. Organic compounds detected in deciduous teeth: A replication study from children with autism in two samples. J. Environ. Public Health 2015:862414.
Park, Y.H., K. Lee, Q.A. Soltow, F.H. Strobel, K.L. Brigham, R.E. Parker, M.E. Wilson, R.L. Sutliff, K.G. Mansfield, L.M. Wachtman, T.R. Ziegler, and D.P. Jones. 2012. High-performance metabolic profiling of plasma from seven mammalian species for simultaneous environmental chemical surveillance and bioeffect monitoring. Toxicology 295(1-3):47-55.
Plowchalk, D.R., and J. Teeguarden. 2002. Development of a physiologically based pharmacokinetic model for estradiol in rats and humans: A biologically motivated quantitative framework for evaluating responses to estradiol and other endocrine-active compounds. Toxicol. Sci. 69(1):60-78.
Pontel, L.B., I.V. Rosado, G. Burgos-Barragan, J.I. Garaycoechea, R. Yu, M.J. Arends, G. Chandrasekaran, V. Broecker, W. Wei, L. Liu, J.A. Swenberg, G.P. Crossan, and K.J. Patel 2015. Endogenous formaldehyde is a hematopoietic stem cell genotoxin and metabolic carcinogen. Mol. Cell 60(1):177-188.
Pottenger, L.H., L.S. Andrews, A.N. Bachman, P.J. Boogaard, J. Cadet, M.R. Embry, P.B. Farmer, M.W. Himmelstein, A.M. Jarabek, E.A. Martin, R.J. Mauthe, R. Persaud, R.J. Preston, R. Schoeny, J. Skare, J.A. Swenberg, G.M. Williams, E. Zeiger, F. Zhang, and J.H. Kim. 2014. An organizational approach for the assessment of DNA adduct data in risk assessment: Case studies for aflatoxin B1, tamoxifen and vinyl chloride. Crit. Rev. Toxicol. 44(4):348-391.
Quinn, C.L., and F. Wania. 2012. Understanding differences in the body burden-age relationships of bioaccumulating contaminants based on population cross sections versus individuals. Environ. Health Perspect. 120(4):554-559.
Rager, J.E., M.J. Strynar, S. Liang, R.L. McMahen, A.M. Richard, C.M. Grulke, J.F. Wambaugh, K.K. Isaacs, R. Judson, A.J. Williams, and J.R. Sobus. 2016. Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environ. Int. 88:269-280.
Rappaport, S.M., D.K. Barupal, D. Wishart, P. Vineis, and A. Scalbert. 2014. The blood exposome and its role in discovering causes of disease. Environ. Health Perspect. 122(8):769-774.
Rebuli, M.E., J. Cao, E. Sluzas, K.B. Delclos, L. Camacho, S.M. Lewis, M.M. Vanlandingham, and H.B. Patisaul. 2014. Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus. Toxicol. Sci. 140(1):190-203.
Reichenberg, F., and P. Mayer. 2006. Two complementary sides of bioavailability: Accessibility and chemical activity of organic contaminants in sediments and soils. Environ. Toxicol. Chem. 25(5):1239-1245.
Regens, J.L., K.R. Obenshain, C.T. Quest, and C. Whipple. 2002. Conceptual site models and multimedia modeling: Comparing MEPAS, MMSOILS, and RESRAD. Hum. Ecol. Risk Asses. 8(2):391-403.
Ritter, R., M. Scheringer, M. MacLeod, C. Moeckel, K.C. Jones, and K. Hungerbühler. 2011. Intrinsic human elimination half-lives of polychlorinated biphenyls derived from the temporal evolution of cross-sectional biomonitoring data from the United Kingdom. Environ. Health Perspect. 119(2):225-231.
Roede, J.R., K. Uppal, Y. Liang, D.E. Promislow, L.M. Wachtman, and D.P. Jones. 2013. Characterization of plasma thiol redox potential in a common marmoset model of aging. Redox Biol. 1:387-393.
Rostami-Hodjegan, A. 2012. Physiologically based pharmacokinetics joined with in vitro-in vivo extrapolation of ADME: A marriage under the arch of systems pharmacology. Clin. Pharmacol. Ther. 92(1):50-61.
Rotroff, D.M., B.A. Wetmore, D.J. Dix, S.S. Ferguson, H.J. Clewell, K.A. Houck, E.L. Lecluyse, M.E. Andersen, R.S. Judson, C.M. Smith, M.A. Sochaski, R.J. Kavlock, F. Boellmann, M.T. Martin, D.M. Reif, J.F. Wambaugh, and R.S. Thomas. 2010. Incorporating human dosimetry and exposure into high-throughput in vitro toxicity screening. Toxicol. Sci. 117(2):348-358.
Rowbotham, A.L., and R.M. Gibson. 2011. Exposure-driven risk assessment: Applying exposure-based waiving of toxicity tests under REACH. Food Chem. Toxicol. 49(8):1661-1673.
Rule, A.D., H.M. Gussak, G.R. Pond, E.J. Bergstralh, M.D. Stegall, F.G. Cosio, and T.S. Larson. 2004. Measured and estimated GFR in healthy potential kidney donors. Am. J. Kidney Dis. 43(1):112-119.
Sadler, N.C., and A.T. Wright. 2015. Activity-based protein profiling of microbes. Curr. Opin. Chem. Biol. 24:139-144.
Sadler, N.C., P. Nandhikonda, B.J. Webb-Robertson, C. An-song, L.N. Anderson, J.N. Smith, R.A. Corley and A.T. Wright. 2016. Hepatic cytochrome P450 activity, abundance, and expression throughout human development. Drug Metabol. Dispos. 44(7):984-991.
Sayes, C.M., K.L. Reed, and D.B. Warheit. 2007. Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci. 97(1):163-180.
Schenker, U., M. Scheringer, M.D. Sohn, R.L. Maddalena, T.E. McKone, and K. Hungerbühler. 2009. Using information on uncertainty to improve environmental fate modeling: A case study on DDT. Environ. Sci. Technol. 43(1):128-134.
Sedykh, A., D. Fourches, J. Duan, O. Hucke, M. Garneau, H. Zhu, P. Bonneau, and A. Tropsha. 2013. Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm. Res. 30(4):996-1007.
Seltenrich, N. 2014. Remote-sensing applications for environmental health research. Environ. Health Perspect. 122(10):A268-A275.
Shankaran, H., and J. Teeguarden. 2014. Improving urine-based human exposure assessment of short-lived chemicals using reverse dosimetry and nhanes physiological and behavior data: A value-of-information approach for bisphenol A. Toxicologist CD138(1).
Shin, H.M.., T.E. McKone, and D.H. Bennett. 2012. Intake fraction for the indoor environment: A tool for prioritizing indoor chemical sources. Environ. Sci. Technol. 46(18):10063-10072.
Shin, H.M., T.E. McKone, and D.H. Bennett. 2014. Attributing population-scale human exposure to various source categories: Merging exposure models and biomonitoring data. Environ. Int. 70:183-191.
Shin, H.M., A. Ernstoff, J.A. Arnot, B.A. Wetmore, S.A. Csiszar, P. Fantke, X. Zhang, T.E. McKone, O. Jolliet, and D.H. Bennett. 2015. Risk-based high-throughput chemical screening and prioritization using exposure models and in vitro bioactivity assays. Environ. Sci. Technol. 49(11):6760-6771.
Snoeys, J., M. Beumont, M. Monshouwer, and S. Ouwerkerk-Mahadevan. 2016. A mechanistic understanding of the non-linear pharmacokinetics and inter-subject variability of simeprevir: A PBPK-guided drug development approach. Clin. Pharmacol. Ther. 99(2):224-234.
Sonne, C., K. Gustavson, R.J. Letcher, and R. Dietz. 2015. Physiologically-based pharmacokinetic modelling of distribution, bioaccumulation and excretion of POPs in Greenland sledge dogs (Canis familiaris). Environ. Res. 142:380-386.
Stapleton, H.M., J.G. Allen, S.M. Kelly, A. Konstantinov, S. Klosterhaus, D. Watkins, M.D. McClean and T.F. Webster. 2008. Alternate and new brominated flame retardants detected in US house dust. Environ. Sci. Technol. 42(18):6910-6916.
Su, J.G., M. Jerrett, Y.Y. Meng, M. Pickett, and B. Ritz. 2015. Integrating smart-phone based momentary location tracking with fixed site air quality monitoring for personal exposure assessment. Sci. Total Environ. 506:518-526.
Sud, M., E. Fahy, D. Cotter, K. Azam, I. Vadivelu, C. Burant, A. Edison, O. Fiehn, R. Higashi, K.S. Nair, S. Sumner, and S. Subramaniam. 2016. Metabolomics Workbench: An international repository for metabolomics data and metadata, metabolite standards, protocols, tutorials and training, and analysis tools. Nucleic Acids Res. 44(D):D463-D470.
Sumner, L.W., Z.T. Lei, B.J. Nikolau, K. Saito, U. Roessner, and R. Trengove. 2014. Proposed quantitative and alphanumeric metabolite identification metrics. Metabolomics 10(6):1047-1049.
Swenberg, J.A., G. Boysen, N. Georgieva, M.G. Bird, and R.J. Lewis. 2007. Future directions in butadiene risk assessment and the role of cross-species internal dosimetry. Chem. Biol. Interact. 166(1-3):78-83.
Swenberg, J.A., E. Fryar-Tita, Y.C. Jeong, G. Boysen, T. Starr, V.E. Walker, and R.J. Albertini. 2008. Biomarkers in toxicology and risk assessment: Informing critical dose-response relationships. Chem. Res. Toxicol. 21(1):253-265.
Tan, Y.M., K.H. Liao, R.B. Conolly, B.C. Blount, A.M. Mason, and H.J. Clewell. 2006. Use of a physiologically based pharmacokinetic model to identify exposures consistent with human biomonitoring data for chloroform. J. Toxicol. Environ. Health A 69(18):1727-1756.
Tan, Y.M., K.H. Liao, and H.J. Clewell, III. 2007. Reverse dosimetry: Interpreting trihalomethanes biomonitoring data using physiologically based pharmacokinetic modeling. J. Expo. Sci. Environ. Epidemiol. 17(7):591-603.
Tan, Y.M., J. Sobus, D. Chang, R. Tornero-Velez, M. Goldsmith, J. Pleil, and C. Dary. 2012. Reconstructing human exposures using biomarkers and other clues. J. Toxicol. Environ. Health B Crit. Rev. 15(1):22-38.
Teeguarden, J.G., and H.A. Barton. 2004. Computational modeling of serum-binding proteins and clearance in extrapolations across life stages and species for endocrine active compounds. Risk Anal. 24(3):751-770.
Teeguarden, J.G., J.M. Waechter, H.J. Clewell, T.R. Covington, and H.A. Barton. 2005. Evaluation of oral and intravenous route pharmacokinetics, plasma protein binding, and uterine tissue dose metrics of bisphenol A: A physiologically based pharmacokinetic approach. Toxicol. Sci. 85(2):823-838.
Teeguarden, J.G., P.M. Hinderliter, G. Orr, B.D. Thrall, and J.G. Pounds. 2007. Particokinetics in vitro: Dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol. Sci. 95(2):300-312.
Teeguarden, J.G., M.S. Bogdanffy, T.R. Covington, C. Tan, and A.M. Jarabek. 2008. A PBPK model for evaluating the impact of aldehyde dehydrogenase polymorphisms on comparative rat and human nasal tissue acetaldehyde dosimetry. Inhal. Toxicol. 20(4):375-390.
Teeguarden, J.G., A.M. Calafat, X. Ye, D.R. Doerge, M.I. Churchwell, R. Gunawan, and M.K. Graham. 2011. Twenty-four hour human urine and serum profiles of Bisphenol A during high-dietary exposure. Toxicol. Sci. 123(1):48-57.
Teeguarden, J.G., S. Hanson-Drury, J.W. Fisher, and D.R. Doerge. 2013. Are typical human serum BPA concentrations measurable and sufficient to be estrogenic in the general population? Food Chem. Toxicol. 62:949-963.
Teeguarden, J.G., V.B. Mikheev, K.R. Minard, W.C. Forsythe, W. Wang, G. Sharma, N. Karin, S.C. Tilton, K.M.
Waters, B. Asgharian, O.R. Price, J.G. Pounds, and B.D. Thrall. 2014. Comparative iron oxide nanoparticle cellular dosimetry and response in mice by the inhalation and liquid cell culture exposure routes. Part. Fibre Toxicol. 11:46.
Teeguarden, J.G., N. Twaddle, M.I. Churchwell, X. Yang, J.W. Fisher, L.M. Seryak, and D.R. Doerge. 2015. 24-hour human urine and serum profiles of bisphenol A: Evidence against sublingual absorption following ingestion in soup. Toxicol. Appl. Pharmacol. 288(2):131-142.
Teeguarden, J.G., C. Tan, S. Edwards, J.A. Leonard, K.A. Anderson, R.A. Corley, M.L. Kile, S.M Simonich, D. Stone, K.M. Waters, S. Harper, and D.E. Williams. 2016. Completing the link between exposure science and toxicology for improved environmental health decision making: The aggregate exposure pathway framework. Environ. Sci. Technol. 50(9):4579-4586.
Thayer, K.A., D.R. Doerge, D. Hunt, S.H. Schurman, N.C. Twaddle, M.I. Churchwell, S. Garantziotis, G.E. Kissling, M.R. Easterling, J.R. Bucher, and L.S. Birnbaum. 2015. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 83:107-115.
Tolonen, A., and O. Pelkonen. 2015. Analytical challenges for conducting rapid metabolism characterization for QIVIVE. Toxicology 332:20-29.
Tonnelier, A., S. Coecke, and J.M. Zaldíva. 2012. Screening of chemicals for human bioaccumulative potential with a physiologically based toxicokinetic model. Arch. Toxicol. 86(3):393-403.
Tumer, T.B., S. Savranoglu, P. Atmaca, G. Terzioglu, A. Sen, and S. Arslan. 2016. Modulatory role of GSTM1 null genotype on the frequency of micronuclei in pesticide-exposed agricultural workers. Toxicol. Ind. Health. 32(12):1942-1951.
Uppal, K., D.I. Walker, K. Liu, S. Li, Y.M. Go, and D.P. Jones. 2016. Computational metabolomics: A framework for the million metabolome. Chem. Res. Toxicol. 29(12):1956-1975.
van Donkelaar, A., R.V. Martin, M. Brauer, and B.L. Boys. 2015. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ. Health Perspect. 123(2):135-143.
Vermeire, T., M. van de Bovenkamp, Y.B. de Bruin, C. Delmaar, J. van Engelen, S. Escher, H. Marquart and T. Meijster. 2010. Exposure-based waiving under REACH. Regul. Toxicol. Pharmacol. 58(3):408-420.
Verner, M.A., A.E. Loccisano, N.H. Morken, M. Yoon, H. Wu, R. McDougall, M. Maisonet, M. Marcus, R. Kishi, C. Miyashita, M.H. Chen, W.S. Hsieh, M.E. Andersen, H.J. Clewell, III, and M.P. Longnecker. 2015. Associations of perfluoroalkyl substances (PFASs) with lower birth weight: An evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK). Environ. Health Perspect. 123(12):1317-1324.
Vineis, P., K. van Veldhoven, M. Chadeau-Hyam, and T.J. Athersuch. 2013. Advancing the application of omicsbased biomarkers in environmental epidemiology. Environ. Mol. Mutagen. 54(7):461-467.
Vrijheid, M., R. Slama, O. Robinson, L. Chatzi, M. Coen, P. van den Hazel, C. Thomsen, J. Wright, T.J. Athersuch, N. Avellana, X. Basagaña, C. Brochot, L. Bucchini, M. Bustamante, A. Carracedo, M. Casas, X. Estivill, L. Fairley, D. van Gent, J.R. Gonzalez, B. Granum, R. Gražulevičienė, K.B. Gutzkow, J. Julvez, H.C. Keun, M. Kogevinas, R.R.C. McEachan, H.M. Meltzer, E. Sabidó, P.E. Schwarze, V. Siroux, J. Sunyer, E.J. Want, F. Zeman, and M.J. Nieuwenhuijsen. 2014. The human early-life exposome (HELIX): Project rationale and design. Environ. Health Perspect. 122(6):535-544.
Wambaugh, J.F., R.W. Setzer, D.M. Reif, S. Gangwal, J. Mitchell-Blackwood, J.A. Arnot, O. Joliet, A. Frame, J. Rabinowitz, T.B. Knudsen, R.S. Judson, P. Egeghy, D. Vallero, and E.A. Cohen Hubal. 2013. High-throughput models for exposure-based chemical prioritization in the ExpoCast Project. Environ. Sci. Technol. 47(15):8479-8488.
Wambaugh, J.F., A. Wang, K.L. Dionisio, A. Frame, P. Egeghy, R. Judson, and R.W. Setzer. 2014. High throughput heuristics for prioritizing human exposure to environmental chemicals. Environ. Sci. Technol. 48(21):12760-12767.
Wania, F., and D. Mackay. 1999. The evolution of mass balance models of persistent organic pollutant fate in the environment. Environ. Pollut. 100(1-3):223-240.
Weaver, V.M., D.J. Kotchmar, J.J. Fadrowski, and E.K. Silbergeld. 2016. Challenges for environmental epidemiology research: Are biomarker concentrations altered by kidney function or urine concentration adjustment? J. Expo. Sci. Environ. Epidemiol. 26(1):1-8.
Webster, E.M., H. Oian, D. Mackay, R.D. Christensen, B. Tietjen, and R. Zaleski. 2016. Modeling human exposure to indoor contaminants: External source to body tissues. Environ. Sci. Technol. 50(16):8697-8704.
Weijs, L., A. Covaci, R.S. Yang, K. Das, and R. Blust. 2012. Computational toxicology: Physiologically based pharmacokinetic models (PBPK) for lifetime exposure and bioaccumulation of polybrominated diphenyl ethers (PBDEs) in marine mammals. Environ. Pollut. 163:134-141.
Weschler, C.J., and W.W. Nazaroff. 2010. SVOC partitioning between the gas phase and settled dust indoors. Atmos. Environ. 44(30):3609-3620.
Weschler, C.J., and W.W. Nazaroff. 2012. SVOC exposure indoors: Fresh look at dermal pathways. Indoor Air 22(5):356-377.
Wetmore, B.A., J.F. Wambaugh, S.S. Ferguson, M.A. Sochaski, D.M. Rotroff, K. Freeman, H.J. Clewell, III, D.J. Dix, M.E. Andersen, K.A. Houck, B. Allen, R.S. Judson, R. Singh, R.J. Kavlock, A.M. Richard, and R.S. Thom-
as. 2012. Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol. Sci. 125(1):157-174.
Wetmore, B.A., J.F. Wambaugh, S.S. Ferguson, L. Li, H.J. Clewell, III, R.S. Judson, K. Freeman, W. Bao, M.A. Sochaski, T.M. Chu, M.B. Black, E. Healy, B. Allen, M.E. Andersen, R.D. Wolfinger, and R.S. Thomas. 2013. Relative impact of incorporating pharmacokinetics on predicting in vivo hazard and mode of action from high-throughput in vitro toxicity assays. Toxicol. Sci. 132(2):327-346.
Wetmore, B.A., B. Allen, H.J. Clewell, III, T. Parker, J.F. Wambaugh, L.M. Almond, M.A. Sochaski, and R.S. Thomas. 2014. Incorporating population variability and susceptible subpopulations into dosimetry for high-throughput toxicity testing. Toxicol. Sci. 142(1):210-224.
Wetzel, T.A., and W.J. Doucette. 2015. Plant leaves as indoor air passive samplers for volatile organic compounds (VOCs). Chemosphere 122:32-37.
WHO (World Health Organization). 2005. Principles of Characterizing and Applying Human Exposure Models. Harmonization Project Document No. 3. Geneva: WHO [online]. Available: http://apps.who.int/iris/bitstream/10665/43370/1/9241563117_eng.pdf [accessed October 24, 2016].
WHO (World Health Organization). 2016. Exposure Assessment. International Programme on Chemical Safety [online]. Available: http://www.who.int/ipcs/methods/harmonization/areas/exposure/en/ [accessed October 24, 2016].
Wild, C.P. 2005. Complementing the genome with an exposome: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomarkers Prev. 14(8):1847-1850.
Wild, C.P. 2012. The exposome: From concept to utility. Int. J. Epidemiol. 41(1):24-32.
Wilk-Zasadna, I., C. Bernasconi, O. Pelkonen, and S. Coecke. 2015. Biotransformation in vitro: An essential consideration in the quantitative in vitro-to-in vivo extrapolation (QIVIVE) of toxicity data. Toxicology 332:8-19.
Wishart, D.S., C. Knox, A.C. Guo, D. Cheng, S. Shrivastava, D. Tzur, B. Gautam, and M. Hassanali. 2008. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 36:D901-D906.
Wu, H., M. Yoon, M.A. Verner, J. Xue, M. Luo, M.E. Andersen, M.P. Longnecker, and H.J. Clewell, III. 2015. Can the observed association between serum perfluoroalkyl substances and delayed menarche be explained on the basis of puberty-related changes in physiology and pharmacokinetics? Environ. Int. 82:61-68.
Wu, J., C. Jiang, D. Houston, D. Baker, and R. Delfino. 2011. Automated time activity classification based on global positioning system (GPS) tracking data. Environ. Health 10:101.
Xie, H. 2008. Activity assay of membrane transport proteins. Acta. Biochim. Biophys. Sin. 40(4):269-77.
Yang, Y., Y.M. Tan, B. Blount, C. Murray, S. Egan, M. Bolger, and H. Clewell. 2012. Using a physiologically based pharmacokinetic model to link urinary biomarker concentrations to dietary exposure of perchlorate. Chemosphere 88(8):1019-1027.
Yeo, K.R., J.R. Kenny, and A. Rostami-Hodjegan. 2013. Application of in vitro-in vivo extrapolation (IVIVE) and physiologically based pharmacokinetic (PBPK) modelling to investigate the impact of the CYP2C8 polymorphism on rosiglitazone exposure. Eur. J. Clin. Pharmacol. 69(6):1311-1320.
Yoon, M., J.L. Campbell, M.E. Andersen, and H.J. Clewell. 2012. Quantitative in vitro to in vivo extrapolation of cell-based toxicity assay results. Crit. Rev. Toxicol. 42(8):633-652.
Yoon, M., A. Efremenko, B.J. Blaauboer, and H.J. Clewell. 2014. Evaluation of simple in vitro to in vivo extrapolation approaches for environmental compounds. Toxicol. In Vitro 28(2):164-170.
Young, B.M., N.S. Tulve, P.P. Egeghy, J.H. Driver, V.G. Zartarian, J.E. Johnston, C.J. Delmaar, J.J. Evans, L.A. Smith, G. Glen, C. Lunchick, J.H. Ross, J. Xue, and D.E. Barnekow. 2012. Comparison of four probabilistic models (CARES(®),Calendex™, ConsExpo, and SHEDS) to estimate aggregate residential exposures to pesticides. J. Expo. Sci. Environ. Epidemiol. 22(5):522-532.
Young, J.F., R.H. Luecke, and D.R. Doerge. 2007. Physiologically based pharmacokinetic/pharmacodynamic model for acrylamide and its metabolites in mice, rats, and humans. Chem. Res. Toxicol. 20(3):388-399.
Yu, R., Y. Lai, H.J. Hartwell, B.C. Moeller, M. Doyle-Eisele, D. Kracko, W.M. Bodnar, T.B. Starr, and J.A. Swenberg. 2015. Formation, accumulation, and hydrolysis of endogenous and exogenous formaldehyde-induced DNA damage. Toxicol. Sci. 146(1):170-182.
Zartarian, V., T. Bahadori, and T. McKone. 2005. Adoption of an official ISEA glossary. J. Expo. Anal. Environ. Epidemiol. 15(1):1-5.
Zhang, D., G. Luo, X. Ding, and C. Lu. 2012. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm. Sinic. B. 2(6):549-561.
Zhang, X., J.A. Arnot, and F. Wania. 2014. Model for screening-level assessment of near-field human exposure to neutral organic chemicals released indoors. Environ. Sci. Technol. 48(20):12312-12319.

































