Poor understanding of disease biology remains the most critical gap hindering the development of new central nervous system (CNS) drugs, said David Michelson. Moreover, even if a target is identified, it may be inaccessible or difficult to move the biology in a way that will be therapeutic. These problems are especially severe for CNS disorders, said Michelson. Consequently, the success rates for development of CNS drugs are among the lowest of all therapeutic areas (TCSDD, 2014). Michelson alluded to the fact that the situation may even be worse than those numbers indicate, given that many of the approved drugs are merely iterative, leading to a false sense of success. To serve patients well and increase the flow of drugs needed to treat the hundreds of millions of people with CNS disorders such as depression, schizophrenia, and Alzheimer’s disease (AD), more efficient discovery and development methods are required, he added. Going back to Michelson’s earlier point, Stevin Zorn summed it up by saying: “We need to find a way to reduce the burden of failure and increase the probability of success.”
CROSSING THE THRESHOLD FOR INVESTMENT
The good news, according to Kim Andersen, senior vice president and head of research at H. Lundbeck A/S, is that industry has demonstrated an interest in investing in neuroscience. Moreover, the substantial investments needed for target discovery and validation are spread across public (e.g., National Institutes of Health), industry, and private funders, lowering the risk to any one entity, said Rita Balice-Gordon. A major inflection point in the drug discovery process comes after safety has been established at the transition from Phase I to Phase II studies, she said. At this point, pharmaceutical companies must decide whether investing substantially more dollars will likely pay off in Phase III and beyond. This,
said Balice-Gordon, requires a compelling package of preclinical and clinical data that senior management weights as priorities are set and decisions are made about which programs to pursue.
She shared two examples of drug discovery programs that illustrate some of the pitfalls in the transition from Phase I to Phase II. One program was for a drug to treat peripheral neuropathy. The target had been genetically validated and preclinical work and natural history studies supported the rationale that regulating this target could impact the disease. However, there was no predictive preclinical model to study efficacy, and no predictive biomarkers of disease progression or efficacy. This created significant risk when considering advancing the project to clinical development. The second example was a clinical trial designed to connect circuit function to domains of dysfunction. Balice-Gordon and colleagues designed a discovery human neuroscience study with multiple endpoints across several modalities, and a parallel study with a clinical functional rating scale as an endpoint. If the clinical endpoint had indicated a positive effect, a strategic decision would have been made to accelerate that program and stop the discovery-based work, even though it would have revealed interesting insights about disease pathophysiology.
Balice-Gordon said these programs illustrate several points: first, that the significance of even a robust clinical package may be insufficient if biomarkers and sensitive outcome measures are not available; and second, that industry must conduct trials that are relatively fast and cheap. Industry, she said, is not the right arena for innovation in clinical trial design, endpoint validation, or execution.
Andersen said that although industry has demonstrated an interest in investing in neuroscience, the threshold for investment in any disease area varies among companies. No clear matrix exists to determine whether investments are warranted, he said. Everything is about judgment and developing a deep understanding of the assets available before making an investment. He cited four criteria that should be fulfilled before making an investment: (1) a very strong biological hypothesis, (2) evidence that the identified mechanism can be manipulated, (3) evidence that target engagement or proof of mechanism can be demonstrated (in humans), and (4) feasibility of proof of concept, that is, evidence that an effect can be seen that is clinically relevant. Bill Martin, head of research and development at BlackThorn Therapeutics, Inc., added that in addition to scientific thresholds, certain clinical and regulatory thresholds must be met: Can a hypothesis be tested in a human subject model or a
targeted clinical population, and is the unmet medical need sufficiently high to justify following a path without regulatory precedent?
Testing hypotheses and demonstrating target engagement and proof of mechanism will require more basic research as well as improved tools for studying humans, said Andersen, noting that the models discussed earlier have facilitated innovation at the preclinical level. For example, iPS cells enable both hypothesis building as well as assessment of safety and toxicology, and offer the potential to address disease heterogeneity, which has proved to be a major hurdle in drug development for nervous system disorders. Nonhuman disease models remain essential for translation, and the nonhuman primates discussed earlier, coupled with novel methods for inducing relevant phenotypes (e.g., Clustered Regularly Interspaced Short Palindromic Repeats [CRISPR] technology), appear particularly promising, said Andersen. Applying -omics technologies—as well as functional genetics, epigenetics, and single-cell sequencing methods—and using advanced bioinformatics approaches further increase the value of these models, he said.
Andersen called for closer collaboration between clinicians and model developers to ensure that phenotypes can be translated from nonhuman models to human systems. This includes aligning endophenotypes that are not directly behavioral, such as electrophysiological responses, with clinical observations. For example, tools for assessing the inflammatory state in the human brain are limited, which makes it difficult to test hypotheses for some diseases such as depression. Andersen said basic research is also needed to better understand how to deliver antibodies and other drug substances into the brain. In addition, updated clinical scales and novel remote assessment tools are needed to test compounds in human subjects.
Frank Yocca added that companies should integrate discovery and biomarker research. He said that discovery always outpaces the biomarker development. Consequently, depending on metrics set by a company, development often proceeds without fully understanding target engagement, dose, or patient selection parameters.
BALANCING INNOVATION AND INVESTMENT AT BIOTECHNOLOGY COMPANIES
Small biotech companies have a track record of pursuing innovative approaches in drug development, including using human models to
evaluate therapies intended for human disorders, according to Martin. This approach follows a path taken more than 50 years ago, when imipramine, the first tricyclic antidepressant, was developed in the absence of a known mechanism of action or any kind of predictive model. Rather, over the course of 3 years, the compound was evaluated in more than 500 people with depression, providing a comprehensive description of the onset of action across different patient populations, diurnal effects, safety, and tolerability, in comparison to the standard of care, electroshock therapy. This study highlights the power of testing agents in humans as soon as reasonably safe and possible, said Martin.
Unfortunately, this serendipitous success has not been replicated in recent years, he noted. In 2010, pharmaceutical companies were developing more than 300 medicines to treat mental illness (PhRMA, 2010), but this number dropped to only about 100 in 2014 (PhRMA, 2014). Martin drew a sports analogy to explain the problem: while drug discovery engines in many companies generated numerous molecules, the process was more akin to “playing darts blindfolded” rather than producing many “shots on goal.”
Martin underscored the importance of risk versus cost trade-offs when considering whether to invest in a particular clinical development program. As illustrated in Figure 5-1, drug developers, especially those working in the CNS space, must ask whether proceeding through the various stages of development can be accomplished within a time frame that, if approved, will allow the molecule to be launched and commercialized before a loss of patent exclusivity. First, this requires answering questions about whether the compound will work, which often leads to a convergence around the same set of molecular targets as companies try to mitigate target validation risk, said Martin. Second, developers must ask whether the compound is sufficiently different from existing treatments to warrant the investment. Finally, they must determine whether there is a predictable path to approval. In answering this last question, companies may be faced with a choice of either following a regulatory precedent or investing in novel endpoints.
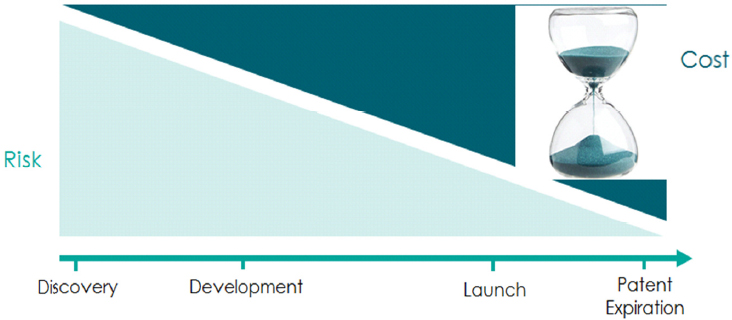
SOURCE: Presentation by Martin, 2016.
Martin provided a few examples of this approach, which have been carried out by smaller biotech companies. For example, Autifony Therapeutics, a U.K.-based company, tested the hypothesis that a certain type of interneuron is dysfunctional in schizophrenia by using a ketamine challenge model (Lewis et al., 2012). By inducing schizophrenia-like symptoms with ketamine, they were able to identify quickly whether particular modulators worked against the dysregulated circuit.
Sage Therapeutics also applied the “learn and confirm” paradigm to study multiple indications for a single molecule that had been shown efficacious in patients with refractory epilepsy. A positive signal from an exploratory study of four patients with postpartum depression led the company to conduct a 21-patient, placebo-controlled Phase II study conducted within regulatory guidelines with a widely used endpoint (the Hamilton Depression Scale). Approximately 15 months later, the results of this study resulted in “breakthrough therapy” designation from the Food and Drug Administration (FDA).
Martin said the learn and confirm approach can also be applied across multiple companies that have converged on a common target, which he said is critical because innovation takes place both in small pharmaceutical and biotechnology companies as well as in large pharmaceutical companies. In recent years, he said, companies have demonstrated a willingness and commitment to continue the cycle of continuous learning across companies. For example, there are more than 1,000 com-
pounds in various stages of discovery and development that target mGluR5 for the treatment of Fragile X syndrome. Seaside Therapeutics, a small biotech company, conducted a very small, 2-week duration trial of one mGluR5 antagonist, while Novartis conducted a larger, 12-week duration study of a similar compound. Although both studies failed and the Novartis study did not achieve its primary endpoints, the company published its data in an article exploring the reasons for the negative results (Berry-Kravis et al., 2016).
Sharing and combining data from multiple studies not only enables the field to learn from successes and failures, but also to identify novel hypotheses, said Martin. For example, he cited a meta-analysis of neuroimaging data from more than 193 studies in nearly 16,000 people with an array of psychiatric diagnoses, including schizophrenia, bipolar disorder, depression, addiction, obsessive-compulsive disorder, and anxiety (Goodkind et al., 2015). This study demonstrated loss of gray matter in three regions of the brain across diagnoses, highlighting the importance of these shared endophenotypes across psychopathology, not explicitly defined by current nosology.
In a later presentation, Doug Cole noted that small companies often do not have access to resources such as clinically annotated tissue, which could help better elucidate disease course and natural history. He suggested that a national repository of clinically annotated tissue, for example, could be enormously enabling for small companies. Cole also agreed that validation must come from patients and humans rather than by trying to recapitulate disease in animal models. Human models are essential to define responder populations of patients, he said. For example, a disease such as AD will inevitably turn out not to be a single disease of 5 million Americans, but many diseases in smaller subgroups, noted Cole.
However, Hyman argued that it would be a mistake to do away with living system models, although they should not be the only prerequisite for going forward. Delivering therapy without understanding the biology, he said, would represent a failure to learn from 60 years of failure in the CNS area. Cole agreed, commenting that the field is at the cusp of being able to engineer animal models in ways that will make them much more useful. Similarly, said Hyman, moving forward without understanding the natural history of a disease also lowers the likelihood of success.
A VENTURE CAPITALIST PERSPECTIVE
Cole said the approach that venture capitalists take is somewhat different from industry. When considering whether to start a new company, investors consider potential for impact, potential to create value, and risks. Scientific success, not market size, should be the key drivers, stated Cole. He added that a new idea in neuroscience is judged against many good ideas outside the field, with the most important question being not whether one has the best idea for tackling a certain disease, but the best chance to succeed.
Cole commented that some of the most innovative ideas are ones that seem least plausible or that approach diseases of the nervous system from an orthogonal perspective. Although the desire to pursue new ideas is understandable, he spoke of the need to maintain the discipline of focusing on mechanistically well-characterized diseases. He added that the odds of success are so low for less well-understood diseases that novelity by itself does not justify allocation of resources. He suggested that while these kinds of ideas may not be ready for venture capitalist investment, it might be worthwhile for funding agencies to test such ideas.
As an example of an area where more basic research can be useful, Cole mentioned that the blood‒brain barrier presents a major roadblock for CNS therapeutics, largely confining drug developers to small molecules as therapeutics. Breaching the blood‒brain barrier, he argued, could enable the use of a wider range of approaches, including antibodies, RNA-based technologies, CRISPR-based technologies, and gene therapy. Cole also noted that moving forward, gene editing, induced pluripotent stem (iPS) cells, organoids, and noninvasive functional monitoring are all technologies that could substantially benefit from additional investment.
PUBLIC‒PRIVATE PARTNERSHIPS AND OTHER PRECOMPETITIVE CONSORTIA
While better tools are needed for dose selection, signal detection, target selection, and validation, John Michael Sauer, executive director of the Critical Path Institute’s (C-Path’s) Predictive Safety Testing Consortium (PSTC), noted that individual companies often do not have the resources to invest in developing these tools. This is where public‒
private partnerships, such as C-Path,1 could play an important role in bringing stakeholders together to share risks and costs. Sauer added that regulatory acceptance of the utility of these tools is essential, noting that C-Path was created in response to the FDA’s Critical Path Initiative,2 and has a Memorandum of Understanding with the FDA that allows them to create a precompetitive neutral ground in which multiple stakeholders can work. For example, PSTC has worked with multiple stakeholders to gather and analyze data and submit qualification dossiers to regulatory agencies for several biomarkers of tissue injury. Sauer said the work conducted by contract research organizations to validate methods should also be integrated into these efforts.
Workshop participants described several other collaborations that have helped to advance drug development for nervous system disorders:
- Sarah Caddick, neuroscience advisor to Lord Sainsbury of Turville at the Gatsby Charitable Foundation, mentioned an example where a nonprofit, disease-focused foundation (The ALS Association) funded a study in partnership with a pharmaceutical company (Ionis Pharmaceuticals) to test whether antisense oligonucleotides can be delivered to motor neurons to prevent the production of toxic proteins. The work completed by this partnership in precompetitive space has since enabled the advancement of antisense oligonucleotide treatments to clinical trials for spinal muscular atrophy, amyotrophic lateral sclerosis, and Huntington’s disease. Balice-Gordon lauded this partnership as a great example of the community bringing resources together to answer key questions required to go from the preclinical stage to the clinic while sufficiently de-risking subsequent investment.
- Yocca presented a different model for drug development, which he has helped to establish in Virginia. The Virginia Bioscience Health Research Corporation is a nonprofit corporation funded by the state as well as by six of Virginia’s research universities; it acts as a catalyst by funding collaborative projects between academia and industry. With an initial focus on neuroscience, it seeks to support the development, validation, and application of new approaches to diagnosing, preventing, and treating neuro-
___________________
1 For more information, go to https://c-path.org (accessed November 18, 2016).
2 For more information, go to http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/ucm076689.htm (accessed November 18, 2016).
-
logic diseases by requiring academic and industry groups to work together to accelerate development and commercialization.
- Jonathan Cohen, co-director of the Princeton Neuroscience Institute, mentioned a complementary model of collaboration between Princeton and Intel that aims to create brain images that correlate every voxel (the three-dimensional elements composing a brain scan) to every other voxel by exploiting the technological capabilities of a big industrial company with the academic expertise at Princeton. Project teams are evenly split between the two institutions. They meet once per week, and hire graduate students and postdoctoral fellows together.
- Yet another effort to de-risk drug development was mentioned by Susan Amara, scientific director at the National Institute of Mental Health (NIMH). She said they have created a translational neuropharmacology task force of individuals from industry, academia, and National Institutes of Health institutes to vet compounds, drugs, and targets with the goal of identifying those that seem most promising, and then matching academic and industry partners interested in pursuing them.
- A final collaborative model for studying neurologic diseases was mentioned by Stuart Hoffman. For some conditions where an adequate animal model is not available, it may be possible to model human populations using longitudinal data. For example, the Chronic Effects of Neurotrauma Consortium3 has been established to follow a population of concussed athletes for many years as a means of better understanding traumatic brain injury.
Data sharing is critical for creating new tools and enabling them to become qualified or endorsed by regulatory agencies; for improved decision making; and for ensuring that lessons are learned from failed studies, said Sauer. These shared data themselves may also become the raw material for the creation of modeling and simulation tools, such as one that was created by C-Path for use in drug development for AD. The tool used de-identified, standardized, and compiled clinical trial data from nine companies, with 6,500 patients in 24 clinical trials. The model has been qualified by the European Medicines Agency and endorsed by the FDA for the purpose of simulating clinical trials to test different trial design parameters.
___________________
3 For more information, go to https://cenc.rti.org (accessed November 18, 2016).
Sharing negative data from clinical trials may be equally important as a means of making more informed investment decisions, added William Potter, senior advisor to the NIMH director. The tools exist to do this, he said, but the question remains whether companies are willing to expand data and information sharing to ensure that developers are aware of toxicity issues observed in one trial, for example, before launching a subsequent trial of a similar compound.
INCREASING THE PROBABILITY OF SUCCESS FOR DRUG DEVELOPMENT
Martin listed five policy considerations that could increase the probability of success, reduce development costs, and increase pay-off and predictability. Commitments to these five considerations are needed from industry, academia, and public‒private partnerships, he said:
- Create risk-sharing models for target validation.
- Improve access to clinical data.
- Simplify clinical study requirements.
- Invest in regulatory science.
- Extend patent protection.
Balice-Gordon predicted that the field will continue to actively work to bring stakeholders together from different sectors to improve the efficiency and productivity of drug development, particularly at the transition from Phase I to Phase II. Sauer commented that creating new tools and infrastructure will require long-term investment. As an example, he pointed to the Innovative Medicines Initiative,4 a public‒private partnership in Europe aimed at expediting drug development through collaborations and networking. In the United States, the 21st Century Cures bill5 originally included a similar partnership approach, although this provision was subsequently stripped out of the bill while making its way through Congress, said Sauer.
Zorn, however, contended that progress will also require ensuring that the right clinical questions are being asked. If we don’t have an idea
___________________
4 For more information, go to https://www.imi.europa.eu (accessed November 18, 2016).
5 For more information, go to https://www.congress.gov/bill/114th-congress/housebill/34 (accessed January 8, 2017).
of how preclinical findings relate to clinical endpoints, it will be difficult to move from preclinical to something that will both reduce the burden of failure and increase the probability of success. Balice-Gordon cited a promising model to bridge preclinical research and relevant clinical endpoints pursued by The Michael J. Fox Foundation. They surveyed the field to identify the four or five most impactful questions in Parkinson’s disease basic biology, natural history, biomarker development, and clinical instrument development and validation. Then they built collaborations with academia and industry to steer efforts aimed at answering these questions.












