7
Research
This committee’s first report was clear that eliminating viral hepatitis as a public health problem is a bold goal, and not one that can be realized without significant improvements in prevention, screening, and treatment (NASEM, 2016a). There are still gaps in our understanding of the viruses that can hold back progress on meeting the targets set in Chapter 2. For this reason, the World Health Organization (WHO) identified research as one of the essential pieces of any country’s national viral hepatitis elimination program. For the United States, a comparative advantage in science and technology compels special attention to such questions. Advancing understanding of the hepatitis B and C viruses and their treatment and prevention in a range of settings is a crucial contribution to the elimination effort in this country and the world.
The National Institutes of Health (NIH) has been criticized for failing to align its funding priorities with measures of disease burden in the United States or the world (Gillum et al., 2011). Viral hepatitis research is no exception. Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection affects 3 to 5 times more Americans than HIV, for example;1 worldwide, it is about 10 times more.2 But NIH funding for viral hepatitis between 2012 and 2017 has been between $195 and $273 million a year,
___________________
1 The Centers for Disease Control and Prevention (CDC) estimates 1.2 million cases of HIV in the United States, 2.7 to 3.9 million chronic hepatitis C, and 850,000 to 2.2 million chronic hepatitis B (CDC, 2016a,b).
2 The WHO estimates 36.7 million cases of HIV, 240 million chronic hepatitis B, and 130 to 150 million chronic hepatitis C (WHO, 2016a,b,c).
compared to nearly $3 billion a year (roughly 12 times more) for HIV (NIH, 2016b).3 Despite being the seventh leading cause of death in the world, viral hepatitis research accounts for less than 1 percent of NIH’s $31 billion research budget (IHME, 2015; NIH, 2016b,c; Stanaway et al., 2016).
Viral hepatitis elimination would do well to copy the success of the fight against HIV, starting with its success in research. Many of the breakthroughs that stopped transmission of HIV, from the development of AIDS therapies to the use of treatment to prevent sexual transmission, came from NIH-funded studies (Cohen et al., 2011b; NCI, n.d.; NIH, 2011). In the early 1980s, most HIV research was within the purview of the National Institute of Allergy and Infectious Diseases and the National Cancer Institute, but as the virus emerged as an international epidemic, NIH leaders realized that no one institute’s mission was sufficient to encompass the necessary research agenda (NIH, n.d.). The Office of AIDS Research was established in response to this problem. The office works across the NIH to ensure that a range of biomedical and behavioral research questions get sufficient attention, coordinating the budget and setting priorities for HIV research (NIH, n.d.).
This report has already argued that eliminating viral hepatitis as a public health threat is a complicated proposition and one that will require attention from various federal, state, and local government offices, as well as coordination with various private sector organizations. Coordinating these organizations will be challenging, and may do well to try to replicate the success of the HIV research program.
The creation of the Office of AIDS Research in 1993 was accompanied by a full review of NIH’s investment in HIV and AIDS research (Cohen, 1996). It could be helpful to conduct a similar review of viral hepatitis activities, considering them against the care continuum introduced in Chapter 4. The diagram shown in Chapter 4 represents an ideal continuum with very few drop-offs. In the real world, patients are frequently lost to follow-up and fail to respond to treatment. Attrition at any step is a barrier to elimination, so each drop along the continuum should be considered for possible research opportunities.
The NIH Division of Program Coordination, Planning, and Strategic Initiatives manages research on topics that naturally straddle more than one institute, including behavioral science, women’s health, and AIDS (NIH, 2016a). The office also handles the Trans-NIH Committee on Viral Hepatitis, a group formed to implement the goals articulated in the Department of Health and Human Services viral hepatitis action plan (HHS,
___________________
3 As indicated by a search for the terms “hepatitis” and “HIV” in 2016 on the NIH’s Research Portfolio Online Reporting Tools.
2015; NIH, 2013). The committee involves members from nine centers and institutes and the Office of AIDS Research (NIH, 2013; Viral Hepatitis Implementation Group, 2011). It is possible that the committee, or some other coordinating entity, might be able to lead an expanded viral hepatitis research program across agencies that fund viral hepatitis research. Special priorities for NIH and other research funding organizations are discussed in the next section.
RESEARCH ACROSS THE CARE CONTINUUM
A diagram of the hepatitis C care continuum shows multiple opportunities for improvement (see Figure 7-1). A recent study in New York City found that only 20 percent of people tested for HCV antibody have a confirmatory HCV RNA test, and among those who received a confirmatory HCV RNA test, the median wait time for testing was 13 days (IQR, 2 to 52 days) (Norton et al., 2016). Figure 7-2 suggests similar gaps in hepatitis B care.
There are new opportunities to better understand where and why people drop off the care continuum. The Department of Veterans Affairs (VA) program to treat all HCV-infected patients in its health system is one such opportunity. The VA has a good clinical records system that is the same across the country (Kane and Chesanow, 2014). VA beneficiaries have relatively reliable access to care, something that one would expect to be maintained after HCV treatment is over. The cohort is also large; over 174,000 veterans have chronic HCV infection (VA, 2014). Therefore, this population is an ideal one in which to study the long-term risk of liver disease in people cured of hepatitis C. It would also be possible to investigate results from different models of care or subpopulations of infected veterans.
Meeting people at every step of the care continuum will get more challenging over time; the patients who are easiest to manage will have already been found. The challenges of the later stages of elimination could be headed off now by developing new tools and clarifying the most efficient strategies for reaching different patient groups. To this end, the committee has identified a set of pressing research questions key to the elimination effort. These topics are divided broadly into mechanistic questions dealing with the basic science of the viruses and ways to diagnose and cure them, and operations and implementation research questions concerned with identifying the best strategies to prevent and treat viral hepatitis. The research questions identified below are not necessarily new, in many cases the body of knowledge described builds off other recent studies. This is, rather, the committee’s best summary of important questions in the field. Attention to these topics would serve the large goal of elimination of hepatitis B and C.
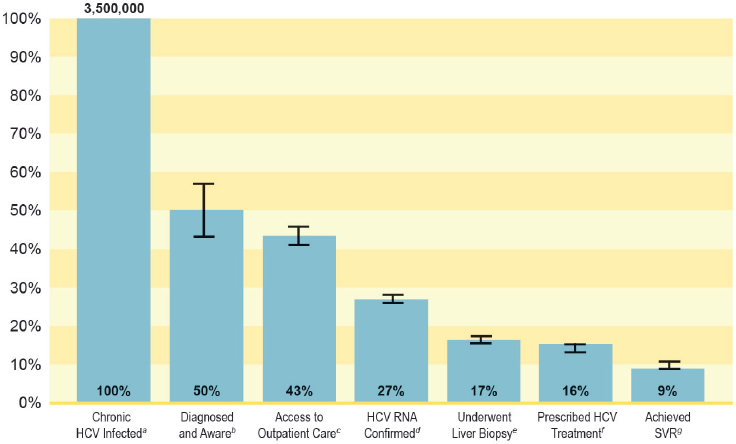
NOTES: Only non-VA studies are included in the above HCV treatment cascade. HCV = hepatitis C virus; RNA = ribonucleic acid; SVR = sustained virologic response; VA = Department of Veterans Affairs. a Chronic HCV infected; n = 3,500,000. b Calculated as estimated number chronic HCV-infected (3,500,000) × estimated percentage diagnosed and aware of their infection (49.8%); n = 1,743,000. c Calculated as estimated number diagnosed and aware (1,743,000) × estimated percentage with access to outpatient care (86.9%); n = 1,514,667. d Calculated as estimated number with access to outpatient care (1,514,667) × estimated percentage HCV RNA confirmed (62.9%); n = 952,726. e Calculated as estimated number with access to outpatient care (1,514,667) × estimated percentage who underwent liver biopsy (38.4%); n = 581,632. f Calculated as estimated number with access to outpatient care (1,514,667) × estimated percentage prescribed HCV treatment (36.7%); n = 555,883. g Calculated as estimated number prescribed HCV treatment (555,883) × estimated percentage who achieved SVR (58.8%); n = 326,859.
SOURCE: Yehia et al., 2014.
Mechanistic Research
There can be no elimination of HBV and HCV infections without enhanced understanding of the fundamental biology of these viruses and novel therapeutic targets (Palese, 2016). To this end, there should more research on viral life cycle, including the entry of the virus into host cells,
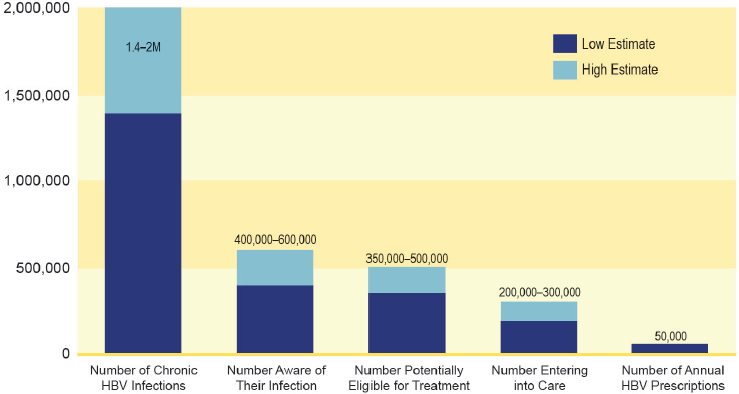
NOTE: HBV = hepatitis B virus.
SOURCE: Cohen et al., 2011a.
host protein interactions, viral replication, persistence, release, and virion maturation. Virology research has always been intertwined with discovery of host biology, particularly immunology, providing dividends that extend well beyond the particular virus studied (Tortorella et al., 2000). Between 1901 and 2014, 14 Nobel Prizes in medicine were awarded for virology research; Table 7-1 gives examples of breakthroughs with broader applications that have come from studying viral hepatitis (Nobelprize.org, n.d.).
Molecular Epidemiology and Phylodynamic Methods
As elimination efforts proceed and viral hepatitis prevalence decreases, case finding will become more difficult and the study of ongoing transmission networks more important. Modern molecular epidemiology, including the use of phylodynamics to describe transmission dynamics and reconstruct transmission histories, would complement classical epidemiological research (Pybus and Rambaut, 2009). Sequencing of HBV and HCV genomes would advance this goal, as would investing in epidemiological and clinical data to link the genomic analysis (Pillay et al., 2015). Understanding the risk of transmission requires linking sequences in the viral genome to clinical and epidemiological data (Pillay et al., 2015). To be successful,
TABLE 7-1 Virology Breakthroughs from Viral Hepatitis Research
| Virus | Discovery | Reference |
|---|---|---|
| HBV | First vaccine to prevent human cancer (the hepatitis B vaccine) | Chang et al., 2009; NAS, 2000 |
| HBV | First radioimmunoassay (RIA) | Ling and Overby, 1972 |
| HBV | First model to elucidate the causative role of inflammation in carcinogenesis | Nakamoto et al., 1998 |
| HCV | Discovery of human polymorphism (initially IL28B, ultimately interferon λ4) governing outcome of acute HCV infection and response to interferon | O’Brien et al., 2014 |
| HCV | Discovery of MAVS, a key adaptor protein in RIG-I signaling | Meylan et al., 2005 |
| HCV | First microRNA dependent virus, first microRNA targeted drug | Lindow and Kauppinen, 2012 |
| HCV | First cure of an infectious disease using an immune checkpoint inhibitor (anti-D-1) | Gardiner et al., 2013 |
NOTE: HBV = hepatitis B virus; HCV = hepatitis C virus; MAVS = mitochondrial antiviral-signaling protein.
elimination efforts will have to adapt to address remaining transmission, which is likely to vary by location, population, and behavior—variables that can be informed by phylodynamics.
Rapid Diagnostic Testing for HBV DNA and HCV RNA
Rapid or point-of-care tests can help avoid attrition on the viral hepatitis care continuum from the start. With rapid tests, people receive counseling and test results all in one interaction, limiting opportunity for loss to follow-up. In some settings, rapid point-of-care tests also enhance the ability to screen family members and other contacts of infected cases.
Hepatitis C antibody testing alone is not sufficient to diagnose hepatitis C. As discussed earlier in this report, rapid tests for HCV RNA in addition to existing antibody tests would improve patient management, especially in places catering to patients at elevated risk for hepatitis C such as syringe exchange centers (Smith et al., 2011).
In addition to individual testing for screening and disease management, there is a need for population-level assessment of progress toward elimination goals. Understanding incidence is crucial to this goal, but the cohort studies needed to document incidence are expensive and logistically
complicated. Recently, serological testing research has demonstrated the accuracy of cross-sectional incidence testing for HIV, and similar work with HCV is promising (Brookmeyer et al., 2013; Patel et al., 2016). Further work with HBV and HCV could result in tests that detect recent infection in a single blood sample, which would greatly enhance efforts to identify new infections.
Immune Response to HBV
More complete understanding of the human immune response to HBV would have broad benefits. Immune responses can protect against transmission of HBV and play a key role in successful antiviral therapy (Bertoletti and Ferrari, 2012). Immune response also contributes to fibrosis progression (Bertoletti and Ferrari, 2012; Bertoletti et al., 2010). Deeper understanding of anti-HBV immune responses could open new avenues for additional vaccine development, informing antigen and adjuvant selection. Of particular interest would be research to simplify the hepatitis B vaccine schedule and reduce vaccine failure, and ways to shorten or enhance success of antiviral therapy, and further reduce mother-to-child transmission of HBV.
Hepatitis B in Pregnancy
Recent evidence suggests that it is possible to prevent chronic hepatitis B in newborns born to highly viremic, HBeAg+ women with prophylactic antiviral therapy in addition to standard newborn prophylaxis with hepatitis B immune globulin and vaccine (Pan et al., 2016). There is considerable uncertainty as to what the HBV DNA threshold should be to start antiviral therapy in pregnant women who would not otherwise require treatment. Suggested cut points range from 200,000 to 10 million IU/ml (ASHM, 2014; Terrault et al., 2016). It is also unclear when in pregnancy the therapy should be started or stopped, or even if the goal of such therapy should be preventing newborn viremia or preventing chronic infection in the newborn. All of these questions warrant wider attention.
Vaccine Against HCV
The well-tolerated direct-acting antiviral treatments for chronic HCV infection make it possible to even consider eliminating viral hepatitis in the United States, but curative tools alone seem limited in service to a goal as ambitious as elimination. Control of other infectious diseases such as tuberculosis and syphilis is still extremely challenging, and cures for these infections have been available for decades. Vaccines are almost invariably
essential for eliminating infectious diseases, and an HCV vaccine has been elusive (Palese, 2016).
It is not surprising that HCV vaccine development has been challenging. HCV is extensively diverse genetically, the virus cannot be cultured (with a few genetically restricted exceptions), and there is no immunocompetent small animal model that supports HCV replication (Bukh, 2016).
In spite of early setbacks, there is growing evidence that it may be possible to make an effective vaccine for HCV, especially if the goal is to enhance spontaneous clearance rather than prevent infection (Man John Law et al., 2013). It is well known that about 25 percent of people acutely infected with HCV clear the infection spontaneously (Grebely et al., 2012; Micallef et al., 2006). People who have cleared HCV infection once are far more likely to do so again, with progressively lower levels and duration of viremia (Grebely et al., 2006; Osburn et al., 2010). People who clear infection spontaneously develop cell-mediated and neutralizing antibody responses (Dowd et al., 2009; Keoshkerian et al., 2016; Pestka et al., 2007). Chimpanzee studies have shown that depletion of CD4+ and CD8+ T cells resulted in HCV persistence (Grakoui et al., 2003; Shoukry et al., 2003). Both findings indicate immune response is an essential element in clearance (Dowd et al., 2009; Grakoui et al., 2003; Keoshkerian et al., 2016; Pestka et al., 2007; Shoukry et al., 2003). Studies are now emerging in humans, but additional vaccine candidates and correlates of protection are likely to be necessary to prevent HCV infection (Swadling et al., 2014).
Studies of humoral and cellular immunity have advanced understanding of HCV and suggest that a vaccine to prevent chronic infection is feasible (Bukh, 2016; Liang, 2013). Though the HCV envelope is highly variable, broadly neutralizing antibody responses have been identified during early infection (Osburn et al., 2014), and some epitopes of broadly neutralizing antibodies are evolutionarily constrained (Rodrigo et al., 2017). Though the crystal structure of the HCV envelope proteins remains elusive, structural studies of core domains are advancing (McCaffrey et al., 2017). Such studies can inform the design of rational humoral vaccine. Recent molecular dynamic studies have also illuminated a mechanistic basis for prior failures to elicit broadly neutralizing antibody responses, opening new avenues for rational design (Kong et al., 2016). As with antibody response, broadly directed T cell responses appear early in natural infection, but are rapidly lost in chronic infection (Schulze Zur Wiesch et al., 2012). A candidate prophylactic vaccine currently in clinical trials elicited strong T cell responses in phase I and II testing, but lacks an envelope protein component (NIAID, 2017; Swadling et al., 2014).
Taken together, the current status of HCV vaccine development is intermediate but promising; given the importance of vaccine prophylaxis in
other elimination campaigns, this area of research should receive additional attention.
Curative Therapy for HBV
The lack of a cure for HBV infection is an obstacle to elimination. Fundamental study of the virus would identify therapeutic targets that might facilitate development of curative rather than suppressive therapy. Such research should aim to clarify the mechanisms of viral persistence, host and viral determinants of the stability of cccDNA, and the role of integrated portions of the HBV genome in the host genome.
The treatment of HBV infection might be improved by novel antiviral combinations and sequential therapy. Clinical trials of such treatments are necessary to determine the best treatment strategy, as well as the best format for answering questions regarding the discontinuation of therapy, treatment of low-level viremia in persons with cirrhosis, and treatment in patients at particular risk of complications, such as HIV patients. Clinical trials would also help determine the best frequency and way to monitor treatment outcomes. Clinical outcomes such as cirrhosis and liver cancer are the gold standard in trials, but it takes a large study size and years of follow-up to track such outcomes. Some key clinical outcomes have been shown to correlate strongly with intermediate outcomes, such as HBV DNA suppression, normalization of liver enzymes, HBeAg to anti-HBe conversion, and regression of cirrhosis (Lok et al., 2016). Therapeutic trials may make faster progress by giving attention to some of these intermediate outcomes, though some authorities have indicated a need for studies on long-term health outcomes, which would require a large prospective cohort study (USPSTF, 2014).
HBV Reactivation During Immunosuppressant Therapy
The mechanisms contributing to reactivation of HBV are poorly understood. Both reactivation of HBV during therapy for autoimmune disease or cancer and reactivation of HBV during or after treatment of HCV infection may occur (Balagopal and Thio, 2015; Collins et al., 2015; Paul et al., 2016; Perez-Alvarez et al., 2011). It will be difficult to eliminate chronic hepatitis B in the face of potential reactivation of HBV infection. Widespread treatment for hepatitis C could trigger complications in people with both infections. Greater understanding of what predicts reactivation at the host, and viral levels, as well as ways to prevent and treat HBV reactivation is necessary.
Detection and Management of Fibrosis, Cirrhosis, Hepatocellular Carcinoma
The complications of chronic HBV and HCV infection arise mainly from progressive liver fibrosis. Even after cure or sustained suppression of the virus, liver fibrosis and cirrhosis continue to be associated with end-stage liver disease and hepatocellular carcinoma (Lok et al., 2016; Tholey and Ahn, 2015). Basic research on the pathogenesis of fibrosis has led to some candidate anti-fibrotic therapies (Lee et al., 2015). Such treatment has the potential to benefit everyone with liver disease, not only viral hepatitis patients, but no treatment for reversal of liver fibrosis has been approved.
As Chapter 1 made clear, everyone with chronic viral hepatitis is at increased risk of hepatocellular carcinoma, which is the second most common cause of cancer death worldwide (Ferlay et al., 2015; Makarova-Rusher et al., 2016; Welzel et al., 2013). The incidence of liver cancer in the United States increased 38 percent between 2003 and 2012; liver cancer deaths increased 56 percent in the same time (Ryerson et al., 2016).
The environmental, host, and viral determinants that predict the transition from chronic viral hepatitis to liver cancer are not completely understood (El-Serag, 2012; Westbrook and Dusheiko, 2014). Guidelines for detection of hepatocellular carcinoma in persons at risk, including those with HBV or HCV infection, are well-established despite limited data, but adherence to the guidelines is poor, even among specialists (Bruix and Sherman, 2011; Hearn et al., 2015; Sharma et al., 2011; Wu et al., 2014). Part of the challenge lies in the available screening tools.
Current screening methods call for liver imaging every 6 months. However, liver ultrasound without bubble contrast has limited sensitivity, particularly in obese patients (Chou et al., 2015; Giannini et al., 2013; Hennedige and Venkatesh, 2013). There are logistical challenges with the more accurate abdominal computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound with contrast imaging (Chou et al., 2015). (CT scan exposes the patient to radiation; both MRI and CT scans are expensive, require special equipment, and, when being used to diagnose hepatocellular carcinoma, both scans require contrast.) In any case, repeated imaging appointments are a burden to patients and providers. In the 2010 update to the AASLD4 guidelines, alpha-fetoprotein testing was abandoned due to poor sensitivity and specificity, and no serum biomarker has replaced it (Bruix and Sherman, 2011).
The effectiveness of current screening methods for hepatocellular carcinoma, in terms of reducing mortality, is disputed (El-Serag and Davila, 2011; Marrero and El-Serag, 2011). A recent meta-analysis in cirrhotic
___________________
4 Officially, the American Association for the Study of Liver Diseases.
patients showed hepatocellular carcinoma surveillance is associated with significant improvements in early tumor detection, curative treatment rates and overall survival (Singal et al., 2014). Additional study of pathogenesis, correlates, and biomarkers of hepatocellular carcinoma could significantly improve the accuracy and availability of screening tests and the ability to diagnose liver cancer at a treatable stage. Longitudinal study of these correlates should help identify people at minimal risk of liver cancer for whom frequent screening may not be necessary.
Hepatocellular carcinoma remains a risk among hepatitis B patients whose HBV DNA is suppressed and in hepatitis C patients with cirrhosis who have not been cured (de Oliveria Andrade et al., 2009; El-Serag, 2012; Fernandez-Rodriguez and Gutierrez-Garcia, 2014). It is not clear if such patients need to be monitored for cancer for the rest of their lives. A prospective cohort study to determine their residual risk over time would be valuable.
New treatments for liver cancer are also needed. Without early detection, most hepatocellular carcinoma patients have poor outcomes. Surgical treatment is associated with 5-year survival rates of up to 70 percent, but only 10 to 20 percent of patients are diagnosed with resectable tumors (Dhir et al., 2012; NCI, 2016; Shah et al., 2011; Sonnenday et al., 2007; Tiong and Maddern, 2011). In patients with unresectable disease, systemic or targeted chemotherapy or embolization with or without radiofrequency ablation yield a median survival of about 1 to 2 years (Tiong and Maddern, 2011). Genetically targeted cancer therapy and immunotherapy should offer more effective avenues for treating hepatocellular carcinoma (Bernicker, 2016; Roychowdhury and Chinnaiyan, 2016; Yang, 2015).
The cure of hepatitis C poses a valuable opportunity to study the long-term risk of complications after the agent causing damage to the liver is removed. Follow-up studies of people cured of chronic hepatitis C, with and without HIV, have revealed reduced, but non-zero, rates of hepatic decompensation, hepatocellular carcinoma, and death (Labarga et al., 2015; Moon et al., 2015; Morgan et al., 2010, 2013; Papastergiou et al., 2013). Cohort studies on cured patients could give insight into the management of liver fibrosis and identify genetic and environmental risks that may guide education, monitoring, and treatment after successful antiviral therapy.
Implementation Research
A better understanding of how and under what circumstances interventions work in the real world is the purview of implementation research (Peters et al., 2013). Much of the challenge of viral hepatitis elimination will lie in ensuring that preventative services and care reach the widest possible audience. This section identifies some implementation science ques-
tions that would serve the viral hepatitis elimination effort, both by adding to the tools available to prevent infection and by clarifying the best manner in which to implement programs that work.
Health in Jails and Prisons
As discussed in Chapter 5, prisons bear a disproportionate burden of viral hepatitis (Dolan et al., 2016; Maurer and Gondles, 2015; Weinbaum et al., 2003). They also have a serious burden of other infectious diseases, as well as mental and behavioral health problems (Freudenberg, 2001). In order for society to reap the full benefit of treating chronic hepatitis C in correctional facilities, better effort must be made to treat substance use disorder among prisoners, as this is the root cause of most viral hepatitis (CDC, 2016c; Grebely et al., 2015; Nelson et al., 2011). Failing to treat substance use risks exposing the cured inmate to reinfection, undermining the investment in his or her treatment. Drug relapse is more common during the transition from prison to civilian life (Vestal, 2016).
Estimates of the prevalence of drug dependence in jails and prisons range from 10 to 60 percent, partly because of varying definitions of drug dependence in this setting (Fazel et al., 2006). The National Institute on Drug Abuse encourages treatment of substance use disorder in prison, with provisions for outpatient treatment made after the inmate’s release, but this is not common practice (NIH, 2014) (see Figure 7-3). Fewer than 0.1 percent of inmates in the United States receive opioid agonist therapy, the modern treatment standard for opioid addiction (Larney et al., 2011; WHO, 2009). Reasons for this inconsistency range from the philosophical (the belief that substance use is a weakness rather than a medical disorder or that the appropriate role of the criminal justice system is to punish offenders, not medicate them) to the practical (a lack of funds or concern that the medicines could not be securely stored in the prison infirmary) (NIDA, 2011; Nunn et al., 2009; Pecoraro and Woody, 2011).
Inconsistency in treatment of drug use in prisons has consequences for society, which routine treatment of hepatitis C in prisons will underscore. Treating substance use disorder in prisons would minimize the threat of reinfection after cure. It is not clear what the best strategies to manage such treatment may be. An evidence base on the value of treating substance use (including opioid dependence), considering outcomes such as blood-borne disease, and also violence and recidivism, would be of value to policy makers, as would information about the comparative effectiveness of modern opioid agonist methods compared to the current standard of care in most states.
Research in incarcerated people poses special challenges. The imprisoned participant is, by definition, being held against his or her will, so ques-
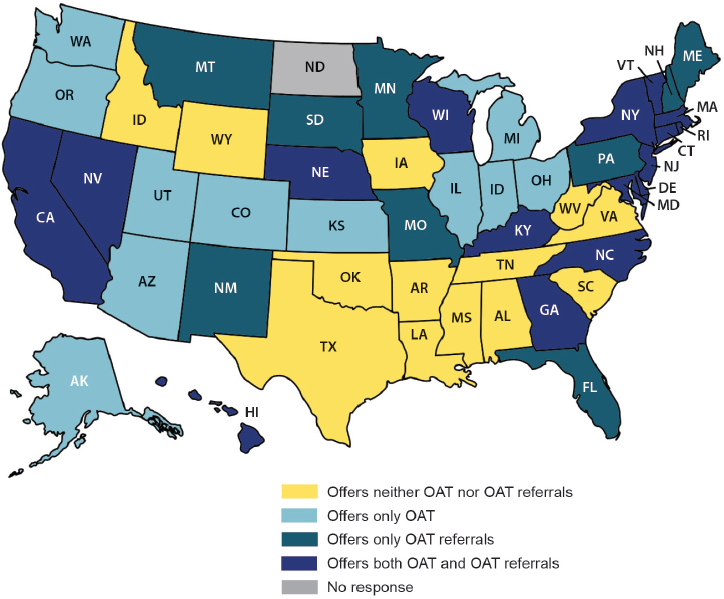
NOTE: OAT = opioid agonist therapy.
SOURCE: Nunn et al., 2009.
tions of coercion and informed consent become paramount (Chandler et al., 2009; Gostin, 2007). Prison research must be designed to ensure benefit to the participant, either individually or to prisoners as a group (IOM, 2007). Research on how best to implement substance use treatment programs in prisons meets both criteria. Prisoners would benefit from having research done on their behalf, as would society benefit from better understanding the safest and most efficient strategies to return inmates to their communities in the best health possible.
The transition from prison to civilian life, particularly the health risks of this transition, would also benefit from research attention. As Chapter 5 discussed, fewer than 20 percent of prison medical directors follow CDC recommendations for discharge planning with inmates (Solomon et al., 2014). Operations research would also be useful to determine the best
ways to ensure a smooth transition from correctional health to Medicaid or another insurance program.
Strategies to Reach Key Populations
Chapter 5 discussed various populations that viral hepatitis services must reach in order for elimination to work. This includes people with substance use problems, mental illness, in unstable housing or living on the streets, and people facing cultural, language, or financial barriers to accessing care, including undocumented immigrants. These groups can be described as sensitive or hard to reach, terms used in the literature for populations that meet three criteria: the exact size of the group is not known, preventing articulation of a clear sampling frame; recognition as a member of the group risks prosecution or stigma; and members in the groups may be distrustful or uncooperative with outsiders (Benoit et al., 2005; Heckathorn, 1997). It is not clear how to best overcome these barriers and ensure that viral hepatitis prevention and care services reach the people who need them most.
Complex adaptive systems research, a field that aims to understand how different parts of a systems work together and influence each other, is a promising way to clarify what strategies work best to reach these populations (Paina and Peters, 2012). The complex adaptive systems strategy has been successful in studying health programs, especially at determining why some strategies work and others do not, as well as modeling the likely outcomes of different strategies (Jordon et al., 2010; Paina and Peters, 2012). On a practical note, connecting with community organizations that have existing, trusting relationships with the groups in question often eases the research logistics (Benoit et al., 2005).
Strategies to Alleviate Stigma
As this committee’s first report discussed, stigma is a barrier to elimination of viral hepatitis (NASEM, 2016a). Stigma is also a difficult problem to tackle, partly because it can occur at many levels ranging from the laws and policies in place in a society, to negative attitudes toward certain groups or behaviors, to a person’s internalized sense of shame or guilt (NASEM, 2016b). The best strategy to reduce stigma depends on the type of stigma targeted.
Part of the challenge relating to viral hepatitis is that it is difficult to disentangle the stigma of the infection from that of substance use often associated with liver disease in general, viral hepatitis in particular. Qualitative research could clarify the relative contributions of each among different
communities and HBV- and HCV-infected people. This information would be a first step to understanding the problem.
Evidence regarding what strategies work best to fight stigma is murkier. There is an emerging consensus in mental health that media campaigns are not effective (Livingston et al., 2014; NASEM, 2016b). It is not clear if the same would be true for infectious disease, or what might work better. Research into this topic is sorely needed, and should consider, as the recent National Academies report concluded, “captur[ing] both direct and indirect effects, [. . .] intended and unintended consequences” (NASEM, 2016b, p. 10).
Harm Reduction in Emerging Settings and Populations
The 2010 Institute of Medicine report on viral hepatitis recommended the expansion of access to sterile injecting equipment for people who inject drugs (IOM, 2010). In the years since that report was published, injection drug use has exploded in rural and suburban areas (Des Jarlais et al., 2015). Less is known about the best strategies for harm reduction outside of cities, as Chapter 5 discussed. The relative merits of mobile syringe services, unstaffed exchange methods (i.e., vending machines), and over-the-counter sale of injecting equipment should all be examined (Duplessy and Reynaud, 2014; Strathdee and Beyrer, 2015). A recent meta-analysis found plausible evidence that combining harm reduction and substance use treatment was effective at preventing transmission of HCV, but the authors acknowledged that very few studies used HCV seroconversion as an outcome measure (Hagan et al., 2011). Future researchers should be encouraged to measure HCV seroconversion among people who inject drugs.
Another feature of the epidemic of injection drug use described in Chapter 5 that warrants particular research attention is injection drug use in people younger than 30. Young adults and adolescents who inject drugs are thought to be fueling spikes in HCV infection in the United States, but less is known about how to reach this group with harm reduction and other health services (Page et al., 2013; Stockings et al., 2016). Research on networks of drug users may be an efficient strategy to understand this population (Bryant, 2014).
Understanding Networks of Drug Users
As this report has made clear, people who inject drugs are a difficult but important population to engage in a viral hepatitis elimination program. Chapter 4 described how opioid drug use is reaching more and different populations than it did a generation ago (Des Jarlais et al., 2015; Havens et al., 2013). Our understanding, albeit limited, of social relationships among
people who inject drugs and how these relationships influence disease transmission may not be relevant to rural or suburban settings.
Social network research aims to understand the influence of a person’s social group on his or her behavior and risk (De et al., 2007; Weeks et al., 2009). The quality and types of relationships members have with other drug users and with the wider community are all thought to affect such behavior (De et al., 2007; Young et al., 2013). Research among drug users has shown that people in large, dense networks are more likely to share injecting equipment (De et al., 2007). It is not clear if or how social dynamics would differ in rural areas. On one hand, there is little in-migration to rural areas, so social ties tend to be long-lasting and strong. At the same time, distance and reliance on cars could contribute to isolation, especially among people already feeling isolated by substance use. A better understanding of how these dynamics unfold could help behavioral scientists tailor interventions to stop transmission of viral hepatitis and bring infected people who inject drugs into care.
Prevention of the Transition to Injecting Drugs
It is not clear what factors influence people who may have an addiction to drugs that are smoked or ingested to switch to injection. The user’s social milieu is thought to play a role: relatives, friends, and sexual partners may encourage the switch, as does living in a neighborhood where injection drug use is common (Neaigus et al., 2006; Sherman et al., 2002). The user’s physiological addiction can also drive him or her to more potent injectable drugs, as does the drug’s cost, and the user’s relative wealth (Mars et al., 2014; Neaigus et al., 2006; Sherman et al., 2002). Homelessness is also a risk factor (Neaigus et al., 2006).
A better understanding of why people start injecting drugs would inform efforts to prevent it. Self-administered questionnaires for people who inject drugs have been used in the past to measure risk of HIV (University of Pennsylvania, n.d.; Ward et al., 1990). A more recent tool, the Behavioral Risk Assessment for Infectious Diseases, looks specifically at drug use other than injection (Dunn et al., 2016). Research using these tools, alone or in combination, may help identify common threads driving the switch to the higher risk injection behavior.
REFERENCES
ASHM (Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine). 2014. 10.0 managing hepatitis B virus infection in pregnancy and children. http://hepatitisb.org.au/10-0-managing-hepatitis-b-virus-infection-in-pregnancy-and-children (accessed November 21, 2016).
Balagopal, A., and C. L. Thio. 2015. Editorial commentary: Another call to cure hepatitis B. Clinical Infectious Diseases 61(8):1307-1309.
Benoit, C., M. Jansson, A. Millar, and R. Phillips. 2005. Community-academic research on hard-to-reach populations: Benefits and challenges. Qualitative Health Research 15(2):263-282.
Bernicker, E. 2016. Next-generation sequencing and immunotherapy biomarkers: A medical oncology perspective. Archives of Pathology & Laboratory Medicine 140(3):245-248.
Bertoletti, A., and C. Ferrari. 2012. Innate and adaptive immune responses in chronic hepatitis B virus infections: Towards restoration of immune control of viral infection. Gut 61(12):1754-1764.
Bertoletti, A., M. K. Maini, and C. Ferrari. 2010. The host-pathogen interaction during HBV infection: Immunological controversies. Antiviral Therapy 15(3):15-24.
Brookmeyer, R., O. Laeyendecker, D. Donnell, and S. H. Eshleman. 2013. Cross-sectional HIV incidence estimation in HIV prevention research. Journal of Acquired Immune Deficiency Syndromes 63(Suppl 2):S233-S239.
Bruix, J., and M. Sherman. 2011. Management of hepatocellular carcinoma: An update. Hepatology 53(3):1020-1022.
Bryant, J. 2014. Using respondent-driven sampling with ‘hard to reach’ marginalised young people: Problems with slow recruitment and small network size. International Journal of Social Research Methodology 17(6):599-611.
Bukh, J. 2016. The history of hepatitis C virus (HCV): Basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. Journal of Hepatology 65(1 Suppl):S2-S21.
CDC (Centers for Disease Control and Prevention). 2016a. Living with HIV. https://www.cdc.gov/hiv/basics/livingwithhiv/index.html (accessed December 15, 2016).
CDC. 2016b. Viral hepatitis–Statistics & surveillance. https://www.cdc.gov/hepatitis/statistics/index.htm (accessed December 15, 2016).
CDC. 2016c. Viral hepatitis surveillance: United States, 2014. http://www.cdc.gov/hepatitis/statistics/2014surveillance/pdfs/2014hepsurveillancerpt.pdf (accessed Setpember 22, 2016).
Chandler, R. K., B. W. Fletcher, and N. D. Volkow. 2009. Treating drug abuse and addiction in the criminal justice system: Improving public health and safety. JAMA 301(2):183-190.
Chang, M. H., S. L. You, C. J. Chen, C. J. Liu, C. M. Lee, S. M. Lin, H. C. Chu, T. C. Wu, S. S. Yang, H. S. Kuo, and D. S. Chen. 2009. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year follow-up study. Journal of the National Cancer Institute 101(19):1348-1355.
Chou, R., C. Cuevas, R. Fu, B. Devine, N. Wasson, A. Ginsburg, B. Zakher, M. Pappas, E. Graham, and S. D. Sullivan. 2015. Imaging techniques for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Annals of Internal Medicine 162(10):697-711.
Cohen, C., S. D. Holmberg, B. J. McMahon, J. M. Block, C. L. Brosgart, R. G. Gish, W. T. London, and T. M. Block. 2011a. Is chronic hepatitis B being undertreated in the United States? Journal of Viral Hepatitis 18(6):377-383.
Cohen, J. 1996. AIDS politics: OAR gets by with a little help from new guard friends. Science 272(5270):1878.
Cohen, M. S., Y. Q. Chen, M. McCauley, T. Gamble, M. C. Hosseinipour, N. Kumarasamy, J. G. Hakim, J. Kumwenda, B. Grinsztejn, J. H. S. Pilotto, S. V. Godbole, S. Mehendale, S. Chariyalertsak, B. R. Santos, K. H. Mayer, I. F. Hoffman, S. H. Eshleman, E. PiwowarManning, L. Wang, J. Makhema, L. A. Mills, G. de Bruyn, I. Sanne, J. Eron, J. Gallant, D. Havlir, S. Swindells, H. Ribaudo, V. Elharrar, D. Burns, T. E. Taha, K. Nielsen-Saines, D. Celentano, M. Essex, and T. R. Fleming. 2011b. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine 365(6):493-505.
Collins, J. M., K. L. Raphael, C. Terry, E. J. Cartwright, A. Pillai, F. A. Anania, and M. M. Farley. 2015. Hepatitis B virus reactivation during successful treatment of hepatitis C virus with sofosbuvir and simeprevir. Clinical Infectious Diseases 61(8):1304-1306.
De, P., J. Cox, J. F. Boivin, R. W. Platt, and A. M. Jolly. 2007. The importance of social networks in their association to drug equipment sharing among injection drug users: A review. Addiction 102(11):1730-1739.
de Oliveria Andrade, L. J., A. D’Oliveira, R. C. Melo, E. C. De Souza, C. A. Costa Silva, and R. Parana. 2009. Association between hepatitis C and hepatocellular carcinoma. Journal of Global Infectious Diseases 1(1):33-37.
Des Jarlais, D. C., A. Nugent, A. Solberg, J. Feelemyer, J. Mermin, and D. Holtzman. 2015. Syringe service programs for persons who inject drugs in urban, suburban, and rural areas—United States, 2013. Morbidity and Mortality Weekly Report 64(48):1337-1341.
Dhir, M., E. R. Lyden, L. M. Smith, and C. Are. 2012. Comparison of outcomes of transplantation and resection in patients with early hepatocellular carcinoma: A meta-analysis. HPB (Oxford) 14(9):635-645.
Dolan, K., A. L. Wirtz, B. Moazen, M. Ndeffo-mbah, A. Galvani, S. A. Kinner, R. Courtney, M. McKee, J. J. Amon, L. Maher, M. Hellard, C. Beyrer, and F. L. Altice. 2016. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet 388(10049):1089-1102.
Dowd, K. A., D. M. Netski, X. H. Wang, A. L. Cox, and S. C. Ray. 2009. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology 136(7):2377-2386.
Dunn, K. E., F. S. Barrett, E. S. Herrmann, J. G. Plebani, S. C. Sigmon, and M. W. Johnson. 2016. Behavioral risk assessment for infectious diseases (BRAID): Self-report instrument to assess injection and noninjection risk behaviors in substance users. Drug and Alcohol Dependence 168:69-75.
Duplessy, C., and E. G. Reynaud. 2014. Long-term survey of a syringe-dispensing machine needle exchange program: Answering public concerns. Harm Reduction Journal 11:16.
El-Serag, H. B. 2012. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142(6):1264-1273.
El-Serag, H. B., and J. A. Davila. 2011. Surveillance for hepatocellular carcinoma: In whom and how? Therapeutic Advances in Gastroenterology 4(1):5-10.
Fazel, S., P. Bains, and H. Doll. 2006. Substance abuse and dependence in prisoners: A systematic review. Addiction 101(2):181-191.
Ferlay, J., I. Soerjomataram, R. Dikshit, S. Eser, C. Mathers, M. Rebelo, D. M. Parkin, D. Forman, and F. Bray. 2015. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 136(5):e359-e386.
Fernandez-Rodriguez, C. M., and M. L. Gutierrez-Garcia. 2014. Prevention of hepatocellular carcinoma in patients with chronic hepatitis B. World Journal of Gastrointestinal Pharmacology and Therapeutics 5(3):175-182.
Freudenberg, N. 2001. Jails, prisons, and the health of urban populations: A review of the impact of the correctional system on community health. Journal of Urban Health 78(2):214-235.
Gardiner, D., J. Lalezari, E. Lawitz, M. DiMicco, R. Ghalib, K. R. Reddy, K. M. Chang, M. Sulkowski, S. O. Marro, J. Anderson, B. He, V. Kansra, F. McPhee, M. Wind-Rotolo, D. Grasela, M. Selby, A. J. Korman, and I. Lowy. 2013. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One 8(5):e63818.
Giannini, E. G., A. Cucchetti, V. Erroi, F. Garuti, F. Odaldi, and F. Trevisani. 2013. Surveillance for early diagnosis of hepatocellular carcinoma: How best to do it? World Journal of Gastroenterology 19(47):8808-8821.
Gillum, L. A., C. Gouveia, E. R. Dorsey, M. Pletcher, C. D. Mathers, C. E. McCulloch, and S. C. Johnston. 2011. NIH disease funding levels and burden of disease. PLoS One 6(2):e16837.
Gostin, L. O. 2007. Biomedical research involving prisoners: Ethical values and legal regulation. JAMA 297(7):737-740.
Grakoui, A., N. H. Shoukry, D. J. Woollard, J. H. Han, H. L. Hanson, J. Ghrayeb, K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302(5645):659-662.
Grebely, J., B. Conway, J. D. Raffa, C. Lai, M. Krajden, and M. W. Tyndall. 2006. Hepatitis C virus reinfection in injection drug users. Hepatology 44(5):1139-1145.
Grebely, J., M. Prins, M. Hellard, A. L. Cox, W. O. Osburn, G. Lauer, K. Page, A. R. Lloyd, and G. J. Dore. 2012. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: Towards a vaccine. The Lancet Infectious Diseases 12(5):408-414.
Grebely, J., G. Robaeys, P. Bruggmann, A. Aghemo, M. Backmund, J. Bruneau, J. Byrne, O. Dalgard, J. J. Feld, M. Hellard, M. Hickman, A. Kautz, A. Litwin, A. R. Lloyd, S. Mauss, M. Prins, T. Swan, M. Schaefer, L. E. Taylor, and G. J. Dore. 2015. Recommendations for the management of hepatitis C virus infection among people who inject drugs. International Journal on Drug Policy 26(10):1028-1038.
Hagan, H., E. R. Pouget, and D. C. Des Jarlais. 2011. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. Journal of Infectious Diseases 204(1):74-83.
Havens, J. R., M. R. Lofwall, S. D. Frost, C. B. Oser, C. G. Leukefeld, and R. A. Crosby. 2013. Individual and network factors associated with prevalent hepatitis C infection among rural Appalachian injection drug users. American Journal of Public Health 103(1):e44-e52.
Hearn, B., R. Chasan, K. Bichoupan, M. Suprun, E. Bagiella, D. T. Dieterich, P. Perumalswami, A. D. Branch, and S. Huprikar. 2015. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clinical Infectious Diseases 61(11):1742-1748.
Heckathorn, D. D. 1997. Respondent-driven sampling: A new approach to the study of hidden populations. Social Problems 44(2):174-199.
Hennedige, T., and S. K. Venkatesh. 2013. Imaging of hepatocellular carcinoma: Diagnosis, staging and treatment monitoring. Cancer Imaging 12:530-547.
HHS (Department of Health and Human Services). 2015. Action plan for the prevention, care, and treatment of viral hepatitis: 2014-2016. HHS, Office of the Assistant Secretary for Health, Office of HIV/AIDS and Infectious Disease Policy. https://www.aids.gov/pdf/viral-hepatitis-action-plan.pdf (accessed October 26, 2016).
IHME (Institute for Health Metrics and Evaluation). 2015. GBD compare. http://vizhub.healthdata.org/gbd-mortality (accessed February 23, 2016).
IOM (Institute of Medicine). 2007. Ethical considerations for research involving prisoners. Washington, DC: The National Academies Press.
IOM. 2010. Hepatitis and liver cancer: A national strategy for prevention and control of hepatitis B and C. Washington, DC: The National Academies Press.
Jordon, M., H. J. Lanham, R. A. Anderson, and R. R. McDaniel, Jr. 2010. Implications of complex adaptive systems theory for interpreting research about health care organizations. Journal of Evaluation in Clinical Practice 16(1):228-231.
Kane, L., and N. Chesanow. 2014. Medscape EHR report 2014: Top rated EHRs by practice situation. http://www.medscape.com/features/slideshow/public/ehr2014#11 (accessed December 15, 2016).
Keoshkerian, E., M. Hunter, B. Cameron, N. Nguyen, P. Sugden, R. Bull, A. Zekry, L. Maher, N. Seddiki, J. Zaunders, A. Kelleher, and A. R. Lloyd. 2016. Hepatitis C-specific effector and regulatory CD4 T-cell responses are associated with the outcomes of primary infection. Journal of Viral Hepatitis 23(12):985-993.
Kong, L., D. E. Lee, R. U. Kadam, T. Liu, E. Giang, T. Nieusma, F. Garces, N. Tzarum, V. L. Woods, Jr., A. B. Ward, S. Li, I. A. Wilson, and M. Law. 2016. Structural flexibility at a major conserved antibody target on hepatitis C virus E2 antigen. Proceedings of the National Academy of Sciences of the United States of America 113(45):12768-12773.
Labarga, P., J. V. Fernandez-Montero, C. de Mendoza, P. Barreiro, J. Pinilla, and V. Soriano. 2015. Liver fibrosis progression despite HCV cure with antiviral therapy in HIV-HCV-coinfected patients. Antiviral Therapy 20(3):329-334.
Larney, S., B. Toson, L. Burns, and K. Dolan. 2011. Opioid substitution treatment in prison and post-release: Effects on criminal recidivism and mortality. Canberra, Australian Capital Territory: National Drug and Alcohol Research Centre, University of New South Wales.
Lee, Y. A., M. C. Wallace, and S. L. Friedman. 2015. Pathobiology of liver fibrosis: A translational success story. Gut 64(5):830-841.
Liang, T. J. 2013. Current progress in development of hepatitis C virus vaccines. Nature Medicine 19(7):869-878.
Lindow, M., and S. Kauppinen. 2012. Discovering the first microRNA-targeted drug. Journal of Cell Biology 199(3):407-412.
Ling, C. M., and L. R. Overby. 1972. Prevalence of hepatitis B virus antigen as revealed by direct radioimmune assay with 125 I-antibody. Journal of Immunology 109(4):834-841.
Livingston, J. D., M. Cianfrone, K. Korf-Uzan, and C. Coniglio. 2014. Another time point, a different story: One year effects of a social media intervention on the attitudes of young people towards mental health issues. Social Psychiatry and Psychiatric Epidemiology 49(6):985-990.
Lok, A. S. F., B. J. McMahon, R. S. Brown, J. B. Wong, A. T. Ahmed, W. Farah, J. Almasri, F. Alahdab, K. Benkhadra, M. A. Mouchli, S. Singh, E. A. Mohamed, A. M. Abu Dabrh, L. J. Prokop, Z. Wang, M. H. Murad, and K. Mohammed. 2016. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 63(1):284-306.
Makarova-Rusher, O. V., S. F. Altekruse, T. S. McNeel, S. Ulahannan, A. G. Duffy, B. I. Graubard, T. F. Greten, and K. A. McGlynn. 2016. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 122(11):1757-1765.
Man John Law, L., A. Landi, W. C. Magee, D. Lorne Tyrrell, and M. Houghton. 2013. Progress towards a hepatitis C virus vaccine. Emerging Microbes & Infections 2:e79.
Marrero, J. A., and H. B. El-Serag. 2011. Alpha-fetoprotein should be included in the hepatocellular carcinoma surveillance guidelines of the American Association for the Study of Liver Diseases. Hepatology 53(3):1060-1061; author reply 1061-1062.
Mars, S. G., P. Bourgois, G. Karandinos, F. Montero, and D. Ciccarone. 2014. “Every ‘never’ I ever said came true”: Transitions from opioid pills to heroin injecting. International Journal of Drug Policy 25(2):257-266.
Maurer, K., and E. F. Gondles. 2015. Hepatitis C in correctional settings: Challenges and opportunities. Coalition of Correctional Health Authorities and American Correctional Association 2(1).
McCaffrey, K., I. Boo, C. M. Owczarek, M. P. Hardy, M. A. Perugini, L. Fabri, P. Scotney, P. Poumbourios, and H. E. Drummer. 2017. An optimized hepatitis C virus E2 glycoprotein core adopts a functional homodimer that efficiently blocks virus entry. Journal of Virology 91(5).
Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437(7062):1167-1172.
Micallef, J. M., J. M. Kaldor, and G. J. Dore. 2006. Spontaneous viral clearance following acute hepatitis C infection: A systematic review of longitudinal studies. Journal of Viral Hepatitis 13(1):34-41.
Moon, C., K. S. Jung, D. Y. Kim, O. Baatarkhuu, J. Y. Park, B. K. Kim, S. U. Kim, S. H. Ahn, and K. H. Han. 2015. Lower incidence of hepatocellular carcinoma and cirrhosis in hepatitis C patients with sustained virological response by pegylated interferon and ribavirin. Digestive Diseases and Sciences 60(2):573-581.
Morgan, R. L., B. Baack, B. D. Smith, A. Yartel, M. Pitasi, and Y. Falck-Ytter. 2013. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Annals of Internal Medicine 158(5 Pt 1):329-337.
Morgan, T. R., M. G. Ghany, H. Y. Kim, K. K. Snow, M. L. Shiffman, J. L. De Santo, W. M. Lee, A. M. Di Bisceglie, H. L. Bonkovsky, J. L. Dienstag, C. Morishima, K. L. Lindsay, and A. S. Lok. 2010. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 52(3):833-844.
Nakamoto, Y., L. G. Guidotti, C. V. Kuhlen, P. Fowler, and F. V. Chisari. 1998. Immune pathogenesis of hepatocellular carcinoma. Journal of Experimental Medicine 188(2):341-350.
NAS (National Academy of Sciences). 2000. Beyond discovery: The hepatitis B story. http://www.nasonline.org/publications/beyond-discovery/hepatitis-b-story.pdf (accessed January 3, 2017).
NASEM (National Academies of Sciences, Engineering, and Medicine). 2016a. Eliminating the public health problem of hepatitis B and C in the United States: Phase one report. Washington, DC: The National Academies Press.
NASEM. 2016b. Ending discrimination against people with mental and substance use disorders: The evidence for stigma change. Washington, DC: The National Academies Press.
NCI (National Cancer Institute). 2016. Adult primary liver cancer treatment (PDQ®)–Health professional version. https://www.cancer.gov/types/liver/hp/adult-liver-treatment-pdq (accessed October 28, 2016).
NCI. n.d. HIV and AIDS Malignancy Branch. https://ccr.cancer.gov/HIV-and-AIDS-Malignancy-Branch (accessed December 15, 2016).
Neaigus, A., V. A. Gyarmathy, M. Miller, V. M. Frajzyngier, S. R. Friedman, and D. C. Des Jarlais. 2006. Transitions to injecting drug use among noninjecting heroin users: Social network influence and individual susceptibility. Journal of Acquired Immune Deficiency Syndromes 41(4):493-503.
Nelson, P. K., B. M. Mathers, B. Cowie, H. Hagan, D. Des Jarlais, D. Horyniak, and L. Degenhardt. 2011. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: Results of systematic reviews. Lancet 378(9791):571-583.
NIAID (National Institute of Allergy and Infectious Diseases). 2017. Staged phase I/II hepatitis C prophylactic vaccine. In: ClinicalTrials.gov. Bethesda, MD: National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01436357 (accessed February 23, 2017).
NIDA (National Institute on Drug Abuse). 2011. More opioid replacement therapy in correctional facilities might yield public safety and health benefits. https://www.drugabuse.gov/news-events/nida-notes/2011/07/prison-use-medications-opioid-addiction-remains-low (accessed December 15, 2016).
NIH (National Institutes of Health). 2011. HIV study named 2011 breakthrough of the year by Science. https://www.nih.gov/news-events/news-releases/hiv-study-named-2011-breakthrough-year-science (accessed December 15, 2016).
NIH. 2013. Committees, working groups, and tasks forces. https://dpcpsi.nih.gov/collaborations/committees.aspx?TID=2&FY=2013 (accessed February 23, 2017).
NIH. 2014. Drug addiction treatment in the criminal justice system: Drug use, crime, and incarceration. https://www.drugabuse.gov/related-topics/criminal-justice/drug-addiction-treatment-in-criminal-justice-system (accessed December 15, 2016).
NIH. 2016a. About DPCPSI. https://dpcpsi.nih.gov/about (accessed February 23, 2017).
NIH. 2016b. Estimates of funding for various research, condition, and disease categories (RCDC). https://report.nih.gov/categorical_spending.aspx (accessed December 15, 2016).
NIH. 2016c. NIH budget history: Total NIH budget authority: FY 2016 enacted. https://report.nih.gov/NIHDatabook/Charts/Default.aspx?showm=Y&chartId=5&catId=1 (accessed March 6, 2017).
NIH. n.d. OAR history. https://www.oar.nih.gov/about_oar/history.asp (accessed December 15, 2016).
Nobelprize.org. n.d. The Nobel Prize in physiology or medicine fields. https://www.nobelprize.org/nobel_prizes/medicine/fields.html (accessed January 3, 2017).
Norton, B. L., W. N. Southern, M. Steinman, J. Deluca, Z. Rosner, A. H. Litwin, B. L. Norton, M. Steinman, J. Deluca, Z. Rosner, A. H. Litwin, W. N. Southern, and B. D. Smith. 2016. No differences in achieving hepatitis C virus care milestones between patients identified by birth cohort or risk-based screening. Clinical Gastroenterology and Hepatology 14(9):1356-1360.
Nunn, A., N. Zaller, S. Dickman, C. Trimbur, A. Nijhawan, and J. D. Rich. 2009. Methadone and buprenorphine prescribing and referral practices in U.S. prison systems: Results from a nationwide survey. Drug and Alcohol Dependence 105(1-2):83-88.
O’Brien, T. R., L. Prokunina-Olsson, and R. P. Donnelly. 2014. IFN- 4: The paradoxical new member of the interferon lambda family. Journal of Interferon & Cytokine Research 34(11):829-838.
Osburn, W. O., B. E. Fisher, K. A. Dowd, G. Urban, L. Liu, S. C. Ray, D. L. Thomas, and A. L. Cox. 2010. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 138(1):315-324.
Osburn, W. O., A. E. Snider, B. L. Wells, R. Latanich, J. R. Bailey, D. L. Thomas, A. L. Cox, and S. C. Ray. 2014. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 59(6):2140-2151.
Page, K., M. D. Morris, J. A. Hahn, L. Maher, and M. Prins. 2013. Injection drug use and hepatitis C virus infection in young adult injectors: Using evidence to inform comprehensive prevention. Clinical Infectious Diseases 57(Suppl 2):S32-S38.
Paina, L., and D. H. Peters. 2012. Understanding pathways for scaling up health services through the lens of complex adaptive systems. Health Policy and Planning 27(5):365-373.
Palese, P. 2016. Profile of Charles M. Rice, Ralf F. W. Bartenschlager, and Michael J. Sofia, 2016 Lasker-DeBakey Clinical Medical Research Awardees. Proceedings of the National Academy of Sciences of the United States of America 113(49):13934-13937.
Pan, C. Q., Z. Duan, E. Dai, S. Zhang, G. Han, Y. Wang, H. Zhang, H. Zou, B. Zhu, W. Zhao, and H. Jiang. 2016. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. New England Journal of Medicine 374(24):2324-2334.
Papastergiou, V., M. Stampori, P. Lisgos, C. Pselas, K. Prodromidou, and S. Karatapanis. 2013. Durability of a sustained virological response, late clinical sequelae, and long-term changes in aspartate aminotransferase to the platelet ratio index after successful treatment with peginterferon/ribavirin for chronic hepatitis C: A prospective study. European Journal of Gastroenterology & Hepatology 25(7):798-805.
Patel, E. U., A. L. Cox, S. H. Mehta, D. Boon, C. E. Mullis, J. Astemborski, W. O. Osburn, J. Quinn, A. D. Redd, G. D. Kirk, D. L. Thomas, T. C. Quinn, and O. Laeyendecker. 2016. Use of hepatitis C virus (HCV) immunoglobulin G antibody avidity as a biomarker to estimate the population-level incidence of HCV infection. Journal of Infectious Diseases 214(3):344-352.
Paul, S., A. Saxena, N. Terrin, K. Viveiros, E. M. Balk, and J. B. Wong. 2016. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: A systematic review and meta-analysis. Annals of Internal Medicine 164(1):30-40.
Pecoraro, A., and G. E. Woody. 2011. Medication-assisted treatment for opioid dependence: Making a difference in prisons. F1000 Medicine Reports 3:1.
Perez-Alvarez, R., C. Diaz-Lagares, F. Garcia-Hernandez, L. Lopez-Roses, P. Brito-Zeron, M. Perez-de-Lis, S. Retamozo, A. Bove, X. Bosch, J. M. Sanchez-Tapias, X. Forns, and M. Ramos-Casals. 2011. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: Analysis of 257 cases. Medicine 90(6):359-371.
Pestka, J. M., M. B. Zeisel, E. Blaser, P. Schurmann, B. Bartosch, F. L. Cosset, A. H. Patel, H. Meisel, J. Baumert, S. Viazov, K. Rispeter, H. E. Blum, M. Roggendorf, and T. F. Baumert. 2007. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proceedings of the National Academy of Sciences of the United States of America 104(14):6025-6030.
Peters, D. H., O. Alonge, T. Adam, I. A. Agyepong, and N. Tran. 2013. Implementation research: What it is and how to do it. BMJ 347:f6753.
Pillay, D., J. Herbeck, M. S. Cohen, T. de Oliveira, C. Fraser, O. Ratmann, A. L. Brown, and P. Kellam. 2015. PANGEA-HIV: Phylogenetics for generalised epidemics in Africa. The Lancet Infectious Diseases 15(3):259-261.
Pybus, O. G., and A. Rambaut. 2009. Evolutionary analysis of the dynamics of viral infectious disease. Nature Reviews Genetics 10(8):540-550.
Rodrigo, C., M. R. Walker, P. Leung, A. A. Eltahla, J. Grebely, G. J. Dore, T. Applegate, K. Page, S. Dwivedi, J. Bruneau, M. D. Morris, A. L. Cox, W. Osburn, A. Y. Kim, J. Schinkel, N. H. Shoukry, G. M. Lauer, L. Maher, M. Hellard, M. Prins, F. Luciani, A. R. Lloyd, and R. A. Bull. 2017. Limited naturally occurring escape in broadly neutralizing antibody epitopes in hepatitis C glycoprotein E2 and constrained sequence usage in acute infection. Infection, Genetics and Evolution 49:88-96.
Roychowdhury, S., and A. M. Chinnaiyan. 2016. Translating cancer genomes and transcriptomes for precision oncology. CA: A Cancer Journal for Clinicians 66(1):75-88.
Ryerson, A. B., C. R. Eheman, S. F. Altekruse, J. W. Ward, A. Jemal, R. L. Sherman, S. J. Henley, D. Holtzman, A. Lake, A. Noone, R. N. Anderson, J. Ma, K. N. Ly, K. A. Cronin, L. Penberthy, and B. A. Kohler. 2016. Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 122(9):1312-1337.
Schulze Zur Wiesch, J., D. Ciuffreda, L. Lewis-Ximenez, V. Kasprowicz, B. E. Nolan, H. Streeck, J. Aneja, L. L. Reyor, T. M. Allen, A. W. Lohse, B. McGovern, R. T. Chung, W. W. Kwok, A. Y. Kim, and G. M. Lauer. 2012. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. The Journal of Experimental Medicine 209(1):61-75.
Shah, S. A., J. K. Smith, Y. Li, S. C. Ng, J. E. Carroll, and J. F. Tseng. 2011. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer 117(5):1019-1026.
Sharma, P., S. D. Saini, L. B. Kuhn, J. H. Rubenstein, D. S. Pardi, J. A. Marrero, and P. S. Schoenfeld. 2011. Knowledge of hepatocellular carcinoma screening guidelines and clinical practices among gastroenterologists. Digestive Diseases and Sciences 56(2):569-577.
Sherman, S. G., L. Smith, G. Laney, and S. A. Strathdee. 2002. Social influences on the transition to injection drug use among young heroin sniffers: A qualitative analysis. International Journal of Drug Policy 13(2):113-120.
Shoukry, N. H., A. Grakoui, M. Houghton, D. Y. Chien, J. Ghrayeb, K. A. Reimann, and C. M. Walker. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. Journal of Experimental Medicine 197(12):1645-1655.
Singal, A. G., A. Pillai, and J. Tiro. 2014. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Medicine 11(4):e1001624.
Smith, B. D., J. Drobeniuc, A. Jewett, B. M. Branson, R. S. Garfein, E. Teshale, S. Kamili, and C. M. Weinbaum. 2011. Evaluation of three rapid screening assays for detection of antibodies to hepatitis C virus. Journal of Infectious Diseases 204(6):825-831.
Solomon, L., B. T. Montague, C. G. Beckwith, J. Baillargeon, M. Costa, D. Dumont, I. Kuo, A. Kurth, and J. D. Rich. 2014. Survey finds that many prisons and jails have room to improve HIV testing and coordination of postrelease treatment. Health Affairs 33(3):434-442.
Sonnenday, C. J., J. B. Dimick, R. D. Schulick, and M. A. Choti. 2007. Racial and geographic disparities in the utilization of surgical therapy for hepatocellular carcinoma. Journal of Gastrointestinal Surgery 11(12):1636-1646; discussion 1646.
Stanaway, J. D., A. D. Flaxman, M. Naghavi, C. Fitzmaurice, T. Vos, I. Abubakar, L. J. Abu-Raddad, R. Assadi, N. Bhala, B. Cowie, M. H. Forouzanfour, J. Groeger, K. M. Hanafiah, K. H. Jacobsen, S. L. James, J. MacLachlan, R. Malekzadeh, N. K. Martin, A. A. Mokdad, A. H. Mokdad, C. J. L. Murray, D. Plass, S. Rana, D. B. Rein, J. H. Richardus, J. Sanabria, M. Saylan, S. Shahraz, S. So, V. V. Vlassov, E. Weiderpass, S. T. Wiersma, M. Younis, C. Yu, M. El Sayed Zaki, and G. S. Cooke. 2016. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet 388(10049):1081-1088.
Stockings, E., W. D. Hall, M. Lynskey, K. I. Morley, N. Reavley, J. Strang, G. Patton, and L. Degenhardt. 2016. Prevention, early intervention, harm reduction, and treatment of substance use in young people. The Lancet Psychiatry 3(3):280-296.
Strathdee, S. A., and C. Beyrer. 2015. Threading the needle—How to stop the HIV outbreak in rural Indiana. New England Journal of Medicine 373(5):397-399.
Swadling, L., S. Capone, R. D. Antrobus, A. Brown, R. Richardson, E. W. Newell, J. Halliday, C. Kelly, D. Bowen, J. Fergusson, A. Kurioka, V. Ammendola, M. Del Sorbo, F. Grazioli, M. L. Esposito, L. Siani, C. Traboni, A. Hill, S. Colloca, M. Davis, A. Nicosia, R. Cortese, A. Folgori, P. Klenerman, and E. Barnes. 2014. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Science Translational Medicine 6(261):261ra153.
Terrault, N. A., N. H. Bzowej, K. M. Chang, J. P. Hwang, M. M. Jonas, and M. H. Murad. 2016. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 63(1):261-283.
Tholey, D. M., and J. Ahn. 2015. Impact of hepatitis C virus infection on hepatocellular carcinoma. Gastroenterology Clinics of North America 44(4):761-773.
Tiong, L., and G. J. Maddern. 2011. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. British Journal of Surgery 98(9):1210-1224.
Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annual Review of Immunology 18:861-926.
University of Pennsylvania. n.d. Risk assessment battery (RAB): Overview. University of Pennsylvania Perelman School of Medicine, Department of Psychiatry, HIV/AIDS Prevention Research Division. http://www.med.upenn.edu/hiv/rab_overview.html (accessed December 14, 2016).
USPSTF (U.S. Preventive Services Task Force). 2014. Final recommendation statement: Hepatitis B virus infection: Screening, 2014. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-b-virus-infection-screening-2014 (accessed October 31, 2016).
VA (Department of Veterans Affairs). 2014. State of care for veterans with hepatitis C 2014. Veterans Health Administration.
Vestal, C. 2016. Helping drug-addicted inmates break the cycle. Pew Charitable Trusts, January 13. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/01/13/helping-drug-addicted-inmates-break-the-cycle (accessed December 15, 2016).
Viral Hepatitis Implementation Group. 2011. Status report on the implementation of the viral hepatitis action plan. https://www.aids.gov/pdf/status-report-on-implementation-of-vhap.pdf (accessed February 24, 2017).
Ward, J., S. Darke, and W. Hall. 1990. The HIV Risk-taking Behavior Scale (HRBS) manual. Technical report no. 10. National Drug and Alcohol Research Centre. https://ndarc.med.unsw.edu.au/sites/default/files/ndarc/resources/TR.010.PDF (accessed December 14, 2016).
Weeks, M. R., M. Convey, J. Dickson-Gomez, J. Li, K. Radda, M. Martinez, and E. Robles. 2009. Changing drug users’ risk environments: Peer health advocates as multi-level community change agents. American Journal of Community Psychology 43(3-4):330-344.
Weinbaum, C., R. Lyerla, and H. S. Margolis. 2003. Prevention and control of infections with hepatitis viruses in correctional settings. Morbidity and Mortality Weekly Report 52(RR-01):1-33.
Welzel, T. M., B. I. Graubard, S. Quraishi, S. Zeuzem, J. A. Davila, H. B. El-Serag, and K. A. McGlynn. 2013. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. American Journal of Gastroenterology 108(8):1314-1321.
Westbrook, R. H., and G. Dusheiko. 2014. Natural history of hepatitis C. Journal of Hepatology 61(1 Suppl):S58-S68.
WHO (World Health Organization). 2009. Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. Geneva, Switzerland: WHO.
WHO. 2016a. Hepatitis B: Fact sheet. http://www.who.int/mediacentre/factsheets/fs204/en (accessed December 6, 2016).
WHO. 2016b. Hepatitis C: Fact sheet. http://www.who.int/mediacentre/factsheets/fs164/en (accessed December 15, 2016).
WHO. 2016c. HIV/AIDS: Factsheet. http://www.who.int/mediacentre/factsheets/fs360/en (accessed December 15, 2016).
Wu, Y., K. B. Johnson, G. Roccaro, J. Lopez, H. Zheng, A. Muiru, N. Ufere, R. Rajbhandari, O. Kattan, and R. T. Chung. 2014. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. American Journal of Gastroenterology 109(6):867-875.
Yang, Y. 2015. Cancer immunotherapy: Harnessing the immune system to battle cancer. Journal of Clinical Investigation 125(9):3335-3337.
Yehia, B. R., A. J. Schranz, C. A. Umscheid, and V. Lo Re III. 2014. The treatment cascade for chronic hepatitis C virus infection in the United States: A systematic review and meta-analysis. PLoS One 9(7):e101554.
Young, A. M., A. B. Jonas, and J. R. Havens. 2013. Social networks and HCV viraemia in anti-HCV-positive rural drug users. Epidemiology & Infection 141(2):402-411.
This page intentionally left blank.


























