2
Targets for Elimination
The World Health Organization’s (WHO’s) hepatitis strategy document set targets for reducing the world’s burden of viral hepatitis (see Table 2-1). The document makes clear, however, that the proposed targets are global and will not necessarily be suitable for any one country. The emphasis on the screening of donor blood and the use of safety-engineered syringes, for example, does not apply to the United States but to countries where such measures are not already required. The proposed reductions in incidence and mortality shown in Table 2-1 are similarly broad. One percent prevalence of hepatitis B surface antigen (HBsAg) among children is an aspirational goal for 2020 in most of the world’s hepatitis B endemic countries but considerably higher than 2006 estimates suggest for the United States (Wasley et al., 2010).
Given differences in epidemiology and disease burden, WHO guidance asks every country to identify its most affected populations and tailor its response accordingly (WHO, 2016b). The organization’s strategy document directs countries to “develop as soon as practicable ambitious national goals and targets for 2020 and beyond [. . .]. Targets should be feasible and developed based on country realities, the best possible data [. . .], trends and responses, and monitored through a set of standard and measurable indicators” (WHO, 2016b, p. 23). Box 2-1, for example, describes the Republic of Georgia’s viral hepatitis strategy in response to the country’s high prevalence of hepatitis C.
As a first step to identifying feasible targets for hepatitis B and C elimination in the United States, the committee commissioned modeling analysis to estimate how different interventions might reduce the national burden of
TABLE 2-1 WHO Targets for Reducing the Global Burden of Viral Hepatitis
| Target Area | Baseline 2015 | 2020 Targets | 2030 Targets |
|---|---|---|---|
| Impact targets | |||
| Incidence: New cases of chronic viral hepatitis B and C infections | Between 6 and 10 million infections are reduced to 0.9 million infections by 2030 (95% decline in hepatitis B virus infections, 80% decline in hepatitis C virus infections) | 30% reduction (equivalent to 1% prevalence of HBsAg among children) |
90% reduction (equivalent to 0.1% prevalence of HBsAg among children) |
| Mortality: Viral hepatitis B and C deaths | 1.4 million deaths reduced to less than 500,000 by 2030 (65% for both viral hepatitis B and C) | 10% reduction | 65% reduction |
| Service coverage targets | |||
| Hepatitis B virus vaccination: childhood vaccine coverage (third dose coverage) | 82% in infants | 90% | 90% |
| Prevention of hepatitis B virus mother-to-child transmission: hepatitis B virus birth-dose vaccination coverage or other approach to prevent mother-to-child transmission | 38% | 50% | 90% |
| Blood safety | 39 countries do not routinely test all blood donations for transfusion-transmissible infections 89% of donations screened in a quality-assured manner |
95% of donations screened in a quality-assured manner | 100% of donations are screened in a quality-assured manner |
| Target Area | Baseline 2015 | 2020 Targets | 2030 Targets |
|---|---|---|---|
| Safe injections: percentage of injections administered with safety-engineered devices in and out of health facilities | 5% | 50% | 90% |
| Harm reduction: number of sterile needles and syringes provided per person who injects drugs per year | 20 | 200 | 300 |
| Viral hepatitis B and C diagnosis | <5% of chronic hepatitis infections diagnosed | 30% | 90% |
| Viral hepatitis B and C treatment | <1% receiving treatment | 5 million people will be receiving hepatitis B virus treatment 3 million people have received hepatitis C virus treatment (Both targets are cumulative by 2020) |
80% of eligible persons with chronic hepatitis B virus infection treated 80% of eligible persons with chronic hepatitis C virus infection treated |
NOTE: HBsAg = hepatitis B surface antigen; WHO = World Health Organization.
SOURCE: WHO, 2016b.
hepatitis B and C, including liver cancer, cirrhosis, and liver-related deaths (see Appendixes A and B). The modelers were chosen on the basis of their prior work in the field; only models that have been extensively validated and peer-reviewed were considered.1
Given the inherent differences in the biology, epidemiology, natural history, and treatment options for hepatitis B and C, the models presented in this chapter are not directly comparable. Broadly, the hepatitis B model considers the effects of varying rates of diagnosis, care, and treatment on
___________________
1 Staff at Center for Disease Analysis can make the hepatitis C model available upon request to employees of government or academic institutions. The hepatitis B model can be re-created using TreeAge software and the information presented in Appendix A.
liver health outcomes. The hepatitis C model, in contrast, considers the absolute number of people treated and diagnosed as inputs, then compares the consequences of different treatment strategies. Both provide useful insight into realistic targets for hepatitis B and C elimination in the United States.
HEPATITIS B MODELS
The author’s full report Population Health Impact and Cost-Effectiveness of Chronic Hepatitis B Diagnosis, Care, and Treatment in the United States is shown in Appendix A. Briefly, the author adapted a Markov model originally developed to study hepatitis B interventions in Shanghai, China
(Toy et al., 2014). Updated data from recent cohort studies and a meta-analysis of mostly North American research were used to estimate disease progression (Campsen et al., 2013; Chen et al., 2010; Chu and Liaw, 2007, 2009; Fattovich et al., 2008; Kanwal et al., 2006; Lin et al., 2005; Raffetti et al., 2016; Thiele et al., 2014) and treatment effectiveness (Heathcote et al., 2011; Lok et al., 2016; Papatheodoridis et al., 2015; Tenney et al., 2009; Wong et al., 2013). The model’s assumptions regarding the likelihood of developing cirrhosis and liver cancer during antiviral treatment are based on recent data (Arends et al., 2015; Marcellin et al., 2013). Background mortality by age and probability of receiving a liver transplant were drawn from the Organ Procurement and Transplant Network (HRSA, 2016).
The model then compared different rates of diagnosis, care, treatment (among the subset of chronic hepatitis B patients for whom treatment is appropriate), and patient adherence to serological monitoring, as shown in Table 2-2. The estimates for current practice are based on peer-reviewed studies (Chotiyaputta et al., 2011; Hu et al., 2013; Juday et al., 2011; Kim et al., 2014; Lin et al., 2007). The next scenario (HHS 2020 Target) showed the effect of meeting the Department of Health and Human Services (HHS) goal of increasing diagnosis from one-third to two-thirds of all patients with chronic hepatitis B (HHS, 2015). A third scenario (HHS 2020 Target + Improved Treatment) added improvements in rates of care and treatment to the stated HHS target. For additional comparison, the modeler then included a scenario of 80 percent diagnosed, 80 percent of those in care, with 80 percent treatment among those eligible, and 80 and 95 percent patient adherence to monitoring and treatment. This scenario is slightly lower coverage than the next scenario, modeling the WHO’s proposed global targets of 90 percent of chronic hepatitis B cases diagnosed, and 80 percent of treatment among those eligible, assuming that 90 percent of diagnosed patients are in care, with (for comparison’s sake) perfect patient adherence (WHO, 2016a). Finally, the analysis considered an ideal scenario where everyone with chronic hepatitis B is diagnosed and adheres perfectly to treatment and monitoring.
The modeled prevalence of chronic hepatitis B in the United States in 2015 was 1.29 million people (95 percent confidence interval [CI]: 855,000 to 2.02 million), similar to the Center for Disease Control and Prevention’s (CDC’s) current estimate of 850,000 to 2.2 million people (CDC, 2016a). This prevalence amounts to 0.4 percent (95 percent CI: 0.27 to 0.63 percent) of the total U.S. population, compared to the most recent NHANES2 estimate of 0.3 percent (95 percent CI: 0.2 to 0.4 percent) (Roberts et al., 2016). Foreign-born blacks and people born in Asia account for 72.6 percent of cases. Appendix A gives more detail on the age breakdown of
___________________
2 Officially, National Health and Nutrition Examination Survey.
TABLE 2-2 Scenario Analysis Rates
| Scenario | Diagnosed | Received HBV Care | Treatment Rate Among Treatment Eligible Patients | Adherence to Monitoring | Adherence to Treatment |
|---|---|---|---|---|---|
| Natural History | — | — | — | — | — |
| Current Practice | 34.6%a | 33.3%b | 45%c | 35.1%d | 85%e |
| HHS 2020 Target | 66% | 33.3% | 45% | 35.1% | 85% |
| HHS 2020 Target + Improved Rx | 66% | 80% | 80% | 35.1% | 85% |
| Hypothetical Scenario | 80% | 80% | 80% | 80% | 95% |
| WHO 2030 Target | 90% | 90% | 80% | 100% | 100% |
| Idealistic (Utopian) | 100% | 100% | 100% | 100% | 100% |
NOTE: HBV = hepatitis B virus; HHS = Department of Health and Human Services; WHO = World Health Organization.
this cohort, about 25 percent of whom are eligible for antiviral treatment because of chronic active hepatitis or cirrhosis.
Table 2-3 shows the cumulative risks in 2030 of hepatocellular carcinoma, cirrhosis, and HBV-related death among chronic hepatitis B patients in the United States, based on the current practice for diagnosis, care, and treatment. Table 2-4 shows cumulative reductions in these outcomes relative to 2015 practice. Improving the rate of diagnosis to the two-thirds level cited in the HHS strategy document would reduce deaths related to hepatitis B by only about 4 percent by 2030 (HHS, 2015). Table 2-4 makes it clear that higher levels of diagnosis, care, and treatment will be necessary to meaningfully reduce morbidity and mortality from chronic hepatitis B. If the United States were to meet the WHO target of 90 percent of chronic hepatitis B patients diagnosed, 90 percent of those in care, and 80 percent treatment among those eligible (hereafter the 90/90/80 scenario), there would be about 50 percent fewer cumulative deaths related to hepatitis B in the United States over the next 15 years. Meeting the same targets would reduce new cases of cirrhosis by about 45 percent and new cases of hepatocellular carcinoma by about one-third.
Under the current practice, about 9.4 percent of HBV-infected people would die by 2030, only 4.7 percent would die if the WHO target levels of diagnosis, testing, and treatment were met. Given the size of the infected population, this would translate into about 60,630 deaths averted by 2030. (Working off the lower bound of the estimate of HBV-infected people in the United States, 40,185 deaths averted; given the higher bound, 94,940 deaths averted.)
At the same time, the model’s analysis of the WHO 2030 target scenario assumed almost perfect adherence to serological monitoring and treatment. This would be a significant improvement over current practice and will require changing the system for delivering hepatitis B care; such changes are discussed more in Chapter 5. Sensitivity analysis shown in Appendix A indicates that increasing care has the strongest effect on the outcomes, followed by increasing diagnosis. Increasing treatment had the least effect on the model’s outcomes.
As with all such analyses, this model has several important limitations. First, it works with a hypothetical cohort of chronic hepatitis B patients in the United States in 2015. This cohort does not include the estimated 23,370 new chronic hepatitis B cases (95 percent CI: 17,800 to 31,660 cases) entering the United States every year from immigration, or the relatively small number (fewer than 2,000 a year) of chronic infections acquired domestically.3 When considering new cases from immigration, the preva-
___________________
3 The CDC estimates about 19,200 acute infections every year, including roughly 900 newborns a year acquiring the infection from their mothers (95 percent CI: 800 to 1,000) (CDC, 2016b; Ko et al., 2014).
TABLE 2-3 Cumulative Risks of Hepatocellular Carcinoma, Cirrhosis, and HBV-Related Deaths by 2030 in the 2015 Cohort of Chronic HBV-Infected Persons in the United States
| Cumulative Risk | Scenario | |||||
|---|---|---|---|---|---|---|
| Current Practice D35/C33/T45 | HHS 2020 Target D66/C33/T45 | HHS + Improved Rx D66/C80/T80 | Hypothetical Scenario D80/C80/T80 | WHO 2030 Target D90/C90/T80 | Idealistic D100/C100/T100 | |
| Hepatocellular carcinoma risk | 6.00% | 5.84% | 5.27% | 4.46% | 3.91% | 3.14% |
| Cirrhosis risk | 10.31% | 10.15% | 9.01% | 6.84% | 5.70% | 3.79% |
| HBV-related deaths | 9.40% | 8.98% | 7.60% | 5.98% | 4.66% | 2.84% |
NOTE: HBV = hepatitis B virus; HHS = Department of Health and Human Services; WHO = World Health Organization.
TABLE 2-4 Cumulative Reduction in Hepatocellular Carcinoma, Cirrhosis, and HBV-Related Deaths in the 2015 Cohort of Chronic HBV-Infected Persons with Various Improved Diagnosis, Care, and Treatment Scenarios Compared with the Base Scenario (D35/C33/T45) in 15 Years
| Cumulative Reduction | Scenario | ||||
|---|---|---|---|---|---|
| HHS 2020 Target D66/C33/T45 | HHS + Improved Rx D66/C80/T80 | Hypothetical Scenario D80/C80/T80 | WHO 2030 Target D90/C90/T80 | Idealistic D100/C100/T100 | |
| Hepatocellular carcinoma cases | 2.66% | 12.16% | 25.66% | 34.83% | 47.66% |
| Cirrhosis cases | 1.55% | 12.60% | 33.65% | 44.71% | 63.23% |
| HBV-related deaths | 4.46% | 19.14% | 36.38% | 50.42% | 69.78% |
NOTE: HBV = hepatitis B virus; HHS = Department of Health and Human Services; WHO = World Health Organization.
lence of chronic hepatitis B increases to about 1.64 million by 2030. (These additional cases would not, however, affect the estimated percent reduction in cumulative risk if they follow the same diagnosis, care, and treatment patterns.) NHANES estimates put the prevalence of chronic hepatitis B around 730,000 from 1999 to 2006 (Wasley et al., 2010). After 2007, when more attention was given to identifying participants of Asian descent, the estimate rose to 850,000 (Roberts et al., 2016). But since people born in HBV endemic countries are still not well-represented in NHANES this figure is likely an underestimate (Cohen et al., 2008; Kim, 2009). If the foreign-born populations in the United States have the same prevalence rates as in their birth countries, as many as 2.2 million additional people may have hepatitis B (Kowdley et al., 2012). At the same time, immigrants are not entirely representative of their native country; the hepatitis B prevalence in an immigrant’s birth country may not apply to the people living in the United States (Uddin et al., 2010). With this is mind, the modeler used age-specific U.S. prevalence rates, reported in studies of various racial and ethnic groups, including people born in the United States and abroad. To accommodate the range in prevalence estimates, the committee based its conclusions on percentage reductions in morbidity and mortality rather than absolute numbers.
Furthermore, the work presented in Appendix A does not model strategies to end mother-to-child transmission of hepatitis B or horizontal transmission, as this is not a question for which the modeler has developed peer-reviewed analytic tools. Both are now rare in the United States. Only about 900 infants a year contract HBV infection at birth (Ko et al., 2014); less than 5 percent of the roughly 19,200 adults and children over five who acquire HBV every year develop chronic hepatitis B (CDC, 2016b; Mast et al., 2006). Therefore, new cases entering the population from horizontal transmission would not substantively change the estimates.
Work in Alaska has shown that it is possible to fully eliminate mother-to-child transmission of HBV. Despite HBV infection being endemic among Alaska Natives and 70 percent of chronic hepatitis B patients living in remote areas without road connection, there has not been a single case of acute hepatitis B in an Alaska Native child since 19924 (FitzSimons et al., 2013; McMahon et al., 2014). This work, coupled with an assessment of the relative rarity of vertical transmission of HBV, may have inspired the support for elimination of mother-to-child transmission of viral hepatitis expressed in the U.S. government’s Viral Hepatitis Action Plan, 2017-2020 (HHS, 2017b). Chapter 4 discusses management of HBsAg+ pregnant women and steps that may be taken to replicate the Alaskan success nation-
___________________
4 Barring one or two international adoptions (McMahon, 2015).
ally. This chapter also describes measures to bring hepatitis B vaccination to more adults, thereby preventing horizontal transmission of HBV.
HEPATITIS C MODELS
The author’s full report, Modeling the Elimination of Hepatitis C in the United States, is shown in Appendix B. The author used a Markov model developed to estimate how morbidity, mortality, and total number of viremic HCV infections would change between 2015 and 2030 (Razavi et al., 2013, 2014). The model has been used previously to project disease burden in 100 countries; results have been validated with a panel of local experts in 59 of these countries (Blach et al., 2016). The model estimates the number of incident cases of hepatitis C, accounting for spontaneous clearance (Razavi et al., 2014). An older version of the model (predating direct-acting antiviral treatment for chronic hepatitis C) was validated with U.S. data (Kershenobich et al., 2011).
The model drew on published data from multiple sources. Estimates of the current U.S. population, including mortality by gender and age, came from United Nations (UN) data (Razavi et al., 2014; UN, 2015). Estimates of the percentage of the population who are HCV antibody positive and HCV RNA positive, the age and sex distribution of this prevalence, and the total number of cases diagnosed in a given year were drawn from peer-reviewed literature (Denniston et al., 2014; Edlin et al., 2015; Seeff, 2002; Volk et al., 2009). CDC data informed estimates of the distribution of HCV genotypes in the population and across newly diagnosed cases, while national reports and drug sale data were used to estimate the number of patients treated each year (Klevens et al., 2009, 2014; NCHS, 2015).
Information on the number of liver transplants, incidence of hepatocellular carcinoma, and deaths from hepatitis C came from published sources (Altekruse et al., 2014; CDC, 2016a; HRSA, 2016; NCI, 2015; Yang et al., 2012). The model’s results were validated against incident cases of hepatocellular carcinoma attributable to chronic hepatitis C as reported to the Surveillance, Epidemiology, and End Results program.
The model compared four scenarios with different assumptions about screening and treatment on hepatitis C incidence and deaths, hepatocellular carcinoma, and decompensated cirrhosis due to hepatitis C. Table 2-5 describes the four scenarios. Briefly, the first scenario (labeled “Base 2013”) reflects historical treatment data before the 2014 introduction of direct-acting antivirals. This scenario assumes 110,000 new patients diagnosed every year between 2013 and 2017. This number is shown to decline after 2017, both because of the preventive effect of removing infectious cases from the population and because over time the treatment-eligible patients remaining will be harder to find. This scenario allows for about 30,000 patients treated a year, and a rate of sustained virologic response5 of 58 percent. The second scenario (labeled “Base 2015”) assumes relatively little change in the number of new infections annually, but the use of current drug therapy, with its vastly better rates of sustained virologic response and far more people eligible for treatment. The model also assumes annual treatment of 260,000 people (about what has been reported since direct-acting agents came on the market) gradually declining to 130,000 between 2020 and 2030, but with current restrictions limiting treatment to patients with fibrosis grade 2 or worse. This model makes the same assumptions as the base scenario regarding the number of cases diagnosed and new infections each year. The third scenario (labeled “Aggressive ≥F0”) assumes no fibrosis restrictions on treatment after 2017, with aggressive efforts made to diagnose new cases, but allows that the annual number diagnosed will begin to decrease around 2020 because there will be fewer infected cases in the population and the cases left will be harder to find. The final scenario (labeled “Aggressive ≥F2”) also assumes 260,000 people will be treated every year and that aggressive measures will be taken to diagnose new cases, but limits treatment only to people with hepatitis fibrosis stage 2 or higher. The drop-off in the number of newly diagnosed cases is less pronounced in this model because HCV transmission will not decline much in a situation where only people with more advanced fibrosis are treated.
Estimates of the percentage change in total viremic HCV infections (meaning cases with detectable HCV RNA) and the percentage change in
___________________
5 Sustained virologic response refers to eradication of HCV from the body, indicated by no detectable viral RNA 24 weeks after therapy. Relapse after sustained virologic response occurs in less than 1 percent of cases (NASEM, 2016a).
TABLE 2-5 Model Assumptions Regarding Numbers of People Treated, Diagnosed, and Newly Infected with HCV, as well as Their Ages and the Effectiveness of Treatment Over Time
| Scenario | Assumption | Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 |
|---|---|---|---|---|---|---|
| Base 2013 | Years | 2013-2015 | 2016-2017 | 2018-2019 | 2020-2024 | 2025-2030 |
| Annual Treated | 32,000 | 32,000 | 32,000 | 32,000 | 32,000 | |
| Annual Newly Diagnosed | 110,000 | 110,000 | 77,780 | 55,000 | 55,000 | |
| Fibrosis Stage | ≥F0 | ≥F0 | ≥F0 | ≥F0 | ≥F0 | |
| Annual New Infections | 29,690 | 30,270 | 30,100 | 29,980 | 29,800 | |
| Treated Age | 15-64 | 15-64 | 15-64 | 15-64 | 15-64 | |
| SVR | 58% | 58% | 58% | 58% | 58% | |
| Base 2015 | Years | 2013-2014 | 2015-2016 | 2017-2019 | 2020-2024 | 2025-2030 |
| Annual Treated | 32,000 | 260,000 | 183,800 | 130,000 | 130,000 | |
| Annual Newly Diagnosed | 110,000 | 110,000 | 77,780 | 55,000 | 55,000 | |
| Fibrosis Stage | ≥F0 | ≥F2 | ≥F2 | ≥F2 | ≥F2 | |
| Annual New Infections | 29,690 | 30,340 | 30,160 | 29,980 | 29,830 | |
| Treated Age | 15-64 | 15-64 | 15-74 | 15-74 | 15-74 | |
| SVR | 58% | 90% | 95% | 95% | 95% |
| Aggressive ≥F0 | Years | 2013-2014 | 2015-2016 | 2017-2019 | 2020-2024 | 2025-2030 |
| Annual Treated | 32,000 | 260,000 | 260,000 | 260,000 | 260,000 | |
| Annual Newly Diagnosed | 110,000 | 110,000 | 110,000 | 88,790 | 71,660 | |
| Fibrosis Stage | ≥F0 | ≥F1 | ≥F0 | ≥F0 | ≥F0 | |
| Annual New Infections | 29,690 | 30,340 | 22,620 | 11,150 | 2,730 | |
| Treated Age | 15-64 | 15-64 | 15-74 | 15-74 | 15-74 | |
| SVR | 58% | 90% | 95% | 95% | 95% | |
| Aggressive ≥F2 | Years | 2013-2014 | 2015-2016 | 2017-2019 | 2020-2024 | 2025-2030 |
| Annual Treated | 32,000 | 260,000 | 260,000 | 260,000 | 260,000 | |
| Annual Newly Diagnosed | 110,000 | 110,000 | 110,000 | 95,940 | 83,670 | |
| Fibrosis Stage | ≥F0 | ≥F2 | ≥F2 | ≥F2 | ≥F2 | |
| Annual New Infections | 29,690 | 30,330 | 30,150 | 29,960 | 29,800 | |
| Treated Age | 15-64 | 15-64 | 15-74 | 15-74 | 15-74 | |
| SVR | 58% | 90% | 95% | 95% | 95% |
NOTE: HCV = hepatitis C virus; SVR = sustained virologic response.
cumulative incident hepatocellular carcinoma, decompensated cirrhosis, and liver-related deaths relative to the 2015 baseline scenario are shown in Figure 2-1. As with the hepatitis B models, the value of the models lies in comparing the different scenarios and identifying important influences on the outcomes.
Removing all disease severity restrictions and taking aggressive efforts to diagnose new cases would reduce cumulative liver deaths between 2015 and 2030 by 10 percent (relative to 2015). As Figure 2-1 shows, the scenario combining aggressive diagnosis and treatment with restriction to only patients with advanced fibrosis results in a 35 percent reduction in deaths over the same time period. This counterintuitive result is partly a function of the model that holds constant the annual number of treated patients. The treatment restriction scenario modeled therefore includes some relatively healthy people in the 260,000 treated each year. Given that chronic hepatitis C infection is usually asymptomatic for decades, many of the people cured in this scenario would not have died by 2030, but they are more likely to transmit the infection (Hagan et al., 2004; Zeiler et al., 2010). For this reason, unrestricted treatment has a pronounced influence on incidence of chronic hepatitis C.
The model indicates considerable public health benefit to unrestricted treatment. Figure 2-1 shows that the total number of infected cases would drop 75 percent by 2030 (relative to the 2015 base scenario) if treatment were allocated without regard for disease stage. The trade-off between a sharp reduction in disease prevalence and a marginally larger reduction in deaths implied by these models is a function of holding the number of patients treated as an input parameter. Data from recent drug sales and national reports inform the model estimate of 260,000 patients treated every year. There is no reason why this number could not increase, especially if the recommendations made in Chapter 5 are implemented. Increasing the number of patients treated annually, so as to treat those with minimal fibrosis and those with more advanced disease at the same time, would allow for larger reductions in both chronic hepatitis C incidence and prevalence and liver-related mortality.
As Appendix B discusses, there were an estimated 21,600 deaths from chronic hepatitis C in the United States in 2015 (95 percent CI: 10,300 to 36,700 deaths). With no change to current practice, about 13,500 deaths related to hepatitis C would be expected in 2030, or 37.5 percent fewer than in 2015. The scenario combining unrestricted treatment with aggressive diagnosis (labeled “Aggressive ≥F0”) would result in 7,100 hepatitis C-related deaths in 2030, about a two-thirds reduction from the 2015 level. The same scenario would reduce the incidence of chronic hepatitis C by 90 percent. Table 2-6 presents this information as well as the reduction in prevalence relative to the 2015 scenario.
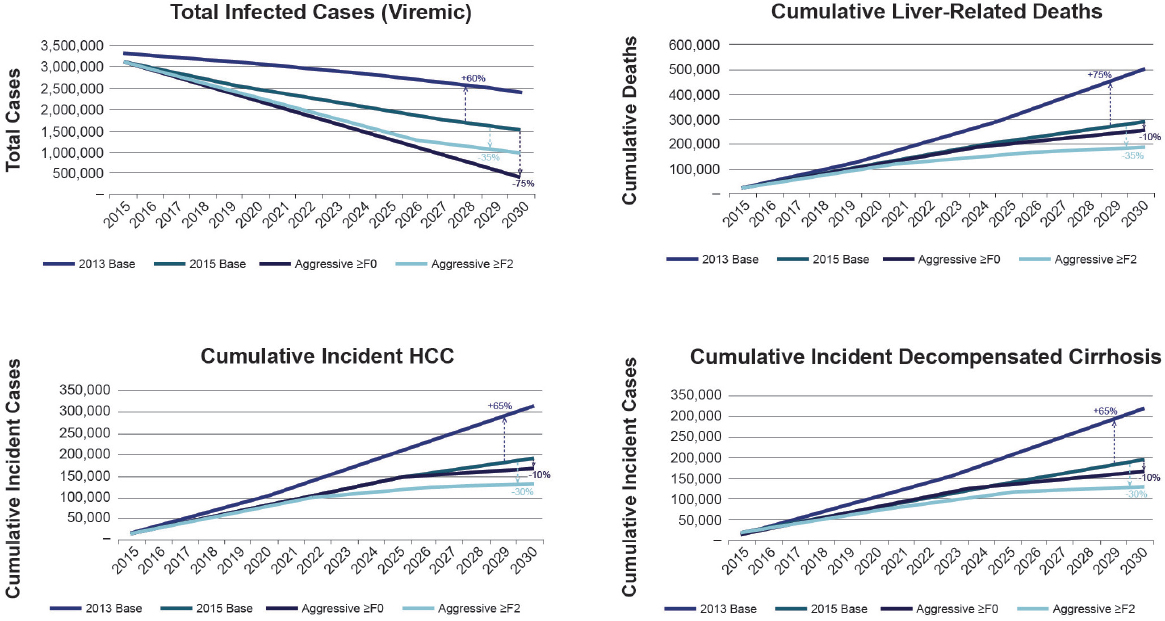
NOTE: HCC = hepatocellular carcinoma.
TABLE 2-6 Reduction in Number of Deaths, Prevalence, and Incidence of Hepatitis C in 2030 Relative to 2015
| No Change from 2015 | Aggressive ≥F0 | Aggressive ≥F2 | |
|---|---|---|---|
| Number of deaths in 2030 | 13,500 | 7,100 | 4,100 |
| Reduction in mortality relative 2015 | 38% | 65% | 80% |
| Total viremic cases in 2030 | 1,495,000 | 390,000 | 980,000 |
| Reduction in prevalence relative 2015 | 50% | 85% | 70% |
| Total incident cases in 2030 | 29,830 | 2,730 | 29,800 |
| Reduction in incidence relative 2015 | 2% | 90% | 2% |
| Cumulative HCV deaths | 289,200 | 260,400 | 190,700 |
| Deaths averted by 2030 | — | 28,800 | 98,500 |
NOTE: HCV = hepatitis C virus.
The committee’s first report discussed a theoretical trade-off in eliminating hepatitis C: ending HCV transmission and ending deaths from hepatitis C are both possible with treatment, but meeting those goals requires attention to different populations (NASEM, 2016a). This model makes the same point. As Table 2-6 shows, more deaths would be averted by 2030 under the scenario where only patients with advanced fibrosis were treated, but unrestricted treatment reduces incident infections by 90 percent. Given that this committee was charged with identifying a strategy for the elimination of viral hepatitis, it favors the scenario that would elicit a sharp reduction in incidence. This incidence reduction, in turn, results in 85 percent fewer viremic cases in the population by 2030.
Replicating the success of this scenario, however, depends on aggressive diagnosis of new infections. As shown in Table 2-5, this scenario depends on diagnosing 110,000 cases a year until 2020, dropping to almost 89,000 from 2020 to 2024 and nearly 72,000 from 2025 to 2030. Diagnosis of 110,000 new infections a year was possible in the past partly because transmission was still so high. Over time, undiagnosed cases will be harder to find. People in contact with the health system will be cured of their HCV infection, and meeting the ambitious elimination target will depend on finding and curing individuals who have so far remained largely hidden.
People who inject drugs account for most HCV transmission in the United States; depending on the setting, hepatitis C prevalence in this group ranges from a third to over 80 percent, and these estimates, for the
most part, predate the opioid epidemic (Alter, 1997; Hagan et al., 2008). Prisoners account for a (somewhat overlapping) third of national hepatitis C cases (Varan et al., 2014). One way to ensure sufficient case finding and treatment to allow for a 90 percent reduction in incidence and 65 percent reduction in mortality is to actively test and treat in these populations. The comprehensive harm reduction and prison health services described in Chapters 4 and 5 will be crucial to meeting these targets.
The main limitations of this model stem from its structure; it considers the number of patients treated every year as a model input. As Appendix B explains, the estimate of the number of patients treated every year comes from published data combined with expert consultation. This number is a bottleneck in the model, and it stands to reason that increasing the capacity of the health system to treat hepatitis C patients would allow a greater reduction in deaths, without compromising the reduction in total viremic cases or incident infections.
The model is also not designed to account for the effects of enhanced harm reduction on disease incidence, or how efforts to diagnose and treat in high-risk populations might have varying effects on morbidity and mortality. (That is, efforts to diagnose and treat clusters of people who inject drugs may elicit a sharper decrease in incidence than the same work spread among the general population.)
As with the hepatitis B models, underestimating the prevalence of hepatitis C in the population may also affect the results. The sensitivity analysis presented in Appendix B cites disease incidence as the variable with the largest effect on the results. It also explains that incidence of HCV infection is mostly driven by injection drug use, and that the opiate epidemic is not distributed evenly across the country. If the HCV incidence rates reported in Massachusetts were applied to the national model there would be considerably more incident infections and a greater prevalence of hepatitis C. At the same time, a greater prevalence of hepatitis C in the population would make it easier to diagnose a sufficient number of new cases every year to provide candidates for treatment.
A CENTRAL COORDINATING OFFICE
The targets suggested in this report are appropriately ambitious for the United States. The committee chose them in consideration of the country’s resources and its responsibility to the global hepatitis elimination program. These targets have motivational value, but caution should be taken to prevent them from becoming purely aspirational. Overly ambitious targets, after all, can have discouraging consequences, as did the WHO’s 2003 pledge to enroll 3 million HIV patients in poor countries on antiretrovirals in 2 years (Rice, 2016). When the goal was not met, Jim Kim, then head of the WHO’s HIV and AIDS program, apologized for the failure, acknowledging the chosen timeline had not been realistic (Morris, 2005). Nevertheless, Kim ventured that the ambition of the pledge drove its eventual realization in 2007 (Rice, 2016).
The indicators and timelines given in this chapter represent the committee’s best effort to balance a compelling public health target against practical constraints. The previous sections make clear, however, that meeting the targets will depend on aggressive testing, diagnosis, and treatment, as well as considerably increased attention to primary prevention methods such as needle exchange. In short, eliminating hepatitis B and C will require a significant departure from the status quo.
The next four chapters of this report discuss the committee’s proposed strategy for viral hepatitis elimination. Recommendations are directed to various federal and state government agencies, as well as to legislators and different private sector organizations. With work spread among so many players, the opportunity for distraction is high. The leadership of a single office would help avoid diffusion of responsibility and ensure efficient and harmonious work.
President Clinton established the Office of National AIDS policy to make AIDS programming more cohesive (ONAP, n.d.). The office coordi-
nates national and global HIV and AIDS programming to ensure that the national program is smoothly integrated at different levels of government and with foreign programs (ONAP, n.d.). Similar leadership would be a boon to the viral hepatitis strategy described in this report.
Recommendation 2-1: The highest level of the federal government should oversee a coordinated effort to manage viral hepatitis elimination.
Successful public health programs are characterized by high political commitment, financial support, and coordination. During the polio epidemics of the mid-20th century, for example, leaders understood that advances in science were necessary to stop children from becoming paralyzed. Pressure from the White House hastened the development of the Salk vaccine necessary for the eventual elimination of polio from the western hemisphere (Juskewitch et al., 2010). Similar cooperation at the highest levels of government has been characteristic of the guinea worm eradication campaign, one of the most successful disease eradication programs to date, and measles elimination (Orenstein, 2006; The Carter Center, n.d.).
History also provides examples of times when failure of high-level coordination prevented success. In the early years of the smallpox eradication campaign, the vaccine, though effective, was not reaching the public in the quantities necessary to stop the spread of infection. Immunizing vulnerable people became the responsibility of a relatively small team at the WHO that managed fieldwork (WHO, 1980).
The importance of central leadership, particularly at the White House level, would be invaluable to the fight against viral hepatitis. Leadership from the White House conveys a certain authority and indicates commitment to the effort across the executive branch; no department would be likely to command the same convening power. If HHS or one of its agencies were to lead the initiative, its ability to direct the Department of Justice or the Department of Veterans Affairs would be hard to establish. Such coordination is of particular concern for viral hepatitis response. The most recent nation action plan for viral hepatitis involved 23 different agencies and offices from 4 departments (HHS, 2017a).
If the president prefers not to create a new office, the coordinating role might also be filled by White House Office of National AIDS Policy mentioned above. This office has experience working across federal and state government agencies to implement the national strategy on HIV and AIDS (ONAP, 2015). Its staff hosted regional HIV forums around the country to encourage open discussion about how to reduce new infections and disparities and increase access to care (Brooks, 2015). Something similar may be appropriate as part of the viral hepatitis elimination strategy.
At the same time, the new administration might prefer that an office in HHS manage viral hepatitis elimination. In this case, the best choice would be the Office of HIV/AIDS and Infectious Disease Policy under the Office of the Assistant Secretary for Health. As the convener for the national viral hepatitis action plan this office already has experience working with the relevant federal agencies on viral hepatitis. Its staff also have technical depth on the topic (HHS, 2015, 2017b). Another viable candidate within HHS would be the Office of the Surgeon General, charged with managing public health practice (HHS, n.d.).
There is also room for active state support of viral hepatitis programming. Ideally, the states would be fully supported by a national office, but motivated state health commissioners may be able to adjust their state’s priorities to make viral hepatitis programming more prominent.
The Problem of Stigma
As this committee’s earlier report made clear, there are aspects of the viral hepatitis epidemic that resist easy remedy (NASEM, 2016a). Stigma is one such problem. Viral hepatitis patients often feel deeply ashamed of their condition, partly because HBV and HCV are commonly acquired through sexual contact or drug use and because liver disease in general is associated with substance use (Butt, 2008; Cotler et al., 2012; Golden et al., 2006; Moore et al., 2008; Wu et al., 2009; Yoo et al., 2012). Both HBV and HCV are infectious, so patients may fear social rejection if their diagnosis is widely known (Marinho and Barreira, 2013). In the face of such stigma, people may avoid testing, preferring not to know if they are infected, and people with chronic infection may avoid situations that force them to think about their condition (namely, medical care) (Vaughn-Sandler et al., 2014). In short, stigma encourages silence and inaction. It could undo the best viral hepatitis elimination campaign.
Stigma alleviation, while challenging, is possible. A recent National Academies of Sciences, Engineering, and Medicine report found mixed evidence on the effectiveness of education programs to change public attitudes toward mental illness, for example (NASEM, 2016b). In reviewing large campaigns, the report promoted legislative and policy change as valuable goals for anti-stigma efforts. These so-called hard goal campaigns may have particular promise for improving quality of life for people with substance use disorders and other mental health problems (NASEM, 2016b). A large central office at the level of the White House might be in the best position to manage such efforts to reduce stigma.
Other aspects of elimination described later in this report will also require a central coordinating office. The models presented in this chapter make it clear that elimination will depend on considerable improvements
to diagnosis and treatment of infected patients. Many such patients were born abroad, and others may have a history of substance use disorders or incarceration. Outreach to such groups can be sensitive and could benefit from a unified plan for communication and service provision with an emphasis on bringing services to the target populations.
Finally, this report discusses key gaps in the research on viral hepatitis and suggests research priorities for different federal agencies. The oversight of a single office could help ensure that various research agendas are balanced efficiently among funding agencies and devoid of unnecessary redundancy.
REFERENCES
Altekruse, S. F., S. J. Henley, J. E. Cucinelli, and K. A. McGlynn. 2014. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. American Journal of Gastroenterology 109(4):542-553.
Alter, M. J. 1997. Epidemiology of hepatitis C. Hepatology 26(3 Suppl 1):62S-65S.
Arends, P., M. J. Sonneveld, R. Zoutendijk, I. Carey, A. Brown, M. Fasano, D. Mutimer, K. Deterding, J. G. Reijnders, Y. Oo, J. Petersen, F. van Bommel, R. J. De Knegt, T. Santantonio, T. Berg, T. M. Welzel, H. Wedemeyer, M. Buti, P. Pradat, F. Zoulim, B. Hansen, and H. L. Janssen. 2015. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: Limited role for risk scores in Caucasians. Gut 64(8):1289-1295.
Blach, S., S. Zeuzem, M. Manns, I. Altraif, A.-S. Duberg, D. H. Muljono, I. Waked, S. M. Alavian, M.-H. Lee, F. Negro, F. Abaalkhail, A. Abdou, M. Abdulla, A. A. Rached, I. Aho, U. Akarca, I. Al Ghazzawi, S. Al Kaabi, F. Al Lawati, K. Al Namaani, Y. Al Serkal, S. A. Al-Busafi, L. Al-Dabal, S. Aleman, A. S. Alghamdi, A. A. Aljumah, H. E. Al-Romaihi, M. I. Andersson, V. Arendt, P. Arkkila, A. M. Assiri, O. Baatarkhuu, A. Bane, Z. Ben-Ari, C. Bergin, F. Bessone, F. Bihl, A. R. Bizri, M. Blachier, A. J. Blasco, C. E. B. Mello, P. Bruggmann, C. R. Brunton, F. Calinas, H. L. Y. Chan, A. Chaudhry, H. Cheinquer, C.-J. Chen, R.-N. Chien, M. S. Choi, P. B. Christensen, W.-L. Chuang, V. Chulanov, L. Cisneros, M. R. Clausen, M. E. Cramp, A. Craxi, E. A. Croes, O. Dalgard, J. R. Daruich, V. de Ledinghen, G. J. Dore, M. H. El-Sayed, G. Ergör, G. Esmat, C. Estes, K. Falconer, E. Farag, M. L. G. Ferraz, P. R. Ferreira, R. Flisiak, S. Frankova, I. Gamkrelidze, E. Gane, J. García-Samaniego, A. G. Khan, I. Gountas, A. Goldis, M. Gottfredsson, J. Grebely, M. Gschwantler, M. G. Pessôa, J. Gunter, B. Hajarizadeh, O. Hajelssedig, S. Hamid, W. Hamoudi, A. Hatzakis, S. M. Himatt, H. Hofer, I. Hrstic, Y.-T. Hui, B. Hunyady, R. Idilman, W. Jafri, R. Jahis, N. Z. Janjua, P. Jarčuška, A. Jeruma, J. G. Jonasson, Y. Kamel, J.-H. Kao, S. Kaymakoglu, D. Kershenobich, J. Khamis, Y. S. Kim, L. Kondili, Z. Koutoubi, M. Krajden, H. Krarup, M.-s. Lai, W. Laleman, W.-c. Lao, D. Lavanchy, P. Lázaro, H. Leleu, O. Lesi, L. A. Lesmana, M. Li, V. Liakina, Y.-S. Lim, B. Luksic, A. Mahomed, M. Maimets, M. Makara, A. O. Malu, R. T. Marinho, P. Marotta, S. Mauss, M. S. Memon, M. C. M. Correa, N. Mendez-Sanchez, S. Merat, A. M. Metwally, R. Mohamed, C. Moreno, F. H. Mourad, B. Müllhaupt, K. Murphy, H. Nde, R. Njouom, D. Nonkovic, S. Norris, S. Obekpa, S. Oguche, S. Olafsson, M. Oltman, O. Omede, C. Omuemu, O. Opare-Sem, A. L. H. Øvrehus, S. Owusu-Ofori, T. S. Oyunsuren, G. Papatheodoridis, K. Pasini, K. M. Peltekian, R. O. Phillips, N. Pimenov, H. Poustchi, N. Prabdial-Sing, H. Qureshi, A. Ramji, D. Razavi-Shearer, K. Razavi-Shearer, B. Redae, H. W. Reesink, E. Ridruejo, S. Robbins, L. R. Roberts, S. K.
Roberts, W. M. Rosenberg, F. Roudot-Thoraval, S. D. Ryder, R. Safadi, O. Sagalova, R. Salupere, F. M. Sanai, J. F. S. Avila, V. Saraswat, R. Sarmento-Castro, C. Sarrazin, J. D. Schmelzer, I. Schréter, C. Seguin-Devaux, S. R. Shah, A. I. Sharara, M. Sharma, A. Shevaldin, G. E. Shiha, W. Sievert, M. Sonderup, K. Souliotis, D. Speiciene, J. Sperl, P. Stärkel, R. E. Stauber, C. Stedman, D. Struck, T.-H. Su, V. Sypsa, S.-S. Tan, J. Tanaka, A. J. Thompson, I. Tolmane, K. Tomasiewicz, J. Valantinas, P. Van Damme, A. J. van der Meer, I. van Thiel, H. Van Vlierberghe, A. Vince, W. Vogel, H. Wedemeyer, N. Weis, V. W. S. Wong, C. Yaghi, A. Yosry, M.-f. Yuen, E. Yunihastuti, A. Yusuf, E. Zuckerman, and H. Razavi. 2016. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. The Lancet Gastroenterology & Hepatology 2(3):161-176.
Brooks, D. M. 2015. Office of National AIDS Policy to host national HIV/AIDS strategy regional forums. https://obamawhitehouse.archives.gov/blog/2015/03/27/office-national-aids-policy-host-national-hivaids-strategy-regional-forums (accessed February 24, 2017).
Butt, G. 2008. Stigma in the context of hepatitis C: Concept analysis. Journal of Advanced Nursing 62(6):712-724.
Campsen, J., M. Zimmerman, J. Trotter, J. Hong, C. Freise, R. Brown, A. Cameron, M. Ghobrial, I. Kam, R. Busuttil, S. Saab, C. Holt, J. Emond, J. Stiles, T. Lukose, M. Chang, and G. Klintmalm. 2013. Liver transplantation for hepatitis B liver disease and concomitant hepatocellular carcinoma in the United States with hepatitis B immunoglobulin and nucleoside/nucleotide analogues. Liver Transplantation 19(9):1020-1029.
CDC (Centers for Disease Control and Prevention). 2016a. Viral hepatitis–Statistics and surveillance. https://www.cdc.gov/hepatitis/statistics (accessed December 20, 2016).
CDC. 2016b. What is viral hepatitis? https://www.cdc.gov/hepatitis/abc/index.htm (accessed December 27, 2016).
Chen, Y. C., C. M. Chu, and Y. F. Liaw. 2010. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology 51(2):435-444.
Chotiyaputta, W., C. Peterson, F. A. Ditah, D. Goodwin, and A. S. Lok. 2011. Persistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis B. Journal of Hepatology 54(1):12-18.
Chu, C. M., and Y. F. Liaw. 2007. HBsAg seroclearance in asymptomatic carriers of high endemic areas: Appreciably high rates during a long-term follow-up. Hepatology 45(5): 1187-1192.
Chu, C. M., and Y. F. Liaw. 2009. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. American Journal of Gastroenterology 104(7): 1693-1699.
Cohen, C., A. A. Evans, W. T. London, J. Block, M. Conti, and T. Block. 2008. Underestimation of chronic hepatitis B virus infection in the United States of America. Journal of Viral Hepatitis 15(1):12-13.
Cotler, S., S. Cotler, H. Xie, B. Luc, T. Layden, and S. Wong. 2012. Characterizing hepatitis B stigma in Chinese immigrants. Journal of Viral Hepatitis 19(2):147-152.
Denniston, M. M., R. B. Jiles, J. Drobeniuc, R. M. Klevens, J. W. Ward, G. M. McQuillan, and S. D. Holmberg. 2014. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Annals of Internal Medicine 160(5):293-300.
Edlin, B. R., B. J. Eckhardt, M. A. Shu, S. D. Holmberg, and T. Swan. 2015. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 62(5):1353-1363.
Fattovich, G., F. Bortolotti, and F. Donato. 2008. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. Journal of Hepatology 48(2):335-352.
FitzSimons, D., B. McMahon, G. Hendrickx, A. Vorsters, and P. Van Damme. 2013. Burden and prevention of viral hepatitis in the Arctic region, Copenhagen, Denmark, 22-23 March 2012. International Journal of Circumpolar Health 72(10).
Golden, J., R. M. Conroy, A. M. O’Dwyer, D. Golden, and J.-B. Hardouin. 2006. Illness-related stigma, mood and adjustment to illness in persons with hepatitis C. Social Science & Medicine 63(12):3188-3198.
Gvinjilia, L., M. Nasrullah, D. Sergeenko, T. Tsertsvadze, G. Kamkamidze, M. Butsashvili, A. Gamkrelidze, P. Imnadze, V. Kvaratskhelia, N. Chkhartishvili, L. Sharvadze, J. Drobeniuc, L. Hagan, J. W. Ward, J. Morgan, and F. Averhoff. 2016. National progress toward hepatitis C elimination—Georgia, 2015-2016. Morbidity and Mortality Weekly Report 65(41):1132-1135.
Hagan, H., H. Thiede, and D. C. Des Jarlais. 2004. Hepatitis C virus infection among injection drug users: Survival analysis of time to seroconversion. Epidemiology 15(5):543-549.
Hagan, H., E. R. Pouget, D. C. Des Jarlais, and C. Lelutiu-Weinberger. 2008. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: The influence of time and place. American Journal of Epidemiology 168(10):1099-1109.
Heathcote, E. J., P. Marcellin, M. Buti, E. Gane, A. Robert, Z. Krastev, G. Germanidis, S. S. Lee, R. Flisiak, and K. Kaita. 2011. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 140(1):132-143.
HHS (Department of Health and Human Services). 2015. Action plan for the prevention, care, and treatment of viral hepatitis: 2014-2016. HHS, Office of the Assistant Secretary for Health, Office of HIV/AIDS and Infectious Disease Policy. https://www.aids.gov/pdf/viral-hepatitis-action-plan.pdf (accessed October 26, 2016).
HHS. 2017a. Federal agencies & offices engaged in the viral hepatitis action plan 2017-2020. https://www.hhs.gov/hepatitis/action-plan/federal-agencies-engaged/index.html (accessed February 24, 2017).
HHS. 2017b. National viral hepatitis action plan 2017-2020. HHS, Office of the Assistant Secretary for Health, Office of HIV/AIDS and Infectious Disease Policy. https://www.hhs.gov/sites/default/files/National%20Viral%20Hepatitis%20Action%20Plan%202017-2020.pdf (February 24, 2017).
HHS. n.d. Duties of the Surgeon General. https://www.surgeongeneral.gov/about/duties/index.html (accessed February 24, 2017).
HRSA (Health Resources and Services Administration). 2016. OPTN (Organ Procurement and Transplant Network). Data. https://optn.transplant.hrsa.gov/data (accessed December 22, 2016).
Hu, D. J., J. Xing, R. A. Tohme, Y. Liao, H. Pollack, J. W. Ward, and S. D. Holmberg. 2013. Hepatitis B testing and access to care among racial and ethnic minorities in selected communities across the United States, 2009-2010. Hepatology 58(3):856-862.
Juday, T., H. Tang, M. Harris, A. Z. Powers, E. Kim, and G. J. Hanna. 2011. Adherence to chronic hepatitis B treatment guideline recommendations for laboratory monitoring of patients who are not receiving antiviral treatment. Journal of General Internal Medicine 26(3):239-244.
Juskewitch, J. E., C. J. Tapia, and A. J. Windebank. 2010. Lessons from the Salk polio vaccine: Methods for and risks of rapid translation. Clinical and Translational Science 3(4):182-185.
Kanwal, F., M. Farid, P. Martin, G. Chen, I. M. Gralnek, G. S. Dulai, and B. M. Spiegel. 2006. Treatment alternatives for hepatitis B cirrhosis: A cost-effectiveness analysis. American Journal of Gastroenterology 101(9):2076-2089.
Kershenobich, D., H. A. Razavi, C. L. Cooper, A. Alberti, G. M. Dusheiko, S. Pol, E. Zuckerman, K. Koike, K. Han, C. M. Wallace, S. Zeuzem, and F. Negro. 2011. Applying a system approach to forecast the total hepatitis C virus-infected population size: Model validation using US data. Liver International 31(Suppl 2):4-17.
Kim, W. R. 2009. Epidemiology of hepatitis B in the United States. Hepatology 49(5 Suppl): S28-S34.
Kim, L. H., V. G. Nguyen, H. N. Trinh, J. Li, J. Q. Zhang, and M. H. Nguyen. 2014. Low treatment rates in patients meeting guideline criteria in diverse practice settings. Digestive Diseases and Sciences 59(9):2091-2099.
Klevens, R. M., J. Miller, C. Vonderwahl, S. Speers, K. Alelis, K. Sweet, E. Rocchio, T. Poissant, T. M. Vogt, and K. Gallagher. 2009. Population-based surveillance for hepatitis C virus, United States, 2006-2007. Emerging Infectious Diseases 15(9):1499-1502.
Klevens, R. M., S. Liu, H. Roberts, R. B. Jiles, and S. D. Holmberg. 2014. Estimating acute viral hepatitis infections from nationally reported cases. American Journal of Public Health 104(3):482-487.
Ko, S. C., L. Fan, E. A. Smith, N. Fenlon, A. K. Koneru, and T. V. Murphy. 2014. Estimated annual perinatal hepatitis B virus infections in the United States, 2000–2009. Journal of the Pediatric Infectious Diseases Society 5(2):114-121.
Kowdley, K. V., C. C. Wang, S. Welch, H. Roberts, and C. L. Brosgart. 2012. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology 56(2):422-433.
Lin, X., N. J. Robinson, M. Thursz, D. M. Rosenberg, A. Weild, J. M. Pimenta, and A. J. Hall. 2005. Chronic hepatitis B virus infection in the Asia-Pacific region and Africa: Review of disease progression. Journal of Gastroenterology and Hepatology 20(6):833-843.
Lin, S. Y., E. T. Chang, and S. K. So. 2007. Why we should routinely screen Asian American adults for hepatitis B: A cross-sectional study of Asians in California. Hepatology 46(4):1034-1040.
Lok, A. S. F., B. J. McMahon, R. S. Brown, J. B. Wong, A. T. Ahmed, W. Farah, J. Almasri, F. Alahdab, K. Benkhadra, M. A. Mouchli, S. Singh, E. A. Mohamed, A. M. Abu Dabrh, L. J. Prokop, Z. Wang, M. H. Murad, and K. Mohammed. 2016. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 63(1):284-306.
Marcellin, P., E. Gane, M. Buti, N. Afdhal, W. Sievert, I. M. Jacobson, M. K. Washington, G. Germanidis, J. F. Flaherty, R. Aguilar Schall, J. D. Bornstein, K. M. Kitrinos, G. M. Subramanian, J. G. McHutchison, and E. J. Heathcote. 2013. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 381(9865):468-475.
Marinho, R. T., and D. P. Barreira. 2013. Hepatitis C, stigma and cure. World Journal of Gastroenterology 19(40):6703-6709.
Mast, E. E., C. Weinbaum, A. E. Fiore, M. J. Alter, B. Bell, L. Finelli, L. Rodewald, J. M. Douglas, Jr., R. S. Janssen, and J. W. Ward. 2006. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Part II: Immunization of adults. Morbidity and Mortality Weekly Report 55(RR16):1-25.
McMahon, B. J. 2015. Management of patients withchronic hepatitis B: The Alaska experience. PowerPoint presentation to the Committee on a National Strategy for the Elimination of Hepatitis B and C, Washington, DC, November 30, 2015. http://www.nationalacademies.org/hmd/~/media/Files/Activity%20Files/PublicHealth/HepatitisBandC/1-November2015/8%20-%20Brian%20McMahon.pdf (accessed January 10, 2017).
McMahon, B. J., L. Bulkow, B. Simons, Y. Zhang, S. Negus, C. Homan, P. Spradling, E. Teshale, D. Lau, and M. Snowball. 2014. Population-based longitudinal study of hepatitis B “e” antigen negative persons with chronic hepatitis B: Level of HBV DNA and liver disease. Clinical Gastroenterology and Hepatology 12(4):701.
Mitruka, K., T. Tsertsvadze, M. Butsashvili, A. Gamkrelidze, P. Sabelashvili, E. Adamia, M. Chokheli, J. Drobeniuc, L. Hagan, A. M. Harris, T. Jiqia, A. Kasradze, S. Ko, V. Qerashvili, L. Sharvadze, I. Tskhomelidze, V. Kvaratskhelia, J. Morgan, J. W. Ward, and F. Averhoff. 2015. Launch of a nationwide hepatitis C elimination program—Georgia, April 2015. Morbidity and Mortality Weekly Report 64(28):753-757.
Moore, G. A., D. A. Hawley, and P. Bradley. 2008. Hepatitis C: Studying stigma. Gastroenterology Nursing 31(5):346-352.
Morris, M. 2005. Apology over missed AIDS target. BBC, November 28.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2016a. Eliminating the public health problem of hepatitis B and C in the United States: Phase one report. Washington, DC: The National Academies Press.
NASEM. 2016b. Ending discrimination against people with mental and substance use disorders: The evidence for stigma change. Washington, DC: The National Academies Press.
NCHS (National Center for Health Statistics). 2015. National Health and Nutrition Examination Survey data, 2003-2014. Hyattsville, MD: Department of Health and Human Services, Centers for Disease Control and Prevention.
NCI (National Cancer Institute). 2015. SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2015 Sub (1973-2013). Surveillance, Epidemiology, and End Results (SEER) Program.
ONAP (Office of National AIDS Policy). 2015. National HIV/AIDS strategy for the United States: Updated to 2020. Federal action plan. Washington, DC: White House Office of National AIDS Policy.
ONAP. n.d. Office of National AIDS Policy. https://clinton2.nara.gov/ONAP/accomp.html (accessed February 24, 2017).
Orenstein, W. A. 2006. The role of measles elimination in development of a national immunization program. The Pediatric Infectious Disease Journal 25(12):1093-1101.
Papatheodoridis, G. V., H. L.-Y. Chan, B. E. Hansen, H. L. Janssen, and P. Lampertico. 2015. Risk of hepatocellular carcinoma in chronic hepatitis B: Assessment and modification with current antiviral therapy. Journal of Hepatology 62(4):956-967.
Raffetti, E., G. Fattovich, and F. Donato. 2016. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: A systematic review and meta-analysis. Liver International 36(9):1239-1251.
Razavi, H., C. Estes, K. Pasini, E. Gower, and S. Hindman. 2013. HCV treatment rate in select European countries in 2004–2010. Journal of Hepatology 58(Suppl 1):S22-S23.
Razavi, H., I. Waked, C. Sarrazin, R. Myers, R. Idilman, F. Calinas, W. Vogel, M. Correa, C. Hézode, and P. Lázaro. 2014. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. Journal of Viral Hepatitis 21(S1):34-59.
Rice, A. 2016. How the World Bank’s biggest critic became its president. Guardian, August 11. https://www.theguardian.com/news/2016/aug/11/world-bank-jim-yong-kim (accessed September 22, 2016).
Roberts, H., D. Kruszon-Moran, K. N. Ly, E. Hughes, K. Iqbal, R. B. Jiles, and S. D. Holmberg. 2016. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology 63(2):388-397.
Seeff, L. B. 2002. Natural history of chronic hepatitis C. Hepatology 36(5B):S35-S46.
Sergeenko, D. 2015. Progress towards hepatitis C elimination in Georgia. World Hepatitis Summit 2015. http://www.worldhepatitissummit.org/docs/default-source/defaultdocument-library/2015/resources/hepatitis-at-a-national-level.pdf?sfvrsn=4 (accessed October 26, 2016).
Stvilia, K., T. Tsertsvadze, L. Sharvadze, M. Aladashvili, C. del Rio, M. H. Kuniholm, and K. E. Nelson. 2006. Prevalence of hepatitis C, HIV, and risk behaviors for blood-borne infections: A population-based survey of the adult population of T’bilisi, Republic of Georgia. Journal of Urban Health 83(2):289-298.
Tenney, D. J., R. E. Rose, C. J. Baldick, K. A. Pokornowski, B. J. Eggers, J. Fang, M. J. Wichroski, D. Xu, J. Yang, and R. B. Wilber. 2009. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 49(5):1503-1514.
The Carter Center. n.d. Guinea worm eradiction program. https://www.cartercenter.org/health/guinea_worm (accessed September 22, 2016).
Thiele, M., L. L. Gluud, A. D. Fialla, E. K. Dahl, and A. Krag. 2014. Large variations in risk of hepatocellular carcinoma and mortality in treatment naïve hepatitis B patients: Systematic review with meta-analyses. PLoS One 9(9):e107177.
Toy, M., J. A. Salomon, H. Jiang, H. Gui, H. Wang, J. Wang, J. H. Richardus, and Q. Xie. 2014. Population health impact and cost-effectiveness of monitoring inactive chronic hepatitis B and treating eligible patients in Shanghai, China. Hepatology 60(1):46-55.
Tsertsvadze, T. 2016. National hepatitis C elimination program in Georgia. Ninth Paris Hepatitis Conference, Paris, France, January 11.
Uddin, G., D. Shoeb, S. Solaiman, R. Marley, C. Gore, M. Ramsay, R. Harris, I. Ushiro-Lumb, S. Moreea, S. Alam, H. C. Thomas, S. Khan, B. Watt, R. N. Pugh, S. Ramaiah, R. Jervis, A. Hughes, S. Singhal, S. Cameron, W. F. Carman, and G. R. Foster. 2010. Prevalence of chronic viral hepatitis in people of South Asian ethnicity living in England: The prevalence cannot necessarily be predicted from the prevalence in the country of origin. Journal of Viral Hepatitis 17(5):327-335.
UN (United Nations). 2015. World population prospects: The 2015 revision, key findings and advance tables. New York: Department of Economic and Social Affairs, Population Division.
Varan, A. K., D. W. Mercer, M. S. Stein, and A. C. Spaulding. 2014. Hepatitis C seroprevalence among prison inmates since 2001: Still high but declining. Public Health Reports 129(2):187-195.
Vaughn-Sandler, V., C. Sherman, A. Aronsohn, and M. L. Volk. 2014. Consequences of perceived stigma among patients with cirrhosis. Digestive Diseases and Sciences 59(3): 681-686.
Volk, M. L., R. Tocco, S. Saini, and A. S. Lok. 2009. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology 50(6):1750-1755.
Wasley, A., D. Kruszon-Moran, W. Kuhnert, E. P. Simard, L. Finelli, G. McQuillan, and B. Bell. 2010. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. Journal of Infectious Diseases 202(2):192-201.
WHO (World Health Organization). 1980. The global eradiction of smallpox: Final report of the Global Commission for the Certification of Smallpox Eradication, Geneva, December 1979. Geneva, Switzerland: WHO.
WHO. 2016a. Combating hepatitis B and C to reach elimination by 2030. Geneva, Switzerland: WHO.
WHO. 2016b. Global health sector strategy on viral hepatitis, 2016-2021: Towards ending viral hepatitis. Geneva, Switzerland: WHO. http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf (July 19, 2016).
WHO Regional Office for Europe. 2015. Georgia sets sights on eliminating hepatitis C. http://www.euro.who.int/en/countries/georgia/news/news/2015/07/georgia-sets-sights-on-eliminating-hepatitis-c (accessed July 11, 2016).
Wong, G. L. H., H. L. Y. Chan, C. W. H. Mak, S. K. Y. Lee, Z. M. Y. Ip, A. T. H. Lam, H. W. H. Iu, J. M. S. Leung, J. W. Y. Lai, and A. O. S. Lo. 2013. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 58(5):1537-1547.
Wu, H., C. Yim, A. Chan, M. Ho, and J. Heathcote. 2009. Sociocultural factors that potentially affect the institution of prevention and treatment strategies for hepatitis B in Chinese Canadians. Canadian Journal of Gastroenterology 23(1):31-36.
Yang, J. D., B. Kim, S. O. Sanderson, J. L. S. Sauver, B. P. Yawn, R. A. Pedersen, J. J. Larson, T. M. Therneau, L. R. Roberts, and W. R. Kim. 2012. Hepatocellular carcinoma in Olmsted County, Minnesota, 1976-2008. Mayo Clinic Proceedings 87(1):9-16.
Yoo, G. J., T. Fang, J. Zola, and W. M. Dariotis. 2012. Destigmatizing hepatitis B in the Asian American community: Lessons learned from the San Francisco Hep B Free campaign. Journal of Cancer Education 27(1):138-144.
Zeiler, I., T. Langlands, J. M. Murray, and A. Ritter. 2010. Optimal targeting of hepatitis C virus treatment among injecting drug users to those not enrolled in methadone maintenance programs. Drug and Alcohol Dependence 110(3):228-233.
This page intentionally left blank.




























