3
Assessment of Therapeutic Trials
After the discussions about clinical trial design (as presented in Chapter 2), scientific and ethics committees convened by the World Health Organization (WHO) published their findings regarding suggested research designs. For example, the Scientific and Technical Advisory Committee on Ebola Experimental Interventions found at their meeting in Geneva on November 11–12, 2014, that “it was likely that for anti-Ebola treatments that did not have large effects, randomized concurrently controlled trials may be needed” (WHO, 2014b). The WHO Ethics Working Group (convened on October 20–21, 2014) also noted the pros and cons of various designs. For example, they noted that single-arm studies that use nonrandomized retrospective control data have “a high risk of bias and may lack internal validity” (WHO, 2014a). However, despite their concerns, the Ethics Working Group concluded, “In principle, so long as standard requirements for human research ethics are met, all scientifically recognized methodologies and study designs should be considered as ethically acceptable—whether they are placebo-controlled randomized trials or trials that don’t involve randomization to control groups” (WHO, 2014a). The group added that the reality of the situation—for example, the scarcity of health care providers, research staff, infrastructure, and other resources—should be taken into account in making design decisions (WHO, 2014a).
Ultimately, formal clinical trials were conducted on five investigational therapeutic agents in the three countries most affected by the epidemic. The five therapeutic agents were
- Favipiravir, developed by Fuji/Toyama (Japan) for pandemic influenza (repurposed);
- Brincidofovir, Chimerix (United States), developed and used for treatment of cytomegalovirus (repurposed);
- TKM-130803,1 developed by Tekmira (Canada);
- Convalescent plasma; and
- ZMapp, developed by MappBio (United States).
Preparation and planning for the trials started in September 2014, and the trials began enrolling participants between December 2014 and March 2015. While the trials were launched rapidly, most began participant enrollment at the tail end of the epidemic (see Table 3-1 and Figure 3-1; see also Table 3-2 for details on the preclinical and, if available, clinical data on investigational Ebola agents as of October 2015–before the launch of the trials).
JIKI Trial: Favipiravir
The French institut national de la santé et de la recherche médicale (Inserm) funded a study of favipiravir (MSF, 2015), a repurposed medicinal product that was originally developed for pandemic influenza virus infection (Furuta et al., 2013). The trial was conducted at four Ebola treatment units (ETUs) in Guinea that were operated by four different organizations: at Guéckédou, by Médecins Sans Frontières (MSF); at Nzerekore, by the Alliance for International Medical Action; at Macenta, by the French Red Cross; and at Conkary, by the French military health service (Sissoko et al., 2016). The study was designed to rapidly gather standardized preliminary data about favipiravir in order to guide further research.
Study Design
The trial was designed as a multicenter, single-arm, proof-of-concept trial. Initially, the plan was to use historical data to establish target success rates, but as the trial began, information became available from a patient database in Guinea, so these data were used instead of gathering data de novo during the epidemic (Sissoko et al., 2016). The trial team opted for
___________________
1 TKM-130803 is a new formulation of TKM-100802, one of the lead experimental agents prioritized by WHO. “TKM-100802 has been administered to five patients with Ebola medically evacuated to the US and Europe, and to one individual as post-exposure prophylaxis (personal communication, Mark Kowalski, Tekmira Pharmaceuticals). Since the product was administered on a compassionate basis to these individuals and because the patients simultaneously received other experimental products, it has not been possible to assess the efficacy or safety of TKM-100802 in the treatment of [Ebola]” (Dunning et al., 2016b).
TABLE 3-1 Timeline of Therapeutic Trials
| Trial Name (investigational agent) | Preparation and Planning | Trial Enrollment Start | Trial Enrollment End | No. of Patients Enrolled | Country |
|---|---|---|---|---|---|
| JIKI (Favipiravir) | September–December 2014 | December 2014 | April 2015 | 126 patients | Guinea |
| RAPIDE-BCV (Brincidofovir) | September 2014–January 2015 | January 2015 | February 2015 | 4 patients | Liberia |
| TKM-Ebola (TKM-130803) | September 2014–January 2015 | March 2015 | June 2015 | 14 patients | Sierra Leone |
| Ebola Tx (Convalescent plasma) | November 2014–January 2015 | February 2015 | August 2015 | 99 patients | Guinea |
| PREVAIL II (ZMapp) | September 2014–February 2015* | March 2015 | November 2015 | 72 patients | Guinea, Liberia, Sierra Leone, United States |
*A sufficient supply of ZMapp was available in September 2015 (Dodd et al., 2016).
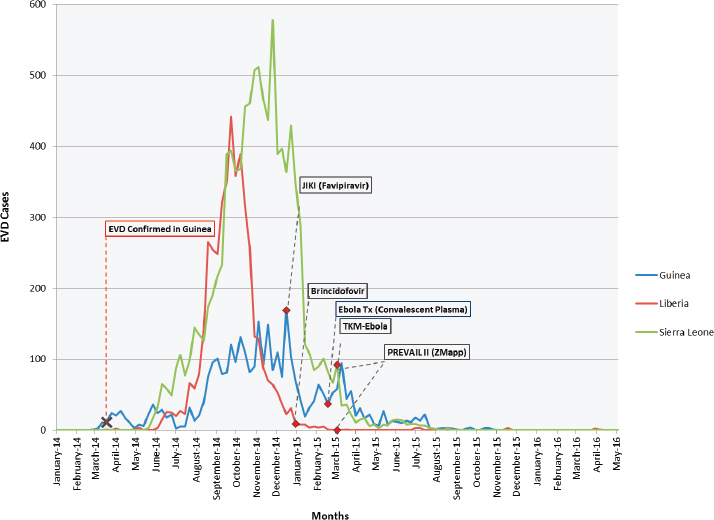
SOURCES: WHO, 2014a, 2016a,b,c.
a nonrandomized design for two main reasons. First, since Ebola strikes in clusters, the team felt that it was “ethically unacceptable to randomize patients from within the same family or village, who appear together to seek care, to receive or not receive an experimental drug” (Sissoko et al., 2016). Second, investigators, noting the already-existing fear and distrust in the community, worried that a randomized design might exacerbate these tensions and make patients more reluctant to seek care.
Advantages and Disadvantages
The multicenter approach allowed for a large number of participants—in fact, this was the largest Ebola treatment trial and the first to be conducted during the epidemic. Assessment of viral load permitted stratification of patients into risk groups. The rapid initiation of this trial meant that this
early experience could potentially inform later efforts: The investigators remarked that the conduct of this trial resulted in lessons learned about how to “quickly set up and run an Ebola trial, in close relationship with the community and nongovernmental organizations,” and they learned how to integrate “research into care so that it improved care” (Sissoko et al., 2016).
Results and Discussion
Between December 2014 and April 2015, 126 participants—children, adolescents, and adults—were enrolled. Subsequently, 15 were excluded from the final analysis, 10 because they had received convalescent plasma in another treatment center prior to enrollment in the trial and 5 because they had no available polymerase chain reaction (PCR) data on virus load available at baseline and could not be classified according to the revised stratification (Sissoko et al., 2016). The trial was inconclusive about the efficacy and tolerance of favipiravir in Ebola patients; the investigators noted that their data on tolerance were encouraging but could not be conclusive due to the lack of randomization. However, the study did provide some new information on biomarkers for evaluating patient prognosis and on the course of the disease. For example, investigators found that PCR cycle threshold (Ct)2 was predictive of patient outcome and served as an effective surrogate of viral load; they suggested that future drug trials should systematically stratify analyses by viral load at baseline Ct value in a semiquantitative Ebola virus reverse transcription (a method the PREVAIL II trial team also used [PREVAIL II Writing Group, 2016]). They also suggested that favipiravir monotherapy “merits further study in patients with medium to high viremia, but not in those with very high viremia” (Sissoko et al., 2016). They reported that a nonsignificant trend in the subgroup of patients with lower viral load might make randomization in a future trial of favipiravir difficult, as the suggestion of possible benefit may limit willingness to allow randomization to an alternative regimen or control group.
Interim data from this trial were released in February 2015 (MSF, 2015); because these data suggested possible benefit, the government of Guinea expanded the use of favipiravir in ETUs (Reuters, 2015). The committee is concerned that the release of interim results could have inappropriately influenced other ongoing clinical trials or caregivers. For example, the coordinator for France’s response to Ebola during the outbreak stated that despite concerns over randomizing patients, they would consider sup-
___________________
2 Lower Ct values indicate high amounts of targeted nucleic acid, while higher Ct values mean lower (and even too little) amounts of the targeted nucleic acid.
| Therapeutic Candidate Trial Name (Producer) | Drug Type | Preclinical Evidence | Early Clinical Evidence/ Known Safety Issues | Availability and Logistical Considerations (as of November 2014) |
|---|---|---|---|---|
| Favipiravir JIKI (Fuji/Toyama, Japan) | Small molecule antiviral with activity against many RNA viruses. Functions through inhibiting viral RNA-dependent RNA polymerase. Approved in Japan for treating novel/ pandemic influenza. |
In vitro inhibition IC50 64 µM; higher than that needed for influenza.
Mice: protected at 300 mg/ kg. Nonhuman primate (NHP): antiviral effect seen; 2 log reduction in viremia. Model limitation due to frequent need to anesthetize NHP to administer drug orally. |
Clinical use in healthy volunteers up to 3.6 g on first day followed by 800 mg twice daily. No safety issues identified. Increased drug exposure in setting of hepatic dysfunction. |
200 mg tablets; dosing at 6 g/first day requires 30 tablets—potentially difficult to swallow.
1.6 million tablets available free (10,000 treatment courses). Thermostable. |
| Brincidofovir RAPIDE-BCV (Chimerix, USA) | Small molecule antiviral with activity against dsDNA viruses. Developed and used for treatment of CMV. In theory, should not work on Ebola (RNA virus), mode of action may be different to that for DNA viruses. |
In vitro EC50 varies by assay from 120 nM to 1.3 µM. Thought to be a concentration readily achieved in clinic. Selectivity index variable depending on assay.
Mice: no therapeutic benefit seen in two separate studies, but no pharmacokinetics (PK); therefore, not known if effective concentration reached. |
Testing in >1,000 patients: main symptom GI tolerability, and liver enzymes AST/ALT Elevations. | PO drug. Twice weekly dosing after initial load. 22,000×100 mg tablets (>3 500 treatment courses) available. Thermostable. |
NHP: Rhesus macaque—not feasible due to PK profile. Guinea pig: study planned to determine PK and efficacy. |
||||
| TKM-100802 RAPIDE-TKM; TKM-Ebola (Tekmira, Canada) | Small inhibitory RNA which catalytically cleaves Ebola RNA once inside the cell. Sequence-specific to this strain of Ebola. | NHP: 67–100% (7 rhesus–macaques challenged) efficacy among NHP given 4 to 7 doses with treatment initiated 30 minutes post-challenge (Geisbert et al., 2010). | A Phase 1 safety study found dose-related side effects including dizziness, chest tightness, raised heart rate. A lower dose was better tolerated. A study in healthy volunteers is on partial clinical hold. | Several hundred doses currently available. Several thousand doses could be available in short time period.
IV infusion. Requires refrigeration. |
| ZMapp PREVAIL II (MappBio, USA) | Cocktail of three monoclonal antibodies produced in tobacco plants. | NHP: 100% survival (18 rhesus macaques) when administered 5 days after virus challenge (Qiu et al., 2014). | Phase 1 safety/PK study conducted in January 2015. | Supply reported to be around 150 treatment courses with another 100 being produced. |
SOURCE: This chart is adapted from the WHO’s chart “Categorization and Prioritization of Drugs for Consideration for Testing or Use in Patients Infected with Ebola” (WHO, 2015a) and provides a summary of the available Ebola treatments that were under evaluation in formal clinical trials in West Africa as of October 2015. The WHO R&D Landscape (WHO, 2015b) captures a more detailed look at additional investigational therapeutics used during the outbreak, both in formal clinical trials as well as those in compassionate use, historical observational studies, and others that lack sufficient protocol details.
SOURCE: WHO, 2015a.
porting the ZMapp trial but “perhaps the standard of care should include favipiravir as part of the control arm in studies of ZMapp and other experimental treatments” (Cohen, 2015). In fact, favipiravir was included as part of optimized standard of care in Guinea for the PREVAIL trial (Davey, 2016), and in June 2016 the Guinean government “formally adopted the administration [of favipriravir] as a part of the standard treatment for [Ebola]” (FujiFilm Corporation, 2016), despite the lack of reliable evidence of efficacy. The JIKI trial experience illustrates the increased risks for biased assessments and prejudgments about interim data occurring when single-arm trials are conducted and when there is early public access to unreliable interim results.
This trial, coupled with the large data set of N >500 patients treated in Guinea early in the course of the outbreak that became available just before the trial’s launch, identified important prognostic factors, thus adding value to the evidence base. However, based on the available evidence at this time, there is no conclusive evidence that the drug had a beneficial effect, and therefore favipiravir will still require further evaluation in a randomized controlled trial (RCT) during a future outbreak to resolve the question of efficacy.
Rapid Assessment of Potential Interventions and Drugs for Ebola: TKM-Ebola (TKM-130803)
The Rapid Assessment of Potential Interventions and Drugs for Ebola (RAPIDE) trials, led by investigators from the University of Oxford and the International Severe Acute Respiratory and Emerging Infection Consortium, investigated two agents, brincidofovir and TKM-Ebola, in two separate trials of similar design (Kroll, 2015; Wellcome Trust, 2015). Brincidofovir was prioritized for Ebola trials because of its oral bioavailability and its known safety in seriously ill patients, in addition to its being stable at room temperature and not requiring cold storage (Haque et al., 2015). The brincidofovir study began in Liberia in January 2015, but was terminated after enrolling only four patients due to changing priorities of the drug sponsor as well as the waning of the epidemic (Chimerix Inc., 2015; Dunning et al., 2016a). This terminated trial is probably most valuable as an example of how commercial and other nonhumanitarian considerations can be barriers to successful evaluation of a new treatment in a challenging setting. The TKM-Ebola (TKM-130803) trial was launched in Sierra Leone at an ETU operated by GOAL Global, an Irish nongovernmental organization, and ran from March to June 2015 (Dunning et al., 2016b; Wellcome Trust, 2015).
Study Design
The trial used a multistage design (Dunning et al., 2016b). The first stage, in which 100 patients would be evaluated, was a single-arm sequential design with three possible decisions: the treatment is effective, promising, or not promising. A survival probability of 50 percent or less was defined as “not promising.” The design had 99 percent power to conclude that a survival probability of 80 percent was effective or promising, with a Type I error rate of 10 percent if the survival proportion was 0.50 (Whitehead et al., 2016). If the treatment was determined to be either effective or promising at the end of Stage 1, it would be subjected to further evaluation: a confirmatory single-arm study if determined effective, or a randomized controlled trial if determined promising. These additional stages provided some protection against both false positive and false negative results; in reality, however, it may have been difficult to do a controlled study of a drug yielding a promising result in Stage 1 as there likely would have been pressure to provide such a drug to everyone if it was not in limited supply. This design was similar to the common approach to drug development for solid tumors, in which a small single-arm Phase 2 trial that sees a response rate greater than a prespecified threshold is followed by a larger randomized Phase 3 trial with a standard treatment comparator (Horby, 2015).
The choice of design was influenced by two factors: (1) a desire to quickly identify highly effective or clearly ineffective treatments due to the high death rate and volatile conditions of the epidemic and (2) a desire to avoid randomization, unless necessary, because of a perception that randomization might not be acceptable or would not be feasible in the setting of a trial. On days that the capacity for trial enrollment was reached, additional patients were to be enrolled into a concurrent observational cohort. This practical approach may have been more acceptable to the community than other approaches to randomization. Patients who died within 48 hours were excluded from the analysis as they were assumed to have been too sick to be potentially responsive to the treatment. A futility bound was established to allow for termination after a small number of patients if the treatment did not appear to be promising, protecting future patients from being exposed to any risks of an ineffective treatment.
Advantages and Disadvantages
The overall strategy of the trial—starting with a single-arm stage and proceeding to a randomized stage if intermediate results were neither clearly positive nor clearly negative—had reasonable operating characteristics (low Type I error rate and high power to identify highly effective treatments). The initial single-arm phase of the trial was likely easier to initiate than a
randomized design, as it was simpler to explain to patients and health care workers who had to administer only a single treatment regimen. However, the overall trial strategy may have been difficult to fully implement in an outbreak environment. Once a treatment is labeled as “promising” in this first stage, caregivers and researchers may be inclined to resist randomizing patients to a standard of care arm, as required by the next stage in the testing strategy.
Results and Discussion
After 14 patients had been treated, the study was terminated because 11 of the 14 patients died, an outcome that was inconsistent with a true survival rate of 50 percent or greater (Dunning et al., 2016b). Unfortunately, without a control group and with the small number of patients involved it is difficult to draw strong conclusions from this experience. The trial used historical controls to define a target probability of survival at 14 days greater than 55 percent as promising (and anything less as futile). The selection of this target stemmed from an analysis of individual-level data on 1,820 adult patients with PCR-confirmed Ebola virus infection from earlier in the outbreak. A major problem with this approach was the lack of data from this population on key prognostic factors (e.g., viral load) to stratify the probability of survival. Other reports have suggested that the probability of survival for patients with high viral load might have been closer to 0.10, as opposed to the original estimate of 0.27. “The probability that a TKM-130803 recipient who survived for 48 h will subsequently survive to day 14 was estimated to be 0.27 (95 percent CI = 0.06–0.58)” (Dunning et al., 2016b). Because no estimates of viral load in earlier patients were available, it cannot be determined if the patients in the trial were more or less severely ill than those treated in the past. In addition, given changes in supportive care over time, these historical controls may not have been a relevant comparator.
Ebola-Tx: Convalescent Plasma
The European Union funded the Ebola-Tx project to investigate the safety and efficacy of convalescent plasma (CP) (ITM, 2016). Previous preclinical evidence supported the use of CP; it was shown that nonhuman primates who were challenged with filoviruses survived after being treated with antibodies from previously exposed primates (Dye et al., 2012). The provision of CP is used to achieve short-term immunization, termed passive immunization (PI), against a pathogen through administering pathogen-specific antibodies present in the survivor’s plasma. “Although antibiotics
have largely supplanted the use of PI in bacterial infections, it remains an important tool in the treatment of many viral infections when vaccines or other specific treatments are not available” (Marano et al., 2016). The Ebola-Tx project was led by the Institute of Tropical Medicine in Antwerp and was conducted at MSF’s Donka ETU in Conakry, Guinea (ITM, 2016). The trial was initiated in February 2015. Patient enrollment was stopped in early July 2015, on the advice of the independent Data Safety and Monitoring Board, primarily because the outbreak had slowed in Conakry (ITM, 2016).
Study Design
The trial was a nonrandomized, open-label (nonblinded) study that used patients who had been admitted to the same ETU prior to the start of the study as historical controls. The trial initially planned for a concurrent standard-of-care control arm in the event that there was a shortage of CP; however, a shortage never materialized (Edwards et al., 2016). The trial team made the decision not to randomize patients because they believed it would not be acceptable to patients or health care workers, given the volatile epidemic and high mortality rate, and because it would mean withholding a potentially lifesaving treatment from patients (Adebamowo et al., 2014). For future trials, however, the trial team suggested that “in-depth anthropological studies should also be conducted to gain a better understanding of community acceptability of randomization during outbreaks of diseases with high case fatality rates” (Edwards et al., 2016, p. 20).
Advantages and Disadvantages
The trial had broad entry criteria, enrolling men and women of all ages, including pregnant women, unless CP was contraindicated. These criteria made it possible to enroll 102 patients, of whom 99 were assigned to receive CP. Of the 102 enrolled, 18 were excluded from analysis (3 died before completion of eligibility assessments, 4 died before the third day of diagnosis, 10 received favipiravir as well, and 1 did not have the required PCR cycle-threshold value), resulting in 84 patients in the primary analysis (van Griensven et al., 2016a). Those who received another treatment were excluded from analysis, potentially introducing a bias since these subjects had to survive long enough to get another treatment and excluding them would lead to an underestimation of the average survival time. In terms of the delivery of treatment and explanations to patients, the trial was also relatively straightforward for the clinic to conduct.
Results and Discussion
The study found that the transfusion of up to 500 ml of CP, with unknown levels of neutralizing antibodies, in 84 patients with confirmed Ebola was not associated with a significant improvement in survival compared with historical controls (van Griensven et al., 2016a). The results are difficult to interpret in the absence of both randomization and a concurrent control arm, and the fact that antibody titers and evidence of virus neutralization are unknown. It is worth noting that the CP may have actually had a modest to moderate effect in patient outcome; it is the lack of a control group that makes it impossible to identify anything other than a very large effect with reasonable confidence. Because of logistical challenges, the antibody levels in the plasma were not evaluated before administration. Ideally, the donor CP would have been screened for antibody levels, and the plasma with the highest levels would have been used for transfusion. Alternatively, the data could have been stratified on the basis of the neutralizing antibody titer of the administered plasma sample before analysis. The serious consequence of this study design is that the inability of the trial to identify a significant but moderate efficacy may result in a rejection of CP as a treatment, despite the possibility that plasma, especially with a sufficiently high antibody level, may be effective. The most important findings that can be drawn from the study are that treatment with convalescent plasma appears to be safe and that the treatment appeared to have a high level of feasibility and acceptability in the midst of an outbreak.
However, caution should be used in the use of passive immunization due to the potential for “antibodies to enhance viral infections via antibody-dependent enhancement mechanisms” (van Griensven et al., 2016a). This increase in infectivity has been observed in vitro for both Ebola virus and Marburg virus as well as for other viruses, including HIV (Beck et al., 2008; Nakayama et al., 2011).
Partnership for Research on Ebola Vaccines in Liberia (PREVAIL) II–ZMapp
The PREVAIL II trial to investigate the use of ZMapp in the treatment of Ebola was sponsored by the U.S. National Institutes of Health and involved the ministries of health of Guinea, Liberia, and Sierra Leone, along with Mapp Biopharmaceuticals, Inserm, and academic medical centers in the United States. It was conducted in Guinea, Liberia, Sierra Leone, and the United States between March and November 2015 (PREVAIL II Writing Group, 2016).
Study Design
The trial was a Phase 1/2, multicenter, randomized, open-label trial. The initial stage of the trial consisted of two arms: ZMapp plus optimized standard of care versus optimized standard of care only. Patients were randomized in a 1:1 ratio to the two groups. ZMapp was given in three intravenous infusions (50 mg per kilogram of body weight) 3 days apart, and optimized standard of care included the provision of intravenous fluids, balancing electrolytes, maintaining oxygen status and blood pressure, and treating concurrent infections. If an investigational treatment were proven to be superior to optimized standard of care alone with respect to survival, it would then become the basis of the new standard of care against which additional investigational Ebola interventions could be tested and compared. The trial also incorporated frequent interim monitoring by an independent data and safety monitoring board to facilitate the early elimination of poorly performing treatments and the introduction of new candidate therapies without influencing those conducting the trial and treating patients. The plan was for each experimental therapy to be studied in up to 100 participants per arm. If investigators were unable to establish a significant benefit of the therapy over optimized standard of care after enrolling 100 participants per arm, then that particular treatment would be declared ineffective, and investigators would begin testing the next therapy.
Advantages and Disadvantages
The antibody combination and dose selection for ZMapp were predicated on strong translational evidence from nonhuman primate studies (Qiu et al., 2014). The clinical study was not blinded because of the burden and potential harm of administering placebo infusions to Ebola patients and because the study outcomes of primary interest—mortality and viral load—were thought to be less susceptible to bias. However, the lack of blinding could have resulted in some bias in interpreting clinical response and adverse events. The use of randomization allowed for an appropriate comparator to assess the safety and efficacy of ZMapp (and other novel interventions that might have been studied later). The trial stratified patients to control for presumed differences in prognosis based on baseline viral burden as well as in potential differences in optimized standard of care based on location. The trial used an innovative barely Bayesian-type design that was more permissive of termination for efficacy or futility than some other standard approaches, without undermining the control of Type I error (Dodd et al., 2016). The study protocol was designed to be adaptive; it included a series
of two-arm comparisons of novel interventions (the first being ZMapp) compared with optimized standard of care to establish a framework that could be used to evaluate multiple potential Ebola treatments in the future. The design could be extended to multiple arms if multiple treatment options were simultaneously available and seemed equally promising.
Results and Discussion
The trial enrolled 72 adults and children with confirmed Ebola infection from Guinea (12 patients), Liberia (5 patients), Sierra Leone (54 patients), and the United States (1 patient); the trial was stopped after 72 of the intended 200 patients were enrolled, due to the winding down of the epidemic. In general, those who received ZMapp appeared to do better, regardless of virus levels, but the results were not statistically significant. The observed posterior probability that ZMapp plus the current standard of care was superior to the current standard of care alone was 91.2 percent, falling short of the prespecified threshold of 97.5 percent. Frequentist analyses yielded similar results (absolute difference in mortality with ZMapp, −15 percentage points; 95 percent confidence interval, −36 to 7). From a safety standpoint, ZMapp appeared to be well tolerated. ZMapp showed promise as a possible effective treatment for Ebola, but the data were insufficient to determine definitively whether it is superior to supportive care alone. Although only 72 patients were enrolled, being the only randomized trial of a therapeutic intervention conducted during the outbreak, it added valuable information on the effects of ZMapp on Ebola (PREVAIL II Writing Group, 2016). Prior to PREVAIL, only animal model and nonhuman primate data existed for ZMapp, but the conduct of this trial has provided important safety data and efficacy data in humans showing a trend toward a ~40 percent reduction in mortality.
DISCUSSION
The end result of the therapeutic trials was a “thin scientific harvest” (Cohen and Enserink, 2016) (see Table 3-3 at the end of the chapter for a summary of the therapeutic trials). Because the epidemic began to wane as the trials were being planned in the fall of 2014, most of the trials were unable to enroll enough patients to meet the desired targets. Due to the problem with sample size, none of the therapeutic trials were able to reach definitive conclusions about treatment efficacy. However, even if the trials had been able to enroll to completion, it is highly unlikely that the single-arm studies would have provided conclusive evidence on the effectiveness of the agents in the absence of concurrent controls. Given the limited pre-
clinical evidence available on the safety and efficacy of the investigational medicinal products, the changing standard of care for Ebola patients, and variable mortality rates in different settings and population subgroups, the case for randomization providing the most robust evidence was strong, and the committee concludes that randomization should have been more widely used. PREVAIL II demonstrated that an RCT was acceptable in all three countries, despite the doubts expressed earlier in the epidemic; this is in large part due to the evolving circumstances on the ground and the social mobilization efforts made by the research team (see Chapter 6 for more detailed discussion on community engagement). ZMapp, initially hoped to be a highly efficacious therapeutic agent for treating Ebola, did not live up to the publicity, although the limited evidence suggests it might have some benefit, even if it is less than uniformly effective. The investigators concluded that “in the event of another outbreak, that experimental niche should probably be filled by one of a small number of other promising, but unproven, treatments that have emerged since the beginning of the recent crisis” (PREVAIL II Writing Group, 2016, p. 1455). However, it should be noted that PREVAIL II successfully used an adaptive randomized, controlled trial design that could facilitate future trials.
Our understanding of treatment options for Ebola is little better than it was before the outbreak due to the fact that none of the trials yielded conclusive results. There is a legitimate concern that inconclusive trials may actually set back the search for an effective therapy. Single-arm trials may have missed moderate and clearly worthwhile effects and thus discounted a potentially beneficial product for future study. Trials that released preliminary results suggesting the experimental intervention was effective may have contributed to perceptions that overestimated the potential benefits, thereby compromising the ability to perform future controlled trials of these products.
Aside from compromising the ability to conduct a future clinical trial, the adoption of investigational medicinal products (or practices) based on inconclusive or preliminary evidence may lead to medical care that is ineffective or even potentially harmful. In many cases, accepted medical practice (therapies and diagnostics) established without the basis of solid evidence from RCTs may be found to be without value when RCTs are eventually conducted (Prasad et al., 2013). While this “phenomenon should be rare in the age of evidence-based medicine, it is ubiquitous” (Prasad and Cifu, 2011, p. 472). For example, hormone replacement therapy was widely used to prevent cardiovascular disease on the basis of nonrandomized evidence before randomized trials showed that such treatment was more likely harmful than beneficial (ACOG, 2013; Writing Group for the Women’s Health Initiative, 2002). These medical reversals can have seri-
ous implications, not just regarding suboptimal care for patients but also regarding a loss of patient trust in the medical system (Prasad et al., 2012). For a disease like Ebola, the potential consequences of promoting an ineffective medical practice can be even more severe. The efficacy of a treatment for Ebola can only be tested during an outbreak, so reversing a perceived benefit would require a repetition of the trial during another outbreak; it is clearly better to get it right in the first place with the right design. In addition, Ebola strikes in countries where trust in the government and authority, including the medical system and health care providers, is already low and where research may not be well understood, which results in a situation in which reversing a common practice (e.g., reversing the decision to include favipiravir as standard of care in Guinea) would risk being perceived as even more suspect.
One of the major goals of conducting clinical research is to generate sufficient evidence to lead to product approval. Early consultations with regulators may help researchers select agents for study and develop trial designs that would generate reliable information with the potential to lead to regulatory approval. Outside the U.S. Food and Drug Administration’s (FDA’s) Investigational New Drug program (FDA, 2016), researchers in the United States and Europe are not required to consult with their regulators, but such consultations for both new investigational drugs and repurposed medicinal products may be beneficial as regulators very often have access to proprietary information that others do not and can use their discretion to inform researchers in a way that can save effort and direct resources to best use. Consultations may take time, but in urgent situations such as the Ebola outbreak, regulators have shown they can be very supportive and responsive in the context of the epidemic. Further, some delay on the front end may result in shorter approval time down the road and provide access to more people more quickly. As was recognized by the regulators involved in the Ebola outbreak, it is essential that regulatory bodies in affected countries are included in these conversations as early as possible.3
Regulators in the United States and Europe also have mechanisms for expedited review that can speed up the review timeline. These regulations strongly advise sponsors participate in early and frequent dialogue with regulators (EMA, 2005; HHS, 2014). The FDA, for example, has four main programs “intended to facilitate and expedite development and review of new drugs to address unmet medical need in the treatment of a serious or life-threatening condition”; these are fast track, breakthrough therapy,
___________________
3 Testimony by Robert Hemmings, Medicines and Healthcare Products Regulatory Agency (MHRA) UK; Peter Marks, U.S. FDA; Edward M. Cox, U.S. FDA; and Marco Cavaleri, European Medicine Agency (EMA). Public Webinar of the Committee on Clinical Trials During the 2014–2015 Ebola Outbreak, May 19, 2016.
accelerated approval, and priority review designation (HHS, 2014). Products can qualify for one or more of these programs depending on the qualifying criteria. During the Ebola outbreak, both TKM-Ebola and ZMapp were given fast track review. Additionally, regulators may aid in selecting products for investigation if the available preclinical evidence is based on animal models. Animal models can help to prioritize the agents most likely to be efficacious, but only if there are good animal models for the medical condition. In rare cases, efficacy in animals might support licensure of a product under the Animal Efficacy Rule rule; however, the animal rule is only applicable when there are validated animal models for the disease (HHS, 2015). Even with the animal rule, researchers would still have to conduct Phase 1 and Phase 2 trials to obtain sufficient efficacy and safety data in humans to determine safety.
Conclusion 3-1 Product regulators can play a useful role in providing advice about trial design and selection of agents to study, and they should be involved in deliberations about these decisions in future epidemic situations.
TABLE 3-3 Summary of Therapeutic Trials Conducted During 2014–2015 Ebola Outbreak
| Investigational Product | Sponsoring Organization, Trial Name | Trial Location | Trial Design and Design Considerations | Timeline | Results |
|---|---|---|---|---|---|
| Convalescent plasma (CP) | Institute of Tropical Medicine, Belgium; Ebola Tx | Guinea |
Trial Design
|
February 2015–August 2015 | The transfusion of up to 500 ml of convalescent plasma with unknown levels of neutralizing antibodies in 84 patients with confirmed Ebola was not associated with a significant improvement in survival. |
| Favipiravir | Institut national de la santé et de la recherche médicale, France (Inserm); JIKI | Guinea |
Trial Design
|
December 2014–June 2015 | Efficacy and tolerance inconclusive. |
Design Considerations
|
| Investigational Product | Sponsoring Organization, Trial Name | Trial Location | Trial Design and Design Considerations | Timeline | Results |
|---|---|---|---|---|---|
| Brincidofovir (BCV)
(Dunning et al., 2016a; Horby, 2015; Whitehead et al., 2016) |
University of Oxford; RAPIDE-BCV | Monrovia, Liberia | Trial Design:
Design Considerations:
|
January 2015 | Drug developer Chimerix decided to discontinue a program to test its lead product against the Ebola virus in Liberia (diminishing patient population).
Chimerix determined to focus on CMV. |
Ongoing humanitarian crisis + trial involves risks
High death rate + volatile conditions
|
| Investigational Product | Sponsoring Organization, Trial Name | Trial Location | Trial Design and Design Considerations | Timeline | Results |
|---|---|---|---|---|---|
| TKM-130803
(Dunning et al., 2016b; Horby, 2015; Whitehead et al., 2016) |
University of Oxford | Port Loko, Sierra Leone | Trial Design
Design Considerations:
|
March–June 2015 | Early results from the study, demonstrated that TKM-130803 was not effective in increasing the survival fraction above 50 percent; unlikely to demonstrate an overall therapeutic benefit to patients. |
Ongoing humanitarian crisis + trial involves risks
High death rate + volatile conditions
|
| Investigational Product | Sponsoring Organization, Trial Name | Trial Location | Trial Design and Design Considerations | Timeline | Results |
|---|---|---|---|---|---|
| ZMapp | National Institute of Allergy and Infectious Diseases (NIAID)–PREVAIL II | Liberia, Guinea, Sierra Leone, United States
The trial enrolled 72 adults and children with confirmed Ebola infection from Guinea (12 patients), Liberia (5 patients), Sierra Leone (54 patients), and the United States (1 patient) |
This intervention trial is a multinational/ multicenter, Phase 1/2 randomized, open-label trial consisting of 2 arms—ZMapp + optimized standard of care (oSOC) versus oSOC only.
|
March 2015–November 2015 | A total of 72 patients were enrolled at sites in Guinea, Liberia, Sierra Leone, and the United States. Of the 71 patients who could be evaluated, 21 died, representing an overall case fatality rate of 30 percent. Death occurred in 13 of 35 patients (37 percent) who received the current standard of care alone and in 8 of 36 patients (22 percent) who received the current standard of care plus ZMapp. The observed posterior probability that ZMapp plus the current standard of care was superior to the current standard of care alone was 91.2 percent, falling short of the prespecified threshold of 97.5 percent. |
Design Considerations:
|
Frequentist analyses yielded similar results (absolute difference in mortality with ZMapp, −15 percentage points; 95 percent confidence interval, −36 to 7). Baseline viral load was strongly predictive of both mortality and duration of hospitalization in all age groups. ZMapp showed promise as a possible effective treatment agent for Ebola but there were insufficient data to determine definitively whether it is a better treatment for Ebola than supportive care alone. |
REFERENCES
ACOG (American College of Obstetricians and Gynecologists). 2013. Hormone therapy and heart disease. Committee opinion no. 565. Washington, DC: American Congress of Obstetricians and Gynecologists. http://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-on-Gynecologic-Practice/Hormone-Therapy-and-Heart-Disease (accessed January 17, 2017).
Adebamowo, C., O. Bah-Sow, F. Binka, R. Bruzzone, A. Caplan, J.-F. Delfraissy, D. Heymann, P. Horby, P. Kaleebu, J.-J. M. Tamfum, P. Olliaro, P. Piot, A. Tejan-Cole, O. Tomori, A. Toure, E. Torreele, and J. Whitehead. 2014. Randomised controlled trials for Ebola: Practical and ethical issues. The Lancet 384(9952):1423–1424.
Beck, Z., Z. Prohászka, and G. Füst. 2008. Traitors of the immune system—Enhancing antibodies in HIV infection: Their possible implication in HIV vaccine development. Vaccine 26(24):3078–3085.
Chimerix Inc. 2015. Chimerix focusing efforts on CMV and adenovirus pivotal trials. http://ir.chimerix.com/releasedetail.cfm?releaseid=893927 (accessed December 21, 2016).
Cohen, J. 2015. Results from encouraging Ebola trial scrutinized. Science Insider, Feb. 26. http://www.sciencemag.org/news/2015/02/results-encouraging-ebola-trial-scrutinized (accessed January 21, 2017).
Cohen, J., and M. Enserink. 2016. As Ebola epidemic draws to a close, a thin scientific harvest. Science 351(6268):12–13.
Davey, R. T. J. 2016. PREVAIL II: A randomized controlled trial of ZMapp™ in acute Ebola virus infection. Paper read at Conference on Retroviruses and Opportunistic Infections 2017, February 22–25, Boston, MA.
Dodd, L. E., M. A. Proschan, J. Neuhaus, J. S. Koopmeiners, J. Neaton, J. D. Beigel, K. Barrett, H. C. Lane, and R. T. Davey, Jr. 2016. Design of a randomized controlled trial for Ebola virus disease medical countermeasures: PREVAIL II, the Ebola MCM study. Journal of Infectious Diseases 213(12):1906–1913.
Dunning, J., S. B. Kennedy, A. Antierens, J. Whitehead, I. Ciglenecki, G. Carson, R. Kanapathipillai, L. Castle, R. Howell-Jones, R. Pardinaz-Solis, J. Grove, J. Scott, T. Lang, P. Olliaro, and P. W. Horby, for the RAPIDE- BCV trial team. 2016a. Experimental treatment of Ebola virus disease with brincidofovir. PLoS ONE 11(9):e0162199.
Dunning, J., F. Sahr, A. Rojek, F. Gannon, G. Carson, B. Idriss, T. Massaquoi, R. Gandi, S. Joseph, H. K. Osman, T. J. G. Brooks, A. J. H. Simpson, I. Goodfellow, L. Thorne, A. Arias, L. Merson, L. Castle, R. Howell-Jones, R. Pardinaz-Solis, B. Hope-Gill, M. Ferri, J. Grove, M. Kowalski, K. Stepniewska, T. Lang, J. Whitehead, P. Olliaro, M. Samai, and P. W. Horby, for the RAPIDE-TKM trial team. 2016b. Experimental treatment of Ebola virus disease with TKM-130803: A single-arm Phase 2 clinical trial. PLoS Medicine 13(4):e1001997.
Dye, J. M., A. S. Herbert, A. I. Kuehne, J. F. Barth, M. A. Muhammad, S. E. Zak, R. A. Ortiz, L. I. Prugar, and W. D. Pratt. 2012. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proceedings of the National Academy of Sciences of the United States of America 109(13):5034–5039.
Edwards, T., M. G. Semple, A. De Weggheleire, Y. Claeys, M. De Crop, J. Menten, R. Ravinetto, S. Temmerman, L. Lynen, E. I. Bah, P. G. Smith, and J. van Griensven, on behalf of the Ebola-Tx Consortium. 2016. Design and analysis considerations in the Ebola-Tx trial evaluating convalescent plasma in the treatment of Ebola virus disease in Guinea during the 2014–2015 outbreak. Clinical Trials (London, England) 13(1):13–21.
EMA (European Medicines Agency). 2005. Guideline on procedures for the granting of a marketing authorisation under exceptional circumstances, pursuant to article 14 (8) of regulation (ec) no 726/2004 release. http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004883.pdf (accessed March 8, 2017).
FDA (U.S. Food and Drug Administration). 2016. Investigational new drug (IND) application. http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/investigationalnewdrugindapplication/default.htm (accessed February 20, 2017).
FujiFilm Corporation. 2016. The anti-influenza drug “Avigan Tablet” selected as one of the supplies to be procured with the Japanese government’s emergency grant aid for countering the Ebola virus disease in Guinea. http://www.fujifilm.com/news/n160614.html (accessed January 17, 2017).
Furuta, Y., B. B. Gowen, K. Takahashi, K. Shiraki, D. F. Smee, and D. L. Barnard. 2013. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Research 100(2): 446–454.
Geisbert, T. W., A. C. H. Lee, M. Robbins, J. B. Geisbert, A. N. Honko, V. Sood, J. C. Johnson, S. de Jong, I. Tavakoli, A. Judge, L. E. Hensley, and I. MacLachlan. 2010. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: A proof-of-concept study. The Lancet 375(9729):1896–1905.
Haque, A., D. Hober, and J. Blondiaux. 2015. Addressing therapeutic options for Ebola virus infection in current and future outbreaks. Antimicrobial Agents and Chemotherapy 59(10):5892–5902.
HHS (U.S. Department of Health and Human Services). 2014. Guidance for industry: Expedited programs for serious conditions – drugs and biologics. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf (accessed March 8, 2017).
HHS. 2015. Product development under the animal rule: Guidance for industry. Silver Spring, MD: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf (accessed January 17,2017).
Horby, P. 2015. Trials of antivirals for Ebola ISARIC experience. http://www.ebolatx.eu/wp-content/uploads/2015/09/Peter-Horby-ECTMIH-Sept-2015.pdf (accessed January 17, 2017).
ITM (Institute of Tropical Medicine). 2016. Ebola-Tx Project. http://www.ebolatx.eu (accessed January 17, 2017).
Kroll, D. 2015. Chimerix ends brincidofovir Ebola trials to focus on adenovirus and CMV. Forbes, January 31. http://www.forbes.com/sites/davidkroll/2015/01/31/chimerix-ends-brincidofovir-ebola-trials-to-focus-on-adenovirus-and-cmv/#5e8d557069d8 (accessed January 21, 2017).
Marano, G., S. Vaglio, S. Pupella, G. Facco, L. Catalano, G. M. Liumbruno, and G. Grazzini. 2016. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfusion 14(2):152–157.
MSF (Médecins sans Frontières). 2015. Preliminary results of the JIKI clinical trial to test the efficacy of favipiravir in reducing mortality in individuals infected by Ebola virus in Guinea. http://www.msf.org/en/article/preliminary-results-jiki-clinical-trial-test-efficacy-favipiravir-reducing-mortality (accessed December 21, 2016).
Nakayama, E., D. Tomabechi, K. Matsuno, N. Kishida, R. Yoshida, H. Feldmann, and A. Takada. 2011. Antibody-dependent enhancement of Marburg virus infection. Journal of Infectious Diseases 204(Suppl 3):S978–S985.
Prasad, V., and A. Cifu. 2011. Medical reversal: Why we must raise the bar before adopting new technologies. Yale Journal of Biology and Medicine 84(4):471–478.
Prasad, V., A. Cifu, and J. A. Ioannidis. 2012. Reversals of established medical practices: Evidence to abandon ship. JAMA 307(1):37–38.
Prasad, V., A. Vandross, C. Toomey, M. Cheung, J. Rho, S. Quinn, S. J. Chacko, D. Borkar, V. Gall, S. Selvaraj, N. Ho, and A. Cifu. 2013. A decade of reversal: An analysis of 146 contradicted medical practices. Mayo Clinic Proceedings 88(8):790–798.
PREVAIL II Writing Group. 2016. A randomized, controlled trial of ZMapp for Ebola virus infection. New England Journal of Medicine 375(15):1448–1456.
Qiu, X., G. Wong, J. Audet, A. Bello, L. Fernando, J. B. Alimonti, H. Fausther-Bovendo, H. Wei, J. Aviles, E. Hiatt, A. Johnson, J. Morton, K. Swope, O. Bohorov, N. Bohorova, C. Goodman, D. Kim, M. H. Pauly, J. Velasco, J. Pettitt, G. G. Olinger, K. Whaley, B. Xu, J. E. Strong, L. Zeitlin, and G. P. Kobinger. 2014. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514(7520):47–53.
Reuters. 2015. Guinea to expand use of experimental anti-Ebola drugs. Reuters, February 7. http://www.reuters.com/article/us-health-ebola-guinea-idUSKBN0LB0Q620150207 (accessed December 21, 2016).
Sissoko, D., C. Laouenan, E. Folkesson, A.-B. M’Lebing, A.-H. Beavogui, S. Baize, A.-M. Camara, P. Maes, S. Shepherd, C. Danel, S. Carazo, M. N. Conde, J.-L. Gala, G. Colin, H. Savini, J. A. Bore, F. Le Marcis, F. R. Koundouno, F. Petitjean, M.-C. Lamah, S. Diederich, A. Tounkara, G. Poelart, E. Berbain, J.-M. Dindart, S. Duraffour, A. Lefevre, T. Leno, O. Peyrouset, L. Irenge, N. F. Bangoura, R. Palich, J. Hinzmann, A. Kraus, T. S. Barry, S. Berette, A. Bongono, M. S. Camara, V. Chanfreau Munoz, L. Doumbouya, H. Souley, P. M. Kighoma, F. R. Koundouno, L. Réné, C. M. Loua, V. Massala, K. Moumouni, C. Provost, N. Samake, C. Sekou, A. Soumah, I. Arnould, M. S. Komano, L. Gustin, C. Berutto, D. Camara, F. S. Camara, J. Colpaert, L. Delamou, L. Jansson, E. Kourouma, M. Loua, K. Malme, E. Manfrin, A. Maomou, A. Milinouno, S. Ombelet, A. Y. Sidiboun, I. Verreckt, P. Yombouno, A. Bocquin, C. Carbonnelle, T. Carmoi, P. Frange, S. Mely, V.-K. Nguyen, D. Pannetier, A.-M. Taburet, J.-M. Treluyer, J. Kolie, R. Moh, M. C. Gonzalez, E. Kuisma, B. Liedigk, D. Ngabo, M. Rudolf, R. Thom, R. Kerber, M. Gabriel, A. Di Caro, R. Wölfel, J. Badir, M. Bentahir, Y. Deccache, C. Dumont, J.-F. Durant, K. El Bakkouri, M. Gasasira Uwamahoro, B. Smits, N. Toufik, S. Van Cauwenberghe, K. Ezzedine, E. Dortenzio, L. Pizarro, A. Etienne, J. Guedj, A. Fizet, E. Barte de Sainte Fare, B. Murgue, T. Tran-Minh, C. Rapp, P. Piguet, M. Poncin, B. Draguez, T. Allaford Duverger, S. Barbe, G. Baret, I. Defourny, M. Carroll, H. Raoul, A. Augier, S. P. Eholie, Y. Yazdanpanah, C. Levy-Marchal, A. Antierrens, M. Van Herp, S. Günther, X. de Lamballerie, S. Keïta, F. Mentre, X. Anglaret, D. Malvy, and JIKI Study Group. 2016. Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): A historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Medicine 13(3):e1001967.
van Griensven, J., T. Edwards, X. de Lamballerie, M. G. Semple, P. Gallian, S. Baize, P. W. Horby, H. Raoul, N. F. Magassouba, A. Antierens, C. Lomas, O. Faye, A. A. Sall, K. Fransen, J. Buyze, R. Ravinetto, P. Tiberghien, Y. Claeys, M. De Crop, L. Lynen, E. I. Bah, P. G. Smith, A. Delamou, A. De Weggheleire, and N. Haba. 2016a. Evaluation of convalescent plasma for Ebola virus disease in Guinea. New England Journal of Medicine 374(1):33–42.
van Griensven, J., T. Edwards, X. de Lamballerie, M. G. Semple, P. Gallian, S. Baize, P. W. Horby, H. Raoul, N. F. Magassouba, A. Antierens, C. Lomas, O. Faye, A. A. Sall, K. Fransen, J. Buyze, R. Ravinetto, P. Tiberghien, Y. Claeys, M. De Crop, L. Lynen, E. I. Bah, P. G. Smith, A. Delamou, A. De Weggheleire, and N. Haba. 2016b. Evaluation of convalescent plasma for Ebola virus disease in Guinea. New England Journal of Medicine 374(1):33–42.
Wellcome Trust. 2015. New trial of TKM-Ebola treatment to start in Sierra Leone. https://wellcome.ac.uk/press-release/new-trial-tkm-ebola-treatment-start-sierra-leone (accessed December 21, 2016).
Whitehead, J., P. Olliaro, T. Lang, and P. Horby. 2016. Trial design for evaluating novel treatments during an outbreak of an infectious disease. Clinical Trials 13(1):31–38.
WHO (World Health Organization). 2014a. Ethical issues related to study design for trials on therapeutics for Ebola virus disease. Geneva, Switzerland: WHO Ethics Working Group Meeting, October 20–21, 2014. http://apps.who.int/iris/bitstream/10665/137509/1/WHO_HIS_KER_GHE_14.2_eng.pdf (accessed January 17, 2017).
WHO. 2014b. WHO meeting of the Scientific and Technical Advisory Committee on Ebola Experimental Interventions—Briefing note. http://www.who.int/medicines/ebola-treatment/scientific_tech_meeting/en (accessed December 21, 2016).
WHO. 2015a. Categorization and prioritization of drugs for consideration for testing or use in patients infected with Ebola. http://www.who.int/medicines/ebola-treatment/2015_0703TablesofEbolaDrugs.pdf?ua=1 (accessed January 21, 2017).
WHO. 2015b. Ebola R&D landscape of clinical candidates and trials. Geneva, Switzerland: World Health Organization. http://www.who.int/medicines/ebola-treatment/EbolaR_D_public-report_updt2015.pdf (accessed January 21, 2017).
WHO. 2016a. Graph of new confirmed cases per epi week for Guinea. http://apps.who.int/gho/data/node.ebola-sitrep.ebola-country-GIN-20160511?lang=en (accessed January 17, 2017).
WHO. 2016b. Graph of new confirmed cases per epi week for Liberia. http://apps.who.int/gho/data/view.ebola-sitrep.ebola-country-LBR-20160511-graph?lang=en (accessed January 17, 2017).
WHO. 2016c. Graph of new confirmed cases per epi week for Sierra Leone. http://apps.who.int/gho/data/view.ebola-sitrep.ebola-country-SLE-20160511-graph?lang=en (accessed January 17, 2017).
Writing Group for the Women’s Health Initiative. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. JAMA 288(3):321–333.
This page intentionally left blank.






























