4
Assessment of Vaccine Trials
Overall, the Ebola vaccine trials displayed better coordination and cooperation among international researchers, regulators, manufacturers, funders, and the national authorities and communities of the Ebola affected countries than did the therapeutic trials. In fact, some candidates were already available and had been tested in nonhuman primates in the decade before the West Africa outbreak began. As a result, the trials were designed, approved, and implemented quickly; in one case, “a first-in-human Phase 1 was authorized in 4 working days by regulators in the UK, including initial assessment and time to review responses by the applicant” (WHO, 2015c). However, as with the design of the therapeutic trials, there were also disagreements, competition, and infighting among the organizations that were carrying out the trials (see Chapter 2 for more detail on the disagreements and Chapter 3 for details on the therapeutic trials conducted). Most notably, while there was consensus that clinical trial data would be necessary in order to support the licensure of any investigational vaccine candidate, there was little agreement concerning the preferred specific design or execution of the trials.
On September 29–30, 2014, with the international response just beginning in earnest, the World Health Organization (WHO) convened a meeting to coordinate the planned clinical trials for candidate Ebola vaccines. At this time, the GlaxoSmithKline (GSK) ChAd3 vaccine and the Newlink rVSV vaccine were the only candidates that met the criteria laid out by the
WHO the previous month.1 These criteria required “availability of good manufacturing practice grade vials after lot release for clinical trials, and 100 percent efficacy had been documented in nonhuman primates with acceptable preclinical safety” (WHO, 2015c).
With these vaccine candidates selected, debate shifted to concerns regarding the best trial designs for testing their efficacy. While determining the best designs for vaccine trials involved many of the same issues complicating the design of therapeutic trials—randomization, community perspectives, and access to potential benefit—the vaccine trials also posed distinct ethical issues. First, a person receiving a vaccine would presumably not be infected with Ebola and therefore not at immediate risk of death. Many of the arguments against randomized controlled trials (RCTs) for Ebola therapeutics centered on the ethics of giving an infected person a placebo or only standard of care when the risk of death was so high without an effective intervention. The risks of adverse effects from an unproven investrigational agent were considered by some to be smaller than the risks of not providing one. However, with a vaccine, the risks of adverse effects may outweigh the risks of not receiving the vaccine since the participant may or may not be exposed to Ebola. Giving a potentially harmful agent to a healthy person has different implications than giving a potentially harmful agent to someone who is at a high risk of death, such as a patient suffering from Ebola, as Dawson (2015) noted: “[I]t is not so clear that when not infected [a person] would or should be willing to accept an unknown risk from an unlicensed preventive vaccine, given that other measures such as good quality protective equipment, if properly used, may reduce the risk of infection to an acceptable level” (Dawson, 2015, p. 108). Second, research participants who have received a vaccine or a placebo may believe that they are protected from infection. They may not even consider the possibility that they may have received the placebo or that even if they got the vaccine it might not be effective, and, as a consequence, they may fail to take all proscribed safety precautions, such as the proper donning and doffing of personal protective equipment while caring for Ebola patients. “Known as risk compensation, this behavioral adjustment draws on the theory of ‘risk homeostasis,’ which has previously been applied to phenomena as diverse as Lyme disease vaccination, insurance mandates, and automobile safety” (Underhill, 2013, p. 115).
At the WHO meeting in September 2014, participants discussed the scientific and ethical issues involved in designing vaccine trials. As reported in Science, a researcher with the Ebola vaccine development program at GSK said, “Going into this meeting, we were told the idea of a controlled
___________________
1 J&J (Ad26/MVA) and Novavax (recombinant protein) met this criteria later in the epidemic (WHO, 2015d).
trial . . . was not going to be acceptable” (Cohen and Kupferschmidt, 2014b). Yet, the trial design he presented included one-to-one randomization between the investigational vaccine and an active control, which would be an approved vaccine for another disease such as hepatitis B. The GSK representative maintained that this design would determine the efficacy of the vaccine much faster than alternative designs. However, some participants—particularly those from Médecins Sans Frontières (MSF)—disagreed and argued that, as with therapeutic agents, any vaccine trial involving a placebo or active control arm would be unethical (Cohen and Kupferschmidt, 2014b).
Randomization was seen as particularly problematic for health care workers, who were at high risk of contracting Ebola. A representative from the Wellcome Trust asked, “If you were there tomorrow and you were a health care worker, would you be willing to be in a control arm, when the next 3 months you will be looking after patients with Ebola?” (Cohen and Kupferschmidt, 2014a, p. 290). One MSF representative, who oversaw experimental Ebola products for MSF, told Science, “Studies on efficacy in affected countries and more so in at-risk populations should not have a placebo or active control arm as this cannot be defended ethically” (Cohen and Kupferschmidt, 2014a). However, at the time there were no in-human data to determine the risk–benefit balance between the benefit of the vaccine and the risk of side effects.
“The meeting was quite tense at moments,” said Marie-Paule Kieny, WHO assistant director-general and vaccine expert (Cohen and Kupferschmidt, 2014a), and determining the choice of control arm proved to be one of the most contentious points in designing Ebola vaccine trials. There were three main options: a placebo control, an active vaccination (with a non-Ebola vaccine), or delayed vaccination (Nason, 2016). The placebo-controlled trial was argued by some to be unethical due to a responsibility of researchers to provide something of value to research participants (Cohen and Kupferschmidt, 2014b). While many at the September 29–30 WHO meeting argued that using an active control would be the fastest method for determining the safety and efficacy of the vaccine, this design did not win over all meeting participants. As a representative from the Wellcome Trust put it, “An RCT may yield results faster, but if it’s simply unacceptable for trial participants, a stepped-wedge design is preferable” (Cohen and Kupferschmidt, 2014a). The stepped-wedge design became the leading alternative trial design and ultimately best addressed the concerns of the meeting participants (Cohen and Kupferschmidt, 2014a). A stepped-wedge trial rolls out the intervention to participants over time, either as individuals or in clusters. By the time the study ends, all participants will have received the intervention, but they will have received it in a random order and in some cases the intervention will have been delayed.
Researchers are then able to learn about efficacy by looking at when participants received the vaccine and if and when they were infected in order to calculate how much protection the vaccine provided (Brown and Lilford, 2006). Stepped-wedge designs do have drawbacks, including an inability to determine long-term harm from vaccination, a difficulty determining how long to wait before vaccine administration to the delay group, and difficulty determining whether an infection-enhancing immune adverse response might be induced, as has been seen with other vaccines, such as respiratory syncytial virus (Openshaw and Tregoning, 2005). On the other hand, since all participants would receive the Ebola vaccine, the design appealed to those opposed to placebos or active controls for ethical reasons. See Box 4-1 for WHO requirements for Ebola vaccine trials.
At the October 23 WHO meeting the U.S. National Institutes of Health (NIH) and the U.S. Centers for Disease Control and Prevention (CDC)
trials, to take place in Liberia and Sierra Leone, respectively (discussed in more detail below), were presented and generally supported. However, at that time there were no trials planned in Guinea (WHO, 2015a). As a result a small group formed at this meeting to discuss options for implementing a vaccine trial in Guinea. A Guinea Ebola vaccine trial working group2 was formed which determined the trial designs to be used in Guinea. The working group consisted of multiple stakeholders, including the WHO, academics, representatives from U.S. government, and representatives from GSK, Newlink, and Merck (WHO, 2015a).
In selecting trial designs, the NIH determined that in order to test safety and efficacy in the most robust way a traditional placebo-controlled RCT should be implemented while the CDC was more geared toward distributing vaccines to the population, which led to the use of a two-arm immediate and delayed vaccination approach with individual randomization. The main motivation for the Guinea ring vaccination approach was that the working group (formed at the October 23 WHO meeting) saw that there was not sufficient capacity for doing a large population-based trial and therefore decided on two other populations: the rings around new cases and the frontline workers. Individual randomization was considered in the ring vaccination trial, but it was decided that cluster randomization would be more feasible given the logistical and capacity issues.
When the public health emergency of international concern (PHEIC) was declared on August 8, 2014, the WHO convened a meeting to be held that month to discuss how to “fast-track the testing and the deployment of promising vaccines in sufficient numbers to use in the field in 2015 to try and impact the Ebola epidemic curve” (WHO, 2015c). At this meeting it was agreed that Phase 1 trials would launch and that before Phase 1 trials were completed, efficacy trials in the affected countries would be initiated. This decision made it difficult for manufacturers, as they were unsure which dose would be required for Phase 2 trials (Mohammadi, 2015). See Table 4-1 for details on the Phase 1 Ebola vaccine trials initiated during the Ebola outbreak.
ASSESSMENT OF TRIALS
Below is an individual assessment of the vaccine trials that were conducted in the Ebola-affected countries during the Ebola epidemic, including assessments of their study designs and conduct, results, and analyses. In-depth descriptions of the different trials are available in the published
___________________
2 Testimonies of Peter Smith and Ana Maria Henao Restrepo at the Public Workshop of the Committee on Clinical Trials During the 2014–2015 Ebola Outbreak. London, UK; March 2016.
TABLE 4-1 October 2015 WHO Summary of the Phase 1 Ebola Vaccine Trials
| Product/Company | Phase | Trial Location | Dates |
|---|---|---|---|
| ChAd3-ZEBOV GlaxoSmithKline and PHAC | Phase 1 |
By VRC at NIH, USA
By Oxford University in the UK |
September 2014 |
|
By CVD in Mali
At the University of Lausanne, Lausanne, Switzerland |
October 2014 | ||
| rVSV-ZEBOV NewLink Genetics and Merck Vaccines USA | Phase 1 |
By WRAIR in the US
By NIAID in the US |
October 2014 |
|
By CTC North GmbH in Hamburg, Germany
At Albert Schweitzer Hospital in Lambarene, Gabon At the University of Geneva, Geneva, Switzerland At the IWK Health Center, Halifax, Canada |
November 2014 | ||
| By KEMRI Wellcome Trust in Kilifi, Kenya | December 2014 | ||
| Ad26.ZEBOV and MVA-BN-Filo Johnson & Johnson and Bavarian Nordic | Phase 1 | By University of Oxford in the UK and NIAID, USA | January 2015 |
|
By University of Nairobi, Kenya
By MRC, Uganda Virus Research Institute, Uganda By Mwanza Intervention Trials Unit, United Republic of Tanzania |
Second half of 2015 | ||
| Recombinant protein Ebola vaccine candidate Novavax | Phase 1 | Australia | February 2015 |
SOURCE: Adapted from WHO, 2015a.
manuscripts for these trials. Similar to the case with the therapeutic trials, preparation and planning for the vaccine trials started in September 2014 and Phase 2 and Phase 3 trials began enrolling participants between February 2015 and October 2015 (see Table 4-2). While the trials were launched rapidly, the Phase 2 and Phase 3 trials began participant enrollment at the tail end of the epidemic (see Figure 4-1).
Ebola ça Suffit–Guinea Ring Vaccination Trial; Guinea (rVSV-ZEBOV)
Among all of the therapeutic and vaccine trials conducted in West Africa during the outbreak, the ring vaccination trial came the closest to fulfilling the hope for a clinical trial “home run” (or a “six,” its cricket equivalent). It was a collaboration among the government of Guinea, WHO, MSF, and the Norwegian Institute of Public Health that demonstrated it was possible to perform a type of randomized study during the outbreak, despite apparent substantial opposition to randomized trials by stakeholders involved in the Ebola response (Ebola ça Suffit Ring Vaccination Trial Consortium, 2015). In an example of excellent communication, coordination, and a willingness to compromise between researchers and public health officials, the study took advantage of the public health contact tracing efforts implemented during the outbreak. Index persons in the immediate vaccination clusters were hospitalized, on average, within 3.9 days after symptom onset; clusters defined for the index person were randomized, on average, within 9.7 days of symptom onset in the index person, with similar numbers in the delayed vaccination clusters (Henao-Restrepo et al., 2016). In appreciation of the success in launching this trial, The Lancet editors observed, “That such a trial was even possible is a testament not only to the skill of the research teams but also to the commitment of communities to defeating an epidemic that has devastated their nation. Over 90 percent of the study’s staff was from Guinea. Before this work, no clinical trial on this scale had ever been performed in the country” (The Lancet, 2015).
Study Design
The trial design was a cluster-randomized controlled study modeled on the ring vaccination approach used in the 1970s to eradicate smallpox (WHO, 2015b). Ring vaccination is a measure used to control the spread of an infection that involves vaccinating individuals who are socially or geographically connected to a known case, thereby creating a protective “ring” of immunity around infected individuals to prevent further spread (Rid and Miller, 2016). In the Ebola ring vaccination trial, participants were enrolled and randomized into two groups, one of which was vaccinated immediately and the other of which was assigned to receive the vaccine
TABLE 4-2 Timeline of Vaccine Trials
| Trial Name (Vaccine) | Phase | Location | Trial Enrollment Starta | Trial Enrollment End | Number of Participants |
|---|---|---|---|---|---|
| Guinea Ring Vaccine VSV-EBOV | Phase 3 | Guinea | April 1, 2015 | July 20, 2015 | 7,284 |
| CDC STRIVE VSV-EBOV | Phase 3 | Sierra Leone | April 9, 2015 | August 21, 2015 | 8,673 |
| PREVAIL I VSV-EBOV/ChAd3 | Phase 2b | Liberia | February 2, 2015 | April 30, 2015 | 1,500 |
| EBOVAC–Salone Ad26.ZEBOV and MVA-BN-Filo | Staged Phase 3 | Sierra Leone | October 8, 2015 | As of February 2017 this study is currently recruiting participantsc | |
NOTE: CDC = U.S. Centers for Disease Control and Prevention; EBOVAC = Ebola vaccine projects; STRIVE = Sierra Leone Trial to Introduce a Vaccine Against Ebola; PREVAIL = Partnership for Research on Ebola Vaccines in Liberia.
a The Phase 2 and Phase 3 trials did not begin earlier in the course of the outbreak because the manufacturers did not have a clear view of the required doses and the investigators were still working on community engagement (Mohammadi, 2015).
b In March 2015, Independent DSMB recommended moving to the Phase 2 PREVAIL trial to Phase 3; however, no cases of Ebola were reported for 2 weeks in Liberia and plans were made to move Phase 3 to other countries if possible.
c Janssen Vaccines & Prevention B.V. 2017. Staged Phase 3 Study to Assess the Safety and Immunogenicity of Ebola Candidate Vaccines Ad26. ZEBOV and MVA-BN-Filo During Implementation of Stages 1 and 2 (EBOVAC-Salone). https://clinicaltrials.gov/ct2/show/NCT02509494 (accessed February 20, 2017).
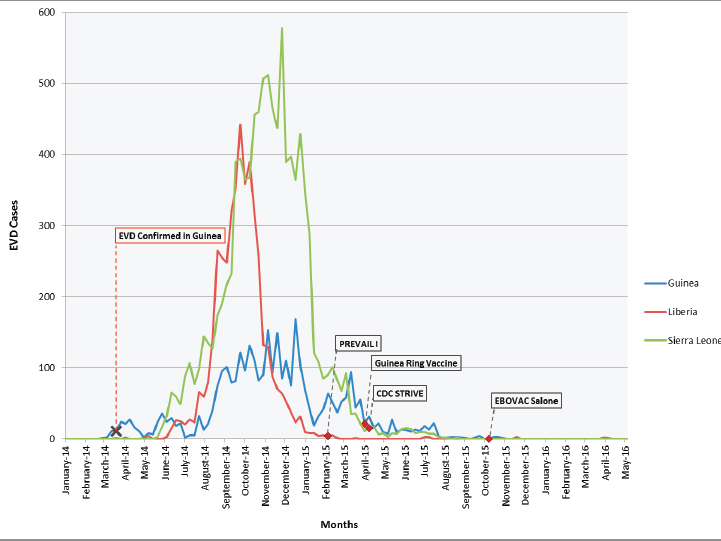
NOTE: The figure plots the trial start dates on the time course of the Ebola epidemic (confirmed cases) in each of the respective countries (Guinea, Liberia, and Sierra Leone) where the trials were conducted.
SOURCES: The confirmed Ebola case number was obtained from WHO incident reports (2014, 2016b,c,d).
21 days after enrollment. Based on the known incubation period of 2–21 days after infection before symptoms appear and on the fact that it takes some time for vaccine-induced protection to develop (if the vaccine actually works), the period of observation for risk of infection—or, conversely, protection from infection—was set for both groups as the 21-day period from 10 to 30 days post-enrollment (Ebola ça Suffit Ring Vaccination Trial Consortium, 2015). This design was chosen at least in part as a pragmatic solution to address the ethical concerns surrounding the use of an unproven vaccine and an unvaccinated control group. As one researcher noted, “A traditional trial with a placebo control would have been contentious and politically unacceptable, given the known mortality of Ebola and the lack of other options for prevention or treatment. To substitute an inert substance for a potentially life-saving vaccine, given the circumstances, would not
have been ethical—but a comparison still needed to be made. So half of the volunteer participants were vaccinated immediately, and the other half after a three-week delay” (Farrar, 2015).
Not all of the scientists and ethicists involved in the conversations agreed with this reasoning, yet to many it appeared to represent an acceptable compromise between scientific rigor and the desire to offer the hoped-for benefits of the vaccine to as many as possible. To others it meant that the results might be difficult to interpret (Rid and Miller, 2016). The investigators planned the primary analysis to “estimate vaccine efficacy against disease [where] vaccine efficacy is defined as . . . the hazard [ratio] of disease for eligible and vaccinated individuals in a ring who receive immediate vaccination and eligible individuals in a ring who receive delayed vaccination” (Ebola ça Suffit Ring Vaccination Trial Consortium, 2015). However, during the course of the trial the data safety monitoring board (DSMB) concluded that the data were sufficiently convincing of vaccine protection and terminated the delayed vaccination arm (see further discussion below). The study continued to enroll additional participants who were all offered immediate immunization; the importance of this, going forward, meant that there was no longer a control arm in the trial (Henao-Restrepo et al., 2016).
Advantages and Disadvantages
The trial focused on persons at elevated risk of contracting Ebola because of contact with an infected individual, such as health care or burial workers—or contacts of such contacts, so fewer persons needed to be enrolled to demonstrate possible efficacy (Henao-Restrepo et al., 2015). However, given that only 21 days passed between the administration of the vaccine to the immediate vaccination group and to the delayed-vaccination control group, there had to be a high enough risk in order for the study to show results—that is, if people in the delayed-vaccination group were not infected soon enough to develop symptoms of Ebola within the 21-day delay period, it would be difficult to show that the vaccine was effective. The delay period began 10 days after the immediate group received vaccine and ended 10 days after the delayed group received the vaccine. The decision on this timing represented a rational attempt to respond to the major challenge to the design and to balance the desire of the investigators to immunize all participants within a reasonable time frame and the desire to have a long enough exposure in the delayed group to increase the likelihood that endpoints might be reached within the 21-day incubation period for Ebola virus (WHO, 2016a). The danger was that not enough events would occur within this window to permit an assessment of vaccine efficacy, the essential goal of the study.
Another drawback to the design was that cluster randomization is less efficient than an individually randomized design and therefore that “the sample size must be inflated for the effect of clustering within rings as the members of a ring share a common exposure to the index case and are not statistically independent”(Ebola ça Suffit Ring Vaccination Trial Consortium, 2015). Moreover, the intrinsic risk of transmission within a cluster is assumed to be similar across clusters, but this may not be the case. As indicated in the study results, cases were documented in only 7 of 42 clusters in the delayed arm, and across these 7 clusters, per-person transmission risk also varied markedly (Henao-Restrepo et al., 2015). (See Table 4-3 below for the interim trial data.) Slight imbalances in intrinsic
| All Vaccinated in Immediate Versus All Eligible in Delayed (primary analysis) | All Eligible and Consented | All Eligible (eligible adults, contacts and contacts of contacts) | All (all contacts and contacts of contacts) | |
|---|---|---|---|---|
| Number of individuals (clusters) | ||||
| Immediate | 2014 (48) | 2048 (48) | 3035 (48) | 4123 (48) |
| Delayed | 2380 (42) | 1930 (42) | 2380 (42) | 3528 (42) |
| Number of cases at <10 days (affected clusters) | ||||
| Immediate | 9 (4) | 10 (5) | 18 (9) | 21 (9) |
| Delayed | 16 (12) | 6 (5) | 16 (12) | 25 (13) |
| Number of cases at ≥10 days | ||||
| Immediate | 0 (0) | 0 (0) | 6a (3) | 8 a (4) |
| Delayed | 16b (7) | 11b (5) | 16b (7) | 21b (7) |
| Vaccine efficacy/ effectivenessc (%; 95% CI) | 100% (74.4 to 100) | 100% (70.8 to 100) | 75.1% (–7.1 to 94.2) | 76.3% (–15.5 to 95.1) |
| p valued | 0.0036 | 0.0194 | 0.1791 | 0.3351 |
a All cases occurred in unvaccinated individuals.
b Four cases were vaccinated and developed symptoms on day 0, 2, 6, or 6 after vaccination.
c From fitting a β-binomial distribution to the cluster-level numerators and denominators and using an inverted likelihood ratio test to identify the lower bound for vaccine efficacy (first two columns); from Cox proportional hazards model to estimate vaccine effectiveness (last two columns).
d From Fisher’s exact test (two-sided).
SOURCE: Henao-Restrepo et al., 2015.
transmission risk across clusters, especially if the number of clusters is low, may lead to the primary comparisons having biased results. The lack of a placebo and the lack of blinding also raise concerns of possible bias in the ascertainment of safety endpoints, which could have significantly influenced the data presented. Additionally, if the study team was convinced that the vaccine was effective, it might at the very least, however unintentional, raise the possibility that efforts might have been less intense to detect and report events in the clusters randomized to immediate vaccination. It should also be noted that the logistical considerations of the ring vaccination trial are complex, with numerous trial sites across a large geographic area (Logistical considerations for the trials are discussed further in Chapter 5.)
Results and Discussion
The results were released in two publications (Henao-Restrepo et al., 2015, 2016). The first, designated the interim analysis, was published in July 2015 and included the data from the original cluster-randomized study design, immediate versus delayed immunization (Table 4-3), which had been collected up to the point at which the DSMB decided to terminate the delayed-immunization arm. The DSMB action was based on the emerging evidence from the trial that the vaccine was safe and effective as well as on the reality that the numbers of new ring-defining index cases were rapidly decreasing, which led the DSMB to conclude that it “would be unethical to deny people access to this life-saving intervention when the interim analysis showed evidence that rVSV-ZEBOV is both safe and effective” (UF, 2015). In the first publication the authors report, “The results of this interim analysis indicate that rVSV-ZEBOV might be highly efficacious and safe in preventing Ebola virus disease and is most likely effective at the population level when delivered during an Ebola outbreak via a ring vaccination strategy” (see data analysis below) (Henao-Restrepo et al., 2015, p. 857).
The second publication, designated the final analysis, appeared in December 2016 and included all data in the interim analysis as well as the additional data collected after the DSMB acted to terminate the delayed arm. Overall, there was a total of 64 laboratory-confirmed Ebola infections among participants eligible for randomization in the 96 randomized clusters. Of these, 41 had symptom onset before day 10 post-randomization (i.e., on days 0–9), including 20 of 3,232 participants in 9 of the 51 clusters randomized to immediate vaccination and 21 of 3,096 participants in 14 of the 47 clusters randomized to delayed vaccination. These data are indicative of a real, though variable, exposure to Ebola infection among contacts of the index person in the clusters (Henao-Restrepo et al., 2016). Among the remaining 23 Ebola cases with symptom onset 10 or more days after randomization (i.e., the primary endpoint of the study), 7 occurred in 4 of
the 51 clusters randomized to immediate vaccination and 16 were identified in 7 of the 47 clusters randomized to delayed vaccination. However, in the immediate clusters, all 7 Ebola primary events occurred among eligible participants who actually did not receive the vaccine, whereas none were seen among the 2,108 persons immediately vaccinated. The additional data collected after the delayed arm was terminated supported the finding of an apparent protective effect. Among 1,677 persons in 19 additional nonrandomized clusters that were immediately vaccinated, there were no cases of Ebola with symptom onset 10 or more days after vaccination (Henao-Restrepo et al., 2016) (see Table 4-4). The investigators used multiple analytic strategies to probe the data, and they included these in the two resulting publications (see Tables 4-3 and 4-4).
While the data indicate that this vaccine provides protection from Ebola, there are differing estimates of the vaccine’s efficacy depending on the analytical approach employed. If all persons eligible for vaccination within each of the clusters were included in the analysis, consistent with the intention-to-treat principle,3 the trial was inconclusive (i.e., 7 of 3,212 eligible persons in immediate clusters with a primary endpoint versus 16 of 3,075 in delayed clusters who were eligible for vaccination and ascertainment of the primary endpoint, for a vaccine effectiveness of 65 percent, 95% confidence interval, –47–91%). In the final report, the investigators concluded, “The results add weight to the interim assessment that rVSV-ZEBOV offers substantial protection against Ebola virus disease, with no cases among vaccinated individuals from day 10 after vaccination in both randomised and non-randomised clusters” (Henao-Restrepo et al., 2016, p. 2). However, in the clusters randomized to immediate vaccination, approximately two-thirds (2,108/3,212) of eligible persons actually got the vaccine (Henao-Restrepo et al., 2016). When an “on-treatment” analysis was applied to those in the immediate vaccination clusters who were actually vaccinated and this subset of participants was compared to all eligible in the delayed vaccination clusters, the trial results now showed statistically significant benefits (0 of 2,108 vaccinated persons in immediate clusters with a primary endpoint versus 16 of 3,075 persons in delayed clusters eligible for vaccination and a primary endpoint; vaccine efficacy 100 percent, 95% confidence interval, 69–100%).
___________________
3 Intention-to-treat (ITT) analysis. ITT analysis includes every subject who is randomized according to randomized treatment assignment. It ignores noncompliance, protocol deviations, withdrawal, and anything that happens after randomization. ITT analysis maintains prognostic balance generated from the original random treatment allocation. In ITT analysis, the estimate of treatment effect is generally conservative. A better application of the ITT approach is possible if complete outcome data are available for all randomized subjects. The per-protocol population is defined as the subset of the ITT population who completed the study without any major protocol violations (Gupta, 2011).
| All Clustersa | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| All vaccinated in immediate (group A) versus all contacts and contacts of contacts in delayed plus all never-vaccinated in immediate or nonrandomized (group B) | All vaccinated in immediate (group A) versus all eligible in delayed plus all eligible never-vaccinated in immediate (group B) | All contacts and contacts of contacts in immediate (group A) versus delayed (group B) | All vaccinated in immediate (group A) versus all eligible never vaccinated in immediate (group B) | |||||
| Group A | ||||||||
| Number of individuals (clusters) | 3775 (70) | 3775 (70) | 7241 (70) | 3775 (70) | ||||
| Cases of Ebola virus disease (clusters affected) | 0 (0) | 0 (0) | 12 (7) | 0 (0) | ||||
| Attack rate | 0% | 0% | 0.17% | 0% | ||||
| Group B | ||||||||
| Number of individuals (clusters) | 7995 (116) | 4507 (104) | 4529 (47) | 1432 (57) | ||||
| Cases of Ebola virus disease (clusters affected) | 34 (15) | 23 (11) | 22 (8) | 7 (4) | ||||
| Attack rate Vaccine effect | 0.43% | 0.51% | 0.49% | 0.49% | ||||
| Vaccine efficacy/ effectivenessc (%, 95% CI) | 100% (77.0 to 100.0) | 100% (79.3 to 100.0) | 70.1% (–4.9 to 91.5%) | 100% (–51.5 to 100.0) | ||||
| p valued | 0.0012 | 0.0033 | 0.2759 | 0.125 | ||||
a Randomly assigned and nonrandomly assigned individuals who were allocated to immediate vaccination were combined.
b Nonrandomized immediate clusters are excluded from this analysis.
c From fitting a β-binomial distribution to the cluster-level numerators and denominators and using an inverted likelihood ratio test to identify the lower bound for vaccine efficacy (columns 1, 2, 5, and 6); from a Cox proportional hazards model (columns 3, 7, and 8); from signed test (two-sided): probability of observing endpoints in control groups among
| Randomized Clustersb | ||||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | |||||
| All vaccinated in immediate (group A) versus all eligible and consented on day 0 visit in delayed (group B) | All vaccinated in immediate (group A) versus all eligible in delayed (group B) | All eligible in immediate (group A) versus all eligible in delayed (group B) | All contacts and contacts of contacts in immediate (group A) versus all contacts and contacts of contacts in delayed (group B) | |||||
| 2108 (51) | 2108 (51) | 3212 (51) | 4513 (51) | |||||
| 0 (0) | 0 (0) | 7 (4) | 10 (5) | |||||
| 0% | 0% | 0.22% | 0.22% | |||||
| 1492 (46) | 3075 (47) | 3075 (47) | 4529 (47) | |||||
| 20 (4) | 16 (7) | 16 (7) | 22 (8) | |||||
| 0.7% | 0.52% | 0.52% | 0.49% | |||||
| 100% (63.5 to 100.0) | 100% (68.9 to 100.0) | 64.6% (–46.5 to 91.4) | 64.6% (–44.2 to 91.3) | |||||
| 0.0471 | 0.0045 | 0.344 | 0.3761 | |||||
treatment–control mismatched pairs and under the null hypothesis that the vaccine has no efficacy (column 4).
d From Fisher’s exact test (two-sided), which is approximate for columns 1 and 2. From signed test (two-sided): the probability of observing endpoints in control groups among treatment–control mismatched pairs and under the null hypothesis that the vaccine has no efficacy (column 4).
SOURCE: Henao-Restrepo et al., 2016.
In late December 2016 the WHO announced, “An experimental Ebola vaccine was highly protective against the deadly virus in a major trial in Guinea, according to results published today in The Lancet. The vaccine is the first to prevent infection from one of the most lethal known pathogens, and the findings add weight to early trial results published last year” (WHO, 2016c). Coverage in the press, however, focused on the statistically significant results from the “on-treatment” analyses, which demonstrated 100 percent vaccine efficacy. For example, Donald McNeil of The New York Times wrote: “In a scientific triumph that will change the way the world fights a terrifying killer, an experimental Ebola vaccine tested on humans in the waning days of the West African epidemic has been shown to provide 100 percent protection against the lethal disease” (McNeil, 2016). Echoing the WHO press release, Sarah Boseley of The Guardian observed that the vaccine was “highly effective against one of the most lethal known pathogens in existence. Ten days after vaccination, none of the trial subjects developed Ebola virus disease” (Boseley, 2016).
It may appear rational to compare only those who were randomized to the immediate group and actually received the vaccine to the entire delayed group because an individual can only be protected if he or she receives the vaccine. However, while this “as-received” analysis is intended to measure vaccine efficacy, it is likely to be a biased estimate of vaccine efficacy, as discussed below. In contrast, the intention-to-treat analysis, which includes the entire “as-assigned” group, provides an unbiased estimate of efficacy; in this case, however, the estimate is substantially diluted due to the inclusion of those who did not receive the vaccine although this is likely more representative of overall clinical effectiveness. Additional observational analyses reported by the investigators—for example, no cases of Ebola having occurred among those vaccinated in nonrandomized clusters—provide further suggestive evidence of vaccine efficacy, although it is pertinent that the epidemic was already waning by the time the nonrandomized clusters were defined, at which point the risk of infection was substantially reduced.
The committee has devoted considerable attention to these different analyses and what they imply. We concur that, taken together, the results suggest that the vaccine most likely provides some protection to recipients—possibly “substantial protection,” as stated in the final report. However, we remain uncertain about the magnitude of its efficacy, which could in reality be quite low or even zero, as the confidence limits around the unbiased estimate include zero. The reason for this uncertainty is that the primary comparison reported by the investigators is no longer protected by randomization because those who accepted vaccination in the immediate clusters are being selected for inclusion post-randomization. The main potential—but unmeasured—bias of this approach is that the individuals who received the vaccine may have had a lower chance of acquisition of
infection (e.g., different risk of exposure to the virus) than those who were not immunized. If those who did not get the vaccine were more likely to be exposed, then by excluding them from the analysis but not excluding a comparable subset from the group assigned to delayed vaccination we would bias our results in favor of the immediate vaccination group. This is why the primary analysis in any RCT, including a cluster-randomized RCT, is almost always intention to treat, following the “once randomized, always analyzed” dictum (Hennekens et al., 1987). In addition, as noted earlier, the small proportion of clusters in which Ebola cases were reported raises a concern about the comparability of risk across clusters. Increasing the number of clusters in future similar types of trials will be required to minimize the possibility of disproportional allocation of clusters with different intrinsic transmission probability.
Due to safety and logistical concerns no serologic data were collected during the conduct of the trial, so no immunological correlate of protection from the vaccine can be determined (Henao-Restrepo et al., 2016). This is unfortunate because the establishment of such a correlate of protection would provide a benchmark, and other existing or newly designed vaccines could be compared with the product used in the current study. Long-term follow-up will also be required to ascertain the duration of protection and the potential need for future booster doses. The PREVAIL study is expected to provide data on the immune responses to this vaccine and their persistence, but not on the correlates of protection. Although there were only two serious adverse events (one febrile reaction and one case of anaphylaxis) attributed to the vaccine among the nearly 10,000 subjects vaccinated in the ring trial, the detection of less common adverse events would require a larger sample size (Henao-Restrepo et al., 2016). Fever, arthritis, and rash were associated with the vaccine in several patients in the initial Phase 1 trial, but there were no such reports in the ring trial (Agnandji et al., 2016). Although the safety profile is encouraging, further studies of the rates of these reactions and their potential pathogenesis are needed. It was not possible to compile longer-term safety data comparing the vaccinated and the control groups in the ring trial since all the control subjects were vaccinated. But because the same vaccine was used in the PREVAIL study; the committee believes that additional useful safety information may become available as those results are analyzed over time (Davey, 2016).
The “on treatment” vaccine efficacy estimate of 100 percent has been widely reported, but the reports generally do not acknowledge the fact that no vaccine is—or ever likely will be—100 percent effective, whether because of such host factors as immunodeficiency states or immunogenetics based antigen unresponsiveness or because of extrinsic factors such as a very high infection inoculum size, which can overcome existing immunity (CDC, n.d.). Once the authors were informed by the DSMB that they had
documented 100 percent vaccine efficacy in July 2015, randomization was discontinued. Immediate vaccination was thereafter offered to an additional 19 subsequently formed clusters, and reported as an observational study (Henao-Restrepo et al., 2016). None of the vaccinated persons developed Ebola disease. Had the DSMB and the authors applied a more conservative interpretation of the preliminary results, along the lines of what this committee thinks the data demonstrate, and thereby continued to randomize all remaining clusters, the power to demonstrate benefit would have increased. That aside, the high level of expected protection, based on the trial results, may make it more difficult to conduct a confirmatory controlled trial of sufficient size in the event of a future outbreak; we can expect that it will be considered unethical to deny the vaccine or delay its administration to any individuals who are at risk of infection. This will be reinforced if the PREVAIL study demonstrates long-lived antibody responses that are protective when studied in either in vitro or in passive immunization animal studies and also adds to the favorable safety profile in the ring vaccination trial. The latter study included persons with a relatively high exposure to the virus, for whom a greater degree of uncertainty regarding potential adverse effects might be more acceptable than in populations at lower or negligible risk. Additional benefit and risk assessments are important for refining the indications for vaccine use during a future Ebola outbreak because the risk–benefit determination may differ for those at high risk (contacts, health care workers, burial teams) versus members of the general public, who are at considerably lower risk. In addition, this vaccine may not be as effective against a different Ebola virus strain, which is another issue that needs to be evaluated. Given these constraints, future vaccine trials during another outbreak could focus on head-to-head comparisons of different dosing schedules of the rVSV-ZEBOV vaccine or on a comparison with other vaccine candidates for which there is sufficient preliminary safety and immunogenicity data. In such trials, the determination of a surrogate measure for protection and long-term follow-up for continued efficacy and safety assessment should be prioritized; and in the event of an epidemic the immediacy of the protection should also be prioritized.
These considerations aside, the ring vaccination study has provided important new information of value for any future response to an Ebola outbreak. To ensure the further development of the rVSV-ZEBOV vaccine, Gavi (previously the Global Alliance for Vaccines and Immunization) and Merck, the company producing the vaccine used in the ring vaccination trial, announced a partnership in January 2016 in which Gavi committed funding to “help Merck take the vaccine through licensure and WHO prequalification. . . . If approved, it would become one of the world’s first licensed Ebola vaccines, and Gavi would be able to begin purchasing the vaccine to create a stockpile for future outbreaks” (Gavi, 2016). This move
helps assure a market to manufacturers working in the rare and neglected disease space and ensures the vaccine will be available to those who need it. “Ensuring a vaccine will be available to protect people who might have missed out due to a market failure lies at the heart of what makes Gavi so important in global health,” said Gavi Board Chair Dr. Ngozi Okonjo-Iweala. “It is our moral duty to ensure that people do not miss out simply because of where they are born or whether they can afford to pay” (Gavi, 2016).
Partnership for Research on Ebola Vaccines in Liberia—PREVAIL I; Liberia, cAD3-EBOZ, VSV-ZEBOV, Placebo
The PREVAIL vaccine trial was a partnership between the Ministry of Health of Liberia and the National Institute of Allergy and Infectious Diseases of the U.S. National Institutes of Health and was conducted in Liberia in early 2015 to compare the safety, efficacy, and immunogenicity of two candidate vaccines, ChAd3-EBO-Z and VSVDG-ZEBOV, versus a saline placebo (Kennedy et al., 2016). The trial was slated to be the largest trial performed during the epidemic, with a planned enrollment of 28,000 participants—however, only 1,500 patients were ultimately enrolled, and it was the only individually randomized, double-blind, placebo-controlled vaccine trial conducted during the outbreak (Doe-Anderson et al., 2016). A timeline of the PREVAIL I trial can be found in Figure 4-2.
Study Design
The study was designed to allow a seamless transition from Phase 2 to Phase 3, and it used a common control group to assess the efficacy of the two candidate vaccines; i.e., the subjects given ChAd3-EBO-Z, VSVDG-ZEBOV, or a saline placebo were in a one-to-one-to-one ratio. Participant follow-up visits were originally planned at 1 week, 1 month, and 2 months, and then at 2-month intervals until the close of the study (Kennedy et al., 2016). However, with the outbreak rapidly coming under control, it was clear that the Phase 3 study to assess vaccine efficacy as well as safety could not be undertaken. Instead, based on the recommendation of the U.S. Food and Drug Administration (FDA) and with the concurrence of the independent DSMB and the two scientific and ethics review boards of record, the Phase 2 substudy was expanded to 1,500 participants; enrollment in it was completed within 3 months. Shortly before the study ended, the protocol was amended to also include a week 2 follow-up visit to specifically evaluate these participants for joint problems. As of December 2016, the study is still ongoing, although not recruiting additional volunteers (NIH and NIAID, 2016). The researchers state,
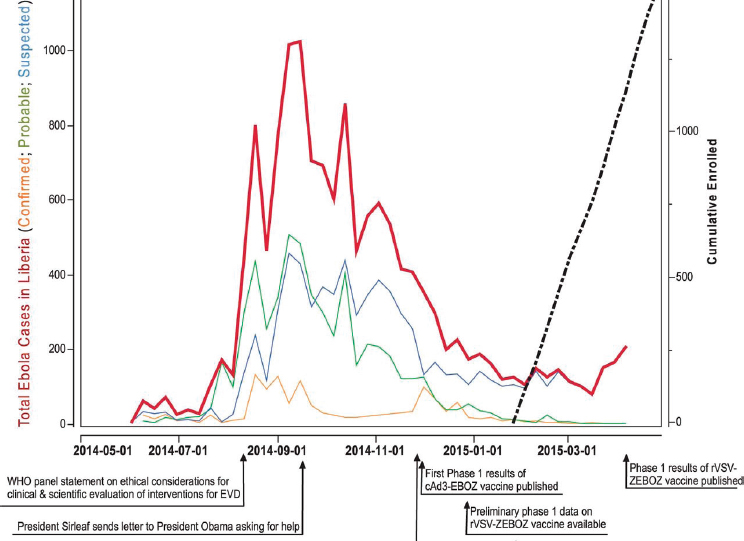
SOURCE: Kennedy et al., 2016.
The plan is to extend the follow-up of the original cohort of PREVAIL study participants to conduct long-term immunogenicity testing and collection of severe adverse events . . . for an additional 1 year after the original 12-month visit with the schedule of these follow-up visits determined from the date of vaccination. In order to understand the durability of the antibody response, follow-up may be continued for an additional 3 years (i.e., 5 total years post-vaccination date) to measure IgG antibody levels against the Ebola surface glycoprotein if after a total of 2 years post-vaccination follow-up, there is evidence that the antibody response has not substantially waned. (PREVAIL, 2016)
An interesting facet of the design is that had the outbreak continued, or if the trial had begun a few months earlier, the Phase 2 trial would have been seamlessly incorporated into a Phase 3 trial. The participants and the
data that were already collected on efficacy and safety would have been included in the continuing Phase 3 study with the sample size enlarged to ensure that sufficient power was available to assess efficacy, investigate the possibility of enhancing antibodies, and evaluate both short- and long-term safety. Information from the Phase 2 laboratory evaluation would also be used to guide which data to capture in this larger cohort (Kennedy et al., 2016).
Advantages and Disadvantages
The target population for the trial was the general population rather than groups of higher-risk individuals such as health care workers, burial workers, or contacts of identified cases, as had been the focus of other studies (Henao-Restrepo et al., 2015; Widdowson et al., 2016). This focus on the general population made the study’s circumstances similar to how the vaccine might be used in the future, and it allowed adverse effects of the vaccine to be detected more easily. In a situation of a larger Ebola outbreak it is likely that the vaccine will be offered more widely than to those at highest risk of contracting the infection. Additionally, given the collection of routine blood analysis and placebo design, it allowed for easier detection of adverse effects of the vaccine. A downside, however, is the larger sample size required when including an overall lower-risk population.
Only adults were enrolled initially because of concerns about safety. Had the trial continued, the data from this trial and others would have been used to evaluate amending the protocol to allow children to participate. Of note, the Phase 3 section of the trial was event driven, so that if transmission within the trial’s cohort was higher than what had been estimated conclusive results might have been achieved with fewer enrollees (Pierson, 2015).
It was decided that the PREVAIL I serology tests would be performed at the Liberian Institute of Biomedical Research (LIBR) as part of a commitment to strengthen research capacity at LIBR. The LIBR laboratory continues to support the PREVAIL research program. PREVAIL analyzed samples with both (1) a commercially available enzyme-linked immunosorbent assay (ELISA) from Alpha Diagnostics International for the detection and quantification of immunoglobulin-G (IgG) against Ebola virus (EBOV) glycoprotein (GP) and (2) the Filovirus Animal Nonclinical Group test for IgG to EBOV GP; however, to date neither of these assays has been validated.4
___________________
4 Personal communication, Jerome F. Pierson, Chief, Regulatory Compliance & Human Subjects Protection Branch, National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH). October 2016.
Results and Discussion
The data from the PREVAIL I trial indicate that the two tested vaccines are safe and immunogenic. Interim data on the long-term serologic responses were presented at the 8th International Symposium on Filoviruses held in Antwerp, Belgium, on September 12–15, 2016 (Davey, 2016). During the first year of the study the rate of follow-up has consistently exceeded 95 percent; this serves to minimize any bias due to drop-outs and loss of information. The serologic data are summarized in Table 4-5 for the noted time intervals. The antibody response peaked 1 month after vaccination and was sustained over the next 11 months, without any clear evidence of decline for the rVSΔG group; 70 to 80 percent of the cohort responded to the vaccination with an antibody response (i.e., more than two standard deviations of response in the placebo group). Although the immune responses in the rVSΔG group were significantly higher than those to the ChAd3 group at all post-immunization time points, these data have only appeared in an abstract form and will need to be reassessed when published. The two actively vaccinated groups reported an excess risk of injection site reactions at 1 week (29 percent and 30 percent versus 7 percent), but not at the 1 month follow-up visit, compared to placebo. No excess risk of other clinical events was noted (Davey, 2016). The results as outlined document a robust antibody response to both of the vaccines tested that is maintained over a 12-month follow-up period, without evidence of adverse reactions other than the expected local injection site reactions.
Interestingly, at the beginning of the trial, 6.3 percent of enrollees were found to have pre-existing Ebola antibodies, possibly indicative of past Ebola infection (Davey, 2016). Additional investigations will be required to assess whether this is cross reactivity with shared antigens of other viruses or actual asymptomatic infections with Ebola virus and, if so, whether these might confer immunity to Ebola. On the basis of this information, Ebola virus may have been circulating in West Africa in advance of the outbreak, either unrecognized as Ebola or perhaps as asymptomatic infections. The follow-up of this is important for understanding the geographic boundaries of Ebola virus and the possibility of subclinical infection.
Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE); Sierra Leone, VSV-ZEBOV
The STRIVE trial was a collaboration among the College of Medicine and Allied Health Sciences of the University of Sierra Leone, the Sierra Leone Ministry of Health and Sanitation, and the CDC. The study involved health care workers and other frontline workers at greater risk of Ebola infection because of their increased exposure. It was also intended to
TABLE 4-5 Preliminary Antibody Responses Following Vaccination—PREVAIL I Trial
| Antibody Responses Following Vaccination | |||||
|---|---|---|---|---|---|
| ChAd3 | rVSVΔG | Placebo | P-value | ||
| ChAd3 Versus rVSVΔG | rVSVΔG Versus Placebo | ||||
| Week 1 Number of participants GMT (95% CI) Responders (%; 95% CI) |
478 88 (82–95) 3.6 (1.9–5.2) | 477 83 (76–89) 2.5 (1.1–3.9) | 471 74 (69–80) 1.5 (0.4–2.6) | <0.001 0.06 | 0.004 0.36 |
| Month 1 Number of participants GMT (95% CI) Responders (%; 95% CI) |
476 621 (565–682) 70.8 (66.7–74.9) | 473 1000 (910–1099) 83.7 (80.4–87.1) | 468 75 (69–80) 2.8 (1.3–4.3) | <0.001 <0.001 | <0.001 <0.001 |
| Month 6 Number of participants GMT (95% CI) Responders (%; 95% CI) |
460 598 (547–654) 72.4 (68.3–76.5) | 447 797 (727–874) 78.5 (74.7–82.3) | 432 88 (81–96) 6.3 (4.0–8.5) | <0.001 <0.001 | <0.001 <0.001 |
| Month 12 Number of participants GMT (95% CI) Responders (%; 95% CI) |
452 478 (442–517) 63.3 (58.8–67.7) | 442 797 (733–867) 78.7 (74.9–82.6) | 435 90 (84–97) 6.9 (4.5–9.3) | <0.001 <0.001 | <0.001 <0.001 |
NOTES: GMT = Geometric Mean Titer.
P-values for group comparisons of GMT based on log10 titer values at visit with baseline log10 titer as a covariate in analysis of covariance.
P-values for group comparisons of % responders based on Fisher’s exact test.
Responders defined as change in log10 titer >2×SD of the change in placebo group at month 1, including participants without elevated antibody levels at entry.
SOURCE: Davey, 2016.
strengthen the existing research capacity of institutions in Sierra Leone by providing training and research experience to hundreds of Sierra Leonean staff. Infrastructure was expanded, including renovating existing structures and building new structures to be able to enroll and vaccinate participants, handle data management, and store the vaccine. New technology was also introduced to maintain the cold chain for vaccine storage (Widdowson et al., 2016).
Study Design
The trial was initially designed as a stepped-wedge study, but it shifted to a more traditional individually randomized trial with a delayed vaccination arm. In the initial design the plan was to offer the vaccine to everyone in the study in a sequential manner, using the unvaccinated time as a comparator for the vaccinated time. However, researchers found that this design was too complex to carry out in the local setting and did not allow for the flexibility required to go into new places as the epidemic moved. The project was therefore converted to an unblinded but individually randomized trial, in which individuals were randomized to receive the vaccine immediately or to receive vaccine 18 to 24 weeks later.5 The primary end point was laboratory-confirmed Ebola infection, and there were no futility stopping rules, although an interim analysis was planned.
Advantages and Disadvantages
Eligible participants included all health care workers or related workers involved in Ebola care and who were 18 years and older; women who were pregnant or breastfeeding were not enrolled. Subjects were followed for 6 months after vaccination. Detailed safety surveillance was prioritized for the first 400 subjects. An additional 500 subjects underwent immunogenicity studies at baseline and at three additional times during the study. During the conduct of the Phase 1 studies in Europe, skin rash and arthralgia were seen in some participants beginning the second week after vaccination (Regules et al., 2017). Given these findings, STRIVE leaders modified the suspected Ebola case definition for trial participants for the first 48 hours to avoid potential confusion with adverse reactions to the
___________________
5 Presentation of Anne Schuchat, CDC, Clinical trial designs for emerging infectious diseases. U.S. Food and Drug Administration, Bethesda, MD. November 9, 2015.
vaccine (Widdowson et al., 2016).6 Given the interim results for the ring vaccination study, 100 individuals who were randomized to the late phase of the study were instead given the vaccine early.
There were several potential ethical issues with the trial, as acknowledged by study staff (Widdowson et al., 2016). First, the widespread fear of Ebola could skew the risk–benefit calculation by health care and frontline workers and push them toward accepting a vaccine of unknown safety and efficacy. Second, participants were reimbursed for participation and received free health care, which could have induced some to enroll. “These ethical and communication concerns were addressed with guidance from Sierra Leone STRIVE leadership and other partners. Active and transparent communication of risks and benefits to participants and the public continued throughout the trial as the risk–benefit balance changed with ebbing Ebola incidence” (Widdowson et al., 2016, p. 100). The rVSV-ZEBOV vaccine is an investigational new drug (IND), and STRIVE was conducted under an approved IND protocol, with the intent to include data from it in a biologics licensing application to the FDA.
Study Results and Discussion
As of November 2016, sera from around 500 STRIVE participants had been collected at baseline and at 1 month, 6 months, and 9–12 months after vaccination, with more than 80 percent follow-up at final time points. As agreed by the CDC and the Sierra Leonean collaborators, these sera have been shipped to the United States for study. Testing of the sera is pending validation of the GP-ELISA assay by the FDA. The CDC decided early on that the STRIVE serology should be conducted using a validated assay so that the results can be included in an application for vaccine licensure.7
Although still incomplete, the safety data are reassuring. “The safety sub-study enrolled 453 participants (227 immediate vaccines and 226 deferred vaccines) in April 2015. As of April 28, 2016, a total of 64 participants had illnesses that were investigated as suspected Ebola, of whom
___________________
6 Standard suspected Ebola case definition: temperature ≥38°C (≥100.4°F) and three or more of the following symptoms: headache, loss of appetite, fatigue, muscle/joint pain, diarrhea, unusual bleeding, difficulty breathing, nausea, vomiting, abdominal pain, difficulty swallowing, or hiccups; OR illness after direct, unprotected Ebola contact or a breach in personal protective equipment in the past 21 days. Modified case definition applied to vaccine recipients in the first 48 hours after vaccination: same as for standard suspected Ebola case except that at least one symptom had to be one of the following symptoms not consistent with a vaccine reaction: diarrhea, unusual bleeding, difficulty breathing, nausea, vomiting, abdominal pain, difficulty swallowing, or hiccups (Widdowson et al., 2016).
7 Personal communication, Barbara Mahon, CDC lead, Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE). October 2016.
60 provided specimens for testing, but none were confirmed as Ebola. No serious adverse events related to vaccination have been reported; the data from the safety sub-study are generally consistent with data found in Phase 1 trials of the vaccine, and no association of vaccine with arthritis has been noted” (Widdowson et al., 2016, p. 104). Because cases were already declining when the trial began recruiting participants, and there were no subsequent cases among them, STRIVE was not able to determine vaccine efficacy or draw any conclusions regarding how well the vaccine would work in this population. The major contribution of the study was to expand safety information available for the rVSV-ZEBOV candidate vaccine, creating the largest safety database available on the vaccine and, ultimately, immunogenicity data.
EBOVAC–Salone; Sierra Leone, Ad26.ZEBOV and MVA-BN-Filo
The EBOVAC–Salone trial was originally designed as a large-cluster, randomized study in Sierra Leone to achieve and assess the efficacy of a prime boost vaccine approach. Phase 1 studies on safety and immunogenicity were conducted in Europe starting in late 2014 and in Africa starting in 2015 (EBOVAC, 2016). Phase 2 studies are also under way, with participants in France, the United Kingdom, Burkina Faso, Uganda, Kenya, Cote d’Ivoire, and Rwanda, including an age deescalation study to include young children (EBOVAC2, 2016). The EBOVAC projects are intended to determine the safety and tolerability of a prime boost vaccine regimen that was developed by Janssen Pharmaceutical Companies of Johnson & Johnson (EBOVAC, 2016). The prime boost approach is a two-step vaccination protocol in which participants are first given a dose of Ad26.ZEBOV vaccine to prime their immune system and then a dose of MVA-BN-Filo at a later point to further enhance the immune response and achieve long-lasting protection (EBOVAC, 2016). With enrollment starting in 2015, the study includes an active control arm, using the meningitis Men ACYW vaccine for one-third of the subjects in a randomized manner, to provide a control group for safety and immunogenicity analysis (NIH and Janssen Vaccines & Prevention B.V., 2016).
Study Design
The Phase 1 trial in the United Kingdom (the most comprehensive study site from which data are available) used a randomized, placebo-controlled, observer-blind design, enrolling only adults. Participants were randomized to receive either a placebo or to receive both Ad26.ZEBOV and MVA-BN-Filo in a different order and time interval, thus generating subgroups to evaluate (Milligan et al., 2016).
Study Results and Analysis
The Phase 1 study in the United Kingdom found no serious vaccine related adverse events. All participants had specific IgG detectable at 21 days after the boost vaccine as well as at the 8-month follow-up. In the group that received Ad26.ZEBOV first, 97 percent showed an immune response after the primary immunization (Milligan et al., 2016). While these data do not provide information on the potential efficacy of the approach and the vaccines used, they indicate that the vaccines are promising candidates for further study in a future outbreak.
OVERALL ASSESSMENT OF VACCINES
One of the most remarkable successes of the vaccine trials was how rapidly they were planned, approved, and implemented. Under the pressure of the outbreak, the timelines for scientific and ethics approval were compressed. Protocol development was completed within a few weeks, and to address the requests from clinical trial investigators in Africa, Phase 1 studies were conducted in high-income countries (the United States and European countries) before the vaccine trials were launched in Africa. In fact, “[f]ive Phase 1 trials of ChAd3 and eight Phase 1 rVSV trials were initiated between September and December 2014 in North America, Europe, and Africa” (WHO, 2015c, p. 10). By February 2015, data were available from the Phase 1 trials to select vaccine dosing and to begin implementing Phase 2 and Phase 3 trials in Ebola-affected countries in Africa. These began only 6 months after the WHO declared the epidemic a PHEIC, with the PREVAIL I trial starting in February 2015 in Liberia and both the STRIVE trial in Sierra Leone and the ring vaccination trial in Guinea starting in March 2015 (WHO, 2015c). Conducting Phase 1 and 2 trials in countries not affected by the outbreak was thought to facilitate acceptance of larger trials in affected countries; however, given the persistent belief in the affected countries that foreign medical teams were possibly testing something dangerous it remained imperative that trial teams also focus on community engagement and communication of the clinical trial process. (Community engagement on the part of the trial teams is discussed further in Chapter 6.)
The vaccine trials conducted during the epidemic indicate there are promising Ebola vaccine candidates in terms of safety and immunogenicity. The study designs selected were generally appropriate for the context and question being explored—for example, implementing ring vaccination trials for high-risk populations and individual RCTs for the general population at lower risk in order to more fully assess safety. While the ring vaccination study showed suggestive efficacy, the trial was not designed to
document long-term safety and efficacy because all participants were ultimately immunized and the protocol only followed participants out to day 84 (Henao-Restrepo et al., 2016). When the immunogenicity data become available, the results of the PREVAIL trial will provide information on the long-term immunogenicity of the vaccines, including the vaccine used in the ring vaccination study (PREVAIL, 2016). The differences in the study designs and the value of the information generated from each trial highlight the importance of collaboration in future trials. For example, if the ring trial had been the only one conducted during the outbreak, an unfortunate situation could have emerged; because of the initial suggestion of its high degree of efficacy equipoise could have been preemptively eliminated despite the estimate of protection from the intention-to-treat analysis being much lower. Additionally, the results from the ring vaccination trial provide limited safety data and no data on the duration of the immune response beyond 84 days; fortunately, the PREVAIL I trial can address these important gaps in knowledge. (For a summary of the vaccine trials conducted during 2014–2015, see Table 4-6 at the end of the chapter.)
Improving the implementation of vaccine trials in a future outbreak will require a mechanism to assess the pros and cons of the different vaccine trial approaches and a process to prioritize what to study among the available candidates. This is particularly important in advance of the next event because the length and severity of future epidemics cannot be predicted ahead of time and, therefore, rapid trial approval and implementation will be critical in order to generate conclusive results. For future outbreaks, it would be valuable to have a portfolio of trial designs in advance that have already been vetted among the key stakeholders and that are designed to suit different populations, including high-risk populations in direct contact with infected individuals as well as the lower-risk general population. Early in the 2014–2015 epidemic, there was insufficient coordination among the trial teams to prioritize vaccines to test in which population with what protocol, to harmonize data collection, or to select assays to analyze sera. As a consequence, with the outbreak winding down when the trials began, there was competition for enrollment and little standardization of data collection (including data on adverse events); standardized data collection is necessary so that information can be combined for the purpose of analysis.
In the event of a future outbreak, given the practical constraints on the ground, it may be more strategic and easier in practice to quickly launch one type of trial at the start of the epidemic in order to obtain initial information on investigational vaccines. The preliminary findings from the first trial could be used to inform the trial protocols of more robust subsequent trials already in development. To do this effectively would require preoutbreak planning and coordination and should also include consideration of better and faster ways to undertake the clinical trial review and approval
mechanisms within at-risk countries, shared experiences on best practices for fostering community engagement, and a discussion of lessons learned about the context in which randomized double-blind placebo or active-control arms can be accepted by the country and the community. There are also different scenarios in which the course of the outbreak and interim trial results may influence the trial designs and, ultimately, vaccine use during an outbreak. For example, during an epidemic with a new pathogen in which the general population is at high risk of infection and a ring trial shows initial efficacy, it may be reasonable to forgo planning a placebo-controlled trial in order to vaccinate the entire population. Alternatively, it may be preferable to move quickly to implement a placebo-controlled trial as an epidemic begins to wane and it becomes clear that a ring trial may not give definitive answers.
Agreement in principle on diverse issues such as sharing data and resources, intercomparability and interpretation of information, the launch of the trials, and standardization of data collection and assays used for analysis would speed up the design, approval, and initiation of well-thought-out studies. Much of this work can be initiated in advance of the next outbreak, pushing ahead to reach consensus among the key players if at all possible. Other issues to be dealt with in advance of an epidemic (which are beyond the scope of this report) include the manufacture of vaccines; access and distribution; affordability and the source of funding; and how to address liability issues and risk management.
Conclusion 4-1 If research during future epidemics is to be conducted in a more efficient and effective manner, funders and sponsors of research need to plan well in advance, ideally during an inter-epidemic period, to coordinate efforts more closely and must agree to initiate clinical research during an outbreak in concordance with an overall research agenda.
TABLE 4-6 Summary of Ebola Vaccine Trials
| Investigational Product | Sponsoring Organization, Trial Name | Trial Location | Trial Design and Design Considerations | Timeline | Results |
|---|---|---|---|---|---|
|
rVSV-ZEBOV
NewLink Genetics and Merck Vaccines USA |
WHO, Médecins Sans Frontiéres (MSF) and Government of Guinea in Conakry, Guinea—Guinea Ring Vaccine Trial
Ebola ça Suffit Trial |
Guinea |
Trial design
|
April 1, 2015, and July 20, 2015 | Summary of main findings: Randomization to assess vaccine efficacy was possible to implement during the Ebola outbreak. If cluster was used as unit for analysis, the trial was inconclusive. If individual persons eligible for vaccination within the clusters (intent to treat) were used as unit for the analysis, the trial was inconclusive (the 95 percent CI overlaps with 0). If an on-treatment analysis was applied (with all eligible used in control arm), trial results provide suggestion |
|
for benefit. Potential harm from vaccination could not be evaluated from a review of the report as safety analyses were ongoing when published. |
| Investigational Product | Sponsoring Organization, Trial Name | Trial Location | Trial Design and Design Considerations | Timeline | Results |
|---|---|---|---|---|---|
| rVSVΔG-ZEBOV
NewLink Genetics and Merck Vaccines USA |
U.S. Centers for Disease Control and Prevention and MOH Sierra Leone; Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE) | Sierra Leone | Trial design
|
April 9–August 21, 2015 | Enrollment complete: 8,673 enrolled
|
|
| Investigational Product | Sponsoring Organization, Trial Name | Trial Location | Trial Design and Design Considerations | Timeline | Results |
|---|---|---|---|---|---|
| ChAd3-EBOZ GlaxoSmithKline and PHAC
rVSV-ZEBOV NewLink Genetics and Merck Vaccines USA (Davey, 2016; NIH and NIAID, 2016; Pierson, 2015; PREVAIL, 2016b) |
By U.S. NIH and MOH Liberia
Partnership for Research on Ebola Vaccines in Liberia (PREVAIL I) |
Monrovia, Liberia | Trial design:
|
February 2, 2015–April 30, 2015 | The trial enrolled 1,500 men and women ages 18 and older with no reported history of Ebola virus disease at Redemption Hospital in Monrovia from February 2 through April 30, 2015. Three equal-sized groups of 500 received either one of the two vaccine candidates or a saline injection. Both vaccines were well tolerated. At 1 month, 87 percent of the volunteers who received the cAd3EBOZ vaccine candidate had measurable Ebola antibodies; 94 percent of the volunteers who received the rVSV-ZEBOV vaccine had demonstrable antibodies after 1 month. |
|
The results as outlined document a robust antibody response to either of the two vaccines tested, that is maintained over a 12-month follow-up period and without evidence of adverse drug reactions other than the expected local injecting site reactions. |
| Investigational Product | Sponsoring Organization, Trial Name | Trial Location | Trial Design and Design Considerations | Timeline | Results |
|---|---|---|---|---|---|
| Ad26.ZEBOV and MVA-BN-Filo
Johnson & Johnson and Bavarian Nordic (EBOVAC2, 2016; EBOVAC, 2016; Milligan et al., 2016; NIH and Janssen Vaccines & Prevention B.V., 2016) |
Crucell Holland BV; MoH/ LSHTM
EBOVAC |
Sierra Leone | Trial design
|
First dose of vaccine was given on October 8, 2015 |
|
| Investigational Product | Sponsoring Organization, Trial Name | Trial Location | Trial Design and Design Considerations | Timeline | Results |
|---|---|---|---|---|---|
|
a Personal communication, Barbara Mahon, CDC lead, Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE). October 2016.
b Personal communication, Jerome F. Pierson, Chief, Regulatory Compliance & Human Subjects Protection Branch, National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH). October 2016.
REFERENCES
Agnandji, S. T., A. Huttner, M. E. Zinser, P. Njuguna, C. Dahlke, J. F. Fernandes, S. Yerly, J.-A. Dayer, V. Kraehling, R. Kasonta, A. A. Adegnika, M. Altfeld, F. Auderset, E. B. Bache, N. Biedenkopf, S. Borregaard, J. S. Brosnahan, R. Burrow, C. Combescure, J. Desmeules, M. Eickmann, S. K. Fehling, A. Finckh, A. R. Goncalves, M. P. Grobusch, J. Hooper, A. Jambrecina, A. L. Kabwende, G. Kaya, D. Kimani, B. Lell, B. Lemaître, A. W. Lohse, M. Massinga-Loembe, A. Matthey, B. Mordmüller, A. Nolting, C. Ogwang, M. Ramharter, J. Schmidt-Chanasit, S. Schmiedel, P. Silvera, F. R. Stahl, H. M. Staines, T. Strecker, H. C. Stubbe, B. Tsofa, S. Zaki, P. Fast, V. Moorthy, L. Kaiser, S. Krishna, S. Becker, M.-P. Kieny, P. Bejon, P. G. Kremsner, M. M. Addo, and C.-A. Siegrist. 2016. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. New England Journal of Medicine 374(17):1647–1660.
Boseley, S. 2016. Ebola vaccine is safe and effective, scientists declare after trials. The Guardian, December 22. https://www.theguardian.com/world/2016/dec/22/ebola-vaccine-is-safe-and-effective-scientists-declare-after-trials-successful?CMP=share_btn_link (accessed January 27, 2017).
Brown, C. A., and R. J. Lilford. 2006. The stepped wedge trial design: A systematic review. BMC Medical Research Methodology 6:54.
CDC (U.S. Centers for Disease Control and Prevention). n.d. What you need to know about vaccine safety. https://www.cdc.gov/media/subtopic/matte/pdf/asd-vaccine-safety-matte.pdf (accessed January 25, 2017).
Cohen, J., and K. Kupferschmidt. 2014a. Ebola vaccine trials raise ethical issues. Science 346(6207):289–290.
Cohen, J., and K. Kupferschmidt. 2014b. Tough choices ahead in Ebola vaccine trials. Science, October 7. http://www.sciencemag.org/news/2014/10/tough-choices-ahead-ebola-vaccine-trials (accessed February 4, 2017).
Davey, R. T. 2016. PREVAIL I: A randomized controlled safety and immunogenicity trial of two different vaccines against Ebola virus. Paper read at 8th International Symposium on Filoviruses, September 12–15, 2016, Antwerp, Belgium.
Dawson, A. J. 2015. Ebola: What it tells us about medical ethics. Journal of Medical Ethics 41(1):107–110.
Doe-Anderson, J., B. Baseler, P. Driscoll, M. Johnson, J. Lysander, L. McNay, W. S. Njoh, M. Smolskis, L. Wehrlen, and J. Zuckerman. 2016. Beating the odds: Successful establishment of a Phase II/III clinical research trial in resource-poor Liberia during the largest-ever Ebola outbreak. Contemporary Clinical Trials Communications 4:68–73.
Ebola ça Suffit Ring Vaccination Trial Consortium. 2015. The ring vaccination trial: A novel cluster randomised controlled trial design to evaluate vaccine efficacy and effectiveness during outbreaks, with special reference to Ebola. BMJ 351:h3740.
EBOVAC. 2016. Developing a vaccine for Ebola. http://www.ebovac.org (accessed January 18, 2017).
EBOVAC2. 2016. 1st EBOVAC2 newsletter. http://www.ebovac2.com/images/EBOVAC2_newsletter_no._1_March_2016.pdf (accessed January 18, 2017).
Farrar, J. 2015. The Ebola vaccine we dared to dream of is here. https://www.theguardian.com/commentisfree/2015/aug/03/ebola-vaccine-trials-diseases (accessed December 21, 2016).
FDA (U.S. Food and Drug Administration). 2015. FDA clinical trial designs for emerging infectious diseases. Paper read at FDA Clinical Trail Designs for Emerging Infectious Diseases, November 9, 2015.
Gavi. 2016. Ebola vaccine purchasing commitment from Gavi to prepare for future outbreaks. http://www.gavi.org/library/news/press-releases/2016/ebola-vaccine-purchasing-commitment-from-gavi-to-prepare-for-future-outbreaks (accessed January 25, 2017).
Gupta, S. K. 2011. Intention-to-treat concept: A review. Perspectives in Clinical Research 2(3):109–112.
Henao-Restrepo, A. M., I. M. Longini, M. Egger, N. E. Dean, W. J. Edmunds, A. Camacho, M. W. Carroll, M. Doumbia, B. Draguez, S. Duraffour, G. Enwere, R. Grais, S. Gunther, S. Hossmann, M. K. Kondé, S. Kone, E. Kuisma, M. M. Levine, S. Mandal, G. Norheim, X. Riveros, A. Soumah, S. Trelle, A. S. Vicari, C. H. Watson, S. Kéïta, M. P. Kieny, and J.-A. Røttingen. 2015. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: Interim results from the Guinea ring vaccination cluster-randomised trial. The Lancet 386(9996):857–866.
Henao-Restrepo, A. M., A. Camacho, I. M. Longini, C. H. Watson, W. J. Edmunds, M. Egger, M. W. Carroll, N. E. Dean, I. Diatta, M. Doumbia, B. Draguez, S. Duraffour, G. Enwere, R. Grais, S. Gunther, P.-S. Gsell, S. Hossmann, S. V. Watle, M. K. Kondé, S. Kéïta, S. Kone, E. Kuisma, M. M. Levine, S. Mandal, T. Mauget, G. Norheim, X. Riveros, A. Soumah, S. Trelle, A. S. Vicari, J.-A. Røttingen, and M.-P. Kieny. 2016. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). The Lancet 389:505–518.
Hennekens, C. H., J. E. Buring, and S. L. Mayrent. 1987. Epidemiology in medicine. Boston: Little, Brown and Co.
Kennedy, S. B., J. D. Neaton, H. C. Lane, M. W. Kieh, M. B. Massaquoi, N. A. Touchette, M. C. Nason, D. A. Follmann, F. K. Boley, M. P. Johnson, G. Larson, F. N. Kateh, and T. G. Nyenswah. 2016. Implementation of an Ebola virus disease vaccine clinical trial during the Ebola epidemic in Liberia: Design, procedures, and challenges. Clinical Trials 13(1):49–56.
The Lancet. 2015. An Ebola vaccine: First results and promising opportunities. The Lancet 386(9996): 830.
McNeil, D. G. 2016. New Ebola vaccine gives 100 percent protection. New York Times, December 22. https://www.nytimes.com/2016/12/22/health/ebola-vaccine.html?_r=0 (accessed January 25, 2017).
Milligan, I. D., M. M. Gibani, R. Sewell, E. A. Clutterbuck, D. Campbell, E. Plested, E. Nuthall, M. Voysey, L. Silva-Reyes, M. J. McElrath, S. C. D. Rosa, N. Frahm, K. W. Cohen, G. Shukarev, N. Orzabal, W. v. Duijnhoven, C. Truyers, N. Bachmayer, D. Splinter, N. Samy, M. G. Pau, H. Schuitemaker, K. Luhn, B. Callendret, J. V. Hoof, M. Douoguih, K. Ewer, B. Angus, A. J. Pollard, and a. M. D. Snape. 2016. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia Ankara–vectored Ebola vaccines: A randomized clinical trial. JAMA 315(15):1610–1623.
Mohammadi, D. 2015. Ebola vaccine trials back on track. The Lancet 385(9964):214–215.
Nason, M. 2016. Statistics and logistics: Design of Ebola vaccine trials in West Africa. Clinical Trials 13(1):87–91.
NIH (U.S. National Institutes of Health) and Janssen Vaccines & Prevention B.V. 2016. Staged Phase 3 study to assess the safety and immunogenicity of Ebola candidate vaccines Ad26.ZEBOV and MVA-BN-Filo during implementation of stages 1 and 2 (EBOVAC-Salone). Clinical Trial No. NCT02509494. https://clinicaltrials.gov/ct2/show/record/NCT02509494 (accessed January 17, 2017).
NIH and NIAID (National Institute of Allergy and Infection Diseases). 2016. Partnership for Research on Ebola Vaccines in Liberia (PREVAIL). Clinical trial no. NCT02344407. https://clinicaltrials.gov/ct2/show/record/NCT02344407 (accessed January 18, 2017).
Openshaw, P. J. M., and J. S. Tregoning. 2005. Immune responses and disease enhancement during respiratory syncytial virus infection. Clinical Microbiology Reviews 18(3):541–555.
Pierson, J. F. 2015. Partnership for Research on Ebola Vaccines in Liberia: Presentation to the Vaccines and Related Biological Products Advisory Committee to the Center for Biologics Evaluation and Research of the Food and Drug Administration. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM448001.pdf (accessed January 17, 2017).
PREVAIL (Partnership for Research on Ebola Vaccines in Liberia). 2016. PREVAIL protocol (version 5.0, 09 May 2016). Bethesda, MD: Office of Clinical Research Operations and Regulatory Compliance, Division of Clinical Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Regules, J. A., J. H. Beigel, K. M. Paolino, J. Voell, A. R. Castellano, P. Muñoz, J. E. Moon, R. C. Ruck, J. W. Bennett, P. S. Twomey, R. L. Gutiérrez, S. A. Remich, H. R. Hack, M. L. Wisniewski, M. D. Josleyn, S. A. Kwilas, N. Van Deusen, O. T. Mbaya, Y. Zhou, D. A. Stanley, R. L. Bliss, D. Cebrik, K. S. Smith, M. Shi, J. E. Ledgerwood, B. S. Graham, N. J. Sullivan, L. L. Jagodzinski, S. A. Peel, J. B. Alimonti, J. W. Hooper, P. M. Silvera, B. K. Martin, T. P. Monath, W. J. Ramsey, C. J. Link, H. C. Lane, N. L. Michael, R. T. J. Davey, S. J. Thomas, and the rVSVΔG-ZEBOV-GP Study Group. 2017. A recombinant vesicular stomatitis virus Ebola vaccine. New England Journal of Medicine 376(4):330–341.
Rid, A., and F. G. Miller. 2016. Ethical rationale for the Ebola “ring vaccination” trial design. American Journal of Public Health 106(3):432–435.
UF (University of Florida). 2015. Biostat researchers discuss roles in ongoing Ebola vaccine trial. epi.ufl.edu/news-and-events/epi-news/biostat-researchers-discuss-roles-in-ongoing-ebola-vaccine-trial.html (accessed February 4, 2017).
Underhill, K. 2013. Study designs for identifying risk compensation behavior among users of biomedical HIV prevention technologies: Balancing methodological rigor and research ethics. Social Science & Medicine 94:115–123.
WHO (World Health Organization). 2014. Ebola virus disease in Guinea. http://www.who.int/csr/don/2014_03_23_ebola/en (accessed January 17, 2017).
WHO. 2015a. Call for proposals: Mapping of the WHO coordinated international effort to develop Ebola vaccines, treatments and diagnostics and process for the Ebola vaccine trial in Guinea. http://www.who.int/medicines/ebola-treatment/call-for-proposals-int-effort-ebola-vax-trial/en (accessed February 20, 2017).
WHO. 2015b. Guinea: Ebola vaccine trial. http://www.who.int/features/2015/guinea-ebola-vaccine/en (accessed December 21, 2016).
WHO. 2015c. Report of the SAGE Working Group on Ebola Vaccines and Vaccination with provisional recommendations for vaccination. Geneva, Switzerland: Section B: Vaccines and vaccination, WHO. http://www.who.int/immunization/sage/meetings/2015/october/2_WHO_SAGE_WG_ebola_vaccines_and_immunization_MPP_VM_AMHR.pdf (accessed January 17, 2017).
WHO. 2016a. Final trial results confirm Ebola vaccine provides high protection against disease. http://www.who.int/mediacentre/news/releases/2016/ebola-vaccine-results/en (accessed January 25, 2017).
WHO. 2016b. Graph of new confirmed cases per epi week for Guinea. http://apps.who.int/gho/data/node.ebola-sitrep.ebola-country-GIN-20160511?lang=en (accessed January 17, 2017).
WHO. 2016c. Graph of new confirmed cases per epi week for Liberia. http://apps.who.int/gho/data/view.ebola-sitrep.ebola-country-LBR-20160511-graph?lang=en (accessed January 17, 2017).
WHO. 2016d. Graph of new confirmed cases per epi week for Sierra Leone. http://apps.who.int/gho/data/view.ebola-sitrep.ebola-country-SLE-20160511-graph?lang=en (accessed January 17, 2017).
Widdowson, M. A., S. J. Schrag, R. J. Carter, W. Carr, J. Legardy-Williams, L. Gibson, D. R. Lisk, M. I. Jalloh, D. A. Bash-Taqi, S. A. S. Kargbo, A. Idriss, G. F. Deen, J. B. W. Russell, W. McDonald, A. P. Albert, M. Basket, A. Callis, V. M. Carter, K. R. C. C. Ogunsanya, J. Gee, R. Pinner, B. E. Mahon, S. T. Goldstein, J. F. Seward, M. Samai, and a. A. Schuchat. 2016. Implementing an Ebola vaccine study—Sierra Leone. Morbidity and Mortality Weekly Review 65(Suppl 3):98–106.










































