5
Strengthening Capacity for Response and Research
Major shifts in our perceptions of our increasingly connected world have occurred over the last several decades as the global health movement has gained traction and the underlying motivations in the international arena have changed from colonial and paternalistic to a shared vision of good health for all, regardless of where you come from. With this, clinical research has also become an increasingly global endeavor, involving populations that have traditionally been underrepresented in research due to the lack of global interest in the health issues they uniquely face, the lack of commercial viability for the products of research, and the dearth of trained local researchers (Ali et al., 2012; Lang and Siribaddana, 2012). As a result of the globalization of clinical trials and accompanying external investment, developing countries have increased their capacity and resources for conducting research, and increasingly they have also tried to ensure the research agenda is relevant to the health challenges they face (Lang and Siribaddana, 2012).
Diseases like Ebola are not highest on the list of targets for research and development, except when there is a global threat such as the Ebola epidemic of 2014–2015 and the need for clinical research to evaluate therapeutics and vaccines seems more urgent. Building clinical research capacity in smaller, poorer developing countries is not a top priority of the international community; it is a particular challenge in the midst of an outbreak when the focus and attention is on helping patients, containing the outbreak, and preventing pandemic spread. However, strengthening research capacity is vital to preventing, responding to, and ending an epidemic. The World Health Organization (WHO) recognizes that “when assessing a new
infectious disease outbreak, it is of utmost importance—but enormously difficult—to quickly estimate its key characteristics, such as clinical severity, clinical presentation, the course of the illness, and the risk factors associated with infection. All such information is critical for decision making” (Williams et al., 2011, p. 63). The knowledge that can be produced through research during an epidemic—and sometimes only during an epidemic—is not only critical to informing ongoing preparedness and response, but it can also inform revisions in treatment protocols to advance patient care in real time, identify at-risk groups, and inform clinical trial protocols if there are products in development at a stage ready to be tested in humans (Lurie et al., 2013). This information can only be generated if there is the capacity in place to conduct robust research that meets acceptable scientific and ethics standards. The inherent problems with top-down “parachute research” are well documented and the alternative advocated by many in the field is for international researchers to partner with local scientists (Aizenman, 2016; Heymann et al., 2016).
Although there have been many programs over the years to help build research capacity in low-income countries, it is a difficult and long-term effort. Just a few countries in Africa have developed significant local research capacity capable of functioning on its own and ready to fully partner with international colleagues. In 1990 the Commission on Health Research for Development recognized that health research is an essential part of the health system and that it plays a critical role in improving health outcomes. The Commission Report concluded that “for the most vulnerable people, the benefits of research offer a potential for change that has gone largely untapped” (Commission on Health Research for Development, 1990, p. vii; see also Tugwell et al., 2006). With this report as the springboard, the concept of essential national health research (ENHR) was introduced (Evans, 1990). Instead of identifying specific research issues to address, ENHR is an integrated strategy for organizing and managing health-related research so that the research can contribute to health and development within a country (AfHRF, 2014). The programs that have been launched in the past 25 years cover a range of approaches, from ENHR capacity building by the Geneva-based Council on Health Research for Development (COHRED) to specific disease research in low- and middle-income countries supported by a variety of international institutions, including the Fogarty International Center at the U.S. National Institutes of Health (NIH), the Canadian International Development Research Centre, the UK Medical Research Council, the Wellcome Trust, the Pasteur Institutes, the European Developing Country Clinical Trials Partnership, and the Special Programme for Research and Training in Tropical Disease at the WHO, among many others around the world.
Despite these efforts, the majority of African countries, including
Guinea, Liberia, and, Sierra Leone have lagged behind. Most capacity-building programs have focused on the training and career development of individual researchers. As important as that is, it is increasingly recognized that a national health research system must be far more than the number of trained researchers in the country and must also include the institutions and activities involved in the generation and dissemination of knowledge. The health research system is an integral part of the health system and should produce evidence to inform the development and strengthening of national health and public health systems1 (Alliance for Health Policy and Systems Research, 2004; WHO, 2002).
The Ebola-affected countries are by no means the only countries that lack the necessary infrastructure and resources to respond to an outbreak. As Ariel Pablos-Mendez, the assistant administrator for global health at the U.S. Agency for International Development (USAID), said, “The state of the health workforce and health systems of the affected countries hampers the ability of these countries to respond to the Ebola outbreak—but these countries are hardly alone in having inadequate training, support and numbers of health workers, especially in the rural areas where this outbreak took hold” (Pablos-Mendez, 2014). Forging resilient health systems within all developing countries is critical so that these countries can rapidly respond to emergencies and prevent epidemics from occurring. Fragile health systems increase the vulnerability of countries to the risk of future epidemics, as seen in cholera outbreaks in Haiti in 2010 (Ivers et al., 2013); with influenza H1N1 in 2009, which disproportionately affected populations in Africa and Asia (Viboud and Simonsen, 2012); and most recently Zika (WHO, 2016c). But it is not just developing countries that stand to gain from global investments in health. It is now widely agreed that high- and middle-income countries have a defendable self-interest to invest in capacity-building in low-income countries affected by potentially pandemic diseases such as Ebola—not as a luxury, nor an act of charity, but as a necessity for protecting their own people from disease that, given the globalized economy, will inevitably spread to them. It is important to continue capacity-building investments even when the world’s attention turns to the next great threat, as Ebola was swept off the front pages of newspapers and funds were diverted to the threat of Zika (Scott, 2016). The case has been clearly made by the Council on Foreign Relations, “Supporting public health worldwide will enhance U.S. national security, increase prosperity at home and abroad, and promote democracy in developing
___________________
1 Public health systems are commonly defined as “all public, private, and voluntary entities that contribute to the delivery of essential public health services within a jurisdiction.” This concept ensures that all entities’ contributions to the health and well-being of the community or state are recognized in assessing the provision of public health services (CDC, 2014).
countries and those in transition. Emerging risks to the health and security of Americans make it prudent policy to grant higher priority to health in these countries. In addition to the threat of the deliberate spread of disease through biological weapons, Americans may now be at greater risk than at any time in recent history from recognized and emerging infectious diseases. These diseases are resurgent everywhere and spread easily across permeable national borders in a globalizing economy” (Kassalow, 2001, p. 4).
CAPACITY CHALLENGES TO CONDUCTING CLINICAL RESEARCH
The highly regulated nature of clinical trials, as well as the scientific and ethical mandates established to ensure that their risks to participants are minimized and the expected benefits are sufficient to justify going forward, can make trials time consuming and expensive to conduct (DiMasi et al., 2016). In the context of an infectious disease outbreak in a low-resource setting it can be even more difficult to meet the logistical, technical, and regulatory requirements of clinical trials, particularly in the narrow window of time that an outbreak affords. In fact, in outbreaks prior to the 2014–2015 Ebola epidemic not a single clinical trial was set up, as evidenced during recent outbreaks of MERS-CoV and influenza H1N1 which originated in middle-income countries—Saudi Arabia and Mexico, respectively (Gray, 2015). In order to conduct a trial in the setting of an outbreak, in addition to the many traditional clinical trial considerations (e.g., hypothesis testing), numerous issues must be considered and solved; the first step is determining whether and when launching a trial would be feasible. This involves (1) predicting when an outbreak can be expected to be large enough in size and long enough in duration to conduct trials, and (2) condensing the time that it takes to design, obtain the necessary approvals, identify the staff and the site, and implement the trials, so that participants can be enrolled while there are still sufficient numbers of new cases occurring to reach an endpoint for analysis. In an ideal situation, this would involve the preparation of an agile trial design in advance of an outbreak, refining the design according to the local circumstances and context, a rapid global response, and extensive collaboration across multiple organizations in multiple countries. In the Ebola epidemic, however, this preplanning did not happen, research was not on the table for the first 6 months of the outbreak, and trial teams were confronted with multiple challenges which they went to great lengths to overcome. The lack of capacity in the affected countries, along with the delayed recognition by the WHO and other key stakeholders of the extent and urgency of the outbreak, also delayed discussions on the need for research until the declaration of the public health emergency of
international concern, and frustrated the ability of researchers and responders alike to adequately plan and respond to the emerging outbreak.
Once the magnitude of the outbreak was appreciated by both national and international stakeholders, shortcomings in local capacity became a roadblock to moving forward with research. For example, inexperience with independent scientific and ethics review of proposed research and limited legal experience in evaluating and negotiating research contracts put the countries at a strategic disadvantage, whether actual or just potential. In order to conduct high-quality research in a timely manner in a resource-poor setting during an emerging epidemic, the following challenges experienced during the Ebola outbreak must be addressed if there is to be a more efficient, rapid, and effective research response:
- poor surveillance and a lack of experience with outbreak investigations in the three countries
- a lack of clinical experience with Ebola-infected patients in West Africa
- a lack of health care personnel and basic and health infrastructure
- a small pool of clinical research experts and research infrastructure in countries
- limited prior experience in the conduct of clinical research
- overwhelmed, understaffed, and poorly supported ethics review boards
- limited experience with contract negotiations and large program project management
Each of these challenges can be addressed by strengthening in-country capacity.
Poor Surveillance and a Lack of Experience with Ebola in Country
The ability to rapidly recognize, coordinate, and respond to outbreaks relies on robust surveillance systems that monitor the incidence of communicable and zoonotic diseases and are able to detect increases and warn of outbreaks. Accurate, timely surveillance data are critical before and during public health emergencies because these data can provide the information needed to identify an outbreak at the earliest possible time as well as for appropriate resource allocation, assessment of the success of response, and planning for staffing and resource needs (McNamara et al., 2016). The increases in international travel and trade, recognition of the emergence and reemergence of communicable disease threats and other health risks, and the need for early and accurate identification of these events was a driving force behind development of the 2005 International Health Regulations
(IHR 2005), which requires every country to develop core capacities to “a) detect events involving disease or death above expected levels for the particular time and place in all areas within the territory of the State Party; and b) to report all available essential information immediately to the appropriate level of health-care response; and c) to implement preliminary control measures immediately” (WHO, 2005, p. 40). Subsequently, a 2009 Institute of Medicine (IOM) report called for the development of sustainable surveillance capacity for emerging infectious diseases rather than the buildup of surveillance when a new threat occurs and then its dismantling when the threat disappears (IOM and NRC, 2009). As Dr. David Nabarro, then the senior United Nations system coordinator for avian and human influenza, said at a meeting during the development of the report, “we are dealing with things that are likely to emerge at some time and that need attention. We have to persuade decision makers to invest in surveillance systems and other actions to deal with these uncertainties in a flexible and responsive way without being able to tell them, with an absolute precision, when they are going to emerge and what their economic or social cost might be” (IOM and NRC, 2009, p. 27).
Despite the establishment of effective global public health surveillance being a key stipulation in the IHR 2005, as of 2014 only 64 of the member states had achieved the required core capacities and 48 failed even to respond to the WHO (Gostin and Katz, 2016; Katz and Dowell, 2015). This is of paramount concern because it means that only about one-third of the world’s health systems are prepared to respond effectively to a public health emergency. At present, there are no enforceable sanctions available to penalize countries for noncompliance past the deadline, which have already been extended several times, or incentives or support for lowincome countries to comply. It is also difficult to see where the financial and other support required will come from, both from the countries themselves and from the international community. This process is slow, steady, and not in the public spotlight; mobilizing the necessary resources is, in fact, very difficult. The 2014–2015 Ebola epidemic revealed several weaknesses in the disease surveillance and response systems in the region.
A Lack of Clinical Experience with Ebola-Infected Patients in West Africa
Despite claims that Ebola was new to West Africa there is some evidence that Ebola was present in the region before the 2014–2015 epidemic. In 1994 the Tai Forest strain of Ebola was identified in an Ebola-infected veterinarian in Cote d’Ivoire who was attending to a colony of chimpanzees affected by a fatal outbreak of Ebola; the veterinarian survived (Formenty et al., 1999). There was speculation that the infection might have been acquired in Liberia where a serological diagnosis of Ebola was made in
another individual; however, this was not confirmed by virus isolation (United Nations, 1995). A retrospective serosurvey of 672 serum samples collected at the Lassa Diagnostic Laboratory at Kenema Government Hospital, Sierra Leone, between 2007 and 2014, primarily from Sierra Leone, identified 35 samples (5.2 percent) positive for Ebola virus IgG antibodies. Virus isolation was not part of the investigation; however, there was no recognized outbreak of Ebola during this period and the authors suggested this might be “the result of a reservoir maintaining Ebola in the environment” (O’Hearn et al., 2016, p. 5). Without prior appreciation that Ebola virus was present in the region, the appearance of Ebola in the index case in Guinea in late December 2013 and its subsequent spread in early 2014 was a surprise. This first cluster of cases and the missed opportunity to realize that an Ebola outbreak had begun was dubbed a Black Swan event by Osterholm et al. (2015). A Black Swan event is defined by three attributes: “First, it is an outlier, as it lies outside the realm of regular expectations, because nothing in the past can convincingly point to its possibility. Second, it carries an extreme ‘impact.’ Third, in spite of its outlier status, human nature makes us concoct explanations for its occurrence after the fact, making it explainable and predictable” (Osterholm et al., 2015).
Outbreak Surveillance
While the first string of related cases in Guinea following the death of the initial index subject was quickly noticed as unusual and was reported to the Ministry of Health in Guinea, as required by IHR 2005, the initial investigation by the ministry reached the conclusion that the likely cause was cholera. This misdiagnosis, which determined the initial response, represented the first serious impediment to a quick and effective clinical and public health response to contain and control the disease. Although this misstep was later alleged to be due to the lack of prior experience with Ebola in West Africa, improved outbreak investigation capacity, backed up by access to diagnostic laboratory expertise, either in country or through established collaborations, preferably in the region, could have resulted in the identification of the true cause of these deaths soon after they were spotted. However, clinicians in West Africa “had never managed cases. No laboratory had ever diagnosed a patient specimen. No government had ever witnessed the social and economic upheaval that can accompany an outbreak of this disease. Populations could not understand what hit them or why” (WHO, 2015a). Regardless of the impetus, be it the lack of awareness of the presence of Ebola in West Africa or lack of experience with Ebola as a clinical and public health challenge, the conditions for propagating a firestorm outbreak were present, awaiting the first spark and the subsequent failure to identify and extinguish it quickly. The 2014–2015 epidemic in
West Africa quickly and tragically provided the health systems and health care workers of Guinea, Liberia, and Sierra Leone with plenty of experience with the devastating nature of the infection.
The slow response and porous borders between the three epicenter countries allowed Ebola patients and contacts to freely move from one to the others during the critical first weeks, spreading and escalating the outbreak as it moved from Guinea to Liberia and Sierra Leone and from villages into more populous city centers (WHO, 2015a). Through the intervention of Médecins Sans Frontières (MSF) the outbreak was successfully identified as Ebola; however, by this point it was already rapidly spreading. For a variety of political and economic reasons which overrode the public health concerns, WHO and the affected countries were not as transparent as they could have been (Associated Press, 2015b; Taddonio, 2015). The WHO, as a member state organization, can be reticent to act vigorously when the affected country resists full reporting (Cheng et al., 2015).
The U.S. Centers for Disease Control and Prevention (CDC), along with many other organizations, eventually managed to conduct effective surveillance in the affected region during the epidemic, but in the process it faced numerous challenges, including
- case data that were underreported or missing altogether,
- a slow adoption of nationwide standardization of case definitions,
- difficulty in linking laboratory results with epidemiological data,
- mistrust and violence toward contact tracers,
- a lack of information technology equipment and staff,
- a lack of digital systems to track and analyze outbreaks,
- a lack of basic computer skills, and
- a lack of isolation facilities and laboratory capacity for diagnosis (McNamara et al., 2016).
While the surveillance efforts on the part of the CDC and partners were critical during the outbreak, it is also essential that the effort be maintained after the epidemic subsides (and before the next one begins). Diagnostic laboratory capacity was brought into the three countries by various international partners during the outbreak, including Belgium, Canada, China, France, Germany, Italy, the Netherlands, Nigeria, South Africa, Spain, the United Kingdom, and the United States (Abayomi et al., 2016). However, many of these laboratories were dismantled and removed since the outbreak was halted.
There is reason to be concerned about whether a sustainable surveillance system within countries at a similar level of core capacity as Guinea, Liberia, and Sierra Leone can be established, although there is some appreciation of the need and an attempt to do so by the European Union
Chemical Biological Radiological and Nuclear Risk Mitigation Centres of Excellence Initiative (or EU CBRN CoE), the CDC, Expertise France, and Public Health England (Abayomi et al., 2016; House of Commons International Development Committee, 2016). The CDC acknowledges the importance of continuing to support strong public health and surveillance capacity in the region in order to be prepared to respond to future outbreaks: “With the establishment of CDC offices in Guinea, Liberia, and Sierra Leone, the CDC is well-positioned to continue supporting the expansion of public health and surveillance capacity infrastructure to improve the response to future epidemics” (McNamara et al., 2016). An alternative may be establishing capacity at the regional level. The lessons learned through the program to eradicate polio in Nigeria can serve to inform other developing countries’ approach to surveillance and emergency public health challenges (Desmarais, 2016; WHO, 2015d). Notably, during the Ebola outbreak, within days of the arrival of the index case in Lagos in July 2014 the well-established African Center of Excellence for Genomics of Infectious Diseases laboratories at Redeemers University in Ogun State, Nigeria, was able to correctly and safely diagnose the index case for the Nigerian outbreak, using polymease chain reaction at biosafety level 2 containment (Salu et al., 2016). The WHO meanwhile is working to strengthen the surveillance systems of the Ebola-affected countries; efforts include
- providing technical support to the West African Health Organization for the establishment of the West African Regional CDC and its network of national coordinating institutions;
- supporting nine West African countries which will participate in the World Bank’s West Africa Regional Diseases Surveillance Systems Enhancement (REDISSE) project, with the preparation of their country profiles;
- supporting the three countries in developing and implementing national surveillance strategies and the National Integrated Disease Surveillance and Response guidelines and tools;
- supporting the Ebola-affected countries with the establishment of national public health institutions (or national CDCs), including study visits to existing national public health institutes in selected countries; and
- assisting the Ebola-affected countries with the development and maintenance of their essential health services situation reports, which monitor the health services recovery progress (WHO, 2017).
Building a viable system for public health surveillance and outbreak response requires training individuals and building the necessary infrastructure along with the sustained support to enable the system to continue to
function and grow in capability. Building such a system is step 1 in emergency preparedness.
Conclusion 5-1 In order to better respond to future outbreaks and recognize an emerging epidemic in time to effectively mount a response, including conduct of clinical trials, it is critical that surveillance, outbreak investigation, and diagnostic capacity be strengthened in low- and middle-income countries. The mandate to ensure compliance with IHR 2005 core capacity for surveillance, reporting, and initial response rests with the WHO; however, two-thirds of countries have not yet reached the minimal required standards, which represents a major gap in global readiness.
Recommendation 1
Support the development of sustainable health systems and research capacities—Inter-epidemic
To better prepare low-income countries to both respond to future outbreaks and conduct foundational research, during the inter-epidemic period (as covered in 2005 International Health Regulations [IHR 2005]), major research funders and sponsors (e.g., U.S. National Institutes of Health and comparable public and private research funders) and development agencies (e.g., U.S. Agency for International Development and comparable public and private development funders) should collaborate with the World Health Organization and regional centers of excellence to
- Assist in monitoring and evaluating the development of national and regional core capacities under IHR 2005, and
- Provide financial and technical assistance to the extent possible or establish a financing mechanism, to help build sustainable core capacities at the intersection of health systems and research (e.g., diagnostics, surveillance, and basic epidemiology).
Lack of Health Personnel and Basic and Health Infrastructure
An effective emergency response relies on the existing health systems having robust capacity before an outbreak occurs. In the specific example of Ebola, the first link in the chain is surveillance and diagnostic capacity, and, as noted above, in the case of the 2014–2015 outbreak it failed at the outset. The second link in the chain is the capacity of the health care system to care for patients and stop transmission. Prior to the 2014–2015 Ebola epidemic, the three epicenter countries most affected by the Ebola outbreak had weak health systems with chronic shortages of human resources, diagnostic capabilities, infection control experience
and supplies, adequate medicines, and basic infrastructure. The epidemic only further strained available resources, and, in turn, this lack of health system capacity dramatically hindered the Ebola response (Kamal-Yanni, 2015). A 2014 survey of health facilities around the world found that over half did not have protocols to deal with an Ebola suspected patient; two-fifths lacked basic infection protection such as gloves, masks, and gowns; and over one-fifth did not have the basic amenities necessary for facility and personnel hygiene, including something as elementary as running water (Wright et al, 2015). This assessment concluded, “There is general agreement that the Ebola crisis was not quickly contained in Guinea, Liberia, and Sierra Leone because their national health systems were dangerously under-resourced, understaffed, and poorly equipped” (Wright et al., 2015, p. 40).
To compound the problem, the outbreak itself was creating further stress, as physicians, health care workers, and ancillary staff became infected and died. In the early phases of the response, the rate of infection in health care workers was 21 to 32 times greater than in the general population. By May 2015, 0.02 percent of Guinea’s population had died due to Ebola, compared with 1.45 percent of the country’s doctors, nurses, and midwives (Evans et al., 2015). The differences in overall versus health care worker mortality were equally dramatic in Liberia and Sierra Leone. In the former, 0.11 percent of the general population died, versus 8.07 percent of health care workers, while in Sierra Leone the corresponding figures were 0.06 percent of the general population and 6.85 percent of the health care workers, with nurses and nursing aides accounting for more than half of these losses. Given the relative paucity of physicians and nurses or midwives in the three countries at the onset of the outbreak, these numbers translated into a 10 percent reduction in the number of doctors in Liberia; an 8 percent reduction in nurses and midwives, and a 5 percent and 7 percent reduction, respectively, in Sierra Leone; and 2 percent and 1 percent for doctors and nurses in Guinea (Evans et al., 2015). By May 2015 the total loss of health professionals to Ebola in the three countries was 78, 83, and 79 doctors, nurses, and midwives in, respectively, Guinea, Liberia, and Sierra Leone. While these numbers are not big, the WHO ranked Guinea, Liberia, and Sierra Leone as 26th, 1st, and 4th from the bottom, respectively, among 193 countries in terms of doctors per capita; any loss of trained health professionals would have had a huge impact on the ability to care for patients within the three countries (WHO, 2015c). These tragic deaths were particularly critical early in the outbreak, when the case load was increasing exponentially, isolation facilities were insufficient, the international mobilization of volunteers was still in its early stage, and the personal protective equipment, when available, was cumbersome and still unfamiliar to the health care workers (WHO, 2014). To make
matters worse, during the outbreak some health care workers in Guinea, Liberia, and Sierra Leone went on strike over salary and incentive pay for the hazardous work they were being asked to do, further interfering with the care of patients at treatment centers (BBC, 2014; Camara, 2015; Telegraph, 2014).
International agencies and nongovernmental organizations (NGOs) can also contribute to human resource crises in Africa, and elsewhere, when they lure government health workers away into more highly paid positions; they may offer 5 to 20 times more than the comparable public-sector salaries (Pfeiffer et al., 2008). While it is difficult to quantify, the internal brain drain of health care workers from local treatment centers providing routine health care to NGOs during the 2014–2015 Ebola outbreak—or, at a later date, to provide skilled local professional and administrative staff to international research projects—would be expected to contribute to an already weakening health care system performance and to adversely affect the environment in which clinical research could be safely conducted (Anderson and Beresford, 2016). Compounded by the realities of migration of trained nationals to richer countries or to higher salaried international positions, what is known is that by the end of 2014 routine hospitalization and health care services in the three countries were dramatically on the decline, including routine immunization campaigns, leading to subsequent outbreaks of preventable childhood diseases such as measles (Bolkan et al., 2014; Brolin Ribacke et al., 2016; Suk et al., 2016; Takahashi et al., 2015). To prevent the negative impacts of siphoning of health care workers out of the national system and into local and international NGOs in the future, it has been suggested that “rather than hiring workers out of the public system to work in a parallel program, NGOs can integrate projects into local systems and fund additional workers in the public system in accordance with local pay structures. NGOs can also support other incentives to retain staff, such as payment for overtime or after-hours service expansion, or stipends for extra training and additional job responsibilities” (Pfeiffer et al., 2008, p. 2137). In addition to the human resource challenges during the epidemic, trial teams also struggled with basic infrastructure needs such as the provision of power, Internet access, and clean water; a reliable cold chain; backup generators; and more—all of which are issues of equal concern for the operation of an effective health care system in nonemergency conditions (Widdowson et al., 2016). It is clear that achieving this level of basal health care infrastructure is neither simple nor inexpensive, but unless it is prioritized during the inter-epidemic period there is little chance that the response to a future epidemic will be any less fraught than it was in 2014.
Conclusion 5-2 To effectively promote the health of a population, every country requires a well-integrated functional health care and health research system. The separation of the responsibility to care for the sick, which is the humanitarian mandate of medicine, from the responsibility to continually learn and improve the quality of care, or the research mandate, adversely affects the potential to fully meet both imperatives. Mechanisms for training (and the stable support of) key personnel, laboratories, and medical care facilities are essential to establishing an effective clinical research environment.
Logistical Considerations
Logistics, much like public health measures, are frequently discounted when things are going well. In a humanitarian crisis in a low-resource setting, logistics play a crucial role in successfully mounting a response and conducting research. Researchers and responders must assess the limited resources on hand and determine how to use those most effectively in order to have the greatest impact. In an outbreak scenario of a rare or novel pathogen, this task can be made even more challenging. For example, when the Ebola outbreak began, the limited knowledge about patient management and prevention made it “nearly impossible to prioritize [the] limited available resources for those who might benefit the most, especially early in the response” (Roshania et al., 2016, p. 402). This deficit at the start of the outbreak made the data collection efforts of humanitarian organizations like International Medical Corps (IMC) and MSF (discussed below) critical because this information fed back to develop standardized clinical protocols, identify at-risk groups, and determine other epidemiological factors for contracting Ebola; in addition, it enabled humanitarian and trial teams to define how to best manage their limited resources.
Through their logistical support, humanitarian organizations contributed greatly to the launch of clinical trials during the Ebola outbreak. Trials were launched out of Ebola treatment units (ETUs) established and run by a multitude of international NGOs. For example, PREVAIL II (ZMapp) partnered with IMC at two sites in Sierra Leone; MSF collaborated with trial teams on the Guinea ring vaccination trial, brincidofovir, favipiravir (JIKI), and convalescent plasma trials (Ebola-Tx); and GOAL Global partnered with the RAPIDE-TKM trial team (MSF, 2016; NIAID, 2017; Wellcome Trust, 2015). As these treatment units were already established and running in country, it allowed trial teams to benefit from the existing relationships between the humanitarian organizations running the ETUs and the local officials and community members hosting the ETUs (Georgetown Journal of International Affairs, 2014; Levine, 2016). “International Medical Corps field staff worked closely with the NIH team, introducing them to local
government and community leadership and helping facilitate numerous town hall presentations of the study, in order to ensure community acceptance before beginning the trial” (Levine, 2016, p. 80). This contributed to the trial team’s ability to quickly launch trial discussions and gain local support. In addition, trial teams benefited from the already established ETU infrastructure established by the NGO (storage space, equipment, chlorinated water, etc.). While this is helpful to the research team, it may strain the NGOs and constrain their ability to carry out their missions and provide patient care.
Any research project carried out in a humanitarian context, however small or non-invasive, will always place a burden on the organization providing the logistical infrastructure for the research study. Even if outside researchers are able to provide for their own staff and the food, housing, transportation, and security of those staff (which will be difficult in many humanitarian contexts), they may still siphon off precious resources from their host organization. These resources include tangibles, such as electricity, water, fuel, and space, as well as intangibles, such as staff time and local political capital. Funding to offset these tangible and intangible overhead costs should be built into any research grant and provided to the humanitarian organization as part of the research partnership. (Levine, 2016, p. 81)
Working with experienced care providers also assisted the trial teams as they were able to learn from the humanitarian medical staff running the ETUs. MSF, for instance, which is widely acknowledged as having expertise in treating Ebola, “took a leadership role in the latest epidemic in ways that it had not before. It taught staff from other organizations—including the WHO and the [CDC]—how to treat people with Ebola” (Hayden, 2015, p. 18). The role of humanitarian organizations in the Ebola outbreak and their crucial contributions to the clinical trials conducted should not be understated. Without their support, it is highly unlikely that any trial would have successfully enrolled patients, or even launched.
For the vaccine trials occurring outside of ETUs and in remote villages, meeting the necessary logistical needs required detailed planning and precision. The fact that the basic infrastructure needed to run trials did not exist in the three countries at the start of the outbreak greatly affected the operational and logistical planning necessary for conducting trials. For example, the Kambia District in northern Sierra Leone is not on the national power grid, which led the EBOVAC team to purchase generators to service their vaccine storage facility, which required 24-hour power. The trial team also had to build or refurbish all of the trial clinics and establish a clinical trials laboratory in Kambia (once the epidemic waned they could no longer make use of the laboratory at the Ebola Treatment Center in
Port Loko because it had been decommissioned and the local hospital did not have the capacity to process trial samples). Furthermore, the curfews and lockdowns employed to help control the outbreak resulted in limited working hours and restricted movement, which affected the schedules of the trials; specifically, they “contributed to the unpredictability and delays in an already time-sensitive project” (Watson-Jones, 2016). The emergency context did, however, bring “some operational benefits to the project, including a blanket exemption to import goods for the trial which expired when the state of emergency was lifted” (Watson-Jones, 2016). The Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) team faced similar challenges, and to move as rapidly as possible, the CDC Foundation raised donor funds for immediate needs such as infrastructure building, supplies, and hiring staff.
It should be noted, however, that some of the responses to the logistical challenges contributed to the negative perceptions of the trials and influenced community trust. For example, storing vaccine at the U.S. Embassy in Monrovia, Liberia, initially undermined the credibility of the trial teams. Additionally, in Sierra Leone, WHO responders did not have transportation available to them in order to monitor the spread of the virus despite a recently purchased fleet of vehicles that sat, unavailable, at UN headquarters in Freetown, Sierra Leone, fostering mistrust. “One WHO official suggested Sierra Leonean responders requesting motorbikes for travel to villages buy bicycles instead” (Associated Press, 2015a). When tackling logistical barriers, perception matters and community engagement, consultation, and partnership remain of the utmost importance. (See Chapter 6 for further discussion on community engagement.)
Trial teams in affected countries came up with creative solutions to the innumerable challenges they encountered. Table 5-1 below captures the experience of the team carrying out the CDC’s STRIVE study—and the challenges and solutions to implement that vaccine trial. The Guinea ring vaccination trial had the complex task of developing remote trial sites at each ring location. This required the tailoring of standard operating procedures to account for challenges that might arise at the different sites. Checklists had to be developed, prepacked boxes of supplies had to be assembled, and generators and backup generators to supply electricity for electronic record monitoring and cold chains had to be purchased and moved into place—these were just a few of the challenges encountered on top of the processes associated with engaging the community and trial conduct (Capital Reporting Company, 2015).
The committee learned that randomized controlled trials are very possible during an outbreak, but it requires funding, logistical support, and a team that reaches far beyond just the researchers and scientists involved, including communication and social mobilization (discussed in more detail
| Challenge | Solution |
|---|---|
| No –80°C (–112° F) freezers or method of transport at –80°C (–112°F) | Purchase and international shipping of freezers; phase-change material transporters |
| No appropriate space for enrollment and vaccination | Identify, negotiate the use of, and renovate some facilities |
| No space for data entry and management | Build and renovate facilities |
| No reliable Internet for data entry, storage, and transmission | Installation of satellite-routed Internet and wireless capacity |
| No reliable power for cold chain, laboratory, and participant follow-up sites | Installation of generators, solar panels, and backup batteries |
| Health status of population unknown; poor and dispersed health care access | Establish free medical care; provide supplies to upgrade intensive care unit at referral hospital |
| Misinformation and misconceptions on vaccines and the motives of the trial organizers | Focus groups, key informant interviews, informational sessions, extensive communication materials |
| Relevant supplies limited in country | Procure and ship supplies internationally |
| No basic equipment (e.g., centrifuges) in country for serology study | Procure and ship equipment internationally |
| No staff GCP training; inexperienced research staff | Conduct large-scale, in-person training; repeated retraining on operating procedures |
NOTE: GCP = good clinical practice; STRIVE = Sierra Leone Trial to Introduce a Vaccine against Ebola.
SOURCE: Widdowson et al., 2016.
in Chapter 6). Capacity was brought in and trial teams admirably launched trials in the most challenging of circumstances, but the question now is what will be left in place, who will maintain it, what will be improved for the future, and where are the resources needed for sustainability to come from?
Conclusion 5-3 Researchers conducting clinical trials during epidemics in low-resource settings will require substantial logistical support from organizations that build and operate treatment centers (including international humanitarian organizations and national health systems), and these organizations should be included in strategic planning for clinical research activities during the inter-epidemic period.
Small Pool of Clinical Research Experts in Countries
It is difficult to find systematic assessments of research capacity for the West African region, but the data that are available suggest that the capacity is low. In 2013 the Economic Community of the West African States (ECOWAS) reported on the state of health research in ministries of health among ECOWAS countries as of January 2011 (Sombié et al., 2013). It reported that just half of West African countries had established directorates for health research with defined terms of reference, the existing funding mechanisms were inadequate to support the research structures within and outside the ministries or to improve the capacity of researchers, networking and monitoring activities were weak, and “just 7 percent of the directors of research units were trained in research management” (Sombié et al., 2013). While 86 percent of the countries had broader national health policies in place, and 57 percent had some form of policy or strategic document for research development, half of them had not established national research priorities. Specific country assessments were not included in the report; instead the authors concluded that “urgent action to improve the research environment in the Ministries of Health in the West African sub-region” was essential (Sombié et al., 2013). This report was updated recently by an independent evaluation sponsored by the West African Health Organization (WAHO). Although there was evidence of increased regional investment and some progress, “high staff turnover, weak institutional capacities, and ineffective collaboration” remained significant challenges (Aidam and Sombié, 2016).
In the affected countries, the WHO has assessed the policy frameworks that facilitate the conduct of health research, such as the availability of a national health research policy, a health research strategic plan, a health research program, and health research laws in place (WHO, 2016a). None of the three affected countries met all four criteria, and Liberia satisfied just one, an available health research program which was funded by support from WAHO. The WHO also observed that in the Africa region,
[o]nly a few countries have successfully coordinated the support and involvement of development partners, the private sector and civil society to improve the research policy environment by developing health research policies, strategic plans, legislation, and programs. Policy-makers and decision-makers are not strongly active in national research agenda priority setting. Only half of the health research institutions surveyed reported having a written policy requiring that researchers obtain the informed consent of research participants. Little or no money is allocated to health research in almost all the countries in region. (WHO, 2016b)
Furthermore, the
[c]ontinued dependence [of the African countries] on external funds for research may not always align to regional priorities and may not be sustainable. Research institutions in the Region have insufficient facilities and infrastructure: less than half have institutional websites, provide email addresses to research staff, and have a library. There is a serious shortage of qualified staff engaged in health research. Although the majority of researchers are full-time staff, significant numbers also leave their institutions for various reasons, leading to shortages of experienced senior researchers. . . . Researchers have also not always been able to push for their evidence to be used to drive policy. (WHO, 2016b)
As international organizations began to plan clinical research on Ebola, the dire lack of broad experience and knowledge in the affected countries became evident. For example, local researchers had limited or no experience in developing collaborative arrangements with international partners, with obtaining approval from local and international authorities, and with negotiating the legal aspects of clinical trial agreements and other legal documents such as clinical trial agreements, material transfer agreements,2 data sharing, and post-trial benefits. In addition to these responsibilities, local researchers were also under pressure to identify suitable research study locations, obtain funding, recruit research staff, conduct training on how to work safely in the context of containment, and ensure that research did not impair clinical care.
Collecting Patient Data
The lack of robust health systems and personnel dramatically impeded the ability of clinicians to collect patient-level data, which could be used to inform treatment protocols for patients in real time. MSF and IMC, for example, encountered numerous challenges in collecting patient data in the high-risk zone of their treatment centers. Due to the concern over the possibility of transmitting Ebola via paper records, MSF staff “had to shout the results of ward rounds across the fence to staff in the low risk zone on the other side who recorded the information on clean paper” (MSF, 2016). It was not until MSF started using personal digital assistants that patient
___________________
2 A material transfer agreement (MTA) is a contract that governs the transfer of tangible research materials between two organizations, when the recipient intends to use it for his or her own research purposes. The MTA defines the rights of the provider and the recipient with respect to the materials and any derivatives. Biological materials, such as reagents, cell lines, plasmids, and vectors, are the most frequently transferred materials, but MTAs may also be used for other types of materials, such as chemical compounds and even some types of software (UC Regents, 2017).
information could be transmitted in real time, thus reducing the staff time spent recording and relaying information. Similarly, IMC noted that ensuring data quality was a complicated process: (1) using data validation settings in Excel reentry documents, (2) using a codebook to ensure that patient data from various types of patient charts were standardized, (3) conducting additional audits by data entry research assistants, and (4) discussing data entry concerns with the principal investigator (Roshania et al., 2016).
For future outbreaks, IMC stresses that “to facilitate data collection and global reporting in future humanitarian responses, standardized data forms and databases, with clear definitions of clinical and epidemiological variables, should be developed and adopted by the international community” (Roshania et al., 2016). With little empirical evidence on Ebola prevention, treatment, or management to guide clinical care, those responding to the Ebola outbreak in West Africa lacked standardized clinical protocols for patient care contributing to the variability of care across ETUs. The lack of standardized protocols combined with the uncertainty regarding how basic supportive critical care could be translated to the setting of an ETU in a limited resource setting in the midst of an outbreak made the collection of patient-level data and real-time learning imperative.
Humanitarian groups made a concerted effort to collect patient data in ETUs during the 2014–2015 Ebola outbreak and as a result of efforts by IMC, MSF, and others, the global community now has a better grasp on Ebola than ever before. Adam Levine, the primary investigator of the IMC’s Ebola research team, was quoted as saying, “At a more fundamental level we have proven that with the right partnerships, the right funding, and the right planning, we can do research in this type of emergency—not just research but high-quality research” (Marshall, 2016). Although the committee did not address the fiscal management systems required to ensure that funds provided through international donors, research institutions, or NGOs are used as intended and are not inappropriately diverted, it is essential to have the necessary systems and audits built into these partnerships. As a team effort this needs to be a shared responsibility between the external and the national members in order to build mutual trust and respect.
Conclusion 5-4 In an epidemic context, particularly with a highly lethal contagious pathogen in a low-resource setting, recording detailed clinical data is a resource-intensive process that may be seen as diverting attention from patient care. However, despite the difficulties, it is imperative to systematically and comprehensively collect basic information on patient characteristics and clinical outcomes in order to document the natural history of the evolving epidemic and to provide clues to better patient management.
Recommendation 2a
Develop memoranda of understanding3 to facilitate data collection and sharing—Inter-epidemic
Research funders, sponsors, national governments, and humanitarian organizations should work together with the World Health Organization to develop memoranda of understanding during the inter-epidemic period to improve capacity to collect and share clinical data, with all necessary provisions to protect the privacy of individuals and anonymize data for epidemiological research.
Recommendation 2b
Provide resources to enable data collection and sharing—Epidemic
At the start of an outbreak, developed countries, research funders, and sponsors should work together with national and international health care providers responding to an outbreak, to provide the additional resources and personnel needed to enable systematic data collection on routine care practices and outcomes. Data collection should begin as soon as possible, and data should be shared and coordinated in a central database to advance an understanding of the natural history of the disease and of the best practices for standard of care. This information should also be used to inform protocols for clinical trials.
Overwhelmed Ethics Review Boards4
Among the many technical capabilities required for assessing clinical research proposals is the availability of a trained and independent research ethics committee and the administrative support necessary for its members to work efficiently in the country where the trial will be conducted. The Declaration of Helsinki addresses the role of ethics committees in the
___________________
3 Memoranda of Understanding: Documents whereby parties entering into a partnership agree to an intended common purpose or set of goals. This is sometimes seen as more of a moral agreement rather than a legally binding agreement, and thus it is usually not intended to have the enforceability of a legal document. Although useful as an overarching agreement that sets out the working principles between parties, other written agreements are necessary to create binding commitments.
4 An ethics review board (ERB)—also known around the world as an independent ethics committee, research ethics committee, research ethics board, or institutional review board—is a type of committee used in research that has been formally designated to approve, monitor, and review biomedical and behavioral research involving humans. The purpose of the review board is to ensure that appropriate steps are taken to protect the rights and welfare of humans participating as subjects in a research study (OHRP, 2017a). The committee is sensitive to the fact that the procedures and guidelines for the protection of human subjects may differ internationally and that some review boards may have more or less capacity for scientific or ethics review, or both (OHRP, 2017b).
review of human subjects research: “The research protocol must be submitted for consideration, comment, guidance, and approval to a research ethics committee before the trial begins. This committee must be independent of the researcher, the sponsor, and any other undue influence. It must take into consideration the laws and regulations of the country or countries in which the research is to be performed as well as applicable international norms and standards, but these must not be allowed to reduce or eliminate any of the protections for research participants set forth in this Declaration” (WMA, 2013). In the context of a public health emergency, ethics oversight of research can pose numerous challenges due to the rapid turnaround needed to initiate research (discussed in more detail in Chapter 2).
An earlier mapping study of research ethics committees conducted by COHRED identified more than 165 committees operating in 34 African countries, but concluded that there was great variability in skills, membership, capacity, and efficiency (Kasule et al., 2016). Although there had been efforts to train individuals in low- and middle-income countries in research ethics and help promote the establishment of functional mechanisms for ethics review of clinical research, a subsequent 2016 report concluded that “most African research institutions do not have—or allocate—adequate financial resources to strengthen the capacity of their own research ethics committees. Many [ethics committee] administrators may not have defined roles and responsibilities, may lack adequate training, and do not have efficient electronic information management systems to assist with their heavy and often complex workloads” (Kasule et al., 2016). While Guinean, Liberian, and Sierra Leonean ethics review committees are all included in the National Institute of Allergy and Infectious Diseases–operated ClinRegs database of country-specific clinical research regulatory information, there is no accompanying assessment of their functional capacities (NIH, 2016). However, it should be noted that variability in skills is not unique to African countries and can be seen across low- and middle-income countries and in the developed world as well (Bhatt, 2011).
During the Ebola epidemic, the demands on national scientific and ethics review committees taxed them far beyond what they were capable of handling. For example, scientific competition burdened committees as multiple research teams raced to be the first to scientific and ethics review boards, local principal investigators, and treatment units with their product and protocol (Heymann et al., 2016). There was a high volume of research proposals put forth for ethics review, including clinical trials, anthropological qualitative studies, expanded access, and diagnostic studies. In Guinea, for example, the number of research proposals that the ethics committee considered increased threefold from 2014 to 2015 (Djénab, 2016). Moreover, the proposed research was complex, involving contextual consider-
ations such as “a highly vulnerable population faced with a deadly disease; research activities spanned over three low-income countries, with fragile health systems, poor infrastructure and little experience of medical research (and in particular for clinical trials); and some research was carried out in collaboration with academic institutions, which required setting up new collaborative research agreements very quickly” (Schopper et al., 2016). The committee heard testimony that some researchers did not go through the scientific and ethics review process because it was perceived as too time consuming.5 If true, this is deeply disturbing and such flagrant violations should not happen in the future. However, despite the numerous challenges, with assistance the in-country ethics committees often fulfilled their responsibilities, and at times could act with remarkable speed. For example, the PREVAIL II trial protocol was submitted in Sierra Leone on March 4, 2015; rewritten for a more resource-limited setting on March 18; and approved by the Pharmacy Board, the relevant body in the country, on April 2 (Davey et al., 2016). This was the result of a close collaboration between the sponsor and national researchers on prior research protocols during the outbreak and of an understanding of the local context and requirements; it also demonstrates the impact of effective collaboration and accrued experience.
In addition to these challenges, most proposals required the review not just of one board, but of multiple scientific and ethics committees in the countries where the studies were slated to take place, at the WHO, and in the country of the trial sponsors (Saxena and Gomes, 2016). Carrying out multiple reviews took time and posed a barrier to trial implementation. While multiple reviews can strengthen trial protocols, without coordination and simultaneous reviews this can contribute to major delays in trial approval and implementation—an unfortunate loss of time in a situation where time is of the essence. The WHO tried unsuccessfully to consolidate ethics reviews, and it has been suggested that a supranational ethics committee be formed, although this would require additional resources to establish and operate (Saxena and Gomes, 2016). MSF has suggested alternative methods to encourage coordinated reviews, including
- establishing ethics committee communication mechanisms well before the emergence of the next outbreak; and
- setting up joint pre-review or review mechanisms that could become feasible via upfront planning and a better use of communication technologies for audio- and videoconferences, which have been seriously underused (Schopper et al., 2016).
___________________
5 Testimony of several participants at the Public Workshop of the Committee on Clinical Trials During the 2014–2015 Ebola Outbreak. Monrovia, Liberia, August 15–16, 2016.
If a “pre-review meeting” was organized at the start of an outbreak, representatives of the several scientific and ethics committees likely to review the clinical trials protocols could come together—using videoconferencing capacities as necessary to bring the international participants into the discussion—to consider general issues and approaches to foreseeable ethical dilemmas.
As is well documented in the literature, there is a need to boost scientific and ethics review capacity and develop not just the normal capacity required in inter-epidemic periods but also a surge capacity for use during epidemics (Eckstein, 2004). Part of this is to upgrade the administration of ethics committees and their ability to review and track proposals during the ethics and scientific review process, which has the capacity to handle multicenter and multicountry reviews as well. One such system has been developed by COHRED, and it is now being introduced into a number of countries in Africa in general and in West Africa in particular (COHRED, 2012) (see Box 5-1). If this were adopted as the standard tool in a country or in a region with multiple scientific and ethics committees it would greatly facilitate the necessary coordination. During Ebola, efforts were made by trial teams to enhance the capacity for ethics review by national ethics committees. For example, the committee heard at its meeting in Monrovia, Liberia, that NIH worked with Liberians to help them establish a national research ethics board; initially there were two distinct ethics committees, and it was unclear which would have oversight of PREVAIL. The NIH team met with ethics committee leaders and other national thought leaders to establish a board chartered through the Ministry of Health with
oversight over all proposals to streamline the process and, at the same time, improve the expertise of the members. Additionally, NIH provided basic human research ethics training to national ethics committee members in order to further expand their ethics knowledge (Kennedy et al., 2006). NIH, through the Fogarty International Center, has now been investing in ethics training for developing country professionals for more than 16 years (Millum et al., 2013). With the need for harmonizing multiple international ethics reviews, it is also critical to have host and sponsor country scientific and ethics review committees and regulatory agencies partner and share information to aid the deliberation of each. These efforts were useful and effective in moving proposals through the approval process during the Ebola epidemic. However, there are limits to how much surge capacity is possible particularly if it is above the local capacity and in-country expertise. It seems apparent that advanced planning for outside assistance when a surge is required, while keeping control over decisions within the affected countries, would be an important step to take. The international community and national institutions would benefit from facilitating strong partnerships—such as, for example, NIH’s partnership with Liberia—that make global resources available to local review committees without supplanting the local committee. Deference to the local committee is important because international scientific and ethics committees from high-income countries coming in to support local committees may not fully understand the culture and context in a developing country in which clinical research is being proposed, and culture and context matter.
In addition to scientific and ethics committees, international regulatory agencies must also defer to local regulators and their knowledge of their own populations in order to identify and agree on common principles for regulatory approvals. During the 2014–2105 Ebola epidemic, U.S. and African regulators established numerous agreements “to help facilitate communications between the two agencies on medical products used, or proposed to be used, for Ebola-related purposes as part of cooperative regulatory activities” (FDA, 2016). These partnerships included the U.S. Food and Drug Administration, the Ministry of Public Health and Hygiene of Guinea, the Liberian Medicines and Health Products Regulatory Authority, the Pharmacy Board of Sierra Leone, and the World Health Organization Department of Essential Medicines and Health Products (FDA, 2016). Similarly, the European Medicines Agency
established a type of rolling review to allow experts to continuously assess the data on new medicines as they became available. Through this process, the Agency was able to develop increasingly robust scientific opinions based on additional data provided during the assessment process. The initial review and subsequent updates were shared with health care decision makers in concerned countries. This enabled them to take informed
decisions on whether and how they wanted to use vaccines and medicines during the latest outbreak, taking into account their specific situation. (European Medicines Agency, 2017)
Preexisting mechanisms for regulatory collaboration were also engaged during the epidemic. For example,
To address the challenge of authorizing clinical trials of Ebola candidate vaccines with limited available data, the WHO African Vaccine Regulatory Forum (AVAREF) [established in 2006] was used as a collaboration platform enabling regulators, ethics committees and sponsors to reach consensus on key ethical and regulatory questions. Given AVAREF’s crucial role in speeding up product development through coordinated regulatory efforts to combat Ebola it is essential that necessary resources are allocated to further strengthen its capacity. (Akanmori et al., 2015)
These types of coordinating activities and collaborative agreements are challenging to establish during an outbreak, so the time to build this capacity is primarily during an inter-epidemic period, when planning, training, and implementation in the countries at risk can be systematically organized and executed in collaboration with the countries and assistance from the international community.
In developing international partnerships to build ethics review and regulatory capacity for clinical trials, it would be advantageous to have experts in clinical research and trial design work together with local research staff and representatives of communities that might be enrolled in these studies. The goal of this collaborative partnership would be to develop model protocols during the inter-epidemic period that meet scientific standards and are acceptable to the local researchers and community representatives. Then, in the event of an outbreak, these model protocols would be available to be rapidly adapted to the specific circumstances of the outbreak and local environment; and trials could begin the implementation phase after the normal review process by the relevant local and international scientific and ethics review committees. An additional benefit that is gained from close partnerships between international ethics and regulatory bodies during the inter-epidemic period is the establishment of a corps of ethics and regulatory experts knowledgeable about different regions or countries, their culture, and the context in which trials would be conducted.
Limited Experience with Contract Negotiations
While the clinical trials conducted during the Ebola outbreak moved at record speed once they were prioritized and through ethics reviews, the actual starts of the trials were unnecessarily slowed by bureaucratic barriers
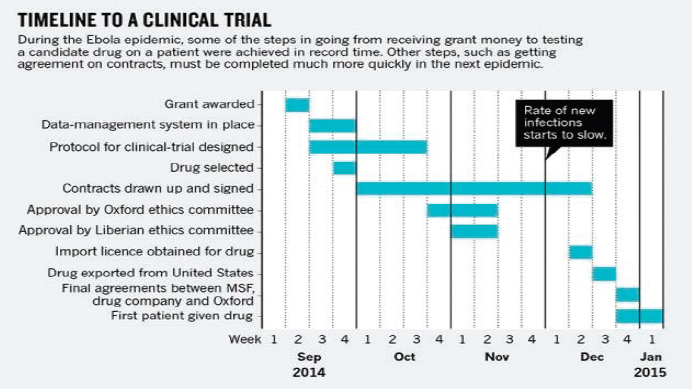
SOURCE: Lang, 2015.
such as the negotiation and approval process of clinical trial contracts—in other words, they could have been implemented even faster (Lang, 2015). As seen in Figure 5-1, most of the steps required to implement a clinical trial during the Ebola outbreak, such as designing the protocol and obtaining ethics committee approval, could be completed relatively quickly when the partners worked closely together (Lang, 2015). However, developing and signing contracts took up considerable additional time in a process that needed to be very time sensitive, and it seriously delayed the beginning of trials. Speeding up this process would require that countries be “research ready” for the next outbreak and that trial teams, governments, research agencies, relevant NGOs, and the WHO assist countries in acquiring competency in drawing up template contracts (Lang, 2015). Despite the undeniable need for speed in implementation, developing fair research contracts and precluding exploitation is imperative, and trial teams will need to be prepared to overcome the various and complex legal and bureaucratic hurdles in the event of a future outbreak. “The basis for a good collaboration should be trust and openness. A well-negotiated contract will ensure that all partners achieve a fair share of both the benefits and the costs. It is worth spending time on, and will help to ensure minimization of problems in project execution further on” (Edwards et al., 2014).
Conclusion 5-5 Helping low- and middle-income countries expand their capacity for the ethics review of human research protocols, regula-
tory oversight, and the legal review of clinical trial agreements, material transfer agreements, data sharing, and post-trial benefits could reduce bottlenecks in the clinical trial setup process during epidemics and greatly speed up the time to enrollment of the first participant.
Recommendation 3
Facilitate capacity for rapid ethics reviews and legal agreements—Inter-epidemic
Major research sponsors should work with key stakeholders in low- and middle-income countries to
- Build relationships between local ethics boards and entities that could provide surge capacity for ethics review in the event of an emergency situation. Such efforts would include strengthening networks of ethics boards in a region or connecting local and outside ethics boards, agencies, or experts. Memoranda of understanding setting forth who will provide what services and how decisions will be made should be executed in the inter-epidemic period.
- Establish banks of experts in negotiation of clinical trial and material transfer agreements, and other essential components of collaboration, who are willing to offer pro bono advice and support to counterparts in countries affected by outbreaks.
- Develop template clinical trial agreements reflecting shared understandings about key issues such as data sharing, post-trial access to interventions, storage and analysis of biospecimens, and investments to build local capacity.
Those who agree to be available during outbreaks to provide surge capacity would review protocols and provide opinions and advice to local scientific and ethics review committees, which would retain control over and accountability for decisions about protocols. Potential sources of experts in ethics review and negotiation of clinical trial and material transfer agreements are schools of medicine and public health with extensive experience conducting clinical trials in low-resource settings; Public Interest Intellectual Property Advisors, a nongovernmental organization that provides pro bono legal advice to low- and middle-income countries regarding health research and contracts; and COHRED, through its program on fair research contracting (Musolino et al., 2015).
A Clinical Research Document Database
In light of the numerous logistical and operational barriers to implementing clinical trials (discussed above) that confronted Guinea, Liberia, and Sierra Leone it is worth considering what steps might be taken to
improve the speed with which research is considered, approved, and implemented. To this end the committee recognizes two capacity-building initiatives that could be useful:
1. The Creation of a Clinical Research Document Database
The database would be meant to provide a framework that can assist national and local researchers in the highly regimented steps required in clinical research. It would be comprised of template documents that are integral parts of the conduct of clinical trials, including trials implemented in low-resource settings—addressing the allocation and provision of scarce resources. The database would include template clinical trial designs for different classes of products (i.e., therapeutics and vaccines); model clinical trial agreements and other contractual arrangements such as material transfer agreements, data sharing, and post-trial expectations; as well as logistical checklists. The numerous logistical tasks that need to be considered and addressed in advance of implementing clinical trials in a low-resource setting and responding to an outbreak are extensive and each task comes with a litany of steps and substeps that must be adapted to the individual situation and followed with precision. These tasks include everything from obtaining sustainable financial assistance, training health care workers and staff, building medical facilities, obtaining reliable power and clean water, to patient monitoring and data collection, cold chain logistics, regulatory document preparation, drug supply accountability, and transportation (road conditions, vehicles, fuel) to name a few. With pre-prepared documents detailing the necessary steps and procedures readily available a country wanting to initiate clinical research would not have to start planning from ground zero and in-country researchers would have a starting point for discussions and planning with international organizations coming in to provide aid. The documents would also be accompanied by explanatory text to help the in-country researcher and Ministry of Health leaders in the affected countries adapt and modify the documents to incorporate pertinent local details, for example, the specific circumstances of the research, the pathogen, and target trial participants.
2. Inter-epidemic Research Partnerships
To be effectively used, the research community and Ministry officials in the countries need to understand firsthand what the documents are and be trained in their use. This can be accomplished best through experience in clinical research. International partners experienced in clinical research can collaborate with low- and middle-income countries to strengthen skills, increase expertise, and prepare in-country experts for rapid, independent
use of the documents in the database when a public health emergency arises. Fogarty, for example, recently launched a program aimed at strengthening research training in Guinea, Liberia, and Sierra Leone, “In the first round of funding, four U.S. institutions received grants to partner with academic centers in two of the West African countries. The support will enable them to design training programs to increase expertise in Ebola, Lassa fever and other emerging viral diseases” (Fogarty International Center, 2016). Initiatives like these, in addition to developing expertise, would help foster trust and partnership between international research stakeholders and would contribute to more rapid implementation of clinical trials.
Developing a readily accessible set of documents for preparedness is not a new idea. A 2012 IOM report detailed a “toolkit” for public engagement in disaster response (IOM, 2012), the U.S. Department of Homeland Security has a Community Preparedness Toolkit to enhance community resilience in an emergency (DHS, 2007), and the WHO regional office in Europe has a toolkit to assess health systems capacity for crisis management (WHO Regional Office for Europe, 2012), while individual institutions have toolkits for clinical researchers interested in doing clinical research in the specific country, on a specific grant, or within a specific institution (National Institute for Health Research, 2017; NIAID, 2015; NIH, 2017; University of Wisconsin, 2017). Having a centralized repository of useful documents to be used on a global scale and linked through inter-epidemic research partnerships between global institutes would be valuable in the event of the next epidemic. The development of this document database paired with training in the application of these pertinent documents would contribute to building national capacity in low- and middle-income countries. The database could help kick-start the research planning phase into motion and focus discussion on the adaptation of generic documents and models to the specific circumstances of the epidemic, what the pathogen is, where it is, who is affected, and which tools should be implemented. Importantly, these templates are only useful if stakeholders know what the tools are, how to use them, and can access an infrastructure to facilitate their application—this is best achieved through training and partnerships with those researchers already well experienced and adept at international trials. The committee has not identified the central repository for this, although it seems to be a WHO function well within their capacity and mandate.
INTEGRATION OF RESEARCH INTO HEALTH SYSTEMS
Building capacity for research cannot—and should not—be separated from building health systems capacity in general; to be most effective clinical research needs to be embedded within the health care system while emphasizing its specialized nature and the need for well-trained practitio-
ners. Efforts to improve clinical research in the circumstances of an evolving epidemic setting are interdependent with efforts to improve the overall response, from the initial identification and reporting of emerging infection events, the care of patients and stopping transmission, and the approval and implementation of necessary research, all the way to obtaining regulatory approval for new therapeutics and vaccines—in short, the integration of health care, public health, and health research into a coordinated system with the close collaboration of national and international partners, and full engagement of the community at risk of the disease. Similar conclusions were drawn at a meeting in October 2015, sponsored by the Wellcome Trust, the WHO, the University of Oxford, and the Special Programme for Research and Training in Tropical Diseases (WHO, 2015b). The attendees concluded that research must be included as an “integral and essential component of epidemic preparedness and response,” that research should be “integrated with clinical care and public health responses,” and that mechanisms “should be established that facilitate efficient and effective joint working” (WHO, 2015b).
The clinical research efforts in the three countries have mobilized resources from the international sponsors to rehabilitate facilities, equip laboratories for clinical and epidemiological research, hire staff, and support salaries, maintain facilities, purchase research supplies and consumables, and upgrade technical and information technology capacity to handle data from collection to storage and on to analysis. Newly refurbished space is often located within deteriorating clinical facilities that remain in dire straits. The difference between the two is obvious, and it is equally obvious that the message it conveys is that research is important but patient care is not. Such observations are not new. In 2015, Daniel Bausch from the Tulane School of Public Health and Tropical Medicine in New Orleans, referring to research projects on Lassa and other hemorrhagic fever viruses in West Africa, noted that externally funded research projects “led to considerable upgrades in the laboratory infrastructure as well as advances in our understanding of Lassa fever, [but] NIH funding restrictions left little room to support patient care. I always felt bad when comparing the shiny new research and diagnostics laboratory at Kenema Government Hospital with the dilapidated, cramped, and poorly resourced Lassa ward only some 50 meters away” (Bausch, 2015, p. 230). Bausch also reflected on ongoing considerations of building a Lassa ward on the part of several international sponsors (European Union, the WHO, and the U.S. Department of Defense); however, frequent logistical issues resulted in lost funding and an unfinished ward in Kenema: “The unfinished shell of the new ward in Kenema still sits collecting rain, a testament to good intentions betrayed by the logistical, bureaucratic, and financial complexities of the world of development, although there are plans now to finally finish it off. . . . How
much better and safer might Ebola care have been in Kenema if these projects were seen through to completion?” (Bausch, 2015, p. 230).
To build the health care, public health, and health research systems countries will also need to enhance the quality of the health professional education that they can deliver locally. The committee’s visit to the A.M. Dogliotti School of Medicine campus in Monrovia showed how limited the educational resources were for the training of physicians; it is hard to imagine how graduates will be able to deliver competent clinical care for routine illnesses, respond to a crisis, and handle complex situations like the Ebola epidemic of 2014–2015, let alone participate in clinical research, except at the direction of well-trained researchers, primarily from the international community. With increased investments in health research strengthening in the three highly affected countries in West Africa focused on a few facilities and a few people, the divide in the quality and capacity of research facilities versus the quality and capacity of the health care and public health facilities is growing. In parallel, the striking limited capacity of health professional education, at least as observed in Liberia, constrains their ability to educate and train the next generation of health professionals to provide competent health care; these individuals also represent the national talent pool from which future health researchers and leaders will emerge.
Conclusion 5-6 When conducting research in settings with weak public health, clinical care, and health research infrastructure, efforts to strengthen research capacity without improving the general public health and clinical care infrastructure may inadvertently create the perception that research is more important than care of patients and will ultimately undermine the acceptance of clinical research by the population.
Recommendation 4
Ensure that capacity-strengthening efforts benefit the local population—Epidemic
When the health care services of a population need to be enhanced or augmented in order to support the conduct of research, development organizations (e.g., USAID), international bodies, and other stakeholders should partner with national governments to ensure that capacity-strengthening efforts are not limited to services that solely benefit study participants.
Because health is now recognized as one of the drivers of economic growth, the concept that improving health contributes to productivity and national wealth generation should be driving country investments to
improve the quality of the health care system (WHO, 2001). While there has been a considerable increase in national investment, supplemented by international assistance, the efforts are falling short of the need and often are not well targeted to maximize the impact. As Sir Nigel Crisp observed,
Even when African health systems’ visions are good, they are often poorly embedded within long-term economic growth plans; like most issues of social development, they have to compete with security and economic priorities of leaders who do not realize that their people’s health is central to the delivery of economic success. For these reasons, aid funding and the demands attached to it often bypass governments, resulting in aid that focuses on short-term projects rather than an overall resilient system. . . . African government’s expenditure on health is mainly spent on recurring costs, such as the training and education of health workers and their salaries, and capital costs such as hospitals, clinics, and transport. Aid funding is mostly focused on the delivery of services, providing life-saving vaccines and basic medicines, and to strengthen accountability processes for the projects they support. . . . The 2008 financial crisis in donor countries has compounded “donor fatigue” among donor countries and their citizens, resulting in the stagnation and reduction of aid to the most vulnerable countries, and increasingly in the combination of aid with trade and military interests which on many occasions prevents it from getting through to the countries that need it the most. (Crisp, 2016, p. 174)
In late 2016 the report of the High-Level Commission on Health Employment and Economic Growth was presented to the Secretary-General of the United Nations. The report argues that there is an urgent need for global investment in the health workforce in low- and lower-middle-income countries to prevent an estimated shortfall of 18 million health workers and maximize the social and economic benefits of improved health, global security, and economic growth (WHO, 2016c,d). Without new commitments from leadership in donor and recipient countries to invest in health systems, facilities, and staff in order to improve health care services and the health status of the population, it is likely that investments in health research systems will not produce the desired sustainable results and that those countries most at risk of emerging infectious disease outbreaks will be no more equipped to deal with the sudden clinical burden and the need to initiate critical research than Guinea, Liberia, and Sierra Leone were in 2014.
Recommendation 5
Enable the incorporation of research into national health systems—Inter-epidemic
National governments should strengthen and incorporate research systems into their emergency preparedness and response systems for
epidemic infectious diseases. The multilateral institutions (the World Health Organization and the World Bank Group), regional and international development agencies, and foundations working in global health, should support national efforts by providing expertise and financing.
Financing National Capacity Strengthening
This committee’s set of recommendations for actions to strengthen capacity for response and research is intended to provide the basis for cooperative initiatives and a rational partition of primary responsibility among national health authorities, the WHO, and other supranational and international partners involved in health care, public health, and research and development for therapeutics and vaccines, including the academic and private sectors; it is now up to these entities to seize the moment to engage and to invest the critical resources needed to strengthen capacity in low- and middle-income countries for the benefit of all in terms of creating national, regional, and global public goods. There is no doubt that a considerable investment in a sustainable manner will be required and that low-income countries have very limited ability to contribute their own funds to the effort; however, these countries still should be investing partners to claim co-ownership. The committee did not have the mandate or the resources to estimate the amount of funding necessary to get these initiatives off the ground. However, the Commission on a Global Health Risk Framework for the Future has provided an informed benchmark (NAM, 2016). The commission states, “[Their] analysis suggests that expected economic losses from potential pandemics could amount to around $60 billion per year. Implementing [their] recommendations, by contrast, would cost about $4.5 billion per year. This figure has three elements: the cost of upgrading public health systems in low- and middle-income countries, which [their] report puts close to $3.4 billion per year; the cost of enhancing the WHO’s pandemic prevention and response capabilities and of financing the WHO and World Bank contingency funds, which [they] assume to be $130 million to $155 million per year; and a proposed incremental investment in research and development of $1 billion per year” (Sands et al., 2016, p. 1284). What seems certain to us is that the actual options are to pay now and prepare in advance, or to pay later when an outbreak occurs, with the likelihood that the cost will be multiple times greater in the latter case.
Many global health initiatives have been financed through global joint funding cooperatives, ranging from the Global Alliance for Vaccines and Immunization on the product procurement side to the International AIDS Vaccine Initiative on the research side, with periodic replenishment of trust accounts to finance a well-thought-out 5-year strategic plan. Generally, these lack meaningful contributions by low-income countries and are sup-
ported by just a handful of high- and middle-income countries. However, there are funding models to finance development projects—which is the right way to characterize the effort to build a better, more comprehensive, and more stable health, public health, and health research system—that would engage the usual donors and the usual recipients in a new partnership. One prominent mechanism to accomplish this is overseen by the World Bank Group, which has recently created the Pandemic Emergency Financing Facility (PEF) to rapidly provide funding for the early response to an epidemic, with the goal of moving quickly to gain early and timely control. As the World Bank notes, “While outbreaks are inevitable, pandemics, if addressed early, are for the most part preventable. Money and support delivered at the right time can save lives and economies. . . . Yet as we saw in the recent Ebola crisis in West Africa, there is currently no fast-disbursing financial mechanism to make available significant funds to resource-constrained countries early enough to help them fight an epidemic outbreak that is escalating. Time and again, the world continues to follow the same pattern: money isn’t brought to the table until a major outbreak hits an explosive point. Without a strong system in place, the world will simply continue to move from crisis to crisis” (World Bank, 2016a). The PEF is organized to provide funding when a potentially pandemic outbreak occurs that meets the threshold criteria for activation in one of the 77 poorest countries eligible for financing from IDA (the International Development Association component of the World Bank Group), which can also “provide funding to qualified international agencies involved in the response to a major outbreak in affected countries” (World Bank, 2016a). Very briefly, IDA provides grants or loans at zero or near-zero interest rates, with repayment of the principal stretched out over 25–40 years (Mcgroarty et al., 2015). In addition to taking on IDA funding, there is a necessary level of country engagement across sectors; generally the ministry of finance for an IDA-eligible country’s government must request the funding and designate its purpose. This means that a health and health research system investment must compete internally for funding with roads, bridges, industrial development, and other national development and investment priorities, requiring the development and articulation of a country strategy to justify the government’s choices. What is important is that this represents an internal government-led initiative, in contrast to a donor-specified agenda.
The World Bank Group has other mechanisms of direct relevance to the funding of these initiatives in the form of direct investment projects, including regional (multicountry) investment projects. One such example recently agreed upon is the West Africa REDISSE project. This was developed with financial support from the Bill & Melinda Gates Foundation and technical support from the WHO and the CDC to “address systemic weaknesses within the human and animal health sectors that hinder effective disease
surveillance and response” (World Bank, 2016b). Under REDISSE I, Guinea and Sierra Leone have each been approved for $30 million in funding, along with $30 million for Senegal, which the World Bank says
has shown regional leadership in developing effective disease detection and response capacity. . . . In addition, the West African Health Organization will receive $20 million from IDA and $4 million in trust fund co-financing from the government of Canada to help improve disease surveillance infrastructure, information sharing, and collaboration across the 15 [member] countries that [make up] the Economic Community of West African States (ECOWAS) and across the health, agriculture, and environmental sectors. (World Bank, 2016b)
In addition to making IDA funds available to individual countries that decide to take the opportunity to improve their capacity to respond to another emerging infectious diseases outbreak, the committee strongly supports the development of regional capacity. It is essential to have mechanisms for supporting countries and their governments during inter-epidemic as well as epidemic periods in their efforts to improve the ability of both regional and national systems to respond to epidemic diseases and to provide the necessary infrastructure to support the health research system. The program is expanding, with the announcement of REDISSE II, which will engage Guinea Bissau, Liberia, Nigeria, and Togo, and with plans for REDISSE III to expand to other ECOWAS member states to ensure that by the end of 2017 countries bordering the original nations (Benin, Burkina Faso, Ivory Coast, and Ghana) will be included and will have the means for cross-border collaboration and exchange (PaulClark, 2017). Similar regional investment projects exist in other regions of the world, funded by the World Bank Group or by the regional development banks (African Development Bank, Asian Development Bank, etc.).
Substantial sums of money are involved in the effort to strengthen national health systems, and yet they may not be sufficient to bootstrap the nations of the world to uniformly reach or exceed a minimum standard of capacity under IHR 2005 and to be capable of partnering in clinical research on epidemic infectious diseases. The Ebola epidemic of 2014–2015 demonstrated that a partnership of affected nations and the international community can meet the challenge, but with serious consequences in loss of life, disruption of society, and economic losses. As a result of efforts by the IMC, MSF, and others that provided the initial health care response and also as a result of the subsequent engagement of the larger global community, the epidemic was brought to a close, but not before 11,350 people in 10 countries died. It could have been worse, however, which is why infrastructure in vulnerable countries should be improved—so that, with their participation, insights into the disease and clues to therapeutic and
vaccine development gleaned from clinical research can be applied so that countries and international organizations are better prepared to act quickly with better tools the next time there is an outbreak of Ebola.
Whether the recent experience will prove to be a strong enough stimulus for sustained action to elevate the clinical research agenda and national capacity in at-risk countries, as the concern about Ebola no longer raises the specter of pandemic spread, displaced by other crises, remains to be seen. In addition to identifying the funds and partnerships needed, the committee is aware that it has not addressed—nor did it have the requisite expertise to address—the financial management systems, procurement, and logistics required to ensure that the relatively large amounts of funding for clinical research provided through international funders, including the global research institutions, multinational organizations, foundations, and other consortia, are used as intended in efficient and accountable ways. External partners can help by aligning and harmonizing their efforts to strengthen local governance, management, and accountability systems, rather than having donor-specific requirements that further fragment and duplicate efforts to build and use capacity within low- and middle-income countries.
Since most international donors have their own expectations of financial systems to account for grant funds it is important that capacity strengthening is extended to acquiring expertise in these financial systems such that grant or investment funds are managed impeccably, with safeguarding audit processes that allow monies also to be controlled nationally rather than exclusively externally and internationally. It would not hurt if these systems were also harmonized and the burden of effort was on managing the funds properly and not on reporting to different donors using different tools with too little administrative support; it is up to the partner institutions to make this happen (in line with the Paris harmonization agenda6).
REFERENCES
Abayomi, A., R. Katz, S. Spence, B. Conton, and S. M. Gevao. 2016. Managing dangerous pathogens: Challenges in the wake of the recent West African Ebola outbreak. Global Security: Health, Science and Policy 1(1):51–57.
AfHRF (African Health Research Forum). 2014. African Health Research Forum Fellowship Program: Module 5: National health research systems. http://www.ccghr.ca/resources/mentorship-and-leadership/afhrf-leadership-development-learning-modules (accessed January 23, 2017).
___________________
6 The Paris Declaration (2005) is a practical, action-oriented roadmap to improve the quality of aid and its impact on development. It gives a series of specific implementation measures and establishes a monitoring system to assess progress and ensure that donors and recipients hold each other accountable for their commitments (OECD, 2016).
Aidam, J., and I. Sombié. 2016. The West African Health Organization’s experience in improving the health research environment in the ECOWAS region. Health Research Policy and Systems 14:30.
Aizenman, N. 2016. Scientists say it’s time to end “parachute research.” http://www.npr.org/sections/goatsandsoda/2016/04/02/472686809/scientists-say-its-time-to-end-parachuteresearch (accessed February 20, 2017).
Akanmori, B., A. Bellah, M. Ward, and L. Rägo. 2015. Regulatory collaboration. WHO Drug Information 29(2):127–132.
Ali, R., and A. E. Finlayson, on behalf of the INDOX Cancer Research Network. 2012. Building capacity for clinical research in developing countries: The INDOX cancer research network experience. Global Health Action 5(17288).
Alliance for Health Policy and Systems Research. 2004. Strengthening health systems: The role and promise of policy and systems research. Geneva, Switzerland: Alliance for Health Policy and Systems Research. http://www.who.int/alliance-hpsr/resources/Strengthening_complet.pdf (accessed January 23, 2017).
Anderson, E.-L., and A. Beresford. 2016. Infectious injustice: The political foundations of the Ebola crisis in Sierra Leone. Third World Quarterly 37(3):468–486.
Associated Press. 2015a. 10 critical mistakes in last year’s Ebola outbreak. http://www.businessinsider.com/ap-10-critical-mistakes-in-last-years-ebola-outbreak-2015-9 (accessed February 20, 2017).
Associated Press. 2015b. Emails: UN health agency resisted declaring Ebola emergency. http://www.dailymail.co.uk/wires/ap/article-3003593/Emails-UN-health-agency-resisted-declaring-Ebola-emergency.html (accessed February 15, 2017).
Bausch, D. G. 2015. The year that Ebola virus took over West Africa: Missed opportunities for prevention. American Journal of Tropical Medicine and Hygiene 92(2):229–232.
BBC (British Broadcasting Corporation). 2014. Ebola crisis: Sierra Leone health workers strike. http://www.bbc.com/news/world-africa-30019895 (accessed January 23, 2017).
Bhatt, A. 2011. Quality of clinical trials: A moving target. Perspectives in Clinical Research 2(4):124–128.
Bolkan, H. A., D. A. Bash-Taqi, M. Samai, M. Gerdin, and J. von Schreeb. 2014. Ebola and indirect effects on health service function in Sierra Leone. PLoS Current Outbreaks 6.
Brolin Ribacke, K. J., D. D. Saulnier, A. Eriksson, and J. von Schreeb. 2016. Effects of the West Africa Ebola virus disease on health-care utilization—A systematic review. Frontiers in Public Health 4:222.
Camara, O. 2015. Guinea labor unions begin general strike over wage demands. https://www.bloomberg.com/news/articles/2015-01-05/guinea-labor-unions-begin-general-strike-overwage-demands (accessed January 23, 2017).
Capital Reporting Company. 2015. Transcripts - Clinical Trial Designs for Emerging Infectious Diseases Workshop. Paper read at FDA Clinical Trail Designs for Emerging Infectious Diseases, Bethesda, MD.
CDC (U.S. Centers for Disease Control and Prevention). 2014. The public health system and the 10 essential public health services. https://www.cdc.gov/nphpsp/essentialservices.html (accessed February 15, 2017).
Cheng, M., R. Satter, and K. Larson. 2015. AP investigation: Too few body bags and bad chlorine marred WHO Ebola response in Sierra Leone. http://www.usnews.com/news/world/articles/2015/09/21/ap-investigation-bungling-by-un-agency-hurt-ebola-response (accessed January 23, 2017).
COHRED (Council on Health Research for Development). 2012. RHInnO (Research for Health and Innovation Organiser): Your research management process at your fingertips. Geneva, Switzerland: COHRED. http://www.cohred.org/wp-content/uploads/2012/05/RHInnO.pdf
COHRED. 2016. The WAHO and COHRED collabortation to facilitate reserach for health in West Africa. http://www.cohred.org/2013/05/the-waho-and-cohred-collaboration-to-facilitate-research-for-health-in-west-africa (accessed January 23, 2017).
Commission on Health Research for Development. 1990. Health reserach: Essential link to equity in development. Cambridge, MA: Oxford University Press.
Crisp, N. 2016. One World Health: An overview of global health. Boca Raton, FL: CRC Press.
Davey, R., M. Proschan, J. Beigel, and L. Dodd. 2016. Background and performance of The MCM RCT (PREVAIL* II): A randomized controlled trial of ZMappTM in acute Ebola virus infection. Presentation at the Public Workshop of the Committee on Clinical Trials During the 2014–2015 Ebola Outbreak. Washington, DC; June 13–15, 2016.
Desmarais, S. 2016. Eradicating polio in Nigeria. http://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/eradicating-polio-in-nigeria (accessed February 16, 2017).
DHS (U.S. Department of Homeland Security). 2007. Community Preparedness Toolkit. https://www.ready.gov/community-preparedness-toolkit (accessed February 20, 2017).
DiMasi, J. A., H. G. Grabowski, and R. W. Hansen. 2016. Innovation in the pharmaceutical industry: New estimates of R&D costs. Journal of Health Economics 47:20-33.
Djénab, S. N. 2016. Experiences and lessons learned. Presentation at the Public Workshop of the Committee on Clinical Trials During the 2014–2015 Ebola Outbreak. Monrovia, Liberia; August 15–16, 2016.
Eckstein, S. 2004. Efforts to build capacity in research ethics: An overview. http://www.scidev.net/global/policy-brief/efforts-to-build-capacity-in-research-ethics-an-ov.html (accessed January 27, 2017).
Edwards, D., J. Toohey, and C. IJsselmuiden. 2014. Negotiating research contracts. Geneva, Switzerland: Council on Health Research for Development. http://www.cohred.org/wp-content/uploads/2014/06/COHRED-negotiationbookletv-web.pdf (accessed January 23, 2017).
European Medicines Agency. 2017. Ebola. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000624.jsp (accessed February 15, 2017).
Evans, D. K., M. Goldstein, and A. Popova. 2015. Health-care worker mortality and the legacy of the Ebola epidemic. The Lancet Global Health 3(8):e439–e440.
Evans, J. R. 1990. Essential national health research. New England Journal of Medicine 323(13):913–915.
FDA (U.S. Food and Drug Administration). 2016. MCM-related cooperative arrangements. http://www.fda.gov/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/MCMLegalRegulatoryandPolicyFramework/ucm465378.htm (accessed January 23, 2017).
Fogarty International Center. 2016. Fogarty provides support to Ebola-affected countries. https://www.fic.nih.gov/News/GlobalHealthMatters/november-december-2016/Pages/research-capacity-in-countries-affected-by-ebola.aspx (accessed February 20, 2017).
Formenty, P., C. Hatz, B. Le Guenno, A. Stoll, P. Rogenmoser, and A. Widmer. 1999. Human infection due to Ebola virus, subtype Côte d’Ivoire: Clinical and biologic presentation. Journal of Infectious Diseases 179(Suppl. 1):S48–S53.
Georgetown Journal of International Affairs. 2014. NGOs, Ebola, and the future of civil actors in international politics: Five minutes with Sam Worthington. http://journal.georgetown.edu/ngos-ebola-and-the-future-of-civil-actors-in-international-politics-fiveminutes-with-sam-worthington (accessed February 25, 2017).
Gostin, L. O., and R. Katz. 2016. The International Health Regulations: The governing framework for global health security. Georgetown Law Faculty Publications and Other Works. http://scholarship.law.georgetown.edu/facpub/1770 (accessed February 15, 2017).
Gray, K. A. 2015. How to conduct a clinical trial during a disease outbreak. https://blog.wellcome.ac.uk/2015/08/11/how-to-conduct-a-clinical-trial-during-a-disease-outbreak (accessed January 23, 2017).
Hayden, E. C. 2015. Ebola outbreak thrusts MSF into new roles. Nature 522:18–19.
Heymann, D. L., J. Liu, and L. Lillywhite. 2016. Partnerships, not parachutists, for Zika research. New England Journal of Medicine 374(16):1504–1505.
House of Commons International Development Committee. 2016. Ebola: Responses to a public health emergency. Second Report of Session 2015–16. London, UK: House of Commons. https://www.publications.parliament.uk/pa/cm201516/cmselect/cmintdev/338/338.pdf (accessed February 15, 2017).
IOM (Institute of Medicine). 2012. Crisis standards of care: A systems framework for catastrophic disaster response: Volume 1: Introduction and CSC framework. Washington, DC: The National Academies Press.
IOM and NRC (National Research Council). 2009. Sustaining global surveillance and response to emerging zoonotic diseases. Washington, DC: The National Academies Press.
Ivers, L. C., J. E. Teng, J. Lascher, M. Raymond, J. Weigel, N. Victor, J. G. Jerome, I. J. Hilaire, C. P. Almazor, R. Ternier, J. Cadet, J. Francois, F. D. Guillaume, and P. E. Farmer. 2013. Use of oral cholera vaccine in Haiti: A rural demonstration project. American Journal of Tropical Medicine and Hygiene 89(4):617–624.
Kamal-Yanni, M. 2015. Never again: Building resilient health systems and learning from the Ebola crisis. Oxfam Briefing Paper No. 203. Oxford, England: Oxfam International. https://www.oxfam.org/sites/www.oxfam.org/files/file_attachments/bp-never-again-resilient-health-systems-ebola-160415-en.pdf (accessed January 23, 2017).
Kassalow, J. S. 2001. Why health is important to U.S. foreign policy. Council on Foreign Relations. http://www.cfr.org/world/why-health-important-us-foreign-policy/p8315 (accessed February 15, 2017).
Kasule, M., D. R. Wassenaar, C. IJsselmuiden, and B. Mokgatla. 2016. Silent voices: Current and future roles of African Research Ethics Committee administrators. IRB: Ethics & Human Research 38(1):13–19.
Katz, R., and S. F. Dowell. 2015. Revising the International Health Regulations: Call for a 2017 review conference. The Lancet Global Health 3(7):e352–e353.
Kennedy, S. B., A. O. Harris, E. Oudemans, L. Young, J. Kollie, E. S. Nelson, R. A. Nisbett, C. Morris, N. Bartee, E. George-Williams, and J. Jones. 2006. Developing capacity to protect human research subjects in a post-conflict, resource-constrained setting: Procedures and prospects. Journal of Medical Ethics 32(10):592–595.
Lang, T. 2015. Ebola: Embed research in outbreak response. Nature 524(7563):29–31.
Lang, T., and S. Siribaddana. 2012. Clinical trials have gone global: Is this a good thing? PLoS Medicine 9(6):e1001228.
Levine, A. C. 2016. Academics are from Mars, humanitarians are from Venus: Finding common ground to improve research during humanitarian emergencies. Clinical Trials 13(1):79–82.
Lurie, N., T. Manolio, A. P. Patterson, F. Collins, and T. Frieden 2013. Research as a part of public health emergency response. New England Journal of Medicine 368(13):1251–1255.
Marshall, T. 2016. Public health experts conclude research conducted at heart of West Africa’s Ebola outbreak can provide valuable guidance in preparing for future emergencies. https://internationalmedicalcorps.org/research-conducted-at-heart-of-west-africas-ebola-outbreak-can-provide-valuable-guidance (accessed January 23, 2017).
Mcgroarty, S., C. E. Rossel, and J. M. Mcfadden. 2015. The World Bank Group A to Z 2016. Washington, DC: World Bank Group. http://documents.worldbank.org/curated/en/896931468189267370/The-World-Bank-Group-A-to-Z-2016 (accessed February 15, 2017).
McNamara, L. A., I. J. Schafer, L. D. Nolen, Y. Gorina, J. T. Redd, T. Lo, E. Ervin, O. Henao, B. A. Dahl, O. Morgan, S. Hersey, and B. Knust. 2016. Ebola surveillance—Guinea, Liberia, and Sierra Leone. Morbidity and Mortality Weekly Report 66(Suppl 3):35–43.
Millum, J., C. Grady, G. Keusch, and B. Sina. 2013. Introduction: The Fogarty International Research Ethics Education and Curriculum Development Program in historical context. Journal of Empirical Research on Human Research Ethics 8(5):3–16.
MSF (Médecins Sans Frontières). 2016. MSF supported research on Ebola. Brussels, Belgium: MSF. https://www.doctorswithoutborders.org/sites/usa/files/msf_ebola_research_en_web.pdf (accessed January 27, 2017).
Musolino, N., J. Lazdins, J. Toohey, and C. IJsselmuiden. 2015. COHRED Fairness Index for international collaborative partnerships. The Lancet 385(9975):1293–1294.
NAM (National Academy of Medicine). 2016. The neglected dimension of global security: A framework to counter infectious disease crises. Washington, DC: The National Academies Press.
National Institute for Health Research. 2017. Clinical trials toolkit. http://www.ct-toolkit.ac.uk (accessed February 20, 2017).
NIAID (National Institute of Allergy and Infectious Diseases). 2015. Trans NIAID clinical research toolkit. https://www.niaid.nih.gov/research/trans-niaid-clinical-research-toolkit (accessed February 20, 2017).
NIAID. 2017. Putative investigational therapeutics in the treatment of patients with known Ebola infection. https://clinicaltrials.gov/ct2/show/NCT02363322 (accessed January 15, 2017).
NIH (U.S. National Institutes of Health). 2016. ClinRegs. https://clinregs.niaid.nih.gov/index.php (accessed January 27, 2017).
NIH. 2017. Toolkit resource. https://www.nhlbi.nih.gov/research/funding/research-support/crg/toolkit-resources.htm (accessed February 20, 2017).
OECD (Organisation for Economic Co-operation and Development). 2016. Paris Declaration and Accra Agenda for Action. https://www.oecd.org/dac/effectiveness/parisdeclarationandaccraagendaforaction.htm (accessed February 15, 2017).
O’Hearn, A. E., M. A. Voorhees, D. P. Fetterer, N. Wauquier, M. R. Coomber, J. Bangura, J. N. Fair, J.-P. Gonzalez, and R. J. Schoepp. 2016. Serosurveillance of viral pathogens circulating in West Africa. Virology Journal 13:163.
OHRP (U.S. Office of Human Research Protections). 2017a. Institutional review board guidebook. https://archive.hhs.gov/ohrp/irb/irb_guidebook.htm (accessed February 20, 2017).
OHRP. 2017b. International Compilation of Human Research Standards 2017 Edition. https://www.hhs.gov/ohrp/sites/default/files/internationalcomp2017-part2.pdf (accessed February 20, 2017).
Osterholm, M. T., K. A. Moore, and L. O. Gostin. 2015. Public health in the age of Ebola in West Africa. JAMA Internal Medicine 175(1):7–8.
Pablos-Mendez, A. 2014. Combating the Ebola threat. http://docs.house.gov/meetings/FA/FA16/20140807/102607/HHRG-113-FA16-Wstate-Pablos-MndezA-20140807.pdf (accessed February 15, 2017).
PaulClark, J. 2017. Appraisal project information document-integrated safeguards data sheet - Regional Disease Surveillance Systems Enhancement (REDISSE) Phase II - P159040. Washington, DC: World Bank Group. http://documents.worldbank.org/curated/en/765151485797373436/Appraisal-Project-Information-Document-IntegratedSafeguards-Data-Sheet-Regional-Disease-Surveillance-Systems-Enhancement-REDISSE-Phase-II-P159040 (accessed February 15, 2017).
Pfeiffer, J., W. Johnson, M. Fort, A. Shakow, A. Hagopian, S. Gloyd, and K. Gimbel-Sherr. 2008. Strengthening health systems in poor countries: A code of conduct for nongovernmental organizations. American Journal of Public Health 98(12):2134–2140.
Roshania, R., M. Mallow, N. Dunbar, D. Mansary, P. Shetty, T. Lyon, K. Pham, M. Abad, E. Shedd, A.-M. A. Tran, S. Cundy, and A. C. Levine. 2016. Successful implementation of a multicountry clinical surveillance and data collection system for Ebola virus disease in West Africa: Findings and lessons learned. Global Health: Science and Practice 4(3):394–409.
Salu, O. B., A. B. James, B. O. Oke, M. R. Orenolu, R. A. Anyanwu, M. A. Abdullah, C. Happi, J. Idris, I. A. Abdus-Salam, A.-S. Nasidi, F. T. Ogunsola, O. Tomori, and S. A. Omilabu. 2016. Biosafety level-2 laboratory diagnosis of Zaire Ebola virus disease imported from Liberia to Nigeria. African Journal of Laboratory Medicine 5(1).
Sands, P., C. Mundaca-Shah, and V. J. Dzau. 2016. The neglected dimension of global security—A framework for countering infectious-disease crises. New England Journal of Medicine 374(13):1281–1287.
Saxena, A., and M. Gomes. 2016. Ethical challenges to responding to the Ebola epidemic: The World Health Organization experience. Clinical Trials 13(1):96–100.
Schopper, D., R. Ravinetto, L. Schwartz, E. Kamaara, S. Sheel, M. J. Segelid, A. Ahmad, A. Dawson, J. Singh, A. Jesani, and R. Upshur. 2016. Research ethics governance in times of Ebola. Public Health Ethics 1–13.
Scott, D. 2016. Millions in Ebola funding, a casualty of Zika virus, may not be replenished. https://www.statnews.com/2016/06/01/ebola-zika-virus-funding (accessed February 16, 2017).
Sombié, I., J. Aidam, B. Konaté, T. D. Somé, and S. S. Kambou. 2013. The state of the research for health environment in the ministries of health of the Economic Community of the West African States (ECOWAS). Health Research Policy and Systems 11(1):35.
Suk, J. E., A. P. Jimenez, M. Kourouma, T. Derrough, M. Baldé, P. Honomou, N. Kolie, O. Mamadi, K. Tamba, K. Lamah, A. Loua, T. Mollet, M. Lamah, A. N. Camara, and V. Prikazsky. 2016. Post-Ebola measles outbreak in Lola, Guinea, January–June 2015. Emerging Infectious Diseases 22(6):1106–1108.
Taddonio, P. 2015. Outbreak: Inside the troubled early days of Guinea’s Ebola response. PBS Frontline, May 5. http://www.pbs.org/wgbh/frontline/article/watch-inside-the-troubled-early-days-of-guineas-ebola-response (accessed January 11, 2017).
Takahashi, S., C. J. E. Metcalf, M. J. Ferrari, W. J. Moss, S. A. Truelove, A. J. Tatem, B. T. Grenfell, and J. Lessler. 2015. The growing risk from measles and other childhood infections in the wake of Ebola. Science 347(6227):1240–1242.
Telegraph. 2014. Liberia health workers strike over Ebola “danger money.” http://www.telegraph.co.uk/news/worldnews/ebola/11159217/Liberia-health-workers-strike-over-Ebola-danger-money.html (accessed January 23, 2017).
Tugwell, P., C. Sitthi-Amorn, J. Hatcher-Roberts, V. Neufeld, P. Makara, F. Munoz, P. Czerny, V. Robinson, Y. Nuyens, and D. Okello. 2006. Health research profile to assess the capacity of low- and middle-income countries for equity-oriented research. BMC Public Health 6:151.
UC Regents. 2017. A quick guide to material transfer agreements at UC Berkeley. http://www.spo.berkeley.edu/guide/mtaquick.html (accessed February 16, 2017).
United Nations. 1995. Suspect cases of Ebola in Liberia: Press release H/2888. http://www.un.org/press/en/1995/19951211.h2888.html (accessed February 15, 2017).
University of Wisconsin. 2017. Clinical research toolkit. https://ictr.wisc.edu/clinical-research-toolkit (accessed February 20, 2017).
Viboud, C., and L. Simonsen. 2012. Global mortality of 2009 pandemic influenza A H1N1. The Lancet Infectious Diseases 12(9):651–653.
Watson-Jones, D. 2016. The Ebola outbreak: The challenge of establishing a clinical trial during an epidemic. http://www.clinicaltrialsarena.com/news/operations/the-ebola-outbreak-the-challenge-of-establishing-a-clinical-trial-during-an-epidemic-4925139 (accessed February 15, 2017).
Wellcome Trust. 2015. New trial of TKM-Ebola treatment to start in Sierra Leone. https://wellcome.ac.uk/press-release/new-trial-tkm-ebola-treatment-start-sierra-leone (accessed December 21, 2016).
WHO (World Health Organization). 2001. Macroeconomics and health: Investing in health for economic development: Report of the Commission on Macroeconomics and Health. Geneva, Switzerland: WHO. http://www1.worldbank.org/publicsector/pe/PEAMMarch2005/CMHReport.pdf (accessed January 27, 2017).
WHO. 2002. National health research systems: Report of an international workshop. Geneva, Switzerland: WHO. http://www.who.int/rpc/summit/documents/en/national_health_research_systems.pdf (accessed February 15, 2017).
WHO. 2005. International Health Regulations (2005), third ed. Geneva, Switzerland: WHO Press, World Health Organization.
WHO. 2014. Barriers to rapid containment of the Ebola outbreak. http://www.who.int/csr/disease/ebola/overview-august-2014/en (accessed January 23, 2017).
WHO. 2015a. Factors that contributed to undetected spread of the Ebola virus and impeded rapid containment. http://www.who.int/csr/disease/ebola/one-year-report/factors/en (accessed January 27, 2017).
WHO. 2015b. Generating evidence for infectious diseases with epidemic potential. Meeting report, October 20, 2015, London, UK. Jointly organized by the World Health Organization, the Wellcome Trust, the University of Oxford, and the Special Programme for Research and Training in Tropical Diseases. http://www.who.int/medicines/ebola-treatment/meetings/MeetingReport_GEIDEP.pdf (accessed January 23, 2017).
WHO. 2015c. Health workforce: Density of physicians (total number per 1,000 population). http://gamapserver.who.int/gho/interactive_charts/health_workforce/PhysiciansDensity_Total/atlas.html (accessed January 23, 2017).
WHO. 2015d. WHO removes Nigeria from polio-endemic list. http://www.who.int/mediacentre/news/releases/2015/nigeria-polio/en (accessed February 16, 2017).
WHO. 2016a. Atlas of African health statistics 2016: Health situation analysis of the African region. Brazzaville, Congo: Africa Regional Office, WHO. https://www.aho.afro.who.int/sites/default/files/publications/5266/Atlas-2016-en.pdf (accessed January 27, 2017).
WHO. 2016b. The health of the people: What works. Chapter 6: Improving access to health care. http://www.who.int/bulletin/africanhealth2014/improving_access_to_health_care/en (accessed January 23, 2017).
WHO. 2016c. Making health systems resilient to changing needs and threats must be a top priority, says PAHO director. http://www.paho.org/hq/index.php?option=com_content&view=article&id=12755%3Amaking-health-systems-resilient-changing-needs-threats-top-priority-paho-director&Itemid=135&lang=en (accessed January 23, 2017).
WHO. 2016d. Working for health and growth: Investing in the health workforce. Geneva, Switzerland: WHO Press. http://apps.who.int/iris/bitstream/10665/250047/1/9789241511308-eng.pdf?ua=1 (accessed January 27, 2017).
WHO. 2017. National and regional surveillance systems. http://www.who.int/csr/disease/ebola/health-systems-recovery/surveillance/en (accessed February 20, 2017).
WHO Regional Office for Europe. 2012. Strengthening health-system emergency preparedness: Toolkit for assessing health-system capacity for crisis management. Copenhagen, Denmark: The Regional Office for Europe of the World Health Organization. http://www.euro.who.int/__data/assets/pdf_file/0008/157886/e96187.pdf?ua=1 (accessed February 20, 2017).
Widdowson, M., S. Schrag, R. Carter, W. Carr, J. Legardy-Williams, L. Gibson, D. R. Lisk, M. I. Jalloh, D. A. Bash-Taqi, S. A. Sheku Kargbo, A. Idriss, G. F. Deen, J. B.W. Russell, W. McDonald, A. P. Albert, M. Basket, A. Callis, V. M. Carter, K. R. Clifton Ogunsanya, J. Gee, R. Pinner, B. E Mahon, S. T. Goldstein, J. F. Seward, M. Samai, and A. Schuchat. 2016. Implementing an Ebola vaccine study—Sierra Leone. Morbidity and Mortality Weekly Report 65(Suppl 3):98–106.
Williams, S., J. Fitzner, A. Merianos, and A. Mounts. 2011. The challenges of global case reporting during pandemic A(H1N1) 2009. Bulletin of the World Health Organization 92:60–67. Geneva, Switzerland: WHO Press.
WMA (World Medical Association). 2013. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194.
World Bank. 2016a. Pandemic emergency facility: Frequently asked questions. http://www.worldbank.org/en/topic/pandemics/brief/pandemic-emergency-facility-frequently-asked-questions (accessed February 15, 2107).
World Bank. 2016b. World Bank contributes to improved disease surveillance and health systems in West Africa following Ebola epidemic. http://www.worldbank.org/en/news/press-release/2016/06/29/world-bank-contributes-to-improved-disease-surveillance-andhealth-systems-in-west-africa-following-ebola-epidemic (accessed February 15, 2017).
Wright, S., L. Hanna, and M. Mailfert. 2015. A wake-up call: Lessons from Ebola for the world’s health systems. London, UK: Save the Children. https://www.savethechildren.net/sites/default/files/libraries/WAKE%20UP%20CALL%20REPORT%20PDF.pdf (accessed January 23, 2017).
This page intentionally left blank.












































