1
Introduction
Ebola has been known since 1976 when two outbreaks occurred, one in the Democratic Republic of the Congo, then known as Zaire, and the other in what is now South Sudan (Johnson et al., 1977). Ebola is a serious illness, transmitted from person to person by direct contact with infected body fluids, with a high mortality rate even with good clinical care (see Box 1-1). Until 2014, however, previous outbreaks had been limited in size and duration, occurring in relatively isolated communities in Central Africa and Uganda, with at most a few hundred cases and deaths in each outbreak but no cross-border or international spread (CDC, 2016c). Much was learned about the virus from previous outbreaks, but there was little knowledge of case management or the clinical sequelae among survivors because these outbreaks affected a limited number of individuals and were contained in isolated settings. The opportunity to make clinical observations was therefore restricted. Research on drugs and vaccines for Ebola was accordingly also limited in support and scope; the research was primarily focused on early preclinical development and was not a particularly high priority for the major international medical research organizations, including military health research institutes in the United States and elsewhere, or for the pharmaceutical industry (Burki, 2011). There was, nonetheless, some steady progress, including nonhuman primate challenge studies on investigational therapeutic agents and vaccines and also some very limited human Phase 1 studies of vaccine candidates at the beginning of 2014 (Gebre et al., 2014). Because of the nature of the disease, there was no way to create an experimental human infection model to test the efficacy of these products. Testing these products would require that a natural outbreak of
sufficient size and duration occurred and that researchers could quickly mobilize products, protocols, and participants and implement clinical trials before the outbreak came under control and transmission was halted. And no one knew when, where, or whether this situation would present itself—until the epidemic of 2014–2015.
Conducting clinical research can often seem secondary to addressing the immediate health needs of patients, if not a distraction and unnecessary impediment to public health control activities and patient care in the midst of a public health emergency. However, research is an essential component to epidemic response, as it is the only way to learn how to improve care for current and future patients and to potentially prevent an epidemic from occurring again (Lurie et al., 2013). This is especially true for a disease like Ebola because there were no proven safe and effective therapeutic products or vaccines when the epidemic began. An epidemic, despite the often chaotic environment, is an opportunity to test the efficacy of vaccines and therapeutics that are currently in development. If research quickly reveals a safe and effective therapeutic agent or one that is superior to any available at the time, current patients could reap the benefits as soon as the interven-
tion could be approved, made available, and delivered to those in need. The discovery of a safe and effective vaccine could protect people who are at immediate risk of infection as well as prevent future generations from contracting the disease.
There are established principles for conducting scientifically sound, ethical clinical research. For many years these have been reviewed and refined for the international context, in particular when research is sponsored by high-income countries for conduct in low-income countries. For example, the Declaration of Helsinki states that researchers must obtain the “freely given informed consent” of research subjects and that medical research “must conform to generally accepted scientific principles” and “be preceded by careful assessment of the predictable risks and burdens to the individuals and groups involved” (WMA, 2013, p. 2192). During an epidemic some of the standard practices of research may need to be accelerated or modified in order to work in the specific context of the community and disease. For example, informed consent procedures may need to be sped up or abbreviated, or consent by proxy may be deemed appropriate in situations in which patients are not able to give consent. However, as discussed in more detail in Chapter 2, the core ethical and scientific principles of research must undergird efforts even in the midst of an epidemic. Doing so helps to ensure that research can “quickly and definitively determine the safety and efficacy of interventions and thus provide access for the greatest number of patients to the most effective therapies in the shortest possible time” (Lane et al., 2016, p. 2). In addition, adhering to ethical principles such as “respect for volunteers and study communities, the value of informed consent, and the need for collaborative partnership with affected communities” helps to ensure that affected communities are not exploited and that the researchers gain the trust and buy-in of the community (Lane et al., 2016, p. 2).
Every epidemic is different in terms of the communities affected, the transmission and mortality rates, and the availability of potential treatments (KFF, 2014). The 2014–2015 Ebola epidemic was different from all previous Ebola outbreaks—it was unpredictable and fast moving, crossed borders, affected large numbers of people, was highly deadly, and was exacerbated by the lack of local experience, resources, infrastructure, and the limited number of experienced researchers (Heymann and Wertheimer, 2014). However, the issues raised by conducting research in the midst of the epidemic were not unique to Ebola. The same scientific and ethical questions have arisen in various other epidemics, ranging from the HIV/ AIDS epidemic in the early 1980s to the current Zika epidemic (Deloffre, 2016; Wainberg et al., 2014). Much can be learned from prior debates about conducting research during public health emergencies, in resource-poor settings, or among a population of desperately ill patients. Researchers should not consider each epidemic to be sui generis; rather, the response to
each new epidemic should take advantage of the fact that various epidemics have common elements and build upon the knowledge gained from previous experiences.
CHARGE TO THE COMMITTEE AND STUDY SCOPE
In October 2015, the Office of the Assistant Secretary for Preparedness and Response, the National Institute of Allergy and Infectious Diseases, and the U.S. Food and Drug Administration of the U.S. Department of Health and Human Services charged the National Academies of Sciences, Engineering, and Medicine (the National Academies) with developing a consensus report that explores and analyzes scientific and ethical issues related to clinical trial design, conduct, and reporting. The sponsors stipulated that particular emphasis be given to clinical trials for investigational therapeutic and vaccine candidates for Ebola conducted by the international community in settings where there is limited health care and research infrastructure, focusing on research conducted in Guinea, Liberia, and Sierra Leone during the 2014–2015 epidemic. The information and analysis presented in this report is meant to inform guidelines and best practices for clinical trials of therapeutics and vaccines conducted in response to future outbreaks and epidemics in low-resource settings (see Box 1-2 for the full Statement of Task).
To respond to this charge, the National Academies convened a 16-member ad hoc committee composed of experts from a range of disciplines. Members of the committee have expertise in clinical trial conduct and design, biostatistics, infectious disease, public health, health systems, and bioethics as well as considerable experience working in low- and middle-income countries. The committee included members from Africa, Europe, and North America.
STUDY METHODOLOGY
The committee deliberated from February to November 2016, during which time it held three 3-day public workshops in Washington, DC; London; and Monrovia; one 2-hour public webinar; and three 2-day closed meetings. The committee also solicited and considered written statements from stakeholders and members of the public, as well as soliciting information regarding the clinical trials conducted from responsible clinical trial teams. Furthermore, the committee conducted an extensive literature review on relevant topics. (See Appendix A for more information.)
CONTEXT OF THE 2014–2015 EBOLA EPIDEMIC
The 2014–2015 Ebola epidemic, the largest in history, began in December 2013 when a toddler in Guinea became ill, likely as a result of contact with a bat (WHO, 2015d). He died on December 28, 2013, and, soon after, members of his family and several health care workers who treated them also became ill and died. By the end of February 2014, the illness had spread to Conakry, the capital, as well as to other villages and cities. On March 22, the cause was confirmed to be the Zaire species of the Ebola family, and the following day the World Health Organization (WHO) publicly announced the outbreak. The WHO’s announcement provided an official count of 49 cases with 29 deaths and noted that reports of suspected cases in Liberia and Sierra Leone, which share a common border with Guinea, were being investigated (WHO, 2014c). Within a few days, Ebola cases were confirmed in both countries (MSF, 2015). By the time the epidemic was nearing its end in early 2016, the epidemic was responsible for 28,616 cases of Ebola, with 11,310 deaths (WHO, 2016a). The WHO declared the emergency phase of the epidemic to be over on March 29, 2016, though flare-ups continued to occur through April (WHO, 2016a).
The three countries at the epicenter of the 2014–2015 epidemic—Guinea, Liberia, and Sierra Leone—were all ill equipped to handle such a serious and quick-moving epidemic. Given its size and rapid spread, the epidemic would have been a challenge for any country to contain. Before the epidemic Liberia and Sierra Leone were in the process of rebuilding
after long civil wars that had spilled over into Guinea, and all three countries suffered from political and social instability, weak health care systems, extreme poverty, and poor infrastructure (International Crisis Group, 2015). In the 2014 United Nations Human Development Index, which ranks 187 countries on the basis of life expectancy, income per capita, and years of schooling, Guinea was ranked 179th, Liberia 175th, and Sierra Leone 183rd (UNDP, 2014). The health systems of each country were weak, with critical shortages of medical doctors and hospital beds (see Table 1-1). Health care facilities were unevenly distributed, inadequately staffed, and lacked the supplies necessary to treat patients and protect health care workers (CIA, 2016; International Crisis Group, 2015). The 2014–2015 Ebola epidemic in the three countries moved quickly, was difficult to contain, and lasted longer than any previous outbreak. In contrast, outbreaks of Ebola in Nigeria, Senegal, and Mali in, respectively, July, August, and October 2014 were contained relatively quickly due to a high state of alert, more robust health systems and public health capacity, and the ability to mobilize and deploy the necessary human and laboratory resources rapidly from within and outside of these countries (WHO, 2015e).
The Ebola epidemic in Guinea, Liberia, and Sierra Leone exposed and strained those countries’ already fragile health care and public health systems, and the situation quickly deteriorated: the shortage of staff was exacerbated when workers became infected or, in some instances, refused
| Country | Medical Doctor Density | Hospital Bed Density |
|---|---|---|
| Guinea | 0.1 physicians/1,000 population (2005) | 0.3 beds/1,000 population (2011) |
| Liberia | 0.01 physicians/1,000 population (2008) | 0.8 beds/1,000 population (2010) |
| Sierra Leone | 0.02 physicians/1,000 population (2010) | 0.4 beds/1,000 population (2006) |
| United States | 2.45 physicians/1,000 population (2011) | 2.9 beds/1,000 population (2011) |
| United Kingdom | 2.81 physicians/1,000 population (2013) | 2.9 beds/1,000 population (2011) |
| France | 3.19 physicians/1,000 population (2013) | 6.4 beds/1,000 population (2011) |
SOURCE: CIA, 2016.
to report to work due to fear of infection; health facilities closed due to a lack of staff or could offer only the most basic care; and, as the number of cases increased, Ebola patients were denied treatment and turned away from facilities (MSF, 2015). As the epidemic progressed, the inability of the countries’ systems to control the epidemic became clear. Foreign medical staff and aid organizations stepped in to provide support and direct care. Médecins Sans Frontières (MSF), as one of the few agencies with direct experience responding to Ebola outbreaks in the past, had staff with established expertise in treating Ebola. As a result of its experience, MSF and its staff was quickly at the epicenter of the outbreak (Hofman and Au, 2017). As the affected area and number of infected patients grew MSF staff and resources were soon spread thin (MSF, 2015). Health care facilities often lacked personal protective equipment to prevent transmission to health care workers, and as workers became infected, the facilities acted as amplifiers of the virus (WHO, 2015b). In addition, the treatment units lacked the staff and the equipment to provide the necessary supportive care—particularly intravenous fluids and electrolyte management—and patient demand far outpaced the availability of treatment beds (WHO, 2015b). The opening of treatment centers was delayed by a lack of funding, and patients traveled for miles over poor roads in attempts to get care.
In addition to the lack of facilities, staff, and equipment, the response to the Ebola outbreak was made more difficult because of such issues as stigma, fear, rumors, traditional practices, mistrust of authorities and foreign response workers, and mistakes made in engaging communities and community leaders. Stigma took on different forms in different communities, but it complicated the response efforts in all three countries. For example, in Guinea, Ebola initially spread among the Forestièrs (people from the Forest region), a marginalized and suppressed ethnic group. Guineans from the rest of Guinea initially saw Ebola as a disease of “immoral” people (Fairhead, 2015). As a result, Guineans were slow to admit that Ebola could infect their communities and resisted taking measures that could prevent the spread of the virus (Taddonio, 2015). Also in Guinea, rumors spread that foreign response workers were deliberately spreading the virus through disinfection campaigns or that they were killing people in order to harvest their organs (WHO, 2015b). Traditional burial practices in the region—which include kissing, touching, and washing the body—were responsible for many secondary infections (Manguvo and Mafuvadze, 2015). One traditional healer’s funeral, which drew hundreds of mourners from miles around, may have been the source of as many as 365 subsequent Ebola deaths (WHO, 2016c).
The mistrust of authorities—which was exacerbated by a heavy-handed government response which included quarantine, mass cremations, and the use of military force—resulted in community resistance to response
workers (Pellecchia et al., 2015). Authorities often failed to establish effective communication with community members and could not answer their concerns, and the community sometimes did not comply with the infection control efforts of authorities and actually avoided contact with health care facilities or workers (RAS, 2015). Patients who entered Ebola treatment units (ETUs) were sequestered away from the outside world and all too often never emerged except for being cremated and buried safely without the involvement of family. The process, while understandable from a public health perspective, served to drive a wedge between the health system and the community, and the result was disastrous. Patients ran from authorities, or families hid them away, and burials took place in secrecy and without precautions—and transmission of Ebola increased (WHO, 2015b). Community resistance even took the form of violence. For example, in Guinea, treatment facilities and equipment were vandalized, foreign epidemiologists were run out of town by an angry mob, and, in one instance, an eight-member response team was murdered in a village (McCoy, 2014; WHO, 2015b). Nearly 1 year into the epidemic, new cases continued to emerge in both new areas and areas that had already been affected, while communities were overwhelmed by unmet needs—bodies lay uncollected on the streets, patients were dying outside of full treatment units, and orphaned children were left to die (WHO, 2015b).
“Unprecedented” and “Out of Control”
The first victim of the outbreak contracted Ebola in the village of Meliandou in December 2013, and the virus soon spread to the nearby towns of Guéckédou and Macenta and beyond as contacts dispersed to other locations. But it was not until early March 2014 that the Ministry of Health seriously confronted the mysterious spreading illness, and not until March 14 that MSF was asked for help (Baize et al., 2014). Upon learning of the outbreak, MSF quickly sent emergency teams into the field, with the first team arriving in Guéckédou, Guinea, on March 18 (MSF, 2015). Concerned about the likelihood that it was Ebola, MSF arranged to obtain and ship samples for diagnosis to the Inserm laboratory in Lyon, France, where the diagnosis was confirmed and announced by MSF on March 22. In the WHO’s first official outbreak report on March 23, the organization reported that the Ministry of Health, WHO, and other partners were taking steps to control the outbreak and that teams had been deployed to search for and manage cases, while MSF actually began to mobilize the capacity to receive affected patients (WHO, 2014b). The WHO country office in Guinea classified the outbreak as a grade 2 emergency: “a single or multiple country event with moderate public health consequences that requires a moderate WHO country office response and/or moderate international
WHO response” (WHO, 2013). However, in late March, a case was confirmed in the capital city of Conakry, 400 miles away from the initial index cases (MSF, 2015). By March 31, MSF declared that the outbreak was unprecedented because of the geographic spread of the cases (MSF, 2014b). One day later, despite the appearance of confirmed or probable cases in Sierra Leone and Liberia, WHO spokesman Gregory Hartl in Geneva downplayed the outbreak, saying that it was relatively small, neither an epidemic nor unprecedented, and that the appearance of Ebola in a capital city was not a new phenomenon (Samb, 2014). MSF continued to warn about the very real potential for a humanitarian disaster, saying that the distribution of cases in Guinea and Liberia showed that the epidemic was already of a magnitude never seen before and characterizing the response of state authorities and international organizations as minimal (Samb, 2014).
By mid-May 2014 the outbreak seemed to be waning: there was a slight decline in cases in Guinea, Liberia had not reported a new case since April 9, and there were no confirmed cases in Sierra Leone (WHO, 2015b). However, the hope that the three-country outbreak was resolving proved to be wishful thinking. On May 26, the first case was confirmed in Sierra Leone, and it soon became clear that the disease had already been present in the country for some time (Williams, 2015). Within 3 days, from May 27 to May 30, the cases of Ebola reported in Sierra Leone tripled from 16 to 50 (Boston Children’s Hospital and Harvard Medical School, 2016). There was also an escalation of new cases in Liberia and Guinea, and by late June, Ebola patients were identified in more than 60 separate locations across the three countries (MSF, 2014a). On June 21, MSF declared that the epidemic was “out of control,” and it warned that it was reaching the limit of what it could do alone; MSF said it could no longer respond or send teams to new outbreak areas and argued that containment would require massive assistance from local governments and international organizations (MSF, 2014a). On later reflection, MSF director of operations Bart Janssens said that it was like “shouting into a desert” as their appeal for help went unanswered (MSF, 2015). The WHO, the U.S. Centers for Disease Control and Prevention (CDC), and other international organizations sent small numbers of experts to help in the response (CDC, 2016a; WHO, 2014a), but the vast majority of the day-to-day treatment of patients was being provided by local health workers and volunteers from private aid organizations such as MSF as well as by smaller groups such as Samaritan’s Purse and SIM (Serving in Mission) (MSF, 2015).
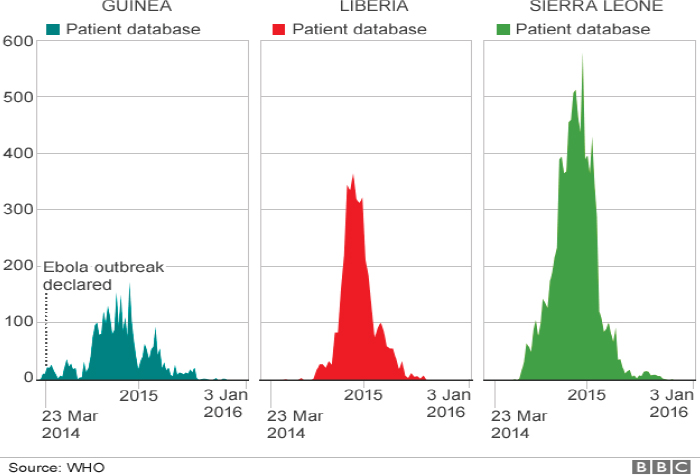
By late June 2014, the West Africa outbreak was officially the largest in history, with 759 confirmed, probable, and suspected cases, including 467 deaths (WHO, 2014d). (See Figure 1-1 for a graphic display of the progression of the epidemic in the three countries.) On July 24, the director-general of the WHO declared the outbreak to be a grade 3 emergency (WHO, 2014c). International attention to the Ebola epidemic was
SOURCE: BBC, 2016.
heightened when people from outside the African continent were infected: an American citizen died of Ebola in Nigeria on July 25, shortly after arriving from Liberia; two American aid workers were infected in Liberia in late July and transported back to the United States for treatment; and a Spanish priest died in Madrid in early August after treating patients in Liberia. These cases sparked fears that Ebola could become an international pandemic and spread far beyond the affected region. Yet it was not until August 6 that the WHO director-general convened an Emergency Committee for Ebola under the International Health Regulations (IHR 2005), and it was not until August 8 that WHO declared the Ebola outbreak a public health emergency of international concern (PHEIC) (WHO, 2014e).
International Response
For the past decade, since the adoption of the updated IHR in 2007,1 there has existed a mechanism to engage the international community in a global public health response. This mechanism resides in the responsibility of the director-general to convene an emergency committee to advise on
___________________
1 The IHR (2005) entered into force, generally, on June 15, 2007, and are currently binding on 194 countries (States Parties) across the globe, including all 193 Member States of WHO (WHO, 2007).
the need to declare a PHEIC, the most urgent designation for a potentially pandemic infectious diseases threat. A PHEIC is an event that constitutes a “public health risk through the international spread of disease” and that “potentially require[s] a coordinated international response” (Murthy, 2016). Four public health crises have been designated PHEICs since PHEIC was defined in IHR 2005: swine flu (2009), polio (2014), Ebola (2014), and Zika virus (2016) (CDC, 2016b).
The declaration of a PHEIC made clear the need for a coordinated international response, and the global community gathered to aid the individuals in the affected communities and countries (WHO, 2015c). The international response was multifaceted, including pledges of money from foreign governments to help build containment facilities to care for the infected and to build laboratory capacity for diagnosis, and the mobilization of foreign volunteers, including health care workers and public health outbreak control experts to provide patients with the best possible level of care and to stop the epidemic from spreading further. However, not much happened until mid-September, when the UN Security Council passed Resolution 2177 (UN, 2014), calling for the creation of a UN Mission for Ebola, and governments began to mobilize the logistics for support. Shortly afterwards, at the High-level Meeting on Response to the Ebola Disease Outbreak at the United Nations on September 25, 2014, Joanne Liu, international president of MSF, said, “Generous pledges of aid and unprecedented UN resolutions are very welcome. But they will mean little, unless they are translated into immediate action. The reality on the ground today is this: the promised surge has not yet delivered” (Liu, 2014). The WHO coordinated outbreak response efforts through the Global Outbreak Alert and Response Network, which “deployed a multidisciplinary workforce of 895 experts in the current Ebola outbreak response operation in West Africa, including doctors, nurses, infection control specialists, logisticians, laboratory specialists; communication, anthropology and social mobilization experts, emergency management and public health professionals among others” (WHO, 2016b).
Clinical Trials
Shortly after the declaration of a PHEIC, the WHO began to convene meetings to discuss the use of potential Ebola therapeutics and vaccines that were in various stages of development. ETUs had little to offer patients because treatments such as supportive care with fluids and electrolytes, monitoring blood pressure and kidney function, and medications for associated secondary infections were unavailable, rudimentary, or limited. The lack of targeted antiviral therapeutics meant that ETUs were limited to providing supportive care for patients, and mortality rates were high
(CDC, 2015a). Some patients—primarily Westerners—had been treated with unproven Ebola therapeutics2 and survived, giving hope that a safe and effective therapeutic could be found in time to stem the tide of the epidemic, while rumors circulated in West Africa that there was a magic or secret serum cure that was not being made available for them (Seay, 2014). The WHO convened several meetings during the fall of 2014, during which stakeholders discussed the scientific, ethical, and regulatory issues involved in conducting clinical trials on these therapeutics and vaccines. By December 2014 the first clinical trials began in the region, as the death toll from Ebola neared 8,000 with over 20,000 reported cases (Dunning et al., 2016; WHO, 2014c).
Key players involved in developing and conducting the clinical trials in West Africa included the WHO; research organizations such as the U.S. National Institutes of Health; public health organizations such as CDC and Institut national de la santé et de la recherche médicale3 (Inserm); academic centers including the University of Oxford, the London School of Hygiene and Tropical Medicine, and the Institute of Tropical Medicine at Antwerp, Belgium; humanitarian groups such as MSF, International Medical Corps, and GOAL International; pharmaceutical companies including GlaxoSmithKline, Merck, and Johnson & Johnson (Janssen Pharmaceuticals); and international funders such as the Wellcome Trust and the Bill & Melinda Gates Foundation (WHO, 2015f). There were many hurdles to overcome, and international research groups and researchers worked in partnership with the ministries of health in Guinea, Liberia, and Sierra Leone to implement their trials.
Conducting the trials took immense effort, from selecting investigational medicinal products to identifying trial sites and setting up appropriate infrastructure to implement trials in the midst of a public health emergency. The success of these groups in launching clinical trials on a compressed time frame, in countries that were unfamiliar with clinical research, and for products that had largely never before been tested in humans, was groundbreaking. However, this success was not without setbacks, which included administrative delays (Lang, 2015) and various disputes regarding the selection of vaccine and therapeutic candidates, trial designs, and other issues. The disparate goals and missions of international partners were displayed when conflict arose over two different perspectives regarding the goals of the clinical trials and how best to design them (Presidential Commission for the Study of Bioethical Issues, 2015). Research organizations
___________________
2 Unproven investigational therapeutics used under expanded access (also known as compassionate use) included ZMapp, brincidoforvir, TKM-Ebola, favipiravir, and convalescent plasma (WHO, 2015a).
3 France’s National Institute for Health and Medical Research.
said that the aim of conducting clinical trials should be to identify safe and effective interventions as efficiently and reliably as possible and that randomized, controlled trials were the best way to achieve this goal. Others said that trials should be conducted in order to provide access to the potential benefits of experimental interventions to as many participants as possible. These stakeholders promoted the use of research designs without randomization or concurrent controls. The conflict between these two perspectives became a central point of contention between stakeholders. These protracted arguments hindered the implementation of robust clinical trials during the 2014–2015 epidemic. (The conflict between researchers and its impact on trial implementation is further discussed in Chapter 2.)
ORGANIZATION OF THE REPORT
This report is organized into six chapters, which follow this introductory chapter. Chapter 2, Conducting Clinical Research During an Epidemic, explores the arguments that arose around clinical trial designs, discusses the ethics and moral principles of conducting clinical research during an epidemic, and examines the ethical arguments made during the 2014–2015 Ebola epidemic. Chapter 3, Assessment of Therapeutic Trials, reviews the formal clinical trials on investigational therapeutic agents conducted in West Africa during the Ebola epidemic, specifically looking at the scientific value of the data generated as a result of the trials. Chapter 4, Assessment of Vaccine Trials, similarly assesses the formal Ebola-specific vaccine trials conducted in West Africa during the Ebola epidemic. Chapter 5, Strengthening Capacity for Research and Response, examines the underlying health systems in West Africa and how a lack of clinical and research capacity influenced clinical research and epidemic response, examines logistical considerations that impacted the conduct of trials, and makes recommendations on how to strengthen capacity to be better prepared for the next epidemic. Chapter 6, Engaging Communities in Research and Response, discusses the social and community context that surrounded the Ebola outbreak and how this influenced clinical trials and explores best practices for community engagement in the event of a future public health emergency. Finally, Chapter 7, Facilitating International Coordination and Collaboration, discusses the need for a coalition of international stakeholders to establish a mechanism that will encourage relationship building and participation of the global research and development and epidemic response communities in addressing key concerns prior to the next epidemic.
REFERENCES
Baize, S., D. Pannetier, L. Oestereich, T. Rieger, L. Koivogui, N. F. Magassouba, B. Soropogui, M. S. Sow, S. Keïta, H. De Clerck, A. Tiffany, G. Dominguez, M. Loua, A. Traoré, M. Kolié, E. R. Malano, E. Heleze, A. Bocquin, S. Mély, H. Raoul, V. Caro, D. Cadar, M. Gabriel, M. Pahlmann, D. Tappe, J. Schmidt-Chanasit, B. Impouma, A. K. Diallo, P. Formenty, M. Van Herp, and S. Günther. 2014. Emergence of Zaire Ebola virus disease in Guinea. New England Journal of Medicine 371(15):1418–1425.
BBC (British Broadcasting Corporation). 2016. Ebola: Mapping the outbreak. http://www.bbc.com/news/world-africa-28755033 (accessed January 11, 2017).
Boston Children’s Hospital and Harvard Medical School. 2016. Ebola outbreaks: Map. http://www.healthmap.org/ebola/#timeline (accessed January 11, 2017).
Burki, T. K. 2011. USA focuses on Ebola vaccine but research gaps remain. The Lancet 378(9789):389.
CDC (U.S. Centers for Disease Control and Prevention). 2015a. Ebola (Ebola virus disease): Treatment. https://www.cdc.gov/vhf/ebola/treatment (accessed January 11, 2017).
CDC. 2015b. Q&As on transmission. https://www.cdc.gov/vhf/ebola/transmission/qas.html (accessed January 11, 2017).
CDC. 2016a. Ebola (Ebola virus disease): CDC’s role. https://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/what-cdc-is-doing.html (accessed January 11, 2017).
CDC. 2016b. Global health security: International Health Regulations (IHR). https://www.cdc.gov/globalhealth/healthprotection/ghs/ihr (accessed January 11, 2017).
CDC. 2016c. Outbreaks chronology: Ebola virus disease. https://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html (accessed December, 2016).
CIA (Central Intelligence Agency). 2016. The world factbook. https://www.cia.gov/library/publications/the-world-factbook/wfbExt/region_afr.html (accessed December 20, 2016).
Deloffre, M. Z. 2016. Three lessons from Ebola can help us fight the Zika virus. Washington Post, February 14. https://www.washingtonpost.com/news/monkey-cage/wp/2016/02/14/heres-why-zika-and-ebola-are-more-than-public-health-crises/?utm_term=.31c928a82973 (accessed January 11, 2017).
Dunning, J., C. Longuet, and A. Salam. 2016. Ebola research: An encounter between science and humanitarian action. Alternatives Humanitaires/Humanitarian Alternatives 1(1):81–93.
Fairhead, J. 2015. Understanding social resistance to Ebola response in Guinea: An anthropological perspective. African Studies Review 59(3):7–31.
Gebre, Y., T. Gebre, and A. Peters. 2014. The Ebola virus: A review of progress and development in research. Asian Pacific Journal of Tropical Biomedicine 4(12):928–936.
Heymann, D., and L. Wertheimer. 2014. Why this Ebola outbreak is different than earlier versions. In Weekend edition Sunday: NPR. http://www.npr.org/2014/08/17/341083551/why-this-ebola-outbreak-is-different-than-earlier-versions (accessed January 11, 2017).
Hofman, M., and S. Au. 2017. The politics of fear: Médecins Sans Frontières and the West African Ebola epidemic. New York: Oxford University Press.
International Crisis Group. 2015. The politics behind the Ebola crisis. Africa report no. 232. Brussels, Belgium: International Crisis Group. https://d2071andvip0wj.cloudfront.net/232-the-politics-behind-the-ebola-crisis.pdf (accessed January 11, 2017).
Johnson, K. M., J. V. Lange, P. A. Webb, and F. A. Murphy. 1977. Isolation and partial characterisation of a new virus causing acute hæmorrhagic fever in Zaire. The Lancet 310(8011):569–570.
KFF (Kaiser Family Foundation). 2014. Ebola characteristics and comparisons to other infectious diseases. http://kff.org/infographic/ebola-characteristics-and-comparisons-to-other-infectious-diseases (accessed January 11, 2014).
Lane, H. C., H. D. Marston, and A. S. Fauci. 2016. Conducting clinical trials in outbreak settings: Points to consider. Clinical Trials 13(1):92–95.
Lang, T. 2015. Ebola: Embed research in outbreak response. Nature 524(7563):29–31.
Liu, J. 2014. MSF president urges UN General Assembly to act now. http://www.msf.org/en/article/msf-president-urges-un-general-assembly-act-now (accessed January 11, 2017).
Lurie, N., T. Manolio, A. P. Patterson, F. Collins, and T. Frieden. 2013. Research as a part of public health emergency response. New England Journal of Medicine 368(13):1251–1255.
Manguvo, A., and B. Mafuvadze. 2015. The impact of traditional and religious practices on the spread of Ebola in West Africa: Time for a strategic shift. Pan African Medical Journal 22(Suppl 1):9.
McCoy, T. 2014. Why the brutal murder of several Ebola workers may hint at more violence to come. Washington Post, September 19. https://www.washingtonpost.com/news/morningmix/wp/2014/09/19/why-the-brutal-murder-of-eight-ebola-workers-may-hint-at-moreviolence-to-come/?utm_term=.7b84f596959b (accessed January 11, 2017).
MSF (Médecins Sans Frontières). 2014a. Ebola in West Africa: Epidemic requires massive deployment of resources. http://www.msf.org/article/ebola-west-africa-epidemic-requires-massive-deployment-resources (accessed January 11, 2017).
MSF. 2014b. Guinea: Mobilisation against an unprecedented Ebola epidemic. http://www.msf.org/en/article/guinea-mobilisation-against-unprecedented-ebola-epidemic (accessed December 20, 2016).
MSF. 2015. Ebola: Pushed to the limit and beyond: A year into the largest ever Ebola outbreak. Geneva, Switzerland: Médecins Sans Frontières. http://www.msf.org/sites/msf.org/files/msf1yearebolareport_en_230315.pdf (accessed January 11, 2017).
Murthy, N. 2016. Public health experts urge World Health Organization to ramp up Zika response. http://news.medill.northwestern.edu/chicago/public-health-experts-urge-worldhealth-organization-to-ramp-up-zika-response/ (accessed January 11, 2017).
Pellecchia, U., R. Crestani, T. Decroo, R. Van den Bergh, and Y. Al-Kourdi. 2015. Social consequences of Ebola containment measures in Liberia. PLoS ONE 10(12):e0143036.
Presidential Commission for the Study of Bioethical Issues. 2015. Ethics and Ebola: Public health planning and response. Washington, DC: Presidential Commission for the Study of Bioethical Issues. http://bioethics.gov/sites/default/files/Ethics-and-Ebola_PCSBI_508.pdf (accessed January 18, 2017).
RAS (Royal African Society). 2015. Ebola: An epidemic of mistrust. http://www.royalafricansociety.org/analysis/ebola-epidemic-mistrust (accessed December 20, 2016).
Samb, S. 2014. WHO says Guinea Ebola outbreak small as MSF slams international response. http://www.reuters.com/article/us-guinea-ebola-idUSBREA301X120140401 (accessed December 20, 2016).
Seay, L. 2014. Ebola, research ethics, and the ZMapp serum. Washington Post, August 6. https://www.washingtonpost.com/news/monkey-cage/wp/2014/08/06/ebola-researchethics-and-the-zmapp-serum/?utm_term=.826de62c2cd9 (accessed January 11, 2017).
Taddonio, P. 2015. Outbreak: Inside the troubled early days of Guinea’s Ebola response. PBS Frontline, May 5. http://www.pbs.org/wgbh/frontline/article/watch-inside-the-troubled-early-days-of-guineas-ebola-response (accessed January 11, 2017).
UN (United Nations). 2014. With spread of Ebola outpacing response, Security Council adopts resolution 2177 (2014) urging immediate action, end to isolation of affected states. https://www.un.org/press/en/2014/sc11566.doc.htm (accessed January 11, 2017).
UNDP (United Nations Development Programme). 2014. Human development report 2014—Sustaining human progress: Reducing vulnerabilities and building resilience. New York: UNDP. http://hdr.undp.org/sites/default/files/hdr14-report-en-1.pdf (accessed January 11, 2017).
Wainberg, M. A., S. Kippax, M. Bras, and P. S. Sow. 2014. HIV and Ebola: Similarities and differences. Journal of the International AIDS Society 17(1):19896.
WHO (World Health Organization). 2007. International Health Regulations (2005): Areas of work for implementation. http://www.who.int/ihr/finalversion9Nov07.pdf (accessed February 16, 2017).
WHO. 2013. Emergency response framework (ERF). Geneva, Switzerland: WHO Press. http://www.who.int/hac/about/erf_.pdf (accessed January 11, 2017).
WHO. 2014a. Ebola challenges West African countries as WHO ramps up response. http://www.who.int/mediacentre/news/notes/2014/ebola-response/en (accessed December 20, 2016).
WHO. 2014b. Ebola response roadmap: Situation report—31 December 2014. Geneva, Switzerland: WHO Press. http://apps.who.int/ebola/en/status-outbreak/situation-reports/ebola-situation-report-31-december-2014 (accessed January 11, 2017).
WHO. 2014c. Ebola virus disease in Guinea. http://www.who.int/csr/don/2014_03_23_ebola/en (accessed December 20, 2016).
WHO. 2014d. Ebola virus disease, West Africa—Update. http://www.who.int/csr/don/2014_07_01_ebola/en (accessed December 20, 2016).
WHO. 2014e. Statement on the 1st meeting of the IHR Emergency Committee on the 2014 Ebola outbreak in West Africa. http://www.who.int/mediacentre/news/statements/2014/ebola-20140808/en (accessed December 20, 2016).
WHO. 2015a. Compassionate use of experimental treatments for Ebola virus disease: Outcomes in 14 patients admitted from August to November, 2014. http://www.who.int/medicines/ebola-treatment/outcomes_experimental_therapies/en (accessed February 17, 2017).
WHO. 2015b. Guinea: The Ebola virus shows its tenacity. http://www.who.int/csr/disease/ebola/one-year-report/guinea/en (accessed December 20, 2016).
WHO. 2015c. Key events in the WHO response to the Ebola outbreak. http://www.who.int/csr/disease/ebola/one-year-report/who-response/en (accessed January 11, 2017).
WHO. 2015d. Origins of the 2014 Ebola epidemic. http://www.who.int/csr/disease/ebola/one-year-report/virus-origin/en (accessed December 20, 2016).
WHO. 2015e. Successful Ebola responses in Nigeria, Senegal and Mali. http://www.who.int/csr/disease/ebola/one-year-report/nigeria/en (accessed January 11, 2017).
WHO. 2015f. WHO Ebola R&D effort—Vaccines, therapies, diagnostics. http://www.who.int/medicines/ebola-treatment/ebola_r_d_effort/en (accessed December 20, 2016).
WHO. 2016a. Ebola virus disease—Fact sheet. http://www.who.int/mediacentre/factsheets/fs103/en (accessed December 21, 2016).
WHO. 2016b. Partners: Global Outbreak Alert and Response Network (GOARN). http://www.who.int/csr/disease/ebola/partners/en (accessed December 20, 2016).
WHO. 2016c. Sierra Leone: A traditional healer and a funeral. http://www.who.int/csr/disease/ebola/ebola-6-months/sierra-leone/en (accessed January 11, 2017).
Williams, C. L. 2015. Leading the charge: Médecins Sans Frontières receives the 2015 Lasker~Bloomberg Public Service Award. Journal of Clinical Investigation 125(10): 3737–3741.
WMA (World Medical Association). 2013. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194.
This page intentionally left blank.


















