4
Scientific Progress and New Research Tools
The scientific knowledge base of Brucella abortus and B. abortus-induced disease pathogenesis has expanded since the previous 1998 National Research Council (NRC) report was issued. This chapter provides a summary of progress since 1998 in understanding B. abortus infection biology; diagnosis of brucellosis in cattle, bison, and elk; and Brucella vaccinology. Coupled with systems biology, there is now the ability to more fully understand the infectious process of B. abortus and enable more rapid discovery of brucellosis vaccines and diagnostics for elk and bison.
1. INFECTION BIOLOGY AND PATHOGENESIS OF B. ABORTUS IN CATTLE, BISON, AND ELK
1.1 Background
When Brucella abortus come in contact with mucous membranes in the alimentary or respiratory tracts of the host, they invade by attaching to host epithelial cells and quickly transmigrate across the mucosa, where they are engulfed by phagocytic cells. The Brucella-containing phagocytic cells disseminate to regional draining lymph nodes and the blood by the lymphatic system, establishing an intermittent bacteremia. They can then colonize the placenta, fetus, mammary glands, testes, and regionaldraining lymph nodes of those tissues or organs; colonization of the placenta and fetus frequently results in abortion.
Brucellae primarily replicate within macrophages, neutrophils, dendritic cells, and placental trophoblasts using different survival strategies; however, the pathogen has the ability to replicate in a wide variety of additional mammalian cell types, including epithelial cells and endothelial cells. The intracellular growth and survival of Brucella in specialized compartments limits exposure to the host immune responses, sequesters the organism from the effects of some antibiotics, and is responsible for the unique features of pathology in infected hosts (Anderson and Cheville, 1986; Myeni et al., 2013; Celli and Tsolis, 2015; de Figueiredo et al., 2015). The pathology in pregnant ruminants is typically divided into three phases: the incubation phase, which is before clinical signs are evident; the acute phase, during which the pathogen is disseminated among host tissues; and the chronic phase, during which massive replication B. abortus occurs in the placental trophoblasts resulting in severe necrotizing placentitis and fetal death (Anderson and Cheville, 1986). Chronic infection results from the ability of the organism to persist in the cells of the host, which is variable for cattle, elk, and bison (Qureshi et al., 1996; Olsen, 2010).
1.2 Infection Biology and Molecular Pathogenesis
While research on the infection biology and molecular pathogenesis of brucellosis has made tremendous progress since the 1998 NRC report, several aspects of the host-Brucella relationship remain to be elucidated (Atluri et al., 2011). For example, it is now known how Brucella enter host cells (Rossetti et al., 2012) and exploit this ability to cross mucosalsurfaces (Rossettiet al., 2012, 2013). However, the receptors involved in binding host cells are only partially understood (Castaneda-Roldán et al., 2004; Seabury et al., 2005). The intracellular invasion and survival of Brucella depends largely on its Type IV secretion system,
although exact targets of its effectors are still elusive (O’Callaghan et al., 1999; Comerci et al., 2001; de Jong and Tsolis, 2012; Chandran, 2013; Lacerda et al., 2013; Myeni et al., 2013; Ke et al., 2015).
After entering the host, Brucella foils host protective responses by evading the so-called innate immune responses (Barquero-Calvo et al., 2007; Carvalho Neta et al., 2008; Gorvel, 2008; de Jong et al., 2010; Gomes et al., 2012; Rossetti et al., 2012; von Bargen et al., 2012). Brucella dampens inflammatory responses, relative to what occurs with other pathogens that infect through the gut mucosa (Oliveira et al., 2008; Rossetti et al., 2013). Brucella also restricts proinflammatory immune responses, including maturation of cells known as dendritic cells, which are crucial for induction of protective adaptive immune responses (Radhakrishnan et al., 2009; Sengupta et al., 2010; Chaudhary et al., 2012; Kaplan-Turkoz et al., 2013; Smith et al., 2013). The curtailed host immune responses together—with Brucella’s ability to live inside host cells and adapt to low oxygen tension—make it a successfulpathogen (Kohler et al., 2002, 2003; Billard et al., 2007; Al Dahouk et al., 2008; Lamontagne et al., 2009; Barbier et al., 2011; Hanna et al., 2013). Because Brucella can persist inside host cells indefinitely, this contributes to its spread within the host, including to placental trophoblasts, fetal lung, male genitalia, skeletal tissues, reticuloendothelial system, and endothelium (Kim et al., 2013; Roop and Caswell, 2013; Xavier et al., 2013).
Because minimal information is available to describe the interaction of Brucella with target cells and tissue, a holistic systems biology analysis of the pathogenesis of brucellosis at the level of the whole host is needed for bison, elk, and cattle (Carvalho Neta et al., 2008; Delpino et al., 2009; Rossetti et al., 2013; Sankarasubramanian et al., 2016). Identification of the most critical components of pathogenesis will enhance the ability to rationally design vaccines, diagnostics, and therapeutics for elk and bison. Fortunately, many of the currently available molecular approaches and methods can be directly applied to in vitro and in vivo research on both the pathogen and the host for a comparative molecular pathogenesis approach.
1.3 Clinical Disease
Under natural conditions, a B. abortus infection is usually acquired by contact with the placenta, fetus, fetal fluids, or vaginal discharges from infected cows in all three host species of interest (cattle, bison, elk). Studies both before and since 1998 have shown that following infection, clinical manifestations of B. abortus infection in bison are largely similar to those of cattle (Nicoletti, 1980; Davis et al., 1990, 1991; Rhyan et al., 1994, 2009; Roffe, 1999a,b; Olsen and Holland, 2003; Olsen et al., 2009; Xavier et al., 2009, 2010; Van Campen and Rhyan, 2010). Since 1998, it has been confirmed that elk are also similar in that systemic clinical signs do not usually occur in the acute stages of infections (Thorne and Morton, 1978; Kreeger et al., 2000; Cook et al., 2002; Kreeger et al., 2002; Van Campen and Rhyan, 2010). In the later stages of infection, the primary clinical disease manifestations are fetal or newborn death, weak calves, metritis with retained placentas—although it is now known that the latter does not occur in elk (Rhyan et al., 2009). Abortions usually occur during the second half of gestation, accompanied by mild mastitis and reduced milk production. After the first abortion, subsequent pregnancies are generally normal, with cows occasionally giving birth to weak calves. But because B. abortus infection may persist, organisms can still be shed in milk and uterine discharges (Meador et al., 1989). In chronic stages of brucellosis, infertility may occur in both sexes due to metritis in cows or orchitis, epididymitis, seminal vesiculitis, and testicular abscesses in bulls, with arthritis and hygromas developing after long-term B. abortus infections.
1.4 Pathology and Pathogenesis
The pathogenesis of brucellosis has been most extensively studied by in vitro and in vivo experiments in nontarget hosts, especially the murine model (Cheers, 1984; Tobias et al., 1993; Grillo et al., 2000; Silva et al., 2011). While studies in models have revealed extensive valuable information on the molecular pathogenesis of brucellosis, these models do not reflect the important differences in the infection biology of brucellosis in elk, bison, and cattle (Olsen and Palmer, 2014). Studies of the molecular pathogenesis in elk, bison, and cattle have been largely limited because of onerous Select Agent requirements, lack of large
animal biocontainment facilities, and costs for large animal experiments. As a consequence, few critical studies have been conducted.
The principal lesions of all three species occur in adult female and male reproductive tracts—the placenta and testes, respectively—and the fetal respiratory tract (Rhyan, 2013). The gross pathology and histopathology of infected elk, bison, and cattle have been described in varying levels of detail (Thorne and Morton, 1978; Rhyan et al., 1997, 2001; Xavier et al., 2009, 2010; Olsen and Palmer, 2014), but the pathological lesions are more similar than they are different among the three host species (Payne, 1959; Thorne and Morton, 1978; Davis et al., 1990; Samartino and Enright, 1993; Williams et al., 1993; Rhyan et al., 1994, 1997, 2001, 2009, 2010; Palmer et al., 1996; Adams, 2002; Xavier et al., 2009; Carvalho Neta et al., 2010; Poester et al., 2013; Rhyan, 2013). Nonetheless, there are some significant differences in animal behavior, disease expression, and susceptibility to brucellosis. For example, elk normally calve in solitary confined conditions in contrast to cattle and bison where parturition is a herd event that attracts other members to sniff and lick the calf or aborted fetus (Van Campen and Rhyan, 2010), potentially affecting the exposure dose of B. abortus and thus the transmission and frequency of disease. Studies on elk have shown they rarely have mastitis or retained placentas compared to cattle and bison, which means they may be less negatively affected by the disease (Rhyan, 2013).
Bison may be considerably more susceptible to brucellosis than cattle, as abortions occurred in <2% of pregnant cattle vaccinated with the B. abortus strain 19 (S19) vaccine compared to 58% of pregnant vaccinated bison (Davis et al., 1991). Higher infection and abortion rates also occurred in experimentally challenged, non-vaccinated bison compared to cattle (Olsen, 2010; Olsen and Johnson, 2011). Additionally, clearance time of the B. abortus RB51 vaccine is twice as long in bison as compared to cattle, yet less bacterial colonization of the udder and less mastitis is seen in bison than in cattle (Cheville et al., 1992; Roffe et al., 1999a,b; Rhyan et al., 2001).
2. DIAGNOSTICS
2.1 Background
Diagnostic assays are vital for the identification of brucellosis in humans and animals for clinical, regulatory, and research purposes (Bricker, 2002a). Bacterial and antibody detection have had key roles in the brucellosis eradication program since its inception in 1934, and there have been numerous advancements in the area of diagnostics in the past few decades.
Multiple diagnostic approaches have been used in both domestic and wild animals. Livestock surveillance initially tested all cattle within a vicinity, but it has evolved to focus on animals at surveillance nodes where cattle are accessible, such as slaughter and first point testing (e.g., markets, shows, and sales), while maintaining wide area testing in communities with known infections. Whole herd follow-up testing has also been a mainstay in surveillance, trace-back, and eradication programs. All have used serological assays in a tiered approach with initial high-sensitivity assays followed by confirmatory testing using assays with greater specificity.
Isolation of the bacteria through culture and subsequent identification of B. abortus has been important for confirmation (the so-called gold standard) in serologically positive animals. However, the success of culture is dependent on a number of variables. For example, in chronically infected animals, B. abortus may only be present in certain lymph nodes and in fewer numbers than in acutely affected animals. Thus, while a culture positive animal is confirmed as infected with brucellosis, false negative culture results can be obtained if inappropriate tissues are collected or if tissues are not properly collected and handled during collection or laboratory processing. Additionally, in an infected population, sera from animals in early stages of infection or with latent infection may not exhibit positive test reactions (O’Grady et al., 2014).
2.2 DNA-Based Identification of B. abortus
Compared to clinical settings, researchers have been able to use a broader repertoire of diagnostic assays in brucellosis research. In particular, multiple DNA amplification and detection methods provide sensitive and rapid identification and quantification of B. abortus in tissues and fluids originating from live and dead animals. Detection of B. abortus by polymerase chain reaction (PCR) is useful for research purposes, where a positive PCR result after experimental infection is definitive evidence of the presence of B. abortus. The specificity of singleplex and quantitative PCR (qPCR) assays used in research are excellent at the genus level, although much lower at the species and subspecies levels without modification of amplification primers and conditions (Bricker, 2002b; Tiwari et al., 2014). However, neither singleplex nor qPCR are a part of routine diagnostic and regulatory program testing.
Other means of specifically identifying various Brucella species, strains, and biovars by DNA-based methods have been developed, including multiplex assays targeting multiple genes and genomic regions, restriction fragment length polymorphisms, and the use of tandem repeat sequences. It can be challenging to determine both analytic and diagnostic sensitivity and specificity for multiplex assays, which impacts the decision-making process for accepting these tests for regulatory purposes. Nevertheless, multiplex PCR assays—such as the “AMOS” test, the “Bruce Ladder,” and more recent modifications of these tests—can differentiate multiple Brucella species and biovars and can differentiate both S19 and RB51 strains from wild type B. abortus (Bricker et al., 2003; López-Goñi et al., 2008; Kang et al., 2011).
2.3 Serology
Obtaining tissues for culture is often infeasible in wildlife populations, and culturing B. abortus is not always successful from infected animals. As a result, antibody detection is used as a proxy for infection (Gilbert et al., 2013). Serological tests reveal past exposure but not necessarily whether an individual is actively infectious, and the interpretation of seropositivity relative to the likelihood of an animal being infected needs to be evaluated relative to the knowledge of the population being tested (Nielsen and Duncan, 1990). In populations where prevalence is high, results are less likely to be false positives and more likely to be accurate indicators of disease (Gilbert et al., 2013). Although some animals may become transiently seropositive yet not infected after exposure, those animals usually do not retain a positive titer for the long term.
A positive serological result is an accurate indicator of infection in bison (Clarke et al., 2014). Brucella abortus biovar 1 was cultured from all but 3 of 36 seropositive bison (91%), and of the 88 seronegative bison, none had positive results of culture from any tissues (Clarke et al., 2014). Furthermore, infected seropositive bison cows likely remain seropositive and infected for a prolonged time, with positive antibody titers to B. abortus remaining remarkably stable over time (Rhyan et al., 2009).
The ability of an infected animal to transmit brucellosis varies depending largely on the reproductive status of that animal. Therefore, predicting “infectiousness” of a particular animal in a known infected population can be difficult. For disease management purposes, all seropositive animals in a known infected population would be considered likely to be infected with the potential to be infectious to other animals at various times.
The types of serological tests and algorithms for identifying B. abortus-infected cattle and bison for regulatory purposes is outlined in the U.S, Department of Agriculture’s Animal and Plant Health Inspection Service’s Uniform Methods and Rules (UM&R) for brucellosis eradication, and the Standard Operating Procedures for Submission and Testing of Brucellosis Serological Specimens (USDA-APHIS, 2003, 2014). The currently accepted testing procedures for serology that are most commonly used in approved brucellosis testing laboratories include the Buffered Acidified Plate Antigen (BAPA), Rapid Automated Presumptive (RAP) Examination, Fluorescence Polarization Assay (FPA), and Complement Fixation (CF) tests. Under certain conditions, the UM&R also allows for the use of other tests, such as Card Agglutination, Particle Concentration Fluorescence Immunoassay (PCFIA), and IDEXX HerdCheck Milk Antibody ELISA, as well as the various brucellosis milk surveillance tests for herd testing.
While the FPA has been much more broadly adopted over the past decade in laboratories approved for brucellosis testing, there have been no new developments for routinely used regulatory tests since the 1998 report. This largely reflects the confidence in the level of validation resulting from decades of use that is the basis for regulatory decision making. As specified in the UM&R, these assays are approved for use in both cattle and bison testing.
Cross-reactions are possible in serological assays due to antibodies directed against other bacteria (e.g., E. coli, Salmonella, Francisella and Yersinia spp.). These cross-reactions appear as false positives and affect the specificity of the diagnostic test. In general, serological tests in cattle have high specificities (>96%), suggesting that false positives are relatively rare in cattle (Nielsen, 2002). Similarly, cross-reactivity does not appear to be a problem in bison (See et al., 2012), and the BAPA, Card, SPT, RIV, and CF tests are of high specificity in elk (Clarke et al., 2015). When the seroprevalence began to increase in some elk herds in Montana, it was suspected that the cause was cross-reactions with Y. enterocolitica O-antigen side chain epitopes (Shumaker et al., 2010). As a result, there was an increased use of the western blot test to rule out potential cross-reactions (Gevock, 2006; Anderson et al., 2009). However, Montana Department of Fish, Wildlife & Parks noted that three of seven culture positive elk samples for which blood samples are also available were incorrectly identified as Yersinia cross-reactions by the western blot test (Anderson et al., 2009). In recent unpublished work provided to the committee, researchers with the Wyoming Game & Fish Department (WGFD) have demonstrated under experimental conditions that routine tests used for B. abortus diagnosis in cattle and bison (such as RAP, FPA, and others) show cross-reactions using serum from Brucella-negative, Yersinia enterocolitica-infected elk. However, elk titers against Y. enterocolitica do not persist beyond an average of 4 months after infection (personal communication, W.H. Edwards, 2015). The lack of persistent titers, together with the relatively few false positives observed in areas without brucellosis, suggest that cross-reactivity observed with Yersinia-infected elk may be minimal (Clarke et al., 2015). Lastly, immunoblot testing is, in general, more difficult to perform and interpret consistently in the diagnostic laboratory, which makes quality management a challenge. For these reasons, the western blot is not the best routine assay for detecting Brucella infected elk.
2.4 Elk Testing and Interpretation
The BAPA test is the best alternative among existing and commonly used Brucella diagnostic assays to screen elk serum for Brucella antibodies, as indicated by data on sensitivity and specificity of various routinely used Brucella antibody detection assays (CT, rivanol, standard plate, CF, and BAPA) (Clarke et al., 2015). Consistent with these data, the United States Animal Health Association passed a resolution in 2011 recommending the use of the BAPA for presumptive testing of elk. A competitive ELISA assay has also been validated for use in elk (Van Houten et al., 2003). The cELISA can differentiate S19-vaccinated from unvaccinated but infected elk, and it has a reasonable degree of overall accuracy if the purpose of testing is to determine seroprevalence in a vaccinated elk herd. However, the cELISA failed to identify approximately 10% of elk from which it was possible to culture B. abortus. All culture positive elk were also positive on conventional Brucella serology assays. Thus, the specificity obtained by using a cELISA, while helpful in differentiating vaccinated from infected animals, was offset by somewhat reduced sensitivity. This is a disadvantage for presumptive testing of individual animals when a high degree of sensitivity is essential. There is no perfect serological test for brucellosis and no single test alone is reliable; thus, the use of multiple tests increases the confidence in diagnosis (Nielsen and Duncan, 1990).
Brucella abortus S19 vaccine has been known to cause positive test results in many animals, especially those recently vaccinated. WGFD began vaccinating elk with S19 in 1985 on the Grey’s River supplemental feedground and gradually expanded the program across all the other feedgrounds except one (Dell Creek). In the supplemental feedgrounds, however, very few elk are identified as vaccine strain positive at 1.5 years or older despite the vaccination of more than 90% of juveniles. In addition, if S19 was creating false positives, one would expect a large fraction of 1.5-year-old individuals to be seropositive on
conventional serological assays. Instead it appears as though S19-induced seroprevalence gradually increases with the age of vaccination as would be expected for field exposures. Therefore, S19 does not appear to induce long-lasting serological titers on the elk feedgrounds (Maichak et al., 2017).
A recent publication describes the use of synthetic oligosaccharides representing the O-polysaccharide side chain of Brucella and related species in an indirect ELISA (McGiven et al., 2015). Initial validation data provide proof of principle that synthetic oligosaccharides representing the capping M-epitope of the side chain can provide excellent specificity in discriminating antibodies against various Brucella species as well as Y. enterocolitica O:9. The use of synthetic oligos also provides a ready source of antigen without the need for culture of B. abortus. While additional validation data are needed to examine analytical sensitivity, diagnostic sensitivity, and diagnostic specificity, the data suggest that a better serological assay for multiple species may be available in the near future.
In 2014, Idaho, Montana, and Wyoming agreed to a uniform testing and interpretation algorithm for serological testing of elk. Both RAP examination and FPA plate tests are run in parallel on each sample. The interpretation algorithm (as shown in Figure 4-1) uses a tiered approach similar to testing of cattle for regulatory purposes. However, the current elk testing and interpretation schematic is rather complex and highlights the challenges with serological testing of elk for Brucella infection.
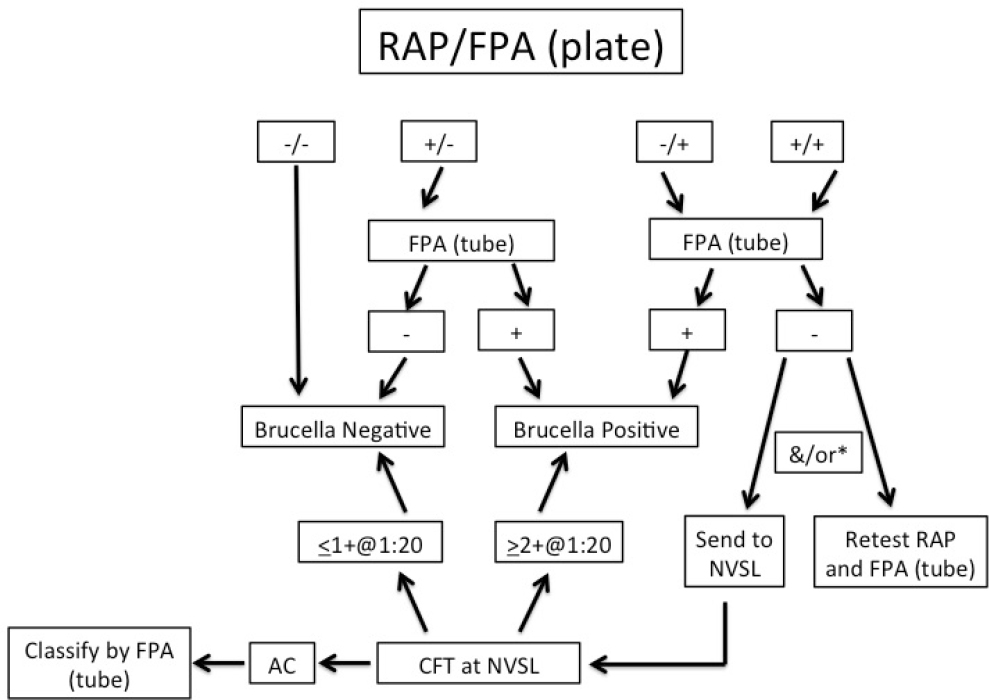
NOTES: If the initial testing results are not interpretable (i.e., a “no test”), the manual card agglutination test is run.
*If initial RAP and FPA (plate) testing is positive and the FPA tube test is negative, submission to the National Veterinary Services Laboratory (NVSL) for CFT is required if the animal is outsidea known brucellosis endemic area.
3. COMMERCIAL VACCINES IN WILDLIFE
B. abortus strain 19 (S19) and B. abortus strain RB51 (RB51) are commercially available live vaccines against B. abortus that are licensed for use in cattle. A number of studies to evaluate their ability to prevent infection and abortion in elk and bison are reviewed here.
3.1 Vaccination of Elk
The S19 vaccine was previously reported to be about 60% effective in preventing abortion in elk when the animals were vaccinated as calves (Thorne et al., 1981; Herriges et al., 1989). This study involved the vaccination of >40,000 elk on feedgrounds by WGFD. However, since 1998, a study found the effective rate to be much lower when a limited number of elk were evaluated in a controlled setting (Roffe et al., 2004). While there were fewer abortions in the vaccinated group relative to the unvaccinated group, the protection rate was considered too low to be efficacious, especially because Brucella was isolated at equal rates from the calves and fetuses in the two groups. There have been questions on whether the number of Brucella organisms used to infect the elk in some studies represents a dose similar to that experienced by elk at feedgrounds that come into contact with aborted fetuses (Roffe et al., 2004). In the field, the estimated exposure would be 4.1 × 106 live organisms for 10 cm diameter of skin contact (Cook, 1999). The challenge dose used in the above study was only about twice as large, making it a realistic dose, though slightly more stringent than a natural infection in the field. In another study, vaccination of feedground elk with S19 delivered via ballistics did not decrease the rate of abortion or stillbirths that occurred following infection. However, if 100% of juveniles were vaccinated, there were fewer abortion events relative to the rate that occurred when none were vaccinated (Maichak et al., 2017). Overall, S19 vaccination is considered to be inadequate for generating protective immunity in elk in the Greater Yellowstone Area.
The RB51 vaccine is composed of a mutant strain of B. abortus that lacks the O-polysaccharide side chain. As a result, animals vaccinated with RB51 do not make antibodies to the O-polysaccharide; the presence of such antibodies is used as an indicator of infection in conventional brucellosis diagnostic tests. Given at one of two dosages (109 live organisms for adults and 1010 for calves), RB51 is considered to be as efficacious in preventing infection and abortions in cattle as the S19 vaccine (Cheville et al., 1996; Olsen, 2000; Olsen et al., 2009). In contrast, experimental trials indicate that the RB51 vaccine is ineffective at protecting elk from brucellosis (Cook et al., 2000) in that the RB51 vaccine resulted in only low levels of protection when administered intramuscularly or by biobullets (Cook et al., 2002; Kreeger et al., 2000). Animals given a booster dose of RB51 1 year after the initial RB51 vaccination aborted at a rate equal to or higher than that of unvaccinated animals, demonstrating that RB51 is not efficacious for elk (Kreeger et al., 2002).
3.2 Immune Responses by Elk to Vaccination
Since neither S19 nor RB51 vaccines protect elk from infection or abortion, it is likely that the immune responses in elk differ from those of cattle. Immune responses are manifested as antibodies and interferon (IFN)-γ, a product of the immune system’s T lymphocytes, known as a cytokine, important for controlling brucellosis. IFN-γ production can be measured using a commercially available kit made for measuring IFN-γ of red deer (Olsen et al., 2006). The expressed IFN-γ gene sequence for red deer is identical to elk except for the last amino acid; therefore, the same kit can be used to detect elk IFN-γ (Sweeney et al., 2001). Antibody responses to the vaccine strains of B. abortus were detected in vaccinated elk, and an expansion of CD4 T lymphocytes was seen after vaccination, but in vitro tests determined that lymphocyte multiplication in response to bacteria was not greater for vaccinated than for unvaccinated elk. In comparison, lymphocyte replication in response to bacteria was detected for cattle and bison cells following vaccination with RB51 (Stevens et al., 1995; Olsen et al., 2002). A similar discrepancy occurred when elk were vaccinated with Mycobacterium bovis (BCG), a vaccine that typically induces a strong IFN-γ response in cattle
but not in elk, indicating that this is not peculiar to Brucella alone. To quantitatively and qualitatively evaluate the differences in immune responses between cattle and elk, it is necessary to first understand the elk immune system. To do this, tools are needed to identify and measure the cells and molecules involved in elk immune responses; however, those tools currently do not exist.
3.3 Vaccination of Bison
Both B. abortus S19 and RB51 vaccines have variable efficacy in bison (see Table 4-1). When S19 was given to adult pregnant bison—either by needle or ballistically using hollow pellets containing freeze-dried S19 organisms—50% aborted (Davis et al., 1991). This demonstrated that pregnant bison are more sensitive to abortion with S19 than pregnant cattle. Nevertheless, when bison were challenged with B. abortus strain 2308 (a fully virulent field isolate) in their second trimester of pregnancy, having been previously vaccinated with S19, 67% of bison were protected from abortion and 39% were protected from infection while only 4% of non-vaccinated bison failed to abort (Davis et al., 1991). These levels of protection in bison following S19 vaccination are only slightly lower than the range found for cattle. However, other studies showed that S19 vaccination of bison calves is inadequate (Davis, 1993; Davis and Elzer, 2002).
In contrast to S19, RB51 vaccination is safe in male bison, pregnant female bison, and bison calves (Elzer et al., 1998). However, a number of studies show that RB51 efficacy varies in bison adults and calves. Protection from abortion using RB51 ranged from 0% to 100%, while protection against fetal infection ranged from 0% to 81% (as summarized in Table 4-1). Calfhood vaccination of bison with RB51 was shown to provide protection from abortion when bison were challenged with virulent B. abortus S2308 mid-gestation in one study (Olsen et al., 2003). RB51 vaccination also reduced the recovery of S2308 from calf tissues, but not maternal tissues. In contrast, another group did not obtain significant efficacy in RB51booster vaccinated bison (Davis and Elzer, 1999).
Studies have also shown mixed results on the efficacy of RB51 booster doses in pregnant bison. Adult pregnant bison given two doses of RB51 did not abort even though RB51 was present in fetal tissues (Olsen and Holland, 2003). Another study demonstrated that the booster dose resulted in higher IFN-γ (the immune system product associated with protective immunity to Brucella) responses, and none had infected fetuses (Olsen and Johnson, 2012a,b). Adult female YNP bison that had been previously vaccinated with 107 or 109 live RB51 organisms were revaccinated during the first trimester and boosted during the second trimester. An additional group of Kansas bison that had been previously vaccinated with 109 live RB51 organisms was also boosted during pregnancy. While abortion rates were slightly lower than for unvaccinated animals, the investigators concluded that RB51 was not significantly protective, and they questioned whether vaccination with the standard dose would be more effective (Davis and Elzer, 1999; Elzer et al., 2002). No significant differences in abortion or calf infection rates were seen among animals vaccinated once, left unvaccinated, or vaccinated twice. Thus, RB51 vaccination did not protect against abortion, and only one-third of all the calves were protected against infection in that study (Elzer et al., 2002).
To address whether booster vaccination with RB51 can enhance protection in yearlings, one group of bison heifer calves was vaccinated subcutaneously while a second group was darted (Olsen and Johnson, 2012b). All animals were naturally bred; some of the subcutaneously RB51 vaccinated animals were boosted with RB51; and pregnant bison were challenged with virulent B. abortus S2308. Unvaccinated controls had an 83% abortion rate compared to 33% for the animals that received a single dose of RB51, 57% for the darted animals, and none of the twice-vaccinated bison aborted. The results again indicate that multiple doses of RB51 has efficacy in bison. However, regardless of vaccination status, 100% of the fetuses/calves had viable wild type B. abortus in their tissues (Olsen and Johnson, 2012b).
TABLE 4-1 Summary of RB51’s Efficacy in Bison
| Study | Bison Source | Vaccine Group | Bison/Group | # of Vaccine Doses | Age of Primary and Booster Vaccinations | Vaccine Dose at 1° and Booster Vaccinations (CFUs) | Strain 2308 Challenge Dose (CFUs)* | Time of Challenge (Days of Gestation) | % Bison Protected Against Abortion | % Bison Protected Against Fetal Infection |
|---|---|---|---|---|---|---|---|---|---|---|
| Olsen et al., 2003 | Iowa | saline | 13 | – | 3-8 months old | 0 | 107 | 150-180 | 38% | 38% |
| Iowa | RB51 | 37 | 1 | 3-8 months old | 1.2-6.1 × 1010 | 107 | 150-180 | 85% | 81% | |
| Davis and Elzer, 1999 | Colorado | saline | 19 | – | adult | 0 | 107 | 150-180 | 21% | Not determined |
| Kansas | RB51 | 8 | 2 | adult | 109/109 | 107 | 150-180 | 50% | Not determined | |
| Yellowstone National Park | RB51 | 20 | 3 | adult/1st trimes./ 2nd trimes. | 107 or 109/109/109 | 107 | 150-180 | 50% | Not determined | |
| Elzer et al, 2002 | South Dakota | – | 27 | – | 6 months old | 0 | 107 | Mid-gestation | 67% | 0% |
| South Dakota | RB51 | 28 | 1 | 6 months old | 1010 | 107 | Mid-gestation | 75% | 0% | |
| South Dakota | RB51 | 28 | 3 | 6/12/18 months old | 1010/1010/1010 | 107 | Mid-gestation | 71.4% | 32% | |
| Olsen and Johnson, 2012b | Brucellosis-free herd | saline | 6 | – | 8-10 months old | 0 | 107 | 170-180 | 17% | 0% |
| Brucellosis-free herd | RB51 | 7 | 1 | 8-10 months old | darted with 1.8 × 1010 | 107 | 170-180 | 43% | 0% | |
| Brucellosis-free herd | RB51 | 6 | 1 | 8-10 months old | 2.2 × 1010 | 107 | 170-180 | 67% | 0% | |
| Brucellosis-free herd | RB51 | 5 | 2 | 8-10/23-25 months old | 1.1 × 1010/2.2 × 1010 | 107 | 170-180 | 100% | 0% | |
| Olsen et al., 2015 | Brucellosis-free herd | saline | 6 | – | 8-11 months old | 0 | 107 | 170-180 | 0% | 17% |
| Brucellosis-free herd | RB51 | 5 | 1 | 8-11 months old | 1.6 × 1010 | 107 | 170-180 | 80% | 40% | |
| Brucellosis-free herd | RB51 | 14 | 2 | 8-11/19-22 months old | 1.6 × 1010/2.8 × 1010 | 107 | 170-180 | 93% | 57% | |
| Olsen et al., 2009 | Brucellosis-free herd | saline | 8 | – | 10 months old | 0 | 107 | 170-180 | 0 | 0% |
| Brucellosis-free herd | RB51 + sodC, wboA | 6 | 1 | 10 months old | 7.4 × 1010 | 107 | 170-180 | 33% | 0% | |
| Brucellosis-free herd | RB51 | 6 | 1 | 10 months old | 4.26 × 1010 | 107 | 170-180 | 66% | 0% |
NOTE: *Bison were challenged via the conjunctival route with virulent wild type B. abortus strain 2308.
Alternative methods for delivering RB51 have been evaluated. Vaccinating bison by darts induced immune responses similar to those achieved by hand vaccination, but neither the dart nor the hand vaccination method protected the bison from abortion when challenged with B. abortus S2308 (Olsen and Johnson, 2012b). However, bison that were given a booster dose showed protection from abortion. A follow-up study demonstrated that giving a booster dose of RB51 results in a greater IFN-γ response as measured by mRNA transcripts, reduced percentage of abortions, and less bacterial colonization of tissues (Olsen et al., 2015).
4. NEW SCIENTIFIC TOOLS INFORMING BRUCELLOSIS INFECTION BIOLOGY, PATHOGENESIS, AND VACCINOLOGY
New molecular tools have recently been developed that link cell biology with genetics and genomics. Bioinformatics has also emerged as an important tool to manage and analyze massive datasets of biological information. The expansion of the “-omics” (fields of study related to the genome, transcriptome, proteome, metabolome), genomics tools, and next-generation sequencing technologies now enable in-depth analyses needed to understand cellular function and behavior of B. abortus and its hosts (including elk, bison, and cattle).
4.1 Brucella Genome
More than 30 complete Brucella genomes have been sequenced since 1998, providing a database for comparative analysis of gene structure and homologies, gene expression, regulatory networks, protein synthesis, and metabolic pathways. Gene variations among strains have been identified by comparative genomics and through speciation. The identified variations only partially explain the differences in virulence among Brucella species and their specificity for certain host species (He, 2012).
Genes of a pathogen can be interrogated by a process known as reverse vaccinology to identify their potential to induce immune responses in their host, and this has been applied to the Brucella genomes (He and Xiang, 2010; He, 2012; Gomez et al., 2013a,b; Vishnu et al., 2015). Candidate gene products have been tested for in vivo efficacy, an approach that could be used to tailor brucellosis vaccines for elk, bison, and cattle (Ko and Splitter, 2003; Wang et al., 2012; Gomez et al., 2013a,b). For example, Vaxign (a Web-based vaccinology tool) identified 14 outer membrane proteins that are conserved in six virulent strains of B. abortus, B. melitensis, and B. suis (He and Xiang, 2010). Some of these proteins were shown to induce antibody and T cell responses in immunized mice (Gomez et al., 2013a,b). This type of information may also be useful for developing new diagnostic tests.
Whole genome sequencing and other sequence-based technologies can show evolutionary relationships of Brucella relative to geography and host origin. This is a particularly relevant tool for understanding the epidemiology of Brucella infections among cattle, elk, and bison in Yellowstone National Park (Beja-Pereira et al., 2009; Higgins et al., 2012; Rhyan et al., 2013; Kamath et al., 2016).
Gene expression analysis of Brucella during host adaptation has identified critical factors for virulence and long-term survival of Brucella (Kim et al., 2013, 2014). Inactivation or knockout of Brucella genes allows gene function to be identified in pathogenesis and virulence. This could also facilitate enhanced vaccine development by producing new attenuated strains of the bacteria (O’Callaghan et al., 1999; Rosinha et al., 2002; Ficht, 2003; Arenas-Gamboa et al., 2008, 2009; Kim et al., 2014).
4.2 Host Genomes
Substantial progress has been made on assembling the Bison bison bison reference genome (NIH, 2016). A bison reference genome provides fundamental information and can eventually help identify any genetic basis for increased susceptibility of bison to B. abortus. A deer reference genome (red deer, Canadian elk) is also being completed and validated (Brauning et al., 2015). Functionalgenomics can detect
host genes that are either expressed or repressed and could further reveal the mechanism by which B. abortus survives. For example, gene silencing (using RNA interference) was used to knock down specific host genes during Brucella infection in model systems, which allowed scientists to identify the genes controlling major infection pathways (Qin et al., 2008; Rossetti et al., 2012). While genetic resistance against brucellosis is a complex polygenic trait in cattle and bison, newer genetic tools can provide the means to better understand the genetic basis for susceptibility to B. abortus in elk and bison and to clone livestock or wildlife for enhanced genetic resistance to B. abortus (Adams and Templeton, 1998; Westhusin et al., 2007; Adams and Schutta, 2010).
Brucella and host gene expression and proteome datasets have been generated in the past decade, which will provide future opportunities for a comprehensive analysis of both host and pathogen responses during infection (Rajashekara et al., 2006; Carvalho Neta et al., 2008; Lamontagne et al., 2009; He et al., 2010; Rossetti et al., 2010, 2012, 2013; Viadas et al., 2010; Weeks et al., 2010; Lin et al., 2011; Wang et al., 2011; Liu et al., 2012; Karadeniz et al., 2015). To date, datasets have been analyzed to understand gene regulatory networks, characterize Brucella stress responses, and understand modulation of host responses (He et al., 2010; He, 2012; Hanna et al., 2013; Kim et al., 2013, 2014; Karadeniz et al., 2015).
5. CONCLUSION
Even though there is now a greater scientific understanding of B. abortus than in 1998, there continue to be major gaps in understanding infection biology and molecular pathogenesis of brucellosis in each host. New tools and reagents are needed to gain a basic understanding of the uniqueness of the elk immune system response to Brucella to develop elk specific vaccines. There has been limited progress in understanding Brucella host preference and genetic resistance to brucellosis to manage transmission between domestic animals and wildlife species (Godfroid et al., 2011, 2014), but new molecular and bioinformatics tools offer greater hope to understand these phenomena (see Chapter 9 on Remaining Gaps for Understanding and Controlling Brucellosis).
REFERENCES
Adams, L.G. 2002. The pathology of brucellosis reflects the outcome of the battle between the host genome and the Brucella genome. Veterinary Microbiology 90(1-4):553-561.
Adams, L.G., and C.J. Schutta. 2010. Natural disease resistance to brucellosis: A review. The Open Journal of Veterinary Science 4:61-71.
Adams, L.G., and J.W. Templeton. 1998. Genetic resistance to bacterial diseases of animals. Revue Scientifique et Technique Office Internationaldes Epizooties 17(1):200-219.
Al Dahouk, S., V. Jubier-Maurin, H.C. Scholz, H. Tomaso, W. Karges, H. Neubauer, and S. Kohler. 2008. Quantitative analysis of the intramacrophagic Brucella suis proteome reveals metabolic adaptation to late stage of cellular infection. Proteomics 8(18):3862-3870.
Anderson, N.J., J.M. Ramsey, and K.D. Hughes. 2009. 2008 Brucellosis Survey Final Report. Montana Fish, Wildlife & Parks, Wildlife Laboratory, Bozeman, MT.
Anderson, T.D., and N.F. Cheville. 1986. Ultrastructural Morphometric Analysis of Brucella abortus-Infected Trophoblasts in Experimental Placentitis. American Journal of Pathology 124(2):226-237.
Arenas-Gamboa, A.M., T.A. Ficht, M.M. Kahl-McDonagh, and A.C. Rice-Ficht. 2008. Immunization with a single dose of a microencapsulated Brucella melitensis mutant enhances protection against wild-type challenge. Infection and Immunology 76(6):2448-2455.
Arenas-Gamboa, A.M., T.A. Ficht, M.M. Kahl-McDonagh, G. Gomez, and A.C. Rice-Ficht. 2009. The Brucella abortus S19 DeltavjbR live vaccine candidate is safer than S19 and confers protection against wild-type challenge in BALB/c mice when delivered in a sustained-release vehicle. Infection and Immunology 77(2):877-884.
Atluri, V.L., M.N. Xavier, M.F. de Jong, A.B. den Hartigh, and R.M. Tsolis. 2011. Interactions of the human pathogenic Brucella species with their hosts. Annual Reviews in Microbiology 65:523-541.
Barbier, T., C. Nicolas, and J.J. Letesson. 2011. Brucella adaptation and survival at the crossroad of metabolism and virulence. FEBS Letters 585(19):2929-2934.
Barquero-Calvo, E., E. Chaves-Olarte, D.S. Weiss, C. Guzmán-Verri, C. Chacon-Diaz, A. Rucavado, I. Moriyón, and E. Moreno. 2007. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLOS ONE 2(7):e631.
Beja-Pereira, A., B. Bricker, S. Chen, C. Almendra, P.J. White, and G. Luikart. 2009. DNA genotyping suggests that recent brucellosis outbreaks in the Greater Yellowstone area originated from elk. Journal of Wildlife Diseases 45(4):1174-1177.
Billard, E., J. Dornand, and A. Gross. 2007. Brucellasuis prevents human dendritic cell maturation and antigen presentation through regulation of tumor necrosis factor alpha secretion. Infection and Immunology 75(10):4980-4989.
Brauning, R., P.J. Fisher, A.F. McCulloch, R.J. Smithies, J.F. Ward, M.J. Bixley, C.T. Lawley, S.J. Rowe, and J.C. McEwan. 2015. Utilization of high throughput genome sequencing technology for large scale single nucleotide polymorphism discovery in red deer and Canadian elk. Proceedings of the Cold Spring Harbor Laboratory.
Bricker, B.J. 2002a. Diagnostic strategies used for the identification of Brucella. Veterinary Microbiology 90(1-4):433-434.
Bricker, B.J. 2002b. PCR as a diagnostic tool for brucellosis. Veterinary Microbiology 90(1-4):435-446.
Bricker, B.J., D.R. Ewalt, S.C. Olsen, and E.E. Jensen. 2003. Evaluation of the Brucella abortus species-specific polymerase chain reaction assay, an improved version of the Brucella AMOS-polymerase chain reaction assay for cattle. Journal of Veterinary Diagnostic Investigation 15:374-378.
Carvalho Neta, A.V., A.P. Stynen, T.A. Paixao, K.L. Miranda, F.L. Silva, C.M. Roux, R.M. Tsolis, R.E. Everts, H.A. Lewin, L.G. Adams, A.F. Carvalho, A.P. Lage, and R.L. Santos. 2008. Modulation of the bovine trophoblastic innate immune response by Brucellaabortus. Infection and Immunology 76(5):1897-1907.
Carvalho Neta, A.V., J.P. Mol, M.N. Xavier, T.A. Paixao, A.P. Lage, and R.L. Santos. 2010. Pathogenesis of bovine brucellosis. Veterinary Journal 184(2):146-155.
Castaneda-Roldán, E.I., F. Avelino-Flores, M. Dall’Agnol, E. Freer, L. Cedillo, J. Dornand, and J.A. Girón. 2004. Adherence of Brucella to human epithelial cells and macrophages is mediated by sialic acid residues. Cellular Microbiology 6(5):435-445.
Celli, J., and R.M. Tsolis. 2015. Bacteria, the endoplasmic reticulum and the unfolded protein response: friends or foes. Nature Reviews Microbiology 13:71-82.
Chandran, V. 2013. Type IV secretion machinery: Molecular architecture and function. Biochemical Society Transactions 41(1):17-28.
Chaudhary, A., K. Ganguly, S. Cabantous, G.S. Waldo, S.N. Micheva-Viteva, K. Nag, W.S. Hlavacek, and C.S. Tung. 2012. The Brucella TIR-like protein TcpB interacts with the death domain of MyD88. Biochemical and Biophysical Research Communications 417(1):299-304.
Cheers, C. 1984. Pathogenesis and cellular immunity in experimental murine brucellosis. Development in Biological Standardization 56:237-246.
Cheville, N.F., A.E. Jensen, S.M. Halling, F.M. Tatum, D.C. Morfitt, S.G. Hennager, W.M. Frerichs, and G. Schurig. 1992. Bacterial survival, lymph node changes, and immunologic responses of cattle vaccinated with standard and mutant strains of Brucellaabortus. American Journal of Veterinary Research 53(10):1881-1888.
Cheville, N.F., S.C. Olsen, A.E. Jensen, M.G. Stevens, M.V. Palmer, and A.M. Florance. 1996. Effects of age at vaccination on efficacy of Brucellaabortus strain RB51 to protect cattle against brucellosis. American Journal of Veterinary Research 57(8):1153-1156.
Clarke, P.R., R.K. Frey, J.C. Rhyan, M.P. McCollum, P. Nol, and K. Aune. 2014. Feasibility of quarantine procedures for bison (Bison bison) calves from Yellowstone National Park for conservation of brucellosis-free bison. Journal of American Veterinary Medical Association 244(5):588-591.
Clarke, P.R., W.H. Edwards, S.G. Hennager, J.F. Block, A.M. Yates, E. Ebel, D.J. Knopp, A. Fuentes-Sanchez, J. Jennings-Gaines, R.L. Kientz, and M. Simunich. 2015. Comparison of buffered, acidified plate antigen to standard serologic tests for the detection of serum antibodies to Brucella abortus in elk (Cervus canadensis). Journal of Wildlife Diseases 51(3):764-768.
Comerci, D.J., M.J. Martinez-Lorenzo, R. Sieira, J.P. Gorvel, and R.A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucellaabortus-containing vacuole. Cellular Microbiology 3(3):159-168.
Cook, W.E. 1999. Brucellosis in Elk: Studies of Epizootiology and Control. Ph.D. dissertation, University of Wyoming, Laramie, WY.
Cook, W.E., E.S. Williams, E.T. Thorne, T.J. Kreeger, G.W. Stout, G. Schurig, L.A. Colby, F. Enright, and P.H. Elzer. 2000. Safety of Brucellaabortus strain RB51 in bull elk. Journal of Wildlife Diseases 36(3):484-488.
Cook, W.E., E.S. Williams, E.T. Thorne, T.J. Kreeger, G. Stout, K. Bardsley, H. Edwards, G. Schurig, L.A. Colby, F. Enright, and P.H. Elzer. 2002. Brucellaabortus strain RB51 vaccination in elk. I. Efficacy of reduced dosage. Journal of Wildlife Diseases 38(1):18-26.
Davis, D.S. 1993. Summary of bison/brucellosis re search conducted at Texas A & M University 1985-93. Pp. 347-361 in Proceedings of North American Public Bison Herds Symposium. H.E. Walker, ed. Denver, CO: National Bison Association.
Davis, D.S., and P.H. Elzer. 1999. Safety and efficacy of Brucella abortus RB51 vaccine in adult American bison (Bison bison). Proceedings of the U.S. Animal Health Association 103:154-158.
Davis, D.S., and P.H. Elzer. 2002. Brucella vaccines in wildlife. Veterinary Microbiology 90(1-4):533-544.
Davis, D.S., J.W. Templeton, T.A. Ficht, J.D. Williams, J.D. Kopec, and L.G. Adams. 1990. Brucella abortus in captive bison. I. Serology, bacteriology, pathogenesis, and transmission to cattle. Journal of Wildlife Diseases 26(3):360-371.
Davis, D.S., J.W. Templeton, T.A. Ficht, J.D. Huber, R.D. Angus, and L.G. Adams. 1991. Brucella abortus in bison. II. Evaluation of strain 19 vaccination of pregnant cows. Journal of Wildlife Diseases 27(2):258-264.
de Figueiredo, P., T.A. Ficht, A. Rice-Ficht, C.A. Rossetti, and L.G. Adams. 2015. Pathogenesis and Immunobiology of Brucellosis: Review of Brucella-Host Interactions. American Journal of Pathology 185:1505-1517.
de Jong, M.F., and R.M. Tsolis. 2012. Brucellosis and type IV secretion. Future Microbiology 7(1):47-58.
de Jong, M.F., H.G. Rolan, and R.M. Tsolis. 2010. Innate immune encounters of the (Type) 4th kind: Brucella. Cellular Microbiology 12(9):1195-1202.
Delpino, M.V., C.A. Fossati, and P.C. Baldi. 2009. Proinflammatory response of human osteoblastic cell lines and osteoblast-monocyte interaction upon infection with Brucella spp. Infection and Immunology 77(3):984-995.
Elzer, P.H., M.D. Edmonds, S.D. Hagius, J.V. Walker, M.J. Gilsdorf, and D.S. Davis. 1998. Safety of Brucellaabortus strain RB51 in bison. Journal of Wildlife Diseases 34(4):825-829.
Elzer, P., S. Hagius, T.J. Roffe, S. Holland, and D.S. Davis. 2002. Failure of RB51 calfhood bison vaccine against brucellosis. Proceedings of U.S. Animal Health Association 106:87-91.
Ficht, T.A. 2003. Intracellular survival of Brucella: Defining the link with persistence. Veterinary Microbiology 92(3):213-223.
Gevock, N. 2006. Brucellosis tests show no spike in Madison elk. Montana Standard, April 27, 2006.
Gilbert, A.T., A.R. Fooks, D.T.S. Hayman, D.L. Horton, T. Müller, R. Plowright, A.J. Peel, R. Bowen, J.L.N. Wood, J. Mills, A.A. Cunningham, and C.E. Rupprecht. 2013. Deciphering serology to understand the ecology of infectious diseases in wildlife. EcoHealth 10(3):298-313.
Godfroid, J., H.C. Scholz, T. Barbier, C. Nicolas, P. Wattiau, D. Fretin, A.M. Whatmore, A. Cloeckaert, J.M. Blasco, I. Moriyon, C. Saegerman, J.B. Muma, S. Al Dahouk, H. Neubauer, and J.J. Letesson. 2011. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Preventive Veterinary Medicine 102(2):118-131.
Godfroid, J., X. DeBolle, R.M. Roop, D. O’Callaghan, R.M. Tsolis, C. Baldwin, R.L. Santos, J. McGiven, S. Olsen, I.H. Nymo, A. Larsen, S. Al Dahouk, and J.J. Letesson. 2014. The quest for a true One Health perspective of brucellosis. Revue Scientifique et Technique Office International des Epizooties 33(2):521-538.
Gomes, M.T., P.C. Campos, L.A. de Almeida, F.S. Oliveira, M.M. Costa, F.M. Marim, G.S. Pereira, and S.C. Oliveira. 2012. The role of innate immune signals in immunity to Brucella abortus. Frontiers in Cellular and Infection Microbiology 2:130.
Gomez, G., L.G. Adams, A. Rice-Ficht, and T.A. Ficht. 2013a. Host-Brucella interactions and the Brucella genome as tools for subunit antigen discovery and immunization against brucellosis. Frontiers in Cellular and Infection Microbiology 3:17.
Gomez, G., J. Pei, W. Mwangi, L.G. Adams, A. Rice-Ficht, and T.A. Ficht. 2013b. Immunogenic and invasive properties of Brucella melitensis 16M outer membrane protein vaccine candidates identified via a reverse vaccinology approach. PLOS ONE 8(3):e59751.
Gorvel, J.P. 2008. Brucella: A Mr. “Hide” converted into Dr. Jekyll. Microbes and Infection 10(9):1010-1013.
Grillo, M.J., N. Bosseray, and J.M. Blasco. 2000. In vitro markers and biological activity in mice of seed lot strains and commercial Brucellamelitensis Rev 1 and Brucella abortus B19 vaccines. Biologicals 28(2):119-127.
Hanna, N., S. Ouahrani-Bettache, K.L. Drake, L.G. Adams, S. Kohler, and A. Occhialini. 2013. Global Rsh-dependent transcription profile of Brucella suis during stringent response unravels adaptation to nutrient starvation and cross-talk with other stress responses. BMCGenomics 14:459.
He, Y. 2012. Analyses of Brucella pathogenesis, host immunity, and vaccine targets using systems biology and bioinformatics. Frontiers in Cellular and Infection Microbiology 2:2.
He, Y., and Z. Xiang. 2010. Bioinformatics analysis of Brucella vaccines and vaccine targets using VIOLIN. Immunome Research 6(Suppl. 1):S5.
He, Y., S. Sun, H. Sha, Z. Liu, L. Yang, Z. Xue, H. Chen, and L. Qi. 2010. Emerging roles for XBP1, a sUPeR transcription factor. Gene Expression 15(1):13-25.
Herriges, J.D., E.T. Thirne, S.L. Anderson, and H.A. Dawson. 1989. Vaccination of elk in Wyoming with reduced dose strain 19 Brucella: Controlled studies and ballistic implant field trials. Proceedings of the U.S. Animal Health Association 93:640-655.
Higgins, J., T. Stuber, C. Quance, W.H. Edwards, R.V. Tiller, T. Linfield, J. Rhyan, A. Berte, and B. Harris. 2012. Molecular epidemiology of Brucella abortus isolates from cattle, elk, and bison in the United States, 1998 to 2011. Applied and Environmental Microbiology 78(10):3674-3684.
Kamath, P., P.C. Cross, and J.T. Foster. 2016. Genomics reveals historic and contemporary transmission dynamics of a bacterial disease among wildlife and livestock. Nature Communications 7:11448.
Kang, S.I., M. Her, J.W. Kim, J.Y. Kim, K.Y. Ko, Y.M. Ha, and S.C. Jung. 2011. Advanced multiplex PCR assay for differentiation of Brucella species. Applied and Environmental Microbiology 77(18):6726-6728.
Kaplan-Turkoz, B., T. Koelblen, C. Felix, M.P. Candusso, D. O’Callaghan, A.C. Vergunst, and L. Terradot. 2013. Structure of the Toll/interleukin 1 receptor (TIR) domain of the immunosuppressive Brucella effector BtpA/Btp1/TcpB. FEBS Letters 587(21):3412-3416.
Karadeniz, I., J. Hur, Y. He, and A. Ozgur. 2015. Literature mining and ontology based analysis of Host-Brucella Gene-Gene Interaction Network. Frontiers in Microbiology 6:1386.
Ke, Y., Y. Wang, W. Li, and Z. Chen. 2015. Type IV secretion system of Brucella spp. and its effectors. Frontiers in Cellular and Infection Microbiology 5:72.
Kohler, S., V. Foulongne, S. Ouahrani-Bettache, G. Bourg, J. Teyssier, M. Ramuz, and J.P. Liautard. 2002. The analysis of the intramacrophagic virulome of Brucellasuis deciphers the environment encountered by thepathogen inside the macrophage host cell. Proceedings of the National Academy of Sciences of the United States of America 99(24):15711-15716.
Kohler, S., S. Michaux-Charachon, F. Porte, M. Ramuz, and J.P. Liautard. 2003. What is the nature of the replicative niche of a stealthy bug named Brucella?Trends in Microbiology 11(5):215-219.
Kreeger, T.J., M.W. Miller, M.A. Wild, P.H. Elzer, and S.C. Olsen. 2000. Safety and efficacy of Brucella abortus strain RB51 vaccine in captive pregnant elk. Journal of Wildlife Diseases 36(3):477-483.
Kreeger, T.J., W.E. Cook, W.H. Edwards, P.H. Elzer, and S.C. Olsen. 2002. Brucella abortus strain RB51 vaccination in elk. II. Failure of high dosageto prevent abortion. Journal of Wildlife Diseases 38(1):27-31.
Kim, H.S., C.C. Caswell, R. Foreman, R.M. Roop, II, and S. Crosson. 2013. The Brucella abortus general stress response system regulates chronic mammalian infection and is controlled by phosphorylation and proteolysis. Journal of Biological Chemistry 288(19):13906-13916.
Kim, H.S., J.W. Willett, N. Jain-Gupta, A. Fiebig, and S. Crosson. 2014. The Brucella abortus virulence regulator, LovhK, is a sensor kinase in thegeneral stress response signalling pathway. Molecular Microbiology 94(4):913-925.
Ko, J., and G.A. Splitter. 2003. Molecular host-pathogen interaction in brucellosis: Current understanding and future approaches to vaccinedevelopment for mice and humans. Clinical Microbiology Reviews 16(1):65-78.
Lacerda, T.L., S.P. Salcedo, and J.P. Gorvel. 2013. Brucella T4SS: The VIP pass inside host cells. Current Opinion in Microbiology 16(1):45-51.
Lamontagne, J., A. Forest, E. Marazzo, F. Denis, H. Butler, J.F. Michaud, L. Boucher, I. Pedro, A. Villeneuve, D. Sitnikov, K. Trudel, N. Nassif, D. Boudjelti, F. Tomaki, E. Chaves-Olarte, C. Guzman-Verri, S. Brunet, A. Cote-Martin, J. Hunter, E. Moreno, and E. Paramithiotis. 2009. Intracellular adaptation of Brucella abortus. Journal of Proteome Research 8(3):1594-1609.
Lin, Y., Z. Xiang, and Y. He. 2011. Brucellosis Ontology (IDOBRU) as an extension of the Infectious Disease Ontology. Journal of Biomedical Semantics 2(1):9.
Liu, Q., W. Han, C. Sun, L. Zhou, L. Ma, L. Lei, S. Yan, S. Liu, C. Du, and X. Feng. 2012. Deep sequencing-based expression transcriptional profiling changes during Brucella infection. Microbal Pathogenesis 52(5):267-277.
López-Goñi, I., D. García-Yoldi, C.M. Marín, M.J. de Miguel, P.M. Muñoz, J.M. Blasco, I. Jacques, M. Grayon, A. Cloeckaert, A.C. Ferreira, R. Cardoso, M.I. Corrêa de Sá, K. Walravens, D. Albert, and B. Garin-Bastuji. 2008. Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. Journal of Clinical Microbiology 46(10):3484-3487.
Maichak, E.J., B.M. Scurlock, P.C. Cross, J.D. Rogerson, W.H. Edwards, B. Wise, S.G. Smith, and T.J. Kreeger. 2017. Assessment of a strain 19 brucellosis vaccination programin elk. Wildlife Society Bulletin 41:70-79.
McGiven, J., L. Howells, L. Duncombe, J. Stack, N.V. Ganesh, J. Guiard, and D.R. Bundle. 2015. Improved serodiagnosis of bovine brucellosis by novel synthetic oligosaccharide antigens representing the capping m epitope elements of Brucella O-polysaccharide. Journal of Clinical Microbiology 53(4):1204-1210.
Meador, V., B. Deyoe, and N. Cheville. 1989. Effect of nursing on Brucella abortus infection of mammary glands of goats. Veterinary Pathology 26:369-375.
Myeni, S., R. Child, T.W. Ng, J.J. Kupko, III, T.D. Wehrly, S.F. Porcella, L.A. Knodler, and J. Celli. 2013. Brucella modulates secretory trafficking via multiple type IV secretion effector proteins. PLOS Pathogens 9(8):e1003556.
Nicoletti, P. 1980. The epidemiology of bovine brucellosis. Advances in Veterinary Science and Comparative Medicine 24:69-98.
Nielsen, K. 2002. Diagnosis of brucellosis by serology. Veterinary Microbiology 90(1-4):447-459.
Nielsen, K., and J.R. Duncan, eds. 1990. Animal Brucellosis. Boca Raton, FL: CRC Press.
NIH (National Institutes of Health). 2016. Bison bison bison (American bison) Reference Genome. Available online at https://www.ncbi.nlm.nih.gov/assembly/GCF_000754665.1 (accessed December 21, 2016).
O’Callaghan, D., C. Cazevieille, A. Allardet-Servent, M.L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacteriumtumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Molecular Microbiology 33(6):1210-1220.
O’Grady, D., W. Byrne, P. Kelleher, H. O’Callaghan, K. Kenny, T. Heneghan, S. Power, J. Egan, and F. Ryan. 2014. A comparative assessment of cultureand serology in the diagnosis of brucellosis in dairy cattle. The Veterinary Journal 199(3):370-375.
Oliveira, S.C., F.S. de Oliveira, G.C. Macedo, L.A. de Almeida, and N.B. Carvalho. 2008. The role of innate immune receptors in the control of Brucella abortus infection: Toll-like receptors and beyond. Microbes and Infection 10(9):1005-1009.
Olsen, S.C. 2000. Responses of adult cattle to vaccination with a reduced dose of Brucella abortus strain RB51. Research in Veterinary Science69(2):135-140.
Olsen, S.C. 2010. Brucellosis in the United States: Role and significance of wildlife reservoirs. Vaccine 28(Suppl. 5):F73-F76.
Olsen, S.C., and S.D. Holland. 2003. Safety of revaccination of pregnant bison with Brucella abortus strain RB51. Journal of Wildlife Diseases 39(4):824-829.
Olsen, S.C., and C. Johnson. 2011. Comparison of abortion and infection after experimental challenge of pregnant bison and cattle with Brucellaabortus strain 2308. Clinical and Vaccine Immunology 18(12):2075-2078.
Olsen, S.C., and C. Johnson. 2012a. Immune responses and safety after dart or booster vaccination of bison with Brucellaabortus strain RB51. Clinical and Vaccine Immunology 19(5):642-648.
Olsen, S.C., and C.S. Johnson. 2012b. Efficacy of dart or booster vaccination with strain RB51 in protecting bison against experimental Brucellaabortus challenge. Clinicaland Vaccine Immunology 19(6):886-890.
Olsen, S.C., and M.V. Palmer. 2014. Advancement of knowledge of Brucella over the past 50 years. Veterinary Pathology 51(6):1076-1089.
Olsen, S.C., T.J. Kreeger, and W. Schultz. 2002. Immune responses of bison to ballistic or hand vaccination with Brucellaabortus strainRB51. Journal of Wildlife Diseases 38(4):738-745.
Olsen, S.C., A.E. Jensen, W.C. Stoffregen, and M.V. Palmer. 2003. Efficacy of calfhood vaccination with Brucella abortus strain RB51 in protecting bison against brucellosis. Research in Veterinary Science 74(1):17-22.
Olsen, S.C., S.J. Fach, M.V. Palmer, R.E. Sacco, W.C. Stoffregen, and W.R. Waters. 2006. Immune responses of elk to initial and booster vaccinations with Brucellaabortus strain RB51 or 19. Clinical and Vaccine Immunology 13(10):1098-1103.
Olsen, S.C., S.M. Boyle, G.G. Schurig, and N.N. Sriranganathan. 2009. Immune responses and protection against experimental challenge after vaccination of bison with Brucella abortus strain RB51 or RB51 overexpressing superoxide dismutase and glycosyltransferase genes. Clinical and Vaccine Immunology 16(4):535-540.
Olsen, S.C., J.L. McGill, R.E. Sacco, and S.G. Hennager. 2015. Immune responses of bison and efficacy after booster vaccination with Brucellaabortus strain RB51. Clinical and Vaccine Immunology 22(4):440-447.
Palmer, M.V., S.C. Olsen, M.J. Gilsdorf, L.M. Philo, P.R. Clarke, and N.F. Cheville. 1996. Abortion and placentitis in pregnant bison (Bison bison) induced by the vaccine candidate, Brucella abortus strain RB51. American Journal of Veterinary Research 57(11):1604-1607.
Payne, J.M. 1959. The pathogenesis of experimental brucellosis in the pregnant cow. Journal of Pathology and Bacteriology 78:447-463.
Poester, F.P., L.E. Samartino, and R.L. Santos. 2013. Pathogenesis and pathobiology of brucellosis in livestock. Revue Scientifique et Technique Office International des Epizooties 32(1):105-115.
Qin, Q.M., J. Pei, V. Ancona, B.D. Shaw, T.A. Ficht, and P. de Figueiredo. 2008. RNAi screen of endoplasmic reticulum-associated host factors reveals a role for IRE1alpha in supporting Brucella replication. PLOS Pathogens 4(7):e1000110.
Qureshi, T., J.W. Templeton, and L.G. Adams. 1996. Intracellular survival of Brucellaabortus, Mycobacteriumbovis BCG, Salmonella dublin, and Salmonella typhimurium in macrophages from cattle genetically resistant to Brucellaabortus. Veterinary Immunology and Immunopathology 50:55-65.
Radhakrishnan, G.K., Q. Yu, J.S. Harms, and G.A. Splitter. 2009. Brucella TIR domain-containing protein mimics properties of thetoll-like receptor adaptor protein TIRAP. Journal of Biological Chemistry 284(15):9892-9898.
Rajashekara, G., L. Eskra, A. Mathison, E. Petersen, Q. Yu, J. Harms, and G. Splitter. 2006. Brucella: Functional genomics and host-pathogen interactions. AnimalHealth Research Reviews 7(1-2):1-11.
Rhyan, J.C. 2013. Pathogenesis and pathobiology of brucellosis in wildlife. Revue Scientifique et Technique Office International des Epizooties 32(1):127-136.
Rhyan, J.C., W.J. Quinn, L.S. Stackhouse, J.J. Henderson, D.R. Ewalt, J.B. Payeur, M. Johnson, and M. Meagher. 1994. Abortion caused by Brucella abortus biovar 1 in a free-ranging bison (Bison bison) from Yellowstone National Park. Journal of Wildlife Diseases 30(3):445-446.
Rhyan, J.C., S.D. Holland, T. Gidlewski, D.A. Saari, A.E. Jensen, D.R. Ewalt, S.G. Hennager, S.C. Olsen, and N.F. Cheville. 1997. Seminal vesiculitis and orchitis caused by Brucellaabortus biovar 1 in young bison bulls from South Dakota. Journal of Veterinary Diagnostic Investigation 9(4):368-374.
Rhyan, J.C., T. Gidlewski, T.J. Roffe, K. Aune, L.M. Philo, and D.R. Ewalt. 2001. Pathology of brucellosis in bison from Yellowstone National Park. Journal of Wildlife Diseases 37(1):101-109.
Rhyan, J.C., K. Aune, T. Roffe, D. Ewalt, S. Hennager, T. Gidlewski, S. Olsen, and R. Clarke. 2009. Pathogenesis and epidemiology of brucellosis in yellowstone bison: Serologic and culture results from adult females and their progeny. Journal of Wildlife Diseases 45(3):729-739.
Rhyan, J.C., P. Nol, C. Quance, A. Gertonson, J. Belfrage, L. Harris, K. Straka, and S. Robbe-Austerman. 2013. Transmission of brucellosis from elk to cattle and bison, Greater Yellowstone area, U.S.A., 2002-2012. Emerging Infectious Diseases 19(12):1992-1995.
Roffe, T.J., S.C. Olsen, T. Gidlewski, A.E. Jensen, M.V. Palmer, and R. Huber. 1999a. Biosafety of parenteral Brucellaabortus RB51 vaccine bisoncalves. Journal of Wildlife Management 63:950-959.
Roffe, T.J., J.C. Rhyan, K. Aune, L.M. Philo, D.R. Ewalt, T. Gidlewski, and S.G. Hennager. 1999b. Brucellosis in Yellowstone National Park bison: Quantitative serology and infection. Journal of Wildlife Management 63:1132-1137.
Roffe, T.J., L.C. Jones, K. Coffin, M.L. Drew, S.J. Sweeney, S.D. Hagius, P.H. Elzer, and D. Davis. 2004. Efficacy of single calfhood vaccination of elk with Brucella abortus strain 19. Journal of Wildlife Management 68(4):830-836.
Roop, R.M., II, and C.C. Caswell. 2013. Bacterial persistence: Finding the “sweet spot.” Cell Host and Microbe 14(2):119-120.
Rosinha, G.M., D.A. Freitas, A. Miyoshi, V. Azevedo, E. Campos, S.L. Cravero, O. Rossetti, G. Splitter, and S.C. Oliveira. 2002. Identification and characterization of a Brucella abortus ATP-binding cassette transporter homolog to Rhizobiummeliloti ExsA and its role in virulence and protection in mice. Infection and Immunity 70(9):5036-5044.
Rossetti, C.A., C.L. Galindo, H.R. Garner, and L.G. Adams. 2010. Selective amplification of Brucella melitensis mRNA from a mixed host-pathogen totalRNA. BMC Research Notes 3:244.
Rossetti, C.A., K.L. Drake, and L.G. Adams. 2012. Transcriptome analysis of HeLa cells response to Brucella melitensis infection:A molecular approach to understand the role of the mucosalepitheliumin the onset of the Brucella pathogenesis. Microbes and Infection 14(9):756-767.
Rossetti, C.A., K.L. Drake, P. Siddavatam, S.D. Lawhon, J.E. Nunes, T. Gull, S. Khare, R.E. Everts, H.A. Lewin, and L.G. Adams. 2013. Systems biology analysis of Brucella infected Peyer’s patch reveals rapid invasion with modest transient perturbations of the host transcriptome. PLOS ONE 8(12):e81719.
Samartino, L.E., and F.M. Enright. 1993. Pathogenesis of abortion of bovine brucellosis. Comperative Immunology, Microbiology and Infectious Diseases 16(2):95-101.
Sankarasubramanian, J., U.S. Vishnu, V. Dinakaran, J. Sridhar, P. Gunasekaran, and J. Rajendhran. 2016. Computational prediction of secretion systems and secretomes of Brucella: Identification of novel type IVeffectors and their interaction with thehost. Molecular Biosystems 12(1):178-190.
Seabury, C.M., N.D. Halbert, P.J. Gogan, J.W. Templeton, and J.N. Derr. 2005. Bison PRNP genotyping and potential association with Brucella spp. seroprevalence. Animal Genetics 36(2):104-110.
See, W., W.H. Edwards, S. Dauwalter, C. Almendra, M.D. Kardos, J.L. Lowell, R. Wallen, S.L. Cain, W.E. Holben, and G. Luikart. 2012. Yersinia enterocolitica: An unlikely cause of positive bruellosis tests in Greater Yellowstone Ecosystembison (Bison bison). Journal of Wildlife Diseases 48(3):537-541.
Sengupta, D., A. Koblansky, J. Gaines, T. Brown, A.P. West, D. Zhang, T. Nishikawa, S.G. Park, R.M. Roop, II, and S. Ghosh. 2010. Subversion of innate immune responses by Brucella through the targeted degradation of the TLR signaling adapter, MAL. Journal of Immunology 184(2):956-964.
Shumaker, B.A., J.A.K. Mazet, B.J. Gonzales, P.H. Elzer, S.K. Hietala, and M.H. Ziccardi. 2010. Evaluation of the western immunoblot as a detection method for Brucella abortus exposure in elk. Journal of Wildlife Diseases 46(1):87-94.
Silva, T.M., E.A. Costa, T.A. Paixao, R.M. Tsolis, and R.L. Santos. 2011. Laboratory animal models for brucellosis research. Journal of Biomedicine and Biotechnology 2011:518323.
Smith, J.A., M. Khan, D.D. Magnani, J.S. Harms, M. Durward, G.K. Radhakrishnan, Y.P. Liu, and G.A. Splitter. 2013. Brucella induces an unfolded protein response via TcpBthat supports intracellular replication in macrophages. PLOS Pathogens 9(12):e1003785.
Stevens, M.G., S.C. Olsen, and N.F. Cheville. 1995. Comparative analysis of immune responses in cattle vaccinated with Brucella abortus strain 19 or strain RB51. Veterinary Immunology and Immunopathology 44(3-4):223-235.
Sweeney, S.J., C. Emerson, and I.S. Eriks. 2001. Cloning, sequencing, and expression of interferon-gamma from elk in North America. Journal of Wildlife Diseases 37(1):164-171.
Thorne, E.T., and J.K. Morton. 1978. Brucellosis in elk. II. Clinical effects and means of transmission as determined through artificial infections. Journal of Wildlife Diseases 14(3):280-291.
Thorne, E.T., T.J. Kreeger, T.J. Walthall, and H.A. Dawson. 1981. Vaccination of elk with strain 19 Brucellaabortus. Proceedings of the U.S. Animal Health Association 85:359-374.
Tiwari, A., P. Afley, D.K. Sharma, C.S. Bhatnagar, B. Bhardwaj, G.P. Rai, and S. Kumar. 2014. Real-time PCR carried out on DNA extracted from serum or blood sample is not a good method for surveillance of bovine brucellosis. Tropical Animal Health and Production 46(8):1519-1522.
Tobias, L., D.O. Cordes, and G.G. Schurig. 1993. Placental pathology of the pregnant mouse inoculated with Brucella abortus strain 2308. Veterinary Pathology 30(2):119-129.
USDA-APHIS (U.S. Department of Agriculture’s Animal and Plant Health Inspection Service). 2003. Brucellosis Eradication: Uniform Methods and Rules, Effective October 1, 2003. APHIS 91-45-013. Available online at https://www.aphis.usda.gov/animal_health/animal_diseases/brucellosis/downloads/umr_bovine_bruc.pdf (accessed January 6, 2017).
USDA-APHIS. 2014. Standard Operating Procedures for Submission and Testing of Brucellosis Serological Specimens. APHIS/Veterinary Services Approved Brucellosis Laboratories. Available online at https://www.aphis.usda.gov/animal_health/animal_diseases/brucellosis/downloads/aphis_approved_br_sero_labs_sop.pdf (accessed January 6, 2017).
Van Campen, H., and J. Rhyan. 2010. The role of wildlife in diseases of cattle. Veterinary Clinics of North America: Food Animal Practice 26(1):147-161.
Van Houten, C.K., E.L. Belden, T.J. Kreeger, E.S. Williams, W.H. Edwards, E.T. Thorne, W.E. Cook, and K.W. Mills. 2003. Validation of a Brucellaabortus competitiveenzyme-linked immunosorbent assay for use in Rocky Mountain elk (CervusElaphusNelsoni). Journal of Wildlife Diseases 39(2):316-322.
Viadas, C., M.C. Rodriguez, F.J. Sangari, J.P. Gorvel, J.M. Garcia-Lobo, and I. Lopez-Goni. 2010. Transcriptome analysis of the Brucellaabortus BvrR/BvrS two-component regulatory system. PLOS ONE 5(4):e10216.
Vishnu, U.S., J. Sankarasubramanian, P. Gunasekaran, and J. Rajendhran. 2015. Novel vaccine candidates against Brucellamelitensis identified through reverse vaccinology approach. OMICS 19(11):722-729.
von Bargen, K., J.P. Gorvel, and S.P. Salcedo. 2012. Internal affairs: Investigating the Brucella intracellular lifestyle. FEMS Microbiology Reviews 36(3):533-562.
Wang, F., S. Hu, W. Liu, Z. Qiao, Y. Gao, and Z. Bu. 2011. Deep-sequencing analysis of the mouse transcriptome response to infection with Brucella melitensis strains of differing virulence. PLOS ONE 6(12):e28485.
Wang, Y., Y. Ke, Z. Wang, X. Yuan, Y. Qiu, Q. Zhen, J. Xu, T. Li, D. Wang, L. Huang, and Z. Chen. 2012. Genome sequences of three live attenuated vaccine strains of Brucella species and implications for patho-genesis and differential diagnosis. Journal of Bacteriology 194(21):6012-6013.
Weeks, J.N., C.L. Galindo, K.L. Drake, G.L. Adams, H.R. Garner, and T.A. Ficht. 2010. Brucella melitensis VjbR and C12-HSL regulons: Contributions of the N-dodecanoyl homoserine lactone signaling molecule and LuxR homologueVjbR to gene expression. BMC Microbiology 10:167.
Westhusin, M.E., T. Shin, J.W. Templeton, R.C. Burghardt, and L.G. Adams. 2007. Rescuing valuable genomes by animal cloning: A case for natural disease resistance in cattle. Journal of Animal Science 85:138-142.
Williams, E.S., E.T. Thorne, S.L. Anderson, and J.D. Herriges, Jr. 1993. Brucellosis in free-ranging bison (Bison bison) from Teton County, Wyoming. Journal of Wildlife Diseases 29(1):118-122.
Xavier, M.N., T.A. Paixao, F.P. Poester, A.P. Lage, and R.L. Santos. 2009. Pathological, immunohisto chemical and bacteriological study of tissues and milk of cows and fetuses experimentally infected with Brucella abortus. Journal of Comperative Pathology 140(2-3):149-157.
Xavier, M.N., T.A. Paixão, A.B. den Hartigh, R.M. Tsolis, and R.L. Santos. 2010. Pathogenesis of Brucella spp. The Open Veterinary Science Journal 4:109-118.
Xavier, M.N., M.G. Winter, A.M. Spees, K. Nguyen, V.L. Atluri, T.M. Silva, A.J. Baumler, W. Muller, R.L. Santos, and R.M. Tsolis. 2013. CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLOS Pathogens 9(6):e1003454.


















