4
Effect of Polybrominated Diphenyl Ethers on Neurodevelopment
Polybrominated diphenyl ethers (PBDEs) are a class of brominated hydrocarbons that have been used as flame retardants in a variety of products, such as textiles, plastics, electronic materials, and polyurethane foams for furniture (EPA 2010). The class is comprised of 209 congeners that share a brominated diphenyl ether molecule with up to 10 bromine atoms attached (see Figure 4-1). Three classes of commercial formulations of PBDE mixtures were produced—the pentaPBDEs, octaPBDEs, and decaPBDEs—but they are no longer produced or used. They are ubiquitous in the environment; they have been shown to be persistent and to bioaccumulate; and human exposure to them has been well documented (Bramwell et al. 2016).
PBDEs can undergo a variety of metabolic changes depending on their structure and the degree of bromine substitution (Stapleton et al. 2009). For example, rodents given 2,2′,4,4′,5-penta-bromodiphenyl ether (BDE-99) produce hydroxylated congeners (OH-BDE) and other metabolites (Chen et al. 2006; Qiu et al. 2007). BDE-99 undergoes more extensive metabolism when compared with either BDE-47, 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE-153), or 2,2′,3,3′,4,4′,5,5′,6,6′-deca-bromodiphenyl ether (BDE-209) (Chen et al. 2006; Staskal et al. 2006). Glucuronide and sulfate conjugates of dibromophenols and tribromophenols are also formed and excreted in urine and feces (Ho et al. 2015).
PBDEs have been measured in the air, soil, dust, and various foods (EPA 2010). The predominant congeners evaluated and detected are BDE-47, BDE-99, and BDE-153. One approach to monitor human exposure to PBDEs involves measurement of BDE, OH-BDEs, and methoxylated (MeO-BDEs) congeners in blood, milk, and other biological samples (EPA 2010; Wiseman et al. 2011; Butryn et al. 2015). The apparent half-lives of octaBDEs and decaBDEs in human serum are less than 3 months (Thuresson et al. 2006), whereas the half-lives for pentaBDEs may be 2 years or more (Geyer et al. 2004).
PBDEs have been linked to effects on the liver and the nervous system (EFSA 2011; Linares et al. 2015). Concerns related to possible endocrine-disrupting effects associated with PBDEs and OH-BDEs also exist (EFSA 2011; Linares et al. 2015). The OH-BDEs have greater endocrine activity than the parent PBDE does (Ptak et al. 2005, 2006). Certain PBDEs and OH-BDEs alter estrogen receptor alpha and estrogen receptor beta activity in cultured cells (Meerts et al. 2001), rats (Ceccatelli et al. 2006), and mice (Mercado-Feliciano and Bigsby 2008). In vitro, OH-BDEs also affect aromatase (CYP19) and steroid 17α-monooxygenase (CYP17) activities, two enzymes involved in estrogen and androgen synthesis (Canton et al. 2005; Canton et al. 2008; He et al. 2008; Karpeta et al. 2013). Changes in female sexual behavior and estrous cyclicity and alterations in expression patterns of estrogen-regulated genes in sexually dimorphic brain regions occur in rats following in utero exposure to BDE-99 (Lilienthal et al. 2006; Faass et al. 2013). The PBDEs also affect thyroid hormone homeostasis in a number of ways. OH-BDEs share structural similarities with thyroid hormones and bind thyroid hormone receptors and serum thyroid hormone binding proteins and lower free and total thyroxine (T4) concentrations in animals (Fowles et al. 1994; Darnerud and Sinjari 1996; Meerts et al. 2000; Siddiqi et al. 2003; Linares et al. 2015).
PBDE effects on the developing brain are receiving considerable attention in the public-health community. The possible association between PBDE exposure and neurodevelopmental effects was originally considered for a topic at the committee’s workshop on February 3, 2016, which was designed to explore potential case studies and to help the committee select the topics for its systematic reviews. During the process of planning the workshop, the committee became aware of a systematic review on the topic that was already under way by Lam et al. (2015, 2016). The focus of the Lam et al. review was the possible association between PBDE exposure and measures of intelligence or attention deficit/hyperactivity disorder (ADHD) and attention-related behavioral conditions in children. The committee judged that a systematic review of animal studies on PBDE exposure and neurodevelopmental outcomes would be an appropriate complement to that review of the human literature. Because the committee’s goal was to identify end points that could be considered relevant for human intelligence and ADHD and other attention-related behavioral conditions, the committee’s systematic review of the animal data focused on PBDE studies that measured learning, memory, attention, and response inhibition. Animal studies that solely evaluated motor function, fear conditioning, and other tests that were not directly linked to learning, memory, attention, or response inhibition were considered outside the scope of the systematic review. This chapter describes the systematic review of the animal evidence on PBDE neurodevelopmental effects that the committee performed. It also uses the review of human evidence to demonstrate how an existing systematic review can be critically evaluated, used, and updated. The separate lines of evidence are subsequently integrated with mechanistic information to draw conclusions about associations.
SYSTEMATIC REVIEW METHODS
The systematic reviews were designed to answer two related questions:
- Is developmental exposure to PBDEs in nonhuman mammals associated with alterations in learning, memory, attention, or response inhibition? This question was addressed through the conduct of an independent systematic review of the relevant animal literature.
- Is developmental exposure to PBDEs in humans associated with alterations in quantitative measures of intelligence or ADHD and attention-related behavioral conditions? The committee updated a recent systematic review of the human literature by Lam et al. (2015, 2016) to address the question.
The PECO (Population, Exposure, Comparator, and Outcome) statements for the systematic reviews of the animal and the human studies are presented in Boxes 4-1 and 4-2, and the protocols used to conduct the systematic reviews are provided in Appendix E (Section E-1) and Appendix F (Section F-1), respectively. The protocols were peer reviewed in accordance with the standard report-review practices of the National Academies of Sciences, Engineering, and Medicine. Most of the peer reviewers of the protocols
were also peer reviewers of this report to ensure that the original protocols were followed and that any revisions or updates have been appropriately documented and justified. (See the Acknowledgments for the list of peer reviewers.) A summary of the methods is briefly described below.
Literature Searches and Screening
Searches for relevant existing systematic reviews were performed first. PubMed was searched for systematic reviews published in 2013 or later, and the systematic-review protocol registries PROSPERO and CAMARADES were searched for relevant protocols on August 3, 2016. Citations found in searching for systematic reviews of PBDEs and measures of intelligence or ADHD and attention-related behavioral conditions were considered a systematic review if they met the following minimum criteria: (1) conducted an explicit and adequate literature search; (2) applied predefined eligibility criteria; (3) considered the quality of included studies or risk of bias assessment; and (4) synthesized (or attempted to synthesize) the findings, either qualitatively or quantitatively. This definition of a systematic review is consistent with previous Institute of Medicine recommendations that defined a systematic review as “a scientific investigation that focuses on a specific question and uses explicit, prespecified scientific methods to identify, select, assess, and summarize the findings of similar but separate studies” (IOM 2011, p. 1).
Animal Evidence
For the independent systematic review of the animal evidence, scientific literature databases were searched for relevant studies on the effects of developmental exposure to PBDEs on measures of learning, memory, attention, or response inhibition in nonhuman mammals. A medical librarian, with specific training and expertise in performing searches for systematic reviews, developed and conducted the searches. Searches to support the systematic review of the animal studies were performed by the librarian in PubMed, Embase, and Toxline on August 15, 2016. The search strategies for the animal data are presented in the protocol provided in Appendix E (Section E-1b).
References were screened at the title and abstract level and at the full-text level by the same two reviewers using DistillerSR (https://www.evidencepartners.com). The screening criteria used are specified in the protocol for the animal systematic review (see Appendix E, Section E-1c). At the title and abstract screening level, if there was disagreement between the reviewers or an abstract was not available, the reference was passed on to the full-text screening level for further review. At the full-text level, disagreements about whether to include a study were discussed by the two reviewers to reach consensus; if consensus could not be reached, a third team member was consulted to resolve the differences.
Human Evidence
Literature searches were performed to update the systematic review performed by Lam et al. (2015, 2016). A librarian used the same literature search methods and databases as the existing review to search for new reports on September 28, 2016, and searched from a year before the last search date of the review so that there was a 1-year overlap between the two searches.
References were screened at the title and abstract level and at the full-text level by the same two reviewers using DistillerSR (https://www.evidencepartners.com). The screening criteria used are specified in protocol for the existing human systematic review. At the title and abstract screening level, if there was disagreement between the reviewers or an abstract was not available, the reference was passed on to the full-text screening level for further review. At the full-text level, disagreements about whether to include a study were discussed by the two reviewers to reach consensus; if consensus could not be reached, a third team member was consulted to resolve the differences.
Evaluating a Systematic Review
The Lam et al. (2015, 2016) systematic review was evaluated for risk of bias using the ROBIS tool (Whiting et al. 2016). The tool has three evaluation phases: phase 1 involves assessing the relevance; phase 2 involves identifying concerns with the review process; and phase 3 involves judging risk of bias. In the first phase, the relevance of the systematic review was assessed by comparing the committee’s tar-
get question with the question being addressed in the review. The PECO framework was used to assess the match between the target question and the question addressed by the review. This phase was informally completed by the entire committee early in the process (immediately following its workshop in February 2016), and the committee’s systematic review protocol was designed to be an update of an existing systematic review.
The next two phases of applying the ROBIS tool involved two committee members who used the tool to identify concerns with the review process and to judge risk of bias in the review. Four domains were used to identify concerns with the review process: (1) study eligibility criteria, (2) identification and selection of studies, (3) data collection and study appraisal, and (4) synthesis and findings. Included for each domain were questions to help assess specific issues with potential biases. The response to the questions helped in judging overall risk of bias for each domain. In the third phase, the overall risk of bias in the review (low, high, or unclear) was determined.
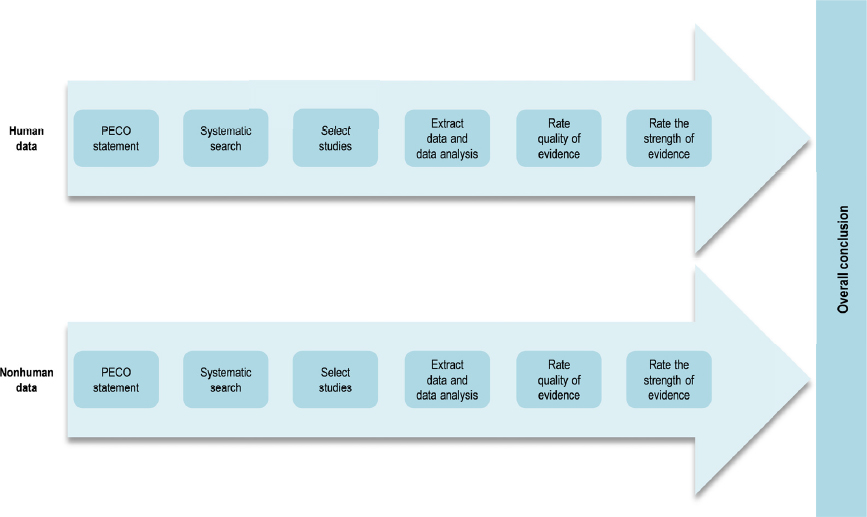
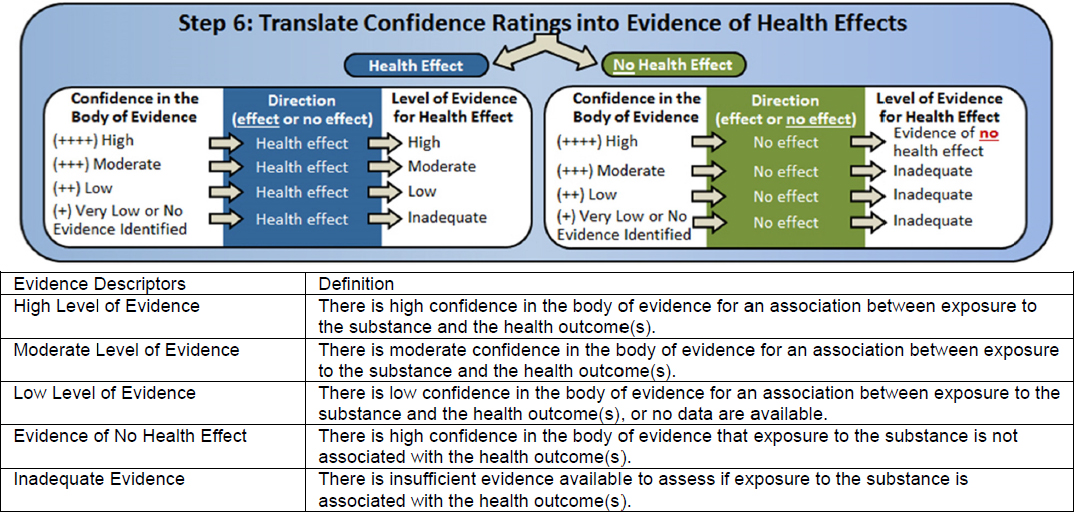
In contrast with the animal systematic review, which followed methods developed by the National Toxicology Program’s (NTP’s) Office of Health Assessment and Translation (OHAT), the Lam et al. (2015, 2016) review of the human evidence used the Navigation Guide methodology (Woodruff and Sutton 2014). Figure 4-2 illustrates the steps of the Navigation Guide method. The two approaches are very similar (see Table 1-1 in Chapter 1 for a side-by-side comparison), and they are based on the same established methodology for the conduct of systematic review and evidence assessment (e.g., Cochrane Collaboration, AHRQ Evidence-based Practice Center Program, and GRADE). Both the OHAT and Navigation Guide methods include the key steps recommended by a previous National Academies committee (NRC 2014) for problem formulation, protocol development, specifying a study question, developing a PECO statement, identifying and selecting the evidence, evaluating the evidence, and integrating the evidence. Different terminology, however, is used to describe the results of analyzing and integrating the evidence. Consequently, it was necessary for the committee to “translate” the findings from the Lam et al. (2015, 2016) systematic review into OHAT ratings for the evidence integration (discussed later in this section).
Data Extraction
Animal data from the included studies were entered into the Health Assessment Workspace Collaborative (HAWC), a Web-based interface application for warehousing data and creating visualizations (https://hawcproject.org). See Appendix E, Section E-1d, for data extraction elements for the animal studies. One person entered data and a second person verified the entries. All data entered into HAWC are available at the following link: https://hawcproject.org/assessment/352/. For the human evidence, new information was summarized and considered in context with the results of the existing systematic review.
Risk of Bias and Study Quality Evaluations
Risk of bias is related to the internal validity of a study and reflects study-design characteristics that can introduce a systematic error (or deviation from the true effect) that might affect the magnitude and even the direction of the apparent effect. Internal validity or risk of bias was assessed for individual animal studies using the OHAT method that outlines an approach to evaluating risk of bias for experimental animal studies (NTP 2015). The criteria were customized from the basic OHAT method and described in the protocol for addressing the specific research question for this review (e.g., appropriate methods for PBDE exposure characterization and use of litter as the unit of analysis) (Appendix E, Section E-1e). Key risk of bias elements for animal studies included reliability of the outcome measure, blinding of researchers to treatment groups, and whether investigators controlled for litter effects in their experimental design or statistical approaches. Two committee members independently assessed each study and answered all applicable risk of bias questions following prespecified criteria detailed in the study protocol. One individual of the pair then reconciled any discrepancies with input from the second committee member.
For the human studies, the committee accepted the risk of bias approach used in the Lam et al. (2015, 2016) review. The review was performed similarly and included risk of bias domains very similar to those used by NTP (2015). The Navigation Guide examines nine risk of bias domains: source population representation, blinding, exposure assessment, outcome assessment, confounding, incomplete outcome data, selective outcome reporting, conflict of interest, and other sources of bias (Woodruff and Sutton 2014). Similar to the OHAT method, each domain was rated as low, probably low, probably high, high, or not applicable.
Data Analysis and Evidence Integration
For the evaluation of the animal evidence, the body of evidence on each outcome was synthesized qualitatively and, where appropriate, a meta-analysis was performed. Summaries of main characteristics for each included study were compiled and reviewed by two committee members to determine comparability between studies, to identify data transformations necessary to ensure comparability, and to determine whether heterogeneity was a concern. The main characteristics considered across all eligible studies included the following:
- Experimental design (e.g., acute, chronic, multigenerational);
- Animal model used (e.g., species, strain, sex, genetic background);
- Age of animals (e.g., at start of treatment, mating, and/or pregnancy status);
- Developmental stage of animals at treatment and outcome assessment;
- Dose levels, frequency of treatment, timing, duration, and exposure route;
- Health outcome(s) and specific measures reported;
- Type of data (e.g., continuous or dichotomous), statistics presented in paper; and
- Variation in the degree of risk of bias at individual study level.
For the human evidence, the expanded body of evidence was synthesized qualitatively, and a determination was made about whether the new information would substantially affect the conclusions drawn
in the Lam et al. (2015, 2016) systematic review. If the data were determined to materially affect the evidence base, any quantitative evaluations performed in the original review were updated.
Confidence Rating and Level of Evidence Conclusions
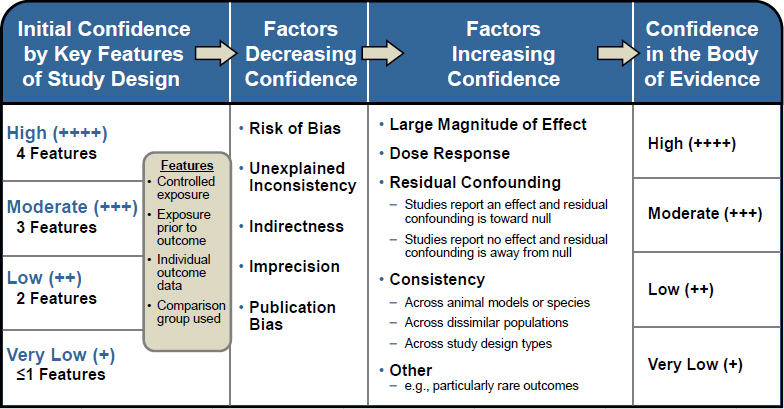
For the animal systematic review, the confidence in the body of evidence for each outcome was evaluated using a grading system based on a modification of the GRADE system for rating certainty in the body of evidence (Guyatt et al. 2011; Rooney et al. 2014). The process for rating confidence in the body of evidence as high, moderate, low, or very low was guided by the OHAT Handbook for Conducting a Literature-Based Health Assessment (NTP 2015) (see Figure 4-3). In brief, studies on a particular outcome were initially grouped by key study design features, and each grouping of studies was given an initial confidence rating by those features. Several factors were then considered to determine whether the initial rating should be downgraded or upgraded. Factors that decrease confidence in results and lead to downgrading are risk of bias, unexplained inconsistency in results, indirectness or lack of applicability, imprecision, and publication bias. Factors that increase confidence in results and can upgrade a rating are a large magnitude of effect; evidence of a dose-response relationship; consistency across study designs, populations, animal models, or species; consideration of residual confounding; and other factors that increase confidence in the association or effect (e.g., rare outcomes). Confidence ratings were independently assessed by two committee members, and discrepancies were resolved by consensus and consultation with a third committee member as needed. After a final confidence rating is determined, the rating is translated into a level of evidence using the scheme presented in Figure 4-4.
The Navigation Guide methodology was used by Lam et al. (2015, 2016) to evaluate the body of evidence from human studies. The approach is similar to the OHAT method in that both methods use the GRADE approach to evaluating confidence in the body of evidence; many groups use different terminology for this step, however, and the terminology has changed from “quality” to “confidence” and most recently to “certainty” within the GRADE framework (Morgan et al. 2016; Rooney et al. 2017). The Navigation Guide uses the term “quality” of the body of evidence, whereas OHAT method uses the term “confidence” in the body of evidence to reflect the GRADE-based evaluation. The basis for determining the ratings is similar in the two methods and involves a similar process of giving an initial rating to the evidence and then considering factors that could upgrade or downgrade the rating. All human observational studies start out with an initial moderate rating in the Navigation Guide method, and the initial confidence rating assigned to studies in the OHAT method depends on aspects of study design (see Figure 4-3). Cohort and case-control studies would also start out as moderate under the OHAT method, but cross-sectional studies would start at low initial confidence because the study design cannot assure that exposure preceded outcomes. Both methods use the same eight factors from GRADE to consider potential upgrades or downgrades to the body of evidence (risk of bias, unexplained inconsistency, indirectness, imprecision, publication bias, large magnitude of effect, dose response, and residual confounding). The OHAT method also considers two additional potential upgrades for consistency across study designs or diverse populations and for other factors, such as particularly rare outcomes. Although these factors are not part of the Navigation Guide method for rating quality/certainty, they are considered in the next step, so the methods differ in the sequence of consideration of these factors rather than any difference in approach.
After rating the quality/confidence in the body of evidence, a determination is made about the “strength” of the evidence using the Navigation Guide (see Figure 4-2) and about the “level” of evidence using the OHAT method (see Figure 4-4). Both determination schemes consider the GRADE quality/confidence rating in the body of evidence. Then the Navigation Guide also evaluates the likelihood that a new study would change the conclusion and other compelling attributes of the data that might influence certainty. Because the systematic review conducted by the committee on the animal evidence followed the OHAT method, the Lam et al. (2015, 2016) review is described below using the closest equivalent OHAT terminology.
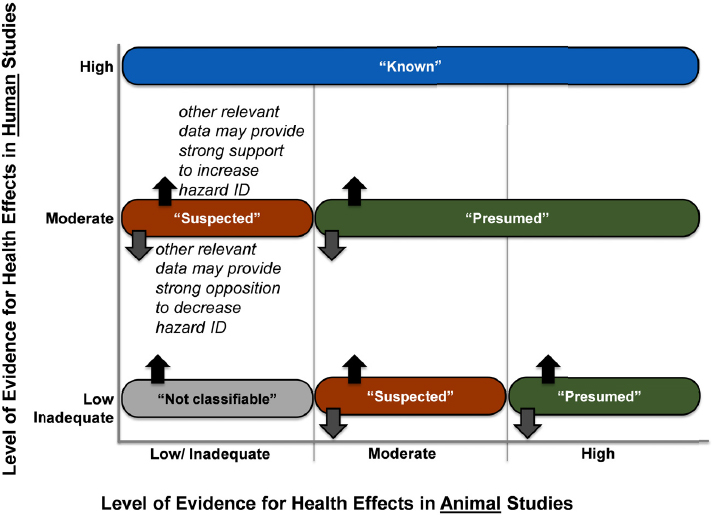
Integration of Evidence and Drawing Hazard Identification Conclusions
The committee used guidance from OHAT to draw hazard identification conclusions (NTP 2015). The procedure involves integrating the levels of evidence ratings for the human and the animal data and considering them within the context of mechanistic information. The five possible hazard conclusions are (1) known, (2) presumed, (3) suspected, (4) not classifiable, or (5) not identified to be a hazard to humans. If either the animal or the human evidence stream has been described as having inadequate evidence, conclusions are drawn on the basis of a single evidence base. The hazard identification scheme is presented in Figure 4-5.
RESULTS
Literature Search and Screening Results
Animal Studies
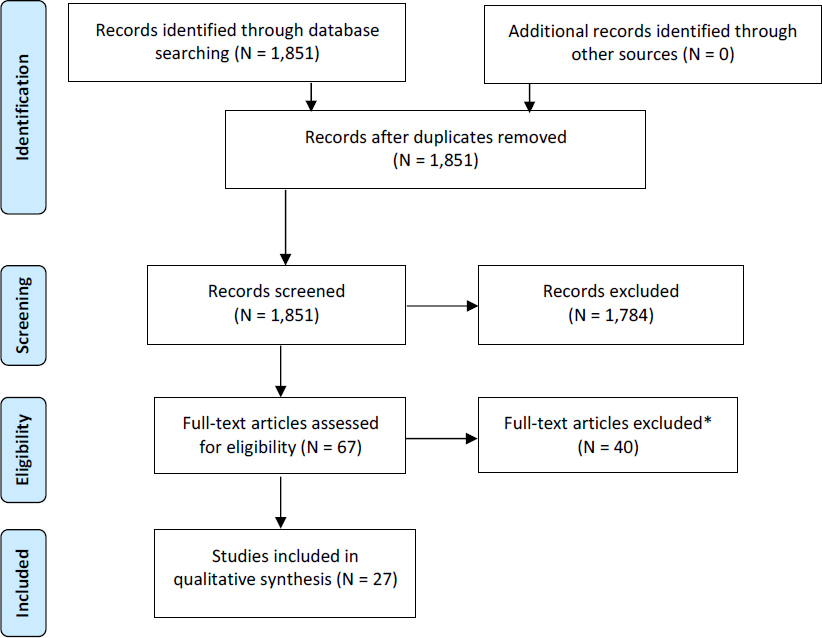
A search for recently published systematic reviews on developmental exposure to PBDEs and alterations in learning, memory, attention, or response inhibition in nonhuman mammals found no relevant reviews. No relevant protocols of ongoing reviews were found in PROSPERO or CAMARADES either, so an independent systematic review of the animal literature was performed. A search of PubMed, Embase, and Toxline for relevant publications to address the PECO statement found 1,851 unique citations (see Appendix E, Section E-2, for breakdown by database). A total of 67 publications met the criteria for full-text review, and 27 of those met the inclusion criteria for data extraction (see Figure 4-6). A review of the reference lists of the 27 included studies found no additional publications that were potentially relevant. Thus, 27 publications were included in the review (see Box 4-3).
Human Studies
A search for existing systematic reviews on developmental exposure to PBDEs and effects on intelligence or attention-related conditions in humans found 18 reports (see Appendix F, Section F-2). After screening at the title and abstract level, two reports were evaluated at the full-text level (Roth and Wilks 2014; Lam et al. 2015). One publication was found in the search of PubMed (Roth and Wilks 2014), but it did not meet the criteria established for an appropriate and relevant systematic review because the literature search was restricted to articles published since January 1, 2006, and no formal risk of bias assessment of the studies was performed. A relevant systematic review protocol was found in PROSPERO (Lam et al. 2015); this was the same review that the committee identified in preparing for its workshop. The authors of this systematic review provided the committee with an interim draft of their systematic review (Lam et al. 2016).
For the update of the Lam et al. (2015, 2016) review, a search of BIOSIS, Embase, PubMed, ToxNet/DART, and Web of Science and relevant websites found 19 publications that met the criteria for full-text review, and three of those met the inclusion criteria (see Figure 4-7). The three reports (Cowell et al. 2015; Sagiv et al. 2015; Zhang et al. 2017) were follow-up assessments of three cohorts already included in the Lam et al. (2015, 2016) review.
Evaluation of the Lam et al. (2015, 2016) Review
The ROBIS tool (Whiting et al. 2016) was used to assess the protocol of the Lam et al. (2015, 2016) review. Two committee members independently assessed the risk of bias for the review and were in agreement in their evaluations. Consequently, it was unnecessary to consult a third committee member to achieve consensus. For each of the four domains, and for the overall assessment of the review, the committee members agreed that the risk of bias was low. No concerns were raised for any of the domains. For instance, there was a comprehensive search, with predefined eligibility criteria applied by two independent committee members.
Two minor issues were identified by the committee members, but neither was judged sufficient to change the overall evaluation. One issue was the possible need to consider updating the risk of bias assessment in the systematic review to the ROBINS-I tool (Sterne et al. 2016). This tool is new, however, and was released only recently, so the committee judged that it should not affect the “data collection and study appraisal”. The second issue was in the choice of model used in the meta-analyses. The systematic review used the DerSimonian-Laird estimator, and concerns have been raised recently about the model potentially providing biased estimates (Cornell et al. 2014). Currently, however, there is no agreement as to the most appropriate model to use for random effects meta-analysis, so the use of this model did not impact the assessment for the “synthesis and findings” domain.
Health Effects Results
Animal Health Effects Results
A wide range of behavioral tests was used to evaluate learning, memory, and attention in rodents exposed to PBDEs during development (see Table 4-1). The most commonly used test of learning and memory in association with PBDEs was the Morris water maze. In this test, animals are typically placed in a large circular pool of water and required to escape by finding and climbing onto a hidden platform. A variety of behaviors can be assessed using this task, including spatial memory (often assessed using a probe trial in which the platform is removed from the pool) or reversal learning (the platform is moved to a different quadrant of the pool). Outcome measures can include escape latency across trials, path length, swim speed, and animal orientation in relation to the platform location. For the purposes of this review, acquisition and reversal learning were considered tests of learning whereas performance in the probe trial was considered an assessment of memory.
Other behavioral tests of learning and memory included the Barnes spatial maze (Koenig et al. 2012); passive avoidance (Zhang et al. 2013); Y maze (Llansola et al. 2009); water T maze (Biesemeier et al. 2011); radial arm maze (Fischer et al. 2008; Verma et al. 2013; de-Miranda et al. 2016); visual discrimination (Dufault et al. 2005; Rice et al. 2009); and operant conditioning test paradigms (Rice et al. 2009). Some studies investigated attention (Driscoll et al. 2009, 2012). For example, Driscoll et al. (2009) provided visual cues with a variable (0-6 sec) pre-cue delay and, in some experiments, also a variable cue duration of 200 to 800 ms. These tasks required the animal to sustain visual attention across five nose poke portals for an indeterminate period of time. There was considerable variability in the animal models used: rats of the Sprague-Dawley, Wistar, and Long Evans strains; mice of the NMRI, C57Bl/6J, apoE2, apoE3, apoE4, Swiss albino, and Mecp2 308+/− strains. The duration of exposure was also highly variable and included acute (single-day) and repeated exposures during gestation and lactation. In general, standardized test methods were not used across studies.
Studies relevant to the systematic review were available on six BDE congeners (BDE-47, -99, -153, -203, -206, and -209) and one technical grade flame retardant mixture (DE-71; a mixture of 24 BDE congeners [Konstantinova et al. 2008]). Studies on learning were available for all these BDEs; studies of memory were available on six of them (BDE-47, -99, -153, -203, and -209 and DE-71); and a study of attention was available on the one mixture (DE-71). No studies on response inhibition were found for any of the BDEs. A variety of different behavioral tests and test parameters were measured; measurements were taken multiple times a day or over several days; and data were presented in different ways. Because of this heterogeneity, the committee found that the PBDE animal data did not lend themselves to creating useful visualizations in HAWC. Thus, summary tables of the evidence were created from the data sets entered into HAWC for evaluation. Confidence in the bodies of evidence on these end points were evaluated for each of the congeners by considering the number of studies available and evaluating factors that would decrease or increase confidence in evidence. All animal studies started with an initial confidence rating of high, because the exposures were controlled, doses were administered before the outcomes were evaluated, individual outcome data were reported, and a comparison group was used. Factors that would upgrade or downgrade confidence in the body of evidence were then considered to determine a final confidence rating. Documentation of how the evidence was evaluated and how the confidence ratings were determined is provided for BDE-47 in this chapter; for the other BDEs, short summaries are provided and are supported by details in Appendix E, Section E-4.
BDE-47 and Learning and Memory
There is moderate confidence in the body of evidence on developmental exposure to BDE-47 and effects on learning in rodents. Six studies of learning were available (see Table 4-2). Two studies in rats found several indications of decreased learning in the Morris water maze (e.g., prolonged latency to find
TABLE 4-1 Studies Included in the PBDE (Animal) Systematic Review
| Study | Chemical | Species (strain) | Life Stage Exposed | Life Stage Assessed | Test(s) | Doses (mg/kg-day) |
|---|---|---|---|---|---|---|
| Eriksson et al. 2001 | BDE-47 | Mouse (NMRI) | PND 10 | 5 months | Morris water maze | 0, 10.5 |
| He et al. 2009 | BDE-47 | Rat (Sprague-Dawley) | PND 10 | 2 months | Morris water maze | 0, 1, 5, 10 |
| He et al. 2011 | BDE-47 | Rat (Sprague-Dawley) | PND 10 | 2 months | Morris water maze | 0, 1, 5, 10 |
| Koenig et al. 2012 | BDE-47 | Mouse (C57Bl/6J) | 4 weeks before breeding to PND 21 | 8 weeks | Barnes spatial maze | 0, 0.03, 0.1, 1 |
| Ta et al. 2011 | BDE-47 | Mouse (C57BL/6J) | GD 0 - PND 21 | 8 weeks | Morris water maze | 0, 0.03, 0.1, 1 |
| Woods et al. 2012 | BDE-47 | Mouse (Mecp2 308+/-) Mouse (wild type) | GD 0 - PND 21 | PND 50-54 | Morris water maze | 0, 0.03 |
| Blanco et al. 2013 | BDE-99 | Rat (Sprague-Dawley) | GD 6 - PND 21 | PND 26-35 | Morris water maze | 0, 1, 2 |
| Cheng et al. 2009 | BDE-99 | Rat (Sprague-Dawley) | GD 6 - PND 21 | PND 34-36 | Morris water maze | 0, 2 |
| Eriksson et al. 2001 | BDE-99 | Mouse (NMRI) | PND 10 | 5 months | Morris water maze | 0, 12 |
| Fischer et al. 2008 | BDE-99 | Mouse (NMRI) | PND 10 | 4 and 6 months | Morris water maze; radial arm maze | 0, 0.8 |
| Llansola et al. 2009 | BDE-99 | Rat (Wistar) | GD 2-9 or GD 11-19 | PND 68–70 | Y maze | 0, 30 (IP) |
| Zhao et al. 2014 | BDE-99 | Rat (Sprague-Dawley) | GD 1 - PND 21 | PND 34-36 | Morris water maze | 0, 0.2 |
| Viberg et al. 2003 | BDE-153 | Mouse (NMRI) | PND 10 | PND 180 | Morris water maze | 0, 0.45, 0.9, 9 |
| Zhang et al. 2013 | BDE-153 | Rat (Sprague-Dawley) | PND 10 | PND 40 and 70 | Morris water maze; passive avoidance | 0, 1, 5, 10 (IP) |
| Viberg et al. 2006 | BDE-203 | Mouse (NMRI) | PND 3 or 10 | PND 90 | Morris water maze | 0, 16.8 |
| Viberg et al. 2006 | BDE-206 | Mouse (NMRI) | PND 10 | PND 90 | Morris water maze | 0, 18.5 |
| Biesemeier et al. 2011 | BDE-209 | Rat (Sprague-Dawley) | GD 6 - PND 21 | PND 22, PND 62 | Water T maze | 0, 1, 10, 100, 1000 |
| Buratovic et al. 2014 | BDE-209 | Mouse (NMRI) | PND 3 | 5 and 7 months | Morris water maze | 0, 3.4, 7.9 |
| Chen et al. 2014 | BDE-209 | Rat (Sprague-Dawley) | GD 1-14 | PND 25 | Morris water maze | 0, 10.0, 30, 50 |
| Reverte et al. 2013 | BDE-209 | Mouse (apoE2) Mouse (apoE3) Mouse (apoE4) | PND 10 | PND 120 and 360 | Morris water maze | 0, 10, 30 |
| Reverte et al. 2014 | BDE-209 | Mouse (apoE2) Mouse (apoE3) Mouse (apoE4) | PND 10 | PND 150-180 | Fear conditioning | 0, 10, 30 |
| Rice et al. 2009 | BDE-209 | Mouse (C57BL6/J) | PND 2-15 | PND 87 or PND 497 | Operant (fixed ratio; fixed interval; visual discrimination) | 0, 6, 20 |
| Study | Chemical | Species (strain) | Life Stage Exposed | Life Stage Assessed | Test(s) | Doses (mg/kg-day) |
|---|---|---|---|---|---|---|
| Verma et al. 2013 | BDE-209 | Mouse (Swiss albino) | PND 3-10 | PND 60-66 | Morris water maze; radial arm maze | 0, 20 |
| Verma et al. 2014 | BDE-209 | Mouse (Swiss albino) | PND 3-10 | NR | Morris water maze | 0, 20 |
| Bowers et al. 2015 | DE-71 | Rat (SpragueDawley) | GD 1 - PND 21 | PND 235 | Morris water maze | 0, 0.3, 3, 30 |
| de-Miranda et al. 2016 | DE-71 | Rat (Wistar) | PND 5-22 | PND 100 | Radial maze learning | 0, 30 |
| Driscoll et al. 2009 | DE-71 | Rat (Long-Evans) | GD 0 - PND 21 | PND 40-95 | Visual discrimination; attention task | 0, 3 or 0, 4.5 |
| Driscoll et al. 2012 | DE-71 | Rat (Long-Evans) | PND 6-12 | PND 40-95 | Visual task; attention task | 0, 5, 15 |
| Dufault et al. 2005 | DE-71 | Rat (Long-Evans) | PND 6-12 | PND 30 | Visual discrimination; attention task | 0, 30 |
NOTES: Unless otherwise noted the studies involved oral exposure. GD, gestation day; IP, intraperitoneal; PND, postnatal day; NR, not reported.
the platform) after developmental exposure to BDE-47. Both studies were from the same laboratory (He et al. 2009, 2011). Three of the four mouse studies reported decreased learning in at least one test, strain, or sex and were conducted by different research groups (Eriksson et al. 2001; Ta et al. 2011; Koenig et al. 2012; Woods et al. 2012). The mouse results were variable depending on the tests administered, and a clear pattern was not identified to explain the heterogeneity in response relative to a susceptible strain, to sex, or to dose.
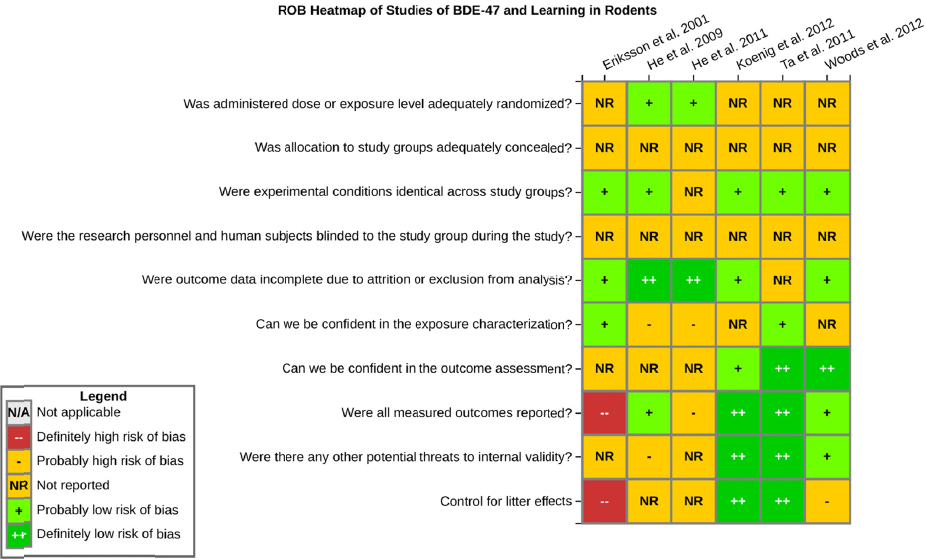
Confidence in the evidence was determined by considering factors that might upgrade or downgrade confidence (see Figure 4-3 for the factors). Confidence was downgraded because of risk-of-bias concerns; all studies had ratings of probably high risk or definitely high risk of bias in at least one of the key issues considered (e.g., lack of randomization of treatment), and most of the studies had multiple risk of bias issues, including not controlling for litter effects in the study design or analysis (see Figure 4-8). Qualitatively, the studies of learning in rodents appeared to have inconsistent results that might warrant a downgrade in confidence because of unexplained inconsistency. Nevertheless, a meta-analysis (presented later in this chapter) of rodent studies on several BDEs, including BDE-47, and latency in the last learning trial of the Morris water maze showed consistent evidence of an association between developmental exposure to PBDEs and decrements in this one measure. Results of the meta-analysis provided another line of evidence to support the consistency of the evidence. Specifically, the meta-analysis found that heterogeneity among studies was low; therefore, the apparent qualitative inconsistency can be explained by differences in precision across studies. No downgrades or upgrades on other factors were made.
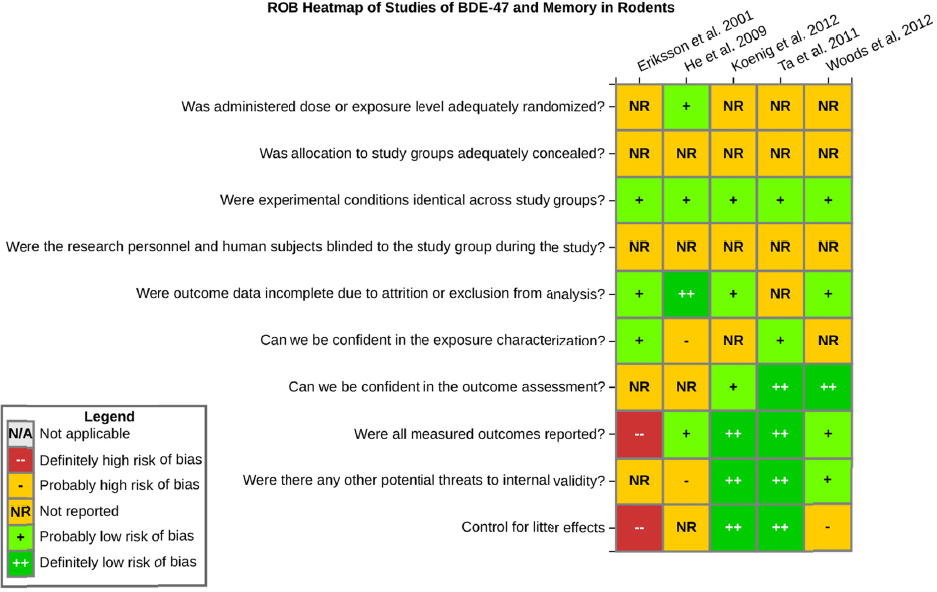
There is low confidence in the body of evidence on developmental exposure to BDE-47 and effects on memory in rodents. There were five studies of BDE-47 that tested memory in rodents (see Table 4-3). The one study in rats (He et al. 2011) reported decreased memory in the Morris water maze. Three of the mouse studies (Eriksson et al. 2001; Ta et al. 2011; Koenig et al. 2012) reported no effects on memory, and a fourth study by Woods et al. (2012) reported decrements in memory in female Mecp2 308+/- mice, with no effects on males or in C57BL6 mice of either sex. The data set is similar and contains some of the same studies discussed above with respect to effects of BDE-47 on learning; however, there were fewer studies that reported an effect and one less study overall. Confidence in the body of evidence on memory was downgraded because of risk of bias concerns. All the studies had a probably high risk of bias rating for at least one major issue (e.g., researchers were not blinded to the study groups during outcome assessment), and most of the studies had multiple risk of bias issues, including not controlling for litter effects in the study design or analysis (see Figure 4-9). Confidence was further downgraded for unexplained inconsistencies in the evidence on memory. No downgrades or upgrades on other factors were made.
TABLE 4-2 Studies of BDE-47 and Learning in Rodents
| Study | Species | Life Stage Exposed | Observation Time | Test | NOAEL (mg/kg-day) | LOAEL (mg/kg-day) |
|---|---|---|---|---|---|---|
| Eriksson et al. 2001 | NMRI mice | PND 10 | 5 months | Morris water maze | 10.5 | None |
| He et al. 2009 | Sprague- Dawley rats | PND 10 | 2 months | Morris water maze | None | 1 |
| He et al. 2011 | Sprague- Dawley rats | PND 10 | 2 months | Morris water maze | None | 1 |
| Koenig et al. 2012 | C57BL/6J mice | GD 0 - PND 21 | 2 months | Barnes maze | None | 0.03 |
| Ta et al. 2011 | C57BL/6J mice | GD 0 - PND 21 | 2 months | Morris water maze | 0.1 | 1 |
| Woods et al. 2012 | Female Mecp2 308+/- mice | GD 0 - PND 21 | PND 50-53 | Morris water maze | None | 0.03 |
| Male Mecp2 308+/- mice | GD 0 - PND 21 | PND 50-53 | Morris water maze | 0.03 | None | |
| Female C57Bl6 mice | GD 0 - PND 21 | PND 50-53 | Morris water maze | 0.03 | None | |
| Male C57Bl6 mice | GD 0 - PND 21 | PND 50-53 | Morris water maze | 0.03 | None |
NOTE: GD, gestation day; LOAEL, lowest-observed-adverse-effect level; NOAEL, no-observed-adverse-effect level; PND, postnatal day.
TABLE 4-3 Studies of BDE-47 and Memory in Rodents
| Study | Species | Life Stage Exposed | Observation Time | Test | NOAEL (mg/kg-day) | LOAEL (mg/kg-day) |
|---|---|---|---|---|---|---|
| Eriksson et al. 2001 | NMRI mice | PND 10 | 5 months | Morris water maze | 10.5 | None |
| He et al. 2009 | Sprague- Dawley rats | PND 10 | 2 months | Morris water maze | None | 1 |
| Eriksson et al. 2001 | NMRI mice | PND 10 | 5 months | Morris water maze | 10.5 | None |
| Koenig et al. 2012 | C57BL/6J mice | GD 0 - PND 21 | 2 months | Barnes maze | 1 | None |
| Ta et al. 2011 | C57BL/6J mice | GD 0 - PND 21 | 2 months | Morris water maze | 1 | None |
| Woods et al. 2012 | Female Mecp2 308+/- mice | GD 0 - PND 21 | PND 54 | Morris water maze | None | 0.03 |
| Male Mecp2 308+/- mice | GD 0 - PND 21 | PND 50-54 | Morris water maze | 0.03 | None | |
| Female C57Bl6 mice | GD 0 - PND 21 | PND 50-54 | Morris water maze | 0.03 | None | |
| Male C57Bl6 mice | GD 0 - PND 21 | PND 50-54 | Morris water maze | 0.03 | None |
NOTE: GD, gestation day; LOAEL, lowest-observed-adverse-effect level; NOAEL, no-observed-adverse-effect level; PND, postnatal day.
BDE-99 and Learning and Memory
There is moderate confidence in the body of evidence on developmental exposure to BDE-99 and effects on learning and memory in rodents. Five studies of BDE-99 and learning were available (see Appendix E, Table E4-3). Two of the three studies in rats reported slower learning in the Morris water maze at a dose of 2 mg/kg-day (Cheng et al. 2009; Blanco et al. 2013). Zhao et al. (2014) reported no effects at a lower dose (0.2 mg/kg-day) under similar exposure and testing conditions. In contrast, developmental exposure of Wistar rats at doses up to 30 mg/kg-day had no effect on learning tested with a Y maze (Llansola et al. 2009). A single study (Fischer et al. 2008) in NMRI mice also reported decrements in learning during the acquisition period in tests using either a radial maze or a Morris water maze at a dose of 0.8 mg/kg-day. As noted earlier for BDE-47, the results of a meta-analysis (presented later in this chapter) of rodent studies on several BDEs, including BDE-99, and latency in the last trial of the Morris water maze lessened the committee’s concerns about unexplained inconsistency. Three studies of BDE-99 and memory found no effects in several memory tests at doses of 0.2-2 mg/kg-day (see Appendix E, Table E4-4).
Confidence in the evidence on both learning and memory was downgraded because of serious concerns about several risk of bias issues. Risk of bias heatmaps of the studies are available in Appendix E (see Figures E4-3 and E4-4). The study with the fewest concerns in study design and conduct (Blanco et al. 2013) was rated probably high risk of bias for at least one key risk of bias issue (e.g., lack of randomization of treatment), and at least one study had a definitely high risk of bias rating for not controlling for litter effects in the study design or analysis. No other downgrades or upgrades on other factors were made.
BDE-153 and Learning and Memory
There is low confidence in the body of evidence on developmental exposure to BDE-153 and effects on learning and memory. Two studies—one in rats (Zhang et al. 2013) and one in mice (Viberg et al. 2003)—evaluated both outcomes (see Appendix E, Tables E4-5 and E4-6). Although the results were inconsistent, a meta-analysis (presented later in this chapter) of data on several BDEs strengthened the evidence for an effect on learning. Confidence was downgraded twice for serious concerns about multiple risk of bias concerns, including lack of randomization of treatment, reduced confidence in outcome assessment due to lack of blinding of outcome assessors, and definitely high risk of bias ratings for not controlling for litter effects in the study design or analysis. A risk of bias heatmap of the studies is available in Appendix E (see Figure E4-5). No other downgrades or upgrades on other factors were made.
BDE-203 and Learning and Memory
There is very low confidence in the body of evidence on developmental exposure to BDE-203 and effects on learning and memory in mice. One study was available on both end points (Viberg et al. 2006), and only a single dose was tested in a single species (mouse). Therefore, confidence was downgraded for both outcomes because it was not possible to evaluate consistency in results (see Appendix E, Tables E4-7 and E4-8). The study did not have elements that would strengthen conclusions from a single study, such as multiple species, strains, or a particularly large sample size. Confidence was also downgraded twice because of multiple risk of bias issues, including lack of randomization of treatment, reduced confidence in outcome assessment due to lack of blinding of outcome assessors, and a definitely high risk of bias rating for not controlling for litter effects in the study design or analysis. A risk of bias heatmap of the study is available in Appendix E (see Figure E4-6). No other downgrades or upgrades on other factors were made.
BDE-206 and Learning
There is very low confidence in the body of evidence on developmental exposure to BDE-206 and learning in mice. Only one study was available (see Appendix E, Table E4-9). Confidence in the body of evidence was downgraded because only a single study in mice was identified (Viberg et al. 2006), and it was not possible to establish or evaluate consistency as the study tested just a single species and a single dose. The study did not have elements that would strengthen conclusions from a single study, such as multiple species, strains, or a particularly large sample size. Confidence was also downgraded twice because of multiple risk of bias issues, including lack of randomization of treatment, reduced confidence in outcome assessment due to lack of blinding of outcome assessors, and a definitely high risk of bias rating for not controlling for litter effects in the study design or analysis. A risk of bias heatmap of the study is available in Appendix E (see Figure E4-6). No other downgrades or upgrades on other factors were made.
BDE-209 and Learning and Memory
There is moderate confidence in the body of evidence on developmental exposure to BDE-209 and effects on learning and low confidence in the body of evidence on memory. Eight studies on learning were available (see Appendix E, Table E4-10). Several studies show effects on learning when it was assessed using the Morris water maze; however, other studies found no effects when BDE-209 was tested at similar doses using other test methods. As noted earlier for BDE-47, a meta-analysis (presented later in this chapter) of data on several BDEs, including BDE-209, strengthened the evidence for an effect on learning. Six studies on memory were available on BDE-209 (see Appendix E, Table E4-11). Several mouse studies showed effects on memory when assessed with the Morris water maze, but other studies found no effects on memory in the same dose range using other methods. Thus, confidence in the memory evidence was downgraded for unexplained inconsistency. Confidence in the evidence on both outcomes was downgraded because of concerns about multiple risk of bias issues, including reduced confidence in outcome assessment due to lack of blinding of outcome assessors and a definitely high risk of bias rating in three studies because of failure to control for litter effects in the study design or analysis. Risk of bias heatmaps of the studies are available in Appendix E (see Figure E4-7 and E4-8).
DE-71 and Learning, Memory, and Attention
There is very low confidence in the body of evidence to evaluate whether developmental exposure to DE-71 affects learning, memory, or attention in rats. There were three studies on learning, two on memory, and three on attention (see Appendix E, Tables E4-12, E4-13, and E4-14). The results of the three studies on learning were inconsistent and used different tests (Morris water maze, radial maze, and visual discrimination) and animals of different ages. One study (Dufault et al. 2005) reported increased
errors in the visual discrimination task at the single dose tested (30 mg/kg-day on postnatal days 6-12). The other two studies reported no effects of DE-71 on learning at the same dose using longer exposure windows (Bowers et al. 2015; de-Miranda et al. 2016); however, the animals in these studies were evaluated at older ages than were the rats tested in the Dufault et al. (2005) study. The two studies of DE-71 effects on memory had inconsistent results and were evaluated in rats of different ages and with different tests (Morris water maze and radial maze). One study (de-Miranda et al. 2016) reported memory deficits in female Wistar rats (but not in males) in the radial maze at the single dose tested (30 mg/kg-day). Studies of DE-71 and attention were from a single laboratory (Dufault et al. 2005; Driscoll et al. 2009, 2012), and the majority of the tests reported no effects at doses up to 30 mg/kg-day across multiple tests (various attention tasks and a visual task).
Confidence in the body of evidence for all three outcomes was downgraded for unexplained inconsistency and downgraded twice for serious concerns about multiple risk of bias issues, such as reduced confidence in outcome assessment due to lack of reporting about whether outcome assessors were blinded and definitely high risk of bias ratings for exposure characterization in two of the studies (Dufault et al. 2005; de-Miranda et al. 2016). Risk of bias heatmaps of the studies are available in Appendix E (see Figures E4-9, E4-10, and E4-11). No downgrades or upgrades on other factors were made.
A summary of the confidence ratings of all the BDEs is presented in an evidence profile in Table 4-4.
Meta-Analysis of Selected Animal Data on Learning
The animal database for effects from PBDEs is both diverse and complex, with studies of varying designs and varying outcome measures. The outcome judged to be most amenable to meta-analysis was the results for latency in the last trial of the Morris water maze. This maze was the test used most often in the PBDE studies. It is a test of spatial learning for rodents that requires them to use distal cues to navigate from starting locations around the perimeter of an open swimming area to locate a submerged platform. Learning is assessed by latency, the amount of time it takes the animal to find the platform across repeated trials; learning is demonstrated by a reduction in latency with an increasing number of trials. For the meta-analysis, latency data on the last trial were used because latency to find the platform on the last trial was always reported in these studies. There are no a priori data that suggest species differences, so results for rats and mice were analyzed together. Given the sparse data on individual PBDEs, all BDEs were initially analyzed together. Additional analyses of individual PBDEs were conducted in cases where there were more than two data points (see Appendix E, Section E-5). All studies were considered except one that used humanized transgenic mice (which had variants of a human APOE gene).
Effect sizes were calculated as the log10 ratio of the mean difference between the treatment group and the concurrent control, multiplied by 100 (y = 100 × ln [mean of treated group ÷ mean of control group]). For small changes, this is approximately equal to the percent change, but the resulting confidence interval is more symmetric and closer to normal (Hedges et al. 1999; Lajeunesse 2011). This normalization allows for treatment groups to be compared across studies and experiments. When normalized in this way, however, treatment groups within a study are correlated. Therefore, in one of the sensitivity analyses, effects were estimated using only the highest treatment group from each study. Additional sensitivity analyses were performed by sequentially excluding each study (all treatment groups for that study). See Appendix E, Section E-5, for the sensitivity analyses.
Both the overall effect of any treatment and the coefficients of meta-regressions were estimated. For meta-regressions, three models were used: a linear model in y = a + b*log10(dose) in order to test for a dose-response trend; a linear model y = b*dose; and a linear-quadratic model y = b*dose + c*dose2 in order to model the dose-response shape. In the linear and linear-quadratic models, the intercept was omitted because the effect measures were already normalized relative to control levels. Additionally for these models, the coefficients were rescaled in terms of the change per 10 mg/kg-day (e.g., y = b*[dose/10] + c*[dose/10]2) for ease of interpretation. In all cases, random effect models were used, as described in the
TABLE 4-4 Profile of the Body of Evidence on PBDEs and Learning, Memory, and Attention
| Factors Decreasing Confidence “---” If No Concern; “↓” If Serious Concern to Downgrade Confidence | Factors Increasing Confidence “---” If Not Present; “↑” I f Sufficient to Upgrade Confidence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| INITIAL CONFIDENCE for each body of evidence (# of studies) | Risk of Bias | Unexplained Inconsistency | Indirectness | Imprecision | Publication Bias | Large Magnitude | Dose Response | Residual Confounding | Consistency Species/Model | FINAL CONFIDENCE RATING | LEVEL OF EVIDENCE FOR HEALTH EFFECT* |
| BDE-47 Learning High (2 rat,a 4 mouseb) | ↓ | --- | --- | --- | --- | --- | --- | --- | --- | Moderate | Moderate |
| BDE-47 Memory High (1 rat,c 4 mouseb) | ↓ | ↓- | --- | --- | --- | --- | --- | --- | --- | Low | Low |
| BDE-99 Learning High (4 rat,d 1 mousee) | ↓ | --- | --- | --- | --- | --- | --- | --- | --- | Moderate | Moderate |
| BDE-99 Memory High (2 rat,f 1 mousee) | ↓ | --- | --- | --- | --- | --- | --- | --- | --- | Moderate | Inadequate |
| BDE-153 Learning High (1 rat,g 1 mouseh) | ↓↓ | --- | --- | --- | --- | --- | --- | --- | --- | Low | Low |
| BDE-153 Memory High (1 rat,g 1 mouseh) | ↓↓ | --- | --- | --- | --- | --- | --- | --- | --- | Low | Low |
| BDE-203 Learning High (1 mousei) | ↓↓ | ↓ | --- | --- | --- | --- | --- | --- | --- | Very Low | Inadequate |
| BDE-203 Memory High (1 mousei) | ↓↓ | ↓ | --- | --- | --- | --- | --- | --- | --- | Very Low | Inadequate |
| BDE-206 Learning High (1 mousei) | ↓↓ | ↓ | --- | --- | --- | --- | --- | --- | --- | Very Low | Inadequate |
| BDE-209 Learning High (2 rat,j 6 mousek) | ↓ | --- | --- | --- | --- | --- | --- | --- | --- | Moderate | Moderate |
| BDE-209 Memory High (1 rat,l 5 mousem) | ↓ | ↓- | --- | --- | --- | --- | --- | --- | --- | Low | Low |
| DE-71 Learning High (3 ratn) | ↓↓ | ↓ | --- | --- | --- | --- | --- | --- | --- | Very Low | Inadequate |
| DE-71 Memory High (2 rato) | ↓↓ | ↓ | --- | --- | --- | --- | --- | --- | --- | Very Low | Inadequate |
| DE-71 Attention High (3 ratp) | ↓↓ | ↓ | --- | --- | --- | --- | --- | --- | --- | Very Low | Inadequate |
* See the section “Determinations of Level of Evidence” later in this chapter for an explanation of how these ratings were determined.
NOTE: Studies were available on six BDE congeners and one technical grade mixture. All the BDEs had studies of learning, six had studies of memory, and only the mixture had studies of attention; no studies of response inhibition were found for any of the congeners.
a He et al. (2009, 2011).
b Eriksson et al. (2001); Ta et al. (2011); Koenig et al. (2012); Woods et al. (2012).
d Cheng et al. (2009); Llansola et al. (2009); Blanco et al. (2013); Zhao et al. (2014).
f Blanco et al. (2013); Zhao et al. (2014).
j Biesemeier et al. (2011); Y. Chen et al. (2014).
k Eriksson et al. (2001); Rice et al. (2009); Reverte et al. (2013); Verma et al. (2013, 2014); Buratovic et al. (2014).
m Eriksson et al. (2001); Reverte et al. (2013); Verma et al. (2013, 2014); Buratovic et al. (2014).
n Dufault et al. (2005); Bowers et al. (2015); de-Miranda et al. (2016).
protocol. All analyses utilized random effects models as implemented in the R “metafor” package. Sensitivity analyses included leaving one study out at a time and using only the highest dose group in each study (see Appendix E, Table E5-1). Benchmark dose (BMD) estimates were calculated for an effect size of 5% (BMD5; see Appendix E, Figure E5-3). The BMD5 was calculated using the linear or the linear-quadratic model, with the model selection based on the lowest AICc (Akaieke information criterion corrected for small sample size). The BMD5 was calculated only for the “fixed effect”—that is, the estimated mean response across studies.
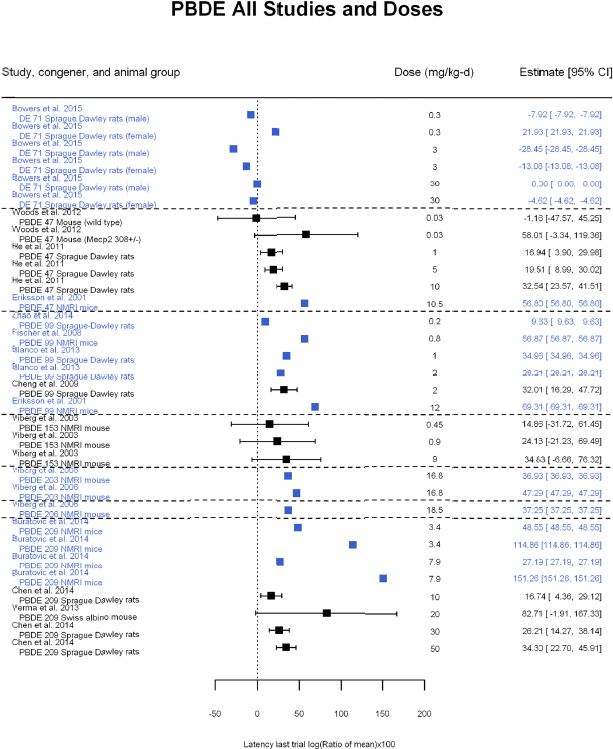
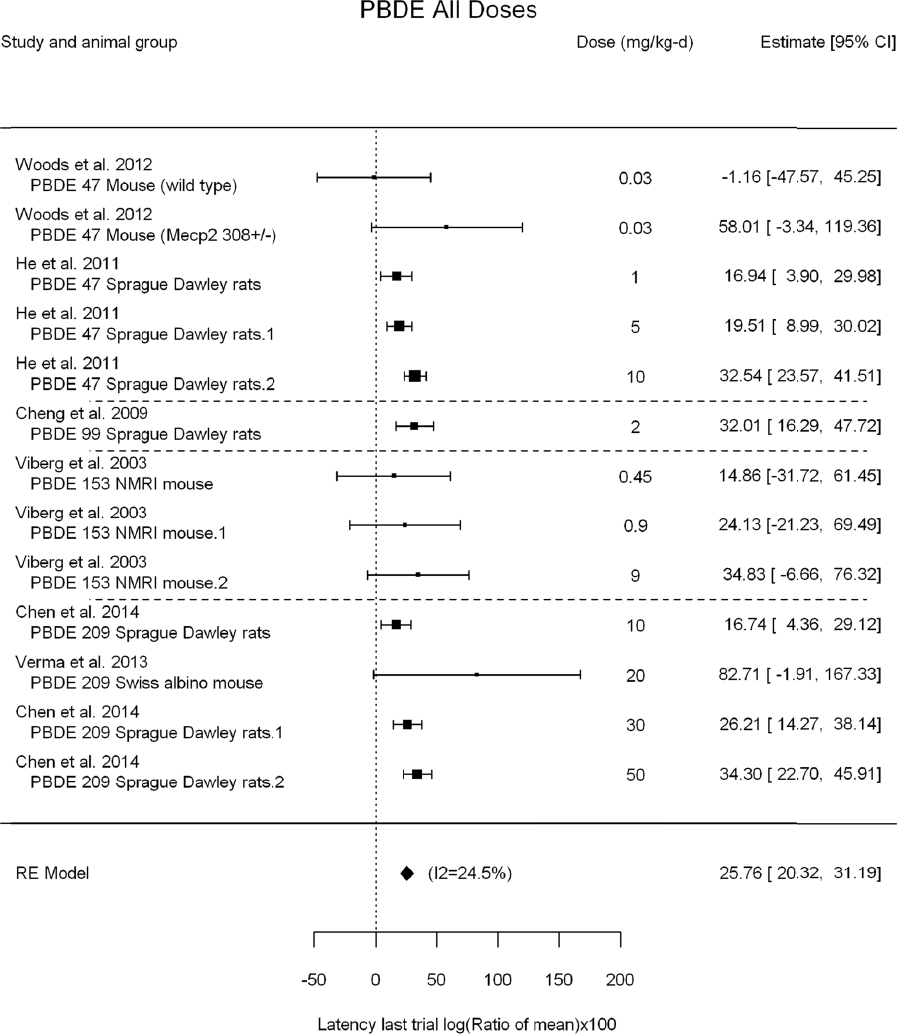
A meta-analysis using all the data is shown in Figure 4-10 and includes data from DE-71, BDE-47, -99, -153, and -209. For some studies, the standard deviations (SDs) were not reported or could not be digitized. These are shown in blue in the figure, and they were not included in the meta-analysis. There was a concern about possible reporting bias because studies that reported an SD might be more likely to show an effect than studies that did not. No such bias is evident from the figure, however, as the data from studies that did not have an SD had similar reported effect sizes as studies that did report an SD. For instance, the unweighted mean for studies reporting an SD was 30, whereas the unweighted mean for studies not reporting an SD was 36. Therefore, excluding studies without reported SDs is not likely to lead to a substantial bias in the meta-analysis results. The results of a meta-analysis of studies for which SDs were available is presented in Figure 4-11.
The results are as follows:
- Statistically significant overall effect of PBDE treatment that is also robust to leaving out individual studies, using only the highest dose group in each study and leaving out individual studies using the highest dose group only. There was also low or no heterogeneity (25%, not statistically significant in primary analysis; and <35%, not statistically significant in all sensitivity analyses).
- There was a positive, but not statistically significant, trend from the meta-regression in log10(dose). Meta-regression using a linear model or a linear-quadratic model resulted in a statistically significant linear term. The estimated BMD5 for change in latency was 5.1 mg/kg-day (95% confidence interval [CI]: 3.2, 13) for the linear model and 1.8 mg/kg-day (95% CI: 1.1, 4.5) for the linear-quadratic model (see Appendix E, Figure E5-3). In both cases, however, heterogeneity was statistically significant with I2 increased to >70%.
Overall, there is consistent evidence of an increase in latency in the last trial of the Morris water maze that is robust to multiple sensitivity analyses. Nevertheless, the evidence of a dose-response gradient across all PBDE congeners is tempered by the fact that there is no statistically significant trend in log10(dose) and that heterogeneity increased under linear and linear-quadratic meta-regression. When accounting for dose, this heterogeneity might be because different PBDEs have different potencies for this effect, different duration of dosing in the studies produces different cumulative doses, and test methods (e.g., number of daily water maze trials) vary among experiments. It is possible that using a different dose metric—such as cumulative dose or cumulative dose during a particular developmental window—would produce a dose-response gradient. Separate analyses of each PBDE for which there was enough data for meta-analysis were subsequently conducted (see Appendix E, Section E-5).
Human-Health Effects Results
Lam et al. (2015, 2016) conducted a systematic review of associations between developmental exposure to PBDEs and measures of intelligence and attention in children. Developmental exposure was defined as exposure that occurred prior to conception in one or both parents, during pregnancy (exposure to offspring in utero), perinatally, or in childhood. Studies were sought that measured exposure in human biological samples (e.g., urine, blood, or other specimens). The committee was provided with a draft of the systematic review in July 2016, and the committee reviewed it and decided to update it. In early 2017, the authors notified the committee that their review had been submitted for publication and that the litera-
ture search had been updated before the draft was submitted. The paper has been accepted for publication in Environmental Health Perspectives (Lam et al. in press). This section describes the committee’s evaluations in the sequence they occurred, and includes a description of the draft systematic review by Lam et al., the committee’s evaluation of it, the committee’s update, and a discussion of the updated Lam et al. review that was accepted for publication.
As described earlier under “Evaluation of the Lam et al. (2015, 2016) Review,” the draft systematic review was relevant to the committee’s topic of interest and was judged appropriate for demonstrating how an existing systematic review could be updated by EPA. The original literature search was performed on March 5, 2015, without any date restrictions. After screening the results, 12 studies met the inclusion criteria of the PECO statement; nine studies measured IQ and seven studies evaluated ADHD and/or attention-related behavioral conditions (see Appendix F, Section F-4). Most of the individual IQ studies were judged to have low or probably low risk of bias, and the authors rated the confidence (or “quality”) in the body of evidence as moderate. Only one study was given a high risk of bias rating in one of the domains (selective outcome reporting [Lin et al. 2010]); the study was part of conference proceedings and
did not provide sufficient detail about effects estimates for all the study outcomes. More concerns about risk of bias were found in the studies of ADHD and attention-related behaviors. Two studies were given high risk of bias ratings, one in the domain of confounding (Gump et al. 2014) and one in the domain of incomplete outcome data (Roze et al. 2009); the latter study was also given probably high risk of bias rating in two domains because of lack of blinding and inadequate adjustment for confounding.
A meta-analysis including four of the nine IQ studies (Herbstman et al. 2010; Gascon et al. 2012; Eskenazi et al. 2013; A. Chen et al. 2014) was performed and found a decrease in IQ in relation to PBDE exposure. Under the Navigation Guide, the confidence (or “quality”) of the body of evidence was rated as moderate and the strength of evidence was considered sufficient1 by the authors and was translated by the committee to a “moderate” level of evidence to support an inverse association between PBDEs and IQ following the OHAT method.
Although two of the individual studies that evaluated ADHD and attention-related behavioral conditions had high risk of bias rating in at least one domain, the other five studies were found to have low or probably low risk of bias, and the authors rated the confidence of the body of evidence as moderate.2 They also judged that there was an insufficient number of combinable studies to perform a meta-analysis. Under the Navigation Guide, the strength of evidence was considered limited by the authors and was translated by the committee to a “low” level of evidence to support an association between PBDEs and ADHD following the OHAT method.
The committee’s search for articles published since the Lam et al. (2015, 2016) literature search found three articles that met the eligibility criteria. All three articles involved cohorts from studies included in the Lam et al. systematic review (see Appendix F, Section F-4). One of the reports (Zhang et al. 2017) assessed full-scale IQ scores at age 9 in the same cohort as Y. Chen et al. (2014). The IQ data from this cohort at earlier ages were included in the meta-analysis of IQ scores. The effect size (a decrease of 5.3 IQ points for each 10-fold increase in PBDE exposure) reported by Zhang et al. (2017) was consistent with the earlier IQ data from the cohort and was very similar in magnitude to the overall effect size in the meta-analysis. The committee concluded that a new meta-analysis would not change the strength of evidence, nor would it alter the overall conclusion reached in the Lam et al. (2015, 2016) systematic review of an inverse association between PBDEs and childhood IQ.
Each of the three newly identified articles assessed attention-related problems and/or ADHD (see Appendix F, Section F-4). Zhang et al. (2017) found that each 10-fold increase in serum PBDE concentration was marginally associated with a 3.5-point increase in externalizing problems scores on the BASC-2 (Behavioral Assessment System for Children-2), and that was consistent with the results of earlier tests conducted in the cohort (Y. Chen et al. 2014). The article by Cowell et al. (2015) reported the results of behavioral assessments of the same cohort of children studied by Herbstman et al. (2010). The CBCL (Child Behavior Checklist) was administered annually from age 3 through age 7. Multivariable regression analyses were performed on the data collected at ages 4 and 6 because they were the oldest ages at which the preschool and school-aged CBCLs were performed in person rather than over the phone. Associations were detected between cord blood concentration of BDE-47 and BDE-153 and increased attention problems in children at age 4 but not age 6. Sagiv et al. (2015) conducted assessments of attention and ADHD in children from the same cohort studied by Eskenazi et al. (2013). Measures included the Conners’ Con-
___________________
1 Lam et al. based their conclusion for IQ on (1) moderate quality/confidence in the body of evidence for IQ, (2) consistent evidence for an effect from BDE-47, other congeners, and overall consistent results in combination of similar studies in a meta-analysis; and (3) support from one or more well-conducted studies; most studies were prospective cohorts that as a group represented diverse populations, were reasonably large, and supported by a statistically significant meta-analysis. The Navigation Guide has additional steps to reach a “strength of evidence” conclusion. There is no equivalent step in the OHAT method.
2 Lam et al. based their conclusion for attention-related behaviors on (1) moderate quality/confidence in the body of evidence for attention and (2) general evidence for an effect from BDE-47 and other congeners, but not consistent evidence overall. There were too few combinable studies to conduct a meta-analysis, so chance, bias, and confounding could not be ruled out with reasonable confidence. The Navigation Guide has additional steps to reach a “strength of evidence” conclusion. There is no equivalent step in the OHAT method.
tinuous Performance Test II (CPT II), the ADHD Confidence Index score, which is derived from the Conners’ CPT II, and the Conners’ ADHD Index (CADS-P), which is derived from the Conners’ Parent Rating Scale. They reported associations of higher prenatal serum concentrations of PBDEs with decrements in attention on the Conners’ CPT II task as well as with increased ADHD Index scores at both 9 and 12 years of age; they also reported an association of higher prenatal PBDE exposure with increased scores on the CADS-P at age 9 but not at age 12. The results were consistent with the earlier findings in this cohort.
The committee concluded that, even with the addition of the three new reports, the data are still not amenable to meta-analysis due to the different assessment measures used across studies that prevent them from being combined. Furthermore, although all three reports found associations between prenatal serum PBDE concentrations and at least one measure of attention-related problems or ADHD, these reports present data collected from study cohorts that were already included in the Lam et al. review. The committee judged that they would not appreciably change the strength of evidence, nor would they change the overall conclusions reached by Lam et al. (2015, 2016) of limited evidence to support an association between PBDEs and ADHD.
After the committee completed its analysis, Lam et al.’s systematic review was accepted for publication (Lam et al. in press). The literature search had been updated from the 2016 draft, and the same three new publications identified as relevant to the systematic review by the committee were also found by the authors and included in their updated systematic review. The inclusion of the new evidence did not change the risk of bias assessments, but an analysis of the ratings in relation to age found that most studies that tested children at a later age (tested with Full Scale IQ) were rated as having low or probably low risk of bias across domains, whereas many studies of children at younger ages (tested with the Bayley Scales of Infant Development) had a rating of probably high risk of bias in at least one domain. The studies included in the meta-analysis remained unchanged, and the overall decrement in IQ points was reported as 3.70 (95% CI: 0.83, 6.56) per 10-fold increase in lipid-adjusted PBDE concentration (range: limit of detection–761 ng/g lipid). The committee’s translation of the Navigation Guide evaluation of the into OHAT ratings remains the same—a moderate level of evidence to support an inverse association between PBDEs and IQ and a low level of evidence to support an association between PBDEs and ADHD.
MECHANISTIC EVIDENCE
The mechanisms of action through which developmental exposure to PBDEs alters neurobehavioral outcomes, such as IQ or attention in children or learning and memory in rodents, are not well understood. Nevertheless, data from mechanistic studies conducted in vitro or in animal models can help establish the biological plausibility of the associations that have been observed between PBDE exposure during the perinatal period and later behavioral outcomes. A large number of molecular, cellular, hormonal, and neurochemical changes have been reported following PBDE exposure (e.g., see Dingemans et al. 2011; Westerink 2014). In vitro, zebrafish and rodent models have been employed. An example of a possible initiating event is thyroid hormone disruption (Ibhazehiebo et al. 2011). Thyroid hormone plays a number of critical roles in brain development (Horn and Heuer 2010), and inadequate concentrations of thyroid hormone during early development have been associated with neurodevelopmental sequelae, including reduced IQ (Ghassabian et al. 2014), and increased risk of ADHD behaviors (Modesto et al. 2015). PBDEs have also been shown to alter intracellular calcium signaling through both the ryanodine and the IP3 receptors (Kim et al. 2011; Gassmann et al. 2014), leading to increased cytosolic calcium concentrations. PBDEs have been shown to increase oxidative stress in neuronal cell cultures (Costa et al. 2015), leading to apoptosis; to alter the expression of various genes involved in neurogenesis (Dingemans et al. 2011); and to decrease the expression of key proteins involved in neurodevelopmental processes (Kodavanti et al. 2015).
Examples of the wide array of potential initiating events are given in Figure 4-12. Although the figure is not exhaustive, it illustrates that there are many possible pathways through which developmental PBDE exposure could affect later cognitive or behavioral function. The large number of alterations that
have been reported at various levels, from molecular to neural systems, makes defining a particular adverse outcome pathway or pathways very difficult. Most studies span only one or at most two levels in the pathway. For example, Costa et al. (2015) have shown that BDE-47 causes oxidative stress leading to apoptosis both in cerebellar granule cells in vitro and in a mouse model. These changes were observed in the absence of any changes in thyroid hormone concentrations. Nevertheless, whether the observed increases in oxidative stress and subsequent apoptosis lead to changes in nervous system connectivity and behavior has not been investigated.
The development of high-throughput approaches to assess the effects of toxicants on the developing nervous system—especially those that may affect learning and memory—is a tremendous challenge. It is clear that many critical cell and molecular processes are involved. The adverse outcome pathways (AOPs) that have been proposed to lead to developmental neurotoxicity not only clearly identify major knowledge gaps in terms of the key events that are involved but also provide a valuable means to organize essential information and to identify research gaps (Bal-Price et al. 2017). Current approaches to fill some of these gaps include platforms based on the use of stem-cell-derived neurons or neuroprogenitor cells (Druwe et al. 2015; Pallocca et al. 2016; Ryan et al. 2016; Singh et al. 2016; Schmidt et al. 2017). End points include the expression of genes that play a role in neurodevelopment, neuroprogenitor cell proliferation and differentiation, neurite outgrowth, cell migration, and apoptosis as well as more generalized stress responses. Because the nervous system, ultimately, must develop a functional, coordinated, neural network, efforts are being expended to capture neural networks on microelectrode arrays (Brown et al. 2016). The usefulness of model species, such as Caenorhabditis elegans and zebrafish, is also being explored (Behl et al. 2015).
EVIDENCE INTEGRATION
Evidence synthesis was conducted in a three-part process. First, the confidence ratings for the animal studies were translated into conclusions about level of evidence of health effects using the procedure in Figure 4-4, and the level of evidence of health effects for the human studies was derived from the Lam et al. (2016, in press) analysis of the evidence. Second, initial hazard identification conclusions were reached by integrating the conclusions about level of evidence for the human and the animal evidence streams using the approach presented in Figure 4-5. Third, the degree of support from mechanistic data was considered and discussed in reaching final hazard identification conclusions.
Determinations of Level of Evidence
In the following sections, the confidence ratings of the evidence on each outcome and BDE congener are considered in context with the direction of the effect and then translated into a determination of level of evidence using Figure 4-4.
Animal Evidence
Learning
There is moderate confidence in the body of evidence on PBDEs and effects on learning in rodents on the basis of studies on BDE-47, -99, and -209. Qualitative analyses suggested effects on learning for each of these congeners. A meta-analysis of learning data found consistent evidence of an effect, measured as latency in the last trial of the Morris water maze. Thus, the moderate confidence rating translates to a moderate level of evidence that developmental exposure to these congeners is associated with decrements in learning in rodents.
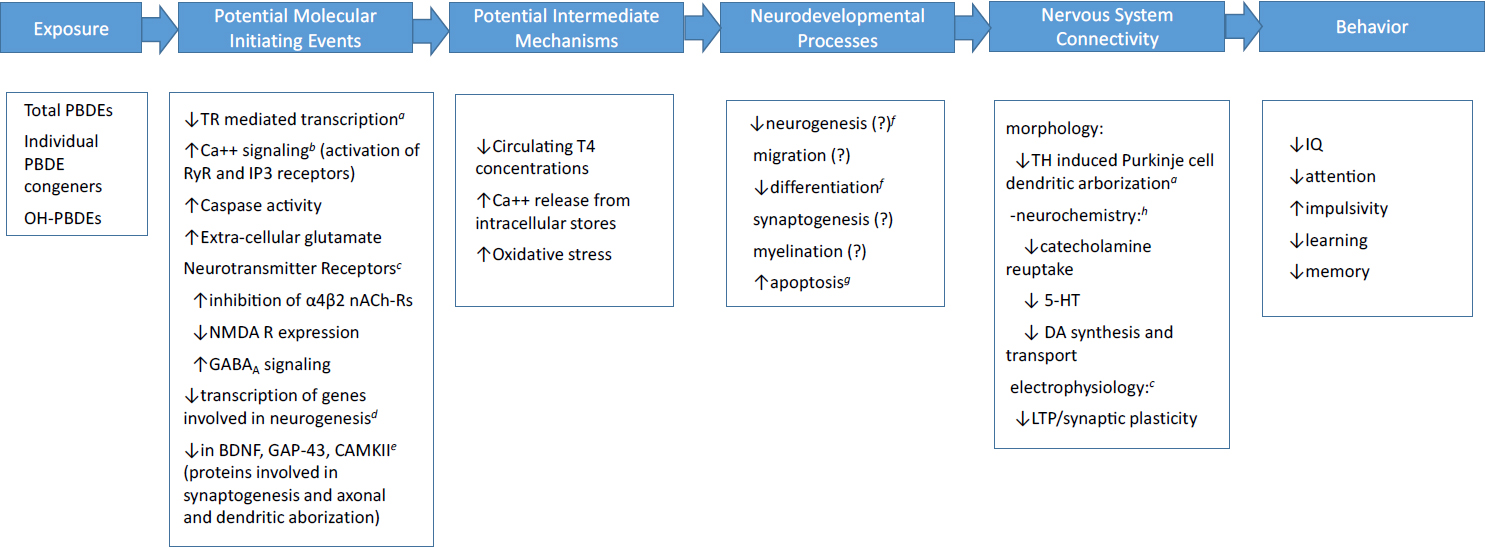
NOTE: 5-HT, 5-hydroxytryptamine (serotonin); BDNF, brain-derived neurotrophic factor; CAMKII, calmodulin-dependent kinase II; DA, dopamine; GABA, gamma-aminobutyric acid; GAP-43, growth-associated protein; IP3, 1,4,5-triphosphate; LTP, long-term potentiation; nACh-R, nicotine acetylcholine; NMDAR, glutamate receptor; RyR, ryanodine receptor; T4, thyroxine; TH, thyroid hormone; TR, thyroid hormone receptor.
a Ibhazehiebo et al. (2011).
b Kim et al. (2011); Gassmann et al. (2014); Westerink (2014).
c Westerink (2014).
d Wang et al. (2016).
e Viberg et al. (2008).
f Li et al. (2013).
g Costa et al. (2015).
h Wang et al. (2015, 2016).
SOURCE: Adapted from Mundy (2016).
There was low confidence in the body of evidence on BDE-153. Only two studies were available, one in rats and one in mice, with differing results. Nevertheless, the meta-analysis strengthened support for an effect on learning, so the low confidence rating was judged to have a low level of evidence (rather than an inadequate level) that developmental exposure to BDE-153 is associated with decrements in learning in rodents.
Very low confidence ratings were given to BDE-203 and -206 and to DE-71, which means there is an inadequate level of evidence to assess whether exposure to these congeners or to the technical mixture is associated with decrements in learning in rodents.
Memory
There is low confidence in the body of evidence on developmental exposure to PBDEs and effects on memory in rodents on the basis of evidence on BDE-47, -153, and -209. The two studies on BDE-153 reported results that were consistent in direction (both decreased performance on a memory test) across species. The results of the studies on BDE-47 and BDE-209 were less consistent, but effects on memory were found in some studies. Thus, the low confidence in the body of evidence was translated to a low level of evidence for these congeners.
DE-71 and BDE-203 had very low confidence ratings, which means there is an inadequate level of evidence to assess whether exposure to them is associated with decrements in learning in rodents.
Confidence in the body of evidence on BDE-99 was rated as being moderate, but the findings in those studies suggested a lack of association. A more robust database is needed to support a finding of a lack of effect (NTP 2015), so the evidence was judged to be inadequate to reach a conclusion that developmental exposure to BDE-99 has no effect on memory.
Attention
The only available data on attention and PBDEs was on DE-71. Confidence in the body of evidence was rated as being very low, which means there is an inadequate level of evidence to assess whether exposure to this technical mixture is associated with effects on attention in rats.
Human Evidence
Intelligence
As described earlier, the evaluation by Lam et al. (2016, in press) was translated into an OHAT level of evidence determination of a moderate level of evidence that exposure to PBDEs is associated with a decrease in IQ.
ADHD/Attention-Related Behavioral Conditions
As described earlier, the evaluation by Lam et al. (2016, in press) was translated into an OHAT level of evidence determination of a low level of evidence that exposure to PBDEs is associated with increased reporting of ADHD symptoms.
Hazard Identification Conclusions
The animal evidence on learning and memory was considered to have the closest parallels to intelligence measured in human studies, and the animal evidence on attention was used in parallel with the human evidence on ADHD. Using the OHAT scheme presented in Figure 4-5, the hazard conclusions drawn
were that (1) developmental exposure to PBDEs is presumed to pose a hazard to intelligence in humans, and (2) it is not possible to draw a conclusion about potential hazards to attention-related behavioral conditions in humans. Because the mechanisms of action involved in developmental neurotoxicity are unknown, this data stream had minimal impact on the hazard identification conclusion.
ANALYSIS OF LOW-DOSE EFFECTS
Human studies provide a moderate level of evidence that PBDEs are associated with decrements in IQ in humans. Lam et al. (in press) found a decrease of 3.70 IQ points in children per 10-fold increase in serum PBDE concentration. The committee’s update of the systematic review likewise did not find evidence that would appreciably change the overall conclusions reached by Lam et al. (2016) of limited evidence (or a low level of evidence according to the OHAT method) to support an association between PBDEs and ADHD.
The committee attempted to compare exposures to PBDEs in humans with those in animal studies (see Table 4-5). Human intakes of PBDEs estimated from levels found in food and dust appear to be about 500,000 times lower than the intakes estimated from animal studies (BMD5 estimated earlier in the chapter). To compare internal doses, blood concentrations of BDE-47 in humans were obtained from the National Health and Nutrition Examination Survey (NHANES) and from one epidemiology cohort included in the systematic review of the human evidence (CHAMACOS cohort). Blood concentrations of BDE-47 in rodents were obtained from studies in which the animals were exposed near the BMD5. Comparisons of internal doses of BDE-47 also showed large disparities in the exposure between humans and animals, but not as large as suggested by the intake data. For lipid adjusted BDE-47 plasma levels, the 95th percentile exposure in humans was about 170 times lower than the level in rats treated near the BMD5; the highest BDE-47 level in any child in the CHAMACOS cohort was 36 times lower than that rat plasma level. Another observation was that internal dose measurements were more similar between rodents and humans on a ng/g serum basis than on a ng/g lipid basis. Thus, there is significant uncertainty associated with the internal dose correspondence between rodents and humans, and the most relevant dose metric is unclear. These observations indicate that it would be helpful to have internal dose measures in rodent studies, preferably measuring the same matrix and metabolites as in human biomonitoring studies to reduce uncertainty in rodent to human extrapolation.
RELEVANCE TO ANIMAL TOXICITY TESTING
Rodent and human outcome measures in the PBDE case studies were less parallel than were those in the phthalate case studies in Chapter 3, where AGD, alterations in testosterone, and hypospadias were shared outcome measures in the studies. In general, measurement of IQ in children relies on well-described test methods that have been validated for broad use in biomedical research. The committee’s evaluation of the animal data found that, unlike the case of IQ testing in people, standardized test batteries, animal models, and exposure regimens were not used in the animal studies. Even within individual tests, different methods for categorizing responses were sometimes used, making it difficult to determine whether different studies shared similar or conflicting results or if they assessed different cognitive functions. Despite these limitations, the committee was able to demonstrate the use of a meta-analysis that combined results from a single type of test and a single outcome measure for several BDEs that showed a statistically significant overall effect of BDE treatment. This particular example represents a case where current toxicity-testing paradigms detect a hazard that is presumed to be of concern to humans but might not be not accurately predicting doses at which effects occur in humans.
TABLE 4-5 Comparison of Human and Rat Intake and Internal Concentrations of BDE-47
| Human Intake or Blood Concentrations of BDE-47 | Rat Administered Dose, Blood BDE Concentration, and BMD5 | ||
|---|---|---|---|
| Description | Value | Description | Value |
| Total PBDE and BDE-47 estimated intake based on levels reported in food and dusta | mg/kg-day Total PBDEs: 7.7 × 10-6 (adults) 4.9 × 10-5 (ages 1-5) 1.4 × 10-5 (ages 6-11) 9.1 × 10-6 (ages 12-19) BDE-47: 2.6 × 10-6 mg/kg-day (adults) | PBDE BMD5 for latency in the last trial of the Morris water mazeb | 1.79-5.08 mg/kg-day |
| BDE-47 in blood (NHANES 2003-2004)c | ng/g serum 0.12 (median) 1.0 (95th percentile) 13 (maximum) | BDE-47 measured levels in whole blood of mouse dam treated with 1 mg/kg-day for about 50 days before and during pregnancyd | 28 ng/g blood |
| BDE-47 measured levels in whole blood of mouse dam treated at 1 mg/kg-day for about 70 days before and during pregnancy and weaninge | 9.6 ng/g blood | ||
| ng/g lipid 19 (median) 163 (95th percentile) 2,350 (maximum) | BDE-47 measured levels in plasma of rats treated at 1 mg/kg-day for 14 days by gavagef | 28,000 ng/g lipid | |
| BDE-47 in serum at age 7 (CHAMACOS cohort)g | ng/g lipid 47.5 (geometric mean) 768 (maximum) | ||
b See Appendix E, Figure E5-3.
FINDINGS AND RECOMMENDATIONS
Systematic Reviews
- Consistency and Transparency: The committee found that the systematic review process was valuable because it provided a framework for identifying, selecting, and evaluating evidence in a consistent and explicit manner; maximized transparency in how the assessments were performed; and facilitated the clear presentation of the basis for scientific judgments.
- Meta-analysis: The committee found that the meta-analyses were valuable in summarizing data from the systematic reviews and in comparing the animal and human evidence in a robust and consistent manner. The meta-analysis of a subset of animal studies that tested learning in rodents exposed to various BDEs provided evidence of a possible relationship between PBDEs and decrements in learning that was not evident when the data sets on the individual BDEs were evaluated qualitatively. The meta-analysis results informed the confidence ratings of the body of evidence and allowed the committee to estimate benchmark doses on the basis of data from multiple studies. Meta-analyses of animal studies have not been commonly performed, but they were found to be useful both to inform confidence ratings in the body of evidence and to support benchmark dose modeling.
-
Recommendation: Systematic reviews should include meta-analyses of the animal and the human evidence, if approprriate. The results of meta-analyses should be used to examine quantitative relationships between EACs and end points of interest, to inform the confidence ratings of the bodies of evidence, and, if possible, to estimate benchmark doses.
-
Risk of Bias Evaluations: Information important to the evaluation of the quality of individual animal studies was often not reported, including whether the study controlled for litter effects, whether animals were randomly allocated to study groups, and whether research personnel were blinded to the study groups during the outcome assessment. Because a lack of adequate reporting could not be distinguished from failure to adhere to practices that minimize bias, failure to report practices that minimize bias often led to higher risk of bias ratings for individual studies, downgrading the overall level of confidence in the body of evidence. These types of problems could be remedied if journals required better reporting of the methods used in animal studies, especially reporting pertaining to issues that might introduce bias into the research. These requirements could build on reporting standards that have been developed by various organizations to improve transparency (e.g., the ARRIVE guidelines [Kilkenny et al. 2010]). For example, studies should be required to report whether animals were assigned to study groups using random allocation and whether researchers were blinded to the study groups during outcome assessment.
-
Using an Existing Systematic Review: The committee critically evaluated a recent systematic review of epidemiologic studies on the effect of developmental exposure to PBDEs on IQ and ADHD and judged it to be adequate for use in the context of the review question. This evaluation allowed the committee to focus its efforts on updating the literature search of the recent review. Because no studies on new cohorts were found, only a qualitative update to the recent review was performed.
Recommendation: The US Environmental Protection Agency (EPA) should develop policies and procedures to allow the agency to use and update existing systematic reviews. It is important that the existing systematic review’s study question directly addresses EPA’s topic of interest and that the methods be critically evaluated before the systematic review is used and updated.
Evidence Integration
- A comparison of doses between the animal and the human studies of PBDEs was challenging and imprecise because the animal studies often report report external administered doses (usually without measures of internal dose), whereas human studies measure biomarkers of internal dose (with estimates of the external administered dose being uncertain). There is some indication that the difference in internal dose between humans and rodents may be less than the difference in administered dose; these estimates are uncertain, however, and additional work is needed to clarify this issue. Toxicology studies that measure internal dose metrics, including metrics that are similar to those used in human biomonitoring, could help address this data gap.
Recommendation: To support animal-to-human extrapolations, pharmacokinetic data should be generated and used to develop pharmacokinetic models that make it possible to infer human internal doses (not just intake) from biomonitoring data and animal internal doses from administered doses.
- Integration of human data that evaluated measures of IQ and ADHD with animal studies that evaluated learning, memory, and attention was challenging since these end points are not identical. The animal studies use different tests of learning and memory and, even when the same type of behavioral test was used, testing methods and data analyses often differed between studies. The heterogeneity in testing methods and data analyses contributed to the challenges for evaluating consistency of the evidence in the animal studies. The committee found it helpful to focus its quantitative analysis on a specific measure of learning that was consistently reported in the animal studies.
Mechanistic Information
- A review of pharmacokinetic and mechanistic data on PBDEs in relation to developmental neurotoxicity provided some biological plausibility of the associations observed between PBDE exposure during the perinatal period and later neurobehavioral outcomes. An attempt to illustrate an adverse outcome pathway from these data was hindered by the complexity and multifactorial nature of how PBDEs affect neurodevelopmental processes.
Hazard Identification
- The committee concluded that developmental exposure to PBDEs is presumed to pose a hazard to intelligence in humans and that it was not possible to draw a hazard conclusion about effects on attention-related behavioral conditions in humans.
Low-Dose Effects
- The committee concluded that the human studies provide a moderate level of evidence that PBDEs are associated with decrements in IQ in humans. Uncertainty in the internal doses of humans relative to experimental animals limited the ability to draw conclusions about the prediction of low-dose effects based on experimental animal studies. The development of pharmacokinetic data and models for extrapolation of data from animal studies or human biomonitoring data could facilitate the evaluation of the potential of PBDEs to cause health effects in humans at low doses.
Other Considerations
- Mixtures: The committee found that humans are exposed to a mixture of PBDEs, whereas the experimental animal evidence was generally from studies with a single congener. This difference between the human mixture exposures and the single chemical animal exposures contributed to the challenges for integrating evidence between the human and the animal studies.
- Expertise: The committee found that the conduct of the systematic review and evidence integration requires a multidisciplinary approach that should be tailored to the specific review question. Experts in the conduct of meta-analyses and benchmark dose modeling will be essential.
REFERENCES
Bal-Price, A., P.J. Lein, K.P. Keil, S. Sethi, T. Shafer, M. Barenys, E. Fritsche, M. Sachana, and M.E. Meek. 2017 Developing and applying the adverse outcome pathway concept for understanding and predicting neurotoxicity. Neurotoxicology. 59:240-255.
Behl, M., J.H. Hsieh, T.J. Shafer, W.R. Mundy, J.R. Rice, W.A. Boyd, J.H. Freedman, E.S. Hunter, III, K.A. Jarema, S. Padilla, and R.R. Tice. 2015. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 52(Pt. B):181-193.
Biesemeier, J.A., M.J. Beck, H. Silberberg, N.R. Myers, J.M. Ariano, A. Radovsky, L. Freshwater, D.W. Sved, S. Jacobi, D.G. Stump, M.L. Hardy, and T. Stedeford. 2011. An oral developmental neurotoxicity study of decabromodiphenyl ether (DecaBDE) in rats. Birth Defects Res. B Dev. Reprod. Toxicol. 92(1):17-35.
Blanco, J., M. Mulero, L. Heredia, A. Pujol, J.L. Domingo, and D.J. Sánchez. 2013. Perinatal exposure to BDE-99 causes learning disorders and decreases serum thyroid hormone levels and BDNF gene expression in hippocampus in rat offspring. Toxicology 308:122-128.
Bowers, W.J., P.M. Wall, J.S. Nakai, A. Yagminas, M. Wade, and N. Li. 2015. Behavioral and thyroid effects of in utero and lactational exposure of Sprague-Dawley rats to the polybrominated diphenyl ether mixture DE71. Neurotoxicol. Teratol. 52(Pt. B):127-142.
Bradman, A., R Castorina, A. Sjödin, L. Fenster, R.S. Jones, K.G. Harley, J. Chevrier, N.T. Holland, and B. Eskenazi. 2012. Factors associated with serum polybrominated diphenyl ether (PBDE) levels among school-age children in the CHAMACOS cohort. Environ. Sci. Technol. 46(13):7373-7381.
Bramwell, L., S.V. Glinianaia, J. Rankin, M. Rose, A. Fernandes, S. Harrad, and T. Pless-Mulolli. 2016. Associations between human exposure to polybrominated diphenyl ether flame retardants via diet and indoor dust, and internal dose: A systematic review. Environ. Int. 92-93:680-694.
Brown, J.P., D. Hall, C.L. Frank, K. Wallace, W.R. Mundy, and T.J. Shafer. 2016. Editor’s highlight: Evaluation of a microelectrode array-based assay for neural network ontogeny using training set chemicals. Toxicol. Sci. 154(1):126-139.
Buratovic, S., H. Viberg, A. Fredriksson, and P. Eriksson. 2014. Developmental exposure to the polybrominated diphenyl ether PBDE 209: Neurobehavioural and neuroprotein analysis in adult male and female mice. Environ. Toxicol. Pharmacol. 38(2):570-585.
Butryn, D.M., M.S. Gross, L.H. Chi, A. Schecter, J.R. Olson, and D.S. Aga. 2015. “One-shot” analysis of polybrominated diphenyl ethers and their hydroxylated and methoxylated analogs in human breast milk and serum using gas chromatography-tandem mass spectrometry. Anal. Chim. Acta 892:140-147.
Canton, R., J. Sanderson, R. Letcher, A. Bergman, and M. van den Berg. 2005. Inhibition and induction of aromatase (CYP19) activity by brominated flame retardants in H295R human adrenocortical carcinoma cells. Toxicol. Sci. 88(2):447-455.
Canton, R.F., D.E. Scholten, G. Marsh, P.C. de Jong, and M. van den Berg. 2008. Inhibition of human placental aromatase activity by hydroxylated polybrominated diphenyl ethers (OH-PBDEs). Toxicol. Appl. Pharmacol. 227(1):68-75.
Ceccatelli, R., O. Faass, M. Schlumpf, and W. Lichtensteiger. 2006. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology 220(2-3):104-116.
Chen, A., K. Yolton, S.A. Rauch, G.M. Webster, R. Hornung, A. Sjödin, K.N. Dietrich, and B.P. Lanphear. 2014. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: The HOME study. Environ. Health Perspect. 122(8):856-862.
Chen, L.J., E.H. Lebetkin, J.M. Sanders, and L.T. Burka. 2006. Metabolism and disposition of 2,2',4,4',5-pentabromodiphenyl ether (BDE99) following a single or repeated administration to rats or mice. Xenobiotica 36(6):515-534.
Chen, Y.H., Z.H. Li, Y. Tan, C.F. Zhang, J.S. Chen, F. He, Y.H. Yu, and D.J. Chen. 2014. Prenatal exposure to decabrominated diphenyl ether impairs learning ability by altering neural stem cell viability, apoptosis, and differentiation in rat hippocampus. Hum. Exp. Toxicol. doi: 10.1177/0960327113509661.
Cheng, J., J. Gu, J. Ma, X. Chen, M. Zhang, and W. Wang. 2009. Neurobehavioural effects, redox responses and tissue distribution in rat offspring developmental exposure to BDE-99. Chemosphere 75(7):963-968.
Cornell, J.E., C.D. Mulrow, R. Localio, C.B. Stack, A.R. Meibohm, E. Guallar, and S.N. Goodman. 2014. Ran-dome-effects meta-analysis of inconsistent effects: A time for change. Ann. Intern. Med. 160(4):267-270.
Costa, L.G., C. Pellacani, K. Dao, T.J. Kavanagh, and P.J. Roque. 2015. The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. Neurotoxicology 48:68-76.
Cowell, W.J., S.A. Lederman, A. Sjödin, R. Jones, S. Wang, F.P. Perera, R. Wang, V.A. Rauh, and J.B. Herbstman. 2015. Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3-7 years. Neurotoxicol. Teratol. 52(Pt B):143-150.
Darnerud, P.O., and T. Sinjari. 1996. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroxine and TSH blood levels in rats and mice. Organohalogen Compd. 29:316-319.
Darnerud, P.O., M. Aune, L. Larsson, and S. Hallgren. 2007. Plasma PBDE and thyroxine levels in rats exposed to Bromkal or BDE-47. Chemosphere 67(9):S386-S392.
de-Miranda, A.S., S.N. Kuriyama, C.S. da-Silva, M.S. do-Nascimento, T.E. Parente, and F.J. Paumgartten. 2016. Thyroid hormone disruption and cognitive impairment in rats exposed to PBDE during postnatal development. Reprod. Toxicol. 63:114-124.
Dingemans, M.M., M. van den Berg, and R.H. Westerink. 2011. Neurotoxicity of brominated flame retardants: (In)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ. Health Perspect. 119(7):900-907.
Driscoll, L.L., A.M. Gibson, and A. Hieb. 2009. Chronic postnatal DE-71 exposure: Effects on learning, attention and thyroxine levels. Neurotoxicol. Teratol. 31(2):76-84.
Driscoll, L.L., J. Kaplan, E. Bucuvalas, H. Allen, J. Kraut, and J. Fitzpatrick. 2012. Acute postnatal exposure to the pentaBDE commercial mixture DE-71 at 5 or 15mg/kg/day does not produce learning or attention deficits in rats. Neurotoxicol. Teratol. 34(1):20-26.
Druwe, I., T.M. Freudenrich, K. Wallace, R.J. Shafer, and W.R. Mundy. 2015. Sensitivity of neuroprogenitor cells to chemical-induced apoptosis using a multiplexed assay suitable for high-throughput screening. Toxicology 333:14-24.
Dufault, C., G. Poles, and L.L. Driscoll. 2005. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol. Sci. 88(1):172-180.
EFSA (European Food Safety Authority). 2011. Scientific opinion on polybrominated diphenyl ethers (PBDEs) in food. EFSA J. 9(5):2156.
EPA (U.S. Environmental Protection Agency). 2010. An Exposure Assessment of Polybrominated Diphenyl Ethers. EPA/600/R-08/086F. National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC [online]. Available: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=210404 [accessed March 14, 2016].
Eriksson, P., E. Jakobsson, and A. Fredriksson. 2001. Brominated flame retardants: A novel class of developmental neurotoxicants in our environment? Environ. Health Perspect. 109(9):903-908.
Eskenazi, B., J. Chevrier, S.A. Rauch, K. Kogut, K.G. Harley, C. Johnson, C. Trujillo, A. Sjödin, and A. Bradman. 2013. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ. Health Perspect. 121(2):257-262.
Faass, O., R. Ceccatelli, M. Schlumpf, and W. Lichtensteiger. 2013. Developmental effects of perinatal exposure to PBDE and PBC on gene expression in sexually dimorphic rat brain regions and female sexual behavior. Gen. Comp. Endocrinol. 188:232-241.
Fischer, C., A. Fredriksson, and P. Eriksson. 2008. Coexposure of neonatal mice to a flame retardant PBDE 99 (2, 2',4, 4',5-pentabromodiphenyl ether) and methyl mercury enhances developmental neurotoxic defects. Toxicol. Sci. 101(2):275-285.
Fowles, J.R., A. Fairbrother, L. Baecher-Steppan, and N.I. Kerkvliet. 1994. Immunologic and endocrine effects of the flameretardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology 86(1-2):49-61.
Gascon, M., M. Fort, D. Martínez, A.E. Carsin, J. Forns, J.O. Grimalt, L. Santa Marina, N. Lertxundi, J. Sunyer, and M. Vrijheid. 2012. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ. Health Perspect. 120(12):1760-1765.
Gassmann, K., T. Schreiber, M.M. Dingemans, G. Krause, C. Roderigo, S. Giersiefer, J. Schuwald, M. Moor, K. Unfried, K. Bergman, R.H. Weterink, C.R. Rose, and E. Fritsche. 2014. BDE-47 and 6-oh-BDE-47 modulate calcium homeostasis in primary fetal human neural progenitor cells via ryanodine receptor-independent mechanisms. Arch. Toxicol. 88(8):1537-1548.
Geyer, H.J., K.W. Schramm, P.O. Darnerud, M. Aune, E.A. Feicht, K.W. Fried, B. Henkelmann, D. Lenoir, P. Schmid, and T.A. McDonald. 2004. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd. 66:3820-3825.
Ghassabian, A., H. El Marroun, R.P. Peeters, V.W. Jaddoe, A. Hofman, F.C. Verhulst, H. Tiemeier, and T. White. 2014. Downstream effects of maternal hypothroxinemia in early pregnancy: Nonverbal IQ and brain morphology in school-age children. J. Clin. Endocrinol. Metab. 99(7):2383-2390.
Gump, B.B., S. Yun, and K. Kannan. 2014. Polybrominated diphenyl ether (PBDE) exposure in children: Possible associations with cardiovascular and psychological functions. Environ. Res. 132:244-250.
Guyatt, G.H., A.D. Oxman, R. Kunz, J. Brozek, P. Alonso-Coello, D. Rind, P.J. Devereaux, V.M. Montori, B. Freyschuss, G. Vist, R. Jaeschke, J.W. Williams, Jr., M.H. Murad, D. Sinclair, Y. Falck-Ytter, J. Meerpohl, C. Whittington, K. Thorlund, J. Andrews, and H.J. Schunemann. 2011. GRADE guidelines 6. Rating the quality of evidence—imprecision. J. Clin. Epidemiol. 64(12):1283-1293.
He, P., A.G. Wang, T. Xia, P. Gao, Q. Niu, L.J. Guo, and X.M. Chen. 2009. Mechanisms underlying the developmental neurotoxic effect of PBDE-47 and the enhanced toxicity associated with its combination with PCB153 in rats. NeuroToxicology 30(6):1088-1095.
He, P., A. Wang, Q. Niu, L. Guo, T. Xia, and X. Chen. 2011. Toxic effect of PBDE-47 on thyroid development, learning, and memory, and the interaction between PBDE-47 and PCB153 that enhances toxicity in rats. Toxicol. Ind. Health 27(3):279-288.
He, Y. M.B. Murphy, R.M. Yu, M.H. Lam, M. Hecker, J.P. Giesy, R.S. Wu, and P.K. Lam. 2008. Effects of 20 PBDE metabolites on steroidogenesis in the H295R cell line. Toxicol. Lett. 176(3):230-238.
Hedges, L.V., J. Gurevitch, and P.S. Curtis. 1999. The meta-analysis of response ratios in experimental ecology. Ecology 80(4):1150-1156.
Herbstman, J.B., A. Sjödin, M. Kurzon, S.A. Lederman, R.S. Jones, V. Rauh, L.L. Needham, D. Tang, M. Niedzwiecki, R.Y. Wang, and F. Perera. 2010. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect. 118(5):712-719.
Ho, K.L., M.S. Yau, M.B. Murphy, Y. Wan, B.M. Fong, S. Tam, J.P. Giesy, K.S. Leung, and M.H. Lam. 2015. Urinary bromophenol glucuronide and sulfate conjugates: Potential human exposure molecular markers for polybrominated diphenyl ethers. Chemosphere 133:6-12.
Horn, S., and H. Heuer. 2010. Thyroid hormone action during brain development: More questions than answers. Mol. Cell Endocrinol. 315(1-2):19-26.
Ibhazehiebo, K., T. Iwasaki, J. Kimura-Kuroda, W. Miyazaki, N. Shimokawa, and N. Koibuchi. 2011. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ. Health Perspect. 119(2):168-175.
IOM (Institute of Medicine). 2011. Finding What Works in Health Care: Standards for Systematic Review. Washington, DC: The National Academies Press.
Karpeta, A., J. Barc, A. Ptak, and E.L. Gregoraszczuk. 2013. The 2,2',4,4'-tetrabromodiphenyl ether hydroxylated metabolites 5-OH-BDE-47 and 6-OH-BDE-47 stimulate estradiol secretion in the ovary by activating aromatase expression. Toxicology 305:65-70.
Kilkenny, C., W.J. Browne, I.C. Cuthill, M. Emerson, and D.G. Altman. 2010. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 8(6):e1000412.
Kim, K.H., D.D. Bose, A. Ghogha, J. Riehl, R. Zhang, C.D. Barnhart, P.J. Lein, and I.N. Pessah. 2011. Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environ. Health Perspect. 119(4):519-526.
Kodavanti, P.R., J.E. Royland, C. Osorio, W.M. Winnik, P. Oritz, L. Pei, R. Ramabhadran, and O. Alzate. 2015. Developmental exposure to a commercial PBDE mixture: Effects on protein networks in the cerebellum and hippocampus of rats. Environ. Health Perspect. 123(5):428-436.
Koenig, C.M., J. Lango, I.N. Pessah, and R.F. Berman. 2012. Maternal transfer of BDE-47 to offspring and neurobehavioral development in C57BL/6J mice. Neurotoxicol. Teratol. 34(6):571-580.
Konstantinova, A., G. Arsenault, B. Chittim, A. McAlees, R. McCrindle, D. Potter, C. Tashiro, and B. Yeo. 2008. Identification of the minor components of Great Lakes DE-71™ technical mix by means of 1H NMR and GD/MS. Chemosphere 73(1 Suppl.):S39-S43.
Lajeunesse, M.J. 2011. On the meta-analysis of response ratios for studies with correlated and multi-group designs. Ecology 92(11):2049-2055.
Lam, J., P. Sutton, J. McPartland, L.I. Davidson, N. Daniels, S. Sen, D. Axelrad, B. Lanphear, D. Bellinger, and T.J. Woodruff. 2015. Applying the Navigation Guide Systematic Review Methodology, Case Study #5. Association Between Developmental Exposures to PBDEs and human Neurodevelopment: A Systematic Review of the Evidence Protocol, April 2015 [online]. Available: http://www.crd.york.ac.uk/PROSPEROFILES/17890_PROTOCOL_20150322.pdf [accessed August 3, 2016].
Lam, J., B. Lanphear, D. Bellinger, D.A. Axelrad, J. McPartland, P. Sutton, L. Davidson, N. Daniels, S. Sen, and T. Woodruff. 2016. Systematic Review and Meta-Analysis of Association between PBDE Exposure and IQ or ADHD. Draft Report, July 2016. University of California, San Francisco, CA.
Lam, J., B.P. Lanphear, D. Bellinger, D.A. Axelrad, J. McPartland, P. Sutton, L. Davidson, N. Daniels, S. Sen, and T.J. Woodruff. In press. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ. Health Perspect.
Li, T., W. Wang, Y.W. Pan, L. Xu, and Z. Xia. 2013. A hudroxylated metabolite of flame-retardant PBDE-47 decreases the survival, proliferation and neuronal differentiation of primary cultured adult neural stem cells and interferes with signaling of ERK5 MAP kinase and neurotrophin 3. Toxicol. Sci. 134(1):111-124.
Lilienthal, H., A. Hack, A. Roth-Härer, S.W. Grande, and C.E. Talsness. 2006. Effects of developmental exposure to 2,2′,4,4′,5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ. Health Perspect. 114(2):194-201.
Lin, D.Y., H.R. Chao, Y.Y. Gou, and C.Y. Huang. 2010. Infants ingesting high breast milk levels of polybrominated diphenyl ethers may have negative impact on their neurodevelopment. Pp. 325-327 in 2010 International Conference on Chemistry and Chemical Engineering (ICCCE 2010) IEEE [online]. Available: http://ieeexplore.ieee.org/document/5560424/#full-text-section [accessed November 10, 2016].
Linares, V., M. Bellés, and J.L. Domingo. 2015. Human exposure to PBDE and critical evaluation of health hazards. Arch. Toxicol. 89(3):335-356.
Llansola, M., M. Hernandez-Viadel, S. Erceg, C. Montoliu, and V. Felipo. 2009. Increasing the function of the glutamate-nitric oxide-cyclic guanosine monophosphate pathway increases the ability to learn a Y-maze task. J. Neurosci. Res. 87(10):2351-2355.
Lorber, M. 2008. Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci.Epidemiol. 18(1):2-19.
Meerts, I.A., J.J. Van Zanden, E.A. Luijks, I. Van Leeuwen-Bol, G. Marsh, E. Jakobsson, Å. Bergman, and A. Brouwer. 2000. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 56(1):95-104.
Meerts, I.A., R.J. Letcher, S. Hoving, G. Marsh, A. Bergman, J.G. Lemmen, B. van der Burg, and A. Brouwer. 2001. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ. Health Perspect. 109(4):399-407.
Mercado-Feliciano, M., and R.M. Bigsby. 2008. Hydroxylated metabolites of the polybrominated diphenyl ether mixture DE-71 are weak estrogen receptor-alpha ligands. Environ. Health Perspect. 116(10):1315-1321.
Modesto, T., H. Tiemeier, R.P. Peeters, V.W. Jaddoe, A. Hofman, F.C. Verhulst, and A. Ghassabian. 2015. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr. 169(9):838-845.
Morgan, R.L., K.A. Thayer, L. Bero, N. Bruce, Y. Falck-Ytter, D. Ghersi, G. Guyatt, C. Hooijmans, M. Langendam, D. Mandrioli, R.A. Mustafa, E.A. Rehfuess, and A.A. Rooney. 2016. GRADE: Assessing the quality of evidence in environmental and occupational health. Environ. Int. 92-93:611-616.
Mundy, W. 2016. Presentation at Fortieth Annual Meeting of the Developmental Neurotoxicology Society, June 26, 2016, San Antonio, TX.
NCHS (National Center for Health Statistics). 2007. National Health and Nutrition Examination Survey 2003-2004 [online]. Available: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L28PBE_C.htm [accessed March 7, 2017].
NRC (National Research Council). 2014. Review of the Environmental Protection Agency’s State-of-the Science Evaluation of Nonmontonic Dose-Response Relationships as They Apply to Endocrine Disruptors. Washington, DC: The National Academies Press.
NTP (National Toxicology Program). 2015. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. Office of Health Assessment and Translation, Division, National Toxicology Program, National Institute of Environmental Health Sciences. January 9, 2015 [online]. Available: http://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf [accessed September 21, 2015].
Pallocca, G., M. Grinberg, M. Henry, T. Frickey, J.G. Hengstler, T. Waldmann, A. Sachinidis, J. Rahnenführer, and M. Leist. 2016. Identification of transcriptome signatures and biomarkers specific for potential developmental toxicants inhibiting human neural crest cell migration. Arch. Toxicol. 90(1):159-180.
Ptak, A., G. Ludewig, H.J. Lehmler, A.K. Wojtowicz, L.W. Robertson, and E.L. Gregoraszczuk. 2005. Comparison of the actions of 4-chlorobiphenyl and its hydroxylated metabolites on estradiol secretion by ovarian follicles in primary cells in culture. Reprod. Toxicol. 20(1):57-64.
Ptak, A., G. Ludewig, M. Kapiszewska, Z. Magnowska, H.J. Lehmler, L.W. Robertson, E.L. Gregoraszczuk. 2006. Induction of cytochromes P450, caspase-3 and DNA damage by PCB3 and its hydroxylated metabolites in porcine ovary. Toxicol. Lett. 166(3):200-211.
Qiu, X., M. Mercado-Feliciano, R.M. Bigsby, and R.A. Hites. 2007. Measurement of polybrominated diphenyl ethers and metabolites in mouse plasma after exposure to a commercial pentabromodiphenyl ether mixture. Environ. Health Perspect. 115(7):1052-1058.
Reverte, I., A.B. Klein, J.L. Domingo, and M.T. Colomina. 2013. Long term effects of murine postnatal exposure to decabromodiphenyl ether (BDE-209) on learning and memory are dependent upon APOE polymorphism and age. Neurotoxicol. Teratol. 40:17-27.
Reverte, I., A. Pujol, J.L. Domingo, and M.T. Colomina. 2014. Thyroid hormones and fear learning but not anxiety are affected in adult apoE transgenic mice exposed postnatally to decabromodiphenyl ether (BDE-209). Physiol. Behav. 133:81-91.
Rice, D.C., W.D. Thompson, E.A. Reeve, K.D. Onos, M. Assadollahzadeh, and V.P. Markowski. 2009. Behavioral changes in aging but not young mice after neonatal exposure to the polybrominated flame retardant decaBDE. Environ. Health Perspect. 117(12):1903-1911.
Rooney, A.A., A.L. Boyles, M.S. Wolfe, J.R. Bucher, and K.A. Thayer. 2014. Systematic review and evidence integration for literature-based environmental health assessments. Environ. Health Perspect. 122(7):711-718.
Rooney, A.A., G.S. Cooper, G.D. Jahnke, J. Lam, R.L. Morgan, A.L. Boyles, J.M. Ratcliffe, A.D. Kraft, H.J. Schunemann, and P. Schwingl. 2016. How credible are the study results? Evaluating and applying internal validity tools to literature-based assessments of environmental health hazards. Environ. Int. 92-93:617-629.
Roth, N., and M.F. Wilks. 2014. Neurodevelopmental and neurobehavioural effects of polybrominated and perfluorinated chemicals: A systematic review of the epidemiological literature using a quality assessment scheme. Toxicol. Lett. 230(2):271-281.
Roze, E., L. Meijer, A. Bakker, K.N. Van Braeckel, P.J. Sauer, and A.F. Bos. 2009. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect. 117(12):1953-1958.
Ryan, K.R., O. Sirenko, F. Parham, J.H. Hsieh, E.F. Cromwell, R.R. Tice, and M. Behl. 2016. Neurite outgrowth in human induced pluripotent stem cell-derived neurons as a high-throughput screen for developmental neurotoxicity or neurotoxicity. Neurotoxicology 53:271-281.
Sagiv, S.K., K. Kogut, F.W. Gaspar, R.B. Gunier, K.G. Harley, K. Parra, D. Villasenor, A. Bradman, N. Holland, and B. Eskenazi. 2015. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9-12 years of age. Neurotoxicol. Teratol. 52(Pt B):151-161.
Schmidt, B.Z., M. Lehmann, S. Gutbier, E. Nembo, S. Noel, L. Smirnova, A. Forsby, J. Hescheler, H.X. Avci, T. Hartung, M. Leist, J. Kobolák, and A. Dinnyés. 2017. In vitro acute and developmental neurotoxicity screening: an overview of cellular platforms and high-throughput technical possibilities. Arch. Toxicol. 91(1):1-33.
Siddiqi, M.A., R.H. Laessig, and K.D. Reed. 2003. Polybrominated diphenyl ethers (PBDEs): New pollutants-old diseases. Clin. Med. Res. 1(4):281-290.
Singh, S., A. Srivastava, V. Kumar, A. Pandey, D. Kumar, C.S. Rajpurohit, V.K. Khanna, S. Yadav, and A.B. Pant. 2016. Stem cells in neurotoxicology/developmental neurotoxicology: Current scenario and future prospects. Mol. Neurobiol. 53(10):6938-6949.
Stapleton, H.M., S.M. Kelly, R. Pei, R.J. Letcher, and C. Gunsch. 2009. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environ Health Perspect. 117(2):197-202.
Staskal, D.F., H. Hakk, D. Bauer, J.J. Diliberto, and L.S. Birnbaum. 2006. Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicol. Sci. 94(1):28-37.
Sterne, J.A., M.A. Hernán, B.C. Reeves, J. Savović, N.D. Berkman, M. Viswanathan, D. Henry, D.G. Altman, M.T. Ansari, I. Boutron, J.R. Carpenter, A.W. Chan, R. Churchill, J.J. Deeks, A. Hróbjartsson, J. Kirkham, P. Jüni, Y.K. Loke, T.D. Pigott, C.R. Ramsay, D. Regidor, H.R. Rothstein, L. Sandhu, P.L. Santaguida, H.J. Schünemann, B. Shea, I. Shrier, P. Tugwell, L. Turner, J.C. Valentine, H. Waddington, E. Waters, G.A. Wells, P.F. Whiting, and J.P. Higgins. 2016. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919.
Ta, T.A., C.M. Koenig, M.S. Golub, I.N. Pessah, L. Qi, P.A. Aronov, and R.F. Berman. 2011. Bioaccumulation and behavioral effects of 2,2',4,4'-tetrabromodiphenyl ether (BDE-47) in perinatally exposed mice. Neurotoxicol. Teratol. 33(3):393-404.
Thuresson, K., P. Höglund, L. Hagmer, A. Sjödin, Å. Bergman, and K. Jakobsson. 2006. Apparent half-lives of hep-ta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ. Health Perspect.114(2):176-181.
Verma, P., P. Singh, and B.S. Gandhi. 2013. Prophylactic efficacy of Bacopa monnieri on decabromodiphenyl ether (PBDE-209)-induced alterations in oxidative status and spatial memory in mice. Asian J. Pharm. Clin. Res. 6(3):242-247.
Verma, P., P. Singh, and B.S. Gandhi. 2014. Neuromodulatory role of Bacopa monnieri on oxidative stress induced by postnatal exposure to decabromodiphenyl ether (PBDE -209) in neonate and young female mice. Iran. J. Basic Med. Sci. 17(4):307-311.
Viberg, H., A. Fredriksson, and P. Eriksson. 2003. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol. Appl. Pharmacol. 192(2):95-106.
Viberg, H., N. Johansson, A. Fredriksson, J. Eriksson, G. Marsh, and P. Eriksson. 2006. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicol. Sci. 92(1):211-218.
Viberg, H., W. Mundy, and P. Eriksson. 2008. Neuonatal exposure to a decabrominated diphenyl ether (PBDE 209) results in changes in BDNF, CaMKII and GAP-43, biochemical substrates of neuronal survival, growth and synaptogenesis. Neurotoxicology 29(1):152-159.
Wang, X., L. Yang, Q. Wang, Y. Guo, N. Li, M. Ma, and B. Zhou. 2016. The neurotoxicity of DE-71: Effects on neural development and impairment of serotonergic signaling in zebrafish larvae. J. Appl. Toxicol. 36(12):1605-1613.
Wang, Z., L. Yang, Y. Wu, C. Huang, Q. Wang, J. Han, Y. Guo, Z. Shi, and B. Zhou. 2015. The developmental neurotoxicity of polybrominated diphenyl ethers: Effect of DE-71 on dopamine in zebrafish larvae. Environ. Toxicol. Chem. 34(5):1119-1126.
Westerink, R.H. 2014. Modulation of cell viability, oxidative stress, calcium homeostasis and votage- and ligand-gated ion channels as common mechanisms of action of (mixtures of) non-dioxin-like plychlorinated biphenyls and polybrominated diphenyl ethers. Environ. Sci. Pollut. Res. 21(10):6373-6383.
Whiting, P., J. Savovic, J.B. Higgins, D.M. Caldwell, B.C. Reeves, B. Shea, P. Davies, J. Kleijnen, and R. Churchill. 2016. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 69:225-234.
Wiseman, S.B., Y. Wan, H. Chang, X. Zhang, M. Hecker, P.D. Jones, and J.P. Giesy. 2011. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: Environmental sources, metabolic relationships, and relative toxicities. Mar. Pollut. Bull. 63(5-12):179-188.
Woodruff, T.J., and P. Sutton. 2014. The Navigation Guide systematic review methodology: A rigorous and transparent method for translating environmental health science into better health outcomes. Environ. Health Perspect. 122(10):1007-1014.
Woods, R., R.O. Vallero, M.S. Golub, J.K. Suarez, T.A. Ta, D.H. Yasui, L.H. Chi, P.J. Kostyniak, I.N. Pessah, R.F. Berman, and J.M. LaSalle. 2012. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum. Mol. Genet. 21(11):2399-2411.
Zhang, H., X. Li, J. Nie, and Q. Niu. 2013. Lactation exposure to BDE-153 damages learning and memory, disrupts spontaneous behavior and induces hippocampus neuron death in adult rats. Brain Res. 1517:44-56.
Zhang, H., K. Yolton, G.M. Webster, A. Sjodin, A.M. Calafat, K.N. Dietrich, Y. Xu, C. Xie, J.M. Braun, B.P. Lanphear, and A. Chen. 2017. Prenatal PBDE and PCB exposures and reading, cognition, and externalizing behavior in children. Environ. Health Perspect. 125(4):746-752.
Zhao, W., J. Cheng, J. Gu, Y. Liu, M. Fujimura, and W. Wang. 2014. Assessment of neurotoxic effects and brain region distribution in rat offspring prenatally co-exposed to low doses of BDE-99 and methylmercury. Chemosphere 112:170-176.