Appendix C
Supporting Materials for the Phthalate (Animal) Systematic Review
SECTION C-1
PHTHALATE (ANIMAL) SYSTEMATIC REVIEW PROTOCOL
August 3, 2016
(Modified on September 15, 2016—See Section C-1f)
BACKGROUND AND INTRODUCTION
Phthalates are high production volume chemicals used primarily as plasticizers in many industrial and consumer products. As a result of their ubiquitous use, there is documented widespread human exposure to them. Because the developing organism has been shown to be particularly vulnerable to endocrine-disrupting chemicals, such as phthalates, the committee decided to focus on studies of in utero exposure. Ortho-phthalates have been linked to effects on male reproductive-tract development after in utero exposure in animal studies.
OBJECTIVE AND SPECIFIC AIMS
Review Question
The overall objective of this systematic review is to answer the question what is the effect of in utero exposure to ortho-phthalates on anogenital distance, hypospadias, or fetal testosterone in nonhuman male mammals?
The specific aims of the review are to:
- Identify literature reporting the effects of in utero phthalate exposure on male anogenital distance, hypospadias, or fetal testosterone in nonhuman mammals.
- Extract data on male effects of in utero phthalate exposure on anogenital distance, hypospadias, or fetal testosterone from relevant studies.
- Assess the internal validity (risk of bias) of individual studies.
- Summarize the extent of evidence available.
- Synthesize the evidence using a narrative approach or meta-analysis (if appropriate) considering limitations on data integration, such as study-design heterogeneity.
- Rate the confidence in the body of evidence for studies in nonhuman mammals according to one of five statements: (1) high, (2) moderate, (3) low, (4) very low/no evidence available, or (5) evidence of lack of effects on male reproductive-tract development.
PECO Statement
A PECO (Population, Exposure, Comparator, and Outcome) statement was developed by the review team as an aid to identify search terms and inclusion/exclusion criteria as appropriate for addressing the review question for the systematic review.
Population: Nonhuman male mammals
Exposure:
- In utero exposure to any of the following ortho-phthalates or the corresponding monoester or oxidative metabolites: benzylbutyl phthalate (CAS no. 85-68-7), dibutyl phthalate (CAS no. 84-74-2), diethyl phthalate (CAS 84-66-2), diethylhexyl phthalate (CAS no. 117-81-7), diisobutyl phthalate (CAS no. 84-69-5), diisononyl phthalate (CAS no. 28553-12-0), diisooctyl phthalate (CAS no. 27554-26-3), dimethyl phthalate (CAS no. 131-11-3), di-n-octyl phthalate (CAS no. 117-84-0), diisodecyl phthalate (CAS no. 26761-40-0), and/or dipentyl phthalate (CAS no. 131-18-0).
- Oral route of exposure.
Comparator: Male nonhuman mammals exposed in utero to different doses of phthalates or vehicle-only treatment.
Outcomes:
- Anogenital distance (AGD): the measured distance between the anus and the genitals. Typically measured from the anus to the base of the scrotum or the base of the phallus. Other measures that might be used:
- Anogenital index (AGI): AGD measurement divided by body weight or by the cube root of body weight
- Anoscrotal distance (ASD): the measured distance between the anus and base of the scrotum
- Anopenile distance (APD): the measured distance from the anus to the base of the penis
- Hypospadias (incidence and severity/grade)
- Fetal testosterone concentration (e.g., measured from testes, serum, or plasma taken in utero)
METHODS
Problem Formulation and Protocol Development
The review question and specific aims were developed and refined through a series of problem formulation steps. The committee considered review articles on endocrine disruptors in surveying the types of chemicals that might make good case examples and held a workshop to explore potential case examples, including phthalates. The committee sought an example of a chemical for which the human and the animal evidence on effects appears to be associated with different exposure levels of that chemical and due to perturbation of the estrogen or androgen hormone system. Phthalates appear to fit this case criterion, and positive feedback was received at the committee’s workshop.
Alterations in male reproductive-tract development are the most sensitive effects from exposure to phthalates (NRC 2008). Because the period during in utero sexual differentiation (i.e., the masculinization programming window) is the most sensitive life stage, the exposure period of interest for the systematic
review is in utero. Animal studies have illustrated a spectrum of effects in male reproductive development after in utero exposure to phthalates, including under developed or absent reproductive organs, malformed external genitalia (hypospadias), undescended testicles (cryptorchidism), decreased AGD, retained nipples, and decreased sperm production (NRC 2008). The systematic review will focus on end points reflecting androgen-dependent adverse effects (AGD and hypospadias), an adverse effect that occurs at relatively low doses (AGD), and a key event in the adverse outcome pathway leading to reduced AGD and hypospadias (fetal testosterone).
Consideration was given to including cryptorchidism as an end point, but the committee decided against it. The mode of action for phthalate-induced cryptorchidism involves reductions in INSL-3 levels in addition to androgen-dependent mechanisms. Important for the committee’s charge, there are few, if any, human studies on dose-response relationships between phthalate exposure and cryptorchidism to compare to animal data. Furthermore, studies have shown that rats exposed to phthalates have similar sensitivity to decreased fetal testosterone and AGD as they do for decreased INSL-3, and that cryptorchidism is a less sensitive end point compared to reductions in AGD. Because the overall objective of the committee is to use this systematic review with the one being conducted on the human evidence to evaluate the coherence between effects and dose-response relationships, the committee judged that it would not be useful to include cryptorchidism in the systematic reviews on phthalates.
The protocol will be peer reviewed by subject-matter and systematic-review experts in accordance with standard report-review practices of the National Academies of Sciences, Engineering, and Medicine. The protocols will be revised in response to peer review comments and will subsequently be published as appendices to the committee’s final report. The identity of the peer reviewers will remain anonymous to the committee until the publication of the final report, when their names and affiliations are disclosed in the Preface.
Committee and Staff
There are 11 committee members, supported by two staff members of the National Academies. The committee members were appointed in accordance with the standard policies and practices of the National Academies on the basis of their expertise in general toxicology, reproductive toxicology, developmental toxicology, endocrinology, neurotoxicology, epidemiology, risk assessment, biostatistics, and systematic-review methods. The membership of the committee and the staff was determined before the topic of the systematic review was selected. It was known, however, that each case study would be on an endocrine-disrupting chemical, so committee members who have relevant expertise were specifically recruited and appointed.
Review Team
The review team for this case study will be a subgroup of the committee (DD, KJ), two National Academies staff members (EM, SM), and an information specialist (JB). If a member of the review team was a coauthor of a study under review, that member will recuse himself or herself from the evaluation of the quality of that study.
The review team will be responsible for performing all aspects of the review, including conducting the literature searches; applying inclusion/exclusion criteria to screen studies; extracting data; assessing risk of bias for included studies; and analyzing and synthesizing data. The roles and responsibilities of the team members will be documented throughout the protocol. Throughout the course of its work, the review team will also engage other members of the committee to provide consultation as needed. The involvement of those individuals will be documented and acknowledged.
Biographical information on the review team is presented in Section C-1a.
Search Methods
Search for Existing Systematic Reviews
The review team will consider using existing systematic reviews to address or help to address its research question. English-language systematic reviews conducted within the last 3 years will be sought. The review team will incorporate prior reviews, update prior reviews, and/or use the reviews as part of its searching, depending on determination of their relevancy and quality (Whitlock et al. 2008). Current guidance on using existing systematic reviews will be used (Robinson et al. 2014, 2015, 2016).
Search
Recent, relevant, high-quality systematic reviews addressing the research question about phthalates and male reproductive-tract development will be searched. PubMed will be searched by adding the qualifier “systematic review”[ti] OR “meta-analysis”[pt] OR “meta-analysis”[ti] OR (“systematic”[ti] AND “review”[ti]) OR (systematic review [tiab] AND review [pt]) OR “meta synthesis”[ti] OR “meta synthesis”[ti] OR “integrative review”[tw] OR “integrative research review”[tw] OR “cochrane database syst rev”[ta] OR “evidence synthesis”[tiab] to the preliminary search strategy (see Section C-1b). Language and date restrictions will be applied (English language; published 2013 to present). The systematic review protocol registries PROSPERO (CRD) and CAMARADES will also be searched using key terms from the preliminary PubMed strategy.
Study Selection
Two team members (SM, EM) will independently screen search results, applying the following exclusion criteria:
- Not a systematic review.1 The minimum criteria for a study to be considered a systematic review are
- conduct of an explicit and adequate literature search,
- application of predefined eligibility criteria,
- consideration of the quality of included studies or risk of bias assessment, and
- synthesis (or attempt at synthesis) of the findings, either qualitatively or quantitatively.
- Not in English.
- Search date prior to 2013.
- Does not match the research question or PECO elements.
For PubMed results, screening will be conducted first using abstracts and then at the full-text level. Results from PROSPERO and CAMARADES will be conducted at one level, using the information in the registry. Disagreements regarding eligibility will be resolved through discussion or, where necessary, by a third team member.
Assessment for Quality
Two investigators (KR, AR) will independently assess the risk of bias of eligible systematic reviews using ROBIS (Whiting et al. 2016). Disagreements in rating will be resolved through discussion or, where necessary, through consultation with a third team member. Systematic reviews rated as low quality will be excluded from further consideration at this stage.
___________________
1 A systematic review “is a scientific investigation that focuses on a specific question and uses explicit, prespecified scientific methods to identify, select, assess, and summarize the findings of similar but separate studies” (IOM 2011, p. 1).
Use of Existing Reviews
Eligible systematic reviews of high quality will be reviewed, considering date of search and match with the PECO statement as well as availability of data from the primary studies, how risk of bias was conducted, and other factors. Current reviews considered a good match will be used to address the research question. Reviews that are a good match but with search dates more than a year ago will be updated. If no relevant systematic reviews are found, an independent systematic review will be performed.
Literature Search for Independent Systematic Review
The review team will collaborate with an information specialist (JB) who has training, expertise, and familiarity with developing and performing systematic review literature searches. A variety of methods will be used to identify relevant data (see below). Literature searches will not be limited by publication date.
Online Databases
Electronic searches of the following three online databases will be performed using the search terms outlined in Section C-1b: PubMed, Embase, and Toxline. The search strategy and search terms will be developed by the information specialist (JB), who will implement the search for relevant studies.
Other Resources
Hand searching the reference lists of all the included studies after full-text review will be conducted using the same study selection process as used for screening records retrieved from the electronic search. Relevant studies identified through these steps will be marked as “provided from other sources” in the study selection flow diagram.
Study Selection
All search results will be imported or manually entered into EndNote (Version x7) reference management software. EndNote will be used to eliminate any duplicate citations before evaluating the eligibility of the citations.
Screening Process
References retrieved from the literature search will be screened for relevance and eligibility against the evidence selection criteria using DistillerSR (Evidence Partners; https://www.evidencepartners.com/). Screeners from the review team will be trained with an initial pilot phase on 25 studies undertaken to improve clarity of the evidence selection criteria and to improve accuracy and consistency among screeners. Screening forms are presented in Section C-1c.
Title and Abstract Screening
Each citation will be independently screened by two reviewers (SM, EM) to determine whether it meets the selection criteria for inclusion that reflect the PECO statement with some additional considerations as listed below. Citations included at the title/abstract screening level will be subject to a full-text review by the same two reviewers. Disagreements regarding citation eligibility will be resolved via consensus and, where necessary, by consulting a committee member.
The title/abstract screening form will be used to screen and EXCLUDE references if at least one of the following criteria is met:
- No original data (e.g., review article, commentary, editorial)
- Study does not include nonhuman mammals
- Study does not report phthalate exposure
- No relevant outcomes
- Incomplete information (e.g., conference abstract, meeting poster)
- Not in English and unable to determine eligibility
- Other (explanation required)
The following types of records will be INCLUDED at the title/abstract level: any English-language study of male humans exposed to phthalates in utero.
Only English-language publications will be included because of time and resource constraints. There appears to be no indication that foreign-language publications would make a contribution that is distinct from what is found in the English-language literature.
Updated details to instructions and interpretations for title and abstract screening will be added to the Section C-1f to document the process of the review team during the screening process.
Full-Text Screening
Citations included at the title/abstract screening level will be subject to a full-text review by the same two reviewers involved in title and abstract screening (SM, EM). Each reference will be screened in duplicate and independently. Disagreements regarding citation eligibility will be resolved via consensus and, where necessary, by consulting a committee member.
Citations will be EXCLUDED at the full-text level if at least one of the following criteria are met:
- No original data (e.g., review article, commentary, editorial)
- Study does not include nonhuman mammals
- Study does not report experimental exposure to one or more of the phthalates listed in the PECO statement
- Study does not report oral exposure to phthalates
- Study does not quantify exposure to phthalates
- Study does not include in utero exposure
- Study does not assess or report anogenital distance, anogenital index, anoscrotal distance, anopenile distance, hypospadias, or fetal testosterone concentrations
- No comparator group (animals exposed to different doses of phthalates or vehicle-only treatment)
- Not in English
- Other reason (explanation required).
The reason for exclusion at the full-text-review stage will be annotated and reported in a study selection flow diagram in the final report (following PRISMA [Moher et al. 2009]). The reasons for exclusion will be documented from the list (1-9) above.
Citations will be INCLUDED if they meet the PECO statement criteria:
- Study includes nonhuman male mammals
- Study includes in utero exposure
- Study includes comparison with animals exposed to different doses of phthalates or vehicle-only treatment
- Study measures anogenital distance, anogenital index, anoscrotal distance, anopenile distance, hypospadias, or fetal testosterone
Updated details to instructions and interpretations for full-text screening will be added to the Section C-1f to document the process of the review team during the screening process.
Data Extraction
Data will be collected and recorded (i.e., extracted) from included studies by one member of the review team and checked by a second member for completeness and accuracy. Any discrepancies in data extraction will be resolved through discussion. The extracted data will be used to summarize study designs and findings and/or to conduct statistical analyses. Section C-1d presents the data extraction elements that will be used.
The review team will attempt to contact authors of included studies to obtain missing data considered important for evaluating key study findings (e.g., level of data required to conduct a meta-analysis). The study extraction files will note whether an attempt was made to contact study authors by email for missing data considered important for evaluating key study findings (and whether or not a response was received).
Multiple publications with overlapping data for the same study (e.g., publications reporting subgroups, additional outcomes or exposures outside the scope of an evaluation, or longer follow-up) are identified by examining author affiliations, study designs, cohort name, enrollment criteria, and enrollment dates. If necessary, study authors will be contacted to clarify any uncertainty about the independence of two or more articles. The review will include all publications on the study, select one publication to use as the primary publication, and consider the others as secondary publications with annotation as being related to the primary record during data extraction. The primary study will generally be the publication with the longest follow-up or, for studies with equivalent follow-up periods, the study with the largest number of cases or the most recent publication date. The review will include relevant data from all publications of the study, although if the same outcome is reported in more than one report, the review team will include a single instance of the data (and avoid more than one—that is, duplicate instances of the data).
Data extraction will be completed using the Health Assessment Workspace Collaborative (HAWC) software, an open source and freely available Web-based interface application, for visualization and warehousing.2
Risk of Bias (Quality) Assessment of Indiviual Studies
Risk of bias is related to the internal validity of a study and reflects study-design characteristics that can introduce a systematic error (or deviation from the true effect) that might affect the magnitude and even the direction of the apparent effect. Internal validity or risk of bias will be assessed for individual studies using a tool developed by the National Toxicology Program’s Office of Health Assessment and Translation (OHAT) that outlines an approach to evaluating risk of bias for experimental animal studies. The risk of bias domains and questions for experimental animal studies are based on established guidance for experimental human studies (randomized clinical trials) (Higgins and Green 2011; Viswanathan et al. 2012, 2013; Sterne et al. 2014) and recent tools for animal studies (Hooijmans et al. 2014; Koustas et al. 2014). The riskof bias tool includes a common set of questions (Section C-1e) that are answered based on the specific details of individual studies to develop risk of bias ratings (using the four options: definitely low risk of bias; probably low risk of bias; probably high risk of bias; or definitely high risk of bias).
___________________
2 HAWC (Health Assessment Workspace Collaborative): A Modular Web-based Interface to Facilitate Development of Human Health Assessments of Chemicals (https://hawcproject.org/portal/).
Information or study procedures that were not reported are assumed not to have been conducted, resulting in an assessment of “probably high” risk of bias. Study design determines the subset of questions that should be used to assess risk of bias for an individual study (see Table C1-1).
Studies are independently assessed by two assessors (DD, KJ) who answer all applicable risk of bias questions with one of four options (see Table C1-2) following prespecified criteria detailed in Section C-1e. The criteria describe aspects of study design, conduct, and reporting required to reach risk of bias ratings for each question and specify factors that can distinguish among ratings (e.g., what separates “definitely low” from “probably low” risk of bias). The instructions and detailed criteria are tailored to the specific type of human study designs. Risk of bias will be assessed at the outcome level because study design or method specifics may increase the risk of bias for some outcomes and not others within the same study. Information or study procedures that were not reported are assumed not to have been conducted, resulting in an assessment of “probably high” risk of bias. Authors will be queried by email to obtain missing information, and responses received will be used to update riskof bias ratings.
Assessors will be trained using the criteria in an initial pilot phase undertaken to improve clarity of criteria that distinguish between adjacent ratings and to improve consistency among assessors. All team members involved in the risk of bias assessment will be trained on the same set of studies and asked to identify potential ambiguities in the criteria used to assign ratings for each question. Any ambiguities and rating conflicts will be discussed relative to opportunities to refine the criteria to more clearly distinguish between adjacent ratings. If major changes to the risk of bias criteria are made based on the pilot phase (i.e., those that would likely result in revision of response), they will be documented in a protocol amendment along with the date modifications were made and the logic for the changes. It is also expected that information about confounding, exposure characterization, outcome assessment, and other important issues may be identified during or after data extraction, which can lead to further refinement of the risk of bias criteria.
After assessors have independently made risk of bias determinations for a study across all risk of bias questions, the two assessors will compare their results to identify discrepancies and attempt to resolve them. Any remaining discrepancies will be considered and resolved with the review team. The final riskof bias rating for each question will be recorded along with a statement of the basis for that rating.
Data Analysis and Evidence Synthesis
The review team will qualitatively synthesize the body of evidence for each outcome and, where appropriate, a meta-analysis will be performed. If a meta-analysis is performed, summaries of main characteristics for each included study will be compiled and reviewed by two team members to determine comparability between studies, to identify data transformations necessary to ensure comparability, and to determine whether heterogeneity is a concern. The main characteristics considered across all eligible studies include the following:
- Experimental design (e.g., acute, chronic, multigenerational)
- Animal model used (e.g., species, strain, sex, genetic background)
- Age of animals (e.g., at start of treatment, mating, and/or pregnancy status)
- Developmental stage of animals at treatment and outcome assessment
- Dose levels, frequency of treatment, timing, duration, and exposure route
- Health outcome(s) reported
- Type of data (e.g., continuous or dichotomous), statistics presented in paper, access to raw data
- Variation in degree of risk of bias at individual study level
The review team expects to require input from subject-matter experts to help assess the heterogeneity of the studies. Subgroup analyses to examine the extent to which risk of bias contributes to heterogeneity will be performed. If there is evidence of species differences, the review team will consider stratifying
by species and performing separate meta-analyses by species. Situations where it may not be appropriate to include a study are when data on exposure or outcome are too different to be combined or other circumstances that may indicate that averaging study results would not produce meaningful results. When considering outcome measures for conducting meta-analyses, benchmark dose (BMD) estimates (and their associated confidence intervals) with a benchmark response (BMR) set to a common percent of control (for continuous outcomes) or extra risk (for dichotomous outcomes) are preferred. A secondary alternative, when there are more than two groups, is the conduct of BMD modeling and the use of the derived BMD estimates. Meta-analyses are not possible with lowest-observed-adverse-effect levels or no-observed-adverse-effect levels, since no confidence interval can be derived for these measures.
If a meta-analysis is conducted, a random effects model will be used for the analysis. Heterogeneity will be assessed using the I-squared statistic. Interpretation of I-squared will be based on the Cochrane Handbook: 0% to 40% (might not be important); 30% to 60% (may represent moderate heterogeneity); 50% to 90% (may represent substantial heterogeneity); and 75% to 100% (considerable heterogeneity). Additionally, as described in the Cochrane Handbook, for the last three categories, the importance of the I-squared will be interpreted considering not only the magnitude of effects but also the strength of the evidence (90% two-tailed confidence interval).
The review team will also perform sensitivity analyses on the exclusion of individual studies in succession.
If sufficient studies are available, subgroup analyses will be performed based on the following characteristics described above: experimental design, animal model used (e.g., species and/or strain), age of animals, and developmental stage of animals at treatment and outcome assessment.
In the event that these proposed methods for data analysis are altered to tailor to the evidence base from included studies, the protocol will be amended accordingly, and the reasons for change will be justified in the documentation.
Confidence Rating: Assessment of the Body of Evidence
The quality of evidence for each male reproductive outcome will be evaluated using the GRADE system for rating the confidence in the body of evidence (Guyatt et al. 2011; Rooney et al. 2014). More detailed guidance on reaching confidence ratings in the body of evidence as “high,” “moderate,” “low,” or “very low” is provided in NTP (2015, see Step 5). In brief, available studies on a particular outcome are initially grouped by key study-design features, and each grouping of studies is given an initial confidence rating by those features.
The initial rating is downgraded for factors that decrease confidence in the results, including
- risk of bias
- unexplained inconsistency
- indirectness or lack of applicability
- imprecision
- publication bias
The initial rating is upgraded for factors that increase confidence in the results, including
- large magnitude of effect
- dose response
- consistency across study designs/populations/animal models or species
- consideration of residual confounding
- other factors that increase confidence in the association or effect (e.g., particularly rare outcomes)
TABLE C1-1 OHAT Risk of Bias Tool
| Risk-of-Bias Questions | Experimental Animal* | Human Controlled Trials** | Cohort | Case-Control | Cross-Sectional*** | Case Series |
|---|---|---|---|---|---|---|
|
X | X | ||||
|
X | X | ||||
|
X | X | X | |||
|
X | X | X | X | ||
|
X | |||||
|
X | X | ||||
|
X | X | X | X | X | |
|
X | X | X | X | X | X |
|
X | X | X | X | X | X |
|
X | X | X | X | X | X |
|
X | X | X | X | X | X |
* Experimental animal studies are controlled exposure studies. Non-human animal observational studies can be evaluated using the design features of observational human studies such as cross-sectional study design.
** Human Controlled Trials are studies in humans with controlled exposure (e.g., randomized controlled trials, non-randomized experimental studies)
*** Cross-sectional studies include population surveys with individual data (e.g., NHANES) and surveys with aggregate data (i.e., ecological studies).
SOURCE: NTP (2015, p. 37).
TABLE C1-2 Answers to the Risk of Bias Questions
| Definitely Low risk of bias: There is direct evidence of low risk-of-bias practices | |
| Probably Low risk of bias: There is indirect evidence of low risk-of-bias practices OR it is deemed that deviations from low risk-of-bias practices for these criteria during the study would not appreciably bias results, including consideration of direction and magnitude of bias | |
| Probably High risk of bias: There is indirect evidence of high risk-of-bias practices (indicated with “-”) OR there is insufficient information provided about relevant risk-of-bias practices (indicated with “NR” for not reported). Both symbols indicate probably high risk of bias. | |
| Definitely High risk of bias: There is direct evidence of high risk-of-bias practices |
SOURCE: NTP (2015, p. 36).
The reasons for downgrading (or upgrading) confidence may not be due to a single domain of the body of evidence. If a decision to downgrade is borderline for two domains, the body of evidence is downgraded once in a single domain to account for both partial concerns based on considering the key drivers of the strengths or weaknesses. Similarly, the body of evidence is not downgraded twice for what is essentially the same limitation (or upgraded twice for the same asset) that could be considered applicable to more than one domain of the body of evidence. Consideration of consistency across study designs, human populations, or animal species is not included in the GRADE guidance (Guyatt et al. 2011); however, it is considered in the modified version of GRADE used by OHAT (Rooney et al. 2014).
Confidence ratings are independently assessed by members of the review team, and discrepancies will be resolved by consensus and consultation with technical advisors as needed. Confidence ratings will be summarized in evidence profile tables.
REFERENCES
Guyatt, G.H., A.D. Oxman, R. Kunz, J. Brozek, P. Alonso-Coello, D. Rind, P.J. Devereaux, V.M. Montori, B. Freyschuss, G. Vist, R. Jaeschke, J.W. Williams, Jr., M.H. Murad, D. Sinclair, Y. Falck-Ytter, J. Meerpohl, C. Whittington, K. Thorlund, J. Andrews, and H.J. Schunemann. 2011. GRADE guidelines 6. Rating the quality of evidence—imprecision. J. Clin. Epidemiol. 64(12):1283-1293.
Higgins, J., and S. Green, eds. 2011. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). The Cochrane Collaboration [online]. Available: http://handbook.cocharne.org [accessed May 6, 2016].
Hooijmans, C.R., M.M. Rovers, R.B. de Vries, M. Leenars, M. Ritskes-Hoitinga, and M.W. Langendam. 2014. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Method. 14:43.
IOM (Institute of Medicine). 2011. Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: The National Academies Press.
Koustas, E., J. Lam, P. Sutton, P.I. Johnson, D.S. Atchley, S. Sen, K.A. Robinson, D.A. Axelrad, and T.J. Woodruff. 2104. The Navigation Guide—evidence-based medicine meets environmental health: Systematic review of nonhuman evidence for PFOA effects on fetal growth. Environ. Health Perspect. 122(10):1015-1027.
Moher, D., A. Liberati, J. Tetzlaff, and D.G. Altman. 2009. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 62(10):1006-1012.
NRC (National Research Council). 2008. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Washington, DC: The National Academies Press.
NTP (National Toxicology Program). 2015. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. Office of Health Assessment and Translation, Division, National Toxicology Program, National Institute of Environmental Health Sciences. January 9, 2015 [online]. Available: http://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf [accessed September 21, 2015].
Robinson, K.A., E.P. Whitlock, M.E. O'Neil, J.K. Anderson, L. Hartling, D.M. Dryden, M. Butler, S.J. Newberry, M. McPheeters, N.D. Berkman, J.S. Lin, and S. Chang S. 2014. Integration of Existing Systematic Reviews. Research white paper. AHRQ Publication No. 14-EHC016-EF. Rockville, MD: Agency for Healthcare Research and Quality [online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK216379/ [accessed May 9, 2016].
Robinson, K.A., R. Chou, N.D. Berkman, S.J. Newberry, R. Fu, L. Hartling, D. Dryden, M. Butler, M. Foisy, J. Anderson, M. Motu'apuaka, R. Relevo, J.M. Guise, and S. Chang. 2015. Integrating Bodies of Evidence: Existing Systematic Reviews and Primary Studies. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Publication No. 15-EHC007-EF. Rockville, MD: Agency for Healthcare Research and Quality [online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK279904/ [accessed May 9, 2016].
Robinson, K.A., R. Chou, N.D. Berkman, S.J. Newberry, R. Fu, L. Hartling, D. Dryden, M. Butler, M. Foisy, J. Anderson, M. Motu'apuaka, R. Relevo, J.M. Guise, and S. Chang. 2016. Twelve recommendations for integrating existing systematic reviews into new reviews: EPC guidance. J. Clin. Epidemiol. 70:38-44.
Rooney, A.A., A.L. Boyles, M.S. Wolfe, J.R. Bucher, and K.A. Thayer. 2014. Systematic review and evidence integration for literature-based environmental health assessments. Environ. Health Perspect. 122(7):711-718.
Sterne, J.A.C., J.P.T. Higgins, and B.C. Reeves. 2014. ACROBAT-NRSI: A Cochrane risk of Bias Assessment Tool for Non-randomized Studies of Interventions. Version 1.0.0. September 24, 2014 [online]. Available: www.riskofbias.info [accessed May 6, 2016].
Viswanathan, M., M. Ansari, N.D. Berkman, S. Chang, L. Hartling, L.M. McPheeters, P.L. Santaguida, T. Shamliyan, K. Singh, A. Tsertsvadze, and J.R. Treadwell. 2012. Assessing the Risk of Bias of Individual Studies when Comparing Medical Interventions. AHRQ Publication No. 12-EHC047-EF. Rockville, MD: Agency for Healthcare Research and Quantitative Methods Guide for Comparative Effectiveness Reviews [online]. Available www.effectivehealthcare.ahrq.gov/ [accessed May 6, 2016].
Viswanathan, M., N.D. Berkman, D.M. Dryden, and L. Hartling. 2013. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank. Methods Research Report. AHRQ Publication No. 13-EHC106-EF. Rockville, MD: Agency for Healthcare Research and Quantitative Methods Guide for Comparative Effectiveness Reviews [online]. Available www.effectivehealthcare.ahrq.gov/reports/final.cfm [accessed May 6, 2016].
Whiting, P., J. Savovic, J.B. Higgins, D.M. Caldwell, B.C. Reeves, B. Shea, P. Davies, J. Kleijnen, and R. Churchill. 2016. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 69:225-234.
Whitlock, E.P., J.S. Lin, R. Chou, P. Shekelle, and K.A. Robinson. 2008. Using existing systematic reviews in complex systematic reviews. Ann. Intern. Med. 148(10):776-782.
SECTION C-1a
REVIEW TEAM BIOGRAPHICAL INFORMATION
Jaime F. Blanck is a clinical informationist at the Welch Medical Library at Johns Hopkins University. She creates and implements systematic review search strategies across multiple databases and provides comprehensive reference, research, and information services to multiple departments within the School of Medicine. She received an MLIS from the University of Pittsburgh and an MPA from the University of Baltimore.
David C. Dorman is a professor of toxicology in the Department of Molecular Biosciences of North Carolina State University. The primary objective of his research is to provide a refined understanding of chemically induced neurotoxicity in laboratory animals that will lead to improved assessment of potential toxicity in humans. Dr. Dorman’s research interests include neurotoxicology, nasal toxicology, pharmacokinetics, and cognition and olfaction in animals. He has chaired or served on several NRC committees, including the Committee on Design and Evaluation of Safer Chemical Substitutions: A Framework to Inform Government and Industry Decisions, the Committee to Review EPA’s Draft IRIS Assessment of Formaldehyde, and the Committee to Review the IRIS Process. He has served on other advisory boards for the US Navy, NASA, and USDA, and is currently a member of the NTP’s Board of Scientific Counselors. He is an elected fellow of the Academy of Toxicological Sciences and a fellow of the American Association for the Advancement of Sciences. He received his DVM from Colorado State University. He completed a combined PhD and residency program in toxicology at the University of Illinois at Urbana-Champaign, and is a diplomate of the American Board of Veterinary Toxicology and the American Board of Toxicology.
Kamin J. Johnson is a lead scientist for The Dow Chemical Company’s Toxicology and Environmental Research and Consulting function, responsible for the scientific conduct and interpretation of developmental and reproductive toxicology studies. He has served on study sections of the National Institutes of Health reviewing reproductive toxicology grants, and he was a counselor for the Reproductive and Toxicology Specialty Section of the Society of Toxicology. His research interests are in the molecular and cellular biology of fetal and postnatal testis function, as well as mechanisms of testicular toxicants. Dr. Johnson received a PhD in molecular biology, cell biology, and biochemistry from Brown University.
Ellen Mantus is a scholar and director of risk assessment on the Board on Environmental Studies and Toxicology at the National Academies of Sciences, Engineering, and Medicine, with more than 20 years of experience in the fields of toxicology and risk assessment. She has served as the study director on numerous projects, including ones that have assessed the health implications of various chemical exposures; developed strategies for applying modern scientific approaches in toxicology and risk assessment; provided guidance to federal agencies on risk-based decision making; and evaluated barriers to deployment of electric vehicles and associated charging infrastructure. Before joining the National Academies, Dr. Mantus was a project manager with ICF Consulting where she served as a primary reviewer for numerous toxicological studies and provided risk assessment and regulatory support on a wide array of projects. Dr. Mantus received a PhD in chemistry from Cornell University.
Susan Martel is a senior program officer in the Board on Environmental Studies and Toxicology at the National Academies of Sciences, Engineering, and Medicine. She has 20 years of experience in supporting toxicology and risk assessment projects for the US Environmental Protection Agency, the US Department of Defense, and the National Aeronautics and Space Administration. Recent projects include working with committees evaluating the toxicological effect of arsenic, developing exposure guidelines for use on spacecraft, and assessing pesticide risks-assessment practices. Before joining the National
Academies, she was the administrator of the Registry for Toxicology Pathology for Animals at the American Registry of Pathology. She received a BA in biology from Skidmore College.
Andrew A. Rooney is deputy director of the Office of Health Assessment and Translation (OHAT) in the National Toxicology Program at the National Institute of Environmental Health Sciences. He has been developing risk assessment methods and guidance throughout his professional career and is a principal author of the 2012 WHO/IPCS Guidance for Immunotoxicity Risk Assessment for Chemicals. Most recently, he has been working on emerging issues in toxicology and environmental health, including methods to address study quality in terms of risk of bias for human, animal, and mechanistic studies and adaptation of systematic review methods for addressing environmental health questions. He led the team that developed the OHAT approach to systematic review. Dr. Rooney has an MS and a PhD in zoology from the University of Florida.
SECTION C-1b
LITERATURE SEARCH STRATEGY
The review team will employ a multi-method process to identify all potentially relevant studies as detailed below.
Electronic Searches
PubMed
A search string employing medical subject heading (MeSH) terms and keyword synonyms will be developed. The PubMed search strategy will be considered the primary search strategy and will provide the basis of the other electronic search strategies. To assist in compiling these terms, the review team will conduct a text analysis of 25 articles known to the authors. These articles were selected because they represent both American and non-American publications and will help identify spelling variants. The search strategies will address each of the following concepts:
- Phthalates—The review team will use the MeSH database (http://www.ncbi.nlm.nih.gov/mesh) to find all MeSH heading and Supplementary Concept headings that relate to the following phthalates: the CAS numbers to these 11 phthalates: benzylbutyl phthalate (CAS no. 85-68-7), dibutyl phthalate (CAS no. 84-74-2), diethyl phthalate (CAS 84-66-2), diethylhexyl phthalate (CAS no. 117-81-7), diisobutyl phthalate (CAS no. 84-69-5), diisononyl phthalate (CAS no. 28553-12-0), diisooctyl phthalate (CAS no. 27554-26-3), dimethyl phthalate (CAS no. 131-11-3), di-n-octyl phthalate (CAS no. 117-84-0), diisodecyl phthalate (CAS no. 26761-40-0), and/or dipentyl phthalate (CAS no. 131-18-0). The review team will mine the “Entry Terms” list for each of the controlled vocabulary terms identified and include all unique keyword synonyms listed for each. CAS registry numbers for each phthalate substance will also be included in the list of search terms. All MeSH terms, Supplementary Concept terms, keyword synonyms, and CAS registry numbers will be searched together as one concept using the Boolean operator “OR.”
- Exposure—The review team will use the MeSH database (http://www.ncbi.nlm.nih.gov/mesh) to find all MeSH heading and Supplementary Concept headings that relate to the exposure concept. The review team will mine the “Entry Terms” list for each of the controlled vocabulary terms identified and include all unique keyword synonyms listed for each. All MeSH terms and keyword synonyms will be searched together as one concept using the Boolean operator “OR.”
- Animal studies—The review team will adapt the search filter published in Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Laboratory Animals. 2010;44(3):170-175 to eliminate nonmammalian animals. doi:10.1258/la.2010.009117.
Each of the above concepts will be searched together using the Boolean operator “AND.” There will not be limitations on date of publication, language, or publication type. All citation records will be exported to EndNote. Additional citations identified through the search processes identified below will also be exported to the project EndNote library. Duplicates will be removed from the citation library using the “Find Duplicates” tool in EndNote as well as a manual review of citations by the project librarian to identify any duplicates not found during the automated process. The number of citations found in each database will be recorded, as well as the number of duplicates and final tally of unique citations. The final library of citations will be uploaded to the Health Assessment Workspace Collaboration Web-based tool (www.hawcproject.org) for systematic reviews where they will be reviewed by the team.
Embase
The controlled vocabulary database Emtree is used by Embase. For each MeSH term identified through the process above, Emtree will be searched for the appropriate corresponding term. Additional keywords will be identified using the list of synonyms from each Emtree record and added to the keywords from the MeSH records. The review team will substitute the animal study search filter used in the PubMed search with the comparable Embase filter published in De Vries RBM, Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M. A search filter for increasing the retrieval of animal studies in Embase. Laboratory Animals. 2011; 45(4):268-270. doi:10.1258/la.2011.011056. This version of the animal filter will also be adapted to remove all nonmammalian animals.
Toxline
The review team will develop the Toxline search strategy by removing any database specific formatting from the PubMed search strategy to create a keyword-only search (Toxline does not employ a controlled vocabulary).
Search Strategies
PubMed
(“butylbenzyl phthalate” [Supplementary Concept] OR “Dibutyl Phthalate”[Mesh] OR “diethyl phthalate” [Supplementary Concept] OR “Diethylhexyl Phthalate”[Mesh] OR “diisobutyl phthalate” [Supplementary Concept] OR “diisononyl phthalate” [Supplementary Concept] OR “diisooctyl phthalate” [Supplementary Concept] OR “dimethyl phthalate” [Supplementary Concept] OR “di-n-octyl phthalate” [Supplementary Concept] OR “benzylbutyl phthalate”[tw] OR “benzyl butyl phthalate”[tw] OR “butyl benzyl phthalate”[tw] OR “butylbenzyl phthalate”[tw] OR “butylbenzylphthalate”[tw] OR “phthalic acid butyl benzyl ester”[tw] OR “butyl-benzyl-phthalate”[tw] OR “BBzP”[tw] OR “BzBP”[tw] OR “BBPHT”[tw] OR “85-68-7”[tw] OR “Dibutyl Phthalate”[tw] OR “Di-n-Butyl Phthalate”[tw] OR “Di n Butyl Phthalate”[tw] OR “Butyl Phthalate”[tw] OR “d n butyl phthalate”[tw] OR “dbp”[tw] OR “di n butyl phthalate”[tw] OR “dibutyl phthalate”[tw] OR “dibutylphthalate”[tw] OR “phthalic acid di n butyl este”[tw] OR “84-74-2”[tw] OR “phthalic acid diethyl ester”[tw] OR “diethyl phthalate”[tw] OR “diethylphthalate”[tw] OR “ethyl phthalate”[tw] OR “di-ethyl phthalate”[tw] OR “DEP”[tw] OR “84-66-2”[tw] OR “bis (2 ethylhexyl) phthalate”[tw] OR “bis (2 ethylhexyl) phthalate”[tw] OR “bis (2 ethylhexyl) phthalate”[tw] OR “bis (2 ethylhexylphthalate)”[tw] OR “Bis(2-ethylhexyl)phthalate”[tw] OR “DEHP”[tw] OR “di (2 ethylhexyl) phthalate”[tw] OR “di 2 ethylhexyl phthalate”[tw] OR “di 2 ethylhexylphthalate”[tw] OR “Di-2-Ethylhexylphthalate”[tw] OR “diethylhexyl phthalate”[tw] OR “Dioctyl Phthalate”[tw] OR “octoil”[tw] OR “phthalic acid di 2 ethylhexyl ester”[tw] OR “phthalic acid diethylhexyl ester”[tw] OR “117-81-7”[tw] OR “di-iso-butyl phthalate”[tw] OR “DiBP”[tw] OR “84-69-5”[tw] OR “di-isononylphthalate”[tw] OR “ENJ 2065”[tw] OR “ENJ-2065”[tw] OR “di-isononyl phthalate”[tw] OR “di-iso-nonyl phthalate”[tw] OR “DINP”[tw] OR “28553-12-0”[tw] OR “Diisooctylphthalate”[tw] OR “27554-26-3”[tw] OR “diamyl phthalate”[tw] OR “dipentyl phthalate”[tw] OR “phthalic acid dipentyl ester”[tw] OR “dipentyl benzene-1,2-dicarboxylate”[tw] OR “di-n-pentyl phthalate”[tw] OR “131-18-0”[tw] OR “Dimethyl phthalate”[tw] OR “Dimethylphthalate”[tw] OR “Avolin”[tw] OR “Citrola”[tw] OR “Dmp”[tw] OR “dmp30”[tw] OR “fermine”[tw] OR “methyl phthalate”[tw] OR “mipax”[tw] OR “mugia”[tw] OR “palatinol m”[tw] OR “sketofax”[tw] OR “131-11-3”[tw] OR “Di-n-octyl phthalate”[tw] OR “di n octyl phthalate”[tw] OR “di n octylphthalate”[tw] OR “dioctyl phthalate”[tw] OR “dioctylphthalate”[tw] OR “di(n-octyl)phthalate”[tw] OR “phthalic acid di n octyl ester”[tw] OR “DNOP”[tw] OR “117-84-0”[tw]) AND (“Maternal Exposure”[Mesh] OR “Environmental Exposure”[Mesh:NoExp] OR “Prenatal Exposure Delayed Effects”[Mesh] OR “Exposure”[tw] OR “Exposed”[tw] OR “exposures”[tw] OR “exposing”[tw] AND (“Genital Diseases, Male”[Mesh] OR
“Genitalia, Male”[Mesh] OR “Testosterone”[Mesh:NoExp] OR “Androgens”[Mesh] OR “Anogenital”[tw] OR “AGD”[tw] OR “AGI”[tw] OR “ASD”[tw] OR “APD”[tw] OR “Urogenital”[tw] OR “Penile”[tw] OR “penis”[tw] OR “Anoscrotal”[tw] OR “Anopenile”[tw] OR “anorectal”[tw] OR “Testosterone”[tw] OR “androgen”[tw] OR “androgens”[tw] OR “Hypospadias”[tw] OR “hypospadia”[tw] OR “Testis”[tw] OR “testes”[tw] OR ((“Anorectal”[tw] OR “genital”[tw] OR “genitals”[tw] OR “testes”[tw] OR “rectum”[tw]) AND (“malformation”[tw] OR “malformations”[tw] OR “development”[tw] OR “abnormalities”[tw] OR “abnormality”[tw] OR “dysplasia”[tw])) OR (“Male”[tw] and (“genital”[tw] OR “genitals”[tw] OR “genitalia”[tw])) OR (“Anus”[tw] AND (“genital”[tw] OR “genitals”[tw] OR “genitalia”[tw]))) AND (“animal experimentation”[MeSH Terms] OR “models, animal”[MeSH Terms] OR “invertebrates”[MeSH Terms] OR “Animals”[Mesh:noexp] OR “animal population groups”[MeSH Terms] OR “mammals”[MeSH Terms:noexp] OR “primates”[MeSH Terms:noexp] OR “artiodactyla”[MeSH Terms] OR “carnivora”[MeSH Terms] OR “cetacea”[MeSH Terms] OR “chiroptera”[MeSH Terms] OR “elephants”[MeSH Terms] OR “hyraxes”[MeSH Terms] OR “insectivora”[MeSH Terms] OR “lagomorpha”[MeSH Terms] OR “marsupialia”[MeSH Terms] OR “monotremata”[MeSH Terms] OR “perissodactyla”[MeSH Terms] OR “rodentia”[MeSH Terms] OR “scandentia”[MeSH Terms] OR “sirenia”[MeSH Terms] OR “xenarthra”[MeSH Terms] OR “haplorhini”[MeSH Terms:noexp] OR “strepsirhini”[MeSH Terms] OR “platyrrhini”[MeSH Terms] OR “tarsii”[MeSH Terms] OR “catarrhini”[MeSH Terms:noexp] OR “cercopithecidae”[MeSH Terms] OR “hylobatidae”[MeSH Terms] OR “hominidae”[MeSH Terms:noexp] OR “gorilla gorilla”[MeSH Terms] OR “pan paniscus”[MeSH Terms] OR “pan troglodytes”[MeSH Terms] OR “pongo pygmaeus”[MeSH Terms] animals[tiab] OR animal[tiab] OR mice[tiab] OR mus[tiab] OR mouse[tiab] OR murine[tiab] OR woodmouse[tiab] OR rats[tiab] OR rat[tiab] OR murinae[tiab] OR muridae[tiab] OR cottonrat[tiab] OR cottonrats[tiab] OR hamster[tiab] OR hamsters[tiab] OR cricetinae[tiab] OR rodentia[tiab] OR rodent[tiab] OR rodents[tiab] OR pigs[tiab] OR pig[tiab] OR swine[tiab] OR swines[tiab] OR piglets[tiab] OR piglet[tiab] OR boar[tiab] OR boars[tiab] OR “sus scrofa”[tiab] OR ferrets[tiab] OR ferret[tiab] OR polecat[tiab] OR polecats[tiab] OR “mustela putorius”[tiab] OR “guinea pigs”[Tiab] OR “guinea pig”[Tiab] OR cavia[Tiab] OR callithrix[Tiab] OR marmoset[Tiab] OR marmosets[Tiab] OR cebuella[Tiab] OR hapale[Tiab] OR octodon[Tiab] OR chinchilla[Tiab] OR chinchillas[Tiab] OR gerbillinae[Tiab] OR gerbil[Tiab] OR gerbils[Tiab] OR jird[Tiab] OR jirds[Tiab] OR merione[Tiab] OR meriones[Tiab] OR rabbits[Tiab] OR rabbit[Tiab] OR hares[Tiab] OR hare[Tiab] OR cats[Tiab] OR cat[Tiab] OR felis[Tiab] OR dogs[Tiab] OR dog[Tiab] OR canine[Tiab] OR canines[Tiab] OR canis[Tiab] OR sheep[Tiab] OR sheeps[Tiab] OR mouflon[Tiab] OR mouflons[Tiab] OR ovis[Tiab] OR goats[Tiab] OR goat[Tiab] OR capra[Tiab] OR capras[Tiab] OR rupicapra[Tiab] OR chamois[Tiab] OR haplorhini[Tiab] OR monkey[Tiab] OR monkeys[Tiab] OR anthropoidea[Tiab] OR anthropoids[Tiab] OR saguinus[Tiab] OR tamarin[Tiab] OR tamarins[Tiab] OR leontopithecus[Tiab] OR hominidae[Tiab] OR ape[Tiab] OR apes[Tiab] OR pan[Tiab] OR paniscus[Tiab] OR “pan paniscus”[Tiab] OR bonobo[Tiab] OR bonobos[Tiab] OR “pan troglodytes”[Tiab] OR gibbon[Tiab] OR gibbons[Tiab] OR siamang[Tiab] OR siamangs[Tiab] OR nomascus[Tiab] OR symphalangus[Tiab] OR chimpanzee[Tiab] OR chimpanzees[Tiab] OR prosimians[Tiab] OR “bush baby”[Tiab] OR prosimian[Tiab] OR bush babies[Tiab] OR galagos[Tiab] OR galago[Tiab] OR pongidae[Tiab] OR gorilla[Tiab] OR gorillas[Tiab] OR pongo[Tiab] OR “pongo pygmaeus”[Tiab] OR orangutans[Tiab] OR lemur[Tiab] OR lemurs[Tiab] OR lemuridae[Tiab] OR horse[Tiab] OR horses[Tiab] OR pongo[Tiab] OR equus[Tiab] OR cow[Tiab] OR calf[Tiab] OR bull[Tiab] OR chicken[Tiab] OR chickens[Tiab] OR squirrel[Tiab] OR squirrels[Tiab] OR chipmunk[Tiab] OR chipmunks[Tiab] OR suslik[Tiab] OR susliks[Tiab] OR vole[Tiab] OR voles[Tiab] OR lemming[Tiab] OR lemmings[Tiab] OR muskrat[Tiab] OR muskrats[Tiab] OR lemmus[Tiab] OR otter[Tiab] OR otters[Tiab] OR marten[Tiab] OR martens[Tiab] OR martes[Tiab] OR weasel[Tiab] OR badger[Tiab] OR badgers[Tiab] OR ermine[Tiab] OR mink[Tiab] OR minks[Tiab] OR sable[Tiab] OR sables[Tiab] OR gulo[Tiab] OR gulos[Tiab] OR wolverine[Tiab] OR wolverines[Tiab] OR minks[Tiab] OR mustela[Tiab] OR llama[Tiab] OR llamas[Tiab] OR alpaca[Tiab] OR alpacas[Tiab] OR camelid[Tiab] OR camelids[Tiab] OR guanaco[Tiab] OR guanacos[Tiab] OR chiroptera[Tiab] OR chiropteras[Tiab] OR bat[Tiab] OR bats[Tiab] OR fox[Tiab] OR foxes[Tiab] OR donkey[Tiab] OR donkeys[Tiab] OR mule[Tiab] OR mules[Tiab] OR zebra[Tiab] OR zebras[Tiab] OR
shrew[Tiab] OR shrews[Tiab] OR bison[Tiab] OR bisons[Tiab] OR buffalo[Tiab] OR buffaloes[Tiab] OR deer[Tiab] OR deers[Tiab] OR bear[Tiab] OR bears[Tiab] OR panda[Tiab] OR pandas[Tiab] OR “wild hog”[Tiab] OR “wild boar”[Tiab] OR fitchew[Tiab] OR fitch[Tiab] OR beaver[Tiab] OR beavers[Tiab] OR jerboa[Tiab] OR jerboas[Tiab] OR capybara[Tiab] OR capybaras[Tiab])
Embase
“phthalic acid benzyl butyl ester”/exp OR “phthalic acid dibutyl ester”/exp OR “phthalic acid diethyl ester”/exp OR “phthalic acid bis(2 ethylhexyl) ester”/exp OR “phthalic acid dimethyl ester”/exp OR “phthalic acid dioctyl ester”/exp OR “benzylbutyl phthalate” OR “benzyl butyl phthalate” OR “butyl benzyl phthalate” OR “butylbenzyl phthalate” OR “butylbenzylphthalate” OR “phthalic acid butyl benzyl ester” OR “butyl-benzyl-phthalate” OR “BBzP” OR “BzBP” OR “BBPHT” OR “85-68-7” OR “Dibutyl Phthalate” OR “Di-n-Butyl Phthalate” OR “Di n Butyl Phthalate” OR “Butyl Phthalate” OR “d n butyl phthalate” OR “dbp” OR “di n butyl phthalate” OR “dibutyl phthalate” OR “dibutylphthalate” OR “phthalic acid di n butyl este” OR “84-74-2” OR “phthalic acid diethyl ester” OR “diethyl phthalate” OR “diethylphthalate” OR “ethyl phthalate” OR “di-ethyl phthalate” OR “DEP” OR “84-66-2” OR “bis (2 ethylhexyl) phthalate” OR “bis (2 ethylhexyl) phthalate” OR “bis (2 ethylhexyl) phthalate” OR “bis (2 ethylhexylphthalate)” OR “Bis(2-ethylhexyl)phthalate” OR “DEHP” OR “di (2 ethylhexyl) phthalate” OR “di 2 ethylhexyl phthalate” OR “di 2 ethylhexylphthalate” OR “Di-2-Ethylhexylphthalate” OR “diethylhexyl phthalate” OR “Dioctyl Phthalate” OR “octoil” OR “phthalic acid di 2 ethylhexyl ester” OR “phthalic acid diethylhexyl ester” OR “117-81-7” OR “di-iso-butyl phthalate” OR “DiBP” OR “84-69-5” OR “di-isononylphthalate” OR “ENJ 2065” OR “ENJ-2065” OR “di-isononyl phthalate” OR “di-isononyl phthalate” OR “DINP” OR “28553-12-0” OR “Diisooctylphthalate” OR “27554-26-3” OR “diamyl phthalate” OR “dipentyl phthalate” OR “phthalic acid dipentyl ester” OR “dipentyl benzene-1,2dicarboxylate” OR “di-n-pentyl phthalate” OR “131-18-0” OR “Dimethyl phthalate” OR “Dimethylphthalate” OR “Avolin” OR “Citrola” OR “Dmp” OR “dmp30” OR “fermine” OR “methyl phthalate” OR “mipax” OR “mugia” OR “palatinol m” OR “sketofax” OR “131-11-3” OR “Di-n-octyl phthalate” OR “di n octyl phthalate” OR “di n octylphthalate” OR “dioctyl phthalate” OR “dioctylphthalate” OR “di(n-octyl)phthalate” OR “phthalic acid di n octyl ester” OR “DNOP” OR “117-84-0” AND (‘male genital system disease’/exp OR ‘male genital system’/exp OR ‘testosterone’/exp OR ‘androgen’/de OR “Anogenital”:ti,ab OR “AGD”:ti,ab OR “AGI”:ti,ab OR “ASD”:ti,ab OR “APD”:ti,ab OR “Urogenital”:ti,ab OR “Penile”:ti,ab OR “penis”:ti,ab OR “Anoscrotal”:ti,ab OR “Anopenile”:ti,ab OR “anorectal”:ti,ab OR “Testosterone”:ti,ab OR “androgen”:ti,ab OR “androgens”:ti,ab OR “Hypospadias”:ti,ab OR “hypospadia”:ti,ab OR “Testis”:ti,ab OR “testes”:ti,ab OR ((“Anorectal”:ti,ab OR “genital”:ti,ab OR “genitals”:ti,ab OR “testes”:ti,ab OR “rectum”:ti,ab) AND (“malformation”:ti,ab OR “malformations”:ti,ab OR “development”:ti,ab OR “abnormalities”:ti,ab OR “abnormality”:ti,ab OR “dysplasia”:ti,ab)) OR (“Male”:ti,ab and (“genital”:ti,ab OR “genitals”:ti,ab OR “genitalia”:ti,ab)) OR (“Anus”:ti,ab AND (“genital”:ti,ab OR “genitals”:ti,ab OR “genitalia”:ti,ab))) AND (‘prenatal exposure’/exp OR ‘environmental exposure’/exp OR ‘exposure’ OR ‘exposed’ OR ‘exposures’ OR ‘exposing’ AND (‘ape’/de OR ‘bat’/exp OR ‘carnivora’/exp OR ‘catarrhini’/de OR ‘cercopithecidae’’/exp OR ‘‘cetacea’’/exp OR ‘chimpanzee’/exp OR ‘chordata’/de OR ‘elephant’/exp OR ‘gorilla’/exp OR ‘haplorhini’/de OR ‘hominid’/de OR ‘hylobatidae’/exp OR ‘hyrax’/exp OR ‘lagomorph’/exp OR ‘mammal’/de OR ‘marsupial’/exp OR ‘monotremate’/exp OR ‘orangutan’/exp OR ‘placental mammals’/de OR ‘platyrrhini’/exp OR ‘primate’/de OR ‘prosimian’/exp OR ‘rodent’/exp OR ‘scandentia’/exp OR ‘simian’/de OR ‘sirenia’/exp OR ‘tarsiiform’/exp OR ‘ungulate’/exp OR ‘vertebrate’/de OR ‘xenarthra’/exp OR animals:ti,ab OR animal:ti,ab OR mice:ti,ab OR mus:ti,ab OR mouse:ti,ab OR murine:ti,ab OR woodmouse:ti,ab OR rats:ti,ab OR rat:ti,ab OR murinae:ti,ab OR muridae:ti,ab OR cottonrat:ti,ab OR cottonrats:ti,ab OR hamster:ti,ab OR hamsters:ti,ab OR cricetinae:ti,ab OR rodentia:ti,ab OR rodent:ti,ab OR rodents:ti,ab OR pigs:ti,ab OR pig:ti,ab OR swine:ti,ab OR swines:ti,ab OR piglets:ti,ab OR piglet:ti,ab OR boar:ti,ab OR boars:ti,ab OR “sus scrofa”:ti,ab OR ferrets:ti,ab OR ferret:ti,ab OR polecat:ti,ab OR polecats:ti,ab OR “mustela putorius”:ti,ab OR “guinea pigs”:ti,ab OR “guinea pig”:ti,ab OR cavia:ti,ab OR callithrix:ti,ab OR marmo-
set:ti,ab OR marmosets:ti,ab OR cebuella:ti,ab OR hapale:ti,ab OR octodon:ti,ab OR chinchilla:ti,ab OR chinchillas:ti,ab OR gerbillinae:ti,ab OR gerbil:ti,ab OR gerbils:ti,ab OR jird:ti,ab OR jirds:ti,ab OR merione:ti,ab OR meriones:ti,ab OR rabbits:ti,ab OR rabbit:ti,ab OR hares:ti,ab OR hare:ti,ab OR cats:ti,ab OR cat:ti,ab OR felis:ti,ab OR dogs:ti,ab OR dog:ti,ab OR canine:ti,ab OR canines:ti,ab OR canis:ti,ab OR sheep:ti,ab OR sheeps:ti,ab OR mouflon:ti,ab OR mouflons:ti,ab OR ovis:ti,ab OR goats:ti,ab OR goat:ti,ab OR capra:ti,ab OR capras:ti,ab OR rupicapra:ti,ab OR chamois:ti,ab OR haplorhini:ti,ab OR monkey:ti,ab OR monkeys:ti,ab OR anthropoidea:ti,ab OR anthropoids:ti,ab OR saguinus:ti,ab OR tamarin:ti,ab OR tamarins:ti,ab OR leontopithecus:ti,ab OR hominidae:ti,ab OR ape:ti,ab OR apes:ti,ab OR pan:ti,ab OR paniscus:ti,ab OR “pan paniscus”:ti,ab OR bonobo:ti,ab OR bonobos:ti,ab OR “pan troglodytes”:ti,ab OR gibbon:ti,ab OR gibbons:ti,ab OR siamang:ti,ab OR siamangs:ti,ab OR nomascus:ti,ab OR symphalangus:ti,ab OR chimpanzee:ti,ab OR chimpanzees:ti,ab OR prosimians:ti,ab OR “bush baby”:ti,ab OR prosimian:ti,ab OR bush babies:ti,ab OR galagos:ti,ab OR galago:ti,ab OR pongidae:ti,ab OR gorilla:ti,ab OR gorillas:ti,ab OR pongo:ti,ab OR “pongo pygmaeus”:ti,ab OR orangutans:ti,ab OR lemur:ti,ab OR lemurs:ti,ab OR lemuridae:ti,ab OR horse:ti,ab OR horses:ti,ab OR pongo:ti,ab OR equus:ti,ab OR cow:ti,ab OR calf:ti,ab OR bull:ti,ab OR chicken:ti,ab OR chickens:ti,ab OR squirrel:ti,ab OR squirrels:ti,ab OR chipmunk:ti,ab OR chipmunks:ti,ab OR suslik:ti,ab OR susliks:ti,ab OR vole:ti,ab OR voles:ti,ab OR lemming:ti,ab OR lemmings:ti,ab OR muskrat:ti,ab OR muskrats:ti,ab OR lemmus:ti,ab OR otter:ti,ab OR otters:ti,ab OR marten:ti,ab OR martens:ti,ab OR martes:ti,ab OR weasel:ti,ab OR badger:ti,ab OR badgers:ti,ab OR ermine:ti,ab OR mink:ti,ab OR minks:ti,ab OR sable:ti,ab OR sables:ti,ab OR gulo:ti,ab OR gulos:ti,ab OR wolverine:ti,ab OR wolverines:ti,ab OR minks:ti,ab OR mustela:ti,ab OR llama:ti,ab OR llamas:ti,ab OR alpaca:ti,ab OR alpacas:ti,ab OR camelid:ti,ab OR camelids:ti,ab OR guanaco:ti,ab OR guanacos:ti,ab OR chiroptera:ti,ab OR chiropteras:ti,ab OR bat:ti,ab OR bats:ti,ab OR fox:ti,ab OR foxes:ti,ab OR donkey:ti,ab OR donkeys:ti,ab OR mule:ti,ab OR mules:ti,ab OR zebra:ti,ab OR zebras:ti,ab OR shrew:ti,ab OR shrews:ti,ab OR bison:ti,ab OR bisons:ti,ab OR buffalo:ti,ab OR buffaloes:ti,ab OR deer:ti,ab OR deers:ti,ab OR bear:ti,ab OR bears:ti,ab OR panda:ti,ab OR pandas:ti,ab OR “wild hog”:ti,ab OR “wild boar”:ti,ab OR fitchew:ti,ab OR fitch:ti,ab OR beaver:ti,ab OR beavers:ti,ab OR jerboa:ti,ab OR jerboas:ti,ab OR capybara:ti,ab OR capybaras:ti,ab)
Toxline
(“117-81-7” OR “117-84-0” OR “131-11-3” OR “131-18-0” OR “27554-26-3” OR “28553-12-0” OR “84-66-2” OR “84-69-5” OR “84-74-2” OR “85-68-7” OR “Avolin” OR “BBPHT” OR “BBzP” OR “bis 2 ethylhexylphthalate” OR “Bis 2-ethylhexyl phthalate” OR “butylbenzylphthalate” OR “butyl-benzyl-phthalate” OR “BzBP” OR “Dbp” OR “DEP” OR “di n octylphthalate” OR “DiBP” OR “diethylphthalate” OR “di-isononylphthalate” OR “Diisooctylphthalate” OR “Dimethylphthalate” OR “DINP” OR “dioctylphthalate” OR “dipentyl benzene-1,2-dicarboxylate” OR “Dmp” OR “dmp30” OR “DNOP” OR “ENJ 2065” OR “fermine” OR “mipax” OR “mugia” OR “octoil” OR “o-phthalate” OR “o-phthalates” OR “palatinol” OR “sketofax”) AND (“Exposure” OR “Exposed” OR “exposures” OR “exposing”) AND (“Anogenital” OR “AGD” OR “AGI” OR “ASD” OR “APD” OR “Urogenital” OR “Penile” OR “penis” OR “Anoscrotal” OR “Anopenile” OR “anorectal” OR “Testosterone” OR “androgen” OR “androgens” OR “Hypospadias” OR “hypospadia” OR “Testis” OR “testes” OR ((“Anorectal” OR “genital” OR “genitals” OR “testes” OR “rectum”) AND (“malformation” OR “malformations” OR “development” OR “abnormalities” OR “abnormality” OR “dysplasia”)) OR (“Male” and (“genital” OR “genitals” OR “genitalia”)) OR (“Anus” AND (“genital” OR “genitals” OR “genitalia”))) AND (animals OR animal OR mice OR mus OR mouse OR murine OR woodmouse OR rats OR rat OR murinae OR muridae OR cottonrat OR cottonrats OR hamster OR hamsters OR cricetinae OR rodentia OR rodent OR rodents OR pigs OR pig OR swine OR swines OR piglets OR piglet OR boar OR boars OR “sus scrofa” OR ferrets OR ferret OR polecat OR polecats OR “mustela putorius” OR “guinea pigs” OR “guinea pig” OR cavia OR callithrix OR marmoset OR marmosets OR cebuella OR hapale OR octodon OR chinchilla OR chinchillas OR gerbillinae OR gerbil OR gerbils OR jird OR jirds OR merione OR meriones OR rabbits OR rabbit OR hares OR hare OR cats OR cat OR felis OR dogs OR dog OR canine OR canines OR
canis OR sheep OR sheeps OR mouflon OR mouflons OR ovis OR goats OR goat OR capra OR capras OR rupicapra OR chamois OR haplorhini OR monkey OR monkeys OR anthropoidea OR anthropoids OR saguinus OR tamarin OR tamarins OR leontopithecus OR hominidae OR ape OR apes OR pan OR paniscus OR “pan paniscus” OR bonobo OR bonobos OR “pan troglodytes” OR gibbon OR gibbons OR siamang OR siamangs OR nomascus OR symphalangus OR chimpanzee OR chimpanzees OR prosimians OR “bush baby” OR prosimian OR bush babies OR galagos OR galago OR pongidae OR gorilla OR gorillas OR pongo OR “pongo pygmaeus” OR orangutans OR lemur OR lemurs OR lemuridae OR horse OR horses OR pongo OR equus OR cow OR calf OR bull OR chicken OR chickens OR squirrel OR squirrels OR chipmunk OR chipmunks OR suslik OR susliks OR vole OR voles OR lemming OR lemmings OR muskrat OR muskrats OR lemmus OR otter OR otters OR marten OR martens OR martes OR weasel OR badger OR badgers OR ermine OR mink OR minks OR sable OR sables OR gulo OR gulos OR wolverine OR wolverines OR minks OR mustela OR llama OR llamas OR alpaca OR alpacas OR camelid OR camelids OR guanaco OR guanacos OR chiroptera OR chiropteras OR bat OR bats OR fox OR foxes OR donkey OR donkeys OR mule OR mules OR zebra OR zebras OR shrew OR shrews OR bison OR bisons OR buffalo OR buffaloes OR deer OR deers OR bear OR bears OR panda OR pandas OR “wild hog” OR “wild boar” OR fitchew OR fitch OR beaver OR beavers OR jerboa OR jerboas OR capybara OR capybaras)
SECTION C-1c
SCREENING FORMS
Title and Abstract Screening Form
INSTRUCTIONS: When a citation is excluded, reason should be specified.
| Exclusion Reasons | |
| No original data (e.g., review article, commentary, editorial) | |
| Study does not include nonhuman mammals | |
| Study does not report phthalate exposure | |
| No relevant outcomes | |
| Incomplete information (e.g., conference abstract, meeting poster) | |
| Not in English and unable to determine eligibility | |
| Other (explanation required) |
Full-Text Screening Form
INSTRUCTIONS: When a citation is excluded, reason should be specified.
| Exclusion Reasons | |
| No original data (e.g., review article, commentary, editorial) | |
| Study does not include nonhuman mammals | |
| Study does not report phthalate exposure to one or more of the phthalates listed in the PECO statement | |
| Study does not report oral exposure to phthalates | |
| Study does not quantify exposure to phthalates | |
| Study does not include in utero exposure | |
| Study does not assess or report anogenital distance, anogenital distance, anoscrotal distance, anopenile distance, hypospadias, or fetal testosterone concentration | |
| No comparator group (different doses or vehicle-only treatment) | |
| Not in English | |
| Other (explanation required) |
SECTION C-1d
DATA EXTRACTION ELEMENTS FOR ANIMAL STUDIES
| Funding | Funding source(s) |
| Reporting of COI by authors (*reporting bias) | |
| Animal Model | Sex |
| Species | |
| Strain | |
| Source of animals | |
| Age or life stage at start of dosing and at health outcome assessment | |
| Definition of gestation age for the day after mating (e.g., GD 0 vs GD 1) | |
| Diet and husbandry information (e.g., diet name/source) | |
| Treatment | Chemical name and CAS number |
| Source of chemical | |
| Purity of chemical (*information bias) | |
| Dose levels or concentration (as presented and converted to mg/kg bw/d when possible) | |
| Other dose-related details, such as whether administered dose level was verified by measurement, information on internal dosimetry (*information bias) | |
| Vehicle used for exposed animals | |
| Route of administration (e.g., oral, inhalation, dermal, injection) | |
| Duration and frequency of dosing (e.g., hours, days, weeks when administration was ended, days per week) | |
| Methods | Study design (e.g., single treatment, acute, subchronic [e.g., 90 days in a rodent], chronic, multigenerational, developmental, other) |
| Guideline compliance (i.e., use of EPA, OECD, NTP, or another guideline for study design, conducted under good laboratory practice [GLP] guideline conditions, non-GLP but consistent with guideline study, non-guideline peer-reviewed publication) | |
| Number of animals per group (and dams per group in developmental studies) (*missing data bias) | |
| Randomization procedure, allocation concealment, blinding during outcome assessment (*selection bias) | |
| Method to control for litter effects in developmental studies (*information bias) | |
| Use of negative controls and whether controls were untreated, vehicle-treated, or both | |
| Report on data from positive controls—was expected response observed? (*information bias) | |
| End point health category (e.g., reproductive) | |
| End point (e.g., infertility) | |
| Diagnostic or method to measure end point (*information bias) | |
| Statistical methods (*information bias) | |
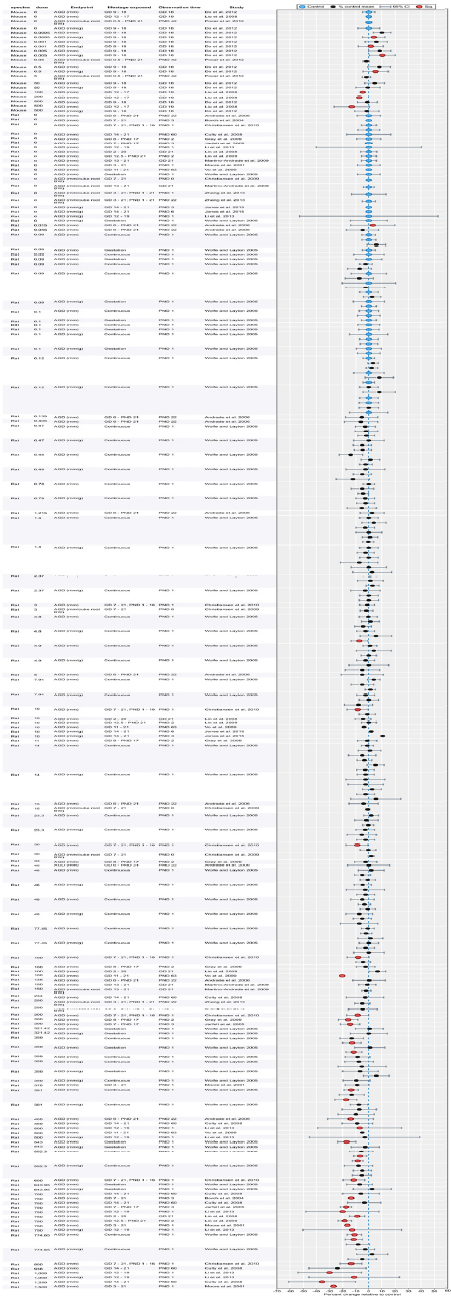
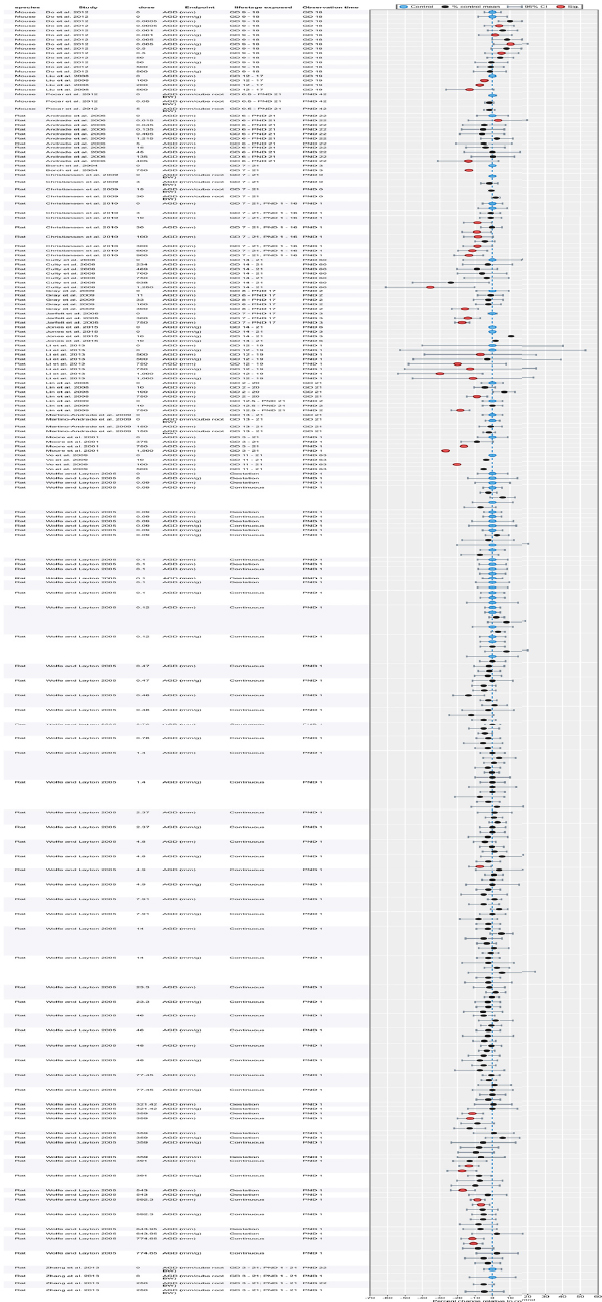
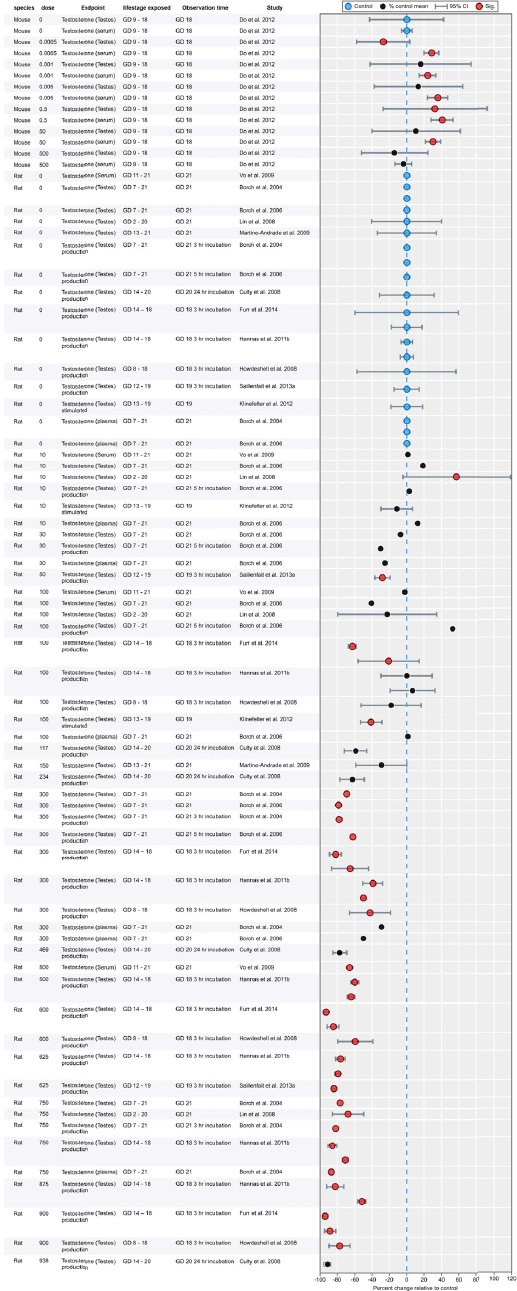
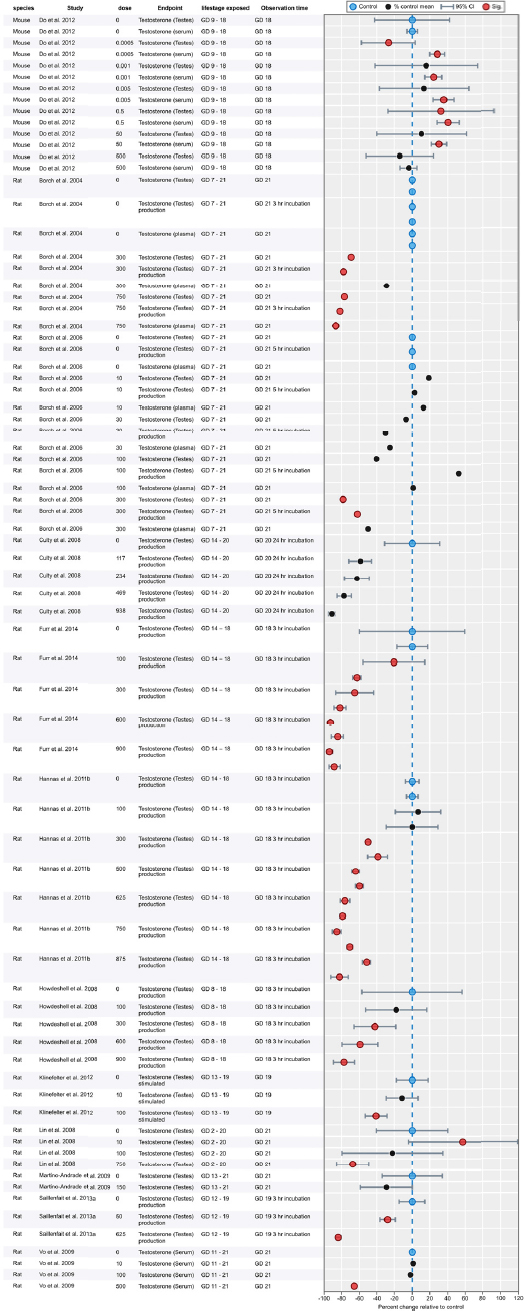
| Results | Measures of effect at each dose or concentration level (e.g., mean, median, frequency, and measures of precision or variance) or description of qualitative results. When possible, measures of effect will be converted to a common metric with associated 95% confidence intervals (CI). Most often, measures of effect for continuous data will be expressed as mean difference, standardized mean difference, and percent control response. Categorical data will be expressed as relative risk (RR, also called risk ratio). |
| No-observed-effect level (NOEL), lowest-observed-effect level (LOEL), benchmark dose (BMD) analysis, statistical significance of other dose levels, or other estimates of effect presented in paper. Note: The NOEL and LOEL are highly influenced by study design; do not give any quantitative information about the relationship between dose and response; and can be subject to author’s interpretation (e.g., a statistically significant effect may not be considered biologically important). Also, a NOEL does not necessarily mean zero response. Ideally, the response rate at specific dose levels is used as the primary measure to characterize the response. | |
| Observations on dose response (e.g., trend analysis, description of whether dose-response shape appears to be monotonic, non-monotonic) | |
| Data on internal concentration, toxicokinetics, or toxicodynamics (when reported) | |
| Other | Documentation of author queries, use of digital rulers to estimate data values from figures, exposure unit, and statistical result conversions, etc. |
Items marked with an asterisk (*) are examples of items that can be used to assess internal validity/risk of bias.
SECTION C-1e
RISK OF BIAS QUESTIONS FOR ANIMAL STUDIES
1. Was administered dose or exposure level adequately randomized?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
2. Was allocation to study groups adequately concealed?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
3. Did selection of study participants result in the appropriate comparison groups? [NA]
4. Did study design or analysis account for important confounding and modifying variables? [NA]
5. Were experimental conditions identical across study groups?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
6. Were the research personnel blinded to the study group during the study?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
7. Were outcome data complete without attrition or exclusion from analysis?
| Definitely Low Risk of Bias (++) |
|
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
8. Can we be confident in the exposure characterization?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
|
| Definitely High Risk of Bias (--) |
|
9. Can we be confident in the outcome assessment?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
10. Were all measured outcomes reported?
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
11. Was litter or litter effects considered appropriately in the statistical analyses and were there no other potential threats to internal validity?
Because this evaluation is focused on developmental exposure, this question was added to address litter effects in data analysis. This question will be used to examine individual studies for appropriate statistical methods (e.g., confirmation of homogeneity of variance for ANOVA and other statistical tests that require normally distributed data). It will also be used for risk of bias considerations that do not fit under the other questions.
| Definitely Low Risk of Bias (++) |
|
| Probably Low Risk of Bias (+) |
|
| Probably High Risk of Bias (-) or (NR) |
|
| Definitely High Risk of Bias (--) |
|
SECTION C-1f
AMENDMENTS TO THE PROTOCOL
Addition of an Exclusion Criteron for Full-Text Screening
The following criterion for excluding studies at the full-text screening level was added on September 15, 2016, after the protocol was peer reviewed:
Study involved rodents exposed to a single high dose (≥500 mg/kg/day).
The committee judged that a study testing only a single high dose level would not be useful for a systematic review intended to address the low-dose toxicity of phthalates.
Additions to the Review Team
The following committee members were added to the review team to supplement expertise:
- Weihsueh Chiu is a professor in the Department of Veterinary Integrative Biosciences in the College of Veterinary Medicine and Biomedical Sciences at Texas A&M University. Before joining the university, he worked at the US Environmental Protection Agency (EPA) for more than 14 years, most recently as chief of the Toxicity Pathways Branch in the Integrated Risk Information System (IRIS) Division of the National Center for Environmental Assessment. His research has focused on human health risk assessment, particularly with respect to toxicokinetics, mechanisms of toxicity, physiologically based pharmacokinetic modeling, dose-response assessment, and characterizing uncertainty and variability. He led the development of EPA’s 2011 IRIS assessment of trichloroethylene, which pioneered the use of probabilistic methods for characterizing uncertainty and variability in toxicokinetics and dose-response. He is currently a member of the NRC’s Committee on Predictive-Toxicology Approaches for Military Assessments of Acute Exposures. Dr. Chiu received a PhD in physics from Princeton University.
- Katrina Waters is deputy director of the Biological Sciences Division at the Pacific Northwest National Laboratory. Her research interests are focused on the integration of genomics, proteomics, metabolomics and high-throughput screening data to enable predictive mechanistic modeling of disease and toxicity pathways. She currently serves on EPA’s Board of Scientific Counselors Subcommittee on Chemical Safety for Sustainability and the US Food and Drug Administration’s Scientific Advisory Board to the National Center for Toxicological Research. She recently served on the NRC’s Committee on Predictive-Toxicology Approaches for Military Assessments of Acute Exposures. Dr. Waters received a PhD in biochemistry from the University of Wisconsin–Madison, and did a postdoctoral fellowship on endocrine disruptors at the Chemical Industry Institute of Toxicology.
SECTION C-2
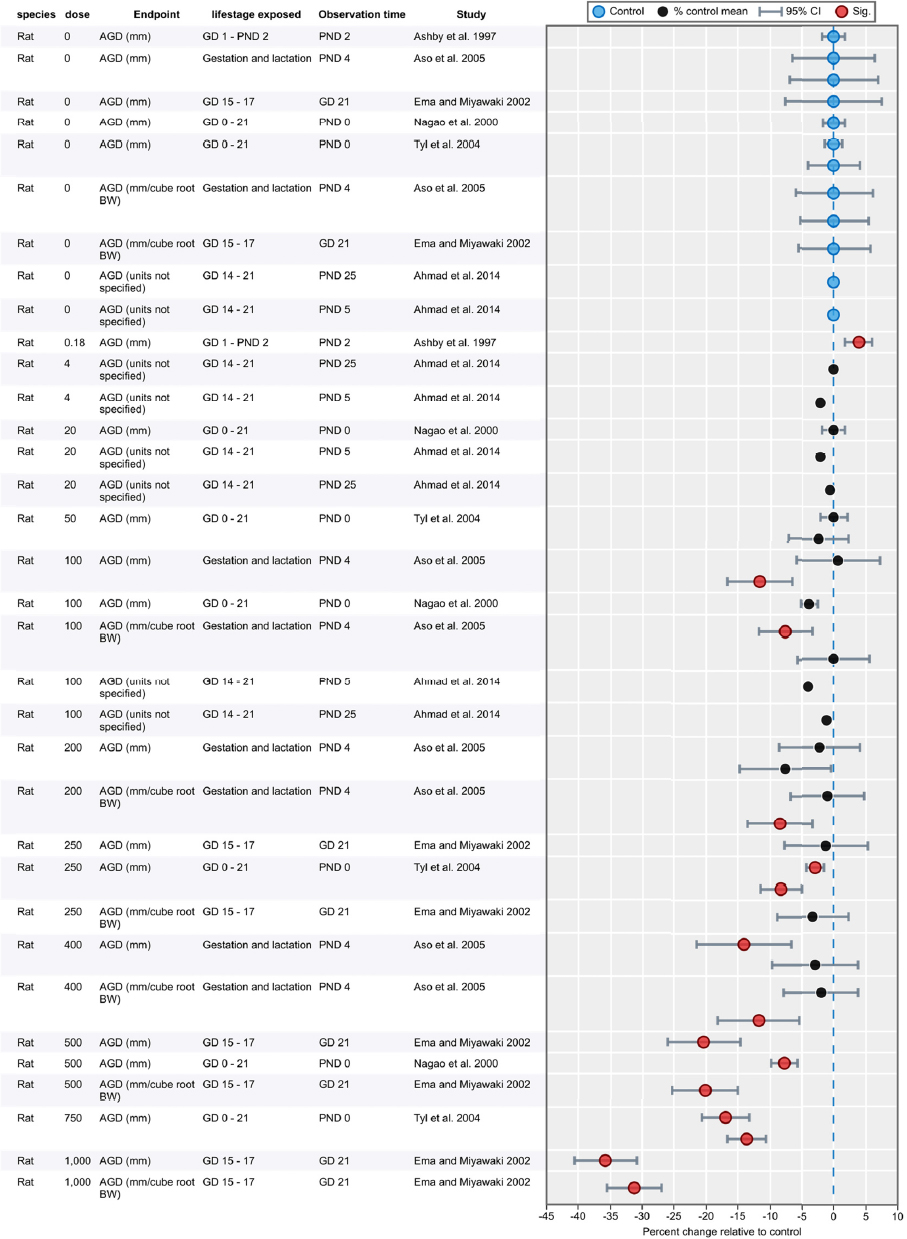
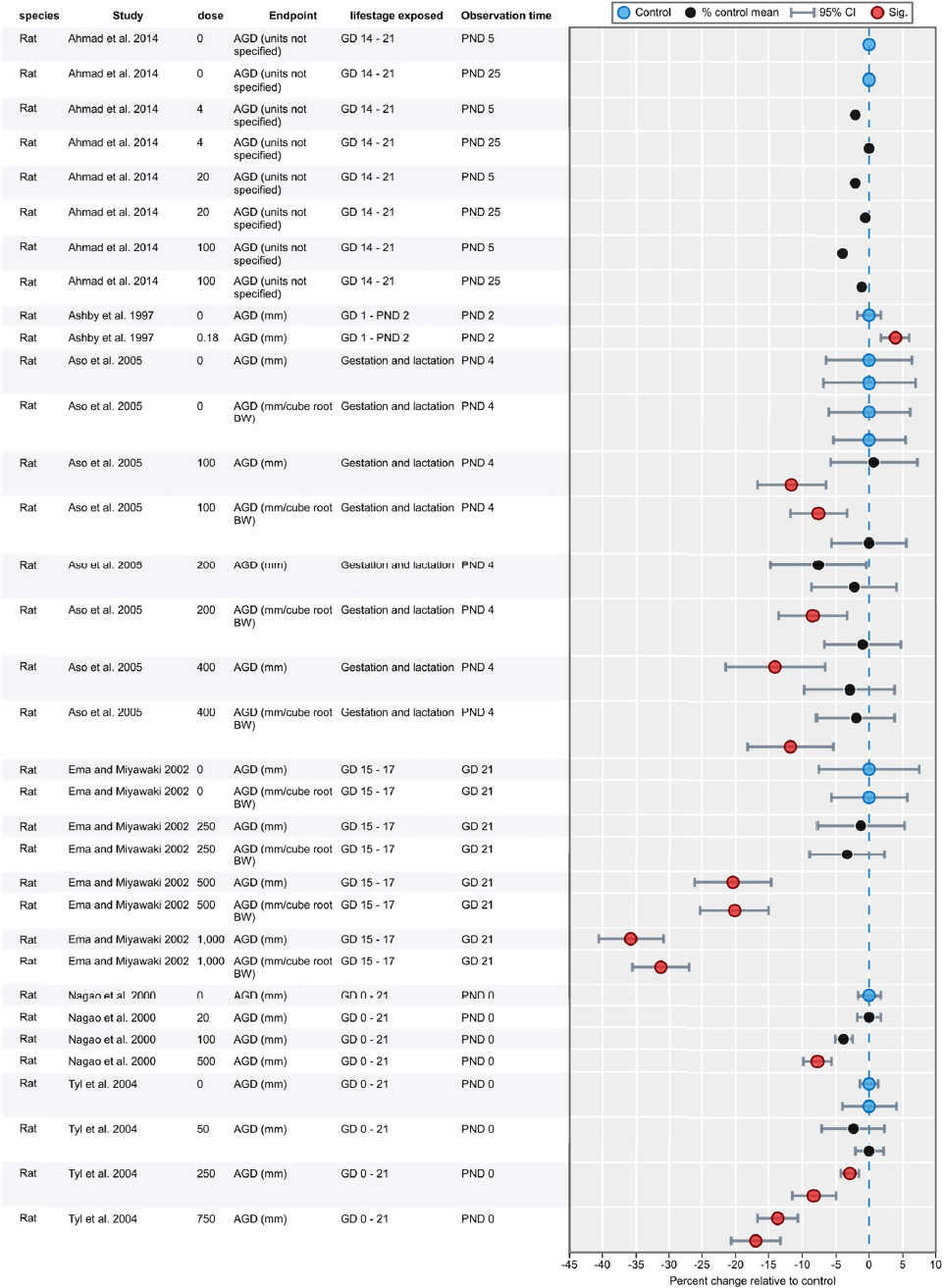
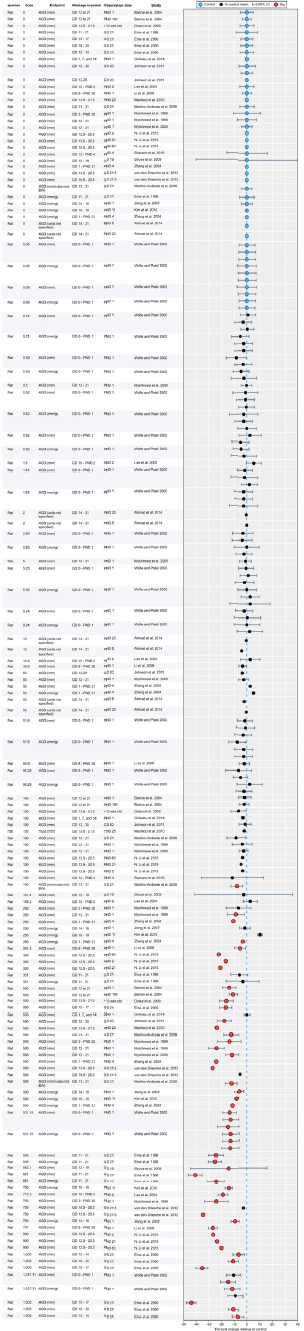
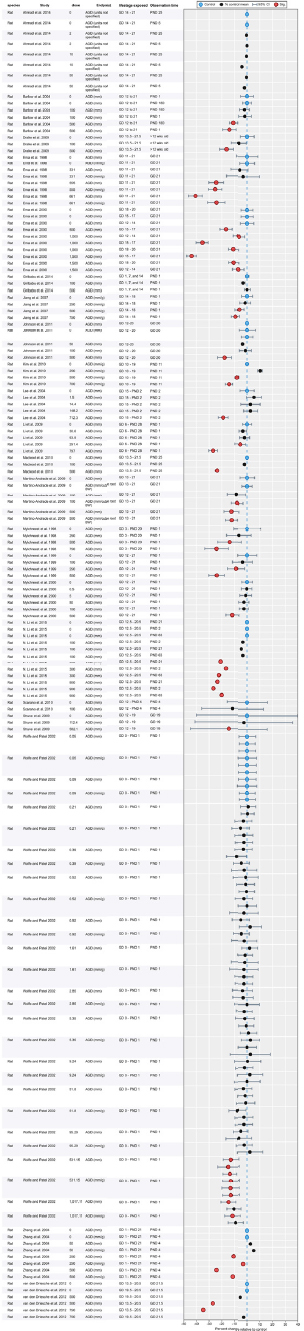
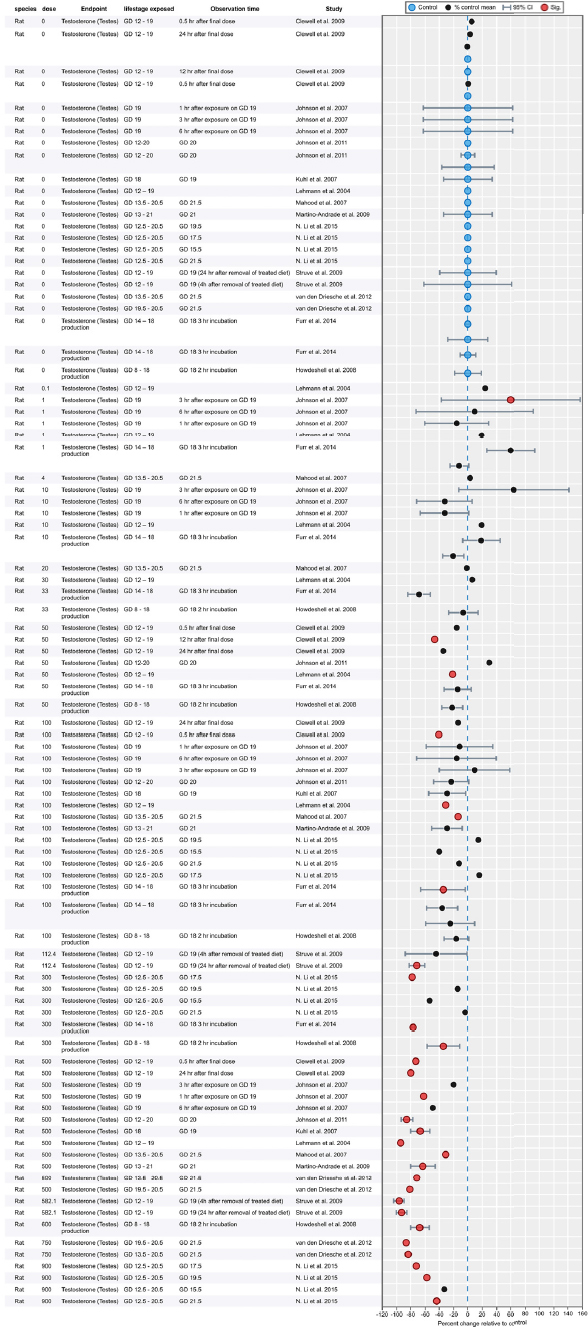
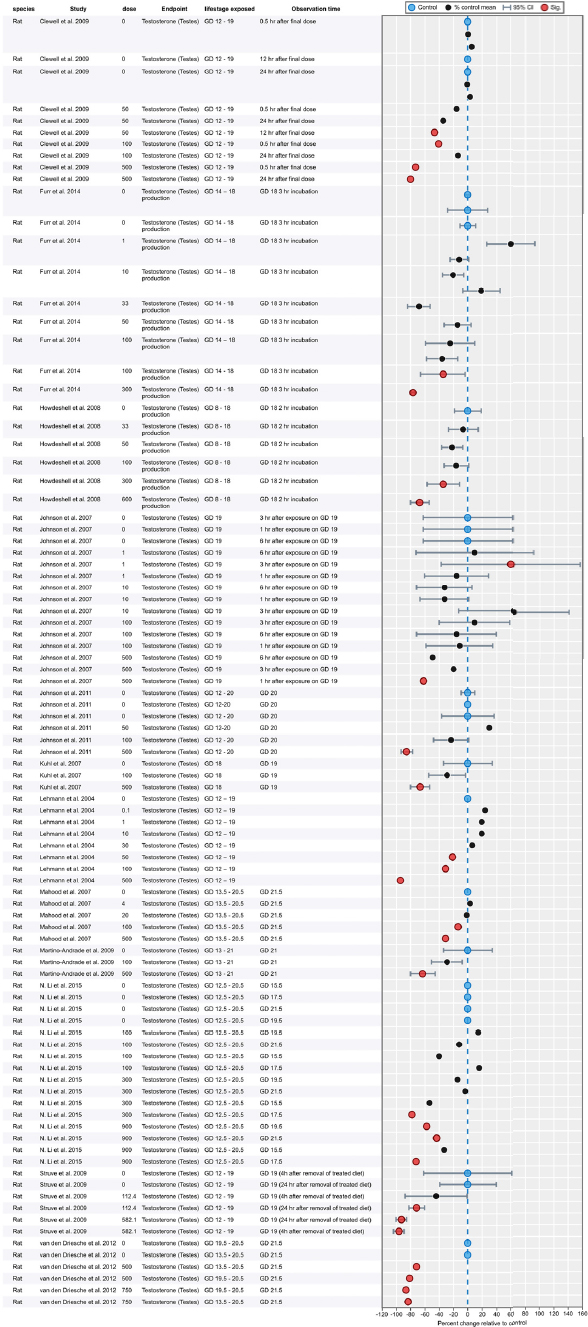
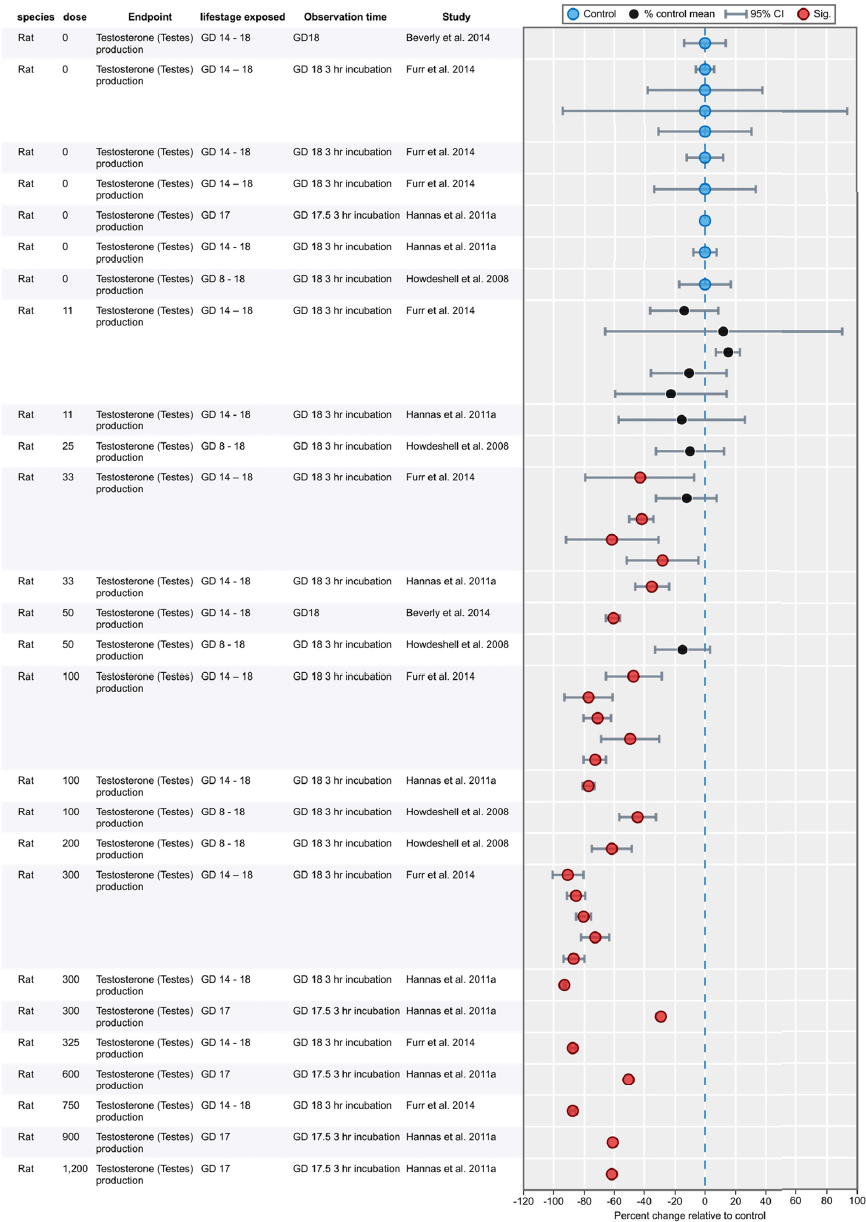
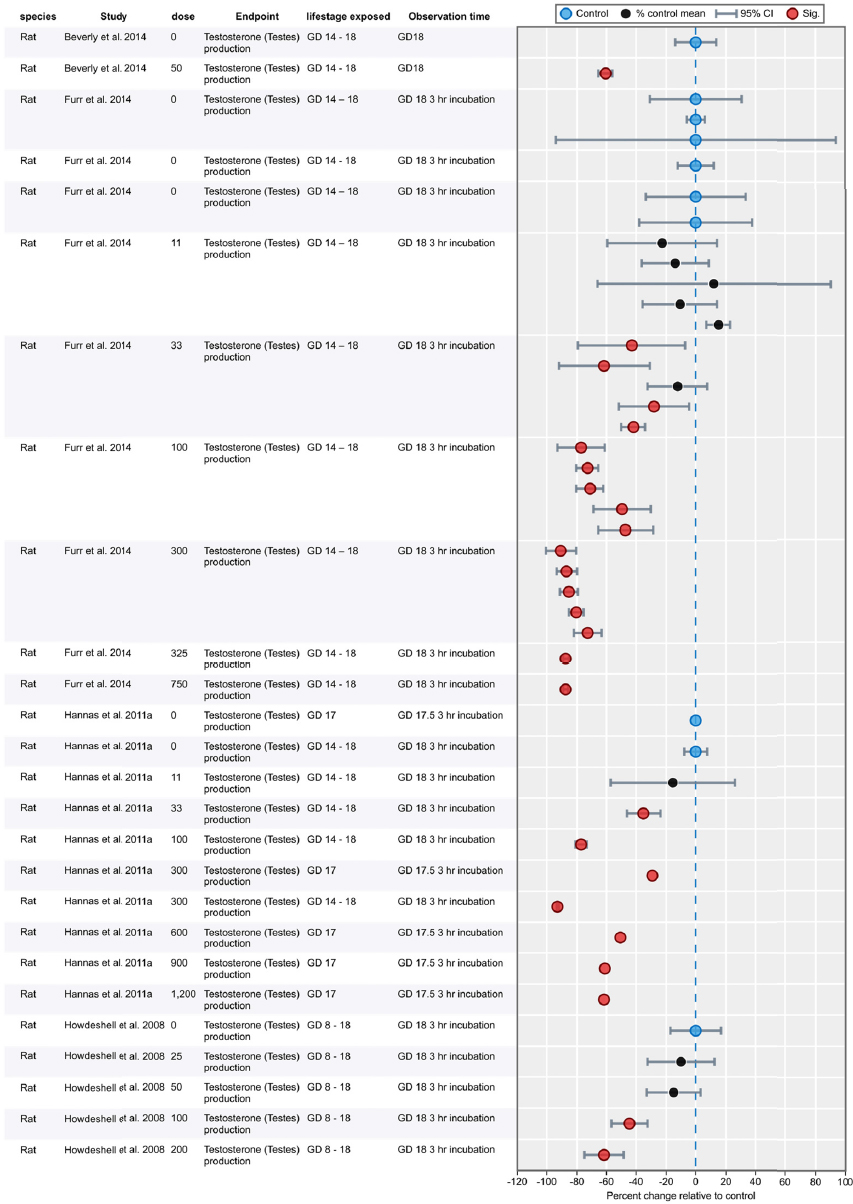
Results of Literature Searches for Animal Studies on the Effects of Phthalates on Male Reproductive-Tract Development
Literature searches were performed on August 15, 2016, using the search strategy presented in the Phthalate (Animal) Systematic Review Protocol (Section C-1). A summary of the results is presented below.
| Embase: | 754 |
| PubMed: | 521 |
| Toxline: | 865 |
|
Total citations found: |
2,140 |
| Duplicates removed: | 613 |
| Total unique citations: | 1,527 |
SECTION C-3
Funding Sources of the Animal Studies on Phthalates and Male Reproductive-Tract Development
Sources of funding were used to evaluate publication bias in terms of whether a particular sector funded more studies than another.
| Reference | Government | Industry | Other | Unknown |
|---|---|---|---|---|
| Adamsson et al. 2009 | X (European Commission) | X (Academy of Finland, Sigrid Juselius Foundation, The Finnish Concordia Fund, Turku University Hospital) | ||
| Ahmad et al. 2014 | X (ICMR-India) | |||
| Andrade et al. 2006 | X (Germany) | |||
| Ashby et al. 1997 | X (Zeneca, UK) | |||
| Aso et al. 2005 | X (Japan) | |||
| Barlow et al. 2004 | X (NIEHS) | X (American Chemistry Council) | ||
| Beverly et al. 2014 | X (EPA, NIEHS) | |||
| Boberg et al. 2011 | X (Nordic Council of Ministers, EU) | |||
| Borch et al. 2004 | X (Denmark) | |||
| Borch et al. 2006 | X (Nordic Council of Ministers, EU) | |||
| Christiansen et al. 2009 | X (Danish EPA, EU) | |||
| Christiansen et al. 2010 | X (EU) | |||
| Clewell et al. 2009 | X (NIEHS) | X (American Chemistry Council) | ||
| Clewell et al. 2013 | X (ExxonMobil Biochemical Sciences) | |||
| Culty et al. 2008 | X (NIEHS) | |||
| Do et al. 2012 | X | |||
| Drake et al. 2009 | X (UK Medical Research Council, EU) | |||
| Ema and Miyawaki 2002 | X (Japan) | |||
| Ema et al. 1998 | X | |||
| Ema et al. 2000 | X (Japan) | |||
| Fujii et al. 2005 | X (Japan) | |||
| Furr et al. 2014 | X (NIEHS, NTP, NIH) | |||
| Giribabu et al. 2014 | X (ICMR-India) | |||
| Gray et al. 2009 | X (NSF) | |||
| Hannas et al. 2011a | X (EPA) | |||
| Hannas et al. 2011b | X (EPA) | |||
| Hannas et al. 2012 | X (EPA, NTP, NAS) | |||
| Howdeshell et al. 2008 | X (EPA) | |||
| Jarfelt et al. 2005 | X (Denmark) | |||
| Jiang et al. 2007 | X (China) | |||
| Johnson et al. 2007 | X (American Chemistry Council) | |||
| Johnson et al. 2011 | X (NIH) |
| Jones et al. 2015 | X (Canada) | |||
| Kim et al. 2010 | X (Korea) | |||
| Klinefelter et al. 2012 | This research didn’t receive any funding. | |||
| Kuhl et al. 2007 | X (NIH) | |||
| Lee et al. 2004 | X (Japan) | |||
| Lehmann et al. 2004 | X (NIH) | |||
| Li et al. 2009 | X (China) | |||
| Li et al. 2013 | X (China) | |||
| L. Li et al. 2015 | X (China) | |||
| N. Li et al. 2015 | There are no funding sources to declare. | |||
| Lin et al. 2008 | X (NIEHS) | |||
| Lin et al. 2009 | X (NIEHS) | |||
| Liu et al. 2008 | X (China) | |||
| MacLeod et al. 2010 | X (UK Medical Research Council, EU) | |||
| Mahood et al. 2007 | X (EU) | |||
| Martino-Andrade et al. 2009 | X (Brazil) | |||
| Masutomi et al. 2003 | X (Japan) | |||
| McKinnel et al. 2009 | X (UK Medical Research Council) | |||
| Moore et al. 2001 | X (NIH) | |||
| Mylchreest et al. 1998 | Chemical Industry Institute of Toxicology | |||
| Mylchreest et al. 1999 | Chemical Industry Institute of Toxicology | |||
| Mylchreest et al. 2000 | Chemical Industry Institute of Toxicology | |||
| Nagao et al. 2000 | X (Japan) | |||
| Pocar et al. 2012 | X (Italy, EU) | |||
| Saillenfait et al. 2008 | X (INRS-France) | |||
| Saillenfait et al. 2009 | X (INRS-France) | |||
| Saillenfait et al. 2011 | X (INRS-France) | |||
| Saillenfait et al. 2013a | X (INRS-France) | |||
| Saillenfait et al. 2013b | X (INSERM, INRS-France) | |||
| Scarano et al. 2010 | X (State of Sao Paulo Research Foundation) | |||
| Struve et al. 2009 | X (American Chemistry Council) | |||
| Tyl et al. 2004 | X (European Council for Plasticisers and Intermediates) | |||
| van den Driesche et al. 2012 | X (UK Medical Research Council, EU) | |||
| Vo et al. 2009 | X (Korea) | |||
| Wolfe and Layton 2005 | X (NTP/NIEHS) | |||
| Wolfe and Patel 2002 | X (DHHS) | |||
| Zhang et al. 2004 | X (China) | |||
| Zhang et al. 2013 | X |
SECTION C-4
Confidence Ratings for the Body of Evidence from Animal Studies of Phthalates and Anogenital Distance (AGD), Fetal Testosterone, and Hypospadias
The confidence in the body of evidence from animal studies on phthalates and male reproductive-tract development was rated in accordance with the OHAT Guidance (NTP 2015) specified in Section C-1. The results for di(2-ethylhexyl) phthalate/diethylhexyl phthalate (DEHP) are presented first, and the remaining phthalates are subsequently presented in alphabetical order.
DEHP and AGD
Nineteen animal studies of DEHP and AGD were available; 3 used the mouse model and 16 used the rat model.
Factors Considered for Downgrading Confidence
- Risk of bias: Downgraded. Two of the three mouse studies did not control or account for litter effects, and the studies had issues with outcome assessment and lack of blinding of the researchers to the study groups during outcome assessment (see Figure C4-1). Six of 16 rat studies did not account for litter effects, and most of the studies also had issues with outcome assessment and blinding of the researchers (see Figure C4-2).
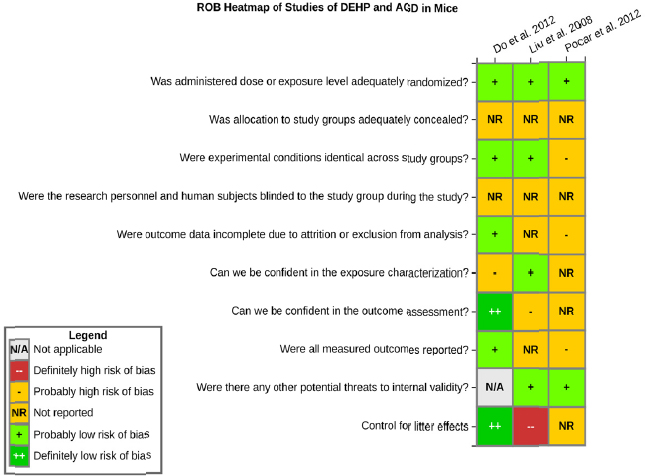
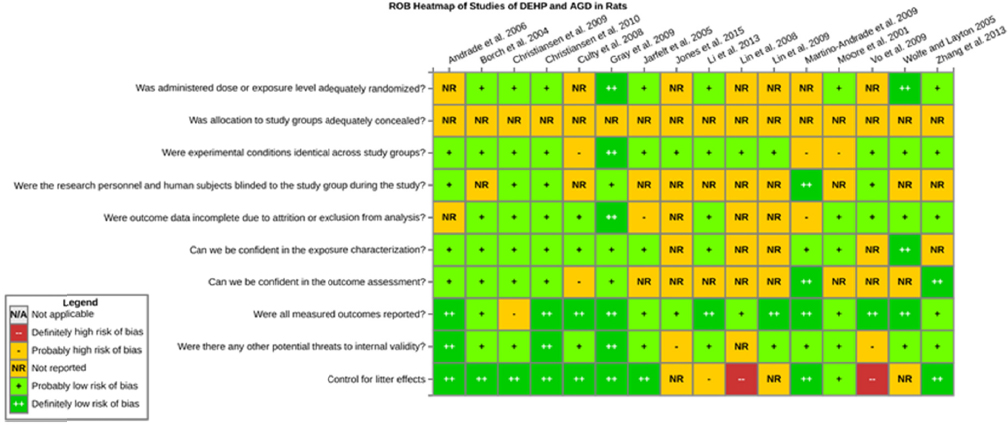
- Unexplained inconsistencies: No downgrade. Although there appeared to be heterogeneity in the results (see Figure C4-3), most of it could be explained by dose, species, or strain differences. Meta-analyses of the data found no important heterogeneity in the rat or the mouse data (see Appendix C, Section C-5), further supporting the decision not to downgrade.
- Indirectness: No downgrade.
- Imprecision: No downgrade. Mean versus standard deviation for most studies reflects reasonable precision (see Figure C4-3). Meta-analyses of the data found a statistically significant summary overall estimate for rats but not for mice (see Appendix C, Section C-5). Because the mouse studies account for a small percentage of the overall body of evidence (3 of 19 studies), confidence was not downgraded for imprecision.
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: Upgraded. Meta-analysis of the data showed that, in rats, the effects could be considered large and robust, with overall summary estimates having z-scores of ≥7.0 (see Appendix C, Section C-5). The effect sizes were robust to multiple sensitivity analyses.
- Dose-response: Upgraded. Although the visualization in Figure C4-4 suggests some inconsistency in dose response across studies, an upgrade is supported by the meta-analysis of the rat data, which found statistically significant linear trends in log10 (dose) or dose. The results were robust to multiple sensitivity analyses.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade.
DEHP and Fetal Testosterone
Twelve animal studies of DEHP and fetal testosterone were available; 11 used the rat model and 1 used the mouse model.
Factors Considered for Downgrading Confidence
- Risk of bias: No downgrade. Most studies accounted for litter effects and used reliable methods of measuring fetal testosterone. See Figure C4-5.
- Unexplained inconsistencies: No downgrade. Consistent decrease in fetal testosterone across studies, with a few exceptions that can be explained by study design features (e.g., examining testosterone in fetal plasma, which might have technical difficulties). See Figure C4-6. A meta-analysis of the data also supported the decision not to downgrade (see Appendix C, Section C-5).
- Indirectness: No downgrade.
- Imprecision: No downgrade. A meta-analysis of the data found a statistically significant summary overall estimate, linear trend in log10(dose), and linear trend in dose, which were robust to multiple sensitivity analyses.
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: Upgraded. High dose groups reflect a relatively large magnitude of change (about 75-90%) across several studies. See Figure C4-6. A meta-analysis of the data also supported the decision to upgrade (see Appendix C, Section C-5).
- Dose-response: Upgraded. Several studies reflect a dose response in the same dose ranges (see Figure C4-7). A meta-analysis of the data also supported the decision to upgrade (see Appendix C, Section C-5).
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only one mouse study was available so cross-species consistency could not be evaluated. Results are generally consistent across studies in the high dose range.
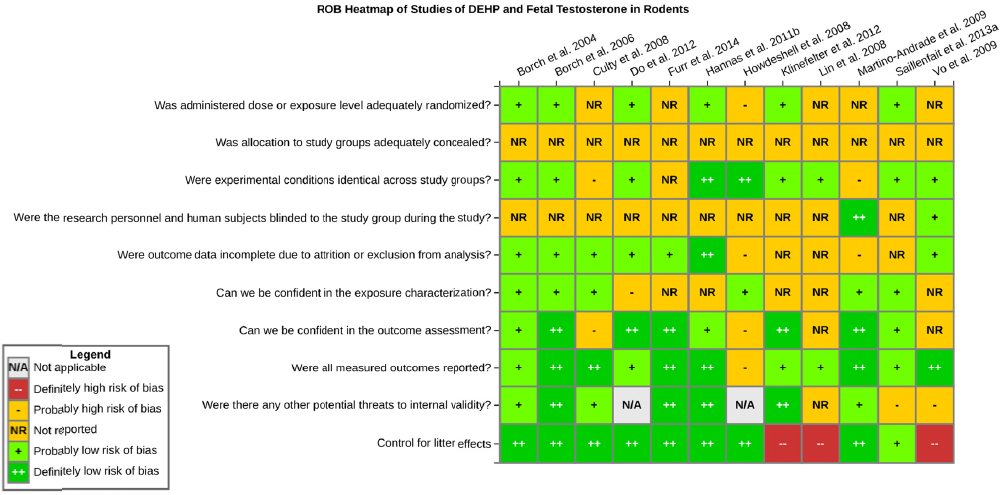
DEHP and Hypospadias
Nine animal studies of DEHP and hypospadias were available; 8 used the rat model and 1 used the mouse model.
Factors Considered for Downgrading Confidence
- Risk of bias: Downgraded. Over half of the studies had a probably high risk of bias rating because they lacked reporting on the outcome assessment. Other concerns were related to whether the researchers were blinded to the study groups during outcome assessment and not controlling for litter effects. See Figure C4-8.
- Unexplained inconsistencies: Downgraded. Incidence of hypospadias is not consistent across studies within similar dose ranges (e.g., Christiansen et al. [2009, 2010] and Jarfelt et al. [2005] show no increased incidence at doses of 750 mg/kg-day or higher). See Figure C4-9.
- Indirectness: No downgrade.
- Imprecision: No downgrade. No confidence intervals for incidence data, but no hypospadias in control groups. See Figure C4-9.
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: No upgrade. Incidence of hypospadias is not consistently large in magnitude across studies for high dose groups. See Figure C4-9.
- Dose-response: No upgrade. Dose response noted for a couple of the studies but not consistently across studies. See Figure C4-10.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgraded. Only one mouse study was available so cross-species consistency could not be evaluated. Results are generally not consistent across studies.
- Rare outcome: Upgraded. Background control incidence of hypospadias was reported as zero across all studies, so any positive finding was considered treatment related.
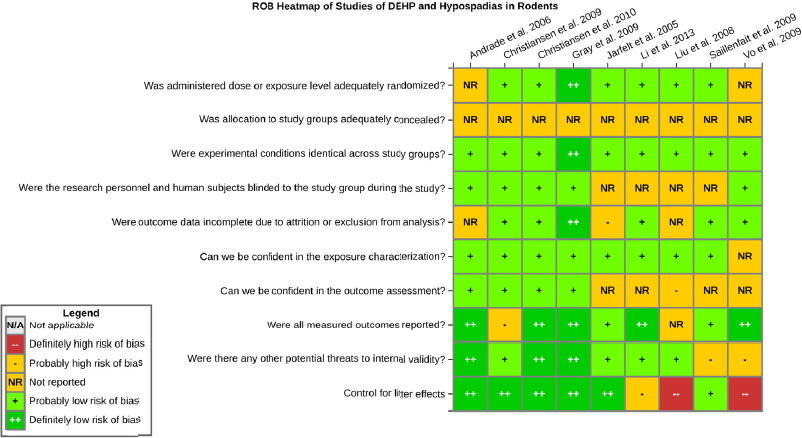
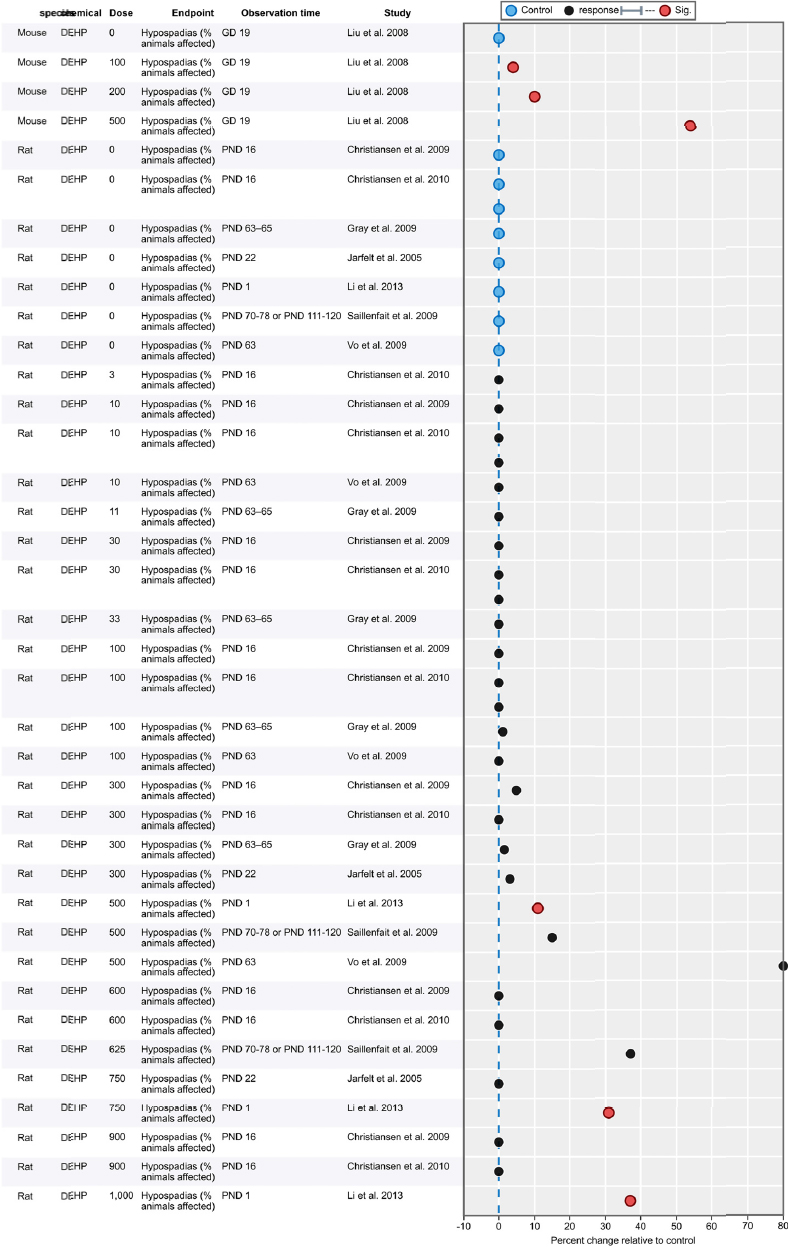
The following links have additional visualizations presenting data on hypospadias in terms of the percentage of litters affected (https://hawcproject.org/summary/data-pivot/assessment/351/dehp-effect-hypospadias-litters-affected/) or litter incidence (https://hawcproject.org/summary/data-pivot/assessment/351/dehp-effect-hypospadias-litter-incidence/).
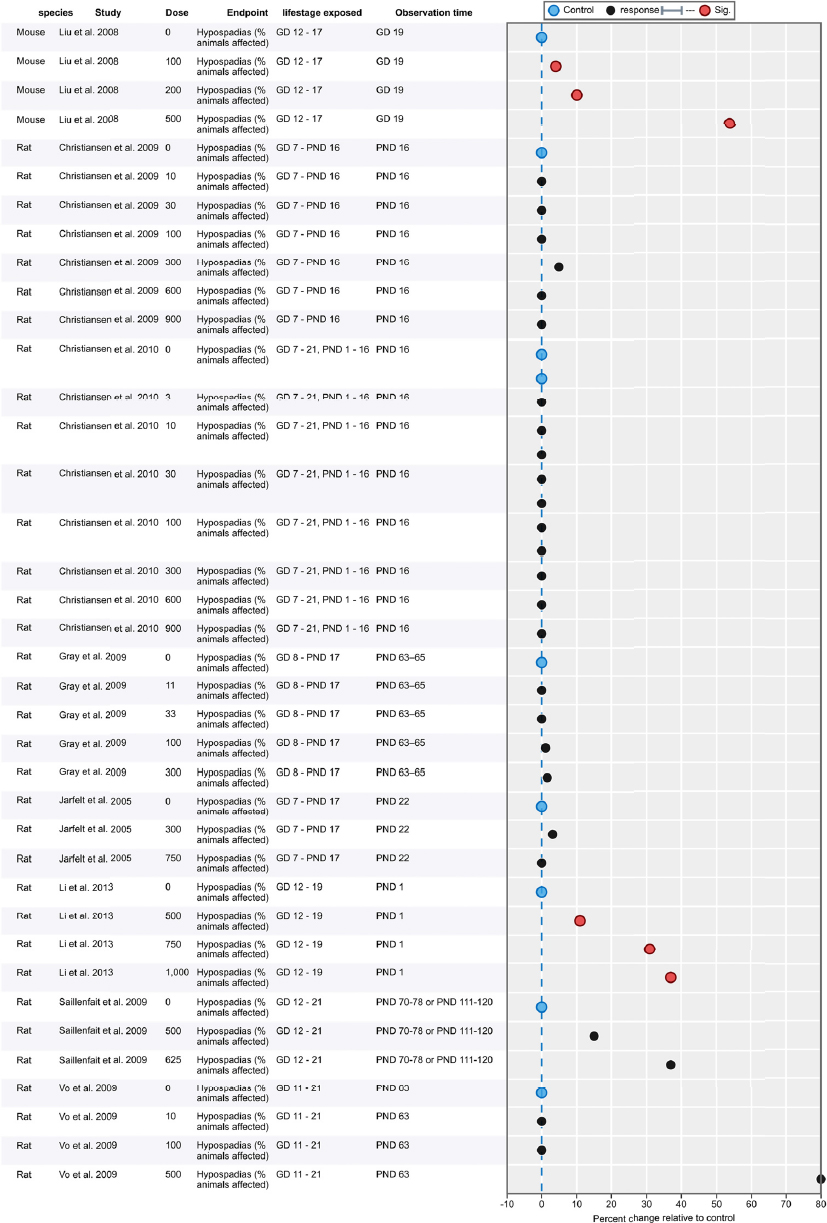
The following links have additional visualizations presenting data on hypospadias in terms of the percentage of litters affected (https://hawcproject.org/summary/data-pivot/assessment/351/dehp-effect-hypospadias-litters-affected-dose-resp/) or litter incidence (https://hawcproject.org/summary/data-pivot/assessment/351/dehp-effect-hypospadias-litterincidence-dose-resp/).
BzBP and AGD
Six studies of BzBP and AGD in rats were available.
Factors Considered for Downgrading Confidence
- Risk of bias: Downgraded. All the studies had ratings of probably high risk of bias or definitely high risk of bias in at least one of the key issues considered, and all had multiple risk of bias issues. See Figure C4-11.
- Unexplained inconsistencies: No downgrade. Consistent dose response across most studies with the exception of the study by Aso et al. (2005), which could be explained by study design features. See Figure C4-12.
- Indirectness: No downgrade.
- Imprecision: No downgrade. Mean versus standard deviation for the studies reflects reasonable precision. See Figure C4-12.
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: Upgraded. Three studies reflect relatively large magnitude of change (about 20-40%) in the same dose range. See Figure C4-12.
- Dose-response: Upgraded. Most studies reflect a dose response in the same dose range. See Figure C4-13.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only studies in rats were available so cross-species consistency could not be evaluated.
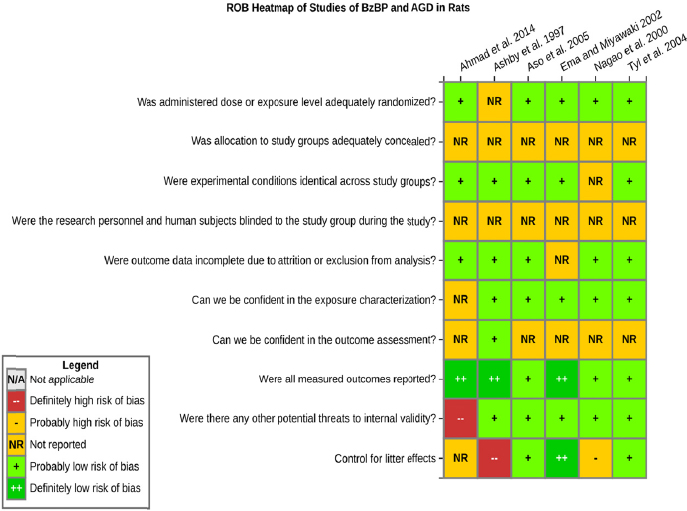
BzBP and Fetal Testosterone
Two studies of BzBP and effects on fetal testosterone in rats were available.
Factors Considered for Downgrading Confidence
- Risk of bias: No downgrade. Both studies accounted for litter effects. See Figure C4-14.
- Unexplained inconsistencies: No downgrade. See Figure C4-15.
- Indirectness: No downgrade.
- Imprecision: No downgrade. Mean versus standard deviation for the studies reflects reasonable precision. See Figure C4-15.
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: Upgraded. Higher dose groups reflect a relatively large magnitude of change (about 80% in both studies). See Figure C4-15.
- Dose-response: Upgraded. Both studies reflect a dose response in the same dose range. See Figure C4-16.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only studies in rats were available so cross-species consistency could not be evaluated.
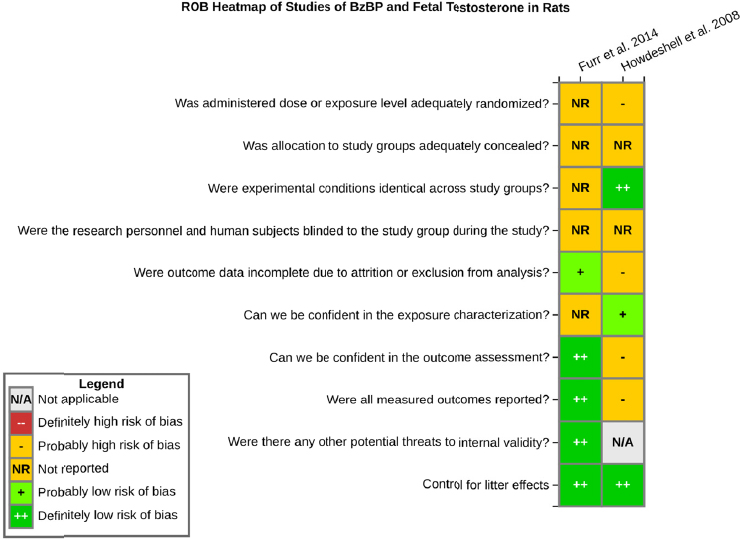
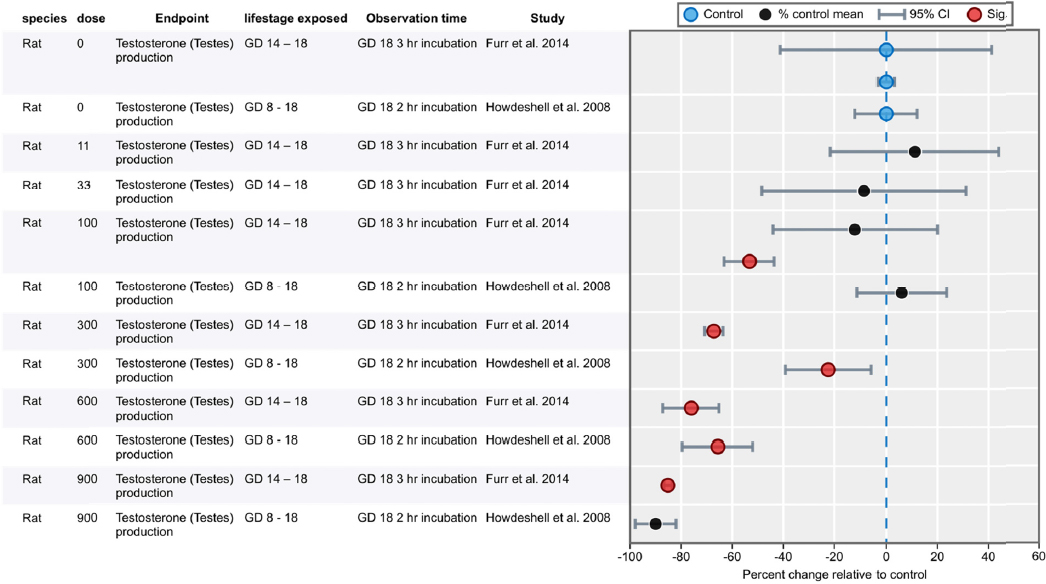
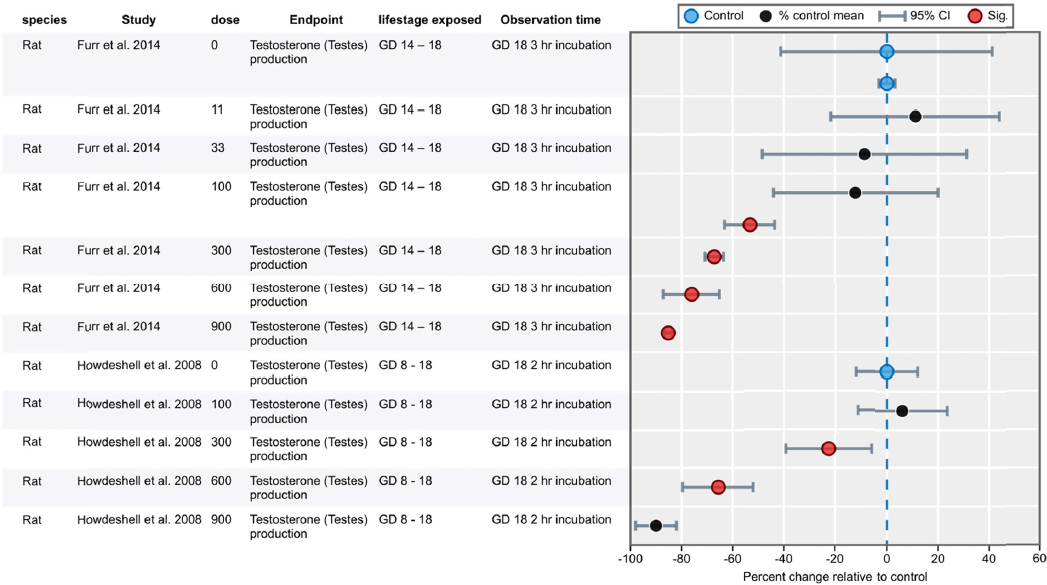
BzBP and Hypospadias
Two studies of BzBP and hypospadias in rats were available.
Factors Considered for Downgrading Confidence
- Risk of bias: Downgraded. Both of the studies had probably high risk of bias ratings because of concerns about whether the researchers were blinded to the treatment groups and concerns about the outcome measures. One study did not control for litter effects, but it reported no hypospadias. See Figure C4-17.
- Unexplained inconsistencies: No downgrade. Little response seen in either study. See Figure C4-18.
- Indirectness: No downgrade.
- Imprecision: No downgrade. See Figure C4-18.
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: No upgrade. Only a single hypospadias case was reported in the highest dose group in one study. See Figure C4-18.
- Dose-response: No upgrade. See Figure C4-18.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only studies in rats were available so cross-species consistency could not be evaluated.
- Rare outcome: Because the data are limited and there were risk of bias concerns regarding the outcome measure, confidence was not upgraded for the finding of a rare effect as was done for other phthalates and hypospadias.
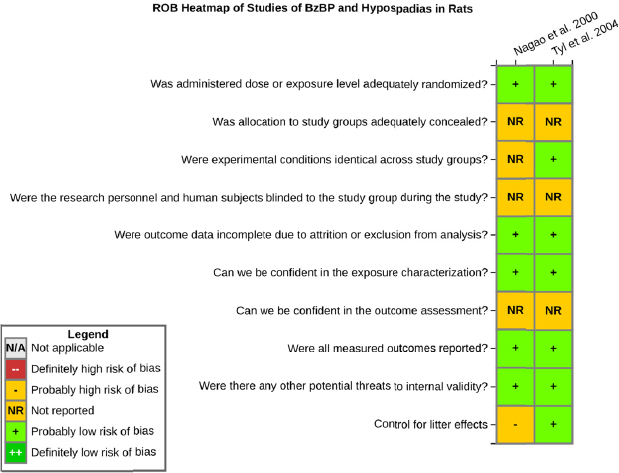
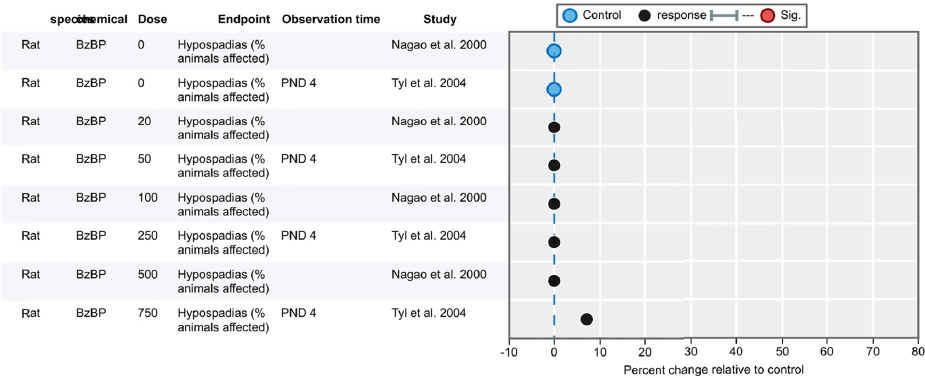
The following links have additional visualizations presenting data on hypospadias in terms of the percentage of litters affected (https://hawcproject.org/summary/data-pivot/assessment/351/bzbp-effect-hypospadias-litters-affected/) or litter incidence (https://hawcproject.org/summary/data-pivot/assessment/351/bzbp-effect-hypospadias-litter-incidence/).
DBP and AGD
Twenty-two studies of DBP and effects on AGD in rats were available.
Factors Considered for Downgrading Confidence
- Risk of bias: Downgraded. All studies had ratings of probably high risk of bias or definitely high risk of bias in at least one of the key issues considered, and most of the studies had multiple risk of bias issues. See Figure C4-19.
- Unexplained inconsistencies: No downgrade. Consistent effects observed across multiple studies. Inconsistencies could be explained by study design features. See Figure C4-20.
- Imprecision: No downgrade. Mean versus standard deviation for most studies reflects reasonable precision, with the exception of the study by Struve et al. (2009). See Figure C4-20.
- Indirectness: No downgrade
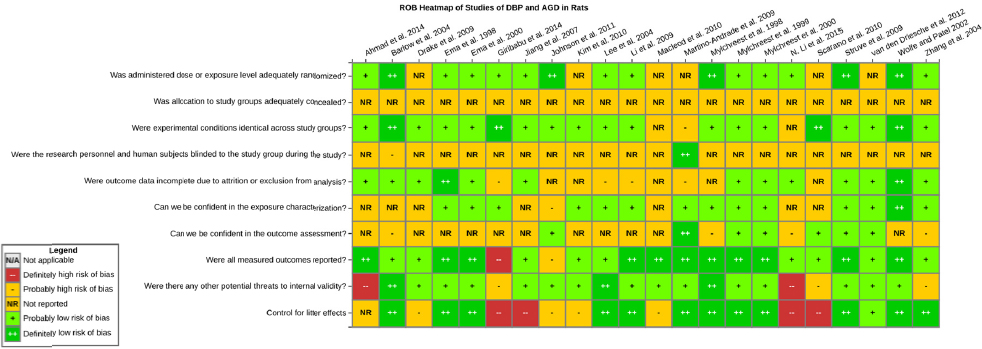
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: No upgrade. Only a few studies demonstrate a large effect (40%) even at higher doses. See Figure C4-20.
- Dose-response: Upgraded. Several studies reflect a dose response. See Figure C4-21.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only studies in rats were available so cross-species consistency could not be evaluated.
DBP and Fetal Testosterone
Twelve studies of DBP and effects on fetal testosterone in rats were available.
Factors Considered for Downgrading Confidence
- Risk of bias: No downgrade. See Figure C4-22.
- Unexplained inconsistencies: No downgrade. Consistent effects observed across multiple studies. Inconsistencies could be explained by study design features. See Figure C4-23.
- Indirectness: No downgrade
- Imprecision: No downgrade. Mean versus standard deviation for most studies reflects reasonable precision. See Figure C4-23.
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: Upgraded. Several studies demonstrate large effects (about 80%) in high dose groups. See Figure C4-23.
- Dose-response: Upgraded. Several studies reflect a dose response. See Figure C4-24.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only studies in rats were available so cross-species consistency could not be evaluated.
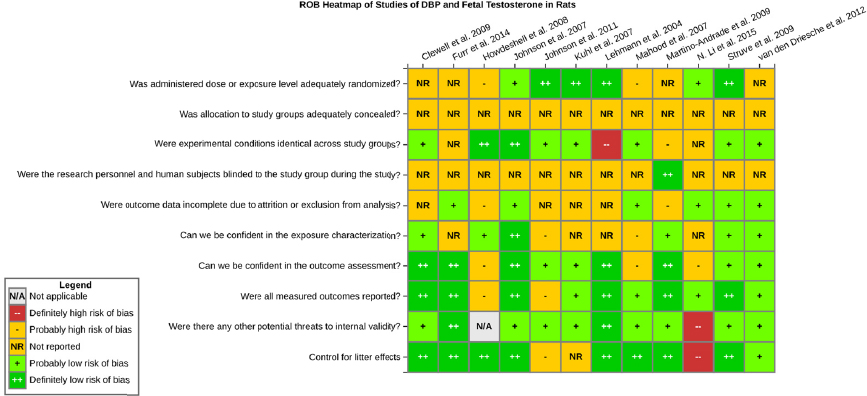
DBP and Hypospadias
Eight studies of DBP and effects on hypospadias in rats were available.
Factors Considered for Downgrading Confidence
- Risk of bias: Downgraded. Risk of bias concerns included confidence in the outcome assessment and whether the researchers were blinded to the treatment groups. See Figure C4-25.
- Unexplained inconsistencies: No downgrade. Incidence of hypospadias appeared to be consistent across studies within similar dose ranges. See Figure C4-26.
- Indirectness: No downgrade.
- Imprecision: No downgrade. No confidence intervals for incidence data, but no hypospadias in control groups. See Figure C4-26.
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: Upgraded. High incidence (about 40%) of hypospadias was found in different studies in high dose groups. See Figure C4-26.
- Dose-response: Upgraded. Dose response was noted for studies and there was general agreement across studies. See Figure C4-27.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only studies in rats were available so cross-species consistency could not be evaluated.
- Rare outcome: Upgraded. Background control incidence of hypospadias was reported as zero across all studies, so any positive finding was considered treatment related.
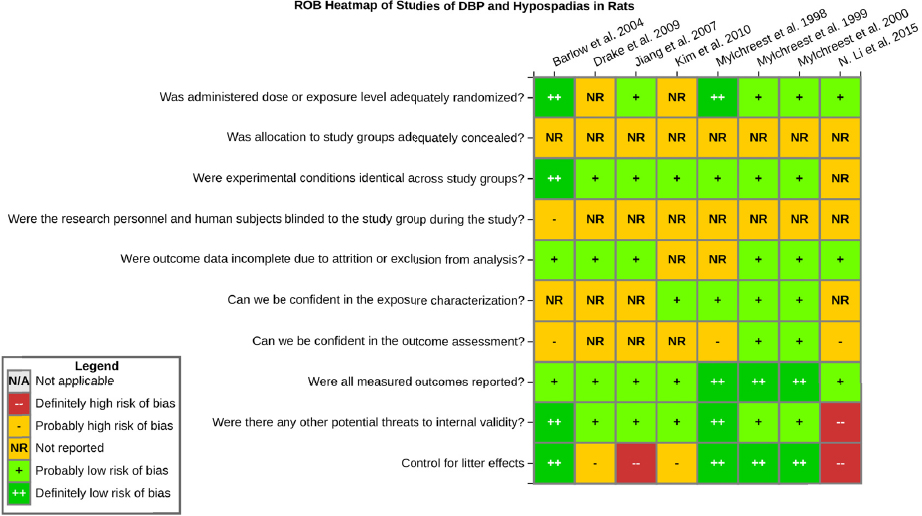
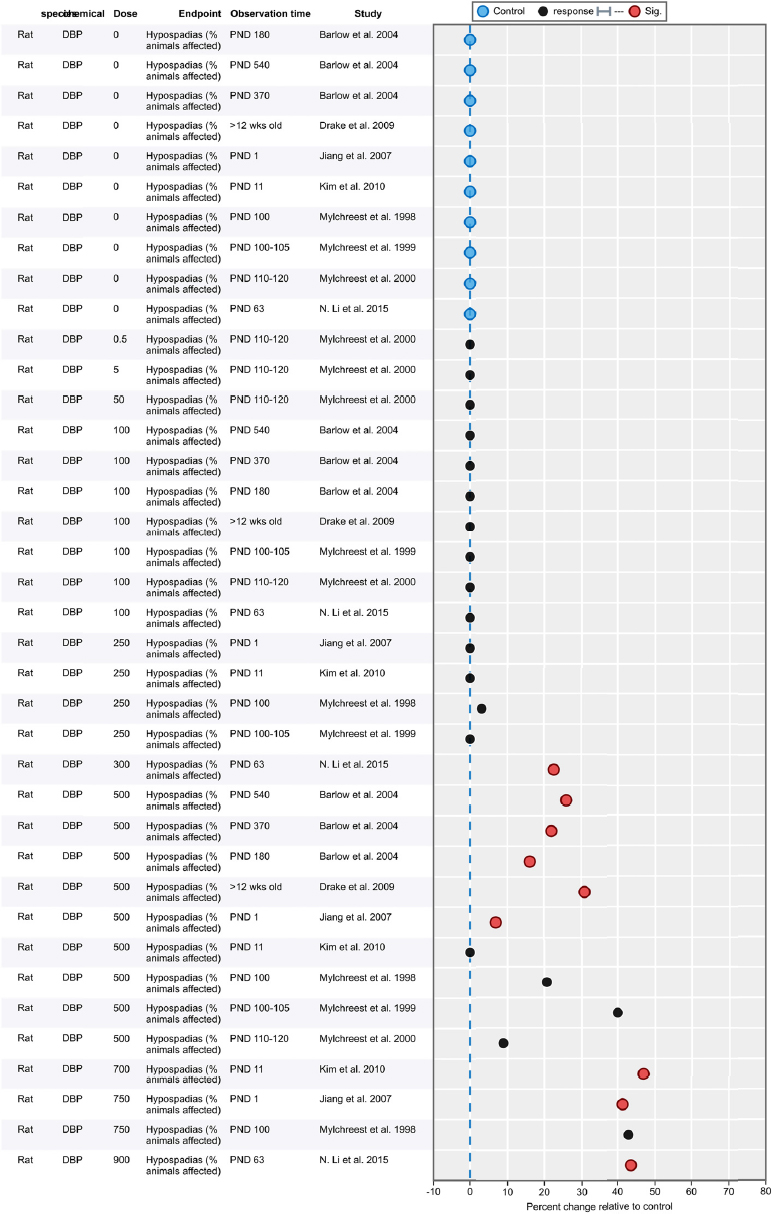
The following links have additional visualizations presenting data on hypospadias in terms of the percentage of litters affected (https://hawcproject.org/summary/data-pivot/assessment/351/dbp-effect-hypospadias-litters-affected/) or litter incidence (https://hawcproject.org/summary/data-pivot/assessment/351/dbp-effect-hypospadias-litter-incidence/).
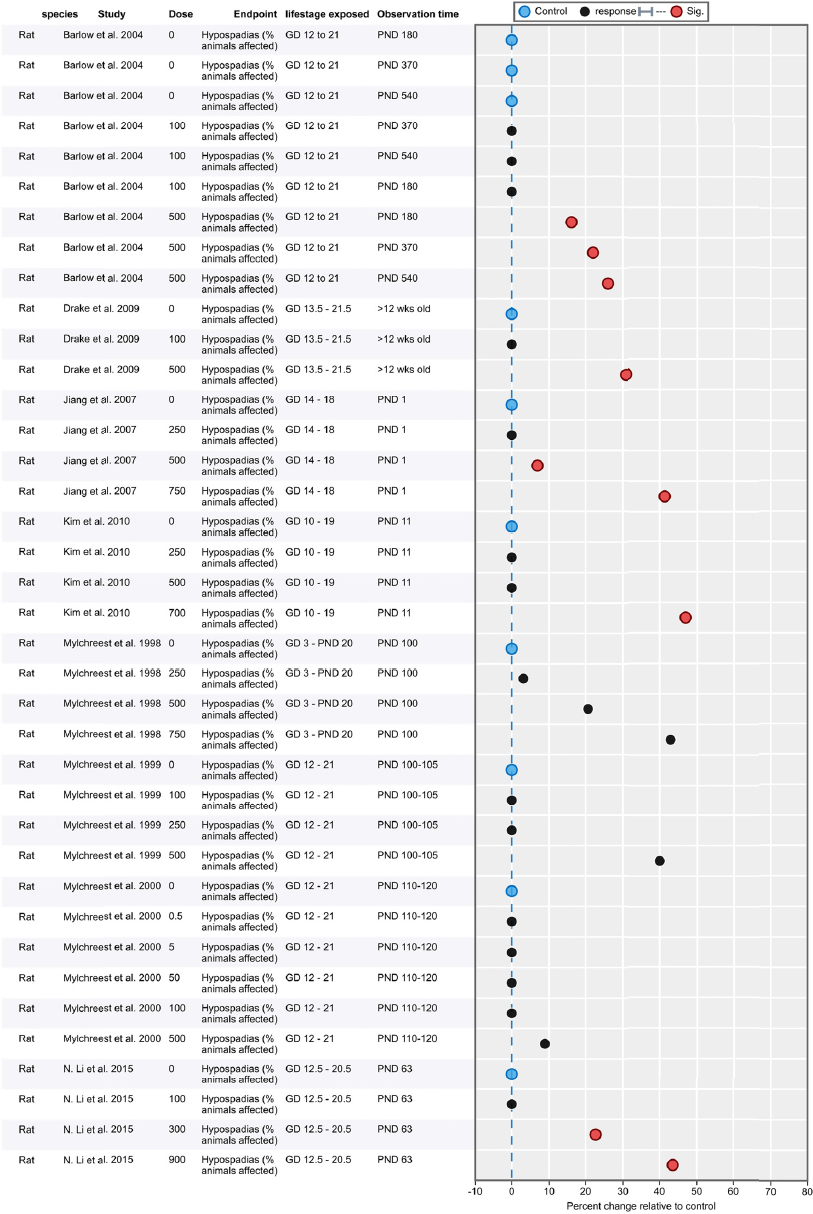
The following links have additional visualizations presenting data on hypospadias in terms of the percentage of litters affected (https://hawcproject.org/summary/data-pivot/assessment/351/dbp-effect-hypospadias-litters-affected-dose-respo/) or litter incidence (https://hawcproject.org/summary/data-pivot/assessment/351/dbp-effect-hypospadias-litterincidence-dose-respo/).
DIBP and Fetal Testosterone
Two studies of DIBP and effects on fetal testosterone in rats were available.
Factors Considered for Downgrading Confidence
- Risk of bias: No downgrade. See Figure C4-28.
- Unexplained inconsistencies: No downgrade. Studies are relatively consistent. See Figure C4-29.
- Indirectness: No downgrade.
- Imprecision: Downgraded. Mean versus standard deviation reflects variable precision across studies, including overlapping error bars between control and significant treatment groups. See Figure C4-29. Meta-analysis of the data supports the downgrade (see Appendix C, Section C-6).
- Publication bias: No downgrade (see Appendix C, Section C-3).
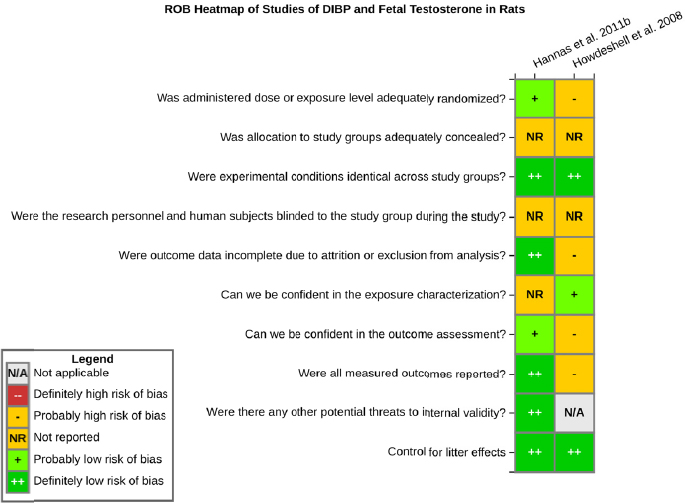
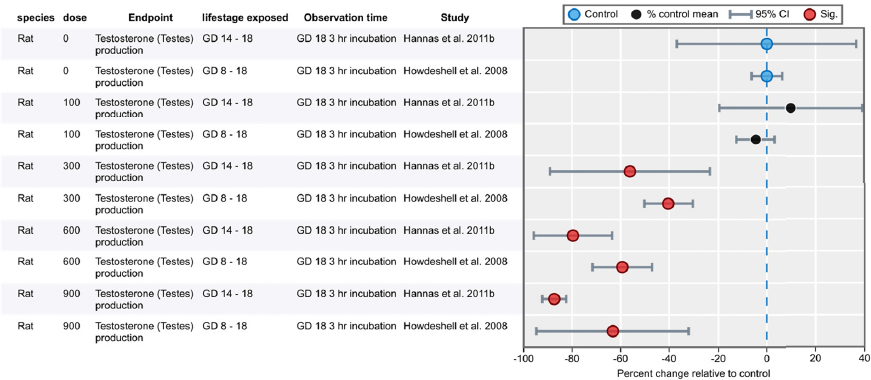
Factors Considered for Upgrading Confidence
- Large magnitude: Upgraded. Consistently large effects of more than 50% are seen in both studies. See Figure C4-29.
- Dose-response: Upgraded. Dose response is evident in both studies. See Figure C4-30.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only studies in rats were available so cross-species consistency could not be evaluated.
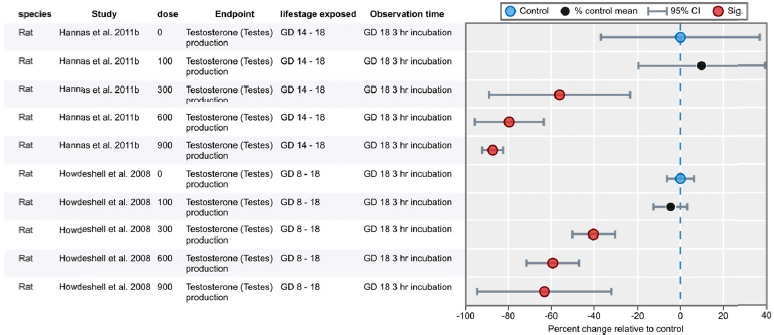
DINP and AGD
Four studies of DINP and effects on AGD in rats were available.
Factors Considered for Downgrading Confidence
- Risk of bias: Downgraded. Two of the studies had a probably high risk of bias rating in two key areas (whether researchers were blinded to the treatment groups or how outcomes were assessed), and one had a probably high risk of bias rating for not controlling for litter effects. See Figure C4-31.
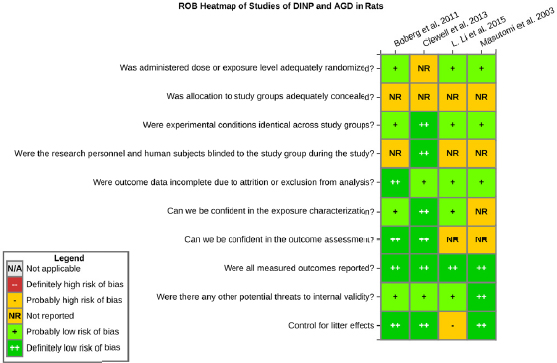
- Unexplained inconsistencies: Downgraded. Responses are inconsistent across the studies and not explained by methodology or other factors. One would expect a treatment-related decrease in AGD at these dose levels given the increase in fetal testosterone; however, only one of the four studies showed a clear treatment-related decrease in AGD (Boberg et al. 2011). The study by Clewell et al. (2013) did not find decreased AGD, nor did L. Li et al. (2015), although the error was huge in that study. Masutomi et al. (2003) data appear to be equivocal; the mean is lower but the error is large. See Figure C4-32.
- Indirectness: No downgrade
- Imprecision: Downgraded. The L. Li et al. (2015) and Masutomi et al. (2003) studies had larger standard deviations than did the effect measured by Clewell et al. (2013) and Boberg et al. (2011). See Figure C4-32.
- Publication bias: No downgrade (see Appendix C, Section C-3).
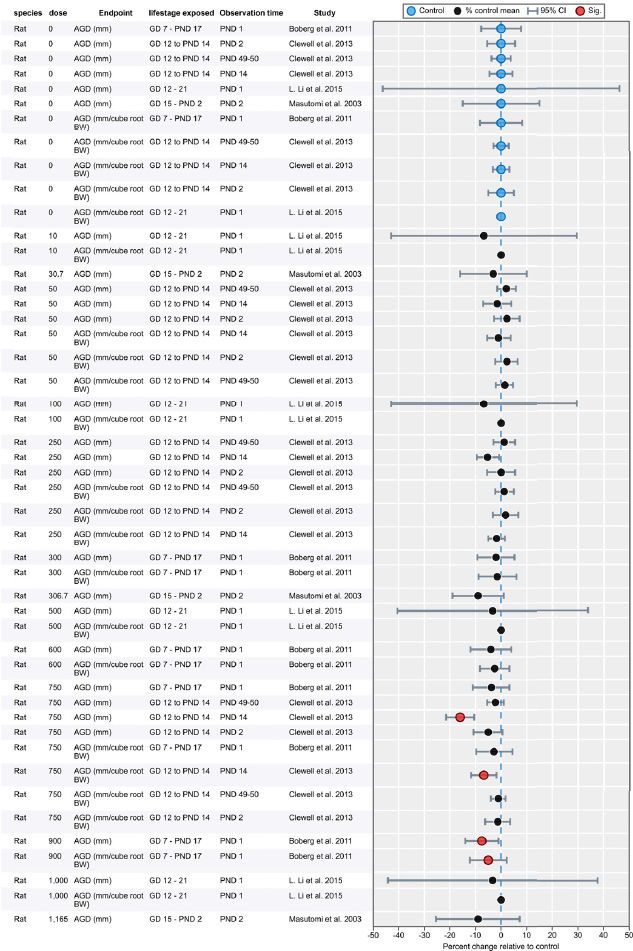
Factors Considered for Upgrading Confidence
- Large magnitude: No upgrade. A large magnitude of effect (less than 20%) was not observed consistently across multiple studies. See Figure C4-32.
- Dose-response: No upgrade. Dose response is not consistent across studies. Only two of four studies show a dose response. The data from Clewell et al. (2013) are internally inconsistent; no effect at PND 2 or PND 49-50, but a statistically-identified decrease in AGD was found at PND 14. See Figure C4-33.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only studies in rats were available so cross-species consistency could not be evaluated.
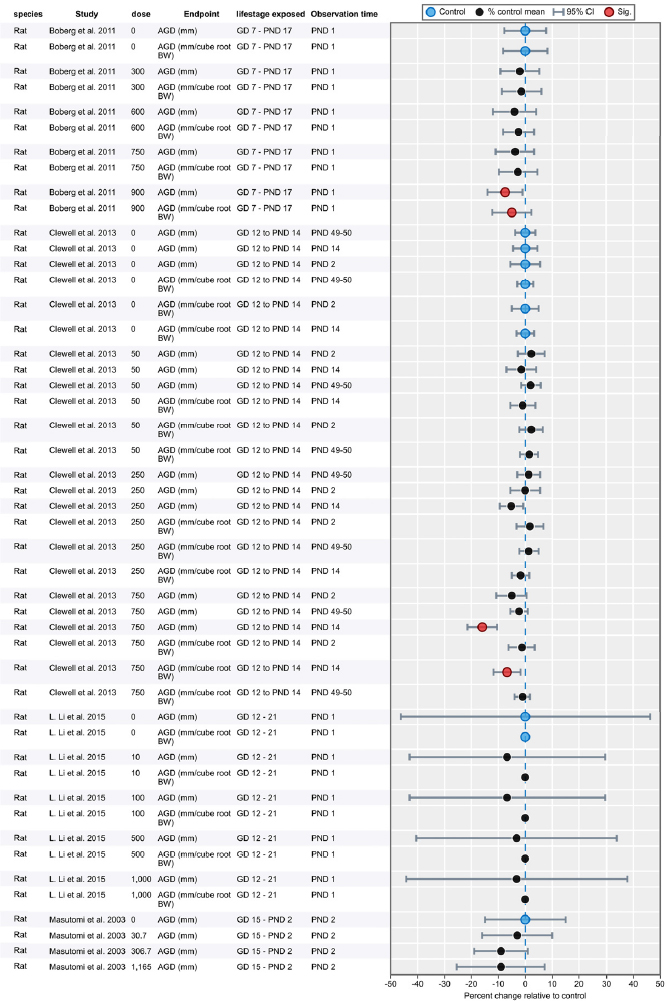
DINP and Fetal Testosterone
Four studies of DINP and effects on fetal testosterone in rats were available.
Factors Considered for Downgrading Confidence
- Risk of bias: No downgrade. See Figure C4-34.
- Unexplained inconsistencies: No downgrade. Inconsistencies can be explained by study design features (exposure window) and differences in measurements (testosterone in plasma is different from testosterone in the testes). See Figure C4-35.
- Indirectness: No downgrade.
- Imprecision: Downgraded. Mean versus standard deviation reflects variable precision across studies, particularly testosterone production and testosterone measurements in plasma. See Figure C4-35. Meta-analysis of the data also supported a downgrade (see Appendix C, Section C-6).
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: Upgraded. Studies show large effects of more than 50% (see Figure C4-35), and meta-analysis of the data found an overall effect that was large in magnitude (see Appendix C, Section C-6).
- Dose-response: Upgrade. Dose response is evident in most studies, although not statistically significant in most cases. See Figure C4-36.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only studies in rats were available so cross-species consistency could not be evaluated.
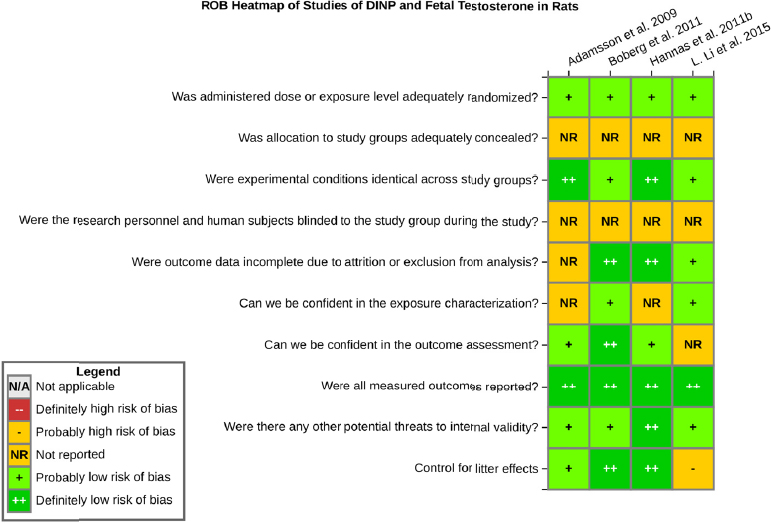
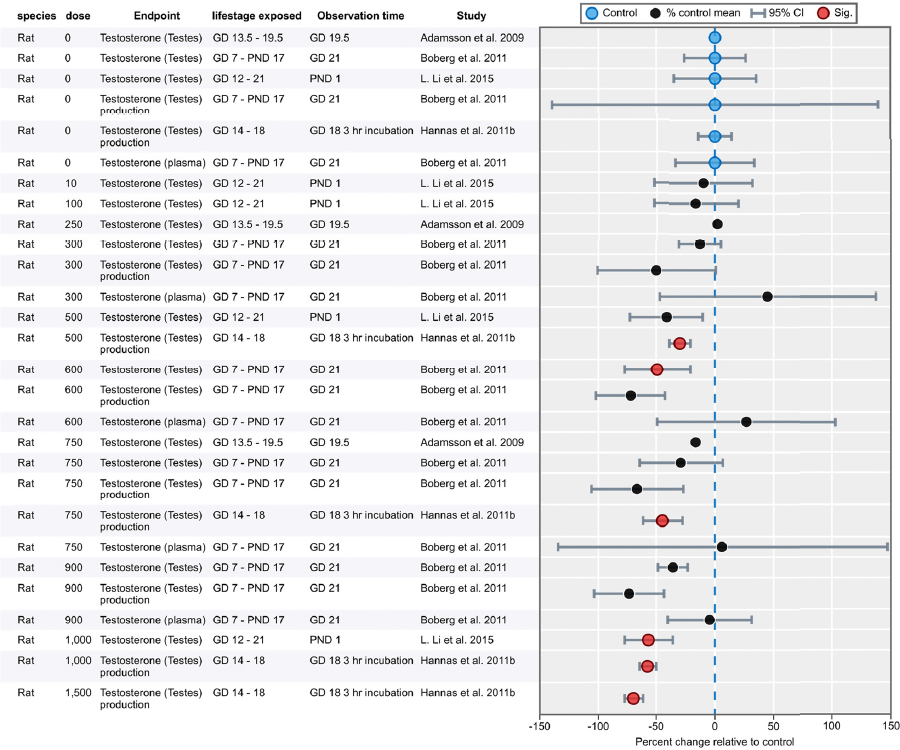
DPP and Fetal Testosterone
Four studies of DPP and effects on fetal testosterone in rats were available.
Factors Considered for Downgrading Confidence
- Risk of bias: No downgrade. See Figure C4-37.
- Unexplained inconsistencies: No downgrade. Data are relatively consistent across studies, and inconsistencies can be explained by study design or measurement features (incubation time). See Figure C4-38.
- Indirectness: No downgrade.
- Imprecision: No downgrade. Mean versus standard deviation reflects reasonable precision across studies. See Figure C4-38.
- Publication bias: No downgrade (see Appendix C, Section C-3).
Factors Considered for Upgrading Confidence
- Large magnitude: Upgrade. Consistently large effects of more than 60% are seen in several studies within the same dose ranges. See Figure C4-38.
- Dose-response: Upgrade. Dose response is evident in most studies. See Figure C4-39.
- Residual confounding: Not applicable.
- Cross-species consistency: No upgrade. Only studies in rats were available so cross-species consistency could not be evaluated.
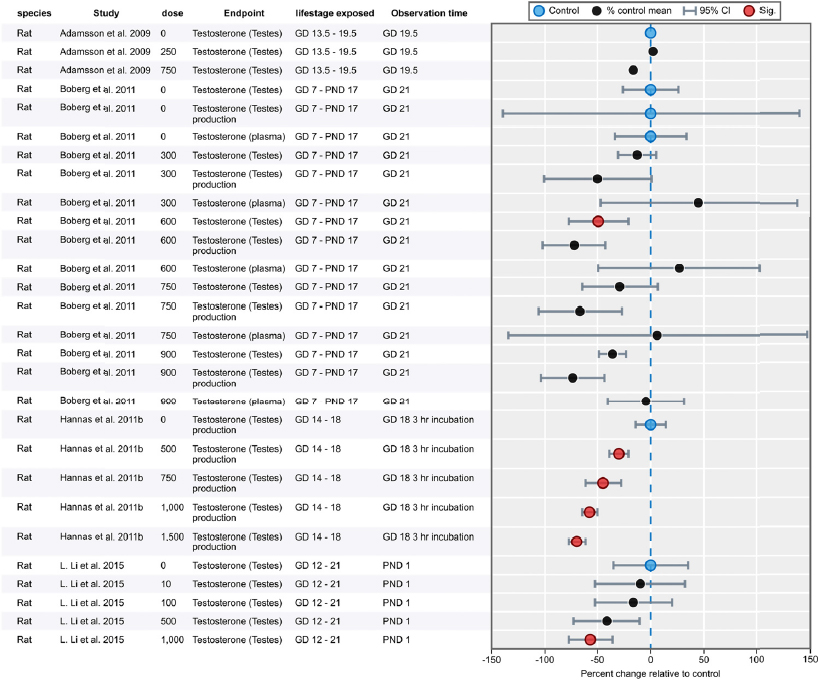
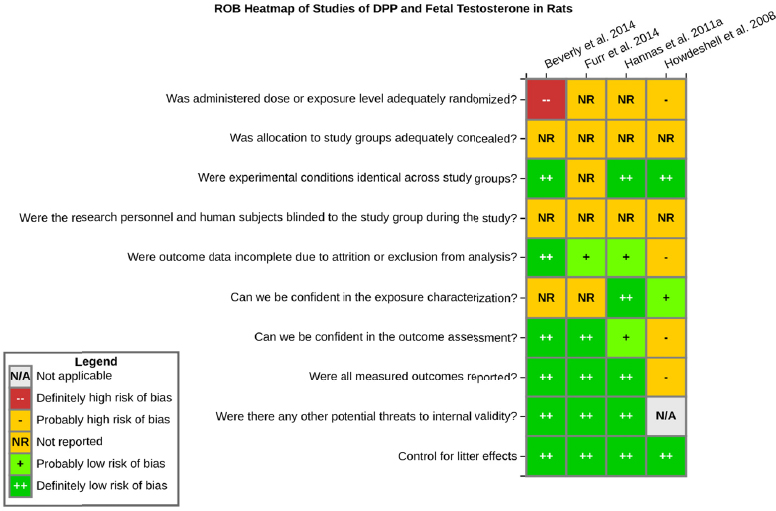
SECTION C-5
Supporting Information for the Meta-analyses of Studies of DEHP
META-ANALYSES OF RAT STUDIES ON DEHP AND AGD
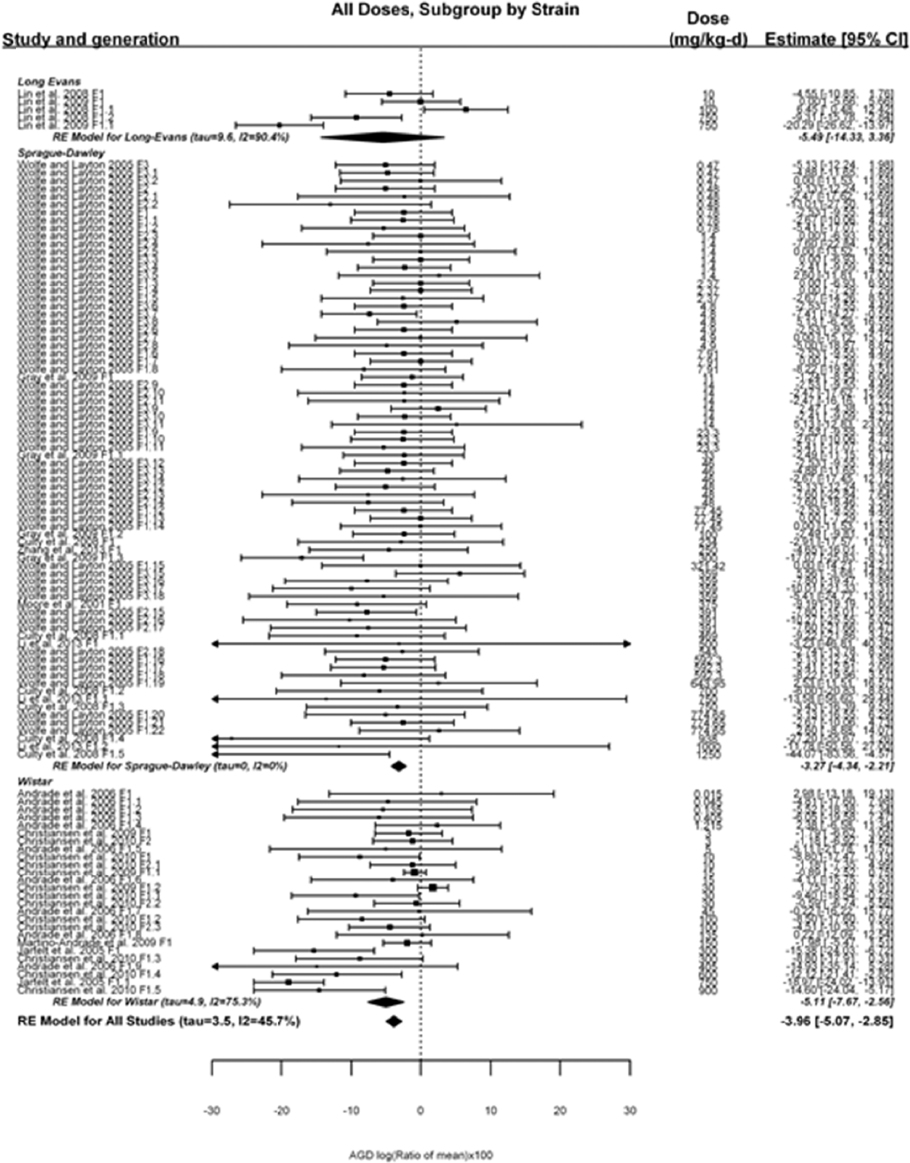
TABLE C5-1 Subgrouping Analyses of Rat Studies on DEHP and AGD
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Long Evans | |||||||||
| Rat DEHP LE Overall | Intrcpt | -5.49 | -14.33 | 3.36 | 0.224 | 9.59 | 90.38 | 0.000 | 45.84 |
| Rat DEHP LE Trend in log10 dose | log10(dose) | -6.46 | -16.21 | 3.29 | 0.194 | 8.78 | 88.76 | 0.000 | 51.91 |
| Rat DEHP LE Linear in dose100 | dose100 | -1.90 | -3.04 | -0.77 | 0.001 | 5.21 | 73.98 | 0.004 | 41.81* |
| Sprague-Dawley | |||||||||
| Rat DEHP SD Overall | Intrcpt | -3.27 | -4.34 | -2.21 | 0.000 | 0.00 | 0.00 | 0.988 | 448.47 |
| Rat DEHP SD Trend in log10 dose | log10(dose) | -0.92 | -1.96 | 0.12 | 0.083 | 0.00 | 0.00 | 0.993 | 442.56* |
| Rat DEHP SD Linear-Quadratic in dose100 | dose100 | -2.40 | -3.78 | -1.01 | 0.001 | 0.00 | 0.00 | 0.899 | 452.31 |
| I(dose100^2) | 0.22 | 0.01 | 0.43 | 0.036 | |||||
| Wistar | |||||||||
| Rat DEHP W Overall | Intrcpt | -5.11 | -7.67 | -2.56 | 0.000 | 4.94 | 75.25 | 0.000 | 168.41 |
| Rat DEHP W Trend in log10 dose | log10(dose) | -3.14 | -5.21 | -1.06 | 0.003 | 3.94 | 65.66 | 0.000 | 157.70 |
| Rat DEHP W Linear-Quadratic in dose100 | dose100 | -3.58 | -5.57 | -1.59 | 0.000 | 1.38 | 22.04 | 0.386 | 143.73* |
| I(dose100^2) | 0.18 | -0.09 | 0.45 | 0.201 | |||||
* Indicates the lowest AICc for each strain.
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | -3.96 | -5.07 | -2.85 | 0.000 | 3.48 | 45.74 | 0.000 | 680.05 |
| Trend in log10(dose) | log10(dose) | -1.97 | -2.98 | -0.96 | 0.000 | 3.00 | 38.46 | 0.000 | 662.96 |
| Linear in dose100 | dose100 | -1.55 | -1.86 | -1.24 | 0.000 | 1.97 | 22.05 | 0.124 | 659.46 |
| Linear-Quadratic in dose100 | dose100 | -2.11 | -3.30 | -0.91 | 0.001 | 2.03 | 22.94 | 0.117 | 654.59* |
| I(dose100^2) | 0.08 | -0.09 | 0.25 | 0.337 | |||||
| Sensitivity Analyses | |||||||||
| Overall minus Christiansen et al. 2009 | intrcpt | -4.23 | -5.37 | -3.09 | 0.000 | 3.43 | 37.58 | 0.001 | 662.79 |
| Overall minus Christiansen et al. 2010 | intrcpt | -3.69 | -4.88 | -2.50 | 0.000 | 3.53 | 46.25 | 0.000 | 618.76 |
| Overall minus Culty et al. 2008 | intrcpt | -3.86 | -4.99 | -2.73 | 0.000 | 3.51 | 47.34 | 0.000 | 634.25 |
| Overall minus Lin et al. 2008 | intrcpt | -4.02 | -5.13 | -2.91 | 0.000 | 3.33 | 43.19 | 0.000 | 658.78 |
| Overall minus Gray et al. 2009 | intrcpt | -3.89 | -5.02 | -2.76 | 0.000 | 3.43 | 45.13 | 0.000 | 653.44 |
| Overall minus Lin et al. 2009 | intrcpt | -3.72 | -4.78 | -2.67 | 0.000 | 2.97 | 37.79 | 0.001 | 656.63 |
| Overall minus Li et al. 2013 | intrcpt | -3.95 | -5.06 | -2.84 | 0.000 | 3.49 | 46.49 | 0.000 | 655.79 |
| Overall minus Jarfelt et al. 2005 | intrcpt | -3.44 | -4.44 | -2.43 | 0.000 | 2.59 | 31.65 | 0.012 | 650.98 |
| Overall minus Moore et al. 2001 | intrcpt | -3.91 | -5.03 | -2.80 | 0.000 | 3.48 | 45.89 | 0.000 | 673.84 |
| Overall minus Zhang et al. 2013 | intrcpt | -3.96 | -5.07 | -2.84 | 0.000 | 3.50 | 46.16 | 0.000 | 674.38 |
| Overall minus Andrade et al. 2006 | intrcpt | -4.04 | -5.20 | -2.89 | 0.000 | 3.59 | 48.97 | 0.000 | 616.81 |
| Overall minus Martino-Andrade et al. 2009 | intrcpt | -4.01 | -5.13 | -2.88 | 0.000 | 3.53 | 45.56 | 0.000 | 675.20 |
| Overall minus Wolfe and Layton 2005 | intrcpt | -5.59 | -7.72 | -3.45 | 0.000 | 5.37 | 72.75 | 0.000 | 314.53 |
| Highest Doses-Overall | intrcpt | -8.08 | -12.31 | -3.86 | 0.000 | 7.26 | 81.76 | 0.000 | 129.90 |
| Highest Doses-Linear in dose100 | dose100 | -1.87 | -2.45 | -1.30 | 0.000 | 4.22 | 65.26 | 0.004 | 120.97 |
| Highest Doses-Trend in log10(dose) | log10(dose) | -11.44 | -19.03 | -3.86 | 0.003 | 5.21 | 63.51 | 0.001 | 120.83 |
| Highest Doses-Linear-Quadratic in dose100 | dose100 | -1.34 | -3.53 | 0.85 | 0.232 | 4.46 | 67.35 | 0.004 | 117.23 |
| I(dose100^2) | -0.07 | -0.37 | 0.22 | 0.623 |
* Indicates the lowest AICc.
TABLE C5-3 Benchmark Dose Estimates for DEHP and AGD in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| All strains Linear in dose100 | -5.1 | 332 | 276 | 415 |
| All strains Linear-Quadratic in dose100 | -5.1 | 271 | 178 | 418 |
| LE Linear in dose100 | -5.1 | 268 | 168 | 659 |
| SD Linear-Quadratic in dose100 | -5.1 | 294 | 166 | NA |
| W Linear-Quadratic in dose100 | -5.1 | 154 | 99 | 282 |
Benchmark dose estimates were calculated for an effect size of 5%. The benchmark dose was calculated using the linear or linear-quadratic model, with the model selection based on the lowest AIC (including correction for small sample size). The benchmark dose was only calculated for the “fixed effect”—the estimated mean response across studies.
TABLE C5-4 Overall Analyses and Sensitivity Analyses of Mouse Studies of DEHP and AGD
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | -1.57 | -4.61 | 1.47 | 0.310 | 3.94 | 82.39 | 0.000 | 68.84 |
| Trend in log10(dose) | log10(dose) | -1.77 | -2.71 | -0.83 | 0.000 | 1.60 | 40.12 | 0.095 | 60.64 |
| Linear in dose100 | dose100 | -2.03 | -3.51 | -0.55 | 0.007 | 2.81 | 69.81 | 0.005 | 64.57 |
| Linear-Quadratic in dose100 | dose100 | -5.71 | -7.15 | -4.27 | 0.000 | 0.00 | 0.00 | 0.185 | 59.68* |
| I(dose100^2) | 0.96 | 0.42 | 1.49 | 0.000 | |||||
| Sensitivity Analyses | |||||||||
| Overall minus Do et al. 2012 | intrcpt | -4.48 | -7.12 | -1.85 | 0.001 | 2.48 | 80.24 | 0.000 | 37.71 |
| Overall minus Pocar et al. 2012 | intrcpt | -1.08 | -5.32 | 3.16 | 0.617 | 5.10 | 85.14 | 0.000 | 59.53 |
| Overall minus Liu et al. 2008 | intrcpt | 0.31 | -2.38 | 3.00 | 0.821 | 2.19 | 38.54 | 0.186 | 47.17 |
| Highest Doses-Overall | intrcpt | -2.27 | -5.10 | 0.55 | 0.115 | 0.00 | 0.00 | 0.319 | 28.25 |
| Highest Doses-Linear in dose100 | dose100 | -1.01 | -2.65 | 0.63 | 0.228 | 1.14 | 22.50 | 0.195 | 27.46 |
* Indicates the lowest AICc.
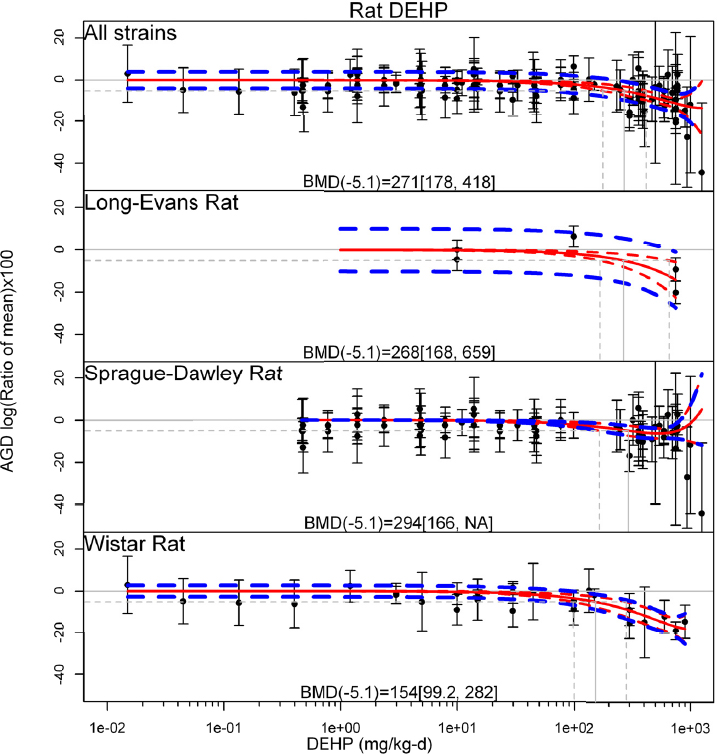
META-ANALYSES OF MOUSE STUDIES ON DEHP AND AGD
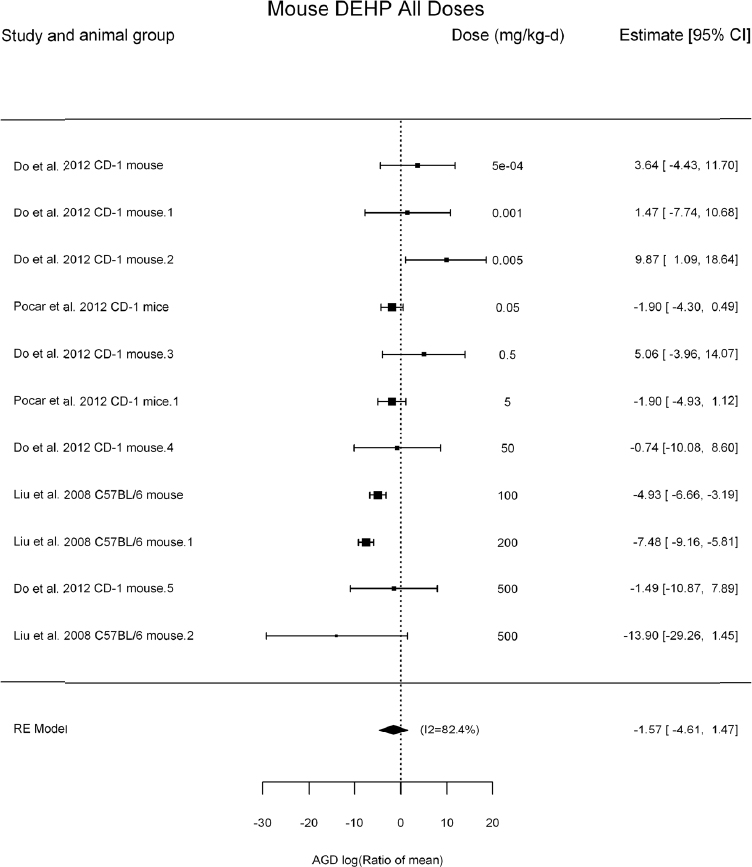
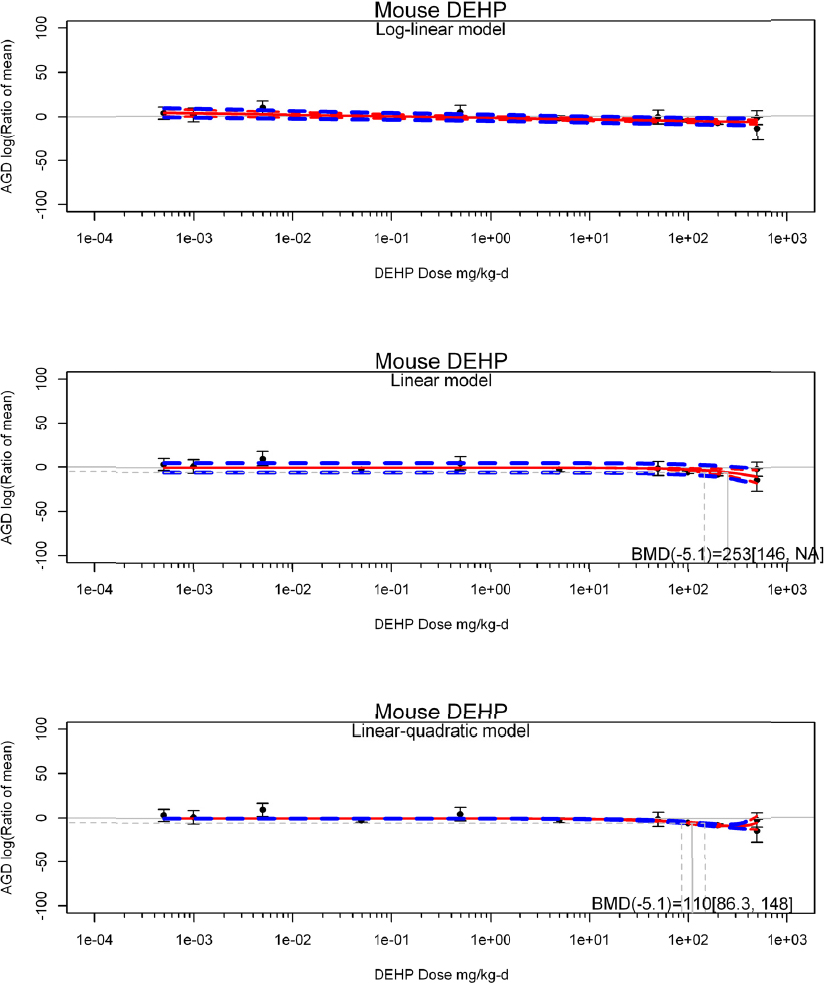
TABLE C5-5 Benchmark Dose Estimates for DEHP and AGD in Mice
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| Linear in dose100 | -5.1 | 253 | 146 | NA |
| Linear-Quadratic in dose100 | -5.1 | 110 | 86 | 148 |
META-ANALYSES OF RAT DATA ON FETAL TESTOSTERONE
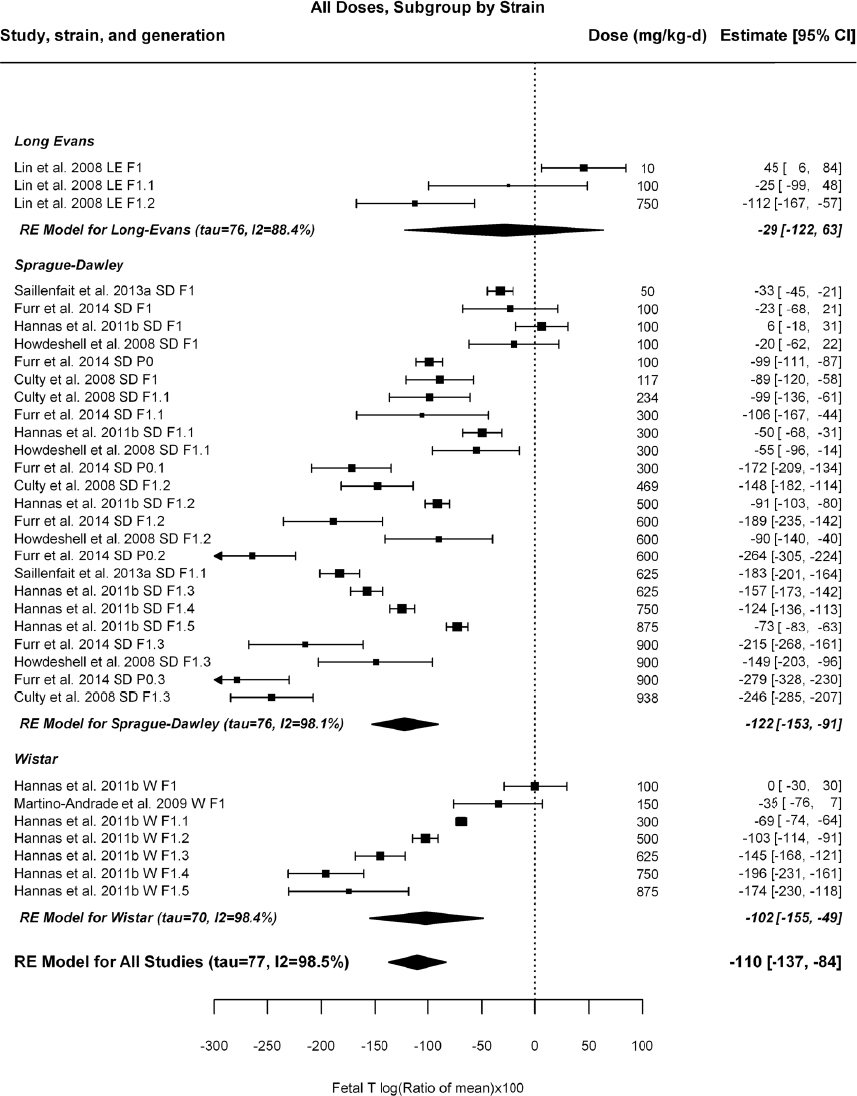
TABLE C5-6 Subgrouping Analyses of Rat Studies on DEHP and Fetal Testosterone
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Long Evans | |||||||||
| Rat DEHP LE Overall | intrcpt | -29.3 | -121.9 | 63.3 | 0.535 | 76.5 | 88.4 | 0.000 | 39.2 |
| Rat DEHP LE Trend in log10 dose | log10(dose) | -83.0 | -118.6 | -47.4 | 0.000 | 0.0 | 0.0 | 0.751 | 39.0 |
| Rat DEHP LE Linear in dose100 | dose100 | -15.0 | -26.0 | -3.9 | 0.008 | 31.8 | 62.0 | 0.061 | 36.3* |
| Sprague-Dawley | |||||||||
| Rat DEHP SD Overall | intrcpt | -121.8 | -153.1 | -90.5 | 0.000 | 76.0 | 98.1 | 0.000 | 270.6 |
| Rat DEHP SD Trend in log10 dose | log10(dose) | -141.8 | -200.9 | -82.6 | 0.000 | 53.7 | 95.9 | 0.000 | 247.2 |
| Rat DEHP SD Linear-Quadratic in dose100 | dose100 | -38.1 | -54.4 | -21.8 | 0.000 | 53.3 | 95.8 | 0.000 | 246.9* |
| I(dose100^2) | 1.9 | -0.2 | 4.0 | 0.075 | 53.3 | 95.8 | 0.000 | 246.9* | |
| Wistar | |||||||||
| Rat DEHP W Overall | intrcpt | -102.0 | -155.2 | -48.9 | 0.000 | 69.8 | 98.4 | 0.000 | 76.4 |
| Rat DEHP W Trend in log10 dose | log10(dose) | -191.7 | -246.1 | -137.3 | 0.000 | 17.2 | 77.4 | 0.010 | 75.1 |
| Rat DEHP W Linear in dose100 | dose100 | -22.3 | -23.9 | -20.7 | 0.000 | 3.8 | 21.2 | 0.203 | 57.3* |
* Indicates the lowest AICc for each strain.
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | -110.14 | -136.73 | -83.54 | 0.000 | 76.76 | 98.49 | 0.000 | 386.87 |
| Trend in log10(dose) | log10(dose) | -132.83 | -171.03 | -94.63 | 0.000 | 47.74 | 96.06 | 0.000 | 349.39 |
| Linear in dose100 | dose100 | -23.01 | -26.24 | -19.77 | 0.000 | 48.52 | 96.55 | 0.000 | 358.32 |
| Linear-Quadratic in dose100 | dose100 | -34.23 | -47.02 | -21.44 | 0.000 | 46.72 | 95.49 | 0.000 | 348.01* |
| I(dose100^2) | 1.53 | -0.16 | 3.21 | 0.076 | |||||
| Sensitivity Analyses | |||||||||
| Overall minus Culty et al. 2008 | intrcpt | -105.46 | -134.09 | -76.82 | 0.000 | 77.66 | 98.64 | 0.000 | 341.26 |
| Overall minus Howdeshell et al. 2008 | intrcpt | -114.34 | -143.48 | -85.21 | 0.000 | 79.30 | 98.72 | 0.000 | 342.30 |
| Overall minus Lin et al. 2008 | intrcpt | -117.28 | -144.05 | -90.51 | 0.000 | 73.90 | 98.49 | 0.000 | 349.80 |
| Overall minus Saillenfait et al. 2013a | intrcpt | -110.31 | -137.82 | -82.80 | 0.000 | 76.92 | 98.40 | 0.000 | 363.95 |
| Overall minus Hannas et al. 2011b | intrcpt | -117.57 | -154.20 | -80.93 | 0.000 | 84.83 | 96.74 | 0.000 | 252.17 |
| Overall minus Martino-Andrade et al. 2009 | intrcpt | -112.40 | -139.42 | -85.37 | 0.000 | 76.87 | 98.52 | 0.000 | 375.37 |
| Overall minus Furr et al. 2014 | intrcpt | -92.95 | -119.61 | -66.28 | 0.000 | 67.05 | 98.30 | 0.000 | 287.66 |
| Highest Doses-Overall | intrcpt | -162.10 | -214.75 | -109.45 | 0.000 | 77.37 | 96.29 | 0.000 | 99.26 |
| Highest Doses-Trend in log10(dose) | log10(dose) | -195.38 | -380.18 | -10.58 | 0.038 | 64.59 | 94.51 | 0.000 | 92.78 |
| Highest Doses-Linear in dose100 | dose100 | -21.03 | -26.26 | -15.79 | 0.000 | 60.17 | 93.59 | 0.000 | 95.30 |
| Highest Doses-Linear-Quadratic in dose100 | dose100 | -21.87 | -73.27 | 29.53 | 0.404 | 64.57 | 92.67 | 0.000 | 92.71 |
| I(dose100^2) | 0.10 | -5.83 | 6.02 | 0.975 | |||||
* Indicate the lowest AICc.
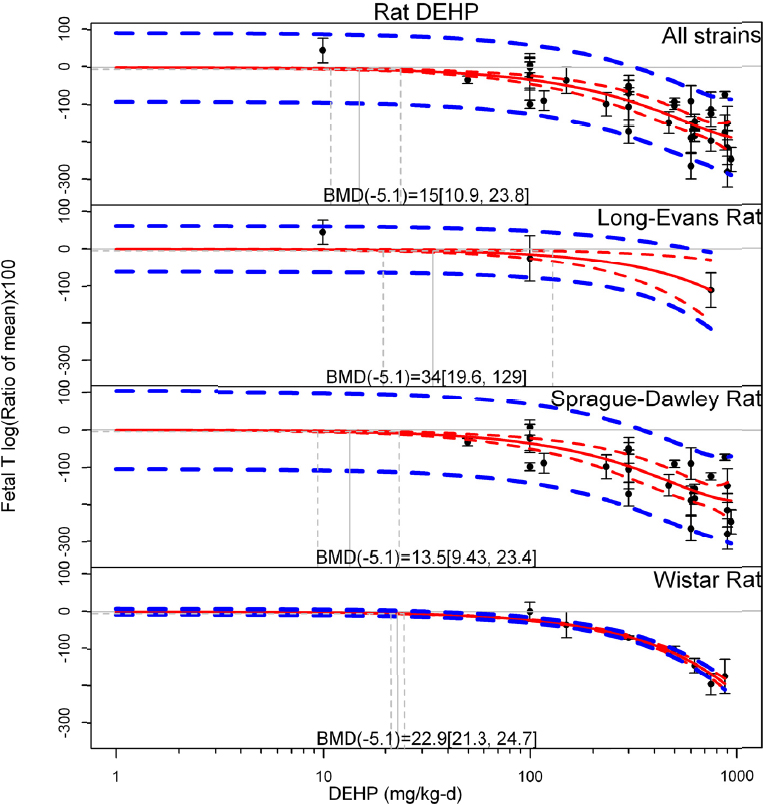
TABLE C5-8 Benchmark Dose Estimates for DEHP and Fetal Testosterone (Effect Size of 5%) in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| All strains Linear in dose100 | -5.1 | 22 | 20 | 26 |
| All strains Linear-Quadratic in dose100 | -5.1 | 15 | 11 | 24 |
| LE Linear in dose100 | -5.1 | 34 | 20 | 129 |
| SD Linear-Quadratic in dose100 | -5.1 | 13 | 9 | 23 |
| W Linear in dose100 | -5.1 | 23 | 21 | 25 |
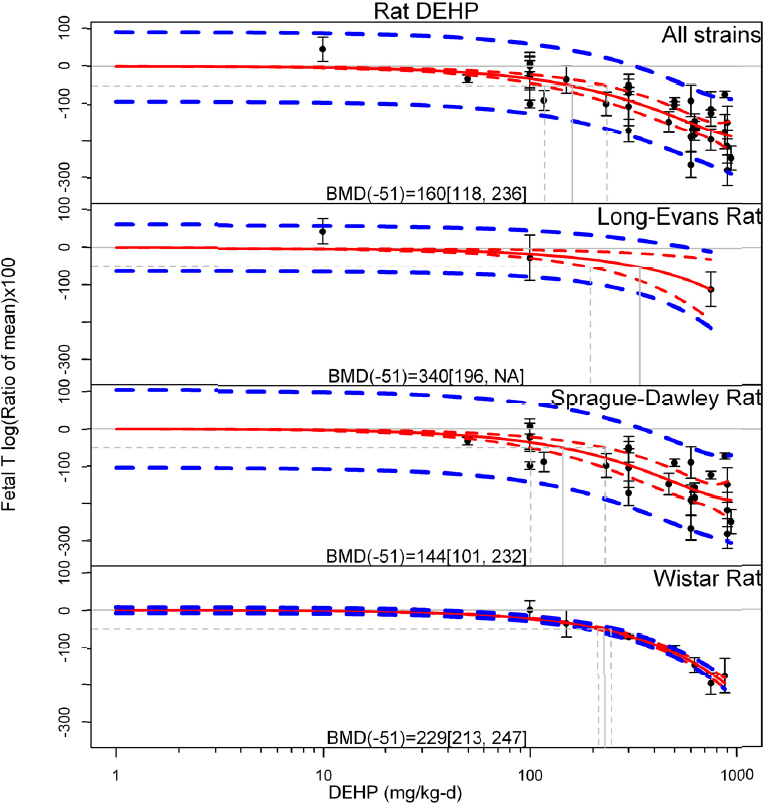
TABLE C5-9 Benchmark Dose Estimates for DEHP and Fetal Testosterone (Effect Size of 40%) in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| All strains Linear in dose100 | -51 | 222 | 195 | 258 |
| All strains Linear-Quadratic in dose100 | -51 | 161 | 118 | 236 |
| LE Linear in dose100 | -51 | 340 | 196 | NA |
| SD Linear-Quadratic in dose100 | -51 | 144 | 101 | 232 |
| W Linear in dose100 | -51 | 229 | 213 | 247 |
SECTION C-6
Meta-Analyses of Studies of Other Phthalates and AGD or Fetal Testosterone
SUPPLEMENTAL INFORMATION ABOUT METHODS
The conversion from log transformed ratio of means (ROM) to a percent change is as follows:
The effect sizes reported are
y = 100 × ROM = 100 × ln (treated response/control response).
Therefore:
Percent change = 100 × (treated − control)/control = 100 × (treated/control − 1)
= 100 × (exp(y/100) − 1)
BENZYLBUTYL PHTHALATE (BzBP)
For anogenital distance (AGD), there was a statistically significant overall effect and linear trends in log10(dose) and dose. There was substantial, statistically significant heterogeneity in all cases (I2 >75%). The statistical significance of these effects was robust to leaving out individual studies and restricting to the highest dose group from each study. The linear-quadratic model had the lowest AICc (Akaike information criterion corrected for small sample sizes), and benchmark dose estimates from this model were 252 [164, 377] for a 5% change (BMR = -5.1).
For fetal testosterone, there was also a statistically significant overall effect and linear trends in log10(dose) and dose, with an overall effect that is large in magnitude (>50% change). There was substantial, statistically significant heterogeneity in all cases (I2 >85%). There were too few studies for sensitivity analyses. The linear-quadratic model had the lowest AICc, and benchmark dose estimates from this model were 23 mg/kg-day [95% CI: 13, 74] for a 5% change (BMR = -5.1) and 230 mg/kg-day [140, 390] for a 40% change (BMR = 51).
Although there was substantial heterogeneity, standard deviation of the random effect (tau) was less than the estimated size of the effect at higher doses. Therefore, the heterogeneity does not affect the conclusion that BzBP exposure affects both AGD and fetal testosterone in the rat.
DIBUTYL PHTHALATE (DBP)
For AGD there was a statistically significant overall effect and linear trends in log10(dose) and dose. There was substantial, statistically significant heterogeneity in all cases (I2 >75%). The statistical significance of these effects was robust to leaving out individual studies and restricting to the highest dose group from each study. The linear-quadratic model had the lowest AICc, and benchmark dose estimate from this model wsd 153 mg/kg-day [95% CI: 115, 216] for a 5% change (BMR = -5.1).
TABLE C6-1 Overall Analyses and Sensitivity Analyses of Rat Studies of BzBP and AGD
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | -5.34 | -8.35 | -2.33 | 0.001 | 5.78 | 94.87 | 0.000 | 102.07 |
| Trend in log10(dose) | log10(dose) | -4.68 | -7.09 | -2.27 | 0.000 | 3.91 | 88.95 | 0.000 | 90.43 |
| Linear in dose100 | dose100 | -2.07 | -2.50 | -1.63 | 0.000 | 2.36 | 76.64 | 0.000 | 82.92 |
| Linear-Quadratic in dose100 | dose100 | -2.01 | -3.45 | -0.56 | 0.007 | 2.50 | 77.70 | 0.000 | 82.14* |
| I(dose100^2) | -0.01 | -0.24 | 0.22 | 0.927 | |||||
| Sensitivity Analyses | |||||||||
| Overall minus Ashby et al. 1997 | intrcpt | -6.00 | -8.92 | -3.08 | 0.000 | 5.36 | 93.79 | 0.000 | 94.06 |
| Overall minus Aso et al. 2005 | intrcpt | -5.41 | -9.65 | -1.16 | 0.012 | 6.68 | 97.37 | 0.000 | 66.33 |
| Overall minus Tyl et al. 2004 | intrcpt | -3.72 | -6.82 | -0.63 | 0.018 | 4.47 | 90.78 | 0.000 | 60.65 |
| Overall minus Nagao et al. 2000 | intrcpt | -5.72 | -9.38 | -2.06 | 0.002 | 6.32 | 93.59 | 0.000 | 84.97 |
| Highest Doses-Overall | intrcpt | -8.57 | -15.41 | -1.72 | 0.014 | 8.24 | 95.86 | 0.000 | 45.55 |
| Highest Doses-Linear in dose100 | dose100 | -2.02 | -2.59 | -1.45 | 0.000 | 3.15 | 79.09 | 0.000 | 38.03 |
| Highest Doses-Trend in log10(dose) | log10(dose) | -4.54 | -7.89 | -1.19 | 0.008 | 5.27 | 86.99 | 0.000 | 55.54 |
| Highest Doses-Linear-Quadratic in dose100 | dose100 | -0.82 | -3.61 | 1.98 | 0.566 | 3.17 | 78.74 | 0.001 | 52.64 |
| Highest Doses-Linear-Quadratic in dose100 | I(dose100^2) | -0.18 | -0.60 | 0.23 | 0.388 | 3.17 | 78.74 | 0.001 | 52.64 |
* Indicates the lowest AICc.
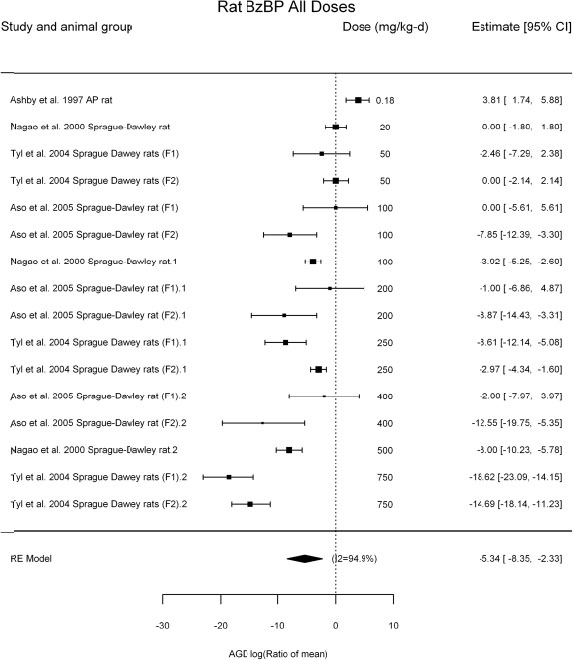
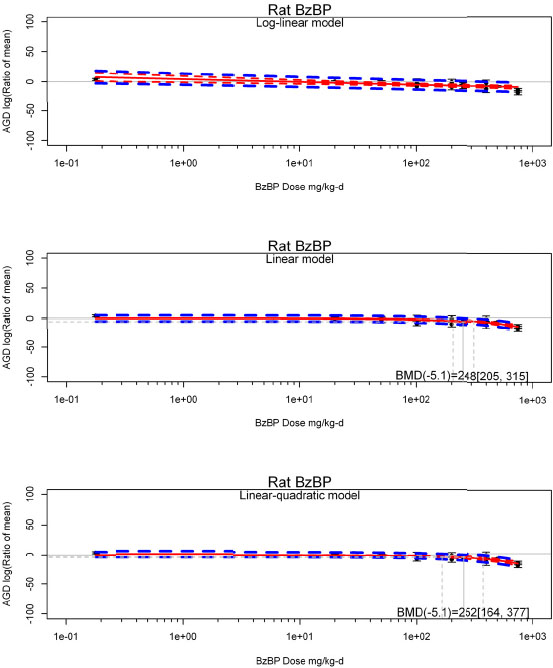
TABLE C6-2 Benchmark Dose Estimates for BzBP and AGD in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| Linear in dose100 | -5.1 | 248 | 205 | 315 |
| Linear-Quadratic in dose100 | -5.1 | 252 | 164 | 377 |
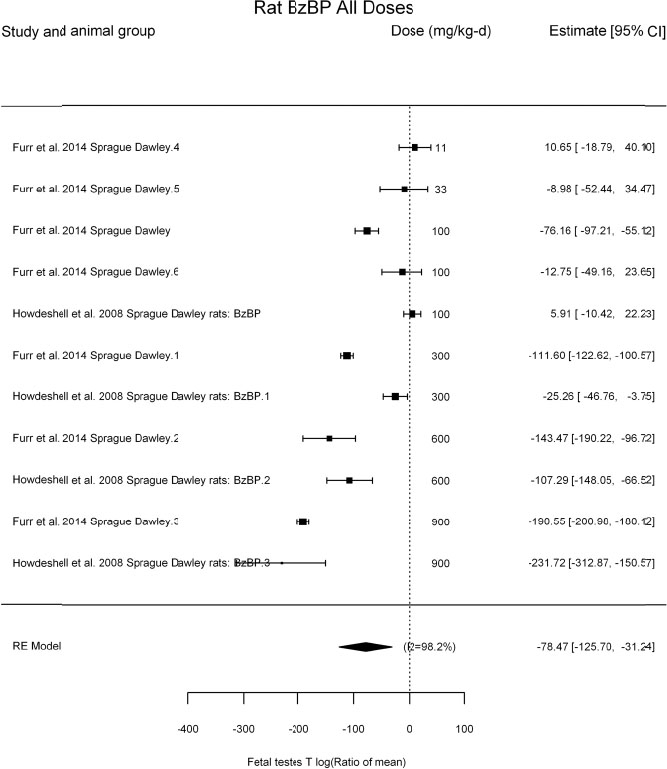
TABLE C6-3 Overall Analyses of Rat Studies of BzBP and Fetal Testosterone*
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Overall | intrcpt | -78.47 | -125.70 | -31.24 | 0.001 | 77.72 | 98.17 | 0.000 | 122.09 |
| Trend in log10(dose) | log10(dose) | -106.74 | -154.77 | -58.71 | 0.000 | 43.83 | 93.93 | 0.000 | 105.93 |
| Linear in dose100 | dose100 | -22.12 | -26.60 | -17.64 | 0.000 | 29.98 | 87.79 | 0.000 | 103.86 |
| Linear-Quadratic in dose100 | dose100 | -22.52 | -39.59 | -5.45 | 0.010 | 31.76 | 86.02 | 0.000 | 100.00** |
| I(dose100^2) | 0.05 | -2.14 | 2.24 | 0.964 |
* Too few studies for sensitivity analyses.
** Indicates the lowest AICc.
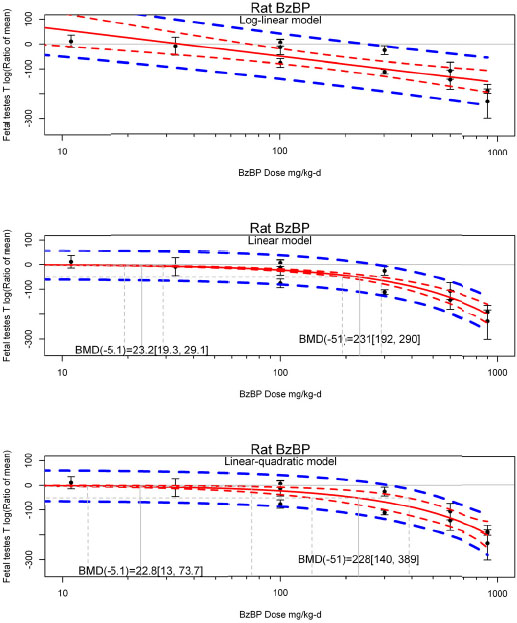
TABLE C6-4 Benchmark Dose Estimates for BzBP and Fetal Testosterone in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| Linear in dose100 | -5.1 | 23 | 19 | 29 |
| Linear in dose100 | -51.1 | 231 | 192 | 290 |
| Linear-Quadratic in dose100 | -5.1 | 23 | 13 | 74 |
| Linear-Quadratic in dose100 | -51.1 | 228 | 140 | 389 |
For fetal testosterone there was also a statistically significant overall effect and linear trends in log10(dose) and dose, with an overall effect that is large in magnitude (>50% change). There was substantial, statistically significant heterogeneity in all cases (I2 >80%). The statistical significance of these effects was robust to leaving out individual studies and restricting to the highest dose group from each study. The linear-quadratic model had the lowest AICc, and benchmark dose estimates from this model were 12 mg/kg-day [95% CI: 8, 22] for a 5% change (BMR = -5.1) and 130 mg/kg-day [95% CI: 85, 210] for a 40% change (BMR = 51).
Although there was substantial heterogeneity, standard deviation of the random effect (tau) was less than the estimated size of the effect at higher doses. Therefore, the heterogeneity does not affect the conclusion that DBP exposure affects both AGD and fetal testosterone in the rat.
TABLE C6-5 Overall Analyses and Sensitivity Analyses of Rat Studies of DBP and AGD
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | -6.88 | -8.94 | -4.83 | 0.000 | 7.84 | 89.12 | 0.000 | 500.28 |
| Trend in log10(dose) | log10(dose) | -4.14 | -5.63 | -2.65 | 0.000 | 6.38 | 84.31 | 0.000 | 471.15 |
| Linear in dose100 | dose100 | -2.42 | -2.80 | -2.04 | 0.000 | 4.95 | 76.58 | 0.000 | 449.24 |
| Linear-Quadratic in dose100 | dose100 | -3.64 | -4.85 | -2.42 | 0.000 | 4.90 | 75.57 | 0.000 | 441.75* |
| I(dose100^2) | 0.18 | 0.01 | 0.35 | 0.039 | |||||
| Sensitivity Analyses | |||||||||
| Overall minus Struve et al. 2009 | intrcpt | -6.87 | -8.94 | -4.80 | 0.000 | 7.86 | 89.45 | 0.000 | 484.30 |
| Overall minus Barlow et al. 2004 | intrcpt | -6.84 | -8.94 | -4.73 | 0.000 | 7.89 | 89.27 | 0.000 | 486.80 |
| Overall minus Li et al. 2009 | intrcpt | -6.61 | -8.64 | -4.59 | 0.000 | 7.35 | 87.25 | 0.000 | 466.34 |
| Overall minus Johnson et al. 2011 | intrcpt | -6.78 | -8.86 | -4.69 | 0.000 | 7.79 | 89.03 | 0.000 | 485.44 |
| Overall minus Mylchreest et al. 1998 | intrcpt | -6.55 | -8.62 | -4.48 | 0.000 | 7.74 | 89.20 | 0.000 | 476.04 |
| Overall minus Jiang et al. 2007 | intrcpt | -6.94 | -9.09 | -4.78 | 0.000 | 8.04 | 89.17 | 0.000 | 481.61 |
| Overall minus Mylchreest et al. 2000 | intrcpt | -7.16 | -9.37 | -4.96 | 0.000 | 8.07 | 89.27 | 0.000 | 467.83 |
| Overall minus Mylchreest et al. 1999 | intrcpt | -6.59 | -8.65 | -4.53 | 0.000 | 7.66 | 88.89 | 0.000 | 476.03 |
| Overall minus Scarano et al. 2010 | intrcpt | -6.86 | -8.93 | -4.79 | 0.000 | 7.86 | 89.30 | 0.000 | 492.79 |
| Overall minus Kim et al. 2010 | intrcpt | -7.01 | -9.07 | -4.95 | 0.000 | 7.60 | 85.07 | 0.000 | 476.65 |
| Overall minus Drake et al. 2009 | intrcpt | -6.71 | -8.81 | -4.62 | 0.000 | 7.86 | 89.26 | 0.000 | 486.40 |
| Overall minus Lee et al. 2004 | intrcpt | -7.15 | -9.21 | -5.09 | 0.000 | 7.54 | 88.37 | 0.000 | 468.14 |
| Overall minus Martino-Andrade et al. 2009 | intrcpt | -6.76 | -8.88 | -4.64 | 0.000 | 7.94 | 89.35 | 0.000 | 487.55 |
| Overall minus Wolfe and Patel 2002 | intrcpt | -9.17 | -12.36 | -5.98 | 0.000 | 9.50 | 94.35 | 0.000 | 297.19 |
| Overall minus Ema et al. 1998 | intrcpt | -6.29 | -8.23 | -4.34 | 0.000 | 7.16 | 87.54 | 0.000 | 468.32 |
| Overall minus Clewell et al. 2013 | intrcpt | -6.82 | -8.91 | -4.73 | 0.000 | 7.90 | 89.25 | 0.000 | 494.00 |
| Highest Doses-Overall | intrcpt | -16.07 | -19.41 | -12.74 | 0.000 | 6.71 | 83.07 | 0.000 | 143.78 |
| Highest Doses-Linear in dose100 | dose100 | -2.49 | -3.03 | -1.95 | 0.000 | 7.00 | 84.14 | 0.000 | 145.84 |
| Highest Doses-Trend in log10(dose) | log10(dose) | -14.44 | -28.07 | -0.81 | 0.038 | 5.99 | 79.18 | 0.000 | 136.70 |
| Highest Doses-Linear-Quadratic in dose100 | dose100 | -5.20 | -6.93 | -3.48 | 0.000 | 5.48 | 76.59 | 0.000 | 134.34 |
| I(dose100^2) | 0.37 | 0.14 | 0.60 | 0.001 | |||||
* Indicates the lowest AICc.
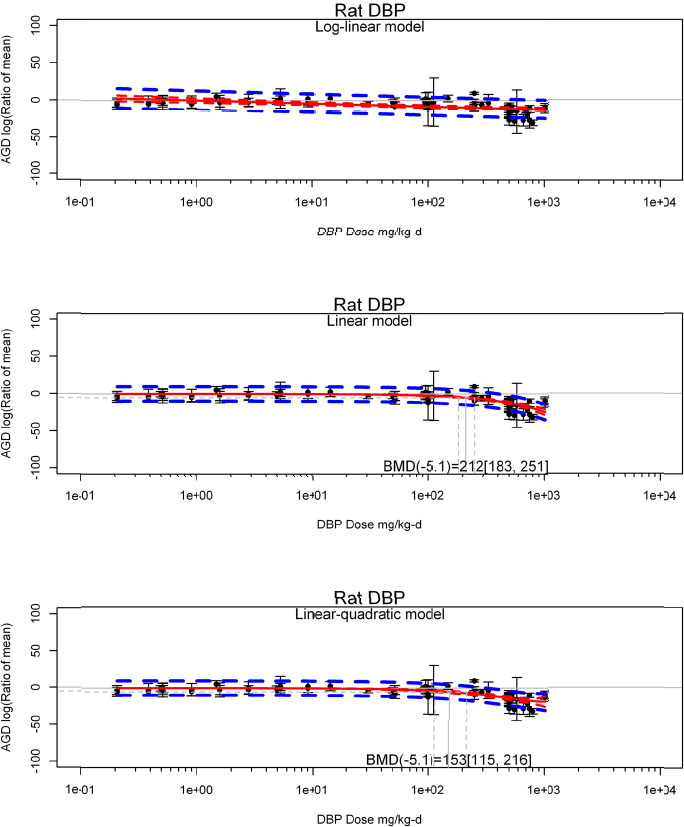
TABLE C6-6 Benchmark Dose Estimates for DBP and AGD in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| Linear in dose100 | -5.1 | 212 | 183 | 251 |
| Linear-Quadratic in dose100 | -5.1 | 153 | 115 | 216 |
TABLE C6-7 Overall Analyses and Sensitivity Analyses of Rat Studies of DBP and Fetal Testosterone
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | -56.97 | -80.64 | -33.31 | 0.000 | 59.25 | 94.78 | 0.000 | 311.44 |
| Trend in log10(dose) | log10(dose) | -53.72 | -74.64 | -32.79 | 0.000 | 39.50 | 88.01 | 0.000 | 285.61 |
| Linear in dose100 | dose100 | -29.43 | -35.83 | -23.04 | 0.000 | 35.25 | 86.12 | 0.000 | 285.72 |
| Linear-Quadratic in dose100 | dose100 | -44.98 | -66.52 | -23.43 | 0.000 | 32.90 | 83.99 | 0.000 | 277.00* |
| I(dose100^2) | 3.29 | -1.05 | 7.63 | 0.137 | |||||
| Sensitivity Analyses | |||||||||
| Overall minus Struve et al. 2009 | intrcpt | -45.23 | -67.11 | -23.35 | 0.000 | 51.20 | 93.92 | 0.000 | 254.23 |
| Overall minus Howdeshell et al. 2008 | intrcpt | -62.19 | -90.88 | -33.50 | 0.000 | 65.13 | 95.26 | 0.000 | 258.67 |
| Overall minus Johnson et al. 2007 | intrcpt | -61.63 | -87.60 | -35.66 | 0.000 | 62.18 | 95.65 | 0.000 | 279.22 |
| Overall minus Johnson et al. 2011 | intrcpt | -52.63 | -75.58 | -29.69 | 0.000 | 54.84 | 94.16 | 0.000 | 286.41 |
| Overall minus Kuhl et al. 2007 | intrcpt | -56.08 | -81.36 | -30.80 | 0.000 | 61.09 | 95.24 | 0.000 | 290.39 |
| Overall minus Martino-Andrade et al. 2009 | intrcpt | -56.61 | -82.07 | -31.15 | 0.000 | 61.58 | 95.28 | 0.000 | 290.63 |
| Overall minus Furr et al. 2014 | intrcpt | -70.79 | -101.69 | -39.89 | 0.000 | 60.74 | 91.61 | 0.000 | 201.05 |
| Highest Doses-Overall | intrcpt | -116.72 | -164.82 | -68.62 | 0.000 | 71.04 | 94.06 | 0.000 | 112.13 |
| Highest Doses-Trend in log10(dose) | log10(dose) | -160.89 | -246.19 | -75.60 | 0.000 | 37.54 | 81.20 | 0.000 | 99.44 |
| Highest Doses-Linear in dose100 | dose100 | -29.77 | -37.50 | -22.05 | 0.000 | 42.45 | 86.55 | 0.000 | 104.16 |
| Highest Doses-Linear-Quadratic in dose100 | dose100 | -49.92 | -86.82 | -13.02 | 0.008 | 37.92 | 77.06 | 0.000 | 99.67 |
| I(dose100^2) | 3.98 | -3.08 | 11.04 | 0.269 | |||||
* Indicates the lowest AICc.
TABLE C6-8 Benchmark Dose Estimates for DBP and Fetal Testosterone in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| Linear in dose100 | -5.1 | 17 | 14 | 22 |
| Linear in dose100 | -51.1 | 174 | 143 | 222 |
| Linear-Quadratic in dose100 | -5.1 | 12 | 8 | 22 |
| Linear-Quadratic in dose100 | -51.1 | 125 | 85 | 205 |
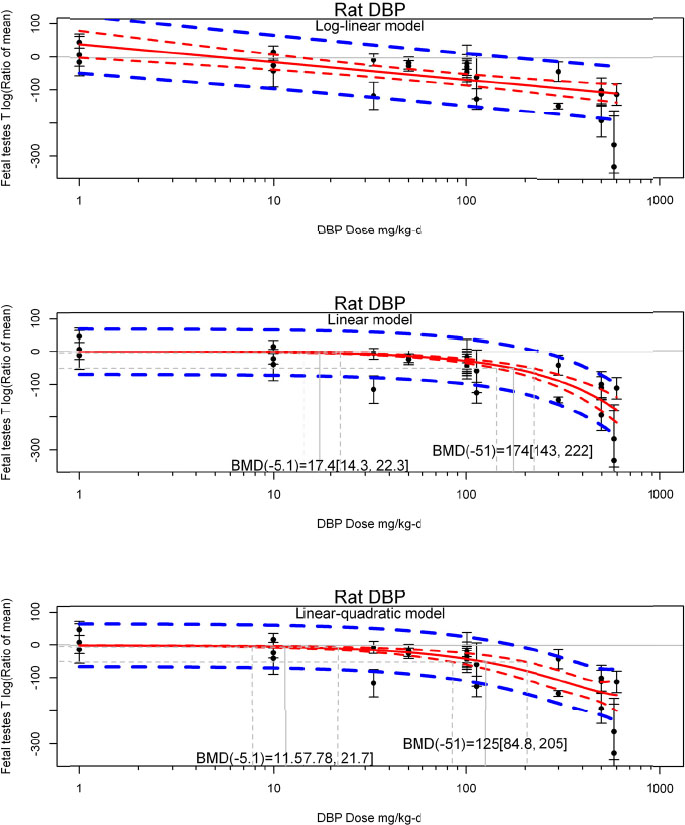
DIPENTYL PHTHALATE (DPP)
For fetal testosterone, there was also a statistically significant overall effect and linear trends in log10(dose) and dose, with an overall effect that is large in magnitude (>50% change). There was substantial, statistically significant heterogeneity in all cases (I2 >90%). The statistical significance of these effects was robust to leaving out individual studies and restricting to the highest dose group from each study. The linear-quadratic model had the lowest AICc, and benchmark dose estimates from this model were 5.6 [95% CI: 4.8, 6.4] for a 5% change (BMR = -5.1) and 58 [95% CI: 50, 70] for a 40% change (BMR = 51).
Although there was substantial heterogeneity, standard deviation of the random effect (tau) was less than the estimated size of the effect at higher doses. Therefore, the heterogeneity does not affect the conclusion that DPP exposure affects fetal testosterone in the rat.
TABLE C6-9 Overall Analyses and Sensitivity Analyses of Rat Studies of DPP and Fetal Testosterone
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | -92.57 | -120.33 | -64.81 | 0.000 | 76.25 | 98.14 | 0.000 | 351.76 |
| Trend in log10(dose) | log10(dose) | -127.64 | -152.92 | -102.36 | 0.000 | 34.29 | 90.42 | 0.000 | 300.49 |
| Linear in dose100 | dose100 | -50.24 | -60.17 | -40.30 | 0.000 | 56.12 | 96.75 | 0.000 | 334.20 |
| Linear-Quadratic in dose100 | dose100 | -93.99 | -107.96 | -80.02 | 0.000 | 32.57 | 90.61 | 0.000 | 298.59* |
| I(dose100^2) | 8.93 | 6.44 | 11.42 | 0.000 | |||||
| Sensitivity Analyses | |||||||||
| Overall minus Howdeshell et al. 2008 | intrcpt | -99.93 | -130.63 | -69.23 | 0.000 | 78.63 | 98.31 | 0.000 | 307.12 |
| Overall minus Beverly et al. 2014 | intrcpt | -92.57 | -121.32 | -63.81 | 0.000 | 77.71 | 98.07 | 0.000 | 341.24 |
| Overall minus Hannas et al. 2011a | intrcpt | -88.02 | -115.86 | -60.18 | 0.000 | 70.84 | 97.62 | 0.000 | 302.42 |
| Overall minus Furr et al. 2014 | intrcpt | -84.01 | -138.21 | -29.81 | 0.002 | 81.93 | 98.71 | 0.000 | 99.72 |
| Highest Doses-Overall | intrcpt | -173.32 | -214.05 | -132.59 | 0.000 | 58.87 | 96.97 | 0.000 | 95.02 |
| Highest Doses-Trend in log10(dose) | log10(dose) | -117.01 | -227.86 | -6.15 | 0.039 | 48.75 | 94.22 | 0.000 | 89.25 |
| Highest Doses-Linear in dose100 | dose100 | -45.89 | -61.35 | -30.43 | 0.000 | 82.45 | 98.43 | 0.000 | 100.18 |
| Highest Doses-Linear-Quadratic in dose100 | dose100 | -87.62 | -111.52 | -63.72 | 0.000 | 48.60 | 94.72 | 0.000 | 89.05 |
| I(dose100^2) | 7.96 | 3.88 | 12.05 | 0.000 | |||||
* Indicates the lowest AICc.
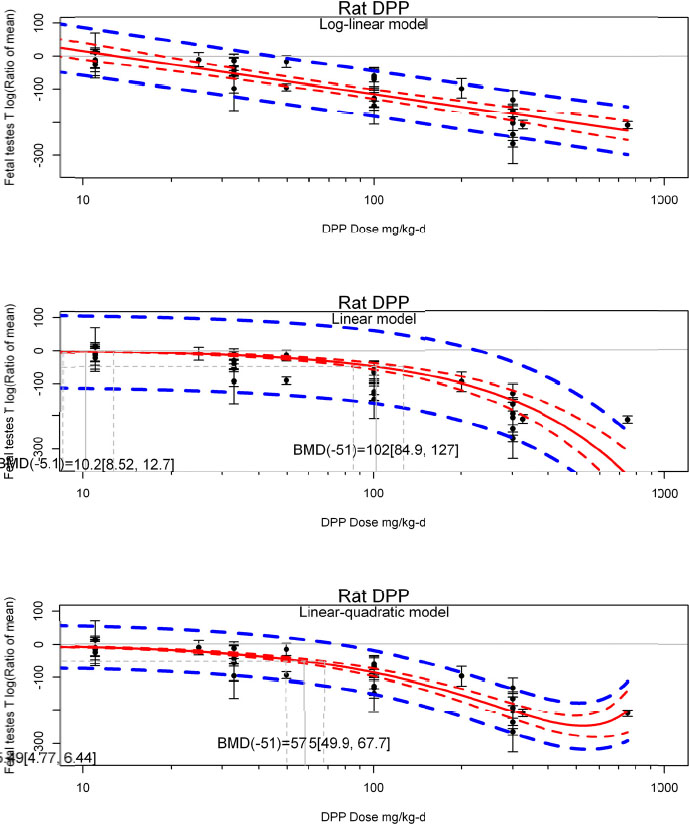
TABLE C6-10 Benchmark Dose Estimates for DPP and Fetal Testosterone in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| Linear in dose100 | -5.1 | 10 | 9 | 13 |
| Linear in dose100 | -51.1 | 102 | 85 | 127 |
| Linear-Quadratic in dose100 | -5.1 | 5.6 | 4.8 | 6.4 |
| Linear-Quadratic in dose100 | -51.1 | 58 | 50 | 68 |
DIISOBUTYL PHTHALATE (DIBP)
For fetal testosterone, there was also a statistically significant overall effect and linear trends in log10(dose) and dose, with an overall effect that is large in magnitude (>50% change). There was substantial, statistically significant heterogeneity in all cases (I2 >60%). There were too few studies to conduct sensitivity analyses. The linear model had the lowest AICc, and a benchmark dose estimate 270 [225,
340] for a 40% change (BMR = 51). (The 5% change was well below the range of the data but will be 10-times lower because a linear model was used.)
Although there was substantial heterogeneity, standard deviation of the random effect (tau) was less than the estimated size of the effect at higher doses. Therefore, the heterogeneity does not affect the conclusion that DIBP exposure affects fetal testosterone in the rat.
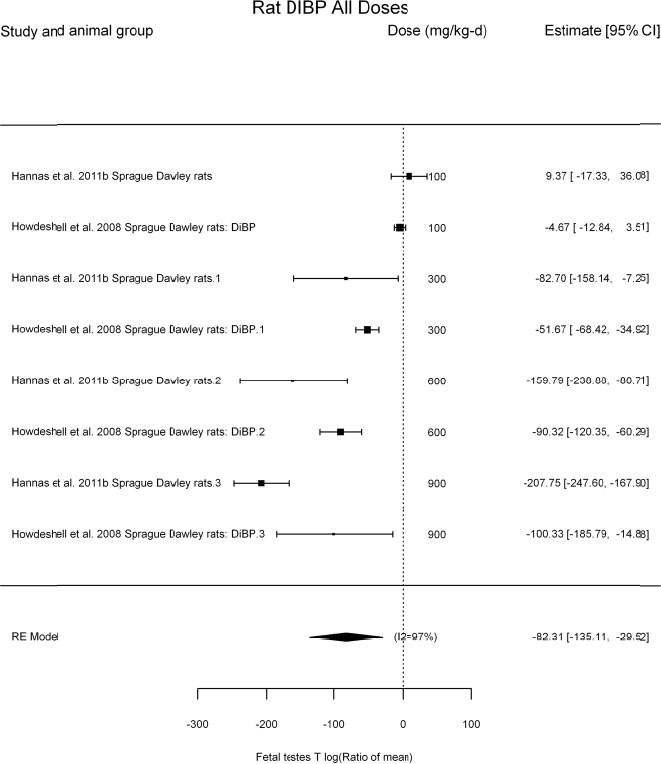
TABLE C6-11 Overall Analyses of Rat Studies of DIBP and Fetal Testosterone*
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Overall | intrcpt | -82.31 | -135.11 | -29.52 | 0.002 | 71.76 | 96.96 | 0.000 | 87.28 |
| Trend in log10(dose) | log10(dose) | -169.23 | -234.13 | -104.33 | 0.000 | 28.14 | 77.83 | 0.001 | 78.52 |
| Linear in dose100 | dose100 | -18.84 | -22.73 | -14.94 | 0.000 | 18.64 | 78.78 | 0.001 | 75.51** |
| Linear-Quadratic in dose100 | dose100 | -11.61 | -22.13 | -1.08 | 0.031 | 12.22 | 57.12 | 0.020 | 77.04 |
| I(dose100^2) | -1.00 | -2.42 | 0.42 | 0.169 |
* Too few studies for sensitivity analyses.
** Indicates the lowest AICc.
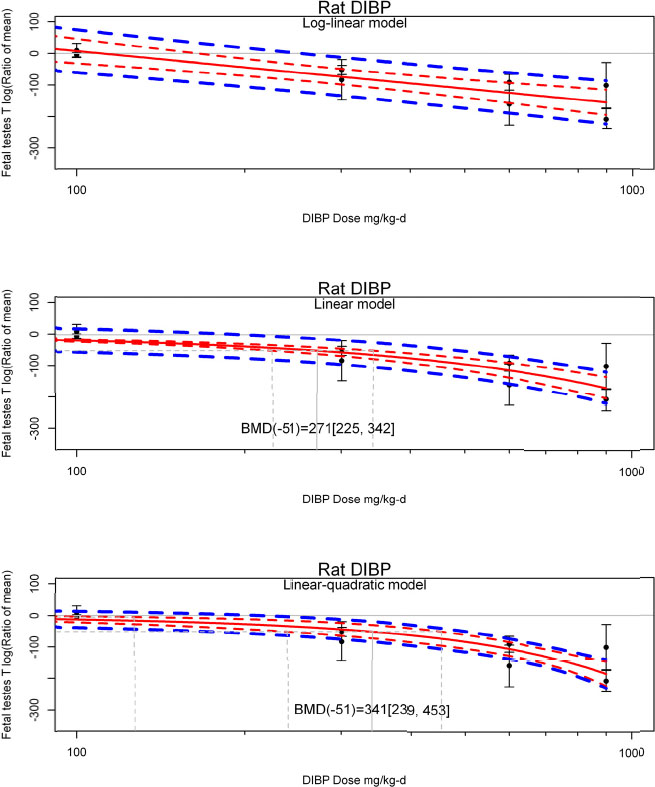
TABLE C6-12 Benchmark Dose Estimates for DIBP and Fetal Testosterone in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| Linear in dose100 | -5.1 | 27 | 23 | 34 |
| Linear in dose100 | -51.1 | 271 | 225 | 342 |
| Linear-Quadratic in dose100 | -5.1 | 43 | 23 | 127 |
| Linear-Quadratic in dose100 | -51.1 | 341 | 239 | 453 |
DIISONONYL PHTHALATE (DINP)
For AGD, there was no statistically significant overall effect, nor were there any statistically significant trends in log10(dose) or dose. There was very little heterogeneity in all cases (I2 <5%). The lack of statistical significance of these effects was robust to leaving out individual studies and restricting to the highest dose group from each study. The linear model had the lowest AICc, and due to the lack of statistically significant trend, the upper confidence limit on the benchmark was unbounded, and only a lower confidence bound of 684 could be derived for a 5% change (BMR = -5.1). In sum, although a small effect
was observed, the precision of the estimate was not sufficient to rule out chance. Thus, the available studies do not support DINP exposure being associated with decreased AGD.
By contrast, for fetal testosterone, there was a statistically significant overall effect and linear trends in log10(dose) and dose, with an overall effect that is large in magnitude (>50% change). There was substantial heterogeneity in the overall effect (I2 = 83%), but this was reduced when the effect of dose was included. I2 = 42% for trend in log10(dose) and I2 of 22-24% under a linear or linear-quadratic model, neither of which was statistically significant. There were too few studies to conduct sensitivity analyses. The linear-quadratic model had the lowest AICc, and benchmark dose estimates from this model were 76 mg/kg-day [95% CI: 49, 145] for a 5% change (BMR = -5.1) and 701 mg/kg-day [94% CI: 552, 847] for a 40% change (BMR = 51).
Although there was substantial heterogeneity, standard deviation of the random effect (tau) was less than the estimated size of the effect at higher doses. Therefore, the heterogeneity does not affect the conclusion that DINP exposure affects both AGD and fetal testosterone in the rat.
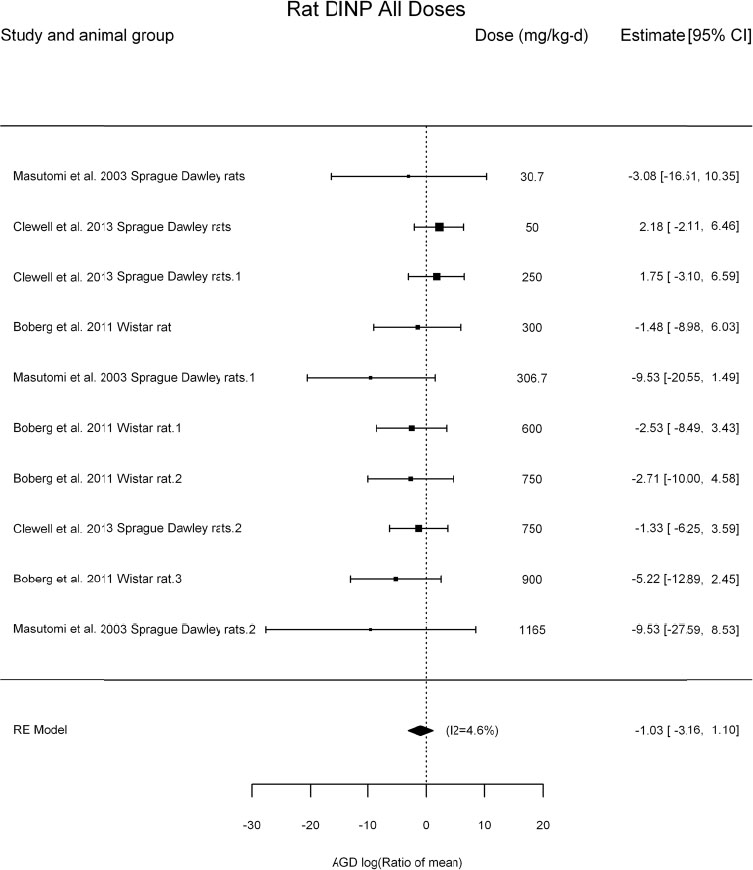
TABLE C6-13 Overall Analyses and Sensitivity Analyses of Rat Studies of DINP and AGD
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Primary Analyses | |||||||||
| Overall | intrcpt | -1.03 | -3.16 | 1.10 | 0.345 | 0.75 | 4.63 | 0.508 | 55.61 |
| Trend in log10(dose) | log10(dose) | -3.86 | -8.11 | 0.39 | 0.075 | 0.00 | 0.00 | 0.748 | 54.13 |
| Linear in dose100 | dose100 | -0.36 | -0.75 | 0.02 | 0.065 | 0.00 | 0.00 | 0.773 | 52.71* |
| Linear-Quadratic in dose100 | dose100 | 0.22 | -1.33 | 1.78 | 0.778 | 0.00 | 0.00 | 0.749 | 53.03 |
| I(dose100^2) | -0.08 | -0.28 | 0.12 | 0.444 | |||||
| Sensitivity Analyses | |||||||||
| Overall minus Boberg et al. 2011 | intrcpt | 0.09 | -2.45 | 2.62 | 0.947 | 0.00 | 0.00 | 0.315 | 39.75 |
| Overall minus Masutomi et al. 2003 | intrcpt | -0.45 | -2.58 | 1.68 | 0.679 | 0.00 | 0.00 | 0.576 | 37.15 |
| Overall minus Clewell et al. 2013 | intrcpt | -3.67 | -6.86 | -0.49 | 0.024 | 0.00 | 0.00 | 0.901 | 40.31 |
| Highest Doses-Overall | intrcpt | -2.82 | -6.85 | 1.22 | 0.171 | 0.00 | 0.00 | 0.533 | 27.40 |
| Highest Doses-Linear in dose100 | dose100 | -0.38 | -0.87 | 0.12 | 0.134 | 0.00 | 0.00 | 0.642 | 26.72 |
| Highest Doses-Trend in log10(dose) | log10(dose) | -45.42 | -125.00 | 34.16 | 0.263 | 0.00 | 0.00 | 0.930 | 34.90 |
| Highest Doses-Linear-Quadratic in dose100 | dose100 | 1.09 | -2.18 | 4.37 | 0.513 | 0.00 | 0.00 | 0.761 | 34.66 |
| I(dose100^2) | -0.17 | -0.56 | 0.21 | 0.373 | |||||
* Indicates the lowest AICc.
TABLE C6-14 Benchmark Dose Estimates for DINP and AGD in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| Linear in dose100 | -5.1 | NA | 684 | NA |
| Linear-Quadratic in dose100 | -5.1 | NA | 706 | NA |
TABLE C6-15 Overall Analyses of Rat Studies of DINP and Fetal Testosterone*
| Analysis | Estimate | Beta | CI, Lower Bound | CI, Upper Bound | P value | tau | I2 | P value for Heterogeneity | AICc |
|---|---|---|---|---|---|---|---|---|---|
| Overall | intrcpt | -63.95 | -86.35 | -41.55 | 0.000 | 31.16 | 83.28 | 0.000 | 118.16 |
| Trend in log10(dose) | log10(dose) | -128.35 | -186.46 | -70.24 | 0.000 | 12.59 | 42.01 | 0.076 | 106.24 |
| Linear in dose100 | dose100 | -7.56 | -8.69 | -6.43 | 0.000 | 7.21 | 21.84 | 0.215 | 107.77 |
| Linear-Quadratic in dose100 | dose100 | -6.74 | -10.51 | -2.96 | 0.000 | 8.04 | 23.81 | 0.182 | 104.59** |
| I(dose100^2) | -0.08 | -0.42 | 0.26 | 0.648 |
* Too few studies for sensitivity analyses.
** Indicates the lowest AICc.
TABLE C6-16 Benchmark Dose Estimates for DINP and Fetal Testosterone in Rats
| Analysis | BMR | BMD | CI, Lower Bound | CI, Upper Bound |
|---|---|---|---|---|
| Linear in dose100 | -5.1 | 68 | 59 | 80 |
| Linear in dose100 | -51.1 | 676 | 588 | 795 |
| Linear-Quadratic in dose100 | -5.1 | 76 | 49 | 145 |
| Linear-Quadratic in dose100 | -51.1 | 701 | 552 | 847 |
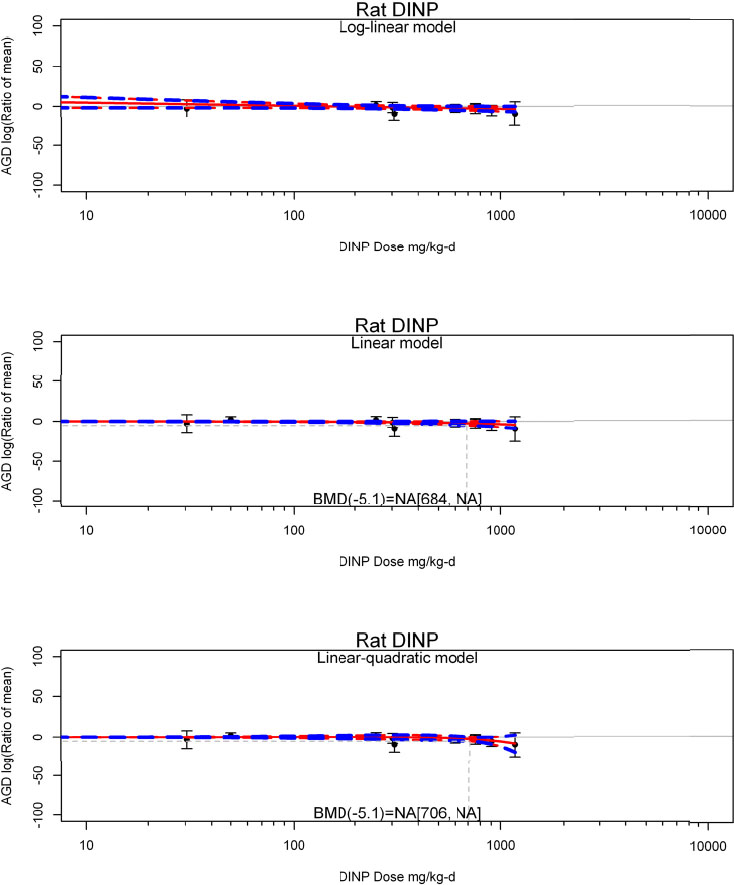
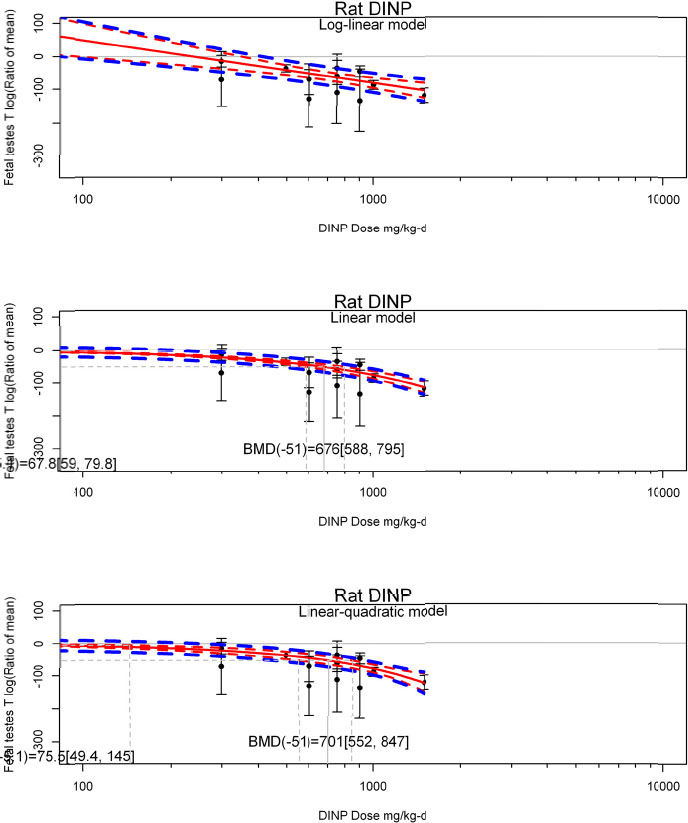
REFERENCES
Adamsson, A., V. Salonen, J. Paranko, and J. Toppari. 2009. Effects of maternal exposure to di-isononylphthalate (DINP) and 1, 1-dichloro-2,2-bis(p-chlorophenyl)ethylene (p,p'-DDE) on steroidogenesis in the fetal rat testis and adrenal gland. Reprod. Toxicol. 28(1):66-74.
Ahmad, R., A.K. Gautam, Y. Verma, S. Sedha, and S. Kumar. 2014. Effects of in utero di-butyl phthalate and butyl benzyl phthalate exposure on offspring development and male reproduction of rat. Environ. Sci. Pollut. Res. Int. 21(4):3156-3165.
Andrade, A.J., S.W. Grande, C.E. Talsness, K. Grote, A. Golombiewski, A. Sterner-Kock, and I. Chahoud. 2006. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): Effects on androgenic status, developmental landmarks and testicular histology in male offspring rats. Toxicology 225(1):64-74.
Borch, J., O. Ladefoged, U. Hass, and A.M. Vinggaard. 2004. Steroidogenesis in fetal male rats is reduced by DEHP and DINP, but endocrine effects of DEHP are not modulated by DEHA in fetal, prepubertal and adult male rats. Reprod. Toxicol. 18(1):53-61.
Borch, J., S.B. Metzdorff, A.M. Vinggaard, L. Brokken, and M. Dalgaard. 2006. Mechanisms underlying the antiandrogenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology 223(1-2):144-155.
Christiansen, S., M. Scholze, M. Dalgaard, A.M. Vinggaard, M. Axelstad, A. Kortenkamp, and U. Hass. 2009. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ. Health Perspect. 117(12):1839-1846.
Christiansen, S., J. Boberg, M. Axelstad, M. Dalgaard, A.M. Vinggaard, S.B. Metzdorff, and U. Hass. 2010. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod. Toxicol. 30(2):313-321.
Clewell, R.A., J.J. Kremer, C.C. Williams, J.L. Campbell, M.A. Sochaski, M.E. Andersen, and S.J. Borghoff. 2009. Kinetics of selected di-n-butyl phthalate metabolites and fetal testosterone following repeated and single administration in pregnant rats. Toxicology 255(1-2):80-90.
Culty, M., R. Thuillier, W. Li, Y. Wang, D.B. Martinez-Arguelles, C.G. Benjamin, K.M. Triantafilou, B.R. Zirkin, and V. Papadopoulos. 2008. In utero exposure to di-(2-ethylhexyl) phthalate exerts both short-term and long-lasting suppressive effects on testosterone production in the rat. Biol. Reprod. 78(6):1018-1028.
Do, R.P., R.W. Stahlhut, D. Ponzi, F.S. Vom Saal, and J.A. Taylor. 2012. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod. Toxicol. 34(4):614-621.
Ema, M., and E. Miyawaki. 2002. Effects on development of the reproductive system in male offspring of rats given butyl benzyl phthalate during late pregnancy. Reprod. Toxicol. 16(1):71-76.
Ema, M., E. Miyawaki, and K. Kawashima. 2000. Critical period for adverse effects on development of reproductive system in male offspring of rats given di-n-butyl phthalate during late pregnancy. Toxicol. Lett. 111(3):271-278.
Fujii, S., K. Yabe, M. Furukawa, M. Hirata, M. Kiguchi, and T. Ikka. 2005. A two-generation reproductive toxicity study of diethyl phthalate (DEP) in rats. J. Toxicol. Sci. 30(Spec.):97-116.
Giribabu, N., S.B. Sainath, and P.S. Reddy. 2014. Prenatal di-n-butyl phthalate exposure alters reproductive functions at adulthood in male rats. Environ. Toxicol. 29(5):534-544.
Gray, L.E., Jr., N.J. Barlow, K.L. Howdeshell, J.S. Ostby, J.R. Furr, and C.L. Gray. 2009. Transgenerational effects of di(2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: Added value of assessing multiple offspring per litter. Toxicol. Sci. 110(2):411-425.
Hannas, B.R., C.S. Lambright, J. Furr, N. Evans, P.M.D. Foster, E.L. Gray, and V.S. Wilson. 2012. Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: A targeted RT-PCR array approach for defining relative potency. Toxicol. Sci. 125(2):544-557.
Jarfelt, K., M. Dalgaard, U. Hass, J. Borch, H. Jacobsen, and O. Ladefoged. 2005. Antiandrogenic effects in male rats perinatally exposed to a mixture of di(2-ethylhexyl) phthalate and di(2-ethylhexyl) adipate. Reprod. Toxicol. 19(4):505-515.
Jones, S., A. Boisvert, S. Francois, L. Zhang, and M. Culty. 2015. In utero exposure to di-(2-ethylhexyl) phthalate induces testicular effects in neonatal rats that are antagonized by genistein cotreatment. Biol. Reprod. 93(4):92.
Klinefelter, G.R., J.W. Laskey, W.M. Winnik, J.D. Suarez, N.L. Roberts, L.F. Strader, B.W. Riffle, and D.N. Veeramachaneni. 2012. Novel molecular targets associated with testicular dysgenesis induced by gestational exposure to diethylhexyl phthalate in the rat: A role for estradiol. Reproduction 144(6):747-761.
Lehmann, K.P., S. Phillips, M. Sar, P.M. Foster, and K.W. Gaido. 2004. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di(n-butyl) phthalate. Toxicol. Sci. 81(1):60-68.
Li, L., T. Bu, H. Su, Z. Chen, Y. Liang, G. Zhang, D. Zhu, Y. Shan, R. Xu, Y. Hu, J. Li, G. Hu, Q. Lian, and R.S. Ge. 2015. In utero exposure to diisononyl phthalate caused testicular dysgenesis of rat fetal testis. Toxicol. Lett. 232(2):466-474.
Li, M., L. Qiu, Y. Zhang, Y. Hua, S. Tu, Y. He, S. Wen, Q. Wang, and G. Wei. 2013. Dose-related effect by maternal exposure to di-(2-ethylhexyl) phthalate plasticizer on inducing hypospadiac male rats. Environ. Toxicol. Pharmacol. 35(1):55-60.
Li, N., X. Chen, X. Zhou, W. Zhang, J. Yuan, and J. Feng. 2015. The mechanism underlying dibutyl phthalate induced shortened anogenital distance and hypospadias in rats. J. Pediatr. Surg. 50(12):2078-2083.
Lin, H., R.S. Ge, G.R. Chen, G.X. Hu, L. Dong, Q.Q. Lian, D.O. Hardy, C.M. Sottas, X.K. Li, and M.P. Hardy. 2008. Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc. Natl. Acad. Sci. U.S.A. 105(20):7218-7222.
Lin, H., Q.Q. Lian, G.X. Hu, Y. Jin, Y. Zhang, D.O. Hardy, G.R. Chen, Z.Q. Lu, C.M. Sottas, M.P. Hardy, and R.S. Ge. 2009. In utero and lactational exposures to diethylhexyl-phthalate affect two populations of Leydig cells in male Long-Evans rats. Biol. Reprod. 80(5):882-888.
Liu, X., D.W. He, D.Y. Zhang, T. Lin, and G.H. Wei. 2008. Di(2-ethylhexyl) phthalate (DEHP) increases transforming growth factor-beta1 expression in fetal mouse genital tubercles. J. Toxicol. Environ. Health A 71(19):1289-1294.
MacLeod, D.J., R.M. Sharpe, M. Welsh, M. Fisken, H.M. Scott, G.R. Hutchison, A.J. Drake, and S. van den Driesche. 2010. Androgen action in the masculinization programming window and development of male reproductive organs. Int. J. Androl. 33(2):279-287.
Mahood, I.K., H.M. Scott, R. Brown, N. Hallmark, M. Walker, and R.M. Sharpe. 2007. In utero exposure to di(nbutyl) phthalate and testicular dysgenesis: Comparison of fetal and adult end points and their dose sensitivity. Environ. Health Perspect. 115(Suppl. 1):55-61.
McKinnell, C., R.T. Mitchell, M. Walker, K. Morris, C.J.H. Kelnar, W.H. Wallace, and R.M. Sharpe. 2009. Effect of fetal or neonatal exposure to monobutyl phthalate (MBP) on testicular development and function in the marmoset. Human Reprod. 24(9):2244-2254.
Moore, R.W., T.A. Rudy, T.M. Lin, K. Ko, and R.E. Peterson. 2001. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer di(2-ethylhexyl) phthalate. Environ. Health Perspect. 109(3):229-237.
NTP (National Toxicology Program). 2015. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. Office of Health Assessment and Translation, Division, National Toxicology Program, National Institute of Environmental Health Sciences. January 9, 2015 [online]. Available: http://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf [accessed September 21, 2015].
Pocar, P., N. Fiandanese, C. Secchi, A. Berrini, B. Fischer, J.S. Schmidt, K. Schaedlich, and V. Borromeo. 2012. Exposure to di(2-ethyl-hexyl) phthalate (DEHP) in utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology 153(2):937-948.
Saillenfait, A.M., J.P. Sabate, and F. Gallissot. 2008. Diisobutyl phthalate impairs the androgen-dependent reproductive development of the male rat. Reprod. Toxicol. 26(2):107-115.
Saillenfait, A.M., J.P. Sabate, and F. Gallissot. 2009. Effects of in utero exposure to di-n-hexyl phthalate on the reproductive development of the male rat. Reprod. Toxicol. 28(4):468-476.
Saillenfait, A.M., A.C. Roudot, F. Gallissot, and J.P. Sabate. 2011. Prenatal developmental toxicity studies on di-nheptyl and di-n-octyl phthalates in Sprague-Dawley rats. Reprod. Toxicol. 32(3):268-276.
Saillenfait, A.M., J.P. Sabate, A. Robert, B. Cossec, A.C. Roudot, F. Denis, and M. Burgart. 2013a. Adverse effects of diisooctyl phthalate on the male rat reproductive development following prenatal exposure. Reprod. Toxicol. 42:192-202.
Saillenfait, A.M., J.P. Sabate, A. Robert, V. Rouiller-Fabre, A.C. Roudot, D. Moison, and F. Denis. 2013b. Dose-dependent alterations in gene expression and testosterone production in fetal rat testis after exposure to di-n-hexyl phthalate. J. Appl. Toxicol. 33(9):1027-1035.
van den Driesche, S., P. Kolovos, S. Platts, A.J. Drake, and R.M. Sharpe. 2012. Inter-relationship between testicular dysgenesis and Leydig cell function in the masculinization programming window in the rat. PLOS One 7(1):e30111.
Vo, T.T., E.M. Jung, V.H. Dang, K. Jung, J. Baek, K.C. Choi, and E.B. Jeung. 2009. Differential effects of flutamide and di-(2-ethylhexyl) phthalate on male reproductive organs in a rat model. J. Reprod. Dev. 55(4):400-411.
Wolfe, G.W., and K.A. Layton. 2005. Diethylhexylphthalate: Multigenerational Reproductive Assessment by Continuous Breeding when Administered to Sprague-Dawley Rats in the Diet. TRC Study No. 7244-200. NTPRACB 98-004. TherImmune Research Corporation, Gaithersburg, MD.
Zhang, L.D., Q. Deng, Z.M. Wang, M. Gao, L. Wang, T. Chong, and H.C. Li. 2013. Disruption of reproductive development in male rat offspring following gestational and lactational exposure to di-(2-ethylhexyl) phthalate and genistein. Biol. Res. 46(2):139-146.
Zhang, Y., X. Jiang, and B. Chen. 2004. Reproductive and developmental toxicity in F1 Sprague-Dawley male rats exposed to di-n-butyl phthalate in utero and during lactation and determination of its NOAEL. Reprod. Toxicol. 18(5):669-676.