2
COMMUNICATING WITH THE PUBLIC ABOUT INTERVENTIONS TO PREVENT COGNITIVE DECLINE AND DEMENTIA
I deally, communications with the public about interventions to delay or slow age-related cognitive decline (ARCD) and prevent, delay, or slow mild cognitive impairment (MCI) and clinical Alzheimer’s-type dementia (CATD) would be informed primarily by evidence from randomized controlled trials (RCTs). However, the Agency for Healthcare Research and Quality (AHRQ) systematic review found—at best—modest RCT evidence for only a few classes of interventions (Kane et al., 2017). Accordingly, it is challenging to specify which intervention domains, if any, are supported by sufficient evidence to justify a public health messaging campaign. To supplement the AHRQ systematic review, the committee considered evidence from relevant studies using other methodologies, including observational studies1 and neurobiological studies that support the biological plausibility of the effectiveness of a class of interventions; information from studies of risk factors; information about intervention effects on intermediate outcomes (e.g., changes in brain structure and function) that may predict cognitive decline and dementia; knowledge about whether and how an intervention would benefit or harm other organ systems; and information about other general harms and costs potentially associated with an intervention (see Chapter 1).
Based on this body of evidence, the committee identified three classes of interventions—cognitive training, blood pressure management for people
___________________
1 The committee uses this term to refer to a study that did not employ random assignment to an intervention. Except where indicated, the committee relied on observational data from longitudinal population-based cohort studies.
with hypertension, and increased physical activity—as being supported by modest but inconclusive evidence at present. This strength of evidence does not, in the committee’s judgment, warrant aggressive public health campaigns but does suggest information that should be made available to the interested public.
The strength of evidence differs for the three interventions. Cognitive training is suggested by the findings of the AHRQ systematic review, bolstered by data from observational studies of cognitively stimulating activities. The suggestion that blood pressure management and increased physical activity be included is not based primarily on evidence from the AHRQ systematic review. For cases in which strong experimental evidence does not exist, Bradford Hill proposed criteria2 for evaluating whether credible causal inferences can be drawn when epidemiologic evidence suggests an association (Hill, 1965; Lucas and McMichael, 2005). Acknowledging that application of these criteria is subjective, the committee found them to be a useful tool for examining the body of evidence from non-RCT data when results from experimental studies were mixed. In the committee’s judgment, when the Bradford Hill criteria are applied to blood pressure management and physical activity, there is sufficient evidence from observational studies and neurobiological understanding to include these interventions in communications with the public. Given the moderate-strength RCT evidence for cognitive training, the committee did not apply the Bradford Hill criteria to this intervention domain. The importance of further research on these interventions is discussed in Chapter 4.
Before proceeding to discuss each of the three interventions in turn, it is important to consider the challenges of making decisions about public health messaging, particularly in a domain so difficult to study and characterized by evidence that is modest at best.
First, different criteria and methodologies may be appropriate for different purposes. The AHRQ methodology is designed to support the work of the U.S. Preventive Services Task Force (USPSTF)—which informs health coverage determinations—and may not be optimally suited to guiding public health messaging on interventions for preventing cognitive decline and dementia or to informing agencies about prioritizing future research topics. Moreover, messaging aimed at encouraging people to adopt an intervention with compelling evidence would be stronger and would use different tactics
___________________
2 The Bradford Hill criteria, described in more detail in the sections of this chapter on blood pressure management and physical activity, include strength of association, consistency, specificity, temporality, biological gradient, plausibility, coherence, experiment, and analogy (Hill, 1965). Analogy is not relevant for the exercises described in this chapter, and experimental data are discussed in the context of the findings from the AHRQ systematic review. Thus, these two criteria are not discussed in the sections on assessing interventions against the Bradford Hill criteria.
relative to the messaging that public health practitioners and health care providers might use to inform individuals interested in taking action. The latter efforts, for example, might be focused more on improving scientific literacy (e.g., understanding of differences between association and causation) and public awareness to inform decision making among consumers and patients.
Second, it is important to emphasize that very little evidence exists about effectiveness in particular populations, such as underrepresented groups, people with a family history of or genetic susceptibility to dementia, and other high-risk groups. This issue may need to be considered in communications with the public about the state of the science and the need for individuals from such groups to participate in future research. Moreover, individual responses to these interventions will vary. These issues are discussed further in Chapter 3.
Third, in keeping with its statement of task (see Box 1-2 in Chapter 1), this study excludes some causes of dementia (e.g., stroke, traumatic brain injury) and therefore does not examine the behaviors that would potentially address these causes. It is widely recognized that CATD, especially late in life, often has mixed findings, including evidence of cerebrovascular disease, as well as neurodegenerative changes. When developing public health messaging, however, it will be important to remember that the public will not make fine distinctions based on specific causes and will want a sense of what could work to prevent any type of dementia.
Lastly, this report represents a snapshot of the state of the science in early 2017, but more data are constantly becoming available. Thus, it will be necessary to continually reassess whether new public health messages should be created or existing communication efforts should be revised.
COGNITIVE TRAINING
In the context of this report, the term cognitive training is used to denote a broad set of interventions, including those aimed at enhancing reasoning (e.g., problem solving), memory, and speed of processing (e.g., speed of identifying visual information on a screen). Such structured training exercises may or may not be computer based. Cognitively stimulating activities, for the purposes of this report, include such interventions as learning a new language and increasing proficiency in daily activities, such as playing bridge and doing crossword puzzles.
Cognitive training has engendered considerable interest and debate in both the academic and commercial sectors, particularly within the past 15 years (Simons et al., 2016). Recently, different groups have publicly released statements with conflicting conclusions on the benefits of cognitive training (Cognitive Training Data, 2015; Stanford Center on Longevity, 2014),
which may stem in part from differing opinions on what constitutes success. There is good evidence to show that cognitive training can improve performance on a trained task, at least in the short term (Simons et al., 2016), but debate has centered on the evidence for long-term benefits and whether training in one domain (e.g., processing speed) yields benefits in others (e.g., memory, reasoning) and can translate to maintaining independence in instrumental activities of daily living (IADLs), such as remembering to take medications and driving.
Findings from the AHRQ Systematic Review
Summary of the AHRQ Systematic Review Findings
The AHRQ systematic review identified 38 RCTs of cognitive training interventions, 11 of which were found to have sufficiently low risk of bias to be included in the analysis. The findings from the AHRQ systematic review on these cognitive training interventions are presented in Box 2-1.
The AHRQ systematic review found no evidence to indicate that cognitive training can reduce the risk of CATD in individuals with normal cognition or those with MCI (Kane et al., 2017). The strongest evidence supporting the potential for cognitive training interventions to delay or slow ARCD was generated by the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial—a long-duration study (10 years) with a large sample size (N = 2,802) and a notable level of diversity (25 percent minority participants). This study showed long-term impacts on reasoning, speed of processing, and maintenance of independence in IADLs in older adults (aged 65 and older) with normal cognition at enrollment (Ball et al., 2002; Rebok et al., 2014; Willis et al., 2006).3 Results from other cognitive training RCTs meeting the systematic review criteria were mixed but showed positive trends (Carretti et al., 2013; Klusmann et al., 2010; Miller et al., 2013; Stine-Morrow et al., 2014; Wolinsky et al., 2013). In each of these studies, significant short-term improvements in cognitive performance over controls were observed for at least one of the domains on which participants had been trained, but impacts on other domains (i.e., transfer effects) were rare. Only the Iowa Health and Active Minds Study (IHAMS), which used a modified version of the computer-based speed-of-processing training employed in the ACTIVE study, observed small but significant effects of training on tests used to evaluate a different cognitive domain (executive functioning) (Wolinsky et al., 2013). All of these studies were of short duration, however, with follow-up periods in the range of 6 to 12 months. The long follow-up period in the ACTIVE trial is an important aspect of that study, as it showed the sustained benefit of the intervention. In contrast, the majority of RCTs on computer-based “brain training” applications identified in the literature search were excluded from the AHRQ analysis because of follow-up periods that were too short to enable assessment of impacts on such long-term outcomes as age-related cognitive decline.
Given the prominence of the ACTIVE trial in the AHRQ systematic review and in the cognitive training literature more broadly, the intervention arms and results of this study—the largest RCT in this intervention domain to date—warrant more detailed description and analysis. It is important to note that the training model employed in the ACTIVE trial was far more complex than most computer-based “brain training” programs marketed commercially. ACTIVE trial participants were random-
___________________
3 Tables summarizing effect sizes for the impacts of the ACTIVE trial’s cognitive training intervention on cognitive testing outcomes and IADLs (among other outcomes) at the 2-, 5-, and 10-year time points can be found in the 2017 AHRQ systematic review, Interventions to prevent age-related cognitive decline, mild cognitive impairment, and clinical Alzheimer’s-type dementia (Kane et al., 2017) (see Appendix A).
ized to one of three training arms—memory, reasoning, and processing speed—or a no-contact control. The intervention included specific guidance on how to improve performance on a cognitive task during in-person and small-group training sessions with certified trainers over 5 to 6 weeks, as well as follow-up “booster sessions” similar to the initial training, administered to participants who adhered to the intervention 11 months after the initial training and again after 3 years. Hence, there was a component of social interaction. Specific training strategies for the three intervention arms (described in Box 2-2) differed, but the intervention conditions shared key features (Jobe et al., 2001) that included
- providing strategies for solving problems, remembering, or responding rapidly to information;
- using trainers to demonstrate the strategy;
- including both individual and group exercises;
- providing feedback to participants on their performance;
- fostering self-efficacy with regard to performance (i.e., individuals’ belief that they could improve their ability to carry out a specific task); and
- applying strategies to real-world tasks, such as recalling a shopping list, understanding medication dosing, and driving.
The ACTIVE trial was not designed to study the impact of cognitive training on the incidence of dementia, but an analysis conducted post hoc found no difference in incidence between intervention (all arms combined) and control arms4 (Unverzagt et al., 2012). The primary outcome for the trial, as specified in the initial Request for Applications, was independence in cognitively demanding daily functions (performance-based and self-reported measures). Cognitive performance was measured as a short- and long-term outcome, and the study showed that participants improved from baseline on tests for the domain in which they had been trained, but not other domains. These domain-specific benefits were sustained for 10 years except in the memory group, where benefits were observed only at the 2- and 5-year follow-up periods (Rebok et al., 2014). Evidence from the 5- and 10-year follow-up periods was considered low strength in the AHRQ report because of high attrition. Importantly, participants with mild memory impairments at baseline profited from reasoning and speed training, indicating that mild impairment in one cognitive domain does not preclude training-related improvements in other domains (Unverzagt et al., 2007). It is difficult to assess the effect of the booster training in the ACTIVE trial since assignment to the booster group was contingent on adherence to the initial training.5 Nonetheless, the results suggest that the periodic refreshing was beneficial for those compliant with the speed-of-processing and reasoning interventions. The beneficial effects of booster training for speed of processing also were observed in a study by Wolinsky and colleagues (2013), which did not condition the booster on compliance.
An unexpected finding from the ACTIVE trial was a substantial lag in the observed effect of training on measures of daily function. Greater
___________________
4 The study had 80 percent power to detect a hazard ratio of 0.75 at a significance level of 0.05 assuming a 30 percent rate of loss to follow-up (Unverzagt et al., 2012).
5 To be eligible to receive the booster, participants had to have attended at least 8 of the 10 initial training sessions. Only 20 percent of participants in the nonbooster group completed the initial training, so the two groups are not comparable (Rebok et al., 2014).
maintenance of independence in IADLs was reported for all intervention arms compared with the control group at 10 years but not at earlier time points (Rebok et al., 2014), with the exception of the reasoning training group, which also showed less decline in IADLs than controls at 5 years (Willis et al., 2006). While improvements are expected in the domain in which participants were trained, the observation of greater maintenance of independence in IADLs is a notable finding because it suggests that results are more broadly generalizable and that benefits of cognitive training are likely to be meaningful in the context of people’s daily lives. This observation is supported by an analysis showing that at-fault motor vehicle collisions were reduced in the speed-of-processing and reasoning groups relative to controls at 6 years post-training (Ball et al., 2010).6
Limitations of the AHRQ Systematic Review Findings
The AHRQ systematic review finding of moderate-strength evidence for a beneficial effect of cognitive training on cognitive performance was drawn largely from a single large trial—the ACTIVE study. Data were insufficient to draw any conclusions regarding effects of cognitive training on the prevention, delay, or slowing of MCI and CATD. Results of the other RCTs meeting the inclusion criteria—short-duration studies with follow-up periods of 12 months or less—are consistent with expectations of short-term improvements in cognitive performance in the domain trained but do not add to the strength of evidence on long-term benefits of cognitive training and generalizability to other areas such as IADLs. The 5- to 10-year lag in training effects on functional outcomes observed in the ACTIVE trial underscores the importance of including long follow-up periods in study designs and highlights the challenge of research aimed at preventing cognitive decline and dementia.
The ACTIVE trial represents a promising model for subsequent studies on cognitive training interventions but still suffered from notable methodological limitations that need to be considered in the discussion of its results. One criticism is the use of a no-contact control, which may not control adequately for potential placebo effects related to motivation and expectations (Boot et al., 2013; Foroughi et al., 2016). One could argue that expectations would be similar across intervention arms, so if the conditions of the intervention rather than the training itself were responsible for the beneficial effects, the observed differences among the intervention arms
___________________
6 It should be noted that the overall crash rate (at-fault and not-at-fault) was similar for the speed-of-processing and control groups, and, for the typical individual, the observed reduction in rates of at-fault crashes translates to approximately one fewer at-fault crash every 62.5 years (Simons et al., 2016).
would not be expected. However, the intervention arms were not directly compared, which is another limitation of the study design. Finally, booster training was contingent on adherence to the initial training, complicating comparisons between booster and nonbooster groups.
A number of questions remain regarding the critical components and optimal form of cognitive training interventions. The ACTIVE trial intervention was complex. Unlike some interventions in which participants completed training exercises while sitting alone at a computer, the intervention arms in ACTIVE included a social aspect. The relative contributions of the socialization and training components to the observed effects are unclear and require further study. A number of studies have suggested that social engagement may be important, independent of cognitive training (IOM, 2015). The effect of instructing participants on the relevance of the training exercises to daily activities that involve similar cognitive demands is also unclear.
Given the unique and complex nature of its intervention, the results from the ACTIVE trial cannot be used to draw conclusions on the cognitive benefits of computerized cognitive training applications (i.e., brain games) and other interventions that fall within this general domain but have different components and forms. Future research on cognitive training interventions (discussed in Chapter 4) may help tease out the effects of different aspects of the ACTIVE intervention and address other questions that arise from the literature included in the AHRQ systematic review, such as the optimal duration of the intervention, the effects of differing levels of participation in cognitively stimulating activities at baseline, and whether training would be better than taking up other cognitively stimulating activities (e.g., reading, chess).
Evidence from Observational Studies
Intervention studies have been focused largely on structured cognitive training activities (computer- and noncomputer-based), which lend themselves to clinical trials, but there also exists a wealth of observational data suggesting that higher levels of educational attainment, literacy, and participation in other cognitively stimulating activities are associated with maintenance of cognitive performance and reduced risk of cognitive impairment and dementia (IOM, 2015). For example, a recently published prospective cohort study of nearly 2,000 adults aged 70 and older with normal cognition at baseline found that participating in a variety of cognitively stimulating activities, including games, craft activities, computer use, and social activities, was associated with a lower risk of MCI in future years (Krell-Roesch et al., 2017). Similarly, Wilson and colleagues (2013) found that the frequency of both early- and late-life participation in cognitive
activities, such as visiting a library and reading books, was associated with slower cognitive decline in later life, even after accounting for the development of neuropathologic findings (e.g., beta-amyloid deposits, tau-positive tangles, neocortical Lewy bodies).
A large number of studies have examined the relationship of education (usually measured by years of formal schooling) to cognitive decline and dementia incidence. A recent systematic review found an association between lower educational attainment and worse cognitive outcomes in 18 of 27 prospective studies and 21 of 25 cross-sectional studies (Beydoun et al., 2014). The potential importance of education for dementia prevention is further underscored by the finding of an analysis focused on known modifiable dementia risk factors that low educational attainment is the single greatest contributor to the risk of Alzheimer’s disease globally, accounting for nearly 20 percent of cases (Barnes and Yaffe, 2011; Norton et al., 2014). Although the mechanistic pathways are not well understood, one hypothesis is that education and sustained cognitive stimulation over the life course may help build “cognitive reserve” through alternative networks or pathways in the brain that enable individuals to better compensate for neurodegeneration and thereby maintain normal cognitive function longer (Meng and D’Arcy, 2012; Stern, 2012; Valenzuela, 2008). This has not been empirically demonstrated, however, and other factors may account for the association with cognitive outcomes.
Potential Harms and Costs
None of the published RCTs on cognitive training interventions reviewed by the committee found adverse effects, and there is little empirical evidence to suggest that participating in these activities has negative consequences. One consideration, however, that should feed into decision making for individuals interested in taking up activities that may help maintain cognitive function and reduce risk of dementia is cost. Opportunity cost is the time and money that could have been used for engaging in other activities (e.g., physical activity), some of which may also benefit cognition and have other positive health effects (e.g., reduced risk of cardiovascular disease). Commercially marketed cognitive training applications generally entail a purchase or subscription cost, which varies across manufacturers.
Conclusions About Cognitive Training
As a whole, the evidence for cognitive training impacts on maintenance of cognitive function is encouraging but inconclusive, and requires further study. Future research on cognitive training interventions, discussed in Chapter 4, may further strengthen the evidence base for the effectiveness
of cognitive training interventions and provide stronger support for specific activities, which need to be robustly evaluated for their effectiveness before they can be recommended to the public.
CONCLUSION: Some RCT evidence suggests that cognitive training can delay or slow ARCD, as measured by performance on cognitive tests and instrumental activities of daily living. This conclusion is based largely on the ACTIVE trial.
CONCLUSION: There is no RCT evidence at this time that cognitive training will prevent, delay, or slow MCI or CATD.
CONCLUSION: At present, there is no evidence to support the notion that the beneficial long-term cognitive effects suggested by the ACTIVE trial apply to computer-based “brain training” applications. The suite of cognitive training interventions in the ACTIVE trial—which included cognitive training and social engagement in a group setting—differ substantially from commercial computer-based “brain training” applications, the effects of which appear to be short term and apply only to the specific cognitive task that is rehearsed.
CONCLUSION: The encouraging evidence on cognitive training interventions from RCTs, bolstered by additional data from prospective observational cohort studies on the benefits of cognitively stimulating activities, supports communicating with the public about cognitive training as a tool for delaying or slowing ARCD. However, existing data did not allow the committee to draw conclusions regarding the relative effectiveness of different cognitive training approaches or techniques.
BLOOD PRESSURE MANAGEMENT FOR PEOPLE WITH HYPERTENSION
Lowering blood pressure in people with hypertension substantially reduces the risk of heart disease and stroke by slowing blood vessel changes that are the key causes of cardiovascular disease (Wang et al., 2005). The most widely used blood pressure management strategies, which aim to maintain blood pressures at levels specified by established clinical guidelines, rely on medications (antihypertensives), although lifestyle-based interventions such as diet, weight loss, and exercise are also effective and can be first-line strategies (Wexler and Aukerman, 2006). Different classes of medications are available for blood pressure management, with a typical
medication lowering systolic blood pressure (SBP) by about 5 to 10 points; greater decreases can be achieved through use of multiple medications (Law et al., 2009). It appears likely that the benefits of lowering blood pressure may relate not to the specific treatment approach or medication class but to the level of lowering achieved (Turnbull, 2003). Some network meta-analyses, however, have suggested that the cognitive benefits of antihypertensives may differ by drug class, with angiotensin receptor blockers possibly being considered most promising (Levi Marpillat et al., 2013). Nonetheless, evidence is insufficient to determine whether certain classes of antihypertensives may be more beneficial with respect to cognitive decline and dementia. Identification of hypertension and improved control of blood pressure in patients with hypertension through lifestyle changes and medication have been linked to temporal declines in stroke incidence and mortality in prospective cohort studies (Koton et al., 2014), and are doubtless key contributors to the major reduction in cardiovascular mortality seen over the last few decades (Ford et al., 2007). Yet of the almost one-third of Americans with hypertension, more than half lack adequate blood pressure control (National Center for Health Statistics, 2016), despite such efforts as those of the National High Blood Pressure Education Program to provide evidence-based guidance on blood pressure management to clinicians (NHLBI, 2004).
Multiple links exist among cerebrovascular disease, Alzheimer’s disease, and dementia. A majority of dementia patients have pathologic (Kovacs et al., 2008, 2013; Rahimi and Kovacs, 2014) or radiographic (Debette and Markus, 2010) evidence of cerebrovascular disease (e.g., large vessel atherosclerosis, small vessel disease, microbleeds, white matter hyperintensities),7 and these conditions are often seen in combination with Alzheimer’s pathology (Iadecola et al., 2016). Moreover, vascular cognitive impairment and vascular dementia are defined cognitive disorders. Epidemiologic data also link cerebrovascular disease and dementia, as both clinical stroke and subclinical cerebrovascular disease (silent strokes) are important risk factors for dementia (Pendlebury and Rothwell, 2009; Vermeer et al., 2003). In addition, decreased cerebral blood flow resulting from cerebrovascular disease may increase the production, or decrease the clearance, of Alzheimer’s disease proteins in the brain (Zlokovic, 2011). It is plausible, then, that antihypertensive treatments, which are the most powerful tools for reducing the risk of stroke and subclinical cerebrovascular disease, would also reduce the risk of dementia.
___________________
7 Vascular pathologies are also common in the brains of older people without dementia (MRC CFAS, 2001).
Findings from the AHRQ Systematic Review
Summary of the AHRQ Systematic Review Findings
The AHRQ systematic review identified 16 unique RCTs of blood pressure management interventions in hypertensive populations, 13 of which were found to have sufficiently low risk of bias to be included in the analysis. Of the 16 RCTs identified, 8 compared antihypertensive treatments with placebo, 8 compared active treatments, and 1 compared intensive versus standard blood pressure management. A summary of the AHRQ findings on blood pressure management interventions is presented in Box 2-3.
The AHRQ systematic review found inconsistent evidence from RCTs for an effect of blood pressure management on cognitive decline and dementia in patients with hypertension. None of the included trials, however, were designed primarily to evaluate effects on cognitive decline and dementia. In secondary analyses, these RCTs demonstrated no effect of
blood pressure management on global measures of cognitive performance (e.g., Brief Cognitive Test results), and data from domain-specific tests (e.g., executive function, attention, memory) were mixed. The review identified four placebo-controlled trials of antihypertensive medications in adults with normal cognition that measured incident dementia as a secondary outcome. Of these, only the Syst-Eur trial showed beneficial effects on dementia incidence (Forette et al., 1998), raising the possibility of a chance effect. The overall results of this trial convincingly demonstrated the efficacy of blood pressure reduction for the primary endpoint of fatal and nonfatal strokes combined. Active treatment reduced all strokes by 42 percent (p = 0.003) and nonfatal strokes by 44 percent (p = 0.007) (Staessen et al., 1997). In a substudy of this trial, 3,162 adults who were over age 60, had SBP ranging from 160 to 219 mm Hg, and were free of baseline cognitive impairment were randomized to placebo or stepped, multiple-agent active treatment targeting SBP below 150 mm Hg. After 2 years, active treatment had lowered SBP by 8 points and reduced dementia incidence by 50 percent (21 versus 11 patients; p = 0.05) relative to the control group, but no change in cognitive performance as measured by scores on the Mini Mental State Exam (MMSE) was noted in either group (Forette et al., 1998). Subsequent data gathered during an open follow-up phase of the main trial showed that the effect of active treatment on SBP (7-point reduction) and dementia incidence (55 percent risk reduction) persisted after a median of 3.9 years of follow-up (Forette et al., 2002).
The three negative blood pressure–lowering trials in cognitively normal adults that reported incident dementia as an outcome were the Study on Cognition and Prognosis in the Elderly (SCOPE) trial (N = 4,937) (Lithell et al., 2003), Action in Diabetes and Vascular Disease: PreterAx and DiamicroN-MR Controlled Evaluation (ADVANCE) trial (N = 11,140) (Patel et al., 2007), and Hypertension in the Very Elderly Trial (HYVET) Cog (N = 3,336) (Peters et al., 2008). Although the AHRQ systematic review did not include a meta-analysis of these trials, prior meta-analyses including similar trial populations demonstrated a positive effect on cognitive outcomes with the use of antihypertensive treatment (Levi Marpillat et al., 2013; Peters et al., 2008). In particular, when the Perindopril Protection against Recurrent Stroke Study (PROGRESS) (N = 6,105)—which compared variable-intensity antihypertensive treatments in a poststroke population (Tzourio et al., 2003)—was included, a meta-analysis of RCT results showed a borderline significant reduction in incident dementia (p = 0.045) (Peters et al., 2008). Another negative study—the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) trial—found no significant benefit of intensive blood pressure management over standard therapy at 40 months in patients with type 2 diabetes as
measured by cognitive test performance (Williamson et al., 2014). Of note, dementia incidence was not included as an outcome measure in this study.
As the AHRQ systematic review notes, the limitations of the existing experimental evidence stem from the fact that few studies have been explicitly designed to measure the impact of blood pressure management on cognitive impairment and dementia. Instead, these trials typically were designed to measure the impact of antihypertensive treatment on cardiovascular outcomes, with cognitive outcomes being addressed in secondary analyses. As a consequence, these trials tended to have limited statistical power, provide less than optimal cognitive assessments, and entail relatively short-duration follow-up. In addition, they were not designed to address the potential heterogeneity resulting from age differences among subjects at the time of treatment, baseline blood pressure and cognitive status, and class of drug.
Limitations of the AHRQ Systematic Review Findings
Although the AHRQ systematic review found the experimental evidence for the effect of antihypertensive treatment on dementia incidence to be inconsistent, the committee found the data from observational prospective cohort studies to be more consistently positive. Several important methodologic issues need to be considered to place these findings in context.
As noted earlier, RCTs are the gold standard for evaluating intervention efficacy. The trials analyzed in the AHRQ systematic review were not optimally designed to detect effects of blood pressure management on dementia incidence. For both SCOPE and ADVANCE, the difference in blood pressure control between study arms was likely too small to result in significant between-group differences in dementia incidence. In contrast, a substantial difference in blood pressure reduction between study arms was observed for the HYVET-Cog trial, but this study was stopped early for efficacy on a noncognitive endpoint; as a result, mean follow-up was only 2.2 years (Peters et al., 2008). It is unknown whether an effect on cognitive function would have been observed had the study continued longer. In addition, these four trials enrolled older patient cohorts and followed them for short durations; the longest mean duration of follow-up (in the ADVANCE trial) was 4.3 years. Based on evidence from observational studies, which suggests that the effects of lowering blood pressure may be greatest in midlife, the optimal trial to quantify the effect of antihypertension treatment on dementia incidence would likely involve enrolling a younger cohort. Given the low baseline risks of dementia in a midlife cohort (Brookmeyer et al., 1998) and the slow progression of neurodegenerative pathology (Bateman et al., 2012), a decade or more of follow-up may be required to identify whether there is a true effect of such treatment on dementia incidence. In
addition, the fact that the primary goal of these four trials was to assess cardiovascular effects may have made them less likely to detect cognitive effects. In ADVANCE, for example, outcomes included multiple cardiovascular events in addition to cognitive performance and dementia (Patel et al., 2007). The latter outcomes were secondary and not systematically assessed as rigorously as the primary cardiovascular outcomes. Moreover, most cognitive performance outcomes relied on such insensitive measures as the MMSE.
Results of these hypertension trials are further complicated by the potential for heterogeneity of treatment effects across different subpopulations, particularly across age strata. For example, the focus of prior trials on late-life hypertension treatment may have resulted in study populations at high risk of treatment-related harm (Protogerou et al., 2007). In the oldest old, for instance, late-life hypertension is associated with a reduced risk of dementia (Corrada et al., 2017). Low diastolic blood pressure may in fact lead to declining cognitive performance through hemodynamic mechanisms in a population with more preexisting small vessel disease. Comorbid conditions that themselves may be dementia risk factors, such as diabetes (Poblador-Plou et al., 2014), pose additional challenges to intervention studies targeting dementia risk reduction through blood pressure management.
Assessing Antihypertensive Treatment Against the Bradford Hill Criteria for Causation
Although RCTs provide at best modest support for a role of blood pressure management in patients with hypertension for dementia prevention, other converging sources of evidence provide additional support. When assessed against the Bradford Hill criteria discussed earlier (Hill, 1965), blood pressure management for dementia prevention generally fares well.
Strength of the Association
Causal explanations are more likely when observational evidence suggests strong associations. The association between hypertension and dementia differs based on the age of the population that is studied (Qiu et al., 2005). Across multiple studies, midlife but not late-life hypertension is consistently associated with increased risk of dementia (odds ratio of 1.61 for midlife hypertension) (Barnes and Yaffe, 2011). The strength of the association between antihypertensive therapy and dementia is more modest based on RCT evidence, as discussed in the prior section. However, two meta-analyses of prospective cohort studies assessing the association between antihypertensive treatment and dementia have identified larger effect sizes
(relative risk [RR]8 = 0.84) (Chang-Quan et al., 2011; Levi Marpillat et al., 2013).
Consistency
Confidence in causal explanations for observed associations is enhanced when independent studies that test different study populations or use different methodologies produce similar results. While the experimental evidence on the effect on dementia incidence of blood pressure management in people with hypertension has varied, evidence from observational studies has shown a relatively consistent effect. In a recent systematic review, for example, 7 of 11 studies found that antihypertensive treatment was associated with reduced risk of dementia or Alzheimer’s disease (Rouch et al., 2015). Meta-analyses of observational longitudinal data also have found a relatively consistent association between lowering blood pressure and improved outcomes on a variety of measures of cognition (Chang-Quan et al., 2011; Levi Marpillat et al., 2013).
Specificity
According to the Bradford Hill criteria, the association between an exposure and the potential outcome is more likely to be causal if the exposure influences predominantly outcomes that are on a hypothesized causal pathway. Animal studies have confirmed the etiological relationship among hypertension, vascular disease in the brain, and cognitive impairment. Spontaneously hypertensive rats develop cerebrovascular disease in a pattern similar to that of humans with vascular dementia and have more severe cognitive deficits than those without hypertension (Saito et al., 1995; Yamori et al., 1976), and antihypertensive treatment limits cognitive and behavioral abnormalities (Wyss et al., 2003). These animal findings suggest a causal link between hypertension and cognitive deficits that can be attenuated by antihypertensive treatment and are broadly concordant with results of observational studies in humans.
Temporality
Analyses using the Bradford Hill criteria assess temporality to demonstrate that an intervention or exposure occurred prior to the change in outcome measures and, therefore, that an observed statistical association
___________________
8 RR, used to compare risk between two groups, is the ratio of the probability of a certain outcome in an exposed/treatment group to the probability of that outcome in an unexposed/no-treatment group.
does not result from reverse causality. In the present case, hypertension and antihypertensive treatment are clearly present prior to dementia in observational studies.
Biological Gradient
The demonstration of a dose–response effect lends additional support to a causal relationship. The degree of blood pressure lowering across RCTs has not varied substantially; thus, the trials offer little opportunity to determine whether differential blood pressure lowering is associated with differential risk of dementia. It is worth noting that the positive Syst-Eur trial (Forette et al., 1998) enrolled subjects with higher baseline SBPs (160 to 219 mm Hg) relative to those of subjects in the other trials. Moreover, several cohort studies have found evidence of a biological gradient, with greater associations between cognitive outcomes and blood pressure lowering among those with the most elevated blood pressures (Elias et al., 2007; Gottesman et al., 2014). In one cohort study with long-term follow-up, the incidence of dementia decreased as the duration of antihypertensive treatment increased (Peila et al., 2006). Similarly, in another long-term cohort study, individuals with more severe hypertension and stronger indications for blood pressure treatment at midlife showed less decline in a variety of long-term cognitive measures after treatment (Gottesman et al., 2014). Although the unpublished Heart Outcomes Preventions Evaluation-3 (HOPE-3) ancillary study showed no overall effect of blood pressure control on cognitive measures, blood pressure lowering was related to better cognitive performance among the subgroup in the highest tertile of baseline SBP (>145 mm Hg) (Bosch, 2016).
Plausibility/Coherence
Plausibility and coherence relate to how a causal explanation aligns with what is known regarding the biology and natural history of a disease. There are two converging arguments for the plausibility of blood pressure management reducing dementia incidence. First, blood pressure management in hypertensives may prevent dementia by preventing or delaying the progression of cerebrovascular disease. Blood pressure management is a powerful tool for preventing stroke (Wang et al., 2005), even in individuals with modest blood pressure elevations (Sipahi et al., 2012). Blood pressure management also reduces the risk of subclinical and silent cerebrovascular disease, such as white matter disease that is associated with small vessel disease (Dufouil et al., 2005). Both stroke and subclinical cerebrovascular disease are strong predictors of dementia (Debette and Markus, 2010; Pendlebury and Rothwell, 2009). Consequently, it is plausible that blood
pressure management may reduce dementia risk through prevention of symptomatic and asymptomatic cerebrovascular disease. Second, vascular disease may be an important causal factor in the neurodegenerative cascade underlying the most common forms of dementia, such as Alzheimer’s disease (Zlokovic, 2011). In patients with MCI and dementia, both neurodegenerative and vascular pathology are commonly identified at autopsy (Kovacs et al., 2008, 2013; Rahimi and Kovacs, 2014; Saito and Murayama, 2007), and a variety of plausible pathophysiologic links between vascular disease and neurodegeneration have been proposed (Jellinger, 2007).
Overall Summary
When measured against the Bradford Hill criteria, the observational data indicating that blood pressure management reduces the risk of dementia are consistent with a causal relationship. Although RCT evidence is inconsistent, the observational data support management of hypertension as a plausible intervention for reducing dementia. As discussed in Chapter 4, opportunities exist for new RCTs focused on optimal targets (blood pressure levels and patient groups) and therapies to reduce risks for CATD that may avoid some of the potential shortcomings of observational studies (e.g., biases related to design and reporting) and add further support for this recommendation. However, given that blood pressure management, like physical activity (discussed below), is broadly recommended for primary, secondary, and tertiary prevention of many chronic conditions, the committee notes that it may not be possible to generate definitive evidence through prospective RCTs.
Other Known Health Benefits and Potential Harms and Costs
In addition to the efficacy and observational data discussed above, two other considerations are relevant to communications with the public on the potential benefits of blood pressure management for prevention of cognitive decline and dementia.
First, there is strong evidence indicating that effective blood pressure management substantially reduces the risk of fatal and nonfatal cardiovascular events and all-cause mortality in hypertensive patients (The SPRINT Research Group, 2015). While the proportion of Americans aware of their hypertension and receiving treatment has increased over time, both under-recognition and undertreatment remain common (Bromfield et al., 2014; Navar-Boggan et al., 2014). In addition, results of the recent Systolic Blood Pressure Intervention Trial (SPRINT) suggest that many patients already receiving antihypertensive treatment may benefit from more intense treatment in terms of cardiovascular outcomes (The SPRINT Research Group,
2015). Given the underutilization of antihypertensive treatment for blood pressure management, broader diffusion of such treatments would likely have important societal benefits with respect to the incidence of cardiovascular disease.
Second, the risks of antihypertensive treatment have been well studied and are generally modest in magnitude. Although some subgroups, including diabetics with low baseline blood pressure (Brunström and Carlberg, 2016), may be at risk for severe adverse events, the majority of patients treated in trials did not have a higher rate of severe adverse events relative to control groups. The four placebo-controlled trials of antihypertensive treatments for dementia prevention summarized above collectively enrolled more than 20,000 patients, and none of the four identified an increase in major cardiac events, microvascular events, or mortality. Importantly, given that these studies focused on late-life populations, they included the groups at highest risk for harm. While antihypertensive treatments do cause problematic idiosyncratic adverse events, these events are relatively rare and rarely severely disabling. For any given antihypertensive agent, approximately 3 to 6 percent of patients will discontinue the medication because of an adverse event, but the majority of these events do not result in irreversible harm (Ross et al., 2001). In addition to their relatively benign adverse event profile, antihypertensive treatments are relatively inexpensive, and their use is highly cost effective for the prevention of cardiovascular disease (Moran et al., 2015). In fact, the World Health Organization has categorized interventions for prevention of cardiovascular disease, including antihypertensive treatment, as one of their “best buys” for reducing noncommunicable diseases (WHO and World Economic Forum, 2011).
Conclusions About Blood Pressure Management
Hypertension is a well-documented risk factor for dementia. Intervention studies suggest the risk of dementia attributable to hypertension can be reduced through blood pressure management strategies, although further research (discussed in Chapter 4) is needed to optimize the effectiveness of this approach.
CONCLUSION: Blood pressure management for people with hypertension, particularly during midlife, is supported by encouraging but inconclusive evidence for preventing, delaying, and slowing CATD. The RCT data do not offer strong support for blood pressure management in patients with hypertension for delaying or slowing ARCD or preventing, delaying, or slowing MCI and CATD, although one trial provides some positive evidence of an impact on the risk of CATD. When prospective cohort studies
and knowledge of the natural history and biology of the disease are considered, however, effects of blood pressure management on incident CATD in patients with hypertension are consistent with a causal relationship. In addition, there are known cardiovascular benefits from blood pressure management.
INCREASED PHYSICAL ACTIVITY
Physical activity has been recognized as a key contributor to healthy aging, with benefits including both physical and cognitive function (IOM, 2015). Physical activity encompasses a diverse set of behaviors, alone or in combination, including aerobic activity (e.g., walking, dancing); resistance training (e.g., weightlifting); and stretching, toning, and balance (e.g., yoga, tai chi). A 2015 Institute of Medicine report identifies engaging in physical activity as one of the specific actions individuals should take to maintain and sustain their cognitive health (IOM, 2015). Importantly, individuals can change their behavior to become more physically active at any age, and doing so does not necessarily require adherence to a structured exercise program. Physical activity levels can be boosted by work or leisure (e.g., hiking) activities, and also may be influenced by community conditions (e.g., availability of neighborhood green space) (Dalton et al., 2016).
Physical activity has consistently been identified as one of the modifiable risk factors that could have the greatest impact on rates of cognitive impairment and dementia (Barnes and Yaffe, 2011; Beydoun et al., 2014). In 2011, Barnes and Yaffe (2011) estimated that nearly 4.3 million Alzheimer’s disease cases globally and more than 1 million cases in the United States alone could be attributed to physical inactivity, and a 25 percent reduction in the prevalence of physical inactivity could potentially have prevented nearly 1 million cases globally and 230,000 in the United States. However, despite evidence accumulated over decades from observational studies showing the significant benefit of physical activity for reducing the risk of cognitive decline and dementia (Blondell et al., 2014; Brown et al., 2013), supportive data from intervention studies have been sparse, with meta-analyses reporting both no benefit (Young et al., 2015) and benefit (Northey et al., 2017; Zheng et al., 2016).
Findings from the AHRQ Systematic Review
Summary of the AHRQ Systematic Review Findings
The AHRQ systematic review identified 43 RCTs of physical activity interventions, 19 of which were rated as having a low or medium risk of
bias and were included in the analysis. A summary of the AHRQ findings on physical activity interventions is presented in Box 2-4.
To date, no RCTs have demonstrated that physical activity can reduce the incidence of MCI or CATD, despite associations reported in observational studies (discussed in more detail below). Dementia and MCI incidence were rarely included as outcomes in physical activity trials analyzed in the AHRQ systematic review, and studies generally were not of sufficient duration or size to detect such changes. However, results from several RCTs of aerobic training interventions or interventions that included an aerobic training component in older adults with MCI suggest that physical activity can delay or slow cognitive decline in this population9 (Baker et al., 2010; Lautenschlager et al., 2008; Suzuki et al., 2012). Langa and Levine (2014) identified two additional studies that provide further evidence supporting the beneficial effects of physical activity in individuals with MCI—Barnes
___________________
9 The Baker et al. (2010) study is misclassified in the AHRQ report as having been conducted in adults with normal cognition.
et al. (2013) and Nagamatsu et al. (2012)—but these trials were excluded from the AHRQ analysis because of short follow-up periods (12 weeks) and a high risk of bias due to attrition, respectively.
Results from clinical trials of physical activity interventions in older adults with normal cognition at the time of enrollment have been mixed. The AHRQ systematic review found some instances of benefits for cognitive outcomes for aerobic activity (Antunes et al., 2015; Muscari et al., 2010) and resistance training (Cassilhas et al., 2007), but the effects were modest and may not be clinically meaningful (Kane et al., 2017). Evidence for both types of physical activity interventions was considered insufficient to conclude whether they were of benefit to adults with normal cognition. The largest physical activity trial, the Lifestyle Interventions and Independence for Elders (LIFE) study, featured a multicomponent intervention focused on walking, resistance training, and flexibility exercises targeting preservation of mobility in sedentary individuals aged 70-89 who had functional limitations (Pahor et al., 2014). Cognitive function, which was a preplanned secondary outcome for the trial, was not improved across 2 years, overall (Sink et al., 2015), although exploratory analyses provided some evidence for benefit among individuals who were relatively older, who had greater physical limitations, or who had diabetes (Espeland et al., 2017; Sink et al., 2015). These findings suggest that multicomponent physical activity interventions in older adults may be effective in preserving cognitive function only in subgroups of individuals. Although the AHRQ systematic review found that multicomponent physical activity interventions demonstrated no clear benefit for cognitive performance in adults with normal cognition, heterogeneity in the elements of multicomponent interventions limits the ability to generalize existing study results to all such interventions.
Limitations of the AHRQ Systematic Review Findings
The reasons for the mixed RCT data and the discrepancy between the results of RCTs and observational studies (discussed below) with respect to benefits of physical activity for reducing rates of cognitive impairment and dementia are not well understood. They may result in part from methodological limitations of physical activity intervention studies, such as insufficient study durations and follow-up periods, failure to include a proper control group, inconsistent and often subjective measures of physical activity, small sample sizes, and associated lack of power to determine efficacy for prevention of MCI or CATD. It is, of course, also possible that physical activity has no real effect on cognitive decline and dementia incidence and that the statistically significant effects on cognitive tests reported in some RCTs are the result of chance, with confounding potentially explaining the association between physical activity and cognitive outcomes in observa-
tional studies. However, with the exception of the LIFE study, all RCTs included in the AHRQ systematic review were of short duration (1 year or less). If the effect of increasing physical activity is to slow cognitive decline rather than increase cognitive performance, this is a relatively short period of time in which to observe decline reliably in most individuals, and any observed rates of change will vary depending on the population’s risk factor profiles. One year or less is likely much too short a time in which to assess intervention effects on the risk of Alzheimer’s disease and related dementias. Larger and longer-term studies are therefore needed to determine whether the short-term effects of physical activity translate into long-term cognitive benefits.
Additionally, given the multitude of potential interactions among pathways that may mediate cognitive decline and progression to dementia, the strength of the effect of any one intervention would be expected to vary across individuals. Further elucidation of the biological mechanisms mediating the protective effects of physical activity and the dose–response relationship may provide some additional explanation. The timing of initiation of interventions may be another factor, as there is increasing evidence that physical activity in midlife is important for reducing MCI and dementia risk in older adults (Hamer and Chida, 2009). These and other areas for future study are discussed further in Chapter 3.
Assessing Physical Activity Against the Bradford Hill Criteria for Causation
The pattern of positive results from RCTs included in the AHRQ systematic review provides an indication of short-term beneficial effects of physical activity, particularly in adults with MCI. However, physical activity interventions are not supported by sufficient evidence from clinical trials alone to justify public health messaging beyond the known physical benefits. Additional data supporting consideration of physical activity for public health messaging derive from the wealth of observational studies, including several large prospective studies (Larson et al., 2006; Lee et al., 2013; Scarmeas et al., 2009; Singh-Manoux et al., 2005; Yaffe et al., 2001), that have found that physical activity is associated with reduced rates of cognitive decline and dementia risk (Beydoun et al., 2014; Blondell et al., 2014; Hamer and Chida, 2009; Zhu et al., 2017). Absent strong experimental evidence, the committee again used the Bradford Hill criteria (Hill, 1965) to evaluate whether the large body of evidence from observational studies supports a causal relationship between physical activity and reduced rates of cognitive decline and dementia.
Strength of the Association
A recent meta-analysis of longitudinal studies demonstrated low- to moderate-strength inverse associations of physical activity with cognitive decline (see Figure 2-1) and dementia (see Figure 2-2), with overall RR
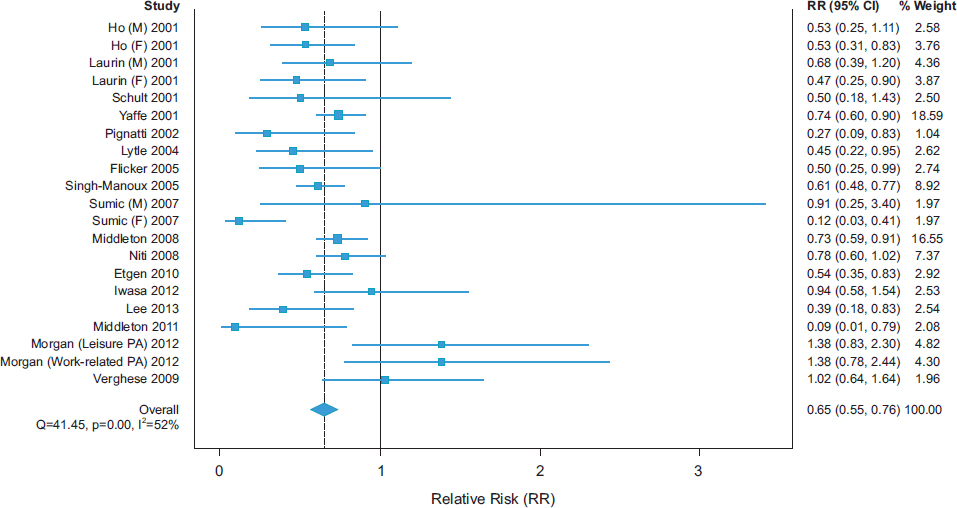
SOURCE: Blondell et al., 2014.
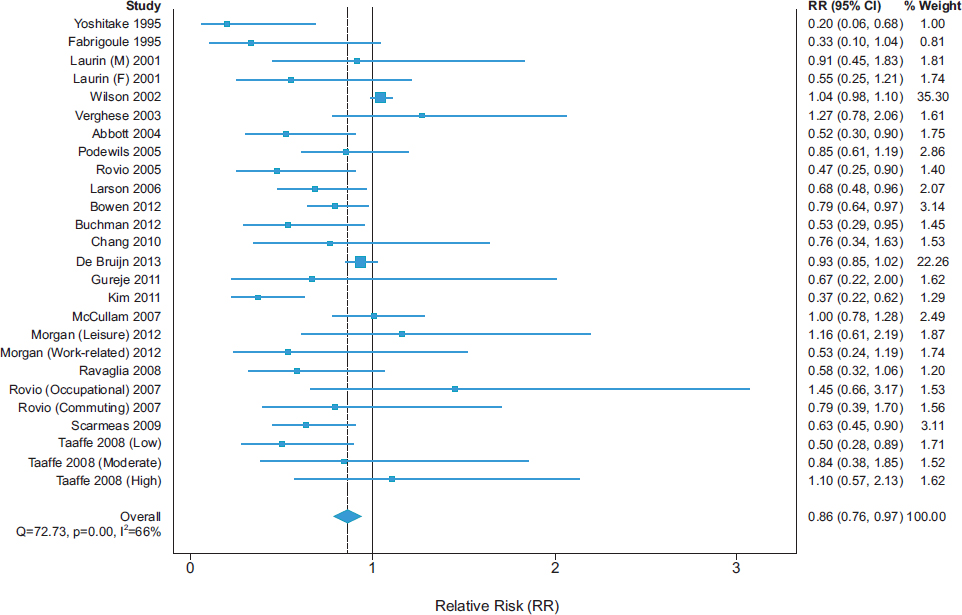
SOURCE: Blondell et al., 2014.
estimates of 0.65 (95 percent confidence interval [CI] 0.55-0.76) and 0.86 (95 percent CI 0.76-0.97), respectively (Blondell et al., 2014).
Consistency
In the meta-analysis by Blondell and colleagues (2014), 18 of 21 studies had point estimates of reduced risk for cognitive decline, and 20 of 26 studies had point estimates of reduced risk for dementia. None of the 95 percent CIs for RR across all studies excluded benefit. In another recent systematic review, 21 of 24 cohort studies and 4 of 4 cross-sectional studies found an association between physical activity and cognitive outcomes (Beydoun et al., 2014). There also is a growing body of evidence showing that sedentary behavior is consistently associated with poor cognitive outcomes. A recent systematic review found that 6 of 8 observational studies reported significant negative associations between sedentary behavior and cognitive function (Falck et al., 2016). Some lack of consistency in findings on the beneficial cognitive effects of physical activity may be explained by methodological limitations and, perhaps, real differences in effects across individuals who have different risk factor profiles or are at different stages of cognitive decline (Tolppanen et al., 2015; Willey et al., 2016).
Specificity
It is difficult to establish specificity for how physical activity may reduce the risks for cognitive decline and dementia. As described in the section on plausibility below, there are many potential biological pathways through which physical activity may provide these benefits.
Temporality
In interpreting results from observational studies, the potential for an observed statistical association to result from reverse causality needs to be considered. In the present case, it is plausible that cognitive decline and dementia lead to reduced physical activity, thereby explaining the inverse relationship. However, RCT data showing improvement in cognitive outcomes in adults with MCI following physical activity interventions (Baker et al., 2010; Lautenschlager et al., 2008; Suzuki et al., 2012) suggest that reverse causality does not explain the association between physical activity and cognitive function (Ahlskog et al., 2011). Furthermore, Middleton and colleagues (2010) examined the effects of physical activity in women across the life course using a cross-sectional study design and found that the negative association between physical activity and late-life cognitive impairment was strongest for those who were physically active early in life. Finally, as discussed below, the temporal relationship between physical activity inter-
ventions and potential biological mediators of cognitive benefits, such as brain perfusion and brain structure, has been documented in some studies (Erickson et al., 2011).
Biological Gradient
Observational studies have provided only mixed evidence of a biological gradient for effects of physical activity on cognitive outcomes (Blondell et al., 2014). In their meta-analysis, Sofi and colleagues (2011) grouped studies according to the intensity level of physical activity (high and low to moderate) and found no indication of a dose–response relationship. However, several large prospective studies, including the Study for Osteoporotic Fractures (Yaffe et al., 2001) and the Reasons for Geographical and Racial Differences in Stroke (REGARDS) study (Zhu et al., 2017), found that objectively measured physical activity had a graded relationship for the risk of cognitive decline. The possibility of an intensity threshold has been raised but is not strongly supported by current evidence (Brown et al., 2013). One challenge to evaluating dose–response relationships is the use of subjective methods (e.g., questionnaires) to assess physical activity levels. The inadequate reliability of such methods can introduce bias into analyses. The use of objective measures of physical activity may help overcome this limitation and lead to evidence-based recommendations on target physical activity levels for cognitive benefit.10 Indeed, when cardiorespiratory fitness was determined by objective measures, including peak oxygen consumption and treadmill exercise duration, Barnes and colleagues (2003) observed a graded association between cardiorespiratory fitness at baseline and level of performance on cognitive tests conducted 6 years later.
Plausibility/Coherence
The underlying mechanisms mediating changes in brain structure and function in response to physical activity are not well understood, but a number of hypotheses on possible direct and indirect effects have been proposed and are biologically plausible. These include increased brain perfusion, protection against brain volume loss (potentially mediated through such neurotrophic factors as brain-derived neurotrophic factor), reduction of inflammation, and reduction of brain beta-amyloid deposition. Indirectly, physical activity may prevent cognitive decline and dementia by reducing atherosclerosis and the associated risk of vascular disease (including stroke) (Ahlskog et al., 2011; Blondell et al., 2014; Brown et al., 2013).
___________________
10 The American Heart Association (AHA, 2016) currently recommends 150 minutes of moderate physical activity per week, but this recommendation is not based on data from studies of cognitive benefit.
In addition to the RCT data summarized in the AHRQ systematic review and epidemiological studies discussed above, studies examining the effects of physical activity on biomarkers—neuroimaging studies in particular—provide additional evidence of the beneficial effects of physical activity on cognition (Ahlskog et al., 2011; Erickson et al., 2012). Brain shrinkage is a normal part of the aging process (Raz et al., 2005), and decreasing hippocampal volume has been associated with cognitive decline (O’Shea et al., 2016). In individuals with MCI, hippocampal atrophy is a predictor of progression to Alzheimer’s dementia (Jack et al., 2010). Erickson and colleagues (2011) found that aerobic exercise was associated with an increase in hippocampal volume, while hippocampal volume decreased in a stretching control group over the same time period. Other intervention and observational studies have similarly found that aerobic exercise was associated with increases in brain matter volume in older adults (Colcombe et al., 2006; Erickson et al., 2010; Ruscheweyh et al., 2011). By attenuating and even reversing declines in brain volume, physical activity may help to protect cognitive function (Erickson et al., 2011) and reduce the risk of dementia (Tan et al., 2017), although the mechanisms for these effects have yet to be fully elucidated. A recent study emphasizes the importance of promoting physical activity in midlife to protect against brain volume loss observed later in life (Spartano et al., 2016). As discussed further in Chapter 3, however, the relationship between such biomarkers as brain volume and cognitive outcomes in intervention studies requires further study.
Overall Summary
When assessed against the Bradford Hill criteria, the existing body of evidence from observational studies lends some support, but is not conclusive, for a causal relationship between physical activity and cognitive decline and dementia. While many of the studies included in this analysis tried to adjust for confounding and assessed physical activity using standardized measures (Blondell et al., 2014), the committee recognizes that even with carefully conducted observational studies, there is a risk of confounding, bias, and reverse causality.
Other Known Health Benefits and Potential Harms and Costs
In considering public health messaging on interventions for reducing the risk of cognitive decline and dementia, it is important to weigh an intervention’s other known health benefits, as well as risks and costs. Physical activity is an important factor in healthy aging (Hamer et al., 2014), with well-documented benefits extending to improvements in quality of life—
e.g., maintaining mobility and independence (Pahor et al., 2014)—and reduced risk of chronic conditions such as depression, hypertension, osteoarthritis, metabolic syndrome, diabetes, stroke, and coronary heart disease, as well as fall-related injuries (Chang et al., 2004; Howard and McDonnell, 2015; Lee et al., 2012).
While uncommon, it has been reported that physical activity may result in musculoskeletal injury and hospitalization in older adults (Marsh et al., 2016), although these risks in older adults do not appear to be greater than those for other age groups (Little et al., 2013), and for most individuals, the benefits are likely to far outweigh the risk of harm. Costs of physical activity interventions vary depending on the type of activity, but are typically minimal. Walking, for example, represents a low-cost, universally available form of physical activity that has been associated with maintaining cognitive performance in later life (Erickson et al., 2010).
The widely acknowledged public health benefits of physical activity beyond cognitive outcomes, along with the low risk of harm and minimal cost, are important factors that, combined with encouraging RCT data and strong epidemiological evidence of cognitive benefit, bolster the argument for public health messaging on interventions that increase physical activity. Even in the absence of RCT data showing positive long-term effects, there appears to be no clear detriment to public health messaging that promotes increased physical activity.
Conclusions About Increased Physical Activity
Physical activity has many known health benefits, some of which (e.g., stroke prevention) are causally related to brain health. There is growing evidence that among these health benefits is reduced risk of cognitive decline.
CONCLUSION: Increased physical activity is supported by encouraging but inconclusive evidence for delaying or slowing ARCD. The pattern of RCT results across different types of physical activity interventions provides an indication of the effectiveness of increased physical activity for delaying or slowing ARCD. These effects are consistent with a causal relationship when prospective cohort studies and knowledge of neurobiological processes are considered. In addition, increased physical activity has known cardiovascular and other health benefits.
CONCLUSION: There is insufficient evidence at this time to conclude that increased physical activity will prevent, delay, or slow MCI or CATD.
CONCLUSION: There is insufficient evidence to determine which specific types of physical activity are particularly effective for preventing cognitive decline and dementia.
RECOMMENDATION
The committee concludes that three classes of interventions—cognitive training, blood pressure management for people with hypertension, and increased physical activity—are supported by encouraging but inconclusive evidence. The lack of consistent results across RCTs for interventions in these three domains (i.e., effects reach statistical significance for some trials but not others) may be expected for interventions that have weak beneficial effects on cognitive outcomes, and the methodological limitations described in the sections above also may contribute to the inconclusive nature of the evidence base. However, it should also be acknowledged that future research may show that one or more of these interventions do not prevent cognitive decline or dementia, but have only short-term or nonspecific effects. Additionally, although it is biologically plausible that the same interventions that help delay or slow ARCD would also be beneficial for MCI and CATD, and vice versa, it is not known whether this extrapolation or generalization can be made in either direction. Therefore, the recommendation below is based on the available evidence. The public, however, will not draw fine distinctions among these conditions, and it will be challenging to convert these statements about the evidence into appropriate communications with the public.
REFERENCES
AHA (American Heart Association). 2016. American Heart Association Recommendations for Physical Activity in Adults. http://www.heart.org/HEARTORG/HealthyLiving/PhysicalActivity/FitnessBasics/American-Heart-Association-Recommendations-for-PhysicalActivity-in-Adults_UCM_307976_Article.jsp (accessed March 8, 2017).
Ahlskog, J. E., Y. E. Geda, N. R. Graff-Radford, and R. C. Petersen. 2011. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic Proceedings 86(9):876-884.
Antunes, H. K., M. T. De Mello, R. F. Santos-Galduroz, J. C. Galduroz, V. A. Lemos, S. Tufik, and O. F. Bueno. 2015. Effects of a physical fitness program on memory and blood viscosity in sedentary elderly men. Brazilian Journal of Medical and Biological Research 48(9):805-812.
Baker, L. D., L. L. Frank, K. Foster-Schubert, P. S. Green, C. W. Wilkinson, A. McTiernan, S. R. Plymate, M. A. Fishel, G. S. Watson, B. A. Cholerton, G. E. Duncan, P. D. Mehta, and S. Craft. 2010. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Archives of Neurology 67(1):71-79.
Ball, K., D. B. Berch, K. F. Helmers, J. B. Jobe, M. D. Leveck, M. Marsiske, J. N. Morris, G. W. Rebok, D. M. Smith, S. L. Tennstedt, F. W. Unverzagt, and S. L. Willis. 2002. Effects of cognitive training interventions with older adults: A randomized controlled trial. Journal of the American Medical Association 288(18):2271-2281.
Ball, K., J. D. Edwards, L. A. Ross, and G. McGwin. 2010. Cognitive training decreases motor vehicle collision involvement of older drivers. Journal of the American Geriatrics Society 58(11):2107-2113.
Barnes, D. E., and K. Yaffe. 2011. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology 10(9):819-828.
Barnes, D. E., K. Yaffe, W. A. Satariano, and I. B. Tager. 2003. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. Journal of the American Geriatrics Society 51(4):459-465.
Barnes, D. E., W. Santos-Modesitt, G. Poelke, A. F. Kramer, C. Castro, L. E. Middleton, and K. Yaffe. 2013. The Mental Activity and eXercise (MAX) trial: A randomized controlled trial to enhance cognitive function in older adults. JAMA Internal Medicine 173(9):797-804.
Bateman, R. J., C. Xiong, T. L. Benzinger, A. M. Fagan, A. Goate, N. C. Fox, D. S. Marcus, N. J. Cairns, X. Xie, T. M. Blazey, D. M. Holtzman, A. Santacruz, V. Buckles, A. Oliver, K. Moulder, P. S. Aisen, B. Ghetti, W. E. Klunk, E. McDade, R. N. Martins, C. L. Masters, R. Mayeux, J. M. Ringman, M. N. Rossor, P. R. Schofield, R. A. Sperling, S. Salloway, and J. C. Morris. 2012. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. New England Journal of Medicine 367(9):795-804.
Beydoun, M. A., H. A. Beydoun, A. A. Gamaldo, A. Teel, A. B. Zonderman, and Y. Wang. 2014. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health 14:643.
Blondell, S. J., R. Hammersley-Mather, and J. L. Veerman. 2014. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 14:510.
Boot, W. R., D. J. Simons, C. Stothart, and C. Stutts. 2013. The pervasive problem with placebos in psychology: Why active control groups are not sufficient to rule out placebo effects. Perspectives on Psychological Science 8(4):445-454.
Bosch, J. 2016. The Heart Outcomes Prevention Evaluation (HOPE)-3 trial: Cognitive & functional outcomes. Paper presented at the American Heart Association Scientific Sessions 2016, New Orleans, LA, November 13.
Bromfield, S. G., C. B. Bowling, R. M. Tanner, C. A. Peralta, M. C. Odden, S. Oparil, and P. Muntner. 2014. Trends in hypertension prevalence, awareness, treatment, and control among U.S. adults 80 years and older, 1988-2010. Journal of Clinical Hypertension (Greenwich, Conn.) 16(4):270-276.
Brookmeyer, R., S. Gray, and C. Kawas. 1998. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health 88(9):1337-1342.
Brown, B. M., J. J. Peiffer, and R. N. Martins. 2013. Multiple effects of physical activity on molecular and cognitive signs of brain aging: Can exercise slow neurodegeneration and delay Alzheimer’s disease? Molecular Psychiatry 18(8):864-874.
Brunström, M., and B. Carlberg. 2016. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: Systematic review and meta-analyses. The BMJ 352:i717.
Carretti, B., E. Borella, M. Zavagnin, and R. de Beni. 2013. Gains in language comprehension relating to working memory training in healthy older adults. International Journal of Geriatric Psychiatry 28(5):539-546.
Cassilhas, R. C., V. A. Viana, V. Grassmann, R. T. Santos, R. F. Santos, S. Tufik, and M. T. Mello. 2007. The impact of resistance exercise on the cognitive function of the elderly. Medicine & Science in Sports & Exercise 39(8):1401-1407.
Chang, J. T., S. C. Morton, L. Z. Rubenstein, W. A. Mojica, M. Maglione, M. J. Suttorp, E. A. Roth, and P. G. Shekelle. 2004. Interventions for the prevention of falls in older adults: Systematic review and meta-analysis of randomised clinical trials. BMJ 328(7441):680.
Chang-Quan, H., W. Hui, W. Chao-min, W. Zheng-Rong, G. Jun-Wen, L. Yong-Hong, L. Yan-You, and L. Qing-Xiu. 2011. The association of antihypertensive medication use with risk of cognitive decline and dementia: A meta-analysis of longitudinal studies. International Journal of Clinical Practice 65(12):1295-1305.
Cognitive Training Data. 2015. Cognitive training data response letter. http://www.cognitivetrainingdata.org/the-controversy-does-brain-training-work/response-letter (accessed March 2, 2017).
Colcombe, S. J., K. I. Erickson, P. E. Scalf, J. S. Kim, R. Prakash, E. McAuley, S. Elavsky, D. X. Marquez, L. Hu, and A. F. Kramer. 2006. Aerobic exercise training increases brain volume in aging humans. Journals of Gerontology Series A: Biological Sciences & Medical Sciences 61(11):1166-1170.
Corrada, M. M., K. M. Hayden, A. Paganini-Hill, S. S. Bullain, J. DeMoss, C. Aguirre, R. Brookmeyer, and C. H. Kawas. 2017. Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 13(2):103-110.
Dalton, A. M., N. Wareham, S. Griffin, and A. P. Jones. 2016. Neighbourhood greenspace is associated with a slower decline in physical activity in older adults: A prospective cohort study. SSM—Population Health 2:683-691.
Debette, S., and H. S. Markus. 2010. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 341:c3666.
Dufouil, C., J. Chalmers, O. Coskun, V. Besançon, M.-G. Bousser, P. Guillon, S. MacMahon, B. Mazoyer, B. Neal, M. Woodward, N. Tzourio-Mazoyer, and C. Tzourio. 2005. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: The PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 112(11):1644-1650.
Elias, M. F., L. M. Sullivan, P. K. Elias, R. B. D’Agostino Sr., P. A. Wolf, S. Seshadri, R. Au, E. J. Benjamin, and R. S. Vasan. 2007. Left ventricular mass, blood pressure, and lowered cognitive performance in the Framingham offspring. Hypertension 49(3):439-445.
Erickson, K. I., C. A. Raji, O. L. Lopez, J. T. Becker, C. Rosano, A. B. Newman, H. M. Gach, P. M. Thompson, A. J. Ho, and L. H. Kuller. 2010. Physical activity predicts gray matter volume in late adulthood: The Cardiovascular Health Study. Neurology 75(16):1415-1422.
Erickson, K. I., M. W. Voss, R. S. Prakash, C. Basak, A. Szabo, L. Chaddock, J. S. Kim, S. Heo, H. Alves, S. M. White, T. R. Wojcicki, E. Mailey, V. J. Vieira, S. A. Martin, B. D. Pence, J. A. Woods, E. McAuley, and A. F. Kramer. 2011. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America 108(7):3017-3022.
Erickson, K. I., A. M. Weinstein, and O. L. Lopez. 2012. Physical activity, brain plasticity, and Alzheimer’s disease. Archives of Medical Research 43(8):615-621.
Espeland, M. A., K. Lipska, M. E. Miller, J. Rushing, R. A. Cohen, J. Verghese, M. M. McDermott, A. C. King, E. S. Strotmeyer, S. N. Blair, M. Pahor, K. Reid., J. Demons, S. B. Kritchevsky, and Life Study Investigators. 2017. Effects of physical activity intervention on physical and cognitive function in sedentary adults with and without diabetes. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 72(6):861-866.
Falck, R. S., J. C. Davis, and T. Liu-Ambrose. 2016. What is the association between sedentary behaviour and cognitive function? A systematic review. British Journal of Sports Medicine. http://bjsm.bmj.com/content/early/2016/05/06/bjsports-2015-095551 (accessed March 2, 2017).
Ford, E. S., U. A. Ajani, J. B. Croft, J. A. Critchley, D. R. Labarthe, T. E. Kottke, W. H. Giles, and S. Capewell. 2007. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. New England Journal of Medicine 356(23):2388-2398.
Forette, F., M. L. Seux, J. A. Staessen, L. Thijs, W. H. Birkenhager, M. R. Babarskiene, S. Babeanu, A. Bossini, B. Gil-Extremera, X. Girerd, T. Laks, E. Lilov, V. Moisseyev, J. Tuomilehto, H. Vanhanen, J. Webster, Y. Yodfat, and R. Fagard. 1998. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. The Lancet 352(9137):1347-1351.
Forette, F., M. L. Seux, J. A. Staessen, L. Thijs, M. R. Babarskiene, S. Babeanu, A. Bossini, R. Fagard, B. Gil-Extremera, T. Laks, Z. Kobalava, C. Sarti, J. Tuomilehto, H. Vanhanen, J. Webster, Y. Yodfat, and W. H. Birkenhager. 2002. The prevention of dementia with antihypertensive treatment: New evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Archives of Internal Medicine 162(18):2046-2052.
Foroughi, C. K., S. S. Monfort, M. Paczynski, P. E. McKnight, and P. M. Greenwood. 2016. Placebo effects in cognitive training. Proceedings of the National Academy of Sciences of the United States of America 113(27):7470-7474.
Gottesman, R. F., A. C. Schneider, M. Albert, A. Alonso, K. Bandeen-Roche, L. Coker, J. Coresh, D. Knopman, M. C. Power, A. Rawlings, A. R. Sharrett, L. M. Wruck, and T. H. Mosley. 2014. Midlife hypertension and 20-year cognitive change: The atherosclerosis risk in communities neurocognitive study. JAMA Neurology 71(10):1218-1227.
Hamer, M., and Y. Chida. 2009. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychological Medicine 39(1):3-11.
Hamer, M., K. L. Lavoie, and S. L. Bacon. 2014. Taking up physical activity in later life and healthy ageing: The English Longitudinal Study of Ageing. British Journal of Sports Medicine 48(3):239-243.
Hill, S. A. B. 1965. The environment and disease: Association or causation? Journal of the Royal Society of Medicine 58(5):295-300.
Howard, V. J., and M. N. McDonnell. 2015. Physical activity in primary stroke prevention: Just do it! Stroke 46(6):1735-1739.
Iadecola, C., K. Yaffe, J. Biller, L. C. Bratzke, F. M. Faraci, P. B. Gorelick, M. Gulati, H. Kamel, D. S. Knopman, L. J. Launer, J. S. Saczynski, S. Seshadri, and A. Zeki Al Hazzouri. 2016. Impact of hypertension on cognitive function: A scientific statement from the American Heart Association. Hypertension 68(6):e67-e94.
IOM (Institute of Medicine). 2015. Cognitive aging: Progress in understanding and opportunities for action. Washington, DC: The National Academies Press.
Jack, C. R., H. J. Wiste, P. Vemuri, S. D. Weigand, M. L. Senjem, G. Zeng, M. A. Bernstein, J. L. Gunter, V. S. Pankratz, P. S. Aisen, M. W. Weiner, R. C. Petersen, L. M. Shaw, J. Q. Trojanowski, and D. S. Knopman. 2010. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain 133(11):3336-3348.
Jellinger, K. A. 2007. The enigma of mixed dementia. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 3(1):40-53.
Jobe, J. B., D. M. Smith, K. Ball, S. L. Tennstedt, M. Marsiske, S. L. Willis, G. W. Rebok, J. N. Morris, K. F. Helmers, M. D. Leveck, and K. Kleinman. 2001. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials 22(4):453-479.
Kane, R. L., M. Butler, H. A. Fink, M. Brasure, H. Davila, P. Desai, E. Jutkowitz, E. McCreedy, V. Nelson, J. R. McCarten, C. Calvert, E. Ratner, L. Hemmy, and T. Barclay. 2017. Interventions to prevent age-related cognitive decline, mild cognitive impairment, and clinical Alzheimer’s-type dementia. Comparative effectiveness review 188. Rockville, MD: Agency for Healthcare Research and Quality.
Klusmann, V., A. Evers, R. Schwarzer, P. Schlattmann, F. M. Reischies, I. Heuser, and F. C. Dimeo. 2010. Complex mental and physical activity in older women and cognitive performance: A 6-month randomized controlled trial. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 65(6):680-688.
Koton, S., A. L. Schneider, W. D. Rosamond, E. Shahar, Y. Sang, R. F. Gottesman, and J. Coresh. 2014. Stroke incidence and mortality trends in U.S. communities, 1987 to 2011. Journal of the American Medical Association 312(3):259-268.
Kovacs, G. G., I. Alafuzoff, S. Al-Sarraj, T. Arzberger, N. Bogdanovic, S. Capellari, I. Ferrer, E. Gelpi, V. Kovari, H. Kretzschmar, Z. Nagy, P. Parchi, D. Seilhean, H. Soininen, C. Troakes, and H. Budka. 2008. Mixed brain pathologies in dementia: The BrainNet Europe consortium experience. Dementia and Geriatric Cognitive Disorders 26(4):343-350.
Kovacs, G. G., I. Milenkovic, A. Wöhrer, R. Höftberger, E. Gelpi, C. Haberler, S. Hönigschnabl, A. Reiner-Concin, H. Heinzl, S. Jungwirth, W. Krampla, P. Fischer, and H. Budka. 2013. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: A community-based autopsy series. Acta Neuropathologica 126(3):365-384.
Krell-Roesch, J., P. Vemuri, A. Pink, R. O. Roberts, G. B. Stokin, M. M. Mielke, T. J. Christianson, D. S. Knopman, R. C. Petersen, W. K. Kremers, and Y. E. Geda. 2017. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the APOE ε4 genotype. JAMA Neurology 74(3):332-338.
Langa, K. M., and D. A. Levine. 2014. The diagnosis and management of mild cognitive impairment: A clinical review. Journal of the American Medical Association 312(23): 2551-2561.
Larson, E. B., L. Wang, J. D. Bowen, W. C. McCormick, L. Teri, P. Crane, and W. Kukull. 2006. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Annals of Internal Medicine 144(2):73-81.
Lautenschlager, N. T., K. L. Cox, L. Flicker, J. K. Foster, F. M. van Bockxmeer, J. Xiao, K. R. Greenop, and O. P. Almeida. 2008. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. Journal of the American Medical Association 300(9):1027-1037.
Law, M. R., J. K. Morris, and N. J. Wald. 2009. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338:b1665.
Lee, I. M., E. J. Shiroma, F. Lobelo, P. Puska, S. N. Blair, and P. T. Katzmarzyk. 2012. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. The Lancet 380(9838):219-229.
Lee, S., A. Yuki, Y. Nishita, C. Tange, H. Kim, R. Kozakai, F. Ando, and H. Shimokata. 2013. Research relationship between light-intensity physical activity and cognitive function in a community-dwelling elderly population: An 8-year longitudinal study. Journal of the American Geriatrics Society 61(3):452-453.
Levi Marpillat, N., I. Macquin-Mavier, A. I. Tropeano, A. C. Bachoud-Levi, and P. Maison. 2013. Antihypertensive classes, cognitive decline and incidence of dementia: A network meta-analysis. Journal of Hypertension 31(6):1073-1082.
Lithell, H., L. Hansson, I. Skoog, D. Elmfeldt, A. Hofman, B. Olofsson, P. Trenkwalder, and A. Zanchetti. 2003. The Study on Cognition and Prognosis in the Elderly (SCOPE): Principal results of a randomized double-blind intervention trial. Journal of Hypertension 21(5):875-886.
Little, R. M. D., D. H. Paterson, D. A. Humphreys, and L. Stathokostas. 2013. A 12-month incidence of exercise-related injuries in previously sedentary community-dwelling older adults following an exercise intervention. BMJ Open 3(6).
Lucas, R. M., and A. J. McMichael. 2005. Association or causation: Evaluating links between “environment and disease.” Bulletin of the World Health Organization 83(10):792-795.
Marsh, A. P., W. B. Applegate, J. M. Guralnik, W. Jack Rejeski, T. S. Church, R. A. Fielding, T. M. Gill, A. C. King, S. B. Kritchevsky, T. M. Manini, M. M. McDermott, A. B. Newman, C. L. Stowe, M. P. Walkup, M. Pahor, and M. E. Miller. 2016. Hospitalizations during a physical activity intervention in older adults at risk of mobility disability: Analyses from the lifestyle interventions and independence for elders randomized clinical trial. Journal of the American Geriatrics Society 64(5):933-943.
Meng, X., and C. D’Arcy. 2012. Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS ONE 7(6):e38268.
Middleton, L. E., D. E. Barnes, L.-Y. Lui, and K. Yaffe. 2010. Physical activity over the life course and its association with cognitive performance and impairment in old age. Journal of the American Geriatrics Society 58(7):1322-1326.
Miller, K. J., R. V. Dye, J. Kim, J. L. Jennings, E. O’Toole, J. Wong, and P. Siddarth. 2013. Effect of a computerized brain exercise program on cognitive performance in older adults. American Journal of Geriatric Psychiatry 21(7):655-663.
Moran, A. E., M. C. Odden, A. Thanataveerat, K. Y. Tzong, P. W. Rasmussen, D. Guzman, L. Williams, K. Bibbins-Domingo, P. G. Coxson, and L. Goldman. 2015. Cost-effectiveness of hypertension therapy according to 2014 guidelines. New England Journal of Medicine 372(5):447-455.
MRC CFAS (Neuropathology Group of the Medical Research Council Cognitive Function and Aging Study). 2001. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. The Lancet 357(9251):169-175.
Muscari, A., C. Giannoni, L. Pierpaoli, A. Berzigotti, P. Maietta, E. Foschi, C. Ravaioli, G. Poggiopollini, G. Bianchi, D. Magalotti, C. Tentoni, and M. Zoli. 2010. Chronic endurance exercise training prevents aging-related cognitive decline in healthy older adults: A randomized controlled trial. International Journal of Geriatric Psychiatry 25(10):1055-1064.
Nagamatsu, L. S., T. C. Handy, C. L. Hsu, M. Voss, and T. Liu-Ambrose. 2012. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Archives of Internal Medicine 172(8):666-668.
National Center for Health Statistics. 2016. Health, United States, 2015: With special feature on racial and ethnic health disparities. Hyattsville, MD: National Center for Health Statistics.
Navar-Boggan, A. M., M. J. Pencina, K. Williams, A. D. Sniderman, and E. D. Peterson. 2014. Proportion of U.S. adults potentially affected by the 2014 hypertension guideline. Journal of the American Medical Association 311(14):1424-1429.
NHLBI (National Heart, Lung, and Blood Institute). 2004. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute.
Northey, J. M., N. Cherbuin, K. L. Pumpa, D. J. Smee, and B. Rattray. 2017. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. British Journal of Sports Medicine [Epub ahead of print].
Norton, S., F. E. Matthews, D. E. Barnes, K. Yaffe, and C. Brayne. 2014. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. The Lancet Neurology 13(8):788-794.
O’Shea, A., R. Cohen, E. Porges, N. Nissim, and A. Woods. 2016. Cognitive aging and the hippocampus in older adults. Frontiers in Aging Neuroscience 8(298).
Pahor, M., J. M. Guralnik, W. T. Ambrosius, S. Blair, D. E. Bonds, T. S. Church, M. A. Espeland, R. A. Fielding, T. M. Gill, E. J. Groessl, A. C. King, S. B. Kritchevsky, T. M. Manini, M. M. McDermott, M. E. Miller, A. B. Newman, W. J. Rejeski, K. M. Sink, and J. D. Williamson. 2014. Effect of structured physical activity on prevention of major mobility disability in older adults: The life study randomized clinical trial. Journal of the American Medical Association 311(23):2387-2396.
Patel, A., A. C. Group, S. MacMahon, J. Chalmers, B. Neal, M. Woodward, L. Billot, S. Harrap, N. Poulter, M. Marre, M. Cooper, P. Glasziou, D. E. Grobbee, P. Hamet, S. Heller, L. S. Liu, G. Mancia, C. E. Mogensen, C. Y. Pan, A. Rodgers, and B. Williams. 2007. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. The Lancet 370(9590):829-840.
Peila, R., L. R. White, K. Masaki, H. Petrovitch, and L. J. Launer. 2006. Reducing the risk of dementia: Efficacy of long-term treatment of hypertension. Stroke 37(5):1165-1170.
Pendlebury, S. T., and P. M. Rothwell. 2009. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. The Lancet Neurology 8(11):1006-1018.
Peters, R., N. Beckett, F. Forette, J. Tuomilehto, R. Clarke, C. Ritchie, A. Waldman, I. Walton, R. Poulter, S. Ma, M. Comsa, L. Burch, A. Fletcher, and C. Bulpitt. 2008. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial Cognitive Function Assessment (HYVET-COG): A double-blind, placebo controlled trial. The Lancet Neurology 7(8):683-689.
Poblador-Plou, B., A. Calderón-Larrañaga, J. Marta-Moreno, J. Hancco-Saavedra, A. Sicras-Mainar, M. Soljak, and A. Prados-Torres. 2014. Comorbidity of dementia: Cross-sectional study of primary care older patients. BMC Psychiatry 14:84.
Protogerou, A. D., M. E. Safar, P. Iaria, H. Safar, K. Le Dudal, J. Filipovsky, O. Henry, P. Ducimetiere, and J. Blacher. 2007. Diastolic blood pressure and mortality in the elderly with cardiovascular disease. Hypertension 50(1):172-180.
Qiu, C., B. Winblad, and L. Fratiglioni. 2005. The age-dependent relation of blood pressure to cognitive function and dementia. The Lancet Neurology 4(8):487-499.
Rahimi, J., and G. G. Kovacs. 2014. Prevalence of mixed pathologies in the aging brain. Alzheimer’s Research & Therapy 6(9):82.
Raz, N., U. Lindenberger, K. M. Rodrigue, K. M. Kennedy, D. Head, A. Williamson, C. Dahle, D. Gerstorf, and J. D. Acker. 2005. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex 15(11):1676-1689.
Rebok, G. W., K. Ball, L. T. Guey, R. N. Jones, H. Y. Kim, J. W. King, M. Marsiske, J. N. Morris, S. L. Tennstedt, F. W. Unverzagt, and S. L. Willis. 2014. Ten-year effects of the Advanced Cognitive Training for Independent and Vital Elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society 62(1):16-24.
Ross, S. D., K. S. Akhras, S. Zhang, M. Rozinsky, and L. Nalysnyk. 2001. Discontinuation of antihypertensive drugs due to adverse events: A systematic review and meta-analysis. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 21(8):940-953.
Rouch, L., P. Cestac, O. Hanon, C. Cool, C. Helmer, B. Bouhanick, B. Chamontin, J. F. Dartigues, B. Vellas, and S. Andrieu. 2015. Antihypertensive drugs, prevention of cognitive decline and dementia: A systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs 29(2):113-130.
Ruscheweyh, R., C. Willemer, K. Kruger, T. Duning, T. Warnecke, J. Sommer, K. Volker, H. V. Ho, F. Mooren, S. Knecht, and A. Floel. 2011. Physical activity and memory functions: An interventional study. Neurobiology of Aging 32(7):1304-1319.
Saito, H., H. Togashi, M. Yoshioka, N. Nakamura, M. Minami, and H. Parvez. 1995. Animal models of vascular dementia with emphasis on stroke-prone spontaneously hypertensive rats. Clinical and Experimental Pharmacology and Physiology 22(1):S257-S259.
Saito, Y., and S. Murayama. 2007. Neuropathology of mild cognitive impairment. Neuropathology 27(6):578-584.
Scarmeas, N., J. A. Luchsinger, N. Schupf, A. M. Brickman, S. Cosentino, M. X. Tang, and Y. Stern. 2009. Physical activity, diet, and risk of Alzheimer’s disease. Journal of the American Medical Association 302(6):627-637.
Simons, D. J., W. R. Boot, N. Charness, S. E. Gathercole, C. F. Chabris, D. Z. Hambrick, and E. A. Stine-Morrow. 2016. Do “brain-training” programs work? Psychological Science in the Public Interest 17(3):103-186.
Singh-Manoux, A., M. Hillsdon, E. Brunner, and M. Marmot. 2005. Effects of physical activity on cognitive functioning in middle age: Evidence from the Whitehall II Prospective Cohort Study. American Journal of Public Health 95(12):2252-2258.
Sink, K. M., M. A. Espeland, C. M. Castro, T. Church, R. Cohen, J. A. Dodson, J. Guralnik, H. C. Hendrie, J. Jennings, J. Katula, O. L. Lopez, M. M. McDermott, M. Pahor, K. F. Reid, J. Rushing, J. Verghese, S. Rapp, and J. D. Williamson. 2015. Effect of a 24-month physical activity intervention vs. health education on cognitive outcomes in sedentary older adults: The LIFE randomized trial. Journal of the American Medical Association 314(8):781-790.
Sipahi, I., A. Swaminathan, V. Natesan, S. M. Debanne, D. I. Simon, and J. C. Fang. 2012. Effect of antihypertensive therapy on incident stroke in cohorts with prehypertensive blood pressure levels: A meta-analysis of randomized controlled trials. Stroke 43(2):432-440.
Sofi, F., D. Valecchi, D. Bacci, R. Abbate, G. F. Gensini, A. Casini, and C. Macchi. 2011. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. Journal of Internal Medicine 269(1):107-117.
Spartano, N. L., J. J. Himali, A. S. Beiser, G. D. Lewis, C. DeCarli, R. S. Vasan, and S. Seshadri. 2016. Midlife exercise blood pressure, heart rate, and fitness relate to brain volume 2 decades later. Neurology 86(14):1313-1319.
The SPRINT Research Group. 2015. A randomized trial of intensive versus standard blood-pressure control. New England Journal of Medicine 373(22):2103-2116.
Staessen, J. A., R. Fagard, L. Thijs, H. Celis, G. G. Arabidze, W. H. Birkenhäger, C. J. Bulpitt, P. W. de Leeuw, C. T. Dollery, A. E. Fletcher, F. Forette, G. Leonetti, C. Nachev, E. T. O’Brien, J. Rosenfeld, J. L. Rodicio, J. Tuomilehto, and A. Zanchetti. 1997. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Lancet 350(9080):757-764.
Stanford Center on Longevity. 2014. A consensus on the brain training industry from the scientific community. http://longevity3.stanford.edu/blog/2014/10/15/the-consensus-on-the-brain-training-industry-from-the-scientific-community-2 (accessed March 2, 2017).
Stern, Y. 2012. Cognitive reserve in ageing and Alzheimer’s disease. The Lancet Neurology 11(11):1006-1012.
Stine-Morrow, E. A., B. R. Payne, B. W. Roberts, A. F. Kramer, D. G. Morrow, L. Payne, P. L. Hill, J. J. Jackson, X. Gao, S. R. Noh, M. C. Janke, and J. M. Parisi. 2014. Training versus engagement as paths to cognitive enrichment with aging. Psychology & Aging 29(4):891-906.
Suzuki, T., H. Shimada, H. Makizako, T. Doi, D. Yoshida, K. Tsutsumimoto, Y. Anan, K. Uemura, S. Lee, and H. Park. 2012. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: A randomized controlled trial. BMC Neurology 12:128.
Tan, Z. S., N. L. Spartano, A. S. Beiser, C. DeCarli, S. H. Auerbach, R. S. Vasan, and S. Seshadri. 2017. Physical activity, brain volume, and dementia risk: The Framingham Study. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences 72(6):789-795.
Tolppanen, A. M., A. Solomon, J. Kulmala, I. Kareholt, T. Ngandu, M. Rusanen, T. Laatikainen, H. Soininen, and M. Kivipelto. 2015. Leisure-time physical activity from mid- to late life, body mass index, and risk of dementia. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 11(4):434-443, e436.
Turnbull, F. 2003. Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. The Lancet 362(9395):1527-1535.
Tzourio, C., C. Anderson, N. Chapman, M. Woodward, B. Neal, S. MacMahon, and J. Chalmers. 2003. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Archives of Internal Medicine 163(9):1069-1075.
Unverzagt, F. W., L. Kasten, K. E. Johnson, G. W. Rebok, M. Marsiske, K. M. Koepke, J. W. Elias, J. N. Morris, S. L. Willis, K. Ball, D. F. Rexroth, D. M. Smith, F. D. Wolinsky, and S. L. Tennstedt. 2007. Effect of memory impairment on training outcomes in ACTIVE. Journal of the International Neuropsychological Society 13(6):953-960.
Unverzagt, F. W., L. T. Guey, R. N. Jones, M. Marsiske, J. W. King, V. G. Wadley, M. Crowe, G. W. Rebok, and S. L. Tennstedt. 2012. ACTIVE cognitive training and rates of incident dementia. Journal of the International Neuropsychological Society 18(4):669-677.
Valenzuela, M. J. 2008. Brain reserve and the prevention of dementia. Current Opinion in Psychiatry 21(3):296-302.
Vermeer, S. E., N. D. Prins, T. den Heijer, A. Hofman, P. J. Koudstaal, and M. Breteler. 2003. Silent brain infarcts and the risk of dementia and cognitive decline. New England Journal of Medicine 348(13):1215-1222.
Wang, J.-G., J. A. Staessen, S. S. Franklin, R. Fagard, and F. Gueyffier. 2005. Systolic and diastolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension 45(5):907-913.
Wexler, R., and G. Aukerman. 2006. Nonpharmacologic strategies for managing hypertension. American Family Physician 73(11):1953-1956.
WHO (World Health Organization) and World Economic Forum. 2011. From burden to “best buys”: Reducing the economic impact of non-communicable diseases in low- and middle-income countries. Geneva, Switzerland: WHO.
Willey, J. Z., H. Gardener, M. R. Caunca, Y. P. Moon, C. Dong, Y. K. Cheung, R. L. Sacco, M. S. V. Elkind, and C. B. Wright. 2016. Leisure-time physical activity associates with cognitive decline: The Northern Manhattan Study. Neurology 86(20):1897-1903.
Williamson, J. D., L. J. Launer, R. N. Bryan, L. H. Coker, R. M. Lazar, H. C. Gerstein, A. M. Murray, M. D. Sullivan, K. R. Horowitz, J. Ding, S. Marcovina, L. Lovato, J. Lovato, K. L. Margolis, C. Davatzikos, J. Barzilay, H. N. Ginsberg, P. E. Linz, and M. E. Miller. 2014. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: A randomized clinical trial. JAMA Internal Medicine 174(3):324-333.
Willis, S. L., S. L. Tennstedt, M. Marsiske, K. Ball, J. Elias, K. M. Koepke, J. N. Morris, G. W. Rebok, F. W. Unverzagt, A. M. Stoddard, and E. Wright. 2006. Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association 296(23):2805-2814.
Wilson, R. S., P. A. Boyle, L. Yu, L. L. Barnes, J. A. Schneider, and D. A. Bennett. 2013. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 81(4):314-321.
Wolinsky, F. D., M. W. Vander Weg, M. B. Howren, M. P. Jones, and M. M. Dotson. 2013. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS ONE 8(5):e61624.
Wyss, J. M., I. Kadish, and T. van Groen. 2003. Age-related decline in spatial learning and memory: Attenuation by captopril. Clinical and Experimental Hypertension 25(7): 455-474.
Yaffe, K., D. Barnes, M. Nevitt, L. Lui, and K. Covinsky. 2001. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Archives of Internal Medicine 161(14):1703-1708.
Yamori, Y., R. Horie, H. Handa, M. Sato, and M. Fukase. 1976. Pathogenetic similarity of strokes in stroke-prone spontaneously hypertensive rats and humans. Stroke 7(1):46-53.
Young, J., M. Angearen, J. Rusted, and N. Tabet. 2015. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews (4):CD005381.
Zheng, G., R. Xia, W. Zhou, J. Tao, and L. Chen. 2016. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis of randomised controlled trials. British Journal of Sports Medicine 6(50):1443-1450.
Zhu, W., V. G. Wadley, V. J. Howard, B. Hutto, S. N. Blair, and S. P. Hooker. 2017. Objectively measured physical activity and cognitive function in older adults. Medicine & Science in Sports & Exercise 49(1):47-53.
Zlokovic, B. V. 2011. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neuroscience 12(12):723-738.








































