5
Measuring Chronic Disease Outcomes
Studying associations between nutrients or other food substances (NOFSs) and health outcomes starts by formulating specific question(s) about the population of interest, the intervention (e.g., a nutrient level), the comparator (e.g., a different nutrient level), and the health outcome. These elements of the PICO (population, intervention, comparator, and outcome) evidence review framework are described in more detail in Chapter 6. Although these four elements seem simple to address, both the groups preparing and answering the questions face challenges unique to each element. Challenges include using consistent terminology, determining the appropriateness of measurement methods, and being able to identify limitations. This chapter focuses on the component that involves measuring the health outcome. In this case, the outcome, or endpoint, is the chronic disease event (e.g., a diagnosis of heart disease, stroke, diabetes, or a person’s death).
The goal of this chapter is to provide Dietary Reference Intakes (DRI) committees with guidance on considerations related to potential biases and limitations in measuring chronic disease outcomes reported in individual research studies and included in systematic reviews and evidence summaries that are used to make judgments about relationships between NOFSs and a chronic disease. Considerations include how well the outcome has been defined and measured, either directly or indirectly with a surrogate marker that is sufficiently specific to reflect the causal pathway of potential interest. The chapter also includes a general section on chronic disease for context and key definitions.
This guidance is provided within the context of the report Options for Basing Dietary Reference Intakes (DRIs) on Chronic Disease Endpoints:
Report from a Joint US-/Canadian-sponsored Working Group (i.e., the Options Report) (Yetley et al., 2017) and the committee’s statement of task as described in Chapter 1. A critical issue raised in the Options Report relates to the decision to use surrogate markers of disease when information on chronic diseases events is not available or as supplementary information. Research studies often include information on biomarkers along a pathway between exposure and disease, and some can serve as surrogate markers of disease when chronic disease events have not been measured or were observed in numbers too small to permit definitive statistical analyses of causal relationships.
CHRONIC DISEASE OUTCOMES
Defining Chronic Disease
Chronic diseases, including diabetes, cardiovascular and neurodegenerative diseases, and many common cancers, manifest over a lifetime and can begin before birth. As such, aging is widely recognized as the leading risk factor for many human diseases (Niccoli and Partridge, 2012). Chronic diseases are complex traits, and susceptibility to their onset and their progression vary among individuals within a population. Risk of developing a chronic disease is associated with both genetic and non-genetic exposures (Fontana et al., 2010). Family history, including genetic endowment, remain the best predictors of longevity potential (Gavrilov and Gavrilova, 2015), and for many people, their susceptibility to chronic disease. Environmental and lifestyle factors, including diet and exercise, are among the leading modifiable risk factors for chronic disease onset and management (Benziger et al., 2016; GBD 2013 Risk Factors Collaborators, 2015).
Definitions of chronic disease1 often encompass distinct anatomic, physiological, or behavioral abnormalities; theories and/or evidence of causation; and usually some degree of human suffering. Research on disease detection and diagnosis reveals numerous challenges (IOM, 2015) that are outside of the scope of this study. However, when exploring relationships between NOFS intake and disease occurrence or other outcomes,2 it is essential to consider the accuracy of the diagnostics and measures as well as
___________________
1 A chronic disease is “a culmination of a series of pathogenic processes in response to internal or external stimuli over time that results in a clinical diagnosis/ailment and health outcomes” (e.g., diabetes) (IOM, 2010, p. 23).
2 The most common use of the term “outcome,” used synonymously with “endpoints,” refers to the clinical results of a particular illness(es), often after particular therapeutic interventions. With regard to DRIs, the outcome might be a change in disease incidence (primary prevention of coronary disease) but also can be improvement of the clinical outcome of patients who have already sustained a heart attack (secondary prevention).
the existence of disease category subtypes that may respond to NOFS exposures differentially. As an example, a World Health Organization (WHO) systematic review (WHO, 2012) that explored the effects of potassium on all cardiovascular disease, stroke, and coronary heart disease events (fatal and non-fatal) considered a composite as a measure of cardiovascular disease, which included some or all of the following: fatal and non-fatal stroke, coronary heart disease, myocardial infarction, and/or congestive cardiac failure, or episode of coronary revascularization, bypass grafting, and/or angioplasty. They also considered all-cause mortality and all other outcomes reported by the authors of the original studies.
The role of nutrition in chronic disease risk can begin in utero, as nutrition exposures early in life can influence fetal and neonatal gene expression patterns. As such, nutrient exposures drive the epigenetic programming of physiological cellular networks, and these programs, which may persist throughout the lifetime of mammals, affecting disease susceptibility (Waterland and Michels, 2007). Likewise, the composition of the gut microbiome is associated with chronic diseases (Shreiner et al., 2015). The relative contribution of modifiable (e.g., dietary factors) and non-modifiable (e.g., genetics) contributions to chronic diseases within populations merits consideration when establishing DRIs using chronic disease endpoints, as does the magnitude of population heterogeneity leading to individual variation in the diet-disease relationship (Ohlhorst et al., 2013; Rappaport, 2016).
Although links between nutrition and chronic diseases are frequently identified for common conditions such as diabetes, cardiovascular diseases, and some types of cancer, the committee cannot predict which chronic diseases could be related to NOFSs in the future. Table 2-3 in Chapter 2 indicates that a range of chronic conditions have been considered by prior DRI committees, not all of which are life-threatening. Which potential nutrient-related health outcomes warrant priority for DRIs and population nutrition policy will continue to be an important issue for federal agencies. Therefore, the committee chose to use a broad definition of chronic disease, which includes (1) diseases that last for months or years, or their outcomes (e.g., longevity, disease-specific mortality, and all-cause mortality), even if the cause is unknown, (2) some infectious organism-induced diseases, such as AIDS, chronic hepatitis, cervical cancer, or gastric cancer, that could be potentially mitigated by nutritional interventions, or (3) clinical conditions that lack a formal disease status, such as chronic pain syndromes of unknown cause, or clinical syndromes difficult to characterize, such as Gulf War Syndrome or chronic fatigue syndrome.
Interpreting the effects of NOFSs may be complicated by the nature of chronic diseases. For example, some chronic conditions that surface late in life (e.g., atherosclerotic diseases) may have their origins at a young age. In addition, many chronic disease outcomes are likely to differ in the age at
onset, progression, and severity. Therefore, it might not be clear when an NOFS intervention may have its effect within the disease pathway. Also, study outcomes associated with an NOFS intervention may differ depending on diagnostic procedures and protocols used. For example, diseases that are detected by screening tests when individuals are asymptomatic (e.g., through mammography and Pap smears) may have different clinical outcomes than those detected in response to patients with clinical signs and symptoms (Hillerdal, 2008; Jensen and Vedsted, 2017). Furthermore, variation in access to medical care and in the types of clinical treatments may have important (and confounding) effects on outcome rates, particularly in observational studies of NOFSs.
Regardless of the definition adopted, it is important to delineate the condition of interest with high specificity. Is the disease truly chronic? Is the disease biologically heterogeneous in etiology and in responsiveness to nutrients? Do standard diagnostic methods exist? Does the disease have sub-conditions based on clinical behavior, pathophysiology, molecular markers and biology, and outcomes (prognosis)?
Measuring Chronic Disease Outcomes
Once the outcomes of interest are designated (see Figure 1-2), acceptable methods for measurement (i.e., for inclusion in the systematic review) should be selected a priori based on recommendations from relevant authoritative clinical guidelines.
Each chronic disease can be measured with a different set of methodologies, which have strengths and limitations. When considered a priori, identifying the strengths and limitations of the measurements will allow a DRI committee to make judgments about the potential risk of bias due to the outcome measurements for each study. Assessing the accuracy of occurrence of particular chronic diseases in research studies can be challenging, however. First, diagnosis of a chronic disease itself may be complicated. As an example, coronary heart disease may be suspected based on medical and family history, risk factors, and tests, but no single test for a definitive diagnosis is currently available. Data from studies relying on self-reporting of chronic diseases depend on having had an opportunity for diagnosis as well as accurate recall of the diagnosis; they may, therefore, be regarded as not suitably accurate when establishing DRIs. The accuracy of self-report data depends on the disease and its details, as some diseases are more accurately reported than others (Navin Christina et al., 2016). Self-report data can vary substantially from data based on disease ascertainment from primary clinical records or from population surveys or research studies that involve diagnostic procedures (e.g., the National Health and Nutrition Examination Survey, clinical trials, or observational studies).
Clinical records, including various formats such as electronic records or registries derived from them, are expected to be more accurate than patient self-report, but for various reasons, clinical records of any type may not be fully accurate or complete. Clinical records depend on comprehensive and accurately coded data and the accuracy and completeness of the information stored in electronic (or paper) patient records varies widely. The International Classification of Diseases (ICD), which is maintained by WHO, is the international standard diagnostic tool and provides a system of codes for classifying diseases, with a variety of signs and symptoms. In the United States, the ICD-10-CM (ICD, 10th Revision, Clinical Modification) is used by hospitals and other health care facilities to better describe the clinical picture of the patient. Cause of death on United States death certificates are also coded in the ICD. One systematic review examined data quality in electronic primary care records and found that the quality of recording in diagnoses of diseases, in particular, varied; completeness was higher when clear diagnostic criteria existed (Thiru et al., 2003). Another systematic review (Jordan et al., 2004) found that the quality of assigning a morbidity code during primary care consultations also varied depending on the condition. Some cited areas where efforts are needed are development of data quality standards and improvements in understanding implementation challenges.
Another critical measure is mortality from the chronic disease. The cause-of-death statistics as reported in the United States by the U.S. Centers for Disease Control and Prevention’s National Vital Statistics System3 are undoubtedly a valuable tool for research and other public health purposes, but accuracy is variable and depends on the disease and other factors, such as (1) potential diagnostic and certification errors, (2) whether autopsies are performed or data are based on medical records, (3) the training of the certifier, and (4) the presence of multiple conditions leading to the death (Lloyd et al., 2017).
Using Surrogate Markers as a Substitute for a Chronic Disease Outcomes
The initial problem formulation (see Figure 1-2) about a general health outcome of interest that may be associated with intake of a given NOFS leads to another fundamental decision point: whether to require evidence based on the chronic disease outcome(s), accept a biomarker(s) of effect that can lead to a valid and reliable test of association between the NOFS and the risk of the chronic disease outcome (i.e., a surrogate marker), or use both types of measures. In regard to establishing DRIs, both biomarkers of functional outcomes (e.g., activity of an enzyme) and biomarkers of effect (e.g., a chronic disease) have been used but they have distinct
___________________
3 See https://www.cdc.gov/nchs/nvss (accessed July 20, 2017).
TABLE 5-1 Characteristics of Biomarkers of Nutrient Function Versus Biomarkers of Effect
| Characteristics | Biomarkers of Nutrient Function | Biomarkers of Effect (e.g., cancer, heart disease) |
|---|---|---|
| Variables that affect the indicator | Intake of the nutrient is the primary modifiable variable affecting the nutrient function endpoint or development of nutrient deficiency symptoms (unmodifiable variables such as age, sex, genetics, also may play a role, e.g., risk of iron deficiency). | Intake of the nutrient may be one of many modifiable variables (e.g., intake of other nutrients or food substances, physical activity, weight status, environmental exposures) and subgroup characteristics (e.g., age, sex, genetics) that affect development of the chronic disease. |
| Time course over which the nutrient influences the endpoint | Relatively short (e.g., weeks to months in most cases). | Very long (potentially over the lifespan). |
| Without adequate intake, proportion of the population who will develop the deficiency or chronic disease | 100 percent (e.g., with very low intakes of vitamin C, everyone will develop scurvy, as vitamin C deficiency is the only cause of scurvy). | Always <100 percent (no chronic diseases occur in 100 percent of the population; for example, the lifetime risk of cancer in the United States is 42 percent, not 100 percent) (ACS, 2017). |
| With adequate intake, proportion of the population who will be protected against the deficiency or the chronic disease | 100 percent (e.g., with enough vitamin C, no one will develop scurvy, because vitamin C deficiency is the only cause of scurvy). | Extremely variable, but always <100 percent. In most cases, the reduction in the absolute risk associated with modifying a single nutrient would be expected to be relatively small (as a hypothetical example, perhaps 40 percent of those with adequate intake of the nutrient would develop the chronic disease, versus 44 percent of those without an adequate intake). |
| Characteristics | Biomarkers of Nutrient Function | Biomarkers of Effect (e.g., cancer, heart disease) |
|---|---|---|
| Relationship between intake and health or functional status with regard to the indicator of adequacy | At intake below the requirement, function would be impaired. No additional increases in function are expected at intakes above the requirement. | In theory, many different types of relationships between nutrient intake and chronic disease risk are possible, and the type of relationship could differ for a single nutrient and different chronic diseases, or for different nutrients and a single chronic disease. Potentially, there could be a broad range of intake over which relative risk of developing a chronic disease could change in a graded manner, but it may not be possible to identify a point at which no further risk reduction occurs. Conversely, it is possible that, beyond a range of intake over which risk is reduced, risk could begin to increase. |
characteristics (see Table 5-1). Recognizing the current efforts to modernize and standardize the terminology about biomarkers in the medical field (e.g., Robb et al., 2016), the committee adopted the terms used in the Options Report, “surrogate disease markers” and “nonqualified disease marker” (see Box 5-1) to refer to biomarkers of effect that are of interest for this chapter. Box 5-1 also includes a general definition for biomarkers to provide context. Although biomarkers of nutrient intake (which have been discussed in Chapter 4) are included in the definition for completeness, the focus here is on biomarkers of disease endpoints.
Surrogate Markers in Studies of Nutrient-Chronic Disease Relationships
As noted in Chapter 3, studies that employ randomized controlled trial (RCT) designs have the greatest likelihood of establishing causation compared to observational study designs. However, using disease events as outcome measures may not always be feasible due to study expense, the rarity of the disease in question, time imperatives, or the complexity of diagnosis. In those situations, some studies may resort to surrogate markers. These are measures deemed to be on the causal pathways to frank illness,
whose occurrence would often lead to that illness should more time and larger sample sizes be available. Furthermore, the status of the surrogate marker, or more generally the surrogate marker process history, should be able to explain a substantial portion of any relationship between the NOFS and the chronic disease risk (Freedman et al., 1992; Prentice, 1989). A surrogate marker may be any type of biomarker. However, in diagnosing a disease, determining a surrogate marker may require extensive resources, such as a comprehensive clinical evaluation, just as is generally needed for the primary illness. For example, if an adenomatous colon polyp is to be considered a surrogate marker for colon cancer, detecting and diagnosing the polyp usually requires a full clinical examination and colonoscopy.
When evaluating studies relevant to nutrition, identifying true surrogate markers may be problematic for several reasons. Surrogate markers are complex and often relate to the natural history of the specific disease in question. Many categories of conditions, such as many cancers, psychiatric conditions, and neurodegenerative diseases, do not have credible surrogate markers relative to any set of treatments or exposures. Some are not true surrogate markers but may just reflect the activity of existing, active conditions, such as prostate-specific antigen (PSA) for prostate cancer or serological markers of inflammation in various infections or rheumatic conditions. Another challenge is the usual modest ability of candidate surrogate markers to predict full disease outcomes. Disease occurrence may be many years or decades in the future and difficult to evaluate. In addition, other positive or adverse health consequences predicted by that candidate surrogate markers may not be well-evaluated. Perhaps most important, the candidate surrogate marker may only be a risk predictor and not related causally to the condition of interest.
Another complication with selecting candidate surrogate markers is reflected in Figure 5-1. The figure shows how candidate surrogate markers can be the result of multiple exposures and biological pathways, illustrating that they may not be on the causal pathway of the disease. The figure also shows that multiple NOFSs can affect one or more biological pathways. When a chronic disease might be the result of multiple biological pathways, extracting and interpreting specific information about the relationship between one NOFS and the chronic disease outcome in human studies requires a substantial understanding of underlying biological mechanisms, and may not be possible depending on the nature of relevant interrelation-
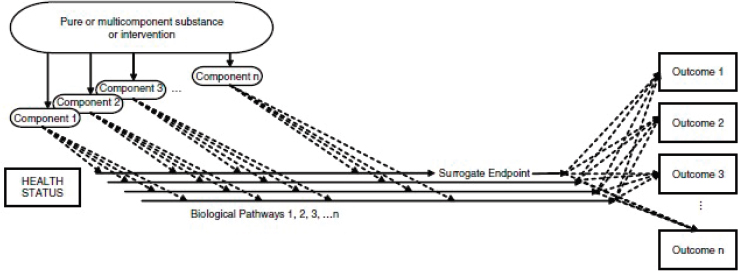
SOURCE: IOM, 2010.
ships. Efforts are ongoing to identify better surrogate markers, which might be important to support causal associations between NOFS and chronic diseases.
One example of the value of surrogate markers is in cancer research where, to be meaningful, studies need to be large and lengthy because specific cancers are not frequent and often take a long time to develop. In exploring the contribution of nutrition to cancer, one area of interest is the use of colorectal adenomas as candidate surrogates because they occur earlier in the disease pathway and relatively frequently compared to the incidence of colorectal cancer. Strong evidence shows a relationship between this marker and colorectal cancer (Fearon and Vogelstein, 1990; Paraskeva et al., 1990; Sugarbaker et al., 1985). The surrogate is still not without challenges, however. Because adenomas occur early in the pathway to disease, if the nutritional intervention has an effect only in the later stages, such an intervention would not have the expected effect on the surrogate marker (false negatives). Another limitation is that only a small proportion of adenomas may ever result in cancer. These considerations need to be accounted for when interpreting the results of interventions. Still, a nutritional intervention reducing the recurrence of adenomas in the large bowel would likely decrease the incidence of colorectal cancer and therefore various nutrition studies have taken advantage of the value of this potential surrogate (Greenberg et al., 1994; Neuhouser et al., 2015; Takata et al., 2014). Another example of the challenges is the ongoing need to find surrogate markers for cardiovascular disease, one of the major causes of death worldwide. The longstanding nutritional guidance of replacing saturated fatty acids (SFAs) in the diet with unsaturated fats has recently been challenged by researchers, who have argued that prospective cohort studies do not show an association between saturated fats, polyunsaturated fatty acids (PUFAs), or monounsaturated fatty acids (MUFAs) with risk of coronary heart disease (Chowdhury et al., 2014). These controversies would be clarified with a reliable surrogate marker. Possible surrogates include blood lipids (low-density lipoprotein [LDL] cholesterol, high-density lipoprotein cholesterol, triglycerides, ApoB, or ApoA1) and fatty acids (PUFA, MUFA, SFA, or omega-3 PUFAs), measured over pertinent subsets of the lifespan. In addition to these more conventional surrogates, the emerging field of metabolomics has recently led to use of plasma metabolites as possible surrogates. Although prospective cohort studies indicate that LDL cholesterol is a good predictor of cardiovascular disease outcome (Lewington et al., 2007), the prediction value of other lipids is questionable (Clarke, 2017). This continued debate shows the difficulties in identifying and interpreting the results when potential surrogate markers are used as predictors of a chronic disease.
New Research on Biomarkers of Chronic Disease
Research on next generation biomarkers of nutrition and chronic disease seeks to (1) identify and classify individuals who are at risk of diet-related chronic disease, the paradigm that currently drives the field of precision medicine (Collins and Varmus, 2015), and (2) quantify the dose-response relationships between individual or groups of nutrients and disease onset and progression (Ohlhorst et al., 2013).
Metabolomics approaches are enabling the identification of comprehensive metabolic signatures in a single assay comprising hundreds of metabolites in serum or other biological fluids (Beger et al., 2016). This snapshot of system biomarkers has the potential to enhance the prediction and identification of disease states at a high level of resolution, as well as inform pharmacological and/or nutrition regimes for chronic disease treatment and prevention. Comprehensive measures of metabolic function can be integrated with other “omics” measures of genetic variation, epigenetic variation, and transcriptional and proteomic measures of gene expression to elucidate the multidimensional biological changes that occur throughout the disease process (Arneson et al., 2017).
Qualified Surrogate Markers
Biomarkers of effect (in this case, a chronic disease) comprise those that are “qualified” (i.e., surrogate marker) and “nonqualified” (i.e., nonqualified disease marker). Therefore, a surrogate marker is, by definition, qualified for its purpose. The concept of “qualified” surrogate markers originated in the medical community, particularly as it applies to pharmaceutical research, but is now being applied to nutrition. The difference between these two types of markers is described in Box 5-1. From a regulatory perspective, qualified surrogate outcomes need to meet certain criteria regarding prediction of clinical outcomes. In this context, the interested parties, such as a pharmaceutical industry, may work collaboratively with FDA’s Center for Drug Evaluation and Research (CDER) Biomarker Qualification Program to guide marker development (FDA, 2017a). The qualification process is initiated by submitters, who are typically interested in a drug approval, once they have (1) a clear understanding of the relationship between the candidate surrogate marker and the clinical outcome, (2) a defined use for the candidate surrogate marker in drug development, and (3) an identified candidate surrogate marker measure, preferably analytically validated. After this initial step, a consultation process is initiated with FDA. The process is similar to that used when a biomarker is intended to be used as evidence for submission of a nutrient health claim. Based on the need for scientific rigor in this qualification process, the IOM published a
2010 report Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease. The report recommends a framework for evaluating biomarkers used as qualified surrogate outcomes that includes three elements: analytical validation,4 evidentiary qualification,5 and utilization analysis6 (IOM, 2010). The report also recommends that FDA use the same degree of scientific rigor to evaluate markers across all regulatory areas—drugs, medical devices, biologics, or foods and dietary supplements.
Examples of biomarkers accepted as qualified surrogate endpoints for the purpose of health claims for specific chronic disease endpoints by FDA’s Center for Food Safety and Nutrition include (1) serum LDL cholesterol concentration, total serum cholesterol concentration, and blood pressure for cardiovascular disease, (2) bone mineral density for osteoporosis, (3) adenomatous colon polyps for colon cancer, and (4) elevated blood sugar concentrations and insulin resistance for type 2 diabetes (FDA/CFSAN, 2009). Although the surrogate is qualified as a marker of the outcome (independent of the NOFS), the NOFS of interest needs to have an effect on the qualified surrogate marker, not on a different marker. The National Institutes of Health (NIH) and/or FDA’s CDER also lists examples of biomarkers of disease risk accepted as “qualified surrogate endpoints” as of December 2015 (FDA, 2017b).
Past DRI committees have attempted to consider chronic disease outcomes when assessing possible indicators of adequacy or excessive intakes of nutrients. Examples of the chronic diseases considered are listed in Table 5-2. Table 5-2 also identifies nutrients for which a DRI was established based on chronic disease risk, as well as whether direct (i.e., disease outcome) or indirect (i.e., biomarker of effect) outcomes were used to set the DRI. Finally, it lists nutrient-disease associations that were considered, but ultimately not used to set DRIs, largely because of insufficient or inconsistent evidence. Although numerous chronic diseases and nutrients were considered, DRIs based on chronic disease risk were set in only a small number of cases (see Chapter 2, Table 2-3). Among those, in most cases an
___________________
4 Analytical validation: Assessment of [an] “assay and its measurement performance characteristics, determining the range of conditions under which the assay will give reproducible and accurate data” (IOM, 2010, p. 3).
5 Evidentiary qualification: “Assessment of available evidence on associations between the biomarker and disease states, including data showing effects of interventions on both the biomarker and clinical outcomes” (IOM, 2010, p. 2).
6 Utilization analysis: “Contextual analysis based on the specific use proposed and the applicability of available evidence to this use. This includes a determination of whether the validation and qualification conducted provide sufficient support for the use proposed” (IOM, 2010, p. 2).
TABLE 5-2 Examples of Chronic Disease Endpoints Considered in Setting Previous Dietary Reference Intakes (DRIs)
| Chronic Disease | Nutrient for Which a Chronic Disease DRI Was Established (DRI) | Basis for DRI | Nutrients for Which No Chronic Disease DRI Was Establisheda |
|---|---|---|---|
| Age-related Macular Degeneration | — | — | Lutein/zeaxanthin |
| Cancer | — | — | Vitamin C, beta-carotene, dietary fiber, vitamin E, selenium, calcium, vitamin D, choline, folate, calcium, vitamin D |
| Cardiovascular Disease | - | — | Magnesium, choline, vitamin B6, calcium, vitamin D |
| Cataracts | — | — | Vitamin C, Vitamin E, riboflavin |
| Coronary Heart Disease | Potassium (AI)b | Blood pressurec (including reduction of salt sensitivity) | |
| Sodium (UL) | Blood pressurec | ||
| Dietary fiber (AI) | Decreased disease risk in prospective cohort studies | ||
| Dental Caries | Fluoride (AI) | Decreased disease risk | Calcium |
| Type 2 Diabetes | Magnesium, vitamin E, chromium, dietary fiber, vitamin D | ||
| Immune Responsed | Vitamin D | ||
| Kidney Stones (Recurrent) | Potassium (AI)b | Reduced risk of kidney stones | |
| Calcium (UL) | Increased risk of kidney stones |
| Chronic Disease | Nutrient for Which a Chronic Disease DRI Was Established (DRI) | Basis for DRI | Nutrients for Which No Chronic Disease DRI Was Establisheda |
|---|---|---|---|
| Neuropsychological Functione | Folate, vitamin D, choline | ||
| Osteoporosis (Bone Health) | Calciumf (EAR/RDA) | Decreased fracture risk, Bone mineral densityc | |
| Vitamin D (EAR/RDA) | Decreased fracture risk, Bone mineral densityc | ||
| Pulmonary Disease | Vitamin C |
NOTES: AI = adequate intake; DRI = Dietary Reference Intake; EAR = Estimated Average Requirement; RDA = Recommended Dietary Allowance; UL = Tolerable Upper Intake Level.
a In most cases, chronic disease outcomes were not used to set DRIs because the evidence was insufficient and/or inconsistent.
b The AI for potassium was based on multiple indicators related to risk of both cardiovascular disease (through the surrogate outcome of blood pressure) and of recurrent kidney stones.
c Indicates that a surrogate outcome qualified by FDA was used to set the DRI.
d Chronic diseases associated with the immune response include asthma, type 1 diabetes, inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis, and other conditions.
e Chronic diseases associated with neuropsychological function include autism, cognitive decline, depression, Alzheimer’s disease, and other conditions.
f Indicators of adequacy used to set the EAR and RDA for calcium varied by age, although they were related to bone health in all cases. For growing children, bone accretion and positive calcium balance were primary indicators; for adults up to age 50 years, calcium balance was the primary indicator; for older adults, bone density and fracture risk were used as indicators of adequacy.
SOURCES: IOM, 2011; Taylor, 2008.
Adequate Intake7 (AI) was set, rather than an Estimated Average Requirement8 (EAR) because of lack of data for chronic disease indicators.
NIH established the Biomarkers of Nutrition for Development (BOND)
___________________
7 Adequate Intake is the average daily nutrient intake observed in apparently healthy individuals in a specific sex and age group. It is based on experimentally derived intake levels or observations of mean nutrient intakes by a group of apparently healthy people who are maintaining a defined criterion of adequacy.
8 Estimated Average Requirement is the average daily intake of a nutrient that is expected to meet the requirement of half of healthy individuals in a group defined by age and sex. The requirement is based on a specific indicator of adequacy.
initiative, which was aimed to harmonize the process for identifying biomarkers for nutrition and development (see Box 5-2), but this initiative is no longer funded and, as of today, no efforts are ongoing to identify qualified surrogate outcomes to be used in establishing chronic disease DRIs (NIH, 2017).
As mentioned in Chapter 2, establishing reference values for adequacy for essential nutrients has been considered a critical task because of their importance to health. Chronic disease DRIs, however, are desirable but not essential. Therefore, despite the movement toward using surrogate markers in nutritional applications to chronic disease prevention, the committee urges caution in applying them, unless they meet a high bar for being considered qualified (as discussed in the next section). Some of the caution in this respect comes from experiences with drug studies in which the intervention affected the surrogate in the expected direction but did not have commensurate benefits on the disease outcome (see Lipska and Krumholz, 2017, for controversies around whether hemoglobin A1c can be used as surrogate in studies of diabetes). This suggests that either that the surrogate was not a true surrogate with respect to the causal pathway of
interest (but perhaps only a correlated disease marker) and/or that harms associated with the mechanism of drug action on the surrogate obviated benefits downstream of the intervention. Other examples are homocysteine as a surrogate for cardiovascular disease (Ganguly and Alam, 2015), HDL-cholesterol as surrogate for cardiovascular disease (Mahdy et al., 2012), and biomarkers of Alzheimer’s disease (Frisoni and Visser, 2015; Sharma and Singh, 2016). Whether this would be the case with a nutrient intervention is uncertain, although examples of unexpected effects could be cited. For example, unexpected adverse effects on lung cancer and cardiovascular disease were found in a large intervention trial testing high-dose beta-carotene plus vitamin A versus placebo conducted in heavy smokers (ATBC Cancer Prevention Study Group, 1994), when previous observational studies at lower intakes from foods had suggested reduced risk of cancer (NRC, 1982; Peto et al., 1981). One potential explanation is that the dose that is given results in a different effect. However, an alternative explanation could be that because study participants were at high risk of developing lung cancer, they possibly already had preneoplastic lesions. Therefore, the treatment could have acted as a promoter of early stage carcinogenesis (versus intake of carotenoids/vitamin A earlier in life seen in observational studies, which might lower cancer initiation). NOFSs may affect candidate surrogate markers by different mechanisms than will various drugs, requiring specific assessment of effects on both surrogates and outcomes as a criterion for qualification. On the other hand, nutrient-based interventions that fall within the established DRI limits for adequacy and toxicity would be presumed safe, unlike pharmacologic interventions for which safety must be established on a case-by-case basis.
RECOMMENDATION FOR OPTIONS AND JUSTIFICATIONS
The Options Report presents a conceptual framework with three scenarios for assessing whether the relationship between an NOFS and a chronic disease is causal: (1) direct assessment, where both the intake and the chronic disease itself are measured, (2) indirect assessment using a qualified surrogate disease marker as a substitute for the measurement of a chronic disease, or (3) indirect assessment using a nonqualified surrogate disease marker. Two options were offered for consideration (see Box 5-3). This committee was tasked with recommending and justifying one of these two options related to selecting chronic disease outcomes or biomarkers of effect when reviewing the evidence related to establishing DRIs based on the chronic disease outcome of interest. The committee supports a variant of option 1 (see Box 5-3), where studies that measure qualified surrogate markers—following the criteria adopted by the committee in Table 5-3—are considered in evaluating the evidence about causal relationships. The
committee does not support option 2, using nonqualified intermediate markers, because they could lead to serious misinterpretation of DRIs by users.
As described in Chapter 3, the evidence base for relationships between NOFSs and chronic disease outcomes includes studies that vary widely in design and health outcome measurement. Observational studies may include direct measurements of chronic disease outcomes in very large populations and over long follow-up periods, but also may include measurements of biomarkers of effect, qualified (i.e., surrogate markers) or nonqualified (i.e., nonqualified intermediate markers). In observational studies with prospective designs (e.g., cohort studies or nested case-control studies),
TABLE 5-3 List of Criteria for Consideration When Making a Decision About Whether a Surrogate Marker Is Qualified to Be Used as an Indicator of a Chronic Disease in the Development of a DRI
| Criteria | Description |
|---|---|
| Analytical Validation | Assessing assays and measurement performance characteristics and determining the range of conditions under which the assays will give reproducible and accurate data. |
| Evidentiary Qualification |
|
| Utilization Analysis | Defining the context of use: population and conditions for use to which the assessment applies, such as purpose and when in the development of the intervention the surrogate applies. Because idealized statistical requirements are rarely or never achievable, subjective assessment is necessary to determine when surrogate endpoints can be used. Other variables, such as morbidities and mortalities associated with the disease are important contextual considerations. For more details, see IOM, 2010. |
SOURCES: Calder et al., 2017; Clarke, 2017; IOM, 2010.
the surrogate disease marker measures may supplement data on disease outcomes in assessing causality. RCTs are also prospective but often shorter in duration, with smaller populations, and therefore may rely on surrogate disease markers alone. That is, data on the relationship between NOFSs and the clinical outcome do not always exist. Recognizing that chronic disease outcomes are the ideal measurement, the committee recommends that when a surrogate marker is used as proxy for a chronic disease outcome, the evidence related to its qualification is reviewed and surrogate markers
that most faithfully reflect a chronic disease outcome for the purpose are identified. Table 5-3 lists criteria the DRI committees should consider when making a decision about whether a surrogate marker is qualified for its purpose. The criteria are based on the IOM report Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease (IOM, 2010), on the work of the International Life Sciences Institute Europe Marker Validation Initiative (Calder et al., 2017), and on the presentation to the committee by Dr. Robert Clarke (Clarke, 2017). Typically, the most challenging evidentiary criterion to meet is #4 under evidentiary qualification in Table 5-3. This criterion requires the surrogate to substantially explain the relationship between the NOFS and the chronic disease, thereby precluding both biological pathways that bypass the surrogate and NOFS effects on disease following surrogate process ascertainment. This is the major statistical criterion needed to ensure that the chronic disease is associated with the NOFS if and only if the surrogate is also associated with the NOFS (Buyse et al., 2000; Frangakis and Rubin, 2002; Prentice 1989; VanderWeele, 2013). Establishment of a suitable surrogate for a specific chronic disease in relation to a specific NOFS is a complex process. DRI committees will need to carefully evaluate the support for surrogacy claims in studies being reviewed. Using nonqualified disease markers or surrogate disease markers qualified for other purposes (e.g., for drug evaluation) in establishing DRIs could have detrimental effects, including misinterpretation of DRIs by users or unintended diet modifications.
REFERENCES
ACS (American Cancer Society). 2017. Lifetime risk of developing or dying from cancer. https://www.cancer.org/cancer/cancer-basics/lifetime-probability-of-developing-or-dyingfrom-cancer.html (accessed April 27, 2017).
Arneson, D., L. Shu, B. Tsai, R. Barrere-Cain, C. Sun, and X. Yang. 2017. Multidimensional integrative genomics approaches to dissecting cardiovascular disease. Front Cardiovasc Med 4:8.
ATBC (Alpha-Tocopherol Beta Carotene) Cancer Prevention Study Group. 1994. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 330(15):1029-1035.
Bailey, L. B., P. J. Stover, H. McNulty, M. F. Fenech, J. F. Gregory, 3rd, J. L. Mills, C. M. Pfeiffer, Z. Fazili, M. Zhang, P. M. Ueland, A. M. Molloy, M. A. Caudill, B. Shane, R. J. Berry, R. L. Bailey, D. B. Hausman, R. Raghavan, and D. J. Raiten. 2015. Biomarkers of nutrition for development-folate review. J Nutr 145(7):1636S-1680S.
Beger, R. D., W. Dunn, M. A. Schmidt, S. S. Gross, J. A. Kirwan, M. Cascante, L. Brennan, D. S. Wishart, M. Oresic, T. Hankemeier, D. I. Broadhurst, A. N. Lane, K. Suhre, G. Kastenmuller, S. J. Sumner, I. Thiele, O. Fiehn, R. Kaddurah-Daouk, M., and for “Precision Medicine and Pharmacometabolomics Task Group”-Metabolomics Society Initiative. 2016. Metabolomics enables precision medicine: “A white paper, community perspective.” Metabolomics 12(10):149.
Benziger, C. P., G. A. Roth, and A. E. Moran. 2016. The Global Burden of Disease Study and the preventable burden of NCD. Glob Heart 11(4):393-397.
Buyse, M., G. Molenberghs, T. Burzykowski, D. Renard, and H. Geys. 2000. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics 1(1):49-67.
Calder, P. C., A. Boobis, D. Braun, C. L. Champ, L. Dye, S. Einother, A. Greyling, C. Matthys, P. Putz, S. Wopereis, J. V. Woodside, and J. M. Antoine. 2017. Improving selection of markers in nutrition research: Evaluation of the criteria proposed by the ILSI Europe Marker Validation Initiative. Nutr Res Rev 30(1):73-81.
Chowdhury, R., S. Warnakula, S. Kunutsor, F. Crowe, H. A. Ward, L. Johnson, O. H. Franco, A. S. Butterworth, N. G. Forouhi, S. G. Thompson, K. T. Khaw, D. Mozaffarian, J. Danesh, and E. Di Angelantonio. 2014. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann Intern Med 160(6):398-406.
Clarke, R. 2017. Pattern of lipid biomarkers to predict disease risk. Presented at the Workshop of the Committee on Development of Guiding Principles for the Inclusion of Chronic Disease Endpoints in Future Dietary Reference Intakes, January 9, 2017, Washington, DC.
Collins, F. S., and H. Varmus. 2015. A new initiative on precision medicine. N Engl J Med 372(9):793-795.
FDA (U.S. Food and Drug Administration). 2017a. Biomarker qualification program. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/default.htm (accessed April 27, 2017).
FDA. 2017b. Biomarkers used as outcomes. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/ucm535926.htm (accessed May 9, 2017).
FDA/CFSAN (FDA/Center for Food Safety and Nutrition). 2009. Guidance for industry: Evidence-based review system for the scientific evaluation of health claims-final. https://www.fda.gov/RegulatoryInformation/Guidances/ucm073332.htm#ftn1 (accessed May 9, 2017).
Fearon, E. R., and B. Vogelstein. 1990. A genetic model for colorectal tumorigenesis. Cell 61(5):759-767.
Fontana, L., L. Partridge, and V. D. Longo. 2010. Extending healthy life span—from yeast to humans. Science 328(5976):321-326.
Frangakis, C. E., and D. B. Rubin. 2002. Principal stratification in causal inference. Biometrics 58(1):21-29.
Freedman, L. S., B. I. Graubard, and A. Schatzkin. 1992. Statistical validation of intermediate endpoints for chronic diseases. Stat Med 11(2):167-178.
Frisoni, G. B., and P. J. Visser. 2015. Biomarkers for Alzheimer’s disease: A controversial topic. Lancet Neurol 14(8):781-783.
Ganguly, P., and S. F. Alam. 2015. Role of homocysteine in the development of cardiovascular disease. Nutr J 14:6.
Gavrilov, L. A., and N. S. Gavrilova. 2015. Predictors of exceptional longevity: Effects of early-life and midlife conditions, and familial longevity. N Am Actuar J 19(3):174-186.
GBD (Global Burden of Disease) 2013 Risk Factors Collaborators. 2015. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet 386(10010):2287-2323.
Greenberg, E. R., J. A. Baron, T. D. Tosteson, D. H. Freeman, Jr., G. J. Beck, J. H. Bond, T. A. Colacchio, J. A. Coller, H. D. Frankl, R. W. Haile, J. S. Mandel, D. W. Nierenberg, R. Rothstein, D. C. Snover, M. M. Stevens, R. W. Summers, R. U. van Stolk, for the Polyp Prevention Study Group. 1994. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med 331(3):141-147.
Hill, A. B. 1965. The environment and disease: Association or causation? Proc R Soc Med 58:295-300.
Hillerdal, G. 2008. Indolent lung cancers—Time for a paradigm shift: A review. J Thorac Oncol 3(3):208-211.
IOM (Institute of Medicine). 2010. Evaluation of biomarkers and surrogate endpoints in chronic disease. Washington, DC: The National Academies Press.
IOM. 2011. Dietary Reference Intakes for calcium and vitamin D. Washington, DC: The National Academies Press.
IOM. 2015. Improving diagnosis in health care. Washington, DC: The National Academies Press.
Jensen, H., and P. Vedsted. 2017. Exploration of the possible effect on survival of lead-time associated with implementation of cancer patient pathways among symptomatic first-time cancer patients in Denmark. Cancer Epidemiol 49:195-201.
Jordan, K., M. Porcheret, and P. Croft. 2004. Quality of morbidity coding in general practice computerized medical records: A systematic review. Fam Pract 21(4):396-412.
King, J. C., K. H. Brown, R. S. Gibson, N. F. Krebs, N. M. Lowe, J. H. Siekmann, and D. J. Raiten. 2016. Biomarkers of Nutrition for Development (BOND)—Zinc review. J Nutr 146(4):858S-885S.
Lewington, S., G. Whitlock, R. Clarke, P. Sherliker, J. Emberson, J. Halsey, N. Qizilbash, R. Peto, and R. Collins. 2007. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. The Lancet 370(9602):1829-1839.
Lipska, K. J., and H. M. Krumholz. 2017. Is hemoglobin A1c the right outcome for studies of diabetes? JAMA 317(10):1017-1018.
Lloyd, J., E. Jahanpour, B. Angell, C. Ward, A. Hunter, C. Baysinger, and G. Turabelidze. 2017. Using national inpatient death rates as a benchmark to identify hospitals with inaccurate cause of death reporting—Missouri, 2009-2012. Morb Mortal Wkly Rep 66(1):19-22.
Mahdy Ali, K., A. Wonnerth, K. Huber, and J. Wojta. 2012. Cardiovascular disease risk reduction by raising HDL cholesterol—Current therapies and future opportunities. Br J Pharmacol 167(6):1177-1194.
Navin Cristina, T. J., J. A. Stewart Williams, L. Parkinson, D. W. Sibbritt, and J. E. Byles. 2016. Identification of diabetes, heart disease, hypertension and stroke in mid- and older-aged women: Comparing self-report and administrative hospital data records. Geriatr Gerontol Int 16(1):95-102.
Neuhouser, M. L., T. Y. Cheng, S. A. Beresford, E. Brown, X. Song, J. W. Miller, Y. Zheng, C. A. Thomson, J. M. Shikany, M. Z. Vitolins, T. Rohan, R. Green, and C. M. Ulrich. 2015. Red blood cell folate and plasma folate are not associated with risk of incident colorectal cancer in the Women’s Health Initiative observational study. Int J Cancer 137(4):930-939.
Niccoli, T., and L. Partridge. 2012. Ageing as a risk factor for disease. Curr Biol 22(17): R741-752.
NIH (National Institutes of Health). 2017. About BOND. https://www.nichd.nih.gov/global_nutrition/programs/bond/Pages/about.aspx (accessed April 27, 2017).
NRC (National Research Council). 1982. Diet, nutrition, and cancer. Washington, DC: National Academy Press.
Ohlhorst, S. D., R. Russell, D. Bier, D. M. Klurfeld, Z. Li, J. R. Mein, J. Milner, A. C. Ross, P. Stover, and E. Konopka. 2013. Nutrition research to affect food and a healthy life span. Am J Clin Nutr 98(2):620-625.
Paraskeva, C., A. P. Corfield, S. Harper, A. Hague, K. Audcent, and A. C. Williams. 1990. Colorectal carcinogenesis: Sequential steps in the in vitro immortalization and transformation of human colonic epithelial cells (review). Anticancer Res 10(5A):1189-1200.
Peto, R., R. Doll, J. D. Buckley, and M. B. Sporn. 1981. Can dietary beta-carotene materially reduce human cancer rates? Nature 290(5803):201-208.
Prentice, R. L. 1989. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med 8(4):431-440.
Raiten, D. J., S. Namaste, B. Brabin, G. Combs, Jr., M. R. L’Abbe, E. Wasantwisut, and I. Darnton-Hill. 2011. Executive summary—Biomarkers of Nutrition for Development: Building a consensus. Am J Clin Nutr 94(2):633S-650S.
Rappaport, S. M. 2016. Genetic factors are not the major causes of chronic diseases. PLOS ONE 11(4):e0154387.
Robb, M. A., P. M. McInnes, and R. M. Califf. 2016. Biomarkers and surrogate endpoints: Developing common terminology and definitions. JAMA 315(11):1107-1108.
Rohner, F., M. Zimmermann, P. Jooste, C. Pandav, K. Caldwell, R. Raghavan, and D. J. Raiten. 2014. Biomarkers of nutrition for development—Iodine review. J Nutr 144(8): 1322S-1342S.
Sharma, N., and A. N. Singh. 2016. Exploring biomarkers for Alzheimer’s Disease. J Clin Diagn Res 10(7):KE01-KE06.
Shreiner, A. B., J. Y. Kao, and V. B. Young. 2015. The gut microbiome in health and in disease. Curr Opin Gastroenterol 31(1):69-75.
Sugarbaker, P. H., L. L. Gunderson, and R. E. Wittes. 1985. Cancer of the anal region. In Cancer: Principles and Practice of Oncology, edited by V. T. J. DeVita, S. Hellman and S. A. Rosenberg. Philadelphia, PA: Lippincott, Co.
Takata, Y., M. J. Shrubsole, H. Li, Q. Cai, J. Gao, C. Wagner, J. Wu, W. Zheng, Y. B. Xiang, and X. O. Shu. 2014. Plasma folate concentrations and colorectal cancer risk: A case-control study nested within the Shanghai Men’s Health Study. Int J Cancer 135(9):2191-2198.
Tanumihardjo, S. A., R. M. Russell, C. B. Stephensen, B. M. Gannon, N. E. Craft, M. J. Haskell, G. Lietz, K. Schulze, and D. J. Raiten. 2016. Biomarkers of Nutrition for Development (BOND)—Vitamin A review. J Nutr 146(9):1816S-1848S.
Taylor, C. L. 2008. Framework for DRI development: Components “known” and components “to be explored.” Washington, DC
Thiru, K., A. Hassey, and F. Sullivan. 2003. Systematic review of scope and quality of electronic patient record data in primary care. BMJ 326(7398):1070.
VanderWeele, T. J. 2013. Surrogate measures and consistent surrogates. Biometrics 69(3): 561-569.
Waterland, R. A., and K. B. Michels. 2007. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr 27:363-388.
WHO (World Health Organization). 2012. Effect of increased potassium intake on cardiovascular disease, coronary heart disease and stroke. Geneva, Switzerland: World Health Organization.
Yetley, E. A., A. J. MacFarlane, L. S. Greene-Finestone, C. Garza, J. D. Ard, S. A. Atkinson, D. M. Bier, A. L. Carriquiry, W. R. Harlan, D. Hattis, J. C. King, D. Krewski, D. L. O’Connor, R. L. Prentice, J. V. Rodricks, and G. A. Wells. 2017. Options for basing Dietary Reference Intakes (DRIs) on chronic disease endpoints: Report from a joint US-/ Canadian-sponsored working group. Am J Clin Nutr 105(1):249S-285S.






















