2
The Current Process to Establish Dietary Reference Intakes
The process that underlies development of Dietary Reference Intakes (DRIs) has evolved over several decades (IOM, 1997, 1998b, 2000b, 2001, 2002/2005, 2005, 2011). In this chapter, the current process is described and an overview of typical applications of the different DRI values is provided. This section is followed by a discussion of the scope of the current DRI process and how chronic disease endpoints have been included as a basis for DRIs to date. This chapter serves as a foundation for understanding the committee’s recommendations and guiding principles to establish chronic disease DRIs found in subsequent chapters of this report.
EVOLUTION OF THE DRI PROCESS TO ITS CURRENT STATUS
Brief Overview of the DRI Process
The DRIs are a set of nutrient reference standards that are used for planning and assessing diets of apparently healthy individuals and groups. For each nutrient, the objective is to establish standards for 22 life-stage groups (infants aged 0-6 and 9-12 months; children aged 1-3 and 4-8 years; males and females separately for ages 9-13, 14-18, 19-30, 31-50, 51-70, and ≥70 years; pregnant women aged 14-18, 19-30, and 31-50 years; and lactating women aged 14-18, 19-30, and 31-50 years). DRIs include reference standards for both nutritional adequacy (Estimated Average Requirement [EAR], Recommended Dietary Allowance [RDA], and Adequate Intake [AI]) as well as potential risk of excess nutrient intake (Tolerable Upper Intake Level [UL]). Two additional DRIs have been established—Acceptable
Macronutrient Distribution Range (AMDR) and Estimated Energy Requirement (EER). These six different types of DRI values are defined in Box 2-1. One or more DRIs may be available for a single nutrient. In particular, many nutrients have an EAR and RDA (or AI) and a UL. If adequate data are available, the DRI may have incorporated considerations for
reducing the risk of chronic disease (IOM, 2003a). The experience to date with incorporating evidence related to chronic disease in DRIs, which is described later in this chapter, is relevant in the context of developing DRIs for reaching adequacy or preventing toxicity but not fully applicable for development of “chronic disease DRIs” with the goal of preventing chronic disease. This report focuses on the potential for developing DRIs for which the primary focus would be on reducing chronic disease risk rather than ancillary to considerations of adequacy or toxicity.
The setting of quantitative nutrient intake reference values in the United States and Canada began in the 1930s. In Canada, the Dietary Standards/Recommended Nutrient Intakes (RNIs) were published from 1938 until 1990, and in the United States, Recommended Dietary Allowances were published from 1941 until 1989 by the Food and Nutrition Board of the National Academy of Sciences. The current DRI process was initiated in the early 1990s, in recognition of the expanded uses of dietary reference values and newer insights into the role of nutrients. With sponsorship primarily from U.S. and Canadian federal agencies, volunteer expert panels (i.e., DRI committees) of Canadian and U.S. scientists and subcommittees in relevant disciplines (e.g., human nutrition, epidemiology, toxicology) are convened based on a selection process that considers suggestions by stakeholders, and conflicts of interests and biases following the policies of the National Academies of Sciences, Engineering, and Medicine (the National Academies). In general, DRI recommendations result from 1 to 2 years of DRI committee deliberations. Table 2-1 lists the DRI reports that have been published to date as well as other key publications related to the development of the DRI process.
A cornerstone of the current thinking of the role of DRIs in nutrition policy was a 2007 Institute of Medicine workshop called The Development of the DRIs 1994-2004 (IOM, 2008). The workshop explored emerging challenges and issues in the process of establishing DRIs with the goal of gathering ideas for improving the process in the future and as scientific knowledge expands.
Traditionally, a major consideration in the DRI process has been nutritional adequacy. For nutrients deemed nutritionally essential for normal physiological functioning (i.e., the nutrient cannot be synthesized in the body, or cannot be synthesized in sufficient amounts to meet needs and thus must be provided in the diet), the scientific literature is reviewed by the DRI committee to determine the most appropriate indicator of adequacy that will be used to set the requirement for the nutrient. Possible indicators of adequacy could include prevention of signs or symptoms of a nutrient deficiency disease, biomarkers of the nutrient’s function (e.g., activity of an enzyme that uses the nutrient as a cofactor), and biomarkers of body stores of the nutrient. Ideally, intake-response data are available for the selected
TABLE 2-1 Chronology of DRI Publications
| Year | DRI Publication Title | Reference |
|---|---|---|
| 1994 | How Should the Recommended Dietary Allowances Be Revised? | IOM, 1994 |
| 1997 | Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride | IOM, 1997 |
| 1998 | Dietary Reference Intakes: A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients | IOM, 1998a |
| 1998 | Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline | IOM, 1998b |
| 2000 | Dietary Reference Intakes: Applications in Dietary Assessment | IOM, 2000a |
| 2000 | Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids | IOM, 2000b |
| 2001 | Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc | IOM, 2001 |
| 2002/2005 | Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids | IOM, 2002/2005 |
| 2003 | Dietary Reference Intakes: Applications in Dietary Planning | IOM, 2003a |
| 2003 | Dietary Reference Intakes: Guiding Principles for Nutrition Labeling and Fortification | IOM, 2003b |
| 2005 | Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate | IOM, 2005 |
| 2006 | Dietary Reference Intakes Research Synthesis: Workshop Summary | IOM, 2006a |
| 2006 | Dietary Reference Intakes: The Essential Guide to Nutrient Requirements | IOM, 2006b |
| 2008 | The Development of DRIs 1994–2004: Lessons Learned and New Challenges: Workshop Summary | IOM, 2008 |
| 2011 | Dietary Reference Intakes for Calcium and Vitamin D | IOM, 2011 |
indicator of adequacy, so that the amount of the nutrient that meets the requirements of half the members of a specified sex and life-stage group can be identified. If such data are available, an EAR is identified and used to establish an RDA (an intake level that meets the needs of nearly all members of a sex and life-stage group, i.e., two standard deviations above the
mean requirement). If intake-response data are not available and an EAR and RDA cannot be established, other types of data (e.g., average intakes of a healthy group of people) are used to set an AI that is used as a recommended intake level.
It is important to recognize that the EAR for a given nutrient could differ depending on the indicator of adequacy that is selected. For example, for iron, possible indicators of adequacy could include prevention of anemia (as identified by a specified hemoglobin level) or maintenance of a certain level of iron stores (as identified by a specified serum ferritin level). If an EAR was established based on maintaining iron stores, it would be higher than an EAR based on prevention of iron-deficiency anemia.
The DRI process also considers risks of adverse effects from excessive intakes. Thus, many nutrients that have an EAR and RDA or AI also have a UL, which represents a maximal daily intake level that is unlikely to lead to adverse health effects when consumed habitually. ULs are set using a risk assessment framework. This involves identifying any adverse effects of high intakes of the nutrient, where “adverse effect” includes impairment of any physiologically important function as well as any detrimental effect of the nutrient on the health benefits of another nutrient (i.e., an adverse nutrient-nutrient interaction). Intake-response data are examined to identify a no-observed-adverse-effect level (NOAEL), which is the highest intake that does not result in adverse effects in any of the individuals studied. If it is not possible to identify a NOAEL, a lowest-observed-adverse-effect level (LOAEL) is identified as the lowest intake at which an adverse effect was observed. An uncertainty factor (UF) reflecting uncertainty associated with extrapolating from the observed data to the general population is then selected. The magnitude of the UF will vary among nutrients, depending on factors such as individual variability in susceptibility to the adverse effect, extrapolation from experimental animals to humans, use of a LOAEL rather than a NOAEL, use of data reflecting subclinical versus chronic exposure, and the severity or irreversibility of the adverse effect. The UL is then set by dividing the NOAEL or LOAEL by the UF. At present, ULs have been identified only for some essential nutrients (i.e., vitamin A, vitamin C, vitamin D, vitamin E, niacin, vitamin B6, folate, choline, calcium, copper, fluoride, iodine, iron, magnesium, manganese, molybdenum, phosphorus, selenium, zinc, sodium, and chloride). ULs also have been set for nickel, vanadium and boron, which are not considered nutritionally essential.
In contrast to the previous method of establishing U.S. RDAs and Canadian RNIs, the current DRI process, which began in the early 1990s also incorporates consideration of chronic diseases. This will be apparent in some of the examples described below.
Derivation of the Six Types of Dietary Reference Intakes
Figure 2-1 illustrates the relationship among the DRIs and the risks of nutrient inadequacy or excess. At extremely low intakes, the risk of inadequacy is 100 percent: Everyone would fail to meet the requirement for the specified indicator of adequacy for the nutrient. As intake increases, the risk of inadequacy decreases: it is 50 percent when intake equals the EAR, and diminishes to near zero (about 2 to 3 percent) when intake equals the RDA. The AI is set when the necessary intake-response data are not available to establish an EAR or an RDA. Although the AI is not shown in Figure 2-1, it is assumed that it would be at or above the RDA if an RDA could be calculated, as it is estimated to meet the needs of almost all healthy individuals. As intakes increase above the RDA (or AI), there is a range of intake that is associated with neither further reductions in risk of inadequacy nor any increase in the potential risk of excess. However, as intake increases above the UL, risk of adverse effects may increase. It should be noted that much less is known about the “shape” of the risk curve for the adverse effects
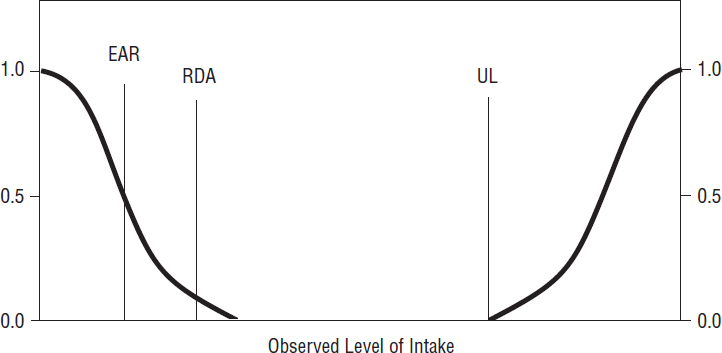
SOURCE: IOM, 2006b.
of excessive intake than the risk curve for inadequacy. The point where risk actually does increase likely varies among nutrients, depending on the magnitude of the UF used to set the UL. Furthermore, it is not possible to identify intake levels where a given proportion of a group experiences the adverse effect used to set the UL (whereas it is possible to identify intake levels where a given proportion of a group do not meet the requirement for the indicator of adequacy used to set the EAR).
The AMDR indicates a range of carbohydrate, protein, or fat intake within which essential nutrient needs could be met without increasing the risk of chronic disease. Finally, the EER indicates the level of energy intake that is predicted to maintain energy balance (or, as appropriate, normal growth). The EAR, RDA, AI, UL, AMDR, and EER can be used to assess the probability of intake adequacy or potential risk of excess or to plan for appropriate intakes of individuals or groups.
Steps in the DRI Process
The DRI process generally involves four overarching steps as part of a risk assessment framework (see also Figure 1-2 in Chapter 1). Each step is described briefly in Box 2-2. These steps describe the ideal DRI process, in which the desired evidence would be available. In reality, the process is typically not so straightforward because of gaps in the data and variation in the type and amount of evidence for each nutrient. However, because of their importance to health, establishing reference values for adequacy for essential nutrients has been considered necessary, regardless of the certainty in the evidence. In the past, therefore, the basis for nutrient adequacy has varied for each nutrient depending, in part, on the availability of data that allowed estimation of an EAR. Further examples and considerations are provided later in this chapter.
Applications of the DRIs
The DRIs are used for a variety of nutrition-related objectives, as summarized in Table 2-2. Applications include providing a reference point for assessing the nutrient intake distribution of populations, as is done to develop the Dietary Guidelines for Americans (HHS and USDA, 2015), and to provide information for consumer evaluation of food products, such as for food labels. The DRIs also may be used to estimate the effect of altering the food supply on population intakes, such as for food fortification or when a new food product or ingredient is proposed for addition to the food supply.
TABLE 2-2 Common Applications of Dietary Reference Intake (DRI) Values for Populations and for Individuals
| Application | Description | Example | DRI |
|---|---|---|---|
| Population-Level Applications | |||
| Food labeling | Use (in part) to calculate the proportion of recommended intake provided by a food product | The % DV (daily value) (FDA, 2013b) | RDA, AI, UL |
| Food fortification | Evaluate the effect of adding a nutrient to a staple food, with the intent of improving population-wide nutrient intakes and reducing prevalence of a health risk | Health Canada is examining whether changes to the current vitamin D fortification policy are needed, and modeling has been used to assess the impact of adding vitamin D to various foods on the percent less than the EAR and greater than the UL (CIHR, 2016) | EAR, UL |
| Federal supplemental food program planning | Assess the nutrient adequacy of specific population groups to determine what supplemental foods should be provided | USDA child nutrition programs (USDA, 2016) | EAR, AI, UL |
| Research | Design and evaluate data from human studies; analyze dietary intake data | Planning diets for intervention studies (individual-level application); NHANES or CCHS data analysis (population-level application) | EAR, AI, RDA, UL |
| Product development or modification of existing foods | Evaluate the effect of adding an ingredient to a food for a non-nutrient purpose, such as preservation | Ascorbic acid (vitamin C) (FDA, 2013a) | RDA, AI, UL |
| Nutrition surveillance | Assess the prevalence of nutrient inadequacy and potentially excessive intake in a population by sex/life-stage group | NHANES (United States), CCHS (Canada) | EAR, UL, AMDR |
| Individual-Level Applications | |||
| Development of dietary guidance | Develop recommended food intake patterns to ensure that individuals meet recommendations for nutrient intake, with consideration of typical food intake patterns | The Dietary Guidelines for Americans (HHS and USDA, 2015); Eating Well with Canada’s Food Guidea (Health Canada, 2007); Army Regulation 40-25 (U.S. Army, 2017) | EAR, AI, RDA, UL, AMDR |
| Clinical assessment | Determine the nutrient adequacy of an individual by using a serum marker of nutrient intake | DRI for serum vitamin D (IOM, 2011) | EAR |
NOTES: AI = adequate intake; AMDR = acceptable macronutrient distribution range; CCHS = Canadian Community Health Survey; DRI = Dietary Reference Intake; EAR = Estimated Average Requirement; NHANES = National Health and Nutrition Examination Survey; NTD = neural tube defect; RDA = Recommended Dietary Allowance; UL = Tolerable Upper Intake Level; USDA = U.S. Department of Agriculture.
a Canada’s Food Guide was developed using a modeling process that enables it to be used to plan diets of groups as well as individuals (Katamay et al., 2007).
SCOPE OF THE CURRENT DRI PROCESS
Food Substances Addressed Using the DRI Process
DRIs have been established for all the nutrients that are considered essential (vitamins, minerals, water, electrolytes, carbohydrate, protein, total fat, linoleic acid, and linolenic acid). Essential nutrients cannot be synthesized by the body (or are synthesized in insufficient amounts), but are required for normal human physiological function.
In contrast to earlier efforts to establish dietary reference values, the DRI committees have also explored the possibility of setting DRIs for non-essential food components that are found naturally in foods (also called non-nutrients), but which may have a meaningful impact on human health. For example, dietary fiber is not an essential nutrient; however, it was included and evaluated in the 2002/2005 DRI report. The DRI com-
mittee found sufficient evidence to set an AI for total fiber in foods, based on the intake level shown to protect against coronary heart disease (IOM, 2002/2005) in men and women.
A wide variety of other non-essential nutrients also have been evaluated by the various DRI committees, but they have been unable to set EAR or AI values for any of them. For example, some evidence suggested that arsenic, boron, nickel, silicon, and vanadium play a beneficial role in human health, but no EAR or AI have been set because no clear and consistent evidence of their metabolic role has been available (IOM, 2001). In the case of saturated fatty acids, monounsaturated fatty acids, trans fatty acids, cholesterol, beta carotene, and other carotenoids, DRI committees have concluded that no EAR or AI values could be determined because of insufficient evidence that these food components are considered essential to human health (IOM, 2000b, 2002/2005). The DRI committee on macronutrients have considered long-chain omega-3 fatty acids (EPA and DHA) and stated that they could contribute to meeting the AMDR for the essential n-3 and n-6 fatty acids (linoleic and α-linolenic acids) (IOM, 2002/2005); however, no reference value has been set for them.
With some exceptions, DRI evaluations and recommendations have been based on intake of the nutrient or food component from foods. In some instances, DRI committees have evaluated intake from fortified foods and/or dietary supplements. The EAR for folate, for example, is based on the amount of folate from foods and folic acid from fortified foods or dietary supplements, measured as dietary folate equivalents, needed to maintain erythrocyte folate (IOM, 1998b). Another example is the vitamin B12 recommendation for individuals older than age 50 years, who may be unable to absorb naturally occurring vitamin B12. The DRI committee advised that this population group meets its requirement by consuming foods fortified with vitamin B12 or by taking a supplement that contains vitamin B12 (IOM, 1998b).
The UL for a number of nutrients has been based on intake from dietary supplements, such as the UL for vitamin E, which applies to intake of all forms of synthetic vitamin E found in dietary supplements, fortified foods, and pharmacological agents (IOM, 2000b). Similarly, the UL for niacin and for folic acid applies to synthetic forms found in dietary supplements and/or fortified foods, and the UL for magnesium has been based only on intake from pharmacologic agents (IOM, 1997, 1998b). Generally, this is done when adverse effects have been observed only with supplemental or synthetic sources of the nutrient (versus sources that occur naturally in foods).
Incorporating Considerations for Chronic Disease
To date, chronic disease endpoints have been used as the indicator to establish a DRI for six nutrients: calcium, fluoride, potassium, sodium, total fiber, and vitamin D. AIs were set for fluoride, potassium, sodium, and total fiber, and EARs were set for calcium and vitamin D. The specific indicators that were selected as well as those that were considered during the DRI process for these nutrients are shown in Table 2-3. It should be noted that, in most cases, it was not possible to identify an EAR based on chronic disease risk, so AIs were set instead. In 2011, EARs and RDAs were set for calcium and vitamin D. However, for most age groups, the calcium EAR and RDA were based on intake-response data for calcium balance, which while clearly related to bone health, is not a specific chronic disease outcome (IOM, 2011). Chronic disease outcomes or surrogates (e.g., fracture risk, bone mineral density) were used to set the EAR and RDA for older adults, but the committee commented that the absence of good intake-response data made it challenging to clearly identify a requirement in these age groups. The vitamin D EAR and RDA also warrant comment, as they were set based on the amounts of dietary vitamin D (in the absence of sunlight exposure) required to achieve a specified serum level of 25(OH)D. Intake-response data on serum 25(OH)D levels and bone health outcomes were used to identify the “required” serum 25(OH)D levels used for the EAR and RDA (IOM, 2011).
USE OF EVIDENCE TO DEVELOP DRIs:
TYPES AND APPLICATIONS
Types of Indicators Used to Develop DRIs
Selecting an appropriate indicator (i.e., a variable for determining adequacy or excess) is the first step in the DRI development process. As previously discussed, indicators may assess nutrient adequacy (EAR or AI) or excess (UL). DRI committees have also taken into account potential reductions or increases in chronic disease outcomes. A wide variety of types of indicators have been used to set the DRIs, including clinical indicators (signs of deficiency, altered body composition, impaired function, or increased morbidity), nutrient balance studies, biochemical measures (blood or urine levels), functional measures (bone health, hormone levels), risk of developmental abnormalities, or risk of chronic disease outcomes (Taylor, 2008). For example, the EAR for vitamin A was based on ensuring adequate liver stores of the vitamin (IOM, 2001). A combination of indicators often has been used. The DRI for copper was based on plasma copper and ceruloplasmin concentrations, erythrocyte superoxide dismutase activity,
TABLE 2-3 Current Dietary Reference Intakes (DRIs) Linked to Chronic Disease and/or Surrogate Endpoints
| Nutrient | Reference Value | Indicator Selected as the Basis for Establishment of Chronic Disease-Related DRI (AI, EAR, UL) | Indicators Considered, But Not Selected | |
|---|---|---|---|---|
| Biomarkers of Nutrient Adequacy | Surrogate Endpoints | |||
| Calcium | EAR | Bone healthc |
Serum calcium Calcium balancec Calcium absorption |
Parathyroid hormone Bone mineral content/bone mineral densityc Hypertension |
| Chronic Disease | Types of Studiesa | Considerations for Other Populationsb |
|---|---|---|
|
Cancer/neoplasms (all cancers, breast cancer, colorectal cancer/colon polyps, prostate cancer) Cardiovascular diseases Diabetes (type 2) and metabolic syndrome Falls Fracture risk Immune responses (asthma, autoimmune disease) Diabetes (type 1) Inflammatory bowel and Crohn’s disease Multiple sclerosis Rheumatoid arthritis Systemic lupus erythematosus Infectious diseases Tuberculosis Influenza/upper respiratory infections Neuropsychological functioning (autism, cognitive function, expression) Physical performance Preeclampsia of pregnancy and other non-skeletal reproductive outcomes Rickets/osteomalacia |
Balance studies RCTs Observational studies (ecological, cross-sectional, case-control, cohort) |
The committee noted that different ethnic/racial groups respond to calcium and vitamin D in some biologically different ways, most notably among those of African American ancestry. However, the available data were too limited to permit the committee to assess whether separate, quantitative reference values for such groups are required. |
| Nutrient | Reference Value | Indicator Selected as the Basis for Establishment of Chronic Disease-Related DRI (AI, EAR, UL) | Indicators Considered, But Not Selected | |
|---|---|---|---|---|
| Biomarkers of Nutrient Adequacy | Surrogate Endpoints | |||
| UL |
Incidence of kidney stones |
Vascular and soft tissue calcification Interactions with iron and zinc |
||
| Vitamin Dd | EAR |
Bone health (operationalized as the intake required, in the absence of sunlight exposure, to achieve serum 25(OH)D levels consistent with desirable changes in bone density and fracture risk) |
Serum 25(OH)D level |
Serum 25(OH)D level |
| Chronic Disease | Types of Studiesa | Considerations for Other Populationsb |
|---|---|---|
|
Hypercalcemia Hypercalciuria Prostate cancer Constipation |
||
|
Cancer/neoplasms (all cancers, breast cancer, colorectal cancer/colon polyps, prostate cancer) Cardiovascular diseases Diabetes (type 2) and metabolic syndrome Falls Fracture riskc Immune responses (asthma, autoimmune disease) Diabetes (type 1) Inflammatory bowel and Crohn’s disease Multiple sclerosis Rheumatoid arthritis Systemic lupus erythematosus Infectious diseases Tuberculosis Influenza/upper respiratory infections Neuropsychological functioning (autism, cognitive function, depression) Physical performance Preeclampsia of pregnancy and other non-skeletal reproductive outcomes Rickets/osteomalacia |
RCTs Observational studies (ecological, cross-sectional, case-control, cohort) |
The committee noted that different ethnic/racial groups respond to calcium and vitamin D in some biologically different ways, most notably among those of African American ancestry; however, the available data were too limited to permit the committee to assess whether separate, quantitative reference values for such groups are required. |
| Nutrient | Reference Value | Indicator Selected as the Basis for Establishment of Chronic Disease-Related DRI (AI, EAR, UL) | Indicators Considered, But Not Selected | |
|---|---|---|---|---|
| Biomarkers of Nutrient Adequacy | Surrogate Endpoints | |||
| ULd |
Hypercalcemia and related toxicity Emerging evidence for all-cause mortality, some cancers, cardiovascular risk, falls and fractures |
|||
| Sodium | AIe |
Replenish losses of sodium needs of moderately active, apparently healthy individuals Based on ensuring adequate intake of other nutrients |
Sodium balance Chloride balance Serum concentration |
Blood pressure Plasma renin activity Blood lipids concentration Insulin resistance |
| Chronic Disease | Types of Studiesa | Considerations for Other Populationsb |
|---|---|---|
|
Hypercalciuria Hypercalcemia (infants) Cancer Cardiovascular risk Falls and fractures All-cause mortality |
Observational studies (ecological, cross-sectional, case-control, cohort) |
For infants, UL was based on retarded linear growth |
|
Balance studies RCTs with feeding or behavioral interventions Observational studies |
AI for 0-12 months was based on mean sodium intake. For 1-18 years, AI is extrapolated down based on energy intake. For those older than 50 years, AI is extrapolated down based on energy intake. Evidence was insufficient to set levels for:
|
| Nutrient | Reference Value | Indicator Selected as the Basis for Establishment of Chronic Disease-Related DRI (AI, EAR, UL) | Indicators Considered, But Not Selected | |
|---|---|---|---|---|
| Biomarkers of Nutrient Adequacy | Surrogate Endpoints | |||
| UL |
Continuous and progressive increase in blood pressure (for CVD) with increases in sodium intake. The LOAEL as applied to dietary sodium (2.3 g) is a point on the continuous relationship with blood pressure that corresponds with the next level above the AI that was tested in dose-response trials. |
Calcium excretion, Bone mineral density |
Left ventricular mass Kidney stones |
|
| Total fiber | AIe |
Intake level found to protect against coronary heart disease. Reduction in risk of diabetes was used as a secondary endpoint to support the AI. |
Fiber intake, satiety and weight maintenance |
Blood pressure Hyperlipidemia Glucose tolerance insulin response |
| No UL set for total fiber | ||||
| Chronic Disease | Types of Studiesa | Considerations for Other Populationsb |
|---|---|---|
|
Stroke Coronary heart disease Pulmonary function Gastric cancer |
||
|
Colon health (constipation, laxation, fecal weight; fiber fermentation products—energy source for colon; prevention of diverticular disease) Colon cancer Breast cancer Other cancers (endometrial, ovarian) Diabetes |
Epidemiological (prospective cohorts), mechanistic, and clinical data, intervention trials |
No AI for infants AI for children is extrapolated from adult AI, based on energy intake. Evidence was insufficient to set levels for:
|
| Nutrient | Reference Value | Indicator Selected as the Basis for Establishment of Chronic Disease-Related DRI (AI, EAR, UL) | Indicators Considered, But Not Selected | |
|---|---|---|---|---|
| Biomarkers of Nutrient Adequacy | Surrogate Endpoints | |||
| Fluoride | AIe |
Prevention of dental caries |
Fluoride balance |
Bone mineral content |
| UL |
UL for infants and children ages 0-8 years is based on risk of enamel fluorosis (two studies from 1937 and 1942). UL for all age groups (>8 years), and pregnant or lactating women is based on risk of skeletal fluorosis (clinical studies). |
Enamel fluorosis |
||
| Nutrient | Reference Value | Indicator Selected as the Basis for Establishment of Chronic Disease-Related DRI (AI, EAR, UL) | Indicators Considered, But Not Selected | |
|---|---|---|---|---|
| Biomarkers of Nutrient Adequacy | Surrogate Endpoints | |||
| Potassium | AIe |
Level of intake from foods that maintains lower blood pressure levels, reduces the adverse effects of sodium chloride intake on blood pressure, reduces risk of kidney stones, and possibly reduces bone loss |
Potassium balance Serum potassium concentration Hypokalemia Bone mineralization |
Blood pressure Kidney stones Bone loss |
| UL |
No UL was set for potassium intake from food. |
|||
NOTES: AI = adequate intake; CVD = cardiovascular disease; DRI = Dietary Reference Intake; EAR = Estimated Average Requirement; LOAEL = lowest-observed-adverse-effect level; RCT = randomized controlled trial; UL = Tolerable Upper Intake Level. Systematic reviews, conducted by an outside contractor, were used by the DRI committee to establish DRIs for calcium and vitamin D (IOM, 2011).
a General statements were made about studies being adequately powered and about the study quality considerations, but no inclusion/exclusion criteria were provided (e.g., studies seem to be included regardless of the dietary intake assessment tool used) or quality assessment included.
| Chronic Disease | Types of Studiesa | Considerations for Other Populationsb |
|---|---|---|
|
CVD Impaired pulmonary function |
Epidemiological studies (observational) Metabolic studies Intervention studies |
AI for 0-12 months was based on mean potassium intake. AI for children age 1-18 years is extrapolated from adult AI based on median energy intakes. Evidence was insufficient to set levels for:
|
b Criteria for nutrition adequacy might differ for different ages and justification is described; it is not basal or normative, as in other reports. Levels are based on reducing the risk of developing a negative condition associated with the nutrient for the apparently healthy population, not for those that are malnourished or, in certain disease states, marked by increased nutrient needs. In those instances, qualified medical and nutrition personnel must tailor the recommendations.
c Bone health was operationalized differently in the age and life-stage groups. For most age groups, calcium balance (positive or neutral) was a criterion, and for older adults, reduced fracture risk was considered.
d This row was revised since prepublication release.
e An EAR could not be established for any of these nutrients due to inadequate data.
and platelet copper concentration (IOM, 2001). More than 400 indicators have been considered by the DRI committees (Taylor, 2008).
Various indicators of chronic disease outcomes for all nutrients and non-nutrients, either measured directly or indirectly, have been considered by the various DRI committees. (See Chapter 5 for a more detailed discussion of chronic disease indicators.) Direct measurement of a chronic disease outcome was used to set the DRI in only a few instances (see Table 2-3); the AI for fluoride was based on prevention of dental caries. The AI for total fiber was based on reduced risk of coronary heart disease. DRIs based on indirect measurement of chronic disease outcomes using intermediate outcomes were established for sodium, vitamin D, calcium, and potassium. The UL for sodium was based on risk of increased blood pressure and cardiovascular outcomes, particularly cardiovascular disease and stroke. The EARs and RDAs for vitamin D and calcium were based on reduced risk of bone loss. Lastly, the AI for potassium was based on maintenance of normal blood pressure, reduced risk of bone loss, and possible reduced risk of recurrent kidney stones.
As is clear from Table 2-3, in most cases, although chronic disease endpoints were considered by the nutrient panels as potential indicators of adequacy for an EAR, ultimately they were not used. Setting an EAR requires intake-response data over a range of intakes that span the requirement range, in order to identify the nutrient intake level where half the group meets the requirement for the specified indicator of adequacy and the other half does not. These types of studies are most feasible to conduct and most easily interpreted when the indicator of adequacy (1) responds only (or to a very large extent) to changes in intake of the nutrient of interest; (2) changes over a relatively short period of time (e.g., weeks versus decades); and (3) can be assessed as having been “met” or “not met.” For example, the indicator of adequacy used to set the EAR for vitamin C was near-maximal neutrophil ascorbate concentrations with minimal urinary loss, as a marker of antioxidant protection (IOM, 2000b; Levine et al., 1996). This indicator responds to changes in individuals’ vitamin C intake, but would not be expected to change if their intake of other nutrients changed. It changes relatively quickly, which allowed investigators to study subjects (adult men) who were first depleted of vitamin C (using a diet with less than 5 mg/d), and then repleted, consecutively, at seven dosage levels, ranging from 30 to 2,500 mg/d. They remained at each repletion dose until steady-state plasma and neutrophil ascorbate concentrations were attained. Urinary ascorbate excretion was also monitored. This design allowed the EAR for adult men to be identified as the average intake at which neutrophils were 80 percent saturated with vitamin C and urine losses were low. EARs for other sex and life-stage groups were extrapolated from the EAR for men, based on differences in body weight, and RDAs were determined
by adding twice the assumed coefficient of variation of 10 percent to the EAR to cover the needs of 97 to 98 percent of the population. Although the DRI panel also considered a number of chronic disease endpoints (e.g., cardiovascular disease, various cancers, cataracts, asthma and chronic obstructive pulmonary disease, and cognitive function) or biomarkers of chronic disease risk (e.g., low density lipoprotein oxidation, cancer biomarkers, DNA damage) as indicators of adequacy, they could not be used to set an EAR because the data were not consistent or specific enough, and in many cases strong intake-response data linking these outcomes to actual disease risk were not available.
Types of Study Designs Used to Develop DRIs
The scientific data used to establish DRIs have been drawn from observational and experimental studies done in humans. For the most part, experimental studies have been used to establish EARs and RDAs, while AIs and ULs have been based more often on evidence from observational studies. The types of observational studies included have been, in descending order of certainty (where “certainty” refers to the ability to make inferences about the possibility of a causal relationship), prospective cohort studies, case-control studies, cross-sectional studies, case series, and case reports. Experimental studies have included randomized and nonrandomized clinical trials, controlled intake-response studies, and balance, turnover, and depletion-repletion physiological studies. Animal studies have been excluded because results are largely not applicable to nutritional deficiencies, chronic diseases, and toxic effects in humans. However, in the absence of human data, animal studies have been considered. The only instances in which animal studies alone were used as the basis for setting a DRI were in the cases of establishing the UL for molybdenum, boron, nickel, and vanadium (IOM, 2001). Study designs that include a determination of the intake-response relationship between intake of the nutrient and the selected indicator are optimal for identifying an EAR. These relationships are necessary to identify intakes that reduce (or increase in some cases) chronic disease risks (see Chapter 7), as well as other outcomes.
Situations in Which Optimal Evidence Is Not Available for All Life-Stage Groups
The need to establish a nutrient recommendation for all 22 life-stage groups often requires extrapolation or interpolation from recommendations based on experimental data that generally come from adults. This circumstance is in contrast to situations when the goal is to establish chronic disease DRIs; preventing chronic disease with diet is highly desirable but
not as necessary as reaching nutrient adequacy; thus, there is no “requirement” to establish a DRI based on chronic disease risk. Experimental data regarding nutrient requirements are limited for infants and children, making it necessary to derive recommendations from adult data. Standards for children are extrapolated from adult data based on a body weight or a metabolic factor and then adjusted for growth or tissue deposition needs. An estimate for tissue deposition is also required for estimating nutrient requirements of pregnant women. Nutrient requirements for lactation are derived from general values for milk nutrient levels that are then adjusted for the bioavailability of each nutrient in a typical maternal diet.
The first step for determining nutrient requirements by extrapolation is to derive reference body weight and height for each age group. Data from a national survey, i.e., the National Health and Nutrition Examination Survey (NHANES), which are based on precise height and weight measurements, are frequently used. Generally, the reference body weights for adults ages 19 to 30 are applied to the older adult age groups. Although body mass index tends to increase with age, this trend is not incorporated into the DRI. If no evidence exists that the metabolic rate influences the nutrient requirement, the nutrient requirement is estimated as being directly proportional to the reference body weight derived from the national surveys. This method is used to determine the nutrient recommendations for children for some, but not all, nutrients. When metabolic rate is thought to influence the requirement, reference values have also been based on metabolic differences related to body weight, estimated as the 0.75 power of body mass. For example, this method was used to extrapolate data from adults to infants and children for all the B vitamins, and to children and adolescents between 1 to 18 years of age for vitamin A. In contrast, sodium and potassium recommendations were adjusted for the combined median energy intakes for adult men and women, i.e., energy intake adults/energy intake younger adolescents and children. Dietary fiber recommendations for children also were derived from the ratio of adult median energy intakes to childhood median energy intakes.
Research on the nutrient requirements of pregnant and lactating women is also limited. To derive recommendations for pregnancy, a factorial approach is used that includes fetal nutrient accretion estimates and additional maternal needs for increased metabolic activity or for fluid or tissue deposition. If known and if it is appropriate, adjustments for insensible fluid losses and physical activity may be added. Although breast milk nutrient composition data are available, the volume produced may vary widely among women. Milk composition also may vary with the mother’s nutritional status. To account for those differences in lactation needs, a reference milk volume is used for months 0-6 (780 mL/d) and months 7-12 (600 mL/d), and the milk composition of a well-nourished woman exclu-
TABLE 2-4 Common Considerations for Adjusting DRI Values When Planning Dietary Intakes for Healthy Individuals or Groups
| Consideration | Nutrient | Adjustment |
|---|---|---|
| Women of childbearing age | Folic acid | Recommended that women capable of becoming pregnant take 400 μg folic acid/d from fortified foods, supplements, or both in addition to meeting the RDA. |
| Individuals older than 50 years of age | Vitamin B12 | Foods fortified with B12 or supplemental B12 should be consumed by those older than 50 years of age due to decreased gastric acid with aging. Persons with any malabsorption syndrome will likely require increased amounts of B12. |
| Smoking | Vitamin C | The vitamin C requirement for smokers is increased by 35 mg/d due to increased vitamin C turnover. This also may be true for nonsmokers who are regularly exposed to tobacco smoke. |
| Bioavailability in vegetarian diets | Iron | The iron requirement is 1.8 times higher for vegetarians due to lower iron bioavailability. |
| Zinc | The zinc requirement may be as much as 50 percent higher, especially for strict vegetarians who consume grains and legumes as the major food staples. | |
| Vitamin A | Individuals who do not consume animal-based foods must meet their requirement by consuming sufficient provitamin A carotenoids or fortified foods. |
| Consideration | Nutrient | Adjustment |
|---|---|---|
| Infants of vegan mothers | Vitamin B12 | Supplemental B12 at the AI should be given at birth due to low stores at birth and in mother’s milk. |
| Age of menstruation | Iron | The RDA for women ages 14 to 18 years includes 2.5 mg iron/d to cover menstrual iron losses. If girls start menstruation before age 14 years, 2.5 mg iron should be added to their RDA; if menstruation starts after age 14 years, 2.5 mg could be subtracted from the RDA until menstruation begins. |
| Women who use oral or patch contraceptives | Iron | Oral or patch contraceptives lower menstrual blood loss, which may lower the requirement. |
| Age at menopause | Iron | Iron needs decrease if menopause occurs before age 50 years, and would be higher in women older than age 50 years who still menstruate. |
| Athletes engaged in regular intense exercise | Iron | Average requirements may range from 30 to 70 percent above those of normally active individuals. |
| Individuals unaccustomed to prolonged physical activity in a hot environment that engage in intense exercise under such environment | Sodium | Due to the excessive loss of sodium, the AI might not apply to individuals in these situations. |
| Recommendation according to reference body weight | Protein | Recommendation for adults is 0.8 g/kg/day. |
| Recommendation set per calorie needs | Fiber | Recommendation is 14g/1,000 kcal. |
| Consideration | Nutrient | Adjustment |
|---|---|---|
| Alcohol consumption | Fatty acids | Significant alcohol intake can depress fatty acid oxidation. Excess may be stored as fat. |
| Zinc | Daily requirement may be higher in individuals who exhibit long-term alcohol consumption. | |
| Multiparous pregnancies | Protein | Women carrying twins should increase their protein intake by an additional 50 g/day beginning in the second trimester and ensure sufficient energy intake. |
| Adolescent mothers, multiparous pregnancies, and mothers nursing multiple infants | Phosphorus, Magnesium | Requirements may be higher due to increased maternal and fetal needs. |
SOURCE: IOM, 2006b.
sively breastfeeding a healthy infant born at term is used as a reference (IOM, 2006b).
Population Groups with Special Concerns
The DRIs are standards for apparently healthy people and are inappropriate for those with acute or chronic disease or for the repletion of nutrient levels in previously deficient individuals (IOM, 2006b). The current DRIs cannot be used to estimate the nutrient requirements for populations with these special concerns or needs. The DRI reports emphasize that only qualified medical or nutritional personnel can make appropriate adjustments for individuals with those specific needs. Other factors, such as nutrient bioavailability and physiological and lifestyle characteristics may alter nutrient requirements (IOM, 2006b). When planning dietary intakes for individuals with such altered nutrient requirements, adjustments in the DRI values can be made. Table 2-4 lists the appropriate adjustments for several common considerations.
As the previous DRI committees began to introduce the idea of chronic disease outcomes as a basis for nutrient adequacy or toxicity, they began to
deal with a number of challenges. These challenges, including the selection of outcomes and the limited availability of longitudinal data with appropriate dietary intake assessments, were discussed in various scientific fora and are further elaborated in Chapter 3. The reminder of the report will delve into the unique issues of including chronic disease outcomes when establishing nutrient reference values.
REFERENCES
CIHR (Canadian Institutes of Health Research). 2016. Best brains exchanges 2015. http://www.cihr-irsc.gc.ca/e/49563.html (accessed April 30, 2017).
FDA (U.S. Food and Drug Administration). 2013a. Everything added to food in the United States (EAFUS). https://www.accessdata.fda.gov/scripts/fcn/fcnNavigation.cfm?rpt=eafusListing (accessed April 26, 2017).
FDA. 2013b. Guidance for industry: A food labeling guide (14. Appendix F: Calculate the percent daily value for the appropriate nutrients). https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm064928.htm (accessed April 26, 2017).
Health Canada. 2007. Eating well with Canada’s Food Guide. http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/index-eng.php (accessed April 30, 2017).
HHS and USDA (U.S. Department of Health and Human Services and U.S. Department of Agriculture). 2015. 2015–2020 Dietary Guidelines for Americans. 8th Edition. https://health.gov/dietaryguidelines/2015/guidelines (accessed July 24, 2017).
IOM (Institute of Medicine). 1994. How should the Recommended Dietary Allowances be revised? Washington, DC: National Academy Press.
IOM. 1997. Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press.
IOM. 1998a. Dietary Reference Intakes: A risk assessment model for establishing upper intake levels for nutrients. Washington, DC: National Academy Press.
IOM. 1998b. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press.
IOM. 2000a. Dietary Reference Intakes: Applications in dietary assessment. Washington, DC: National Academy Press.
IOM. 2000b. Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academy Press.
IOM. 2001. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press.
IOM. 2002/2005. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: The National Academies Press.
IOM. 2003a. Dietary Reference Intakes: Applications in dietary planning. Washington, DC: The National Academies Press.
IOM. 2003b. Dietary Reference Intakes: Guiding principles for nutrition labeling and fortification. Washington, DC: The National Academies Press.
IOM. 2005. Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate. Washington, DC: The National Academies Press.
IOM. 2006a. Dietary Reference Intakes research synthesis: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2006b. Dietary Reference Intakes: The essential guide to nutrient requirements. Washington, DC: The National Academies Press.
IOM. 2008. The development of DRIs 1994–2000: Lessons learned and new challenges: Workshop summary. Washington, DC: The National Academies Press.
IOM. 2011. Dietary Reference Intakes for calcium and vitamin D. Washington, DC: The National Academies Press.
Katamay, S. W., K. A. Esslinger, M. Vigneault, J. L. Johnston, B. A. Junkins, L. G. Robbins, I. V. Sirois, E. M. Jones-Mclean, A. F. Kennedy, M. A. Bush, D. Brule, and C. Martineau. 2007. Eating well with Canada’s Food Guide (2007): Development of the food intake pattern. Nutr Rev 65(4):155-166.
Levine, M., C. Conry-Cantilena, Y. Wang, R. W. Welch, P. W. Washko, K. R. Dhariwal, J. B. Park, A. Lazarev, J. F. Graumlich, J. King, and L. R. Cantilena. 1996. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A 93(8):3704-3709.
Taylor, C. L. 2008. Framework for DRI development: Components “known” and components “to be explored.” Washington, DC.
U.S. Army. 2017. Army Regulation 40-25: Nutrition and menu standards for human performance optimization. Washington, DC: Headquarters, Departments of the Army, the Navy, and the Air Force.
USDA (U.S. Department of Agriculture). 2016. School meals: Child nutrition programs. https://www.fns.usda.gov/school-meals/child-nutrition-programs (accessed April 26, 2017).
This page intentionally left blank.


































