1
Introduction1
Those involved in the drug development process face challenges of efficiency and overall sustainability due in part to high research costs, lengthy development timelines, and late-stage drug failures. Novel clinical trial designs that enroll participants based on their genetics represent a potentially disruptive change that could improve patient outcomes, reduce costs associated with drug development, and further realize the goals of precision medicine (Nelson et al., 2015). On March 8, 2017, the Forum on Drug Discovery, Development, and Translation (the forum) and the Roundtable on Genomics and Precision Health (the roundtable) of the National Academies of Sciences, Engineering, and Medicine (the National Academies) hosted the workshop Enabling Precision Medicine: The Role of Genetics in Clinical Drug Development (see Box 1-1 for the Statement of Task).2 This workshop, co-chaired by Esteban Burchard, professor in the Departments of Bioengineering and Therapeutic Sciences and Medicine at the University of California, San Francisco, and Laura Nisenbaum, advisor at Chorus Clinical Development of Eli Lilly and Company, examined successes, chal-
___________________
1 This workshop was organized by an independent planning committee whose role was limited to identification of topics and speakers. This Proceedings of a Workshop was prepared by the rapporteurs as a factual summary of the presentations and discussion that took place at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants, and are not endorsed or verified by the National Academies of Sciences, Engineering, and Medicine, and they should not be construed as reflecting any group consensus.
2 The workshop statement of task and agenda, speaker biographical sketches, and list of registered attendees can be found in Appendixes B, C, and D, respectively.
lenges, and possible best practices for effectively using genetic information in the design and implementation of clinical trials to support the development of precision medicines, including exploring the potential advantages and disadvantages of such trials across a variety of disease areas. Workshop participants were asked to take a general, high-level approach in their examination of these issues, to take a pulse of the successes that have been realized and the challenges that have been encountered, and to consider how these experiences might inform the advancement of precision medicine.
Geoffrey Ginsburg, co-chair of the roundtable and director of the Duke Center for Applied Genomics and Precision Medicine, began by explain-
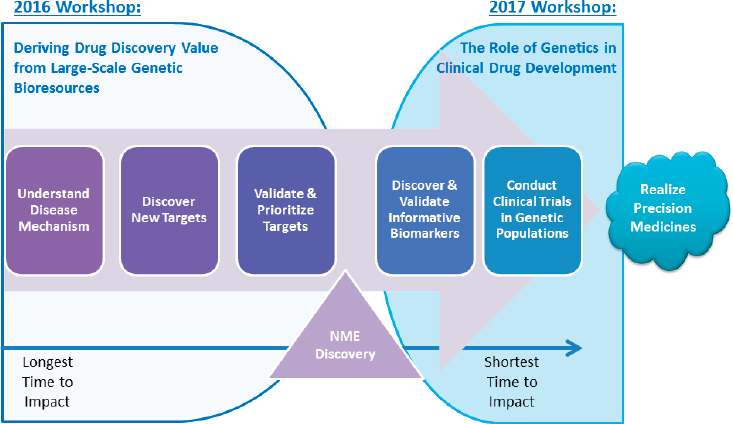
NOTE: NME = new molecular entity (a drug that contains an active moiety that has never before been approved or marketed within the United States).
SOURCE: Laura Nisenbaum, National Academies of Sciences, Engineering, and Medicine workshop presentation, March 8, 2017.
ing that this workshop follows on a 2016 workshop co-convened by the forum and the roundtable, Deriving Drug Discovery Value from Large-Scale Genetic Bioresources3 (NASEM, 2016a). The 2016 workshop, he summarized, focused on genetics in drug discovery, exploring how large genetic bioresources could be leveraged as a way to discover novel pathways and drug targets. Nisenbaum highlighted the role of genetic information in each of the stages across the drug discovery and development lifecycle (see Figure 1-1), from understanding the biological mechanisms of disease to discovery and validation of new therapeutic targets, identification and validation of biomarkers, and, finally, carrying out clinical trials in genetically defined populations. The purpose of the March 2017 workshop, Nisenbaum said, was to focus on the latter stages of drug development and explore how genetically identified populations can be integrated into clinical trials.
___________________
3 Resources from the Deriving Drug Discovery Value from Large-Scale Genetic Bioresources workshop are available online at http://www.nationalacademies.org/hmd/Activities/Research/GenomicBasedResearch/2016-MARCH-22.aspx (accessed May 10, 2017).
WORKSHOP OBJECTIVES
The workshop, developed by an independent planning committee, was intended to
- Explore how clinical trials with genetically identified participants can enable more efficient and effective drug development and advance precision medicine;
- Highlight ongoing genetics-enabled clinical trials across a variety of diseases, examining best practices and lessons learned;
- Learn about the logistical challenges and successes associated with genetics-enabled clinical trial design; and
- Examine possible mechanisms to engage participants and improve enrollment in clinical trials based on genetic characteristics.
Nisenbaum charged workshop participants with considering tangible next steps for achieving effective integration of genetics into the drug development process. What, she asked, might serve as a disruptive force for translating knowledge of human genetics into new precision medicines within the next 3 to 5 years?
OVERVIEW OF CROSS-CUTTING TOPICS HIGHLIGHTED DURING PRESENTATIONS AND DISCUSSIONS4
A number of themes emerged across workshop presentations and discussions as speakers and participants considered various aspects of integrating genetics into the drug development process. The themes and opportunities highlighted below, drawn from individual presentations and open discussions, are discussed in more detail throughout this proceedings.
Engaging Patients in Precision Medicine Clinical Trials
The topic of engaging patients in precision drug development was considered from many angles over the course of the workshop. In order for patients to become effective partners in the drug development process, they need to be able to understand the scientific and health information being conveyed to them by providers (Green et al., 2011). This can be especially challenging because patients often receive an overwhelming amount of scientific information that may include the use of complex terminology. In
___________________
4 The rapporteurs’ summary of the main topics and recurring themes is drawn from the presentations, discussions, and summary remarks by the moderators. Items on this list should not be construed as reflecting any consensus of the workshop participants or any endorsement by the National Academies of Sciences, Engineering, and Medicine.
addition to strategies that increase provider education on this issue, another potential solution to increasing patient engagement discussed at the workshop was for patient advocacy organizations to provide easy-to-understand explanations of complex topics, such as genetic variation or benefit–risk assessment, that could be adapted to different disorders.
A challenge to clinical trial accrual is a lack of awareness among providers and patients about the existence of information about ongoing trials. According to some workshop participants, developing educational tools and establishing new resources, such as a more user-friendly clinical trials database, may help empower providers to identify patients with genetic diseases or diseases with an underlying genetic component, discuss clinical trials as treatment options when necessary, and assist in enrolling patients in trials.
Discussions at the workshop also focused on the idea that patients should be partners in the research process, providing input on where research efforts and clinical trial development are most needed to address unmet needs. For example, a case study was discussed in which patient input was solicited through participation in a live simulation of a clinical trial protocol, which improved patient and investigator experience, optimized trial protocols, and demonstrated the benefits of patient engagement and of providing input to trial sponsors (see Chapter 5).
There are many tools and strategies for engaging patients in drug development and enhancing clinical trial recruitment, enrollment, and retention. These strategies include a variety of communications approaches such as targeted social media outreach, webinars and promotional videos, and direct outreach to networks of disease advocacy groups, patients, and physicians. Another unique approach to increasing trial enrollment is the use of sites that can be activated “just in time” when a patient is identified. Finally, technology can be used through mobile applications for scheduling, reminders, and post-study engagement.
Providing Access to Personal Genetic Information
The issue of returning genetic data to clinical trial participants was raised several times during the workshop. A large percentage of research participants would like to have their genetic testing results returned to them in a timely fashion (Ruiz-Canela et al., 2011). One option for managing genetic test results generated during a clinical trial is to return them directly to patients so that the patients have ownership of their data and easy access to the information as they move between treatment and clinical trial settings over the course of their lifetime. However, this approach will likely present challenges due to a lack of data standards, difficulty communicating results, and insufficient operational resources to manage the
return of genetic data (Prucka et al., 2015). Furthermore, in those cases where patients do receive their genetic information, they may not be fully aware of the implications of the results, further providing a rationale for increased patient and provider education.
Increasing Genetic Diversity in Clinical Trial Populations
Racial and ethnic minorities are vastly underrepresented in clinical research in the United States (Oh et al., 2015; Yancey et al., 2006). Traditionally, the majority of individuals included in genome-wide association studies (GWASs) have been of European descent and the populations that are oftentimes at highest risk for particular disease outcomes are not being included in these studies (Need and Goldstein, 2009). Furthermore, the prevalence of genetic variants can vary markedly by geographic area. To address this issue, some workshop participants expressed interest in recruiting patients from health systems (which might serve a more diverse population) and in leveraging information in electronic health records (EHRs). Several workshop participants noted that socioeconomic status and environmental contributors should also be considered when analyzing the link between genetic data and disease status.
Optimizing Biomarker Development
There are potential benefits to identifying genetic biomarkers early in drug development, and some workshop participants suggested that the greatest impact of precision medicine will be in leveraging genetics and genomics to guide drug development from the earliest possible stage. Examples were also provided of late-stage and retrospective biomarker development, where there is more statistical power to detect a pharmacogenomic association. However, it may be practically difficult or technically unfeasible to identify a biomarker in late-stage development, and participants also noted that it can be difficult to adjust a sponsor’s development strategy in late-stage clinical research if associations are found.
Establishing Innovative Public–Private Partnerships
An increasing number of critical players are working together to find treatments for genetic diseases, and the establishment of new public–private partnerships could further accelerate progress. Some workshop participants noted a number of potential partners for collaborative efforts, including disease consortia that house registries and biorepositories, biopharmaceutical companies, academic medical centers, health care providers, researchers, disease advocacy and patient-focused groups, government and regulatory
agencies, and, importantly, patients, caregivers, and their families. There is interest among many stakeholder groups in participating in integrated precompetitive genetics research, said Rebecca Blanchard, a workshop speaker and head of clinical genetics at Merck Research Laboratories. Partnerships between researchers and patient groups can help facilitate research on patient biospecimens, which could accelerate the development of assays necessary for drug development.
Engaging Regulatory Authorities Early in Development
Interacting with regulatory agencies as early as possible in the drug development pathway could potentially streamline genetics-enabled drug development, said Michael Pacanowski, a workshop speaker and associate director for genomics and targeted therapy in the Center for Drug Evaluation and Research at the U.S. Food and Drug Administration. To further accelerate the process, clinical trials could be designed to gather data that would support the development and approval of both the drug and the companion in vitro diagnostic test (IOM, 2014).
ORGANIZATION OF THE WORKSHOP AND PROCEEDINGS
This report summarizes the presentations and discussions that took place at the workshop. In the first session of the workshop, speakers highlighted overarching considerations for implementing successful genetics-driven drug development (see Chapter 2). Panelists in the second session described case studies in precision drug development and shared lessons learned that could be applied across disease fields (see Chapter 3). The third session focused on integrating genetics into drug development for complex diseases (see Chapter 4). The final panel session considered innovative ways to integrate genetics research into the drug development process (see Chapter 5). In the final discussion, session moderators reflected on the presentations and highlighted key takeaway messages (see Chapter 6).
This page intentionally left blank.








