NOTE: These points were made by the individual speakers identified above; they are not intended to reflect a consensus among workshop participants.
Many targets and neuroimaging biomarkers of neuroinflammation have been approved for human use, said Anna Katrin Szardenings, director of biomarker operations and imaging at Janssen Research & Development (see Figure 5-1). These include nuclear imaging methods such as PET and SPECT, as well as MRI approaches. Szardenings said both approaches are useful to monitor the pathological consequences of neuroinflammation. Nuclear imaging methods are better equipped to image efflux proteins, while MRI enables the visualization of a compromised BBB and human immune cell trafficking, as well as global atrophy, she said. However, while all of these approaches have proved useful in clinical and research laboratories, particularly in diseases such as MS where there is overt neuroinflammation, Brian Campbell questioned whether they have adequate
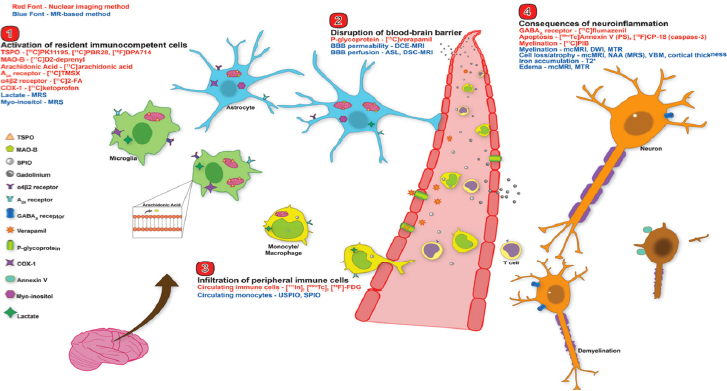
SOURCES: Presentation by Szardenings, March 21, 2017; Albrecht et al., 2016.
sensitivity in diseases where neuroinflammatory changes are more subtle, such as depression, or if these methods are sensitive enough to discriminate subpopulations and their changing microenvironments in diseases such as AD.
NUCLEAR IMAGING APPROACHES
TSPO is a mitochondrial protein that is highly expressed in macrophages, activated microglia, and reactive astrocytes, and thus is a putative biomarker for the activation of the immune system in the brain, said Robert Innis. The first-generation TSPO PET ligand, PK11195, had a low signal-to-noise ratio and gave conflicting results in terms of imaging neuropathology in AD and mild cognitive impairment (MCI). However, Innis said that a second-generation radioligand, PBR28, has a much higher signal-to-noise ratio and shows a significant and widespread increase in TSPO binding in the inferior parietal cortex of patients with AD but not MCI, as well as a correlation with symptom severity (Kreisl et al., 2013). Innis noted that this contrasts with amyloid load as demonstrated with PET imaging, which has never been shown to correlate with cognitive impairment. Moreover, a small longitudinal study in patients with AD and MCI showed that an increase in the amount of TSPO binding correlated with disease progression, suggesting its use as a biomarker of disease progression, said Innis.
Having shown that PBR28 can work as a biomarker in AD, Innis and colleagues investigated its use in MDEs, following up on an earlier study that showed a correlation between TSPO volume and the presence of MDE (Setiawan et al., 2015). Unlike AD, where the neuropathological characteristics have been well defined, MDE has no known neuropathology despite the fact that there is strong evidence that in at least a subset of patients, depression may be linked to peripheral or brain inflammation. The National Institute of Mental Health team demonstrated that unmedicated MDE patients show increased TSPO density compared to healthy controls or patients treated with selective serotonin reuptake inhibitors (SSRIs), suggesting that SSRI treatment itself may influence TSPO density.
Although TSPO may thus be a biomarker of inflammation and might have potential for stratifying patients for clinical trials, Innis said it has limitations because of its high variability. He added that it may not be useful as a tool to demonstrate whether an anti-inflammatory drug is hit-
ting its target because TSPO ligands also act as agonists. However, his team has developed PET ligands for components of the cyclooxygenase system—COX-1 and COX-2—which may be useful in trials of non-steroidal anti-inflammatory drugs because they act as inhibitors of either COX-1 or COX-2. The cyclooxygenase system is responsible for synthesizing inflammatory mediators called prostanoids from arachidonic acid, said Innis. COX-1 and COX-2 are present in different cell types and have different functions. Innis believes that because of its cellular localization, COX-1 may be more useful as a biomarker in neuroinflammatory disorders. Uptake of COX-1 radioligands is specific and is blocked even by low doses of highly selective COX-1 antagonists. Innis and colleagues will soon be starting first-in-human studies of these various radioligands.
Szardenings, along with Hartmuth Kolb and colleagues at Janssen Neuroscience and the University of Leuven, have focused efforts on developing the purinergic receptor P2X7R as a PET tracer to be used in conjunction with the development of a therapeutic compound. In rat models, P2X7R is expressed at low levels throughout the brain, primarily in microglia and astrocytes (Choi et al., 2007). According to Szardenings, P2X7R expression drives microglia activation, activating the inflammasome and interleukin (IL)-1β secretion, thus making it a relevant target for monitoring neuroinflammation. Preclinical studies indicate that the ligand in development, known as 739, hits the target with minimal non-specific binding and can be blocked with an antagonist, she said. Following primate studies that demonstrated dose-dependent target occupancy, the team tested the ligand in an inflammatory rat model, where they showed that inducing neuroinflammation by injecting lipopolysaccharide locally into the striatum was associated with an increased uptake of 739, and that the antagonist blocked this effect, indicating that 739 may be useful to measure target engagement of P2X7R therapeutics and to image neuroinflammation. However, Szardenings noted that because P2X7R is evenly distributed throughout the brain, there is no reference region to allow calculation of occupancy and aid in image analysis. Future efforts will compare PET images using 739 to other markers of inflammation, said Szardenings.
MAGNETIC RESONANCE IMAGING APPROACHES
As mentioned in Chapter 4, MRI has become the predominant tool for diagnosing MS. Indeed, said Amit Bar-Or, axial brain MRI in pa-
tients with MS shows bright hyperintensity on T2-weighted images, demonstrating over time that the disease is dynamic, multifocal, and diffuse. Moreover, gadolinium enhancement demonstrates a breach of the integrity of the BBB, which is thought to reflect perivascular inflammation; MRI also is used to assess brain atrophy as a measure of global injury, although this measure is affected by an individual’s level of hydration, he said. In addition, Bar-Or said that standard MRI sequences fail to show a substantial degree of injury that is interlesional.
Martina Absinta of the Translational Neuroradiology Section at the National Institute of Neurological Disorders and Stroke showed time-lapse MRI from more than 100 gadolinium-enhanced scans from MS patients collected over 24 years. These scans demonstrate the highly inflammatory nature of MS, the progressive enlargement of the ventricles suggesting ongoing atrophy over time, and neurodegeneration. However, in addition to the acute inflammation seen in these images, which is associated with the opening of the BBB, Absinta said that chronic inflammation occurs behind a partially intact BBB at the plaque level and within the leptomeninges. This chronic inflammation is invisible to gadolinium-based MRI, although it is likely responsible for progressive disease.
Pathological studies show that chronic, active demyelinated lesions can be further visualized using iron staining to show the accumulation of activated microglia at their edges, said Absinta. She noted that chronic inactive lesions, in contrast, are still completely demyelinated, but are devoid of inflammatory cells. To see this pathology in living patients, she and her colleagues are using 7-Tesla susceptibility-based MRI, which is sensitive to paramagnetic substances such as iron (Absinta et al., 2013). These images show a dark rim around the lesion indicative of microglial accumulation. In a longitudinal study of newly formed lesions imaged over 18 months, this imaging modality allowed them to identify three different scenarios of lesion evolution as well as the propensity to repair. Then, using postmortem MRI, they confirmed that the worst of these lesions, where the dark rim persists, are completely demyelinated and surrounded by activated microglia (Absinta et al., 2016). They concluded that chronic inflammation develops within the first 3 months of lesion onset and marks the failure of early lesion repair.
In another series of studies, Absinta and colleagues are using MR techniques to image chronic inflammation within the leptomeningeal compartment because pathology studies have shown that meningeal inflammation is a key and persistent driver of MS pathogenesis both early
in the disease and at later progressive stages. The technique they use is called postcontrast 3D T2-FLAIR (fast fluid-attenuated inversion recovery). In a study of 299 MS patients, they demonstrated that focal areas of perivascular leptomeningeal enhancement are much more prevalent in the progressive phase of disease, and that patients with leptomeningeal enhancement were more disabled, older, had long disease duration, and had lower brain and cortical volumes (Absinta et al., 2015). Two autopsy cases, in combination with the in vivo data, confirmed the role of leptomeningeal enhancement as a biomarker for meningeal inflammation associated with the opening of the BBB, said Absinta. She added that other chronic neuroinflammatory conditions show a similar pattern of blood‒meningeal barrier impairment due to meningeal inflammation using this technique, suggesting that it may be useful for patient selection and stratification in clinical trials of disease-modifying treatments for chronic inflammation. Correlating this imaging biomarker with cytokine profiles in the cerebrospinal fluid might also lead to a better understanding of what drives cortical demyelination in MS, she said.
Katerina Akassoglou and colleagues, in collaboration with Roger Tsien and Michael Whitney at the University of California, San Diego, also developed molecular probes that may be used with MRI to monitor thrombin activity in neuroinflammatory disease. As described in Chapter 3, soluble fibrinogen acts with thrombin to form insoluble fibrin, which plays a role in blood clotting and is highly proinflammatory, said Akassoglou. In the experimental autoimmune encephalomyelitis mouse model of MS, the thrombin probe they developed accumulates at the site of demyelinating lesions and correlates with demyelination as well as activation of innate immunity and neurodegeneration (Davalos et al., 2014) (see Figure 5-2). The technology is currently being tested in clinical applications in cancer, and could provide a sensitive tool to be able to detect early changes in clotting and fibrin deposition that could indicate very early BBB leakage in the course of MS, said Akassoglou.
The importance of fibrin deposition during the course of MS is also substantiated by proteomic analysis in human MS lesions, which demonstrate excessive and persistent fibrin deposition in pre-active, active, chronic, and chronic inactive MS lesions (Claudio et al., 1989; Han et al., 2008; Kirk et al., 2003; Marik et al., 2007; Vos et al., 2005). Akassoglou added that numerous neuropathological studies also show the presence of fibrin in AD (Cortes-Canteli, 2015; Lee at al., 2007; Ryu et al., 2009),
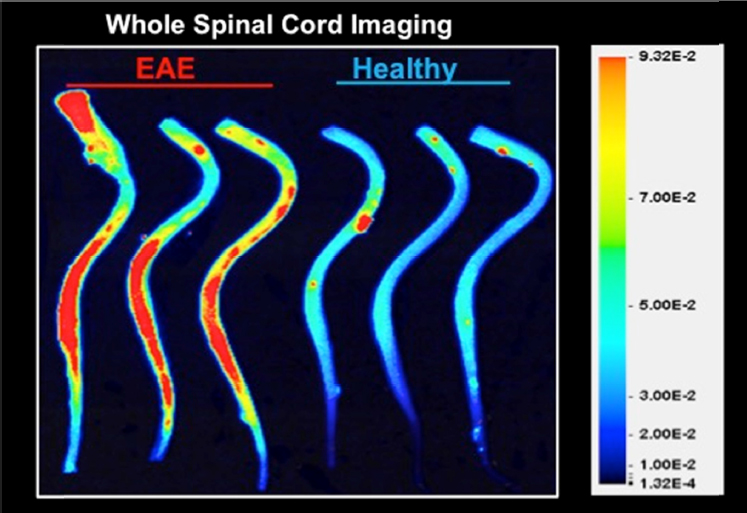
SOURCE: Presentation by Akassoglou, March 21, 2017.
which correlates with microglia activation and dystrophy, a sign of microglial senescence in the aging brain (Streit et al., 2004). Fibrin and activation of coagulation could be a common thread and therapeutic target among neurological diseases with vascular alterations, she concluded.
This page intentionally left blank.








