5
Current Process for Developing the Dietary Guidelines for Americans: Key Findings
This chapter discusses the current process for developing the Dietary Guidelines for Americans (DGA),1 as well as key findings from an assessment of the processes used to develop the 2005 DGA, 2010 DGA, and 2015–2020 DGA.
CURRENT PROCESS
The process to update the DGA involves a number of steps, beginning with administrative tasks and culminating in the release and implementation of the new edition of the DGA (see Figure 5-1).
Administrative Tasks to Begin the DGA Cycle
Typically the first step to establish a given cycle of the DGA is the execution of a memorandum of understanding between the U.S. Department of Agriculture (USDA) and the U.S. Department of Health and Human Services (HHS). The memorandum indicates which agency will serve as the administrative lead for that particular DGA cycle,2 states the intent to establish the Dietary Guidelines Advisory Committee (DGAC),
___________________
1 Refer to Chapter 1, Box 1-1, for an explanation of how the term DGA is used throughout this National Academies report.
2 Responsibility for administrative lead and operational costs rotates between USDA and HHS. For the 2015–2020 DGA, HHS was the lead agency, while USDA will be the lead for the 2020–2025 DGA (USDA/HHS, 2017a).
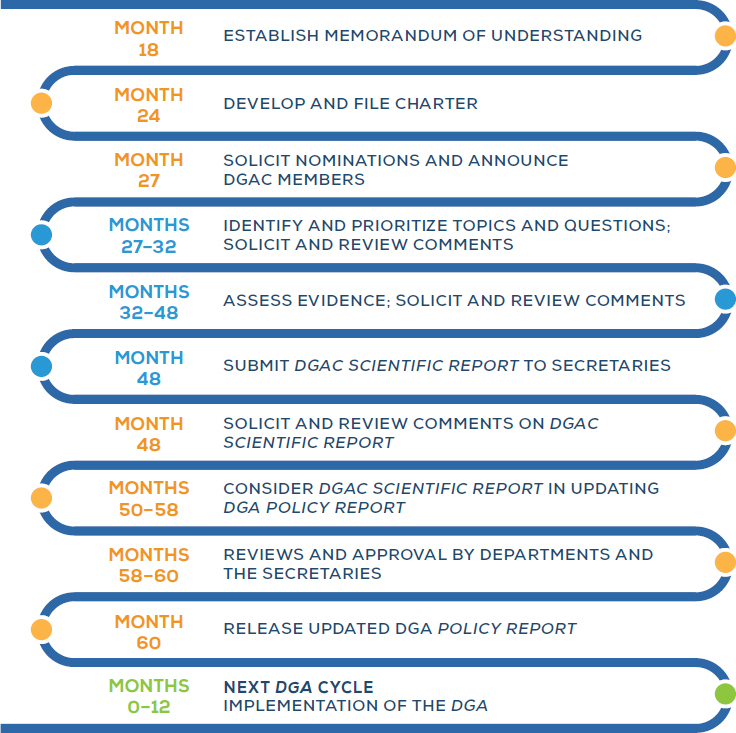
NOTES: Process and timeline based on the 2015–2020 DGA. “Month” values indicate the approximate number of months after release of the previous edition of the DGA, based on an analysis of the 2005, 2010, and 2015 DGA cycles. Orange dots indicate USDA and HHS steps; blue dots indicate DGAC steps; and the green dot indicates steps for government and nutrition and health professionals.
and describes the plan to identify co-executive secretaries. In the past three editions, the memorandum of understanding was executed between 18 and 29 months after the prior DGA Policy Report was released.
The next major step is to establish the DGAC. The DGAC is set up as a federal advisory committee, governed by the Federal Advisory Committee Act of 1972 (Public Law 92-463). Before the DGAC can begin its work, a charter must be developed and filed with Congress that states
the specific duties and general operational characteristics of the federal advisory committee (GSA, 2011). The charter also lists the categories of expertise sought for on the DGAC. In accordance with the Federal Advisory Committee Act, meetings of federal advisory committees are open to the public unless an exception is granted. Discretionary federal advisory committees generally meet for a period of 2 years after the charter is filed unless (1) otherwise specified, (2) the charter is renewed, or (3) the group completes its work, whichever comes first. The federal advisory committee serves as an independent body for the purpose of providing advice to the government (GSA, 2016).
In establishing the 2015 DGAC, HHS and USDA developed the charge: “Examine the previous edition of the DGA and determine topics for which new scientific evidence is likely to be available that may inform revisions to the current guidance or suggest new guidance” (USDA/HHS, 2016a). The DGAC’s advice is provided to the secretaries in the form of a report called the Scientific Report of the Dietary Guidelines Advisory Committee, referred to in this report as the DGAC Scientific Report. The 2015 DGAC comprised 14 individuals selected and appointed by the secretaries of HHS and USDA, representing a broad array of scientific expertise necessary to conduct the work to be performed.
In recent cycles, the charter to establish the DGAC has been filed approximately 2 to 3 years following the release of the prior DGA Policy Report. Before the 2010 cycle, the charter was filed prior to the call for DGAC member nominations. The 2010 and 2015 DGA cycles followed a slightly different process, where the charter was filed after the call for nominations to give the DGAC more time to conduct its work. For a more detailed discussion of the DGAC selection process, please see this National Academies of Sciences, Engineering, and Medicine (the National Academies) committee’s first report (NASEM, 2017). For the 2005, 2010, and 2015 DGACs, members were sworn in and able to begin their work between 6, 4, and 4 months, respectively, into the 2-year timeline, leaving between 19 and 21 months to complete their work. Although five meetings are typically scheduled, additional meetings can be held; the 2010 DGAC met six times, and the 2015 DGAC met seven times.
Identify and Prioritize Topics and Questions
For the 2015 DGAC, USDA and HHS provided some initial guidance for identifying topics, proposing that the DGAC focus on food groups and/or dietary patterns, with an emphasis on food-based recommendations to help promote health and prevent disease. The departments also suggested that specific nutrients only be considered when (1) discussing nutrients of public health concern or (2) advising on how previously
established Dietary Reference Intakes (set by the Institute of Medicine) ought to be implemented. Additional guidance stated that topics could be explored if they potentially enhanced how the DGA Policy Report was implemented, such as the social, behavioral, and food environmental factors related to diet outcomes such as intake of foods, food groups, and dietary patterns. USDA and HHS also suggested that health outcomes of public health concern ought to be considered by the DGAC, including cardiovascular disease, body weight status, cancer, diabetes, bone health, and prevention of food-borne illness, among others (HHS/USDA, 2013a,b).
To identify topics to consider and address in their scientific reports, DGACs typically divide into smaller groups upon their appointment. These topics are then brought back to the full DGAC for final selection and prioritization. The following paragraphs describe the working structure, which prescribes the preliminary themes for these smaller groups.
The process for developing the DGAC’s working structure has changed over time. For the 2005 and 2010 DGACs, USDA and HHS named subcommittees and assigned members to initial subcommittees before the DGAC’s first meeting. This initial subcommittee structure was based on a review of the previous DGA Policy Report (e.g., guidelines categories from the 2005 DGA became the initial subcommittees for the 2010 DGAC). Over the course of their deliberations, the DGACs had the opportunity to recast and rename subcommittees or identify additional subcommittees as needed. For example, the 2010 DGAC broadened the scope of the initial subcommittees: the “carbohydrate subcommittee” became the “carbohydrates and protein subcommittee.” The 2005 DGAC added subcommittees, such as the macronutrient subcommittee (USDA/HHS, 2016b). The subcommittees for the 2005 and 2010 DGACs had multiple roles, spanning from identifying topics and developing questions to be answered by evidence assessments, to creating plans for reviewing the evidence and drafting conclusions and recommendations for consideration by the full DGAC (USDA/HHS, 2016b).
HHS and USDA recommended that the process be modified for the 2015 cycle by having the DGAC, in collaboration with the DGAC’s designated federal officer and co-executive secretaries from HHS and USDA, determine its own working structure with the goals of more efficiently allocating resources and time, and more effectively staffing the DGAC (Millen, 2017; USDA/HHS, 2016b) (see Table 5-1). To accomplish this, the designated federal officer and co-executive secretaries assisted the chair and vice chair of the 2015 DGAC to convene the Science Review Subcommittee, consisting of the chair, vice chair, and two DGAC members who were also part of the 2010 DGAC. This Science Review Subcommittee identified initial themes and assigned the members of the 2015 DGAC
TABLE 5-1 Roles of the Various Working Structures from the 2005, 2010, and 2015 DGACs
| DGAC Cycle | Working Structure | Roles |
|---|---|---|
| 2005 and 2010 DGACs | Subcommittees suggested by USDA and HHS, finalized by DGACs | Identify and prioritize topics; develop questions to be answered by evidence assessments; create plans for reviewing the evidence; draft conclusions and recommendations |
| 2015 DGAC | Three initial work groups | Identify and consider topics; develop questions and topic briefs |
| Five subcommittees and additional working and writing groups | Create plans for reviewing the evidence; draft conclusions and recommendations |
NOTES: DGAC = Dietary Guidelines Advisory Committee; HHS = U.S. Department of Health and Human Services; USDA = U.S. Department of Agriculture.
to one of three initial work groups: (1) environmental determinants of food, diet, and health; (2) dietary patterns and quality and optimizations through lifestyle behavior change; and (3) foods, beverages, and nutrients and their effect on health outcomes (HHS/USDA, 2015b). The task for each work group was to (1) consider topics of public health concern, informed by the 2010 DGA Policy Report and 2010 DGAC Scientific Report, and (2) develop a set of questions based on the importance and likelihood of informing the next edition of the DGA (Millen, 2017). In an iterative process, the Science Review Subcommittee edited the work groups’ questions, and requested that the three work groups develop topic briefs for each area to help prioritize the many topics. Topics were prioritized through discussion and voted on by all the 2015 DGAC members. These efforts culminated in tiers of topics for consideration that were presented to the full DGAC during public meetings, which also provided the public with an opportunity to comment. Generally, topics and questions assigned the highest priority are then taken up in the next step: evidence assessment (Millen, 2017).
During the 2015 DGAC, the Science Review Subcommittee disbanded the work groups after questions were finalized. The Science Review Subcommittee, in consultation with the designated federal officer and co-executive secretaries, reassigned the 2015 DGAC members to five subcommittees to assess the evidence in regard to specific questions. Subcommittees for the 2015 DGAC included (1) food and nutrient intakes and health: current status and trends; (2) dietary patterns, foods and nutrients, and health outcomes; (3) diet and physical activity behavior change; (4) food and physical activity environments; and (5) food sustainability and safety.
HHS and USDA provided a draft of the topic selection criteria for the 2015 DGAC to consider, which included
- Target populations;
- Potential effect on food and nutrition-related outcomes of public health concern, such as health outcomes and diet-related behaviors; and
- Likelihood of informing recommendations, whether it be to suggest new guidance, inform a revision to current guidance, or address urgent public health concerns (HHS/USDA, 2013a).
These criteria, in addition to a description and rationale for each proposed topic, were included in each topic brief.
Next, the 2015 DGAC considered a number of factors to prioritize among the identified topics. In the committee’s first public meeting, HHS and USDA suggested seven criteria for prioritization for the 2015 DGAC:
- A review of the current evidence on the topic may inform the development of new dietary guidance for Americans ages 2 years and older.
- A review of the current evidence on the topic may result in a change or elaboration in existing recommendations.
- The topic represents important uncertainty or a knowledge gap for decision makers.
- The topic addresses a dilemma in public health nutrition.
- The topic represents an area where there is a degree of urgency for guidance (e.g., significant area of public health concern, emerging area for public health action).
- The topic addresses a common practice in public health nutrition for which there is no government guidance.
- The topic has the potential to inform the development of dietary guidance that is public health oriented (i.e., the promotion of health and the prevention of disease at the population/community level) and not the development of clinical guidelines to use for the treatment and care of individuals with specific diseases and conditions (USDA/HHS, 2017a).
Members of the public were invited to comment throughout the DGAC process through the public comments database. In this way, input on the topics and questions presented during public meetings could be gathered.
The identification, final selection, and prioritization of topics and questions took approximately 5 months for the 2015 DGAC to complete.
Assess Evidence
This section focuses on the general use of the aforementioned subcommittees to assess the evidence. DGACs typically complete their work of evaluating scientific evidence through the use of subcommittees, thereby allowing a number of issues to be discussed at the same time. DGAC members all serve on multiple subcommittees. The 2015 DGAC identified and invited consultants to partake in subcommittee deliberations. These consultants were not members of the DGAC and did not participate in discussions or decisions made by the full DGAC.3 Two subcommittees of the 2015 DGAC supplemented their own expertise by inviting a total of three consultants to inform their deliberations.
In general, subcommittees conduct their work through conference calls and webinars. For the 2015 DGAC, each subcommittee met on average approximately 35 times. During most subcommittee meetings, members could communicate directly with federal staff who supported the data gathering and analysis efforts of the 2015 DGAC; additional work between the subcommittee and federal staff members, including the Nutrition Evidence Library (NEL), occurred through email.4 While the subcommittees received support from federal staff and the public in their collection of evidence, the subcommittees independently evaluated the evidence (USDA/HHS, 2017b).
The subcommittees produce assessments of the evidence and drafts of conclusions for consideration by the full DGAC (USDA/HHS, 2016b). Members of the DGAC then work together to finalize conclusions and develop the final report. Additional subgroups of the 2015 DGAC were formed to further advance the DGAC’s efforts, such as working on crosscutting issues. The scope of the subcommittees is subject to change with each cycle. As discussed in the previous section, DGAC subcommittees prior to 2015 were tasked with both developing topics and evaluating the scientific evidence. In contrast, the 2015 DGAC subcommittees focused on examining the evidence, because the topics were identified by the work groups.
The subcommittees’ work has also changed as the types of evidence it considers has evolved. The 2010 and 2015 DGACs considered four types of
___________________
3 While consultants received training and were cleared through the federal process like the DGAC members, they were not members of the full committee and could not vote on decisions made by the DGAC (USDA/HHS, 2016b).
4 Multiple types of federal support staff were involved with the 2015 DGAC. In addition to the co-executive secretaries who represented USDA and HHS throughout the 2015 DGAC process, the Dietary Guidelines Management Team provided administrative support to the DGAC and its subcommittees, the NEL staff helped the DGAC conduct systematic reviews according to NEL systematic review methods, and the Data Analysis Team presented analyses and summaries of data from USDA and HHS as requested by the DGAC.
evidence: (1) original systematic reviews with support from USDA’s NEL; (2) existing systematic reviews, meta-analyses, and reports; (3) descriptive data analyses (e.g., intakes of foods and nutrients); and (4) food pattern modeling analyses. The types of evidence have grown with changes in nutrition science. For example, food pattern modeling was first formally introduced for inclusion during the 2005 DGAC, and the NEL was first employed by the 2010 DGAC (USDA/HHS, 2016a). Plans for evaluating the evidence are decided early on by the subcommittees based on the question being asked, some of which require a combination of methods to address. More information about the methods and standards used to assess the evidence can be found in Chapter 6.
Other sources of information that the DGAC considers include expert speakers and public comments. DGACs typically invite expert speakers to their second and/or third meetings. Speakers are also invited by subcommittees to discuss a particular topic during subcommittee meetings; those speakers are announced during the public session of the full DGAC. Public comments are also solicited over the course of the DGAC’s work through various channels. Spoken comments can also be made directly to the DGAC, typically during its second meeting; in the past, upward of 53 comments have been made in person. Comments can also be submitted through an online public comments application at any time, where individuals are able to select a topic area under which they feel their comments belong. The 2015 DGAC also issued a call for public comments to ask for submission of literature and evidence related to specific topics, which were to be received early in the DGAC process. Federal staff summarize comments submitted for the DGAC’s consideration, typically by topic area. All public comments are also available for general viewing through the online comments database. In total, the 2015 DGAC received 918 comments from the public before the release of its report (USDA/HHS, 2016b).
Submit DGAC Scientific Report
The DGAC prepares its findings and conclusions in the form of the DGAC Scientific Report. The scientific report is submitted to the secretaries of HHS and USDA and publicly released by the departments. The DGAC creates the report, which has historically been a consensus-based document, with the secretaries of USDA and HHS as the target audience for its advice. The DGAC Scientific Report is written by the DGAC itself, with support from a science writer and federal staff. If consensus is not reached, it is up to each DGAC to determine the processes for addressing the differences.
The DGAC Scientific Report generally includes an executive summary
and a methods section, and the remaining science-based chapters generally describe the evidence assessments. Structurally, the science-based chapters reflect the subcommittee structure. The DGAC chair and co-chair lead the development of the introductory materials and executive summary. Each of the science-based chapters typically includes an introduction, a list of questions examined, a description of methods used, a summary, a list of future research needs, and references. In response to each question addressed, the 2010 and 2015 DGACs included both conclusion statements and implications statements. Conclusion statements directly respond to the questions and summarize the evidence reviewed. Implications statements provide context for the conclusion and generally describe how the DGAC believes its conclusions can be implemented, whether through an action, policy, or other initiative. Research recommendations are also included in the DGAC Scientific Report. These generally include emerging issues, research gaps, and limitations of the current body of evidence. In addition to the explanations provided in the scientific report, the DGAC produces online-only appendices to describe its evaluations. Supplementary materials, such as the literature reviewed by the DGAC and detailed descriptions of how food pattern modeling is conducted, are also made available on the departments’ websites to promote transparency.
The subcommittees write and review the science-based chapters, which are then edited by a science writer. If a NEL-conducted systematic review is used, NEL staff can review the draft for accuracy of the description. Other DGAC members who are not part of the specific subcommittee authoring the chapter serve as peer reviewers for each chapter. The full draft of the report is typically discussed during the DGAC’s final public meeting; only the substantive changes discussed at the meeting and minor editorial changes can be made after the final meeting (USDA/HHS, 2016b). Upon finalizing the scientific report, the DGAC submits it to the secretaries of USDA and HHS; it is then posted on dietaryguidelines.gov. Upon submission of the report, the DGAC disbands.
Solicit and Review Comments on the DGAC Scientific Report
No official peer review takes place of the DGAC Scientific Report, but after it is submitted, the report is subject to a formal public comment period and a federal interagency review. HHS and USDA also hold a public meeting, announced in the Federal Register, about 1 month after the scientific report’s release to receive oral comments. Commenters are allowed 3 minutes to address HHS and USDA officials and the co-executive secretaries. Seventy-three individuals provided oral comments in response to the 2015 DGAC Scientific Report (USDA/HHS, 2016b).
Comments on the DGAC Scientific Report are also received through the
aforementioned online application and can be accessed by the public at any time. Federal staff process and summarize every comment and filter out any duplicate, blank, or irrelevant comments. In the case of the 2015 DGAC, the public comment period lasted for a total of 75 days during which more than 29,000 public comments were received, 21,000 of which were form letters or petitions (USDA/HHS, 2016b).
A federal interagency review takes place simultaneously with the public comment period, during which any federal departments or agencies with nutrition expertise are encouraged to comment, not just those within USDA and HHS. The purpose of the interagency review is to provide feedback and advice to the federal staff who use the DGAC Scientific Report as the scientific underpinning of the DGA Policy Report. USDA and HHS suggest that review comments submitted by other agencies be based on science, be the consensus view of that agency to facilitate processing of comments, and also provide insight on how the DGAC’s recommendations can affect that agency’s programmatic policies. Emphasis is placed on comments with scientific justification to ensure that the focus of the DGA Policy Report is founded on science, not the number of comments for or against a topic. All comments are considered by USDA and HHS in the next step: the DGAC Scientific Report informing the development of the DGA Policy Report by USDA and HHS.
Moving from the DGAC Scientific Report to the DGA Policy Report
Upon publication of the DGAC Scientific Report, a joint USDA and HHS writing team is appointed and examines that report as it develops the DGA Policy Report. After the report is drafted, it undergoes a series of reviews before release. The amount of time from the release of the DGAC Scientific Report to the release of the DGA Policy Report has ranged from 5 months, to 8 months, to 11 months for the past three editions respectively.
Dietary Guidelines Writing Team
The DGA writing team’s role is to accurately translate the “preponderance of scientific evidence”5—based on the DGAC Scientific Report, public comments, and federal interagency review comments—into language for health professionals and policy makers to advance the scientific basis of federal nutrition programs. The product of the writing team’s efforts is a set of guidelines, presented in the new edition of the DGA Policy Report.
___________________
5 National Nutrition Monitoring and Related Research Act of 1990, Public Law 101-445, 101st Cong. (October 22, 1990), 7 U.S.C. 5341, 104 Stat. 1042–1044.
To develop the policy report, the writing team reviews the previous edition of the DGA Policy Report, the latest DGAC Scientific Report, and public and agency comments on the scientific report. Since the 2005 edition, the DGA Policy Report has been developed as a technical document with policy makers and health professionals as the primary audience to inform the development of federal food, nutrition, and health policies and programs (USDA/HHS, 2017a). Previous editions were created as consumer-focused brochures (see Table 5-2).
The 2015 writing team consisted of 12 federal employees selected by the co-executive secretaries from the USDA Center for Nutrition Policy and Promotion and the HHS Office of Disease Prevention and Health Promotion, in consultation with agency leadership. A science writer/editor was also a member of the 2015 DGAC writing team. DGA writing team members are experts from USDA and HHS selected for both their understanding of the evidence being considered and the DGAC’s work, as well as policy applications within the federal government. The writing team is designed to include equal representation from HHS and USDA, and its members have backgrounds in nutrition science, policy, and communications, and are directed not to represent their own personal interests or opinions. The membership of the federal writing team is kept confidential until the new edition is published to minimize any intentional or unintentional attempts to influence the report. During their participation on the DGA writing team, members are asked to recuse themselves from participating in activities that could be or could be perceived to be a conflict of interest (USDA/HHS, 2017a).
In conducting its work, the writing team identifies major themes in the DGAC Scientific Report and builds on previous editions of the DGA Policy Report. The major themes serve as the basis for chapters of the next edition. Central tenets of the writing process include the following:
- Base the policy report on the totality of scientific evidence, not just on individual studies or opinions (USDA/HHS, 2017a).
- Address the needs of federal programs and the details needed to allow the program to transform the evidence base into actions focusing on public health (USDA/HHS, 2017a).
- Consider unintended consequences and how the public might respond and change their behaviors given proposed advice (USDA/HHS, 2017a).
- Refine language, and use plain language whenever possible to make sure the document is clear and is not misinterpreted. Designers are also consulted in developing the layout and graphic elements to enhance reader comprehension of main concepts (USDA/HHS, 2017a).
TABLE 5-2 Evolution of the Dietary Guidelines for Americans
| Year | Method for Reviewing the Evidence | Audience | Focus of Guidance | Type and Length of Publication | Number of Guidelines or Key Recommendations |
|---|---|---|---|---|---|
| 1980 | Review of current science by select scientists from USDA and HHS, along with collective expertise of scientific community | Consumers | Healthy Americans (age not specified) | Brochure; 19 pages | 7 guidelines |
| 1985 | Creation of Dietary Guidelines Advisory Committee (DGAC) outside the federal sector; relied on their collective knowledge of nutrition | Consumers | Healthy Americans (age not specified) | Brochure; 23 pages | 7 guidelines |
| 1990 | DGAC’s collective knowledge of nutrition | Consumers | Healthy Americans, ages 2 years and older | Technical report (48 pages) used as basis for creation of 27-page DGA consumer brochure | 7 guidelines |
| 1995 | DGAC’s collective knowledge of nutrition | Consumers | Healthy Americans, ages 2 years and older, to help promote health and prevent disease | Technical report (52 pages) used as basis for creation of 43-page DGA consumer brochure | 7 guidelines |
| 2000 | DGAC’s collective knowledge of nutrition | Consumers, policy officials, nutritionists, nutrition educators | Healthy Americans, ages 2 years and older, to help promote health and decrease risk of certain diseases | Technical report (87 pages) used as basis for creation of 40-page DGA consumer brochure | 10 guidelines (clustered into 3 messages) |
| 2005 | DGAC’s search and review of the scientific literature, data analyses, food pattern modeling analyses, and other scientific reports | Policy officials, nutritionists, nutrition educators | Americans, ages 2 years and older, to help promote health and decrease risk of major chronic diseases | Technical report (364 pages) and online appendices (124 pages) used as basis for creation of 84-page DGA policy document | 41 key recommendations (23 for general population, 18 for specific population groups) |
| 2010 | DGAC’s systematic review of scientific literature using USDA’s newly established Nutrition Evidence Library (NEL), data analyses, food pattern modeling analyses, and other scientific reports | Policy officials, nutritionists, nutrition educators | Americans ages 2 years and older, including those at risk of chronic diseases, to help promote health and decrease risk of major chronic diseases | Technical report (453 pages), online appendixes (266 pages), and supplementary information at NEL.gov used as basis for creation of 108-page DGA policy document | 29 key recommendations (23 for general population, 6 for specific population groups) |
| 2015 | DGAC’s systematic review of scientific literature using USDA’s NEL, data analyses, food pattern modeling analyses, and other scientific reports | Policy officials, nutritionists, nutrition educators | Americans ages 2 years and older, including those at risk of chronic diseases, to help promote health and decrease risk of major chronic diseases | Technical report (567 pages), online appendixes (600 pages), and supplementary information at NEL.gov used as basis for creation of 144-page DGA policy document | 5 overarching guidelines with 13 supporting key recommendations |
NOTES: DGA = Dietary Guidelines for Americans; HHS = U.S. Department of Health and Human Services; USDA = U.S. Department of Agriculture.
SOURCE: USDA/HHS, 2016a.
The writing team also considers the scope and purview of the DGA Policy Report. For example, while the guidelines are to be promoted by each federal agency in carrying out federal food, nutrition, or health programs, how the guidelines are implemented is at the discretion of each agency. As such, conclusions or recommendations suggested by the DGAC proposing how federal programs, policies, or regulations outside the purview of the DGA should be changed are not carried forward in the DGA Policy Report. To that end, the policy report is developed with the intent of stating not only what Americans should eat to support health, but also why a particular guideline is supported by the science, as well as providing suggestions to help identify how everyone can play a role in making these ideals a reality (USDA/HHS, 2017a). Considerations are also made regarding how a proposed change might affect the food supply, because changes to better align with a proposed recommendation might affect the overall nutritional profile of a food product (Casavale, 2016).
The DGA Policy Report includes different types of guidance. Past editions have included guidelines and/or key recommendations. Although there are no official definitions, the term guidelines is generally used in DGA Policy Reports to highlight overarching guidance, while key recommendations further articulate how to meet the guidelines. Key recommendations are generally used to make statements with the strongest scientific evidence or rationale that will not likely result in substantial changes in the face of new evidence. In the 2015–2020 edition, five guidelines (e.g., “limit calories from added sugars and saturated fats and reduce sodium intake”) were supported by 13 key recommendations (e.g., “consume less than 10 percent of calories per day from added sugars; consume less than 10 percent of calories per day from saturated fats; consume less than 2,300 milligrams of calories per day of sodium”) (USDA/HHS, 2017a). One principle of developing key recommendations is that they ought to be viewed and applied together. For federal agencies, key recommendations can be considered authoritative statements and can therefore be the basis of policies. The guidelines and key recommendations are discussed in the text of the policy report, which also presents the scientific and public health rationale for those statements, as well as any context of technical specifications necessary for explanation or implementation. The chapters of the policy report contain additional context and technical details, while the appendices contain information on both specific topics and technical, often quantitative, details (USDA/HHS, 2017a).
Incorporating Evidence into the DGA
The DGA Policy Report is informed by the totality of the science described in the DGAC Scientific Report. To that end, if a topic was dis-
cussed across several chapters of the scientific report, the writing team considered the implications of all of those statements and how to address them in the new edition. The DGA Policy Report is written to accurately depict the strength of evidence, degree of certainty, relevance, and the relationship between nutrition and health. The writing team also takes into account the difference between association and causation, as studies directly determining causes and health outcomes are not always available. Ever since graded conclusions were included in the DGAC Scientific Report, the policy report has been able to incorporate specific statements describing the strength of evidence. The body of evidence described in the scientific report underlies the strength of evidence in the key recommendations (see Box 5-1 for definitions of strength of evidence). Statements supporting the key recommendations describe both how much evidence exists and how consistent that body of evidence is.
Neither the guidelines nor the key recommendations are graded. This is in large part because they are based on the underlying body of evidence. The relationship between systematic reviews and key recommendations does not stem from a direct one-to-one ratio. Multiple systematic reviews inform the guidelines, addressing the topic of the guidance from different perspectives. Other sources of evidence for the key recommendations include food pattern modeling and descriptive data analyses; however, the grading rubrics for establishing strength of evidence does not apply to questions answered using these approaches. A focus of the guidance, particularly for the 2015–2020 DGA, has been on “overall healthy eating patterns supported by evidence evaluating the eating pattern rather than the individual components of patterns; thus, the evidence grade cannot be applied to each individual component within the eating pattern out of the context of the total pattern” (USDA/HHS, 2017a).
Review of the DGA Policy Report
After a draft of the DGA Policy Report is compiled by the writing team, the document undergoes three distinct types of review and revision to ensure clarity and technical accuracy: federal expert technical review, external peer review, and departmental clearances.
First, the draft is reviewed by federal scientists with the goal of building consensus across federal agencies with nutrition policies and/or programs. Experts are selected by USDA and HHS officials based on their subject-matter expertise, familiarity with the DGAC Scientific Report, and knowledge of federal nutrition programs and policies. The collective expertise of the federal reviewers is intended to cover the array of topics in the draft, as well as the population groups to whom the DGA will apply. Names of commenters are removed before the writing team reviews and discusses the scientific merit of proposed edits, although reviewer names and a summary of the comments are made publicly available on the lead department’s website. Sections with major substantive changes made in response to reviewer comments can be sent back to reviewers to verify that proposed changes are appropriately made and no new concerns are inadvertently introduced. For the 2015–2020 edition, more than 100 federal subject-matter experts commented on the draft, including staff that supported the DGAC—a process that occurred over 4 months.
The next round of review invites a select panel of external experts to provide a peer review of the draft, as required by the Information Quality Act of 2001 for influential documents not published in peer-reviewed journals. Reviewers provide independent responses to the draft. Steps are taken to ensure reviews are confidential and anonymous. For example, reviewers sign a confidentiality agreement and do not know who the
other reviewers are; reviewers’ names are also removed when comments are collated before the writing team assesses and discusses the comments. Typically, 4 to 10 individual reviewers familiar with the role of the DGA Policy Report are selected from the fields of human nutrition, health promotion, chronic disease prevention, nutrition education, public health, health policy, and systematic review methodology (HHS, 2015; USDA, 2010). Individuals are generally asked to review the draft for clarity and technical accuracy and are directed to refer back to the DGAC Scientific Report if any substantive science-based questions arise. Of the seven reviewers for the 2015–2020 edition, three were members of the 2015 DGAC and three others were members of previous DGACs (HHS, 2015).
Once external reviewers identify needed substantive revisions, affected sections of the report can be sent for a second review to federal staff to make sure no new issues were introduced. For the 2015–2020 DGA, three major revisions were made after the version was externally peer reviewed (HHS, 2016). Names of external peer reviewers and a summary of unattributed comments are available on the lead agency’s website after the report is released, per Office of Management and Budget policies (USDA/HHS, 2017a).
The third and final round of review consists of two parts. First, the agency review secures departmental clearances, and then the administration review culminates with approval by the secretaries of USDA and HHS. During the agency review, representatives from each agency within USDA and HHS are asked to indicate the agency’s concurrence with the draft; if the agency does not concur, action must be taken before the new edition can be released. If major revisions are made to the draft at this stage, additional reviews and clearances can be required, although one rationale for having the first round of interagency review is to engage relevant agencies before the final clearance process begins. The administration reviews are begun after agency reviews are completed. Generally for USDA, the Office of the USDA Under Secretary of Food, Nutrition, and Consumer Services and the Under Secretary of Research, Education, and Economics formally review the draft, in addition to the Office of the Secretary of Agriculture. HHS reviews typically include the HHS Assistant Secretary for Health and the Office of the Secretary of HHS. Departmental communications and government relations staff from both USDA and HHS are also involved in the final review.
Release
By statute, a DGA report must be released every 5 years. The most recent edition remains the definitive nutrition guidance for federal agencies until the next edition is released. The activities around a release differ
with each edition, but the release is generally communicated to nutrition and health professionals within and outside of the federal government, the news media, and the DGAC through a variety of channels. Webinars are also held with relevant federal agencies to describe the new edition.
Federal agencies then implement the guidelines and accompanying key recommendations through food, nutrition, health policies and programs, as well as education materials. One major vehicle for disseminating the guidelines is Choose MyPlate, an online resource used to help Americans align their daily food and beverage choices with the DGA recommendations.
To ensure consistency across the federal government regarding science-based nutrition information, HHS and USDA maintain a federal interagency working group called the Dietary Guidance Review Committee. This group meets periodically and reviews federally developed materials that contain guidance to the public on diet to ensure their consistency with the DGA Policy Report.
Resources
The cost of developing the DGA can be separated into the costs related to supporting the DGAC and developing the next edition. Resources for these activities can be further broken down into operating costs and staff support.
The operating costs associated with supporting the 2015 DGAC totaled approximately $905,000. These funds covered travel and per diem, meeting logistics (e.g., meeting space, webcasting), science writer/editor, management of the public comments application, technical support of the public website, and technical support for the NEL. DGAC members served as volunteers and were not paid for their service; however, travel and per diem were provided for the public meetings. The cost of staff support must also be included. In total, 55 federal staff and contractors were listed in the 2015 DGAC Scientific Report, equating to an estimated 22.2 full-time equivalents (FTEs) over the 2-year period to support the DGAC. The bulk of this support included work by nutritionists, systematic review methodologists, and public health advisors from both USDA and HHS (USDA/HHS, 2017a).
Operating costs related to development of the 2015–2020 DGA Policy Report largely covered design and production of final products and materials. Activities included making the final product accessible to people with disabilities and producing HTML and PDF versions. Other operating expenses included the use of a science writer/editor and the hosting of a public comment meeting to receive feedback about the DGAC Scientific Report. These operating costs totaled $410,000 for the 2015–2020 DGA
Policy Report. Staff support generally includes the DGA writing team and interagency reviewers. Although approximately 10 FTEs drafted the 2015–2020 DGA Policy Report, the total number of FTEs involved in the development of that report is unknown because of the extent of federal agency reviews and advisors who contributed (USDA/HHS, 2017a).
INCLUSION OF PREGNANT WOMEN AND CHILDREN FROM 0 TO 24 MONTHS OF AGE
Traditionally, the DGA have targeted populations over 2 years of age, leaving guidance for pregnant women, infants, and young children to professional societies, such as the American Academy of Pediatrics and the American College of Obstetrics and Gynecology. A change occurred when the Agricultural Act of 2014 mandated that the 2020–2025 DGA Policy Report include pregnant women, infants, and young children 0–24 months. The inclusion of dietary guidance for pregnancy and infancy is timely because the emerging evidence that supports the developmental origins of disease (Hanson and Gluckman, 2015) has led to a global call to action to reflect the importance of the first 1,000 days of life in order to ensure normal growth and development, to reduce future chronic disease burden, and to promote future health (Shrimpton, 2012; WHO, 2013). For this reason, there is a critical need for optimizing dietary guidance for pregnant women, infants, and children from birth to 2 years. In 2012, HHS and USDA began to evaluate the evidence to support nutrition guidance for infants from birth to 24 months. It is important to understand the process to date to implement this change. The intent of this work was to release a scientific foundation to develop dietary recommendations for infants and young children from birth to 24 months (the B–24 project); this guidance was to have been separate from the DGA Policy Report (USDA, 2017b).
The B–24 project was launched in 2012 with plans for four phases. In phase I, the USDA NEL was to be responsible for launching the B–24 project (USDA, 2017a). The objective of the first phase was to identify topics. In phase II of the B–24 project, systematic reviews on the selected topics were to be conducted. In phase III, the systematic reviews were to form the evidence base for the development of unified dietary guidelines for B–24 by early 2018. In turn, the federal agencies could incorporate this guidance into their programs in the final phase. In the original plan, this guidance could be used by the 2020 DGAC for the purpose of including the B–24 population in its scientific report. The B–24 project process was to “be transparent and public input would be collected and considered throughout” (Obbagy et al., 2014). As outlined below, the original plans for the four phases of the project were subsequently revised.
As the first step, the NEL systematic review program convened a workshop in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with the goal of informing the process to identify key topics for evaluation. Broad stakeholder input was included through use of a workshop planning group and working groups. After the workshop, the NEL continued liaising with the working groups via teleconference, email, and face-to-face meetings to (1) develop topic nominations, (2) refine systematic review questions, (3) identify crosscutting issues, and (4) create topic briefs that outline key elements of a systematic review framework. This framework was intended as a basis for potential systematic reviews on each topic (USDA, 2017a). The NEL efforts resulted in identification of six topics and questions to undergo trial NEL systematic reviews. The process for topic identification and refinement was published in 2014 (Obbagy et al., 2014; Raiten et al., 2014).
In February 2014, the Agricultural Act of 2014 mandated that the dietary guidance for B–24 be expanded to include pregnant women, along with infants from birth to 24 months (renamed P/B–24) in the 2020–2025 DGA. USDA and HHS accordingly adjusted their plan, such that topics and questions of public health importance would be explored and some systematic reviews would be conducted for these population subgroups and made publicly available. However, contrary to original plans (Obbagy et al., 2014) specific dietary recommendations would not be developed by early 2018 (USDA, 2017b). Rather, an evidence-based document that addresses the six identified topics to undergo systematic review would be produced and be publicly available (USDA/HHS, 2017b). The P/B–24 project is not a formal step of the 2020–2025 DGA process; the results of the project may be considered by the 2020 DGAC, just as it considers any other systematic review. Calls for public comment have been deferred to the 2020–2025 DGA process.
The six identified topics for the P/B–24 project to address are
- What is the relationship between infant milk feeding practices and (1) growth, size, and body composition; (2) food allergies and other atopic allergic diseases; (3) chronic disease; and (4) childhood leukemia?
- What is the relationship between complementary feeding and (1) micronutrient status; (2) growth, size, and body composition; (3) developmental milestones; (4) food allergies and other atopic allergic disease; and (5) bone health?
- What is the relationship between exposure to foods and early food acceptance?
- What is the relationship between maternal diet and infant/toddler food acceptance and dietary intake?
- What is the relationship between parental and caregiver feeding practices and growth, size, and body composition?
- What is the relationship between dietary patterns during preconception/pregnancy and (1) risk of gestational diabetes; (2) risk of hypertensive disorders during pregnancy; (3) gestational age at birth; and (4) birth weight standardized for gestational age and sex? (USDA, 2017b).
Responses to these questions are to be made public through the NEL website by early 2018 (USDA/HHS, 2017b).
KEY FINDINGS FROM AN ASSESSMENT OF THE PROCESSES USED TO DEVELOP THE 2005, 2010, AND 2015 EDITIONS OF THE DGA POLICY REPORTS
In its first report, this National Academies committee delineated a set of values, which, if taken together, can enhance the integrity of the selection process: enhance transparency, promote diversity of expertise and experience, support a deliberative process, manage biases and conflicts of interest, and adopt state-of-the-art processes and methods (NASEM, 2017). These values are also central to the process of developing the DGA and have been adapted for this broader goal (see Chapter 2).
These values were compared to the current process for developing the DGA; it is important to note that not all five values are applicable at every step of the process. As a result of this comparison, this National Academies committee found that the integrity of the process could be strengthened. These findings (summarized in Box 5-2) can be categorized into the purpose of the DGA; cycle time and component tasks; and transparency and participation. The following sections describe each key finding.
Purpose of the DGA
The first key finding is how the overall purpose of the DGA is interpreted. There is no clear indication of the considerations used by USDA and HHS to interpret the National Nutrition Monitoring and Related Research Act, or when the purpose of each particular DGA edition was developed. As depicted in Table 5-2, USDA and HHS have seemingly taken careful, deliberate steps to infer the purpose of the DGA with each cycle, resulting in an evolution of the methods, audience, focus, and type of publication over time.
For example, prior to 2005, the primary audience of the DGA Policy Report was consumers, but consumers were no longer an audience after the 2005 edition. Instead, the DGA Policy Report was written for policy officials,
nutritionists, and nutrition educators. Similarly, the focus of the guidance has shifted. In 1995, the focus of the guidelines broadened from just a target population (e.g., healthy Americans ages 2 years and older) to also include effect on health. The focus changed again in 2000, when “decrease risk of certain diseases” was added, which was changed to “decrease risk of major chronic diseases” in subsequent editions (USDA/HHS, 2016a).
While this evolution is understandable in the face of increased chronic disease-related morbidity in the United States and knowledge of the diet–health relationships has advanced, it has led to inconsistencies in the
process used to update the DGA. For example, this National Academies committee identified more than 10 different statements of purpose in materials related to the 2015–2020 DGA Policy Report’s purpose and goals (see Box 5-3). Additionally, although the National Nutrition Monitoring and Related Research Act specifies that the guidelines are for the general public, the stated audience of the DGA Policy Report after 2000 does not include the general public. This array of purpose statements and audiences could lead to confusion and potentially mistrust in the process to update the DGA.
Cycle Time and Component Tasks
Another key finding is the reconsideration of the timeline under which the DGAC has conducted its work. As a federal advisory committee, the DGAC is limited to a 2-year term by the Federal Advisory Committee Act. Unless actions are taken by the departments to extend the DGAC, it is terminated when it produces its report or when its 2 years is completed, whichever comes first. The number of tasks for the DGAC to complete in this fixed time frame has increased over the decades. For example, the scope of the DGAC has expanded (e.g., inclusion of pregnant women and children from birth to 24 months), and there are more types of evidence to assess (e.g., addition of systematic reviews and food pattern modeling), which take time to produce in and of themselves.
The current process has required that the DGAC spends nearly 25 to 30 percent of its available time completing background and preliminary work, such as topic identification and question prioritization. The amount of time between filing the charter and development of systematic review questions for the 2015 DGAC totaled approximately 8 months. As a result, there was less time for the DGAC to focus on assessing the evidence and creating the scientific report.
This time pressure, in the face of the complicated and time-intensive tasks of the DGAC, can be at odds with the goal of producing a final report, potentially reducing the opportunity for a truly deliberative process. While DGACs to date have completed their tasks on time, future DGACs run the risk of not doing so.
Transparency and Participation
A need for greater transparency was identified in six key findings. This National Academies committee recognizes the process for how categories of expertise are selected for the composition of the DGAC as an opportunity to improve the current process. A Federal Register notice is published that announces the departments’ intent to establish the DGAC
and also solicits nominations for the DGAC. Importantly, this notice lists the areas of expertise the departments are considering for DGAC membership, but there is no opportunity for the public to offer comments on the areas of expertise and experience that ought to be included. Selection of DGAC members also occurs prior to the identification of topics for the DGAC to consider. This sequence is questionable in that it is unclear whether the topics selected are indeed the most appropriate topics to be addressed, thus leading to potential uncertainty of the suitability of the DGAC’s expertise. The current process is not as transparent as it could be, and does not sufficiently explain how diversity of expertise and experience is achieved. Additionally, as concluded in this National Academies committee’s first report, more transparency is needed throughout the selection process and an emphasis ought to be placed on managing both financial and nonfinancial conflicts of interest (NASEM, 2017).
The process by which topics are identified and questions are prioritized can also be questioned. USDA and HHS have encouraged DGACs to explore specific outcomes (e.g., the 2015 DGAC was encouraged to include topics that have the potential to affect food- and nutrition-related health outcomes), without explanation for how or why these outcomes were selected. Currently, only limited public input is gathered, either through the online database or oral statements made during the DGAC’s second meeting. No proposed list of topics to be discussed by the DGAC is shared publicly, meaning that the burden is on the public to follow the DGAC’s deliberations and public meetings, potentially limiting the ability of the DGAC to engage in a deliberative process with the public about one of the most critical steps in the process. Similar arguments can be made about the process by which the DGAC develops and prioritizes questions to consider. In addition to not being easily accessible for public input, this National Academies committee found it difficult to identify exactly how questions were developed and the criteria against which questions were prioritized, because this work seemed to occur at the workgroup and/or subcommittee level. This lack of public input into the process for selecting topics and questions to address does not take full advantage of expertise within the nutrition community, thus creating the possibility of subject matter imbalance in the composition of the DGAC. This creates the possibility of enhanced bias, both real and perceived.
The identification of consultants is another point where the integrity of the current process can be questioned. Consultants were introduced during a public meeting of the full DGAC, which provided an opportunity for the public to comment off line. The 2015 DGAC was the first DGAC to use consultants. The need for the three consultants was determined by the two subcommittees that used them, and each comment was discussed with the full DGAC. However, consultants were identified by
the subcommittees themselves without an opportunity for the public to make comments or suggest other individuals for consideration, nor was an explanation given of the specific purpose and role of the consultants. Although consultants are vetted for financial conflicts of interest and do not vote on decisions made by the DGAC, they have a unique opportunity to influence the deliberations of subcommittees and the DGAC. This National Academies committee concludes that the consultant selection process is not as transparent as it could be, and may lead to the process being unduly influenced by an individual.
This National Academies committee also recognizes the need for transparency in the development of the DGA recommendations themselves and the DGA Policy Report. The federal writing team is composed of experts with equal representation from USDA and HHS who are selected by the co-executive secretaries and department leadership (USDA/HHS, 2017a). As described previously, writing team members are experts in nutrition science, policy, and communications. However, other considerations regarding how these individuals are selected (e.g., understanding of scientific methods used, political biases, conflicts of interest) is not clear. Five central tenets for writing the DGA Policy Report are also outlined, but detailed information on how the tenets are applied and implemented, as well as how the process of developing the updated guidelines based on the DGAC Scientific Report, is not readily available. In a typical guideline development process, one group completes the review of evidence, assesses the quality, and develops the subsequent guidelines. Notably, the DGA Policy Report differs in that the DGAC is responsible for reviewing and assessing the quality of the evidence while the federal writing team develops the guidelines. The separation in the DGA process stems from the Federal Advisory Committee Act and the National Nutrition Monitoring and Related Research Act.6 The federal writing team ought not be exempted from adhering to explicit and transparent standards for developing clinical practice guidelines. Several groups have established guidance for evaluating and developing clinical practice guidelines that could be consulted as models for the DGA process (Brouwers et al., 2010; Guyatt et al., 2008; IOM, 2011; Schünemann et al., 2013, 2014). The process for developing the DGA recommendations is not as transparent as it could be, leading to questions about how the evidence was considered and whether the federal writing team was influenced by politics or other factors.
___________________
6 The Federal Advisory Committee Act allows federal advisory committees to provide advice to the executive branch; in this case the DGAC can advise USDA and HHS. However, the DGAC would not be allowed to author the guidelines because the National Nutrition Monitoring and Related Research Act requires that the secretaries of USDA and HHS publish the DGA every 5 years.
Another limitation of the current process is the lack of transparency regarding how and why decisions were made in the consideration of the DGAC Scientific Report when updating the DGA Policy Report. The process is internal to USDA and HHS, without an accounting of differences between the two reports or an independent referee to assure the public that reviewers’ concerns were appropriately addressed. This opens the process up to criticism that it is subject to undue influences. For example, in the 2015 cycle, while issues regarding sustainability and a proposed sugar tax were determined by the secretaries to be outside the scope, suggestions from the DGAC related to cholesterol were modified. The process for considering each DGAC Scientific Report is not as transparent as it should be and does not encourage a deliberative process.
The final key finding of this National Academies committee regarding transparency is that the P/B–24 project process has not been as clear as it could be. The original B–24 project utilized a process to allow for expert and stakeholder input in the identification of key public health outcomes related to nutrition of infants from birth to 24 months. Experts and stakeholders engaged in the process through working groups to help develop and refine questions, and identified research papers that might provide evidence to address those questions. A seemingly similar process was developed for the expanded P/B–24 project. However, specific details of the P/B–24 project were not available to this National Academies committee. For example, the USDA P/B–24 website states that USDA and HHS nutritionists prioritized the aforementioned systematic review questions to be addressed, but there was no mention of input from the broader stakeholder community. Additionally, the NEL is noted to be “collaborating with programmatic and scientific experts in nutrition during pregnancy and early childhood to conduct systematic reviews” (USDA, 2017b), but it is unclear who these experts are or what their roles are in conducting the systematic review. Importantly, it is not immediately evident who will be responsible for grading the evidence from the systematic reviews—NEL staff or these programmatic and scientific experts. Any plans for peer review prior to publication on line are also not shared publicly. Lastly, it is unclear how the goals from the original B–24 project shifted from dietary guidelines to identification of topics and conduct of systematic reviews. The lack of a clear description of how the public has been engaged since completion of work by the B–24 working groups leaves this National Academies committee to conclude that the current P/B–24 project does not take full advantage of all stakeholder expertise within the nutrition community. Articulation of the process for public and stakeholder participation can help reduce uncertainty and strengthen trust and support for the deliberations.
CONCLUSION
The process to update the DGA has evolved over time. The approach to evaluating the evidence has been revised to address changes in the health of Americans and the state of nutrition science. New types of science have been introduced, and a new focus of guidance has been addressed; however, the limitations of doing so given the constraints of the process have not been adequately considered. The process needs to be deliberately reviewed and redesigned so that it can adapt to changes in the future. This National Academies committee concludes there are multiple opportunities to improve the process to update the DGA, but a comprehensive approach needs to be taken to most effectively achieve the promise of the DGA.
REFERENCES
Brouwers, M., M. E. Kho, G. P. Browman, J. S. Burgers, F. Cluzeau, G. Feder, B. Fervers, I. D. Graham, J. Grimshaw, S. Hanna, P. Littlejohns, J. Makarski, and L. Zitzelsberger, for the AGREE Next Steps Consortium. 2010. AGREE II: Advancing guideline development, reporting and evaluation in healthcare. Canadian Medical Association Journal 182(18):E839-E842.
Casavale, K. 2016 (unpublished). Process steps to update and release the Dietary Guidelines for Americans. Presentation to the Committee to Review the Process to Update the Dietary Guidelines for Americans, Washington, DC, October 17, 2016.
GSA (General Services Administration). 2011. Preparing federal advisory committee charters. http://www.gsa.gov/portal/mediaId/169411/fileName/Preparing_Federal_Advisory_Committee_Charters_v_2 (accessed March 8, 2017).
GSA. 2016. When is the Federal Advisory Committee Act applicable? https://www.gsa.gov/portal/content/100794 (accessed August 9, 2017).
Guyatt, G. H., A. D. Oxman, R. Kunz, Y. Falck-Ytter, G. E. Vist, A. Liberati, and H. J. Schünemann. 2008. Going from evidence to recommendations. BMJ 336(7652):1049-1051.
Hanson, M. A., and P. D. Gluckman. 2015. Developmental origins of health and disease—global public health implications. Best Practice & Research Clinical Obstetrics & Gynaecology 29(1):24-31.
HHS (U.S. Department of Health and Human Services). 2015. Office of the assistant secretary of health peer review agenda: Dietary Guidelines for Americans, 2015. https://aspe.hhs.gov/system/files/pdf/136266/2015%20DGA%20peer%20review%20agenda%20submitted%2012%2016%2015.pdf (accessed February 27, 2017).
HHS. 2016. Summary of peer-reviewed comments on DGA. https://aspe.hhs.gov/pdf-document/summary-peer-reviewed-comments-dga (accessed February 27, 2017).
HHS/USDA (U.S. Department of Agriculture). 2013a. 2015 DGAC meeting 1: Topic selection criteria and approaches for examining the evidence. https://health.gov/dietaryguidelines/2015-BINDER/meeting1/topicSelectionCriteria.aspx (accessed March 12, 2017).
HHS/USDA. 2013b. 2015 Dietary Guidelines Advisory Committee first meeting transcript. https://health.gov/dietaryguidelines/2015-BINDER/meeting1/docs/Transcript-Day-2.pdf (accessed May 30, 2017).
HHS/USDA. 2015a. Dietary Guidelines for Americans 2015–2020: Eighth edition. https://health.gov/dietaryguidelines/2015/guidelines (accessed July 18, 2017).
HHS/USDA. 2015b. Scientific report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: USDA, Agricultural Research Service.
IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
Millen, B. 2017 (unpublished). Understanding the committee’s methodology for topic formation and reviewing the evidence base. Presentation to the Committee to Review the Process to Update the Dietary Guidelines for Americans, Washington, DC, January 10, 2017.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Optimizing the process for establishing the Dietary Guidelines for Americans: The selection process. Washington, DC: The National Academies Press.
Obbagy, J. E., D. M. Blum-Kemelor, E. V. Essery, J. M. Lyon, and J. M. Spahn. 2014. USDA Nutrition Evidence Library: Methodology used to identify topics and develop systematic review questions for the birth-to-24-mo population. American Journal of Clinical Nutrition 99(3):692S-696S.
Raiten, D. J., R. Raghavan, A. Porter, J. E. Obbagy, and J. M. Spahn. 2014. Executive summary: Evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans—“the B-24 project.” American Journal of Clinical Nutrition 99(3):663s-691s.
Schünemann, H., J. Brožek, G. Guyatt, and A. Oxman, editors. 2013. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. The GRADE Working Group. http://gdt.guidelinedevelopment.org/app/handbook/handbook.html#h.33qgws879zw (accessed August 3, 2017).
Schünemann, H., W. Wiercioch, I. Etxeandia, M. Falavigna, N. Santesso, R. Mustafa, M. Ventresca, R. Brignardello-Petersen, K.-T. Laisaar, S. Kowalski, T. Baldeh, Y. Zhang, U. Raid, I. Neumann, S. L. Norris, J. Thornton, R. Harbour, S. Treweek, G. Guyatt, P. Alonso-Coello, M. Reinap, J. Brožek, A. Oxman, and E. A. Akl. 2014. Guidelines 2.0: Systematic development of a comprehensive checklist for a successful guideline enterprise. Canadian Medical Association Journal 186(3):E123-E142.
Shrimpton, R. 2012. Global policy and programme guidance on maternal nutrition: What exists, the mechanisms for providing it, and how to improve them? Paediatric and Perinatal Epidemiology 26:315-325.
USDA. 2010. Peer review plan for the 2010 Dietary Guidelines for Americans. https://www.cnpp.usda.gov/sites/default/files/DGPeerReviewPlan.pdf (accessed February 18, 2017).
USDA. 2017a. Nutrition Evidence Library—birth to 24 months (B-24) topic identification project. https://www.cnpp.usda.gov/nutrition-evidence-library-birth-24-months-b-24-topic-identification-project (accessed January 8, 2017).
USDA. 2017b. Pregnancy and birth to 24 months project. https://www.cnpp.usda.gov/birthto24months (accessed January 8, 2017).
USDA/HHS. 2016a (unpublished). Dietary Guidelines for Americans: Process brief, sections 1–3. Prepared for the Committee to Review the Process to Update the Dietary Guidelines for Americans.
USDA/HHS. 2016b (unpublished). Dietary Guidelines for Americans: Process brief, sections 4–5. Prepared for the Committee to Review the Process to Update the Dietary Guidelines for Americans.
USDA/HHS. 2017a (unpublished). Dietary Guidelines for Americans: Process brief, sections 6–7. Prepared for the Committee to Review the Process to Update the Dietary Guidelines for Americans.
USDA/HHS. 2017b (unpublished). HMD follow up questions for USDA. Response to Committee to Review the Process to Update the Dietary Guidelines for Americans.
WHO (World Health Organization). 2013. Essential nutrition actions: Improving maternal, newborn, infant and young child health and nutrition. Geneva, Switzerland: World Health Organization.






























