Appendix B
Selected Prior Analyses Used to Inform the Framework
Prior biodefense analyses and other sources were reviewed in developing the factors and elements that form the framework presented in this report. This appendix provides further summary information about several of these sources to illustrate different approaches to assessing potential synthetic biology concerns. It is not intended to be a comprehensive compendium of all prior risk governance and biotechnology assessment approaches.
CONSIDERATIONS FROM GLOBALIZATION, BIOSECURITY, AND THE FUTURE OF THE LIFE SCIENCES
The report Globalization, Biosecurity, and the Future of the Life Sciences (also sometimes referred to as the “Lemon-Relman” report from the names of its committee co-chairs) classified emerging technologies into categories based on their characteristics as concerning and warranting particular attention for further risk assessment (IOM and NRC, 2006). These four groupings were:
(1) technologies that seek to acquire novel biological or molecular diversity; (2) technologies that seek to generate novel but pre-determined and specific biological or molecular entities through directed design; (3) technologies that seek to understand and manipulate biological systems in a more comprehensive and effective manner; and (4) technologies that seek to enhance production, delivery, and ‘packaging’ of biologically active materials. (IOM and NRC, 2006, p. 4)
This categorization is wholly focused on features of the technology itself in terms of capabilities it might generate.
CAPABILITIES-BASED WEAPON DEVELOPMENT FRAMEWORK FROM NATIONAL DEFENSE UNIVERSITY
This approach, developed at National Defense University (2016) indicates the points at which potential impacts in the age of synthetic biology could be achieved. Beginning at the far left and working across each step of the bioweapon development pathway, one may determine the steps at which synthetic biology could have an impact on the development pathway (see Figure B-1).
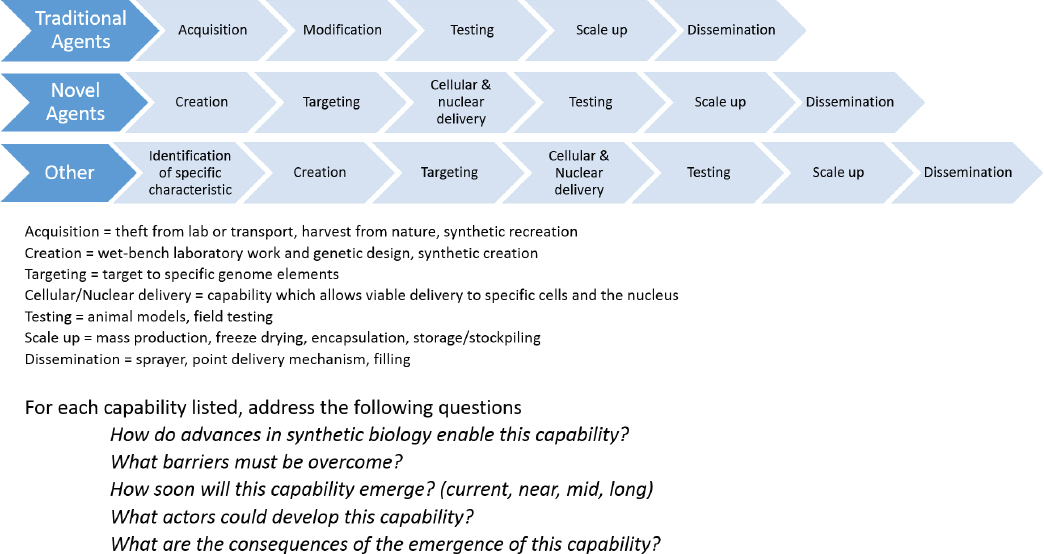
This model was used by National Defense University at a tabletop exercise to assess where gene editing technology (such as CRISPR/Cas) provides heightened capability for creating bioweapons. The approach provides insight into where synthetic biology may have an impact, rather than defining specific characteristics of the technologies themselves.
DECISION FRAMEWORK FROM INNOVATION, DUAL USE, AND SECURITY
Jonathan Tucker’s “Decision Framework” published in Innovation, Dual Use, and Security (Tucker, 2012) suggests a number of attributes that are relevant to the study charge, as restated below:
- Characteristics of the technology:
- Accessibility
- Ease of misuse
- Characteristics of governability:
- Embodiment (material “tangibility” of technologies)
- Maturity
- Convergence (number of technologies that come together to create new technology)
- Rate of advance
- International diffusion
- Level(s) amenable to mitigation
- State
- Institution
- Individual
- Product
- Knowledge
This framework encompasses a variety of features that touch on features of the technology (level of difficulty, maturity, speed of advance, and convergence with other technologies), who has access, and the severity of the outcome if it is misused. This framework also considers options for mitigation, as well as how the cost compares to the benefit of the technology. It is used primarily to assess technology in terms of relative risk on these levels.
EXPERIMENTAL AIMS FROM BIOTECHNOLOGY RESEARCH IN AN AGE OF TERRORISM
In 2004, the National Academies produced the report Biotechnology Research in an Age of Terrorism (NRC, 2004), known as the “Fink report” after its chairman, geneticist Gerald R. Fink, which made the case that scientists have an “affirmative moral duty to avoid contributing to the advancement of biowarfare or bioterrorism.” The Fink report highlights a list of specific experimental aims that that should trigger additional safety and security examination, even if performed for valid scientific reasons. These include experiments that would
- Render a vaccine ineffective,
- Confer resistance to antibiotics or antivirals (countermeasures),
- Enhance virulence of a pathogen or make a nonpathogen virulent,
- Increase transmissibility of a pathogen,
- Alter the host range of a pathogen,
- Enable evasion of detection or diagnostic, or
- Enable weaponization of an agent or toxin.
The report features broad recommendations for mitigation of negative outcomes, to include community outreach, research review (including creation and use of a review board), focused research on mitigation, and international cooperation and outreach. This framework primarily focused on the creation of mitigation tools, but also the creation of a core backbone for biosecurity policy development. The Fink report also led to the creation of the National Science Advisory Board for Biosecurity, a federal advisory committee administered by the U.S. Department of Health and Human Services, which has produced a number of influential reports on dual-use research.
NATIONAL INSTITUTES OF HEALTH CONTAINMENT GUIDELINES
The National Institutes of Health Guidelines (NIH, 2016), conceived initially with the advent of recombinant DNA, provide risk assessment frameworks that enable decision making about the level of biocontainment that can best protect laboratory workers, along with suggestions for mitigation plans. Formal risk groups were developed with respect to particular pathogens.
These guidelines focus on capabilities of particular agents, potential adverse outcomes (accidental infection of laboratory workers or the public), and mitigation strategies. Perhaps most relevant to this study are the characteristics identified for consideration with respect to containment, which include
Virulence;
Pathogenicity;
Potency;
Environmental stability;
Route of spread/communicability;
Availability of vaccine or treatment;
Gene product effects such as toxicity, physiological activity, and allergenicity; and
Any strain that is known to be more hazardous than the parent (wild-type) strain.
CATEGORIES OF EXPERIMENTS HIGHLIGHTED BY THE DURC PROCESS
The Dual Use Research of Concern (DURC) process was initially triggered by concerns over the publication of sequence manipulation information that could map out the creation of a potentially dangerous virus; however, the DURC policies that resulted are more focused on experiments of concern rather than control of information per se. The DURC policies for government and institutions (U.S. Government, 2012, 2014) utilize the Federal Select Agent Program Select Agents and Toxins list and highlight categories of experiments similar to those in the Fink report. These categories include experiments that
- Enhance the harmful consequences of the agent or toxin;
- Disrupt immunity or the effectiveness of an immunization against the agent or toxin without clinical and/or agricultural justification;
- Confer to the agent or toxin resistance to clinically and/or agriculturally useful prophylactic or therapeutic interventions against that agent or toxin or facilitates their ability to evade detection methodologies;
- Increase the stability, transmissibility, or the ability to disseminate the agent or toxin;
- Alter the host range or tropism of the agent or toxin;
- Enhance the susceptibility of a host population to the agent or toxin; or
- Generate or reconstitute an eradicated or extinct agent or toxin listed.
Similar to the Fink report, this list is focused on capabilities that the technology provides to produce a harmful biological entity. The DURC policy is intended to be used to make decisions about funding dual-use experiments.
SOCIETAL RISK EVALUATION SCHEME (SRES)
The SRES approach developed by Cummings and Kuzma (2017) was applied to a set of four case studies of synthetic biology applications. The suggested characteristics for assessing risks of synthetic biology applications are based primarily on outcomes of an adverse event and whether or not mitigation exists. It also includes a novel consideration of society’s attitude toward a potentially adverse outcome, which include considerations such as
- Human health risks,
- Environmental health risks,
- Unmanageability,
- Irreversibility,
- Likelihood that a technology will enter the marketplace,
- Lack of human health benefits,
- Lack of environmental benefits, and
- Anticipated level of public concern.
Since this approach was a risk-benefit framework, it goes beyond the scope of the study charge for this committee, which did not attempt to address the benefits of synthetic biology capabilities.
GRYPHON ANALYSES
In a presentation to the committee, a representative from Gryphon Scientific described an approach for considering how advances in synthetic biology may change the landscape for acquisition of biological threat agents. For example, synthetic biology advances might enable particular threat agents to be synthesized or for a less pathogenic microorganism to be modified into a threat agent, in comparison to alternative acquisition routes such as culturing from clinical or environmental samples or theft. The approach taken by the analysis was comparative and was motivated by the guiding question, “What advantages (or disadvantages) do synthetic biology acquisition routes provide to a malicious actor, relative to alternative acquisition routes?” (Casagrande et al., 2017). The
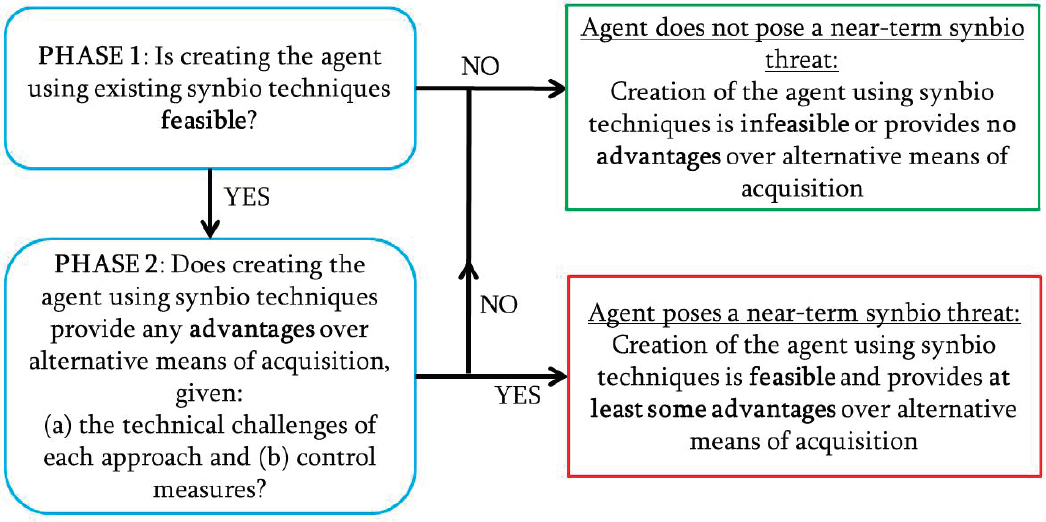
framework used in the analysis, depicted in Figure B-2, included two phases. The first phase asked whether creating a particular biological threat agent was possible using synthetic biology. If so, the second phase asked whether the use of synthetic biology provided acquisition advantages over alternative approaches to obtaining that agent. The results of these two phases informed the determination of whether the agent did or did not pose a near-term threat.
Prior work by Gryphon Scientific, described in the presentation, also considered whether novel biotechnologies, including synthetic biology, have the potential to influence and streamline classical weaponization steps for biological agents. For example, the presenter noted that agents developed using synthetic biology might be developed with increased potency, increased ability to grow to larger numbers, enhanced environmental persistence, increased transmissibility, and the ability to overcome host resistance. However, the use of synthetic biology tools might not be the most effective means to achieve these objectives because of intrinsic factors (such as a lack of knowledge) as well as extrinsic factors such as the need for continual testing of weapons products along a development pathway.
REFERENCES
Casagrande, R., C. Meyer, and K. Berger. 2017. Assessing Biodefense Vulnerabilities Posed by Synthetic Biology: Insights from Related Gryphon Studies. Presentation to the Committee by Gryphon Scientific, January 26.
Cummings, C.L., and J. Kuzma. 2017. Societal Risk Evaluation Scheme (SRES): Scenario-based multi-criteria evaluation of synthetic biology applications. PLoS ONE 12(1):e0168564.
IOM and NRC (Institute of Medicine and National Research Council). 2006. Globalization, Biosecurity, and the Future of the Life Sciences. Washington, DC: The National Academies Press.
National Defense University. 2016. Challenge or Crisis: Security Risk Posed by Gene Editing and Synthesis. Workshop Report, September 14.
NIH (National Institutes of Health). 2016. NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules. April. Available at: https://osp.od.nih.gov/wp-content/uploads/2013/06/NIH_Guidelines.pdf. Accessed November 8, 2017.
NRC (National Research Council). 2004. Biotechnology Research in an Age of Terrorism. Washington, DC: The National Academies Press.
Tucker, J.B. 2012. Decision framework. Pp. 67–84 in Innovation, Dual Use, and Security: Managing the Risks of Emerging Biological and Chemical Technologies. Cambridge, MA: MIT Press.
U.S. Government. 2012. United States Government Policy for Oversight of Life Sciences Dual Use Research of Concern. March 29. Available at: https://www.phe.gov/s3/dualuse/Documents/us-policy-durc-032812.pdf. Accessed May 11, 2017.
U.S. Government. 2014. United States Government Policy for Institutional Oversight of Life Sciences Dual-Use Research of Concern. September 24. Available at: https://www.phe.gov/s3/dualuse/Documents/durc-policy.pdf. Accessed May 11, 2017.






