3
Environmental Dispersal of Lead
Chapter 2 described many possible sources of lead that could cause environmental contamination. To assess whether a lead source might be contributing to observed contamination, one must first consider possible dispersal pathways. As shown in Figure 2-1, there are natural and intentional transport mechanisms; in this chapter, the committee discusses those mechanisms and how they might promote the dispersal of lead into the environment. Aqueous processes and transport are described first, then air transport, and finally intentional transport.
AQUEOUS PROCESSES AND TRANSPORT
A primary natural transport mechanism is water. In this section, the committee discusses lead solubility and species in water, lead partitioning between water and solid phases, and sediment-associated dispersal in rivers.
Lead Solubility and Species in Water
Dissolved lead concentrations can vary by orders of magnitude but are generally lower than those of other dissolved species. In uncontaminated fresh and saline waters, concentrations typically range from around 0.2 to 200 ppt (about 10-12–10-9 mol/L). In contaminated waters, lead concentrations typically rise into the range of a few hundred parts per billion (about 10-6 mol/L). For example, a river contaminated by acid mine drainage and dissolved metals had a maximum lead concentration of 698 ppb (3.4 x 10-6 mol/L) and a mean concentration of 121 ppb measured over 2 years (Nieto et al. 2007); in contrast, mine runoff and discharge waters at near-neutral pH in a lead mining area had lead concentrations that averaged 2.6–14 ppb over 1.5 years (Schaider et al. 2014). For comparison, in a 7-month period in 1966-1967, the mean total lead concentration in rainfall in the United States was 34 ppb, and lead concentrations were higher near cities because of emissions from industrial activities and gasoline (Lovering 1976). As a result of lead accumulation in soils related to lead-containing precipitation before 1976, lead concentrations as high as 330 ppb were measured in runoff water from soils in some parts of New England (Hem 1976). In the United States, emissions of lead to the atmosphere and later deposition on land decreased substantially after the early 1980s, when the addition of lead to gasoline was phased out (Callender 2005). In highly contaminated surface waters and groundwater, lead concentrations can exceed 1,000 ppb (or 1 ppm), but this is generally rare, and most waters with high dissolved lead have concentrations well below parts per million. Away from contamination sources, lead is typically reduced to low concentrations by mixing and dilution with uncontaminated waters and by adsorbing to particles in the water column as described in the next section.
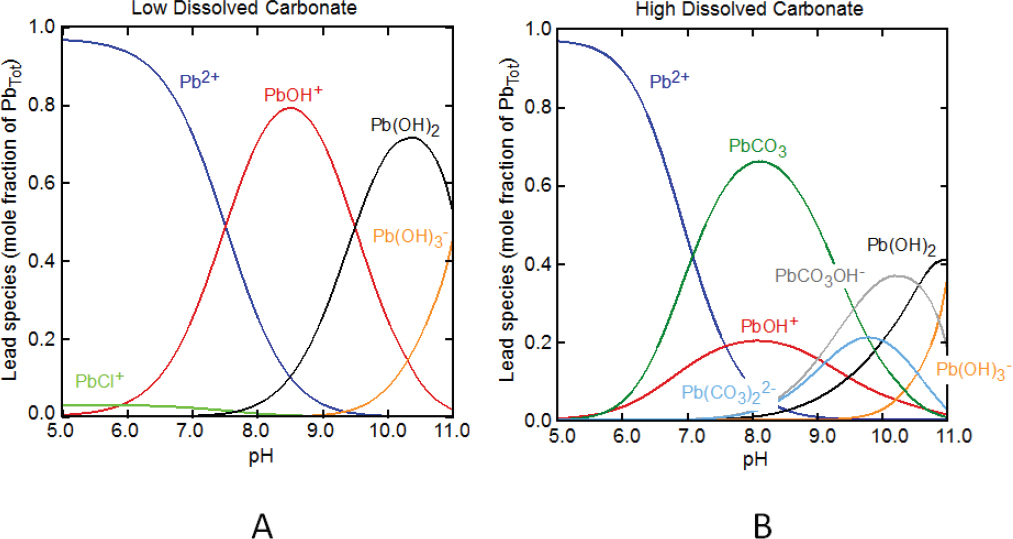
In most surface environmental settings, the lead in water is predominantly divalent lead, Pb2+. In the absence of dissolved organic matter, Pb2+ has a strong tendency to form hydroxo species, such as PbOH+, in solution with increasing pH (Figure 3-1A). In common surface waters and groundwater, the dissolved carbonate is typically about 10-5–10-3 mol/L total carbonate, and lead-carbonate complexes will dominate over lead-hydroxo species over the typical pH range of natural waters, about 6.5–9.5 (Figure 3-1B). In contaminated waters that contain high dissolved sulfate, such as those associated with acid mine drainage, free Pb2+ is the dominant species below a pH of about 7, and the aqueous complex with sulfate, PbSO4(aq), is a minor species (Powell et al. 2009). In seawater and high dissolved-chloride systems, lead-chloride complexes—PbCl+, PbCl3–, and PbCl2(aq)—dominate over free Pb2+ below a pH of about 7.5. At alkaline pH and high chloride, dissolved lead is present mostly as aqueous lead-carbonate complexes, PbCO3(aq) and Pb(CO3)22-, rather than free Pb2+ or mixed carbonate-chloride and carbonate-hydroxo complexes (Powell et al. 2009).
Dissolved organic matter (DOM) forms strong complexes with Pb2+ that can dominate speciation in water, depending on the DOM concentration (Town and Fillela 2000a,b; Rozan et al. 2003). Because natural DOM is a heterogeneous and complex mixture that contains dif-
ferent organic functional groups, many binding-site concentrations and stability constants for lead complexation have been reported (Town and Fillela 2000b). Researchers have attempted to use simple organic acids and ligands as proxies for DOM to describe lead–DOM interactions, but those attempts have had mixed success (Rozan et al. 2003; Vega and Weng 2013; Stockdale et al. 2015).
As discussed in the next section, lead can also be transported in surface waters or groundwater in association with colloidal or suspended particles. After a water sample is collected, it might or might not be filtered before the lead concentration is analyzed. If filtered, the pore size of the filter used can influence the measured lead concentration by removing fine particles that contain lead. A standard filter pore size is 0.45 µm (EPA 1996). The lead concentration measured in a sample after passing through a 0.45-μm filter is typically referred to as the dissolved concentration, whereas total lead generally refers to lead measured in unfiltered water samples after acid digestion. The two measurements differ if filtration removes particles that contain lead larger than the filter pore diameter. For example, in one study of more than 100 groundwater samples from private domestic water wells in Pennsylvania, total (unfiltered) lead concentrations varied from 0 to 100 ppb. When dissolved (filtered) lead concentrations were compared with total lead in the water samples, the authors determined that dissolved lead concentrations were lower by 6–18 ppb (Swistock et al. 1993).
Lead Partitioning
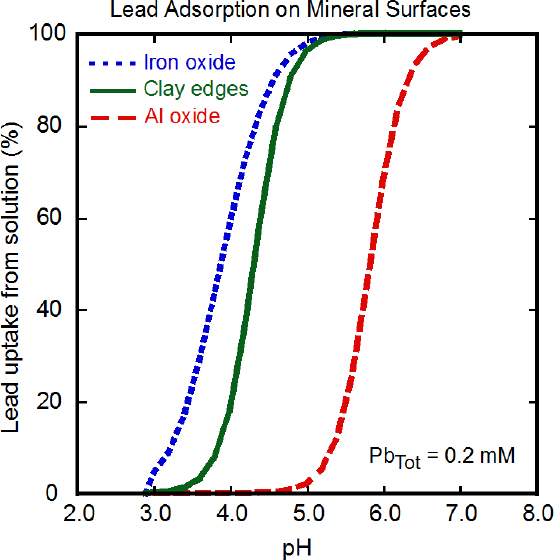
Given the low concentrations of dissolved lead found in most natural waters, surface sorption (adsorption or ion exchange) of Pb2+ onto mineral surfaces or organic matter will likely be the most common mechanism of lead removal from solution to solid phases in surface environments rather than precipitation of new lead species. Precipitation of Pb2+ salts, however, occurs in the surface weathering of lead minerals, in evaporative scenarios, and from high-lead surface waters or groundwater near, for example, mining sites or industrial lead sources (see below). Sorption of lead from solution onto mineral surfaces is controlled by pH, solution concentration, and type of mineral surface (Stumm 1992). For example, dissolved lead has a stronger binding affinity to iron hydroxide mineral surfaces than to aluminum hydroxide surfaces, and this results in greater lead adsorption to surfaces of iron hydroxide than to aluminum hydroxide at lower pHs if
the solution concentration and mineral surface area are the same (Figure 3-2) (Serrano et al. 2009). The high affinity of lead for iron and manganese oxides also allows it to outcompete other ions for sorption. Clay minerals can bind Pb2+, particularly at acidic pH, but the bound lead can be displaced by cations, such as Ca2+, when they are present at concentrations similar to that of the Pb2+. Pb2+ sorbs to calcite, CaCO3, and other carbonate minerals at alkaline pH (Rouff et al. 2005).
Sorption of Pb2+ on mineral surfaces is relatively fast, typically minutes to hours. Depending on the mineral type and aging time, desorption of lead can be mostly reversible (that is, lead is completely removed from the surface and goes back into solution) or only partly reversible (that is, some lead remains bound to the surface) if pH or solution conditions change. Longer reaction or aging time can lead to incorporation of Pb2+ into a mineral structure or irreversible binding. For example, a minor amount of Pb2+ might be substituted for the divalent cation in carbonate mineral phases, or it might be incorporated into the structure of hydrated iron oxide phases (ferrihydrite or hydrous ferric oxide); this requires dissolution of the solid phase to release lead into solution. The observed slow release of lead from soils in leaching experiments has been attributed to the strong binding of Pb2+ to oxoanions in the interlayer of clay minerals and the resulting low rates of desorption from those sites (Serrano et al. 2013).
In soil and sediment weathering, lead-ore minerals, such as galena (PbS), might oxidize, dissolve, or change to form new minerals that might (or might not) be lead-rich solids, such as mixed-element salts or mineral solid solutions. The amount of weathering, rainfall, and leaching in a given environment determines whether lead minerals dissolve completely, change to other solids, or adsorb to other mineral surfaces if dissolved (Hayes et al. 2009). For example, in environments with high rainfall, such ions as lead tend to leach from soils to water, where they are transported as dissolved species, or to adsorb to particles or organic matter in water and migrate as particle-associated species (Schaider et al. 2014; Gutierrez et al. 2016). Organic matter in soils, which is typically more abundant in temperate and tropical environments, tends to retain lead by adsorption and hinder its release to water (Marzouk et al. 2013). In contrast, in arid and semiarid regions where soils undergo intermittent wetting and drying, weathering might result in the formation of carbonate, clay, hydrous oxides, sulfate, or mixed sulfate–hydroxide phases that incorporate lead into their structure or adsorb lead on their surfaces (Dutrizac and Jambor 2000; Jambor et al. 2000).
The pH of water—either surface water or water in the pores of soils and sediments—exerts a strong influence on the dissolution of lead solids derived from original sources (primary phases), the formation of new lead--
containing solids by precipitation (secondary phases), and lead adsorption to the surfaces of other soil minerals. The presence of carbonate minerals, such as calcite, in soils and sediments buffers pH near neutral or above (about 7.2–8.2). Given those conditions, lead is often retained by the precipitation of new carbonate minerals or by adsorption. For example, cerussite, PbCO3, was noted as a major secondary weathering phase in sediments that were affected by a historic mining district of Wanlockhead, Scotland (Hillier 2001). Studies of the weathering of lead munitions illustrated that cerussite and hydrocerussite, Pb3(CO3)2(OH)2, were primary products that likely control dissolved lead concentrations in soil-pore water (Jørgensen and Willems 1987; Lin et al. 1995; Cao et al. 2003a; Hardison et al. 2004; Rooney et al. 2007). Similarly, formation of cerussite and mixed lead hydroxide–carbonate phases—including hydrocerussite and plumbonacrite, Pb5(CO3)3O(OH)2—have been reported as products of corrosion of lead pipes (Xie and Giammar 2011; Masters et al. 2016) or products of direct weathering of galena at alkaline pH (Hudson-Edwards et al. 1996; Root et al. 2015). Soils or sediments that have high phosphate concentrations, such as agricultural soils that receive high concentrations of fertilizer, might contain pyromorphite, Pb5(PO4)3Cl (Cao et al. 2003b). As a result of weathering or alteration, secondary lead phases form as crusts or scales on surfaces of galena, lead shot, or lead pipe where lead concentrations are relatively high (Cao et al. 2003a; Pieper et al. 2017). However, at lower concentrations as lead is dispersed, adsorption on clay minerals or amorphous iron oxides or aluminum oxides in soils and sediments is often the primary mechanism for retaining lead and limiting its mobility in water (Hillier 2001; Yang et al. 2006; Rooney et al. 2007).
In areas that have lead-bearing ore minerals and wastes from deposits that contain abundant iron sulfide minerals that produce sulfuric acid during weathering (see Chapter 2), extensive weathering and leaching result in progressively more acidic waters as carbonate is removed and buffer capacity is lost (Hayes et al. 2009; Nordstrom et al. 2015). In acidic conditions in soils and water, the concentrations of iron, aluminum, and sulfate are important controls on lead dispersal or retention in surface weathering or evaporative scenarios. Assemblages of anglesite, PbSO4, and plumbojarosite, PbFe6(SO4)4(OH)12, with a wide variety of mixed-metal salts that contain iron, aluminum, and sulfate are common products of lead-rich weathered ore deposits and mine wastes (Dutrizac and Jambor 2000; Jambor et al. 2000). Soils and evaporated sediments that have high dissolved lead and sulfate concentrations, such as those associated with tailings impoundments or mine drainage, might contain anglesite; possibly linarite, PbCu(SO4)(OH)2(s), if copper concentrations are high; or lead substituted in many other hydrated-sulfate salts (Jørgensen and Willems 1987; Bigham and Nordstrom 2000; Jambor et al. 2000). The sulfate–hydroxide phases often precipitate and dissolve quickly as conditions change, but reaction rates in different environments will depend on particle size, mineral crystallinity, pH, and solution composition.
Sediment-Associated Lead Dispersal in Rivers
As noted above, lead has a high propensity to be sorbed (attached) to particulate matter under the geochemical conditions observed in most rivers. The concentration of lead associated with particulate matter also tends to be orders of magnitude higher than the concentrations of dissolved lead in surface water or groundwater. Given the concentrations of lead associated with particles and the ability of rivers to move large quantities of sediment, rivers are highly efficient in transporting lead considerable distances downstream from a natural or anthropogenic source. The movement of sediment-associated lead from historical mining and milling operations can be particularly pronounced because waste materials were often stored within impounded river valleys and allowed to enter a river channel unabated.
The downstream transport of sediment-associated lead is a function of the size of the sediment that is being moved and the energy provided by the flow to perform mechanical work. In general, the higher the flow (discharge), the more sediment and sediment-associated lead can be transported through a given stream segment, although the relationship between flow and sediment transport is complicated. Transport of suspended sediment depends on factors—such as the existing soil moisture conditions; rainfall volume, duration, and intensity; and vegetation cover—that influence the movement of sediment from upland areas to the river. As a result, concentrations of suspended sediment and sediment-associated lead vary widely between floods and nonlinearly during a single event, and quantification of suspended sediment and particulate lead transported through a stream segment requires the monitoring of numerous floods over multiple years and an understanding of how concentrations of particulate lead change during a runoff event.1
The downstream dispersal of sediment-associated lead (and other trace metals) from a point source generally creates a distinctive systematic downstream decrease in lead concentrations in the sediments in the channel and its associated valley. Figure 3-3 provides an example of the spatial trends commonly encountered for metals downstream of point sources of contamination along the Rio Chilco–Rio Tupiza drainage system of southern Bolivia. Lead, zinc, and antimony concentrations are relatively low at site RC-3 and are comparable with local background values. Concentrations of the metals or metalloids abrupt-
___________________
1 See Richards (2001) for a summary of methods to estimate pollutant loads.
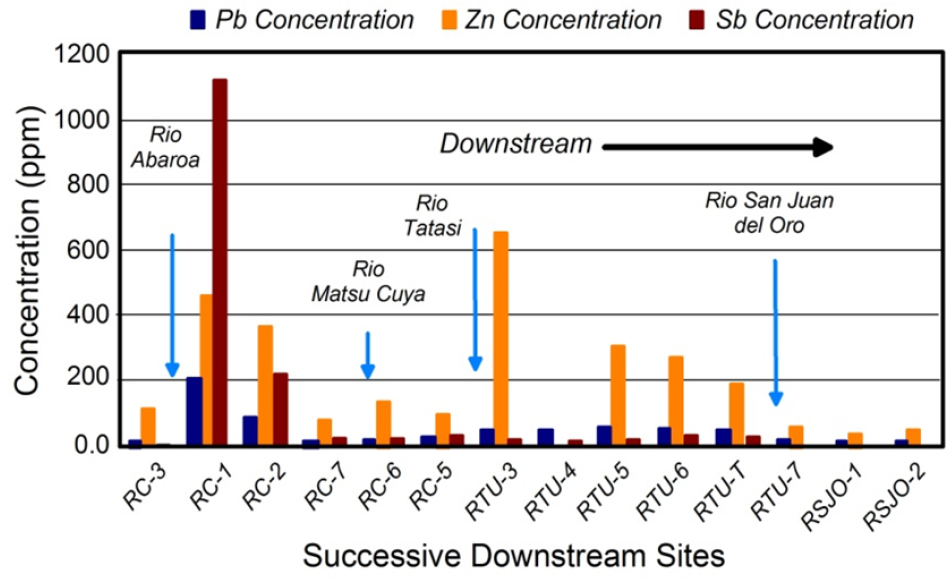
ly increase immediately downstream of the Rio Abaróa, a tributary contaminated by antimony mining and a tailings impoundment failure (Villarroel et al. 2006). The abrupt increase is presumed to result from the influx of contaminated sediment from the mine site. Concentrations of all three elements then decline systematically downstream. However, zinc concentrations abruptly increase again on reaching the confluence of the Rio Tatasi and to a lesser degree the Rio Matsu Cuya. Both drainages contain large-scale mining operations where tailings have entered the channel. Thus, the increased concentrations reflect the additional input of zinc-contaminated tailings into the river.
Longitudinal decreases in lead concentrations, such as those documented downstream of the Rio Abaróa, result from the interaction of multiple processes (Figure 3-4) (Lewin and Macklin 1987; Macklin 1996; Miller and Orbock Miller 2007). Those processes include (1) dilution associated with the dispersal of sediment-associated lead from a relatively small source area over a much longer segment of the river, (2) dilution caused by the mixing of contaminated particles with clean sediment from tributaries and eroded bank deposits, (3) the deposition and storage of lead in the channel bed and other alluvial deposits (a process that removes sediment-associated lead from further transport), and (4) the hydraulic sorting of contaminated particles as a result of the selective erosion, transport, and deposition of larger or denser grains, such as galena. In addition, geographic patterns in lead concentrations can be influenced by the geochemical processes described earlier. Although the downstream dispersal of sediment-associated lead can be systematic, a number of processes complicate geographic patterns in dispersal. The most important are described below.
Lead Partitioning between Particles That Have Different Physical Properties
ln most rivers, lead is largely associated with chemically reactive, fine-grained sediments (Horowitz 1991). During their downstream transport, the sediments might be hydraulically separated by particle size and density in localized areas of the riverbed and its floodplain; each area has a distinctive range of particle sizes. Such areas in the river include pools, riffles, point bars, and glides (Figure 3-4a). Within the floodplain or bank, the particles form packages of sediment referred to as vertical accretion deposits, lateral accretion deposits, crevasse-splay deposits, and oxbow lake deposits, and others (Figure
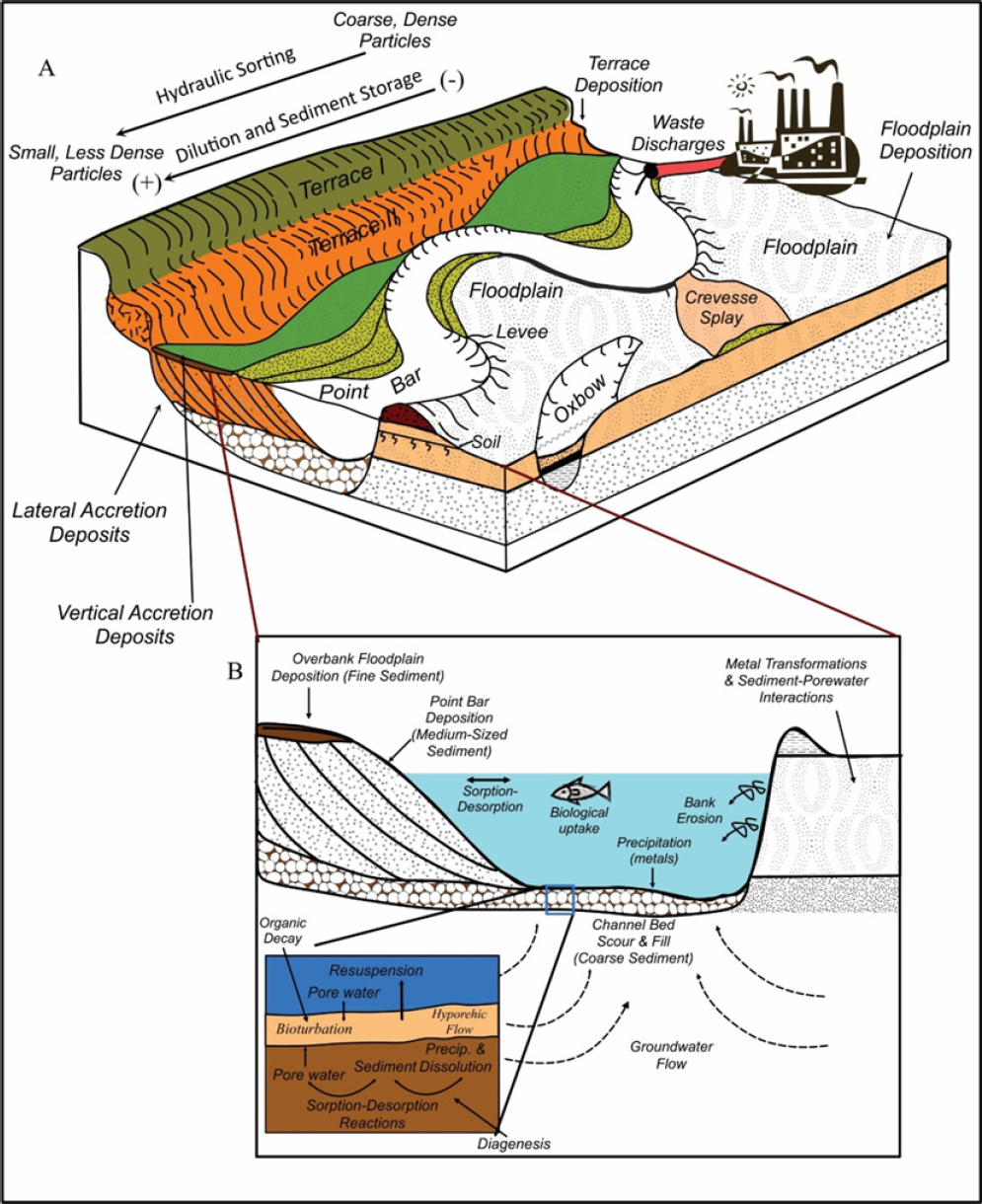
3-4a). Such particle partitioning results in the concentration of lead in specific areas of the drainage network that contain fine-grained, lead-enriched particles (Macklin et al. 1999; Macklin et al. 2006; Miller and Orbock Miller 2007).
Along rivers that are affected by the influx of mining and milling debris, lead is associated not only with fine-grained sediment but with coarse-grained sediment (Moore et al. 1989; Miller et al. 1998). The exact distribution depends largely on the milling process used and on how metals were incorporated into the tailings (waste) materials. For example, Pavlowsky et al. (2010) found that high lead concentrations in tailings from the Leadwood and National Piles in Southeast Missouri occurred in the sediment fraction measuring less than 63 µm and in the fraction measuring 4–8 mm. Lead concentrations associated with particles of an intermediate size were relatively low because the milling process was more efficient in recovering lead (and zinc) from these particle sizes. Thus, the geographic distribution of sediment-associated lead is likely to reflect the spatial distribution of particle sizes in the river valley.
In addition to particle size, grain density might affect partitioning of sediment, particularly in mineralized areas. For example, some minerals, such as gold and galena, have a relatively high density. Those dense mineral grains are often concentrated in localized zones in which the denser but smaller metallic grains coexist with larger but less dense minerals, such as quartz, feldspar, and carbonate minerals. The zones of concentration, which are called placer deposits, are created by the repeated processes of erosion and redeposition of particles of a given size range (Slingerland and Smith 1986). Placers tend to form upstream of natural protrusions that are caused by resistant strata where bedrock is present and upstream of manmade structures, such as road crossings and small dams. Placers might also form along rivers where the channel perimeter is composed of sediments. Those types of placers are formed in areas where the repeated erosion and redeposition of sediment concentrates the denser particles, such as at the base of point bars, the head of midchannel bars or islands, and the confluence of tributaries (Guilbert and Park 1986).
Not all differences in the concentration of sediment-associated metals observed between the depositional units of a river can be attributed to particle size and density (Graf et al. 1991; Hudson-Edwards et al. 2001). Areas in the channel that are inundated or flooded more often commonly exhibit higher sediment-associated metal concentrations (Graf et al. 1991). The influence of inundation frequency on metal concentrations is related to two primary factors. First, the more an area of the channel is flooded, the greater the opportunity for sediment-associated lead to be incorporated into the area’s channel sediments. Second, the frequency of inundation depends on flood size: topographically higher and less frequently inundated areas are flooded during larger runoff events. In many contaminated rivers, sediment-associated metal concentrations decrease with increasing flow because there is an increased influx of clean sediments to the channel from upland areas of the basin. The clean sediments decrease the concentrations of the metals in the sediments that are incorporated into the less frequently inundated channel areas (Marron 1987).
Localized Downstream Variations in Sediment-Associated Lead Transport and Deposition
Many river systems exhibit abrupt changes in their capacity to transport sediment along the drainage network (Macklin and Lewin 1989; Miller and Villarroel 2011; Miller et al. 2012). For example, segments of the stream characterized by higher gradients and narrower than average valleys tend to transport sediment and sediment-associated lead with little deposition. In essence, flood waters are confined by the narrowness of the valley to a smaller area, which when combined with higher gradients creates more energetic and erosive flows. In contrast, sediment transport is reduced in sedimentation zones that are characterized by wide valleys and shallowness (Mackin and Lewin 1989; Mackin and Dowsett 1989; Mackin 1996; Miller et al. 2009). As a result, systematic downstream trends in the concentration and storage (deposition) of sediment-associated lead might be locally disrupted by changes in river and valley gradient and width. The influence of river or valley gradient and width on geographic patterns of sediment-associated metal concentrations is most pronounced along rivers in mountainous terrain but can also be important along lowland rivers.
Influence of River Transformations on Sediment-Associated Lead Dispersal
Lewin and Macklin (1987) argued that the dispersal of sediment-associated metals differs between rivers in a stable, equilibrium state and those in an unstable, disequilibrium state. The latter are characterized by continuing changes or transformations in channel width, depth, slope, and sinuosity caused by localized erosion or deposition. Transformations in river structure are important because they influence the rate at which sediment-associated metals are transported to downstream areas and are important determinants of the sites along the river where sediment-associated metals are deposited and stored. A relevant example is the influx of large quantities of sediment and tailings into a river from historical mining or milling operations. The increased sediment load often overwhelmed the transport capacity of the river near the site of sediment input and caused deposition on the riverbed and on the adjacent floodplain. Deposition was often substantial and in
some cases measured several feet to tens of feet in thickness (Gilbert 1917; James 1989; Knighton 1991). Once the influx of sediment from the mines or mills decreased, the river was able to transport the previously deposited sediment downstream, but some remained in the stream segment and was exposed within the riverbanks, where it could be eroded and re-enter the river. The sediment that was transported downstream often overwhelmed that portion of the river and caused deposition there until sediment loads declined, at which point the riverbed was eroded and the cycle repeated itself further downstream. When viewed in its entirety, the cycle of deposition and erosion formed a kind of sediment wave that migrated downstream from the site of sediment input (Gilbert 1917; Knighton 1991; Nicholas et al. 1995). The downstream passage of the wave typically took decades to complete and strongly influenced the rate at which sediment-associated metals were transported along the river.
The influx of sediment from historical mining and milling operations is not the only type of disturbance that can cause such transformations in river structure and sediment transport rates. In fact, such changes in river function are associated with a wide array of natural disturbances (such as landslides and wildfires) and anthropogenic activities (such as deforestation and land-use change). One of the most widespread and extensively documented disturbances in the United States that led to such transformations in river form was land-use change initiated by European settlement during the late 19th and early 20th centuries (Trimble 1974; Knox 1989; Orbock Miller et al. 1993; Price and Leigh 2006; Leigh 2010). Specifics of the documented changes in the river basins vary between sites; however, they were generally characterized by (1) large-scale devegetation of hillslope and valley bottoms that resulted in an increase in runoff to the drainage network, (2) gullying and accelerated erosion of upland soils that led to an increase in sediment loads into the channel, (3) deposition of sediment on floodplains or riverbeds that produced what are commonly called legacy sediments, and (4) a period of reforestation and improved watershed management practices that resulted in reduced runoff, sediment yield, and, in many cases, channel erosion (Trimble 1974; Knox 1987).
During the above transformations in rivers and river valleys, lead associated with natural soil particles might be incorporated into the sediments that are deposited along the river, particularly the floodplain. After erosion of the riverbed, those sediments (including any lead that they contain) will be exposed in the riverbanks (see Figure 3-5), where they might continue to be eroded and reintroduced to the river (James 1989; Miller et al. 1998; Bain et al. 2012; Niemitz et al. 2013). Such processes might be particularly important along rivers that drain mineralized rocks, such as in Southeast Missouri.

The erosion and lowering of the river bed also forms an alluvial terrace. Terraces are old floodplains that because of erosion and lowering of the river bed now exist topographically above and adjacent to the river and its floodplain (Figure 3-4). Terraces often contain sediment-associated lead that was deposited by the river but is now spatially removed from the modern channel. Geographic patterns in sediment-associated lead concentrations in terraces are influenced by the timing of lead inputs into the river, terrace height above the modern channel, and the frequency with which the terraces are flooded. During the period of lead influx, lower elevation terraces are likely to have higher concentrations and larger quantities because they are inundated more frequently. After the influx of lead, contaminated sediments on the lower terraces are likely to be buried more rapidly by sediments that have lower lead concentrations. Thus, lead-contaminated soils can be at or closer to the surface on higher terraces than on lower terraces or the floodplain (Brewer and Taylor 1997).
AIR TRANSPORT
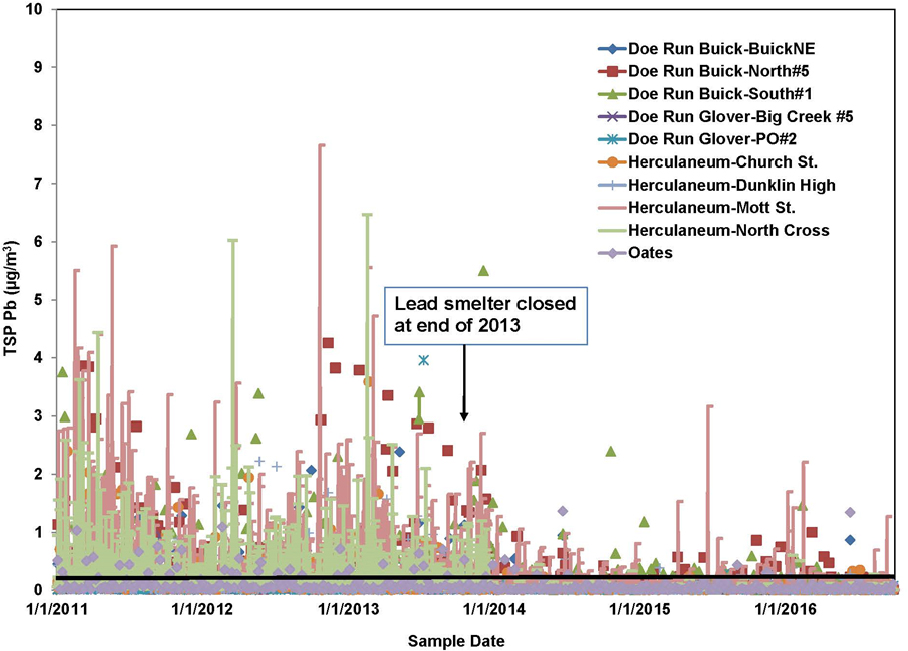
Airborne transport has the greatest potential to disperse lead emissions because the air is not confined by geographic barriers. Furthermore, rates of transport of contaminants are often higher for the air pathway than for groundwater and surface-water pathways (Prabhakar et al. 2014). The potential for lead transport via the air pathway can be illustrated with data from Southeast Missouri. Specifically, lead concentrations that were reported as part of monitoring for National Ambient Air Quality Standard (NAAQS) compliance indicate that lead is transported via the air pathway, and the measurements provide some indication of the sources responsible for the airborne lead. Nonattainment occurred in three counties—Iron, Dent, and Reynolds—in the Southeast Missouri Lead Mining District. One site includes the nearby secondary lead smelter (Boss, MO) that reported a 3-month running lead average of 0.17–0.18 µg/m3 in April–August 2016.2 Some monitoring sites in the facility fence line reported higher 3-month average values of 0.24–0.33 µg/ m3 from December 2016 through March 2017 (MO DNR 2017). An example of long-term variation in ambient lead concentrations in 2011–2016 is shown in Figure 3-6 and summarizes US Environmental Protection Agency (EPA) data. Although large decreases in total suspended particulate (TSP) lead concentrations in the Herculaneum and Doe Run areas were found after the smelter closed at the end of 2013, 588 samples showed that 24-hr lead concentrations exceeded the NAAQS threshold, 0.15 µg/m3, in 2014–2016 (EPA 2017).
A fundamental characteristic of suspended particulate matter (PM) that affects transport is its size distribution, often expressed as a function of the particle’s aerodynamic diameter.3 The size distribution affects aerosol physics, chemistry, and transport, and environmental and health effects. This section first describes particle-size distributions and how they affect transport and then fugitive dust suspension and deposition, which are probably the most relevant to lead dispersal from mining-associated sites and other sources. The section concludes with an example of modeling of air transport.
Particle-Size Distribution and Implications for Transport
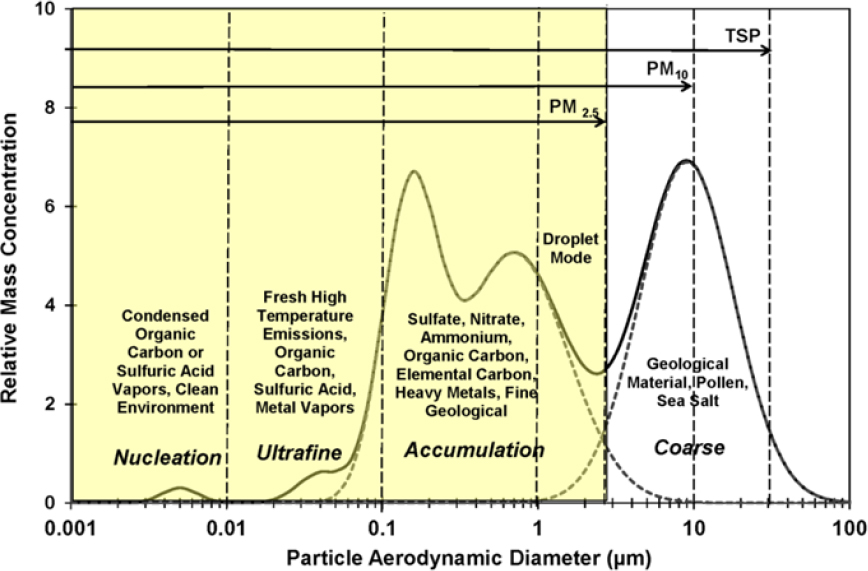
Airborne PM can range in diameter from a few nanometers to a few hundred micrometers, a span of 5 orders of magnitude, and follows a size distribution like that shown in Figure 3-7. Particles that have an aerodynamic diameter of greater than about 2.5 µm are called the coarse fraction, and particles that have an aerodynamic diameter of less than about 2.5 µm are called the fine fraction, which includes the nucleation, ultrafine, and accumulation fractions. Because mass scales with the cube of the diameter, PM mass concentrations are often dominated by the presence of large particles. Conversely, PM number concentrations are dominated by the smallest particles. The different size fractions have different sources, removal mechanisms, transport characteristics, atmospheric residence times, and deposition patterns in the environment and the respiratory tract.
Coarse particles (greater than 2.5 μm) are generated by mechanical action, such as crushing, grinding, material handling, wind erosion, or vehicular traffic (Watson et al. 2000). Large particles have high gravitational settling velocities and can deposit onto a surface within a few minutes to a few hours after suspension. For example, chat piles in Southeast Missouri consist of large particles, which are unlikely to become suspended and transported long distances by direct wind action except during extreme weather events, such as tornadoes. Particles that have diameters of a few micrometers, however, can remain suspended for several days and can therefore be transported long distances, even across continents or oceans. Atmospheric turbulence plays an important role in lofting suspended particles from near the ground to higher altitudes, thereby facilitating long-distance transport (Pye 1987).
Fine particles (less than 2.5 μm) are typically generated by condensation of hot vapors and by diffusion and coagulation of gases and other fine particles. Like smaller particles described above, fine particles can be transport-
___________________
2 Nonattainment occurs when air quality is not in compliance with the NAAQS; that is, when the rolling 3-month average exceeds 0.15 µg/m3.
3 Aerodynamic diameter (da) varies inversely with the square root of the density. It is different from the geometric diameter (dg); dg=da/√density.
ed long distances. For example, lead-smelting activities in Southeast Missouri probably emitted lead-containing vapors that nucleated and condensed into fine particles (Moravec et al. 2015) or that condensed onto the surfaces of pre-existing fine particles; these particles were then transported long distances and deposited on the Earth’s surface through both dry and wet deposition where they might still be present in soils.
Fugitive Dust Suspension and Mechanisms
Fugitive dust (Chow and Watson 1992; Kinsey and Cowherd 1992; Watson et al. 2000; Amato 2001; Cowherd 2001) consists of small particles that are suspended in the atmosphere after emission from such sources as mining pits, open fields and parking lots, paved and unpaved roads, agricultural fields, construction sites, dry lakes (playas), unenclosed storage piles, and material transfer systems. Visible dust plumes are often noticed over those sources when wind speeds are high or when vehicles are moving. Studies show that, on the average, fugitive dust contributes about 40–60% of PM10 and about 5–20% of PM2.5 measured in the atmosphere (Watson and Chow 2000). However, fugitive dust contributions are often overestimated when published emission rates are used in dispersion models that simulate contributions to receptor concentrations (Watson et al. 2000) because the meteorologic, physical, and chemical factors on which fugitive dust emission rates are based can vary widely on local, regional, and national scales.4 Resolving discrepancies between emission estimates and ambient measurements is an important consideration in designing, applying, and evaluating control strategies that are intended to reduce fugitive dust emissions.
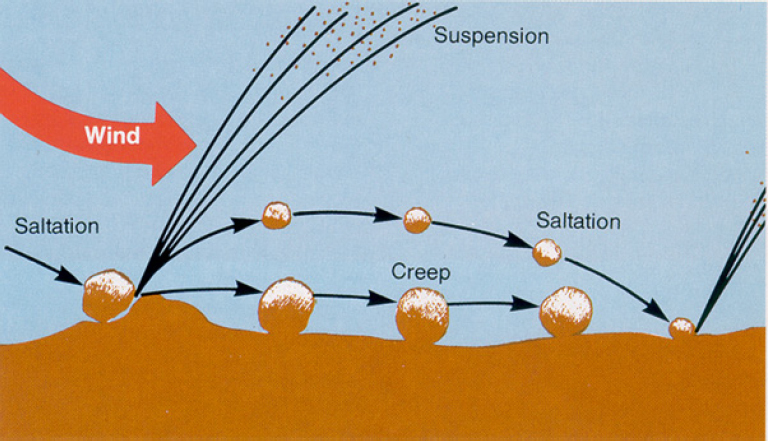
As illustrated in Figure 3-8, dust suspension involves a complex three-step process: creep, saltation, and suspension. Creep is the rolling of the largest particles (about 500–1,000 µm) that are not suspended without extreme wind events; as the wind pushes them along, they can roll over and disaggregate other particles. Saltation is the movement of large particles (about 100–500 µm) that bounce a few centimeters above the surface and then fall back down, thereby bombarding the surface; upon impact, they dislodge smaller particles (less than 100 µm) from the surface, which can be entrained in the prevailing airflow. Creep, saltation, and suspension are inherently chaotic processes that are difficult to model (Shao 2008; Kok et al. 2012; Valance et al. 2015). Instead, semi-empirical models are used to estimate dust production from soils and stockpiles, such as chat piles and mine-tailings impoundments.
The suspension of fugitive dust particles depends on particle sizes, surface loadings, surface conditions, wind speeds, atmospheric and surface moisture, and the existence of dust-generating activities, such as vehicular traffic. Windblown dust occurs when the wind exceeds a threshold velocity. The minimum threshold wind velocity is often found to be about 5–10 m/s when extrapolated to 10 m above the surface (the standard height for reporting wind speed). However, the threshold can vary widely because of such factors as surface roughness, moisture content, vegetative cover, and particle size. Portable wind tunnels are increasingly used in the field to measure dust emissions and threshold wind velocities directly
___________________
4 For further information on emission factors, see https://www.epa.gov/air-emissions-factors-and-quantification/basic-information-air-emissions-factors-and-quantification#About Emissions Factors.
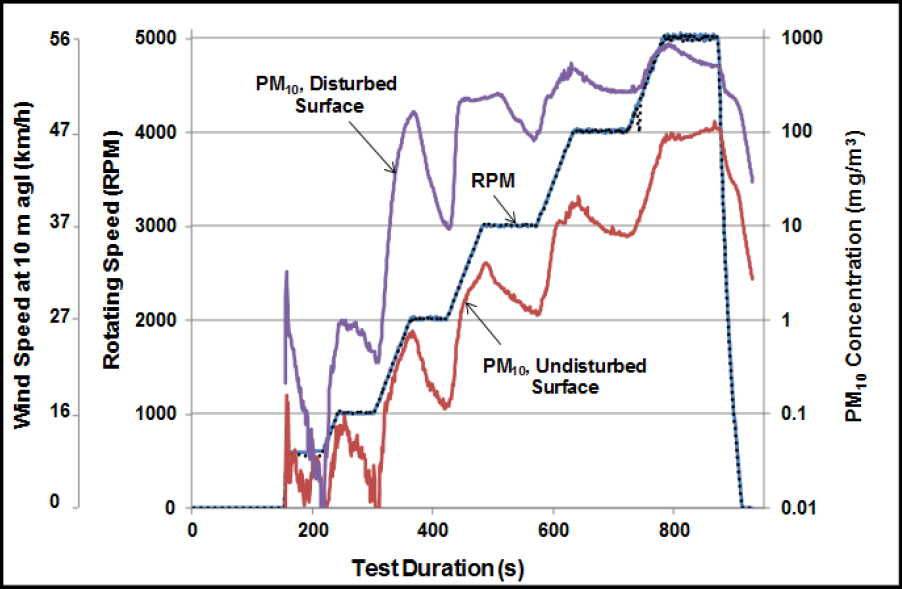
(Etyemezian et al. 2007). Figure 3-9 illustrates how the PM10 emissions can vary as a function of wind speed and surface conditions. Such emission measurements can be used in computational fluid-dynamics models to simulate atmospheric dispersion of dust (Stovern et al. 2014). Those models, for example, can be coupled to lead-isotope data to improve attribution of lead to various sources (Stovern et al. 2016).
Mechanical suspension processes are dominated by moving vehicles, wherein the contact of a tire with the surface of a roadway grinds the dust to smaller sizes and injects it from the road surface into the atmosphere. Vehicle wakes that create turbulence are also sources of dust suspension, although they can also be classified as wind-induced. Other mechanical suspension methods involve transfer of bulk materials through uncovered conveyors, bulldozers, shovels, and open trucks.
Modeling Air Transport: An Example from Southeast Missouri
An example from Southeast Missouri illustrates the potential for air transport of lead-containing particles from chat piles and tailings. Abbott (1999) modeled the erosion and transport of airborne PM from chat piles and tailings impoundments at Bonne Terre, Desloge, Leadwood, National, Elvins, and Federal in Southeast Missouri. The model was evaluated using data from air-quality monitors—presumably TSP monitors, but that is not stated—at four locations and downwind soil sampling. As expected, predicted transport from coarse chat piles (0.5–1.0 km) was less than transport from the fine tailings (3–5 km). The highest deposition rates were predicted for areas east and southeast of the sources, which would be consistent with the prevailing wind direction. The maximum annual average calculated lead deposition rate 1 km downwind from tailings impoundments was about 1–30 µg/m2-hr. Although model predictions and atmospheric observations varied from site to site, the predicted airborne lead at the Desloge site was reasonably close to observations with respect to both annual average (0.1 µg/m3) and maximum 24-h average (1 µg/m3) concentrations. Soil sampling showed the expected rapid decrease in lead concentration with distance that reached more typical concentrations at about 2 km downwind from the Bonne Terre sources, which were mainly tailings and a small chat pile.
A decade later, the Missouri Department of Natural Resources reported that airborne lead concentrations monitored near a chat pile during earth-moving operations could rise to 0.3 µg/m3 (24-hr average) despite the coarse particle diameter of chat (MO DNR 2009). High lead observations from TSP monitors were also reported at St. Joe State Park (up to 0.9 µg/m3) and from personal monitors (up to 6 µg/m3, time-weighted average) worn by motorcycle riders in the vicinity of the disturbed surface of the chat pile (MO DNR 2009). Such observations near chat sources show that in spite of the very large particle diameter, chat can be an important lead source where anthropogenic mechanical disturbance occurs. That observation is important because chat was spread on roads, where the coarse particles can be ground up by traffic, and then suspended by the passage of vehicles or wind erosion. The grinding and resuspension of chat could be an important lead source; it has been reported that some road dust in Southeast Missouri contains higher lead concentrations than are measured elsewhere in the region (Witt et al. 2013, 2014, 2016).
The Abbott (1999) study used meteorologic observations from the St. Louis airport 80–100 km away; wind was predominantly toward the east and southeast, but these wind directions probably do not reflect local conditions, where the topography might channel the flow in a more northerly or southerly direction. The study demonstrates the importance of making local meteorologic observations for source dispersion modeling, as discussed further in Chapter 4.
INTENTIONAL TRANSPORT
Intentional transport includes all the human activities that move materials within and off a site. For example, intentional transport at mining sites in the Southeast Missouri Lead Mining District would include the mechanical, truck, or rail transport of lead ores, lead concentrates, or lead-containing mine wastes (waste rock, tailings, and slags) for further processing, disposal, or beneficial use, such as use of chat as soil amendments on agricultural fields and use of tailings as railroad-bed material. McHenry (2006) compiled a history of the mining companies and chat dumps of the Missouri Lead Belt in St. Francois County and reported considerable anecdotal information about transport of mine wastes, particularly chat, for beneficial uses. Before 1922, chat was offered “free for the asking and many thousands of tons were hauled away by local citizens, city, county and state government, and other industries in the area which had uses for it.” As noted in Chapter 2, one primary use was as an agricultural lime; in 1922, chat began to be sold to limestone dealers for use as a soil amendment. Chat sales continued until 1972, when one chat pile that originally contained 3,522,000 tons was entirely gone (Karsch 1973). Other uses noted by McHenry (2006) were as a component of blacktop, concrete, fill material in building construction and road grades, sandbox sand, and grit for deicing streets and sidewalks.
The intentional transport of mine wastes for beneficial use is of concern as a potential vector for public-health and environmental problems. The Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) remedial investigations (RIs) for mining sites and other industrial sites that have generated large quantities of potentially valuable byproducts do not systematically consider intentional transport mechanisms. Because intentional transport is a business activity, the process of identifying and quantifying intentional transport processes involves locating and auditing business records, such as transport manifests, invoices and receipts, inventory records, and informal communications (oral and written). The systematic consideration of intentional transport mechanisms then falls under the purview of accountants, economists, and historians, who are rarely engaged in such activity for CERCLA RIs, perhaps because of an absence of EPA guidance that articulates the need for and role of qualified practitioners in these professions in systematically considering intentional transport mechanisms.5
One approach for encouraging systematic consideration of intentional transport would be to eliminate the use of the term migration to describe the transport processes to be considered because its use implies that contaminant transport is governed only by air and water dispersion processes. Furthermore, a recommendation to consider intentional transport mechanisms could be added to guidance documents.
REFERENCES
Abbott, M.L. 1999. Air Dispersion Modeling Of Mine Waste in the Southeast Missouri Old Lead Belt. INEEL/EXT-99-00235. Idaho National Engineering and Environmental Laboratory and Environmental Laboratory Integrated Earth Sciences Department, Idaho Falls, ID. October 1999 [online]. Available: https://inldigitallibrary.inl.gov/sites/sti/sti/3156874.pdf [accessed July 11, 2017].
Amato, J.A. 2001. Dust: A History of the Small and the Invisible. Berkeley, CA: University of California Press.
Bain, D.J., M.B. Green, J.L. Campbell, J.F. Chamblee, S. Chaoka, J.M. Fraterrigo, S.S. Kaushal, S.L. Martin, T.E. Jordan, A.J. Parolari, W.V. Sobczak, D.E. Weller, W.M. Wolheim, E.R. Boose, J.M. Duncan, G.M. Gettel, B.R.
___________________
5 The committee notes that archeologists and historians are routinely enlisted at CERCLA sites to locate and document historical artifacts, as required to comply with the National Historic Preservation Act, but social scientists are not routinely enlisted to consider intentional transport mechanisms and their contribution to contaminant distribution systematically.
Hall, P. Kumar, J.R. Thompson, J.M. Vose, E.M. Elliott, and D.S. Leigh. 2012. Legacy effects in material flux: Structural catchment changes predate long-term studies. BioScience 62(6):575-584.
Bigham, J.M., and D.K. Nordstrom. 2000. Iron and aluminum hydroxysulfates from acid sulfate waters. Rev. Mineral. Geochem. 40(1):351-403.
Brewer, P.A., and M.P. Taylor. 1997. The spatial distribution of heavy metal contaminated sediment across terraced floodplains. Catena 30(2-3):229-249.
Callender, E. 2005. Heavy metals in the environment—Historical trends. Pp. 67-105 in Environmental Geochemistry, Treatise on Geochemistry Vol. 9, B. Sherwood Lollar, ed. Amsterdam: Elsevier.
Cao, X., L.Q. Ma, M. Chen, D.W. Hardison, Jr., and W.G. Harris. 2003a. Weathering of lead bullets and their environmental effects at outdoor shooting ranges. J. Environ. Qual. 32:526-534.
Cao, R.X., L.Q. Ma, M. Chen, S.P. Singh, and W.G. Harris. 2003b. Phosphate-induced metal immobilization in a contaminated site. Environ. Pollut. 122(1):19-28.
Chow, J.C. 1995. Measurement methods to determine compliance with ambient air quality standards for suspended particles. J. Air Waste Manage. Assoc. 45(5):320-382.
Chow, J.C., and J.G. Watson. 1992. Fugitive emissions add to air pollution. Environ. Protect. 3:26-31.
Cowherd, C. 2001. Fugitive dust emissions. Pp. 845-857 in Aerosol Measurement: Principles, Techniques, and Applications, 2nd Ed., P. Baron, and K. Willeke, eds. New York: John Wiley & Sons.
Dutrizac, J.E., and J.L. Jambor. 2000. Jarosites and their application in hydrometallurgy. Rev. Mineral. Geochem. 40(1):405-452.
EPA (U.S. Environmental Protection Agency). 1996. Method 1669. Sampling Ambient Water for Trace Metals at EPA Water Quality Criteria Levels. July 1996 [online]. Available: https://www3.epa.gov/caddis/pdf/Metals_Sampling_EPA_method_1669.pdf [accessed August 4, 2017].
EPA. 2017. Interactive Map of Air Quality Monitors [online]. Available: https://www.epa.gov/outdoor-air-quality-data/interactive-map-air-quality-monitors [accessed June 22, 2017].
Etyemezian, V.R., G. Nikolich, S. Ahonen, M.L. Pitchford, M. Sweeney, R. Purcell, J.A. Gillies, and H.D. Kuhns. 2007. The Portable In Situ Wind Erosion Laboratory (PI-SWERL): A new method to measure PM10 potential for windblown dust properties and potential for emissions. Atmos. Environ. 41(18): 3789-3796.
Gilbert, G.K. 1917. Hydraulic-Mining Debris in the Sierra Nevada. U.S. Geological Survey Professional Paper 105. Washington, DC: U.S. Government Printing Office [online]. Available: https://pubs.usgs.gov/pp/0105/report.pdf [accessed June 22, 2017].
Graf, W.L., S.L. Clark, M.T. Kammerer, T. Lehman, K. Randall, R. Tempe, and A. Schroeder. 1991. Geomorphology of heavy metals in the sediments of Queen Creek, Arizona, USA. Catena 18(6):567-582.
Guilbert, J.M., and C.F. Park, Jr. 1986. The Geology of Ore Deposits. New York: WH Freeman.
Gutierrez, M., K. Mickus, and L.M. Camacho. 2016. Abandoned Pb-Zn mining wastes and their mobility as proxy to toxicity: A review. Sci. Total Environ. 565:392-400.
Hardison Jr., D.W., L.Q. Ma, T. Luongo, and W.G. Harris. 2004. Lead contamination in shooting range soils from abrasion of lead bullets and subsequent weathering. Sci. Total Environ. 328(1-3):175-183.
Hayes, S.M., S.A. White, T.L. Thompson, R.M. Maier, and J. Chorover. 2009. Changes in lead and zinc lability during weathering-induced acidification of desert mine tailings: Coupling chemical and micro-scale analyses. Appl. Geochem. 42(12):2234-2245.
Hem, J.D. 1976. Inorganic chemistry of lead in water. Pp. 5-11 in Lead in the Environment, T.G. Lovering, ed. Geological Survey Professional Paper 957. Washington, DC: U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/pp/0957/report.pdf [accessed June 22, 2017].
Hillier, S. 2001. Particulate composition and origin of suspended sediment in the R. Don, Aberdeenshire, UK. Sci. Total Environ. 265(1-3):281-293.
Horowitz, A. 1991. A Primer on Sediment-Trace Element Chemistry, 2nd Ed. Chelsea, MI: Lewis.
Hudson-Edwards, K.A., M.G. Macklin, C.D. Curtis, and D.J. Vaughan. 1996. Processes of formation and distribution of Pb-, Zn-, Cd-, and Cu-bearing minerals in the Tyne Basin, northeast England: Implications for metal-contaminated river systems. Environ. Sci. Technol. 30(1):72-80.
Hudson-Edwards, K.A., M.G. Macklin, J.R. Miller, and P.J. Lechler. 2001. Sources, distribution and storage of heavy metals in the Rio Pilcomayo, Bolivia. J. Geochem. Explor. 72: 229-250.
Jambor, J.L., D.K. Nordstrom, and C.N. Alpers. 2000. Metal-sulfate salts from sulfide mineral oxidation. Rev. Mineral. Geochem. 40(1):305-350.
James, L.A. 1989. Sustained storage and transport of hydraulic gold mining sediment in the Bear River, California. Ann. Assoc. Am. Geogr. 79(4):570-592.
Jørgensen, S.S., and M. Willems. 1987. The fate of lead in soils: The transformation of lead pellets in shooting-range soils. Ambio 16(1):11-15.
Karsch, A. 1973. Chat dumps of St. Francois County, Missouri, lead mining capital of the World: 1864-1972. The Farmington News, January. P. xv in Chat Dumps of the Missouri Lead Belt, St. Francois County, with an Illustrated History of the Lead Companies that Built Them, 2006. Park Hills, MO: Missouri Department of Natural Resources, Missouri Mines State Historic Site.
Kinsey, J.S., and C. Cowherd. 1992. Fugitive emissions. Pp. 133-146 in Air Pollution Engineering Manual, A.J. Buonicore, and W.T. Davis, eds. New York: Van Nostrand Reinhold.
Knighton, D. 1991. Channel bed adjustment along mine-affected rivers of northeast Tasmania. Geomorphology, 4:205-219.
Knox, J.C. 1987. Historical valley floor sedimentation in the Upper Mississippi valley. Ann. Assoc. Am. Geogr. 77(2):224-244.
Knox, J. C. 1989. Valley floor sedimentation in the Upper Mississippi Valley: Reply. Ann. Assoc. Am. Geogr. 79(4):601-608.
Kok, J.F., E.J.R. Parteli, T.I. Michaels, and D.B. Karam. 2012. The physics of wind-blown sand and dust. Rep. Prog. Phys. 75:106901 [online]. Available: https://arxiv.org/ftp/arxiv/papers/1201/1201.4353.pdf [accessed June 23, 2017].
Leigh, D.S. 2010. Morphology and channel evolution of small streams in the southern Blue Ridge Mountains of Western North Carolina. Southeast. Geogr. 50(4):397-421.
Lewin, J., and M.G. Macklin. 1987. Metal mining and floodplain sedimentation in Britian. Pp. 1009-1027 in International Geomorphology, V. Gardiner, ed. Hoboken, NJ: John Wiley and Sons.
Lin, Z., B. Comet, U. Qvarfort, and R. Herbert. 1995. The chemical and mineralogical behavior of Pb in shooting range soils from central Sweden. Environ. Pollut. 89(3):303-309.
Lovering, T.G. 1976. Lead in the environment – Summary. Pp. 1-4. in Lead in the Environment, T.G. Lovering, ed. Geological Survey Professional Paper 957. Washington, DC: U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/pp/0957/report.pdf [accessed June 22, 2017].
Mackin, M.G. 1996. Fluxes and storage of sediment-associated heavy metals in floodplain systems: Assessment and river basin management issues at a time of rapid environmental change. Pp. 441-460 in Floodplain Processes, M.G. Anderson, D.E. Walling, and P.D. Bates, eds. Hoboken, NJ: John Wiley and Sons.
Macklin, M.G., and R.B. Dowsett. 1989. The chemical and physical speciation of trace metals in fine grained overbank flood sediments in the Tyne Basin, north-east England. Catena 16(2):135-151.
Macklin, M.G., and J. Lewin. 1989. Sediment transfer and transformation of an alluvial valley floor: The river South Tyne, Northumbria, U.K. Earth. Surf. Proc. Land. 14(3):233-246.
Macklin, M.G., K.A. Hudson-Edwards, H.E. Jamieson, P. Brewer, T.J. Coulthard, A.J. Howard, and V.H. Renenda. 1999. Physical stability and rehabilitation of sustainable aquatic and riparian ecosystems in the Rio Guadiamar, Spain, following the Aznalcóllar mine tailings dam failure. Pp. 271-278 in Mine, Water and Environment: Proceedings of the International Mine Water Association Congress, Seville, Spain [online]. Available: http://www.mwen.info/docs/imwa_1999/IMWA1999_Macklin_271.pdf [accessed June 23, 2017].
Macklin, M.G., P.A. Brewer, K.A. Hudson-Edwards, G. Bird, T.J. Coulthard, I.A. Dennis, P.J. Lechler, J.R. Miller, and J.N. Turner. 2006. A geomorphological-geochemical approach to river basin management in mining-affected rivers. Geomorphology 79:423-447.
Marron, D.C. 1987. Floodplain storage of metal-contaminated sediments downstream of a gold mine at Lead, South Dakota. Pp. 193-209 in Chemical Quality of Water and the Hydrologic Cycle, R.C. Averett, and D.M. McKnight, eds. Chelsea, MI: Lewis.
Marzouk, E.R., S.R. Chenery, and S.D. Young. 2013. Predicting the solubility and lability of Zn, Cd, and Pb in soils from a minespoil-contaminated catchment by stable isotopic exchange. Geochim. Cosmochim. Acta 123:1-16.
Masters, S., G.J. Welter, and M. Edwards. 2016. Seasonal variations in lead release to portable water. Environ. Sci. Technol. 50(10):5269-5277.
McHenry, R.E. 2006. Chat Dumps of The Missouri Lead Belt, St. Francois County, With an Illustrated History of the Lead Companies that Built Them. Park Hills, MO: Missouri Department of Natural Resources, Missouri Mines State Historic Site.
Miller, J.R., and S.M. Orbock Miller. 2007. Contaminated Rivers: A Geomorphological-Geochemical Approach to Site Assessment and Remediation. Berlin: Springer.
Miller, J.R., and L.F. Villarroel. 2011. Bolivia: Mining, river contamination and human health. Pp. 421-441in Encyclopedia of Environmental Health, J. Nriagu, ed. Amsterdam: Elsevier.
Miller, J.R., P.J. Lechler, and M. Desilets. 1998. The role of geomorphic processing in the transport and fate of mercury in the Carson River basin, west-central Nevada. Environ. Geol. 33(4):249-262.
Miller, J.R., D. Germanoski, L.F. Villarroel, and P. Lechler. 2009. Spatial and temporal variations in the transport and storage of trace metal contaminants in the Upper Río Pilcomayo, Southern Bolivia. Int. J. Environ. Health 3(4):334-361.
Miller, J.R., M. Lord, L. Villarroel, D. Germanoski, and J. Chambers. 2012. Structural organization of process zones in upland watersheds of central Nevada and its influence on basin connectivity, dynamics, and wet meadow complexes. Geomorphology 139-140:284-402.
MO DNR (Missouri Department of Natural Resources). 2009. Air Pollution Control Program: Missouri Lead Monitoring Network Plan, October 2009 [online]. Available: https://dnr.mo.gov/env/apcp/monitoring/moleadplan-09.pdf [accessed June 23, 2017].
MO DNR. 2017. Air Quality Analysis for Lead [online]. Available: https://dnr.mo.gov/env/apcp/docs/leadmonitordata.pdf [accessed July 12, 2017].
Moore, J.N., E.J. Brook, and C. Johns. 1989. Grain size partitioning of metals in contaminated, coarse-grained river floodplain sediments: Clark Fork River, Montana, USA. Environ. Geol. Water S. 14(2):107-115.
Moravec, P., J. Smolik, J. Ondracek, P. Vodicka, and R. Fajgar. 2015. Lead and/or lead oxide nanoparticle generation for inhalation experiments. Aerosol Sci. Technol. 49(8):655-665.
Nicholas, A.P., P.J. Ashworth, M.J. Kirkby, M.G. Macklin, and T. Murray. 1995. Sediment slugs: Large-scale fluctuations in fluvial sediment transport rates and storage volumes. Prog. Phys. Geogr. 19(4):500-519.
Niemitz, J., C. Haynes, and G. Lasher. 2013. Legacy sediments and historic land use: Chemostratigraphic evidence for excess nutrient and heavy metal sources and remobilization. Geology 41(1):47-50.
Nieto, J.M., A.M. Sarmiento, M. Olías, C.R. Canovas, I. Riba, J. Kalman, and T.A. Delvalls. 2007. Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva Estuary. Environ. Int. 33(4):445-455.
Nordstrom, D.K., D.W. Blowes, and C.J. Ptacek. 2015. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 57:3-16.
Orbock Miller, S.O., D.F. Ritter, R.C. Kochel, and J.R. Miller. 1993. Fluvial responses to land-use changes and climatic variations within the drury creek watershed, southern Illinois. Geomorphology 6(4):309-329.
Pavlowsky, R.T., M.R. Owen, and D.J. Martin. 2010. Big River Mining Sediment Assessment Project: Distribution, Geochemistry, and Storage of Mining Sediment in Channel and Floodplain Deposits of the Big River System in St. Francois, Washington, and Jefferson Counties, Missouri, Final Report. OEWRI EDR-10-002. Missouri State University, Ozarks Environmental and Water Resources Institute, Springfield, MO. June 18, 2010.
Pieper, K.J., M. Tang, and M.A. Edwards. 2017. Flint water crisis caused by interrupted corrosion control: Investigating “ground zero” home. Environ. Sci. Technol. 51(4):2007-2014.
Powell, K.J., P.L. Brown, R.H. Byrne, T. Gajda, G. Hefter, A.K. Leuz, S. Sjöberg and H. Wanner. 2009. Chemical speciation of environmentally significant metals with inorganic ligands, Part 3. The Pb2+ + OH-, Cl-, CO32-, SO42-, and PO43-–systems. IUPAC Technical Report. Pure Appl. Chem. 81(12):2425-2476.
Prabhakar, G., A. Sorooshian, E. Toffol, A.F. Arellano, and E.A. Betterton. 2014. Spatiotemporal distribution of airborne particulate metals and metalloids in a populated arid region. Atmos. Environ. 92:339-347.
Price, K., and D. Leigh. 2006. Morphological and sedimentological responses of streams to human impact in the southern Blue Ridge Mountains, USA. Geomorphology 78(1-2):142-160.
Pye, K. 1987. Aeolian Dust and Dust Deposits. London, UK: Academic Press.
Richards, R.P. 2001. Estimation of Pollutant Loads in Rivers and Streams: A Guidance Document for NPS Programs. Report to the Environmental Protection Agency, Region VIII, Grant No. X998397-01-0 [online]. Available: http://www.ccamp.us/ccamp_regional_report/_Documents/PeteLoad_Est1.pdf [accessed June 26, 2017].
Rooney, C.P., R.G. McLaren, and L.M. Condron. 2007. Control of lead solubility in soil contaminated with lead shot: Effect of soil pH. Environ. Pollut. 149(2):149-157.
Root, R.A., S.M. Hayes, C.M. Hammond, R.M. Maier, and J. Chorover. 2015. Toxic metal(loid) speciation during weathering of iron sulfide mine tailings under semi-arid climate. Appl. Geochem. 62:131-149.
Rouff, A.A., E.J. Elzinga, R.J. Reeder, and N.S. Fisher. 2005. The influence of pH on the kinetics, reversibility and mechanisms of Pb(II) sorption at the calcite-water interface. Geochim. Cosmochim. Acta 69(22):5173-5186.
Rozan, T.F., G.W. Luther, D. Ridge, and S. Robinson. 2003. Determination of Pb complexation in oxic and sulfidic waters using pseudovoltammetry. Environ. Sci. Technol. 37(17):3845-3852.
Schaider, L.A., D.B. Senn, E.R. Estes, D.J. Brabander, and J.P. Shine. 2014. Sources and fates of heavy metals in a mining-impacted stream: Temporal variability and the role of iron oxides. Sci. Total Environ. 490:456-466.
Serrano, S., P.A. O’Day, D. Vlassopoulos, M.T. Garcia-Gonzalez, and F. Garrido. 2009. A surface complexation and ion exchange model of Pb and Cd competitive sorption on natural soils. Geochim. Cosmochim. Acta 73:543-558.
Serrano, S., D. Vlassopoulos, F. Garrido, and P.A. O’Day. 2013. A combined site-specific metals sorption and transport model for intact soil columns. Vadose Zone J. 12(4), doi:10.2136/vzj2012.0152.
Shao, Y.P. 2008. Physics and Modelling of Wind Erosion. New York: Springer.
Slingerland, R., and N.D. Smith. 1986. Occurrence and formation of water-laid placers. Annu. Rev. Earth Planet Sci. 14:113-147.
Stockdale, A., E. Tipping, and S. Lofts. 2015. Dissolved trace metal speciation in estuarine and coastal waters: Comparison of WHAM/Model VII predictions with analytical results. Environ. Sci. Technol. 34(1):53-63.
Stovern, M., O. Felix, J. Csavina, K.P. Rine, M.R. Russell, R.M. Jones, M. King, E.A. Betterton, and A.E. Sáez. 2014. Simulation of windblown dust transport from a mine tailings impoundment using a computational fluid dynamics model. Aeolian Res. 14:75-83.
Stovern, M., H. Guzmán, K.P. Rine, O. Felix, M. King, W.P. Ela, E.A. Betterton, and A.E. Sáez. 2016. Windblown dust deposition forecasting and spread of contamination around mine tailings. Atmosphere 7(2):16.
Stumm, W. 1992. Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems. New York: Wiley [online]. Available: http://www.jlakes.org/config/hpkx/news_category/2015-12-11/ChemistryOfTheSolidWaterInterface_Stumm1992.pdf [accessed August 4, 2017].
Swistock, B.R., W.E. Sharpe, and P.D. Robillard. 1993. A survey of lead, nitrate and radon contamination of private individual water systems in Pennsylvania. J. Environ. Health 55:6-12.
Town, R.M., and M. Filella. 2000a. A comprehensive systematic compilation of complexation parameters reported for trace metals in natural waters. Aquat. Sci. 62(3):252-295.
Town, R.M., and M. Filella. 2000b. Dispelling the myths: Is the existence of L1 and L2 ligands necessary to explain metal ion speciation in natural waters? Limnol. Oceanogr. 45(6):1341-1357.
Trimble, S.W. 1974. Man-induced Soil Erosion in the Southern Piedmont 1700-1970. Ankeny, IA: Soil Conservation Society of America. 180pp.
USDA (U.S. Department of Agriculture). 2015. Wind erosion simulation models. U.S. Department of Agriculture, Wind Erosion Research, Fort Collins, CO [online]. Available: https://infosys.ars.usda.gov/WindErosion/simmodels/simmodels.shtml [accessed June 26, 2017].
Valance, A., K.R. Rasmussen, A.O. El Moctar, and P. Dupont. 2015. The physics of Aeolian sand transport. C. R. Phys. 16(1):105-117.
Vega, F.A., and L.P. Weng. 2013. Speciation of heavy metals in River Rhine. Water Res. 47(1):363-372.
Villarroel, L.F., J.R. Miller, P.J. Lechler, and D. Germanoski. 2006. Lead, zinc, and antimony contamination of the Rio Chilco-Rio Tupiza drainage system, southern Bolivia. Environ. Geol. 51(2):283-299.
Watson, J.G., and J.C. Chow. 2000. Reconciling Urban Fugitive Dust Emissions Inventory and Ambient Source Contribution Estimates: Summary of Current Knowledge and Needed Research. Desert Research Institute Document No. 6110.4F. Prepared for EPA, Research Triangle Park, NC. May 2000 [online]. Available: http://www.epa.gov/ttn/chief/efdocs/fugitivedust.pdf.
Watson, J.G., J.C. Chow, and T.G. Pace. 2000. Fugitive dust emissions. Pp. 117-135 in Air Pollution Engineering Manual 2nd Ed., W.T. Davis, ed. New York: John Wiley & Sons.
Watson, J.G., J.C. Chow, X. Wang, S.D. Kohl, and L.N.R. Yatavelli. 2014. Windblown Fugitive Dust Characterization in the Athabasca Oil Sands Region. Prepared by Desert Research Institute, Reno, NV, for Wood Buffalo Environmental Association, Ft. McMurray, AB, Canada.
Witt, E.C., D.J. Wronkiewicz, R.T. Pavlowsky, and H.L.Shi. 2013. Trace metals in fugitive dust from unsurfaced roads in the Viburnum Trend resource mining District of Missouri-Implementation of a direct-suspension sampling methodology. Chemosphere 92:1506-1512.
Witt, E.C., H.L. Shi, D.J. Wronkiewicz, and R.T. Pavlowsky. 2014. Phase partitioning and bioaccessibility of Pb in suspended dust from unsurfaced roads in Missouri-A potential tool for determining mitigation response. Atmos. Environ. 88:90-98.
Witt, E.C., M.J. Pribil, J.P. Hogan, and D.J. Wronkiewicz. 2016. Isotopically constrained lead sources in fugitive dust from unsurfaced roads in the southeast Missouri mining district. Environ. Pollut. 216:450-459.
Xie, Y., and D.E. Giammar. 2011. Effects of flow and water chemistry on lead release rates from pipe scales. Water Res. 45(19):6525-6534.
Yang, J.Y., X.E. Yang, Z.L. He, I.Q. Li, J.L. Shentu, and P.J. Stoffella. 2006. Effect of pH, organic acids and inorganic ions on lead desorption from soils. Environ. Pollut. 143(1):9-15.

















