4
Investigative Strategies for Lead-Source Attribution
During the last several decades, a wide array of analytic techniques have been used, with varied degrees of success, to determine the relative contributions of lead from natural and anthropogenic sources in soils, sediments, water, and air. The US Environmental Protection Agency has used several methods in its investigations, including using elemental concentrations and ratios, isotopic analysis, and other soil-metal characterizations (Burgess 2016). Most techniques rely on a fingerprinting approach that attempts to identify unique physical or chemical characteristics that can distinguish lead or lead-associated materials between suspected sources (Box 4-1). Once the sources have been assessed, the distinguishing source characteristics can be compared with those of samples of soil, sediment, water, and air to assess the origin of the lead. Given the variety of analytic methods that are available, integration of the most appropriate techniques is challenging (Gallego et al. 2016).
In this chapter, the committee provides a series of investigative strategies that are stratified by media type. The strategies are intended to provide a logical framework for determining the source of lead while minimizing the time, effort, and financial resources required to do so. They are not intended to replace procedures associated with the remedial investigation and feasibility study but are meant to serve as a general guide to lead-source attribution. Given the highly variable nature of lead contamination near and adjacent to Superfund sites, not all the methods that are listed in the strategies will be applicable to all sites. In some cases, additional methods not cited in the strategies might be useful. Furthermore, the strategies might require modification to fit the specific characteristics of an individual site or operable unit.
The suggested strategies are based on a multiple-lines-of-evidence approach, in which data are systematically collected, analyzed, and interpreted until there is sufficient confidence that a method (or combination of methods) has resulted in a sound understanding of the sources of lead. The methods inherent in the strategies represent tested techniques that generate results that are widely accepted by the scientific community (unless otherwise stated). Preference was also given to techniques that generate quantifiable degrees of uncertainty.
SOIL
The committee’s investigative strategy for soil is shown in Figure 4-1 and provides a guiding framework for deducing lead sources in soil. In this section, the committee focuses on soils derived from underlying rock strata as opposed to materials that are transported by river, gravity, or wind. The section that follows focuses on river sediments and sites within active floodplains where alluvial deposition is occurring regularly or has occurred over the duration in question for lead deposition.
The committee provides a possible sequence of analyses for soil based on typical successful outcomes, but the sequence might be adjusted on the basis of site-specific conditions, availability of instrumentation, and resource (cost and time) considerations. Before investigation of the source of lead, total soil concentrations need to be measured; sites (or samples) exceeding a regulatory threshold can then be examined for the lead source.
Site Characterization and History
Before a detailed chemical or mineralogic analysis is conducted, a basic site characterization and history should be developed that leads to a site conceptual model that can guide investigations toward likely lead sources; potential contributors of lead noted in Chapter 2 should be specifically identified. The landform of the soil or yard should be noted in terms of its current geology and likely geologic past. The US Department of Agriculture soil survey should be consulted for information on soils and landforms of the area. A history of land use at the site should also be constructed from public records and on-site observations to address appropriate questions, such as, Are there or have there been buildings constructed before the 1980s on the site that could have contributed lead-based paint to the
soil? Are there obvious atmospheric point sources, such as a smelter or mine, in the near vicinity? Has mine-waste material with high lead concentrations been deposited on the site as backfill or roadway material? Is there evidence of storage or disposal of lead-acid batteries on the site? Once basic questions have been considered, a detailed soil sampling protocol can be developed for the specific site. As part of the development of a conceptual model, a handheld x-ray fluorescence (XRF) analyzer can be used to survey the surface-soil lead concentrations and guide soil sampling for additional analyses.
The field characterization should then proceed to determine whether the soil has been disturbed by human activity, whether the site is in an active floodplain and is a location where recent alluvial sediments have been deposited or eroded, and whether the soil was developed in situ (the nature of the underlying parent rock material). Disturbance by human activities is likely to alter spatial and depth trends in lead concentrations and might have led to the direct incorporation of anthropogenic lead into the soil. In Southeast Missouri, for example, coarse-grained tailings (chat) that contain lead were used extensively as fill and thus have been observed to be incorporated into disturbed soils in the region. Similarly, dispersal and source alteration from, for example, weathering processes described in Chapter 3 should be considered.
The distinction between soils composed of alluvial (riverine) and nonalluvial parent materials is an important consideration in site assessment because of differences in vertical patterns of lead concentration. Anthropogenic lead in soils of floodplains that were recently active (in the last century) often extends to depths measured in meters; anthropogenic lead in soils where the parent material was not deposited by the recent action of rivers is generally confined to within a few tens of centimeters of the ground surface. Thus, the topographic setting and type of soil parent material dictate the nature of the sampling program required to assess lead attribution. The following discussion of depth sampling and spatial analysis is for soil developed in place without active deposition where diagnostic vertical distributions tend to be retained as opposed to soils that are subject to recent alluvial deposits (active floodplains).
Spatial and Depth Sampling and Analysis
The spatial distribution of lead in a location is often a clue to its source. Anthropogenic sources of lead in soils are commonly deciphered by using two types of spatial analysis: an assessment of geographic patterns in lead concentration in the topsoil and a spatial analysis of lead as a function of depth below the ground surface. Geographic patterns in lead and other trace-metal concentrations are usually determined by collecting and analyzing composite samples of the topsoil with a gridded sampling scheme (Stafilov et al. 2010) or along transects that radiate from a suspected source, often corresponding to wind direction (Ettler 2016). Alternatively, the data might correspond to the distribution of residences that are sampled in urban areas. With the advent of hand-held XRF units, assessing spatial variation in lead can be easy and rapid and provide key information on lead sources. For example, high concentrations along the dripline of a home suggest that the source is paint or lead particles that have washed off the roof. Materials used for roads or driveways might contribute to high lead concentrations in nearby soils where concentrations decline with distance. Lead distribution, however, might also have nonunique source signals whereby natural, alluvial, or aerially deposited material might all show similar patterns that require further analysis.
Depth sampling and analysis might help to identify the lead concentration in soil parent material and layers that have been enriched by human activities and thus aid in source attribution. Here, the parent material for a soil is simply the rock (or unconsolidated rock material) that now underlies the soil. Soils develop through interaction of parent material with water, gas, living organisms, and organic matter in a given climate over an extended period. Parent material is generally determined through geologic inspection and chemical, mineralogic, and particle-size analysis of samples collected from the bottom of the soil profile and adjacent to the underlying bedrock. To confirm parent composition, multiple elements can be analyzed, and the measured composition compared with the expected weathering behavior of the underlying bedrock derived from modeling (Brantley and Lebedeva 2011). It is gener-
ally helpful to collect multiple samples of parent material if possible to estimate compositional variability, including sampling of local outcrops or drilling material.
Once parent material is identified, the source of lead in the soil can be investigated. Lead that is present in uncontaminated soils derives from the parent material or from natural inputs of dust or sediment. In contaminated soils, lead derives from those sources and from anthropogenic inputs. By sampling and analyzing the depth distribution of lead and, for example, the isotopic signatures, specific minerals, or elemental ratios in the soil, one might be able to determine a scenario that most likely explains the source of the lead. If the physical or chemical characteristics of the lead in the soil are identical with those in the parent material and the depth distribution is consistent with this natural source of the lead, the simplest explanation is that all the lead in the soil is derived from the parent material. If the chemical or physical characteristics of the soil lead instead differ from those of the lead in the parent material and its weathering products, the lead has probably been added to the soil. The analytic methods described below help to define the chemical and physical characteristics of the lead to distinguish its source.
In areas of potential ore deposits, separating natural from anthropogenic lead can be complicated. However, for most cases of soils outside a recently active floodplain, anthropogenic lead will generally be concentrated at or near the soil surface and decrease downward to concentrations similar to those of the parent material. In some cases, if topsoil has been placed over mine wastes or chat, vertical distributions might document a layer that has a high lead concentration between layers that have low lead concentrations. Depth sampling of soil profiles can investigate such diagnostic distributions. Such sampling tends to be conducted at uniform increments or to be stratified according to naturally defined soil horizons.
Most commonly, the magnitude of anthropogenic lead is assessed by comparing concentrations in the surface materials with those in deeper horizons, including parent material. The analyses can be complicated by the downward migration of lead, which can occur by leaching (Miller and Friedland 1994; Prapaipong et al. 2008); the translocation of colloids and clay-sized particles (Citeau et al. 2003); biologic activity caused, for example, by earthworms, gophers, ground squirrels, and plant uprooting; and human activities, including gardening and plowing. The depth and rate of downward migration depend not only on the mechanism and timing of mixing but on the chemical form or speciation of lead in the soil; soil characteristics, such as pH, type and amount of organic matter, and clay content and mineralogy; climate conditions, such as rainfall and temperature; and the nature of the particles with which lead is associated (as discussed in Chapter 3).
Interpretation of lead concentrations as a function of depth in soils can also be complicated because even if lead is not added to or lost from a soil, its concentration can increase upward in a soil if high concentrations of other elements have been dissolved and transported out of the soil by natural processes over geologic time. In other words, lead that is not removed from a soil can become more concentrated near the surface when more soluble materials have been removed. To account for weathering, soil scientists generally normalize concentrations of the element of interest, such as lead, to that of another element that is not present in soluble minerals, such as titanium and zirconium. One such normalization, which has been used extensively in the recent literature, is the mass transfer coefficient1 (Brimhall and Dietrich 1987; Anderson et al. 2002; Brantley and Lebedeva 2011). The mass transfer coefficient of lead is calculated by dividing two ratios. The first is the ratio of the lead concentration in a bulk soil sample to the concentration of an element, such as titanium or zirconium, that is present in the soil as an insoluble mineral. The second is the ratio of those same two elements in the parent material. When the mass transfer coefficient is analyzed as a function of depth, it can reveal patterns of addition or depletion in the soil that can be interpreted in terms of the lead source (Brantley and Lebedeva 2011). Observed depth distributions can be modeled in terms of processes, such as addition, depletion, or biologic cycling of elements into upper soil layers. Using those approaches, several investigators have modeled the addition of lead to soils from atmospheric deposition (Johnson et al. 1995; Drivas et al. 2011), and depth distributions coupled with isotopic signatures of various potential lead sources have been used to determine sources and migration of lead in soils (Prapaipong et al. 2008).
Lead Isotopic Analysis
Measurement of lead isotopic composition can potentially identify a lead source rapidly if the isotopic signature is unique. The effectiveness of isotopic composition as a fingerprinting tool is related to several factors: stable isotopes of lead can be measured with a high degree of precision and accuracy, lead is unreactive and immobile over a wide range of environmental (geochemical) conditions, and fractionation of lead isotopes by physical, chemical, and biologic processes is minimal in contrast with that of stable isotopes of light elements, such as sulfur, carbon, and oxygen (Keinonen 1992; Komárek et al. 2008; Balcaen et al. 2010; Bird 2011; Miller et al. 2012). Thus, isotopic composition of lead of natural and anthropogenic sources (including ore deposits) does not change appreciably as it is dispersed through the environment. In
___________________
1 The use of the term mass transfer coefficient by the authors is different from its use in engineering where it is used widely to characterize the transfer rate of mass between phases.
most cases, the variation in a soil will be small relative to the differences between the soil and external inputs; thus, observed differences in the isotopic composition of the soil in question can be attributed to its mixing with lead from other sources (Cheng and Hu 2010). Finally, lead isotopic composition of ore deposits on a global scale is highly variable (Bird 2011; Miller et al. 2015), and lead derived from ore deposits tend to differ from lead typically found in non-ore rocks and soils (Hopper et al. 1991). See Chapter 5 for a detailed case study.
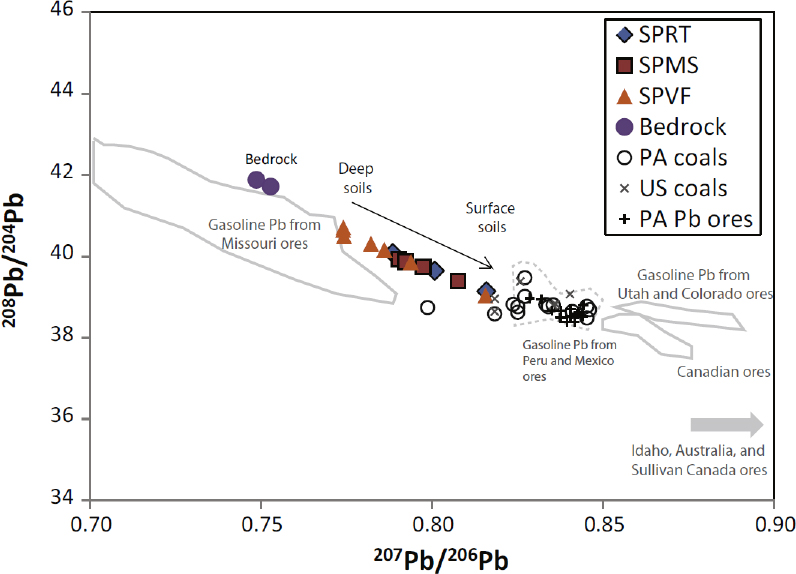
Studies conducted over the last several decades demonstrate that lead isotopes are often effective in determining lead sources in soils if the isotopic values of the source materials are unique (Rabinowitz and Wetherill 1972; Gulson et al. 1981; Steinmann and Stille 1997; Steinnes et al. 2005; Clark et al. 2006; Mihaljevic et al. 2006; Prapaipong et al. 2008; MacKinnon et al. 2011; Ma et al. 2014). In many cases, analyzing the depth distribution and isotopic signatures of lead in the soil allows source determination. If the isotopic character of the lead in the soil is identical with that of the parent rock, the simplest explanation is that all the lead in the soil results from the parent rock material. If, however, the isotopic character of the lead differs from that of the parent material, the lead has probably been added to the soil. As an illustrative example, Ma et al. (2014) completed an investigation in Pennsylvania where lead was enriched in soil relative to the parent bedrock. The isotopic signature of the lead varied significantly and consistently upward from the bedrock to the land surface (Figure 4-2). Using the lead isotopic signature, concentration–depth profiles, knowledge of the history of anthropogenic practices in the area, and models, the researchers attributed the added lead to atmospheric deposition between the 1850s and 1920s, when lead was emitted to the atmosphere from coal-burning and ore-smelting in the area (Ma et al. 2014).
Similarly, Steinnes et al. (2005) examined lead concentrations and sources throughout Norway. Their findings illustrate a similar 206Pb/207Pb ratio for C-horizons (unconsolidated parent rock material) throughout the country and are indicative of the undisturbed soil baseline lead signature. Using known 206Pb/207Pb ratios in atmospheric deposition, they were able to determine the fraction of the lead attributed to atmospheric inputs for lead-enriched surface soils. Gulson et al. (1981) provide another illustrative example. They examined lead sources
in soils near Adelaide, South Australia, by comparing concentrations and isotopic signatures between surface (upper 10 cm) and subsoil (30-40 cm) collected along a path that included urban and rural land use. Subsoil isotopic values were similar throughout the traversed sampling path and were consistent with parent rock; lead in the subsoil was therefore attributed almost entirely to that derived from the parent rock. In contrast, lead from parent rock made up a minor fraction of the total lead in surface soils. Using the isotopic signatures along the transect with the depth comparison, the authors were able to eliminate pesticides, power stations, and smelters as major sources of soil lead; instead, they attributed the lead to gasoline.
Rabinowitz and Wetherill (1972) used isotopic signatures, lead concentrations, and general mineralogic observations to assess sources of soil lead in shallow soil samples (0–2.5 cm and 12.5 cm) in Southeast Missouri and Benicia, California; their work illustrates the benefits and limitations of isotopic analysis for source attribution. In the majority of sites examined from St. Louis to rural areas away from any known ore bodies and sites near ore-processing or gasoline-refining, lead enrichment in surface soil layers could be attributed to specific sources on the basis of comparison with deeper soil layers and measured isotopic values of potential sources. However, a slight enrichment of 25-ppm lead in a surface soil layer near the ore-crushing and beneficiation plant in Indian Creek Mill, Missouri, provided an equivocal isotopic signature. In that case, mineralogy, particle-size distribution, and lead isotopic signature were not sufficient to resolve the source of high lead concentrations in the surface soil. In the other 11 sites examined in Southeast Missouri and Benicia, California, the combination of lead isotopic signature, concentration profiles, and mineralogic analysis allowed source attribution of lead enrichment in surface soil layers.
Using spatially resolved depth variation in lead concentration and lead isotopic signatures, Prapaipong et al. (2008) determined lead sources for soils potentially affected by smelting in Southeast Missouri. Lead isotopes of the smelting ore, which originated from the Viburnum Trend, were distinct from those of the parent materials of soils developed on Bonneterre dolomite or St. Francois rhyolite, and they also differed from the isotopic signature of lead derived from burning of leaded gasoline. The unique signature of each potential lead source allowed apportionment across the study area and with sampling depth. Lead concentrations attributed to smelting decreased with depth to a consistent value (typically 10–30 ppm) and decreased with distance from the smelter. However, within 2 km of the smelter, up to 90% of the lead was derived from smelting operations to depths of 25 cm. Over 4 km away from the smelter, smelter emissions accounted for 10–50% of the soil lead in the upper 15–25 cm. Isotopic analysis revealed that aerially deposited lead (from smelting and automotive exhaust) was present even at depths where concentrations were consistent with natural background. That finding suggests that the naturally occurring lead was displaced from the soil and that a maximum retention capacity was reached; any new lead that entered the soil would therefore either move through the soil or displace lead already present in the soil. To establish temporal trends in soil lead inputs, Prapaipong et al. (2008) examined lead concentrations and isotopic signatures of White Oak tree cores by using tree rings to provide concentration–time profiles. Lead concentration rose sharply 7 years after onset of smelting operations, increased within the tree rings over the following 25 years, and increased by a factor of 10 in trees within 1 km of the smelter. Isotopic analysis substantiated that the rise in lead concentrations was from the uptake of smelter-derived lead. The combined use of tree cores to determine temporal trends with spatial and depth profiles of soils to determine lead concentrations and isotopic signatures provides a powerful means of identifying lead sources.
Thus, if the lead isotopic compositions of the potential sources are unique and the lead isotopic values in the soil of concern fall within the range of the source materials, the contribution of each source to lead contamination can be determined. Given the robust nature of the technique, it might obviate additional analyses for source attribution, and this would greatly reduce the time, effort, and costs involved in determining lead sources. If the lead isotopic compositions of the source materials are similar (that is, they overlap), the method cannot unequivocally assess lead sources, and additional techniques are required.
Mineralogic Analysis and Particle Morphology
Mineralogic analysis of soils, such as optical microscopic identification of sand particles and x-ray diffraction (XRD) analysis of silts and clays, might provide signatures that allow source attribution. Minerals unique to specific sources can help with source attribution. The presence of galena, PbS, and other heavy minerals, for example, might be a clear signature of lead source (Ettler et al. 2005; Cabala and Teper 2007), whereas hydrocerussite, Pb3(CO3)2(OH)2(s), might precipitate as a soil mineral after weathering of the original lead mineral. As lead disperses in the environment, soil concentrations tend to decrease as distance from the source increases, and lead is more likely to be adsorbed to other soil minerals or to be present as a minor substituent in secondary phases in soils and sediments. As a result, source attribution through mineralogic analyses, although often effective, can be inconclusive in other cases.
XRD has been used extensively for clay mineral identification (see Whittig and Allardice 1986) and to elucidate soil and sediment sources (Eberl 2004; Benedetti et al. 2006). It is often used in conjunction with particle-size
or density separation to identify minerals specific to a lead source (Olson and Skogerboe 1975). The mineralogy and particle structure determined with optical microscopy and scanning electron microscopy (SEM) of sand particles has been used extensively on alluvial and dust deposits to track sediment source (Arribas et al. 2000; Abu-Zeid et al. 2001; Cardona et al. 2005; Benedetti et al. 2006) and might serve to attribute lead sources in soil.
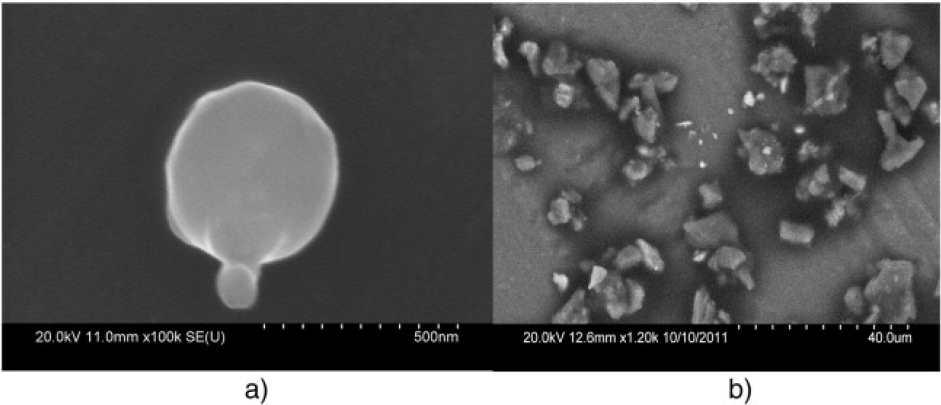
SEM coupled with elemental analysis—conducted with energy dispersive x-ray spectroscopy (EDS) or wave-length dispersive x-ray spectroscopy—is an additional means of obtaining chemical information and information on particle structure and texture. A description of the attributes and limitations of SEM and elemental analysis for identifying particle sources is provided in Pye (2004). Specific elemental ratios and morphologic characteristics of particles can be used to infer mineral identity, and combining mineralogy, particle size, and elemental composition has proved successful in source attribution (de Boer and Crosby 1995; Sterling et al. 1998). For example, Linton et al (1980) used specific particle structure and chemical composition to distinguish lead sources between automotive exhaust and paint chips, and Sobanska et al. (1999) used spherical particle structure and elemental composition to identify lead originating from smelters. Demonstrative features, such as spherical structure associated with smelters, can provide definitive source identification, and more subtle etching of sand grains might provide important clues to particle source (Abu-Zeid et al. 2001; Cardona et al. 2005). Figure 4-3 illustrates the use of SEM and EDS to distinguish between spherical lead-containing particles that are indicative of high-temperature processing, such as smelting, and particles that are more characteristic of airborne dust originating from vehicle activity or wind. Transmission electron microscopy (TEM) coupled with EDS and electron diffraction might also be useful for source identification. Mineralogy and particle-surface morphology can be deduced for isolated particles with TEM, which might be particularly useful for small, well-crystallized particles that have a propensity for deposition via wind-borne transport (Buseck and Posfai 1999).
Lead concentrations also tend to depend on particle size in material deposited as dust, with larger particles deposited closer to the source (NEPC 1998). Thus, particle-size analyses that include dividing particles into fine and coarse fractions can help to determine source. Size separation is typically conducted with wire-mesh sieves for particles larger than about 0.05 mm; finer particles are measured by sediment rate separation, suspension density (hydrometer), or laser light scattering. An example of particle-size variation is illustrated by Taylor et al. (2010), who found that lead concentrations within 2 km of mining and smelting activities in Mount Isa, Queensland, Australia, were higher above more distant soils and depended on particle size. High lead concentrations associated within finer particles (<180 μm) extended further from the site. Such geographic concentration data are often combined with dispersion models that predict the downwind deposition and surface concentrations of atmospheric lead from known sources (see, for example, Small et al. 1995).
Heavy minerals—such as pyrite, galena, cerussite, anglesite, and sphalerite—can be separated from soils by using fluids of different density. Such fractionated samples (separates) can often be used to associate soil grains with sources of lead contamination. Heavy minerals are particularly effective in tracing airborne lead and other metals derived from smelting and mining operations (see review by Ettler et al. 2016). Heavy mineral assemblages are often examined in combination with particle structure for attribution purposes. For example, the combination of ilmenite, FeTiO3, and particle structure allowed successful determination of particle origin (Basu and Molinaroli 1991), and heavy minerals with quartz stress fractures provided evidence on wind-transported particles (Abu-Zeid et al. 2001).
If heavy minerals are isolated with density separation methods that rely on high-viscosity fluids, the assemblages can be characterized by using mineralogic tools noted above, such as XRD (Burt et al. 2003; Ettler et al. 2005) and SEM–EDS (Cabala and Teper 2007). Density separation has been used extensively for the analysis of minerals in both consolidated and unconsolidated rocks (see, for example, Morton 1985), but it has not been applied much to source attribution of anthropogenic lead in soils. Nevertheless, the method holds promise in that it allows additional mineralogic, geochemical, or isotopic analysis (described above) to be performed directly on heavy minerals.
The attribution of airborne lead and other metals to mining and smelting operations by using heavy minerals has generally been based on spatial variations in the quantity with which they are found in soils relative to the source and the area’s predominant wind directions. Calaba and Teper (2007), for example, found that the proportion of heavy minerals, particularly iron sulfides associated with ore minerals, reached concentrations as high as 6–8% within several kilometers of ore-processing sites in southern Poland. Similarly, Ettler et al. (2005) found that anglesite occurred commonly in the dust and fly ash of lead-smelting operations in the Příbram district of the Czech Republic. Thus, they were able to argue that their presence in local soils was a clear indicator of airborne contamination from the nearby lead smelters.
Lead Chemical Speciation
Lead speciation can provide additional means for source attribution, but chemical speciation of lead in soils can be challenging because lead can be dispersed or poorly crystallized or be associated with small particles, all of which makes it difficult to conduct XRD or SEM analyses. Furthermore, weathering, alteration, and dissolution of primary lead compounds can change lead speciation and often result in adsorption of lead on mineral or organic-matter surfaces or its substitution as a minor element in newly precipitated phases. To overcome those challenges, selective extractions have been used commonly to assess the chemical speciation of lead.2 Most series of selective extractions are based on a method developed by Tessier et al. (1979); various methods are reviewed and summarized by Ure et al. (1993). One potential problem is that although the extracting medium might target lead associated with specific materials (such as organic matter, carbonates, or iron or manganese oxide), it might also capture other material; that is, the separation is not clean. Coupling that problem with changes in the lead species leads to imprecision in the method. The accuracy of a series of selective extractions has been assessed by comparing them with direct spectroscopic analyses—such as x-ray absorption spectroscopy, x-ray fluorescence, Raman spectroscopy, and other advanced analytic methods (Scheckel et al. 2005; Hayes et al. 2009; Hayes et al. 2012)—that provide information on chemical bonding of lead. In a study of mine tailings from Leadville, CO, coupling of direct characterization of lead speciation that used x-ray absorption spectroscopy with a series of selective extractions revealed discrepancies between lead fractions targeted in extractions and lead speciation determined spectroscopically and demonstrated substantial redistribution of lead after extraction treatment (Ostergren et al. 1999). However, despite their limitations, selective extractions are useful for assessing the relative reactivity of lead in different soil fractions that can then be compared with other soil layers or other sites and sources. For example, particle-size separations and selective extractions were used with x-ray absorption spectroscopy and x-ray fluorescence to determine lead speciation and mineral phases in core samples from weathered mine tailings to assess the potential for wind transport of particles and their relative bioaccessibility (Hayes et al. 2012). More advanced spectroscopic methods for chemical speciation might also be useful but can be difficult to access or can require specific expertise for data acquisition or analysis; a comprehensive summary of potential techniques is provided in D’Amore et al. (2005).
Source-Associated Tracers
The final source-attribution approach for soils encompasses a wide array of methods, including multielement analysis and methods that involve stable isotopes other than lead; they have been used to determine the contribution of lead from multiple sources on the basis of composition of the geologic materials with which lead is associated. The methods hold considerable promise but have not been extensively applied to the specific problem of lead-source attribution. For example, cadmium and zinc are commonly associated with and recovered during the
___________________
2 Selective extraction is an analytic process in which lead is leached from a sample with a solution to recover only a specific form of lead.
processing of lead ores (Rehkämper et al. 2011). As the ore is smelted, light isotopes of zinc and cadmium preferentially exit with the exhaust; heavy isotopes of zinc and cadmium are preferentially retained in the smelting residue (Sivry et al. 2008; Mattielli et al. 2009; Miller et al. 2015). The differences might then be used to track the deposition of emitted smelter particles, including lead-containing particles, over the region by analyzing the zinc and cadmium isotopic composition of the soils (see, for example, Cloquet et al. 2006; Mattielli et al. 2006, 2009; Shiel et al. 2010; Thapalia et al. 2010). Similarly, stable zinc and copper isotopes were used by Thapalia et al. (2010) to identify smelter emissions in lake sediments and thus trace lead to the smelter emissions.
In other cases, elemental ratios have been an easy means of tracing lead sources. Zota et al. (2011) used the association of lead, zinc, cadmium, and arsenic to identify particles originating from chat, and manganese was used to identify particles from uncontaminated soil. To elucidate whether pesticides or paints were responsible for enriched soil lead, Nezat et al. (2017) used the As/ Pb ratio in particles to denote pesticide contributions and Pb/Ba/Zn ratios for paint contributions. Given the use of antimony in alloying processes at specific smelters, Eckel et al. (2002) found Sb/Pb ratios, as opposed to As/Pb or Zn/Cd ratios, to correlate with soil lead contributions from smelting operations.
Analytic methods have also been paired with modeling to attribute sources, and the following discussion provides several examples of such approaches for source attribution. An example of combined analysis and modeling is the use of SEM–EDS to develop a hierarchical cluster analysis for household-dust apportionment based on particle size and element ratio (Hunt et al. 1992). A combination of multiple analytic methods, extractions, and geospatial modeling was used to characterize iron oxide phases, fine-grained mine waste, and organic material that adsorbed lead (and other metals) and to distinguish relative contributions from two geochemically distinct sources, mine drainage and mine-waste pile runoff (Schaider et al. 2014). Simple mass-balance models—an example of which is provided in Chapter 5—can also help to constrain potential source contribution and aid in source attribution. More sophisticated predictive statistical models can also be useful in lead-source attribution. Contributions of soil lead from an automotive-battery recycling facility were compared with lead-based house paint and other background sources by Small et al. (1995); they used scaled deposition estimates from atmospheric dispersion to develop a statistical model that characterized observed soil-lead concentrations and attributed sources relative to the urban background. Multielement models noted in the next section on sediments might also be applicable to lead-source attribution in soils.
SEDIMENTS
The committee’s investigative strategy for sediment is shown in Figure 4-4 and provides a guide to the effective determination of the natural and anthropogenic sources of lead found in river sediments. Similar analytic methods can be applied to nearly any type of sediment, such as those associated with lakes, wetlands, peat bogs, and coastal or estuarine environments. In fact, it is common to analyze sediments from different types of settings to provide a more holistic assessment of inputs of lead to the environment. Nonetheless, the focus here is on rivers and river sediments.
The sequence of analytic methods is intended only as a guide and might need to be adjusted to fit the site or operable unit of interest given specific site conditions, availability of instrumentation, and considerations of cost and time (see Box 4-2). The discussion that follows provides a general description of the methods shown in the figure and highlights some advantages, disadvantages, and sources of uncertainty. Additional information on each method can be obtained from the references provided.
Site Characterization and History
As applied here, site characterization is the process of collecting data to describe the river basin in terms of its physical, hydrologic, physiographic, and biotic characteristics at the time of the analysis and in the past. The exact list of measures to be used will depend on the site conditions but should include the identification of all natural and anthropogenic lead sources in the site and in the basin upstream of the site. The objective is to document the location and spatial distribution of each source, the form of the lead and associated materials in each source, the pathways and processes through which lead could enter a river from each source, and, for anthropogenic sources, the history and methods of lead use or processing. Most of the data required for site characterization will probably have been collected through the Superfund remedial investigation and feasibility study.
Fluvial Geomorphic Analysis and Sampling
Rivers can be viewed, simplistically, as networks of channels that transport water and sediment from upstream areas to downstream areas of a basin. Rather than being simple conduits, however, they are highly dynamic features in which water is continuously eroding and depositing sediment in the river channel and on the floodplain during floods. Erosion and deposition of sediment can incorporate sediment-associated lead that enters the drainage network into riverbed and floodplain (bank) deposits. The deposits vary widely along the river valley in their areal extent, age, and sedimentologic characteristics, such
as particle size, shape, lithology, and color. The sources of sediment and lead in the deposits also vary along the river and on a local (stream segment) scale.
The primary objective of analyzing a river’s erosional and depositional history is to identify, characterize, and map the distribution of distinct, time-correlated sedimentary deposits along the river valley. The analysis provides an understanding of the transport pathways through which sediment-associated lead is dispersed from suspected lead sources and the potential sites where lead might have accumulated along the river during specific times. It also provides a framework that is essential for developing a sound sampling program for which the physical and chemical analyses described below can be effectively conducted. For example, sediments deposited before European settlement might provide information on natural concentrations of lead associated with the sediments in the river basin.
Lead Isotopic Analysis
When applied to river sediments, the assumption is that lead in riverbed, floodplain, or terrace deposits represents a mixture of lead from all the sources that contribute lead to the river at the time of sediment deposition. Comparison of the lead isotopic composition of the sediment mixture with the isotopic composition of the suspected sources allows an estimation of the relative contribution of lead to the sediments from each potential source. The analysis is often complicated because the lead in river sediment is derived partly from the bedrock that underlies the basin. Depending on the basin’s geology, the lead isotopic composition of the underlying bedrock might vary substantially between rock units. Thus, in addition to the suspected anthropogenic sources of lead, it might be necessary to consider multiple bedrock lead sources.
Nonetheless, many studies have used lead isotopic methods effectively to identify the source of lead in river deposits directly (Church et al. 2004; Miller et al. 2007; Shepherd et al. 2009; Bird et al. 2010a,b; Ayrault et al. 2012). Lead isotopic methods have also been applied to various other sediment types, such as those associated with reservoirs and lakes (Shirahata et al. 1980; Petit et al. 1984; Graney et al. 1995; Chiaradia et al. 1997; Foster et al. 2002; Callender 2005), wetlands, and peat bogs (Shotyk et al. 1998; Weiss et al. 1999; Marcantonio et al.
2000; Cloy et al. 2008). Lead isotopes have been particularly useful in deciphering the contributions of lead from nonmineralized rocks and mining and milling wastes and contributions of lead from distinct mining districts when the lead isotopic characteristics vary between ores (see, for example, Bird et al. 2010a,b). However, when the isotopic characteristics of the mining wastes are similar to those found in the local, mineralized, but unmined bedrock, it will not be possible to attribute lead to those sources by using lead isotopes.
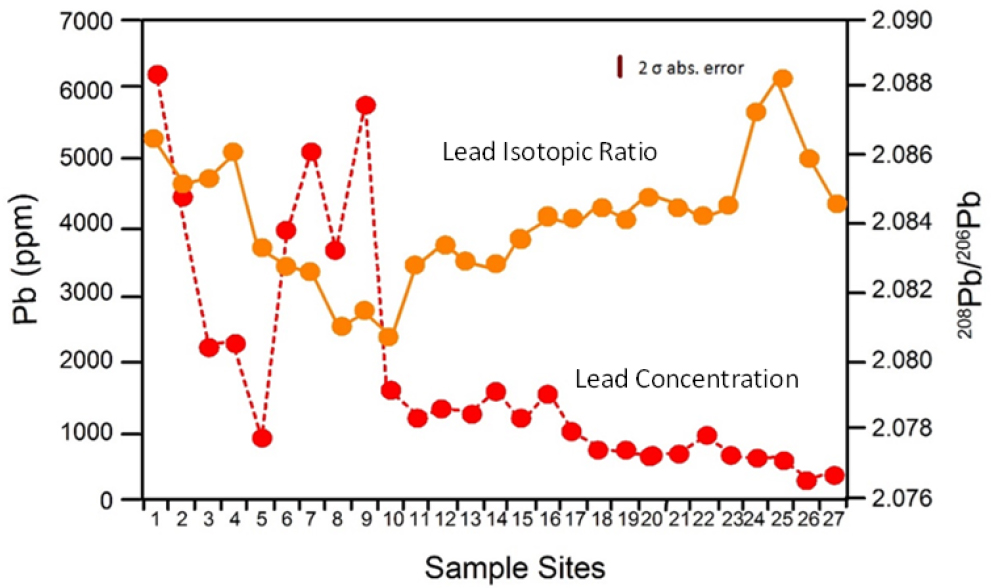
The analysis of river deposits differs from the analysis of upland soils (described in the previous section) in two ways. First, the isotopic composition of river deposits of known age can be determined and compared with suspected lead sources in the basin for that time. In doing so, it might be possible to construct a history of the changes in lead source contributions to the river over periods of decades to centuries. Second, spatial variations in lead concentration and isotopic data can be combined to assess changes in lead source longitudinally along the river valley. For example, Shepherd et al. (2009) analyzed a variety of geologic materials along an 86.5-km segment of the River Wear in northeast England (Figure 4-5). At Site 1, the 208Pb/206Pb ratio in riverbed sediment was comparable with that in geologic materials found in a mineralized ore body and was therefore interpreted as reflecting the relatively high lead concentrations associated with historical mining operations. The decline in downstream concentrations between Sites 1 and 5 was thought to result from dilution and the deposition of dense, finely milled lead particles in the riverbed. Farther downstream, 208Pb/206Pb ratios continue to decline until reaching Site 9, whereas lead concentrations increased. The decrease in 208Pb/206Pb ratios presumably reflected the influx of mine waste from two tributary valleys where lead ore was derived from a second mineralized zone in the ore body; this second mineralized zone was characterized by a lower 208Pb/206Pb ratio. Shepherd et al. (2009) used changes in lead concentration and 208Pb/206Pb ratios between Sites 10 and 27 to assess the influx and importance of additional sources of
lead to the river. The key point is that combined spatial analysis of lead concentration and lead isotopic composition of the river sediments might provide a more detailed understanding of source contributions of lead to a river than is possible by using lead concentration data alone.
Spatial and Temporal Analyses
If the use of lead isotopes fails to determine the source of lead in river sediments completely, it will be necessary to apply one or more additional methods. Spatial and temporal analyses have been effective in determining lead sources qualitatively in many river basins and are described below.
Spatial Analysis
One of the most extensively used methods for determining sources of lead in rivers is examination of the spatial distribution of lead concentrations or elemental ratios, such as Pb/Zn, in alluvial deposits. The initial interpretation is that an abrupt downstream increase in lead concentration or the ratio of lead to another element will correspond geographically to a predominant source. There are several advantages to the approach, including that it can be applied to both riverbed and floodplain deposits, it can be completed relatively rapidly by using samples collected during a single sampling campaign, it relies on standard analytic procedures for the analysis of total lead concentrations, and the data are relatively easy to interpret.
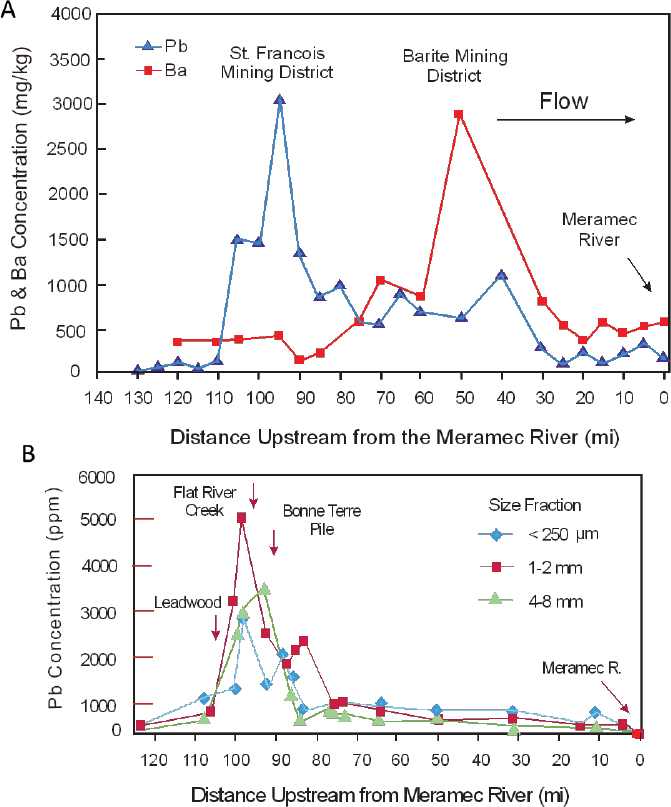
An example was provided in Chapter 3 (Figure 3-3) for lead, zinc, and antimony concentrations along the Rio Chilco–Rio Tupiza drainage system of southern Bolivia (Villarroel et al. 2006). In the United States, Integral (2014) and Pavlowsky et al. (2010) used spatial variations in lead concentrations in channel sediments along the Big River to assess lead sources. Figure 4-6A shows that lead concentrations increase abruptly downstream of river mile 110, presumably in response to the influx of mining-related waste materials as the river passes through the St. Francois County Mining Area. Lead concentrations decrease downstream until reaching the Meramec River. However, the general decrease in concentration is disrupted locally by minor increases in lead concentrations (Figure 4-6A). Integral (2014) argued that those minor increases in lead
concentration downstream of river mile 85 reflect less important additions of lead from other mining operations, including lead from the Barite Mining District.
Although the analysis of spatial patterns in lead concentrations and elemental ratios provides insights into the sources of lead in river sediments in the drainage basin, it has several limitations as described below.
- Multiple sources might exist in a specific area, and it might not be possible to differentiate inputs between multiple overlapping sources by using lead concentration data alone. For example, the input of mining wastes might spatially correspond to areas of mineralized rock where relatively large quantities of natural lead might also enter the channel. Exploration geochemists have applied similar spatial analyses to rivers to identify the location and grade of ore deposits. To help alleviate the problem, downstream variations in other elements or in lead ratios (such as Pb/Zn) might be used to gain additional insights into the source of the lead if the concentrations of such elements differ between the overlapping sources. Figure 4-6A, for example, shows that barium concentrations increase substantially downstream of river mile 85; a trend that Integral (2014) argues is related to the influx of barite from mining operations in the Barite Mining District. Alternatively, the analysis of downstream trends in elemental concentration might focus on a specific sediment size fraction, as shown in Figure 4-6B, which is associated with a known lead source, such as tailings.
- The resolution of the downstream trends in lead concentrations reflects the number of samples that are collected along the river and the location from which the samples were obtained. Differences in sampling frequencies and locations can yield differing results. For example, Figure 4-6 shows the downstream trends in lead concentrations developed by Intergral (2014) (Figure 4-6A) and Pavlowsky et al. (2010) (Figure 4-6B). Differences in the trends in lead concentration are related primarily to differences in the sampling scheme and approach used, which lead to differences in interpretation of the primary source of lead to the Big River.
- Abrupt downstream variations in lead concentrations might reflect erosional and depositional patterns along the river rather than a location of lead influx. Stream segments that are characterized, for example, by relatively wide valleys, low gradients, and gentle flows are often areas of sediment-associated lead deposition. That deposition can lead to zones of high lead concentrations; thus, abrupt increases in lead concentration are not always a perfect indicator of a lead source.
- The method cannot be used to determine the quantity of lead inputs into a system from a specific source. Rather, it provides a means of assessing the relative importance of lead inputs from a given source.
Temporal Analysis
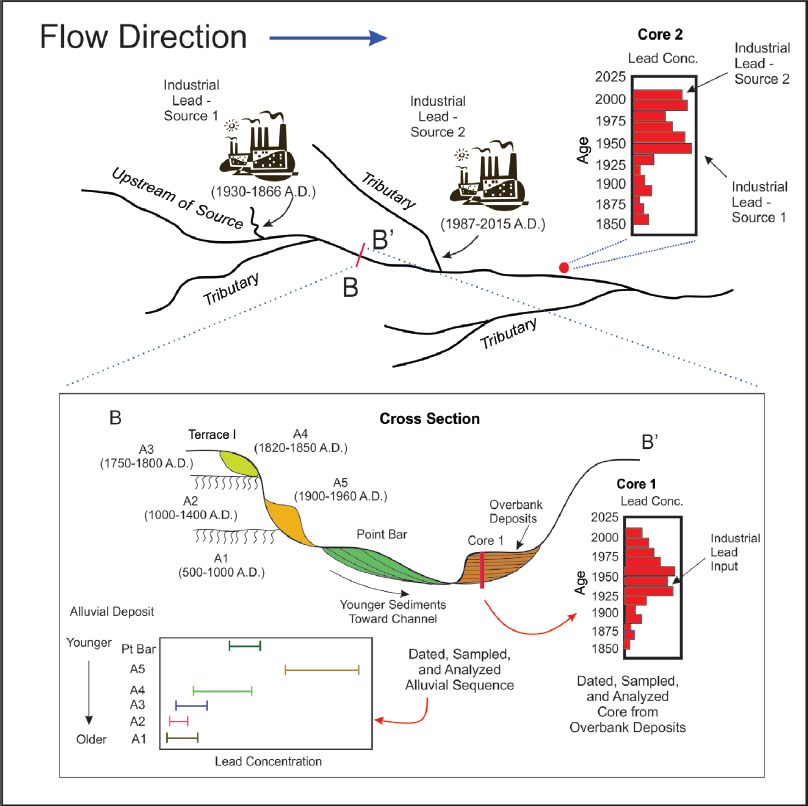
Floodplains and terraces are composed of sediments that are deposited directly by the river adjacent to the channel (Figure 3-4). In contrast with upland areas, anthropogenic lead in floodplains and terraces often extends to depths measured in meters. The spatial distribution of sediment-associated lead in these alluvial deposits reflects the depositional processes responsible for floodplain formation and the sources and concentrations of lead in the river at the time of deposition. The floodplain and terrace deposits therefore contain a historical record of lead inputs (Macklin et al. 1999, 2006). Once deciphered, this stratigraphic record can be compared with documented variations in lead-related human activities to help to determine the potential role of anthropogenic lead sources in contaminating the river environment. The lead-related human activities should be described in terms of their time of operation, spatial extent and location, quantity of potential lead inputs into the river, and the geochemical and physical character of the contaminated sediments (Heim and Schwarzbauer 2013), all of which are to be collected as part of the site characterization described above.
The methods, assumptions, and uncertainties inherent in developing a chronology of trace-metal inputs (including lead) into a river are outlined in Macklin and Klimek (1992), Hudson-Edwards et al. (1997), Tobin et al. (2000), Miller and Orbock Miller (2007), Pease et al. (2007), Du Laing et al. (2009), Bábek et al. (2011), Matys Grygar et al. (2011, 2012, 2013), and Ferrand et al. (2012). In general, it involves documenting the physical characteristics of the river deposits, sampling and analyzing the deposits for lead and other substances, and applying absolute dating methods to estimate the age of the deposits in calendar years. Absolute dating methods include a wide array of techniques, such as measurement of radioactive isotopes (such as 137Cs, 210Pb, and 14C), optically stimulated luminescence, dendrochronology, and identification of historical artifacts; see Stokes and Walling (2003) for a more detailed discussion of these and other dating methods.
The most detailed assessment of lead and trace-metal attribution is based on the analysis of overbank deposits. Overbank floodplain deposits—also referred to as vertical accretion deposits—are produced by the deposition of suspended sediments from floodwaters that inundate the floodplain surface (Figure 3-4). The process results in layers of younger materials that bury older sediments; thus, the sediment age varies systematically with depth. Because deposition occurs only during floods, there are temporal gaps in the depositional record, and the relationship between depth and unit age is not perfect. Nonetheless, such depositional sequences often result in the construction of a relatively high-resolution history of lead loadings into the river.
The distance of a specific stream segment from a source and the number of potential upstream lead sources vary along the river valley (Figure 4-7). Therefore, it is important to develop a chronology of lead inputs into the river at multiple locations. Sites should be selected to ensure that all potential lead sources will be reflected in the constructed chronology and to minimize the number of locations needed to differentiate inputs between all potential lead sources. The historical record should also extend back hundreds to a few thousand years, if possible, to ensure that natural variations in lead loadings (those characteristic of the basin before substantial human activities) are adequately characterized.
A potential disadvantage of the approach is that multiple human activities that have the potential to increase lead influx in a river can overlap temporally. For example, early mining operations in Southeast Missouri during the late 1800s and early 1900s correlate temporally with regional land-use change, which allowed large quantities of eroded upland soil to enter the drainage network. Increases in sediment-associated lead during that time might therefore have been associated with both mining operations and regional erosion of mineralized soil that contained natural lead. In some cases, it might be possible to separate the significance of lead from such diffuse sources from that of point sources by examining spatial trends in the data.
The method also assumes that lead in the sedimentary deposits is immobile, an assumption that is typically met (Hudson-Edwards et al. 2001). Provided that the migration of lead after deposition is negligible, the resolution of the record depends on the degree to which the sediments can be dated; when the ages of the sediments are poorly constrained, only general statements about lead sources and contributions can be made. Other causes of uncertainty are associated with spatial differences in the deposition of overbank sediments across the floodplain, variations in concentration related to flow conditions at the time of sediment deposition, and variations in the grain-size distribution and organic matter content of the sediment
(both of which influence lead concentrations). In some areas, such as sites located downwind of smelters and tailings piles, the atmospheric deposition of lead might also complicate vertical trends in lead concentrations. Various methods have been developed to reduce those uncertainties, such as analyzing multiple floodplain cores at a site and normalizing metal concentrations on the basis of a specific grain size, organic matter content, or conservative element3 (Horowitz 1991; Harrison et al. 2003; Bonotto and de Lima 2006; Miller and Orbock Miller 2007).
In addition to analyzing floodplain deposits, the approach has often been applied to sediments in the bed of a reservoir (Von Gunten et al. 1997; Kähkönen et al. 1998; Müller et al. 2000; Audry et al. 2004; Miller and Mackin 2013). The disruption of stream flow and sediment transport by a dam typically results in higher rates of nearly continuous sediment accumulation in a reservoir (Müller et al. 2000; Arnason and Fletcher 2003) and makes it easier to create a high-resolution record of historical changes in sediment and sediment-associated metal loadings into a river. In addition, thicker depositional units reduce the potential influences of the postdeposition migration of lead in the deposits (Callender 2000; Audry et al. 2004). In other cases, other types of sediments might be examined to complement the historical sedimentary record (Box 4-3).
Mineralogic and Morphologic Analyses of Bulk Samples
It is not uncommon for the geologic materials associated with a lead source to contain a unique set of miner-
___________________
3 A conservative element is one that is uniformly distributed throughout the sampled core. If the ratio of another element to a conservative element changes with depth in the core, the change is a signal that the other element is being depleted from or being supplied to the system.
als. Unique minerals or mineral assemblages have been used effectively to determine the origin of sediment from point and nonpoint (diffuse) sources along contaminated and uncontaminated rivers. Two of the most common analyses focus on clay minerals or heavy-mineral assemblages (Jeong and McDowell 2003; Eberl 2004; Gingele and DeDeckker 2005; Benedetti et al. 2006; Chen et al. 2009; Hardy et al. 2010; Bernárdez et al. 2012), although other mineral groups might also be useful depending on the nature of the source.
The effectiveness of the use of clay minerals stems from the fact that they reflect the composition and weathering characteristics of rock types that underlie the basin. Different rock types generally yield different clay minerals on weathering. Of importance to lead attribution in and around Superfund sites, the use of clay minerals has the potential to differentiate between sediments derived from nonmineralized rocks and mineralized or mined rock units. For example, differences in clay mineralogy were used by Jeong and McDowell (2003) to differentiate between mine tailings (enriched in the clay mineral chlorite), Ontonagon River sediments (with low chlorite and high illite), and a Wisconsin red-clay deposit (with low illite and high expandable-clay content) along the south-central coast of Lake Superior.
Heavy minerals, particularly sulfide minerals, have also been used to assess the origin of sediment and sediment-associated trace metals (including lead) in mining districts (Schreck et al. 2005; Bernárdez et al. 2012). Schreck et al. (2005), for example, found that minerals as-
sociated with the Kupferschiefer ores, which outcropped in the Mansfeld mining district of central Germany, were dominated by covellite, chalcopyrite, and sphalerite. The mineral assemblage differed from the waste materials that were associated with metalliferous flue dust and smelter wastes, which were dominated by slag particles; the differences allowed their contributions to the river to be determined. However, mineral assemblages were similar between mining wastes composed of low-grade ore and natural outcrops of the ore deposits. Determining the importance of the latter sources depended on spatial variations in the waste piles in the basin. A potential shortcoming associated with the use of heavy minerals is that weathering of the particles after deposition might alter the mineralogic characteristics of the source sediments.
Other minerals might also be used to assess sediment contributions from mining operations. In Southeast Missouri, mineral analyses showed that chat particles sampled from the Leadwood, Federal, and National tailings piles were composed almost exclusively of dolomite, whereas sediments sampled from selected control sites were composed of greater than 95% chert and feldspar. Pavlowsky et al. (2010) argued that the quantity of dolomite in the Big River was an indicator of the relative contribution of mine tailings to the river.
The structure of fine-grained sediment is often examined to help to identify minerals. In other cases, the shape of such fine-grained sediments might provide direct insights into sediment and sediment-associated lead sources. As noted above, particles emitted from smelters are often spherical; that knowledge combined with an understanding of their composition can determine their source.
The structure of larger particles in rivers is typically the product of their degree of weathering at their source, abrasion during transport, and modifications after deposition. In general, the farther a particle has been moved along the river, the more rounded (smooth) it is. Thus, particle shape provides information on transport distance and the potential source in the basin (de Boer and Crosby 1995; Cardona et al. 2005). In mining areas, the mechanical grinding and crushing processes associated with the production of mill tailings might create particles characterized by a broken, jagged appearance that can differ from that of sediment derived from natural sources in the basin and allow their identification.
Mineralogic and Morphologic Analysis of Particle Separates
Because of the processes involved in the erosion, transport, and deposition of sediment in river systems, it is possible that sand-sized or dense particles will be derived from a source different from that of the light and fine-grained particles, such as silt and clay (see, for example, Miller et al. 2013b). Thus, the identification of lead source by using mineralogic, chemical, or isotopic methods might be complicated by analyzing mixed population of particles that differ in size or density. It might be possible to alleviate the problem by collecting and analyzing separates of the bulk sediment, which are characterized by a narrow range of particle size or density.
Multielement Fingerprinting and Modeling
The use of multielement geochemical fingerprinting techniques has increased in recent years (Franks and Rowan 2000; Collins et al. 2012, 2013; Rowan et al. 2012; Miller et al. 2015). For example, multielement methods have been applied to suspended sediments (Walling and Woodward 1995; Collins et al. 1997, 2001; Massoudieh et al. 2013), riverbed sediments (Collins et al. 2013; Evrard et al. 2013), floodplain deposits (Collins et al. 2012), and sediments in lakes, reservoirs, and wetlands (Miller et al. 2005, 2013b; Pittam et al. 2009; Rowan et al. 2012). The increased use has been promoted by recent advances in analytic techniques, such as inductively coupled plasma–mass spectrometry, that allow the rapid analysis of large numbers of elements at a reduced cost and by the ability to determine the source of contemporary and historic sediments and sediment-associated lead.
Conceptually, it is assumed that as sediments (and their associated lead) are eroded and transported from a source or source area they are combined with sediments from all other sources in the basin to form a mixture. The geochemical composition of the mixture depends on the composition of the source materials and the relative proportions of sediment derived from each source, provided that the physical and chemical characteristics of the sediment are not altered during transport, as might occur by elemental leaching or changes in the sediment grain size. If the compositions of the source materials and the mixture in question are determined (measured), it is possible to estimate the relative proportions that each source contributed to the mixture (Box 4-1).
Single and multielement geochemical fingerprinting in rivers has been used most extensively to determine nonpoint sources of sediment and sediment-associated contaminants in a river, whose sources might be defined by soil type, underlying geologic units, land-use categories, or tributaries (Russell et al. 2001; Douglas et al. 2003; Miller et al. 2005, 2015; Collins et al. 2009). In mining areas, the approach might provide insights into the quantity of lead eroded from mineralized upland soils. Multielement fingerprinting has not often been applied to point sources of contamination, such as a tailings pile, but it has been shown to be effective when it has been applied (Bird et al. 2010a,b).
The application of statistically based mixing models to river sediments has been criticized because the models can generate vastly different results that have similar
levels of uncertainty, particularly when contributions from a source approach 0 or 100 % (Collins et al. 2010; Rowan et al. 2012). Much of the uncertainty is associated with variability inherent in the physical and geochemical characteristics of the source materials and the river sediment. To reduce and quantify uncertainty inherent in the modeling results, recent studies have explored the use of an increasing suite of statistical approaches, including the use of Monte Carlo sampling frameworks and Bayesian methods (Franks and Rowan 2000; Small et al. 2002, 2004; Collins et al. 2010, 2012, 2013; Rowan et al. 2012; Cooper et al. 2014; Nosrati et al. 2014; Miller et al. 2015; Palazón et al. 2015). The analyses have led to a general agreement that current attempts to apportion sediment and sediment-associated metals that use inverse (unmixing) models should include a sensitivity analysis for the input parameters and a detailed assessment of model uncertainty.
Other Source-Associated Tracers
In addition to the methods described above, a number of other characteristics have been used effectively to attribute sediment and sediment-associated metals in river sediments. They include magnetic properties (Yu and Oldfield 1993; Maher et al. 2009; Armstrong et al. 2010; Guzmán et al. 2010), particle color (Croft and Pye 2004; Martinez-Carreras et al. 2010), chemical speciation (Macklin and Dowsett 1989; Shikazono et al. 2012), and isotopic analyses, such as analyses of isotopes of strontium and neodymium (Weldeab et al. 2002; Padoan et al. 2011; Rosenbauer et al. 2013), copper (El Azzi et al. 2013), mercury (Blum and Bergquist 2007; Foucher et al. 2009; Sonke et al. 2010; Mil-Homens et al. 2013; Blum et al. 2014) and zinc (Sivry et al. 2008; Chen et al. 2009). Although the latter isotopes have not been applied extensively to the problem of lead-source attribution, their application holds considerable promise. Elements that have unique isotopic characteristics as a result of smelting, such as cadmium and zinc, might be particularly useful.
Modeling of Upland Erosion, Sediment Yields, and Transport
A wide array of surface-water modeling routines of varied degrees of complexity have been developed and applied to rivers to understand and predict sediment and sediment-associated contaminant-dispersal processes from both point and nonpoint sources. In general, the models are not designed specifically to determine the source of lead in river basins, and their successful application often depends on the availability and quality of topographic, hydrologic, geologic, and other forms of data. Given those constraints, the wide array of models available, and the ability to combine different models for specific purposes, the committee chose to exclude them from its investigative strategy. Nonetheless, such models might provide important insights into the source of sediment-associated lead. For example, in addition to known point sources of lead in and around a Superfund site, diffuse sources of natural lead exist in a river basin. The occurrence of lead in mineralized soils, which are eroded and delivered to a river during runoff events, might be particularly important in Superfund sites associated with mining districts. It might therefore be important to assess the contributions of lead derived from diffuse upland sources by erosion and mobilization processes. That objective might be addressed partly through the application of spatially distributed models that determine the location and magnitude of upland erosion and the amount of sediment that leaves the basin mouth. The distributed models typically partition the drainage basin into small parcels, each characterized by its own conditions, such as soil type, slope, and vegetation. Those data can then be used to assess soil mobilization and delivery to the river from specific parcel types, such as a specific type of soil or bedrock unit.
GROUNDWATER AND SURFACE WATERS
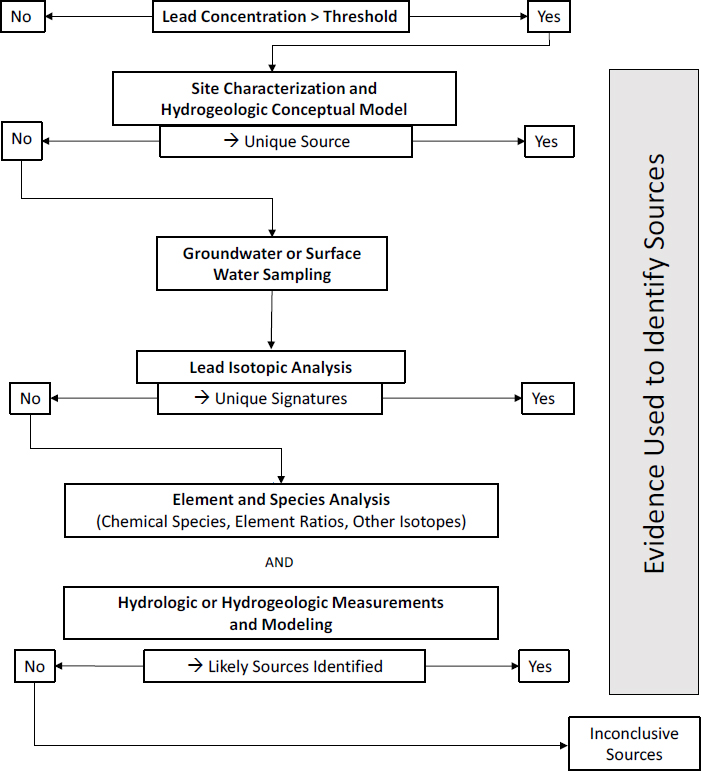
As noted in Chapter 3, concentrations of dissolved lead are typically low in surface waters or groundwater with a pH of 6-8. Thus, concentrations of dissolved lead tend to be relatively low in areas characterized by carbonate rocks (limestone and dolomite), such as in Southeast Missouri, that buffer pH near neutral and limit acid generation associated with the weathering of sulfide minerals, such as pyrite and galena, found in lead-bearing ores. The concentrations can be high in acidic water in other mining areas where limestone and other rock types that can neutralize acid water are generally not present. Here, the committee presents its investigative strategy for groundwater and surface waters (see Figure 4-8) and provides a brief discussion of the methods. The analysis of lead associated with water particles has been discussed in previous sections on soils and sediments.
Site Characterization and Hydrologic Conceptual Model
A first step in determining lead sources in water is to identify and characterize possible sources in and adjacent to the site. For example, if the focus is on river water, potential sources upstream of the site should be identified with potential on-site sources. Once possible sources are located, a conceptual model of the potential dispersal pathways via surface waters and groundwater can be developed by using existing data and standard hydrologic and hydrogeologic approaches. The conceptual model should address questions whose answers might depend on whether lead was found in groundwater from a well,
in surface water from a river, stream, lake, or pond, or in both. Possible questions to consider include, What surface-water bodies are downgradient of the potential lead sources and thus might receive dissolved lead from the source? Do the adjacent streams receive groundwater that originates from the vicinity of the lead source? Is river water derived primarily from surface-water runoff, tributaries, or groundwater, and do these contain lead sources? What geologic materials come into contact with the groundwater and its flow path, and how do they influence flow (for example, limestone vs sandstone)? What are the depth to groundwater and the slope of the groundwater surface, and what are the sources of groundwater recharge? What are the vertical and lateral directions and rates of groundwater flow? Are there important anthropogenic sources of lead, such as lead-containing paint or plumbing, at the site that might dissolve and contribute to lead in water?
Once the conceptual model has been developed, it should be possible to define which sources can contribute lead to groundwater and surface waters. In some cases, such a hydrogeologic assessment might lead to a confident conclusion about the source of the dissolved lead that obviates further study. For example, a suspected lead source might be downgradient of a water-sampling location and be eliminated as a possible source, whereas an obvious upgradient source might be implicated if other lead sources are eliminated.
Groundwater and Surface-Water Sampling
If the conceptual hydrogeologic analysis does not elucidate the source with sufficient confidence, additional investigations are necessary. The sampling of groundwater should reflect the directions, depths, and rates of flow relative to the suspected lead sources and use accepted sampling protocols (see, for example, USGS 2006). Wells selected for sampling should accurately reflect the water chemistry of the hydrogeologic system under investigation and yield samples suitable for water analysis. At a given site, a sufficient number of appropriate wells for sampling or sufficient historical sampling data might not be available. Various factors can influence the integrity of a groundwater sample, and such characteristics as well
type, depth to groundwater, water-sampling depth based on the location of the well screen, pumping rate, water yield, temperature, pressure, and amount of suspended particulate matter (PM) should be considered in the sampling strategy. Wells that are not frequently pumped, such as monitoring wells, should be purged of standing water by pumping before sampling. If dissolved concentrations are of interest, water samples are filtered through a 0.45-μm pore-size membrane and analyzed for lead and other constituents. If total concentrations are desired, unfiltered and acid-digested samples are analyzed. Given the generally low lead concentrations in surface waters and groundwater, all sampling and filtration equipment should be lead-free. All water-sample handling, preservation methods, and storage before analysis should follow standard protocols for trace metals to avoid changes after sampling that might affect measured concentrations (EPA 1996a; Lurry and Kolbe 2000).
In rivers, two approaches to sampling are often used to characterize concentrations and sources of dissolved lead. First, samples are collected at multiple times at fixed locations to characterize temporal variations in lead inputs into the river, particularly during changes in flow or flood events. A monitoring station established along a river has the potential to collect multiple samples as water levels rise and fall during a flood event. Second, one or more synoptic sampling campaigns might be conducted along river segments of interest; synoptic sampling is sampling of a river at many locations at the same time. The samples are obtained from the main channel and all known inflow points—such as tributaries, culverts, irrigation ditches, and urban sewer systems—along the river during a relatively short period of stable flow. River flow (discharge) is also measured so that the amount of lead (mass/time) that passes any point along the river can be calculated from the product of lead concentration (mass/m3) in the river and flow (m3/time). Thus, the analysis provides a snapshot of the variations in elemental concentrations and load along the river that can then be interpreted by using mass balances and inferred or observed lead inputs.
Methods for sampling standing bodies of water, such as lakes and ponds, are generally similar to those used for rivers although strategies for the timing and location of sample collection might differ. The number of samples to collect, the distribution of sampling locations, and the interval between sampling events should be determined on the basis of the size, depth, inflow and outflow points, water residence time, seasonal variations, and study objectives for the particular water body. For example, samples collected at the inflows and outflows of the lake or pond might be compared with representative samples collected from the interior. Sampling as a function of depth in different locations might provide information about water mixing or settling of PM that might affect concentrations. Depth sampling is usually done with a sampling device that is dropped on a weight to a desired depth and opened or closed with an activator. If it is important to assess changes in water chemistry as a function of time, time between samplings of lakes or ponds might be much longer than times between samplings of rivers and will depend on the residence time of the water and possibly on seasonal variations.
Lead Isotopic Analysis
As discussed above, lead isotopic abundances have been used extensively to determine the potential sources of lead in a wide array of media, including water. Specifically, a quantitative comparison is conducted between the isotopic signature of lead in water and possible sources, such as ores, tailings, nonmineralized rocks, and other lead-containing materials in the area. Some lead isotopic data might be available in the published literature. As noted, the method requires that the isotopic signatures of the possible lead sources be sufficiently different to warrant measurement of lead isotopes in water samples. That is, if all the possible sources of lead have the same isotopic signature, an expensive time-intensive isotopic analysis might not be appropriate (Aberg 2001; Komárek et al. 2008; Norrström and Knutsson 2012). If the known isotopic signatures of sources show that isotopic analysis might be useful, an unfiltered water sample could be digested for analysis of lead concentration and isotope ratios to determine the sources of lead in the water. The investigation might include application of a mixing model or other computational analysis. There might not be a unique isotopic signature, but the analytic results could add to the body of evidence and help to eliminate some sources. A total lead measurement includes dissolved lead and lead associated with suspended particles (Graham et al. 2006). A more in-depth analysis of lead isotopes in a filtered water sample (0.45 μm or smaller filter pore size) vs the particles retained on the filter might provide additional source information if the two fractions differ in their lead isotopic signatures.
Element and Species Analysis
If the isotopic signatures of the total, dissolved, or particulate lead do not yield a unique match to one or more of the lead sources or if more data are desired to build confidence in the lead-source attribution, further analysis of constituents in water will need to be undertaken. A first-order assumption is that high lead concentrations will occur at or immediately adjacent to a source and decrease with distance from the source as a result of dilution and a variety of attenuation processes. As noted in the soil and sediment sections, spatial variations in element concentrations and concentration ratios can provide qualitative insights into lead sources by identifying characteristic
associations or ratios. Single-element and multielement analyses and measurement of dissolved chemical species and water-quality characteristics can be undertaken on surface waters and groundwater. For example, total, dissolved, and particulate samples could be analyzed for major, minor, and trace elements; for species, such as sulfate or phosphate; and for total dissolved carbon and organic carbon content. Standard measurements of water include temperature, conductivity, pH, dissolved oxygen, oxidation–reduction potential, and alkalinity (acid-neutralizing capacity) or acidity (base-neutralizing capacity). Stable isotopes of water (18O, 16O, 1H, and 2H), dissolved noble gases (4He, 20Ne, and 40Ar), tritium (3H), sulfur hexafluo-ride (SF6), and radiocarbon (14C) are among the tracers used to determine groundwater sources, flow paths, and water age (Clark and Fritz 1997; Cook and Bohlke 2000). Measurements of elements, species, isotopes, and water characteristics help to bound geochemical and hydrologic models that can predict water sources, flow paths, mixing, and reactions.
The chemical composition of particulate samples can be determined directly as discussed above or calculated on the basis of the difference between the measured total concentration and the dissolved concentration. Element ratios and element associations can then be compared with possible sources. Lead that is associated with particulate organic matter, for example, might point to an organic-rich water-body source. If filtered particles can be recovered, the particle size, structure, mineralogy, and composition could be investigated with SEM, XRD, or other techniques discussed above to provide insight into suspended-particle sources.
Hydrologic and Hydrogeologic Measurements and Modeling
Modeling of River Samples
Analysis of synoptic samples and temporal variations in lead concentrations can help to identify sources along rivers (Kimball 1997; Boughton 2001; Nimick and Cleasby 2001; Wirt et al. 2001; Walton-Day et al. 2005; Kimball et al. 2002, 2005, 2006, 2007, 2010; Church et al. 2007; Kimball and Runkel 2009). In most cases, the samples are obtained from the river channel and all known inflow points—such as tributaries, culverts, irrigation ditches, and urban sewer systems—during relatively short intervals of stable flows, which are also measured. The analysis provides a snapshot of the variations in elemental concentrations and loads along the river that reflect points of substantive elemental (lead) inputs. Instantaneous load is calculated as the product of constituent (lead) concentrations and river discharge at the sample point at the time of collection. Downstream variations in the loads in the river channel are used to determine the importance of each inflow point as a lead source and to determine the attenuation of lead along the river. Sources are identified by using a mass-balance approach that compares the cumulative load within the river channel with the cumulative load that enters the channel from upstream inflows (see, for example, Kimball et al. 2002). In many cases, the approach is combined with the use of elemental tracers, such as lead isotopes, to quantify more fully the contributions of a pollutant from a specific source.
An advantage of the synoptic approach is that the location and importance of unsampled, diffuse sources of lead from groundwater, which are often poorly documented, can be determined. A disadvantage of synoptic sampling is that it is difficult to apply during some periods of high river flow, but methods are being developed to overcome such issues (see, for example, Kimball et al. 2010). In some cases, the data collected during synoptic sampling can be used for in-stream modeling of solute transport, such as that of dissolved lead (Gooseff et al. 2008). An understanding of temporal variations in lead concentration at a site during a flood event can provide insights into the primary sources of contaminants (McDiffett et al. 1989; Evans and Davies 1998; House and Warwick 1998; Lloyd et al. 2016).
Geochemical, Hydrologic, and Hydrogeologic Models
Developing an overall assessment of sources of lead in water at a given site requires integration of multiple lines of evidence from isotopic and chemical measurements with hydrologic or hydrogeologic data. The data are typically analyzed and evaluated by using various statistical, numerical, and modeling approaches of varied complexity. Multivariate statistical methods, such as principal-components analysis or factor analysis, might be used to identify correlations or characteristic patterns among elements or species. Mixing models use differences in chemical or isotopic composition among the potential sources to determine whether the composition of a specific water can be represented by a mixture of the potential water-source compositions. Inverse mass-balance models compare differences in mass composition to deduce whether a specific water was derived from another along a flow path in contact with minerals and gases (Zhu and Anderson 2002). That model approach assumes a chemical steady state (no net change in chemical species) and is based strictly on mass differences and mass transfer between water and the minerals and gases that are assumed to be in contact with it. Mass-balance models also typically ignore hydrodynamic dispersion or diffusion processes that might affect solute concentrations (Zhu and Anderson 2002). Although potentially informative, mass-balance models consider net chemical mass differences and do not produce a unique solution; that is, multiple combinations of water types, minerals, and gases
might fit the water composition in question and depend on the water, mineral, and gas phases assumed in the model.
Models that rely solely on mass differences are distinguished from chemical-speciation models that rely on mass balances and thermodynamics to establish the dominant chemical reactions and chemical species that are present. On the basis of thermodynamic equilibrium constants and kinetic parameter estimates, chemical-speciation models are able to calculate the dominant aqueous species and complexes, the amount of dissolution and precipitation of minerals, the solubility of gases in water, and the extent of surface sorption reactions. Several programs are available and include free packages, such as PHREEQC (Parkhurst and Appelo 1999) and MINTEQA2 (EPA 1999a). Chemical equilibrium calculations, particularly ones that include sorption reactions, can help to elucidate possible chemical reactions that lead to mobilization of lead from sources or how lead might be attenuated along a flow path. For example, streams contaminated by lead, zinc, and cadmium from mine waste runoff and mine-drainage discharge in the Tri-State district in Oklahoma, Missouri, and Kansas were studied with a combination of runoff and discharge measurements by using a conservative tracer, statistical analyses, mass-balance calculations, and equilibrium chemical modeling to evaluate lead sources, metal fluxes, and chemical reactions that mobilize or attenuate lead downstream of waste piles and mine-drainage input (Carroll et al. 1998; Schaider et al. 2014).
A variety of water-quality modeling routines might be applied to rivers, such as the Water Quality Assessment Simulation (Ambrose et al. 1988), which includes methods to model advection, dispersion, and point and diffuse mass loading processes and can be linked to hydrodynamic and sediment transport models to assess flow and particulate transport. For groundwater, hydrologic gradients, pump tests to calculate aquifer storage and characteristics, tracer studies to map flow paths and determine residence time, and geologic and hydraulic information are used to support aquifer and groundwater basin models. A widely used package is MODFLOW, the US Geological Survey three-dimensional finite-difference groundwater model (Harbaugh 2005). Originally developed solely as a groundwater-flow simulation code, MODFLOW has been greatly expanded to include simulation of coupled groundwater and “surface-water systems, solute transport, variable-density flow (including saltwater), aquifer-system compaction and land subsidence, parameter estimation, and groundwater management” (Brovelli and Moreno 2014). Surface-water or groundwater models are increasingly used in conjunction with analysis and modeling of chemical and isotopic data to provide a comprehensive evaluation of contaminant sources, transport, and transformation at a study site (Appelo and Postma 2005; Bedekar et al. 2016).
AIR
Like the investigative approaches presented for soils, sediments, and water, attribution of sources of airborne lead typically requires field assessment, laboratory analysis, and modeling. The laboratory analysis of lead-containing PM that might be collected by air monitors is similar to the laboratory analysis of soils described above. Therefore, the committee focuses here on field assessment and modeling approaches that are used to distinguish sources of airborne lead. The committee’s investigative strategy is a tiered approach illustrated in Figure 4-9; each tier increases in complexity to achieve source attribution. Tier I begins by conducting chemical speciation on archived filter remnants after lead analysis has been conducted, Tier II includes field sampling to develop site-specific source signatures for input into receptor modeling, and Tier III includes more complete ambient and source sampling to provide additional evidence via a combination of source-dispersion and receptor modeling. Although the focus here is on traditional particle analysis, other approaches are possible. As noted above, Prapaipong et al. (2008) used oak trees to determine dispersion of lead emissions from a smelter, and other researchers have used lichen, rainfall, and snowpack sampling to understand spatial gradients of emissions from smelters and other sources (see, for example, Carignan and Gariepy 1995; Simonetti et al. 2000; Spiro et al. 2004; Telmer et al. 2004; Aznar et al. 2008; Graney et al. 2012; Graney and Landis 2013; Sherman et al. 2015).
Two types of models are used in the committee’s investigative strategy. First, receptor models start with pollutant concentrations measured at receptors and attempt to identify and quantify contributions of different sources on the basis of source characteristics (Watson et al. 2016). Specifically, receptor models analyze patterns of chemical composition, particle size, temporal variability, and spatial gradients that are related to specific sources and take into consideration measurement uncertainty and source variability to determine contributions from specific sources that reach the receptors (Watson et al. 2002; Watson and Chow 2005, 2013, 2015; Watson et al. 2016). Second, source-dispersion models start with emission estimates related to potential sources—such as surface soils, road dust, and mine tailings—and calculate meteorologic transport of pollutants to receptors. The receptors are usually neighborhood, urban, or regional air-quality monitoring sites that are established to determine compliance with ambient air-quality standards. The US Environmental Protection Agency (EPA) maintains the Web site Support Center for Regulatory Atmospheric Modeling (SCRAM), which describes EPA-accepted air-quality models in great detail and provides free downloads for the accepted model software.4
___________________
Tier I: Analyze Composition of Archived Filters
The first tier in the committee’s investigative strategy involves further analysis of samples that have already been collected from air-monitoring stations. As noted in Chapter 3, high airborne lead concentrations in total suspended particles (TSP) at monitoring sites of the Missouri Department of Natural Resources (see Figure 3-6) indicate the presence of lead-contaminated resuspended dust in the Southeast Missouri Lead Mining District (MO DNR 2016). Archived filter-sample remnants for which 24-hour TSP lead concentrations exceeded 0.15 μg/m3 could be submitted for PM composition analyses, which could include multielement analysis (Watson et al. 1999) with XRF, inductively coupled plasma–atomic emission spectroscopy, and inductively coupled plasma–mass spectrometry; analysis of anions (such as chloride, nitrate, and sulfate) and cations (such as water-soluble sodium, magnesium, potassium, calcium, and ammonium) with ion chromatography; and thermal-optical carbon analysis of organic and elemental-carbon and thermal-carbon fractions. Lead concentrations should be compared with those reported by the Missouri Department of Natural Resources for quality assurance. Material balances of measured species should be performed to ensure that all major PM components are measured, that is, that the sum of the measured chemical species adequately explains the gravimetric mass (Chow et al. 2015).
High ambient PM lead concentrations might have various sources. Watson et al. (2016) recommend that additional indicators be obtained from archived filter-sample remnants to reduce source apportionment uncertainties. Such indicators include commonly found mineral-related elements (aluminum, silicon, potassium, calcium, and iron), elements related to mining activities (such as lead, zinc, copper, arsenic, and silver), stable lead isotopes (204Pb, 206Pb, 207Pb, and 208Pb) that can be analyzed after hot-block acid digestion procedures (with a combination of nitric, hydrochloride, and hydrofluoric acids) established by EPA for lead analysis with inductively coupled plasma-mass spectrometry (EPA 1996b, 1999b). Contaminated fugitive dust might be the main contributor to atmospheric lead concentrations, and additional measurements—such as the carbonate carbon and rare earth elements (such as cerium, dysprosium, erbium, europium, gadolinium, holmium, lanthanum, lutetium, neodymium, praseodymium, samarium, terbium, thulium, and ytterbium) and particle structure—can also be considered.
Descriptive data analysis can be conducted to understand PM composition and to examine temporal and spatial variations. Comparison of similarities and differences between lead-isotope ratios and abundances of rare earth elements among sites serves as the starting point for examining site-to-site and month-to-month variations (Felix et al. 2015). Because chemical composition varies with geologic source as illustrated in Figure 4-10, that information can be used in source attribution to define source categories. Receptor modeling of about 200–300 samples with the Effective Variance Positive Matrix Factorization and UNMIX solutions to the chemical-mass balance equations can be conducted to identify and quantify major source contributions of PM and lead (EPA 2016).
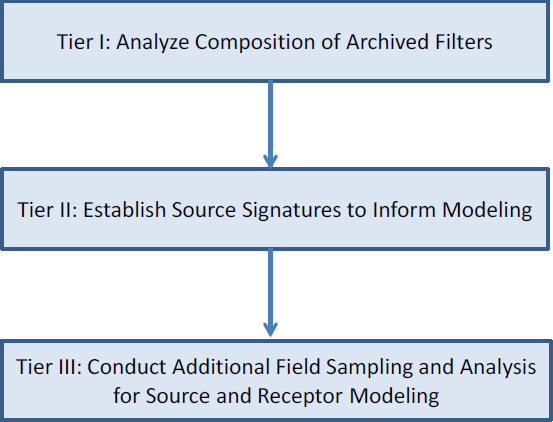
Preliminary source contributions can serve as a guide in determining the type of source signatures that need to be acquired in Tier II and the extent of field sampling needed for Tier III.
Tier II: Establish Source Signatures for Receptor Model Input
Figure 4-11 illustrates the strategy for establishing source signatures. Source signatures of major source types—such as vehicle exhaust, aircraft fuel combustion, lead smelting, and glass manufacturing—from the EPA source-profile library (SPECIATE; EPA 2017a) can be compiled with the available local source signatures to evaluate the adequacy of signatures for receptor modeling. Geological materials are often a major source of PM mass, so site-specific source signatures probably need to be defined. Surface-soil and paved-road dust surrounding sampling sites that are not in compliance with air-quality standards for lead should be collected, processed, and resuspended in a laboratory resuspension chamber into TSP, PM10, PM2.5, and PM1 size fractions by using multiple filter substrates that will accommodate the same chemical speciation as that of archived filter-sample remnants for Tier I identification. For residential areas that have high lead concentrations, soil samples and house lead-based paint chips should also be collected, processed, and resuspended. Additional bulk-material sampling and analysis of storage piles, tailings piles, and mine haul roads that are near sites that have high lead concentration should also be considered. The source signatures identified here in Tier II serve as model input and verification for receptor modeling and as the basis of development of a speciated PM emission inventory for source-dispersion modeling (EPA 2016).
Tier III: Conduct Additional Field Sampling and Analysis for Source-Dispersion and Receptor Modeling
EPA’s National Emissions Inventory (EPA 2017b) shows that lead from mining constitutes only 10% of Missouri emissions, but processes or activities that result in resuspended dust emissions are not well represented in current emission factors (EPA 1998). Because source-dispersion models use emission factors to translate emissions into ambient concentrations, the modeling results can be variable. To improve the accuracy of receptor and source--
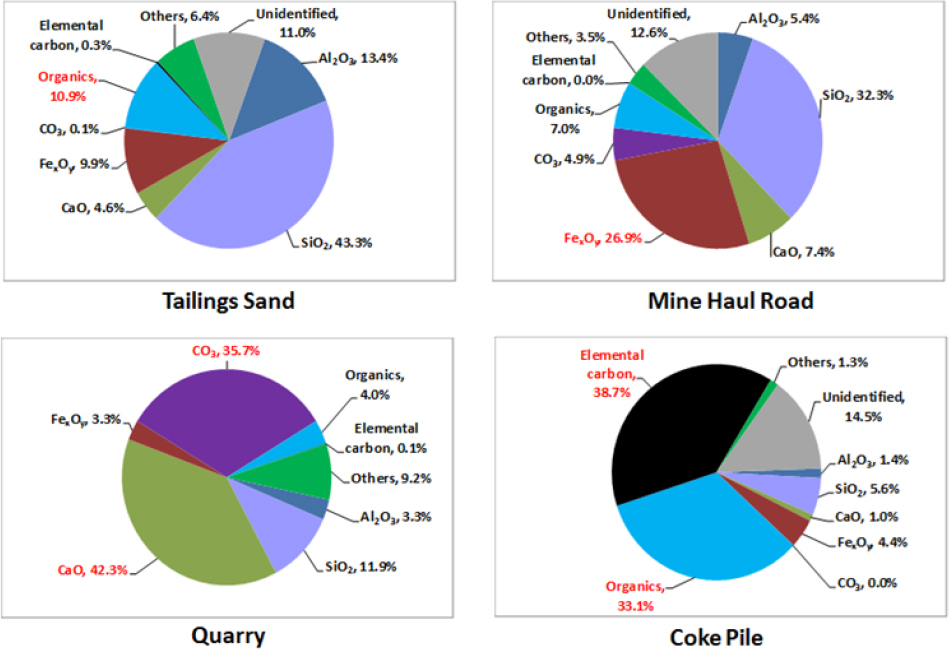
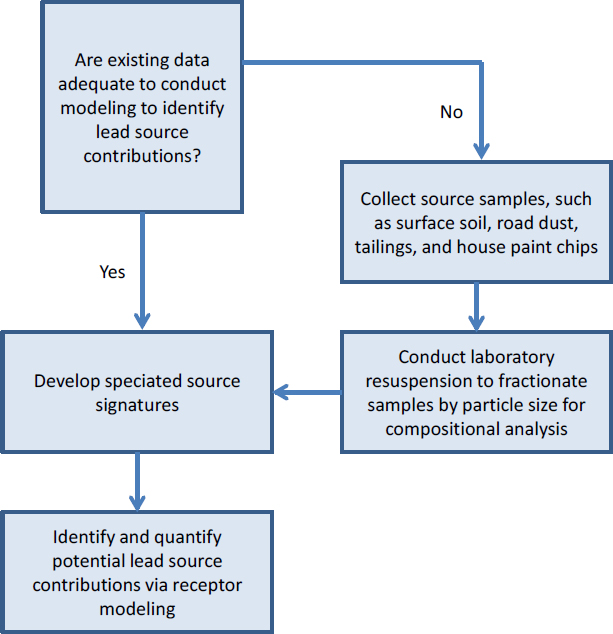
dispersion models, field sampling is necessary to provide site-specific inputs. Thus, the committee’s strategy for Tier III that is illustrated in Figure 4-12 includes varied field sampling to improve model inputs. For source-dispersion models, sensors that are mounted on-board a moving vehicle can be used to determine mechanical suspension and surface loadings (Kuhns et al. 2001), and portable wind tunnels can be used to measure wind erosion potential in various PM size fractions (Etyemezian et al. 2007; Sweeney et al. 2008; Wang et al. 2015). Source-dispersion and receptor models can both benefit from use of site-specific meteorologic variables, such as wind speed and direction, temperature, and relative humidity. Particle-size distributions illustrated in Figure 4-13 vary by geologic source categories and can be obtained by using an optical particle counter that provides particle-number concentrations in several size ranges, such as 0.01–10 µm. Finally, particle mass and chemical composition of lead and other geologically abundant elements (such as aluminum, silicon, potassium, calcium, iron, zinc, and titanium) can be determined by using a cascade impactor.
As indicated in Figure 4-12, model reconciliation can be used to identify uncertainties and limitations in receptor and source-dispersion modeling results and to improve model input datasets (EPA 2007). The goal here is to establish how well the receptor concentrations predicted by a source-dispersion model agree with the measurements collected at receptors and to assess whether receptor-model output compares favorably with the available information on source locations and signatures. Another approach is to use source-dispersion models in a parametric mode to predict chemical patterns at a receptor and then to use receptor models to identify the patterns that best reproduce the ambient measurements (Watson and Chow 2014).
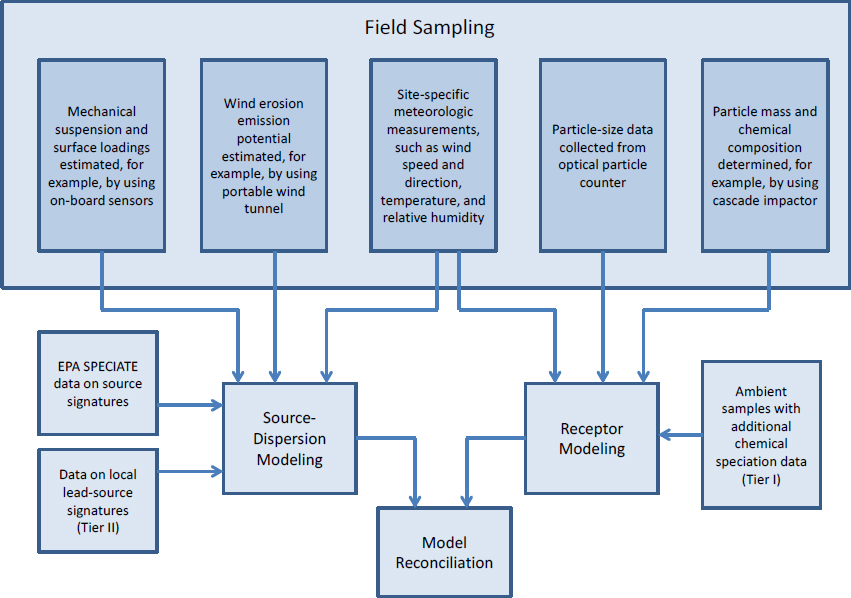
REFERENCES
Åberg, G.E. 2001. Tracing pollution and its sources with isotopes. Water Air Soil Pollut. 130(1):1577-1582.
Abu-Zeid, M., A. Baghdady, and H. El-Etr. 2001.Textural attributes, mineralogy and provenance of sand dune fields in the greater Al Ain area, United Arab Emirates. J. Arid. Environ. 48(4):475-499.
Aebischer, S., C. Cloquet, J. Carignan, C. Maurice, and R. Pientiz. 2015. Disruption of the geochemical metal cycle during mining: Multiple isotope studies of lake sediments from Schefferville, subarctic Québec. Chem. Geol. 412:167-178.
Ambrose, R.B., T.A. Wool, J.P. Connolly, and R.W. Schanz. 1988. WASP4, A Hydrodynamic and Water Quality Model - Model Theory, User’s Manual, and Programmer’s Guide. EPA/600/3-87-039. Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC.
Anderson, S.P., W.E. Dietrich, and G.H. Brimhall. 2002. Weathering profiles, mass balance analysis, and rates of solute loss: Linkages between weathering and erosion in a small, steep catchment. Geol. Soc. Am. Bul. 114(9):1143-1158.
Appelo, C.A.J., and D. Postma. 2005. Geochemistry, Groundwater and Pollution, 2nd Ed. Leiden, The Netherlands: A.A. Balkema.
Armstrong, A., B. Maher, and J. Quinton. 2010. Enhancing the magnetism of soil: The answer to soil tracing? P. 2805 in EGU General Assembly 2010, May 2-7, 2010, Vienna, Austria.
Arnason, J.G., and B.A. Fletcher. 2003. A 40+ year record of Cd, Hg, Pb, and U deposition in sediments of Patroon Reservoir, Albany County, NY, USA. Environ. Pollut. 123(3):383-391.
Arribas, J., S. Critelli, E. Le Pera, and A. Tortosa. 2000. Composition of modem stream sand derived from a mixture of sedimentary and metamorphic source rocks (Henares River, Central Spain). Sediment Geol. 133:27-48.
Audry, S., J. Schäfer, G. Blanc, and J.M. Jouanneau. 2004. Fifty-year sedimentary record of heavy metal pollution (Cd, Zn, Cu, Pb) in the Lot River reservoirs (France). Environ. Pollut. 132(3):413-426.
Ayrault, S., M. Roy-Barman, M.F. Le Cloarec, C. Rianti Priadi, P. Bonté, and C. Göpel. 2012. Lead contamination of the Seine River, France: Geochemical implications of a historical perspective. Chemosphere 87(8):902-910.
Aznar, J.C., M. Richer-Lafleche, and D. Cluis. 2008. Metal contamination in the lichen Alectoria sarmentosa near the copper smelter of Murdochville, Quebec. Environ. Pollut 156(1):76-81.
Bábek, O., M. Famĕra, K. Hilscherová, J. Kalvoda, P. Dobrovolný, J. Sedláček, J. Machát, and I. Holoubek. 2011. Geochemical traces of flood layers in the fluvial sedimentary archive: Implications for contamination history analyses. Catena 87(2):281-290.
Balcaen, L., L. Moens, and F. Vanhaecke. 2010. Determination of isotope ratios of metals and metalloids by means of ICP mass spectrometry for provenanceing purposes. Spectrochim. Acta Part B At. Spectrosc. 65(9):769-786.
Basu, A., and E. Molinaroli. 1991. Reliability and application of detrital opaque Fe-Ti oxide minerals in provenance determination. Pp. 55-65 in Developments in Sedimentary Provenance Study. Special Publication Vol. 57. London: Geological Society.
Bedekar, V., E.D. Morway, C.D. Langevin, and M. Tonkin. 2016. MT3D-USGS Version 1: A U.S. Geological Survey Release of MT3DMS Updated with New and Expanded Transport Capabilities for Use with MODFLOW. U.S. Geological Survey Techniques and Methods 6-A53. Reston, VA: U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/tm/06/a53/tm06a53.pdf [accessed September 21, 2017].
Benedetti, M., M. Raber, M. Smith, and L. Leonard. 2006. Mineralogical indicators of alluvial sediment sources in the Cape Fear River basin, North Carolina. Phys. Geogr. 27(3):258-281.
Bernárdez, P., R. Prego, S. Giralt, J. Esteve, M. Caetano, S. Parra, and G. Francés. 2012. Geochemical and mineralogical characterization of surficial sediments from the Northern Rias: Implications for sediment provenance and impact of the source rocks. Mar. Geol. 291-294:63-72.
Bindler, R. 2006. Mired in the past-looking to the future: Geochemistry of peat and the analysis of past environmental changes. Glob. Planet. Chang. 53(4):209-221.
Bird, G. 2011. Provenancing anthropogenic Pb within the fluvial environment: Developments and challenges in the use of Pb isotopes. Environ. Int. 37(4):802-819.
Bird, G., P.A. Brewer, M.G. Macklin, M. Nikolova, T. Kotsev, M. Mollov, and C. Swain. 2010a. Quantifying sediment-associated metal dispersal using Pb isotopes: Application of binary and multivariate mixing models at the catchment-scale. Environ. Pollut. 158(6):2158-2169.
Bird, G., P.A. Brewer, M.G. Macklin, M. Nikolova, T. Kotsev, M. Mollov, and C. Swain. 2010b. Pb isotope evidence for contaminant-metal dispersal in an international river system: The lower Danube catchment, Eastern Europe. Appl. Geochem. 25(7):1070-1084.
Blum, J.D., and B.A. Bergquist. 2007. Reporting of variations in the natural isotopic composition of mercury. Anal. Bioanal. Chem. 388(2):353-359.
Blum, J.D., L.S. Sherman, and M.W. Johnson. 2014. Mercury isotopes in earth and environmental science. Annu. Rev. Earth Planet. Sci. 42:249-269.
Bonotto, D., and J. de Lima. 2006. 210Pb-derived chronology in sediment cores evidencing the anthropogenic occupation history at Corumbatai river basin, Brazil. Environ. Geol. 50:595-611.
Boughton, G.K. 2001. Metal Loading in Soda Butte Creek Upstream of Yellowstone National Park, Montana and Wyoming: A Retrospective Analysis of Previous Re-
search; and Quantification of Metal Loading, August 1999. Water Resources Investigation Report 01-4170. U. S. Geological Survey [online]. Available: https://pubs.usgs.gov/wri/wri014170/pdf/wri004170.pdf [accessed June 23, 2017].
Brantley, S.L., and M. Lebedeva. 2011. Learning to read the chemistry of regolith to understand the Critical Zone. Annu. Rev. Earth Planet. Sci. 39:387-416.
Brimhall, G., and W.E. Dietrich. 1987. Constitutive mass balance relations between chemical composition, volume, density, porosity, and strain in metasomatic hydro-chemical systems: results on weathering and pedogenesis. Geochim. Cosmochim. Acta 51(3):567-587.
Brovelli, M., and R. Moreno. 2014. Free and Open Souce Software and Web Services Specializing in the Water Resources Domain. Webminar, September 2014 [online]. Available: http://www.asprs.org/a/webinarseries/Brovelli_Moreno_ASPRS_Webminar_Part1_9_26_2014.pdf [accessed June 23, 2017].
Burgess, M. 2016. National Academy of Sciences Sources of Lead Contamination at or Near Superfund Sites. Presentation at the First Meeting on Sources of Lead Contamination at or near Superfund Sites, November 21-22, 2016, Washington, DC.
Burt, R., M.A. Wilson, T.J. Keck, B.D. Dougherty, D.E. Strom, and J.A. Lindahl. 2003. Trace element speciation in selected smelter-contaminated soils in Anaconda and Deer Lodge Valley, Montana, USA. Adv. Environ. Res. 8(1):51-67.
Buseck, P.R., and M. Posfai. 1999. Airborne minerals and related aerosol particles: Effects on climate and the environment. Proc. Nat. Acad. Sci. USA 96(7):3372-3379.
Cabala, J., and J. Teper. 2007. Metalliferous constituents of rhizospere soils contaminated by Zn-Pb mining in southern Poland. Water Air Soil Pollut. 178(1):351-362.
Callender, E. 2000. Geochemical effects of rapid sedimentation in aquatic systems: Minimal diagenesis and the preservation of historical metal signatures. J. Paleolimnol. 23(3):243-260.
Callender, E. 2005. Heavy metals in the environment—Historical trends. Pp. 67-105 in Environmental Geochemistry, Treatise on Geochemistry Vol. 9, B. Sherwood Lollar, ed. Amsterdam: Elsevier.
Cardona, J.P.M., J.M.G. Mas, A.S. Bellon, S. DomiinguezBella, and J.M. Lopez. 2005. Surface textures of heavy mineral grains: A new contribution to provenance studies. Sediment Geol. 174(3-4):223-235.
Carignan, J., and C. Gariepy. 1995. Isotopic composition of epiphytic lichens as a tracer of the sources of atmospheric lead emissions in southern Quebec, Canada. Geochim Cosmochim. Acta 59(21):4427-4433.
Carroll, S.A., P.A. O’Day, and M. Piechowski. 1998. Rock-water interactions controlling zinc, cadmium, and lead concentrations in surface waters and sediments, U.S. Tri-State Mining District. 2. Geochemical interpretation. Environ. Sci. Technol. 32(7):956-965.
Chen, J.B., J. Gaillardet, P. Louvat, and S. Huon. 2009. Zn isotopes in the suspended load of the Seine River, France: Isotopic variations and source determination. Geochim. Cosmochim. Acta 73(14):4060-4076.
Cheng, H., and Y. Hu. 2010. Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: A review. Environ. Pollut. 158(5):1134-1146.
Chiaradia, M., B.E. Chenhall, A.M. Depers, B.L. Gulson, and B.G. Jones. 1997. Identification of historical lead sources in roof dusts and recent lake sediments from an industrialized area: Indications from lead isotopes. Sci. Total. Environ. 205(2-3):107-128.
Chow, J.C., J.G. Watson, J.E. Houck, L.C. Pritchett, C.F. Rogers, C.A. Frazier, R.T. Egami, and B.M. Ball. 1994. A laboratory resuspension chamber to measure fugitive dust size distributions and chemical compositions. Atmos. Environ. 28(21):3463-3481.
Chow, J.C., D.H. Lowenthal, L.W.A. Chen, X. Wang, and J.G. Watson. 2015. Mass reconstruction methods for PM2.5: A review. Air Qual. Atmos. Health 8(3):243-263.
Church, S.E., D.M. Unruh, D.L. Fey, and T.C. Sole. 2004. Trace Element and Lead Isotopes in Streambed Sediment in Streams Affected by Historical Mining. Chapter D8 of Integrated Investigations of Environmental Effects of Historical Mining in the Basin and Boulder Mining Districts, Boulder River Watershed, Jefferson County, Montana, D.A. Nimick, S.E. Church, and S.E. Finger, eds. U.S. Geological Survey Professional Paper 1652. Washington, DC: U.S. Government Printing Office [online]. Available: https://pubs.usgs.gov/pp/2004/1652/pdf/ChapD8.pdf [accessed June 23, 2017].
Church, S.E., P.B. von Guerard, and S.E. Finger. 2007. Integrated Investigations of Environmental Effects of Historical Mining in the Animas River Watershed, San Juan County, Colorado. Professional Paper 1651. U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/pp/1651/ [accessed June 23, 2017].
Citeau, L. I. Lamy, F. van Oort, and F. Elsass. 2003. Colloidal facilitated transfer of metals in soils under different land use. Colloid. Surf. A 217(1-3):11-19.
Clark, H.F., D.J. Brabander, and R.M. Erdil. 2006. Sources, sinks, and exposure pathways of lead in urban garden soil. J. Environ. Qual. 35(6):2066-2074.
Clark, I., and P. Fritz. 1997. Environmental Isotopes in Hydrogeology. Boca Raton: Lewis.
Cloquet, C., J. Carignan, and G. Libourel. 2006. Isotopic composition of Zn and Pb atmospheric depositions in an urban/periurban area of northeastern France. Environ. Sci. Technol. 40(21): 6594-6600.
Cloy, J.M., J.G. Farmer, M.C. Graham, A.B. MacKenzie, and G.T. Cook. 2008. Historical records of atmospheric Pb deposition in four Scottish ombrotrophic peat bogs:
An isotopic comparison with other records from Western Europe and Greenland. Global Biogeochem. Cycles 2(2):GB2016.
Collins, A., D. Walling, and G. Leeks. 1997. Source type ascription for fluvial suspended sediment based on a quantitative composite fingerprinting technique. Catena 29(1):1-27.
Collins, A.L., D.E. Walling, H.M. Sichingabula, and G.J. Leeks. 2001. Suspended sediment source fingerprinting in a small tropical catchment and some management implications. Appl. Geogr. 21(4):387-412.
Collins, A., D. Walling, L. Webb, and P. King. 2009. Particulate organic carbon sources and delivery to river channels in the Somerset levels ECSFDI priority catchment, southwest UK. Int. J. River Basin Manag. 7(3):277-291.
Collins, A., D. Walling, L. Webb, and P. King. 2010. Apportioning catchment scale sediment sources using a modified composite fingerprinting technique incorporating property weightings and prior information. Geoderma 155(3-4):249-261.
Collins, A., Y. Zhang, D. McChesney, D. Waling, S. Haley, and P. Smith. 2012. Sediment source tracing in a lowland agricultural catchment in southern England using a modified procedure combining statistical analysis and numerical modelling. Sci. Total Environ. 414:301-317.
Collins, A., Y. Zhang, D. Duethmann, D. Walling, and K. Black. 2013. Using a novel tracing-tracking framework to source fine-grained sediment loss to watercourses at sub-catchment scale. Hydrol. Process. 27(6):959-974.
Cook, P.G., and J.K. Bohlke. 2000. Determining timescales for groundwater flow and solute transport. Pp. 1-30 in Environmental Tracers in Subsurface Hydrology, P.G. Cook, and A. Herczeg, eds. Boston: Kluwer.
Cooper, R.J., T. Krueger, K.M. Hiscock, B.G. Rawlins, 2014. Sensitivity of fluvial sediment source apportionment to mixing model assumptions: A Bayesian model comparison. Water Resour. Res. 50(11):9031–9047.
Croft, D.J., and K. Pye. 2004. Colour theory and the evaluation of an instrumental method of measurement using geological samples for forensic applications. Pp. 49-62 in Forensic Geosciences: Principles, Techniques, and Applications, K. Pye, and D.J. Croft, eds. Special Publication 232. London: The Geological Society of London.
Csavina, J., M.P. Taylor, O. Félix, K.P. Rine, A.E. Sáez, and E.A. Betterton. 2014. Size-resolved dust and aerosol contaminants associated with copper and lead smelting emissions: Implications for emissions management and human health. Sci. Total. Environ. 493:750-756.
Damman, A.W.H. 1986. Hydrology, development, and biogeochemistry of ombrogenous peat bogs with special reference to nutrient relocation in a western Newfoundland bog. Can. J. Bot. 64(2):384-394.
D’Amore, J.J., S.R. Al-Abed, K.G. Scheckel, and J.A. Ryan. 2005. Methods for speciation of metals in soils: A review. J. Environ. Qual. 34(5):1707-1745.
de Boer, D.H., and G. Crosby. 1995. Evaluating the potential of SEM/EDS analysis for fingerprinting suspended sediment derived from 2 contrasting topsoils. Catena 24(4):243-258.
D’Haen, K., G. Verstraeten, and P. Degryse. 2012. Fingerprinting historic fluvial sediment fluxes. Prog. Phys. Geogr. 36(2):154-186.
Douglas, G., M. Palmer, and G. Caitcheon. 2003. The provenance of sediments in Moreton Bay, Australia: A synthesis of major, trace element and Sr-Nd-Pb isotopic geochemistry, modeling and landscape analysis. Hydrobiologia 494(1):145-152.
Drivas, P., T. Bowers, and R. Yamartino. 2011. Soil mixing depth after atmospheric deposition. I. Model development and validation. Atmos. Environ. 45(25):4133-4140.
Du Laing, G., J. Rinklebe, B. Vandencasteele, E. Meers, and F.M.G. Tack. 2009. Trace metal behavior in estuarine and riverine floodplain sediments: A review. Sci. Total Environ. 407(13):3972-3985.
Eberl, D.D. 2004. Quantitative mineralogy of the Yukon River system: Changes with reach and season, and determining sediment provenance. Am. Miner. 89(11-12):1784-1794.
Eckel, W.P., M.B. Rabinowitz, and G.D. Foster. 2002. Investigation of unrecognized former secondary lead smelting sites: Confirmation by historical sources and elemental ratios in soil. Environ. Pollut. 117(2):273-279.
El Azzi, D., J. Viers, M. Guiresse, A. Probst, D. Aubert, J. Caparros, F. Charles, K. Guizien, and J.L. Probst. 2013. Origin and fate of copper in a small Mediterranean vineyard catchment: New insights from combined chemical extraction and δ65Cu isotopic composition. Sci. Total Environ. 463-464:91-101.
EPA (U.S. Environmental Protection Agency) 1996a. Method 1669: Sampling Ambient Water for Trace Metals at EPA Water Quality Criteria Levels. U.S. Environmental Protection Agency, Office of Water, Washington, DC [online]. Available: https://www.epa.gov/sites/production/files/2015-10/documents/method_1669_1996.pdf [accessed August 18th, 2017].
EPA. 1996b. EPA Method 3050B: Acid Digestion of Sediments, Sludges, and Soils; Revision 2. U.S. Environmental Protection Agency, Washington D.C. [online]. Available: https://www.epa.gov/homeland-security-research/epa-method-3050b-acid-digestion-sediments-sludges-and-soils [accessed August 18th, 2017].
EPA. 1998. Locating and Estimating Air Emissions from Sources of Lead and Lead Compounds. EPA-454/R-98-006. Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, Research Triangle Park, NC [online]. Available: https://www3.epa.gov/ttnchie1/le/lead.pdf [accessed July 3, 2017].
EPA. 1999a. MINTEQA2/PRODEFA2, A Geochemical Assessment Model for Environmental Systems: User Manual Supplement for Version 4.0 [online]. Available:
https://www.epa.gov/sites/production/files/documents/SUPPLE1.PDF [accessed July 3, 2017].
EPA. 1999b. Compendium of Methods for the Determination of Inorganic Compounds in Ambient Air. Compendium Method IO-3.5: Determination of Metals in Ambient Particulate Matter Using Inductively Coupled Plasma/ Mass Spectrometry (ICP/MS). EPA/625/R-96/010a. US Environmental Protection Agency, Cincinnati, OH. June 1999 [online]. Available: https://www.epa.gov/sites/production/files/2015-07/documents/epa-io-3.5.pdf [accessed June 21, 2017].
EPA. 2007. Short Sheet: Estimating the Soil Lead Concentration Term for the Integrated Exposure Uptake Biokinetic (IEUBK) Model. OSWER 9200.1-78. U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response, U.S. Environmental Protection Agency, Washington, DC. September 2007 [online]. Available: https://semspub.epa.gov/work/HQ/175344.pdf [accessed June 21, 2017].
EPA. 2016. Receptor Modeling [online]. Available: https://www3.epa.gov/ttn/scram/receptorindex.htm [accessed July 3, 2017].
EPA. 2017a. Air Emission Modeling. SPECIATE Version 4.5 Through 4.0 [online]. Available: https://www.epa.gov/air-emissions-modeling/speciate-version-45-through-40 [accessed July 3, 2017].
EPA. 2017b. Air Emissions Inventories [online]. Available: https://www.epa.gov/air-emissions-inventories [accessed July 3, 2017].
Ettler, V. 2016. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 64:56-74.
Ettler, V., A. Vaněk, M. Mihaljevič, and P. Bezdička. 2005. Contrasting lead speciation in forest and tilled soils heavily polluted by lead metallurgy. Chemosphere 58(10):1449-1459.
Ettler, V., Z. Johan, B. Kříbek, F. Veselovský, M. Mihaljevič, A Vaněk, V. Penížek, V. Majer, O. Sracek, B. Mapani, F. Kamona, and I. Nyambe. 2016. Composition and fate of mine- and smelter-derived particles in soils of humid subtropical and hot semi-arid areas. Sci. Total Environ. 563-564:329-339.
Etyemezian, V.R., G. Nikolich, S.M. Ahonen, M.L. Pitchford, R. Sweeney, J. Purcell, J.A. Gillies, and H.D. Kuhns. 2007. The Portable In Situ Wind Erosion Laboratory (PI-SWERL): A new method to measure PM10 windblown dust properties and potential for emissions. Atmos. Environ. 41(18):3789-3796.
Evans, C., and T.D. Davies. 1998. Causes of concentration/discharge hysteresis and its potential as a tool for analysis of episode hydrochemistry. Water Resour. Res. 34(1):129-137.
Evrard, O., J. Poulenard, J. Nemery, S. Ayrault, N. Gratiot, C. Duvert, C. Prat, I. Lefevre, P. Bonte, and M. Esteves. 2013. Tracing sediment sources in a tropical highland catchment of central Mexico by using conventional and alternative fingerprinting methods. Hydrol. Process. 27(6):911-922.
Félix, O.I., J. Csavina, J. Field, K.P. Rine, E. Sáez, and E.A. Betterton. 2015. Use of lead isotopes to identify sources of metal and metalloid contaminants in atmospheric aerosol from mining operations. Chemosphere 122:219-226.
Ferrand, E., F. Eyrolle, O. Radakovitch, M. Provansal, S. Dufour, C. Vella, G. Raccasi, and R. Gurriaran. 2012. Historical levels of heavy metals and artificial radionuclides reconstructed from overbank sediment records in lower Rhône River (South-East France). Geochim. Cosmochim. Acta 82:163-182.
Foster, I.D.L., J.A. Less, A.R. Jones, A.S. Chapman, S.E. Turner, and R. Hodgkinson. 2002. The possible role of agricultural land drains in sediment delivery to a small reservoir, Worcestershire UK: A multiparameter tracing study. Pp. 443-442 in Structure, Function and Management Implications of Fluvial Sedimentary Systems, F.J. Dyer, M.C. Thoms, and J.M. Olley, eds. Wallingford, UK: IAHS Publications [online]. Available: https://www.researchgate.net/profile/Ian_Foster2/publication/268355050_The_possible_role_of_agricultural_land_drains_in_sediment_delivery_to_a_small_reservoir_Worcestershire_UK_A_multiparameter_fingerprint_study/links/5682ebec08ae19758391bd9d.pdf [accessed June 23, 2017].
Franks, S.W., and J.S. Rowan. 2000. Multi-parameter fingerprinting of sediment sources: Uncertainty estimation and tracer selection. Pp. 1067-1074 in Computational Methods in Water Resources, L.R. Bentley, C.A. Brebbia, W.G. Gray, G.F. Pinder, and J.F. Sykes, eds. Rotterdam: Balkema.
Foucher, D., N. Ogrinc, and H. Hintelmann. 2009. Tracing mercury contamination from the Idrija mining region (Slovenia) to the Gulf of Trieste using Hg isotope ratio measurement. Environ. Sci. Technol. 43(1):33-39.
Gallego, J.R., E. Rodreguez-Valdes, N. Esquinas, A. Fernandez-Brana, and E. Afif. 2016. Insights into a 20-ha multicontaminated brownfield megasite: An environmental forensics approach. Sci. Total Environ. 563-564:683-692.
Gingele, F.X., and P. De Deckker. 2005. Clay mineral, geochemical and Sr-Nd isotopic fingerprinting of sediments in the Murray-Darling fluvial system, southeast Australia. Aust. J. Earth Sci. 52(6):965-974.
Gooseff, M.N., S.M. Wondzell, and K.E. Bencala. 2008. Solute transport along stream and river networks. Pp. 395-418 in River Confluences, Tributaries and the Fluvial Network, S. Rice, A. Roy, and B. Rhodes, eds. New York: John Wiley & Sons.
Graham, M.C., S.L. Vinogradoff, A.J. Chipchase, S.M. Dunn, J.R. Bacon, and J.G. Farmer. 2006. Using size fractionation and Pb isotopes to study Pb transport in the waters of an organic-rich upland catchment. Environ. Sci. Technol. 40(4):1250-1256.
Graney, J.R., and M.S. Landis. 2013. Coupling meteorology, metal concentrations and Pb isotopes for source attribution in archived precipitation samples. Sci. Total Environ. 448:141-150.
Graney, J.R., A.N. Halliday, G.J. Keeler, J.O. Nriagu, J.A. Robbins, and S.A. Norton. 1995. Isotopic record of lead pollution in lake sediments from the northeastern United States. Geochem. Cosmochim. Acta 59(9):1715-1728.
Graney, J.R., M.S. Landis, and S. Krupa. 2012. Coupling lead isotopes and element concentrations in epiphytic lichens to track sources of air emissions in the Athabasca Oil Sands Region. Pp. 343-372 in Alberta Oil Sands: Energy, Industry and the Environment, K. Percy ed., Amsterdam: Elsevier.
Gulson, B.L., K.G. Tiller, K.J. Mizon, and R.H. Merry. 1981. Use of lead isotope in soils to identify the source of lead contamination near Adelaide, South Australia. Environ. Sci. Technol. 15(6):691-696.
Guzmán, G., V. Barrn, and J.A. Gómez. 2010. Evaluation of magnetic iron oxides as sediment tracers in water erosion experiments. Catena 82:126-133.
Guzmán, G., J.N. Quinton, M.A. Nearing, L. Mabit, and J.A. Gómez. 2013. Sediment tracers in water erosion studies: Current approaches and challenges. J. Soil Sediment. 13(4):816-833.
Harbaugh, A.W. 2005. MODFLOW-2005, the U.S. Geological Survey Modular Ground-Water Model: The GroundWater Flow Process. U.S. Geological Survey Techniques and Methods 6-A16 [online]. Available: https://pubs.usgs.gov/tm/2005/tm6A16/PDF.htm [accessed July 3, 2017].
Hardy, F., L. Bariteau, S. Lorrain, I. Theriault, G. Gagnon, D. Messier, D. Messier, and J.F. Rougene. 2010. Geochemical tracing and spatial evolution of the sediment bed load of the Romaine River, Quebec, Canada. Catena 81(1):66-76.
Harrison, J., H. Heijnis, and G. Caprarelli. 2003. Historical pollution variability form abandoned mine sites, Greater Blue Mountains World Heritage Area, New South Wales, Australia. Environ. Geol. 43(6):680-687.
Hayes, S.M., S.A. White, T.L. Thompson, R.M. Maier, and J. Chorover. 2009. Changes in lead and zinc lability during weathering-induced acidification of desert mine tailings: Coupling chemical and micro-scale analyses. Appl. Geochem. 42(12):2234-2245.
Hayes, S.M., S.M. Webb, J.R. Bargar, P.A. O’Day, R.M. Maier, and J. Chorover. 2012. Geochemical weathering increases lead bioaccessibility in semi-arid mine tailings. Environ. Sci. Technol. 46(11):5834-5841.
Heim, S., and J. Schwarzbauer. 2013. Pollution history revealed by sedimentary records: A review. Environ. Chem. Lett. 11(3):255-270.
Hopper, J.R., H.B. Ross, W.T. Sturges, and L.A. Barrie. 1991. Regional source discrimination of atmospheric aerosols in Europe using the isotopic composition of lead. Tellus 43(1):45-60.
Horowitz, A. 1991. A Primer on Sediment-Trace Element Chemistry, 2nd Ed. U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/of/1991/0076/report.pdf [accessed June 28, 2017].
House, W.A., and M.S. Warwick. 1998. Hysteresis of the solute concentration/discharge relationship in rivers during storms. Water Res. 32(8):2279-2290.
Hudson-Edwards, K.A., M. Macklin, and M. Taylor. 1997. Historic metal mining input to Tees River sediment. Sci. Total Environ. 194-195:437-445.
Hudson-Edwards, K.A., M.G. Macklin, J.R. Miller, and P.J. Lechler. 2001. Sources, distribution and storage of heavy metals in the Rio Pilcomayo, Bolivia. J. Geochem. Explor. 72:229-250.
Hunt, A., D.L. Johnson, J.M. Watt, and I. Thornton. 1992. Characterizing the sources of particulate lead in house dust by automated scanning electron microscopy. Environ. Sci. Technol. 26(8):1513-1523.
Integral Consulting Inc. 2014. Sources of Lead to Residential Soils of St. Francois County. Report prepared for The Doe Run Company, May 15, 2014.
Jeong, J., and S.D. McDowell. 2003. Characterization and transport of contaminated sediments in the southern central Lake Superior. J. Miner. Mater. Charact. Eng. 2(2):111-135.
Johnson, C.E., T.G. Siccama, C.T. Driscoll, G.E. Likens, and R.E. Moeller. 1995. Changes in lead biogeochemistry in response to decreasing atmospheric inputs. Ecol. Appl. 5(3):813-822.
Kähkönen, M.A., K.P. Suominen, P.K. Manninen, and M.S. Salkinoja-Salonen. 1998. 100 years of sediment accumulation history of organic halogens and heavy metals in recipient and nonrecipient lakes of pulping industry in Finland. Environ. Sci. Technol. 32(12):1741-1746.
Keinonen, M. 1992. The isotopic composition of lead in man and the environment in Finland 1966–1987—Isotope ratios of lead as indicators of pollutant source. Sci. Total Environ. 113(3):251-268.
Kimball, B.A. 1997. Use of Tracer Injections and Synoptic Sampling to Measure Metal Loading From Acid Mine Drainage. U.S. Geological Survey Fact Sheet FS-245-296 [online]. Available: https://pubs.usgs.gov/fs/1996/0245/report.pdf [accessed June 28, 2017].
Kimball, B.A., and R.L. Runkel. 2009. Spatially detailed quantification of metal loading for decision making: Metal mass loading to American Fork and Mary Ellen Gulch, Utah. Mine Water Environ. 28(4):274-290.
Kimball, B.A., R.L. Runkel, K. Walton-Day, and K.E. Bencala. 2002. Assessment of metal loads in watersheds affected by acid mine drainage by using tracer injection and synoptic sampling: Cement Creek, Colorado, USA. Appl. Geochem. 17(9):1183-1207.
Kimball, B.A., R.L. Runkel, T.E. Cleasby, and D.A Nimick. 2005. Quantification of metal loading by tracer injection and synoptic sampling, 1997–1998. Pp. 191-261in Integrated Investigations of Environmental Effects of Historical Mining in the Basin and Boulder Mining Districts, Boulder River Watershed, Jefferson County, Montana, D.A. Nimick, S.E. Church, and S.E. Finger, eds. Professional Paper 1652. U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/pp/2004/1652/pdf/ChapD6.pdf [accessed June 28, 2017].
Kimball, B.A., D.K. Nordstrom, R.L. Runkel, K.R. Vincent, and P.L. Verplanck. 2006. Questa Baseline and Pre-mining Groundwater Quality Investigation. 23. Quantification of Mass Loading from Mined and Unmined Areas along the Red River, New Mexico. Scientific Investigations Report 2006-5004. Salt Lake City, UT:U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/sir/2006/5004/PDF/SIR2006_5004.pdf [accessed June 28, 2017].
Kimball, B.A., K. Walton-Day, and R.L. Runkel. 2007. Quantification of metal loading by tracer injection and synoptic sampling, 1996–2000. Pp. 417-495 in Integrated Investigations of Environmental Effects of Historical Mining in the Animas River Watershed, San Juan County, Colorado, S.E. Church, P.B. von Guerard, and S.E. Finger, eds. Professional Paper 1651. U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/pp/1651/downloads/Vol1_combinedChapters/vol1_chapE9.pdf [accessed June 28, 2017].
Kimball, B.A., R.L. Runkel, and K. Walton-Day. 2010. An approach to quantify sources, seasonal change, and biogeochemical processes affecting metal loading in streams: Facilitating decisions for remediation of mine drainage. Appl. Geochem. 25(5):728-740.
Komárek, M., V. Ettler, V. Chrastny, and M. Milhaljevic. 2008. Lead isotopes in environmental sciences: A review. Environ. Int. 34(4):562-572.
Kuhns, H., V. Etyemezian, D. Landwehr, C. MacDougall, M. Pitchford, and M. Green. 2001. Testing re-entrained aerosol kinetic emissions from roads (TRAKER): A new approach to infer silt loadings on roadways. Atmos. Environ. 35(16):2815- 2825.
Kylander, M.E., D.J. Weiss, and B. Kober. 2009. Two high-resolution terrestrial records of atmospheric Pb deposition from New Brunswick, Canada, and Loch Laxford, Scotland. Sci. Total Environ. 407(5):1644-1657.
Linton, R.W., D.F.S. Natusch, R.L. Solomon, and C.A. Evans. 1980. Physicochemical characterization of lead in urban dusts. A microanalytical approach to lead tracing. Environ. Sci. Technol. 14(2):159-164.
Lloyd, C.E.M., J.E. Freer, P.J. Johnes, and A.L. Collins. 2016. Using hysteresis analysis of high-resolution water quality monitoring data, includng uncertainty, to infer controls on nutrient and sediment transfer in catchments. Sci. Total Environ. 543(Pt. A):388-404.
Lurry, D.L., and C.M. Kolbe. 2000. Interagency Field Manual for the Collection of Water-Quality Data. U.S. Geological Survey, Open-File Report 00-213 [online]. Available: https://pubs.usgs.gov/of/2000/ofr00-213/index.html [accessed September 21, 2017].
Ma, L., J. Konter, E.M. Herndon, L. Jin, G. Steinhoefel, D. Sanchez, and S.L. Brantley. 2014. Quantifying an early signature of the industrial revolution from lead concentrations and isotopes in soils of Pennsylvania, US. Anthropocene 7:16-29.
MacKinnon, G., A.B. MacKenzie, G.T. Cook, I.D. Pulford, H.J. Duncan, and E.M. Scott. 2011. Spatial and temporal variations in Pb concentrations and isotopic composition in road dust, farmland soil and vegetation in proximity to roads since cessation of use of leaded petrol in the UK. Sci. Total Environ. 409 (23):5010-5019.
Macklin, M.G., and R.B. Dowsett. 1989. The chemical and physical speciation of trace metals in fine grained overbank flood sediments in the Tyne Basin, north-east England. Catena 16(2):135-151.
Macklin, M.G., and K. Klimek. 1992. Dispersal, storage and transformation of metal-contaminated alluvium in the upper Vistula basin, southwest Poland. Appl. Geogr. 12(1):7-30.
Macklin, M.G., K.A. Hudson-Edwards, H.E. Jamieson, P. Brewer, T.J. Coulthard, A.J. Howard, and V.H. Renenda. 1999. Physical stability and rehabilitation of sustainable aquatic and riparian ecosystems in the Rio Guadiamar, Spain, following the Aznalcóllar mine tailings dam failure. Pp. 271-278 in Mine, Water and Environment, International Mine Water Association Congress, Seville, Spain [online]. Available: http://www.mwen.info/docs/imwa_1999/IMWA1999_Macklin_271.pdf [accessed June 28, 2017].
Macklin, M.G., P.A. Brewer, K.A. Hudson-Edwards, G. Bird, T.J. Coulthard, I.A. Dennis, P.J. Lechler, J.R. Miller, and J.N. Turner. 2006. A geomorphological approach to the management of rivers contaminated by metal-mining. Geomorphology 79(3-4):423-447.
Maher, B.A., S.J. Watkins, G. Brunskill, J. Alexander, and C.R. Fielding. 2009. Sediment provenance in a tropical fluvial and marine context by magnetic ‘fingerprinting’ of transportable sand fractions. Sedimentology 56(3):841-861.
Marcantonio, F., G.C. Flowers, and N. Templin. 2000. Lead contamination in a wetland watershed: Isotopes as fingerprints of pollution. Environ. Geol. 39(9):1070-1076.
Martínez-Carreras, N., T. Udelhoven, A. Krein, F. Gallart, J. Iffly, J. Ziebel, L. Hoffmann, L. Pfister, and D. Walling. 2010. The use of sediment colour measured by diffuse reflectance spectrometry to determine sediment sources: Application to the Attert River catchment (Luxembourg). J. Hydrol. 382(1-4):49-63.
Massoudieh, A., A. Gellis, W. Banks, and M. Wieczorek. 2013. Suspended sediment source apportionment in Chesapeake Bay watershed using Bayesian chemical mass balance receptor modeling. Hydrol. Process. 27(24):3363-3374.
Mattielli, N., J. Rimetz, J. Petit, E. Perdrix, K. Deboudt, P. Flament, and D. Weis. 2006. Zn-Cu isotopic study and speciation of airborne metal particles within a 5-km zone of a lead/zinc smelter. Geochim. Cosmochim. Acta 70(18):A401-A401 [online]. Available: https://goldschmidtabstracts.info/2006/1372.pdf [accessed June 28, 2017].
Mattielli, N., J.C. Petit, K. Deboudt, P. Flament, E. Perdrix, A. Taillez, J. Rimetz-Planchon, and D. Weis. 2009. Zn isotope study of atmospheric emissions and dry depositions within a 5 km radius of a Pb-Zn refinery. Atmos. Environ. 43(6):1265-1272.
Matys Grygar, T., T. Novakoa, M. Mihaljevic, L. Strnad, and L. Koptikova. 2011. Surprisingly small increase in the sedimentation rate in the floodplain of Marova river in the Straznice area, Czech Republic, in the last 100 years. Catena 86:461-471.
Matys Grygar, T., J. Sedlacek, O. Bábek, T. Novakoa, L. Strnad, and M. Mihaljevic. 2012. Regional contamination of Moravia (south-eastern Czech Republic): Temporal shift of Pb and Zn loading in fluvial sediments. Water Air Soil Pollut. 223(2):739-753.
Matys Grygar, T.M., T. Nováková, O. Bábek, J. Elznicová, and N. Vadinová. 2013. Robust assessment of moderate heavy metal contamination levels in floodplain sediments: A cast study on the Jizera River, Czech Republic. Sci. Total Environ. 452-453:233-245.
McDiffett, W.F., A.W. Beidler, T.F. Dominick, and K.D. McCrea. 1989. Nutrient concentration-stream discharge relationships during storm events in a first order stream. Hydrobiologia 179(2):97-102.
Mihaljevič, M., V. Ettler, O. Šebek, and V. Chrastný. 2006. Lead isotopic signatures of wine and vineyard soils - Tracers of lead origin. J. Geochem. Explor. 88(1):130-133.
Mil-Homens, M., J. Blum, J. Canário, M. Caetano, A.M. Cos-ta, S.M. Lebreiro, M. Trancoso, T. Richter, H. de Stigter, M. Johnson, and V. Branco. 2013. Tracing anthropogenic Hg and Pb input using stable Hg and Pb isotope ratios in sediments of the central Portuguese Margin. Chem. Geol. 336(16):62-71.
Miller, E.K., and A.J. Friedland. 1994. Lead migration in forest soils: Response to changing atmospheric inputs. Environ. Sci. Technol. 28(4):662–669.
Miller, J.R., and G. Mackin. 2013. Concentrations, sources, and potential ecological impacts of selected trace metals on aquatic biota within the Little Tennessee River Basin, North Carolina. Air Water Soil Pollut. 244:1613-1637.
Miller, J.R., and S.M. Orbock Miller. 2007. Contaminated Rivers: A Geomorphological-Geochemical Approach to Site Assessment and Remediation. Berlin: Springer.
Miller, J.R., M. Lord, S. Yurkovich, G. Mackin, and L. Kolenbrander. 2005. Historical trends in sedimentation rates and sediment provenance, Fairfield Lake, western North Carolina. JAWRA 41(5):1053-1075.
Miller, J.R., P.J. Lechler, G. Mackin, D. Germanoski, and L.F. Villarroel. 2007. Evaluation of particle dispersal from mining and milling operations using lead isotopic fingerprinting techniques, Rio Pilcomayo Basin, Bolivia. Sci. Total Environ. 384(1-3):355-373.
Miller, J.R., M. Lord, L.F. Villarroel, D. Germanoski, and J. Chambers. 2012. Structural organization of process zones in upland watersheds of Central Nevada and its influence on basin connectivity, dynamics, and wet meadow complexes. Geomorphology 139-140:384-402.
Miller, J.R., L. Wilson, P.J. Lechler, and L. Villarroel. 2013a. Utilization of geochemical tracers including Pb and Sb isotopes to determine trace metal sources within the Rio Loa Basin, Chile. Proceedings of the EnivonMetal Isotopes EMI2013, August 18-23, 2013, Ascona, Switzerland.
Miller, J.R., G. Mackin, P. Lechler, M. Lord, and S. Lorentz. 2013b. Influence of basin connectivity on sediment source, transport, and storage within the Mkabela Basin, South Africa. Hydrol. Earth Syst. Sci. 17:761-781.
Miller, J.R., G. Mackin, and S.M. Orbock Miller. 2015. Application of Geochemical Tracers to Fluvial Sediment. SpringerBriefs in Earth Sciences. Springer.
MO DNR (Missouri Department of Natural Resources). 2016. Air Pollution Control Program: 2016 Monitoring Network Plan [online]. Available: https://dnr.mo.gov/env/apcp/docs/2016monitoringnetworkplan.pdf [accessed June 28, 2017].
Morton, A.C. 1985. Heavy minerals in provenance studies. Pp. 249-277 in Provenance of Arenites, G.G. Zuffa, eds. Dordrecht, The Netherlands: Springer.
Müller, J., H. Ruppert, Y. Muramatsu, and J. Schneider. 2000. Reservoir sediments—A witness of mining and industrial development (Malter Reservoir, eastern Erzgebirge, Germany). Environ. Geol. 39(12):1341-1351.
NEPC (National Environmental Protection Council). 1998. National Environment Protection (Ambient Air Quality) Measure – Revised Impact Statement. National Environment Protection Council Service Corporation, Adelaide, Australia [online]. Available: http://www.ephc.gov.au/sites/default/files/AAQ_ImpStat__AAQ_NEPM_Revised_Impact_Statement_Final_199806.pdf [accessed June 28, 2017].
Nezat, C.A., S.A. Hatch, and T. Uecker. 2017. Heavy metal content in urban residential and park soils: A case study in Spokane, Washington, U.S.A. Appl. Geochem. 78:186-193.
Nimick, D.A., and T.E. Cleasby. 2001. Quantification of Metal Loads by Tracer Injection and Synoptic Sampling in Daisy Creek and the Stillwater River, Park County, Montana. Water-Resources Investigation Report 2000--
4261. U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/wri/wri004261/ [accessed June 28, 2017].
Norrström, A., and G. Knutsson. 2012. Stable lead isotopes as tracers of groundwater pollution in the water supply for a small village. Environ. Earth Sci. 67(4):1085-1095.
Norton, S.A., E.R. Perry, T.A. Haines, and A.C. Dieffenbacher-Krall. 2004. Paleolimnological assessment of Grove and Plow Shop Ponds, Ayer, Massachusetts, USA – a superfund site. J. Environ. Monit. 6(5):457-465.
Nosrati, K., G. Govers, B.X. Semmens, and E.J. Ward. 2014. A mixing model to incorporate uncertainty in sediment fingerprinting. Geoderma. 217–218:173-180.
Olson, K.W., and R.K. Skogerboe. 1975. Identification of soil lead compounds from automotive sources. Environ. Sci. Technol. 9(3):227-230.
Ostergren, J.D., G.E. Brown, G.A. Parks, and T.N. Tingle. 1999. Quantitative speciation of lead in selected mine tailings from Leadville, CO. Environ. Sci. Technol. 33(10):1627-1636.
Padoan, M., E. Garzanti, Y. Harlavan, and I.M. Villa. 2011. Tracing Nile sediment sources by Sr and Nd isotope signatures (Uganda, Ethiopia, Sudan). Geochim. Cosmochim. Acta 75(12):3627-3644.
Palazón, L., B. Latorre, L. Gaspar, W.H. Blake, H.G. Smith, and A. Navas. 2015. Comparing catchment sediment fingerprinting procedures using an auto-evaluation approach with virtual sample mixtures. Sci. Total Environ. 532:456-66.
Parkhurst, D.L., and C.A.J. Appelo. 1999. User’s Guide to PHREEQC (Version 2). A Computer Program for Speciation, Batch-reaction, OneDimensional Transport, and Inverse Geochemical Calculations. US Geological Survey Water-Resources Investigations Report 99-4259. U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/wri/1999/4259/report.pdf [accessed August 10, 2017].
Pavlowsky, R.T., M.R. Owen, and D.J. Martin. 2010. Big River Mining Sediment Assessment Project: Distribution, Geochemistry, and Storage of Mining Sediment in Channel and Floodplain Deposits of the Big River System in St. Francois, Washington, and Jefferson Counties, Missouri, Final Report. OEWRI EDR-1-002. Ozarks Environmental and Water Resources Institute, Missouri State University, Springfield, MO.
Pease, P., S. Leece, P. Gares, and C. Rigsby. 2007. Heavy metal concentrations in sediment deposits on the Tary River floodplain following Hurricane Floyd. Environ. Geol. 51:1003-1111.
Petit, D., J.P. Mennesier, and L. Lamberts. 1984. Stable Pb isotopes in pond sediments as tracer of past and present atmospheric Pb pollution in Belgium. Atmos. Environ. 18(6):1189-1193.
Pittam, N., I. Foster, and T. Mighall. 2009. An integrated lake-catchment approach for determining sediment source changes at Aqualate Mere, Central England. J. Paleolimnol. 42(2):215-232.
Prapaipong, P., C.W. Enssle, J.D. Morris, E.L. Shock, and R.E. Lindvall. 2008. Rapid transport of anthropogenic lead through soils in southeast Missouri. Appl. Geochem. 23(8):2156-2170.
Pye, K. 2004. Forensic examination of rocks, sediments, soils, and dust using scanning electron microscopy and X-ray chemical microanalysis. Pp. 103-122 in Forensic Geosciences: Principles, Techniques, and Applications, K. Pye, and D.J. Croft, eds. Special Publication 232. London: Geological Society.
Rabinowitz, M.B., and G.W. Wetherill. 1972. Identifying sources of lead contamination by stable isotope techniques. Environ. Sci. Technol. 6(8):705-709.
Rehkämper, M., R. Wombacker, T.J. Horner, and Z. Xue. 2011. Natural and anthropogenic Cd isotope variations. Pp. 124-154 in Handbook of Environmental Isotope Geochemistry, M. Baskaran, ed. Heidelberg: Springer.
Rosenbauer, R.J., A.C. Foxgrover, J.R. Hein, and P.W. Swarzenski. 2013. A Sr-Nd isotopic study of sandsized sediment provenance and transport for the San Francisco Bay coastal system. Mar. Geol. 345:143-153.
Rowan, J.S., S. Black, and S.W. Franks. 2012. Sediment fingerprinting as an environmental forensics tool explaining cyanobacteria blooms in lakes. Appl. Geogr. 32(2):832-843.
Russell, M., D. Walling, and R. Hodgkinson. 2001. Suspended sediment sources in two small lowland agricultural catchments in the UK. J. Hydrol. 252(1-4):1-24.
Schaider, L.A., D.B. Senn, E.R. Estes, D.J. Brabander, and J.P. Shine. 2014. Sources and fates of heavy metals in a mining-impacted stream: Temporal variability and the role of iron oxides. Sci. Total Environ. 490:456-466.
Scheckel, K.G., J.A. Ryan, D. Allen, and N.V. Lescano. 2005. Determining speciation of Pb in phosphate-amended soils: Method limitations. Sci. Total Environ. 350(1-3):261-272.
Schreck, P., M. Schubert, K. Freyer, H.C. Treutler, and H. Weiss. 2005. Multi-metal contaminated stream sediment in the Mansfeld mining district: Metal provenance and source detection. Geochem. Expl. Environ. Anal. 5:51-57.
Shepherd, T.J., S.R.N. Chenery, V. Pashley, R.A. Lord, L.E. Ander, N. Breward, S.F. Hobbs, M. Horstwood, B.A. Klinck, and F. Worral. 2009. Regional lead isotope study of a polluted river catchment: River Wear, Northern England, UK. Sci. Total Environ. 407(17):4882-4893.
Sherman, L.S., J.D. Blum, J.T. Dvonch, L.E. Gratz, and M.S. Landis. 2015. The use of Pb, Sr, and Hg isotopes in Great Lakes precipitation as a tool for pollution source attribution. Sci. Total Environ. 502:362-274.
Shiel, A.E., D. Weis, and K.J. Orians. 2010. Evaluation of zinc, cadmium and lead isotope fractionation during smelting and refining. Sci. Total Environ. 408(11):2357-2368.
Shikazono, N., K. Tatewaki, K.M. Mohiuddin, T. Nakano, and H.M. Zakir. 2012. Sources, spatial variation, and speciation of heavy metals in sediments of the Tamagawa River in central Japan. Environ. Geochem. Health 34(Suppl. 1):13-26.
Shirahata, H., R.W. Elias, and C. Patterson. 1980. Chronological variations in concentrations and isotopic compositions and anthropogenic atmospheric Pb in sediments of a remote subalpine pond. Geochim. Cosmochim. Acta 44(2):149-162.
Shotyk, W. 1988. Review of the inorganic geochemistry of peats and peatland waters. Earth Sci. Rev. 25(2):95-176.
Shotyk, W., and M. Krachler. 2010. The isotopic evolution of atmospheric Pb in central Ontario since AD 1800, and its impacts on the soils, waters, and sediments of a forested watershed, Kawagama Lake. Geochim. Cosmochim. Acta 74(7):1963-1981.
Shotyk, W., D. Weiss, P.G. Appleby, A.K. Cheburkin, R. Frei, M. Gloor, J.D. Kramers, S. Reese, and V.O. Knaap. 1998. History of atmospheric lead deposition since 12,370 14C yr BP from a peat bog, Jura Mountains, Switzerland. Science 281(5383):1635-1640.
Simonetti A., C. Gariepy, and J. Carignan. 2000. Pb and Sr isotopic evidence for sources of atmospheric heavy metals and their deposition budgets in northeastern North America. Geochim. Cosmocim. Acta 64(20):3439-3452.
Sivry, Y., J. Riotte, J.E. Sonke, S. Audry, J. Schäfer, J. Viers, G. Blanc, R. Freydier, and B. Dupré. 2008. Zn isotopes as tracers of anthropogenic pollution from Zn-ore smelters The Riou Mort-Lot River system. Chem. Geol. 255(3-4):295-304.
Small, M.J., A.B. Nunn, B.L. Forslund, and D.A. Daily. 1995. Source attribution of elevated residential soil lead near a battery recycling site. Environ. Sci. Technol. 29(4):883-895.
Small, I.F., J.S. Rowan, and S.W. Franks. 2002. Quantitative sediment fingerprinting using a Bayesian uncertainty estimation framework. Pp. 443-450 in The Structure, Functions and Management Implications of Fluvial Sedimentory Systems, F.J. Dyer, M.C. Thoms, and J.M. Olley, eds. IAHS Publication no. 276. Wallingford, UK: IAHS Press.
Small, I.F., J.S. Rowan, S.W. Franks, A. Wyatt, and R.W. Duck. 2004. Bayesian sediment fingerprinting provides a robust tool for environmental forensic geosciences applications. Pp. 207-213 in Forensic Geosciences: Principles, Techniques, and Applications, K. Pye, and D.J. Croft. eds. Special Publication 232. London: The Geological Society of London.
Sobanska, S., N. Ricq, A. Laboudigue, R. Guillermo, C. Bremard, J. Laureyns, J.C. Merlin, and J.P. Wignacourt. 1999. Microchemical investigations of dust emitted by a lead smelter. Environ. Sci. Technol. 33(9):1334-1339.
Sonke, J.E., J.A. Hoogewerft, S.R. van der Laan, and J. Vangronsveld. 2002. A chemical and mineralogical reconstruction of Zn-smelter emissions in the Kempen region (Belgium), based on organic pool sediment cores. Sci. Total Environ. 292(1-2):101-119.
Sonke, J.E., Y. Sivry, J. Viers, R. Freydier, L. Dejonghe, L. Andre, J.K. Aggarwal, F. Fontan, and B. Dupre. 2008. Historical variations in the isotopic composition of atmospheric zinc deposition from a zinc smelter. Chem. Geol. 252(3-4):145-157.
Sonke, J.E., J. Schäfer, J. Chmeleff, S. Audry, G. Blanc, and B. Dupré. 2010. Sedimentary mercury stable isotope records of atmospheric and riverine pollution from two major European heavy metal refineries. Chem. Geol. 279(3-4):90-100.
Spiro, B., D.J. Weiss, O.W. Purvis, I. Mikhailova, B.J. Williamson, B.J. Coles, and V. Udachin. 2004. Lead isotopes in lichen transplants around a Cu smelter in Russia determined by MC-ICP-MS reveal transient records of multiple sources. Environ. Sci. Technol. 38(24):6522-6528.
Stafilov, T., R. Sajn, Z. Pencevski, B. Boev, M.V. Frontasyeva, and L.P. Strelkova. 2010. Heavy metal contamination of topsoils around a lead and zinc smelter in the Republic of Macedonia. J. Hazard. Mater. 175(1-3):896-914.
Steinmann, M., and P. Stille. 1997. Rare earth element behavior and Pb, Sr, Nd isotope systematics in a heavy metal contaminated soil. Appl. Geochem. 12(5):607-623.
Steinnes, E., T.E. Sjøbakk, C. Donisa, and M.L. Brännvall. 2005. Quantification of pollutant lead in forest soils. Soil Sci. Soc. Am. J. 69(5):1399-1404.
Sterling, D.A., D.L. Johnson, A.M. Murgueytio, and R.G. Evans. 1998. Source contribution of lead in house dust from a lead mining waste superfund site. J. Expo. Anal. Environ. Epidemiol. 8(3):359-379.
Stokes, S., and D. Walling. 2003. Radiogenic and isotopic methods for the direct dating of fluvial sediments. Pp. 231-267 in Tools in Fluvial Geomorphology, G.M. Kondolf, and H. Piégay, eds. Chichester, UK: John Wiley & Sons.
Sweeney, M., V.R. Etyemezian, T. Macpherson, W.G. Nick-ling, J.A. Gillies, G. Nikolich, and E. McDonald. 2008. Comparison of PI-SWERL with dust emission measurements from a straight-line field wind tunnel. J. Geophys. Res. 113:F01012.
Taylor, M.P., A.K. Mackay, K.A. Hudson-Edwards, and E. Holz. 2010. Soil Cd, Cu, Pb and Zn contaminants around Mount Isa city, Queensland, Australia: Potential sources and risks to human health. Appl. Geochem. 25(6):841-855.
Telmer, K., G.F. Bonham-Carter, D.A. Kliza, and G.E.M. Hall. 2004. The atmospheric transport and deposition of smelter emissions: Evidence from the multi-element geochemistry of snow, Quebec, Canada. Geochim. Cosmochim. Acta 68(14):2961-2980.
Tessier, A., P.G.C. Campbell, and M. Bisson. 1979. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 51(7):844-851.
Thapalia, A., D.M. Borrok, P. Van Metre, M. Musgrove, and E.R. Landa. 2010. Zn and Cu isotopes as tracers of anthropogenic contamination in a sediment core from an urban lake. Environ. Sci. Technol. 44(5):1544-1550.
Tobin, G.A., R. Brinkmann, and B.E. Montz. 2000. Flooding and the distribution of selected metals in floodplain sediments in St. Maries, Idaho. Environ. Geochem. Health 22(3):219-232.
Ure, A.M., P. Quevauviller, H. Muntau, and B. Griepink. 1993. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 51(1-4):135-151.
USGS (U.S. Geological Survey). 2006. National Field Manual for the Collection of Water-Quality Data. Chapter A4. Collection of Water Samples. Book 9 Handbooks for Water-Resources Investigations [online]. Available: https://water.usgs.gov/owq/FieldManual/chapter4/pdf/Chap4_v2.pdf [accessed July 3, 2017].
Villarroel, L.F., J.R. Miller, P.J. Lechler, and D. Germanoski. 2006. Lead, zinc, and antimony contamination of the Rio Chilco-Rio Tupiza drainage system, southern Bolivia. Environ. Geol. 51(2):283-299.
Von Gunten, H.R., M. Sturm, and R.N. Moser. 1997. 200-year record of metals in lake sediments and natural background concentrations. Environ. Sci. Technol. 31(8):2193-2197.
Walling, D.E., and J.C. Woodward. 1995. Tracing sources of suspended sediment in river basins: A case study of the river Culm, Devon, UK. Mar. Freshwater Res. 46(1):327-336.
Walton-Day, K., J.L. Flynn, B.A. Kimball, and R.L. Runkel. 2005. Mass Loading of Selected Major and Trace Elements in Lake Fork Creek Near Leadville, Colorado, September–October 2001. Scientific Investigation Repopt 2005-5151. U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/sir/2005/5151/pdf/WaltonDay508.book.pdf [accessed June 28, 2017].
Wang, X., J.C. Chow, S.D. Kohl, K.E. Percy, A.H. Legge, and J.G. Watson. 2015. Characterization of PM2.5 and PM10 fugitive dust source profiles in the Athabasca Oil Sands Region. J. Air Waste Manage. Assoc. 65(12):1421-1433.
Watson, J.G., and J.C. Chow. 2005. Receptor models. Pp. 455-501 in Air Quality Modeling -Theories, Methodologies, Computational Techniques, and Available Databases and Software, Vol. II – Advanced Topics, P. Zannetti, ed. Pittsburgh, PA: Air and Waste Management Association and the EnviroComp Institute.
Watson, J.G., and J.C. Chow. 2013. Source apportionment. In Encyclopedia of Environmetrics, A.H. El-Shaarwi, and W.W. Piegorsch, eds. Chichester, UK: John Wiley & Sons.
Watson, J.G., J.C. and Chow. 2014. Source Apportionment. Wiley StatsRef: Statistics Reference Online.
Watson, J.G., and J.C. Chow. 2015. Receptor models and measurements for identifying and quantifying air pollution sources. Pp. 677-706 in Introduction to Environmental Forensics, 3rd Ed., B.L. Murphy, and R.D. Morrison, eds. Amsterdam, The Netherlands: Elsevier.
Watson, J.G., J.C. Chow, and C.A. Frazier. 1999. X-ray fluorescence analysis of ambient air samples. Pp. 67-96 in Elemental Analysis of Airborne Particles, Vol. 1, S. Landsberger, and M. Creatchman, eds. Amsterdam: Gordon and Breach Science.
Watson, J.G., T. Zhu, J.C. Chow, J.P. Engelbrecht, E.M. Fujita, and W.E. Wilson. 2002. Receptor modeling application framework for particle source apportionment. Chemosphere 49(9):1093-1136.
Watson, J.G., J.C. Chow, X. Wang, S.D. Kohl, and L.N.R. Yatavelli. 2014a. Windblown Fugitive Dust Characterization in the Athabasca Oil Sands Region. Prepared by Desert Research Institute, Reno, NV, for Wood Buffalo Environmental Association, Ft. McMurray, AB, Canada.
Watson, J.G., J.C. Chow, X. Wang, and S.D. Kohl. 2014b. Chemical Source Profiles for Geological Dust Samples From the Athabasca Oil Sands Region. Prepared by Desert Research Institute, Reno, NV, for Wood Buffalo Environmental Association, Ft. McMurray, AB, Canada.
Watson, J.G., J.C. Chow, G. Engling, L.W.A. Chen, and X.L. Wang. 2016. Source apportionment: Principles and methods. Pp. 72-125 in Airborne Particulate Matter: Sources, Atmospheric Processes and Health, R.M. Harrison, ed. London, UK: Royal Society of Chemistry.
Weiss, D., W. Shotyk, P.G. Appleby, J.D. Kramers, and A.K. Cheburkin. 1999. Atmospheric lead deposition since the industrial revolution recorded by five Swiss peat profiles: Enrichment factors, fluxes, isotopic composition, and sources. Environ. Sci. Technol. 33(9):1340-1352.
Weiss, D., W. Shotyk, S.E. Page, J.O. Rieley, S. Reese, and A. Martinez-Cortizas. 2002. The geochemistry of major and selected trace elements in a forested peat bog, Kalimantan, SE-Asia, and its implications on past atmospheric dust deposition. Geochim. Cosmochim. Acta 66(13):2307-2323.
Weiss, D.J, N. Rausch, T.F.D. Mason, B.J. Coles, J.J. Wilkinson, L. Ukonmaanaho, T. Arnold, and T.M. Nieminen. 2007. Atmospheric deposition and isotope biogeochemistry of zinc in ombrotropic peat. Geochim. Cosmochim. Acta 71(14):3488-3517.
Weldeab, S., K.C. Emeis, C. Hemleben, and W. Siebel. 2002. Provenance of lithogenic surface sediments and pathways of riverine suspended matter in the eastern Mediterranean Sea: Evidence from 143Nd/144Nd and 87Sr/86Sr ratios. Chem. Geol. 186(1-2):139-149.
Weltje, G. 2012. Quantitative models of sediment generation and provenance: State of the art and future developments. Sediment. Geol. 280:4-20.
Whittig, L.D., and W.R. Allardice. 1986. X-ray diffraction techniques. Pp. 331-362 in Methods of Soil Analysis, Part I. Physical and Mineralogical Methods, A. Klute, ed. Madison, WI: American Society of Agronomy-Soil Science Society of America.
Wirt, L., K.J. Leib, R. Melick, and D.J. Bove. 2001. Metal Loading Assessment of a Small Mountainous Sub-basin Characterized by Acid Drainage- Prospect Gulch, Upper Animas River Watershed, Colorado. Open-File Rep. 01-0258. U.S. Geological Survey [online]. Available: https://pubs.usgs.gov/of/2001/ofr-01-0258/ofr-01-0258.pdf [accessed June 28, 2017].
Yu, L.Z., and F. Oldfield. 1993. Quantitative sediment source ascription using magnetic measurements in a reservoir-catchment system near Nijar, S.E. Spain. Earth Surf. Proc. Land. 18(5):441-454.
Zhang, Y., A. Collins, and A. Horowitz. 2012. A preliminary assessment of the spatial sources of contemporary suspended sediment in the Ohio River basin, United States, using water quality data from the NASQAN programme in a source tracing procedure. Hydrol. Process. 26(3):326-334.
Zhu, C., and G. Anderson. 2002. Environmental Applications of Geochemical Modeling. Cambridge: Cambridge University Press.
Zota, A.R., L.A. Schaider, A.S. Ettinger, R.O. Wright, J.P. Shine, and J.D. Spengler. 2011. Metal sources and exposures in the homes of young children living near a mining-impacted Superfund site. J. Expo. Sci. Environ. Epidemiol. 21(5):495-505.





































