5
Implications for Southeast Missouri and Research Needs
In this report, the committee has described potential sources of lead contamination, transport mechanisms for environmental dispersal of lead, and investigative strategies for determining the sources of lead in environmental media. Here, the committee addresses specifically the implications of that information for the Southeast Missouri Lead Mining District, provides some research recommendations for optimizing approaches, and considers other opportunities for using some of the analytic techniques or methods for identifying potential sources of environmental lead contamination.
IMPLICATIONS FOR SOUTHEAST MISSOURI
Given the extensive mining history of Southeast Missouri, determining the sources of lead at sites in this region is complex. The presence of lead-ore deposits at or near the surface means that there are natural sources of lead at sites. Mining activities have produced waste materials, and the materials have been dispersed over wide areas, have been deposited many miles from their sources, and have come into contact with residential areas. A further complication is that lead can come from consumer products, including lead paint, gasoline, agricultural chemicals, batteries, and plumbing materials. In Chapter 4, the committee described various analytic methods and approaches for identifying sources of lead at a site and presented investigative strategies for implementing the various methods and approaches for soil, sediments, water, and air. As noted, the steps undertaken can depend on site characteristics and available resources. In the pages that follow, the committee first presents its overall conclusions regarding implementation of its strategies for Southeast Missouri and then provides two case studies that use data from the area to support its conclusions.
Overall Conclusions
Isotopic analytic techniques hold great potential to distinguish commercially processed lead or non-native ores from natural or locally mined materials in Southeast Missouri.1 For example, compilation of published 206Pb/207Pb ratios in North American ore deposits, gasoline, and paints is described in the next section. On the basis of those results, it is likely that lead-isotope ratios will be able to be used to apportion the sources of lead in soils, sediments, or house dust between those commercial sources and natural or mined materials in the case of Southeast Missouri. One strength of using isotopic analysis is that it can pinpoint a source with high accuracy if there is a unique signature, as is the case, for example, in the lead-paint data described in the next section.
The use of the analytic methods in the investigative approaches to distinguish natural sources of lead from mined material is much more challenging and might not be possible, particularly for soil, in the absence of a unique isotopic signature. The natural and mined material often contains lead that exhibits similar isotopic values, and this inhibits the use of lead isotopes to determine source. It might therefore be necessary to use other methods alone or in combination as shown in the committee’s investigative strategies to make a judgment about potential sources. Accordingly, determining the grain size, structure, and composition of soil particles might link them to a source. For example, analyzing structure and chemical composition could link soil particles to a smelting operation inasmuch as smelter-derived soil particles often contain spherical grains composed of sulfides, oxides, or glassy particles (Ettler 2016). The spatial distribution of the particles is also likely to vary as a function of wind direction and distance from the smelter; sulfides are relatively dense, and their concentration in soil is therefore likely to be higher closer to the smelter. Furthermore, differences in particles from tailings and chat piles might be determined partly on the basis of grain size; the former consists of relatively small grains (less than 74 μm), and the latter
___________________
1 The committee uses the term natural materials to refer to the geologic background that includes the mineral deposits that have not been mined.
much larger grains (often 4–8 mm). In addition, concentrations of barium and barite might distinguish lead from the Southeast Missouri Barite District and the St. Francois County Mining Area. With time, however, weathering of soil minerals derived from primary ore or smelter sources might alter or dissolve them and potentially disperse lead as a sorbed species or substituted in minor amounts in new precipitated phases. The point is that the selection of specific characteristics for attribution must be based on physical, compositional, mineralogic, or geochemical differences in the various source materials, and it might be necessary to use multiple methods to differentiate between all sources present. Even when multiple analyses are used, however, source identification might not be definitive.
It might be possible to estimate the relative contributions of lead from sources by using data analysis. Data analysis is a catchall term for mathematical manipulations designed to extract useful patterns and narratives from empirically derived collections of numbers, or data sets. The assessment activities undertaken early in the Superfund process described in Chapter 1 typically generate substantial collections of such data. The field collection and laboratory analysis of samples follow quality-assurance protocols; consequently, the consistent quality of the resulting data makes them well suited to systematic analyses.
A concrete example of data analysis to help to establish the extent to which lead-based paint contributes to environmental lead contamination in the Southeast Missouri Lead Mining District is described as the second case study. The mass-balance analysis revealed that much less total lead is likely to reside in exterior paint on older houses than in the top 1 inch of soil in many Southeast Missouri residential yards. Consequently, the contribution of exterior paint is expected to be a small fraction of the average lead content measured in typical Southeast Missouri residential yards. Such data analysis can be used to determine the dominant contributors to the mass balance of lead at a given site. However, the committee emphasizes that lead paint could still be the most important source for human exposure. The committee did not examine exposure pathways to determine the most important source for human exposure.
Lead-Isotope Techniques: A Southeast Missouri Case Study
To use lead-isotope ratios effectively for source attribution, one must first determine the lead isotopic composition of the various potential sources. The technique works well if there is an isotopic contrast between the sources that one wishes to distinguish but can yield equivocal results if there are overlapping isotopic compositions in the various potential sources. In most cases, some potential sources can be eliminated, and the investigation can focus on fewer possible sources. Thus, the lead-isotope method should work for source attribution of lead in soil near residences in mining or smelting areas if the isotopic composition of the ores and tailings is distinct from that of other possible sources.
For this case study, the committee selected lead paint and gasoline as possible sources because widespread environmental contamination has resulted from their use. The committee assessed whether they could be distinguished from natural ores or tailings from Southeast Missouri. It is well known that the lead isotopic composition differs between various ore deposits (Stanton and Russel 1959) and between lead ingots and slags and contaminated soils associated with different mines (Rabinowitz 2002). Different gasoline producers and paint manufacturers used lead mined from different ore bodies and in some cases changed their sources through time (Rabinowitz and Hall 2002). Therefore, it is expected that in some specific cases the lead isotopic composition of gasoline and paint might differ from that of local ore bodies but in other cases might be the same and therefore not useful for source attribution. The lead ores from Southeast Missouri have an extremely unusual lead isotopic composition (Rabinowitz and Wetherill 1972; Goldhaber et al. 1995). Thus, the isotopic composition of lead mined from Southeast Missouri (and adjacent areas in the tristate region) appears to be distinct from lead mined in nearly all other major mines (and products made with that lead) in North America (Doe 1970). However, the local geologic background lead in Southeast Missouri also contains unusual lead, so it might not be possible to differentiate between background and mine-derived lead in Southeast Missouri on the basis of isotopes alone.
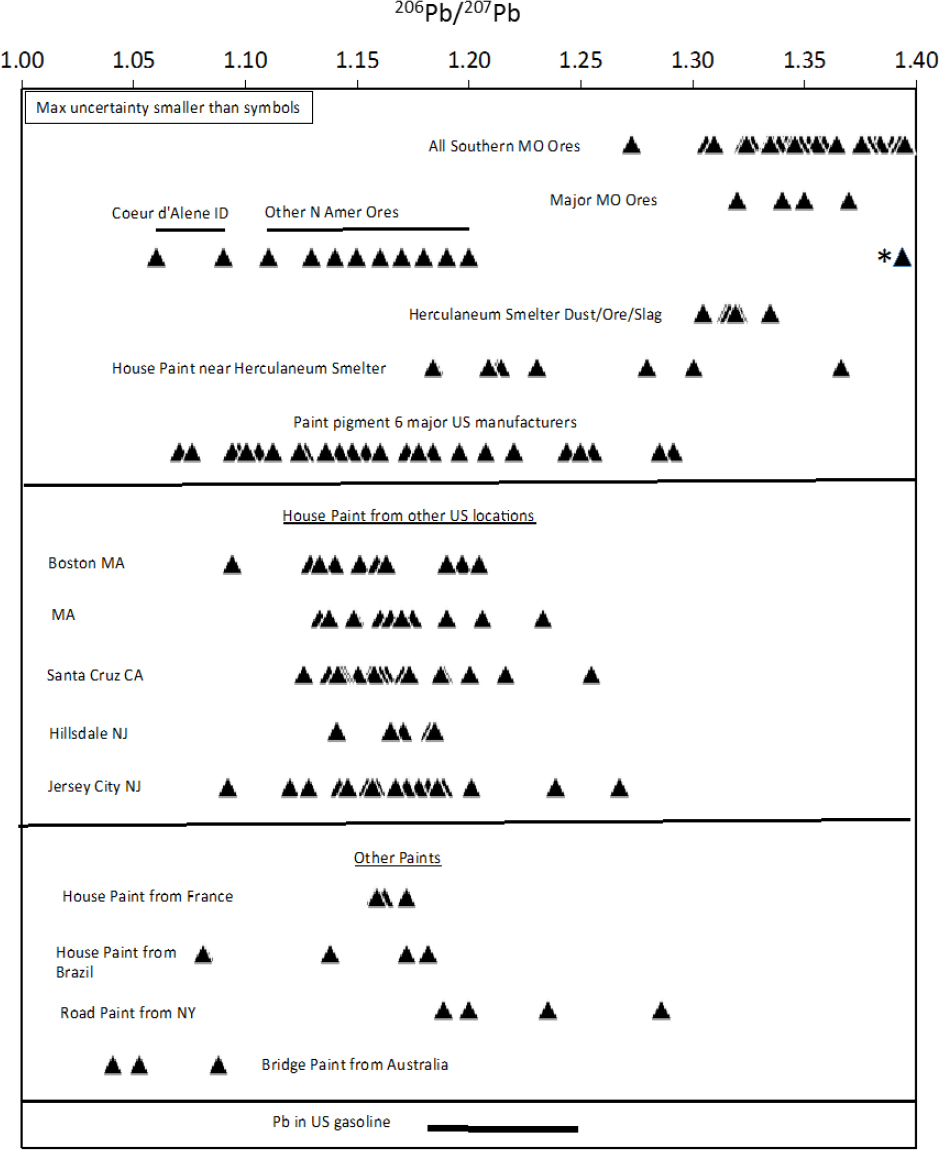
The committee compiled 206Pb/207Pb ratios for North American lead ores, gasoline, and paints from around the world. The compilation is shown in Figure 5-1. All the data are from peer-reviewed published articles except those from the Herculaneum smelter and nearby houses, which come from an exposure-investigation report prepared by the Agency for Toxic Substances and Disease Registry (Orloff 2002). In that report, two houses that were within 1 mile of the smelter were studied. The upper panel in the figure shows that Southeast Missouri ores, especially those from the Old Lead Belt, have considerably higher 206Pb/207Pb ratios than nearly all other North American ores that are plotted; only ores from the Upper Mississippi Valley District have overlapping lead-isotope ratios. In addition, 11 of the 12 samples of paint from the two houses sampled near the Herculaneum smelter in Southeast Missouri had 206Pb/207Pb ratios lower than dust, ore, and slag sampled at the nearby smelter, and one had a higher ratio. Thirty-one samples of paint pigment produced by six major US lead-paint manufacturers had ratios lower than dust, ore, and slag sampled at the
smelter. The single high-ratio paint sample was from the exterior of the back door of a house within 1 mile of the Herculaneum smelter. Either that paint contained lead that was produced from Southeast Missouri ores or the back door was contaminated by lead emitted from the smelter. Lead concentrations of the paint were not given in Orloff (2002), so the possibility that the back-door paint had low lead concentration and that the isotopic composition reflects the addition of lead from the nearby smelter cannot be eliminated.
The lead-isotope ratio measurements of materials from near the Southeast Missouri smelter suggest that the source of lead in most or possibly all lead paint was not Southeast Missouri lead. The middle panel in Figure 5-1 shows the 206Pb/207Pb ratios for six studies of lead in paint sampled from houses in other US locations, including Massachusetts, California, and New Jersey. All the samples have 206Pb/207Pb ratios lower than those of Southeast Missouri ores; this supports the suggestion that most lead paint manufactured in the United States used lead from ore deposits that did not include Southeast Missouri ores. Finally, the lower panel of Figure 5-1 shows the 206Pb/207Pb ratios of house paint from France and Brazil, road paint from New York City, bridge paint from Australia, and gasoline; these samples have 206Pb/207Pb ratios below those of Southeast Missouri ores.
Figure 5-1 shows that the Southeast Missouri ores, especially Old Lead Belt ores, have higher 206Pb/207Pb ratios than other ore deposits, gasoline, or paints compiled for North America. As noted, the only exceptions are the ores from the Upper Mississippi Valley District, which were probably not produced in large quantities in the 20th century compared with those from Southeast Missouri, and the single paint sample from the house near the Herculaneum smelter. Thus, in the case of the Southeast Missouri Lead Mining District, lead-isotope ratios will likely be able to attribute the sources of lead in soils between mined materials and paints and gasoline in most cases. As discussed below, additional analyses of lead concentrations and isotopic compositions of exterior paints in older houses in Southeast Missouri will be needed to verify the range of lead isotopic composition of paints in the area and the origin of the single back-door paint that had a high 206Pb/207Pb ratio.
The committee emphasizes that Figure 5-1 contains 206Pb/207Pb ratios from a variety of sources that were measured with a variety of mass spectrometry methods. Thus, the accuracy of the measurements varies considerably between about 0.01% and 0.5%. However, the highest estimated error is still smaller than the symbol sizes on the diagram; thus, it is clear that even without high-precision measurements, lead in most paints should be easily distinguishable from Southeast Missouri ores. High-precision lead-isotope analyses have uncertainties that are about one-fiftieth of the value reflected by the size of the symbols in Figure 5-1; thus, it is likely that at specific sites near other ore bodies, there will be measurable differences in lead-isotope ratios between paint and ore materials. In Figure 5-1, the committee showed only 206Pb/207Pb ratios because only they were available in some data sets. However, it is well known that if plots of 206Pb/204Pb, 207Pb/204Pb and 208Pb/204Pb are included, there can be greater resolving power for determining isotopic source contributions.
The committee notes that lead-isotope ratio measurements can be classified into three categories according to their precision: low-precision analyses of digests without chromatographic separation using quadrupole inductively coupled plasma mass spectrometry (ICP–MS), medium-precision analyses of digests without chromatographic separation using magnetic-sector ICP–MS, and high-precision analyses of digests after chromatographic separation using either multicollector magnetic-sector ICP–MS or thermal ionization mass spectrometry. Analysis costs vary widely among laboratories but increase from low to medium to high precision. The estimated cost per analysis is $50–500 and depends on the sample type and the analytic method. In the case of the Old Lead Belt in Southeast Missouri, low- and medium-precision analyses are likely to be adequate because of the large difference in 206Pb/207Pb between paint and ore. However, high-precision analyses might in some cases allow differentiation between ores from the various different mineral deposits in the mining district. Furthermore, measurement of 204Pb with high precision might be helpful for source apportionment in some cases. In general, the committee suggests that preliminary studies with medium precision should be conducted first to determine where there would be a benefit from high-precision analyses.
The committee notes that the utility of the lead isotopic methods in other mining and smelting regions in the United States has not been evaluated adequately and will depend on the lead isotopic composition of individual ore deposits and the isotopic contrast between the ores and other exposure sources, including paint. Ores from the Coeur d’Alene Idaho mining district have an unusually low 206Pb/207Pb ratio of about 1.06, which is lower than any of the paint or gasoline 206Pb/207Pb ratios that the committee compiled from the literature (Figure 5-1). Thus, in addition to the Southeast Missouri ores, Coeur d’Alene ores have 206Pb/207Pb ratios that contrast with those of paint and gasoline and should allow source-attribution studies. Furthermore, Vázquez Bahéna et al. (2017) found that in the Taxco mining district of southern Mexico the lead isotopic composition could be used to differentiate between local ores, glazed pottery, and paints. They concluded that women of childbearing age who had high blood lead and lived in the area had acquired lead predominantly from a mixture of local ores and pottery glaze.
Data Analysis to Estimate Lead-Based Paint Contribution to Environmental Lead Contamination: A Southeast Missouri Case Study
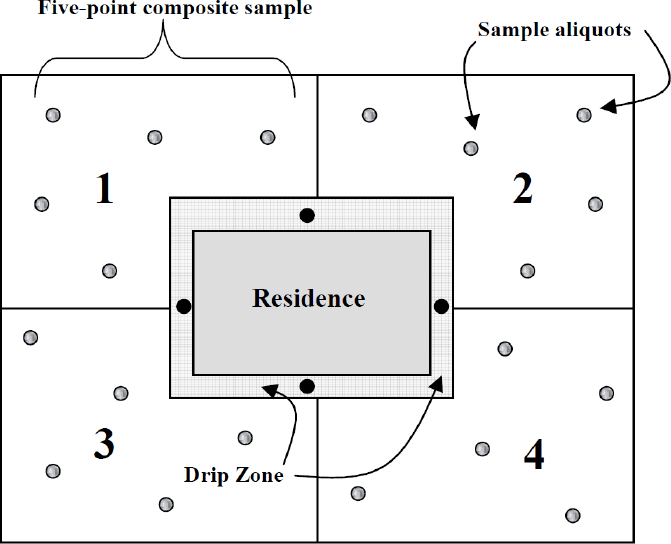
As noted in Chapter 2, lead-based paints were heavily used in the early decades of the previous century (Mielke 1999), particularly to protect the exterior surfaces of houses and other structures from the effects of weathering. Detritus from lead-based paints is well recognized as a potential contaminant of soils in older neighborhoods, and protocols for the collection of residential soil samples distinguish drip zones where such debris is likely to collect adjacent to painted surfaces (see Figure 5-2). In addition to paint flakes and residues, drip zones can be enriched in airborne lead-containing particles deposited initially on roofs and walls and then washed off by precipitation. As Figure 5-2 indicates, samples collected in quadrants of the larger yard are intended to represent average concentrations in soils free from those influences.
Lead is considered to be relatively immobile as a constituent of soil in Southeast Missouri. Consequently, the spatial distribution of soil lead concentrations offers some indication of where exogenous lead might have been introduced and provides a strategy for attributing soil lead to point sources where releases are concentrated at fixed locations. The contributions of removal of lead-based paint in preparation for repainting and of weathering and wear of lead-based exterior paint are expected to manifest themselves as differentials in soil lead concentrations between the drip zone and the outer yard.
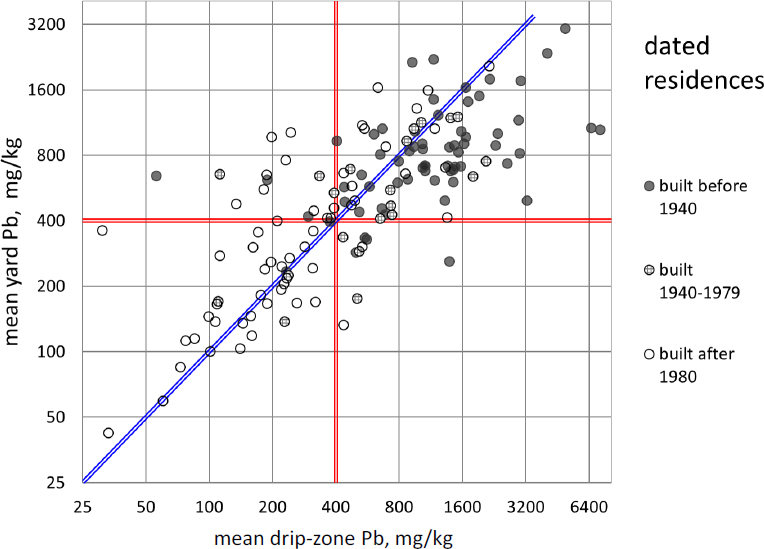
Figure 5-3 directly compares drip-zone with yard-average soil lead concentrations measured at 145 Southeast Missouri lots in the Focused Residential Sampling program described by Integral Consulting Inc. (2014). Drip-zone concentrations were found to be higher than yard concentrations at about 75% of homes (48 of 65) built before 1940, when lead content in house paint started to decline (Mielke 1999). The observation of higher drip-zone concentrations at older houses provides evidence that lead-based paint contributes to environmental contamination. Many other homes of all ages showed yard-average lead concentrations well above those in their drip zones, and these data indicate the presence of sources other than house paint. The mixture of positive and negative spatial concentration gradients near homes suggests that exterior paint does not generally overwhelm other sources of soil lead in the sampled neighborhoods.
A different kind of gradient that is evident in Figure 5-3 is the tendency of older houses to have higher soil lead concentrations outside their drip zones. Of the 60 residences built after 1979, 35 (58%) have yard lead concentrations below 400 mg/kg, whereas only six of 65 built
before 1940 (9%) are below this threshold. The differential could represent nothing more than the circumstance that construction at different times took place in different neighborhoods with different levels of prior contamination. Alternatively, it can be interpreted as evidence that lead-based paint debris is not confined to the drip zones of exterior walls. The higher lead concentrations recorded in older yards might be attributable in some part to the lead-based exterior paint used on the older houses, as suggested by Integral Consulting Inc. (2014, pp. 3-2, 4-1).
A mass balance can place bounds on source contributions of lead. Figure 5-3 compared concentrations in the outer yard with concentrations in the drip zone. The average lead concentration in the outer yard represents a much greater total quantity of lead, however, than would a similar concentration in the narrow drip zone. To assess the possibility raised above that lead-based paint is a major contributor to lead in the outer yard and not just to lead in the drip zone, the committee first considered the total lead contained in a yard’s surface soil.
The total lead content of a yard’s surface soil can be estimated from the average mass concentration and an estimate of soil volume and density. An arithmetic mean lead concentration of 677 mg/kg of soil was reported outside the drip zone for all Southeast Missouri yards sampled in 2013 (Integral Consulting Inc. 2014, Figure 3-8). The committee used the bulk soil density for the relevant density (Timm et al. 2005), which accounts for the volume contributed by interstitial air spaces. Surface soils vary in their composition and compaction; reported residential bulk soil densities typically are 1.2–1.6 g/cm3. The committee took 1.5 g/cm3 as a representative value, and this converts the mean lead concentration of 677 mg/kg reported for sampled Southeast Missouri yards to about 1 mg/cm3 of soil. The committee assumed conservative values (lower bounds) of one quarter-acre for Southeast Missouri residential lot sizes, and 40% as an estimate of the portion covered with soil. A quarter-acre is about 107 cm2, and the 40% soil-cover factor is offset by the sampling depth of 1 in (2.54 cm) rather than 1 cm. Those order-of-magnitude calculations yield average amounts of lead in the top inch of yard soil in a quarter-acre of at least 10 kg ([107 cm3][1 mg/cm3]). The 10-kg total lead burden estimated to reside in the top inch of an average Southeast Missouri yard serves as a meaningful benchmark with which the contents of other potential lead reservoirs can be compared. In the case of lead-based exterior paint, three methods described below can be used to estimate a house’s total lead burden.
- The lead content of house paints has varied, but a representative formulation might be the following (HUD 2012, Appendix A): 12% Pb × 50% solids × 12 lb/gal = lead at 0.7 lb/gal. A square house 40 ft on a side with walls that extend 9 ft above the foundation would have at most 1,440 ft2 of paintable exterior surface. At coverages of 350–400 ft2/gal, this house would take about 4 gal of paint, containing 2.8 lb = 1.3 kg. The 10-kg estimated in the surface yard soil would correspond to seven or eight full-coverage coats of lead-based paint.
- Mielke et al. (2001) studied a two-story wood frame house that was built in the 1920s and had deteriorating lead-based paint. The investigators oversaw an exterior restoration in which care was taken to collect and quantify all removed paint and reported 41 kg of paint scrapings that contained 3.7 kg of lead. They estimated that a similar amount remained bound intact to the wood, for a total of 7.4 kg of lead accumulated in paint layers over many decades.
- A third set of estimates comes from systematic national surveys undertaken for the Department of Housing and Urban Development and the US Environmental Protection Agency (EPA) by field inspectors using portable x-ray fluorescence analyzers (EPA 1995a). Table 5-1 summarizes the survey for exterior surfaces of occupied and privately owned housing units grouped into three construction periods. The historical decline of lead-based paint use is clear in the survey results. For the maximally leaded “pre-war” houses, the committee estimated total lead contents of
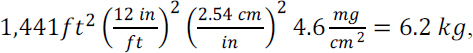
which is close to the value that Mielke et al. (2001) obtained by collecting paint scrapings.
The three estimates indicate that less total lead is likely to reside in exterior paint, even on an older house, than the 10-kg average lead burden that sampling indicates now sits in the top inch of sampled Southeast Missouri yards. Table 5-1 shows that the high-lead-content paints applied before 1940 were largely still in place when inspectors visited them more than 50 years later; that finding suggests that most of the paint ever applied to the exterior remains there. Some portion of the applied paint is lost to weathering, maintenance, and wear, but most of this debris is expected to accumulate in the drip zone, as discussed earlier.
The committee’s mass-balance findings imply that the great majority of the surface lead in a randomly selected sample of surface yard soil outside the drip zone cannot reasonably be attributed to contamination by paint debris in typical Southeast Missouri yards. However, they do not preclude that paint debris might be primarily responsible for the soil lead content in some areas of some yards or that the paint debris is the primary source of human exposure. Again, the committee emphasizes that one must examine exposure pathways to identify the sources important for human exposure.
RESEARCH NEEDS
Additional research provides an opportunity to enhance the performance of the analytic methods incorporated into the investigative strategies proposed by the committee and to reduce uncertainty during implementation. The committee concluded that high research priority should be given to further characterizing potential sources but notes that the research described below is not essential for implementation of its investigative strategies. The research needs are not listed in order of priority.
Characterizing Lead Isotopic Composition of Historical Paints
The committee has discussed the use of lead-isotope ratios for attributing sources of lead in contaminated soils to mining and smelting activities. The committee pointed to examples of previous studies that showed an isotopic contrast between lead from paint and lead from mining and smelting activities in Southeast Missouri and suggested that the two sources of lead in soils could be identified by using lead isotopes. However, to evaluate the utility
| Construction Era | |||
|---|---|---|---|
| Before 1940 | 1940–1959 | 1960–1979 | |
| Number of sampled units | 77 | 87 | 120 |
| Area of lead-based paint per unit (ft2) | 1,441 | 758 | 482 |
| Mean lead-based paint loading (Pb in mg /cm2) | 4.6 | 1.5 | 0.6 |
Source: Extracted from EPA (1995b), Appendix II, Tables 2-1, 2-12, 2-13.
of the approach fully, additional study of lead isotopes should be conducted. In particular, knowledge of the lead isotopic composition of house paint in Southeast Missouri is limited to 11 analyses related to a single house and one analysis related to a second house, and lead concentrations in these samples were not reported. The committee recommends systematic sampling and lead isotopic analysis of paint in houses built before 1980 throughout the Southeast Missouri Lead Mining District. A broad sampling and analysis of paint in houses of various ages and locations should be conducted with at least five interior and five exterior paint samples collected from each house. That approach would give a much clearer indication of the range in lead isotopic composition of paint used in Southeast Missouri and would clarify the degree to which lead isotopes can be used in attributing lead in soil to paint vs mining and smelting sources. If there are other lead-containing consumer products at the site—such as plumbing materials, batteries, and pesticides—studies of their lead isotopic composition could establish or refute their role as a source of lead contamination at a site.
Characterizing Lead-Bearing Geologic Materials and Mining Wastes
The distribution, concentration, and geologic context of potentially key elements need to be better characterized to provide a baseline for interpretation of natural contributions to surface lead. There is a need to move away from just making bulk lead measurements and to provide greater detail on the materials and characteristics of the lead in the geologic and mining sources. Characterization would include chemical data, such as concentrations and isotopic ratios, mineralogy, grain size and texture, and host rock types. Spatial characterization would include vertical distribution, particularly in weathering (soil) profiles, and the lateral (areal) distribution over appropriate areas. Such information will improve confidence in investigative strategies for source attribution introduced in Chapter 4.
Recent advances in analytic chemistry, such as multicollector ICP–MS, have opened the possibility that a wide array of nontraditional stable isotopes can be used to determine the source of trace metals in the environment. Many metals—such as cadmium, copper, iron, mercury, and zinc—occur as sulfides and are associated with lead in geologic materials. Thus, they can potentially be used to attribute lead from geologic and mining sources in the Southeast Missouri Lead Mining District.
Variations in lead isotopic abundance in geologic materials are due mainly to radioactive decay over geologic timescales in that lead undergoes little change in isotopic ratios during other types of physical, chemical, and biologic processes. In contrast, shifts in the isotopic abundances of nontraditional stable isotopes2 are small but are due entirely to physical, chemical, or biologic processes, including ones that occur during industrial processing or during metal dispersal at the Earth’s surface (Wombacher et al. 2004; Cloquet et al. 2006; Shiel et al. 2010; Rehkämper et al. 2011; Blum et al 2014). It is that shift in the isotopic ratio that allows those isotopes to be of use in studies of source attribution. However, their effective use requires a detailed understanding of the processes that cause changes in the isotopic ratios in the environment of interest. They are more likely to change because of chemical reactions in the environment and are therefore more complicated to use for source attribution than lead isotopes. However, several such isotopes—including cadmium, mercury, and zinc—have proved useful as tracers of particles in soils and river sediments. Their application to the Southeast Missouri situation will require additional investigation but might prove to be highly useful, particularly for distinguishing between particles derived from ore deposits and smelter emissions.
Developing Capabilities to Model Metal Concentrations in Soils as a Function of Depth
As described in Chapter 4, the science of modeling sediment distributions in river floodplains is relatively advanced. Advantages of multielement, geochemical fingerprinting for attribution of sources of sediment and sediment-associated metals in rivers include (1) the ability to incorporate a wide array of elements into the analysis, thereby increasing the probability that the sources of sediment (and particulate lead) can be uniquely distinguished; (2) the establishment of widely accepted sampling and statistical analysis protocols; and (3) the increasing ability to quantify uncertainty in the attribution results (see, for example, Collins et al. 2012; Miller et al. 2015). The approach might prove applicable for determining particulate lead in sediments of the Big River with little modification of existing methods. Modeling of metal movement through soils is less developed. The application of multielemental, geochemical fingerprinting to determine sources of particulate lead in soils is more challenging because of potential alteration and dissolution of particulate lead. Theoretically, the approach is applicable to soils and might yield highly useful results, but the method will require additional testing when applied to soils. Studies could be completed on soils near known metal-emitting sources to map and model how the metals, especially lead, have moved through soils as a function of time.
___________________
2 Nontraditional stable isotopes include elements other than carbon, hydrogen, oxygen, nitrogen, and sulfur. Examples are zinc, copper, molybdenum, mercury, iron, lithium, and cadmium.
Characterizing Size Distribution of Suspended Particles in Air Samples
Tailings impoundments, chat piles, and smelting emissions are important sources of airborne lead in a mining district; however, little is known about the size of suspended particles and their mobility. Knowledge of the size distribution of lead-containing airborne particulate matter is important for controlling particle transport and deposition. The largest particles have high terminal velocities and can settle within a few minutes to a few hours whereas particles whose diameters are a few micrometers can remain suspended for several days and can therefore be transported long distances. Size-selective sampling of air samples in the mining district should be conducted to determine the particle-size distribution and establish the PM2.5 and PM10 levels. Size-selective sampling can be conducted upwind or downwind of a source, such as smelter or tailings pile, to evaluate contributions from the source. In addition to particle-size measurements, particle analysis by elemental, mineralogic, isotopic, and microscopic methods can be used to help to relate airborne and deposited particles to their sources.
Characterizing Rates of Emission of Airborne Particles from Mining Wastes
Little is known about rates of emission of airborne lead from stockpiles of mining wastes. Portable wind tunnels can be used to estimate the airborne particle emission rates and dust production from stockpiles of mining wastes, such as chat piles and tailings impoundments. Portable wind tunnels can also be useful in measuring the threshold wind velocity at a specific site that results in particle suspension and later transport downwind. This information can help to establish whether airborne lead from mining wastes is an important source of lead contamination downwind.
Identifying Sources with Investigative Tools in Industrial Archeology and Affiliated Disciplines
Industrial archeology is the systematic study of material evidence associated with historical industrial activities, such as resource extraction and raw-material processing; refining and manufacturing; storage, transport, and distribution; uses throughout product and byproduct life cycles; and waste disposal. Although historical industrial processes were generally on a smaller scale than modern industry, they operated less efficiently, were more likely to use processes and materials that produced hazardous byproducts, and lacked pollution controls. Thus, the relative pollution effect of historical industrial activities can be highly disproportionate relative to the scale of production. Understanding historical practices sheds light on their relative importance as a source of legacy pollution that persists long after the industries have disappeared.
Industrial archeology provides scientific methods for investigating the industrial life cycle of lead and drawing objective conclusions about how past practices throughout the industrial life cycle contributed to the present abundance, distribution, and forms of lead in the built and natural environments. Those investigative tools could help to identify sources of lead contamination, especially at large Superfund sites, including the Southeast Missouri Lead Mining District, and involving industrial archeologists and historians at the earliest stages of a remedial investigation could provide valuable insight on sources, transport, and fate of contaminants. The committee notes that the investigative tools of industrial archeology evolve as do the tools of any other discipline. A good resource is IA, the Journal of the Society for Industrial Archeology, which publishes on industrial archeology of industrial waste.
OTHER APPLICATIONS OF RECOMMENDED ANALYTIC TECHNIQUES: A CASE STUDY OF BLOOD LEAD AND ISOTOPIC ANALYSIS
The analytic techniques and methods incorporated into the committee’s investigative strategies can be used for applications other than simply soil, sediments, water, and air. For example, isotopic analysis can be used for assessing sources of lead in biologic samples, such as blood and urine. When humans are exposed to lead, the lead isotopic composition in their blood and urine reflects the sources of lead exposure. Lead isotope investigations to determine the sources of lead in residents near the Herculaneum Lead Smelter in Southeast Missouri were reported by the Agency for Toxic Substances and Disease Registry and published as two exposure investigations (Orloff 2001, 2002). The committee summarized and interpreted the data in those reports as a case study to demonstrate both the power and the complexity of the use of lead isotopes for source attribution.
Two houses within 1 mile of the Herculaneum Lead Smelter in Southeast Missouri and their occupants were sampled, and the samples were analyzed for lead isotopic composition (Orloff 2001, 2002). The various environmental media and human specimens that were sampled and their lead isotopic compositions are shown in Figure 5-4. The committee focused on the data from house 1 because the sampling included 11 paint samples, compared with only one at house 2. The patterns in lead isotopic composition and the general conclusions are similar for the two houses.
As discussed above, lead ores and mining waste from the Southeast Missouri Lead Mining District have unusually high 206Pb/207Pb ratios compared with most other mining districts and therefore most commercial
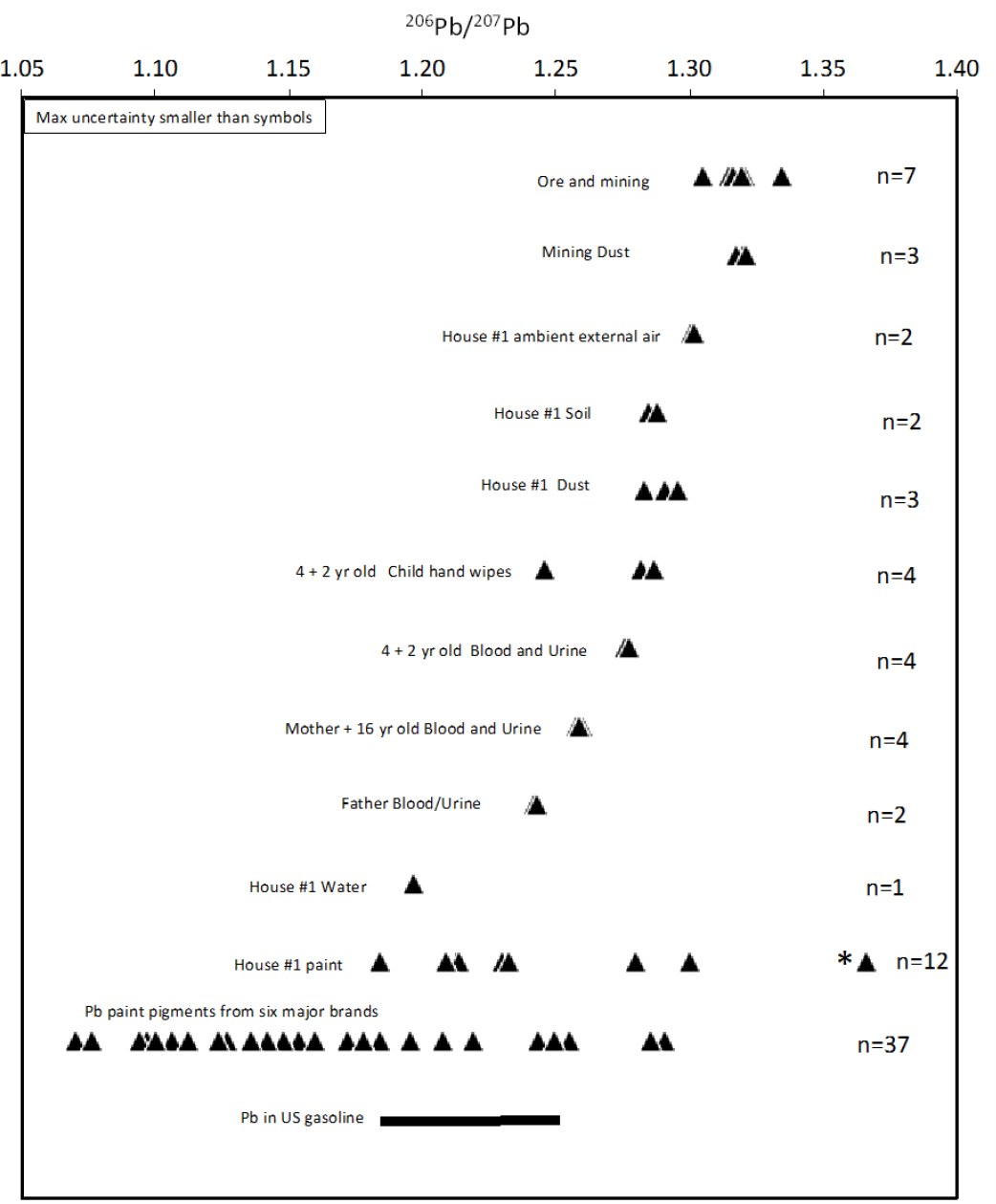
products that contain lead. Ore, mining waste, and dust generated from ore-processing at the Herculaneum lead smelter have the highest 206Pb/207Pb ratios measured (Orloff 2001, 2002), 1.31–1.34. Ambient exterior-air particles larger than 0.8 μm sampled 4–5 ft above the ground in the house 1 yard have similar but slightly lower 206Pb/207Pb ratios, 1.30, and this suggests that the lead in atmospheric dust is generated mainly from the smelter but with the addition of some lower 206Pb/207Pb lead. Lead from leaded gasoline is a ubiquitous component of soils throughout North America. Although lead was phased out of automobile gasoline by the middle 1990s, it remains in soils, especially near roadways. As discussed in Chapter 2, leaded gasoline is still used in aviation fuel, which is currently the largest source of lead emitted to the atmosphere in the United States (Wolfe et al. 2016). The 206Pb/207Pb ratio of lead in gasoline in the United States has been estimated at 1.19–1.25 (Sherrell et al. 1992; Teutsch et al. 2001). The lead-isotope ratio of a mixture of sources whose lead-isotope ratios are known scales linearly with the proportion of lead from each source. As an example of how mixing calculations can be done, the committee used data from Figure 5-1 and applied a mixing calculation to explore the origin of the atmospheric dust samples analyzed by Orloff (2001). Using average 206Pb/207Pb ratios for each sample type, the committee found that the 206Pb/207Pb ratio can be explained by a mixture of about three-fourths smelter emissions and one-fourth lead from gasoline. However, the proportion of lead derived from gasoline could be much lower if the 206Pb/207Pb ratio of smelter emissions during the period of atmospheric sampling was at the lower end of the range of ratios of Southeast Missouri ores.
Soil from the yard and dust from the interior of house 1 have lower 206Pb/207Pb ratios than atmospheric dust, 1.28–1.30. The lower values could be produced by mixing smelter lead with lead from house paint. Eleven samples of interior and exterior house paint from house 1 were analyzed. Ten had values that overlap the previously reported range of ratios of commercial paint pigments (Rabinowitz and Hall 2002), 1.19–1.30. One paint sample has a 206Pb/207Pb ratio above the range of major lead ores; it is an exterior sample, and its lead concentration was not reported, so its lead isotopic composition is difficult to interpret.
As expected from other studies, blood and urine samples from each family member yielded nearly identical 206Pb/207Pb ratios. The 2- and 4-year-old children have 206Pb/207Pb ratios of 1.28—at the lower range of soil, dust, and hand-wipe values—except for the one hand-wipe outlier that might be related to paint exposure, perhaps from a windowsill. That result suggests that the 2-year-old and 4-year-old are exposed to lead mainly via ingestion of soil and dust; this result has been found to be the case in many other studies (see, for example, Gulson et al. 1994; Gwiazda and Smith 2000; Manton et al. 2000; Laidlaw et al. 2016). The 16-year-old and the mother have lower 206Pb/207Pb ratios, 1.26, and the father has a still lower ratio, 1.24. Those results are not unexpected in that even in an adolescent blood lead can reflect a mixture of past and present lead exposure. That is, lead is stored in bone material, and blood lead can be derived partly from lead mobilization from bone over year-long timescales (Manton 1985; Manton et al. 2000).
This case study demonstrates the power of lead isotopes in differentiating between lead sources in several types of environmental and biologic media. The identification of the lead sources for a particular sample is sometimes equivocal, but some sources can be eliminated in most cases, thus narrowing the possible combination of sources that need to be considered. In the Southeast Missouri Lead Mining District examined here, the largest source of lead isotopic uncertainty is the multiple values in paint at different locations in house 1. Many paint samples analyzed in Orloff (2002) were from locations where a child would not typically be exposed to them, including above a ceiling, on a baseboard molding, and in a parent’s bedroom closet. By limiting paint sampling to areas where children have access and to where paint dust is generated by friction on door jambs or window sashes, the 206Pb/207Pb ratio of paint in a given house can be estimated more narrowly. Peeling or flaking paint that can be ingested by a child presents a different problem: the amount ingested can lead to frank lead poisoning or, in a worst-case scenario, encephalopathy that results in permanent brain damage or death. Even with the uncertainties and imperfect sampling strategy, Orloff (2001, 2002) demonstrates systematic differences of lead sources from different environmental sources and allows the testing and narrowing of hypotheses for the sources of human lead exposure.
Other studies that have used lead-isotope ratios to determine potential sources of lead in children in the United States (Viczián et al. 1990; Rabinowitz 1995; Gwiazda and Smith 2000; Manton et al. 2000; Glorennec et al. 2010) have been conducted in urban areas, not near mining or smelter sites. Researchers measured lead isotopes in children’s blood and then sought to distinguish between potential sources, including house paints, household dust, and soils. Most of the studies involved some individual houses where children’s blood-lead isotopes match with paint or soil and some other houses where there is such a large range in paint lead-isotope ratios that no single source can be identified. One such study was carried out in Santa Cruz, CA, by Gwiazda and Smith (2000). They measured the lead isotopic composition of children’s blood, household dust, exterior soil, and interior and exterior paints. In one house, paint could be eliminated as a major source of lead exposure, and exterior dust and soil were implicated. In two other houses, paint from some areas could be eliminated as possible sources, but vari-
ous mixtures of paints from other areas with dust and soil could explain the children’s blood-lead isotope values, and no single source could be identified.
If EPA or others want to pursue blood-lead isotopic investigations for the Southeast Missouri Lead Mining District, the type of investigation described in this case study would need to be replicated in houses of different ages and at different locations in Southeast Missouri to determine where and under what conditions the lead isotope method can provide unambiguous attribution of lead sources for children who have high blood lead concentrations. The approach is promising in Southeast Missouri because the lead associated with mining and smelting has an unusually high 206Pb/207Pb ratio, about 1.34, which in most cases is distinct from lead used to make paint in the United States.
REFERENCES
Adgate, J.L., G.G. Rhoads, and P.J. Lioy. 1998. The use of isotope ratios to apportion sources of lead in Jersey City, NJ, house dust wipe samples. Sci. Total Environ. 221(2-3):171-180.
Blum, J.D., L.S. Sherman, and M.V. Johnson. 2014. Mercury isotopes in earth and environmental science. Annu. Rev. Earth Planet. Sci. 42:249-269.
Cloquet, C., J. Carignan, and G. Libourel. 2006. Isotopic composition of Zn and Pb atmospheric depositions in an urban/periurban area of northeastern France. Environ. Sci. Technol. 40(21):6594-6600.
Collins, A., Y. Zhang, D. McChesney, D. Waling, S. Haley, and P. Smith. 2012. Sediment source tracing in a lowland agricultural catchment in southern England using a modified procedure combining statistical analysis and numerical modelling. Sci. Total Environ. 414:301-317.
Doe, B.R. 1970. Lead Isotopes. Berlin: Springer.
Duzgoren-Aydin, N.S., and E.A.L. Weiss. 2008. Use and abuse of Pb-isotope fingerprinting technique and GIS mapping data to assess lead in environmental studies. Environ. Geochem. Health 30(6):577-588.
EPA (U.S. Environmental Protection Agency). 1995a. Report on the National Survey of Lead-Based Paint in Housing, Base Report. EPA 747-R95-003. U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics, Washington, DC [online]. Available: https://www.epa.gov/sites/production/files/documents/r95-003.pdf [accessed June 21, 2017].
EPA. 1995b. Report on the National Survey of Lead-Based Paint in Housing, Appendix II. Analysis. EPA 747-R95-005. U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics, Washington, DC [online]. Available: https://www.epa.gov/sites/production/files/documents/r95-005.pdf [accessed June 21, 2017].
EPA. 2003. Superfund Lead-Contaminated Residential Sites Handbook. OSWER 9285.7-50. U.S. Environmental Protection Agency, Lead Sites Workgroup [online]. Available: https://semspub.epa.gov/work/HQ/175343.pdf [accessed June 21, 2017].
Ettler, V. 2016. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 64:56-74.
Glorennec, P., C. Peyr, J. Poupon, Y. Oulhote, and B. Le Bot. 2010. Identifying sources of lead exposure for children, with lead concentrations and isotope ratios. J. Occup. Environ. Hyg. 7(5):253-260.
Goldhaber, M., S.E. Church, B.R. Doe, I.N. Aleinikoff, J.C. Brannon, F.A. Podosek, E.L. Mosier, C.D. Taylor, and C.A. Gent. 1995. Lead and sulfur-isotope investigations of Paleozoic sedimentary rocks from the southern midcontinent of the United States: Implications for the paleohydrology and ore genesis of the southeast Missouri lead-belt. Econ. Geol. 90(7):1875-1910.
Gulson, B., K.J. Mizon, A.J. Law, M.J. Korsch, J.J. Davis, and D. Howarth. 1994. Source and pathways of lead in humans from the Broken Hill Mining Community – An alternative use of exploration methods. Econ. Geol. 89(4):889-908.
Gulson, B., M. Chiaradia, J. Davis, and G. O’Connor. 2016. Impact on the environment from steel bridge paint deterioration using lead isotopic tracing, paint compositions and soil deconstruction. Sci. Total Environ. 550:69-72.
Gwiazda, R.H., and D.R. Smith. 2000. Lead isotopes as a supplementary tool in the routine evaluation of household lead hazards. Environ. Health Perspect. 108(11):1091-1097.
Heyl, A.V., G.P. Landis, and R.E. Zartman. 1974. Isotopic evidence for the origin of Mississippi Valley type mineral deposits: A review. Econ. Geol. 69(6):992-1006.
HUD (U.S. Department of Housing and Urban Development). 2012. Guidelines for the Evaluation and Control of Lead-Based Paint Hazards in Housing, 2nd Ed., Appendix 1. Units of Measure. U.S. Department of Housing and Urban Development, Office of Healthy Homes and Lead Hazard Control [online]. Available: https://portal.hud.gov/hudportal/HUD?src=/program_offices/healthy_homes/lbp/hudguidelines [accessed June 21, 2017].
Integral Consulting, Inc. 2014. Sources of Lead to Residential Soils of St. Francois County, Appendix A. Lead Copper and Zinc Concentrations in Residential Soils, 2013 Focused Residential Sampling. Report prepared for The Doe Run Company, May 15, 2014.
Jaeger, R.J., A.L. Weiss, and W.I. Manton. 1998. Isotopic ratio analysis in residential lead-based paint and associated surficial dust. Clin. Toxicol. 36(7):691-703.
Laidlaw, M.A.S., G.M. Filippelli, R.C. Sadler, C.R. Gonzales, A.S. Ball, and H.W. Mielke. 2016. Children’s blood lead seasonality in Flint, Michigan (USA), and soil-sourced lead hazard risks. Int. J. Environ. Res. Public Health 13(4):358; doi:10.3390/ijerph13040358.
Manton, W.I. 1985. Total contribution of airborne lead to blood lead. Br. J. Ind. Med. 42(3):168-172.
Manton, W.I., C.R. Angle, K.L. Stanek, Y.R. Reese, and T.J. Kuehnemann. 2000. Acquisition and retention of lead by young children. Environ. Res. 82(1):60-80.
Mielke, H.W. 1999. Lead in the inner cities: Policies to reduce children’s exposure to lead may be overlooking a major source of lead in the environment. Am. Sci. 87(1):62-73.
Mielke, H.W., E.T. Powell, A. Shah, C.R. Gonzales, and P.W. Mielke. 2001. Multiple metal contamination from house paints: Consequences of power sanding and paint scraping in New Orleans. Environ. Health Perspect. 109(9):973-978.
Miller, J.R., M. Mackin, and S.M. Orbock Miller. 2015. Application of Geochemical Tracers to Fluvial Sediment. New York: Springer. 142 pp.
Mirlean, N., D. Robinson, K. Kawashita, M.L. Vignol, R. Conceição, and F. Chemale. 2005. Identification of local sources of lead in atmospheric deposits in an urban area in Southern Brazil using stable lead isotope ratios. Atmos. Environ. 39(33):6204-6212.
Orloff, K.G. 2001. Exposure Investigation: Herculaneum Lead Smelter Site, Herculaneum, Jefferson County, Missouri, EPA Facility MOD006266373. September 14, 2001.
Orloff, K.G. 2002. Exposure Investigation: Herculaneum Lead Smelter Site, Herculaneum, Jefferson County, Missouri, EPA Facility MOD006266373. December 12, 2002 [online]. Available: http://health.mo.gov/living/environment/hazsubstancesites/pdf/Leadsourcesinchildren2002.pdf [accessed June 21, 2017].
Rabinowitz, M.B. 1995. Stable Isotopes of lead for source identification. Clin. Toxicol. 33(6):649-655.
Rabinowitz, M. 2002. Isotopic characterization of various brands of corroding grade refined lead metal. Bull. Environ. Contam. Toxicol. 69(4):501-508.
Rabinowitz, M.B 2005. Lead isotopes in soils near five historic American lead smelters and refineries. Sci. Total Environ. 346(1-3):138-148.
Rabinowitz, M.B., and G.S. Hall. 2002. Isotopic characterization of six major brands of white basic lead carbonate paint pigments. Bull. Environ. Contam. Toxicol. 69(5):617-623.
Rabinowitz, M.B., and G.W. Wetherill. 1972. Identifying sources of lead contamination by stable isotope techniques. Environ. Sci. Technol. 6(8):705-709.
Rehkämper, M., R. Wombacker, T.J. Horner, and Z. Xue. 2011. Natural and anthropogenic Cd isotope variations. Pp. 124-154 in Handbook of Environmental Isotope Geochemistry, M. Baskaran, ed. Heidelberg: Springer.
Sherrell, R.M., E.A. Boyle, and B. Hamelin. 1992. Isotopic equilibration between dissolved and suspended particulate lead in the Atlantic Ocean: Evidence from 210Pb and stable Pb isotopes. J. Geophys. Res. 97(C7):11,257-11,268.
Shiel, A.E., D. Weis, and K.J. Orians. 2010. Evaluation of zinc, cadmium and lead isotope fractionation during smelting and refining. Sci. Total Environ. 408(11):2357-2368.
Stanton, R.L., and R.D. Russell. 1959. Anomalous leads and the emplacement of lead sulfide ores. Econ. Geol. 54(4):588-607.
Teutsch, N., Y. Erel, L. Halicz, and A. Banin. 2001. Distribution of natural and anthropogenic lead in Mediterranean soils. Geochim. Cosmochim. Acta 65(17):2853-2864.
Timm, L.C., L.F. Pires, K. Reichardt, R. Roveratti, J. Oliveira, and P Bacchi. 2005. Soil bulk density evaluation by conventional and nuclear methods. Aust. J. Soil Res. 43:97-103.
Vázquez Bahéna, A.B., O. Talavera Mendoza, M.E. Moreno Godínez, S.A. Salgado Souto, J. Ruiz, and G. Huerta Beristain. 2017. Source apportionment of lead in the blood of women of reproductive age living near tailings in Taxco, Guerrero, Mexico: An isotopic study. Sci. Total Environ. 583:104-114.
Viczián, M., A. Lásztity, and R.M. Barnes. 1990. Identification of potential environmental sources of childhood lead poisoning by inductively coupled plasma mass spectrometry. Verification and case studies. J. Anal. At. Spectrom. 5(4):293-300.
Wolfe, P.J., A. Giang, A. Ashok, N.E. Selin, and S.R.H. Barrett. 2016. Costs of IQ loss from leaded aviation gasoline emissions. Environ. Sci. Technol. 50(17)9026-9033.
Wombacher, F., M. Rahkämper, and K. Mezger. 2004. Determination of the mass-dependence of cadmium isotope fractionation during evaporation. Geochim. Cosmochim. Acta 68(1):2349–2357.
Zillow. 2017. How much is My Home Worth? [online]. Available: https://www.zillow.com/how-much-is-my-home-worth/ [accessed June 21, 2017].
This page intentionally left blank.














