6
Prospects for Molybdenum-99 Future Supply
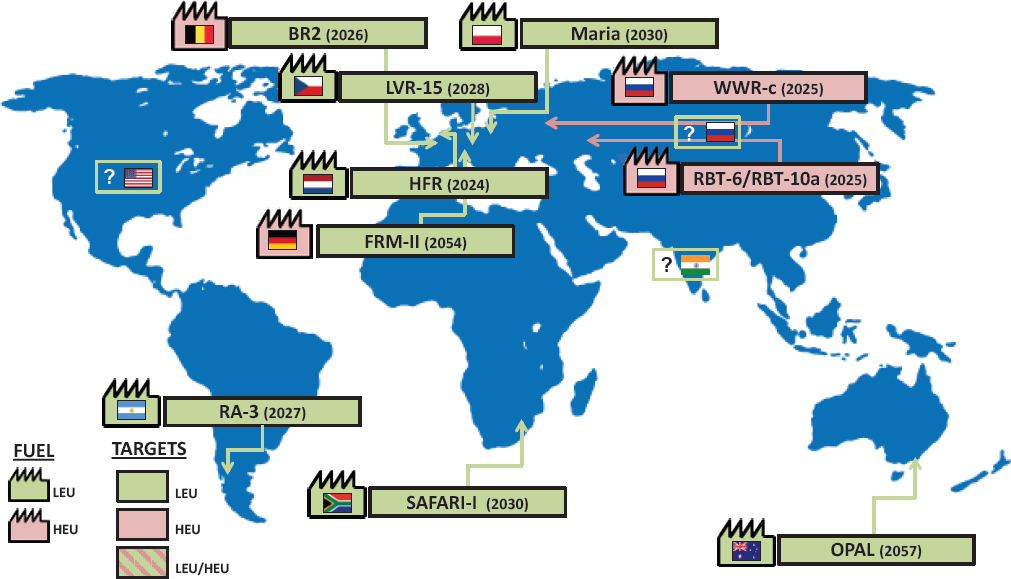
Dr. Ourania Kosti (National Academies) presented a series of speculative maps to illustrate the changes that could occur in molybdenum-99 (Mo-99) supply in the next 10-15 years (see Figures 6.1 and 6.2). The maps presented at the symposium were created using information primarily collected in the process of preparing the 2016 National Academies report and were revised for publication in this proceedings using updated information presented at the symposium. Most of the updates involved delays in Mo-99 production by several projects. Specifically:
- The Jules Horowitz Reactor (JHR), France, is expected to start operation in 2022, pushed back from 2020. The update was provided by Mr. Bernard Ponsard (SCK•CEN).
- The Kijang Research Reactor (KJRR), South Korea, is expected to start operation in 2022, pushed back from 2020. The update was provided by Dr. Ul-Jae Park (KAERI).
- The RA-10 reactor, Argentina, is expected to start operation after 2020; the reactor was expected to start operation in 2020. The update was provided by Dr. Pablo Cristini (National Atomic Energy Commission, Argentina).
- The FRM-II reactor, Germany, is expected to start producing Mo-99 in 2019, pushed back from 2018. The information was provided by Dr. Riane Stene (FRM-II).
Dr. Kosti noted several changes in the Mo-99 supply that could occur by 2020. For example,
- Irradiation facilities that currently irradiate HEU targets for Mo-99 production for medical use (i.e., BR-2, HFR, LVR-15, Maria) may only irradiate LEU targets.
- Existing reactor FRM-II, Germany, could start producing Mo-99 for medical use.
- One or more companies in the United States could start producing Mo-99.
- One or more companies in Canada could start producing Mo-99 or Tc-99m.
- Russia could increase Mo-99 production by introducing additional capacity from existing production facilities and/or from new projects.
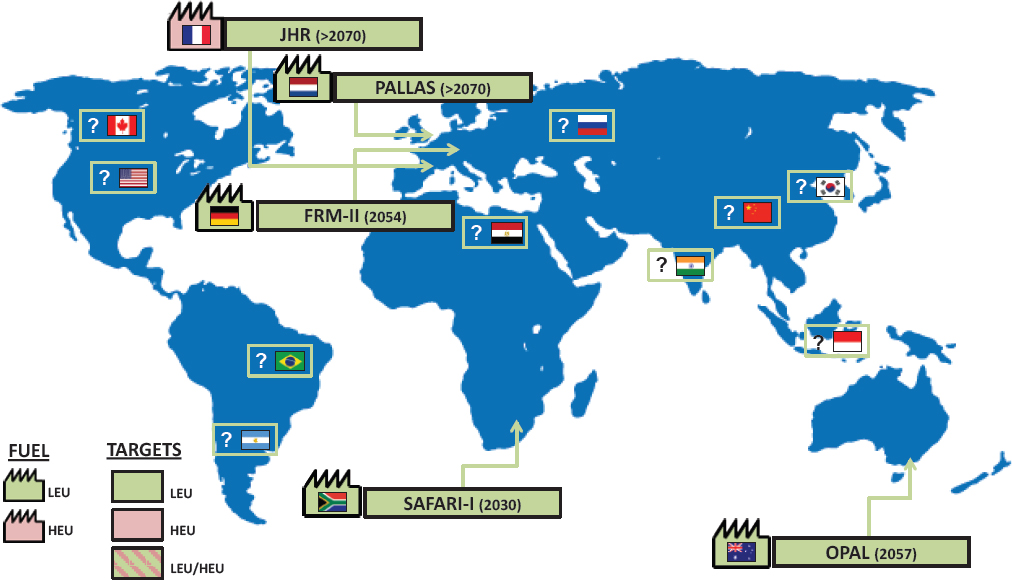
Dr. Kosti presented additional changes that could occur by 2030 and further change the Mo-99 supply chain map. For example, by 2030, it is possible that
- Several changes in the European reactor inventory could occur:
- BR2,1 Maria, LVR-15, and HFR will have reached the end of their operating lives and permanently shut down.
- JHR (France), a reactor currently under construction, could start production of Mo-99 in 2022.
- New reactor PALLAS (Netherlands) could start producing Mo-99 around 2026.
- SAFARI-I2 in South Africa could stop producing Mo-99 when its current operating license expires in 2030.
- Several new projects could be completed and producers in Argentina, Brazil, China, Egypt, India, Japan, and South Korea could start producing Mo-99 for domestic and regional supplies.
POTENTIAL NEW MOLYBDENUM-99 SUPPLIERS
Several countries are planning to develop new capabilities to produce Mo-99. The symposium organizing committees invited many of these potential new producers to provide brief presentations on the status of the Mo-99 production projects. Updates on the production plans of the projects represented at the symposium are summarized by country in the sections that follow.
___________________
1 As noted in Chapter 5, it is possible that the reactor’s operating license can be extended by 10 years, that is, to 2036.
2 Mr. Gavin Ball (NTP) noted that the reactor’s management team is investing in a plant-life extension program. It is possible that the reactor will operate beyond 2030.
Representatives from Algeria, Brazil, Japan, Indonesia, and the United States’ Niowave and Eden Radioisotopes were unable to participate; an invited potential producer from Poland responded that the Mo-99 production project has been halted; a representative from Nordion, Canada, was unable to provide an update on the collaborative project with General Atomics and the University of Missouri Research Reactor Center. Updates on the production plans of those projects that were not represented at the symposium are not summarized in this proceedings.
The United States
Several private-sector companies in the United States are planning to produce Mo-99 for medical use. Three of these companies (General Atomics, NorthStar Medical Radioisotopes, and SHINE Medical Technologies) have signed cooperative agreements with the Department of Energy’s National Nuclear Security Administration (DOE-NNSA). Each project carried out by these companies has been awarded $25M in cost sharing for work that contributes directly to the establishment of Mo-99 production capability. NorthStar and SHINE representatives provided updates on their Mo-99 production projects. As noted earlier, the General Atomics project was not represented at the symposium. In addition to the two cooperative agreement projects, representatives of three private companies working toward establishing U.S.-based production of Mo-99 without DOE-NNSA funding presented their projects at the symposium.
NorthStar Medical Radioisotopes
NorthStar, a company located in Beloit, Wisconsin, is pursuing two processes to establish capabilities to supply Mo-99 to the U.S. and global markets. These are:
- A neutron capture of molybdenum-98 using neutrons produced in a research reactor and
- A photon-induced transmutation of molybdenum-100 [reaction: 100Mo(γ,n)99Mo] using photons produced with electron accelerators.
Dr. James Harvey who represented NorthStar noted that the company intends to run both of these production processes in parallel in the future because “they provide redundancy and have different strengths in terms of approaching and serving the market.” NorthStar’s neutron capture process is the most advanced in terms of market readiness and could be market ready for Mo-99 production in early 2018. NorthStar’s photon-induced transmutation process could be market ready for Mo-99 production at the end of 2019.3
The Mo-99 produced by NorthStar’s two processes has low specific activity and cannot be loaded directly to conventional technetium generators. NorthStar has developed a new technetium generator system, the RadioGenix Tc-99m Generating System, to utilize low-specific-activity Mo-99. The RadioGenix system is a platform technology that NorthStar has used for other isotopes such as actinium/bismuth and tungsten/rhenium, and has been specifically developed for Mo-99/Tc-99m production. Tc-99m generated from the RadioGenix system meets the U.S. and European pharmacopeia standards for Tc-99m.
The RadioGenix Tc-99m Generating System was subject to a New Drug Application (NDA) by the U.S. Food and Drug Administration (U.S. FDA). Dr. Harvey noted that the regulatory approval process has been long: NorthStar met with the U.S. FDA to outline a path to NDA submission in 2010 and submitted the NDA in 2013. As of July 2017 the company was awaiting approval of a resubmission of the NDA to start producing Mo-99. He noted that the 7-year regulatory approval process compared in length with the conversion from the HEU to LEU production process, which was estimated by global producers to also take 6-7 years.
The RadioGenix system is not intended to be a “mobile” system similar to today’s conventional technetium generators, which are shipped to nuclear pharmacies daily. Instead, nuclear pharmacies certified to use the RadioGenix system will be equipped with the system, will be trained to use it, and will be receiving Mo-99 solution from NorthStar to load on the generator for Tc-99m elution. Dr. Harvey recognized that the RadioGenix system’s footprint is much larger than today’s conventional technetium generators but noted that it occupies about the same footprint as four of the conventional generators in their respective secondary shields. He also noted that it is intended for the U.S. centralized nuclear pharmacies, which are typically larger than hospital-based nuclear pharmacies. Mr. David Pellicciarini (Cardinal Health), who represented the largest centralized nuclear pharmacy chain in the United States, agreed that larger Cardinal Health facilities would likely be able to accommodate the RadioGenix system but added that smaller facilities within the Cardinal Health chain may be challenged.
SHINE
SHINE, a Wisconsin-based company, plans to use deuterium/tritium accelerator technology to induce sub-critical fissioning of U-235 in an LEU uranyl sulfate solution. SHINE plans to build eight accelerator-driven operating assemblies to produce up to 4,000 six-day Ci/week of Mo-99 for U.S. and global supply. The company also plans to supply I-131 and Xe-133.
Ms. Katrina Pitas, who represented SHINE, noted that the proposed process is cost-effective and generates less nuclear waste than conventional Mo-99 production methods. She added that the proposed process produces high-specific-activity Mo-99, compatible with existing technetium generators, without the need for a nuclear reactor.
SHINE’s facility in Janesville, Wisconsin, will be an integrated facility for medical isotope production, processing, and target (uranyl sulfate solution) production. The target in the proposed production method is expected
___________________
3 The information was provided by Mr. Peter Kartz (Department of Energy) at the 2017 Mo-99 Topical Meeting in Montreal, Canada, in September 2017.
to last the lifetime of the facility, but if this ends up not being the case, Ms. Pitas noted that SHINE will have the capability to remove impurities from the solution or to create a new solution.
SHINE submitted a facility construction permit application to the U.S. Nuclear Regulatory Commission (U.S. NRC) in 2013 and received approval in 2016. SHINE has entered into supply agreements with GE Healthcare, Lantheus Medical Imaging, and the Chinese company HTA Co., Ltd. The company anticipates starting medical isotope production in 2020.
Dr. Boris Zhuikov (Russian Academy of Sciences) commented on SHINE’s proposed production technology being an attractive alternative to uranium fission in a nuclear reactor, but noted that the company has not yet demonstrated the capability of the proposed system to produce high-specific-activity Mo-99. He added that developers of the SHINE technology would benefit from discussions with Russian investigators at Kurchatov Institute who used the Argus Reactor4 to test small-scale production of Mo-99 using LEU solution in 2014.
Northwest Medical Isotopes
Northwest Medical Isotopes (NWMI) plans to produce Mo-99 by irradiating LEU targets in a network of existing university research reactors. Ms. Carolyn Haass, who represented the company at the symposium, said that two of these reactors have been identified publicly and are the University of Missouri Research Reactor and Oregon State University’s TRIGA Reactor. A third reactor is being explored. For NWMI’s project, targets would be processed in a radioisotope production facility (RPF). The RPF will be located in Columbia, Missouri. In addition, the RPF will be used to recover uranium from the dissolved LEU solution and recycle it back into new LEU target material. A construction permit application for the RPF was submitted to the U.S. NRC in two parts: Environmental Report in January 2015 and Safety Report in July 2015. A decision is expected in January 2018. NWMI’s production capacity is 3,500 six-day Ci of Mo-99/week with the potential to increase it to 5,000 six-day Ci/week.
NWMI’s project proposes recycling and recovering LEU to minimize radioactive waste generation. The recycling process is proprietary. Ms. Haass outlined a high-level summary of the LEU recycling and recovery process.
- First-stage recovery. Mo-99 anion exchange (IX) column LEU stream is held in lag storage tanks to allow decay of select radionuclides. The decayed uranium solution is diluted and pumped through first-stage IX columns to separate bulk fission product contaminants. Uranium is then eluted from IX column, and concentrator/condenser is then used to concentrate eluate for second-stage IX uranium recovery. Waste from the dilution is sampled and sent to a high-dose liquid waste accumulation tank. Condensate is sent to a low-dose liquid waste accumulation tank.
- Second-stage recovery. Interim uranium product solution is processed through a second-stage IX column to remove trace contaminants. Uranium is then eluted from the IX columns and a concentrator/condenser is used to control the volume of recycled uranium product. The final uranium product solution is sampled to confirm that it meets recycle specifications. Waste generated from the second-stage recovery is sampled and sent to the high-dose liquid waste accumulation tank. Condensate is sent to a low-dose liquid waste accumulation tank.
- Product uranium lag storage. Product uranium lag storage allows for U-237 decay so that the recovered LEU can be contact-handled during LEU target fabrication.
Coquí RadioPharmaceuticals
Coquí RadioPharmaceuticals, a Puerto Rico–based company, plans to produce Mo-99 by irradiating LEU targets in two 10-megawatt (MW) LEU-fueled research reactors similar to Australia’s INVAP-designed OPAL reactor. Ms. Carmen Bigles noted that the company plans to build these reactors and associated Mo-99 production
___________________
4 The Argus Reactor was the first solution reactor designed in the former Soviet Union in the 1980s. Currently this reactor is used for testing and experiments and not for regular medical isotope production.
facility on the “Duct Island” site at Oak Ridge, Tennessee. Coquí aims to produce Mo-99 to meet greater than 50 percent of U.S. demand and for exports. The company’s plan calls for start of construction of the reactors in 2020 and production of Mo-99 in 2023.
Flibe Energy
Flibe Energy, located in Huntsville, Alabama, aims to develop a 250-MWe liquid fluoride thorium reactor (LFTR) for the main purpose of electrical power generation and a secondary purpose of medical isotope production. Thorium in the reactor fuel captures a neutron and is transmuted to uranium-233 (U-233), which is fissile. The fission of U-233 with neutrons produces a spectrum of fission products including noble metals, molybdenum, and noble gases such as xenon. Removal of noble metals from liquid-fluoride reactors was demonstrated during the operation of the Molten-Salt Reactor Experiment at Oak Ridge National Laboratory in the mid-1960s, but separation of Mo-99 has not yet been demonstrated. Because LFTRs are designed to operate continuously for the lifetime of the plant, Mo-99 can be removed from the liquid fuel stream along with other noble metal fission products as part of the normal operation of the reactor’s chemistry management system.
Dr. Matthew Lish, who represented Flibe Energy, described the company’s proposed production method as a long-term strategic plan for securing future Mo-99 supply. Upon funding, the company is targeting application for licensing of a demonstration reactor in approximately 5 years (around 2022). The demonstration reactor would be 2-5 megawatts thermal (MWT) capable of producing about 7,000 six-day Ci/week per MWT.
Russia
Representatives of the Russian State Atomic Energy Corporation, Rosatom, Mr. Risovaniy (Rosatom Headquarters), Mr. Vakulenko (JSC Isotope), and Dr. Kononov (Karpov Institute), discussed three prospects for producing Mo-99 from non-HEU sources in Russia. The goal of these projects carried out by Rosatom is to expand supply to the global market to reach up to a 20 percent share:
- Production in an aqueous homogeneous reactor (or solution reactor). In 2015, Rosatom made a decision to construct a proof-of-concept solution reactor in Sarov (Nizhniy Novgorod region) dedicated to Mo-99 production. Construction started in 2017 and is expected to be completed in 2018; operation of the reactor is expected to start in 2019. Mo-99 production capacity from one solution reactor is about 250 six-day Ci/week and can be scaled up by installing more reactors. The advantages of solution reactors for medical isotope production were not discussed at the symposium but they are comprehensively discussed elsewhere (IAEA, 2008).
- Production in nuclear power plants with RBMK reactors. Karpov Institute is exploring the possibility of producing Mo-99 in RBMK nuclear power plant reactors by irradiating LEU targets. Mr. Vakulenko and Dr. Kononov noted that key features of RBMK reactors allow the production of medical isotopes without affecting the reactor’s main purpose of operation, which is production of electricity. One of these key features is the ability to take the targets that are irradiated at periphery channels of the reactor for processing while the reactor is still operating to produce electricity.
The technology for producing radionuclides in an RBMK reactor was demonstrated at the Leningrad Nuclear Power Plant in the early 1990s, and starting in 2001 several targets have been irradiated at that site to produce medical isotopes including Mo-98 targets for small-scale production of Mo-99. To date, irradiation of uranium targets for Mo-99 has not been demonstrated in an RBMK.
Rosatom is planning to test production at the RBMK reactor at Smolensk Nuclear Power Plant, the youngest of the three RBMK reactors5 currently operating in Russia. Karpov Institute is working on designing LEU targets for irradiation at Smolensk and on optimizing the target chemical processing. Dr. Kononov noted that validation of LEU target irradiation could start in early 2018. In his view this is
___________________
5 The RBMK reactor at Smolensk is expected to be decommissioned in 2034.
-
a promising non-HEU-sourced Mo-99 production method with the potential to produce large amounts of Mo-99, about 2,000 six-day Ci/week.
- Production via neutron capture in high-flux reactors. Rosatom is exploring the possibility of irradiating enriched Mo-98 targets in high-flux reactors such as the SM-3 reactor at RIAR (flux up to 5.0 × 1015/(cm2 s)) for the production of Mo-99. This project is based on a new method of Mo-98 activation, currently at early stages of investigation, which uses nanopowder and the Szilard-Chalmers effect. Preliminary calculations have shown that the specific activity of Mo-99 produced with this method is around 50-70 Ci/g, which is higher than that achieved by conventional neutron activation. Mr. Risovaniy appeared to favor this production method of Mo-99 production because of some benefits it offers, including relatively low production costs and low waste generation.
Mr. Vakulenko explained that Rosatom’s goal to capture a large (up to 20 percent) share of the global Mo-99 market relies on the success of one or more of these three projects. He added that all projects are at initial stages of development, and it is too early to predict their success and provide timelines for completion. When asked by several symposium participants to provide further clarity on the schedule for deciding which project Rosatom will pursue further, he said that he anticipates JSC Isotope will make a decision in 2018 on which method of production from non-HEU sources appears to be the most promising from technological, economic, and market perspectives, and focus on the market readiness of that production method.
A fourth prospect for Mo-99 production in Russia is under development at Tomsk Polytechnic University and was presented by Prof. Victor Skuridin and Dr. Evgeniy Nesterov. This project aims to build on the university’s existing Mo-99 production capabilities (see Chapter 3) by irradiating enriched Mo-98 targets and activating both thermal and resonance neutrons to increase the reaction cross-sections and achieve higher-specific-activity Mo-99 than conventional neutron activation. Tomsk Polytechnic University has tested this method at its IRT-T 6 reactor, a 6-MW reactor with neutron flux equal to 1.7 × 1014/(cm2 s). Mo-99 of specific activity up to 15 Ci/g (at the end of bombardment) has been achieved. The group is working on optimizing the reflector geometry to increase activity to 50 Ci/g or higher.
Mo-99 produced with this method can be loaded on generators that are not much larger than conventional generators, and breakthrough is within limits for authorization of distribution within Russia. (It was later noted that Mo-99 produced via neutron activation methods would not be suitable for loading on conventional generators for distribution in Europe or the United States because of failure to meet U.S. or European pharmacopoeia specifications for technetium due to breakthrough.) Tomsk Polytechnic University, in collaboration with the IAEA, is developing regional production using this method, estimated to be market ready in a few years.
Dr. Nesterov noted that, in an effort to reduce costs of purchasing Mo-98,6 Tomsk Polytechnic University is exploring a procedure for reusing Mo-98 so that only 100g of enriched material is expended over a 5-year operation. The radioactive waste generated during the process is only a small fraction (1/10,000) of the produced material activity as opposed to uranium fission, which produces waste that is of 10 times higher activity than that of the product.
Argentina
In 2010, CNEA announced the decision to carry out a project that involves the design, construction, licensing, and operation of a new multipurpose nuclear reactor, RA-10. RA-10 will be a 30-MW reactor, dedicating about half of its operation to medical isotope production. The reactor will be able to produce 2,500 six-day Ci/week of Mo-99 to cover domestic supplies and expand international supplies. The reactor will also be producing 400 Ci of I-131 per week via fission. Dr. Pablo Cristini (CNEA) noted that construction of the RA-10 reactor started in May 2017 and the reactor is expected to start producing Mo-99 after 2020.
___________________
6 The cost is about $200 per gram of Mo-98.
Brazil
Dr. Cristini (CNEA) and Mr. Osso (IAEA) were asked to comment on the status of the Brazilian reactor RMB (Brazilian Multipurpose Research Reactor), a “twin” reactor in terms of design to the RA-10 reactor in Argentina. They noted that the project had been proceeding slowly for 2-3 years because of lack of financing from the Brazilian government. The project was recently revived, and the RMB reactor may start operating around 2023. The reactor’s capacity will be 1,000 six-day Ci/week intended to cover domestic demand (estimated to be around 350-400 six-day Ci/week) and some regional supplies.
Canada
The Canadian government has invested in four projects to produce Mo-99 and Tc-99m via nonfission to cover Canadian demand for Mo-99 (and Tc-99m) which is currently about 500 six-day Ci/week. Two projects were discussed at the symposium:
- TRIUMF’s project, which aims to produce Tc-99m via the 100Mo(p,2n)99mTc reaction using cyclotrons and
- Canadian Isotope Innovations Corporation’s (CIIC’s) project, which aims to produce Mo-99 via the 100Mo(γ,n)99Mo reaction using linear accelerators.
Mr. Ken Buckley (TRIUMF) and Dr. Kennedy Mang’era (CIIC) said that the approaches Canada is currently exploring have several advantages over the current fission-based Mo-99 production and supply approaches. These advantages include
- Diversification of technologies used for Mo-99/Tc-99m supply,
- Decentralization of Mo-99/Tc-99m supply,
- Lower costs associated with construction and operation of Mo-99/Tc-99m facilities, and
- Scalability of supply.
Some project-specific information is provided in the following sections.
TRIUMF
TRIUMF collaborates with four other institutions (University of British Columbia, British Columbia Cancer Agency, Centre for Probe Development and Commercialization, and Lawson Health Research Institute) to produce Tc-99m in cyclotrons. The consortium was tasked with
- Demonstrating routine, reliable commercial-scale production of Tc-99m,
- Obtaining regulatory approval for clinical use of the produced Tc-99m in humans,
- Establishing a business plan for Tc-99m distribution, and
- Commercializing the technology to other producers.
Mr. Buckley noted that the TRIUMF consortium alone, which includes three cyclotrons of suitable energy for Tc-99m production, can provide about 134,000 GBq (3,600 Ci) Tc-99m7 annually. There are 15 cyclotrons in Canada that in total could provide 1,388,000 GBq (37,500 Ci) Tc-99m annually from enriched Mo-100, which is over half the demand for Tc-99m in Canada. He added that Tc-99m produced using the TRIUMF technology may be priced differently depending on the size of the cyclotron used for production. For example, Tc-99m produced using a small, 16-MeV/100 µA cyclotron, which has a lower production yield, will be more expensive compared to that produced using a 24-MeV/500 µA cyclotron, which has a higher production yield.
___________________
7 The concept of the six-day curie of Mo-99 is not relevant for direct Tc-99m production by cyclotrons where Mo-99 plays no role.
Cyclotrons are routinely used for production of positron emission tomography (PET) medical isotopes, but commercial-scale production for Tc-99m was first demonstrated by TRIUMF. Because this is a novel production method for direct production of Tc-99m, TRIUMF is required to file a new drug submission to Health Canada. For the submission, TRIUMF will utilize data on the performance of cyclotron-produced Tc-99m from two studies:
- A small clinical trial to show that the clinical results of technetium produced directly from cyclotrons are not inferior to the clinical results obtained from generator-derived technetium and
- A kit labeling study to validate the labeling performance of cyclotron-produced technetium.
The project is anticipated to be market-ready in 2018. The TRIUMF consortium formed the company ARTMS Products to disseminate and commercialize the technology to other producers. In May 2017, Alliance Medical in the UK entered into an agreement with ARTMS to receive the required products and procedures for the production of Tc-99m using cyclotrons.
Canadian Isotope Innovations Corporation
CIIC proposes to use a linear accelerator (LINAC) to produce Mo-99 from enriched Mo-100 at a facility in Saskatoon, Canada. CIIC is currently validating the technology, and key processes are complete or in advanced development stages. Dr. Mang’era noted that a pilot facility has been operational since 2015 with a capacity for 100 Ci/week of Mo-99 (calibrated 1 day post-end of bombardment [EOB]). CIIC has completed the design and developed a business plan for a larger facility with capacity to produce about 1,100 Ci/week of Mo-99 that would be operational 3 years after funding is secured. The company’s business approach relies on the company shipping Mo-99 solution to its customers for extraction of Tc-99m at the customer’s site using the CIIC-supplied separation generator, the Next-Gen LSA Generator. CIIC would then reclaim and recover the Mo-100, which will be recycled into fresh Mo-100 enriched targets.
Dr. Mang’era noted that the success of CIIC’s business plan relies on
- Secure supply of enriched Mo-100 for target preparation,
- Development of the Next-Gen LSA Generator,
- Health Canada and U.S. FDA approvals of the Next-Gen LSA Generator,
- Market acceptance of alternative nonfission Mo-99/Tc-99m sources,
- Private-investment fundraising for business plan execution, and
- Implementation of full cost recovery by Mo-99/Tc-99m producers.
China
China imports Mo-99 from NTP, ANSTO, and IRE to cover its demand, which is currently estimated at 280-300 six-day Ci/week but is growing at a rate of approximately 5 percent per year. (Mr. Joao Osso [IAEA] pointed out that demand for Mo-99 in China remains very low, if one accounts for the country’s large population.) Dr. Jin Du (China Isotope & Radiation Corporation) noted that the growing demand has led the China National Nuclear Corporation (CNNC) to explore domestic production of Mo-99.
CNNC is a large state-owned enterprise under the direct management of the central government of China. Several radioisotope producers within CNNC have attempted to establish Mo-99 production using different methods, but plans were abandoned in the early 2000s due to high production costs.
Dr. Du discussed two current projects in China aiming to produce Mo-99 to cover domestic needs:
- China Advanced Research Reactor (CARR), a 60-MW reactor located in Fangshan District, Beijing. CARR started operating in 2010 and aims to produce 1,000 six-day Ci/week of Mo-99. Testing of Mo-99 production at CARR is expected to start in 2020. The reactor will also produce iodine-131 (by irradiating tellurium targets) and other isotopes used in nuclear medicine.
- Research and development of the Medical Isotope Production Reactor (MIPR) project, an aqueous homogeneous reactor to be operated by NPIC. The production capacity for Mo-99 will be about 2,000 six-day Ci/week. NPIC applied for a construction permit in May 2017, but a schedule for starting Mo-99 production has not been made public. The reactor will also produce iodine-131 and strontium-89.
Egypt
Egypt has produced about 70-75 six-day Ci/week of Mo-99 every 2 weeks since 2015 by irradiating LEU targets at the Egyptian Atomic Energy Authority’s ETRR-2 complex located at Inshas (a suburb of Cairo). Production at ETRR-2 covers part of Egypt’s Mo-99 demand estimated to be 40-80 six-day Ci/week. Dr. Mostafa Abd Elaal, who represented the Egyptian Atomic Energy Authority, noted that the agency aims to increase production to 200 six-day Ci/week within the next 1-3 years and—with IAEA support—to 400 six-day Ci/week after that.
India
India currently imports Mo-99 from Russia and Belgium to cover most of the country’s demand and produces small amounts of low-specific-activity Mo-99 by neutron capture in the Dhruva research reactor owned and operated by Bhabha Atomic Research Centre (BARC). Dr. Anupam Mathur (Department of Atomic Energy) noted that BARC is currently working with the Argentinian company INVAP to develop capability to produce about 300 six-day Ci/week of high-specific-activity Mo-99 by irradiating LEU targets at the new Apsara reactor or Dhruva reactor. Mo-99 produced at the Apsara or Dhruva reactors will cover domestic needs (estimated at about 200 six-day Ci/week) and for supply to the international market (100 six-day Ci/week).
LEU target design has been completed by BARC, and some natural uranium “dummy” targets have been prepared and sent to INVAP for testing. BARC faces a technical challenge related to different irradiation protocols between INVAP and the Indian reactors (Apsara and Dhruva), which may affect Mo-99 production yield. Production of Mo-99 at Apsara or Dhruva reactors is estimated to start in 2019.
Netherlands
The PALLAS reactor in Netherlands, is an initiative for the future replacement of the HFR when it reaches its end of operations around 2025. The PALLAS reactor is a separate legal entity from NRG (the operator of the HFR), conceptualized in 2013. It will be a 20- to 25-MW reactor, built specifically for medical isotope production on the same site as HFR in Petten, 50 kilometers north of Amsterdam. Mr. Hermen van der Lugt noted that PALLAS has contracted with the designer of the nonnuclear facilities and buildings. PALLAS issued an award to the designer of the nuclear island in January 2018. A licensable design is expected to be completed by 2019 and construction by 2025. The PALLAS reactor will have a capacity for Mo-99 production at least at the level of the HFR capacity. It will also produce a range of other isotopes for medical use.
South Korea
South Korea currently imports Mo-99 from Australia, South Africa, and Russia to cover all of its demand, which is about 150 six-day Ci/week. The Korean government launched the new research reactor project in 2012 to build the 15-MW Kijang Research Reactor (KJRR) to produce Mo-99, I-131, Ir-192, and other medical isotopes. Dr. Ul-Jae Park (Korea Atomic Energy Research Institute) noted that production of Mo-99 at KJRR is expected to start in 2022, a delay of about 2 years since the previous schedule estimate, because of additional seismic specifications imposed by the South Korean regulatory authority following the September 2016 earthquake near the site. These additional regulatory specifications have also added to the cost of construction of the proposed reactor.
Dr. Park noted that South Korea aspires to become the first large-scale Mo-99 producer in Asia and change the existing Asian supply chain, which relies on reactors in Europe, Australia, and South Africa. Initially KJRR
will produce about 500 six-day Ci/week and gradually increase to 2,000 by 2025. Most of the Mo-99 produced at KJRR will be available to the global market.
MARKET-READINESS AND PENETRATION CHALLENGES
Mr. Gavin Ball (NTP) commented on the often ambitious schedules of new potential producers entering the Mo-99 market. Specifically, he thought that many potential producers underestimate the time needed for development, industrialization, and validation of both conventional and alternative technologies. He added that receiving regulatory approvals to construct, operate, and sell medical isotopes can also be long processes. Therefore, many of the projects presented at the symposium are unlikely to be market ready by the dates presented at the symposium (and summarized in Table 6.1).
In addition to the market-readiness challenge, other symposium participants commented on the market penetration challenge that potential new producers will likely face. This is because potential new producers will have to compete with existing global producers who currently can meet demand and the additional 30 percent outage reserve capacity recommended by the Organisation for Economic Co-operation and Development’s Nuclear Energy Agency (OECD-NEA) and have marketing and management experience in doing so. Some additional production capacity has been recognized as needed by organizations such as the OECD-NEA to minimize risks of shortages, especially after the NRU reactor in Canada permanently shuts down in March 2018; however, there has not been an analysis on how much added capacity is needed to ensure a long-term sustainable Mo-99 supply.
U.S. National Academies’ committee member Dr. Gene Peterson (Los Alamos National Laboratory) noted that with the large number of new projects aiming to produce Mo-99 within the next 5-10 years, production capacity could increase to over three times the current available capacity (see Table 6.1). Without an indication that global demand for Mo-99 is likely to increase substantially in the next few years this additional production capacity cannot possibly be absorbed by the market. IRE’s Jean-Michel Vanderhofstadt agreed with the comment and added that the excess capacity would disrupt the dynamics of the Mo-99 market, a market that is already “not greatly profitable.” He could not elaborate further to avoid violating antitrust policies.
Representatives of two new Mo-99 projects responded to the comment on market penetration raised by Dr. Peterson and Mr. Vanderhofstadt. Dr. Cristini (CNEA) said that any potential producer and its investors are free to use funds as they judge appropriate. Argentina’s CNEA, for example, has built what the company considers a viable business plan by investing in the RA-10 reactor to supply Mo-99 domestically and regionally. Dr. Harvey noted that NorthStar’s investment team is committed to taking appropriate actions to overcome the market penetration challenge.
The challenges that new producers might face to build a technically and commercially competitive business were recognized by several symposium participants. Others noted that it is a mistake to conclude that some aspiring domestic/regional and global producers will not succeed in establishing production capacity. For example, a participant noted that it is possible that smaller local or regional producers could be preferred over global producers when selling Mo-99 in the local or regional markets because of favorable logistics of transportation, possible cost advantages, and other factors. As several countries become self-sufficient in terms of Mo-99 supply or rely on regional supplies (for example the representative from South Korea noted that the country aims to become the first large-scale supplier in Asia), existing global producers could lose part of their current supply share.
A potential solution to existing and new producers not having to compete for a share of the existing market is to increase demand for Mo-99. Mr. Vanderhofstadt, in his capacity as president of the Association of Imaging Producers and Equipment Suppliers (AIPES), said that AIPES engages with the European and U.S. nuclear medicine associations to stimulate development of new Tc-based compounds for single-photon emission computed tomography (SPECT) and SPECT/CT. However, this is a long-term (maybe 20-year or so) project and will not provide a solution to the market-sharing problem in the short run. In a separate discussion at the symposium Mr. Vanderhofstadt admitted that IRE follows the trend in nuclear medicine and is investing in PET radiopharmaceuticals. Other companies such as Lantheus Medical Imaging are also investing in development of PET (and not SPECT) radiopharmaceuticals.
TABLE 6.1 Current Mo-99 Producers and New Projects Presented at the Symposium
| Company/Project | Method | Reactor | Production Capacity (6-day Ci/week) | Anticipated Production Year | Plans for Expansion (Mo-99 expressed in 6-day Ci/week) |
|---|---|---|---|---|---|
| Existing Global Producers | |||||
| ANSTO | Fission of U-235 (LEU) | OPAL | 2,150 | Producing currently | 2,650 by Q4 2017 3,500 by Q1 2018 |
| Curium | Fission of U-235 (HEU) | HFR, BR2, Maria | 4,500 | Producing currently; converting to LEU | 5,000 in Q4 2017 ~4,500 in 2018 |
| IRE | Fission of U-235 (HEU) | HFR, BR2, and LVR-15 | 3,600 | Producing currently; converting to LEU | 3,500 in 2018 |
| NTP | Fission of U-235 (LEU) | SAFARI-I | 3,500 | Producing currently | N/A |
| Existing Smaller Producers | |||||
| RIAR | Fission of U-235 (HEU) | RBT-6 and RBT-10a | 1,000 | Producing currently | 2,000 by 2019 |
| Karpov Institute | Fission of U-235 (HEU) | WWR-c | 350 | Producing currently | 700 by 2020 |
| Argentina | Fission of U-235 (LEU) | RA-3 | 400 | Producing currently | N/A |
| Tomsk Polytechnic University | Neutron capture | IRT-T 6 | ~10 | Resuming production in 2018 | N/A |
| Khlopin Radium Institute | Neutron capture | RBMK, Leningrad Nuclear Power Plant | ~10 | Producing currently | N/A |
| Egypt | Fission of U-235 (LEU) | ETRR-2 | 75 | Producing currently | 200-400 after 2020 |
| New Projects | |||||
| United States | |||||
|
NorthStar |
Neutron capture | MURR | ~3,000 six-day Ci | 2018 | >3,000 six-day Ci |
|
NorthStar |
Photon-induced transmutation of Mo-100 | N/A | ~3,000 six-day Ci | 2019 | >3,000 six-day Ci |
|
SHINE |
Fission of U-235 (accelerator-driven) (LEU) | N/A | ~4,000 | 2020 | – |
|
NWMI |
Fission of U-235 (LEU) | MURR, Oregon State University | 3,500 | Not provided | 5,000 |
|
Coquí |
Fission of U-235 (LEU) | To be built in Oak Ridge, Tennessee | 2,500 | 2023 | |
|
Flibe Energy |
Fission of U-233 generated from Th-232 | To be built | >9,000 | After 2022 | |
| Russia | |||||
|
Rosatom |
Fission of U-235 in aqueous homogeneous reactor (LEU) | Sarov | 250 | Not provided | Scaled-up production |
|
Rosatom |
Neutron capture | SM-3 | Not provided | Not provided | |
|
Rosatom |
Fission of U-235 (LEU) | RBMK | 2,000 | Not provided | |
| Canada | |||||
|
TRIUMF |
Direct production of Tc-99m (cyclotron) | N/A | Equivalent of about 250 | 2018 | |
|
CIIC |
Photon-induced transmutation of molybdenum-100 (LINAC) | N/A | 1,200 | Not provided | |
| Argentina | Fission of U-235 (LEU) | RA-10 | 2,500 | 2021 | |
| Brazil | Fission of U-235 (LEU) | RMB | 1,000 | 2023 | |
| China | Fission of U-235 (LEU) | CARR | 1,000 | After 2020 | |
| Fission of U-235 in aqueous homogeneous reactor (LEU) | MIPR | 2,000 | Not provided | ||
| India | Fission of U-235 (LEU) | Apsara or Dhruva | 300 | 2019 | |
| Netherlands | Fission of U-235 (LEU) | PALLAS | ≥6,200 | 2025 | |
| South Korea | Fission of U-235 (LEU) | KJRR | 500 | 2022 | 2,000 by 2025 |
NOTES: New projects aiming to produce Mo-99 that were not presented at the symposium are not summarized on this table.
For acronyms and abbreviations, see same-title section at the beginning of this proceedings.
This page intentionally left blank.














