4
Applying Social and Behavioral Sciences to Combating Antimicrobial Resistance
In session II of the workshop, speakers and discussants explored possibilities for applying social and behavioral sciences to address antimicrobial resistance. The first half of the session, moderated by Franck Berthe, senior livestock specialist in the Agriculture Global Practice of the World Bank, focused on reducing the use of antimicrobials and on strategies for achieving desired behavior change through stewardship programs, incentives, and policy for the responsible use of antimicrobials. Helen Boucher, professor of medicine and director of the infectious diseases fellowship program at Tufts Medical Center, discussed effective guidance for reducing antimicrobials use in health care. David Sjeklocha, operations manager of animal health and welfare at Cattle Empire, surveyed guidelines for antimicrobial usage in the beef industry, and Randall Singer, professor of epidemiology at the University of Minnesota, explored the changing paradigm of antimicrobial use in veterinary medicine. Bruce Stewart-Brown, senior vice president of food safety, quality, and live operations at Perdue Farms, provided retailer and consumer perspectives on eliminating antibiotic use in the broiler industry. Mary Wilson of the University of California, San Francisco, moderated the second half of the session, which focused on reducing the need for antibiotics by exploring ways to achieve desired behavior change through prevention measures and education. The concept of bacterial stewardship in production animal agriculture and companion animal medicine was presented by H. Morgan Scott, professor of veterinary pathobiology at Texas A&M University. Jeffrey Linder, professor of medicine and chief of general internal medicine and geriatrics at the Northwestern University Feinberg School of Medicine, discussed strategies
for leveraging behavioral interventions to achieve appropriate antibiotic prescribing practices. Andrew Maccabe, chief executive officer of the Association of American Veterinary Medical Colleges (AAVMC), surveyed the role of academic veterinary medicine in combating antimicrobial resistance. Darrell Kirch, president and chief executive officer of the Association of American Medical Colleges, concluded the session by reflecting on the changing paradigm of medical education and its impact on the next generation of health professionals.
REDUCING ANTIMICROBIAL USE: STEWARDSHIP PROGRAMS, INCENTIVES, AND POLICY
Effective Guidance for Reducing the Use of Antimicrobials in Health Care
Boucher focused on effective guidance for reducing antimicrobial use in health care settings. She began by defining antimicrobial stewardship as involving
the optimal selection, dose, and duration of an antibiotic resulting in the cure or prevention of infection with minimal unintended consequences to the patient including emergence of resistance, adverse drug events, and cost.1
The goals of antimicrobial stewardship, said Boucher, are patient focused: improving care and health care outcomes. Decreasing antibiotic resistance is also a goal, she said, although there is debate over how to measure and report resistance. Progress is being made, she said, but more research is needed about how to most effectively approach antibiotic stewardship, how to influence prescriber behavior, and how to prevent the spread of antibiotic resistance in acute care, long-term care, and ambulatory settings. Boucher highlighted some promising regulatory developments in the United States, such as the Joint Commission’s new antimicrobial stewardship standard applicable in all health care settings (Joint Commission, 2016). The Centers for Medicare & Medicaid Services (CMS) has proposed a rule for stewardship in long-term care,2 she said, and organizations such
___________________
1 The definition is from collaborative guidelines created by the Infectious Diseases Society of America (IDSA) and the Society for Health Care Epidemiology of America (SHEA), updated in 2016 (Barlam et al., 2016).
2 For more information on the proposed rule by CMS, see www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2016-Fact-sheets-items/2016-06-13.html (accessed July 30, 2017).
as the Leapfrog Group3 and others monitor antimicrobial stewardship practices in health care settings.
Boucher directs the infectious diseases fellowship program at Tufts Medical Center, which started an antimicrobial stewardship program nearly 15 years ago. She said that the program’s patient-focused approach is focused on ensuring appropriate empirical therapy via antimicrobial choice, dosage, route, and duration. Prescribers are educated on the importance of prudent antimicrobial prescribing to reduce medication errors and costs, as well as switching from intravenous (IV) to oral treatment when possible, she added. Formulary restriction, preauthorization protocols, and a system of prospective auditing are also in place. The program’s aim is better outcomes for patients, including better survival rates, fewer adverse drug events, shorter hospital stays, lower rates of resistance, and reduced “collateral damage” of antibiotics, such as Clostridium difficile infections.
Effects of Improved Antibiotic Stewardship
Boucher remarked that for many diseases, involving infectious disease professionals early on leads to lower costs and better outcomes for patients. According to a nationwide survey on antimicrobial stewardship program characteristics, Boucher said, institutions with formal antimicrobial stewardship programs are more likely to have antibiograms,4 infectious disease consultation services, fellowship programs, and higher admissions (Doron et al., 2013; see Box 4-1 for more about the survey). Current IDSA and SHEA guidelines report that comprehensive stewardship programs have consistently saved inpatient health care institutions between $200,000 to $900,000 per year by decreasing antimicrobial use, she said (Doron and Davidson, 2011). At Tufts Medical Center, she added, savings in drug costs alone are estimated at $400,000 annually and at more than $5.6 million since the program’s inception. Published studies confirm the economic benefits of stewardship program interventions, she noted. Restricting cephalexin in a municipal hospital decreased costs for that antibiotic by nearly 30 percent (Seligman, 1981). Implementing a full-service antimicrobial management team at an academic medical center generated savings in use costs of $3 million over 3 years (Standiford et al., 2012). In another hospital, IV to oral conversion of fluoroquinolones saved $4 million over 4 years (Jones et al., 2012).
___________________
3 The Leapfrog Group is a nonprofit watchdog organization for health care consumers that scores hospitals based on their commitment to antimicrobial stewardship principles. For more information, see www.leapfroggroup.org/ratings-reports/antibiotic-stewardship (accessed July 30, 2017).
4 An antibiogram is a periodic summary of antimicrobial susceptibility testing results of a specific microorganism to certain antimicrobial drugs.
Recent studies demonstrate that antibiotic stewardship interventions can have other types of effects, she said, such as decreasing infection and colonization of Clostridium difficile and antibiotic-resistant bacteria (Baur et al., 2017). Another study reported that among hospitalized patients who receive 1 or more days of antibiotic treatment, 20 percent develop an adverse drug event linked to that antibiotic and 20 percent of those events are attributable to antibiotics prescribed for conditions for which they are not indicated (Tamma et al., 2017). Therefore, every 10 days of antibiotic treatment conferred a 3 percent additional risk of an adverse event, Boucher said.
Core Elements of Hospital Antibiotic Stewardship Programs
Boucher summarized guidance from the U.S. Centers for Disease Control and Prevention (CDC) regarding core elements of hospital antibiotic stewardship programs, which she urged all programs to implement (CDC, 2014). The guidance includes
- Establishing leadership commitment—from the C-suite in the hospital or health care system down to the physician’s office—to ensure that the necessary human, financial, and information technology resources are dedicated.
- Ensuring accountability through a single leader who is responsible for program outcomes; an infectious-disease trained physician is in a uniquely qualified position to be an effective leader.
- Providing drug expertise, ideally through a pharmacist leader trained in infectious disease, who is responsible for working to improve antibiotic use.
- Implementing at least one recommended action, such as systemic evaluation of ongoing treatment need after a set period of initial treatment (e.g., an “antibiotic time out” after 48 hours).
- Tracking and monitoring patterns of prescriptions and resistance using, for example, the antibiotic use module from the National Healthcare Safety Network of CDC.
- Reporting information on antibiotic use and resistance on a regular basis to doctors, nurses, and relevant staff.
- Educating clinicians about antibiotic resistance and optimal prescribing.
The One Health Approach to Antibiotic Stewardship
The first report from the Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria (PACCARB, 2016) advocated a One Health approach for stewardship, said Boucher. It recommends implementing efforts to promote adoption of antibiotic stewardship in curricula by faculty in colleges of human and veterinary medicine. It aims to promote a culture of stewardship as an integral part of continuing education and clinical practice, she said. Ensuring an adequate stewardship workforce of trained infectious disease physicians and pharmacists is important, according to Boucher, as is collaboration between CMS (which develops all the quality improvement tools and metrics), CDC, and state and local infection prevention programs. She highlighted the need to examine and improve good stewardship practices in outpatient settings.
Guidelines for Antimicrobial Usage in the Beef Industry
Programs Addressing Antimicrobial Use in the Beef Industry
Sjeklocha explained that programs addressing antimicrobial use have been in place since the mid-1980s in the beef industry. For example, the Beef Quality Assurance (BQA) began as a food safety and residue avoidance
program, then transitioned into a meat quality program that now incorporates animal stewardship and antimicrobial stewardship. He said that BQA requires participants to demonstrate a veterinarian client–patient relationship and to provide written treatment plans and disease descriptions. BQA also offers periodic reports on injection site lesions, he said, which has attracted many more producers to buy into that program. Although BQA guidelines have been widely adopted in the industry, he said, BQA lacks “teeth” because it is voluntary and producer driven.
Progressive Beef, a comprehensive third-party audit, is a commercial program that puts more teeth into BQA program guidelines, according to Sjeklocha. Participants pay a fee and must have a veterinarian client–patient relationship and documented prescriptions, treatment schedule, treatment records, and observed withdrawal times. He suggested that this may open up more upscale beef markets for producers who are part of the program, such as higher-end restaurants.
The Veterinary Feed Directive (VFD), effective January 1, 2017, is a law set out by the U.S. Food and Drug Administration (FDA) aimed at reducing antibiotic use, said Sjeklocha. However, it has not been well received by producers, he said. The VFD does track sales data, but Sjeklocha was unsure of its value because there is not enough verifiable data to use as a benchmark base. For example, knowing the amount of antibiotics sold does not capture the effect on animal health and welfare, he said.5
Gaining Producer Buy-In to Antimicrobial Use Guidelines
Beef producers can be reluctant to buy into guidelines, Sjeklocha explained, for both economic and cultural reasons. Beef producers typically value their independence very highly, and many resent being forced to commit to guidelines of any kind, he said; many producers also feel like they are being unfairly cast as scapegoats for the entire problem of antimicrobial resistance. Sjeklocha explained that the BQA guidelines have been the most widely adopted because producers’ participation is voluntary rather than required. He further noted that younger producers are more likely to accept new concepts, while older, more seasoned producers may feel encroached upon when asked to try new practices. He explained that most producers manage disease on their own with basic diagnostic skills and past experience, with some veterinary input and guidance. New technologies are plentiful, including disease diagnostics, new stethoscopes, high-frequency electronic identification tags, rapid blood tests, and pedometers, but they
___________________
5 If a cow herd is severely reduced in number because of drought, said Sjeklocha, then the remaining cows may be under nutritional stress, causing the amount of antibiotics sold to increase despite the smaller number of cattle.
need further development and research, he said. They can also be expensive to adopt, he warned, and may be less feasible for smaller farms than larger farms that can spread their use over more production units.
To obtain buy-in, producers must have a return on their investment while also maintaining a level of independence, said Sjeklocha. He used the practice of calf preconditioning to illustrate why it can be difficult to gain producer buy-in to antimicrobial stewardship programs and practices (see Box 4-2). Creating an economically sustainable market will require incentivizing producers, he said, to help in convincing them to accept guidance and restrictions. The sales price for preconditioned calves is inconsistent, so weight gain is the primary financial driver for preconditioning. Even value-added programs such as antibiotic-free and other upscale markets are still considered to be niche markets that producers are reluctant to commit to, he said. While specialty and niche markets can provide some incentive, he said, they are also inconsistent. Niche markets also require producers to
sign contracts to commit to the program, he said, even though the practice may have a negative effect on animal welfare. Many producers are leery of organic, natural, and antibiotic-free markets because the return on investment is inconsistent, said Sjeklocha. When he was told that he would be surprised by how many consumers want antibiotic-free beef or organic beef, his reply was:
You’d be surprised how many consumers say they want antibiotic-free beef or organic beef, but they get into the grocery store and they have sticker shock, and they say, “I’m going do my good deed and buy it this one time, and then I’m back to the stuff that’s a little cheaper.”
He emphasized that producers must receive a premium to bring them to these niche markets, and he predicted that ultimately, the majority of producers will provide what consumers want, even if those are niche markets, but only if the return on investment makes it worth their time and effort.
Changing Paradigm of Antimicrobial Use in Veterinary Medicine
Antimicrobial use practices in animal agriculture are changing rapidly, said Singer, due in part to FDA policy changes that Sjeklocha discussed earlier, but also to consumer and customer demands. The actual on-the-farm practices need to be linked to antimicrobial resistance, Singer said, to help veterinarians and production companies understand the effects of antimicrobial use on resistance as they are being asked to rapidly change their production systems. Singer said that improved antimicrobial stewardship and reductions in antimicrobial use are important, but not the end goal, which is to reduce antimicrobial resistance. He suggested that efforts should focus on evaluating changes in antimicrobial resistance as a function of change in antimicrobial use.
On-farm antimicrobial use can be quantified in two ways, said Singer. A bottom-up approach balances samples with antibiotic use data for a relatively small number of farms, he said, and a top-down approach captures industry-wide estimates of on-farm usage. Singer emphasized that the terms no-antibiotics-ever (NAE) and antibiotic-free do not mean that a company never uses antibiotics in any of its animals. NAE labeling means that antibiotics were not used in meat being sold under that label, he explained, and meat treated with antibiotics is marketed elsewhere. “It is really hard to raise 100 percent of your animals without them ever getting sick and needing treatment,” he said, but warned that there is no single best approach to maintaining animal health and welfare.
Regulatory Changes on Antimicrobial Use in Veterinary Medicine
Singer discussed the implications of the 2017 FDA policy changes on growth promotion. In 2012, FDA published Guidance for Industry #209, describing the overall policy change regarding the use of antimicrobials in animal agriculture. Singer explained that it specifically limited the use of medically important drugs in food-producing animals to those that are considered necessary for ensuring animal health and that include veterinary oversight or consultation. Guidance for Industry #213 followed in 2013 and was fully enacted in January 2017, he said, providing more detail on implementing those key principles. It defines the seven classes of medically important antimicrobials that are illegal to use for growth promotion or feed efficiency, and it is illegal to use them without the authorization of a licensed veterinarian.6 It also stipulates that manufacturers voluntarily remove claims relating to production uses (growth promotion and feed efficiency) off the label, he said, or take them off the market. He emphasized that veterinarian use of those antimicrobials is not voluntary; it brings remaining therapeutic uses under veterinary oversight by changing the marketing status from over-the-counter to veterinary feed directive status or prescription status. This veterinary oversight component constitutes stewardship in action, said Singer. The VFD regulation sets requirements related to the distribution and use of VFD drugs, he said, and represents a critical step for facilitating the transition to veterinary oversight.
Field Investigation of Antimicrobial Use and Resistance
Singer described a recently completed study funded by the U.S. Department of Agriculture (USDA) and the National Institute of Food and Agriculture that used a bottom-up approach.7 It was designed to quantify the effect of antimicrobials used for preventing necrotic enteritis in broiler meat chickens on antimicrobial resistance in the broiler environment. Necrotic enteritis is a serious disease problem (related to clostridial overgrowth) in broiler chicken production, he said, especially as companies change how they use antibiotics. Antimicrobials approved for disease prevention are typically given in feed, said Singer, but the effect on resistance of applying them for an extended duration is yet unknown. A pen trial was conducted over three successive flocks raised on the same litter (a common practice
___________________
6 Other antimicrobials deemed not medically important are still allowed for use in growth promotion and feed efficiency.
7 Further information about the study can be found at portal.nifa.usda.gov/web/crisprojectpages/1005062-systems-approach-to-identifying-targeted-interventions-for-minimizingantibiotic-resistance-in-the-poultry-production-system.html (accessed July 30, 2017).
in the industry),8 to determine if antibiotics metabolites being excreted by the birds was building up in that litter over time. He explained that weekly composite litter samples were collected from each pen and cultured for Salmonella and Escherichia coli. The researchers also performed DNA extraction to perform microbiome and metagenomic analysis, as well as quantitative polymerase chain reaction analysis on 48 genes to search for selection pressure. Antimicrobial metabolites in the litter were measured to assess accumulation as a function of feeding these antibiotics to the birds, he said.
Early analysis revealed that the genes do not have a clear pattern of gene amplification under the selection of the antibiotic, according to Singer. For example, the tetA gene, associated with resistance to tetracycline, is very noisy when analyzed over time across the treatment groups. Singer emphasized more agricultural studies are needed to better understand the effect of antibiotic use under a variety of different conditions. However, pen trials are too limited in scope because they do not necessarily represent all the diversity found on farms and are restricted to the litter used in the pens.
Broiler Meat On-Farm Antimicrobial Resistance Surveillance Program
Singer also runs an on-farm antimicrobial resistance surveillance and monitoring program in the poultry sector, initially funded by FDA as a pilot. It aims to collect on-farm samples and antimicrobial use data from broiler meat farms throughout the United States. It was restructured in 2016 to make it more longitudinal, with every flock cycle sampled for Salmonella and Campylobacter and DNA banked from litter from each sample. Participation is voluntary and anonymous, with current enrollment between 50 and 75 percent of annual production. More than 350 sampling efforts have been carried out on 118 farms followed over time.
Singer described a sample report generated from the data of a single company’s four farms over five different flocks; Salmonella was present in all but one farm on one visit. Isolates were also serotyped to demonstrate the common resistance patterns of streptomycin-sulfadiazine-tetracycline, and quantitative data were collected on how antibiotics were used (in this case, no treatments were given). While it may seem that there is matched use data to resistance patterns for Salmonella, he said, data from a different complex that used no antibiotics during the entire year had Salmonella
___________________
8 The birds were raised for 35 days in each flock cycle, with a 7-day downtime between the flocks. Six of the seven treatment groups were given narasin, a nonmedically important ionophore that is a growth-promotion, feed-efficiency compound—plus one of five antibiotics to prevent necrotic enteritis (a bacitracin, a bambermycin, two oxytetracycline groups, and a virginiamycin). The seventh group received no narasin and no antibiotic.
present in all houses at every visit and the same resistance patterns of streptomycin-sulfadiazine-tetracycline. In Singer’s opinion, Salmonella and its resistances do not track with antibiotic use. He also expressed concern about labeling salmonellas as multidrug resistant, because streptomycin, sulfadiazine, and tetracycline are not typically used to treat an invasive case of salmonellosis. The focus should be on the resistances in Salmonella that are related to the problem of treatment failure, argued Singer.
National Industrywide On-Farm Antimicrobial Use Data Collection Effort
Singer discussed an industry-wide effort to collect on-farm antimicrobial use data throughout the United States. He noted that each commodity group—layer hen, turkey, broiler meat, swine, beef, and dairy—is now actively initiating these efforts. The poultry effort began in 2014 with support from FDA and U.S. Poultry & Egg Association cooperative agreements, he said. The survey is designed to capture on-farm usage data on indication, route, dose, and duration,9 and it is organized by how the antibiotic is used (in the hatchery, for growth promotion, for disease prevention, and for treatment and control). Broiler meat surveys are based on 6-month periods, he said, and data from 2013 and beyond are requested in order to capture trends prior to the 2017 FDA policy changes.
Consumer and Retailer Perspectives in the Poultry Industry
In 2002, Perdue Farms realized that the public health implications of antibiotic use in animals would be a matter of ongoing debate for years to come, said Stewart-Brown, and predicted that regulatory efforts would be forthcoming at the local, state, and national levels. Furthermore, consumer concerns about antibiotic use—specifically, that it was being used to cover up bad husbandry practices—also began to increase significantly, he said. To address these issues, Perdue Farms began a 15-year process of reducing with the goal of eventually eliminating the use of antibiotics in raising their chickens.
Antibiotic Use in the Poultry Industry
The chicken industry has traditionally used antibiotics in four different ways, explained Stewart-Brown. The first way is the use of antibiotics for
___________________
9 A survey instrument was designed collaboratively with USDA’s Animal and Plant Health Inspection Service (APHIS) and survey responses coded and analyzed as a composite of the industry; participation is voluntary and confidential. APHIS also serves an auditing role.
growth promotion in the chicken feed; however, using medically important antibiotics in feeds for growth promotion is no longer permitted by producers in the United States. Second, in the hatchery, antibiotics are generally mixed with a vaccine and injected directly into eggs at 18 days of embryonation, but some farms inject it into day-old chicks. The third is feed application of ionophores, which are animal-only antibiotics, and the fourth is treatment of sick or soon-to-be sick animals that are in the same house with sick birds.
Stewart-Brown differentiated between four categories of programs used by chicken producers, noting that consumers may not clearly understand the distinctions. In the industry “all in” category, human-use-approved—also called shared-use—antibiotics are used in hatcheries and in feed for growth promotion or disease prevention; this category also uses ionophores as well as shared-use antibiotics for treatment, control, and targeted prevention in sick flocks. In the gray area (“no human”) category, only ionophores are used. He noted that there are not many choices for treating sick flocks that are not shared-use products, but if shared-use products are required, the flocks are taken out from the remaining two programs—organic and NAE10—where no antibiotics are used at all.
Raising Chickens Without Antibiotics
Perdue Farms now has extensive experience in the organic market, Stewart-Brown said, with 1 million organic chickens among the 13 million they process each week. He related some of their experiences in the process of eliminating all uses of antibiotics in organic chicken production.
Eliminating hatchery use Through a process of gradual transition beginning in 2002, said Stewart-Brown, Perdue eliminated hatchery antibiotic use in 2014. He explained that to eliminate the use of hatchery antibiotics, breeders require cleaner eggs. In a breeder farm, chicken houses have three levels. The females generally stay on the upper level, coming down to the bottom level to mate with males and then lay their eggs in nests in the middle level, he said. At night, a bar is lowered in the nest level that prevents the females from spending the night in the nest and dirtying the nests. Dirty nests contaminate the eggs, and if the farm is not using hatchery antibiotics, then the eggs must be cleaned as they travel down the conveyer belt and out of the nest level. To avoid spreading contamination from manure from using the same wet rag to clean all the eggs, he said, their breeders now use disposable baby wipes. Another development, said Stewart-Brown, build-
___________________
10 This program was formerly known as antibiotic-free, but this caused confusion about whether it refers to the meat or to the production of chickens.
ing separate rooms in the hatchery to mix vaccines under a laminar-flow chemistry hood; this avoids contaminating the vaccines by mixing them in the hatchery area.
Eliminating use of antibiotics for growth promotion and use of any shared-use antibiotics Eliminating the antibiotic use for growth promotion and the use of human-approved antibiotics of any kind in chicken feed was a quicker process, Stewart-Brown said, which was completed by 2007. Chickens were provided with a regimen of probiotics and prebiotics and a vegetable-only diet, he said. It is much harder to run a no-antibiotics-ever program and still use animal by-products in feed, he noted, because any feed items that might be prone to causing gut irritation threaten the long-term success for reducing and eliminating antibiotics.
Since 2010, Perdue has adopted an aggressive approach to vaccination relative to the industry average, said Stewart-Brown. Vaccinating a hen protects the egg yolk as well as provides some protection to the chick, he explained, and low-reaction vaccines are used in the hatchery to prevent the need for antibiotics. When chickens become sick, the entire house is treated with the best possible antibiotic under veterinary supervision, he said, and outcomes are measured. When a house repeatedly needs treatment, the cause is identified and addressed, he added.
Success of the no-antibiotics-ever program at Perdue Farms Stewart-Brown reported that since 2009, the percentage of flocks treated with antibiotics has ranged between 1.1 percent to 5.4 percent per year, with 3.2 percent treated in 2017. The percentage of birds per week starting in the NAE program increased from 20 percent in 2013 to 100 percent in March 2017, he said. The success of the NAE program illustrates that antibiotics are not needed to raise chickens, Stewart-Brown emphasized. He advised that in order to accomplish this across the broader spectrum of animal agriculture, changes in animal care need to be implemented. Perdue Farms is currently rolling out organic animal husbandry rules to their nonorganic flocks, he said. He concluded by encouraging a focus on engaging farmers: “Those are the ones that are raising the animals, they’ve got to buy in . . . you’ve got to spend a lot of time with farmers.”
DISCUSSION
Peter Daszak, president of EcoHealth Alliance, asked if coccidia ever recur when ionophores are removed in establishing NAE programs. Stewart-Brown explained in those situations, there are two options for coccidiosis control in chickens—either a vaccine or a nonantibiotic product, such as nicarbazin—although the latter is not suitable for organic programs.
Daszak asked if Perdue Farms has surveyed the public about why they do not want antibiotics in their chickens. Stewart-Brown said that reasons cited include public health implications (although they might not be well understood by the public), environmental effects, and, according to many consumers, the poultry industry is being selfish and not doing the right thing by using antibiotics to raise animals.
David Relman, professor of medicine at Stanford University, asked about the potential for manipulating gut microbial communities through means other than antibiotics. Stewart-Brown noted that migrating chickens around different parts of the chicken house (during brooding, for example) can be very difficult on the birds. He said that keeping the chickens on feed while they migrate is an example of a management practice that helps to maintain microbial balance in the chickens’ guts. Relman asked if there are good data that the chickens’ difficulty during migration is a result of a disturbance in the gut microbial ecosystem, but Stewart-Brown did not know of any. Singer said that ongoing studies are examining what makes a gut healthy and the effect of practices that try to alter the gut microbiome to make an animal healthier. Sjeklocha noted that adding yeast to the diet of cattle, to deal with the frequent problem of liver abscesses, has lowered the abscess formation rate and reduced reliance on tylosin and antibiotics, the traditional treatments.
Lonnie King, professor and dean emeritus of The Ohio State University College of Veterinary Medicine, asked Boucher about the core element of leadership in stewardship programs. According to Boucher, the leadership component of stewardship cannot be overemphasized because leaders are responsible for negotiating for high-level support, running the programs, and influencing people not to use antibiotics. As stewardship becomes more sophisticated, she said, IDSA and SHEA have put initiatives in place to help develop and train the next generation of leaders. George Poste, chief scientist of the Complex Adaptive Systems Initiative at Arizona State University-SkySong, asked Boucher about pressures to discharge patients prematurely because of concerns about hospital-acquired infections. Boucher said such pressure has never been worse, from her perspective, but has actually increased the value of her institution’s stewardship program because it can be leveraged as an opportunity to, for example, send patients home with an oral rather than IV antibiotic or with no antibiotic at all.
ACHIEVING DESIRED BEHAVIOR CHANGE THROUGH PREVENTION MEASURES AND EDUCATION
Strategies to Enhance Antimicrobial Prescribing Practices Food and Companion Animal Veterinarians
Scott opened his presentation by comparing and contrasting between antimicrobial stewardship for humans and animals. He stated that the definition of antibiotic stewardship from the perspective of human clinical medicine, as presented earlier by Boucher, may be relatively aspirational and challenging to translate to production animal medicine from the veterinary perspective.
In an ideal clinical setting, Scott explained, a physician treating a patient with streptococcal pneumonia would test the bacteria and follow all relevant stewardship guidance to choose the correct antibiotic. However, if individual patients hospitalized in the same ward are receiving different antibiotics, he said, then the potential unintended effect on the nontarget bacteria is a major concern, because most patients carry bacteria as part of their natural microbiome. Scott observed:
We use the term antibiotic stewardship, but if you were talking about stewardship of other natural resources like forests, you wouldn’t talk about chainsaw stewardship. You would actually refer to the trees . . . we are actually talking about bacterial stewardship and, in particular, populations of bacteria that are under our care.
Scott said that Enterococcus normally makes up a very small fraction of the total bacteria in a person’s microbiome, but in human hospital intensive care units (ICUs), antibiotic use can expand those fractions of Enterococcus in the patients. The same effect occurs in animals in veterinary ICU units, Scott said. The top three uses of antibiotics in companion animal veterinary practice are for dermatologic, urinary, and respiratory infections, he reported. It can be difficult to get an animal to ingest a pill, said Scott, so long-duration antibiotics have been developed to treat common conditions like chronic skin infections. He said that some of those antibiotics—such as third-generation cephalosporins—can have dramatic effects on the gut microflora in animals.
To address the problem of resistance, said Scott, animal referral hospitals have developed stewardship policies, like Texas A&M University’s Drugs of Last Resort Policy, and an ongoing CDC-funded project is examining use and resistance in referral veterinary hospitals. He noted that WHO’s 2012 revision of its List of Critically Important Antimicrobials (WHO, 2012) recommended that when a new class of human drug comes on the market, it should be considered critically important from the outset
unless strong evidence suggests otherwise. It also recommends that existing drugs such as carbapenems, linezolid, and daptomycin (not currently used in food production) should not be used in small animals, plants, or aquaculture in the future. This raises the question of how to prevent small-animal practitioners from using those products in an extra-label manner, Scott said, or at least how to ensure that the products are used according to good stewardship principles.
Applying Social Psychology to Antimicrobial Prescribing Practices
To frame antimicrobial prescribing and use in a theoretical context, Scott applied the theory of planned behavior (TPB) from social psychology to intensive production animal agriculture, noting that the TPB model has already been applied to physician prescribing behavior and patient compliance. The basic TPB framework (Ajzen, 1991) describes that intentions, the most important determinant of behavior, is determined by three constructs: attitude (“is the behavior good to do?”); subjective norms (“what do other salient actors expect me to do, and do I care what they think?”); and perceived behavioral controls (“how easy or difficult is the behavior for me to do?”). Perceived behavioral controls can motivate reflexive-type behavior that is not necessarily mediated by intentions, said Scott. Subjective norms can be difficult to analyze, he said, because they are qualitative and span a complex social network of potential influences on decision making.
To explore changes in antibiotic use in intensive production agriculture, Scott’s team expanded the TPB framework to include the factors: “trust or confidence in others,” “behavioral beliefs and belief importance,” and “moral norms and salient obligations to others.” They surveyed feedlot operators and veterinarians, Scott reported, and almost all respondents overwhelmingly agreed with the statement, “I have a moral duty to treat acutely ill feeder cattle with antimicrobials.” When respondents were asked if they had a moral duty to use subtherapeutic antimicrobials to promote growth, the majority of feedlot operators felt they had an obligation to others (for example, to contribute to the financial stability of the business), but the majority of veterinarians did not report having any such moral duty. For Scott, exploring this type of subjective disagreement among stakeholders provides the opportunity to design interventions more effectively to drive improvements in antibiotic stewardship.
Motivating Antibiotic Stewardship in Production Agriculture
Scott explained that target bacterial pathogens such as pneumonia and bovine respiratory disease complex are the most common reason for
antibiotic use in cattle feedlots. Pneumonia, for example, can be caused by different bacterial strains (or combinations of strains) that have normal commensals in the upper respiratory tract, he said. However, stress can lead to a pneumonia outbreak in the animals through the epidemiologic triad of host, agent, and environment. Treatment with antibiotics can drive resistance in those bacteria and motivate changes in prescribing and use, said Scott, but his primary concern is the unintended consequences of antibiotic use on gut bacteria and on Salmonella and Escherichia coli. He said that antibiotic stewardship can be difficult to motivate among producers because it really has no bottom-line economic impact, short of consumer demand. The debate around using antibiotics to treat individual sick cattle versus therapeutic use of antibiotics in groups of animals to control disease (metaphylaxis) or prevent disease (prophylaxis), he noted, is echoed in similar debates around prudent use in human clinical medicine. Scott suggested broadening the focus of stewardship to “what leaves a farm,” both in terms of animals going to slaughter and in terms of preventing the release of resistance determinants from the farm and into the environment at higher than background levels. Scott cited an experiment that applied behavioral science to try to change farmers’ use of antibiotics in dairy farms in the Netherlands (Speksnijder et al., 2017). The intervention was a program called structural animal health planning, which involves veterinary nutritionists exploring ways to reduce antibiotic use with cohorts of farmers. After three years, the use of antibiotics among the group that received the structural animal health intervention was shown to have declined over the entire study period.
Leveraging Social and Behavioral Interventions to Achieve Appropriate Antibiotic Prescribing Practices in Health Care
Linder opened by arguing that improved diagnostics will not resolve the problem of inappropriate antibiotic use in human health. Stewardship has traditionally been an inpatient-focused activity, he said, even though 60 percent of antibiotic use in humans occurs in the outpatient setting (CDC, 2017). In the United States, he reported, there are 34 million hospitalizations each year, but there are 130 million emergency department visits and 1 billion ambulatory visits—with an antibiotic prescribed at 12 percent of those visits (National Center for Health Statistics, 2016). He reported that acute respiratory infection, which includes ear infections, pharyngitis, sinusitis, acute bronchitis, pneumonia, the flu, and nonspecific upper respiratory tract infections, accounts for 10 percent of all annual ambulatory visits in the United States and 44 percent of all the antibiotics prescribed in ambulatory settings. About half of those antibiotic prescriptions are inappropriate, he said, such as the prescription of an antibiotic for a nonspecific
upper respiratory tract infection or acute bronchitis (The Pew Charitable Trusts, 2016). He warned that these practices are causing huge problems in terms of rising costs, increases in antibiotic-resistant bacteria, more frequent adverse drug events, and changing the microbiome. He warned that “many of these antibiotics are given to people who have viral illnesses and the antibiotic has no chance of helping them and a very real chance of hurting them.”
Education is important but not sufficient to address this problem, Linder said. Despite the amount of clinician education provided over the past 20 years, the antibiotic prescribing rate for some infections has not changed much at all, he reported. Four decades of randomized controlled trials have demonstrated that antibiotics do not work for acute bronchitis, he said, yet the antibiotic prescribing rate for acute bronchitis among adults in the United States remains just shy of 80 percent (Barnett and Linder, 2014). For adults with sore throat, the appropriate antibiotic prescribing rate is about 20 percent, but 60 percent of adults with a sore throat are prescribed an antibiotic in the United States (Barnett and Linder, 2014). He reported that for ambulatory visits, there are 506 antibiotic prescriptions per 1,000 people in the United States; at least 30 percent of overall antibiotic prescribing is unnecessary and half of the antibiotics prescribed for acute respiratory infections are unnecessary (Fleming-Dutra et al., 2016). When other types of prescribing are included, such as prescribing over telephone, retail clinics, and at the Veterans Affairs facilities, Linder said, the antibiotic prescribing rate in the United States jumps to 833 per 1,000 people (Hicks et al., 2013).
Linder argued that the focus should be shifted from diagnostics to increasing clinicians’ knowledge about what is happening with a patient. CDC’s Etiology of Pneumonia in the Community study included 2,259 adults hospitalized for pneumonia, who were intensively investigated for pathogens; the etiology was not identified in 62 percent of cases (Jain et al., 2015). Even with improved technology, he said, testing a patient who presents with a respiratory infection will not provide a single answer.
Behavioral Science of Prescribing
Prior interventions to prevent inappropriate prescribing to outpatients have had limited success, Linder reported. The implicit model holds that clinicians are reflective, rational, and deliberate, but attempts to educate and remind them about appropriate prescribing practices at the time of care have continued to fail. Linder argued that “doctoring” needs to be reconstrued as an emotional, social activity carried out by people who are subject to a range of biases and other social factors that drive prescribing behavior. He suggested supplanting the implicit model with a behavioral
model under which clinicians make fast, automatic decisions influenced by emotional and social factors as well as a range of cognitive biases.
The factors that drive antibiotic prescribing are immediate and emotionally salient, Linder explained. In an outpatient setting, he said, the social and emotional factors that promote inappropriate prescribing practices often outweigh the factors that promote appropriate prescribing. For example, a clinician may believe that a patient wants antibiotics and have the perception that it is easier and quicker just to prescribe them, he said. Alternatively, a clinician may prescribe antibiotics out of ingrained habit or prescribe them “just to be safe” out of concern for potential serious complications. The factors that deter antibiotic prescribing are more remote and less emotionally salient, explained Linder. These include the risk of adverse reactions or drug interactions, the need for stewardship, the desire to deter low-value care, and a preference for following guidelines. The effect of nonclinical factors on antibiotic prescribing behavior has been borne out by evidence, Linder said. An analysis of antibiotic prescribing over the course of the day—stratified by diagnoses for which antibiotics were sometimes indicated or antibiotics were never indicated—found a 5 percent absolute increase in antibiotic prescribing at the end of the day versus the beginning of the day with a small drop during lunch (Linder et al., 2014). He noted that this effect was replicated by athenahealth, in a study that found the antibiotic prescribing rate for acute respiratory infections increased over the course of the day as the number of appointments accrued (see Figure 4-1).
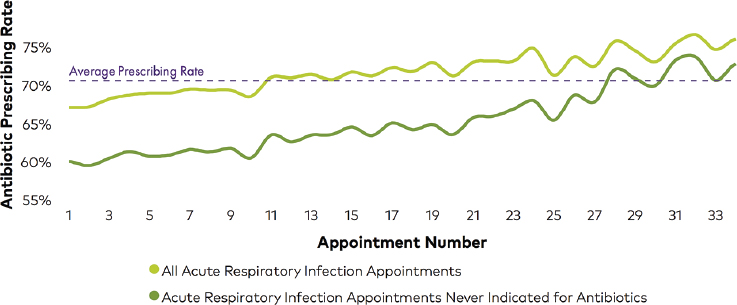
SOURCES: Linder presentation, June 20, 2017; athenahealth, 2016. Image courtesy of athenahealth research.
Social and Behavioral Interventions to Reduce Inappropriate Antibiotic Prescribing in Primary Care
To illustrate the application of behavioral science concepts toward decreasing inappropriate antibiotic prescribing in primary care, Linder reviewed some results from the Behavioral Economics to improve treatment of Acute Respiratory Infection (BEARI) trial (Meeker et al., 2016). Interventions using clinical decision support and health information technology often yield disappointing results, he said. Electronic health records (EHRs) with clinical decision support have been touted as a solution to problems of medical safety, cost, and quality, he said, but they often fail to achieve expected improvements because they implicitly assume that clinicians follow a standard economic and behavioral model. The aim of the BEARI project was to evaluate three behavioral interventions in three different health systems using three different electronic health records, he said.
The three interventions Linder explained that they looked at an 18-month baseline period and an 18-month intervention period. The first type of behavioral intervention—suggested alternatives—is similar to traditional reminder-based clinical decision support. When clinicians seek to prescribe certain types of antibiotics through the EHR system for certain acute respiratory infections, the system triggers a pop-up reminder that antibiotics are not generally indicated for nonspecific upper respiratory infections, he said, and it suggests several nonantibiotic alternatives before the clinician can proceed with the prescription. The second intervention—accountable justification—builds on suggested alternatives with an element of social motivation. Linder explained that an attempted antibiotic prescription in the EHR system triggers advice that an antibiotic is not indicated for likely viral diagnosis, and requires the clinician to input a reason for prescribing the antibiotic. If no justification for prescribing antibiotics is given, he said, there is an implicit understanding that clinician’s colleagues will see that in the EHR system. Linder noted that the first two interventions can be combined easily. The third intervention—peer comparison—involves a different form of social motivation, he explained. Everyone receives a monthly email that stratifies clinicians into top performers with the lowest inappropriate antibiotic prescribing rate, and non-top-performers (all others). Linder explained that this simple, emotionally laden message tends to “pull in the tails on the bell curve”: bad performers do a bit better and good performers may backslide slightly. But keying interventions on top performers, he said, can shift the whole mean toward top performance.
Outcomes and implications of the interventions Linder reported that over 18 months, nearly 250 clinicians enrolled in the BEARI trial from 47
primary care practices. The study’s primary outcome was the antibiotic prescribing rate for nonantibiotic-appropriate diagnoses. He reported that during the 18-month baseline period prior to the intervention, the antibiotic prescribing rate decreased for the control group and that being enrolled in the trial itself had the largest absolute decrease in antibiotic prescription of any intervention, which he attributed to the participants’ knowing that their prescribing was being watched. The antibiotic prescribing rate for the control group continued to decrease during the 18-month intervention period. The antibiotic prescribing rate decreased for the suggested alternative group through both the baseline and intervention periods, but the change was not statistically significantly different from the control group by the end of the study. The accountable justification intervention group had a statistically significantly lower prescribing rate by the end, with a relative 7 percent drop.11 Linder reported an even bigger drop in the peer comparison intervention group, which started off lower and then dropped down to a 4 percent inappropriate antibiotic prescribing rate by the end of the study, for a relative decrease of 5 percent compared to the control practices. In summary, Linder noted that these studies suggest that doctors’ behaviors are influenced by social factors so insights from social and behavioral sciences can facilitate the development of interventions to influence doctors’ prescribing behavior for acute respiratory infections.
The Role of Academic Veterinary Medicine in Combating Antimicrobial Resistance
Maccabe described initiatives to combat antimicrobial resistance by AAVMC, an association of all veterinary medical colleges in Canada and the United States, as well as colleges of veterinary medicine worldwide, facilitating an international approach with a global footprint. The AAVMC felt that academia was not responding actively enough to the need for a One Health approach to address antimicrobial resistance, he said. To help address this gap, Maccabe explained that AAVMC partnered with the Association of Public and Land-grant Universities (APLU) to create a task force focusing on antimicrobial resistance in production medicine.12 He said that the task force also includes the American Veterinary Medicine Association and representation from government (CDC, FDA, and USDA), and industry (Animal Health Institute, National Cattle-
___________________
11 He explained that this estimate calculates the effect of the intervention by comparing the changes in outcomes over time between the intervention group and the control group.
12 APLU is North America’s oldest higher education association, which represents 237 public universities and 49 veterinary medical colleges accredited by AVMA Council on Education and agricultural experiment stations.
men’s Beef Association, National Chicken Council, and National Pork Producers Council).
Task Force Activities to Combat Antimicrobial Resistance
The AAVMC-APLU task force was charged with proposing recommendations and activities for academic institutions related to antibiotic resistance in production agriculture, said Maccabe. The resulting AAVMC-APLU task force report, Addressing Antibiotic Resistance, focuses on education and research (AAVMC, 2017), but Maccabe focused on recommendations related to education specifically. A working group developed learning outcomes for competency-based training and education across six domains: healthy animals, global impact, antimicrobial stewardship, antimicrobial drugs and antimicrobial resistance, roles and relationships, and critical analysis. Individual institutions develop the rubrics and curricular materials that link to those learning outcomes, he explained. Table 4-1 shows a representative page detailing the learning outcomes related to antimicrobial stewardship for novice, intermediate, and advanced students.
TABLE 4-1 Antimicrobial Stewardship Learning Outcomes
| Developmental Level | |||
|---|---|---|---|
| Novice (4-H/FFA/Youth) | Developing (animal science undergraduate or graduate) | Advanced (veterinary medical students) | |
| Definition | Define antimicrobial drug stewardship | ||
| Societal resource | Recognize that there is increasing societal concern about bacterial resistance to antimicrobials and potential reduction or loss of effectiveness | Recognize that there is increasing societal concern about bacterial resistance to antimicrobials and potential reduction or loss of effectiveness. Cite examples of antimicrobial stewardship that might be helpful | Describe specific examples of resistance in pathogenic and nonpathogenic bacteria that are commonly found in a specific animal species and in important human pathogens |
| Developmental Level | |||
|---|---|---|---|
| Novice (4-H/FFA/Youth) | Developing (animal science undergraduate or graduate) | Advanced (veterinary medical students) | |
| Definition | Define antimicrobial drug stewardship | ||
| Common uses of antimicrobial drugs | Identify common situations in which antimicrobials are needed to address animal health and welfare and minimize suffering | Recognize that there are common situations in which antimicrobials are needed to address animal health and welfare and minimize suffering and those in which antimicrobial drugs will not make a difference | Distinguish common or important situations in which antimicrobials are needed to address animal health and welfare and minimize suffering and those in which antimicrobial drugs will not make a difference |
| Complexity of bacterial infections | Recognize that infectious diseases can be caused by a variety of microorganisms, and that disease risks can vary among different animals | Recognize that infectious diseases can be caused by a variety of microorganisms, and describe how disease risks can vary among different animals | Describe the epidemiology and pathogenesis of the most common and the most significant bacterial disease challenges in major domestic species of animals; describe the organism or patient factors that may effect treatment options |
| Need for antimicrobial drugs | Recognize that there may be a need to use antimicrobial drugs in cases of infectious disease where subsequent health and life or lives of animals are threatened | Recognize that there is a need to use antimicrobial drugs in cases of infectious disease where subsequent health and life or lives of animals are threatened, and understand that antimicrobial drugs may not be required | Explain to animal owner or manager why an antimicrobial drug is or is not recommended based on the perceived need and benefit to the animal, including differentiating an infection requiring treatment and a contaminant not requiring treatment |
NOTE: 4-H = U.S. youth organization focused on personal development in “head, heart, hands, and health”; FFA = U.S. student organization, formerly the Future Farmers of America.
SOURCES: Maccabe presentation, June 20, 2017; from AAVMC, 2017.
In another activity, Maccabe explained, the joint task force partnered with the Association for Prevention Teaching and Research, an association composed primarily of faculty from medical schools that teach preventive medicine and public health, to develop the One Health Interprofessional Education Initiative. It seeks to integrate One Health concepts into the degree programs of health profession students through the case study method of instruction, he said, and 15 peer-reviewed case studies have already been published through a competitive process that financially incentivizes faculty members to contribute.13 The same process will be used to solicit the development of curricular materials for dissemination. Maccabe explained that the Antimicrobial Resistance Learning Site is a project that provides open-source teaching modules for instructors in veterinary medical education on antimicrobial resistance with modules in pharmacology, microbiology, public health, and species-specific medicine.14
According to Maccabe, the AAVMC’s education and outreach efforts also involve developing key messages and communication strategies for engaging decision makers at all levels. Informational materials will be made available to producers and veterinarians (e.g., FDA VFD guidance, disease prevention strategies, and antimicrobial stewardship), to agricultural youth groups, and to the general public. Looking forward, said Maccabe, the AAVMC will continue to build its coalition of partners and stakeholders to further develop its strategic communication strategy.
The Medical Curriculum Meets Microbial Threats
Kirch considered the challenge of translating the goal of improved antibiotic stewardship into the educational process. He commended the One Health initiative for bringing together the disciplines of human, animal, and environmental health toward a common cause, but he noted that individuals within those disciplines have traditionally been educated in silos. This is one of the consequences of the traditional model of medical education that, he warned, is rapidly becoming outdated and ineffective. To illustrate, he provided three perspectives on the continuum of medical education. The aspirational model is a true seamless continuum, he said, that creates the mythic “master” clinician. It begins at the premedical stage, continues through medical or veterinary school, residency, and fellow-
___________________
13 For example, a case study related to an antimicrobial resistance effort focuses on the interplay of methicillin-resistant Staphylococcus aureus (MRSA) between companion animals and humans; it discusses infection prevention measures with emphasis on the interaction that is needed between human and veterinary medical professionals in resolving recurrent household MRSA infections.
14 Produced and operated by Michigan State University and the University of Minnesota, in cooperation with CDC.
ships, and on through practice, with continuous learning and assessment throughout the process. When Kirch became a dean, he said, this fantasy was soon dispelled. He explained that the reality is a process that involves a series of sealed and autonomously controlled compartments along the path to becoming a specialist and then subspecialist. The wide array of entities involved—in education, training, certification, program accreditation, assessment, and licensure—form an educational path that is hugely fragmented rather than integrated, said Kirch.
From Facts to Competencies in Learning and Assessment
Better coordination among the different “compartments” is not the answer, said Kirch. He highlighted what he considers to be a major paradigm shift in education. Traditionally, the acquisition of knowledge and facts has driven learning and assessment in medical education, he said, as well as in education writ large. Standardized fact-based assessment is used at various stages to assess the students’ accumulation of facts. Kirch said that the problem today, however, is that the evolution of science has hugely increased the size of the available fact base, such as structural genetics, functional genetics, and now proteomics and other effector molecules. Therefore, the number of facts required per decision has also increased exponentially, he said, and now vastly exceeds human cognitive capacity.
The complexity of clinical decision making will only continue to accelerate, said Kirch, while human cognitive capacity remains static. Unlike decades ago, he said, a single clinician can no longer accumulate all the relevant knowledge required to make clinical decisions. He said, “We need to wean ourselves from the notion that our task is to fill people with facts and rather say that our task is to help people develop foundational knowledge.” He conceded that facts remain important, especially those that constitute key conceptual notions. However, he said that helping health professionals develop the competencies to use those facts is more important and has become a topic of intense interest over the last decade.
Health Profession Competency Domains
Kirch cited a paper that collated more than 150 systems articulating health professional competencies from around the world (Englander et al., 2013). The authors found that virtually all of those competencies map onto eight fundamental domains:
- medical knowledge,
- patient care,
- interpersonal and communication skills,
- professionalism,
- practice-based learning and improvement,
- systems-based practices,
- personal and professional development, and
- interprofessional collaboration.
Kirch noted that the Accreditation Council for Graduate Medical Education core competencies for residency training in the United States emphasize the first six of those domains. He explained that traditional medical knowledge, together with all other competencies, amount to what are called core entrustable professional activities. He defined this as a set of activities that entering residents should be expected (entrusted) to perform on day 1 of residency without direct supervision (AAMC, 2014). He noted that this has been expanded to establish core competencies for Interprofessional Collaborative Practice,15 spanning the domains of values and ethics for interprofessional practice, roles and responsibilities, interprofessional communication, and teamwork.
Creating a Clear Educational Pathway to Mastery
Kirch emphasized that the classroom is “flipped” and learning becomes asynchronous in the pedagogical shift from facts to competency-based learning. Traditional classrooms are being replaced by online tools for acquiring core knowledge, he said, which are coupled with smaller facilitated learning groups that focus on how the facts translate to challenges in day-to-day practice. Along the continuum of educational development, a health professional’s competencies move from novice to expert level. However, he said that a barrier is the poor standard of general health literacy within the undergraduate medical curricula. Preprofessionals often lack the basic foundational knowledge and competencies, he said, that would enable them to move through progressive stages of mastery. Kirch suggested that technology-based tools have great potential to help close this gap and move pedagogy toward the competency-focused flipped classrooms, case studies, and problem-based learning.
The Path to Mastery in Addressing Antimicrobial Resistance
Practice-based learning and communication are particularly relevant to mastery in antibiotic stewardship, Kirch said. He offered a sketch of a four-step pathway to mastery in addressing antimicrobial resistance. The
___________________
15 For more information on the core competencies for Interprofessional Collaborative Practice, see www.ipecollaborative.org/resources.html (accessed July 30, 2017).
first step is fundamental “health literacy” in the One Health framework. The second is acquiring basic science foundational concepts of prevention, epidemiology, microbiology, pharmacology, and genetics. The third, Kirch said, is the appropriate prevention and treatment of uncomplicated infections. The fourth step is to acquire the competencies to manage population health threats, complex infections, and treatment resistance in humans, the food supply, and the environment.
DISCUSSION
Wilson asked Linder if the BEARI trial followed up to see if the prescriber behavior persisted after the intervention phase. Linder said there was a significant persistent effect in the peer comparison group, but the suggested alternative group had migrated back to control practices after 12 months, while the peer comparison group had a smaller amount of backsliding.
Suerie Moon, director of research at the Global Health Centre, Graduate Institute of International and Development Studies, Geneva, asked Linder if the BEARI interventions would work as well in resource-poor, lower-technology settings. Linder noted that the peer comparison intervention was the most effective—and the lowest-tech—of the interventions tested, using email rather than the EHR system. Linder continued that in a resource-poor setting, determining with confidence that an antibiotic prescription is inappropriate would be challenging and the consequences of getting that wrong could be much deeper. He remarked, “we can only improve what we are measuring,” so creating a paradigm to measure the quantity and quality of antibiotic prescribing is a needed first step. Jeffrey Duchin, health officer and chief of Communicable Disease Epidemiology and Immunization Section for Public Health for Seattle and King County, Washington, asked Linder to clarify whether the peer comparison intervention was actually the second largest effect, if the largest effect was simply from being enrolled in the study. Linder agreed that in absolute terms, the strongest effect of any intervention was just being in the trial, but he noted that participants had to actively enroll to take part. After looking at the difference between people who did and did not enroll in the trial, he found that the people who enrolled had a lower baseline antibiotic prescribing rate than those that did not. Linder said that this set the bar higher for the interventions that they tested, as well as highlighting the need to engage people who are not prescribing well and may be aware that they are not.
Gerald Keusch, associate director of the National Emerging Infectious Diseases Laboratory at Boston University, asked if it takes longer to make the decision not to prescribe an antibiotic than to make the decision to prescribe and, given the pressures on time with patients, that itself may
make a difference in the ultimate outcome. Linder replied that according to data he has analyzed, the difference is not huge. However, there are also downstream effects, he said; for example, people who get an antibiotic are much more likely to think they need one in the future and to come back again. Northern European countries have used this tendency to cut down on visits, he said, but in the United States, such visits tend to be perceived as an easy and quick way to satisfy patients and ensure that they return. This is an ongoing issue in urgent care clinics, he said.
Peter Sands, senior fellow at the Mossavar-Rahmani Center for Business and Government, Harvard Kennedy School, commented that experience in the United Kingdom with peer comparison intervention is consistent with Linder’s findings. Antibiotic use data for every general practitioner (GP) in the country is measured monthly and the data are made public. Clinical commissioning groups—who buy services from GPs—receive a financial incentive from the government to manage that performance, he said, which serves as an incentive to shift behavior by naming and shaming. Linder added that the chief medical officer in England sent single letters to high-prescribing GPs, stating their practice was prescribing more antibiotics than others in their region, which resulted in a measurable decrease in antibiotics dispensed overall (Hallsworth et al., 2016). However, he reported that a study in the United States involving letters sent by CMS, which compared the most frequent prescribers of opioids to their peers, had no impact on behavior (Sacarny et al., 2016). This illustrates that the message, the data, and the messenger are all important in the way feedback is delivered, said Linder. Scott commented that there are similar examples on the veterinary side from Denmark and the Netherlands. Both countries have sufficient granularity in their capture of antimicrobial prescribing, both by veterinarians as well as at the producer level, to use annual evaluations and resetting based on standard deviations.
Kirch drew a distinction between the education of future professionals and the problem of remediating clinicians who develop prescribing patterns over many years. A practical experiment is ongoing with the Choosing Wisely campaign,16 he said. Medical societies in the United States have developed specialty-specific consensus lists consisting of five practices that reflect overutilization or misutilization of diagnostic tests and treatments. He said that many of the lists include inappropriate antibiotic prescribing. However, he reported that although these lists are generally ignored by practitioners, they are prominent in medical school and residency curricula, reflecting the difference between imprinting and behavioral change in adults. He suggested adopting a two-pronged approach to address practitioners who have
___________________
16 For more information on the Choosing Wisely campaign, see www.choosingwisely.org (accessed July 30, 2017).
established habits with dedicated strategies on the one hand, and to imbue stewardship as a basic concept in health education on the other.
Kumanan Rasanathan, chief of the Implementation Research and Delivery Science Unit at the United Nations Children’s Fund, asked if any of the programs aimed at changing prescribing behavior have been paired with synergistic interventions aimed at demand, such as by changing expectations around antibiotic prescribing in both medical and veterinary settings. Kirch reported that the Choosing Wisely campaign also has a patient-facing side, Consumers Union, that is engaging with the issue of antibiotic overprescribing. Linder commented that intervening to prevent unnecessary patient visits is a next frontier, albeit a complicated one in terms of patient education and prescreening. He also commented that decades ago, studies showed that the greatest predictor of antibiotic prescribing was not a patient’s demand for an antibiotic, or a patient’s desire for an antibiotic, but the physician’s perception of the patient’s demand for an antibiotic. Linder carried out a study on precommitment, which asked physicians to sign a letter committing to only prescribe antibiotics when a patient needs it, which was displayed prominently in their waiting rooms (Meeker et al., 2014). They found that the intervention reduced inappropriate antibiotic prescribing by around 20 percent, he said. According to Linder, physicians are often reluctant to even bring up the topic of antibiotics with a patient out of fear that the patient will request a prescription. The precommitment letter, he said, is a simple intervention that short-circuits that thought and lets the patient know that the physician will not prescribe an antibiotic unless it is necessary: “The doctor knows the patient knows and the patient knows the doctor knows.”
Lonnie King, professor and dean emeritus of The Ohio State University College of Veterinary Medicine, remarked that a change in production agriculture, especially in food animals, arose out of food safety issues when producers accepted that responsibility and accountability extended past the farm gate. He asked Scott how to extend that into the shifting paradigm of appropriate antibiotic use. Scott said that the clinical side of treating individual animals is fairly straightforward, but the appropriate use of antibiotics for prevention and control is less clear. FDA and pharmaceutical companies facilitated the shift away from growth promotion uses in feed efficiency using a cost-benefit economic model, he said, but the economic benefits around antibiotic stewardship are less tangible. Scott said that the quality assurance program and labeling that focus on residue avoidance have paved the way, but many of the costs of microbial safety have thus far been borne by the processing and slaughter industry. However, he warned that stewardship needs to move away from simply following labels and to be extended to the concept of broader antimicrobial safety in the preharvest environment.
This page intentionally left blank.






























