5
Personal Implants and Medical Devices
A brief summary of the fundamentals of operation of implantable medical devices is described below. The committee reviewed information available for patients, as well as physicians, and discussed manufacturer’s recommendations regarding the use of these devices when proceeding through millimeter wave advanced imaging technology (AIT) scanners. Many of these devices are preemptive in nature, that is, they are preventing a condition from happening such as arrhythmia or for a defibrillator.
PACEMAKERS
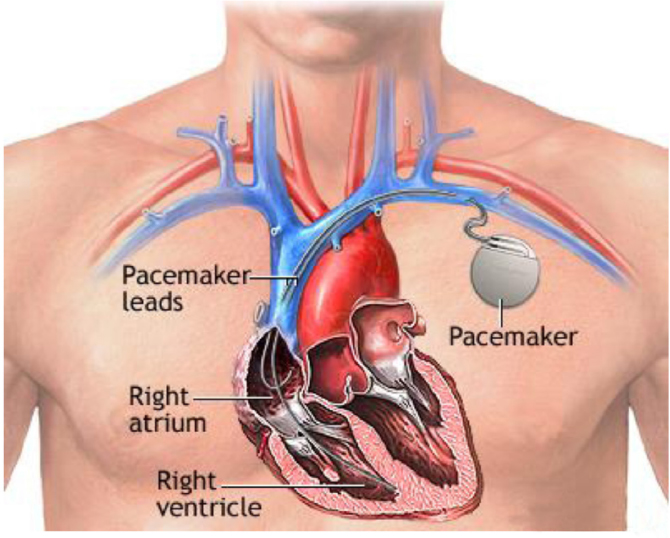
Pacemakers are small electrical devices inserted under the skin with electrodes inserted into the heart via either the right or left subclavian vein (see Figure 5.1). They are used to control rhythm disturbances of the heart. The most commonly used utilized devices monitor and pace both the right atria and right ventricle. These are known as dual-chamber pacemakers; they are very susceptible to magnetic fields and forms of electromagnetic radiation, and earlier versions are contraindicated in use through magnetometers and are sensitive in electromagnetic fields of lower wavelengths. A limited number of newer models of pacemakers are safe for use in magnetic resonance imaging (MRI) scanners, but patients can verify that information with their health care provider.
According to data from the U.S. federal government’s National Inpatient Sample, from 1993 to 2009, there were 2.9 million implants of permanent pacemakers
in the United States.1 Overall, the use of pacemakers increased by 55.9 percent during that time period, and the aging population continues to show an increase in utilization.
Under local anesthesia, a needle is inserted into the subclavian vein. Wire leads are then passed into the heart and active electrode leads are implanted in the right atrum and right ventricle. They are connected to the programmable control module inserted under the skin.
The information regarding pacemakers mentions that the patient will have limitations with respect to magnetic and electromagnetic fields. There is a Frequently Asked Question regarding the safety of being screened at the airports. The response says that due to the short duration of scanning, it is unlikely that the device will be affected by full-body imaging scanners or metal detectors. A recommendation is made to not stop or linger within the scanning area. This, however, is not practical, because the millimeter wave scanner does require the traveler to
___________________
1 A.J. Greenspon, J.D. Patel, E. Lau, J.A. Ochoa, D.R. Frisch, R.T. Ho, B.B. Pavri, and S.M. Kurtz, 2012, Trends in permanent pacemaker implantation in the United States from 1993 to 2009: Increasing complexity of patients and procedures, Journal of the American College of Cardiology 60(16): 1540-1545.
remain stationary while being screened. It is suggested that if the traveler has any questions or concerns, to show their ID card given with the device and ask for a hand screening. The following product warnings are available from Boston Scientific and Medtronic, respectively:
Advise patients to avoid sources of EMI. The pulse generator may inhibit pacing due to oversensing, or may switch to asynchronous pacing at the programmed pacing rate or at the magnet rate in the presence of EMI.2
Changes in a patient’s disease and/or medications may alter the efficacy of the device’s programmed parameters. Patients should avoid sources of magnetic and electromagnetic radiation to avoid possible underdetection, inappropriate sensing and/or therapy delivery, tissue damage, induction of an arrhythmia, device electrical reset or device damage.3
IMPLANTABLE CARDIAC DEFIBRILLATORS
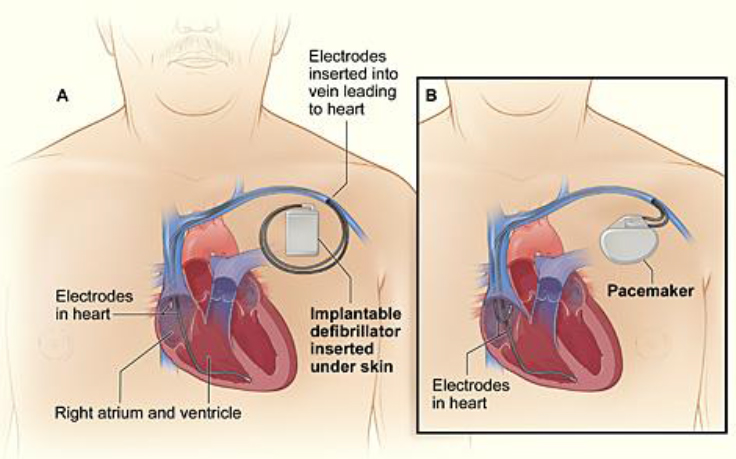
Implantable cardiac defibrillators (ICDs) are similar to pacemakers (see Figures 5.2 and 5.3) but also have an additional function of supplying an electric shock to patients with a life-threatening arrhythmia, such as ventricular tachycardia or ventricular fibrillation. This type of device is indicated in patients with prior history of heart disease to help prevent SCD (sudden cardiac death) from ventricular tachycardia or ventricular fibrillation. The ICD monitors the heart rhythm; if it detects a life-threatening abnormal rhythm, it will supply either a low-power or high-power electric shock to the heart to restore a normal rhythm. Warnings on ICDs are similar to pacemakers and state that patients should avoid sources of magnetic and electromagnetic radiation to avoid possible under detection, inappropriate sensing, and/or therapy. The 11th World Survey of Cardiac Pacing and Implantable Cardioverter Defibrillators estimated in 2009 that 133,262 new implants per year were implanted in the United States on an incidence of 434 new implants per 1 million in population per year.4
See the following product warning from Medtronic for ICDs (similar to pacemakers):
___________________
2 Boston Scientific, “ACCOLADE™ MRI Pacemakers,” CRM-324101-AB, APR2015, https://www.bostonscientific.com/content/dam/bostonscientific/Rhythm%20Management/portfolio-group/Pacemakers/ACCOLADE/ACCOLADE_MRI_Pacemaker_Spec_Sheet.pdf, accessed March 21, 2016.
3 Medtronic, “SureScab™ Portfolio for 1.5T and 3T MR Conditional Use,” brief statement, http://www.medtronic.com/mrisurescan-us/brief_statement_rad.html, accessed March 21, 2016.
4 H.A. Mood and A. Proclemer, 2011, The 11th World Survey of Cardiac Pacing and Implantable Cardioverter-Defibrillators: Calendar Year 2009—A World Society of Arrhythmia’s Project, Pacing and Clinical Electrophysiology 34(8):1013-1027.
Changes in a patient’s disease and/or medications may alter the efficacy of the device’s programmed parameters. Patients should avoid sources of magnetic and electromagnetic radiation to avoid possible underdetection, inappropriate sensing and/or therapy delivery, tissue damage, induction of an arrhythmia, device electrical reset or device damage. Do not place transthoracic defibrillation paddles directly over the device.5
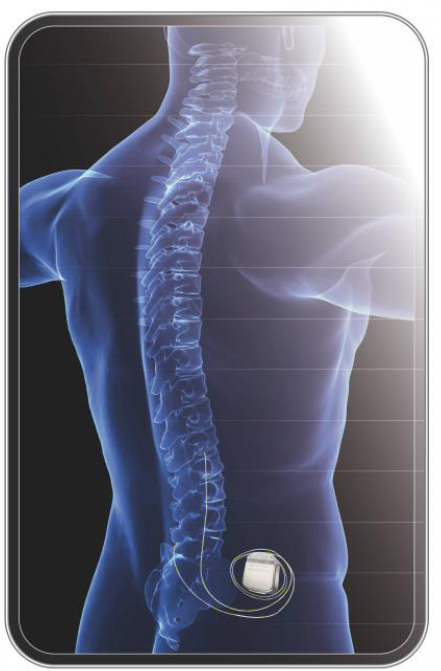
SPINAL CORD NEUROSTIMULATOR
A spinal cord stimulator (Figure 5.4) is an electronic device with a pulse generator implanted under the skin that can be used to control chronic pain as well as treatment of various motor disorders, such as spasticity and assistance in
___________________
5 Medtronic, “Implantable Cardioverter-Defibrillators (ICDs),” backgrounder, http://www.medtronic.com/protectapr/downloads/ICD-Backgrounder-101910.pdf, accessed March 21, 2016.
standing, stepping, or walking. The stimulating electrodes are surgically implanted in the epidural space, and the pulse generator is implanted in the lower abdomen or adjacent area.
Patients with pain from numerous causes, such as failed-back surgery, can greatly benefit from implantable neurostimulators. There is evidence of improved patient outcomes with stimulators, compared to conventional medical management.6
Programming the device involves the use of various frequencies usually between 20 and 120 Hz. Adjusting the amplitude of electrical pulses controls the amount of stimulation.
An example of a Medtronic patient warning is as follows:
Sources of strong electromagnetic interference (eg, defibrillation, diathermy, electrocautery, MRI, RF ablation, and therapeutic ultrasound) can interact with the neurostimulation system, resulting in serious patient injury or death. These and other sources of EMI can also result in system damage, operational changes to the neurostimulator or unexpected changes in stimulation. Rupture or piercing of the neurostimulator can result in severe burns. An implanted cardiac device (eg, pacemaker, defibrillator) may damage a neurostimulator, and the electrical pulses from the neurostimulator may result in an inappropriate response of the cardiac device.7
TRANSCUTANEOUS ELECTRICAL NERVE STIMULATION
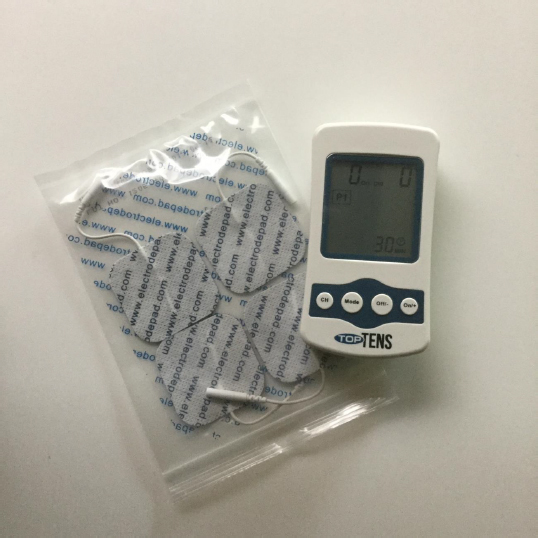
Transcutaneous electrical nerve stimulation, also known as TENS, involves the use of small amounts of electrical currents applied to the skin for the purpose of transcutaneously stimulating the nerves to reduce chronic and acute pain (see Figure 5.5). These devices are usually operated at frequencies from <10 Hz for muscle contraction or >50 Hz for nerve stimulation. It is applied to the skin with at least two electrodes, and the power supply can either be fixed or a battery so that the TENS unit can be operated in a nonmobile fashion with a self-contained battery.
A review of numerous manufacturers’ warnings did not reveal any precautions for using the device when undergoing airport screening. There was no indication of any adverse effect from electromagnetic interference (EMI), but there were ample warnings that the TENS units can interfere with pacemaker and ICD function.
___________________
6 K. Kumar, R.S. Taylor, L. Jacques, S. Eldabe, M. Meglio, J. Molet, S. Thomson, et al., 2007, Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome, Pain 132(1-2):179-188.
7 Medtronic, “Spinal Cord Stimulation: Indications, Safety, and Warnings,” http://professional.medtronic.com/pt/neuro/scs/ind/index.htm?cmpid=URL_Neuro_HCP_YouTube_SCSsafety#.Vu7gfcLmpOE, accessed March 21, 2016.
IMPLANTABLE INSULIN PUMPS
Implantable insulin pumps deliver into the body precise and accurate levels of rapidly acting insulin to maintain the blood glucose within the normal ideal range in patients with diabetes mellitus. Newer approved technology also offers the opportunity for the patient’s blood glucose level to be constantly monitored. The devices are small in size and, while worn externally with an insulin reservoir, there is a needle held in place by an infusion set (see Figure 5.6).
Numerous scientific studies, such as one from the American Diabetes Association, state that insulin pumps have consistently functioned safely in patients with type 1 diabetes. There has been no documentation of over delivery or stoppage. The most frequent complications were lower delivery rates of insulin related to insulin precipitation, which was resolved with lavage by an alkaline solution. Examples of device warnings are provided below.
- Patient warning from the Medtronic website.
Strong sources of electromagnetic interference (EMI), such as short wave (RF) diathermy and MRI, can negatively interact with the pump and cause heating of the implanted pump,
system damage, or changes in pump operation or flow rate, that can result in patient injury from tissue heating, additional surgical procedures, a return of underlying symptoms, and/or a clinically significant or fatal drug underdose or overdose. Avoid using shortwave (RF) diathermy within 30 cm of the pump or catheter. Effects of other types of diathermy (microwave, ultrasonic, etc.) on the pump are unknown. Drug infusion is suspended during MRI; for patients who cannot safely tolerate suspension, use alternative drug delivery method during MRI. Patients receiving intrathecal baclofen therapy are at higher risk for adverse events, as baclofen withdrawal can lead to a life threatening condition if not treated promptly and effectively. Confirm pump status before and after MRI. Reference product labeling for information on sources of EMI, effects on patient and system, and steps to reduce risks from EMI.8
- Precautions from the Medtronic website.
Monitor patients after device or catheter replacement for signs of underdose/overdose. Infuse preservative-free (intraspinal) saline or, for vascular applications, infuse heparinized solutions therapy at minimum flow rate if therapy is discontinued for an extended period of time to avoid system damage. EMI may interfere with programmer telemetry during
___________________
8 Medtronic, “Important Safety Information,” http://www.medtronic.com/patients/cancer/important-safety-information/, accessed March 21, 2016.
pump programming sessions. EMI from the SynchroMed programmer may interfere with other active implanted devices (e.g., pacemaker, defibrillator, neurostimulator).9
- Insulin pump warnings for travelers.
It is important that you test your blood glucose (BG) more frequently while you are traveling. The routine hassle of travel, including stress, changes in time zones, schedules and activity levels, meal times and types of food, can all affect your diabetes control. Be extra attentive to monitoring your BG frequently, and be prepared to respond if needed. Our tips on flying and airport security guidelines apply to travel within the United States. These tips are subject to change, so please also check with the Transportation Security Administration (TSA). International passengers should consult their individual air carriers for international regulations.
- Insulin Pumps and Blood Glucose Meters
When on an airplane, you should go to Utilities > Connect Devices > Meters pump screen, select OFF, and press ACT to unlink your meter from your insulin pump. Manually test your glucose levels using a blood glucose meter.
- Personal Continuous Glucose Monitoring (CGM) device
If you wear a CGM device, it is safe for use on U.S. commercial airlines. If questioned by airline personnel about the use of your device, please show them your Airport Information Card. If they still request that you turn off your CGM device, you must comply.
If you are asked to turn off your CGM device, you will have a “data gap” when uploading data into CareLink® Personal Software, where information is missing from the period of time when your CGM system was turned off.
- Federal Aviation Administration (FAA) requirements
The Federal Aviation Administration (FAA) requires that devices with radio frequency capabilities should not be used on an aircraft.10
Finding 5.1: There seems to be no available documented evidence of any deviation or effect from normal operation of preemptive medical devices when being scanned with a millimeter wave AIT. However, there seem to be many cautionary warnings of potential deviations.
Worth noting is that the current limit of 10 W/m2 is based on heating of biological tissue and does apply to a person being screened no matter whether or not they have an implanted device. Furthermore, there is no separate limit for medical devices even though they very often contain electronics where heating does not make sense to use as a guide.
___________________
9 Medtronic, http://www.medtronic.co.kr/wcm/groups/mdtcom_sg/@mdt/@neuro/documents/documents/ttp-lw-fy10-201003281a.pdf, accessed March 21, 2016.
10 Medtronic, “Traveling with an Insulin Pump or Device,” http://www.medtronicdiabetes.com/customer-support/traveling-with-an-insulin-pump-or-device, accessed March 21, 2016.
Based on knowledge from Chapter 5, the committee also finds the following:
Finding 5.2: The frequency range for current millimeter wave portals are such that the power is mostly deposited in the outer surface or skin of the body; therefore, devices that are not deeply embedded or only partially embedded might be of greater concern.
TSA SCREENING PROCEDURES FOR MEDICAL DEVICES
According to TSA any traveler has a choice, TSA states that:
If you cannot or choose not to be screened by advanced imaging technology or a walk-through metal detector, you will undergo a pat-down procedure instead. 11
Additionally TSA makes the following statements related to internal and external medical devices in how the traveler can inform a TSA officer and how the screening will be conducted for:
- External devices.
Inform the TSA officer if you have a bone growth stimulator, spinal stimulator, neurostimulator, port, feeding tube, insulin pump, ostomy or other medical device attached to your body and where it is located before the screening process begins. You may provide the officer with the TSA notification card or other medical documentation to describe your condition. Submit the device for X-ray screening if you can safely disconnect. Consult with the manufacturer of the device to determine whether it can pass through the X-ray, metal detector or advanced imaging technology for screening.12
- Internal devices.
Inform the TSA officer that you have an artificial knee, hip, other metal implant or a pacemaker, defibrillator or other internal medical device. You may provide the officer with the TSA notification card or other medical documentation to describe your condition. Advanced imaging technology can facilitate your screening and reduces the likelihood of a pat-down. You should not be screened by a walk-through metal detector if you have an internal medical device such as a pacemaker. Consult with your physician prior to flying.13
___________________
11 Department of Homeland Security, “Airport Screening Procedures,” https://www.dhs.gov/ airport-screening-procedures, accessed March 21, 2017.
12 Ibid.
13 Ibid.
In 2010 TSA introduced a medical notification card14 that travelers can use to identify any relevant health conditions or medical devices. Additionally TSA states that:
Passengers with a disability or medical condition or their traveling companion may consult a TSA officer about the best way to relieve any concerns during the screening process. Individuals may provide an officer with a TSA notification card or other medical documentation to describe the condition in a discrete manner. Travelers also may request an accommodation to the security screening process.15
Finding 5.3: The TSA gives travelers with implanted electronic devices, whether life sustaining or therapeutic, a choice between hand screening and millimeter wave AIT. Travelers may weigh the advice of their physicians and the device manufacturers against the absence of evidence of any deviation or effect from normal operation of preemptive medical devices when being scanned with millimeter wave AIT.
Finding 5.4: Travelers with implanted medical electronic devices may wish to carry with them a proper medical identification card or a TSA notification card describing their device and to keep up to date with the latest physician and manufacturer recommendations as well as guidelines from the TSA.
___________________
14 S. Schlichter, “TSA Introduces New Medical Notification Cards for Travelers,” Independent Traveler, December 16, 2010, http://www.independenttraveler.com/blog/index.php/2010/12/16/tsaintroduces-new-medical-notification-cards-for-travelers/, March 21, 2017.
15 Transportation Security Administration, 2016, “TSA Shares Tips for Travelers with Disabilities, Medical Devices, Medical Conditions,” November 15. https://www.tsa.gov/news/releases/2016/11/15/tsa-shares-tips-travelers-disabilities-medical-devices-medical-conditions.











