8
Dependence and Abuse Liability
Studies on the health effects of combustible tobacco have focused on physical disease endpoints (e.g., cancer, cardiovascular disease, respiratory disease). However, combustible tobacco use also has important effects on mental health, including tobacco dependence syndrome. Tobacco use disorder, which is a medical condition recognized by the World Health Organization’s International Classification of Diseases (ICD), had a past-year prevalence of 20 percent among all U.S. adults in 2012–2013 (Chou et al., 2016). It produces clinically significant distress and impairment to those affected. As with other substance use disorders, tobacco dependence1 is characterized by unpleasant withdrawal symptoms and loss of behavioral control over use, which result in dependent individuals spending considerable time obtaining or using combustible tobacco cigarettes, interfering with the ability to fulfill important social or occupational role obligations and having a variety of other social and physical consequences (Fiore et al., 2008; Volkow et al., 2016). As with other psychiatric disorders, the
___________________
1 The committee uses the term “dependence” to describe the constellation of behavioral symptoms associated with the problematic use of tobacco and nicotine products. While earlier versions of the Diagnostic and Statistical Manual of Mental Disorders (DSM) used the term “dependence” to describe the mental health syndrome caused by problematic tobacco use, DSM-5 no longer uses the term dependence and now uses “tobacco use disorder,” which includes many of the symptoms previously identified for the DSM-IV nicotine dependence disorder. Much of the field uses the term “dependence” to describe the mental health symptoms caused by the compulsive use of tobacco, which includes but is not limited to the DSM-IV nicotine dependence operationalizations of the construct.
symptoms of tobacco dependence are experienced by the user as subjectively distressing (Hughes, 2006) and are linked to neurobiological adaptations in the brain’s circuitry underpinning emotion, motivation, and cognition (Markou, 2008). While the amount of tobacco use is associated with risk and severity of tobacco dependence, the correlation is typically of moderate magnitude, and dependence symptoms are reported by an appreciable portion of infrequent and low-intensity tobacco users (Japuntich et al., 2009; Reyes-Guzman et al., 2017), indicating that dependence is a unique outcome in and of itself that is influenced by a combination of the amount of tobacco exposure and other factors. Overall, the tobacco dependence syndrome is an important primary health endpoint to consider.
Nicotine is the principal pharmacological agent that causes dependence on combustible tobacco cigarettes (Benowitz, 2008). Because nicotine is delivered via a pulmonary route, the speed, efficiency, and magnitude of nicotine delivered in “bolus” form produces a higher addiction potential of nicotine relative to other nicotine-delivery devices with slower pharmacokinetics (see Chapter 4 for a detailed review of nicotine pharmacokinetics). While nicotine is necessary, the pharmacological action of nicotine is not sufficient to account for the high addiction potential of combustible tobacco cigarettes (Rose, 2006). “Non-nicotine factors” associated with tobacco self-administration (e.g., taste, smell, and sensations associated with the act of smoking) are critical to the establishment and maintenance of dependence on combustible tobacco cigarettes (Fagerström, 2012). Habitual combustible tobacco cigarette smokers will continue smoking “denicotinized cigarettes” (i.e., cigarettes made with engineered tobacco leaves that contain only trace amounts of nicotine) or very low nicotine-containing cigarettes (i.e., engineered cigarettes with roughly 2–3 percent of the amount in a normal cigarette) for extended periods of time (Donny et al., 2007, 2015). Like other drugs of abuse, denicotinized cigarette smoking can cause a significant release of dopamine in the brain’s reward circuit of regular combustible tobacco cigarette smokers, albeit at lower levels (Domino et al., 2013). Behaviors that have no direct pharmacological effects produce symptoms of addiction (e.g., gambling) and may be associated with dysregulation in brain reward circuits (Quester and Romanczuk-Seiferth, 2015). For these reasons, it is now established that combustible tobacco cigarette dependence is not merely addiction to the nicotine, per se (Rose, 2006). This has prompted experts to call for the reframing and relabeling of the tobacco use disorder concept and measurement away from terms that prioritize nicotine, such as “nicotine dependence,” to conceptualizations and terms that acknowledge the role of non-nicotine factors, such as the term “cigarette dependence” or “tobacco dependence” (Fagerström, 2012).
Given this background, this section focuses on “e-cigarette dependence,” the constellation of behaviors and symptoms that are distressing to the user and promote the compulsive use of e-cigarettes due to nicotine and non-nicotine factors (Strong et al., 2017). Like combustible tobacco cigarettes, if e-cigarette use were to cause dependence symptoms, the symptoms would be strongly influenced by, but not entirely caused by, nicotine per se. Preclinical researchers attempting to uncover the reasons why combustible tobacco cigarettes have such a high addiction potential struggled for decades because animal models were challenged by the fact that, unlike other drugs of abuse, rodents did not easily acquire habitual self-administration of nicotine intravenously (Caggiula et al., 2009). Ultimately, it was discovered that when intravenous nicotine administration was paired with other non-pharmacological sensory stimuli that are pleasant and rewarding (e.g., a sound paired with sucrose) (Caggiula et al., 2009), rats would more easily acquire habitual nicotine self-administration in a manner similar to other drugs of abuse. Based on such research and other studies, it is now established that addiction potential of tobacco products is dependent on the stimulus context that coincides with nicotine administration. The combination of pleasant stimuli associated with the tobacco self-administration ritual (e.g., the taste, smells, sight, and sensations of inhaling and exhaling as well as the hand-to-mouth movements) and the drug itself synergize to account for the high addiction potential of combustible tobacco cigarettes.
Given what is known about the role of nicotine and non-nicotine factors in tobacco product dependence, it is plausible that e-cigarette use may cause dependence symptoms, and the reason may not be explained merely by the fact the e-cigarettes are a nicotine delivery device. Most e-cigarette products are available in desirable flavors and have other characteristics that generate aerosols with a unique profile of pleasurable sensory stimuli due to the taste, sights, smells, and airway sensations, that (like combustible tobacco cigarettes) could have synergistic effects with nicotine on dependence risk. Such enjoyable sensory stimuli in combination with the delivery of “boluses” of nicotine via a pulmonary route (as in combustible tobacco cigarettes) may produce a dependence potential with e-cigarette use. However, it is also possible that e-cigarettes may not produce symptoms of dependence, or that they produce dependence, but at a risk that is significantly lower than combustible tobacco cigarettes. Unlike these combusitble tobacco cigarettes that reliably and quickly deliver nicotine to the brain, the efficiency, speed, and magnitude of nicotine delivery to the user varies widely across different e-cigarette products and user characteristics (see Chapter 4 for a detailed review of nicotine delivery). Relative to a combustible tobacco cigarette, variations in e-cigarette product characteristics and other conditions have been shown
to produce plasma nicotine levels that are below, equal to, or exceed those (Breland et al., 2017). In addition, non-nicotine pharmacological components of combustible tobacco smoke (e.g., monoamine oxidase inhibitors) and other additives may also contribute to the dependence risk caused by combustible tobacco cigarettes (Fagerström, 2012); these compounds may not be present in e-cigarette aerosol. Hence, whether e-cigarettes cause dependence and what the relative magnitude of risk is relative to combustible tobacco cigarettes are questions that cannot be answered solely by the translation of knowledge about nicotine and combustible cigarettes and necessitate a review of the empirical evidence. Furthermore, given the wide variety of products that may alter the nicotine delivery and sensory experience of e-cigarettes, it is plausible that variations in e-cigarette product characteristics affect risk of dependence. Because combustible tobacco cigarette dependence symptoms are known to produce distress as well as social and functional impairment (APA, 2013; Hughes, 2006), independent of the impact of smoking on physical disease, evidence that e-cigarette use causes dependence symptoms would warrant consideration in regulatory policies directed toward e-cigarette manufacture, distribution, and sales.
CHARACTERIZATION OF DISEASE ENDPOINTS AND INTERMEDIATE OUTCOMES
The strongest evidence to characterize the potential association between e-cigarette use and dependence would include methodologically rigorous epidemiological studies with e-cigarette dependence symptoms as an endpoint. While there is no widely agreed-upon method of assessing and diagnosing e-cigarette dependence yet, the initial efforts to operationalize dependence as a health outcome of e-cigarettes have adapted methods of assessing combustible tobacco cigarette dependence to e-cigarettes (Foulds et al., 2015; Strong et al., 2017). Essentially many of the same survey or interview questions aimed at assessing symptom presence or severity are used, but the term “e-cigarettes” is substituted for “cigarettes” on the measure. For instance, the U.S. Population Assessment of Tobacco and Health (PATH) study, a nationally representative survey of tobacco use, adapted dependence measures based on the American Psychiatric Association’s (APA’s) Diagnostic and Statistical Manual of Mental Disorders (DSM) definition of cigarette use disorder. PATH also employed other validated questionnaires that collectively assess various symptoms recognized to be part of the nicotine dependence syndrome, including compulsion to smoke, intensity of smoking (e.g., cigarettes per day), distressing withdrawal symptoms upon abstinence, typical time to first use after awakening each day, and craving for the product. The key manifestations of the DSM and the ICD drug dependence classification
system, which are common to tobacco products and all other substances of abuse, and are summarized in Box 8-1.
E-cigarette dependence can be operationalized as a category (e.g., having at least one or more symptoms, surpassing a “clinical” threshold of two symptoms or more [APA, 2013]), or on a continuum with a score
reflecting a gradient of severity of dependence from none to mild, moderate, or severe. Additional well-established measures of tobacco dependence include the Fagerström Test for Cigarette Dependence (FTCD) (Heatherton et al., 1991), the Heaviness of Smoking Index, the Hooked on Nicotine Checklist (DiFranza et al., 2002), the Nicotine Dependence Syndrome Scale (NDSS) (Shiffman et al., 2004), and the Wisconsin Inventory of Smoking Dependence Motives (Piper et al., 2004). These measures assess symptoms similar to APA and ICD symptoms (e.g., tolerance, withdrawal) and evaluate other domains reflecting other motives for tobacco use or manifestations of habitual smoking (e.g., strong motive to use tobacco to alleviate negative emotions, smoking automatically and instinctually without thinking about it).
Supportive evidence comes from human laboratory investigations that apply “abuse liability” testing methods to e-cigarettes and reflect important intermediate outcomes. Abuse liability tests typically involve human laboratory behavioral pharmacology experiments that test the acute effects of controlled drug administration on indicators that are suspected to be proxies of the likelihood that the drug will produce dependence, including subjective effects (e.g., mood enhancement, drug liking) or behavioral choices indicating the motivational value of the drug (e.g., amount of money willing to trade for the drug, willingness to execute a demanding behavior to obtain the drug) (Henningfield et al., 2011). Abuse liability testing is a long-used paradigm relied on by public health regulatory agencies, such as the Food and Drug Administration (FDA), to indicate whether a novel compound is likely to produce dependence. It is particularly useful for screening the potential for dependence of novel psychoactive compounds (e.g., sedatives, stimulants) prior to obtaining epidemiological data on reports of dependence in the population. Laboratory evidence of abuse liability may not be an exact replication of what occurs in the natural ecology, yet cross-drug differences in laboratory-obtained abuse liability data are in concordance with cross-drug differences in population-level dependence risk among use initiators (Griffiths and Wolf, 1990; Kollins, 2003; Wagner and Anthony, 2002). There is a well-developed literature applying the abuse liability paradigm to combustible tobacco cigarettes and, more recently, emerging literature on the abuse liability of non-traditional tobacco products with specific methodological guidelines put forth from tobacco product abuse liability testing experts (Carter et al., 2009; Henningfield et al., 2011).
OPTIMAL STUDY DESIGN
Primary Endpoint: Epidemiological Evidence of Dependence Symptoms Caused by E-Cigarettes
The optimal epidemiological study would be a longitudinal cohort investigation that follows individuals who initiate e-cigarette use and tracks the development, escalation, and persistence of e-cigarette dependence symptoms in a nationally representative sample. In such a design, descriptive population-level estimates of the speed, likelihood, and duration of dependence symptoms among e-cigarette–ever users would permit inferences regarding the dependence potential of e-cigarettes, with estimates of greater prevalence, speed, and duration of dependence symptoms being indicative of greater dependence risk caused by e-cigarettes. In addition, studies of the association between levels of e-cigarette exposure and likelihood of dependence would also provide key data, with evidence of a dose–response being supportive of greater dependence risk caused by e-cigarette use.
A critical confounder is the use of other tobacco products (namely, combustible tobacco cigarettes), which is strongly associated with e-cigarette use (Kasza et al., 2017; Schoenborn and Gindi, 2015). A large portion of adults in the United States age 25 or older who use e-cigarettes are current or prior combustible tobacco cigarette smokers (CDC, 2016), many of whom have tobacco use disorder (Chou et al., 2016). Individuals with considerable histories of smoking report using e-cigarettes to alleviate nicotine withdrawal caused by their cessation of combustible tobacco cigarettes or to satisfy cravings for such cigarettes (Etter and Bullen, 2014). For current or recent ex-smokers, any behavioral signs or symptoms indicative of dependence on e-cigarettes (e.g., short duration between awakening and time of first e-cigarette) could be attributed merely as an artifact of dependence-like behavior produced by smoking. The confounder of smoking is particularly problematic for dual users; statistical adjustment of smoking behavior may be insufficient for making inferences regarding whether dependence is produced by e-cigarettes. In former smokers who transitioned to using only e-cigarettes, their dependence-like habits with e-cigarettes may be driven by a desire to regulate nicotine levels carried over from when they were smoking. In such cases, statistical adjustment of total combustible tobacco cigarette exposure (e.g., pack-years), age of smoking onset, duration of smoking, and severity and duration of combustible tobacco cigarette dependence could provide some insight into determining whether dependence-like symptoms are the result of e-cigarette use or whether they reflect transference of nicotine dependence from prior combustible tobacco use. Although both reflect forms of dependence, as described above, the committee’s interest is in
whether e-cigarette use may cause dependence on e-cigarettes apart from dependence on nicotine alone.
The optimal epidemiological design would follow a nationally representative sample of never users of tobacco products who initiate use of e-cigarettes and never go on to start using other tobacco products; it would assess the prevalence and association between e-cigarette exposure and e-cigarette dependence symptoms to determine if there is a dose–response association, and if thresholds of exposure that increase risk are comparable to exposure thresholds for combustible tobacco cigarettes. However, the majority of never smokers who use e-cigarettes are youth and young adults (Jamal et al., 2017; Kasza et al., 2017), and a significant portion of them transition to become combustible tobacco cigarette users within several years of e-cigarette use (Soneji et al., 2017). Thus, the incidence of “pure” cases of e-cigarette dependence in the absence of exposure to other tobacco products is likely to be low even if e-cigarettes were to cause dependence.
Supplementary Intermediate Endpoint: Abuse Liability Evidence
For the abuse liability literature used to provide secondary evidence, the optimal design would involve a within-subject, crossover counterbalanced design in which each participant provides data on abuse liability indexes in response to a laboratory “challenge” of at least two conditions, one involving e-cigarettes. Randomized between-subject designs would also provide strong evidence. For example, designs may involve controlled e-cigarette administration challenges with pre- versus post-measures of subjective pleasant effects, with, ideally, comparison data on these measures with no challenge or a sham challenge (e.g., puffing from an unlit combustible tobacco cigarette; see Vansickel et al., 2010). Additional strong designs have an active comparator, such as the comparison of abuse liability indexes across two e-cigarette products that vary on an important dimension of product diversity (e.g., nicotine concentration, flavoring), the comparison of an e-cigarette to a combustible tobacco cigarette, or the comparison of an e-cigarette to an alternative nicotine delivery product (e.g., nicotine gum). Null findings by studies with active controls (or evidence that e-cigarettes have less abuse liability than combustible tobacco cigarettes) should not be interpreted as evidence that e-cigarettes do not produce dependence. However, positive findings from active control studies would provide supportive evidence that e-cigarettes produce dependence to some degree and can address questions regarding the relative dependence risk caused by e-cigarettes compared with combustible tobacco cigarettes or across e-cigarettes with differing product characteristics. From a practical and scientific perspec-
tive, the ideal comparator in an abuse liability study would be a nicotine product known to have low abuse liability (e.g., nicotine lozenge, gum, or transdermal patch).
For the majority of the research, the ideal challenge in laboratory abuse testing involves an experimentally controlled administration whereby the number and pace of puffs is standardized to control the dose administered (e.g., Goldenson et al., 2016). Less ideal (but perhaps more ecologically valid), the participant is permitted to self-administer the product ad libitum (ad lib), which can result in systematic differences in the “dose” of exposure across experimental conditions. For instance, when comparing the pleasant effects of a high- versus low-nicotine e-cigarette, condition challenge involving 5 minutes of ad lib use and the participants self-administering an average of twice as many puffs with the high dose will leave unclear whether differences between conditions are caused by the nicotine level or the number of puffs taken. Thus, how e-cigarettes are used will influence their abuse liability, and patterns of use vary substantially. For example, some users cluster their puffs in cigarette-like sessions or use intermittently throughout the day in short clusters. Large clusters of puffs in relatively quick succession result in a near-bolus dose of nicotine, rapid rise in blood nicotine levels, and likely greater nicotine-related effects (positive reinforcement). This type of use may be associated with greater abuse liability of e-cigarettes. On the other hand, intermittent vaping in short clusters of puffs results in gradual increase in blood nicotine levels throughout the day. This type of use may be done for negative reinforcement (to alleviate nicotine withdrawal symptoms).
Because it is unethical to expose tobacco-product–naïve subjects to e-cigarettes, the majority of research includes either e-cigarette–naïve or inexperienced combustible tobacco cigarette smokers willing to try e-cigarettes or experienced e-cigarette users. E-cigarette–naïve smokers may be unfamiliar with proper use of e-cigarettes, and therefore may produce levels of nicotine exposure that are lower than those of experienced users of the same product (due to differences in puffing topography; see Chapter 3) (Farsalinos et al., 2014; Vansickel and Eissenberg, 2013). Thus, studies using e-cigarette–naïve smokers without proper training in use may result in underestimation of the abuse liability of the product.
An important consideration is the type of outcomes that could be considered evidence of abuse liability in studies that conduct controlled tests of e-cigarette administration. Several controlled laboratory studies of combustible tobacco cigarette smokers who have been acutely deprived of nicotine test the effects of e-cigarette use administration on nicotine withdrawal symptoms, combustible tobacco cigarette craving, and other factors believed to maintain smoking behavior. Such studies are not considered to provide evidence regarding whether e-cigarettes produce
dependence. The suppression of withdrawal and combustible tobacco cigarette craving is known to be caused by a number of products with little or no abuse liability, including FDA-approved smoking cessation medications. In contrast, subjective euphoria, liking, sensory satisfaction, and willingness to exert effort to obtain e-cigarettes are considered evidence of abuse liability, consistent with guidelines provided by FDA and the National Institute on Drug Abuse (ADAMHA, 1989). These particular outcomes generally are not affected by FDA-approved smoking cessation medications.
Ancillary Evidence: Clinical Trials Involving Product Exposure Outside a Laboratory
A number of research studies provide participants (usually e-cigarette–naïve smokers) with an e-cigarette product to use ad lib in the natural ecology for a multiday period. At the end of the period, retrospective reports of the rewarding effects of the product are sometimes collected. While these types of clinical trials may have relevant comparison conditions (e.g., e-cigarette products with differing levels of nicotine strength), which strengthens causal inference, the uncontrolled conditions allow for a number of systematic differences in level of exposure to the product, use of other tobacco product, and other factors that may confound comparisons across conditions.
QUESTIONS ADDRESSED BY THE LITERATURE
Given that e-cigarettes have been widely available for only the past several years, long-term data on whether dependence symptoms emerge among never-smoking e-cigarette users is unavailable. Hence, in the epidemiological data, cross-sectional evidence using e-cigarette dependence symptom measures were considered. Such studies were required to report data on e-cigarette dependence symptoms (e.g., craving for e-cigarettes, short time to first e-cigarette after awakening, difficulty refraining from e-cigarette use in situations when vaping is not allowed; see the section on the characterization of disease endpoints, above); mere reporting on the frequency of use was not considered relevant to dependence. The abuse liability literature was used as supportive evidence. Clinical trials were considered ancillary evidence.
Several epidemiological studies report the prevalence, distribution, and correlates of e-cigarette dependence, including whether frequency of e-cigarette use is associated with symptoms of e-cigarette dependence (Dawkins and Corcoran, 2014; Dawkins et al., 2016; Etter, 2015, 2016; Etter and Eissenberg, 2015; Foulds et al., 2015; Goldenson et al., 2016; Gonzalez-
Roz et al., 2017; Hobkirk et al., 2017; Johnson et al., 2017; Liu et al., 2017; Nichols et al., 2016; Rostron et al., 2016; Strong et al., 2017; Yingst et al., 2015). Descriptive epidemiological reports on base rates and the distribution of e-cigarette dependence symptoms that show that a meaningful portion of e-cigarette users report symptoms of e-cigarette dependence provide evidence to address the question: Does use of e-cigarettes have an effect on e-cigarette dependence risk? Additional epidemiological evidence that the level of exposure to e-cigarettes has a dose–response association with e-cigarette dependence symptom outcomes further addressed that question. In certain experimental studies, data on the prevalence or severity of e-cigarette dependence scores are presented for the purpose of describing the sample used. Because such studies are typically in smaller and non-representative samples, they were used as additional epidemiological evidence. Human laboratory studies of the effects of e-cigarettes (versus a comparator other than combustible tobacco cigarettes) were also supportive evidence.
Some epidemiological studies compared the dependence severity of e-cigarettes to other tobacco products for the typical user (Strong et al., 2017). Some human laboratory studies compared the effects of e-cigarettes to combustible tobacco cigarettes. Collectively, these two streams of evidence address the question: Is the effect of e-cigarette use on e-cigarette dependence risk weaker than the effect of combustible tobacco cigarette use on cigarette dependence?
Finally, there is an emerging epidemiological literature on whether e-cigarette users of products with certain characteristics (e.g., high nicotine concentration) report different levels of e-cigarette dependence than e-cigarette users of products without such characteristics (e.g., low nicotine concentration). Furthermore, there is a human laboratory literature that compares the effects of e-cigarettes with varying product dimensions (e.g., nicotine concentration, flavor) on abuse liability outcomes. Collectively, these streams of evidence address the question: Do e-cigarettes with certain product characteristics have stronger effects on e-cigarette dependence risk than those with other product characteristics?
For each study reviewed, the committee took into account the methodological rigor to grade the strength of evidence. As described above in the Optimal Study Design section, for epidemiological data factors such as the representativeness of the sampling strategy, incorporation of particular exclusions (e.g., excluding current smokers) and covariate adjustment, if relevant, were used to grade the weight of evidence provided by each study. For abuse liability studies, issues such as the inclusion of a comparison condition and sample size were considered.
EPIDEMIOLOGY
The search resulted in 15 studies that reported epidemiological data that matched the requirements above. Review of the studies revealed a natural clustering of different types of studies distinguished by their methodology and rigor: three studies that used nationally representative samples; six online survey studies that did not use a systematic sampling method; two in-person studies that used a non-representative sampling (e.g., recruited users at an e-cigarette convention); and four additional laboratory-based studies that incidentally reported data on e-cigarette dependence symptoms to describe the sample. A brief description of each study’s finding and whether the result provides evidence that is in support of, against, or inconclusive are reviewed in Tables 8-1 and 8-2.
Nationally Representative Studies
Rostron and colleagues (2016) analyzed reports of dependence symptoms among those who were exclusive daily users of e-cigarettes (n = 124), combustible tobacco cigarettes (n = 3,963), or cigars (n = 131) within the past 30 days as well as dependence symptoms of poly-product users in the past 30 days. Data were drawn from the 2012–2013 National Adult Tobacco Survey (NATS), a nationally representative cross-sectional telephone survey. For each product used and each dependence symptom, participants were asked whether they experienced the symptom within the past 30 days. The questions were worded identically across the different products—a strength of the study, which facilitated cross-product comparisons. Among daily e-cigarette users, there were appreciable prevalence rates of various dependence symptoms, including use within 30 minutes of awakening (46.1 percent; 95% CI = 35.1–57.4), strong cravings (46.2 percent; 95% CI = 35.2–57.5), need to use (46.2 percent; 95% CI = 35.2–57.5), and withdrawal symptoms upon abstinence (22.8 percent; 95% CI = 14.8–33.4). Prevalence rates for each dependence symptom were significantly lower among exclusive daily e-cigarette users as compared with exclusive combustible tobacco cigarette smokers and were not significantly different from symptom prevalence estimates for exclusive daily cigar users. Poly-product users of e-cigarettes and combustible tobacco products reported higher prevalence of most symptoms than exclusive e-cigarette, combustible tobacco cigarette, and cigar smokers.
Given the representative sampling, this study provides strong evidence on dependence symptom prevalence estimates in the United States. The separation of exclusive e-cigarette users from poly-product users facilitates inferences that dependence symptoms are not manifestations of dependence toward use of any form of nicotine or tobacco that are driven by dependence on another tobacco product. A limitation is that
comparisons across different groups of users did not statistically adjust for possible confounding factors, such as prior history of tobacco use and demographic factors. In addition, the data were collected from 2012 to 2013 when prevalence of e-cigarette use was low and the marketplace was saturated with early model devices (e.g., cigalikes) and products, which may have had fairly poor nicotine delivery and lacked variety in flavorings (Breland et al., 2017). Modern e-cigarette devices and e-liquids with greater appeal and nicotine delivery effectiveness have become more widely available and more popular within the past few years, but were uncommon when this study was performed. Hence, the generalizability to the current environment is questionable and there is a possibility that e-cigarette prevalence estimates may be different than what would be observed today. In sum, this study provides strong evidence that dependence symptoms are common among daily e-cigarette users and suggestive evidence that the probability of experiencing dependence symptoms is lower for e-cigarettes compared with combustible tobacco cigarettes and not different in comparison to cigars.
Liu and colleagues (2017) analyzed the relative level of dependence among adult participants in the Wave 1 of the PATH study in 2013–2014 who were exclusive everyday users of e-cigarettes (n = 156) and combustible tobacco cigarettes (n = 3,430) in the past 30 days. Four binary dependence symptoms were examined (yes/no), which included identical wording for assessment of e-cigarette and combustible tobacco cigarette dependence:
- “Do you consider yourself addicted to cigarettes/e-cigarettes?”
- “Do you ever have strong cravings to smoke cigarettes/use e-cigarettes?”
- “In the past 12 months, did you find it difficult to keep from smoking cigarettes/using e-cigarettes in places where it was prohibited?”
- “Have you ever felt like you really needed to smoke cigarettes/use e-cigarettes?”
In addition, time to first product use after awakening was also assessed as a quantitative outcome. Results showed high prevalence for both e-cigarettes and combustible tobacco cigarettes for most dependence symptoms—consider yourself addicted (e-cigarettes = 77.2 percent versus combustible tobacco cigarettes = 94.0 percent), strong cravings (e-cigarettes = 72.8 percent versus combustible tobacco cigarettes = 86.9 percent), difficulty refraining from use where prohibited (e-cigarettes = 5.6 percent versus combustible tobacco cigarettes = 28.6 percent), feel need to use (e-cigarettes = 71.5 percent versus combustible tobacco cigarettes = 88.5 percent), time to first use after awakening (grand mean e-cigarettes = 23.46, 95% CI =
TABLE 8-1 Epidemiological Studies on E-Cigarettes and Dependence
| Reference | Study Population | Dependence Measure |
|---|---|---|
| Nationally Representative Studies | ||
| Liu et al., 2017 | Wave 1 adult interview group of PATH database: 156 e-cigarette users; 3,430 combustible tobacco cigarette users | Self-reported time-to-first-use (minutes), and questionnaire: “Do you consider yourself addicted to cigarettes/e-cigarettes?” “Do you ever have strong cravings to smoke cigarettes/use e-cigarettes?” “In the past 12 months, did you find it difficult to keep from smoking cigarettes/using e-cigarettes in places where it was prohibited?” “Have you ever felt like you really needed to smoke cigarettes/use e-cigarettes?” |
| Rostron et al., 2016 | National Adult Tobacco Survey (2012–2013): 60,192 total respondents, daily single tobacco product users: n = 124 e-cigarettes n = 131 cigars n = 3,963 combustible tobacco cigarettes | Average number of cigarettes smoked per day, time to first tobacco use after waking, whether or not respondents sometimes wake at night to use a tobacco product, have had a strong craving to use any tobacco product in the past 30 days, have felt like they really needed to use a tobacco product in the past 30 days, have had a time when they wanted to use a tobacco product so much that it was difficult to think of anything else in the past 30 days, if the statement that they feel restless or irritable after not using tobacco for a while was “not at all true,” “sometimes true,” “often true,” or “always true.” |
| Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|
|
Moderate to high endorsement of e-cigarette dependence symptoms.
E-cigarette dependence in e-cigarette–exclusive users was lower than combustible tobacco cigarette dependence in combustible tobacco cigarette–exclusive smokers (e.g., after adjusting for potential confounders, combustible tobacco cigarette smokers were significantly more likely to have strong cravings, believe they really needed to use the product, and consider themselves addicted). Time-to-first-use: 15% of e-cigarette users said 5 minutes; 24% of combustible tobacco cigarettes users said the same. After adjustment, e-cigarette users had significantly longer time to first use than combustible tobacco cigarette smokers. |
+ | + | |
|
Sizable rates of dependence symptoms endorsed in e-cigarette–only users (23–46%). E-cigarette–users were less likely than users of other products to report withdrawal/craving symptoms, still reported dependence symptoms (e.g., craving for tobacco).
Dual combustible tobacco cigarette and e-cigarette users and e-cigarette poly-product users (cigarette, cigar, e-cigarette) were significantly more likely to report strong craving for tobacco in past 30 days compared with exclusive combustible tobacco cigarette smokers. Symptoms were less prevalent in users of only e-cigarettes and only cigars than people who used both combustible tobacco cigarettes and cigars (e.g., exclusive e-cigarette users reported longer median time to first use than exclusive combustible tobacco cigarette smokers). |
+ | + | |
| Reference | Study Population | Dependence Measure |
|---|---|---|
| Strong et al., 2017 | Adult, established users of a tobacco product from Wave 1 PATH study: combustible tobacco cigarette–only respondents (n = 8,689), e-cigarette–only respondents (n = 437), cigar-only respondents (n = 706), hookah-only respondents (n = 461), smokeless-only respondents (n = 971) | Used four tools (the Hooked on Nicotine Checklist [3 items], WISDM [12 items], NDSS [4 items], the Diagnostic and Statistical Manual criteria [4 items], and Time to First Tobacco Use [1 item]) to obtain 24 tobacco dependence symptoms |
| Studies Using Non-Representative Sampling | ||
| Gonzalez-Roz et al., 2017 | 39 experienced e-cigarette users, 36% of whom were dual users | FTND and NDSS, CO and urinary cotinine |
| Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|
|
With levels of tobacco dependence anchored at 0.0 (SD = 1.0) among combustible tobacco cigarette–only users, mean tobacco dependence was more than a full standard deviation lower for e-cigarette–only users (mean = −1.37, SD = 2.36), cigar-only users (mean = −1.92, SD = 2.11), and hookah-only users (mean = −1.71, SD = 0.53).
Higher level of tobacco dependence among daily groups when compared with non-daily e-cigarette–only users (mean difference = 0.40, SE = 0.07, F(1,10) = 35.1, p < 0.002). |
+ | + | |
| E-cigarette users were dependent on e-liquids containing nicotine, but were less nicotine dependent than current tobacco smokers (FTND 4.38 ± 1.93 versus 5.57 ± 1.48 and NDSS-T 26.26 ± 5.29 versus 40.50 ± 8.14, respectively). This trend was true for all NDSS measures (impulsivity, priority, tolerance, continuity, and stereotyping). | + | + | |
| Reference | Study Population | Dependence Measure |
|---|---|---|
| Johnson et al., 2017 | 131 current e-cigarette users who attended Orlando Vape Convention (October 17, 2015) | FTND and select questions from PSECDI |
| Anonymous Web Surveys of E-Cigarette Users | ||
| Dawkins et al., 2013 | Never (n = 6, 4%), current (n = 218, 16%), and former (n = 1,123, 83%) combustible tobacco cigarette smokers, and current e-cigarette users | Author-constructed survey |
| Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|
| Most users did not wake up during the night to use their device. One-quarter of users reported time to first use within 5 minutes of waking; another 20% reported within 6–15 minutes. More than two-thirds of users would rather forgo other e-cigarette sessions throughout the day than give up their morning session. 50% of respondents used their product 30 times per day for at least 10 minutes. More than 50% said they had ever experienced moderate to extremely strong cravings and 60% had such urges over the past week. 31% reported irritability and 27% reported anxiety if they could not use their device. 60% of users received an FTND score of at least 5. Presence of nicotine in e-liquid and length of e-cigarette use (less than or more than 1 year) were significantly associated with nicotine dependence scores. More than 70% of those who had used an e-cigarette for more than 1 year were classified as moderately or highly nicotine dependent compared with 45% of those who were users for less than 1 year. | + | + | |
| 68% of respondents said “very much so” to “E-cigarette use is as satisfying as tobacco smoking”; 13.3% answered “not at all” to the question “I crave e-cigarettes as much as I do/did tobacco”; 18.4% said “very much so” in response to the same question. | + | + | |
| Reference | Study Population | Dependence Measure |
|---|---|---|
| Etter, 2015 | 374 adult daily users of e-cigarettes who had quit smoking in the previous 62 days |
Online non-representative survey
Used adapted FTND, NDSS, CDS tools to assess dependence on e-cigarettes; also measured urge to use e-cigarette with MPSS (2 items); used modified version item of craving subscale of WSWS |
| Etter and Eissenberg, 2015 | 1,284 adult daily users of e-cigarettes | Used adapted FTND, NDSS, CDS tools to assess dependence on e-cigarettes and nicotine gum; also measured unsuccessful attempts to quit product, and perceptions of likeliness to succeed if stopped using product and addiction to e-cigarette or nicotine gum compared with combustible tobacco cigarette |
| Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|
| Median time to first e-cigarette ranged from 15 to 45 minutes. Users who said e-cigarettes “definitely” decreased tobacco cravings were more likely to report e-cigarettes also alleviated withdrawal symptoms such as anxiety, nervousness, anger, irritability, frustration, depressed mood, sadness, restlessness, impatience, mood swings compared with those who said e-cigarettes had a weak effect on craving. | + | ||
|
Ex-smokers who used only e-cigarettes reported significantly lower time to first cigarette when smoked combustible tobacco cigarettes versus time to first e-cigarette; time to first e-cigarette less than 30 minutes on average. Lower time to first e-cigarette associated with nicotine versus placebo use. 62% of daily dual users said their current dependence on e-cigarettes was weaker than dependence on combustible tobacco cigarettes.
Daily e-cigarette users who used nicotine-containing devices had higher e-FTND scores than those who used non-nicotine–containing devices. Some evidence that gum dependence was more severe (not adjusted for confounding). |
+ | + | + |
| Reference | Study Population | Dependence Measure |
|---|---|---|
| Etter, 2016 | 1,672 adult current users of e-cigarettes (daily and occasionally) | Online non-representative survey |
| Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|
|
Median time to first e-cigarette ranged from 15 to 30 minutes and was lower for those who reported greater throat hit.
Strength of throat hit was associated with satisfaction and dependence variables: “Like the taste of the vapor produced by e-cigarette” (% agree: very weak = 75%; rather weak = 78%; average = 88%; rather strong = 90%; very strong = 88%; r2 = 64.9; p < 0.001). “Likes the sensation when inhales vapor” (% agree: very weak = 60%; rather weak = 81%; average = 86%; rather strong = 92%; very strong = 91%; r2 = 99.6; p < 0.001). “It feels so good to vape” (% agree: very weak = 59%; rather weak = 68%; average = 75%; rather strong = 81%; very strong = 91%; r2 = 41.8; p < 0.001). “Addiction to the e-cigarette” (scale of 0 to 100: very weak = 50%; rather weak = 50%; average = 65%; rather strong = 70%; very strong = 65%; KW = 32.9; p < 0.001). “I am a prisoner of the electronic cigarette” (% agree: very weak = 17%; rather weak = 21%; average = 26%; rather strong = 28%; very strong = 19%; r2 = 43.3; p < 0.001). “I am unable to stop vaping” (% agree = average: 25%; r2 = 41.4; p < 0.001). “If decided to stop using e-cigarette, likely to succeed” (% agree: very weak = 55%; rather weak = 36%; average = 30%; rather strong = 28%; very strong = 42%; r2 = 51.5; p < 0.001). “Stopping using e-cigarette for good would be very difficult” (% agree: very weak = 6%; rather weak = 23%; average = 28%; rather strong = 30%; very strong = 35%; r2 = 56.7; p < 0.001). “Felt the urge to vape today” (% a lot of the time + almost all the time + all the time: very weak = 15%; rather weak = 27%; average = 32%; rather strong = 35%; very strong = 31%; r2 = 46.5; p = 0.001). “Use the e-cigarette because they are addicted to it” (% very true: very weak = 2%; rather weak = 6%; average = 8%; rather strong = 9%; very strong = 9%; r2 = 31.2; p = 0.002). “Former smokers: addiction to e-cigarette compared with former addiction to tobacco cigarette” (% same or stronger: very weak = 12%; rather weak = 15%; average = 25%; rather strong = 25%; very strong = 23%; r2 = 49.7; p < 0.001). |
+ | + | + |
| Reference | Study Population | Dependence Measure |
|---|---|---|
| Etter, 2016 |
| Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|
|
“Stopping using e-cigarette for good would be very difficult” (% agree: very weak = 6%; rather weak = 23%; average = 28%; rather strong = 30%; very strong = 35%; r2 = 56.7; p < 0.001). “Felt the urge to vape today” (% a lot of the time + almost all the time + all the time: very weak = 15%; rather weak = 27%; average = 32%; rather strong = 35%; very strong = 31%; r2 = 46.5; p = 0.001). “Use the e-cigarette because they are addicted to it” (% very true: very weak = 2%; rather weak = 6%; average = 8%; rather strong = 9%; very strong = 9%; r2 = 31.2; p = 0.002). “Former smokers: addiction to e-cigarette compared with former addiction to tobacco cigarette” (% same or stronger: very weak = 12%; rather weak = 15%; average = 25%; rather strong = 25%; very strong = 23%; r2 = 49.7; p < 0.001). |
| Reference | Study Population | Dependence Measure |
|---|---|---|
| Foulds et al., 2015 | 3,609 adult former combustible tobacco cigarette smokers who currently use e-cigarettes | Penn State Cigarette Dependence Index and PSECDI |
| Yingst et al., 2015 | Current advanced-generation e-cigarette device users (n = 3,373); Current first-generation e-cigarette device users (n = 1,048) | Online survey asking, “Did you switch to your current preferred type of e-cigarette because it gives you a more satisfying “hit” than previous e-cigarettes your tried?” (Yes/No); also PSECDI |
| Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|
|
The overall PSECDI for e-cigarette users was significantly lower than their Cigarette Dependence Index, as was the individual score on every other item. More than 90% reported they had experienced strong urges to smoke and withdrawal symptoms when a smoker, but only 25–35% reported experiencing these symptoms of dependence as an e-cigarette user. Those who have used e-cigarettes for a longer time, who have previously tried more e-cigarette models, who currently use an e-cigarette larger than a combustible tobacco cigarette, with a button, with more than one battery, that cost more than $50, and who use a higher concentration of nicotine liquid tend to have a higher e-cigarette dependence index (all p < 0.05).
Those using zero nicotine liquid had a significantly lower e-cigarette dependence index than those using 1–12 mg/ml (p < 0.001), who were significantly lower than those using 13 or greater mg/ml nicotine liquid (p < 0.001). |
+ | + | + |
|
Advanced-generation versus first-generation device users: significantly more dependence on e-cigarettes (despite liquid with lower nicotine concentration) than first-generation device users; also shorter time to first use.
Advanced-generation device user was less likely to be a current smoker. Reported switching to current device because it delivered a more satisfying throat hit. |
+ | + |
| Reference | Study Population | Dependence Measure |
|---|---|---|
| Descriptive Data on E-Cigarette Dependence Symptoms in Small Laboratory Studies | ||
| Dawkins et al., 2016 | 11 experienced male e-cigarette users completed 60 minutes of ad lib use under low (6 mg/ml) and high (24 mg/ml) nicotine liquid conditions in two separate sessions | Adapted FTND; CDS |
| Goldenson et al., 2016 | 20 e-cigarette users (*1 day per week for *1 month; smoking)15 combustible tobacco cigarettes per day; no use of smoking cessation medication) | PSECDI; FTCD |
| Hobkirk et al., 2017 | 9 adult past-month (*20 in the past 28 days) e-cigarette users | PSECDI |
| Nichols et al., 2016 | 7 e-cigarette users | PSECDI |
NOTES: + = positive evidence; − = no positive evidence; +/− = mixed results (some outcomes or analyses yielded positive evidence and others did not yield positive evidence); 0 = inconclusive evidence to determine whether the results are positive or not; CDS = Cigarette Dependence Scale; FTCD = Fagerström Test for Cigarette Dependence; FTND = Fagerström Test for Nicotine Dependence; MPSS = Mood and Physical Symptoms Scale; NDSS = Nicotine Dependence Syndrome Scale; PSECDI = Penn State Electronic Cigarette Dependence Index; WISDM = Wisconsin Inventory of Smoking Dependence Motives; WSWS = Wisconsin Smoking Withdrawal Scale.
| Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|
|
eFTND: mean = 4.73 (SD = 1.35) (range = 2–7).
e-cigarette self-rated addiction item rating: mean = 3.18 (SD = 1.17) (range = 1–5). |
+ | ||
|
PSECDI: mean = 8.4 (95% CI = 6.4–10.4).
FTCD in past 30-day smokers: mean = 6.3 (95% CI = 5.8–6.8). |
+ | ||
| The sample’s average self-reported dependence on e-cigarettes was low based on PSECDI total scores, which ranged from 3 to 8 (mean = 6.33, SD = 1.80) out of a possible score range of 0–20. | + | ||
| PSEDCI: low to medium levels of e-cigarette dependence (mean = 7, SD = 3). | + | ||
TABLE 8-2 Laboratory/Experimental Studies on Dependence and Abuse Liability
| Reference | Study Design | Study Population | Device Measure |
|---|---|---|---|
| Studies Testing the Effects of Flavor | |||
| Audrain-McGovern et al., 2016 | Laboratory | 32 young adult combustible tobacco cigarette smokers who used e-cigarettes at least once |
“e-GO” tank-style e-cigarette with a 2.4-ml refillable e-liquid tank
2 flavored e-liquid options: fruit-flavored (green apple), and dessert-flavored (chocolate), with 6, 12, or 18 mg/ml of nicotine depending on the nicotine content of the participant’s usual smoking rate |
| Dependence Measure | Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|---|
| Modified satisfaction subscale of the Cigarette Evaluation Scale for e-cigarette use, relative reinforcing value of flavor, and number of flavored versus unflavored e-cigarette puffs consumed | Fruit- and dessert-flavored e-cigarettes had a significantly higher reward value than unflavored e-cigarettes; fruit flavor preferred. Users took significantly more flavored puffs than unflavored. Menthol combustible tobacco cigarette smokers took significantly more (at least three times as many) e-cigarette puffs as non-menthol combustible tobacco cigarette smokers. | + | + | |
| Reference | Study Design | Study Population | Device Measure |
|---|---|---|---|
| Goldenson et al., 2016 | Laboratory and used epidemiological data | 20 e-cigarette users (*1 day per week for *1 month; smoking)15 combustible tobacco cigarettes per day; no use of smoking cessation medication) |
Joyetech “Delta 23 Atomizer” tanks connected to a Joyetech “eVic Supreme” battery
20 e-cigarette solutions in 10 flavors were either 0 or 6 mg/ml nicotine (10 flavors included 6 sweet-flavored [peach, watermelon, blackberry, cotton candy, cola, and sweet lemon tea], 3 non-sweet-flavored [mint, tobacco, and menthol], and a single flavorless solution) |
| Rosbrook and Green, 2016, Experiment #1 | Laboratory | 18–45 years of age (n = 32) | Challenge study, controlled e-cigarette use |
| Dependence Measure | Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|---|
| Visual analogue scale assessing “How much did you like it?”, “Would you use it again?”, “How much would you pay for a day’s worth of it?”, “How sweet was it?”, “How strong was the throat hit?”, and “What flavor is it?” |
Significant effect of flavor on each appeal outcome: sweet-flavored solutions produced higher appeal ratings than non-sweet and flavorless solutions. No significant main effects of nicotine or flavor × nicotine interaction effects.
Ratings of sweetness positively associated with each appeal outcome: sweeter associated with increased liking, willingness to use again, and amount willing to pay for a day’s worth of solution. Throat hit not associated with willingness to use again and subjective value and were inversely associated with liking. |
+ | + | |
| General Labeled Magnitude Scale and Labeled Hedonic Scale | No significant effects of menthol or nicotine on liking. Liking was low and did not vary significantly across menthol or nicotine concentrations. | − | − |
| Reference | Study Design | Study Population | Device Measure |
|---|---|---|---|
| Rosbrook and Green, 2016, Experiment #2 | Laboratory | 18–45 years of age (n = 32) | Challenge study, controlled e-cigarette use |
| St.Helen et al., 2017 | Laboratory | 14 e-cigarette users | Inpatient crossover study with strawberry, tobacco, and user’s usual flavor e-liquid. Nicotine levels were nominally 18 mg/ml in the strawberry (pH = 8.29) and tobacco (pH = 9.10) e-liquids and ranged between 3–18 mg/ml in the usual brands (mean pH = 6.80). |
| Dependence Measure | Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|---|
| General Labeled Magnitude Scale and Labeled Hedonic Scale |
Average liking ratings of the e-liquid flavors did not exceed “like slightly” on the Labeled Hedonic Scale.
A trend toward higher ratings for liking of the menthol and menthol–mint flavors over the unflavored e-liquid was supported by a main effect of flavor (F2,60 = 8.11, p < 0.001). |
+/− | +/− | |
| Minnesota Nicotine Withdrawal Scale, Questionnaire for Smoking Urges modified for e-cigarettes, Positive and Negative Affect Schedule, and modified Cigarette Evaluation Questionnaire |
No difference in mCEQ reward or satisfaction subscale between strawberry and tobacco e-liquids, except ratings of sensations in throat and chest (significantly higher with tobacco).
Usual brand e-liquids had significantly more satisfaction and enjoyment of sensations than experimenter-provided liquids. |
+/− | +/− |
| Reference | Study Design | Study Population | Device Measure |
|---|---|---|---|
| Studies Testing the Effects of Nicotine Concentration | |||
| Baldassarri et al., 2017 | Laboratory and epidemiological | Adult experienced e-cigarette users (n = 4) and cigarette smokers (n = 3) | “e-Go type e-cigarette”; nicotine concentrations with a linear range of 0.5–50 μg/ml |
| Dawkins et al., 2016 | Laboratory | 11 experienced male e-cigarette users completed 60 minutes of ad lib use under low (6 mg/ml) and high (24 mg/ml) nicotine liquid conditions in two separate sessions | “eVic™ supreme” e-cigarette from Joyetech, fitted with a “Nautilus Aspire” tank e-cigarette with 6 mg/ml (low) and 24 mg/ml (high) nicotine Halo Smokers’ Angels e-liquid |
| Dependence Measure | Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|---|
| Fagerström Test for Nicotine Dependence adapted for e-cigarettes |
Ratings of product liking were similar after each e-cigarette use (0 mg/ml = 80 ± 28; 8 mg/ml = 75 ± 38; 36 mg/ml = 74 ± 26).
Liking following use of the combustible tobacco cigarette was (37 ± 40); this did not differ compared with the e-cigarette at either liquid strength (8 mg/ml: 75 ± 38; 36 mg/ml: 74 ± 26). |
− | − | |
|
Change in craving and withdrawal symptoms (Mood and Physical Symptoms Scale)
Visual analogue scale assessing positive (hit and satisfaction) and adverse effects associated with nicotine and e-cigarette use |
Mean (SD) percentage hit and satisfaction levels were 61.86 (31.50) and 60.70 (17.30), respectively, in the high condition and 44.73 (23.00) and 46.89 (16.93) in the low condition. These differences did not reach statistical significance (hit: Z = −1.60, p = 0.11; satisfaction: Z = −1.69, p = 0.09). | 0 | ||
| Reference | Study Design | Study Population | Device Measure |
|---|---|---|---|
| Goldenson et al., 2016 | Laboratory and used epidemiological data | 20 e-cigarette users (*1 day per week for *1 month; smoking)15 combustible tobacco cigarettes per day; no use of smoking cessation medication) | Joyetech “Delta 23 Atomizer” tanks connected to a Joyetech “eVic Supreme” battery; 20 e-cigarette solutions in 10 flavors were either 0 or 6 mg/ml nicotine (10 flavors included 6 sweet-flavored [peach, watermelon, blackberry, cotton candy, cola and sweet lemon tea], 3 non-sweet-flavored [mint, tobacco and menthol] and a single flavorless solution) |
| Perkins et al., 2015 | Laboratory | Adult dependent combustible tobacco cigarette smokers (n = 28) in a fully within-subjects design | E-cigarettes with as much as 36 mg/ml nicotine; “rawhide red (tobacco)” for non-menthol and “Freeport (menthol)” for menthol flavors |
| Rosbrook and Green, 2016, Experiment #1 | Laboratory | 18–45 years of age (n = 32) | Challenge study, controlled e-cigarette use |
| Dependence Measure | Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|---|
| Visual analogue scale assessing “How much did you like it?”, “Would you use it again?”, “How much would you pay for a day’s worth of it?”, “How sweet was it?”, “How strong was the throat hit?”, and “What flavor is it?” |
Significant effect of flavor on each appeal outcome. No significant main effects of nicotine or flavor × nicotine interaction effects.
Significant effect of nicotine on throat hit: a stronger throat hit in nicotine versus placebo solutions. Throat hit not associated with willingness to use again and subjective value and were inversely associated with liking. |
+ | + | |
| Reward reinforcement task | Nicotine: significantly greater liking compared with the placebo e-cigarette. | + | + | |
| General Labeled Magnitude Scale and Labeled Hedonic Scale | No significant effects of menthol or nicotine on liking. Liking was low and did not vary significantly across menthol or nicotine concentrations. | − | − |
| Reference | Study Design | Study Population | Device Measure |
|---|---|---|---|
| Rosbrook and Green, 2016, Experiment #2 | Laboratory | 18–45 years of age (n = 32) | Challenge study, controlled e-cigarette use |
| Dependence Measure | Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|---|
| General Labeled Magnitude Scale and Labeled Hedonic Scale |
Average liking ratings of the e-liquid flavors did not exceed “like slightly” on the Labeled Hedonic Scale.
A trend toward higher ratings for liking of the menthol and menthol–mint flavors over the unflavored e-liquid was supported by a main effect of flavor (F2,60 = 8.11, p < 0.001). Significant effect of nicotine on coolness/cold perceptions. |
+/− | +/− |
| Reference | Study Design | Study Population | Device Measure |
|---|---|---|---|
| Comparison of E-Cigarette to Combustible Tobacco Cigarettes and Other Products | |||
| Stiles et al., 2017 | Laboratory | 59 e-cigarette–naïve combustible tobacco cigarette smokers | Vuse Solo e-cigarettes were evaluated in this study, containing either 14, 29, or 36 mg of nicotine. Vuse Solo e-cigarettes are composed of a battery, heating element, microchips, sensor, and a cartridge containing propylene glycol, glycerol, nicotine, flavorings, and water. The three devices were presented without brand style information and were visually indistinguishable by subjects. |
| Dependence Measure | Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|---|
| Questionnaires: Product Liking, Urge to Smoke, Urge for Product, Intent to Use Product Again, Product Effects | The mean maximum scores (Emax) on the Product Liking questionnaire were substantially lower for the three Vuse Solo e-cigarettes compared with the combustible tobacco cigarette condition (LS [least square] mean Emax scores ranging from 4.13 to 4.57, LS mean Emax = 9.06, p < 0.001 for all, respectively), and somewhat higher than nicotine gum (LS mean Emax = 3.21, p < 0.05 for all). A similar pattern was seen with the Intent to Use Again questionnaire. The mean Emax intent to use again scores were substantially lower for the three Vuse Solo e-cigarettes (LS mean Emax scores ranging from 4.07 to 4.75) compared with the combustible tobacco cigarette condition (LS mean Emax = 6.81, p < 0.001 for all), and higher than nicotine gum (LS mean Emax = 3.29, p < 0.006 for all). A similar pattern was also shown for the Liking of Positive Effects measure. | + | + | |
| Reference | Study Design | Study Population | Device Measure |
|---|---|---|---|
| Strasser et al., 2016 | Trial | 28 e-cigarette–naïve current combustible tobacco cigarette smokers | 5 first-generation design brands: NJOY, 18 mg nicotine; V2, 18 mg nicotine; Green Smoke, 18.9–20.7 mg nicotine; blu, 20–24 mg nicotine; and White Cloud, 23–24 mg nicotine |
| Vansickel et al., 2010 | Laboratory | 32 e-cigarette–naïve combustible tobacco cigarette smokers | 16–18 mg/ml first-generation devices that didn’t give nicotine yield in blood. Users’ own brand of combustible tobacco cigarettes versus sham (unlit combustible tobacco cigarette) versus “NPRO” e-cigarette versus “HYDRO” e-cigarette |
| Dependence Measure | Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|---|
| Withdrawal Symptom Checklist and questionnaire of Smoking Urges | Compared with combustible tobacco cigarette smoking, e-cigarettes provided significantly lower nicotine levels (25–50%), reduced CO exposure, and lower ratings of liking (p < 0.05). No differences by brand detected. E-cigarette use on day 5 significantly reduced levels of craving and withdrawal; similar results at day 10. | + | ||
| Questionnaire of Smoking Urges Brief (QSU Brief); visual analogue scale | Significant condition by time interactions were observed for ratings of “satisfying,” “pleasant,” “taste good,” “dizzy,” “calm,” “concentrate,” “awake,” and “reduce hunger.” | + |
| Reference | Study Design | Study Population | Device Measure |
|---|---|---|---|
| Vansickel et al., 2012 | Laboratory | 20 e-cigarette–naïve combustible tobacco cigarette smokers | “Vapor King” (KR808 model) automatic e-cigarette, 18 mg |
| Clinical Trials | |||
| Meier et al., 2017 | Laboratory/Crossover | 24 adult combustible tobacco cigarette smokers, no vaping in past 6 months |
blu cigarette starter kit with up to seven cartridges prefilled with 16-mg nicotine solution
Within a double-blind randomized crossover design, smokers (n = 24; 75% male; mean age = 48.5 years) smoked as usual for 1 week, followed by two counterbalanced naturalistic (i.e., ad lib use) weeks of either placebo or active first-generation e-cigarettes |
| Dependence Measure | Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|---|
| Questionnaire of Smoking Urges Brief (QSU Brief); visual analogue scale |
Effects of the highest magnitude were observed for ratings of “pleasant” (F6,114 = 21.1, p < 0.0001), “satisfying” (F6,114 = 19.5, p < 0.0001), and “taste good” (F6,114 = 20.2, p = 0.0001).
Crossover values were greater in the own brand versus money choice condition relative to the e-cigarette versus money choice condition. Collapsed across time, the average crossover value was $1.06 (SD = $0.16) in the e-cigarette versus money choice condition and $1.50 (SD = $0.26) in the own brand versus money choice condition. |
+ | + | |
| Minnesota Nicotine Withdrawal Scale; Brief Wisconsin Inventory of Smoking Dependence Motives; Glover-Nilsson Smoking Behavioral Questionnaire; and modified Cigarette Evaluation Scale | Modified Cigarette Evaluation Scale scores for e-cigarette use did not differ between active and placebo e-cigarettes. | − | − | |
| Reference | Study Design | Study Population | Device Measure |
|---|---|---|---|
| Steinberg et al., 2014 | Clinical trial | 41 e-cigarette–naïve combustible tobacco cigarette smokers | Device type unknown. Each participant used e-cigarette and nicotine inhaler each for 3 days, in random order, with a washout period between each one. |
NOTE: + = positive evidence; − = no positive evidence; +/− = mixed results (some outcomes or analyses yielded positive evidence and others did not yield positive evidence); 0 = inconclusive evidence to determine whether the results are positive or not.
19.47–28.27 minutes versus grand mean combustible tobacco cigarettes = 19.25, 95% CI = 18.25–20.30 minutes). Of note, as described in Chapter 1, overall prevalence of e-cigarette use is low in the PATH study relative to other nationally representative surveys. Regression analyses adjusted for demographics showed that, relative to exclusive daily combustible tobacco cigarette users, exclusive daily e-cigarette users reported lower prevalence for each dependence symptom and longer time to first use.
A strength of this study was the report on the product characteristics used among the e-cigarette users, which provides information generalizability on a key source of potential variability in dependence risk (i.e., device type). Among e-cigarette users, 96.3 percent reported that the
| Dependence Measure | Results | E-Cigarettes Have Some Dependence Risk? | E-Cigarettes Have Lower Dependence Potential Than Combustible Tobacco Cigaretteh? | Product Characteristics Alter Dependence Risk? |
|---|---|---|---|---|
| Modified Cigarette Evaluation Questionnaire | The total Psychological Rewards scores were higher for the combustible tobacco cigarette and e-cigarette compared with the inhaler. E-cigarettes scored significantly lower on aversion scores than combustible tobacco cigarettes. Compared with inhaler, e-cigarettes scored higher on measures of perception such as helpful for not smoking and effective for quitting, similar to combustible tobacco cigarettes, acceptable to smokers, and cool image. | + |
e-cigarette they used most of the time was rechargeable, 76.5 percent reported that they were able to refill their e-cigarette or e-cigarette cartridges with e-liquid, and 95.8 percent reported using e-cigarettes that usually contained nicotine. The analyses excluded those who used more than one product in the past 30 days, which reduces the impact of current exposure to other products on reports of e-cigarette dependence symptoms. Comparisons in dependence symptoms between e-cigarette and combustible tobacco cigarette users were adjusted for sociodemographics, which helps to rule out some confounding effects.
Prior tobacco use history characteristics were not adjusted for in the analysis, leaving unclear whether chronicity and level of prior tobacco
product exposure, which may directly influence risk of dependence on any tobacco product, may differ between e-cigarette and combustible tobacco cigarette users and explain group differences in dependence. It is possible that one of the groups consumed more tobacco or had greater total exposure to nicotine in their lifetime prior to the past 30 days. The authors reported 92.9 percent of exclusive daily e-cigarette users were former regular combustible tobacco cigarette smokers; hence, both groups had chronic combustible tobacco cigarette exposure. Previous tobacco consumption could produce chronic neurobiological alterations that may increase liability dependency on any product, including e-cigarettes. Consequently, the prevalence estimates reported may be less than what would be observed for e-cigarette users who have little history of use of other tobacco products.
Finally, some the symptoms are likely to be less valid indicators of the underlying addiction to e-cigarettes as compared with combustible tobacco cigarettes. For example, the symptom “difficulty refraining from use in places where prohibited,” which is a well-validated symptom of combustible tobacco cigarette dependence, may be less relevant to e-cigarettes because there are fewer restrictions on where e-cigarettes may be used. Indeed, the authors reported that the majority of e-cigarette users reported living in a place that allows the use of their product anywhere and at any time inside their home (61.9 percent), compared with only 26.5 percent of the combustible tobacco cigarette smokers. In sum, this study provides strong evidence that the prevalence and severity of e-cigarette dependence symptoms in exclusive users are fairly high overall in the U.S. population, but not as high as what is found in exclusive combustible tobacco cigarette smokers.
A separate analysis of PATH Wave 1 2013–2014 data looked at whether responses to dependence symptom questions mapped onto a common “latent dimension” of dependence severity for various tobacco products (Strong et al., 2017). Like the other studies, survey questions for each dependence symptom were worded identically across different tobacco products, and a primary goal was to compare results across mutually exclusive past-year tobacco user groups, including combustible tobacco cigarette only (n = 8,689), e-cigarette only (n = 437), cigar only (traditional, cigarillo, or filtered) (n = 706), hookah only (n = 461), smokeless tobacco only (n = 971), combustible tobacco cigarette plus e-cigarette (n = 709), and multiple tobacco product users (n = 2,314). Wording of each symptom interview question is listed in Table 8-3. To satisfy the study inclusion criteria for current established use, for combustible tobacco cigarettes, a current established user is defined as an adult who has smoked at least 100 cigarettes in his/her lifetime and now smokes every day or some days. For all other tobacco products, a current established user is defined
as an adult who has ever used the product “fairly regularly” and now uses it every day or some days.
Though both Liu and colleagues (2017) and Strong and colleagues (2017) use PATH Wave 1 data, the samples are only partially overlapping, because Strong and colleagues included both daily and non-daily users, whereas Liu and colleagues included daily users. Hence, the results from the two studies provide results from non-redundant data sources. Another difference between the studies was the data analysis approach. Liu and colleagues used regression modeling. A unique strength of the Strong and colleagues study was the application of item response–based statistical modeling, which permitted assessment of whether the extent to which each symptom was a valid indicator of the underlying latent dependence syndrome and whether its validity differed depending on whether it was being reported for one product versus another (i.e., differential item functioning [DIF]). The latent dimension is empirically estimated upon a common-dimension intersymptom association using factor analytic techniques. Once a common latent dimension is ascribed and only items that are equally valid indicators of the dimension are retained to estimate the dimension, comparisons of the relative “severity” of dependence on the dimension can be made with greater rigor and assurance of a common metric. Without doing so, any differences in the relative prevalence or severity of a particular dependence symptom across different user groups could be ascribed to the symptom being a less valid indicator for use of one product versus another. For example, the study found that reporting difficulty refraining from using the product in places where it was prohibited was less strongly associated with the latent dependence dimension for exclusive e-cigarette users than for combustible tobacco cigarette users. This may be due in part to less comprehensive indoor air quality restrictions against e-cigarette use than combustible tobacco cigarette use, making this particular symptom a less relevant indicator of e-cigarette dependence than of combustible tobacco cigarette dependence. The study then used the empirically validated latent dimension to compare the average severity of dependence across different tobacco product user groups.
DIF analyses supported use of 16 of the 24 examined tobacco dependence (TD) indicators for comparisons across different tobacco product users. Three items were omitted from further analyses because they were invalid indicators of the latent dependence dimension in multiple users (i.e., “most of the people I spend time with are tobacco users”; “tobacco use is causing a health problem”; “giving up activities as tobacco use not allowed”); others were retained or eliminated based on DIF analysis and the authors’ judgment, including retaining symptom indicators that may have yielded statistically significant DIF that were not of clinical or practical significance. Using the item response–based model with the
| Item Number | Original Instrument | Domain | Question Text | Final Common Instrument |
|---|---|---|---|---|
| 1 | HONC | Loss of control | Do you consider yourself addicted to [product]? | No |
| 2 | HONC | Craving | Do you ever have strong cravings to [product]? | No |
| 3 | HONC | Craving | Have you ever felt like you really needed [product]? | No |
| 4 | WISDM: Primary | Automaticity | I find myself reaching for [product] without thinking about it. | Yes |
| 5 | WISDM: Primary | Craving | I frequently crave [product]. | Yes |
| 6 | WISDM: Primary | Craving | My urges keep getting stronger if I don’t use [product]. | Yes |
| 7 | WISDM: Primary | Loss of control | Tobacco products control me. | Yes |
| 8 | WISDM: Primary | Loss of control | My [product] use is out of control. | Yes |
| 9 | WISDM: Primary | Tolerance | I usually want to use [product] right after I wake up. | Yes |
| 10 | WISDM: Primary | Craving | I can only go a couple of hours without using [product]. | Yes |
| 11 | WISDM: Primary | Automaticity | I frequently find myself almost using [product] without thinking about it. | Yes |
| 12 | WISDM: Secondary | Negative reinforcement | Using [product] would really help me feel better if I’ve been feeling down. | Yes |
| 13 | WISDM: Secondary | Cognitive enhancement | Using [product] helps me think better. | Yes |
| 14 | WISDM: Secondary | Social reinforcement | Most of the people I spend time with are tobacco users. | No |
| 15 | WISDM: Secondary | Affiliative attachment | I [would] feel alone without my [product]. | Yes |
| 16 | NDSS | Loss of control | I would find it really hard to stop using [product]. | Yes |
| 17 | NDSS | Loss of control | I would find it hard to stop using [product] for a week. | Yes |
| 18 | NDSS | Withdrawal | After not using [product] for a while, I need/I would like to use [product] in order to feel less restless and irritable. | Yes |
| 19 | NDSS | Withdrawal | After not using [product] for a while, I need to use [product] in order to keep myself from experiencing any discomfort. | Yes |
| 20 | DSM: Risky Use | Use despite consequences | Do you believe that [product] is causing a health problem or making it worse? | No |
| 21 | DSM: Social impairment | Give up activities | In the past 12 months, did you give up or cut down on activities that were enjoyable or important to you because [product] was not permitted at the activity? | No |
| 22 | DSM: Impaired control | Loss of control | In the past 12 months, did you find it difficult to keep from using [product] in places where it was prohibited? | Yes |
| 23 | DSM: Withdrawal | Withdrawal | Withdrawal syndrome. | No |
| 24 | Time to first tobacco | Tolerance | On days that you smoke, how soon after you wake up do you typically smoke your first cigarette of the day? Please enter the number of minutes or hours. | No |
NOTES: The Final Common Instrument identifies as “Yes” the 16 items used to compare levels of tobacco dependence (TD) across product users. Items labeled “No” were set aside due to evidence of poor relation to overall levels of TD or differences in how the items measured TD symptoms across products. DSM = Diagnostic and Statistical Manual of Mental Disorders; HONC = Hooked on Nicotine Checklist; NDSS = Nicotine Dependence Syndrome Scale; WISDM = Wisconsin Inventory of Smoking Dependence Motives.
SOURCE: Strong et al., 2017.
validated 16 item cross-product dependence index to estimate the latent dependence severity across all groups, mean tobacco product dependence severity scores were 1.37 standard deviation units lower for e-cigarette–only users than combustible tobacco cigarette–only users (see Figure 8-1). E-cigarette–only users were comparable to cigar-only users and slightly higher than hookah-only users. Poly-product users of e-cigarettes and other products were comparable to combustible tobacco cigarette–only users. Among e-cigarette–only users, the 70.1 percent (SE ± 2.12 percent) of exclusive e-cigarette users who were daily users scored significantly higher on the latent dependence dimension than non-daily exclusive e-cigarette users (mean difference in standard deviation units = 0.40, SE = 0.07). Overall, e-cigarette–only users did have a lower level of TD, but increased frequency of use was significantly associated with increasing levels of TD (Strong et al., 2017).
The results of this study highlight the importance of considering the relative validity of symptom indicators across different tobacco products. Given that certain measurements of dependence symptoms differ in their relative validity, the prevalence and mean severity estimates may be less accurate and perhaps biased for one product versus another. Nonetheless, the bulk of the indicator symptoms (21 of 24) in this study exhibited consistent relationships with the primary dependence dimension for each product, suggesting that any error or bias across products may be modest, and 16 of the 24 were deemed to have minimal or no differential validity across products after substantial empirical scrutiny. The highly rigorous approach of estimating a well-vetted index with a comprehensive set of items is a major strength of the study, as was the use of a large nationally representative sample and separation of multiple mutually exclusive single- and poly-product user groups. In addition to providing precise mean dependence severity estimates of e-cigarette users relative to other user groups, this study shows that frequency of e-cigarette use is significantly associated with severity of dependence. This provides additional evidence that, as with combustible tobacco cigarettes and other drugs of abuse, dependence severity is higher among those who use more frequently. Limitations include the use of a cross-sectional design, which leaves unclear whether the association between level of e-cigarette use and dependence is a result of greater exposure to the product increasing severity of dependence, more frequent use as a consequence of the strong drive to use, or other confounding influences. The omission of other covariates in these analyses and comparisons of dependence severity across different product user groups further leaves unclear the role of alternative explanations for observed associations other than a causal effect. In sum, this study provides robust evidence that the typical level of dependence symptoms among exclusive e-cigarette users is comparable
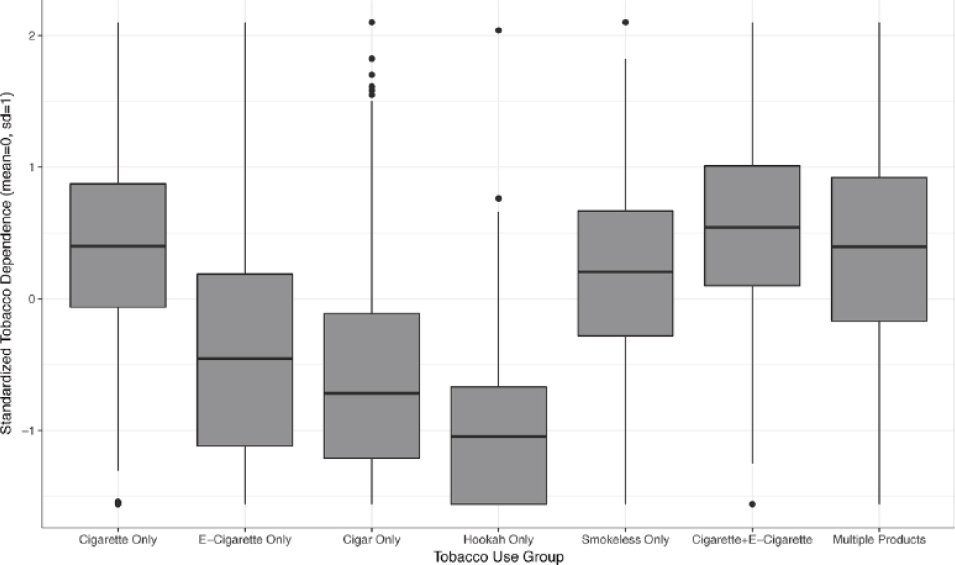
SOURCE: Strong et al., 2017.
to cigar users and lower than combustible tobacco cigarette users in the U.S. population. In addition, the association between frequency of use and dependence among exclusive e-cigarette users further suggests that dependence symptoms are directly linked to e-cigarette exposure.
Studies Using Non-Representative Sampling
Johnson and colleagues (2017) surveyed 117 e-cigarette users attending a large southeastern e-cigarette convention in fall 2015. Modified questions from the FTCD adapted for e-cigarette use and other questions were administered via a paper and pencil survey at the convention center lobby. Total scores were then categorized into one of four categories to approximate the clinical cutoffs for the FTCD. These categories were “low dependence” (score = 1–2, n = 20, 17.1 percent of respondents), “low to moderate dependence” (score = 3–4, n = 26, 22.2 percent), “moderate dependence” (score 5–7; n = 53, 45.3 percent), and “high dependence” (score = 8 or higher; n = 18, 15.4 percent of respondents). Hence, a significant proportion of the sample was classified as moderate or high dependence. Of the sample, 10 percent also used combustible tobacco cigarettes. This low prevalence may reflect a selection bias. Although smokers were not removed from the analysis, current or past smoking status were not
significantly different across the modified-FTCD e-cigarette dependence severity categories, suggesting that the confounder of current smoking was modest. Length of e-cigarette use was positively associated with e-cigarette dependence category. More than half of respondents who have used e-cigarettes for more than a year were ranked as moderately or highly nicotine dependent (70.5 percent). Fewer than half (45.7 percent) who have used e-cigarettes for less than a year were ranked as moderately to highly nicotine dependent. There is a statistical trend in differences between those who used e-liquid with (versus without) nicotine and modified-FTCD dependence level (p = 0.054). Among those who used e-liquid without nicotine, 36.4 percent were classified as low, 22.7 percent were low-to-moderate, 36.4 percent were moderate, and 4.6 percent were highly dependent. In those who used e-liquid with nicotine, the distribution was shifted toward more severe dependence, such that 12.8 percent were low, 22.3 percent were low-to-moderate, 46.8 percent were moderate, and 18.1 percent were high. The idiosyncratic and highly selected sample limits the generalizability of the findings and raises considerable questionability regarding the generalizability of the prevalence estimates. Furthermore, the sample was modest and statistical comparisons did not adjust for confounders. In sum, this study provides weak suggestive evidence that dependence symptoms are of appreciable prevalence, associated with chronicity of use, and are higher among those who use nicotine.
In a letter to the editor, Gonzalez-Roz and colleagues (2017) reported nicotine dependence levels in a sample of “experienced e-cigarette users” (n = 39, men = 77 percent) and current combustible tobacco cigarette smokers (n = 42, men = 57 percent). The authors administered adapted and non-adapted versions of both the FTCD and the NDSS to e-cigarette and combustible tobacco cigarette users, respectively. The authors also collected and analyzed samples for biochemical markers of carbon monoxide and urinary cotinine. Based on the mean scores of each group, the authors concluded that “(1) e-cigarette users were dependent on e-liquids containing nicotine, [and] (2) e-cigarette users were found to be less nicotine dependent than current tobacco cigarette smokers [on all self-reported measures]” (Gonzalez-Roz et al., 2017, pp. 136–137). Cotinine values did not significantly differ between the groups, while CO was higher in smokers than e-cigarette users. This study is subject to the same limitations that all cross-sectional studies using dependence symptom measures that are not psychometrically validated via item-response modeling. Furthermore, because details regarding the recruitment strategy, population, and other variables (e.g., demographics) were not provided nor were adjusted analyses performed, clear conclusions regarding the contribution of this study to the evidence base could not be drawn. This study was judged to provide very weak evidence that e-cigarette dependence
symptoms are of appreciable prevalence and severity in e-cigarette users at levels lower than combustible tobacco cigarette users.
Anonymous Web Surveys of E-Cigarette Users
Foulds and colleagues (2015) collected data on the prevalence and correlates of e-cigarette dependence symptoms among e-cigarette users who completed an online survey. Participation in the survey was voluntary and anonymous; data were collected from December 2012 to August 2014. Participants were recruited by following links to the survey, which the investigators posted on a range of medical websites and those popular among e-cigarette users such as http://www.webMD.com and http://www.e-cigaretteforum.com. Visitors to these sites could also send or post a link to the survey to friends and other websites. The analysis was limited to 3,609 respondents who were exclusive current daily e-cigarette users who had not smoked combustible tobacco cigarettes in the past 30 days. Participants were asked to report on 10 dependence symptoms that compose the Penn State Electronic Cigarette Dependence Index (PSECDI), which assesses frequency of use, time to first use after awakening, difficulty refraining from use when prohibited, craving, and other related symptoms. An analogously worded cigarette dependence index was also completed. Because participants were all past smokers, they were asked “Think back to a time when you were primarily a traditional cigarette smoker . . . before you used e-cigs. To the best of your ability, answer the following questions regarding your cigarette smoking at that time.” Within-person comparisons of the dependence symptoms showed that for nearly all questions, symptoms were more likely and reported at higher levels when participants were asked to recall their experience with combustible tobacco cigarettes than their current experience with e-cigarettes. The mean (SD) composite dependence score for e-cigarettes was 8.1 (3.5), which would be classified as between “low” and “medium” severity dependence, which was significantly lower than the corresponding mean (SD) dependence score for combustible tobacco cigarettes 14.5 (3.7), which would be classified as “high” severity dependence. The e-cigarette versus combustible tobacco cigarette comparison was a “within-subject” comparison that rules out systematic confounders that occur across different populations. However, given that recall errors and other reporting biases for historical information were present only for e-cigarette use, these results are highly impacted by potential methodological confounding. The authors conducted a regression model in which number of demographic and e-cigarette and combustible tobacco cigarette use characteristics were included as simultaneous predictors of PSECDI score. PSECDI was significantly higher in women (versus men), whites (versus other races),
those without (versus with) a college education, those who are older (versus younger), those who have used e-cigarettes for a longer time, those who have previously tried more e-cigarette models, those who currently use a device larger than a combustible tobacco cigarette (versus a cigalike model), those who use a more advanced device with a button (versus other models), those who use a device that costs greater versus less than $50, and those who use a higher concentration of nicotine liquid (see Figure 8-2).
Because participation was anonymous and the recruitment method allowed anyone to complete the survey, the representativeness of the sample is uncertain. The authors note that “those who found out about the survey on specialist websites and took the time to complete the survey are a particularly experienced and likely ‘pro-e-cig’ sample of e-cig users, and it is possible their answers were designed to make e-cigs look ‘good’ relative to traditional cigarettes” (Foulds et al., 2015, p. 191). The authors attempted to address this via sensitivity analyses adjusting for and restricting to self-reported public advocacy for e-cigarettes online (which was reported by 42 percent of participants) and being an e-cigarette retailer (3 percent), which did not affect the main results. The non-representative sample is a limitation, but the fairly large sample is a strength. In sum, this study provides suggestive evidence that e-cigarette dependence symptoms are of appreciable severity and lower than for combustible tobacco cigarettes. Higher nicotine concentration and other device characteristics typically associated with greater power and nicotine yield (e.g., newer generation, higher price) are associated with more severe e-cigarette dependence symptoms.
A study by Yingst and colleagues (2015) drew from the same dataset as in Foulds and colleagues (2015), and compared dependence symptoms among participants using “first-generation” devices (n = 1,048; same size as a combustible tobacco cigarette with no button) and “advanced-generation” devices (n = 3,373; larger than a cigarette with a manual button); participants were combustible tobacco cigarette–ever smokers who reported using an e-cigarette at least 30 days in their lifetime. Results showed that participants currently using an advanced- (compared with first-) generation device exhibited higher scores on the PSECDI dependence symptom composite index (mean [SD] = 8.3 [3.3] versus 7.1 [4.0]) and short time to first e-cigarette after wakening (mean [SD] = 38.7 [60.0] versus 67.3 [116.1] minutes) despite using a liquid with a lower nicotine concentration (mean [SD] = 15.1 [6.6] versus 19.1 [12.7] mg/ml). These results were not adjusted for potential confounding covariates, although device type was also associated with dependence scores in the Foulds and colleagues (2015) analysis, which did adjust for many relevant confounding factors. While subject to the same limitations as Foulds and
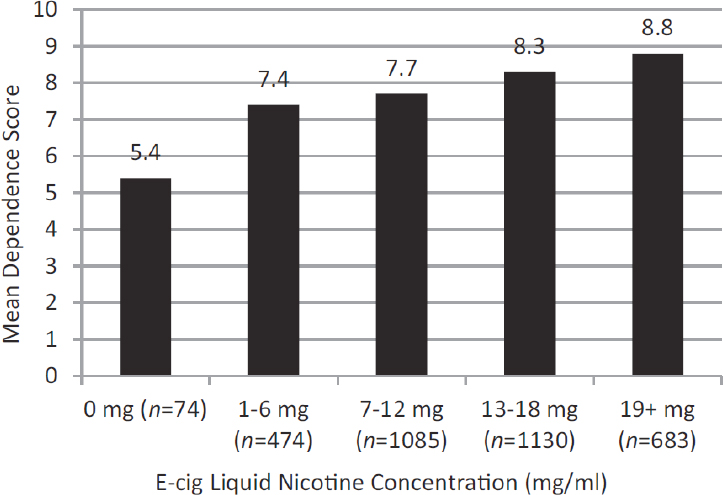
NOTES: Penn State Electronic Cigarette Dependence Index was adjusted for gender, age, race, education level, days used an e-cigarette, e-cigarette size, e-cigarette button, battery, and number of e-cigarettes. All between-group p values < 0.003 except between (1) 1–6 and 7–12 mg groups, and (2) 13–18 and 19+ mg groups.
SOURCE: Foulds et al., 2015.
colleagues (2015) and providing some replicatory findings, this study provides confirmatory evidence that advanced- (compared with first-) generation devices are associated with higher dependence, and this association is clearly not driven by differences in the nicotine concentration of the liquid. The authors speculate that because advanced-generation devices provide more power and greater nicotine delivery per equivalent nicotine composition in e-liquid (Shihadeh and Eissenberg, 2015), greater nicotine exposure to the user may account for the higher dependence levels in advanced- versus first-generation device users.
Dawkins and colleagues (2013) conducted a study of never (n = 6; 4 percent), current (n = 218; 16 percent); and former (n = 1,123; 83 percent) smokers who were also current e-cigarette users. Participants recruited on e-cigarette retailer websites completed a Web survey on e-cigarette dependence and use characteristics, including several survey questions addressing factors relevant to dependence and abuse liability. In the whole sample, the proportion of survey responses indicating the highest level of endorse-
ment (i.e., “very much so”) was 56.2 percent for an item indicative of abuse liability (“I get a definite nicotine hit from the e-cigarette”) and 18.4 percent for an item indicative of possible dependence (“crave e-cigarettes as much as I do/did tobacco”). The representativeness of this study is questionable given the recruitment method and the cursory survey. In sum, this study provides weak suggestive evidence in support of dependence symptoms (and abuse liability to some degree) in e-cigarette use that is lower than corresponding dependence in combustible tobacco use.
In a series of three papers reporting on an overlapping sample, Etter (2015, 2016) and Etter and Eissenberg (2015) reported the prevalence and correlates of dependence symptoms among nicotine- and tobacco-product–using respondents in Internet surveys. The investigators posted links to the e-cigarette survey on health-related websites, smoking cessation websites, and websites selling e-cigarettes or with information about them from October 2012 to September 2014. They collected data on nicotine gum users between 2004 and 2007, also on the Internet. The FTCD, the NDSS, the Cigarette Dependence Scale, and adaptations of these scales for e-cigarettes and nicotine gums were used. Additional questions assessing correlates were also included.
In Etter and Eissenberg (2015), users of nicotine-containing e-cigarettes reported higher dependence ratings than users of nicotine-free e-cigarettes. The authors also found that, among former smokers, those who had used e-cigarettes for more than 3 months (long-term users) were less dependent on e-cigarettes than those who had used nicotine gum for more than 3 months were dependent on the gum. Dependence ratings between short-term (3 months or less) users of gums or e-cigarettes had few differences. Cross-product findings were judged to carry little weight given the dramatic difference in sampling, methodology, and time frame (2004–2007 versus 2012–2014) across the gum and e-cigarette use groups. The nicotine strength comparisons among e-cigarette users were judged to provide weak evidence, given the non-representative sample and the lack of adjustment for confounders.
In Etter (2015), 374 daily users of e-cigarettes who had quit smoking in the previous 2 months had a median time to first e-cigarette that ranged from 15 to 45 minutes across groups, depending on whether participants’ response to the question “Does e-cigarette relieve desire or craving to smoke?” was definitely (median = 15 min), a lot (median = 20), or somewhat/no/maybe (median = 45). This suggests mild to moderate levels of dependence for this particular symptom in the sample and that dependence is higher among those who report that e-cigarettes alleviate combustible tobacco cigarette cravings. No additional relevant analyses were reported. This provides additional weak suggestive evidence of mild to moderate levels of dependence in a sample of e-cigarette users.
In Etter (2016), answers from 1,672 current users of e-cigarettes were obtained. Across sample subgroups, responses to dependence- and abuse liability–relevant questions differed by how respondents rated the strength of the throat hit (“very weak,” “rather weak,” “average,” “rather strong,” and “very strong”). The “throat hit” is the specific sensation felt in the back of the throat by users when they inhale e-cigarette aerosol that is also reported with combustible tobacco cigarettes and is believed to be a pleasant sensation of slight irritation of the airways. Unadjusted comparisons indicated that the time of the first e-cigarette tended to be shorter among users who reported a stronger throat hit (indicating more severe dependence), and the median time across the groups ranged from 15 to 30 minutes, indicating medium levels of dependence. High prevalence estimates for survey questions assessing rewarding effects and euphoria, indicative of product abuse liability, were found overall, including “like the taste of the vapor” (range 75–90 percent across groups differentiated by strength of throat hit), “like sensation of vapor when inhaling” (60–92 percent), and “feels so good to vape” (59–91 percent). For each of these questions, the prevalence tended to be higher among e-cigarette users reporting stronger throat hit in unadjusted comparisons. Overall, this study provides additional suggestive evidence that dependence symptoms and experiences indicative of abuse liability are of moderate to high prevalence and severity and may be higher in those who obtain a stronger throat hit from their product.
In sum, the collective papers across these three studies provide suggestive evidence that e-cigarette dependence symptoms and subjective effects of vaping indicative of abuse liability are of appreciable prevalence and severity in samples of users and may be associated with nicotine concentration and user characteristics.
Additional Descriptive Data on E-Cigarette Dependence Symptoms
In four small laboratory studies of current e-cigarette users (Dawkins et al., 2016 [n = 11]; Goldensen et al., 2016 [n = 20]; Hobkirk et al., 2017 [n = 9]; Nichols et al., 2016 [n = 7]), mean dependence symptom reports were incidentally reported to provide descriptive data on the sample. For the three studies that reported PSECDI composite scores the range was 6.0 to 8.4, indicating low to moderate levels of nicotine dependence. Using a modified FTCD for e-cigarettes, Dawkins and colleagues (2016) reported a mean score of 4.73 and a mean self-rated addiction to e-cigarettes on a 1–5 scale of 3.18 (1.17) in their sample, indicating moderate nicotine dependence (Dawkins et al., 2016). These data provide additional suggestive confirmatory data to reports in the epidemiological data reviewed
above that e-cigarette dependence symptoms are non-negligible in various samples of users.
HUMAN LABORATORY STUDIES
The search resulted in 9 articles that reported original data from 12 separate studies that matched the requirements above (see Table 8-2 for a summary of these studies). Review of the articles revealed that five of the studies compared the effects of e-cigarette products varying in e-liquid flavoring on abuse liability outcomes. Three of these five studies as well as three additional studies also addressed the effect of varying e-cigarette nicotine concentration on abuse liability. Four studies compared the effects of e-cigarette administration with combustible tobacco cigarette administration among smokers.
Studies Testing the Effects of Flavor
Goldenson and colleagues (2016) conducted a double-blind, crossover design study among young adults who reported using e-cigarettes in the past 30 days (n = 20, ages 19–34, 80 percent current smokers). Participants used e-cigarette devices with Joyetech “Delta 23 Atomizer” tanks connected to a Joyetech “eVic Supreme” battery (recent-generation device) filled with e-cigarette solutions (Dekang Biotechnology Co., Ltd., 50/50 propylene glycol [PG]/glycerol) in 10 flavors (6 sweet: peach, watermelon, blackberry, cotton candy, cola, and sweet lemon tea; 3 non-sweet: mint, tobacco, and menthol; and 1 flavorless). The participants self-administered 20 standardized 2-puff doses of aerosolized e-cigarette solutions in 3 flavors (sweet versus non-sweet versus flavorless), either with nicotine (6 mg/ml) or without (0 mg/ml [placebo]). After each administration, participants rated three abuse liability indicators (liking, willingness to use again, and perceived monetary value), perceived sweetness, and throat hit strength. Each flavor was presented twice (once in 6 mg/ml and once in placebo) resulting in 20 total administrations all occurring on a single visit. Before testing, participants were trained on how to follow the standardized puffing procedure that was used for each trial to equalize the “dose” of product administered for each condition, which involved a 10-second preparation, 4-second inhalation, 1-second hold, and 2-second exhale—approximating typical vaping topography.
Results showed that sweet-flavored solutions produced significantly greater abuse liability rating for each index compared with non-sweet and flavorless (p < 0.0001). Throat hit ratings were greater for nicotine than placebo, but did not significantly increase abuse liability or interact with flavor effects on abuse liability outcomes. Controlling for flavor
and nicotine, perceived sweetness was positively associated with each abuse liability outcome. To account for the influence of preexisting flavor preferences, the authors examined results in a subsample of participants who reported regularly using non-sweet flavors (n = 9). Consistent with results in the overall sample, all outcomes were positively associated with sweetness ratings (p < 0.0001). As in the overall sample, results among the subsample showed higher mean abuse liability ratings for sweet flavored solutions compared with non-sweet and flavorless solutions. However, for each appeal rating, the main effects for flavors (p = 0.09–0.17) and pairwise contrasts of sweet-flavored to non-sweet or flavorless solutions (p = 0.06–0.23) did not reach statistical significance. Additional tests of whether participants could correctly guess the characterizing flavor of each liquid administered after each administration indicated that participants’ accuracy was not significantly better than chance guessing, suggesting upholding of the study blind to participants regarding the flavor they received.
The study strengths include the use of three to five different flavors per flavor category and analyses correlating sweetness ratings with abuse liability outcomes, suggesting a more generalized phenomenon across multiple different types of products that e-liquids with flavors that produce perceptions of sweetness also were of higher abuse liability. The standardized puffing procedure to equate the dose of administration was also a strength of the study, because it can prevent confounding of flavor or nicotine condition with the duration of puff taken. The null nicotine finding should be interpreted with the caveat that the study design was not well suited to detect and isolate nicotine’s pharmacological effects, given that participants were rapidly exposed to multiple products with and without nicotine in a short time frame and that participants were not required to be deprived of nicotine to participate in the test session. Therefore, the participants likely had to base their ratings on the acute sensory response rather than a more generalized pharmacological effect that may take several minutes to generate. In addition, outcomes were limited to self-reporting, which reflects one aspect of abuse liability that may or may not be parallel to other indicators (e.g., willingness to work for vaping). In sum, this study provides fairly strong evidence that sweet flavorings enhance subjective abuse liability indexes in young adults and provides limited evidence regarding the impact of nicotine on abuse liability.
Using a within-subjects design, Audrain-McGovern and colleagues (2016) conducted three human laboratory sessions among young adult daily smokers who had previously tried e-cigarettes at least once, but used e-cigarettes less often than daily (n = 32). Participants used an “e-GO” tank-style e-cigarette with a single 2.2- to 2.4-Ω resistance coil that could not be adjusted, 650-mAh rechargeable lithium ion battery, and
a 2.4-ml refillable e-liquid tank. The first session asked participants to rate unflavored and sweet (green apple and chocolate)-flavored e-cigarettes with nicotine on how satisfying and good they tasted to evaluate the rewarding value of flavoring. The sweet flavor that produced the higher reward rating for each respective participant was selected as the “flavored” product to use over the next two sessions for comparison with the unflavored e-cigarette. To assess the relative reinforcing value of a sweet-flavored e-cigarette compared with an unflavored e-cigarette, the second session applied a choice task that evaluated the willingness to “work” in the form of moving a computer mouse to hit targets on one of two computer screens, to earn points toward flavored or unflavored e-cigarette puffs. Session 3 measured the absolute reinforcing value of sweet-flavored versus unflavored e-cigarettes via a 90-minute ad lib e-cigarette use session where puffs from each e-cigarette product (sweet-flavored versus unflavored) were counted.
Results of the study were clear and consistent. Rating on a 1–7 scale, the average subjective rewarding value rating was significantly higher for the chocolate-flavored (mean [SD] = 3.69 [1.78]), and green apple–flavored (mean [SD] = 4.22 [1.55]) product than the unflavored (mean [SD] = 3.11 [1.55]) product. Participants worked harder for flavored e-cigarette puffs than for unflavored e-cigarette puffs (p < 0.0001). Total work was 596.31 responses (mouse clicks on targets) for the flavored e-cigarette (SD = 520.25; range 0–1,375) and 126.66 for the unflavored e-cigarette). During ad lib use over a 90-minute period, participants took twice as many flavored puffs than unflavored e-cigarette puffs (40 versus 23 puffs; incidence rate ratio [IRR] = 2.028; 95% CI = 1.183–3.475; p = 0.01).
The study strengths include the use of three different abuse liability outcomes, each of which provides unique information about abuse liability (i.e., one addressing the subjective experience, one addressing the motivation to obtain the product, one addressing self-administration under unconstrained conditions) and each yielding convergent results. A limitation was e-cigarette exposure eligibility criteria in the sample—all were ever users who had not progressed to become daily users—which may restrict generalizability to users who may be most prone to dependence (i.e., those who have already become daily e-cigarette users). At the same time, because all had experience using e-cigarettes, the likelihood that inability to use e-cigarettes properly had an impact on findings is low. In addition, the subjective reward finding should be interpreted with the caveat that one of the two items in the subjective reward index was “tasted good,” which would be expected to be highly dependent on flavor. A more ideal subjective reward outcome would involve the inclusion of multiple elements indicative of self-reported reward value (e.g., product liking, mood elevation, desire to use again) to parse whether
the result depended entirely on the fact that the sweet-flavored products tasted better than the unflavored product. Because all products contained nicotine, whether the effects of flavor on abuse liability would generalize across different nicotine concentrations (including no nicotine) is unknown. Overall, the study provides clear and consistent evidence across three different types of abuse liability outcomes indicating that sweet-flavored products produced higher abuse liability than unflavored products in young adult smokers.
Rosbrook and Green (2016) conducted two experiments testing the effects of e-cigarette administration varying in menthol and nicotine concentration on subjective abuse liability ratings and sensory effects. Each experiment involved 32 adult smokers age 18–45 (6 subjects participated in both experiments). In both experiments, the majority of subjects were self-reported menthol cigarette smokers (25 in experiment 1 and 26 in experiment 2). Five subjects in experiment 1 and 12 subjects in experiment 2 reported using e-cigarettes regularly. Both studies used the V2 Standard E-Cigarette device (79 mm; VMR Products, LLC) and V2 blank cartridges. In the first experiment, cartridges were filled with 15 different e-liquids (Pace Engineering Concepts, LLC) with 5 different concentrations of nicotine (0, 6, 12, 18, or 24 mg/ml) and 3 different concentrations of menthol (0.0 percent, 0.5 percent, or 3.5 percent l-menthol) in a 70/30 PG/glycerol base. In the second experiment, the cartridges were filled with six different e-liquids, each at 0 or 24 mg/ml nicotine: two menthol and two menthol–mint commercial flavors (70/30 PG/glycerol; AmericaneLiquidStore) and two unflavored e-liquids (PG/glycerol base only; Pace Engineering Concepts, LLC). Participants were trained in the puffing and rating procedure prior to the testing, which involved taking two “priming puffs” into the mouth only, then to fully inhale the third puff as they normally would when smoking a combustible tobacco cigarette and to exhale through the mouth. After exhalation the subject was prompted to rate liking or disliking the flavor on a scale with 11 semantic labels, ranging from “most dislike imaginable” to “most like imaginable” with “neutral” in the middle and other intermediate descriptors. Participants also rated three other sensory effects. Testing occurred on a single day for both experiments, and participants were required to be deprived of tobacco overnight. The study was double blind.
For both experiments, the e-liquids were only “slightly liked” on average. For the first experiment, the degree of liking did not vary significantly across nicotine or menthol concentrations. For the second experiment, the main effect of flavor showed higher ratings for liking of the commercial menthol and menthol–mint flavors over the unflavored e-liquid (p < 0.001). Nicotine and nicotine–flavor interactions were not significant. Sensory effect ratings of nicotine and menthol were reported,
suggesting independent and interactive effects of nicotine and menthol in an expected direction on outcomes like coolness and harshness/irritation. The sensory effect results were consistent with the known effects of these substances from the combustible tobacco cigarette literature and validate the robustness of the menthol and nicotine manipulations.
The results of these experiments should be interpreted within the following caveats. All participants were combustible tobacco cigarette smokers, most of whom did not report frequent use of e-cigarettes. Hence, most participants may have been unfamiliar with e-cigarettes and how to use them, which could impact sensitivity to manipulations in flavor and nicotine. Also, such individuals would be expected to be less likely to be prone to dependence on e-cigarettes given that most were not (yet) users. The use of a relatively low-powered device that likely delivers less nicotine and flavor constituents than do more powerful devices leaves unclear whether these results would generalize to other popular products. Critically, all e-liquids for the first experiment and the unflavored liquid for the second experiment were created by a private engineering company and were merely PG, glycerol, and l-menthol. E-liquids available in the marketplace generally contain numerous other additives to enhance the sweetness and remove aversive tastes and sensory qualities (see Chapter 5 for discussion of flavorings). Hence, the absence of effects of menthol flavoring on liking in the first experiment may bear modest relevance to the mentholated e-liquids used in the population. Indeed, in the second experiment when commercial menthol and menthol-mint flavorings were used, the liking ratings were significantly higher relative to the unflavored solution containing only PG and glycerol. Finally, the use of a single item rating of liking is a very narrow indicator of abuse liability. In sum, this study provides moderately strong controlled evidence that commercially available menthol and menthol-mint flavors produce greater subjective product liking than unflavored e-liquids among smokers.
In a study by St.Helen and colleagues (2017), 11 men and 3 women participated in a 3-day inpatient crossover study with strawberry, tobacco, and their usual flavor e-liquid on subjective product liking ratings indicative of abuse liability and other outcomes. Exclusive e-cigarette users or dual users of fewer than five combustible tobacco cigarettes per day, who used second- and/or third-generation e-cigarettes at least 25 days per month for the past 3 months or more and had saliva cotinine levels at least 30 ng/ml were eligible. Commercially available strawberry and tobacco test e-liquids (Bulkejuice.com) with 60/40 and 56/44 PG/glycerol and with 19–20 mg/ml nicotine were used in the two test e-liquid conditions, The participant’s own e-liquid was used for the comparison condition, each of which had sweet characterizing flavor names (with the exception of one participant who used a flavor that was “tobacco/vanilla”), with a
range from 1.6 mg/ml to 186.7 mg/ml across participants (mean [SD] = 7.4 [5.3]) and a mean (SD) PG/glycerol ratio of 63/37 (18/18). For each session from 4:00 to 10:00 pm, subjects could use e-cigarettes ad lib to become acclimatized to the assigned flavor for the next day. Participants were abstinent overnight until the morning standardized session of 15 puffs, which was followed by 4 hours of abstinence, and then a 90-minute ad lib use session followed by subjective measures. For the standardized puffing procedure, participants took 15 puffs, one puff every 30 seconds, from the e-cigarette. Puff duration was not controlled by the study. Multiple blood draws were taken, and subjective questionnaires were administered 5 to 15 minutes post-puffing.
Results showed that for the standardized session, the tobacco and strawberry test e-liquids were not significantly different for mood enhancement or any subjective satisfaction or reward rating. While statistical tests for comparisons to the usual brand e-liquid were not reported, positive mood change mean (SD) scores from pre- to post-administration were 2.8 (1.6) for usual brand compared with 0.2 (1.1) and 0.4 (1.6) for strawberry and tobacco, respectively, which are suggestive of greater mood enhancement for usual brand than the test e-liquids. A similar pattern was found for mean (SD) satisfaction ratings (usual brand = 17.1 [0.9], strawberry = 12.4 [1.2], tobacco = 13.2 [1.5]).
After the ad lib session, mean ± SEM for the “taste good” ratings of the strawberry, tobacco, and usual e-liquids were 3.4 ± 0.4, 3.1 ± 0.5, and 5.9 ± 0.3, respectively (maximum possible score of this item is 7). The usual flavor was rated significantly higher than the strawberry and tobacco e-liquids (p < 0.001), while the strawberry and tobacco e-liquids were not significantly different for this outcome. For average satisfaction, subjects reported ratings with the strawberry (p = 0.002) and tobacco (p < 0.001) e-liquids compared with the usual brand e-liquids. Ratings of enjoyment of sensations in chest and throat were lower for both the strawberry (p = 0.022) and tobacco (p = 0.019) e-liquids compared with the usual brand e-liquids.
The findings of the study should be interpreted with the caveat that the primary goal was to determine effects of flavorings on nicotine pharmacokinetics, and subjective measures were secondary outcomes. Hence, the study sample size, although appropriate for studying effects on nicotine blood yield, was underpowered to detect meaningful effects for subjective abuse liability–relevant outcomes. Nonetheless, the controlled design, inclusion of both standardized and ad lib testing conditions, and inclusion of regular e-cigarette users with experimentally controlled tobacco product deprivation enhances the internal validity of the study, particularly for the standardized session test results. The ad lib session subjective ratings are subject to between-condition variations in the “dose” of product self-
selected by the participants. Because the e-liquids self-selected by the user varied widely in nicotine concentration, PG/glycerol, and characterizing flavor, the particular product characteristics driving differences between usual brand and test e-liquids cannot be determined. At the same time, there is ecological validity to be gained by the using the participants’ own e-liquids given their ability to self-select the product likely to be highly rewarding to their own preferences. In sum, the study provides tentative evidence that self-selected e-liquids produce greater satisfaction and potential other indicators of abuse liability than experimenter-provided e-liquids in experienced e-cigarette users.
Studies Testing the Effects of Nicotine Concentration
Using a double-blind within-participants design, counterbalanced design with two conditions (low and high nicotine), Dawkins and colleagues (2016) conducted a study of experienced e-cigarette users who completed 60 minutes of ad lib use in two separate sessions. The participants were 11 experienced male e-cigarette users (reported using e-cigarettes daily for more than 3 months) who currently used a second- or third-generation e-cigarette, and used 24 mg/ml at least once in the past 6 months. Participants abstained from nicotine use (including from e-cigarettes) for 12 hours prior to study commencement and were tested individually. In the laboratory, the investigators provided the participants with the study device—a Joyetech “eVic™ supreme” e-cigarette with a “Nautilus Aspire” tank, 3.9 V (8.5 W, 1.8-Ω resistance), adjusted to the largest airflow and filled with Halo Smokers’ Angels brand e-liquid (50/50 PG/glycerol, 6 mg/ml [low] or 24 mg/ml [high] labeled nicotine). The researchers asked study participants to use e-cigarettes ad lib for 60 minutes, after which they completed a visual analogue scale rating assessing positive effects indicative of abuse liability (e.g., hit and satisfaction) and other effects for the preceding product self-administered.
Hit and satisfaction levels (mean percentage [SD]) were higher in the high nicotine condition (hit = 61.86 [31.50], satisfaction = 60.70 [17.30]) than in the low nicotine condition (hit = 44.73 [23.00], satisfaction = 46.89 [16.93]), but these differences did not reach statistical significance. Given that the sample size was small (n = 11), it is likely that the study was underpowered to detect effects, which raises the possibility that the non-significant differences may be type-II errors. Liquid consumption, puff number, and puff duration were significantly higher in the low nicotine condition compared with the high nicotine condition (all p < 0.01), which the authors interpreted engaging in compensatory puffing behavior in order to increase nicotine yield toward titration to achieve equal nicotine exposure in the two conditions. Approximately twice the overall
puff consumption was recorded from the 6-mg/ml versus the 24-mg/ml conditions.
Despite the fact that the amount of product consumed was clearly more in low versus high nicotine condition, the evidence trended toward greater subjective abuse liability indicators addressing a pharmacological drug effect (i.e., “hit” rating) and subjective satisfaction in the higher nicotine condition. Thus, even though the study design allowing consumption to be uncontrolled was likely biased toward larger effects for low nicotine due to more consumption in this condition, the results tended to show the opposite. Strengths include the inclusion of experienced users and experimentally controlled deprivation from nicotine prior to the test session, which is a strong design for detecting abuse liability effects due to the pharmacological effects of nicotine exposure. Limitations include very small sample limited to men only. Taken together, these findings provide tentative evidence that nicotine may enhance some subjective effects indicative of abuse liability; however, no firm conclusion can be drawn due to the absence of statistically significant results (p = 0.09 to 0.11).
In a fully within-subjects design involving adult DSM-IV diagnosed nicotine-dependent smokers (n = 28), Perkins and colleagues (2015) examined the effect of controlled administration of e-cigarettes with 36 mg/ml nicotine concentration compared with a placebo on subjective abuse liability ratings and other measures. None of the participants reported using e-cigarettes weekly either currently or in the past, and none had used within the prior 2 weeks of participating, suggesting relatively little e-cigarette use experience. In two counterbalanced laboratory sessions, each following overnight abstinence, participants self-administered e-cigarettes from PrimeVapor LLC, with prefilled cartridges containing a glycerol-based e-liquid (labeled nicotine concentration 36 mg/ml or 0 mg/ml) in either the rawhide red (tobacco) non-menthol flavor and Freeport (menthol) flavor. A KR808D-1 type automatic e-cigarette battery was used. The procedure involved self-administration of 10 four-second puffs over 5 minutes. To control the “dose” of exposure, the researchers employed computer-presented instructions to guide and standardize the precise timing and duration of each puff inhalation. After the first set of 10 puffs, subjects indicated on a 0–100 visual analog scale (anchored by “not at all” and “extremely”) several ratings relevant to abuse liability (e.g., “liking”).
Results showed that participants provided significantly higher ratings on an indicator of strength of drug effect (e.g., “how much nicotine”) and on two indicators of subjective reward (i.e., “liking” and “satisfied”) for the nicotine e-cigarette than the placebo product (see Figure 8-3). Other outcomes were studied that are not considered within the scope of the review.
The highly controlled tight design with an adequately sized sample
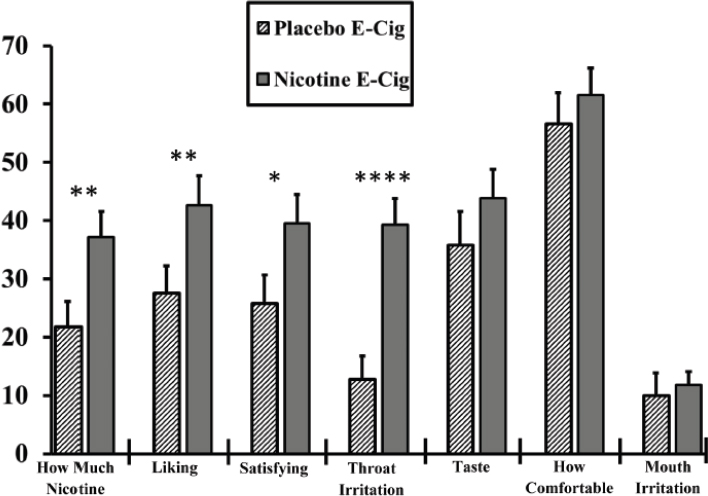
NOTE: *p < 0.05 between e-cigarettes; **p = < 0.01 between e-cigarettes; ****p = < 0.001 between e-cigarettes.
SOURCE: Adapted from Perkins et al., 2015.
for a within-subject laboratory study makes this study highly rigorous. Because subjective abuse liability reports were not a primary outcome, the data collected were fairly cursory and do not address multiple manifestations of abuse liability. Outside of this factor and the use of what would be considered a less powerful device, the methods were very strong. In sum, this study provides rigorous evidence that e-cigarettes with a high dose of nicotine versus placebo increase abuse liability ratings among combustible tobacco cigarette-dependent smokers.
A study conducted by Baldassarri and colleagues (2017) included four daily e-cigarette users who had been using e-cigarettes for 1 month or longer and three smokers who had consumed more than 10 combustible tobacco cigarettes per day for the past year. The goal was to study nicotine receptor occupancy using a positron emission tomography neuroimaging protocol examining responses to an e-cigarette or combustible tobacco cigarette challenge. Self-reported product liking ratings were collected. However, inspection of the study showed that four e-cigarette users participated in two scans each (8-mg/ml and 36-mg/ml e-cigarette), and
only two of the users underwent a third scan with a placebo (0-mg/ml e-cigarette). Hence, the sample was too small to permit meaningful within-person comparisons across e-cigarette nicotine doses. The three healthy smokers participated in one scan with the combustible tobacco cigarette challenge, but did not participate in the e-cigarette challenge, making cross-product comparisons confounded by between-subject group differences. Thus, this study could not be used to make any conclusions regarding the evidence.
As reviewed above in the section on studies testing the flavor effects, Goldenson and colleagues (2016) and Rosbrook and Green (2016) each examined the effects of varying nicotine concentrations on study outcomes and found no significant effect of nicotine variation on abuse liability–relevant measures. However, both studies used a multicondition exposure paradigm in which conditions of varying nicotine levels were administered within a short time frame and in small doses (e.g., either a single puff or two puffs). These designs are aimed to address the sensory effects of manipulations and are poorly suited for isolating the effect of a single pharmacologically active dose of nicotine, which requires a sufficient dosage amount (e.g., likely at least 10 puffs), following a period of nicotine deprivation. Hence, the nicotine effect findings from these studies are considered to provide little weight to the evidence determinations regarding whether nicotine concentration alters the abuse liability of e-cigarettes.
Comparisons of E-Cigarettes to Combustible Tobacco Cigarettes and Other Products
In 28 e-cigarette–naïve current smokers, Strasser and colleagues (2016) compared the effects of own-brand combustible tobacco cigarette smoking on abuse liability outcomes versus an e-cigarette product as a within-subject design factor. As an additional between-subject factor, when the participants were challenged with an e-cigarette, subjects were randomized to receive one of five brands of e-cigarette cigalike brands to determine whether brand variation within the e-cigarette class affected study outcomes: (1) NJOY = 18 mg nicotine; (2) V2 = 18 mg nicotine; (3) Green Smoke = 18.9–20.7 mg nicotine; (4) blu = 20–24 mg nicotine; and (5) White Cloud = 23–24 mg nicotine. On day 1, participants were allowed to smoke their own regular brand of combustible tobacco cigarette for a 10-minute period and then provided subjective rewarding effects of the combustible tobacco cigarette (e.g., satisfying, calming, pleasant, smoke another right now). Participants were then provided with their supply of e-cigarettes based on randomization and instructed to refrain from any tobacco/nicotine use aside from the e-cigarette provided for the
remaining 9 study days. Participants were instructed to use their assigned e-cigarette as much as desired. Participants returned to lab on days 5 and 10 for two identical testing sessions that followed the exact procedures as described for day 1, except that participants used the e-cigarette ad lib during a 10-minute vaping period, and ratings were based on the e-cigarette challenge.
The main finding of the study in regard to the abuse liability outcome was that when comparing the relative self-reported liking assessed at day 1 (mean [SE] = 627.0 [43.0]; in reference to their own combustible tobacco cigarette), and later, reports of liking of the e-cigarette were significantly lower at day 5 (mean [SE] = 340.4 [31.2]) and day 10 (mean [SE] = 343.6 [39.6]). There was no main effect for e-cigarette brand or an interaction effect for e-cigarette liking (p > 0.05). The study result should be interpreted with the caveat of having a very small sample size for brand versus brand between-group comparisons (n = 6 per group). All participants were current daily combustible tobacco cigarette smokers who had no or minimal prior e-cigarette use experience and who were willing to switch to e-cigarettes for 10 days, making this particular group perhaps not generalizable to certain segments of the population at risk for e-cigarette dependence. As noted by the author, the study used an older cigalike model and results may not extend to newer-generation devices. The use of only tobacco flavor also tempered the authors’ conclusions. Hence, the test might have been biased toward detecting lower product liking for e-cigarettes relative to the standard brand. In addition, the ad lib uncontrolled puff administration resulted in the participants using their own combustible tobacco cigarette for a longer period of time during the 10-minute self-administration interval than the duration of use of the e-cigarette products in the 10-minute interval. In sum, this study provides fairly weak evidence regarding lower abuse liability of first-generation e-cigarette devices relative to own-brand combustible tobacco cigarettes among e-cigarette–naïve smokers and inconclusive evidence whether or not product variation within the e-cigarette product class affects abuse liability.
Stiles and colleagues (2017) evaluated the abuse liability of three e-cigarettes (Vuse Solo brand, labeled nicotine concentrations of 14, 29, or 36 mg per e-liquid cartridge; solvent, flavoring additives, or characterizing labels and device properties not reported) relative to “high- and low-abuse liability” comparator products (usual brand combustible tobacco cigarettes and nicotine gum, respectively) among 45 e-cigarette–naïve smokers. For inclusion in the study, subjects were required to be adults age 21–60, smoke 10 or more non-menthol 83-mm (king size) to 100-mm combustible tobacco cigarettes per day for at least 6 months, and typically smoke their first combustible tobacco cigarette of the day within 30 minutes of waking. Products used as comparators were any combustible,
filtered, non-menthol brand style, 83 mm (king size) to 100 mm in length for the high-abuse liability comparator and Nicorette® White Ice Mint nicotine polacrilex gum, 4 mg (GlaxoSmithKline Consumer Healthcare, L.P.) for the low-abuse liability product. Subjects participated in a 7-day ambulatory home use trial of each product before each of five test visits to allow subjects to become accustomed to using the new products. Subjects were required to abstain from smoking for 12 hours prior to reporting to the clinic on the morning of each test visit. The testing consisted of up to 10 minutes use of Vuse Solo or smoking of one combustible tobacco cigarette, or up to 30 minutes using nicotine gum according to the package instructions. Five questionnaires were administered to assess subjective endpoints: Product Liking, Intent to Use Product Again, Product Effects, Urge to Smoke, and Urge for Product measured at multiple time points out to 2 hours following use.
Results showed that product liking was lower for the three Vuse Solo e-cigarettes (least square [LS] mean peak scores ranging from 4.13 to 4.57) compared with combustible tobacco cigarettes (LS mean peak score value = 9.06, p < 0.001 for all), and higher than nicotine gum (LS mean peak score value = 3.21, p < 0.05 for all). Ratings of Intent to Use Again followed a similar pattern. Whether the three different doses of nicotine were different from one another on abuse liability outcomes was not reported, though inspection of mean scores across the conditions suggests the differences are smaller among the different e-cigarette products than relative difference from combustible tobacco cigarette and gum conditions (see Table 8-4).
Subjects used the greatest e-liquid in the Vuse Solo 14-mg device (0.061 g), followed by Vuse Solo 29-mg (0.048 g), and Vuse Solo 36-mg (0.026 g) based on the average difference in the weights of the e-liquid cartridges.
Strengths of the study include use of multiple doses of nicotine to elucidate pharmacological dose–response effects and inclusion of both combustible tobacco cigarette and nicotine gum as active comparator conditions. However, the failure to control the amount of product administered across visits due to the ad lib design for the test session as well as uncontrolled exposure during the 7-day ambulatory period leaves the confounding effects of exposure on study outcomes unclear. Furthermore, the study did not provide data on the flavoring additives, vehicle compound, and device parameters (e.g., voltage, resistance) used. Hence, the generalizability beyond the product to other e-cigarettes that vary in nicotine concentration is unclear. In sum, this study provides suggestive evidence that an e-cigarette product may have intermediate abuse liability relative to nicotine gum (low abuse liability) and combustible tobacco cigarettes (higher abuse liability) among e-cigarette–naïve smokers.
| Parameter | LS Mean | ||||
|---|---|---|---|---|---|
| Vuse Solo 14 mg | Vuse Solo 29 mg | Vuse Solo 36 mg | Usual Brand Cigarette | Nicotine Gum | |
| Product Liking (AUEC15–360) | 1,396.68a,b | 1,430.66 a,b | 1,190.01 a,b | 3,116.52 | 799.38 |
| Emax | 4.36 a,b | 4.57 a,b | 4.13 a,b | 9.06 | 3.21 |
| Intent to Use Again (AUEC15–360) | 1,619.43 a,b | 1,635.82 a,b | 1,400.99 a,b | 2,369.30 | 1,091.84 |
| Emax | 4.71 a,b | 4.75 a,b | 4.07 a,b | 6.81 | 3.29 |
| Liking of Positive Effects (AUEC15–360) | 727.42 | 800.57 a | 673.67 | 889.74 | 444.17 |
| Emax | 6.71 a,b | 6.51 a,b | 5.99 a | 8.31 | 5.47 |
| Disliking of Negative Effects (AUEC15–360) | 502.66 | 827.42 | 740.85 | 423.38 | 422.14 |
| Emax | 6.03 | 6.41 | 6.67 | 5.80 | 6.28 |
NOTE: AUEC = area under the effect curve; LS = least square.
a = Significantly different from usual brand cigarette, p < 0.05.
b = Significantly different from nicotine gum, p < 0.05.
SOURCE: Stiles et al., 2017.
Vansickel and colleagues (2012) conducted a study of e-cigarette–naïve current smokers. Participants completed a behavioral choice abuse liability task evaluating the relative reinforcing value of e-cigarette and usual brand combustible tobacco cigarettes versus money; subjective abuse liability ratings were also collected. Participants were given a “Vapor King” (KR808 model) e-cigarette with a rechargeable 3.7-V battery and airflow sensor with a lighted display end and disposable cartomizer to use. WOW cowboy or WOW cowboy menthol tobacco–flavored cartomizers (18 mg/ml nicotine; commonly used nicotine strength; Vapor4Life) were matched to participants’ combustible tobacco cigarette flavor preference (i.e., non-menthol or menthol). The first of four, within-subject sessions was an e-cigarette administration session that involved six, 10-puff bouts (30-second interpuff interval) with each bout separated by 30 minutes. In the remaining three sessions, participants made choices among 10 e-cigarette puffs and varying amounts of money, 10 own-brand puffs and varying amounts of money, and 10 e-cigarette puffs and a varying number of own-brand combustible tobacco cigarette puffs, respectively, using a standardized multiple-choice procedure. The primary outcome for three choice sessions was the “crossover value,” the point at which participants chose to receive (1) money over 10 puffs from the e-cigarette; (2) money over 10 puffs of their own-brand combustible tobacco cigarette; or (3) own-brand puffs over 10 puffs from the e-cigarette, for each respective session.
Results showed that after the first administration session, e-cigarette administration increased ratings on these measures with each successive sampling session, for ratings of “pleasant” (F6,114 = 21.1, p < 0.0001), “satisfying” (F6,114 = 19.5, p < 0.0001), “taste good” (F6,114 = 20.2, p = 0.0001), and “use another right now.” For the choice procedure sessions, crossover values were greater in the own-brand combustible tobacco cigarettes versus money choice condition relative to the crossover or the e-cigarette versus money condition. Collapsed across time, the average crossover value was $1.06 (SD = $0.16) for choosing money versus e-cigarette, but was $1.50 (SD = $0.26) for choosing money over own-brand combustible tobacco cigarette, indicating greater reinforcing effects of smoking. For the task of pitting choices of e-cigarette and own-brand combustible tobacco cigarette puffs, the average crossover value, collapsed across time, was 3 own-brand puffs (SD = 0.4 puffs), indicating that 10 e-cigarette puffs were equivalent to 3 own-brand puffs. It can be concluded that the e-cigarette carried some abuse liability (albeit lower than combustible tobacco cigarettes) because probability of choosing vaping systematically increased as monetary values decreased, suggesting there was a significant reward value ascribed to e-cigarettes, and participants were willing to forgo a meaningful amount of money for e-cigarette puffs.
The use of multiple operationalizations of abuse liability and a rigor-
ous behavioral choice procedure to ascribe a relative value of e-cigarettes versus both money and combustible tobacco cigarettes are key strengths. The study also showed that the e-cigarette administration significantly increased plasma nicotine, verifying that the manipulation was robust. However, the nicotine boost was lower than what is typically observed via a standard combustible tobacco cigarette. Hence, the abuse liability estimates could reflect conditions and products that may underestimate what regular smokers may choose to use. In sum, this study provides strong evidence that e-cigarettes possess abuse liability in regular smokers and suggestive evidence that the relative abuse liability is lower than the smoker’s usual combustible tobacco cigarette brand used.
In a study by Vansickel and colleagues (2010), 32 e-cigarette–naïve smokers took 10 standardized puffs from one of four conditions in a within-subject crossover design: own brand combustible tobacco cigarette, “NPRO” e-cigarettes (NPRO, NJOY; 18-mg cartridge), “Hydro” e-cigarettes (Hydro, Crown 7; 16-mg cartridge), or sham (unlit combustible tobacco cigarette) conditions. Participants were daily smokers of 15 or more cigarettes per day and e-cigarette–naïve. Flavor (tobacco or menthol) of the product was matched to the preferred flavor of participants’ own combustible tobacco cigarette brand. Participants responded to the subjective effect questionnaires 5, 15, 30, and 45 minutes after the 10 puffs of the respective product (including puffs of the unlit “sham” combustible tobacco cigarette). This cycle was repeated twice for each study visit/product condition.
The authors found significant condition-by-time interactions for ratings of “satisfying,” “pleasant,” and “taste good.” In particular, ratings of “satisfying” and “pleasant” increased significantly at all time points with use of the Hydro e-cigarette, NPRO e-cigarette, and own-brand combustible tobacco cigarette. Ratings of “satisfying” and “pleasant” increased significantly higher for own-brand combustible cigarettes than those for Hydro e-cigarette or NPRO e-cigarette (see Figure 8-4).
This study had strengths in that a detailed four-condition comparison was made, including two separate products with a strong inactive control condition (i.e., sham) and an active comparison condition (i.e., usual brand combustible tobacco cigarette). The multi-time-point detailed assessment strategy increased statistical power. One strength was the assessment of biomarkers and physiological outcomes sensitive to nicotine. These results indicated that, within the first 5 minutes of administration, smoking own-brand combustible tobacco cigarettes significantly increased plasma nicotine and heart rate, but use of the NPRO e-cigarette, Hydro e-cigarette, and sham smoking did not. Thus, the first-generation products used in this study were likely ineffective at delivering nicotine and thus reflect an insensitive test of abuse liability relative to the prod-
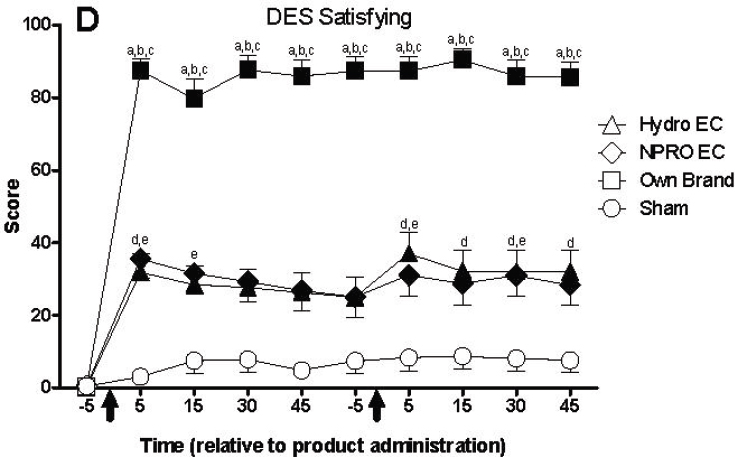
NOTES: An “a,” “b,” or “c” indicates that own brand was significantly different from sham, Hydro EC, or NPRO EC at that time point. A “d” indicates that Hydro EC was significantly different from sham at that time point. An “e” indicates that NPRO EC was significantly different from sham at that time point (Tukey’s HSD, p < 0.05). Unidirectional error bars, one standard error. DES = direct effects of smoking; EC = e-cigarette.
SOURCE: Vansickel et al., 2010.
ucts available in the marketplace today. Furthermore, the e-cigarette–naïve participants were likely not well versed in proper use of e-cigarettes for obtaining efficient nicotine yield. Nonetheless, there were still some differences between these products and the sham condition. In sum, this study provides additional suggestive evidence that e-cigarette products may carry some abuse liability, but not at levels as high as combustible tobacco cigarettes.
Clinical Trials
The search revealed two clinical trials in which smokers were provided products to use at their own leisure. This section describes secondary outcomes, which involved ratings of e-cigarette and other comparison products based on recall of use experiences.
In a crossover trial, 38 current smokers (age 18 and older) used e-cigarettes or nicotine oral inhalers each for 3 days, in random order, with a washout period in between (Steinberg et al., 2014). The researchers provided the participants with three e-cigarettes (disposable, regular-flavor blu e-cigarettes with 20–24 mg/ml nicotine) and nicotine inhalers (plastic, pen-shaped containers with cartridges containing 10 mg nicotine and that deliver up to 2 mg nicotine each; Pfizer). Participants were instructed on how to use each device. As recommended by the blu instruction manual, the researchers instructed the participants to puff the device as they would their usual combustible tobacco cigarettes; participants were also instructed to use a new device each day. As described in the package insert for the inhalers, participants were instructed to inhale deeply into back of throat or puff in short breaths, trying to use 80 inhalations over 20 minutes. Participants were instructed to use the first product assigned as they wished for a 3-day period, which provided sufficient time for participants to learn how to use the devices. After the first product-use period, subjects participated in a post-use visit during which researchers collected product ratings. This was followed by a 3-day washout period, during which participants were instructed to smoke their usual combustible tobacco cigarettes as they wished before using the next product. To gain insight into craving and satisfaction during the product use periods, subjects were instructed to use the e-cigarettes and nicotine inhalers as combustible tobacco cigarette substitutes, but were told that cigarette smoking was permissible if absolutely necessary. The researchers collected retrospective ratings at three time points: baseline, after the 3-day e-cigarette use period, and after the 3-day inhaler use period. The e-cigarette had a higher total satisfaction score (13.9 versus 6.8 [p < 0.001], range for responses = 3–21) and higher reward score (15.8 versus 8.7 [p < 0.001], range for responses = 5–35) than the inhaler. Ratings of combustible tobacco cigarettes and e-cigarette did not differ significantly.
In a study, Meier and colleagues (2017) used a double-blind randomized crossover design, smokers (n = 24, 75 percent male; mean age = 48.5 years) smoked as usual for 1 week, followed by 2 counterbalanced weeks of ad lib use of first-generation e-cigarettes (blu) with up to seven prefilled cartridges containing either 16 mg or 0 mg nicotine (regular tobacco flavor or menthol flavor available only). Participants were instructed “this e-cig may or may not contain nicotine; we ask that you try it at least once, but use it however you like; smoke regular cigarettes as you wish.” At the end of each visit, participants reported no differences between the active and placebo e-cigarettes in satisfaction (nicotine mean [SD] = 3.49 [0.3] versus placebo mean [SD] = 3.18 [0.3]) or rewarding effects (mean [SD] = 2.38 [0.2] versus placebo mean [SD] = 2.36 [0.2]).
Collectively these findings provide little additional weight to conclu-
sions given the uncontrolled nature of e-cigarette exposure and use of early-generation products.
CONCLUSIONS
Conclusion 8-1. There is substantial evidence that e-cigarette use results in symptoms of dependence on e-cigarettes.
Finding: There are several supportive findings from good-quality observational studies with very few or no credible opposing findings that (1) dependence symptoms are of appreciable prevalence or severity or higher in epidemiological studies of users; and (2) greater frequency or chronicity of use is associated with greater likelihood or severity of dependence symptoms. These are supported by well-designed abuse liability studies that e-cigarette use increases abuse liability, with less credible studies also providing supportive evidence. A firm conclusion can be made, but minor limitations, including chance, bias, and confounding factors, cannot be ruled out with reasonable confidence.
Conclusion 8-2. There is moderate evidence that risk and severity of dependence are lower for e-cigarettes than combustible tobacco cigarettes.
Finding: There are several supportive findings from fair-quality studies with very few or no credible opposing findings. A general conclusion can be made, but limitations, including chance, bias, and confounding factors, cannot be ruled out with reasonable confidence.
Conclusion 8-3. There is moderate evidence that variability in e-cigarette product characteristics (nicotine concentration, flavoring, device type, and brand) is an important determinant of risk and severity of e-cigarette dependence.
Finding: Some findings support that nicotine concentration, flavoring, device generation, and brand are associated with outcomes indicative of level of dependence risk, with very few or no credible opposing findings. A general conclusion can be made, but limitations, including chance, bias, and confounding factors, cannot be ruled out with reasonable confidence.
REFERENCES
ADAMHA (Alcohol, Drug Abuse, and Mental Health Administration). 1989. Testing for abuse liability of drugs in humans. https://archives.drugabuse.gov/sites/default/files/monograph92.pdf (accessed October 9, 2017).
APA (American Psychiatric Association). 2013. Diagnostic and statistical manual of mental disorders, 5th edition. Washington, DC: American Psychiatric Association.
Audrain-McGovern, J., A. A. Strasser, and E. P. Wileyto. 2016. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug and Alcohol Dependence 166:263–267.
Baldassarri, S. R., A. T. Hillmer, J. M. Anderson, P. Jatlow, N. Nabulsi, D. Labaree, K. P. Cosgrove, S. S. O’Malley, T. Eissenberg, S. Krishnan-Sarin, and I. Esterlis. 2017. Use of electronic cigarettes leads to significant beta2-nicotinic acetylcholine receptor occupancy: Evidence from a PET imaging study. Nicotine & Tobacco Research 20(4):425–433.
Benowitz, N. L. 2008. Neurobiology of nicotine addiction: Implications for smoking cessation treatment. American Journal of Medicine 121(4 Supplement 1):S3–S10.
Breland, A., E. Soule, A. Lopez, C. Ramoa, A. El-Hellani, and T. Eissenberg. 2017. Electronic cigarettes: What are they and what do they do? Annals of the New York Academy of Sciences 1394(1):5–30.
Caggiula, A. R., E. C. Donny, M. I. Palmatier, X. Liu, N. Chaudhri, and A. F. Sved. 2009. The role of nicotine in smoking: A dual-reinforcement model. Nebraska Symposium on Motivation 55:91–109.
Carter, L. P., M. L. Stitzer, J. E. Henningfield, R. J. O’Connor, K. M. Cummings, and D. K. Hatsukami. 2009. Abuse liability assessment of tobacco products including potential reduced exposure products (PREPs). Cancer Epidemiology, Biomarkers and Prevention 18(12):3241–3262.
CDC (Centers for Disease Control and Prevention). 2016. Quickstats: Cigarette smoking status among current adult e-cigarette users, age group—National Health Interview Survey, United States, 2015. Morbidity and Mortality Weekly Report 65(42):1177.
Chou, S. P., R. B. Goldstein, S. M. Smith, B. Huang, W. J. Ruan, H. Zhang, J. Jung, T. D. Saha, R. P. Pickering, and B. F. Grant. 2016. The epidemiology of DSM–5 nicotine use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions—III. Journal of Clinical Psychiatry 77(10):1404–1412.
Dawkins, L., and O. Corcoran. 2014. Acute electronic cigarette use: Nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl) 231(2):401–407.
Dawkins, L., J. Turner, A. Roberts, and K. Soar. 2013. “Vaping” profiles and preferences: An online survey of electronic cigarette users. Addiction 108(6):1115–1125.
Dawkins, L. E., C. F. Kimber, M. Doig, C. Feyerabend, and O. Corcoran. 2016. Self-titration by experienced e-cigarette users: Blood nicotine delivery and subjective effects. Psychopharmacology (Berl) 233(15–16):2933–2941.
DiFranza, J. R., J. A. Savageau, K. Fletcher, J. K. Ockene, N. A. Rigotti, A. D. McNeill, M. Coleman, and C. Wood. 2002. Measuring the loss of autonomy over nicotine use in adolescents: The DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Archives of Pediatrics & Adolescent Medicine 156(4):397–403.
Domino, E. F., L. Ni, J. S. Domino, W. Yang, C. Evans, S. Guthrie, H. Wang, R. A. Koeppe, and J. K. Zubieta. 2013. Denicotinized versus average nicotine tobacco cigarette smoking differentially releases striatal dopamine. Nicotine & Tobacco Research 15(1):11–21.
Donny, E. C., E. Houtsmuller, and M. L. Stitzer. 2007. Smoking in the absence of nicotine: Behavioral, subjective and physiological effects over 11 days. Addiction 102(2):324–334.
Donny, E. C., R. L. Denlinger, J. W. Tidey, J. S. Koopmeiners, N. L. Benowitz, R. G. Vandrey, M. al’Absi, S. G. Carmella, P. M. Cinciripini, S. S. Dermody, D. J. Drobes, S. S. Hecht, J. Jensen, T. Lane, C. T. Le, F. J. McClernon, I. D. Montoya, S. E. Murphy, J. D. Robinson, M. L. Stitzer, A. A. Strasser, H. Tindle, and D. K. Hatsukami. 2015. Randomized trial of reduced-nicotine standards for cigarettes. New England Journal of Medicine 373(14): 1340–1349.
Etter, J. F. 2015. Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters. Drug and Alcohol Dependence 148:102–108.
Etter, J. F. 2016. Throat hit in users of the electronic cigarette: An exploratory study. Psychology of Addictive Behaviors 30(1):93–100.
Etter, J. F., and C. Bullen. 2014. A longitudinal study of electronic cigarette users. Addictive Behaviors 39(2):491–494.
Etter, J. F., and T. Eissenberg. 2015. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug and Alcohol Dependence 147:68–75.
Fagerström, K. 2012. Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine & Tobacco Research 14(1):75–78.
Farsalinos, K. E., D. Tsiapras, S. Kyrzopoulos, M. Savvopoulou, and V. Voudris. 2014. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: Comparison with the effects of regular cigarettes. BMC Cardiovascular Disorders 14:78. https://doi.org/10.1186/1471-2261-14-78 (accessed February 5, 2018).
Fiore, M. C., C. R. Jaen, and T. B. Baker. 2008. Treating tobacco use and dependence: 2008 update, Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service. https://bphc.hrsa.gov/buckets/treatingtobacco.pdf (accessed February 5, 2018).
Foulds, J., S. Veldheer, J. Yingst, S. Hrabovsky, S. J. Wilson, T. T. Nichols, and T. Eissenberg. 2015. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine & Tobacco Research 17(2):186–192.
Goldenson, N. I., M. G. Kirkpatrick, J. L. Barrington-Trimis, R. D. Pang, J. F. McBeth, M. A. Pentz, J. M. Samet, and A. M. Leventhal. 2016. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology. Drug and Alcohol Dependence 168:176–180.
Gonzalez-Roz, A., R. Secades Villa, and S. Weidberg. 2017. Evaluating nicotine dependence levels in e-cigarette users. Adicciones 29(2):136–138.
Griffiths, R. R., and B. Wolf. 1990. Relative abuse liability of different benzodiazepines in drug abusers. Journal of Clinical Psychopharmacology 10(4):237–243.
Heatherton, T. F., L. T. Kozlowski, R. C. Frecker, and K. O. Fagerström. 1991. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction 86(9):1119–1127.
Henningfield, J. E., D. K. Hatsukami, M. Zeller, and E. Peters. 2011. Conference on abuse liability and appeal of tobacco products: Conclusions and recommendations. Drug and Alcohol Dependence 116(1–3):1–7.
Hobkirk, A. L., T. T. Nichols, J. Foulds, J. M. Yingst, S. Veldheer, S. Hrabovsky, J. Richie, T. Eissenberg, and S. J. Wilson. 2017. Changes in resting state functional brain connectivity and withdrawal symptoms are associated with acute electronic cigarette use. Brain Research Bulletin. https://doi.org/10.1016/j.brainresbull.2017.05.010 (accessed February 5, 2018).
Hughes, J. R. 2006. Clinical significance of tobacco withdrawal. Nicotine & Tobacco Research 8(2):153–156.
Jamal, A., A. Gentzke, S. Hu, K. A. Cullen, B. J. Apelberg, D. M. Homa, and B. A. King. 2017. Tobacco use among middle and high school students—United States, 2011–2016. Morbidity and Mortality Weekly Report 66(23):597–603.
Japuntich, S. J., M. E. Piper, T. R. Schlam, D. M. Bolt, and T. B. Baker. 2009. Do smokers know what we’re talking about? The construct validity of nicotine dependence questionnaire measures. Psychological Assessment 21(4):595–607.
Johnson, J. M., J. L. Muilenburg, S. L. Rathbun, X. Yu, L. P. Naeher, and J.-S. Wang. 2017. Elevated nicotine dependence scores among electronic cigarette users at an electronic cigarette convention. Journal of Community Health. 43:164–174.
Kasza, K. A., B. K. Ambrose, K. P. Conway, N. Borek, K. Taylor, M. L. Goniewicz, K. M. Cummings, E. Sharma, J. L. Pearson, V. R. Green, A. R. Kaufman, M. Bansal-Travers, M. J. Travers, J. Kwan, C. Tworek, Y. C. Cheng, L. Yang, N. Pharris-Ciurej, D. M. van Bemmel, C. L. Backinger, W. M. Compton, and A. J. Hyland. 2017. Tobacco-product use by adults and youths in the United States in 2013 and 2014. New England Journal of Medicine 376(4):342–353.
Kollins, S. H. 2003. Comparing the abuse potential of methylphenidate versus other stimulants: A review of available evidence and relevance to the ADHD patient. Journal of Clinical Psychiatry 64(Supplement 11):14–18.
Liu, G., E. Wasserman, L. Kong, and J. Foulds. 2017. A comparison of nicotine dependence among exclusive e-cigarette and cigarette users in the PATH study. Preventive Medicine 104:86–91.
Markou, A. 2008. Review. Neurobiology of nicotine dependence. Philosophical Transactions of the Royal Society B: Biological Sciences 363(1507):3159–3168.
Meier, E., A. E. Wahlquist, B. W. Heckman, K. M. Cummings, B. Froeliger, and M. J. Carpenter. 2017. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine & Tobacco Research 19(2):176–182.
Nichols, T. T., J. Foulds, J. M. Yingst, S. Veldheer, S. Hrabovsky, J. Richie, T. Eissenberg, and S. J. Wilson. 2016. Cue-reactivity in experienced electronic cigarette users: Novel stimulus videos and a pilot FMRI study. Brain Research Bulletin 123:23–32.
Perkins, K. A., J. L. Karelitz, and V. C. Michael. 2015. Reinforcement enhancing effects of acute nicotine via electronic cigarettes. Drug and Alcohol Dependence 153:104–108.
Piper, M. E., T. M. Piasecki, E. B. Federman, D. M. Bolt, S. S. Smith, M. C. Fiore, and T. B. Baker. 2004. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). Journal of Consulting and Clinical Psychology 72(2):139–154.
Quester, S., and N. Romanczuk-Seiferth. 2015. Brain imaging in gambling disorder. Current Addiction Reports 2(3):220–229.
Reyes–Guzman, C. M., R. M. Pfeiffer, J. Lubin, N. D. Freedman, S. D. Cleary, P. H. Levine, and N. E. Caporaso. 2017. Determinants of light and intermittent smoking in the United States: Results from three pooled national health surveys. Cancer Epidemiology, Biomarkers & Prevention 26(2):228-239.
Rosbrook, K., and B. G. Green. 2016. Sensory effects of menthol and nicotine in an e-cigarette. Nicotine & Tobacco Research 18(7):1588–1595.
Rose, J. E. 2006. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology 184(3):274–285.
Rostron, B. L., M. J. Schroeder, and B. K. Ambrose. 2016. Dependence symptoms and cessation intentions among US adult daily cigarette, cigar, and e-cigarette users, 2012–2013. BMC Public Health 16(1):814.
Schoenborn, C. A., and R. M. Gindi. 2015. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief (217):1–8.
Shiffman, S., A. J. Waters, and M. Hickcox. 2004. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research 6(2):327–348.
Shihadeh, A., and T. Eissenberg. 2015. Electronic cigarette effectiveness and abuse liability: Predicting and regulating nicotine flux. Nicotine & Tobacco Research 17(2):158–162.
Soneji, S., J. L. Barrington-Trimis, T. A. Wills, A. M. Leventhal, J. B. Unger, L. A. Gibson, J. Yang, B. A. Primack, J. A. Andrews, R. A. Miech, T. R. Spindle, D. M. Dick, T. Eissenberg, R. C. Hornik, R. Dang, and J. D. Sargent. 2017. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: A systematic review and meta-analysis. JAMA Pediatrics 171(8):788–797.
St.Helen, G., D. A. Dempsey, C. M. Havel, P. Jacob, 3rd, and N. L. Benowitz. 2017. Impact of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes. Drug and Alcohol Dependence 178:391–398.
Steinberg, M. B., M. H. Zimmermann, C. D. Delnevo, M. J. Lewis, P. Shukla, E. J. Coups, and J. Foulds. 2014. E-cigarette versus nicotine inhaler: Comparing the perceptions and experiences of inhaled nicotine devices. Journal of General Internal Medicine 29(11):1444–1450.
Stiles, M. F., L. R. Campbell, D. W. Graff, B. A. Jones, R. V. Fant, and J. E. Henningfield. 2017. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: Implications for abuse liability. Psychopharmacology (Berl) 234(17):2643–2655.
Strasser, A. A., V. Souprountchouk, A. Kaufmann, S. Blazekovic, F. Leone, N. L. Benowitz, and R. A. Schnoll. 2016. Nicotine replacement, topography, and smoking phenotypes of e-cigarettes. Tobacco Regulatory Science 2(4):352–362.
Strong, D. R., J. Pearson, S. Ehlke, T. Kirchner, D. Abrams, K. Taylor, W. M. Compton, K. P. Conway, E. Lambert, V. R. Green, L. C. Hull, S. E. Evans, K. M. Cummings, M. Goniewicz, A. Hyland, and R. Niaura. 2017. Indicators of dependence for different types of tobacco product users: Descriptive findings from Wave 1 (2013–2014) of the Population Assessment of Tobacco and Health (PATH) study. Drug and Alcohol Dependence 178:257–266.
Vansickel, A. R., and T. Eissenberg. 2013. Electronic cigarettes: Effective nicotine delivery after acute administration. Nicotine & Tobacco Research 15(1):267–270.
Vansickel, A. R., C. O. Cobb, M. F. Weaver, and T. E. Eissenberg. 2010. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes:” Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology, Biomarkers & Prevention 19(8):1945–1953.
Vansickel, A. R., M. F. Weaver, and T. Eissenberg. 2012. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction 107(8):1493–1500.
Volkow, N. D., G. F. Koob, and A. T. McLellan. 2016. Neurobiologic advances from the brain disease model of addiction. New England Journal of Medicine 374(4):363–371.
Wagner, F. A., and J. C. Anthony. 2002. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology 26(4):479–488.
Yingst, J. M., S. Veldheer, S. Hrabovsky, T. T. Nichols, S. J. Wilson, and J. Foulds. 2015. Factors associated with electronic cigarette users’ device preferences and transition from first generation to advanced generation devices. Nicotine & Tobacco Research 17(10): 1242–1246.




















































































