9
Cardiovascular Disease
Active smoking of combustible tobacco cigarettes and exposure to secondhand tobacco smoke are established causes of clinical cardiovascular disease. Prior Surgeon General reports concluded that the evidence is sufficient to infer that active combustible tobacco cigarette smoking causes coronary heart disease, stroke, atherosclerotic peripheral artery disease, and aortic aneurysm and early abdominal aortic atherosclerosis, and that for secondhand tobacco smoke, the evidence is sufficient to infer that it causes coronary heart disease and stroke (HHS, 2014). Evidence on the cardiovascular effects of active smoking and cardiovascular disease is derived from multiple epidemiological and experimental studies, from studies showing the relatively short-term benefits on the cardiovascular system of quitting smoking, and from the reduction in cardiovascular hospitalizations following the implementation of smoke-free legislation in multiple countries and communities around the world.
When evaluating the potential cardiovascular effects of e-cigarette use, it is important to consider what is known about the dose–response or the exposure–response relationship between exposure to airborne fine particulate matter and cardiovascular disease (Pope et al., 2009). Data combined from multiple studies to estimate adjusted relative risks of cardiovascular mortality plotted against the estimated average daily dose of fine particulate matter from combustible tobacco cigarette smoke, secondhand tobacco smoke, and ambient air pollution showed that the exposure–response relationship between fine particulate matter and cardiovascular disease mortality is relatively steep at low levels of exposure and
it plateaus at higher levels. Because the particle characteristics and composition of e-cigarettes differ from those emitted by combustible tobacco cigarettes (see Chapter 3), it is not possible to extrapolate at this time whether the ultrafine particles and liquid particles emitted by e-cigarettes are toxic to the cardiovascular system. The possibility that they could be toxic, however, makes research in this area very important.
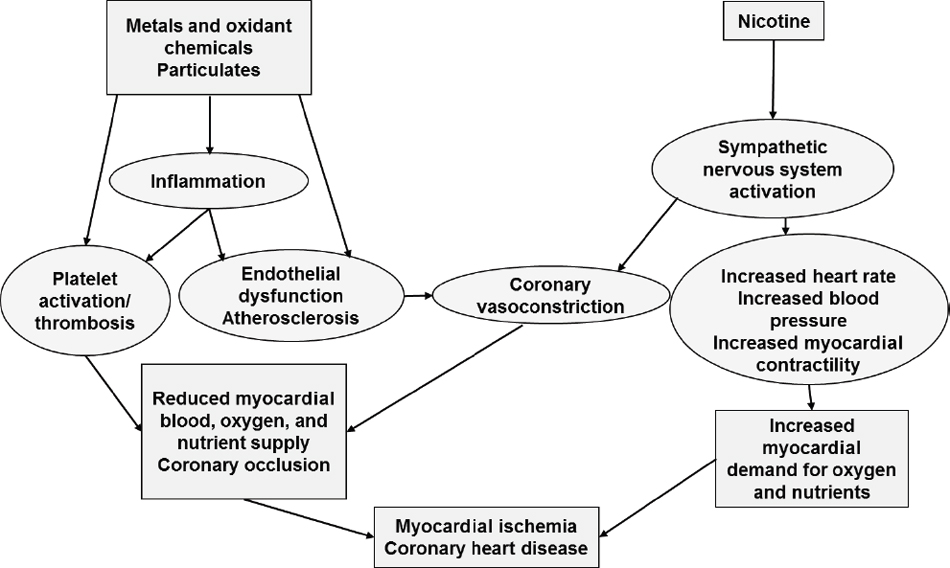
In addition to the particles, some toxicants in combustible tobacco cigarette smoke have been specifically related to cardiovascular disease risk, in particular metals, such as lead, nickel, and cadmium (Cosselman et al., 2015; Nigra et al., 2016). Because increasing evidence supports that e-cigarettes, particularly the heating coil, are a source of metals (see Chapter 5), the cardiotoxicity of e-cigarettes that use metallic coils to heat the e-liquid should be evaluated. Nicotine, moreover, as it has been reviewed in Chapter 4, stimulates the sympathetic nervous system, which results in short-term increases in heart rate, blood pressure, and myocardial contractility (see Figure 9-1). These nicotine mechanisms have been involved in the short-term effects of tobacco as a trigger for myocardial ischemia and myocardial infarction (HHS, 2014), although currently there is no consensus about the health effects of nicotine. While some investigators
SOURCE: Adapted from HHS, 2014.
have minimized potential effects on cardiovascular disease (Benowitz and Fraiman, 2017), others see greater risk (Bhatnagar, 2016). Possible mechanistic pathways for particulates, metals, and other toxic chemicals, which are also found in e-cigarette aerosols and could thus be by which exposure to e-cigarettes influences cardiovascular disease related to atherosclerosis and coronary heart disease, are summarized in Figure 9-1. This figure is inspired from the well-established evidence of the toxicity of combustible tobacco products on the cardiovascular system, as summarized in the Surgeon General’s report (HHS, 2014). A major difference among the potentially cardiotoxic substances that are found in combustible tobacco cigarettes, but not in e-cigarettes, is the lack of combustion chemicals such as polycyclic aromatic hydrocarbons and carbon monoxide (see Chapter 5). The possibility that e-cigarettes may increase the risk of cardiovascular disease must be evaluated carefully given the high burden of cardiovascular disease worldwide and the importance of the burden of disease in the estimation of attributable risk.
CHARACTERIZATION OF DISEASE ENDPOINTS AND INTERMEDIATE OUTCOMES
Relatively few studies have investigated the cardiovascular effects of e-cigarette products. In particular, there are no epidemiological studies evaluating clinical outcomes such as coronary heart disease, stroke, or atherosclerotic peripheral artery disease, or established subclinical outcomes of underlying atherosclerosis such as carotid intima-media thickness or coronary artery calcification. Clinical outcomes such as coronary heart disease (including myocardial infarction and sudden cardiac death), stroke, and peripheral artery disease have been the cornerstone of prospective epidemiological studies evaluating the vascular effects of combustible tobacco cigarettes. Subclinical measures of atherosclerosis, such as carotid intima-media thickness or coronary artery calcification, are also considered excellent measures of cardiovascular risk that can inform on relevant mechanistic pathways (see Figure 9-1). Importantly, these can be measured in cross-sectional designs, allowing for some early assessment as compared with the long-term follow-up needed for clinical cardiovascular outcomes. None of the studies on e-cigarettes and cardiovascular disease conducted so far and summarized below, however, have measured either clinical cardiovascular outcomes or subclinical atherosclerotic outcomes. This lack of data on e-cigarettes and clinical and subclinical atherosclerotic outcomes represents a major research need.
Conclusion 9-1. There is no available evidence whether or not e-cigarette use is associated with clinical cardiovascular outcomes (coro
nary heart disease, stroke, and peripheral artery disease) and subclinical atherosclerosis (carotid intima-media thickness and coronary artery calcification).
The evidence available on the possible cardiovascular effects of e-cigarettes can be classified as studies conducted in vitro, evaluating the cytotoxicity of e-cigarette aerosols and other alterations in myocardial cells and human vascular cells; studies conducted in vivo, evaluating relevant mechanistic pathways for cardiovascular toxicity in mice; and clinical experiments, generally crossover experiments that have assessed short-term cardiovascular effects, such as changes in heart rate, blood pressure, and arterial stiffness, of e-cigarettes as compared with combustible tobacco cigarettes and to not smoking. A few studies have evaluated the associations between e-cigarette use and heart rate, heart rate variability, blood pressure levels, and markers of oxidative stress over longer periods, including a cohort study of patients with hypertension who were using e-cigarettes (Polosa et al., 2016), a randomized clinical trial evaluating the effect of switching from smoking to e-cigarette use analyzed also as a cohort study (Farsalinos et al., 2016), and a cross-sectional study comparing heart rate variability and oxidative stress measures in e-cigarette users versus non-users (Moheimani et al., 2017).
Heart rate, controlled by the autonomic nervous system, is a powerful measure of cardiovascular function (Koskela et al., 2013; Poirier, 2014). Slower average resting heart rate is related to higher cardiovascular health and longer life span. Endurance physical exercise can reduce resting heart rate and promote cardiovascular health. The increase in cardiovascular risk associated with high resting heart rate maybe due to elevated blood pressure or sympathetic overactivity (Koskela et al., 2013). Elevated brachial blood pressure is one of the best established contributors to clinical cardiovascular disease and mortality, including myocardial infarction, stroke, and renal failure, when not detected early and treated appropriately (James et al., 2014). Hypertension diagnosis, treatment, and control are critical for cardiovascular disease prevention and control. Hypertension can be defined when either systolic or diastolic blood pressure (SBP or DBP) is elevated. While there are blood pressure cutoffs that are used clinically, the increase in cardiovascular risk is continuous along blood pressure levels. The short-term effects of combustible tobacco cigarettes on both heart rate and blood pressure levels are well established, resulting in short-term elevations that could be related to the effects of nicotine. Long term, however, the effect of combustible tobacco cigarettes on both heart rate and brachial blood pressure levels are less clear, although chronic smoking has been associated with elevated central systolic blood pressure in smokers (Mahmud and Feely, 2003). The short-term effects of
smoking in heart rate and brachial blood pressure could also play a role in triggering acute events. Arterial elasticity is essential for blood flow, and the hardening or stiffening of the arteries plays an important role in the development of cardiovascular disease. Arterial stiffness, which can be also defined as arteriosclerosis, or the hardening of the artery wall, can be assessed non-invasively measuring the pulse wave velocity, which measures the speed of the blood pressure wave along the arterial system. Pulse wave velocity can be measured at the carotid, aortic, or brachial levels and it is a strong predictor of clinical cardiovascular events (Mattace-Raso et al., 2006; Willum Hansen et al., 2006). Combustible tobacco cigarette smoking has been associated with arterial stiffness both in short-term experiments and in studies evaluating chronic effects (Levenson et al., 1987; Mahmud and Feely, 2003). In healthy individuals without established cardiovascular disease or major cardiovascular risk factors, endothelial function is also related to increased arterial stiffness. Because endothelial function is an early marker of atherosclerosis (narrowing of the arteries because of the presence of plaque) and clinical cardiovascular disease characterized by a reduced bioavailability of endothelium-derived nitric oxide (NO), it shows the close interrelatedness between these well-established markers of cardiovascular disease (McEniery et al., 2006).
In the sections below, the committee reviews the clinical experiments evaluating the short-term cardiovascular effects of e-cigarettes as well as the few studies that have evaluated the effects of e-cigarettes on the cardiovascular system over longer periods of time or in a cross-sectional setting. The primary focus of this chapter is understanding the cardiovascular effects of e-cigarettes compared with no use, although we also report on findings compared with combustible tobacco cigarettes when those are available in the studies evaluated. A more detailed comparison of the cardiovascular effects of e-cigarettes versus combustible tobacco cigarettes is found in Chapter 18 on harm reduction. In the absence of clinical or subclinical studies on the long-term cardiovascular effects of e-cigarettes, evaluating the potential harm reduction of e-cigarettes is preliminary.
EVIDENCE FROM HUMAN STUDIES OF CARDIOVASCULAR EFFECTS
A total of 13 clinical intervention studies published between 2010 and 2017 have evaluated acute cardiovascular effects of e-cigarette use, such as changes in blood pressure levels, heart rate, arterial stiffness and endothelial function, cardiac geometry and function, and oxidative stress measured in minutes to hours following the intervention (see Table 9-1). Among them, 11 studies were crossover studies in which all participants
TABLE 9-1 Clinical Studies of Short-Term Effects of E-Cigarette Use on Cardiovascular Endpoints
| Reference | Study Design | Population | Mean Age % Men % C-Smoker % F-Smoker % Naïve E-Cigarette | E-Cigarette Device Characteristics and E-Liquid |
|---|---|---|---|---|
| St.Helen et al., 2017 | Not blinded, 3-arm randomized crossover trial over 3 consecutive days (inpatient) | Healthy sole and dual e-cigarette user (≤5 cigarettes per day) from colleges campuses in San Francisco, CA | 32.3 years 79% 14.3% 71.4% 0% |
KangerTech Mini ProTank 3 clearomizer (1.5 1) connected to a KangerTech 3.7-V, 1,000-mAh battery; 3 flavors: Bulk e-liquid strawberry (pH 8.29) Bulk e-liquid tobacco (pH 9.10) Own flavor (mean pH 6.80) with 50/50 PG/glycerol and 18 mg/ml nicotine (for strawberry and tobacco) |
| Spindle et al., 2017 | Not blinded, 2-arm ordered crossover trial with a minimum of 48-hour washout period | Healthy sole and dual e-cigarette users (≤5 combustible tobacco cigarettes per day) from Richmond, VA, using e-cigarettes for at least 3 months | 29.6 years 76% 7% NR 0% |
Own e-cigarette device and e-liquid (≥12 mg/ml nicotine) |
| Intervention Pattern and Cotinine Levels | Comparison Groups | na | Study Endpoints | Results |
|---|---|---|---|---|
| Ad libitum (ad lib) acclimatization from 4 to 10 pm, abstinent overnight, 15 puffs/session (1 every 30 seconds) followed by 4 hours of abstinence, and then 90 minutes ad lib. Mean max nicotine concentration (SEM) was 12.1 (2.0), 9.5 (1.2) and 6.2 (1.0) ng/ml for strawberry, tobacco, and own flavor, respectively. |
Before and at 5, 10, 15, 20, and 30 minutes after the final puff of: Strawberry Tobacco Own flavor | 14 | HR (bpm) | Mean max change (SEM) in HR after 15 puffs versus baseline was: 17.2 (2.5) (strawberry), 12.3 (2.3) (tobacco), 9.4 (2.4) (own flavor). Mean maximum increase (95% CI) was 4.6 (0.8, 8.5) bpm higher for strawberry e-liquid than for tobacco e-liquid. Mean (SEM) of HR area under the curve (AUC) after 15 puffs was 245 (37) (strawberry), 210 (45) (tobacco), 169 (53) (own flavor). Mean difference (95% CI) in HR AUC: 34 (−43, 111) comparing strawberry to tobacco. No difference in HR by pH of the e-liquid, mean (SEM) 183 (85) versus 154 (69) for usual acidic and usual basic pH (p = 0.85). HR not reported for the ad lib session. |
| 10 puffs, 30-second interpuff interval, and 90-minute ad lib bout. Plasma nicotine increased up to 4.6 ng/ml during ad lib. |
Before and continually every 20 seconds for 2.5 hours comparing same device and e-liquid with or without a mouthpiece | 29 | HR (bpm) | Mean (SEM) HR increased to 73.3 (1.3) bpm after the directed bout and to 73.9 (1.5), 73.6 (1.6), and 74.4 (1.7) at 30, 60, and 90 minutes, respectively, after the onset of the ad lib but compared with baseline of 66.3 (1.3) bpm. |
| Reference | Study Design | Population | Mean Age % Men % C-Smoker % F-Smoker % Naïve E-Cigarette | E-Cigarette Device Characteristics and E-Liquid |
|---|---|---|---|---|
| St.Helen et al., 2016 | 1-arm trial | Healthy sole and dual e-cigarette users (≤5 cigarettes per day) | 38.4 years 54% 23% NR 0% |
Usual brand of device and e-liquid |
| Carnevale et al., 2016 | Single blinded 2-arm ordered crossover trial with 1-week washout | Healthy smoking and never smoking participants from Rome, Italy (recruitment dates NR) | 28.0 years 47.5% 50% 0% NR |
NR leading brand charged; 16-mg nicotine cartridge (~250 puffs) |
| Intervention Pattern and Cotinine Levels | Comparison Groups | na | Study Endpoints | Results |
|---|---|---|---|---|
| 15 puffs/session, 30-second interpuff interval, followed by 4 hours of abstinence, and then 90 minutes ad lib. Mean plasma nicotine after 15 puffs was 8.4 ng/ml. |
Before and 5, 10, 15, 20, and 30 minutes after the final puff | 13 | HR (bpm) | Compared with baseline HR increased a mean of 8.0 (p < 0.001) and 5.2 (p = 0.04) bpm after 5 and 10 minutes, respectively, and was not significantly different after 15 minutes. |
| 1 cigarette, 9 puffs (equivalent to 0.6 mg of nicotine). Cotinine NR. | Just before and 30 minutes after - 1 combustible tobacco cigarette - 9 e-cigarette puffs | 40 | Serum sNOX2-dp (pg/ml), 8-iso-PGF2_α(pmol/L), Serum NO (μM), Serum vitamin E (μmol/mmol), Brachial artery FMD (%) | Mean (SD) before and after combustible tobacco cigarette/e-cigarette 23.6 (7.8) 38.2 (9.9)/21.6 (6.8) 30.2 (6.2) 135 (56) 203 (81)/133 (54) 187 (62) 35.3 (12.0) 19.5 (9.9)/35.5 (12.5) 25.9 (12.1) 4.6 (1.8) 3.1 (1.9)/3.8 (1.6) 2.8 (1.2) 6.7 (4.3) 3.4 (3.9)/6.7 (3.6) 4.3 (2.2) Stratified results for smokers and non-smokers similar with worse profile for smokers. |
| Reference | Study Design | Population | Mean Age % Men % C-Smoker % F-Smoker % Naïve E-Cigarette | E-Cigarette Device Characteristics and E-Liquid |
|---|---|---|---|---|
| Antoniewicz et al., 2016 | Single blinded 2-arm randomized crossover trial with 1-week washout | Healthy sporadic smokers from Stockholm, Sweden, not smoking in the last 7 days (recruitment dates NR) | 28 years 64.3% 100% 0% 100% |
eGo XL (2nd-generation), 1,100-mAh, 3.7-V, dual-coil CE5 atomizer. E-liquid with nicotine 12 mg/ml, 44.4/49.4% PG/glycerol without flavors (Valeo laboratories GmbH). |
| Intervention Pattern and Cotinine Levels | Comparison Groups | na | Study Endpoints | Results |
|---|---|---|---|---|
| 10 puffs in 10 minutes in semisupine position. Median (IQR) plasma cotinine after 4 hours was 4.1 (3.5, 4.7) ng/ml. | Before and 1, 4, and 24 hours after: - E-Cigarette - Control (resting) |
16 | EPC (leukocytes, events) Microvesicles (number) all and by origin (endothelial, platelet or leukocytes) and inflammation markers (HMGB1, P-selectin, CD40, and E-selectin [CD62E]) FeNO (only pre and 24 hours) | EPCs increased after e-cigarette use at 1 hour (p = 0.003) and 4 hours (p = 0.036) and returned to normal at 24 hours. No changes were observed for control periods. Median (IQR) pre, 1, 4, 24 hours e-cigarette/control 1,725 (731, 4,012), 2,600 (1,264, 7,668), 5,102 (2,164, 7,858), 5,731 (1,402, 7,176)/1,557 (1,020, 4,997), 3,277 (2,038, 4,987), 3,700 (2,545, 4,494), 2,724 (2,012, 4,858) p = 0.683. NS associations for MVs by origin and inflammation markers except for E-selectin: 8 (2, 17), 14 (8, 43), 28 (17, 65), 20 (15, 40)/9 (4, 22), 19 (12, 40), 23 (14, 42), 23 (11, 37) (p = 0.038). Median (IQR) pre, 24 hours e-cigarette/controls 10 (7, 15), 11 (8, 14)/10 (7, 15), 11 (8, 14), p = 0.88. |
| Reference | Study Design | Population | Mean Age % Men % C-Smoker % F-Smoker % Naïve E-Cigarette | E-Cigarette Device Characteristics and E-Liquid |
|---|---|---|---|---|
| Fogt et al., 2016 | Double blinded, 2-arm ordered crossover trial with 1-week washout | Healthy participants from San Antonio, TX (recruitment dates NR) | 23.1 (±2.5) years 50% 0% NR 100% |
GreenSmartLiving e-cigarette (details not described). E-liquid with 0 and 18 mg/ml nicotine. |
| Cooke et al., 2015 | Double blinded, randomized, 2-arm crossover trial with 1-week washout | Healthy nonsmoking participants from San Antonio, TX (recruitment dates NR) | 23 (±1) years 50% 0% NR 100% |
GreenSmartLiving e-cigarette (details not described). E-liquid with 0 and 18 mg/ml nicotine. |
| Intervention Pattern and Cotinine Levels | Comparison Groups | na | Study Endpoints | Results |
|---|---|---|---|---|
| 20 puffs in 10 minutes inhaling as deeply as possible. Urine cotinine 0–10 and 30–100 ng/ml for 18 and 0 mg/ml e-cigarette, respectively. |
40 minutes post-exposure: - E-cigarette 0 mg/ml - E-cigarette 18 mg/ml Exercise test starts 55 minutes post–e-cigarette exposure |
20 | Resting: SBP (mmHg) DBP (mmHg) HR (bpm) RMR (kcal/min) VO2 (L/min) RQ (energy exp.) Exercise test: SBPpeak (mmHg) DBPpeak (mmHg) (VO2)peak (L/min) Power (W)peak | Mean (SD) 0/18 mg/ml e-cigarette 115.8 (8.0)/112.1 (6.8), p = 0.04 73.6 (8.3)/76.6 (6.0), p = 0.04 61 (10)/61 (10), p = 0.47 1.19 (0.2)/1.18 (0.2), p = 0.39 0.25 (0.1)/0.25 (0.2), p = 0.5 0.79 (0.01)/0.78 (0.1), p = 0.15 Numbers NR, p = 0.14 74.9 (8.3)/79.4 (7.6), p = 0.02 2.3 (0.7)/2.3 (0.8), p = 0.77 204.8 (57.8)/201.0 (53.8), p = 0.29 |
| 20 puffs in 10 minutes. Urine cotinine 0–10 and 30–100 ng/ml for 18 and 0 mg/ml e-cigarette, respectively. |
Before and 10–20 (seated), 20–25 (supine), 25–30 (70° head-up tilt), and 30–35 (supine) minutes post-exposure: - E-cigarette 0 mg/ml - E-cigarette 18 mg/ml 1-week washout period |
20 | Seated: SBP (mmHg) DBP (mmHg) HR (pbm) Supine, tilt, and recovery positions (5 minutes each): SBP (mmHg) DBP (mmHg) Autonomic control: R-R RRISD | Change pre-post 0/18 mg/mlb −2/2 (p ≤ 0.03) −2/4 (p = 0.001) −4/1.2 (p ≤ 0.03) Mean BP in each position 0/18 mg/mlb 109/117 p = NR, 99/108 (p = 0.03), 110/118 (p = NR) 62/69 (p = 0.02), 61/67 (p = 0.02), 64/71 (p = 0.04). R-R and RRISD decreased with tilt (p) 0.01), but reductions were similar by treatment group. |
| Reference | Study Design | Population | Mean Age % Men % C-Smoker % F-Smoker % Naïve E-Cigarette | E-Cigarette Device Characteristics and E-Liquid |
|---|---|---|---|---|
| Yan and D’Ruiz, 2015 | Single blinded, randomized 6-arm crossover trial with 36-hour washout period | Healthy participants smoking in past 12 months from Lincoln, NE, and after a lead-in period for 7 days to get accustomed to using e-cigarette products and abstaining from nicotine for 36 hours | 38.7 years 48% 100% 0% 0% |
blu e-cigarettes with the following e-liquid formulations: A: classic e-cigarette 2.4% nicotine 75% glycerol B: classic e-cigarette 2.4% nicotine 50/20% glycerol/PG C: menthol e-cigarette, 2.4% nicotine 75% glycerol D: classic e-cigarette 1.6% nicotine 75% glycerol E: classic e-cigarette 1.6% nicotine 50/20% glycerol/PG |
| Szołtysek-Bołdys et al., 2014 | Single blinded, 2-arm ordered crossover trial with 1-day washout period | Healthy students of University of Silesia, Poland, smoking >5 cigarettes per day for 2 years and used e-cigarettes at least 10 times | 23 (±2) years 0% 100% 0% 0% |
Ego-3 (clearomizer Crystal 2 with coil, 2.4-1 voltage battery 900-mAh, 3.4-V) nicotine 24 mg/ml |
| Intervention Pattern and Cotinine Levels | Comparison Groups | na | Study Endpoints | Results |
|---|---|---|---|---|
| E-cigarette: 50 5-second puffs at 30-second intervals. Combustible tobacco cigarette: usual puff duration at 30-second intervals. E-cigarette and combustible tobacco cigarette: 1 hour ad lib use. Plasma nicotine (ng/ml) ranged from 2.0 (D) to 3.0 (B) at 5 minutes, from 10.0 (D) to 17.1 (B) at 30 minutes and from 13.7 (D) to 22.4 (B) after 1 extra hour ad lib. For cigarettes, it was 14.4, 7.9, and 29.2 at the same times. |
30 minutes pre and 20 minutes following the end of the ad lib period of e-cigarette A, B, C, D, E, and F (Marlboro cigarette) | 23 | SBP (mmHg) DBP (mmHg) HR (bpm) |
Change (SD) post versus pretreatment: A: 1.1 (11.1), p = 0.63/ B: 2.8 (11.3), p = 0.24/ C: 4.0 (10.0), p = 0.07/ D: 5.8 (10.0), p = 0.02/ E: 3.8 (10.7), p = 0.10/ F: 5.7 (12.4), p = 0.04. A: 6.8 (6.7), p < 0.001/ B: 6.8 (6.5), p < 0.001/ C: 3.2 (7.3), p = 0.05/ D: 6.8 (3.8), p < 0.001/ E: 4.4 (4.7), p < 0.001/ F: 6.8 (7.1), p < 0.001. A: 2.3 (5.5), p = 0.06/ B: 3.6 (6.0), p = 0.008/ C: 4.1 (5.7), p = 0.002/ D: 1.9 (7.4), p = 0.24/ E: 2.2 (5.9), p = 0.08/ F: 4.3 (5.4), p = 0.001. Plasma nicotine positively correlated with HR change with a mean increase of 0.16 bpm for 1 ng/ml increase in plasma nicotine (R2: 0.64). |
| 1-hour sessions: Combustible tobacco cigarette 10–12 puffs (personal brand) E-Cigarette 15 puffs Cotinine NR |
10 minutes after: - Combustible tobacco cigarette - E-Cigarette |
15 | Arterial stiffness: SI (m/s) RI (%) SBP (mmHg) DBP (mmHg) HR (bpm) |
Mean (95% CI) before and after cigarette/e-cigarette SI: 6.75 (6.66, 6.85), 6.56 (6.46, 6.65), p = 0.006/6.73 (6.62, 6.84), 6.75 (6.66, 6.83) p = NS. RI: 54.0 (51.5, 56.7), 49.6 (47.5, 51.8), p = 0.01/52.0 (49.3, 54.7), 50.8 (48.2, 53.3), p = NS. Both cigarettes and e-cigarette showed a small increase in SBP, DBP, and HR, but it was not significant (only figure) and was hard to see. |
| Reference | Study Design | Population | Mean Age % Men % C-Smoker % F-Smoker % Naïve E-Cigarette | E-Cigarette Device Characteristics and E-Liquid |
|---|---|---|---|---|
| Farsalinos et al., 2014 | Not blinded, 2-arm ordered trial with no smoking or nicotine use in the 4 hours before the intervention | Healthy consecutive smokers at Onassis Cardiac Surgery Center, Greece (>14 cigarettes per day for ≥5 years) and e-cigarette users who quit smoking and used 9–12 mg/ml nicotine e-liquid for ≥1 month (mean 6 months). Smokers and e-cigarette users similar at baseline except e-cigarette users formerly smoked 10 combustible tobacco cigarettes per day more when they smoked than current smokers | 35 (±5) years 90% 47% 53% NA |
eGO-T battery (Nobacco, Greece) with an eGo-C atomizer (2nd generation) 650-mAh rechargeable lithium battery, 3.5 V, manually activated 11 mg/ml nicotine PG >60%, linalool <5%, tobacco essence <5%, methyl vanillin <1%. |
| Intervention Pattern and Cotinine Levels | Comparison Groups | na | Study Endpoints | Results |
|---|---|---|---|---|
| Combustible tobacco cigarette smoked ad lib. E-cigarette ad lib for 7 minutes. Cotinine NR. Experiments for e-cigarette and combustible tobacco cigarettes were done in different rooms. | Before and after the experiments - Combustible tobacco cigarette smokers - E-cigarette users |
36 40 |
SBP (mmHg) DBP (mmHg) HR (bpm) Echocardiography E (cm/s) A (cm/s) E/A DT (ms) IVRT (ms) IVRTc (ms) MPI Sm (cm/s) Em (cm/s) Am (cm/s) Em/Am E/Em MPIt GS (%) SRs (s-1) SRe (s-1) SRa (s-1) |
Mean (SD) change before after cigarette/e-cigarette Before: 6.6 (5.2) p < 0.001/0.7 (4.6) p = 0.37 After: 4.4 (3.3) p < 0.001/3.0 (3.6) p < 0.001 5.9 (4.7) p < 0.001/0.4 (4.8) p = 0.649 −0.6 (6.1) p = 0.57/1.2 (5.0) p = 0.13 2.9 (5.7) p = 0.007/1.6 (5.6) p = 0.08 −0.10 (0.16) p = 0.001/−0.03 (0.14) p = 0.17 3 (10) p = 0.09/1 (8) p = 0.58 5.6 (9.2) p < 0.001/−1.0 (5.7) p = 0.28 10.4 (10.1) p < 0.001/−1.2 (6.9) p = 0.29 0.03 (0.04) p = 0.002/−0.01 (0.04) p = 0.330 −0.8 (1.1) p = 0.57/0.2 (0.7) p = 0.17 −0.7 (1.4) p < 0.001/0.2 (0.7) p = 0.10 0.1 (0.6) p = 0.80/0.2 (0.8) p = 0.12 −0.08 (0.13) p = 0.004/−0.01 (0.13) p = 0.54 0.29 (0.74) p = 0.02/0.01 (0.47) p = 0.87 0.03 (0.05) p = 0.004/−0.01 (0.04) p = 0.08 0.2 (1.7) p = 0.441/−0.4 (1.2) p = 0.06 −0.2 (0.1) p = 0.15/−0.01 (0.07) p = 0.36 –0.08 (0.12) p < 0.001/0.01 (0.08) p = 0.35 0.03 (0.09) p = 0.11/0.01 (0.08) p = 0.462 Also run analysis for the effect of combustible tobacco cigarette versus e-cigarette on changes of echocardiographic measures after adjustment for changes in SPB and HR (IVRT, IVRTc, MPI, Em, MPIt, SRe remained significantly associated). |
| Reference | Study Design | Population | Mean Age % Men % C-Smoker % F-Smoker % Naïve E-Cigarette | E-Cigarette Device Characteristics and E-Liquid |
|---|---|---|---|---|
| Czogała et al., 2012 | Not blinded, 2-arm ordered crossover study with 1-week washout | Healthy daily cigarette smokers (≥5 cigarettes per day) from Sosnowiec, Poland | 34.9 (15.3) years 50% 100% 0% 100% |
MILD M2001 (1st generation); 14 mg/ml nicotine L&M blu label PM cigarette |
| Eissenberg, 2010 | Not blinded, 4-arm ordered trial with washout period of 48 hours | Healthy smokers from Richmond, VA, with 12 hours of tobacco/nicotine abstinence confirmed with CO <10 ppm | 29.8 years 69% 100% 0% 100% |
NPRO (NJOY) and Hydro (Crown Seven) 16-mg nicotine cartridge menthol or non-menthol (choice of participant) |
| Intervention Pattern and Cotinine Levels | Comparison Groups | na | Study Endpoints | Results |
|---|---|---|---|---|
| Also run analysis for the effect of combustible tobacco cigarette versus e-cigarette on changes of echocardiographic measures after adjustment for changes in SPB and HR (IVRT, IVRTc, MPI, Em, MPIt, SRe remained significantly associated). | ||||
| Ad lib e-cigarette use (minimum amount of puffs NR) | - Combustible tobacco cigarette - E-cigarette |
42 | SBP (mmHg) DBP (mmHg) HR (bpm) |
Mean (SD) before and after combustible tobacco cigarette/e-cigarette SBP: 127.1 (15.4) to 131.4 (NS)/122.6 (11.4) to 122.5 (12.6) (NS) DBP: 78.8 (11.0) to 84.1 (10.4) (p = 0.02)/76.7 (9.5) to 78.6 (10.8) (NS) HR: 78.5 (12.0) to 90.9 (15.4) (p < 0.001)/77.9 (79.4) to 79.4 (13.6) (NS) |
| Instructed to puff and then puffed ad lib 10 times (30-second intervals) for each product, cycle was repeated 60 minutes later. Plasma nicotine (ng/ml) for own cigarette, NPRO, and Hydro, respectively, were 16.8, 3.5, and 2.5 at 5 minutes and 8.7, 2.6, and 2.2 at 30 minutes | Before and up to 30 minutes after 1st puff: Cigarette (own) Sham puffing NPRO Hydro | 16 | HR (bpm) | HR increased only after own cigarette (p < 0.05). Numbers are not shown in the paper for either combustible tobacco cigarette or e-cigarette. |
| Reference | Study Design | Population | Mean Age % Men % C-Smoker % F-Smoker % Naïve E-Cigarette | E-Cigarette Device Characteristics and E-Liquid |
|---|---|---|---|---|
| Vansickel et al., 2010 | Not blinded, randomized 4-arm trial with washout period of *48 hours | Healthy smokers from Richmond, VA, with 12 hours of tobacco/nicotine abstinence confirmed with CO < ppm | 33.6 years 59% 100% 0% 100% |
NPRO (18 mg, NJOY) Hydro (16 mg) |
a Final sample size used in the analyses. For Antoniewicz and colleagues (2016), 2 participants were excluded from the 16 initially recruited because cotinine levels were compatible with recent smoking. For Yan and D’Ruiz (2015), initially 38 participants were recruited but only 23 participants completed the study.
b Numbers approximated because abstracted from a figure.
NOTES: 8-iso-PGF2_ = 8-iso-prostaglandin F2_; EPC = endothelial progenitor cells; FMD = flow-mediated dilation; HR = heart rate; LA = left atrial; LV = left ventricle; MSNA = efferent
| Intervention Pattern and Cotinine Levels | Comparison Groups | na | Study Endpoints | Results |
|---|---|---|---|---|
| Instructed to puff 10 times with 30-second intervals at 2 separate times during the session (1 hour between them). Plasma nicotine increased for own brand but not for NPRO, Hydro, and sham experiments. | Before and up to 1 hour after: - Combustible tobacco cigarette (own) - Sham puffing - NPRO - Hydro |
32 | HR (bpm) | HR increased from 66 ppm before the experiment to 80, 75, and 70 ppm 5, 15, and 30 minutes, respectively, after the first experiment and to 74, 73, and 70 ppm after the second experiment with the own-brand cigarette. For NPRO and Hydro, only small changes not statistically significant were observed (from 66 ppm before to a maximum of 69 ppm at 5 minutes after the first experiment and 67 ppm at 5 minutes after the second experiment with NPRO; and even smaller changes with Hydro). |
muscle sympathetic nerve activity from the right peroneal nerve; NA = not applicable; nic. = nicotine, NO = nitric oxide; NR = not reported; NS = not significant; Ox = oxidative; PG/VG = propylene glycol/glycerol; RI = reflection index; RMR = resting metabolic rate; R-R = intervals to assess vagal-cardiac control in the time domain; RRISD = R-R interval standard deviations to assess respiratory sinus arrhythmia, SI = stiffness index; sNOX2-dp = soluble NOX2-derived peptide, a marker of nicotinamide adenine dinucleotide phosphate (reduced form) oxidase activation.
The literature search also identified 3 studies evaluating cardiovascular-related outcomes over a longer period than the 13 acute clinical studies (see Table 9-2), including a cross-sectional study of e-cigarette users compared with non-users conducted in Los Angeles, California (n = 34) (Moheimani et al., 2017); a cohort of smokers not intending to quit from Catania, Italy, who were randomized to one of three types of e-cigarette use (0 percent nicotine, 2.4 percent nicotine for 12 weeks, and 2.4 percent nicotine for 6 weeks plus 1.8 percent nicotine for 6 weeks) (n = 183 with complete follow-up) and also analyzed as a cohort study comparing sole e-cigarette users (called quitters in the original publication), dual users
(called reducers), and smokers (called failures) according to their continuation of combustible tobacco smoking (n = 145 for participants with continuous e-cigarette/smoking status over time) (Farsalinos et al., 2016); and a cohort of hypertensive patients who were e-cigarette users compared with hypertensive patients who smoked cigarettes (n = 89) also in Catania, Italy (Polosa et al., 2016).
The sample size across the 15 studies ranged from 13 (St.Helen et al., 2016) to 183 (Farsalinos et al., 2016) participants, for a total of 662 participants across the 15 studies (356 in the short-term clinical studies and 306 in the epidemiological studies). Study participants were recruited from Catania (Italy), Khallithea (Greece), Los Angeles (California), Lincoln (Nebraska), Richmond (Virginia), Rome (Italy), San Antonio (Texas), San Francisco (California), Silesia (Poland), Sosnowiec (Poland), and Stockholm (Sweden). In the short-term clinical studies, participants were relatively young (mean age ranged from 23 to 39 years old), required to be healthy (including no hypertension or diabetes risk factors in most studies), and included a balanced number of men and women, except in one study restricted to women (Szołtysek-Bołdys et al., 2014) and another study that included 90 percent men (Farsalinos et al., 2014). Mean age of the participants in the epidemiological studies ranged from 33 to 54 years. In one study, all participants had hypertension at baseline. In a total of seven studies, all participants were current smokers (Antoniewicz et al., 2016; Czogała et al., 2014; Eissenberg, 2010; Farsalinos et al., 2016; Szołtysek-Bołdys et al., 2014; Vansickel et al., 2010; Yan and D’Ruiz, 2015), ranging from sporadic smokers to heavy smokers; five studies included some current smokers; and the remaining were former smokers (Farsalinos et al., 2014; St.Helen et al., 2017) or it was not specified if they were former or never smokers (Polosa et al., 2016; Spindle et al., 2017; St.Helen et al., 2016); one study included half of the participants being current smokers and half never smokers (Carnevale et al., 2016); one study included 65 percent never smokers and 35 percent former smokers; and in two studies the participants were not current smokers, although it is unclear if former smokers were included (Cooke et al., 2015; Fogt et al., 2016). Among the short-term clinical studies, in six studies the participants were e-cigarette–naïve users (Antoniewicz et al., 2016; Cooke et al., 2015; Czogała et al., 2012; Eissenberg, 2010; Fogt et al., 2016; Vansickel et al., 2010); in one study participants were trained to use e-cigarettes during a 7-day period (Yan and D’Ruiz, 2015); in five studies participants were experienced e-cigarette users (Farsalinos et al., 2014; Szołtysek-Bołdys et al., 2014); and one study did not report whether participants were naïve e-cigarette users (Carnevale et al., 2016).
The e-cigarette device used in the experiments included a tank-style device in one study (St.Helen et al., 2017); second-generation devices
in three studies (different eGO models) (Antoniewicz et al., 2016; Farsalinos et al., 2014; Szołtysek-Bołdys et al., 2014); cigalikes in six studies (GreenSmartLiving in two studies [Cooke et al., 2015; Fogt et al., 2016]; blu in one study [Yan and D’Ruiz, 2015]; Mild in one study [Czogała et al., 2012]; NJOY NPRO and Hydro in two studies [Eissenberg, 2010; Vansickel et al., 2010]); one leading brand of an unspecified device in one study (Carnevale et al., 2016); and the personal devices of the study participants in two studies (Spindle et al., 2017; St.Helen et al., 2016). Nicotine or cotinine biomarkers were reported in 10 studies and were generally lower than those that would be reached with combustible tobacco cigarettes (Antoniewicz et al., 2016; Cooke et al., 2015; Eissenberg, 2010; Fogt et al., 2016; Yan and D’Ruiz, 2015), except maybe for studies using tank-style devices and the personal e-cigarettes of the participants (Spindle et al., 2017; St.Helen et al., 2016, 2017). Few studies provided details on actual wattage and resistance (Antoniewicz et al., 2016; Farsalinos et al., 2014; St.Helen et al., 2017; Szołtysek-Bołdys et al., 2014) and no studies provided details on the coils. The e-liquid concentration of nicotine ranged from 0 mg/ml (Cooke et al., 2015; Fogt et al., 2016) to 24 mg/ml (Szołtysek-Bołdys et al., 2014), although some studies reported the total amount of nicotine in the cartridge, but not the actual concentration (Carnevale et al., 2016; Eissenberg, 2010). Only one study tested multiple propylene glycol (PG)/glycerol concentrations (Yan and D’Ruiz, 2015), and only two other studies actually reported the concentrations of other constituents beyond nicotine (Antoniewicz et al., 2016; Farsalinos et al., 2014). Regarding flavors, only one study used a vanillin flavor (Farsalinos et al., 2014); two studies mentioned menthol, one allowing the choice of a menthol flavoring (Eissenberg, 2010), and another study specifically tested menthol (Yan and D’Ruiz, 2015); and one study compared strawberry flavor, tobacco flavor, and the personal flavor used by the participant (St.Helen et al., 2017).
The interventions tested were substantially different across the short-term clinical studies. Seven studies compared the short-term effects of one or more e-cigarettes versus combustible tobacco cigarettes (Carnevale et al., 2016; Czogała et al., 2012; Eissenberg, 2010; Farsalinos et al., 2014; Szołtysek-Bołdys et al., 2014; Vansickel et al., 2010; Yan and D’Ruiz, 2015) (one of those also included one arm with sham puffing [Eissenberg et al., 2010]). One study compared e-cigarettes to a resting period in the same conditions as the e-cigarette use period (Antoniewicz et al., 2016). Two studies compared the same e-cigarette with e-liquids with and without nicotine (Cooke et al., 2015; Fogt et al., 2016) and with different flavors. One study compared the same e-cigarette and e-liquid with and without a mouthpiece (Spindle et al., 2017). The washout periods ranged from less than 24 hours (St.Helen et al., 2017) to 1 week in crossover studies (Antoniewicz et al., 2016; Carnevale et al., 2016; Cooke et al., 2015;
TABLE 9-2 Epidemiological Studies on Chronic E-Cigarette Use and Cardiovascular Endpoints
| Reference | Study Design | Population | Age Range % Men % C-Smoker % F-Smoker | E-Cigarette Device Characteristics | E-Cigarette Pattern of Use and Cotinine Levels |
|---|---|---|---|---|---|
| Moheimani et al., 2017 | XS | Los Angeles, CA, recruited in 2015–2016 (source or recruitment methods NR) | 21–45 years 59% 0% 35% |
NR | Mean = 241 minutes per day Mean = 1.6 years Plasma cotinine range = 2.6–27.3 mg/L |
| Comparison Groups | na | Study Endpoints | Results | Adjustmentb |
|---|---|---|---|---|
| - E-cigarette users - Non-users E-cigarette users asked not to use the e-cigarette the day of the study |
16/18 12/18 |
SBP (mmHg) DBP (mmHg) MAP (mmHg) HR (bpm) HRV: HF (non-user) - LF (nonuser) - LF/HF HRV-controlled breathing oxLDL (user) paraxonase-1 (nmol) HDLantiox. index (user) Fibrinogen (mg/dl) CRP (number abnormal) |
Mean user/non-user 115.8/109.0 (p = 0.07) 73.5/70.0 (p = 0.27) 87.6/83.0 (p = 0.15) 64.0/63.0 (p = 0.73) 46.5/57.8 (p = 0.04) 52.7/39.9 (p = 0.03) 1.37/0.85 (p = 0.05) NS (no. not shown) 3,801/2,413 (p = 0.01) 649.9/892.8 (p = 0.17) 0.42/0.38 (p = 0.55) 270.9/251.9 (p = 0.24) 3/1 (p = 0.15) Correlations of plasma cotinine with: HF (−0.34, p = 0.04) LF (0.35, p = 0.03) LF/HF (0.36, p = 0.03) oxLDL (0.35, p = 0.05) other biomarkers (NS) |
None (e-cigarette users more likely to be men and former smokers) |
| Reference | Study Design | Population | Age Range % Men % C-Smoker % F-Smoker | E-Cigarette Device Characteristics | E-Cigarette Pattern of Use and Cotinine Levels |
|---|---|---|---|---|---|
| Farsalinos et al., 2016 | RCT also analyzed as a CO | Smokers not attempting to quit from Catania, Italy, followed for 52 weeks, recruited in 2010–2011 through a smoking cessation clinic and offered to use e-cigarettes | 44.0 years (mean) 63% 100% 0% |
“Categoria” e-cigarette model 401, Arbi Group Srl (disposable cartridge and 3.7-V, 90mAh lithium ion battery). E-liquid nicotine: - 2.4% 12 weeks - 2.4% 6 weeks + 1.8% 6 weeks - 0% 12 weeks |
NR |
| Polosa et al., 2016 | CO | Regular smokers on treatment for hypertension at an outpatient clinic in Catania, Italy (period of recruitment NR) | 53.9 years (mean) 56% 48% (some dual users) 52% |
NR | Daily use from 10 to 14 months (83.7% more than 12 months) |
| Comparison Groups | na | Study Endpoints | Results | Adjustmentb |
|---|---|---|---|---|
| RCT arms: - 0% nicotine - 1.8% - 2.4% CO groups: - Smokers - Dual users - Sole users (called failures, reducers, and quitters in the paper) |
63/ 66/ 61 93/ 34/ 18 |
SBP (mmHg) DBP (mmHg) HR (bpm) at baseline and at 8 follow-up visits over 52 weeks |
RCT: mean (SD) SBP decreased from 128 (15) at baseline to 123 (14) mmHg at 52 weeks (p = 0.004) with no difference by treatment group. CO: adjusted mean change (95% CI) in SBP over time compared with smokers: Dual users: −6.76 (−13.39, −0.13) mmHg e-cigarette users: −14.25 (−23.70, −4.81) mmHg Stratified analysis by baseline BP:c Elevated (n = 66): mean (SD) change in SBP (mmHg) over time was 6.0 (12.5) (p = 0.002), 10.8 (10.1) (p < 0.001), and 16.3 (11.3) (p = 0.005) for smokers, dual users, and sole users, respectively. Normal (n = 79): No difference by group. No differences over time were observed for HR or for DBP by RCT treatment and CO group overall or stratified by baseline BP (elevated or normal). |
Some analyses adjusted for sex, age, and weight change |
| - Smokers - Dual users - Single users |
46/ 23/ 20 |
SBP (mmHg) DBP (mmHg) HR (bpm) Measured at baseline, 6 and 12 months % HT control smokers/e-cigarette users | Median (IQR) 145 (137, 152)/137 (132, 144)/134 (130, 142) 87 (85, 90)/83 (80, 92)/81 (74, 84) 78 (72, 85)/77 (70, 83)/80 (75, 86) 145 (136, 150)/130 (121, 140)/130 (123, 138) 85 (85, 90)/80 (71, 90)/80 (75, 87) 79 (72, 84)/76 (71, 92)/80 (76, 90) p-value comparing e-cigarette users versus smokers from baseline to 12 months < 0.001 for SBP and DBP and 0.71 for HR 20/37 at 6 months and 22/49 at 12 months |
Sex, age, weight, changes in SBP between pre-baseline and baseline <10 mmHg |
NOTES: C-smoker = current smoker; CO = crossover; DBP = diastolic blood pressure; F-smoker = former smoker; HF = high frequency; HR = heart rate; HRV = heart rate variability; HT = hypertension; LF = low frequency; MAP = mean arterial pressure; NR = not reported; NS = not significant; RCT = randomized controlled trial; SBP = systolic blood pressure; XS = cross-sectional.
a Final sample size used in the analyses. For Moheimani and colleagues (2017), the sample size was initially larger, but 1 participant among non-users of e-cigarettes was excluded because of active smoking, and 2 and 5 e-cigarette users were excluded because of active smoking or because of e-cigarette use the day of the study, respectively. Also, only 12 e-cigarette users had sufficient bio-specimens available to measure biomarkers. For Farsalinos and colleagues (2016), 300 (100 in each group) were initially recruited for the RCT, but 183 completed the study at 52 weeks (61 percent response rate with no difference by treatment group, so the estimated sample size is 61 participants in each treatment group available for the statistical analysis).
b Adjustment for potential confounding through regression modeling, matching, stratification, or other strategy.
c Elevated BP defined as SBP/DBP greater than or equal to 130/85 mmHg.
Czogała et al., 2012; Fogt et al., 2016). The two-arm separate comparison groups study (Farsalinos et al., 2014) and the one-arm before/after study (St.Helen et al., 2016) required no smoking or e-cigarette use several hours prior to the interventions.
In the 13 short-term clinical studies, outcomes were measured before and after the interventions. These studies contribute to assessing the short-term effect of using an e-cigarette regardless of the comparison group. In the remaining studies, the outcomes were measured crosssectionally with the assessment of e-cigarette exposure in one study, and over 1 year in two studies. The following study outcomes were measured:
- heart rate in 14 studies (Cooke et al., 2015; Czogała et al., 2012; Eissenberg, 2010; Farsalinos et al., 2014, 2016; Fogt et al., 2016; Moheimani et al., 2017; Polosa et al., 2016; Spindle et al., 2017; St.Helen et al., 2016, 2017; Szołtysek-Bołdys et al., 2014; Vansickel et al., 2010; Yan and D’Ruiz, 2015);
- blood pressure in 9 studies (Cooke et al., 2015; Czogała et al., 2012; Farsalinos et al., 2014, 2016; Fogt et al., 2016; Moheimani et al., 2017; Polosa et al., 2016; Szołtysek-Bołdys et al., 2014; Yan and D’Ruiz, 2015);
- hypertension control in 1 study (Polosa et al., 2016);
- biomarkers of oxidative stress in 2 studies (Carnevale et al., 2016; Moheimani et al., 2017);
- biomarkers of inflammation in 1 study (Moheimani et al., 2017);
- endothelial function based on brachial artery flow–mediated dilation in 1 study (Carnevale et al., 2016);
- arterial stiffness in 1 study (Szołtysek-Bołdys et al., 2014);
- endothelial progenitor cells and microvesicles in 1 study (Antoniewicz et al., 2016);
- autonomic control and heart rate variability in 2 studies (Cooke et al., 2015; Moheimani et al., 2017); and
- cardiac geometry and function in 1 study (Farsalinos et al., 2014).
The summary of the main results for these outcomes is presented below.
Heart Rate
Among the 11 studies that have evaluated short-term changes in heart rate, 10 studies measured heart rate before and after the intervention and 1 study measured heart rate only at the end of the intervention (Fogt et al., 2016). Five studies found higher heart rate levels after versus before e-cigarette use (Cooke et al., 2015; Spindle et al., 2017; St.Helen et al., 2016, 2017; Yan and D’Ruiz, 2015), all of them published between 2015 and 2017, while five studies published between 2010 and 2014 found no difference in heart rate after versus before e-cigarette use (Czogała et al., 2012; Eissenberg, 2010; Farsalinos et al., 2014; Szołtysek-Bołdys et al., 2014; Vansickel et al., 2010). The study by Fogt and colleagues (2016) also found similar heart rate levels after using an e-cigarette with 0 versus 18 mg/ml nicotine. The studies that found increases in heart rate were characterized by using tank-style devices, own devices, and/or confirmed that nicotine or cotinine biomarkers had increases following e-cigarette use. In those studies, the change in heart rate after versus before e-cigarette use ranged from an increase in 1.2 beats per minute (bpm) in a study of a GreenSmartLiving e-cigarette with nicotine 18 mg/ml (Cooke et al., 2015) to 17.2 bpm in a study of a tank-style e-cigarette device with strawberry flavoring with nicotine 18 mg/ml that closely evaluated the maximum change, which occurred at 5 minutes after completing a 15-puff session (St.Helen et al., 2017). Studies that found no changes generally used first- and second-generation e-cigarette devices and had no or small changes in nicotine-related biomarkers. Studies that compared changes in heart rate levels before and after smoking a combustible tobacco cigarette found marked increases in heart rate, generally larger than those found with e-cigarettes. However, most of the studies comparing e-cigarettes with combustible tobacco cigarettes have been done using first- and second-generation devices that did not markedly increase nicotine or cotinine levels in plasma. In the Yan and D’Ruiz (2015) study comparing a blu
e-cigarette to a Marlboro cigarette (plasma nicotine levels ranged from 13.7 ng/ml to 22.5 ng/ml plasma nicotine after 1 hour of ad lib e-cigarette use depending on the e-liquid formulation compared with 29.5 ng/ml after 1 hour of ad lib use of Marlboro cigarettes), the change in heart rate after versus before e-cigarette ranged from a mean (SD) of 1.9 (7.4) bpm (p = 0.24) for a blu e-cigarette with classic e-liquid with 1.6 percent nicotine and 75 percent glycerol to 4.1 (5.7) bpm (p = 0.002) for a blu e-cigarette with menthol e-liquid, 2.4 percent nicotine and 75 percent glycerol, which compared with a change of 4.3 (5.4) bpm (p = 0.001) following a Marlboro cigarette. These results indicate that in some instances the changes in heart rate induced by e-cigarettes are similar to those induced by combustible tobacco cigarettes.
Short-term effects of e-cigarette use on heart rate do not necessarily mean that chronic e-cigarette use increases resting heart rate, which is an established predictor of poor clinical cardiovascular health. In a cross-sectional study of daily e-cigarette users from Los Angeles, resting heart rate was similar among e-cigarette users compared with non-users (Moheimani et al., 2017) (see Table 9-2). An important limitation of this study is the lack of adjustment for sociodemographic characteristics and cardiovascular disease risk factors between e-cigarette users and nonusers. Resting heart rate was also similar over a 52-week period comparing e-cigarettes “Categoria model 401” with different levels of nicotine (0 percent, 2.4 percent + 1.8 percent, and 2.4 percent) randomly assigned to smokers in a cessation clinic (Farsalinos et al., 2016), as well as in a group of hypertensives using e-cigarettes as single or dual use compared with smoking.
Synthesis
Recent intervention studies using tank-style devices and devices owned by e-cigarette users and with confirmation of nicotine intake have consistently found increases in heart rate shortly after e-cigarette use. Earlier studies, using first- and second-generation devices, found no changes in heart rate following e-cigarette use. However, those studies were characterized by small or no increase in nicotine or cotinine biomarker levels. The crossover design, including randomization of the intervention order in several studies, is an ideal experimental design to evaluate short-term effects minimizing interindividual sources of variability in heart rate. The effect estimates, although generally smaller than those observed for tobacco cigarettes, get closer in value for some types of e-cigarettes, generally related to higher nicotine intake. It is well known that nicotine increases heart rate, which provides biological plausibility to these findings. For studies evaluating the association between e-cigarette use and
heart rate over longer-term periods, the three studies available found no association, although the studies did not adjust for sociodemographic variables and the type of e-cigarettes was not well characterized.
Blood Pressure
A total of six clinical studies measured short-term changes in SBP/DBP following e-cigarette use, five of them including measures before and after the experiments (Cooke et al., 2015; Czogała et al., 2012; Farsalinos et al., 2014; Szołtysek-Bołdys et al., 2014; Yan and D’Ruiz, 2015). All the studies indicated that they had recruited healthy participants without hypertension. Some studies had confirmed that SBP/DBP were less than or equal to 140/90 mmHg or even lower. In a crossover study assessing GreenSmartLiving e-cigarettes (Cooke et al., 2015), the mean (SD) change in SBP before and 10 minutes after the intervention was approximately −2.0 (3.0) and 2.0 (3.0) mmHg for 0 and 18 mg/ml nicotine concentrations, respectively, and the differences between those two groups were significant (p ≤ 0.03). The corresponding changes for DBP were −2.0 (3.0) and 4.0 (6.0) mmHg (p = 0.001). SBP and DBP in that experiment were also higher with nicotine compared with no nicotine during supine, tilt, and recovery experiments in addition to the rest measures. In the cross-over trial using blu e-cigarettes with five different e-liquids (Yan and D’Ruiz, 2015), the increase in mean (SD) SBP measured before and after the intervention (which included an ad lib period) ranged from 1.1 (11.1) mmHg (p = 0.63) for Classic Tobacco with 2.4 percent nicotine and 75 percent glycerol to 5.8 (10.0) mmHg (p = 0.02) for Classic Tobacco with 1.6 percent nicotine and 75 percent glycerol. The corresponding increase after smoking a Marlboro cigarette was 5.7 (12.4) mmHg (p ≤ 0.04). The corresponding changes for DBP ranged from 3.2 (7.3) mmHg (p = 0.05) for blu with menthol and 2.4 percent nicotine and 75 percent glycerol to 6.8 mmHg for three other types of blu cigarettes with different compositions (p < 0.001). The increase in DBP for a Marlboro cigarette was also 6.8 (7.1) mmHg (p < 0.001). Consistent with these findings, in the study by Farsalinos and colleagues (2014), DBP increased both after exposure to a cigarette (mean change [SD] = 4.4 [3.3], p < 0.001) and to an e-cigarette (3.0 [3.6], p < 0.001), while SBP increased after a cigarette (6.6 [5.2], p < 0.001) but not after an e-cigarette (0.7 [4.6], p = 0.37). In the study that compared blood pressure levels before and 10 minutes after a personal cigarette or an e-cigarette (Ego-3) in female students from Silesia, Poland (Szołtysek-Bołdys et al., 2014), the investigators reported small, statistically insignificant increases in SBP and DBP after both e-cigarettes and cigarettes, but the numbers are not shown. In another study in Poland, a first-generation e-cigarette was not associated with short-term changes in SBP/DBP, while a combustible
tobacco cigarette was associated with increases in DBP. In the study that reported blood pressure levels only at the end of the experiments (and thus does not allow assessment of the effect of using the e-cigarette compared with baseline) (Fogt et al., 2016), mean (SD) SBP was lower for the e-cigarette with 18 versus 0 mg/ml, 112.1 (6.8) versus 115.8 (8.0), p = 0.04, while mean (SD) DBP was higher at 76.6 (6.0) versus 73.6 (8.3), p = 0.04. During the exercise test, peak SBP was similar for both levels of nicotine, while peak DBP was higher for those with nicotine.
Short-term effects of e-cigarette use on SBP/DBP do not necessarily mean that chronic e-cigarette use increases resting blood pressure levels. In a cross-sectional study of daily e-cigarette users from Los Angeles, mean SBP was borderline significantly higher in e-cigarette users versus non-users (115.8 versus 109.0 mmHg, p = 0.07), while DBP was similar (Moheimani et al., 2017) (see Table 9-2), although the study did not adjust for sociodemographic characteristics and cardiovascular disease risk factors between e-cigarette users and non-users. In the studies from Catania, Italy, SBP and DBP decreased over time in participants who switched from tobacco cigarettes to e-cigarettes, especially those who achieved sole use (Farsalinos et al., 2016; Polosa et al., 2016). In the group of hypertensives, there was an improvement in hypertension control at 6 months and 12 months (Polosa et al., 2016). The study without hypertensives is limited by
- a relatively large loss of study participants during follow-up;
- lack of detailed reporting for the initial study design based on three treatment groups; and
- the observational design comparing sole and dual e-cigarette user to smokers in the secondary analyses, although the three groups were comparable at baseline by sex, age, pack-years, and blood pressure levels.
The study among hypertensives is limited by
- small sample size;
- unclear description of how many participants with hypertension were available initially and if they were selected using a random sampling strategy;
- lack of details on the e-cigarette devices and the e-liquid used by the participants; and
- the retrospective data collection based on clinical records.
The study matched for age, sex, and lack of fluctuation in SBP comparing a pre-baseline visit occurring 6–13 months prior with the baseline
visit. It is unclear how the authors ensured recruitment of participants who have not had changes in their blood pressure levels of more than 10 mmHg for 6–12 months, but studied the change in the following year. It is possible that the study has been done completely retrospectively.
Synthesis
Overall, for SBP, there are some inconsistent findings, with the majority of studies finding weak positive increases or no changes with the use of e-cigarettes, while experiments with combustible tobacco cigarettes found consistent increases. From studies with different levels of nicotine, it seems that lower nicotine concentrations resulted in weaker increases in SBP or even lower SBP levels than no nicotine. For DBP, on the other hand, the studies consistently show short-term increases in DBP following the use of an e-cigarette that delivers nicotine with a magnitude of the effect similar to the increase observed when smoking a cigarette. The crossover design, including randomization of the intervention order in several studies, is an ideal experimental design to evaluate short-term effects minimizing interindividual sources of variability in SBP/DBP. These findings are consistent with other studies in humans supporting short-term effects of e-cigarette use on markers of endothelial function and arterial stiffness (see below). The short-term effect of nicotine from e-cigarettes on SBP and DBP is consistent with findings from other nicotine delivery products including tobacco cigarettes and even nicotine replacement products. Regarding chronic health effects on blood pressure levels, the evidence is very limited as there is only one study comparing e-cigarette use to non-use, and two studies comparing e-cigarette use to smoking, one including patients with hypertension.
Oxidative Stress, Inflammation, Endothelial Function, and Arterial Stiffness (Arteriosclerosis)
Two studies have measured biomarkers of oxidative stress, one evaluating short-term changes in a study of 20 current smokers and 20 never smokers exposed to a cigarette or an e-cigarette in a non-randomized crossover design (all participants exposed first to the cigarette and 1 week later to the e-cigarette) (Carnevale et al., 2016), and the other a cross-sectional study of e-cigarette users compared with non-users from Los Angeles (Moheimani et al., 2017). In the crossover study, the following biomarkers of oxidative stress were measured in serum before and 30 minutes after exposure to a cigarette or an e-cigarette: soluble NOX2derived peptide (sNOX2-dp), a marker of nicotinamide adenine dinucleotide phosphate (reduced form) oxidase activation, nitric oxide bio-
availability, a signaling molecule with a major role in the regulation of vasodilation and endothelial function, and 8-iso-prostaglandin F2α (8-iso-PGF2α). The study reported the mean (SD) before and after the cigarettes and the e-cigarettes. The mean change in serum before and after cigarette and e-cigarette exposure was 14.6 (p < 0.001) and 8.6 (p < 0.001) pg/ml for sNOX2-dp, 68 (p < 0.001) and 54 (p < 0.001) pmol/L for 8-iso-PGF2α, −15.8 (p < 0.001) and −9.6 (p < 0.001) µM for NO bioavailability, and −1.5 (p < 0.001) and −1.0 (p < 0.001) µmol/mmol for vitamin E, respectively. Although the magnitude of the effect was weaker compared with the changes induced by a combustible tobacco cigarette, these experiments suggest that e-cigarettes can also increase levels of oxidative stress and reduce the levels of antioxidants. A major limitation of this study is the lack of information on the type of e-cigarette device and e-liquid used. Additional research would be needed to confirm these short-term effects and for which types of devices, as well as to evaluate the long-term effects of e-cigarette use on biomarkers of oxidative stress. These findings are consistent with in vitro and in vivo studies that are discussed in more detail in Chapter 7. In summary, several studies in vitro have shown that human vascular endothelial cells show increased reactive oxygen species with e-cigarette extract compared with controls (Anderson et al., 2016). Mice exposed to e-cigarette aerosol for several weeks showed increased levels of oxidative stress, macrophage-mediated inflammation, and inflammatory cytokines including interleukin-6 (Lerner et al., 2015).
In the cross-sectional study from Los Angeles (see Table 9-2), oxidized LDL was higher in e-cigarette users versus non-users, while there were no differences in other biomarkers of oxidative stress or inflammation, although the sample size was small (Moheimani et al., 2017). The same crossover study that measured oxidative stress biomarkers also assessed endothelial function by measuring flow-mediated dilation (FMD) (Carnevale et al., 2016), a marker of vascular reactivity in large arteries that measures the change in arterial diameter following reactive hyperemia. FMD was measured based on ultrasound assessment of basal brachial diameter and endothelial-dependent FMD of the brachial artery following established guidelines. FMD is expressed as a change in post-stimulus diameter evaluated as a percentage of the baseline diameter, with a lower percentage reflecting worse endothelial function. Mean (SD) brachial artery FMD changed from 6.7 (4.3) percent to 3.4 (3.9) percent (p < 0.001) and from 6.7 (3.6) percent to 4.3 (2.2) percent (p = 0.001) before and after, respectively, a cigarette and an e-cigarette. Although the change was larger after a cigarette (−3.3 percent change) than an e-cigarette (−2.4 percent change), both were statistically significant. The study did not provide detailed information on changes in pulse-wave velocity. The
implications of these findings for long-term endothelial function in long-term e-cigarette users need to be evaluated.
The short-term effect of e-cigarettes on endothelial function has also been evaluated based on changes in endothelial progenitor cells (EPCs) measured with flow cytometry and reported as EPC events (Antoniewicz et al., 2016). EPCs are stem cells mainly derived from the bone marrow that have been proposed as a biomarker of endothelial function as they play a critical role in the maintenance, differentiation, and regeneration of endothelial cells following vascular injury or neogenesis (Lekakis et al., 2011). In experiments comparing EPC levels before and 1 hour, 4 hours, and 24 hours after exposure to an eGoXL e-cigarette with nicotine 12 mg/ml and 49.4 percent/44.4 percent PG/glycerol without flavors, EPC events increased at 1 hour and 4 hours and returned to normal at 24 hours (see Figure 9-2). No changes were observed for control periods conducted with 1-week washout in a randomized crossover manner and in the same conditions as the e-cigarette experiment. These short-term effects of e-cigarettes on EPCs could be related to nicotine, as nicotine has been shown to increase short-term increases of EPCs. In addition to EPCs, the same experiment also measured microvesicles (MVs) from the cell membrane. The MVs consist of a lipid bilayer that can be released from all cell types in the circulation, such as leukocytes, erythrocytes, endothelial cells, and platelets. No differences were found in MVs overall, by cell origin (endothelial, platelet, or leukocyte) or by markers of inflammation (high-mobility group protein B1 [HMGB1], P-selectin, CD40 ligand), but a statistically significant difference was found for endothelial MVs with E-selectin (CD144 + CD62E), with higher levels at 4 hours after the experiment (median = 28 [IQR = 17, 65] versus 23 [14, 42]) that returned to normal at 24 hours (20 [15, 40] versus 23 [11, 37]), p = 0.038.1 More research is needed to understand the short-term effects of e-cigarettes on endothelial function and the long-term implications of these findings. Indeed, a short-term increase in EPCs does not necessarily translate to acute endothelial injury. In epidemiological studies, lower rather than higher EPC levels are associated with higher risk of coronary heart disease. The use of novel, relatively easy-to-obtain biomarkers such as EPCs and MVs could be useful to assess both the short-term and the long-term effects of e-cigarettes on cardiovascular disease.
Arterial elasticity is essential for blood flow. The hardening or stiffening of the arteries, which is also called arteriosclerosis, plays an important role in the development of cardiovascular disease. The term arterial
___________________
1Chapter 7 also includes this study in its review and presents effects of e-cigarette exposure on overall MVs. The committee finds no conflict between the evidence presented in this chapter and the evidence presented in Chapter 7.
stiffness is commonly used when arteriosclerosis is measured based on the pulse wave graph using photoplethysmography. One study has measured arterial stiffness at the height of the phalanges artery before and 10 minutes after a personal cigarette or an e-cigarette (Ego-3) exposure in female students from Silesia, Poland (Szołtysek-Bołdys et al., 2014). The main study outcomes are the stiffness index (SI) measured in meters per second and the reflection index (RI) measured in percentage. SI is the ratio of the patient height in meters and the time between peaks of the systolic and diastolic components in the pulse wave graph. The RI is the ratio of diastolic and systolic component heights, expressed as percentage. In those experiments, in which SI and RI were measured before and 10 minutes after smoking a cigarette, and 1 week later after using an e-cigarette (Ego-3) with nicotine 24 mg/ml, SI was reduced from 6.75 to 6.56 (p = 0.006) after the cigarette but remained similar (6.73 and 6.75, changes not statistically significant) after an e-cigarette. RI was reduced (54.0 to 49.6 percent, p = 0.01) after a cigarette. The reduction after an e-cigarette (52.0 percent to 50.8 percent) was not statistically significant, although the exact p-value was not reported. The findings of this experiment would indicate that e-cigarettes would not induce short-term changes in arterial stiffness, contrary to combustible tobacco cigarettes. Given the findings
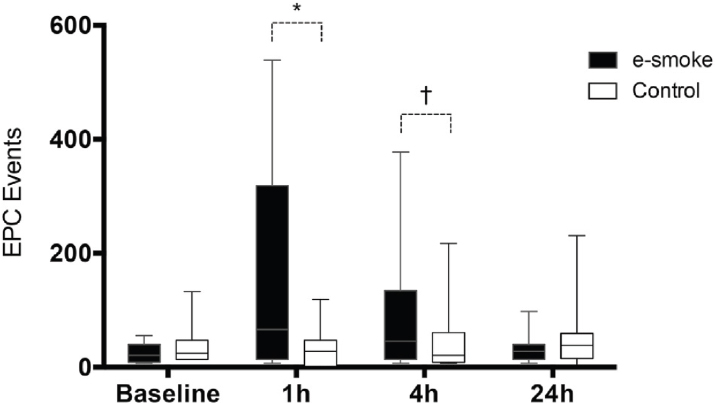
NOTES: Two-way, multiple measures ANOVA were significant for the interaction of exposure and time (p = 0.002). Separate time-point analysis was significant for 1 hour versus baseline, *p = 0.003; and 4 hours versus baseline, †p = 0.036.
SOURCE: Antoniewicz et al., 2016.
for DBP as well as some of the findings reported for endothelial dysfunction, it is important to further evaluate the short- and long-term effects of e-cigarette smoking on arterial stiffness in larger studies.
Synthesis
Although the number of studies evaluating the effects of e-cigarettes on measures of oxidative stress, endothelial dysfunction, and arterial stiffness is small, these outcomes are interrelated and are considered in the underlying pathophysiological pathway toward clinical cardiovascular disease, including coronary heart disease, stroke, and peripheral artery disease. A major limitation is that these outcomes were evaluated short term rather than long term and it is unknown if these short-term findings have long-term consequences for the cardiovascular system. Research further evaluating these subclinical measures of cardiovascular disease is needed.
Cardiac Geometry and Function
The two-arm intervention study comparing the short-term effects of combustible tobacco cigarettes in smokers and e-cigarettes in e-cigarette users conducted measures of echocardiography before and 5 minutes after smoking a cigarette or using an e-cigarette (Farsalinos et al., 2014). During the echocardiography measures of flow diastolic velocities (E, A), their ratio (E/A), deceleration time (DT), isovolumetric time (IVRT), and corrected-to-heart rate IVRT (IVRTc) were measured. Mitral annulus systolic (Sm) and diastolic (Em, Am) velocities were estimated. Myocardial performance index was calculated from Doppler flow (MPI) and tissue Doppler (MPlt). Longitudinal deformation measurements of global strain (GS), systolic (SRs) and diastolic (SRe, SRa) strain rate were also performed. While the study focused its presentation of the findings comparing the effects of smoking a cigarette in smokers to vaping an e-cigarette among e-cigarette users, the comparability of those two groups is unclear. A better study design would be to evaluate the changes that occur before and after within each group. For e-cigarette users, none of the changes in the echocardiograph measures were statistically significant. However, some were borderline. For example, there was a mean (SD) change of 1.6 (5.6) cm/second in A flow diastolic velocity (p = 0.08), which was in the same direction as that observed for combustible tobacco cigarette smokers. The change in Em of 0.2 (0.7) cm/second MPIt (−0.01 [0.04], p = 0.08) was in the opposite direction from that among smokers. For GS the change (SD) was −0.4 (1.2) and almost statistically significant (p = 0.06), although also in the opposite direction from that among smokers. Overall,
the implications of this study are unclear. First, because the study is not using a crossover design, the interventions were not randomized, and the comparability of smokers and e-cigarette users is unclear. Second, the usefulness of echocardiographic measures to assess short-term changes is unclear. Cardiac function and echocardiographic measures can be difficult to obtain and it is unclear if changes in those measures can be observed so quickly. These measures, moreover, are user dependent and if the examiner is aware of the intervention and the before and after status of the participant, the results may be influenced. Finally, this study used an early-generation e-cigarette device, so the relevance for currently used e-cigarettes is also unknown.
Autonomic Control
One study measured short-term effects of e-cigarette use on autonomic cardiovascular control under conditions of orthostatic stress (Cooke et al., 2015). No differences were observed by treatment group. In the cross-sectional study of e-cigarette users from Los Angeles compared with non-users, heart rate variability was measured with an echo-cardiogram (ECG) obtained during 5 minutes of quiet rest and during 5 minutes of controlled breathing at 12 breaths per minute (stimulus for the vagal tone). Three main spectral components were distinguished: high frequency (HF = 0.15–0.4 Hz, indicator of vagal activity), low frequency (LF = 0.04–0.15 Hz, a mixture of both vagal and sympathetic activity), and the ratio of LF to HF, reflecting cardiac sympathetic balance. Time-domain analysis was not applied because the ECG recording was too short.
In a second study, Moheimani and colleagues (2017) found the HF component decreased significantly in e-cigarette users compared with non-users (mean 46.5 versus 57.8, p = 0.04) while the LF and the LF/HF ratio increased significantly (52.7 versus 39.9, p = 0.03 and 1.37 versus 0.85, p = 0.05). No differences were observed between e-cigarette users and non-users in the changes of HF, LF, and LF/HF ratio during the controlled breathing maneuver. Study limitations include the small sample size, unclear description of the sources and forms of recruitment and response rate, the lack of adjustment or matching for sociodemographic and lifestyle risk factors (in particular given the imbalance by sex, former smoking status, and pack-years), and the lack of details on the e-cigarette devices and the e-liquid used by the participant. Outcome assessment was conducted using high-quality protocols and is described in detail.
CONCLUSIONS
The level of evidence regarding the association between e-cigarette use and biomarkers of cardiovascular disease risks varies:
Conclusion 9-2. There is substantial evidence that heart rate increases shortly after nicotine intake from e-cigarettes.
Conclusion 9-3. There is moderate evidence that diastolic blood pressure increases shortly after nicotine intake from e-cigarettes.
Conclusion 9-4. There is limited evidence that e-cigarette use is associated with a short-term increase in systolic blood pressure, changes in biomarkers of oxidative stress, increased endothelial dysfunction and arterial stiffness, and autonomic control.
Conclusion 9-5. There is insufficient evidence that e-cigarette use is associated with long-term changes in heart rate, blood pressure, and cardiac geometry and function.
REFERENCES
Anderson, C., A. Majeste, J. Hanus, and S. Wang. 2016. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicological Sciences 154(2):332–340.
Antoniewicz, L., J. A. Bosson, J. Kuhl, S. M. Abdel-Halim, A. Kiessling, F. Mobarrez, and M. Lundback. 2016. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis 255:179–185.
Benowitz, N. L., and J. B. Fraiman. 2017. Cardiovascular effects of electronic cigarettes. Nature Reviews Cardiology 14(8):447–456.
Bhatnagar, A. 2016. E-cigarettes and cardiovascular disease risk: Evaluation of evidence, policy implications, and recommendations. Current Cardiovascular Risk Reports 10(7):24.
Carnevale, R., S. Sciarretta, F. Violi, C. Nocella, L. Loffredo, L. Perri, M. Peruzzi, A. G. M. Marullo, E. De Falco, I. Chimenti, V. Valenti, G. Biondi-Zoccai, and G. Frati. 2016. Acute impact of tobacco vs. electronic cigarette smoking on oxidative stress and vascular function. Chest 150(3):606–612.
Cooke, W. H., A. Pokhrel, C. Dowling, D. L. Fogt, and C. A. Rickards. 2015. Acute inhalation of vaporized nicotine increases arterial pressure in young non-smokers: A pilot study. Clinical Autonomic Research 25(4):267–270.
Cosselman, K. E., A. Navas-Acien, and J. D. Kaufman. 2015. Environmental factors in cardiovascular disease. Nature Reviews Cardiology 12(11):627–642.
Czogała, J., M. Cholewinski, A. Kutek, and W. Zielinska-Danch. 2012. [Evaluation of changes in hemodynamic parameters after the use of electronic nicotine delivery systems among regular cigarette smokers]. Przegląd Lekarski 69(10):841–845.
Czogała, J., M. L. Goniewicz, B. Fidelus, W. Zielinska-Danch, M. J. Travers, and A. Sobczak. 2014. Secondhand exposure to vapors from electronic cigarettes. Nicotine & Tobacco Research 16(6):655–662.
Eissenberg, T. 2010. Electronic nicotine delivery devices: Ineffective nicotine delivery and craving suppression after acute administration. Tobacco Control 19(1):87–88.
Farsalinos, K. E., D. Tsiapras, S. Kyrzopoulos, M. Savvopoulou, and V. Voudris. 2014. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: Comparison with the effects of regular cigarettes. BMC Cardiovascular Disorders 14:78. https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/1471-2261-14-78 (accessed January 31, 2018).
Farsalinos, K., F. Cibella, P. Caponnetto, D. Campagna, J. B. Morjaria, E. Battaglia, M. Caruso, C. Russo, and R. Polosa. 2016. Effect of continuous smoking reduction and abstinence on blood pressure and heart rate in smokers switching to electronic cigarettes. Internal and Emergency Medicine 11(1):85–94.
Fogt, D. L., M. A. Levi, C. A. Rickards, S. P. Stelly, and W. H. Cooke. 2016. Effects of acute vaporized nicotine in non-tobacco users at rest and during exercise. International Journal of Exercise Science 9(5):607–615.
HHS (U.S. Department of Health and Human Services). 2014. The health consequences of smoking—50 years of progress: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
James, P. A., S. Oparil, B. L. Carter, W. C. Cushman, C. Dennison-Himmelfarb, J. Handler, D. T. Lackland, M. L. LeFevre, T. D. MacKenzie, O. Ogedegbe, S. C. Smith, L. P. Svetkey, S. J. Taler, R. R. Townsend, J. T. Wright, A. S. Narva, and E. Ortiz. 2014. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth Joint National Committee (JNC 8). Journal of the American Medical Association 311(5):507–520.
Koskela, J. K., A. Tahvanainen, A. Haring, A. J. Tikkakoski, E. Ilveskoski, J. Viitala, M. H. Leskinen, T. Lehtimaki, M. A. Kahonen, T. Koobi, O. Niemela, J. T. Mustonen, and I. H. Porsti. 2013. Association of resting heart rate with cardiovascular function: A cross-sectional study in 522 Finnish subjects. BMC Cardiovascular Disorders 13:102.
Lekakis, J., P. Abraham, A. Balbarini, A. Blann, C. M. Boulanger, J. Cockcroft, F. Cosentino, J. Deanfield, A. Gallino, I. Ikonomidis, D. Kremastinos, U. Landmesser, A. Protogerou, C. Stefanadis, D. Tousoulis, G. Vassalli, H. Vink, N. Werner, I. Wilkinson, and C. Vlachopoulos. 2011. Methods for evaluating endothelial function: A position statement from the European Society of Cardiology Working Group on Peripheral Circulation. European Journal of Cardiovascular Prevention and Rehabilitation 18(6):775–789.
Lerner, C. A., I. K. Sundar, H. Yao, J. Gerloff, D. J. Ossip, S. McIntosh, R. Robinson, and I. Rahman. 2015. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 10(2):e0116732. https://doi.org/10.1371/journal.pone.0116732 (accessed February 8, 2018).
Levenson, J., A. C. Simon, F. A. Cambien, and C. Beretti. 1987. Cigarette smoking and hypertension. Factors independently associated with blood hyperviscosity and arterial rigidity. Arteriosclerosis, Thrombosis, and Vascular Biology 7(6):572.
Mahmud, A., and J. Feely. 2003. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension 41(1):183.
Mattace-Raso, F. U. S., T. J. M. van der Cammen, A. Hofman, N. M. van Popele, M. L. Bos, M. A. D. H. Schalekamp, R. Asmar, R. S. Reneman, A. P. G. Hoeks, M. M. B. Breteler, and J. C. M. Witteman. 2006. Arterial stiffness and risk of coronary heart disease and stroke. Circulation 113(5):657.
McEniery, C. M., S. Wallace, I. S. Mackenzie, B. McDonnell, Yasmin, D. E. Newby, J. R. Cockcroft, and I. B. Wilkinson. 2006. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 48(4):602.
Moheimani, R. S., M. Bhetraratana, F. Yin, K. M. Peters, J. Gornbein, J. A. Araujo, and H. R. Middlekauff. 2017. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: Implications for cardiovascular risk. JAMA Cardiology 2(3):278–284.
Nigra, A. E., A. Ruiz-Hernandez, J. Redon, A. Navas-Acien, and M. Tellez-Plaza. 2016. Environmental metals and cardiovascular disease in adults: A systematic review beyond lead and cadmium. Current Environmental Health Reports 3(4):416–433.
Poirier, P. 2014. Exercise, heart rate variability, and longevity: The cocoon mystery? Circulation 129(21):2085–2087.
Polosa, R., J. B. Morjaria, P. Caponnetto, E. Battaglia, C. Russo, C. Ciampi, G. Adams, and C. M. Bruno. 2016. Blood pressure control in smokers with arterial hypertension who switched to electronic cigarettes. International Journal of Environmental Research and Public Health 13(11):E1123.
Pope, C. A., 3rd, R. T. Burnett, D. Krewski, M. Jerrett, Y. Shi, E. E. Calle, and M. J. Thun. 2009. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: Shape of the exposure–response relationship. Circulation 120(11):941–948.
Spindle, T. R., M. M. Hiler, A. B. Breland, N. V. Karaoghlanian, A. L. Shihadeh, and T. Eissenberg. 2017. The influence of a mouthpiece-based topography measurement device on electronic cigarette user’s plasma nicotine concentration, heart rate, and subjective effects under directed and ad libitum use conditions. Nicotine & Tobacco Research 19(4):469–476.
St.Helen, G., K. C. Ross, D. A. Dempsey, C. M. Havel, P. Jacob, 3rd, and N. L. Benowitz. 2016. Nicotine delivery and vaping behavior during ad libitum e-cigarette access. Tobacco Regulatory Science 2(4):363–376.
St.Helen, G., D. A. Dempsey, C. M. Havel, P. Jacob, 3rd, and N. L. Benowitz. 2017. Impact of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes. Drug and Alcohol Dependence 178:391–398.
Szołtysek-Bołdys, I., A. Sobczak, W. Zielińska-Danch, A. Bartoń, B. Koszowski, and L. Kośmider. 2014. Influence of inhaled nicotine source on arterial stiffness. Przegląd Lekarski 71(11):572–575.
Vansickel, A. R., C. O. Cobb, M. F. Weaver, and T. E. Eissenberg. 2010. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology, Biomarkers & Prevention 19(8):1945–1953.
Willum Hansen, T., J. A. Staessen, C. Torp-Pedersen, S. Rasmussen, L. Thijs, H. Ibsen, and J. Jeppesen. 2006. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 113(5):664.
Yan, X. S., and C. D’Ruiz. 2015. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regulatory Toxicology and Pharmacology 71(1):24–34.










































