11
Respiratory Diseases
Smoking of combustible tobacco products is the number one cause of chronic obstructive pulmonary disease (COPD) worldwide. Although the proportion of smokers has decreased over the past 25 years, approximately 1.1 billion people continue to smoke as of 2015 (Rabe and Watz, 2017). COPD leads to more than 3 million deaths per year worldwide, with only ischemic heart disease and cerebrovascular disease causing more deaths. Individuals who smoke also have an increased risk of sleep apnea and asthma exacerbations (Jayes et al., 2016). Respiratory complications from smoking can be further confounded by the increase in cardiovascular disease in individuals who smoke (Rabe and Watz, 2017).
In addition to the adverse respiratory health effects caused by smoking combustible tobacco products, secondhand smoke exposure has been reported to be associated with significant respiratory morbidities in non-users (Jayes et al., 2016). Tobacco smoke exposure has been shown to increase the severity of asthma exacerbations in children exposed to secondhand smoke (Merianos et al., 2016). Exposure to tobacco smoke in utero has been associated with abnormalities in lung development and small airway dysfunction in school-age children, manifested by reductions in forced expiratory volume in 1 second (FEV1) and forced expiratory flow 25–75 percent (FEF25–75 percent) (den Dekker et al., 2015; Duijts et al., 2012; Hayatbakhsh et al., 2009). A study in China found that school-age children exposed to secondhand smoke had increased cough and decreased lung function compared with children not exposed to secondhand smoke (He et al., 2011), and a study from Finland found that
children of mothers who smoked combustible tobacco cigarettes during pregnancy were more likely to have increased airway resistance than children of mothers who did not smoke (Kalliola et al., 2013). Postnatal exposure to tobacco smoke also has been associated with an increased risk of wheeze and upper and lower respiratory tract illnesses in exposed children compared with unexposed children (Jayes et al., 2016).
Currently there is a lack of information regarding the short- and long-term effects of e-cigarettes on the respiratory system. This is due in part to the relative newness of the delivery system, the vast assortment of devices being used, and the variety of nicotine concentrations and flavorings that are currently available. Nevertheless, exposure of the lungs to various components of the e-cigarette aerosol could potentially damage the respiratory system or worsen preexisting lung disease through a variety of mechanisms (see Figure 11-1). For example,
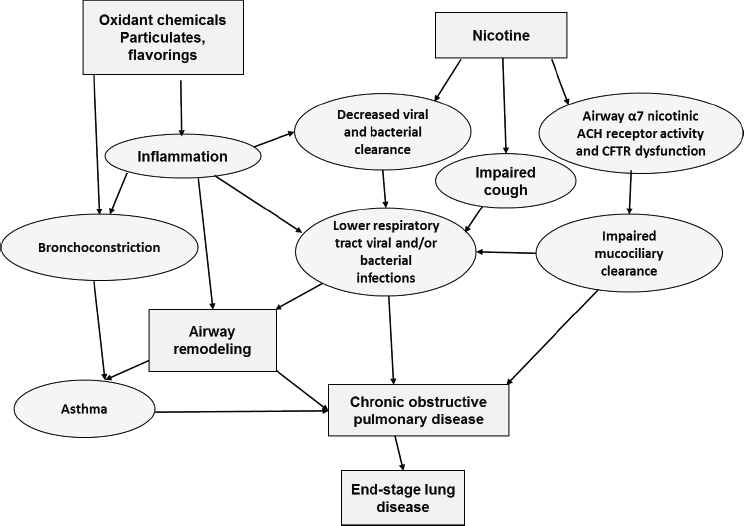
NOTE: ACH = acetylcholine receptors; CFTR = cystic fibrosis transmembrane conductance regulator.
nicotine-containing e-cigarette aerosols have the potential to adversely impact several host defense mechanisms in the lungs. In a murine model, α7 nicotinic acetylcholine receptors (α7 nAChRs) were shown to regulate cystic fibrosis transmembrane conductance regulator (CFTR) activity in the airways. Exposure to nicotine downregulated α7 nAChR activity, which in turn impaired CFTR function, causing impaired mucociliary clearance (MCC) (Maouche et al., 2013). In humans, CFTR dysfunction has been shown to be associated with the development of COPD and asthma hyperresponsiveness (Saint-Criq and Gray, 2017). Exposure to nicotine in tobacco smoke and e-cigarette aerosols also has been reported to impair cough (Dicpinigaitis, 2017; Dicpinigaitis et al., 2006; Sitkauskiene and Dicpinigaitis, 2010). Furthermore, nicotine has been shown to downregulate Th1 immune responses to lipopolysaccharide (Yanagita et al., 2012), consistent with an immunomodulatory effect of nicotine on viral and bacterial clearance.
Independent of nicotine, exposure to particulates and flavorings in e-cigarette aerosols could also potentially impair lung function. The presence of ultrafine particles has been measured in the aerosols of e-cigarettes (Laube et al., 2017), and particulates in the submicron range have the potential to damage airways and lung parenchyma. As noted in Chapter 3, the health risks from exposure to particles will depend on their nature, not simply their size. Nevertheless, certain ultrafine particles, which encompass particle sizes less than 100 nm, can cause DNA damage, induce pro-inflammatory cytokine expression, and adversely affect the immune system through the production of free oxygen radicals (Li et al., 2016). In addition, inhalation of ultrafine particles has been reported to increase the rate of asthma exacerbations (Li et al., 2016). Flavorings in e-cigarettes may also alter cellular redox balances in the airways by increasing pro-inflammatory cytokines (Lerner et al., 2015), and high temperatures generated by e-cigarette devices may cause formation of formaldehyde, leading to toxic effects on the lungs (Geiss et al., 2015).
In established smokers who are trying to quit or reduce combustible tobacco use, e-cigarettes may be less deleterious to the respiratory system when compared with exposure to combustible tobacco smoke (see Chapter 18). However, initiation of e-cigarette use by a person who has never smoked may cause harm to the respiratory system compared with never using e-cigarettes, particularly if initiation of e-cigarettes occurs at a young age. Therefore, understanding the health effects of e-cigarettes is dependent on the context of age, current and prior use of combustible tobacco products, and whether the user has preexisting lung conditions such as asthma and COPD. In addition, there is a need to examine the short- and long-term effects of secondhand and thirdhand e-cigarette aerosols on the respiratory health of non-users, who may inhale or come
in contact with exhaled mainstream aerosol, which can settle on hard surfaces. Infants and preschool children who live with e-cigarette users may be at higher risk for secondary exposures because this age group spends much of their time in the residence of the e-cigarette user. Finally, exposure of the dual user to both combustible tobacco products and e-cigarette aerosols may cause unique health risks to the respiratory system.
CHARACTERIZATION OF DISEASE ENDPOINTS AND INTERMEDIATE OUTCOMES
In studying the effects of e-cigarette use on respiratory disease endpoints, an important question is whether or not e-cigarette use by itself can lead to the development of chronic respiratory conditions such as asthma and COPD or if e-cigarette use can worsen preexisting lung conditions compared with people who do not smoke. Additionally, researchers should determine if substitution of e-cigarettes for combustible tobacco use lessens the development of chronic respiratory conditions or lessens progression of preexisting lung conditions compared with people who continue to smoke. Because these respiratory disease endpoints may take years or even decades to realize, it becomes necessary to measure intermediate outcomes that may predict a disease state. The intermediate outcomes most relevant to the clinician include measurements of lung function and lung structure. The most common measurements of lung function include forced vital capacity (FVC), FEV1, FEV1/FVC ratio, and FEF25–75 percent, with the latter three the most useful in detecting presence and progression of obstructive lung diseases, such as asthma and COPD. These measurements are easily obtainable using spirometry. Body plethysmography can be used to detect an increase in residual volume, which can correlate with worsening airflow obstruction. In addition, impulse oscillometry can be used to detect changes in large and small airway resistance, and may be more sensitive than spirometry in detecting reversibility of airway obstruction in people with COPD (Saadeh et al., 2015). Structural changes in the lung such as the development of emphysematous changes or mucus plugging can be determined using computed tomography (CT) of the chest. Ultra-low-dose CT (Messerli et al., 2017), and more recently, MRI of the chest, has been shown to be an alternative modality to conventional chest CT in assessing COPD changes (Saadeh et al., 2015; Washko et al., 2012). Finally, standardized respiratory questionnaires can be helpful in evaluating outcomes; however, instrument responsiveness may differ among questionnaires (Puhan et al., 2006). Research on other intermediate outcomes in respiratory health should include the effect of e-cigarette aerosols, with and without nicotine, on cough reflex sensitivity, urge to cough, and nasal MCC because
cough and MCC are integral defense mechanisms that help clear pathogens and environmental pollutants from the lungs and sinuses (Chatwin et al., 2003; Lee et al., 2017; Tarrant et al., 2017).
Quantification of inflammatory cell numbers from bronchoalveolar lavage (BAL) (Levanen et al., 2016; Siew et al., 2017) and measurement of pro-inflammatory cytokines from bronchial biopsies (Shields et al., 2017) could be used as intermediate respiratory endpoints to assess inflammation in the lower respiratory tract inflammation caused by e-cigarette use. In addition, combustible tobacco smoke has been shown to alter microbiome diversity; therefore, examination of sputum, nasal, and pharyngeal microbiome diversity may also help predict the impact of e-cigarette use on respiratory health (Diao et al., 2017). Other intermediate outcomes that could be used as markers of respiratory health include self-reported wheeze, bronchitis, shortness of breath, mucus production, other respiratory symptoms, and quality of life measurements.
OPTIMAL STUDY DESIGN
Since the potential health effects of e-cigarettes on the respiratory system are not completely understood, randomized controlled trials (RCTs) would not be appropriate at this time. Alternatively, prospective cohort studies that assess respiratory health outcomes in e-cigarettes users compared with combustible tobacco users and dual users could help determine the risks and benefits of using e-cigarettes. In addition, RCTs testing the efficacy of e-cigarette substitution as a method of smoking cessation in smokers unable to quit using nicotine replacement therapy (NRT) could concurrently measure lung function, lung structure, lung symptoms, and quality of life in individuals substituting e-cigarettes for combustible tobacco products. These additional studies could provide valuable information regarding the respiratory health effects of e-cigarette substitution on established smokers and help determine if switching completely or partly to e-cigarettes from combustible tobacco products in people with preexisting lung disease can alter progression or stability of lung disease.
Prospective cohort studies in adolescents and young adult e-cigarette users without a history of combustible tobacco product use should be performed to determine the likelihood of e-cigarette use leading to the development of chronic respiratory symptoms or decline in lung function. Furthermore, since asthma is a common respiratory disease of childhood, it is also important to determine if adolescents and young adults with asthma are at increased risk for asthma exacerbations and a more rapid decline in lung function when using e-cigarettes. Potential confounding factors, such as dual tobacco or cannabinoid use, exposure to secondhand smoke, and prior history of tobacco use, could introduce bias into the
comparisons across exposure groups and need to be considered. Rigorous, objective assessment of the spectrum of endpoints, including lung function, respiratory symptoms, and cardiovascular and other comorbidities would also be essential to these studies.
QUESTIONS ADDRESSED BY THE LITERATURE
Due to the relatively recent widespread acceptance of e-cigarettes, there is a lack of understanding regarding the positive and negative effects of e-cigarettes on respiratory health. This is due in part to the paucity of long-term observational studies of adolescent/young adult never smokers who initiate e-cigarette use and observational studies and RCTs of adult smokers who switch to e-cigarettes for smoking cessation. Human studies are also needed that examine how exhaled mainstream aerosols affect the respiratory system of non-users when inhaled. As previously noted, exposure to these aerosols may disproportionately impact infants and children in the homes of indoor e-cigarette users because the very young often spend the majority of their time in this environment. However, since e-cigarettes, unlike combustible tobacco products, lack substantial sidestream emissions, it is unclear how detrimental exposure to secondhand e-cigarette emissions is to the non-user.
Further investigations into the effects of e-cigarette aerosols on the lung defense mechanisms such as cough, MCC, and the innate and adaptive immune system are needed. In addition, a better understanding of the impact of particle size on the development of DNA damage in respiratory cells is needed as is the relationship between flavorings and development of reactive oxygen species.
CLINICAL AND EPIDEMIOLOGICAL STUDIES IN HUMANS
Effects on Users of Combustible Tobacco Products
The literature search identified 17 studies that examined respiratory or pulmonary outcomes in people using e-cigarettes (see Table 11-1). Subjects in these studies include adult users of combustible products who switch to e-cigarettes completely or become dual users and include subjects with or without preexisting respiratory disease. Outcomes in the studies include standard measures of function ranging from self-reported symptoms of cough to asthma to exhaled carbon monoxide or nitric oxide. Six of these studies were from the same study group (Campagna et al., 2016; Cibella et al., 2016; Polosa et al., 2014a,b, 2016a,b). Three of these studies were observational studies in which the subject population included smokers not intending to quit. These subjects were invited to
switch to first-generation e-cigarettes (Campagna et al., 2016; Cibella et al., 2016; Polosa et al., 2014b). Cibella and colleagues (2016) reported significant improvement in self-reported respiratory symptoms of cough/phlegm at 52 weeks in smokers who switched completely to e-cigarettes (18 of 130 subjects) and a significant increase in FEF25–75 percent, but not in FEV1 or FVC. No difference in lung function was found in dual users at 52 weeks (Cibella et al., 2016). In a similar study population of smokers not intending to quit, Campagna and colleagues (2016) found significant decreases in the fractional concentration of carbon monoxide in exhaled breath (FeCO) in smokers who switched completely to e-cigarettes (18 of 134 subjects) and significant increases in the fractional concentration of nitric oxide in exhaled breath (FeNO) at 52 weeks. Polosa and colleagues (2014b) reported on 40 smokers not intending to quit, 17 of whom were lost to follow-up, and found that when invited to use e-cigarettes, 5 of the 40 switched completely to e-cigarettes at 24 months.
In two studies from Polosa and colleagues (2014a, 2016a), they identified retrospectively 18 mild to moderate asthmatic smokers who switched to e-cigarettes (either single or dual users). They reported an improvement in FEV1, performance in the methacholine challenge test, and asthma control questionnaire but no change in asthma exacerbations when these subjects were followed prospectively over a 12-month period (Polosa et al., 2014a, 2016a). In a similar study design, Polosa and colleagues (2016b) identified patients with COPD from medical records who switched to e-cigarettes (single or dual users) and reported that they had significantly fewer COPD exacerbations. D’Ruiz and colleagues (2017) reported on pulmonary function tests in smokers who were switched to e-cigarettes for 5 days and found no significant difference in lung function between the groups.
These studies suggest that smokers with preexisting lung conditions such as asthma and COPD may experience some benefits from switching to e-cigarettes. As reported in the Polosa and colleagues (2016a,b) studies, such benefits may include an increase in FEV1, improved performance in a methacholine challenge test and in asthma control, and a decrease in COPD exacerbations. However, a limitation of these studies is that they were performed in a small number of subjects selected retrospectively. In addition, a reasonably high-quality RCT was negative: Cravo and colleagues (2016), in a clinical study that recruited subjects from two centers in the United Kingdom, reported no difference in lung function in subjects who switched to e-cigarettes. Their study had two cohorts. In both cohorts, smokers were randomized to either change to e-cigarettes containing 2 percent nicotine (with or without menthol flavoring) or to continue smoking. The authors reported no significant changes in pulmonary function tests after 12 weeks between the two groups. In this study,
TABLE 11-1 Clinical and Epidemiological Studies in Humans
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Effects in Users of Combustible Tobacco Products | |||||
| Campagna et al., 2016 | n = 134 | 3-arm, double-blind, controlled, randomized, clinical trial; longitudinal. Return Rate 75% at week 12, 70.3% at week 24, and 61% at week 52. No difference in characteristics between those who remained or dropped out, except gender (71% of those lost to followup were male). No difference in dropout rate among the three experimental groups. | “Categoria” e-cigarette (model “401”). E-cigarette kit with either “original” (2.4% nicotine—Group A), or “Categoria” (1.8% nicotine—Group B), or “original” without nicotine (“sweet tobacco” aroma—Group C) cartridges | Those participants receiving e-cigarettes with 0% nicotine | (1) FeNO in ppb from 10-second exhalation; (2) eCO in ppm from a single expiratory breath; (3) adverse event symptom score for 8 different symptoms |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Demographic characteristics, smoking reduction, and quit rates were not significantly different among study groups | (1) FeNO showed significant changes over the time: at baseline (BL), FeNO ppb (medians and interquartile range) were 6.6 (4.3–8.4), 5.9 (5.0–7.8), and 5.5 (4.5–6.9) for failures, reducers, and quitters (as per continuous classification at week 52), respectively. At week 52, it was 7.0 (5.5–9.9), 7.9 (6.0–10.8) and 17.7 (13.3–18.9) ppb, respectively. Repeated-measures ANOVA showed that effect of smoking phenotype was significant (p < 0.0001). No significant difference in FeNO changes from baseline was observed in quitters who stopped using e-cigarettes [+11.8 (7.4–13.4) ppb] compared with quitters who were still using e-cigarettes [+14.3 (9.9–15.3) ppb] at any study time points; (2) Significant within-subject effect (i.e., time, p < 0.0001) was found for changes in eCO. Exhaled CO ppm (medians and interquartile range) were 21 (14–29), 20 (15–26), and 17 (12–20) at BL for failures, reducers, and quitters (as per continuous classification at week 52), respectively. The same figures at week 52 were 20 (14–30), 13 (6–19), and 3 (1–4) ppm. Repeated-measures ANOVA showed a significant between-subject effect (i.e., smoking phenotype, p < 0.0001). Linear regression analysis showed that changes in FeNO were significantly correlated (p < 0.0001) with those in eCO at all time points; (3) High prevalence of respiratory symptoms was reported at baseline and virtually disappeared very quickly in both quitters and reducers. Among failures and reducers, the slopes were flat or not significant. Significant and steeper slopes (positive for eCO and negative for FeNO) were found among quitters. Differences among slopes were significant for both eCO and FeNO (p < 0.0001, ANCOVA). |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Polosa et al., 2016a | n = 16 | Review of longitudinal medical records | Varied | N/A | (1) Juniper’s ACQ score, spirometry for FEV1, FVC, and FEF25–75%, bronchial provocation tests assessing AHR for methacholine PC20. (2) Number of exacerbations from previous visit. (3) eCO monitoring and self-reported cigarette consumption. (4) E-cigarette smoking patterns. |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Not stated; missing measurements were not included in the analyses | (1) At follow-up 1, there were significant improvements in ACQ scores; at follow-up 2 and follow-up 3, significant improvements were observed on ACQ scores, and all lung function parameters including methacholine PC20. Improvements at 12 months were still present at 24 months. Similar improvements were also observed in the dual users. At follow-up 1, there were significant improvements in ACQ scores and FEF25–75%. At follow-up 2, and follow-up 3, significant improvements from baseline (except for FVC at follow-up 3) were observed on ACQ scores, lung function parameters, and methacholine PC20. Deterioration in objective and subjective asthma outcomes noted in the two patients who relapsed to exclusive tobacco smoking. The normal FEV1/FVC of 79.5% at 12 months (follow-up 2) decreased to 71.0% at 24 months (follow-up 3). Their methacholine PC20 was reduced threefold from 2.95 mg/ml to 1.05 mg/ml and their ACQ score increased substantially from 1.45 to 2.3. (2) No significant differences in number of respiratory exacerbations throughout the study. Average number of exacerbations at baseline of 1.13 were not significantly different from 0.93 exacerbations at follow-up 1, 0.87 exacerbations at follow-up 2, and 0.81 exacerbations at follow-up 3. Of note, exacerbation rate increased from 0 at 12 months (follow-up 2) to 2 at 24 months (follow-up 3) in the two patients who relapsed to exclusive tobacco smoking. (3) Marked reduction in combustible tobacco cigarette use among e-cigarette users, the mean cigarette/day consumption of 21.9 at baseline decreasing to 2.3 at follow-up 1, 1.9 at follow-up 2, and 1.5 at follow-up 3. Substantial reduction in combustible tobacco cigarette use also observed in dual users; their mean cigarette/day consumption at baseline decreasing from 20.7 to 5.3 at follow-up 1, 3.7 at follow-up 2, and 3.5 at follow-up 3. Out of 16 asthmatics, 10 were still exclusively using e-cigarettes at 24 months and not smoking combustible tobacco cigarettes throughout the study (single users). (4) Duration of regular e-cigarette use ranged from 20 to 26 months, with 10 patients using them for at least 2 years. All participants were using standard refillable e-cigarettes by the end the study. The preferred nicotine strength of their e-liquid was 9 mg/ml and 18 mg/ml, which was consumed by 62.5% and 18.8% of e-cigarette users respectively. Most of the participants preferred tobacco flavors over other flavors. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Cibella et al., 2016 | Varied, depending on outcome (generally 103+) | 3-arm, double-blind, controlled, randomized, clinical trial; longitudinal. Return rate 75% at week 12, 70.3% at week 24, and 61% at week 52. No difference in characteristics between those who remained or dropped out, except gender (71% of those lost to followup were male). No difference in dropout rate between three experimental groups. | “Categoria” e-cigarette (model “401”). E-cigarette kit with either “original” (2.4% nicotine—Group A), or “Categoria” (1.8% nicotine—Group B), or “original” without nicotine (“sweet tobacco” aroma—Group C) cartridges | Participants receiving e-cigarette with 0% nicotine (n varied depending on outcome) | (1) Subjective respiratory problems (frequency of cough/phlegm, wheezing, shortness of breath, or difficulty breathing). (2) Spirometry metrics (FEV1, FVC, FEF25–75%, and FEV1/FVC ratio). |
| Cravo et al., 2016 | n = 419 | Randomized, parallel group clinical study; combustible tobacco cigarette smokers switched to e-cigarettes for 12 weeks | E-cigarette with rechargeable battery, atomizer, capsule with e-liquid; 2% nicotine; subjects in combustible tobacco cigarette arm smoked own usual brand | Combustible tobacco cigarette smokers | Primary outcomes: AEs, vital signs, 12-lead ECG, lung function tests, hematology, clinical biochemistry, urinalysis |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Demographic characteristics, smoking reduction, and quit rates were not significantly different among study groups. | (1) Cough/phlegm was significantly more frequent at BL among those resulting quitters (64%) with respect to reducers (55%) and failures (36%). No reported wheezing or chest tightness. High prevalence of cough/phlegm and shortness of breath (SoB) reported at BL: frequency of cough/phlegm decreased at each follow-up visit with respect to BL regardless of subjects’ smoking phenotypes classification. SoB showed a similar frequency. Symptoms of cough/phlegm and SoB disappeared completely in quitters during the study. Significant effect of smoking phenotype on the reduction in cough/phlegm and SoB with time. Of note, changes in respiratory symptoms from BL were greater for both reducers and quitters with respect to failures (p < 0.0001). The presence/absence of respiratory symptoms at all time points (BL, week 12, week 24, and week 52) was not associated with significant differences in any of evaluated spirometric variables. (2) Significant within-subject effect was found for changes in FEV1, FVC, and FEF25–75% over the time (at BL, and at week 12, week 24, and week 52, p < 0.0001). No effect of smoking phenotype classification was evident for FEV1, FVC, and FEV1/FVC. Effect of smoking phenotype classification was evident on FEF25–75% that significantly (p = 0.034) increased over time among quitters. FEF25–75% was (mean ± SD) 80.6 ± 18.2, 78.3 ± 19.3, and 85.7 ± 15.6 at BL for failures, reducers, and quitters (as per continuous classification at week 52), respectively. The same figures at week 52 were 83.1 ± 18.4, 87.0 ± 20.0, and 100.8 ± 14.6 (p < 0.0001). |
| Not stated. |
No clinically significant findings in vital signs, electrocardiogram, lung function tests and standard clinical laboratory parameters.
AEs reported: more frequent during the first week and then reduced; 1,515 reported AEs, 495 related to nicotine withdrawal symptoms. Most frequent were headache, sore throat, desire to smoke, and cough; 6% judged as probably or definitely related to the e-cigarette. Additional observations: up to 33.8% decrease in level of urine nicotine equivalents, and decreases in the level of benzene, acrolein, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| D’Ruiz et al., 2017 | n = 105 | Randomized, open-label, forced-switch parallel-arm study (exclusive e-cigarette use group, dual-use group, cessation group) |
3 closed-system blu™
E-cigarette products: Rechargeable tobacco flavor, rechargeable cherry flavor, and disposable cherry flavor; all contained 24 mg/mL (2.4%) nicotine |
Complete tobacco and nicotine product cessation | Pulmonary function (FVC, FEV1, and exhaled CO and NO); safety and tolerability |
| Polosa et al., 2014a | n = 18 | Review of longitudinal medical records | Varied | N/A | (1) FEF25–75%, BHR, and ACQ scores; (2) Combustible tobacco cigarette use; (3) Exacerbations; (4) Safety and tolerability |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Not stated. |
Use of the e-cigarettes for 5 days did not lead to negative respiratory health outcomes or serious AEs.
Pulmonary function tests: small but not significant improvements in FVC and FEV1 measurements in most use groups. Statistically significant benefits associated with smoking reduction were also noted in exhaled CO and NO levels. |
| Not stated; Missing measurements were not included in the analyses. | No significant differences in the parameters of lung function, BHR, or ACQ scores between the pre-baseline and baseline visits (except for a small change in FEF25–75%). (1) Compared with baseline, at 6 months, there were significant improvements in FEF25–75% and ACQ scores; at 12 months significant improvements were observed on all asthma outcomes measures. At 12 months both dual and single users had considerable improvements compared with baseline in all parameters (except for FVC in single users). (2) There was a reduction in combustible tobacco cigarette use amongst all e-cigarette users from a mean combustible tobacco cigarette/day use of 21.9 at baseline decreasing to 1.7 at followup visit 2 (p < 0.001). Similar reduction in combustible tobacco cigarette smoking was observed in dual users as well (22.4 at baseline to 3.9 at follow-up visit 2; p < 0.001). Importantly, 10 asthmatics gave up combustible tobacco cigarette use in favor of the e-cigarette (single users). (3) Prior to e-cigarette use in the 18 patients the average number of exacerbations was 1.06 (at pre-baseline) and 1.17 (at baseline). Over the period of observation none of the subjects in the cohort reviewed had a hospital or intensive care unit admission. (4) No severe adverse reactions or acute exacerbation of asthma symptoms were reported during the period of observation with e-cigarette use. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Polosa et al., 2014b | n = 40 | Observational prospective study following a cohort of smokers in a naturalistic setting after a 24-week intervention phase during which participants were issued Categoria e-cigarettes. Used a “Categoria” e-cigarette 6 months and followed prospectively for 2 years. After an initial 6-month intervention phase using the e-cigarette, participants attended two follow-up visits, at 18 and 24 months. | Categoria e-cigarette, “original” flavor, 7.4-mg nicotine cartridges (no more than 4 cartridges per day) | None | (1) >50% reduction in number of cigarettes from baseline and corresponding eCO level (reducers). (2) >80% reduction in number of cigarettes from baseline, with corresponding eCO (heavy reducers). (3) Abstinence from smoking with corresponding eCO (quitters). Failure to meet any of those benchmarks was defined as smoking cessation failure. (4) Product usage. (5) Adverse smoking-related events or symptoms. |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Not stated. | (1) Sustained 50% reduction in the number of cigarettes per day at 24 months was shown in 11/40 subjects, with a median of 24 cigarettes per day decreasing significantly to 4 cigarettes per day (p = 0.003). (2) Of these 11 combustible tobacco cigarette reducers, 6 could be classified as sustained heavy reducers at 24 months. They had a median consumption of 27.5 cigarettes per day at baseline, decreasing significantly to 4 cigarettes per day by 24 months (p = 0.012). (3) There were 5/40 quitters by the end of the study. (4) Mean of 1.82 (±1.44) cartridges/day was used at 6 months. At 24 months, some e-cigarette users were not using the product (and stayed quitters), some relapsed back to tobacco smoking, and four upgraded their entry-level e-cigarette to better performing intermediate products using e-liquid nicotine from refill bottles (all categorized as heavy reducers). (5) At 6 months, mouth irritation, throat irritation, and dry cough were reported, respectively, by 14.8%, 7.4%, and 11.1% of the participants. Dry mouth, dizziness, headache, and nausea were infrequent. Overall, these symptoms remained stable during the whole duration of the observation phase, with the exception of dizziness and nausea, which disappeared by 24-month study visit. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Polosa et al., 2016b | n = 48 | Reviewed clinical notes of COPD patients attending clinics; 2 followup visits (12, 24 months after baseline). Analyses include data from the 3 visits. | Not stated; varied | Age- and sex-matched COPD patients who smoked combustible tobacco cigarettes but not e-cigarettes | (1) Changes in smoking behavior and e-cigarette use. (2) COPD exacerbations. (3) Lung function assessments and COPD staging. (4) CAT scores and 6-MWD. |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Not stated; no significant differences in baseline characteristics between e-cigarette and control groups. | (1) Significant reduction in combustible tobacco cigarette consumption in COPD e-cigarette users. Complete abstinence from tobacco smoking in 13/24 (54.2%) of COPD e-cigarette users. Dual usage was reported by 11/24 (45.8%) COPD e-cigarette users. Significant reduction in combustible tobacco cigarette consumption in dual users. More than 75% reduction from baseline in cigarettes per day consumption reported by all COPD e-cigarette dual users at both follow-up visits. (2) Significant reduction in annual COPD exacerbations within the COPD e-cigarette user group but not in control group. Significant reduction in COPD exacerbations observed in dual users, but only at 24 months. In the single users there was significant reduction in exacerbations at both follow-ups. (3) Compared with baseline there were no significant differences in the post-bronchodilator FEV1, FVC, and % FEV1/FVC between study groups. Significant difference in the rate of FEV1 decline at the 24-month follow-up visit in COPD e-cigarette users than in the control group. A few COPD patients in the e-cigarette study group downstaged from GOLD Stage 4 to GOLD Stage 3 and 2. (4) COPD symptoms, as assessed using the CAT, at both followup visits decreased statistically and clinically significantly in the e-cigarette group, but no change in control group. Over the 24-month observation period, the median 6-MWD improved more than 60 minutes in the e-cigarette user group compared with just over a median of 3 minutes in the control group. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Acute Exposures | |||||
| Ferrari et al., 2015 | n = 20 | Laboratory-based, randomized crossover design. | The NF e-cigarette used in this study: elips-C Series (steel shell, microprocessor powered by a battery, a filter, and a removable cartridge); nicotine-free liquid with hazelnut flavor (“Natur Smoke aroma Nocciola Antistress 0 mg/ml nicotina”). The commercial combustible tobacco cigarette (Marlboro® Red) contained 0.8 mg nicotine. | Crossover design (both smokers and nonsmokers were randomized to smoke both the NF e-cigarette and a commercial combustible tobacco cigarette ad lib for 5 minutes in 2 different sessions) | (1) FeNO (2) FeCO (3) FVC (4) FEV1 (5) FEF (6) PEF |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Not stated (except that smoking habit and crossover design were considered as factors in the ANOVA). | (1) No significant changes of FeNO were observed in the two groups. (2) Baseline FeCO values were significantly higher in smokers than in non-smokers. The combustible tobacco cigarette significantly increased FeCO values; this effect was significant in both groups of subjects. The e-cigarette did not have any significant effects on FeCO. The increase of FeCO values observed after smoking the combustible tobacco cigarette was significantly different from the effect of the e-cigarette. (3) Smoking a combustible tobacco cigarette significantly decreased the FEV1/FVC in non-smokers. (4) Both types of cigarettes significantly decreased FEV1 values in smokers while the decreases in non-smokers were not significant; thus FEV1 decreased significantly in the overall population after smoking a combustible tobacco cigarette while the effect of the e-cigarette did not reach a statistically significant level. (5) The combustible tobacco cigarette significantly decreased FEF25, FEF50, and FEF75 in the overall population, particularly due to the significant reductions of FEF25 in smokers and FEF75 in non-smokers while the reduction of FEF50 did not reach the significant levels in either smokers or non-smokers. The only significant effect of the e-cigarette was a reduction of FEF25 in smokers. Comparing the effects of combustible tobacco and e-cigarette smoking, only a significantly greater reduction of FEF50 was found after combustible tobacco cigarette smoking in non-smokers. Higher values of FEF75 were found after smoking an e-cigarette than after smoking a combustible tobacco cigarette, whereas the inverse was the case in smokers. (6) The combustible tobacco cigarette significantly decreased PEF values in the overall population due to effect in the smokers. The changes in FEV1, FVC, FEV1/FVC, and PEF between the two types of cigarettes were not significantly different in either smokers or non-smokers or in the overall population. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Vardavas et al., 2012 | n = 30 | Laboratory-based, intervention design. Two groups: experimental group (n = 30) and control group (n = 10). Control group randomly selected from experimental group to participate in an extra session at a separate time. The role of using an e-cigarette was assessed through: (1) comparing the changes noted among control group participants with changes noted among experimental group participants after the intervention (intragroup comparison); and (2) comparing pre- versus post-respiratory function among experimental group participants (intergroup comparison). | NOBACCO e-cigarettes, black line. Medium cartridge, 11 mg nicotine. The subjects in the experimental group were instructed to use the e-cigarette ad lib for 5 minutes as they would usually smoke. | Control group subjects were asked to use the e-cigarette ad lib for 5 minutes, but without the e-cigarette cartridge included (not blinded). | (1) FeNO, ppb. (2) Dynamic lung volumes. (3) Total respiratory resistance. |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Adjustments for the group (control versus experimental) and the relative baseline measurement (pre versus post). After controlling for baseline responses in linear regression, results are strengthened compared with the simple bivariate associations. | (1) FeNO in the experimental group decreased by 16% after the use of an e-cigarette, but not in control group. (2) Pulmonary function assessed via spirometry did not change in either group. (3) Airway impedance at 5 Hz increased in the experimental group by 0.033 kPa/(L/s), whereas no differences were noted among control group participants. Lung resistance in the experimental group also increased at 5 Hz, 10 Hz, and 20 Hz by an average of 0.031 kPa/(L/s), 0.029 kPa/(L/s), and 0.030 kPa/(L/s), respectively. Peripheral pulmonary resistance also increased significantly from 0.22 kPa/(L/s) to 0.25 kPa/(L/s). |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Cough and Mucociliary Clearance | |||||
| Dicpinigaitis et al., 2016a | n = 30 | Pre-post cough test (before e-cigarette exposure, and after) | 30 puffs from a disposable e-cigarette (blu, classic tobacco flavor; approximately 1.5–1.8 mg nicotine) | No control group (instead, pre-post analysis) | (1) C5, measured by the number of coughs following a capsaicin challenge. (2) Secondary analysis with non-nicotine e-cigarette in 8 subjects who demonstrated large degrees of inhibition of cough reflex sensitivity. |
| Dicpinigaitis et al., 2016b | n = 17 | Pre-post cough test (before e-cigarette exposure, and after) | 30 puffs from a disposable e-cigarette (blu, classic tobacco flavor; approximately 1.5–1.8 mg nicotine) | No control group (instead, pre-post analysis) | (1) C5, measured by the number of coughs following a capsaicin challenge. (2) Cu. |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Not stated. | (1) After e-cigarette exposure, cough reflex sensitivity was significantly diminished compared with baseline. This effect was transient. Mean log C5 at baseline was 0.50 ± 0.09 (SEM); 15 minutes after e-cigarette exposure it was 0.79 ± 0.11; and 24 hours subsequently it was 0.55 ± 0.10. Difference between log C5 at baseline and post–e-cigarette exposure was significant as was the difference between post–e-cigarette use and 24 hours later. Twenty-three of 30 subjects demonstrated an inhibition of cough reflex sensitivity after e-cigarette exposure; 5 subjects had no change, and 2 subjects had a one-doubling concentration decrease in C5. Twenty-six of the 30 subjects coughed to some degree. The median number of coughs for the study group was 15.5 (range 0–114) coughs. No correlation was found between the number of coughs induced by e-cigarette inhalation and subsequent change in cough reflex sensitivity. (2) No inhibition of cough reflex sensitivity was observed after exposure to the non-nicotine–containing e-cigarette, by contrast to the change in C5 after use of the nicotine-containing e-cigarette. Significantly less coughing was observed after 30 puffs of the non-nicotine–containing e-cigarette compared with the nicotine-containing product. |
| Not stated. | Seventeen subjects had a demonstrable Cu and formed the subject population: (1) after e-cigarette exposure, C5, and (2) the Cu was significantly diminished compared with baseline. Mean log C5 at baseline was 0.60 ± 0.11 (SEM) and 0.92 ± 0.16, 15 minutes after e-cigarette exposure. Mean log Cu was −0.035 ± 0.08 at baseline and 0.21 ± 0.12 at 15 minutes after e-cigarette exposure. The difference between log C5 at baseline and 15 minutes post–e-cigarette exposure was significant as was the difference in log Cu. This effect was transient. Fourteen of the 17 subjects coughed to some degree in response to inhalation. The median total number of coughs for the study group was 9 with a range of 0–30 coughs. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Kumral et al., 2016 | n = 98 | Prospective randomized single-blind clinical trial. | Participants selected brand of device and flavor of the cartridge; 11–12 mg/ml e-liquid for all e-cigarettes. | Non–e-cigarette users (n = 40) were the smokers who quit smoking without the aid of medical therapy or a device, although they were provided cognitive behavioral treatment. | (1) SNOT-22 for subjective symptoms. (2) Saccharin transit test to evaluate nasal MCC function. |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Not stated. | (1) SNOT-22 scores were insignificant between groups before the cessation of cigarette smoking; there was a significant difference between the groups at the third-month measurements. Comparison of SNOT-22 results of groups at the beginning of the study and after 3 months revealed statistically significantly lower scores after the 3 months. (2) MCC measurements were insignificant between groups before the cessation of cigarette smoking; there was a significant difference between the groups at the third-month measurements. Comparison of MCC results of group 2 at the beginning of the study and after 3 months revealed statistically significantly lower scores after the 3 months. Group 1 did not show any significant difference after 3 months. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Respiratory Symptoms in Adolescents | |||||
| Cho and Paik, 2016 | n = 35,904 | Cross-sectional survey study | E-cigarette use assessed by “Have you ever used an e-cigarette in your life?” (yes/no). Answering no: “never user.” Answering yes: asked a followup question “Have you used e-cigarettes in the past 30 days?” (yes/no). Answering yes: “current user” and answering no: “former user.” Cigarette smoking assessed by question “Have you ever smoked, even one puff in your life?” (yes/no). Answering no: “never smoker.” Answering yes: asked a followup question “In the past 30 days, how many days did you smoke?” Answering “one or more days”: “current smoker,” answering “none:” “former smoker.” | “Current e-cigarette users” are compared with “former e-cigarette users” and “never e-cigarette users” as well as those who had used combustible tobacco cigarettes. | (1) Asthma based on student’s self-reported doctor’s diagnosis of asthma. (2) Severe asthma based on days of missing school due to symptoms. |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Seven variables were included in the model: gender, city size, multicultural family status, overweight status, secondhand smoking at home, atopic dermatitis history, allergic rhinitis history. A variable for combustible tobacco cigarette smoking was added. Multiple logistic regression analyses performed for each potential confounder. | (1) Prevalence rates of asthmatics in “current e-cigarette users,” “former e-cigarette users,” and “never e-cigarette users,” were 3.9%, 2.2%, and 1.7%, respectively. Comparing “current e-cigarette” users with “never e-cigarette” users, the unadjusted OR for asthma was 2.36. Comparing “current e-cigarette” users with “never e-cigarette” users, the adjusted OR for gender only was 2.09, and the adjusted OR for combustible tobacco cigarette smokers only was 1.73. The combustible tobacco cigarette smoking was the highest factor that affected the effect of e-cigarettes on asthma. Gender was the second factor. For all other factors, the changes in estimate of the effect of e-cigarettes on asthma were comparable to that of the unadjusted model. (2) Within the “never combustible tobacco cigarette” group, the OR for “more than 4 day absence from school due to asthma symptoms” was 18.59 in Model A, 13.21 in Model B, and 15.42 in Model C. Differences were not significant for the “former combustible tobacco cigarette” group and “current combustible tobacco cigarette” group. Within the “never combustible tobacco cigarette” group, the OR for “1–3 day absence from school due to asthma symptoms” was 6.81 in Model A, 5.67 in Model B, and 5.04 in Model C. Within the “current combustible tobacco cigarette” group, the OR for “1–3 day absence from school due to asthma symptoms” was 2.48 in Model A, 2.46 in Model B, and 2.23 in Model C. Differences were not significant for the “former combustible tobacco cigarette” group. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Choi and Bernat, 2016 | n = 36,085 | Cross-sectional survey | E-cigarettes described to students as “battery-operated devices that look, feel, and taste like a [combustible] tobacco cigarette.” Students were asked about e-cigarette use (asked if had ever tried using e-cigarettes [yes/no] and if had used e-cigarettes in past 30 days [yes/no]). | N/A | (1) Asthma status (determined by asking if currently had asthma [never diagnosed, currently has asthma, does not currently have asthma, unsure] and if had an asthma attack in last 12 months [yes/no]). (2) E-cigarette use. (3) Susceptibility to combustible tobacco cigarette smoking (asked about number of days smoked in past 30 days; if said never tried, assessed for susceptibility to combustible tobacco cigarette smoking). |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Analyses were weighted to account for cluster sampling and were stratified by county-level metropolitan status. Additional associations adjusted for demographic variables, living with combustible tobacco cigarette smokers, days smoked in the past 30 days, positive social norms toward smoking, and exposure to secondhand combustible tobacco cigarette smoking. |
The weighted prevalence of ever e-cigarette use was 8.2% (8.0% among students in metropolitan counties and 11.0% in non-metropolitan/rural counties). Students in metropolitan counties who reported currently having asthma were significantly more likely to have ever used e-cigarettes compared with those never diagnosed with asthma. The prevalence of ever e-cigarette use in students with current asthma was significantly higher among students in non-metropolitan/rural counties (18.2%) compared with those students with current asthma in metropolitan areas (9.9%).
The weighted prevalence of past 30-day e-cigarette use was 3.3% (3.2% in students in metropolitan counties and 4.8% in students in non-metropolitan/rural counties). The prevalence of past 30-day e-cigarette use in students with current asthma was significantly higher among students in non-metropolitan/rural counties (9.5%) compared with those students with current asthma in metropolitan areas (5.1%). Among students with current asthma who had never smoked combustible tobacco cigarettes, ever e-cigarette use was associated with higher odds of being susceptible to combustible tobacco cigarette smoking (AOR = 3.96) compared with those who never used e-cigarettes. Past 30-day use of e-cigarettes was associated with an asthma attack in the last 12 months (AOR = 1.78) among those with current asthma. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| McConnell et al., 2017 | n = 2086 | Cross-sectional survey with past data included. Logistic regression used to evaluate the association of bronchitic symptoms and current wheeze with e-cigarette use. Dummy variables were created to assess effects of past and current use, compared with never use, and of frequency of use among current users. The linear trend in effects of frequency of current e-cigarette use assessed across 3 categories of use (never users, 1–2, and 3 or more days in the previous 30 days). | Students were asked the age at which they first tried cigarettes or e-cigarettes and number of days they used the product in the past 30 days. Participants who had “never tried” a product were classified as “never users.” Those who had used a product, but not in the last 30 days, were classified as “past users.” Participants who had used a product on at least 1 of the past 30 days were classified as “current users” of that product. Frequency of current e-cigarette use was categorized as 1–2 days or 3 or more days. Students reported number of cigarettes smoked in the previous month and the lifetime number of cigarettes smoked. Lifetime number of cigarettes smoked was categorized as 0 (never smokers), >0–10, 11–99, and >99 cigarettes. | N/A | (1) Chronic bronchitis symptoms (daily cough for 3 months, congestion or phlegm other than when accompanied by a cold, or bronchitis in the previous 12 months). (2) Wheeze assessed based on a report of wheezing or whistling in the chest during the previous 12 months. Analysis based on subjects with complete information on e-cigarette use. |
| Confounders or Factors Adjusted for | Results |
|---|---|
| Asthma was based on student’s self-report of ever having had asthma. Parent-completed questionnaire assessed sociodemographic characteristics. Confounding assessed by including covariates in model. Models were adjusted for lifetime number of cigarettes. In sensitivity analyses, associations of e-cigarettes with bronchitic symptoms and wheeze were adjusted for these same conditions in 2010 and were restricted to children without symptoms in 2010. Twenty-three interaction terms of e-cigarette use with a dog or cat at home were examined for this outcome. For each outcome, the interactions of gender, ethnicity (Hispanic and non-Hispanic white) and asthma (in separate models) with e-cigarette use were also evaluated by calculating a likelihood ratio test for models with and without the interaction across categories of e-cigarette use. In all models, missing data were assumed to occur at random. | (1) Survey included 502 participants (24.0%) who had ever used e-cigarettes; 301 (14.4%) were past and 201 (9.6%) current users. Among current users, 107 (53.3%) used e-cigarettes on 1–2 days monthly and 94 (46.8%) on 3 or more days. Among past and current e-cigarette users, 132 (44.2%) and 81 (40.5%), respectively, were never cigarette users). Compared with Hispanic participants, non-Hispanic white youth were more likely to have bronchitic symptoms or wheeze. Parental education greater than high school was associated with greater risk of both outcomes. Secondhand smoke exposure in the home was associated with increased risk of bronchitic symptoms but not of wheeze. Current and non-current use of cigarettes was associated with greater risk of each outcome. Bronchitic symptoms were associated with both past (OR = 1.85) and current use of e-cigarettes (OR = 2.02). They were attenuated by additional adjustment for lifetime number of cigarettes smoked and secondhand smoke exposure in the home (OR = 1.71, for past and 1.41 for current use). There were no statistically significant interactions of e-cigarette use with gender, ethnicity (Hispanic and non-Hispanic white), asthma, and presence of a dog or cat in the home. The risk of bronchitic symptoms increased with number of days used in the previous 30 days (OR = 1.66 for 1–2 days and OR = 2.52 for 3 or more days) compared with e-cigarette–never users. This association with e-cigarette use frequency was not confounded by demographic characteristics, but was attenuated by additional adjustment for secondhand smoke exposure and lifetime number of cigarettes smoked (OR = 1.37 for 1–2 days and OR = 1.64 for 3 or more days of use) and the trend was no longer significant. (2) Wheeze was associated with current (OR = 1.86) but not with past use of e-cigarettes (OR = 1.02). The effect of current e-cigarette use was not confounded by sociodemographic characteristics but was markedly attenuated by adjustment for secondhand smoke exposure and lifetime number of cigarettes smoked (OR = 1.24), and after adjustment the association of past use of e-cigarettes with wheeze became negative (OR = 0.70). The magnitude of effect estimates for e-cigarette exposure in analyses restricted to never smokers were similar to those found in the entire population after adjustment for sociodemographic characteristics, smoking history, and secondhand smoke exposure. |
| Reference | Sample Size | Study Design | E-Cigarette Product | Control Conditions | Operationally Defined Outcomes |
|---|---|---|---|---|---|
| Wang et al., 2016 | n = 45,128 | Cross-sectional survey (current and past smoking status, e-cigarette use status, respiratory symptoms, demographic characteristics, secondhand smoke exposure) | Not stated; varied | N/A | (1) E-cigarette use. (2) Respiratory symptoms. |
NOTES: 6-MWD = 6-minute walk distance; ACQ = asthma control questionnaire; AE = adverse event; AHR = airway hyperresponsiveness; ANCOVA = analysis of covariance; ANOVA = analysis of variance; AOR = adjusted odds ratios; BHR = bronchial hyperresponsiveness; BL = baseline; C5 = cough reflex sensitivity; CAT = COPD Assessment Test; CO = carbon monoxide; COPD = chronic obstructive pulmonary disease; Cu = urge to cough; eCO = exhaled carbon monoxide; ECG = echocardiogram; FeCO = fractional concentration
smokers with respiratory conditions were excluded from the study and subjects in the e-cigarette randomized arm, although encouraged not to use combustible tobacco cigarettes, did often report dual use (Cravo et al., 2016).
Taken together the majority of studies in the literature examining respiratory outcomes of e-cigarette use and their benefit on respiratory function in current smokers come from the same region of Italy, which limits generalizability of their results. In addition, with the exception of the study by Cravo and colleagues (2016), the sample sizes were generally small and subjects were selected retrospectively.
Acute Exposures
Two studies focused on the short-term effects of e-cigarettes on exhaled breath measurements (FeCO and FeNO) and pulmonary function tests. The first study examined the effects of nicotine-free e-cigarettes on lung function and exhaled breath measurements. Ferrari and col-
| Confounders or Factors Adjusted for | Results |
|---|---|
| AORs of respiratory symptoms due to e-cigarette use calculated using logistic regression for all students and by smoking status, adjusting for sociodemographic characteristics, SHS exposure, school clustering effects, and smoking status. | (1) Only 1.1% of all students, 0.1% of never smokers, 5.8% of ever smokers, 2.0% of experimenters, 9.6% of ex-smokers, and 9.6% of current smokers had used e-cigarettes in the past 30 days. (2) Respiratory symptoms were reported by 18.8% of all students, 17.7% of never smokers, 25.8% of ever smokers, 21.7% of experimenters, 27.2% of ex-smokers, and 34.3% of current smokers. E-cigarette use was significantly associated with respiratory symptoms (AOR = 1.28). The corresponding AORs were 2.06 in never smokers, 1.39 in ever smokers, and 1.40 in ex-smokers. Positive but non-significant associations were observed in experimenters and current smokers. |
of carbon monoxide; FeNO = fractional concentration of nitric oxide; FEF25–75% = forced expiratory flow at 25–75 percent of the pulmonary volume; FEV1 = forced expiratory volume; FVC = forced vital capacity; GOLD Stages 1–4 = Global Initiative for Chronic Obstructive Lung Disease Stages of COPD (1 = mild, 2 = moderate; 3 = severe; 4 = very severe); MCC = mucociliary clearance; NF = nicotine free; NO = nitric oxide; PEF = peak expiratory flow; SHS = secondhand smoke; SNOT-22 = Sino-Nasal Outcome Test; SoB = shortness of breath.
leagues (2015) recruited 10 smokers and 10 non-smokers and found no significant decline in lung function after 5 minutes in the subjects using nicotine-free e-cigarettes by contrast to subjects who smoked combustible tobacco cigarettes. Vardavas and colleagues (2012) recruited healthy smokers and found that after 5 minutes of using a nicotine-containing e-cigarette, airway flow resistance increased and FeNO decreased from baseline. Although the mechanisms underlying the lower FeNO in e-cigarette users are unclear, smokers also have been shown to have low FeNO levels compared with non-smokers (Malinovschi et al., 2012; Torén et al., 2006). This suggests that the mechanisms that cause lower FeNO in e-cigarette users are similar to those that cause lower FeNO levels in smokers. Although higher FeNO levels have been demonstrated in people with eosinophilic-induced asthma and are considered a marker of airway inflammation (Malinovschi et al., 2012), studies of subjects with other respiratory conditions including cystic fibrosis (CF) have reported lower FeNO levels, possibly associated with impaired CFTR function (Korten et al., 2018).
Cough and Mucociliary Clearance
Airway exposure to nicotine has also been implemented as a causal factor in inhibiting cough and MCC defenses (Dicpinigaitis et al., 2016a; Laube et al., 2017; Maouche et al., 2013). Specifically, nicotine may modulate perceptual and motor responses to irritant cough stimulants (capsaicin), inhibiting the urge to cough (Davenport et al., 2009). Four studies reported on the effects of e-cigarettes on cough and nasal MCC (Dicpinigaitis, 2017; Dicpinigaitis et al., 2016a,b; Kumral et al., 2016). In a randomized single-blind clinical trial, Kumral and colleagues (2016) used Sino-Nasal Outcome Test (SNOT-22) scores and measured nasal MCC of subjects recruited from a smoking cessation clinic in which they were assigned to either e-cigarettes or non-e-cigarette cessation therapy. At 3 months, subjects assigned to the e-cigarette group had significantly worse sino-nasal symptoms and nasal MCC than subjects assigned to the non–e-cigarette group (Kumral et al., 2016). Two studies from Dicpinigaitis and colleagues (2016a,b) recruited healthy adult nonsmokers. Subjects were challenged with capsaicin at baseline and then at 15 minutes and 24 hours after a short exposure to nicotine-containing or non–nicotine-containing e-cigarettes. They found that urge to cough as measured by capsaicin challenge was depressed at 15 minutes following nicotine-containing e-cigarettes, but not nicotine-free e-cigarettes. At 24 hours after nicotine-containing e-cigarette use, cough reflex sensitivity returned to baseline (Dicpinigaitis et al., 2016b). Dicpinigaitis and colleagues (2016a) also reported that nicotine-containing e-cigarettes caused a decrease in cough reflex sensitivity (C5), analyzed using mixed-effects modeling, at 15 minutes after nicotine-containing e-cigarette use but not after nicotine-free e-cigarette use. Dicpinigaitis (2017) again highlighted the role of nicotine causing centrally mediated suppression of cough in a study in which he reported suppression of cough at 15 minutes after capsaicin challenge in both combustible tobacco cigarette users and users of nicotine-containing e-cigarettes. Following cessation of combustible tobacco cigarette smoking, this centrally mediated cough reflex returned (Dicpinigaitis, 2017).
Respiratory Symptoms in Adolescents
Four studies examined respiratory symptoms in adolescents using or who have used e-cigarettes. Using self-reported questionnaires from participants in the Southern California Children’s Health Study, McConnell and colleagues (2017) found a significant association between increased rates of chronic bronchitis symptoms among past, but not current, e-cigarette users over the previous 12 months. Cho and Paik (2016), using a Web-based questionnaire in a population of high school students from
South Korea, found that students who used e-cigarettes were more likely to have a self-reported clinical diagnosis of asthma and were more likely to have been absent from school due to severe asthma symptoms. Using an anonymous questionnaire with Chinese adolescents in Hong Kong, Wang and colleagues (2016) reported a higher rate of respiratory symptoms in those who used e-cigarettes regardless of previous or current history of smoking and observed that adolescents who used e-cigarettes had more days absent from school because of asthma. Choi and Bernat (2016) examined the prevalence of ever and past 30-day use of e-cigarettes by adolescents, using the 2012 Florida Youth Tobacco Survey. They reported an association between past 30-day e-cigarette use and having an asthma exacerbation in adolescents with asthma. Interestingly, adolescents with asthma in this study were more likely to have used e-cigarettes ever and in the past 30 days compared with adolescents not diagnosed with asthma.
IN VIVO ANIMAL STUDIES AND IN VITRO MECHANISTIC STUDIES
Animal studies in combination with in vitro studies have provided some unique insights into the potential health effects associated with e-cigarette use. Larcombe and colleagues (2017) exposed 4-week-old female BALB/c mice to 8 weeks of either tobacco smoke or propylene glycol (PG) or glycerol e-cigarette solutions with and without nicotine. They found that mice exposed to tobacco smoke had increased pulmonary inflammation and changes in pulmonary function, including methacholine hyperresponsiveness. Although inflammation was not increased in the e-cigarette–exposed mice, pulmonary function abnormalities were found. A limitation to the study is that they excluded male mice from analysis.
Garcia-Arcos and colleagues (2016) examined the effects of aerosolized nicotine-free and nicotine-containing e-cigarette fluid via inhalation in mice and normal human airway epithelial cells. Exposure in mice was for 1 hour per day for 4 months. Human bronchial epithelial (HBE) cells were cultured at an air–liquid interface with exposure to e-cigarette aerosols or nicotine solutions. Exposure to inhaled nicotine-containing e-cigarette fluids triggered effects normally associated with the development of COPD, including increased airway hyperreactivity, distal airspace enlargement, mucin production, and cytokine and protease expression. Exposure to nicotine-free e-cigarettes did not affect these lung parameters, suggesting effects were nicotine dependent in the mouse lung. These effects were also nicotine dependent in human airway cells in culture, further suggesting that inhaled nicotine contributes to airway and lung
disease in addition to its addictive properties. Exposure of HBE cells to nicotine-containing e-cigarette fluids also demonstrated impaired ciliary beat frequency, airway surface liquid volume, cystic fibrosis transmembrane regulator, and ATP-stimulated K+ ion conductance and decreased expression of FOXJ1 and KCNMA1. The major concerns for this study include the matter of aerosolization and the dose delivered to animals by inhalation compared with human use, as well as the dose delivered to cells in culture versus actual exposure conditions in vivo (Garcia-Arcos et al., 2016).
Acute exposure to e-cigarettes compared with combustible tobacco cigarette smoke has been studied by Husari and colleagues (2016). Mice were exposed for 6 hours per day to air, e-cigarette, or combustible tobacco cigarette smoke for 3 days with higher particulate levels for e-cigarettes compared with combustible tobacco cigarette smoke. Human alveolar cells (A549) in culture were also exposed to various concentrations of e-cigarette aerosol and combustible tobacco cigarette smoke extracts. The authors found a significant increase in interleukin-1β (IL-1β) with exposure to e-cigarette, while combustible tobacco cigarette smoke resulted in significant increases in IL-1β, IL-6, TNF-α expression, and oxidative stress. TUNEL staining demonstrated significant cell death with combustible tobacco cigarette smoke, but not with exposure to e-cigarettes. Concerns about this study include the manner of exposure delivery to animals and the relevance of the A549 cell test results to the assessment of human implications for health (Husari et al., 2016).
Lim and Kim (2014) examined e-cigarette cartridge solution and its potential to aggravate allergen-induced airway inflammation and hyperresponsiveness in BALB/c mice. These investigators used diluted e-cigarette cartridge solution, which was delivered to mice by intratracheal instillation two times a week for 10 weeks. The mice had been previously sensitized to ovalbumin (OVA) by intratracheal, intraperitoneal, and aerosol allergen challenge. E-cigarette exposure increased infiltration of inflammatory cells, including eosinophils, into airways; enhanced the asthmatic AI and airway hyperresponsiveness; and stimulated cytokine production of IL-4, IL-5, and IL-13, as well as OVA-specific IgE production. These data suggest e-cigarette solutions can exacerbate allergy-induced asthma symptoms. This study is limited by its use of intratracheal instillation of dilute e-cigarette solution rather than true delivery of e-cigarette exposure by inhalation.
Hwang and colleagues (2016) examined the effects of e-cigarette inhalation on immune function. Mouse inhalation of e-cigarette aerosols was done 1 hour daily for 4 weeks, leading to alterations in inflammatory markers within the airways and elevation of an acute-phase reactant in serum. Exposure of human epithelial cells at the air–liquid interface
to aerosols from an e-cigarette device resulted in dose-dependent cell death; in mice, reduced antimicrobial activity against Staphylococcus aureus in epithelial cells, alveolar macrophages, and neutrophils were observed. The authors concluded that inhalation of e-cigarette aerosols alters immunomodulatory cytokines in the airways of mice and increases markers of inflammation in BAL and serum, thus enhancing the virulence of Staphylococcus aureus. Although observations of e-cigarette impact are similar in mice and cells in culture, the actual mechanisms based on dose are difficult to ascertain (Hwang et al., 2016).
Additional studies, by Sussan and colleagues (2015), also questioned how e-cigarettes may impair antibacterial and antiviral defenses in mice. They found e-cigarette aerosol exposure for 2 weeks produced a significant increase in oxidative stress and moderate macrophage-mediated inflammation, and significantly impaired pulmonary bacterial clearance, compared with air-exposed mice, following an intranasal infection with Streptococcus pneumonia. For mice infected with influenza A virus, e-cigarette exposure was associated with increased lung viral titers and enhanced virus-induced illness and mortality. These findings demonstrate that e-cigarettes may impair the immune response and enhance susceptibility to bacterial and viral infections (Sussan et al., 2015).
Laube and colleagues (2017) exposed 10-week-old male mice to e-cigarette aerosol containing PG alone or PG in combination with nicotine for 20 minutes per day for either 1 or 3 weeks. Following exposure, mice were examined for MCC using technectium-labeled sulfur colloid with clearance of the colloid determined using an X-SPECT gamma camera. The research showed that daily exposure for 3 weeks to PG and nicotine slowed MCC compared with exposure to PG alone. This finding supports the potential biological plausibility of the previous study by Sussan and colleagues (2015), which also used mice and showed impaired bacterial clearance in the lungs of mice. Together, these studies provide evidence that exposure to e-cigarette aerosols during adolescence and early adulthood is not harmless to the lungs and can result in significant impairments in lung function even in the absence of lung inflammation.
Toxicity, oxidative stress, and inflammatory response in mice and human airway epithelial cells were examined by Lerner and colleagues (2015). E-cigarette exposure in C57BL/6J mice increased pro-inflammatory cytokines, while diminishing glutathione levels in the lungs, critical in maintaining a balance of cellular redox in the lungs. E-cigarette aerosol exposure of human airway epithelial cells (H292) in an air–liquid interface system resulted in increased secretion of inflammatory cytokines IL-6 and IL-8. Delivery of unaerosolized e-liquids was also found to be oxidative dependent on flavor additives. They found sweet or fruit flavors to be stronger oxidizers than tobacco flavors. Thus, exposure to
e-cigarette aerosols/e-liquids produces measurable oxidative and inflammatory responses in lung cells and tissues that might lead to unrealized health consequences. Concerns about this study are minimal, but include the methods of delivery to cells in culture and the extrapolation of in vitro results to humans.
E-cigarette exposure has been found to have potential implications on the larynx as well. Salturk and colleagues (2015) found that exposure of Wistar albino rats to e-cigarette aerosol for 1 hour per day for 4 weeks caused hyperplasia and metaplasia of the laryngeal mucosa of rats, but this finding was not statistically significant. This study, although interesting, is inconclusive as to the relevance of how possible health effects to the larynx should be considered in e-cigarette use. Laube and colleagues (2017) examined MCC changes in C57BL/6 mice after 3 weeks of daily exposure and found that young adult male mice exposed to PG alone had significantly higher MCC than mice exposed to nicotine/PG aerosol. This study suggested that chronic exposure to nicotine-containing e-cigarette aerosols can impair airway MCC.
A 90-day inhalation study in rats, followed by a 42-day recovery period, was conducted by Werley and colleagues (2016). Exposure was done with low-, mid-, and high-dose levels of aerosols composed of vehicle (glycerol and PG mixture); vehicle and 2.0 percent nicotine; or vehicle, 2.0 percent nicotine, and flavor mixture. Daily targeted aerosol total particulate matter (TPM) doses of 3.2, 9.6, and 32.0 mg/kg/day were achieved by exposure to 1 mg/L aerosol for 16, 48, and 160 minutes, respectively. Treatment-related effects following 90 days of exposure included changes in body weight, food consumption, and respiratory rate. Also observed were dose-related decreases in thymus and spleen weights, and increased BALF lactate dehydrogenase, total protein, alveolar macrophages, neutrophils, and lung weights. This study in rats provides some insight for establishing a threshold level based on body-weight decreases at the mid-dose level for each formulation, equivalent to a daily TPM exposure dose of approximately 9.6 mg/kg/day. Histopathology changes appear to be isolated to the nasal mucosa. Concerns for this study include how to extrapolate these findings to human exposure and the relevance of the e-cigarette device used and non-respiratory parameters used for comparison. Further, lung weights and body weights are crude measures of effect.
One study reported that neonatal exposure to aerosol from nicotine-containing e-cigarettes was associated with diminished alveolar cell proliferation and impairment in postnatal lung growth (McGrath-Morrow et al., 2015).
SYNTHESIS AND CONCLUSIONS
The human observational studies examining the effect of switching to e-cigarettes (single or dual use) provide support for a finding of beneficial health effects relative to continued use of combustible tobacco products, with most favoring that conclusion. These studies were judged to be of fair quality. A major limitation of them, however, is that they are primarily from a single study group. In addition, the one RCT was negative, finding no improvement in lung function after 12 weeks in subjects who switched to e-cigarettes compared with people who continued to smoke combustible tobacco cigarettes (Cravo et al., 2016). Therefore, the committee concludes that there is limited evidence supporting improvements in lung function in smokers who switch to e-cigarettes.
Studies examining the long-term effects of e-cigarettes on the development of chronic respiratory symptoms are completely lacking due to the newness of the product. It is of importance to know whether chronic e-cigarette use by itself can cause COPD and if substitution of e-cigarettes for combustible tobacco products can prevent or slow the development of COPD in smokers who quit or reduced use of combustible tobacco products. At this time, there is a lack of well-designed epidemiological studies examining either question.
Studies examining the short-term effects of e-cigarettes indicate that nicotine-containing e-cigarettes, but not nicotine-free e-cigarettes, can have short-term adverse effects on lung defense mechanisms, including MCC, urge to cough, and cough sensitivity. These studies are of fair quality. They include subjects with and without a history of smoking and there are few-or-no credible opposing findings. These studies provide moderate evidence supporting short-term adverse effects of nicotine-containing e-cigarettes on lung defense mechanisms.
The committee identified four studies examining the effects of e-cigarette use on adolescent respiratory health—all are cross-sectional and use self-reported questionnaires. They include large groups of adolescents from three countries and reach similar results, thus providing moderate evidence of an association between respiratory symptoms in adolescents and e-cigarette use.
In non-users who are exposed to secondhand smoke and in healthy adolescents and young adult users, common respiratory endpoints can include an increase in asthma symptoms and severity and a higher prevalence of upper and greater lower respiratory tract symptoms and infections (Liu et al., 2016; Shargorodsky, 2016; Shargorodsky et al., 2015; Wilson et al., 2013). Currently, there is a lack of rigorously designed epidemiological studies examining the relationship between chronic e-cigarette use in adolescents and young adults and increased prevalence of respiratory symptoms and respiratory illnesses. There are also no epidemio-
logical studies reporting on the respiratory effects of exposure to exhaled mainstream smoke from an e-cigarette user on a non-user.
The animal studies that have examined the effects of e-cigarettes on respiratory outcomes have used different e-cigarette devices, pumps, solutions, and exposures, limiting the ability to compare results among studies. Confounding factors such as aerosol temperature and particle size have not been taken into account. These methodological differences among studies can result in differences in particle deposition in the lungs and differences in systemic absorption of particles, nicotine, and toxins, resulting in different respiratory outcomes. In addition, not all studies evaluating the effects of nicotine aerosols on lung inflammation, MCC, and lung immune responses have included biomarkers of systemic nicotine absorption, which would help to standardize exposures in animal studies. The utility of studies using whole-body exposures in animal models when examining health effects of e-cigarette aerosols is limited because this type of exposure may overestimate or underestimate an exposure in the human condition. Furthermore, in vitro cell studies would be more informative and representative of the human condition if aerosols rather than liquid e-cigarette solutions are used and if primary, instead of immortalized, cell lines are used. Despite these limitations, the animal and in vitro studies described provide additional evidence of adverse effects of e-cigarette exposure on the respiratory system and do not change the committee’s conclusions regarding the evidence of human health effects.
There is coherence across studies in humans, animals, and in vitro systems regarding the effect of e-cigarette exposure and respiratory symptoms. This adverse effect on respiratory symptoms is likely associated with an increase in cellular inflammation and oxidative stress and decreased cough reflexes and MCC. The observation that past e-cigarette use was associated with an increase in chronic bronchitic symptoms in adolescents and an increase in school absenteeism from asthma symptoms in current e-cigarette users is potentially concerning since a more rapid decline in lung function in later life has been linked to asthma and chronic bronchitis in early life (Bernal et al., 1989; Vestbo and Lange, 2016). In addition, there is limited evidence to indicate that e-cigarette substitution for tobacco product use in established smokers is associated with a decrease in cellular oxidative stress and improved respiratory symptoms and lung function.
Conclusion 11-1. There is no available evidence whether or not e-cigarettes cause respiratory diseases in humans.
Conclusion 11-2. There is limited evidence for improvement in lung function and respiratory symptoms among adult smokers with asthma who switch to e-cigarettes completely or in part (dual use).
Conclusion 11-3. There is limited evidence for reduction of chronic obstructive pulmonary disease (COPD) exacerbations among adult smokers with COPD who switch to e-cigarettes completely or in part (dual use).
Conclusion 11-4. There is moderate evidence for increased cough and wheeze in adolescents who use e-cigarettes and an association with e-cigarette use and an increase in asthma exacerbations.
Conclusion 11-5. There is limited evidence of adverse effects of e-cigarette exposure on the respiratory system from animal and in vitro studies.
VULNERABLE/SUSCEPTIBLE POPULATIONS
Chronic Obstructive Pulmonary Disease
Despite a number of studies, the results are unclear about whether use of e-cigarettes as a substitute for combustible tobacco use in people with COPD may be beneficial, neutral, or harmful. Harm may occur if e-cigarette use prevents the smoker from quitting entirely and instead prolongs the use of combustible tobacco products through dual use. Harm may also occur in an individual with COPD if single use of an e-cigarette as a substitute for combustible tobacco cigarettes causes additional airway inflammation in already damaged lungs. No studies have examined whether e-cigarette use alone can cause lower respiratory tract (LRT) inflammation in healthy adults or increase or decrease existing LRT inflammation in adults with COPD. If the use of e-cigarettes by a smoker with COPD can reduce use of combustible tobacco products and can decrease lung inflammation secondary to the reduction of exposure to toxicants found in combustible tobacco smoke but not e-cigarettes, this could be beneficial to the patients with COPD. In addition, individuals with COPD who failed or who are resistant to conventional NRT or other cessation strategies may be more willing to use e-cigarettes to quit smoking.
Asthma and Other Respiratory Diseases of Childhood
Asthma is one of the most common chronic respiratory diseases in the United States and is prevalent in young children and adolescents. In recent years since the introduction of e-cigarettes in the United States, substantial numbers of adolescents have tried and have used e-cigarettes (Backinger, 2017; HHS, 2016; Jamal et al., 2017; Kann et al., 2016; Miech et al., 2017).
Recent longitudinal studies have shown that children with asthma may have an accelerated decline in lung function as they age (Martinez, 2016). Smoking and other environmental exposures, including air pollution, can increase severity of asthma symptoms and these exposures have been associated with a more rapid decline in lung function in children (Gautier and Charpin, 2017; Schultz et al., 2017; Vanker et al., 2017). An area that remains unclear is if e-cigarette use can cause neutrophilic inflammation, similar to that which can be found in the asthmatic smoker and the smoker with COPD (Andelid et al., 2015; Siew et al., 2017). If so, then e-cigarette use by people with asthma may further exacerbate lower airway inflammation regardless of whether their asthma phenotype is predominately allergic or neutrophilic in origin. As discussed above, adolescents with asthma—a disease characterized by reversible airway obstruction—who use e-cigarettes may be more likely to have an increase in respiratory symptoms and exacerbations compared with adolescent non-users, as indicated by one cross-sectional study (Cho and Paik, 2016).
Cystic Fibrosis
Children and adolescents with other respiratory diseases who use e-cigarettes may also be at increased risk for worsening of respiratory symptoms. CF and primary ciliary dyskinesia (PCD) are respiratory diseases also characterized by lower respiratory tract neutrophilic inflammation. In the United States, the carrier frequency of CF mutations is 1/36, with whites having a carrier rate of 1/27 and African Americans with a carrier rate of 1/79 (Zvereff et al., 2014). It is unclear whether adolescents with CF or PCD would be more likely to try e-cigarettes if they perceive them to be less harmful than combustible tobacco cigarettes.
Nicotine alone has been shown to cause dysregulation of the CFTR chloride channel in the airways in animal studies, causing impaired airway MCC (Maouche et al., 2013). Another study found an association between secondhand smoke exposure in children with CF and lower FEV1 and weight percentile (Ong et al., 2017). Nicotine exposure from e-cigarette use could potentially cause a higher rate of respiratory symptoms in CF carriers if nicotine causes dysregulation of CFTR in the airways.
Preterm Infants
Exposure to nicotine in utero may have long-lasting negative effects on lung function in vulnerable populations such as preterm infants. Recently, maternal smoking during pregnancy has been associated with the development of bronchopulmonary dysplasia and later respiratory morbidities in preterm infants (Morrow et al., 2017). No studies have
examined the respiratory health effects of e-cigarettes on the preterm infants of mothers who used e-cigarettes during pregnancy.
REFERENCES
Andelid, K., A. Andersson, S. Yoshihara, C. Ahren, P. Jirholt, A. Ekberg-Jansson, and A. Linden. 2015. Systemic signs of neutrophil mobilization during clinically stable periods and during exacerbations in smokers with obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease 10:1253–1263.
Backinger, C. L. 2017. Youth use of electronic cigarettes. http://c.ymcdn.com/sites/www.srnt.org/resource/resmgr/conferences/2017_annual_meeting/FDA_PreCon_Slides/Backinger_youth_e-cig_SRNT20.pdf (accessed September 18, 2017).
Bernal, J. E., P. Sarmiento, I. Briceno, and S. S. Papiha. 1989. Polymorphism of serum proteins (C3, BF, HP and TF) of six populations in Colombia. Human Hereditary 39(2):94–98.
Campagna, D., F. Cibella, P. Caponnetto, M. D. Amaradio, M. Caruso, J. B. Morjaria, M. Malerba, and R. Polosa. 2016. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. European Journal of Clinical Investigation 46(8):698–706.
Chatwin, M., E. Ross, N. Hart, A. H. Nickol, M. I. Polkey, and A. K. Simonds. 2003. Cough augmentation with mechanical insufflation/exsufflation in patients with neuromuscular weakness. European Respiratory Journal 21(3):502–508.
Cho, J. H., and S. Y. Paik. 2016. Association between electronic cigarette use and asthma among high school students in South Korea. PLoS ONE 11(3):e0151022. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0151022 (accessed January 31, 2018).
Choi, K., and D. Bernat. 2016. E-cigarette use among Florida youth with and without asthma. American Journal of Preventive Medicine 51(4):446–453.
Cibella, F., D. Campagna, P. Caponnetto, M. D. Amaradio, M. Caruso, C. Russo, D. W. Cockcroft, and R. Polosa. 2016. Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clinical Science 130(21):1929–1937.
Cravo, A. S., J. Bush, G. Sharma, R. Savioz, C. Martin, S. Craige, and T. Walele. 2016. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regulatory Toxicology and Pharmacology 81:S01-S14.
Davenport, P. W., A. Vovk, R. K. Duke, D. C. Bolser, and E. Robertson. 2009. The urge-to-cough and cough motor response modulation by the central effects of nicotine. Pulmonary Pharmacology and Therapeutics 22(2):82–89.
den Dekker, H. T., A. Voort, J. C. de Jongste, I. K. Reiss, A. Hofman, V. W. V. Jaddoe, and L. Duijts. 2015. Tobacco smoke exposure, airway resistance, and asthma in school-age children: The Generation R study. Chest 148(3):607–617.
Diao, W., N. Shen, Y. Du, K. Qian, and B. He. 2017. Characterization of throat microbial flora in smokers with or without COPD. International Journal of Chronic Obstructive Pulmonary Disease 12:1933–1946.
Dicpinigaitis, P. V. 2017. Effect of tobacco and electronic cigarette use on cough reflex sensitivity. Pulmonary Pharmacology and Therapeutics 47:45–48.
Dicpinigaitis, P. V., B. Sitkauskiene, K. Stravinskaite, D. W. Appel, A. Negassa, and R. Sakalauskas. 2006. Effect of smoking cessation on cough reflex sensitivity. European Respiratory Journal 28(4):786–790.
Dicpinigaitis, P. V., A. L. Chang, A. J. Dicpinigaitis, and A. Negassa. 2016a. Effect of e-cigarette use on cough reflex sensitivity. Chest 149(1):161–165.
Dicpinigaitis, P. V., A. L. Chang, A. J. Dicpinigaitis, and A. Negassa. 2016b. Effect of electronic cigarette use on the urge-to-cough sensation. Nicotine & Tobacco Research 18(8):1763–1765.
D’Ruiz, C. D., G. O’Connell, D. W. Graff, and X. S. Yan. 2017. Measurement of cardiovascular and pulmonary function endpoints and other physiological effects following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Regulatory Toxicology and Pharmacology 87:36–53.
Duijts, L., V. W. V. Jaddoe, R. J. P. van der Valk, J. A. Henderson, A. Hofman, H. Raat, E. A. P. Steegers, H. A. Moll, and J. C. de Jongste. 2012. Fetal exposure to maternal and paternal smoking and the risks of wheezing in preschool children: The Generation R study. Chest 141(4):876–885.
Ferrari, M., A. Zanasi, E. Nardi, A. M. M. Labate, P. Ceriana, A. Balestrino, L. Pisani, N. Corcione, and S. Nava. 2015. Short-term effects of a nicotine-free e-cigarette compared to a traditional cigarette in smokers and non-smokers. BMC Pulmonary Medicine 15(1):120.
Garcia-Arcos, I., P. Geraghty, N. Baumlin, M. Campos, A. J. Dabo, B. Jundi, N. Cummins, E. Eden, A. Grosche, M. Salathe, and R. Foronjy. 2016. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 71(12):1119–1129.
Gautier, C., and D. Charpin. 2017. Environmental triggers and avoidance in the management of asthma. Journal of Asthma and Allergy 10:47–56.
Geiss, O., I. Bianchi, F. Barahona, and J. Barrero-Moreno. 2015. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. International Journal of Hygiene and Environmental Health 218(1):169–180.
Hayatbakhsh, M. R., S. Sadasivam, A. A. Mamun, J. M. Najman, G. M. Williams, and M. J. O’Callaghan. 2009. Maternal smoking during and after pregnancy and lung function in early adulthood: A prospective study. Thorax 64(9):810–814.
He, Q. Q., T. W. Wong, L. Du, Z. Q. Jiang, T. S. Yu, H. Qiu, Y. Gao, A. H. Wong, W. J. Liu, and J. G. Wu. 2011. Environmental tobacco smoke exposure and Chinese schoolchildren’s respiratory health: A prospective cohort study. American Journal of Preventive Medicine 41(5):487–493.
HHS (U.S. Department of Health and Human Services). 2016. E-cigarette use among youth and young adults: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
Husari, A., A. Shihadeh, S. Talih, Y. Hashem, M. El Sabban, and G. Zaatari. 2016. Acute exposure to electronic and combustible cigarette aerosols: Effects in an animal model and in human alveolar cells. Nicotine & Tobacco Research 18(5):613–619.
Hwang, J. H., M. Lyes, K. Sladewski, S. Enany, E. McEachern, D. P. Mathew, S. Das, A. Moshensky, S. Bapat, D. T. Pride, W. M. Ongkeko, and L. E. C. Alexander. 2016. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. Journal of Molecular Medicine 94(6):667–679.
Jamal, A., A. Gentzke, S. Hu, K. A. Cullen, B. J. Apelberg, D. M. Homa, and B. A. King. 2017. Tobacco use among middle and high school students—United States, 2011–2016. Morbidity and Mortality Weekly Report 66(23):597–603.
Jayes, L., P. L. Haslam, C. G. Gratziou, P. Powell, J. Britton, C. Vardavas, C. Jimenez-Ruiz, J. Leonardi-Bee, and Tobacco Control Committee of the European Respiratory Society. 2016. Smokehaz: Systematic reviews and meta-analyses of the effects of smoking on respiratory health. Chest 150(1):164–179.
Kalliola, S., A. S. Pelkonen, L. P. Malmberg, S. Sarna, M. Hamalainen, I. Mononen, and M. J. Makela. 2013. Maternal smoking affects lung function and airway inflammation in young children with multiple-trigger wheeze. Journal of Allergy and Clinical Immunology 131(3):730–735.
Kann, L., T. McManus, W. A. Harris, S. L. Shanklin, K. H. Flint, J. Hawkins, B. Queen, R. Lowry, E. O. Olsen, D. Chyen, L. Whittle, J. Thornton, C. Lim, Y. Yamakawa, N. Brener, and S. Zaza. 2016. Youth risk behavior surveillance—United States, 2015. Morbidity and Mortality Weekly Report 65(6):1–174.
Korten, I., M. Liechti, F. Singer, G. Hafen, I. Rochat, P. Anagnostopoulou, D. Müller-Suter, J. Usemann, A. Moeller, U. Frey, P. Latzin, and C. Casaulta. 2018. Lower exhaled nitric oxide in infants with cystic fibrosis compared to healthy controls. Journal of Cystic Fibrosis 17(1):105–108.
Kumral, T. L., Z. Salturk, G. Yildirim, Y. Uyar, G. Berkiten, Y. Atar, and M. Inan. 2016. How does electronic cigarette smoking affect sinonasal symptoms and nasal mucociliary clearance? B-ENT 12(1):17–21.
Larcombe, A. N., M. A. Janka, B. J. Mullins, L. J. Berry, A. Bredin, and P. J. Franklin. 2017. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. American Journal of Physiology—Lung Cellular and Molecular Physiology 313(1):L67–L79.
Laube, B. L., N. Afshar-Mohajer, K. Koehler, G. Chen, P. Lazarus, J. M. Collaco, and S. A. McGrath-Morrow. 2017. Acute and chronic in vivo effects of exposure to nicotine and propylene glycol from an e-cigarette on mucociliary clearance in a murine model. Inhalation Toxicology 29(5):197–205.
Lee, A. L., B. M. Button, and E. L. Tannenbaum. 2017. Airway-clearance techniques in children and adolescents with chronic suppurative lung disease and bronchiectasis. Frontiers of Pediatrics 5:2. https://www.frontiersin.org/articles/10.3389/fped.2017.00002/full (accessed January 31, 2018).
Lerner, C. A., I. K. Sundar, H. Yao, J. Gerloff, D. J. Ossip, S. McIntosh, R. Robinson, and I. Rahman. 2015. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 10(2):e0116732. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0116732 (accessed January 31, 2018).
Levanen, B., P. Glader, B. Dahlen, B. Billing, I. Qvarfordt, L. Palmberg, K. Larsson, and A. Linden. 2016. Impact of tobacco smoking on cytokine signaling via interleukin-17A in the peripheral airways. International Journal of Chronic Obstructive Pulmonary Disease 11:2109–2116.
Li, N., S. Georas, N. Alexis, P. Fritz, T. Xia, M. A. Williams, E. Horner, and A. Nel. 2016. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. Journal of Allergy and Clinical Immunology 138(2):386–396.
Lim, H. B., and S. H. Kim. 2014. Inhalation of e-cigarette cartridge solution aggravates allergen-induced airway inflammation and hyper-responsiveness in mice. Toxicological Research 30(1):13–18.
Liu, A. H., D. C. Babineau, R. Z. Krouse, E. M. Zoratti, J. A. Pongracic, G. T. O’Connor, R. A. Wood, G. K. Khurana Hershey, C. M. Kercsmar, R. S. Gruchalla, M. Kattan, S. J. Teach, M. Makhija, D. Pillai, C. I. Lamm, J. E. Gern, S. M. Sigelman, P. J. Gergen, A. Togias, C. M. Visness, and W. W. Busse. 2016. Pathways through which asthma risk factors contribute to asthma severity in inner-city children. Journal of Allergy and Clinical Immunology 138(4):1042–1050.
Malinovschi, A., V. Backer, H. Harving, and C. Porsbjerg. 2012. The value of exhaled nitric oxide to identify asthma in smoking patients with asthma-like symptoms. Respiratory Medicine 106(6):794–801.
Maouche, K., K. Medjber, J. M. Zahm, F. Delavoie, C. Terryn, C. Coraux, S. Pons, I. Cloez-Tayarani, U. Maskos, P. Birembaut, and J. M. Tournier. 2013. Contribution of α7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure. Proceedings of the National Academy of Sciences of the United States of America 110(10):4099–4104.
Martinez, F. D. 2016. Early-life origins of chronic obstructive pulmonary disease. New England Journal of Medicine 375(9):871–878.
McConnell, R., J. L. Barrington-Trimis, K. Wang, R. Urman, H. Hong, J. Unger, J. Samet, A. Leventhal, and K. Berhane. 2017. Electronic cigarette use and respiratory symptoms in adolescents. American Journal of Respiratory and Critical Care Medicine 195(8):1043–1049.
McGrath-Morrow, S. A., M. Hayashi, A. Aherrera, A. Lopez, A. Malinina, J. M. Collaco, E. Neptune, J. D. Klein, J. P. Winickoff, P. Breysse, P. Lazarus, and G. Chen. 2015. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS ONE 10(2):e0118344. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0118344 (accessed January 31, 2018).
Merianos, A. L., C. A. Dixon, and E. M. Mahabee-Gittens. 2016. Secondhand smoke exposure, illness severity, and resource utilization in pediatric emergency department patients with respiratory illnesses. Journal of Asthma 54(8):798–806.
Messerli, M., T. Ottilinger, R. Warschkow, S. Leschka, H. Alkadhi, S. Wildermuth, and R. W. Bauer. 2017. Emphysema quantification and lung volumetry in chest x-ray equivalent ultralow dose CT—intra-individual comparison with standard dose CT. European Journal of Radiology 91:1–9.
Miech, R., M. E. Patrick, P. M. O’Malley, and L. D. Johnston. 2017. What are kids vaping? Results from a national survey of U.S. adolescents. Tobacco Control 26(4):386–391.
Morrow, L. A., B. D. Wagner, D. A. Ingram, B. B. Poindexter, K. Schibler, C. M. Cotten, J. Dagle, M. K. Sontag, P. M. Mourani, and S. H. Abman. 2017. Antenatal determinants of bronchopulmonary dysplasia and late respiratory disease in preterm infants. American Journal of Respiratory and Critical Care Medicine 196(3):364–374.
Ong, T., M. Schechter, J. Yang, L. Peng, J. Emerson, R. L. Gibson, W. Morgan, M. Rosenfeld, and E. S. Group. 2017. Socioeconomic status, smoke exposure, and health outcomes in young children with cystic fibrosis. Pediatrics 139(2). http://pediatrics.aappublications.org/content/139/2/e20162730.long (accessed January 31, 2018).
Polosa, R., J. Morjaria, P. Caponnetto, M. Caruso, S. Strano, E. Battaglia, and C. Russo. 2014a. Effect of smoking abstinence and reduction in asthmatic smokers switching to electronic cigarettes: Evidence for harm reversal. International Journal of Environmental Research and Public Health 11(5):4965–4977.
Polosa, R., J. B. Morjaria, P. Caponnetto, D. Campagna, C. Russo, A. Alamo, M. D. Amaradio, and A. Fisichella. 2014b. Effectiveness and tolerability of electronic cigarette in real-life: A 24-month prospective observational study. Internal and Emergency Medicine 9(5):537–546.
Polosa, R., J. B. Morjaria, P. Caponnetto, M. Caruso, D. Campagna, M. D. Amaradio, G. Ciampi, C. Russo, and A. Fisichella. 2016a. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discovery Medicine 21(114):99–108.
Polosa, R., J. B. Morjaria, P. Caponnetto, U. Prosperini, C. Russo, A. Pennisi, and C. M. Bruno. 2016b. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respiratory Research 17(1):116.
Puhan, M. A., I. Soesilo, G. H. Guyatt, and H. J. Schunemann. 2006. Combining scores from different patient reported outcome measures in meta-analyses: When is it justified? Health and Quality of Life Outcomes 4:94. https://hqlo.biomedcentral.com/articles/10.1186/1477-7525-4-94 (accessed January 31, 2018).
Rabe, K. F., and H. Watz. 2017. Chronic obstructive pulmonary disease. Lancet 389(10082): 1931–1940.
Saadeh, C., B. Cross, M. Gaylor, and M. Griffith. 2015. Advantage of impulse oscillometry over spirometry to diagnose chronic obstructive pulmonary disease and monitor pulmonary responses to bronchodilators: An observational study. SAGE Open Medicine 3:2050312115578957. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4679284 (accessed January 31, 2018).
Saint-Criq, V., and M. A. Gray. 2017. Role of CFTR in epithelial physiology. Cellular and Molecular Life Sciences 74(1):93–115.
Salturk, Z., C. Cakir, G. Sunnetci, Y. Atar, T. L. Kumral, G. Yildirim, G. Berkiten, and Y. Uyar. 2015. Effects of electronic nicotine delivery system on larynx: Experimental study. Journal of Voice 29(5):560–563.
Schultz, E. S., A. A. Litonjua, and E. Melen. 2017. Effects of long-term exposure to traffic-related air pollution on lung function in children. Current Allergy and Asthma Reports 17(6):41.
Shargorodsky, J. 2016. Secondhand smoke and rhinitis. Current Opinion in Otolaryngology and Head and Neck Surgery 24(3):241–244.
Shargorodsky, J., E. Garcia-Esquinas, I. Galan, A. Navas-Acien, and S. Y. Lin. 2015. Allergic sensitization, rhinitis and tobacco smoke exposure in U.S. adults. PLoS ONE 10(7):e0131957. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0131957 (accessed January 31, 2018).
Shields, P. G., M. Berman, T. M. Brasky, J. L. Freudenheim, E. Mathe, J. P. McElroy, M. A. Song, and M. D. Wewers. 2017. A review of pulmonary toxicity of electronic cigarettes in the context of smoking: A focus on inflammation. Cancer Epidemiology, Biomarkers & Prevention 26(8):1175–1191.
Siew, L. Q. C., S. Y. Wu, S. Ying, and C. J. Corrigan. 2017. Cigarette smoking increases bronchial mucosal IL-17A expression in asthmatics, which acts in concert with environmental aeroallergens to engender neutrophilic inflammation. Clinical & Experimental Allergy 47(6):740–750.
Sitkauskiene, B., and P. V. Dicpinigaitis. 2010. Effect of smoking on cough reflex sensitivity in humans. Lung 188(Supplement):S29–S32.
Sussan, T. E., S. Gajghate, R. K. Thimmulappa, J. Ma, J. H. Kim, K. Sudini, N. Consolini, S. A. Cormier, S. Lomnicki, F. Hasan, A. Pekosz, and S. Biswal. 2015. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS ONE 10(2):e0116861. http://dx.plos.org/10.1371/journal.pone.0116861 (accessed January 31, 2018).
Tarrant, B. J., C. Le Maitre, L. Romero, R. Steward, B. M. Button, B. R. Thompson, and A. E. Holland. 2017. Mucoactive agents for chronic, non-cystic fibrosis lung disease: A systematic review and meta-analysis. Respirology 22(6):1084–1092.
Torén, K., M. Palmqvist, O. Löwhagen, B. Balder, and A. Tunsäter. 2006. Self-reported asthma was biased in relation to disease severity while reported year of asthma onset was accurate. Journal of Clinical Epidemiology 59(1):90–93.
Vanker, A., R. P. Gie, and H. J. Zar. 2017. The association between environmental tobacco smoke exposure and childhood respiratory disease: A review. Expert Review of Respiratory Medicine 11(8):661–673.
Vardavas, C. I., N. Anagnostopoulos, M. Kougias, V. Evangelopoulou, G. N. Connolly, and P. K. Behrakis. 2012. Short-term pulmonary effects of using an electronic cigarette: Impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141(6):1400–1406.
Vestbo, J., and P. Lange. 2016. Natural history of COPD: Focusing on change in FEV1. Respirology 21(1):34–43.
Wang, M. P., S. Y. Ho, L. T. Leung, and T. H. Lam. 2016. Electronic cigarette use and respiratory symptoms in Chinese adolescents in Hong Kong. JAMA Pediatrics 170(1):89–91.
Washko, G. R., G. Parraga, and H. O. Coxson. 2012. Quantitative pulmonary imaging using computed tomography and magnetic resonance imaging. Respirology 17(3):432–444.
Werley, M. S., D. J. Kirkpatrick, M. J. Oldham, A. M. Jerome, T. B. Langston, P. D. Lilly, D. C. Smith, and W. J. McKinney. 2016. Toxicological assessment of a prototype e-cigarette device and three flavor formulations: A 90-day inhalation study in rats. Inhalation Toxicology 28(1):22–38.
Wilson, K. M., J. C. Pier, S. C. Wesgate, J. M. Cohen, and A. K. Blumkin. 2013. Secondhand tobacco smoke exposure and severity of influenza in hospitalized children. JAMA Pediatrics 162(1):16–21.
Yanagita, M., R. Kobayashi, Y. Kojima, K. Mori, and S. Murakami. 2012. Nicotine modulates the immunological function of dendritic cells through peroxisome proliferator-activated receptor-gamma upregulation. Cellular Immunology 274(1–2):26–33.
Zvereff, V. V., H. Faruki, M. Edwards, and K. J. Friedman. 2014. Cystic fibrosis carrier screening in a North American population. Genetics in Medicine 16(7):539–546.


















































