16
Combustible Tobacco Cigarette Smoking Among Youth and Young Adults
The context surrounding e-cigarette use is markedly different in middle-aged and older adults as compared to adolescents and young adults. The proportion of U.S. adults age 25 or older who reported e-cigarette use in the past 30 days is 5.0 percent, much lower than observed among youth (Kasza et al., 2017). Of these adults, nearly all started vaping after having been a regular smoker (CDC, 2016) and most report that quitting smoking and health improvement are major reasons for starting e-cigarette use (Patel et al., 2016; Zhuang et al., 2016). By contrast, use among never smokers is common in adolescents and young adults age 24 or younger. Indeed, past 30-day use of e-cigarettes (use on one or more days in the past 30 days) by U.S. high school students rose from 1.5 percent in 2011 to a high of 16.0 percent in 2015, before declining to 11.3 percent in 2016 (HHS, 2016; Jamal et al., 2016). Among U.S. adults age 18 and older, past 30-day e-cigarette use (any use, even one or two times) is higher among those age 18–24 (12.5 percent) compared to those age 25 and older (5.8 percent) (Kasza et al., 2017). Among U.S. high school student past 30-day e-cigarette users in 2014, 55 percent used an e-cigarette at least 3 days and more than one-quarter (27.4 percent) used an e-cigarette on 10 or more days (Neff et al., 2015). About one-third to one-half of youth and young adult e-cigarette users report no history of regular combustible tobacco product use (CDC, 2016; HHS, 2016). Young populations are more likely to cite enjoyment of flavors and social factors as reasons for vaping, in contrast with adults who typically use e-cigarettes with the intention of reducing or quitting smoking (Ambrose et al., 2015; Bold et al., 2016).
Given the sizable population of adolescents and young adults who initiate e-cigarette use without previously having been a regular user of combustible tobacco cigarettes, it is important to understand the health effects of e-cigarette use, per se, in this population. Apart from any inherent direct effects from exposure to e-cigarette aerosols, the use of e-cigarettes among adolescent and young adult never smokers may affect health by changing combustible tobacco use behavior.
CONCEPTUAL FRAMEWORK: PATTERNS OF USE AMONG YOUTH AND YOUNG ADULTS
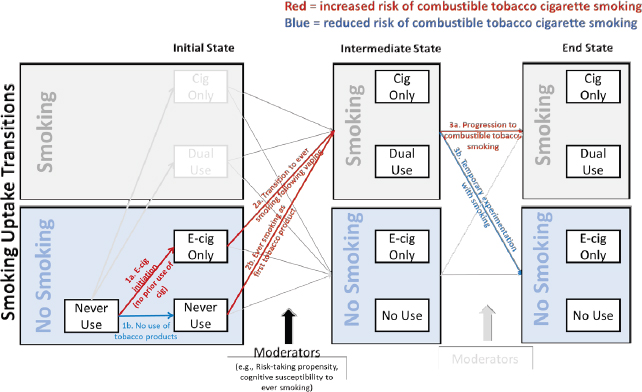
Among the population of teens and young adults with no history of smoking, several possible transitions in combustible tobacco use behavior may occur as a result of e-cigarette use. To illustrate this, the overarching tobacco product transitions conceptual model posed in Figure 16-1 has been adapted to address the possible effects of e-cigarette use on combustible tobacco cigarette use among adolescents and young adults with no history of cigarette smoking. The committee recognizes that there are four distinct tobacco product use states (no use, e-cigarette only, combustible tobacco cigarette only, dual use) that each may have unique health consequences. However, the current section amalgamates tobacco product use outcomes into two possible states, as depicted in the adapted model in Figure 16-1: (1) smoking (including combustible tobacco cigarette use only and dual use with e-cigarettes), and (2) no smoking (including e-cigarette use only and no use of either tobacco product). Outcomes were collapsed for this section of the chapter to simplify the conceptual model and because there is a paucity of longitudinal data on adolescents and young adults that distinguishes subtypes of smoking outcomes based on concomitant e-cigarette use.
Concentrating primarily on the population of adolescents and young adults with no substantive history of combustible tobacco cigarette smoking, this section focuses on whether those who become e-cigarette users (Path 1a, Figure 16-1) versus those who do not (Path 1b, Figure 16-1) exhibit different patterns of combustible tobacco cigarette use behavior (Paths 2a, 2b, 3a, and 3b). Two types of combustible tobacco product use outcomes are of interest, which correspond to two key research questions: (1) ever use (i.e., starting any level of cigarette smoking—even merely a few puffs versus sustained abstinence), and (2) progression, defined as increases in smoking frequency (i.e., number of days used in past 30), intensity (i.e., cigarettes smoked per day on smoking day), and duration (i.e., length of time in which smoking behavior continues versus ceases following initiation). Collectively, these two transitions are necessary for the overall initiation process, which involves transition from never
combustible cigarette smoker to established or regular cigarette use (i.e., not merely temporary experimentation that does not progress to regular smoking).
Transition 1: Among Adolescents and Young Adults with No History of Combustible Tobacco Use, Does E-Cigarette Use Affect Risk of Combustible Tobacco Cigarette Ever Use?
The extent to which factors implicated in ever smoking impact public health is qualified by the fact that a subset of those who become ever users progress to smoke at greater levels of smoking frequency, intensity, or duration (Path 3a, Figure 16-1) and hence become a regular smoker, whereas others may smoke infrequently, smoke very little on each smoking day, and discontinue use shortly following initiation (Path 3b, Figure 16-1) (HHS, 2014). Studying the effect of e-cigarette use on the likelihood of ever smoking is important because ever use is a necessary precursor to progression to regular use. Hence, if e-cigarette use impacts ever smoking, it may have downstream effects on the prevalence of health-damaging courses of smoking. Studying ever use is a particularly useful outcome measure at this time because e-cigarettes have been widely available in the United States only for a short period, leaving the majority of studies lacking sufficient duration of follow-up to study the naturalistic cigarette smoking progression sequence, which can involve a
lengthy period between ever use and reaching daily smoking (Chassin et al., 2009; HHS, 2012).
There are three potential ways in which e-cigarette use may impact ever use of combustible tobacco use in young populations. These ways are discussed in the paragraphs below.
Preventive Effect
E-cigarette use could have a preventive effect that deters ever combustible tobacco cigarette use (i.e., probability of transition is lower for Path 2a than 2b, holding all external confounds constant). Some have proposed that for “high-risk” youth with a disposition toward risk-taking behavior (e.g., impulsive personality, novelty-seeking tendency) who are susceptible to smoking initiation, e-cigarettes may provide a diversion that prevents them from experimenting with harmful combustible tobacco products (Etter, 2017; Kozlowski and Warner, 2017). Known sometimes as the “diversion hypothesis,” this concept proposes that because some youth possess an elevated drive to engage in exploratory and risk-taking behavior, the availability of e-cigarettes allows such young people to satisfy their curiosity and drive for novelty seeking without needing to resort to combustible tobacco products to satisfy the desire for exploration (Etter, 2017; Kozlowski and Warner, 2017). The diversion hypothesis also proposes that if e-cigarettes were otherwise unavailable, youth prone to risk-taking behavior would be more likely to use combustible tobacco products due to the absence of a suitable non-combustible tobacco substitute (Etter, 2017; Kozlowski and Warner, 2017). In such a case, regulatory policies that reduce e-cigarette use in the population of adolescent and young adult never smokers could indirectly increase the prevalence of smoking and perpetuate the epidemic of tobacco-related illness.
Increased Risk
E-cigarette use could also increase risk of ever smoking (i.e., probability of transition is higher for Path 2a than 2b, holding all external confounds constant). Sometimes referred to as the “catalyst hypothesis,”1 this concept proposes a two-step process (Schneider and Diehl, 2016). First,
___________________
1 Some have used the term “gateway” to describe the potential risk-enhancing effect of e-cigarette use on combustible tobacco use initiation (Etter, 2017). Because the term “gateway” has historically been used in colloquial, non-scientific settings and lacks a clear definition, it is not used in this report (Schneider and Diehl, 2016). Instead, this report refers to this potential effect of e-cigarette use on increased smoking initiation as the “catalyst” hypothesis or model as in Schneider and Diehl (2016), which has a clear definition and lends itself to scientifically oriented investigation.
e-cigarettes are suspected to attract “low-risk” teens who would otherwise be deterred from combustible tobacco cigarettes because e-cigarettes possess unique attractive qualities that cigarettes lack, causing e-cigarettes to appeal to a wider segment of the youth population. Relative to combustible tobacco cigarettes, e-cigarettes are perceived to be healthier, be more socially acceptable, be easier to conceal from authority figures, have appealing flavors, have appealing technological features, lack detectable odors, and be easier to access due to inconsistent restriction of sales to youth (Kong et al., 2015; Schneider and Diehl, 2016). Such teens may have a low or moderate risk-taking propensity and may report believing they are not susceptible to trying cigarettes in the future. Second, the exposure to e-cigarettes in this group is suspected to increase proclivity to try combustible tobacco cigarettes (Schneider and Diehl, 2016) for several reasons: (1) the pleasurable sensations caused by nicotine’s pharmacological effects or the sensations to the airways and taste may cause adolescent or young adult never smokers to develop more favorable expectations that other tobacco products will also be enjoyable; (2) after successfully engaging in one risky act (i.e., vaping), courage to engage in other risky acts (i.e., smoking) may build; (3) the environments surrounding the procurement of e-cigarettes may increase opportunity to obtain and use combustible tobacco products (e.g., peers who use e-cigarettes may be more likely to smoke and offer cigarettes to their friends; certain e-cigarette retailers may also advertise and sell cigarettes). If e-cigarette use acts as a catalyst for combustible tobacco ever use, regulatory policies that reduce use of e-cigarettes in never smoking adolescents and young adults could ultimately prevent current generations of youth and young adults from developing tobacco-related illness later in life.
No Effect
E-cigarette use could have no effect on combustible tobacco cigarette ever use in adolescents and young adults. That is, if one were to hold constant all possible external confounding influences on the association between e-cigarette use and smoking initiation, there may be an equal probability of transition for Paths 2a and 2b (see Figure 16-1). No effect would be represented by the lack of a statistical association between e-cigarette use and smoking. Alternatively, there could be a statistical association, but the association is entirely due to confounds. For example, often referred to as the “common liability hypothesis” (Etter, 2017; Kozlowski and Warner, 2017), some have proposed that any positive association between e-cigarette use and smoking initiation is due to shared risk factors, such as impulsive and novelty-seeking personality traits or exposure to pro-smoking peers and family members. Under any scenario
in which e-cigarette use is deemed not to be associated with smoking combustible tobacco cigarettes (or other substance use and other risky behaviors), the health effects of e-cigarette use on the health of current generations of youth and young adults would be isolated to the direct health effects of exposure to e-cigarette aerosols.
Transition 2: Among Adolescents and Young Adults, Does E-Cigarette Use Affect Risk of Progression to Combustible Tobacco Use Patterns of Greater Frequency, Intensity, or Duration?
Existing evidence on typical smoking trajectories indicate that an appreciable portion of ever users never become frequent smokers, will discontinue, and are temporarily experimenting with smoking (i.e., Path 3b, Figure 16-1) (Dutra et al., 2017; HHS, 2012; Sargent et al., 2017). Other ever users progress to become frequent, heavy, and chronic smokers (i.e., Path 3a, Figure 16-1)—a trajectory that is increasingly more likely the longer someone continues to smoke (Dutra et al., 2017; HHS, 2012; Sargent et al., 2017). A key question is whether adolescents and young adults who become ever smokers after e-cigarette use versus those who become ever smokers with no prior history of e-cigarette use differ in risk of progression in frequency (i.e., days used in the past 30), intensity (i.e., cigarettes per day on smoking day, sometimes termed “heaviness”), or duration of smoking. In Figure 16-1, this question is depicted by whether the probability of progression (versus temporary experimentation) in Path 3a differs as a function if one started smoking through vaping (2a) or not (2b). If e-cigarette use affects post-initiation progression of smoking, the impact of e-cigarette use among never smoking adolescents and young adults on the tobacco-related public health burden will be dependent on e-cigarette effects on both ever use and progression to more frequent, heavy, and chronic smoking.
There are three ways in which e-cigarette use may subsequently impact post-initiation adolescent and young adult smoking trajectories.
Preventive Effect
E-cigarette use may reduce risk of smoking progression, such that vapers who become ever smokers may be more likely to be temporarily experimenting with cigarettes, whereas non-vapers who become smokers may be more apt to progress to become more frequent, heavy, and chronic smokers. The “common liability” hypothesis proposes that youth who use e-cigarettes and then transition to combustible tobacco cigarettes are overrepresented by teens with a preference for exploring novel experiences (Etter, 2017; Kozlowski and Warner, 2017). Such youth may be prone
to patterns of brief experimentation, wishing to try new activities for the sake of novelty, but they may become bored quickly with tobacco products and move on to other new activities. The common liability hypothesis would predict that young vapers (versus non-vapers) who try cigarettes may be less likely to progress to regular smoking.
Increased Risk
E-cigarette use may increase the risk and speed of progression to more frequent, heavy, and chronic smoking after first trying combustible tobacco cigarettes. For never smokers, the first experience with cigarette smoking can be aversive due to the harsh sensations and bitter taste of cigarette smoke, awkwardness of the smoking self-administration sequence (e.g., puffing, hand-to-mouth movements), and nicotine’s aversive pharmacological effects (e.g., nausea, dizziness, airway irritation, bitterness). E-cigarette users may habituate to the aversive pharmacological effects of nicotine and become sensitized to nicotine’s addictive effects (e.g., pleasure, anxiolysis) due to nicotine-induced neurobiological changes (Lydon et al., 2014), which would enhance the first few combustible tobacco cigarette smoking experiences. Furthermore, the smoking self-administration ritual may feel more familiar and less awkward to those with previous experience vaping (Wills et al., 2016c). Hence, e-cigarette use could increase the likelihood and speed of progression of the frequency, intensity, and duration of combustible tobacco use.
No Effect
E-cigarette use may have no impact on smoking progression, such that vapers and non-vapers who start smoking show similar likelihood and speed of progression in the frequency, intensity, and duration of smoking. In such a case, youth who start smoking following e-cigarettes may have a risk of tobacco-related illness similar to that of youth who start smoking without a history of e-cigarettes.
EVIDENCE REVIEW: LEVELS OF EVIDENCE AVAILABLE
Because randomized controlled trials to test this research question are not possible and preclinical data testing this question are not entirely relevant, the committee gave extensive consideration to what types of observational data were capable of supporting causal inferences. To this end, the committee applied a framework for determining causality that takes into account multiple streams of evidence to make inferences regarding the causal effect of e-cigarette use on smoking among adolescents and
young adults, as in other published work on this topic (Etter, 2017). The framework considered five key criteria in interpreting the principal epidemiological observational evidence as described in greater detail below: (1) strength of the association; (2) consistency across studies, investigators, individuals, research methods, and replications; (3) temporal precedence of e-cigarette use relative to combustible tobacco cigarette smoking; (4) comprehensiveness by which potential confounding effects were addressed and ruled out by covariate adjustment or other methods; and (5) dose responsivity in the association whereby incremental differences in e-cigarette use are associated with proportional differences in smoking initiation and progression outcomes. Supportive evidence addressing the plausibility and concordance with other streams of data were also considered by the committee and are described further below.
Considerations for Observational Data on the Association of E-Cigarette Use with Combustible Tobacco Cigarette Ever Use and Progression
Most evidence addressing this question comes from observational studies of the association between e-cigarette use and ever use and progression of smoking. Although randomized controlled trials are generally considered the strongest study design, randomized studies examining the effects of e-cigarette exposure on never-smoking youth are unethical. A challenge of observational studies is potential confounding, because without randomization, certain factors (confounders) may be systematically and unequally distributed across e-cigarette users and non-users. If these factors are associated with both e-cigarette use and combustible tobacco cigarette smoking initiation and are not in the causal pathway, they could statistically bias the study results and create a spurious effect of e-cigarette use on a combustible tobacco cigarette use outcome. For example, if e-cigarettes attract youth who already have a strong interest in smoking, then e-cigarette use may serve as an indicator of an underlying proclivity to smoke rather than playing a causal role in cigarette initiation. Studies that control for known confounders would be stronger evidence than studies that do not.
In the evidence review of the observational data, studies varied in the breadth of covariates included as confounders for which to statistically control. While residual confounding from unmeasured factors is always possible, the committee considered studies that adjusted for a more comprehensive set of covariates as stronger evidence. These plausible confounders include (1) sociodemographic factors that may address nonspecific shared risk factors; (2) environmental factors that may increase the opportunity, willingness, or interest to use both products (e.g., low
monitoring of youth by parents, use of tobacco products by peers or family, permissiveness of tobacco product use by peers or family, exposure to tobacco product advertising); and (3) an endogenous propensity to engage in risk-taking behavior assessed via measures of psychological disposition (e.g., sensation-seeking personality traits, rebelliousness, depression) or risky behaviors (e.g., use of products other than e-cigarettes or combustible tobacco cigarettes, use of non-tobacco drugs of abuse, delinquent behaviors). See Tables 16-1, 16-2, and 16-3 for a listing of the covariates adjusted for in studies that were included in the evidence review.
Among observational study designs, longitudinal studies were considered stronger evidence compared with cross-sectional studies. Given the high plausibility of reverse causality such that combustible tobacco cigarette smoking may also impact e-cigarette use, longitudinal cohort studies that assessed e-cigarette use at baseline and smoking at a future follow-up assessment would provide the strongest evidence to rule out potential reverse causality. Removal of ever smokers at baseline assessment rules out the possibility of reverse causality; however, this approach selects a portion of the population and therefore may not generalize to the entire population of youth and young adults, including those who start smoking at an early age. Alternatively, statistical control of baseline smoking can also address this issue to some extent. The strongest design would follow an entire population of youth beginning at an age at which risk of use of any product is negligible (e.g., 10 years old) and investigate time-varying associations between e-cigarette ever use and later combustible tobacco cigarette use at multiple developmental stages throughout the entire period of risk (e.g., up until age 29), while using multiple methods to establish temporal precedence of vaping relative to smoking. If consistent results are observed across all of the time-varying associations across development through adolescence and young adulthood, a stronger conclusion can be made with confidence in both internal validity and generalizability. Given the brief period in which e-cigarettes have been available, studies with such designs are not available. Hence, the committee considered the body of evidence and whether results were consistent across studies following youth from different regions (e.g., Europe versus North America), youth of different age ranges (e.g., early adolescence versus late adolescence versus emerging adulthood), and those applying different methods (e.g., eliminating baseline smokers versus statistical controls).
Among the longitudinal observational data that are currently available, for the first research question—Among adolescents and young adults, does e-cigarette use impact risk of ever smoking?—the committee considered studies that removed ever smokers at baseline from the analytical sample and assessed ever use of smoking at follow-up to reflect the strongest evi-
| Reference | Location | Cohort Size | Cohort Age at Baseline | Follow-Up Duration |
|---|---|---|---|---|
| Barrington-Trimis et al., 2016a | Southern California, USA | 298 | Median = 17.4 years | Mean = 16 months |
| Best et al., 2017 | Scotland, UK | 2,125 | 11–18 years | 12 months |
| Conner et al., 2017 | England, UK | 1,726 | 13–14 years | 12 months |
| Leventhal et al., 2015 | Los Angeles, CA, USA | 2,530 never users of any combustible tobacco product at baseline | Mean = 14 years | 12 months |
| Loukas et al., 2018 | Texas, USA (24 colleges) | 2,558 | Mean = 19.7 years | 18 months |
| Miech et al., 2017 | USA (Monitoring the Future study) | 347 | High school: 12th grade | Mean = 13.4 months |
| Primack et al., 2015 | USA (Media, Advertising and Health Study) | 694 | Mean = 20 years | 12 months |
| Primack et al., 2016 | USA (nationally representative sample) | 915 | Mean = 23.5 years | 18 months |
| Measurement Tobacco | OR; 95% CIc,d | Adjustments | |
|---|---|---|---|
| E-Cigarettea | Cigaretteb | ||
| Ever use | Ever use | 5.48; 2.69–11.2 |
Sex, ethnicity, grade, parental education, and use of hookah, cigar, or pipe at baseline |
| Ever use | Ever use | 2.42; 1.63–3.60 |
Age, sex, SES, ethnic group, school, smoking susceptibility, peer and family smoking status |
| Ever use | Ever use | 4.06; 2.94–5.60 |
Age, sex, SES, ethnic group, school, smoking susceptibility, peer and family smoking status |
| Ever use | Past 6-month use at 6-month and 12-month follow-ups | 1.75; 1.10–2.77 |
Age, sex, ethnicity, parental education, family living situation, peer and family smoking, smoking susceptibility, smoking expectancies, impulsivity, depression, substance use, delinquent behavior |
| Ever use | Ever use | Overall sample: 1.36; 1.01–1.83. Among baseline never users of any tobacco product: 2.26; CI = 1.35–3.76 |
Age, sex, race/ethnicity, school, smoking susceptibility, peer smoking, family-of-origin tobacco use, other tobacco use |
| Ever use | Past 12 months | 4.78; 1.91–11.96 |
Sex, race, baseline marijuana use and binge drinking |
| Ever use | Ever use | 8.3; 1.2–58.6 |
Age, sex, race/ethnicity, maternal education, peer and parental smoking, sensation-seeking tendency |
| Ever use | Ever use | 6.8; 1.2–58.6 |
Age, sex, race/ethnicity, relationship status, living situation, education, self-esteem, sensation seeking, rebelliousness |
| Reference | Location | Cohort Size | Cohort Age at Baseline | Follow-Up Duration |
|---|---|---|---|---|
| Spindle et al., 2017 | Virginia Commonwealth University | 3,757 | Mean = 18.5 years | 12 months |
| Wills et al., 2016b | Oahu, Hawaii, USA | 1,141 | Mean = 14.7 years | 12 months |
a Independent variable.
b Dependent variable.
c Comparing incidence of combustible tobacco cigarette smoking in baseline users of e-cigarettes compared with the referent category of baseline non-users of e-cigarettes (OR = 1.0).
| Reference | Location | Cohort Size | Cohort Age at Baseline | Follow-Up Duration |
|---|---|---|---|---|
| Barrington-Trimis et al., 2016a | Southern California, USA | 298 never smokers | Median = 17.4 years | Average = 16 months |
| Conner et al., 2017 | England, UK | 318 ever smokers | 13–14 years | 12 months |
| Doran et al., 2017 | California, USA | 391 non-daily smokers | 18–24 years | 12 months, follow-up every 3 months |
| Measurement Tobacco | OR; 95% CIc,d | Adjustments | |
|---|---|---|---|
| E-Cigarettea | Cigaretteb | ||
| Ever use | Ever use | 3.37; 1.91–5.94 |
Age, sex, race/ethnicity, depression, anxiety, impulsivity, stressful life events, peer deviance, other (non-cigarette) tobacco use |
| Ever use | Ever use | 2.87; 2.03–4.05 |
Age, sex, race/ethnicity, parental education, family structure, parental support, parental monitoring, rebelliousness |
d The OR presented for each report is the one adjusted for the largest number of covariates.
NOTE: OR = odds ratio; SES = socioeconomic status.
| Measurement Tobacco | OR; IRR, β 95% CIc,d |
Adjustments | |
|---|---|---|---|
| E-Cigarettea | Cigaretteb | ||
| Ever use | Past 30-day use | OR = 7.50; 2.41–23.4 |
Sex, ethnicity, grade, parental education, and use of hookah, cigar, or pipe at baseline |
| Ever use | Increased use of cigarettes at follow-up among baseline ever users | OR = 1.89; 0.82–4.33 |
Age, sex, SES, ethnic group, school, smoking susceptibility, peer and family smoking status |
| Frequency in past 6 months | Total cigarettes smoked | At first follow-up: IRR = 1.13; 1.06–1.21 Interaction with time: IRR = 1.16; 1.09–1.23 |
A propensity score accounting for sex, race/ethnicity, student status, significant other who smoked, smokers in participant’s household, intent to quit |
| Reference | Location | Cohort Size | Cohort Age at Baseline | Follow-Up Duration |
|---|---|---|---|---|
| Hornik et al., 2016 | USA (nationally representative sample) | 944 baseline non-smokers (total cohort = 1,026) | Mean = 18.3 years | 6 months |
| Leventhal et al., 2016 | Los Angeles, CA, USA | 2,966 | Mean = 15.5 years | 6 months |
| Selya et al., 2017 | Chicago, IL, USA | 1,007 sample enriched for early adolescent smoking | 19–23 years | 36 months |
| Spindle et al., 2017 | Virginia Commonwealth University | 3,757 never smokers | Mean = 18.5 years | 12 months |
| Unger et al., 2016 | Los Angeles, CA, USA | 1,056 (all Hispanic) non-smokers | Mean = 22.7 years | 12 months |
a Independent variable.
b Dependent variable.
c Extent of tobacco cigarette smoking in baseline users of e-cigarettes conditional on ecigarette use. Odds ratio is reported as estimate of association unless otherwise noted.
| Measurement Tobacco | OR; IRR, β 95% CIc,d |
Adjustments | |
|---|---|---|---|
| E-Cigarettea | Cigaretteb | ||
| Past 30-day use | Past 30-day use | OR = 5.43; 2.59–11.38 | Age, sex, race/ethnicity, parental education, ever cigarette use, peer and household smoking, sensation seeking, grades |
| Four-level use frequency continuum: Never. Prior = ever use, but no use in past 30 days. Infrequent = use 1–2 days in past 30 days. Frequent = used ≥3 days in past 30 days. | Smoking frequency in past 30 days: 0 days, 1–2 days, ≥3 days. Smoking intensity (cigarettes per day on smoking days) in past 30 days: No smoking, <1 cigarette, 1 cigarette, >2 cigarettes. | Frequency proportional odds: OR = 1.37; 1.16–1.31 Intensity proportional odds: OR = 1.26; 1.07–1.48 |
Age, sex, ethnicity, household structure, parental education, peer and family smoking, smoking susceptibility, smoking expectancies, ever use of alcohol or drugs, ever use of any combustible tobacco product, depression, lack of premeditation, sensation seeking, delinquent behavior |
| Past 30 day frequency | Past 30 day frequency | β coefficient from a path analysis of prior e-cigarette frequency in relation to later smoking frequency: 0.021 (p = 0.08) | Prior-wave e-cigarette frequency, smoking frequency, nicotine dependence |
| Ever use | Past 30-day use | OR = 3.30; 1.20–9.05 | Age, sex, race/ethnicity, depression, anxiety, impulsivity, stressful life events, peer deviance, other (non-cigarette) tobacco use |
| Past 30 day use status | Past 30-day use | OR = 3.32; 1.55–7.10 | Age, sex, past month use of alcohol, hookah, cigars, little cigars, and smokeless tobacco |
d The estimate of association presented for each report is the one adjusted for the largest number of factors.
NOTE: IRR = incidence rate ratio; OR = odds ratio; SES = socioeconomic status.
| Reference | Location | Cohort Size | Cohort Age at Baseline | Follow-Up Duration |
|---|---|---|---|---|
| Wills, 2016b | Oahu, Hawaii, USA | 1,141 never smokers | Mean = 14.7 years | 12 months |
| Leventhal et al., 2016 | Los Angeles, CA, USA | 2,966 | Mean = 15.5 years | 6 months |
a Independent variable.
b Dependent variable.
c Comparing incidence of tobacco cigarette smoking in baseline users of e-cigarettes compared with the referent category of baseline non-users of e-cigarettes (OR = 1.0).
d The OR presented for each report is the one adjusted for the largest number of factors.
| Measurement Tobacco | OR; 95% CIc,d | Adjustments | |
|---|---|---|---|
| E-Cigarettea | Cigaretteb | ||
| Number of times used at baseline | Ever (versus never) use | Never: 1.0; 95% CI = referent 1–2 times: 2.88; 95% CI = 1.96–4.22 3–4 times: 2.29; 95% CI = 1.35–3.87 Yearly/monthly: 4.17; 95% CI = 2.03–8.57 Weekly/daily: 4.09; 95% CI = 2.43–6.88 |
Age, sex, race/ethnicity, parental education, parental support, rebelliousness |
| Frequency: Never. Prior = ever use, but no use in past 30 days. Infrequent = use 1–2 days in past 30 days. Frequent = used ≥3 days in past 30 days. |
Smoking frequency in past 30 days: 0 days, 1–2 days, 3 or more days. Smoking intensity (cigarettes per day on smoking days) in past 30 days: No smoking, <1 cigarette, 1 cigarette, >2 cigarettes. | Proportional odds of smoking frequency level versus never use of e-cigarettes: Prior user = 1.51; 95% CI = 0.78–2.93 Infrequent user: 1.94; 95% CI = 0.97–3.91 Frequent user: 2.64; 95% CI = 1.43–4.87 Proportional odds of smoking intensity level versus never use of e-cigarettes: Prior user: 1.44; 95% CI = 0.79–2.64 Infrequent user: 2.02; 95% CI = 1.16–3.53 Frequent user: 1.96; 95% CI = 1.12–3.41 |
Age, sex, ethnicity, household structure, parental education, peer and family smoking, smoking susceptibility, smoking expectancies, ever use of alcohol or drugs, ever use of any combustible tobacco product, depression, lack of premeditation, sensation seeking, delinquent behavior |
NOTES: Additional studies examining associations of e-cigarette use frequency variables with combustible tobacco smoking that did not present pairwise contrasts of varying levels of e-cigarette use are not presented and can be found in Table 16-2. OR = odds ratio.
dence of temporal precedence. For the second research question—Among adolescents and young adults, does e-cigarette use impact risk of progression in smoking frequency, intensity, and duration?—the strongest study design for establishing temporal precedence would include at least three time points (see Figure 16-1): a wave 1 in which ever smokers would be eliminated from the sample and e-cigarette use would be assessed as the primary exposure variable. The outcome would address trends in smoking status over time to capture the persistence of use (e.g., smoked since the previous wave of assessment or in the past 30 days leading up to the assessment [yes/no]) as well as trends in frequency (e.g., days smoked in the past 30) or intensity (e.g., cigarettes smoked per day on smoking days). In the case of multiple waves of follow-up data, evidence of a positive baseline e-cigarette use by time interaction term for either smoking status, frequency, or intensity would indicate that e-cigarette use is associated with increased likelihood or speed of progression in the respective outcome, whereas a negative interaction term would indicate that e-cigarette use is associated with reduced likelihood or speed of progression.
Other strategies employed for addressing whether e-cigarette use was associated with progression include two time points, but by contrast with evidence addressing initiation, studies with only two waves would not merely assess ever use at follow-up. Given that youth who ultimately progress to become frequent, heavy, and chronic smokers in adulthood are of greatest public health importance, the weakest evidence of progression to regular use in the two time-point design includes studies that measure recent (e.g., past 30-day) smoking status (yes/no) at follow-up. While past 30-day use typically involves a higher level of smoking than the smoking ever use outcome, the past 30-day outcome is considered a weak indicator of likelihood of progression. Among U.S. high school student past 30-day smokers in 2014, 37.0 percent smoked only 1 or 2 days per month, 22.6 percent smoked every day, and 40.4 percent smoked intermittently (Neff et al., 2015), which underscores the uncertainty of past 30-day use status. Instead, studies that include more detailed measures of smoking frequency that distinguish among monthly, weekly, and daily use and smoking intensity that identify the number of cigarettes smoked per smoking day were considered to provide stronger evidence to address smoking progression. Among studies including only two time points that addressed smoking progression, those that did not eliminate baseline ever smokers and used an alternate strategy for addressing reverse causation (e.g., statistical adjustment of baseline combustible tobacco use, elimination of baseline current combustible tobacco users without eliminating baseline past combustible tobacco users) were considered to provide less robust evidence of temporal precedence of an association of e-cigarette use with smoking progression (Etter, 2017).
Studies in which more frequent or chronic use of e-cigarettes is associated with proportional differences in combustible tobacco use initiation or progression in frequency, intensity, or duration provided further support of causation. Studies using e-cigarette ever use as an assessment of e-cigarette exposure amalgamate youth who may have used e-cigarettes on only one occasion with those who are regular vapers. Such studies were deemed to provide weaker evidence than studies that provide more fine-grained differentiation along a continuum of e-cigarette exposure (e.g., temporary use versus recent use on a monthly basis, recent weekly use versus recent daily use) capable of testing dose–response effects.
Supportive Evidence
While the major emphasis of the evidence review focused on the association of e-cigarette use with smoking initiation and progression in observational data, supplementary lines of evidence were used to provide additional information to address the research questions. Just as studies on the effects of e-cigarettes on health outcomes drew upon in vivo animal and in vitro studies as evidence supporting the biological plausibility of a hypothesized disease pathway, study results that address the specificity of hypothesized biological and psychological mechanisms proposed by the diversion and catalyst hypotheses, respectively, were considered by the committee as supportive lines of evidence of the plausibility that an association identified in the primary review was causal.
Tests of whether the association of e-cigarette use with ever smoking and smoking progression differs by baseline smoking risk status captured by the moderator variables of psychological traits (e.g., rebelliousness) and cognitive susceptibility to smoking (e.g., reported interest and willingness in trying smoking in the future; see Figure 16-1) reflect one line of evidence. That is, evidence of a statistical interaction between e-cigarette use and a moderating variable indicative of a general liability to smoking that is outside of the putative causal pathway from e-cigarette use to changes in smoking was considered. In such research, results consistent with the diversion hypothesis that e-cigarette use prevents ever smoking and smoking progression would demonstrate that there would be a negative association between e-cigarette use and smoking that becomes stronger in higher (versus lower) risk youth in stratified analyses whereby the e-cigarette–combustible tobacco cigarette association is estimated in subgroups differing in level of liability for smoking captured by other moderator variables outside of the causal pathway (e.g., rebelliousness). Results consistent with the catalyst hypothesis would show a positive association between e-cigarette use and smoking initiation that becomes stronger in youth who score lower (versus higher) on such moderator variables.
Also considered were studies examining whether e-cigarette use is associated with changes in intermediate psychosocial mediators that putatively link e-cigarette use with subsequent changes in smoking risk. The mediator variables would include those that are based on concepts proposed in either the diversion or catalyst hypothesis. For instance, evidence that a positive association between baseline e-cigarette use and later combustible tobacco cigarette smoking is mediated by increases in beliefs that smoking is enjoyable would be interpreted as evidence consistent with the catalyst hypothesis and that e-cigarette use increases risk of smoking initiation. Studies demonstrating that e-cigarette use is associated with a subsequent reduction in curiosity or interest in trying cigarettes would support the diversion hypothesis and that e-cigarette use reduces smoking risk. As enjoyment of nicotine’s pleasurable effects are hypothesized mechanisms in the catalyst model, studies of whether e-cigarette nicotine concentration is associated with smoking were also considered as relevant to determining plausibility. Qualitative research involving direct testimonials of youth e-cigarette users who explained why they did or did not start smoking were also considered in determining plausibility.
Finally, the committee drew upon additional forms of evidence that provided relevant data to help draw inferences, including ecological studies and studies of other non-cigarette tobacco products. Ecological studies of whether the slope of changes in the prevalence of e-cigarette use and smoking over time are in parallel or opposing directions would cohere with interpretations that e-cigarette use increases or reduces smoking, respectively, and were considered by the committee. As randomized controlled experiments addressing the research questions are unavailable, naturalistic experiments comparing smoking in communities with restrictive e-cigarette use policies for youth (e.g., restrictions against sales to minors) compared with those with permissive youth policies (e.g., no sales restrictions) were considered to provide indirect evidence of the causal effect. Analogous evidence of associations among use of tobacco products popular among youth that are similar to e-cigarettes and cigarette smoking could also provide indirect evidence of causal mechanisms. Like e-cigarettes, hookah and cigarillos are available in characterizing flavors preferred by youth, have not been subject to federal regulation, and have increased in popularity among youth over the past decade. Thus, the committee considered whether analogous associations of relevant, non-cigarette tobacco product use and cigarette smoking initiation existed to interpret the causal effect of e-cigarettes on smoking. Furthermore, the committee reviewed evidence of analogous associations between e-cigarette use and uptake of non-cigarette combustible tobacco products. The committee also reviewed evidence regarding whether e-cigarette
use was associated with other risk behavior outcomes (e.g., cannabis use). Among the putative psychosocial and biological mechanisms linking e-cigarette use and changes in smoking proposed by the diversion and catalyst hypotheses are non-specific mechanisms that would also be expected to change risk of other risk behavior outcomes; other mechanisms would be expected to be specific to nicotine and tobacco products. For example, e-cigarette use resulting in satiation of the desire for novelty in the diversion hypothesis and in feeling emboldened to take risks in the catalyst hypothesis are non-specific mechanisms. The enjoyment of the pharmacological effects of nicotine via e-cigarettes in the catalyst hypothesis is a specific mechanism expected to directly increase risk of nicotine and tobacco products more strongly than other risk behaviors. Thus, evidence that the (positive or negative) association between e-cigarette use and subsequent combustible tobacco cigarette use is stronger than the corresponding association of e-cigarette use with another risk behavior, such as cannabis use, would suggest specificity of the association and further strengthen the conclusion from the primary review.
For this supportive evidence, the committee considered study results consistent with the epidemiological data on the association and plausibility as confirmatory evidence to strengthen the weight of conclusions. However, because a causal effect can exist in the absence of supporting ecological or analogous evidence due to potential methodological or conceptual limitations that restrict causal inferences from such studies, causal conclusions could be made in the presence of inconsistent evidence across epidemiological studies and these additional forms of evidence.
EVIDENCE REVIEW: METHODS
The primary evidence review was limited to studies of associations between (1) e-cigarette use and (2) ever smoking and progression, including adolescents and young adults age 29 or younger, as risk of smoking onset peaks in adolescence and young adulthood and becomes very low at or after age 30 (HHS, 2012). Because of the difficulty in determining the directionality of associations between e-cigarette and combustible tobacco cigarette use in cross-sectional studies and the presence of a sufficient number of high-quality longitudinal studies, literature review was limited to original primary reports and reviews of studies with a longitudinal design that had a minimum follow-up period of 6 months (i.e., a period in which meaningful changes in youth tobacco use has been demonstrated in prior work). The search strategy is described in Appendix B.
The search identified 5 review papers (4 narrative and commentary papers [Etter, 2017; Kozlowski and Warner, 2017; Phillips, 2015; Schneider and Diehl, 2016] and 1 meta-analysis [Soneji et al., 2017]) and 15 empirical
papers that matched these criteria (including those in the review by Soneji and colleagues [Barrington-Trimis et al., 2016a; Best et al., 2017; Conner et al., 2017; Doran et al., 2017; Hornik et al., 2016; Leventhal et al., 2015, 2016; Loukas et al., 2018; Miech et al., 2017; Primack et al., 2015, 2016; Selya et al., 2017; Spindle et al., 2017; Unger et al., 2016; Wills et al., 2016b]).2 Because the sole review that used meta-analysis (Soneji et al., 2017) was published recently, was deemed to be of high quality based on the ROBIS criteria (Whiting et al., 2016), and included the majority of empirical studies identified (nine studies), the committee reviewed the findings from this meta-analysis. The Soneji and colleagues (2017) paper reviewed and meta-analyzed only the ever and past 30-day use status data at one follow-up from each of the nine studies; the paper does not address dose–response effects and some outcomes indicative of progression in smoking frequency, intensity, or duration. Yet, two of the nine published studies also reported alternative e-cigarette exposure and smoking outcome results of interest, such as smoking or e-cigarette use frequency or multiple follow-up time points, and were reviewed in addition to the overarching results of the Soneji and colleagues (2017) meta-analysis (Leventhal et al., 2015; Wills et al., 2016b). The committee also reviewed the findings from four other studies that fit the criteria for the current evidence review, but were not included in the Soneji and colleagues (2017) meta-analysis because they were published after the publication date (Best et al., 2017; Conner et al., 2017; Doran et al., 2017; Selya et al., 2017). An additional study (Leventhal et al., 2016) that was published prior to, but not included in, the Soneji and colleagues (2017) paper was included in the committee’s review. This paper did not meet the Soneji and colleagues inclusion criteria because it included baseline smokers in the sample but was nonetheless relevant to addressing the committee’s research question regarding smoking progression.
For the supplemental evidence review, the committee conducted a targeted review of papers based on the premises described in the section above on levels of evidence available. This review included studies of moderators and mediators of the vaping–smoking association that addressed plausibility; ecological evaluations of trends in vaping and smoking over time; effects of age restrictions on e-cigarette sales on smoking that addressed coherence; and studies on the analogous association of non-cigarette tobacco product use with cigarette smoking and e-cigarette use with other tobacco product use.
___________________
2 Two studies are abstracts and therefore do not meet the committee’s inclusion criteria, but are included here because they were included in the systematic review and meta-analysis by Soneji and colleagues (Hornik et al., 2016; Primack et al., 2016).
EVIDENCE REVIEW: RESULTS
Systematic Review
Method
The Soneji and colleagues (2017) systematic review and meta-analysis included nine studies of U.S. participants age 14 to 26 at baseline based on a search of PubMed, EMBASE, Cochrane Library, Web of Science, the 2016 Society for Research on Nicotine and Tobacco 22nd Annual Meeting abstracts, the 2016 Society of Behavioral Medicine 37th Annual Meeting & Scientific Sessions abstracts, and the 2016 National Institutes of Health Tobacco Regulatory Science Program Conference between February 7 and February 17, 2017. Studies that evaluated the association of ever e-cigarette use among never cigarette smokers at baseline with cigarette ever smoking by follow-up were included. The meta-analysis also included studies that evaluated the association between past 30-day e-cigarette use at baseline with past 30-day smoking at follow-up among baseline non-smokers. Only longitudinal studies were included; cross-sectional studies were excluded. Three independent investigators reviewed the title, abstract, and text of the studies to determine whether the studies met inclusion criteria for the meta-analysis. The interrater agreement among the three reviewers, measured by Fleiss’ κ, was 86.1 percent, which is considered to be adequate. The quality of the included studies was evaluated using the Newcastle-Ottawa Scale, which assesses the quality of non-randomized studies and the risk of bias using the Risk of Bias in Non-randomized Studies of Interventions tool, which considers biases from confounding, selection of participants into the studies, missing data, and measurement of outcomes by two independent investigators.
For the meta-analysis, the exposure variables were ever e-cigarette use (yes/no) and past 30-day e-cigarette use status (1 day or more versus 0). The outcome variables were ever smoking status (yes/no) and past 30-day smoking status (1 day or more versus 0). Two parallel sets of associations were analyzed to study the risk of transition from e-cigarette to cigarette use: (1) the relation of baseline ever e-cigarette use with subsequent ever use of combustible tobacco cigarettes at follow-up among baseline never users of combustible cigarettes, and (2) the relation of baseline past 30-day e-cigarette use with subsequent past 30-day use of combustible tobacco cigarettes among those reporting no use of combustible tobacco cigarettes in the past 30 days at baseline. For each outcome, two overall odds ratio (OR) estimates based on a random effects model were reported that combined each study’s (1) unadjusted OR and (2) OR after adjusting for covariates in the respective primary literature article. For both analyses, statistical heterogeneity of effect estimates was tested
using the I2 statistic. For the cigarette smoking initiation analysis, the source of heterogeneity between studies was examined by conducting subgroup analyses based on age of the participants, baseline year of study, and whether the sample was a nationally representative sample or regional sample.
Results
Nine studies met inclusion criteria (n = 16,621): seven provided data to address the association with smoking initiation and two addressed the association with past 30-day smoking.
Ever-use analysis The ever-use analysis in Soneji and colleagues (2017) can be used for evaluation of the first research question in this section: Among adolescents and young adults, does e-cigarette use affect risk of ever smoking? Each of the seven ever-use studies eliminated ever smokers of combustible tobacco cigarettes at baseline, providing strong evidence of temporal precedence and eliminating some reverse causation explanations (i.e., past smokers seek out e-cigarette use by baseline and then subsequently return to smoking at follow-up). The combined unadjusted and adjusted ORs for the association of e-cigarette–ever use with ever cigarette use across the seven studies were OR = 3.83 (95% CI = 3.74–3.91) and OR = 3.50 (95% CI = 2.38–5.16), respectively, which reflect strong magnitudes of association. Results are shown in Table 16-4. The seven studies were performed by four independent research groups, included a variety of methodologies (i.e., paper-and-pencil surveys, the Internet, phone), sampling regions and strategies (i.e., three national samples, two from southern California, one from Hawaii, and one from Virginia), and age ranges (mean age at baseline range = 14.1–23.5 years). Given the differences across studies and that each study individually found a statistically significant positive association between e-cigarette ever use and smoking initiation, strong evidence in consistency of the association was determined.
The probability of transition from never use to use of combustible tobacco cigarettes during the follow-up period for baseline–e-cigarette–ever users ranged from a low of 7.9 percent in a sample of 9th-grade students (Leventhal et al., 2015) to 40.4 percent in a sample of 11th- and 12th-grade students (Barrington-Trimis et al., 2016a). These estimates of ever use are high compared to previous results for smoking over 12-month follow-up periods in youth and young adults (HHS, 2014) and the comparison groups of e-cigarette–never users in each study for which use over the follow-up period ranged from 3.0 percent (Leventhal et al., 2015) to 10.6 percent (Spindle et al., 2017) across studies.
| Reference | Probability of Combustible Tobacco Smoking During Follow-Up Period, % | |||
|---|---|---|---|---|
| E-Cigarette–Ever Users | E-Cigarette–Never Users | Unadjusted OR; 95% CI | Adjusted OR; 95% CI | |
| Miech et al., 2017 | 31.1 | 6.8 | 6.23; 1.57–24.63 | 4.78; 1.91–11.96 |
| Spindle et al., 2017 | 29.4 | 10.6 | 3.50; 2.41–5.09 | 3.37; 1.91–5.94 |
| Primack et al., 2016 | 37.5 | 9.0 | 6.06; 2.15–17.10 | 6.82; 1.65–28.22 |
| Barrington-Trimis et al., 2016a | 40.4 | 10.5 | 5.76; 3.12–10.66 | 6.17; 3.29–11.57 |
| Wills et al., 2016b | 19.5 | 5.4 | 4.25; 2.74–6.61 | 2.87; 2.03–4.05 |
| Primack et al., 2015 | 37.5 | 9.6 | 5.66; 1.99–16.07 | 8.30; 1.19–58.00 |
| Leventhal et al., 2015 | 8.8 | 3.1 | 2.65; 1.73–4.05 | 1.75; 1.10–2.78 |
| First follow-up | 9.7 | 3.0 | ||
| Second follow-up | 7.9 | 3.3 | ||
| TOTAL | 23.2 | 7.2 | 3.83; 3.74–3.91 | 3.50; 2.38–5.16 |
NOTES: Heterogeneity: τ2 = 0.13; Q6 = 13.79; p = 0.03; I2 = 56%. Test for overall effect: z = 6.34; p < 0.001. The odds ratios (ORs) for the studies are adjusted for a study-specific set of demographic, psychosocial, and behavioral risk factors (see Table 16-1 for covariates).
SOURCE: Adapted from Soneji et al., 2017.
The associations in each of the seven studies were statistically significant and there was heterogeneity in the combined estimate, suggesting variation in effect magnitude across the studies. The authors attempted to explain heterogeneity via subgroup analysis, which found non-significant heterogeneity estimates when the set of studies was limited to the six with young adults only, three studies conducted after 2014, and three nationally representative studies. A limitation of this finding is that a formal interaction test was not conducted to determine whether age, time of publication, or regional versus nationally representative sampling significantly moderated the combined OR estimate across the two groups of studies, perhaps because of the small number of studies precluding formal interaction tests.
This review used a standardized assessment of risk of bias of the individual studies, all of which were deemed as moderate due to potential con-
founding. There was variability in the types and number of confounding covariates included in the primary articles. Some papers included a more comprehensive set of covariates addressing plausible shared risk factors across demographic, environmental, and intrapersonal/endogenous dis-positional domains, providing a more rigorous evaluation of the degree to which an observed association between e-cigarette use and subsequent smoking is direct (i.e., not due to confounding), whereas some included demographic factors and excluded important environmental or intrapersonal factors. The relative difference in ORs between unadjusted and adjusted estimates varied across the seven studies included in the meta-analysis (see Table 16-4). Some studies found larger ORs for unadjusted than adjusted estimates (Leventhal et al., 2015; Miech et al., 2017; Wills et al., 2016b). Others found larger ORs for the adjusted than the unadjusted estimates (Primack et al., 2015, 2016). For two studies, the unadjusted and adjusted ORs did not meaningfully differ (Barrington-Trimis et al., 2016a; Spindle et al., 2017). The net results of this pattern across studies were combined estimates of unadjusted OR = 3.83 (95% CI = 3.74–3.91) and adjusted OR = 3.50 (95% CI = 2.38–5.16) that did not markedly differ from one another as evidenced by highly overlapping confidence intervals for the two estimates. Variation in the disparity in OR estimates between unadjusted and adjusted results across studies could be due to cross-study differences in covariate adjustment. However, inspection of the covariates adjusted for in each study failed to reveal a systematic effect (i.e., the studies with more comprehensive covariate adjustment did not necessarily show reductions in OR estimates from unadjusted to adjusted models). Given this pattern of results, it is difficult to determine the potential influence of residual confounding by unmeasured variables on the associations observed.
An important limitation was the high loss to follow-up in six of the studies (greater than 20 percent). The studies addressed attrition in a number of ways, including (1) comparing associations with complete case analysis to results from analysis using maximum likelihood estimation methods (Wills et al., 2016b), (2) using alternative assumptions that all lost to follow-up are either initiators or non-initiators (Leventhal et al., 2015), and (3) using auxiliary variables to estimate and impute outcome data (Primack et al., 2015). In no cases did the results substantively differ when comparing differing methods for addressing attrition. Because youth and young adults who are lost to follow-up are typically more likely to possess risk factors for cigarette use in previous research (Young et al., 2006) as well as being e-cigarette users at baseline (Barrington-Trimis et al., 2016a; Leventhal et al., 2015; Wills et al., 2016c), the differential loss to followup would have resulted in selection bias that most likely would have
suppressed the magnitude of the association of the individual articles reviewed for the meta-analysis.
Past 30-day use analysis The combined unadjusted and adjusted ORs for the association of past 30-day e-cigarette use with past 30-day cigarette use were OR = 5.68 (95% CI = 3.49–9.24) and OR = 4.28 (95% CI = 2.52–7.27), respectively (see Figure 16-2). For the past 30-day use analysis, only two primary literature studies were included (Hornik et al., 2016; Unger et al., 2016), raising questions about the generalizability and stability of the combined OR estimate for past 30-day associations. Additionally, youth with a prior history of smoking were permitted in the past 30-day use analysis, which was not adjusted for in one of the studies, allowing for reverse causation. Furthermore, past 30-day use does not address ever use, per se, and provides an imprecise estimate of progression to more frequent, heavy, or chronic smoking. Hence, the past 30-day use analysis in Soneji and colleagues (2017) did not provide rigorous evidence to evaluate either the ever use or progression research questions.
Other Original Studies
Leventhal and colleagues (2015) examined the association between baseline ever use of e-cigarettes with past 6-month smoking status at 6- and 12-month follow-ups among 2,530 baseline never smoker ninth-grade students in Los Angeles. Although this article was reviewed by Soneji and colleagues (2017), they focused on the bivariate association between e-cigarette use and ever-smokers averaged across follow-up, without considering the effects across multiple time points reported in Leventhal and colleagues (2015), which addresses the question of whether e-cigarette use is associated with duration of smoking. Hence,
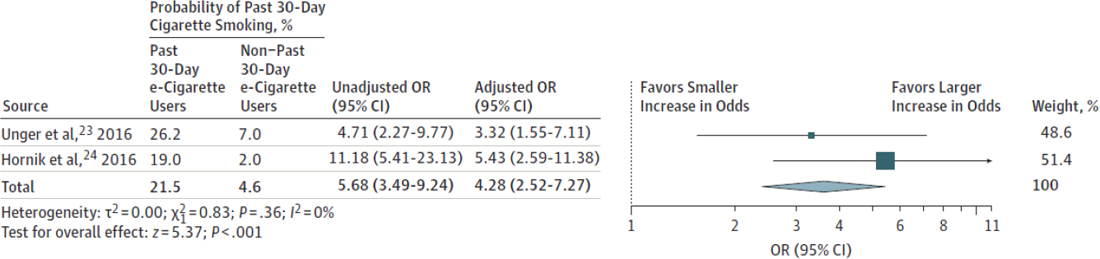
NOTE: OR = odds ratio.
SOURCE: Soneji et al., 2017.
the multiple-time-point analysis in Leventhal and colleagues (2015) was reviewed by the committee. The exposure variable was ever versus never e-cigarette use at baseline in this study. The outcome was smoking status over the previous 6 months (any smoking versus no smoking). The study found no significant interactions between e-cigarette ever use and time in the prediction of combustible tobacco cigarette smoking (interaction adjusted OR = 0.74; 95% CI = 0.34–1.61, p = 0.44), which suggests that the likelihood of persistence (versus discontinuation) of smoking across the 6- and 12-month follow-ups was non-significantly lower among e-cigarette users. Of baseline e-cigarette–ever users compared to e-cigarette–never users, 9.7 percent versus 3.0 percent smoked in months 1 through 6 of follow-up and 7.9 percent compared to 3.3 percent had smoked in months 7 through 12 of follow-up, respectively. An additional analysis found that baseline e-cigarette–ever users (versus never) were more likely to be sustained users of any combustible tobacco product across both follow-ups (10.0 percent versus 3.6 percent). This study had a high follow-up rate (98 percent), adjusted for a comprehensive set of covariates and potential confounders, and removed baseline smokers to provide temporal precedence. The study was limited because dose–response effects could not be evaluated given the e-cigarette ever use exposure variable. In sum, no conclusive evidence of whether e-cigarette use is associated with duration of smoking among initiators was found in this study.
Wills and colleagues (2016b) reported associations of e-cigarette use with smoking in 9th and 10th graders in Hawaii who were surveyed in 2013 (baseline) and followed up 1 year later. While included in the Soneji meta-analysis, this study was individually reviewed by the committee because it included supplementary analyses of e-cigarette and combustible tobacco cigarette use frequency estimates not addressed in Soneji and colleagues (2017). In analyses of dose–response associations with smoking initiation among 1,070 adolescent never smokers at baseline, the probability of ever smoking at 1-year follow-up was 5 percent among baseline never vapers, which was significantly lower than the probability of ever smoking for youth who had vaped one to two times in their life (14 percent versus 5 percent; adjusted OR = 2.88; 95% CI = 1.96–4.22), three to four times in their life (11 percent versus 5 percent; adjusted OR = 2.29; 95% CI = 1.35–3.87), yearly/monthly (19 percent versus 5 percent; adjusted OR = 4.17; 95% CI = 2.03–8.57), or weekly/daily (19 percent versus 5 percent; adjusted OR = 4.09; 95% CI = 2.43–6.88) at baseline after adjusting for demographic-, environmental-, and psychological/personality-related covariates. Although these estimates suggest a possible threshold effect for association between e-cigarette use frequency and smoking initiation for the two higher versus two lower e-cigarette exposure groups, pairwise comparisons with the Tukey-Kramer adjust-
ment indicated that the four frequency levels of e-cigarette use did not differ significantly from each other in smoking initiation likelihood. Pairwise tests may have been limited in power due to smaller sample sizes for individual group pairs. In the same subsample, analyses examined dose–response associations with probability of initiating and progressing to smoking at least a few times per month or more (versus no initiation or initiating, but not progressing to at least a monthly smoking frequency). The probability of initiating and progressing to monthly smoking was not different between adolescents who vaped one to four times versus never in their life at baseline (0.6 percent versus 1.0 to 1.2 percent, adjusted OR < 1.88), but was higher among those who vaped yearly/monthly (4.2 percent versus 0.6 percent, adjusted OR = 7.13; 95% CI = 1.28–39.73) or weekly/daily (9.7 percent versus 0.6 percent: OR = 17.19; 95% CI = 7.24–40.79) versus never vaped at baseline. These estimates suggest a possible threshold effect whereby at higher frequency levels of vaping, the likelihood of initiating and progressing to more frequent smoking is increased, but at low levels of vaping frequency the likelihood of progression in smoking frequency is not changed. However, the small sample size for the individual groups, and resulting wide confidence intervals of the estimates, tempers conclusive inferences regarding possible threshold effects. In sum, Wills and colleagues (2016b) show suggestive evidence that “dose” of e-cigarette exposure (i.e., use frequency) may differentiate likelihood of smoking initiation and progression in frequency.
Leventhal and colleagues (2016) examined the association between baseline e-cigarette use frequency with progression of combustible tobacco cigarette use frequency and intensity at a 6-month follow-up in 3,084 10th-grade high school students in Los Angeles. This paper included the same cohort as Leventhal and colleagues (2015), but used different time points. This study was not included by Soneji and colleagues (2017) because it was published after their analysis. The e-cigarette use exposure variable was defined as a four-level gradient variable (never versus ever use with no use in past 30 days [prior use] versus 1–2 days in past 30 [current infrequent use] versus ≥3 days in past 30 [current frequent use]). The combustible tobacco cigarette use frequency outcome was a three-level variable based on days used in past 30 (0 versus 1–2 days versus 3 days of use or more). The intensity of combustible cigarette use outcome was characterized with a four-level variable based on the amount of smoking per smoking day (no smoking versus less than one cigarette versus a whole cigarette versus two cigarettes or more). Adjusting for baseline smoking and other covariates, ordinal logistic regression found that each increment higher on the four-level baseline vaping level was associated with proportionally higher odds of smoking at a greater level of frequency (OR = 1.37; 95% CI = 1.16–1.61) and intensity (OR = 1.26; 95%
CI = 1.07–1.48) by follow-up. Adjusting for baseline smoking, pairwise ordinal logistic regression results suggested a dose–response association for smoking frequency, yielding ORs of 4.61 (95% CI = 2.49–8.53) for prior (versus never) baseline vaping, 6.60 (95% CI = 3.48–12.51) for current infrequent (versus never) baseline vaping, and 10.62 (95% CI = 6.46–17.46) for current frequent (versus never) baseline vaping. This study also demonstrated that the positive association between baseline e-cigarette using and follow-up smoking frequency and intensity was stronger among baseline non-smokers than baseline infrequent and frequent smokers in the form of a statistical interaction. In baseline non-smokers, there was evidence of a dose–response association such that smoking frequency at follow-up increased proportionately across baseline vaping frequency, as follows: baseline never users (infrequent smokers = 0.7 percent; frequent smokers = 0.5 percent); prior vapers (infrequent = 3.9 percent; frequent = 2.3 percent); infrequent vapers (7.1 percent, 3.6 percent); and frequent vapers (5.4 percent, 9.7 percent). In baseline smokers, vaping was not significantly associated with smoking frequency at follow-up. Lower strength of vaping–smoking associations in baseline smokers (versus nonsmokers) has been reported elsewhere (Conner et al., 2017; Miech et al., 2016; Wills et al., 2016b). As reported in Leventhal and colleagues (2016), this result could suggest that e-cigarette use is more strongly associated with the onset (or return) to smoking as well as the progression to higher levels of cigarette use over the follow-up period, but may have less of an effect among those who are already smokers—many of whom may have started vaping after smoking initiation. In several other studies that report results by baseline smoking status (Conner et al., 2017; Leventhal et al., 2016; Miech et al., 2016; Wills et al., 2016b), the association between vaping and subsequent smoking was null or weakly positive among baseline smokers, which suggests that e-cigarette use is not associated with smoking reduction or cessation in youth and young adults. A strength of the study was the comprehensive adjustment of covariates, which included 15 demographic, environmental, and intrapersonal factors, and high participation (80 percent or more) and retention (99 percent) rates. A limitation was the brief follow-up and the inclusion of youth with a history of smoking, which treated ever smoking as a covariate and baseline current smoking frequency as a moderator rather than eliminating baseline ever-smokers from the analysis to prevent reverse causation. Overall, this study provided fairly strong evidence of dose–response associations between e-cigarette use and progression in smoking frequency and intensity for non-smokers at baseline.
In a sample of 391 non-daily combustible tobacco cigarette smokers age 18 to 24 in California, Doran and colleagues (2017) conducted a longitudinal study that examined whether e-cigarette use frequency was
associated with changes in use of combustible tobacco cigarettes over a 12-month follow-up period. This study was published after the Soneji and colleagues (2017) analysis. E-cigarette use frequency was initially assessed via a five-level variable indicative of use over the previous 6 months (never; one to three times; one to two times per month; weekly; two to four times per week; and daily/almost daily). At each follow-up evaluation, the investigators assessed the number of days smoked (i.e., frequency) and total amount of cigarettes smoked (i.e., intensity) over the previous 14 days. Negative binomial regression models adjusting for baseline cigarette use and a propensity score based on a prediction model involving a number of demographic, environmental, and intrapersonal covariates was used. There was a positive association between initial e-cigarette use frequency with cigarette use frequency and intensity at follow-up. For smoking intensity, the incidence rate ratio (IRR) was 1.13 (95% CI = 1.06–1.21), indicating that each one-category increase in initial e-cigarette frequency (e.g., from one to three uses in 6 months to monthly use) was associated with a 13 percent greater number of total cigarettes reported at the second assessment. Analyses of trends in smoking over time across multiple follow-up time points showed a significant interaction for smoking quantity, indicating that this gap widened over time (IRR = 1.16; 95% CI = 1.09–1.23), as illustrated by the higher slope in cigarette intensity across the follow-ups for individuals who used e-cigarettes at a greater frequency at the initial assessment. For smoking frequency outcomes, the interaction between e-cigarette use frequency and time was not significant. A strength of this study was the assessment strategy using frequent follow-ups and short interassessment intervals (i.e., once every 3 months for five total assessments), detailed characterization of exposure and outcome variables to show dose–response relations, and high retention rate (95 percent). In addition, the covariates used to generate a propensity score were comprehensive. Because all participants were smokers at baseline, it is possible that most started smoking before using e-cigarettes, leaving the temporal precedence of the association unclear. It is possible that there is a selection bias whereby those most at risk who are likely to escalate their smoking start using e-cigarettes as a means to help with cravings or because of unsuccessful efforts to quit (rather than a causal effect whereby using e-cigarettes intensifies smoking progression). However, the authors included intention to quit in the propensity score that was adjusted for in the analysis. In sum, this study provides suggestive evidence that e-cigarette use may be associated with increased progression in smoking intensity and in frequency to some degree.
Best and colleagues (2017) examined the association of baseline e-cigarette–ever use (versus never) with cigarette use initiation at 1-year follow-up among 11- to 18-year-old baseline never smokers (n = 2,125)
enrolled in four schools in Scotland from 2015 to 2016. This study was published after the Soneji and colleagues (2017) analysis. In an unadjusted model, the OR for ever smokers at follow-up in e-cigarette–ever users versus e-cigarette–never users was 4.62 (95% CI = 3.34–6.38). Adjusting for demographics, susceptibility to smoking, and family/peer smoking, the OR remained significant and was 2.42 (95% CI = 1.63–3.60). A limitation of this study was that other intrapersonal covariates indicative of a propensity toward risk-taking behavior were not included. Also, there was a modest retention rate (70.8 percent), which may reduce the magnitude of the association due to the possibility of dropouts being disproportionately likely to use e-cigarettes at baseline and smoke at follow-up. Finally, e-cigarette exposure level was not reported, precluding investigation of dose–response associations. Strengths are elimination of baseline never smokers from the analytical sample, which permits temporal conclusions about the association that e-cigarette use at baseline was related to an increased probability of transition to becoming an ever smoker at followup. In sum, the study methods and results are highly similar to the seven original studies included in the Soneji and colleagues (2017) meta-analysis of initiation and suggests generalization of e-cigarette use–smoking initiation associations to a sample outside the United States.
Conner and colleagues (2017) investigated the association of e-cigarette use at baseline and smoking at a 1-year follow-up among 2,836 adolescents (age 13 to 14 years at baseline) in 20 schools in England from 2014 to 2015. In baseline never smokers (n = 1,726), probability of transitioning from never to ever combustible cigarette use at follow-up was associated with ever (compared to never) use of e-cigarettes at baseline (OR = 5.38; 95% CI = 4.02–7.22), which remained significant when controlling for covariates (OR = 4.06; 95% CI = 2.94–5.60). In adolescents who had tried cigarettes but were not current smokers at baseline (n = 318), ever use was associated with progression in smoking frequency at follow-up (OR = 2.16; 95% CI = 1.01–4.62), which became non-significant when controlling for covariates (OR = 1.89; 95% CI = 0.82–4.33). A unique strength of this study was that smoking was biochemically verified by breath carbon monoxide (CO) readings, and those who had recently smoked or who were defined as having progressed showed significantly higher CO than non-smokers. Due to its half-life, CO is only detected after recent smoking and is thus an insensitive measure for detecting ever use or infrequent smoking. Also, there was a comprehensive set of covariates adjusted for that help to rule out confounding effects. There was a moderate 21 percent attrition rate speculated by the authors to be due to a failure to correctly match anonymous code numbers for students across assessments. Attrition analyses found modest differences between those with and without follow-up data on key variables and no differences in baseline e-cigarette use, sug-
gesting that the impact of attrition on association estimates was small. Adolescents who indicated “I have only tried smoking once” or “I used to smoke sometimes, but I never smoke cigarettes now” at baseline and then selected one of the following, “I sometimes smoke cigarettes now, but I don’t smoke as many as one a week,” “I usually smoke between one and six cigarettes a week,” and “I usually smoke more than six cigarettes a week” at follow-up were classified as having progressed in their smoking. This definition is highly circumscribed and fails to differentiate among low-, moderate-, or high-frequency levels. Also, due to a small number of cases of frequent e-cigarette use, the e-cigarette exposure variables were based on ever use, leaving unclear possible dose–response effects. In sum, this study provides evidence that e-cigarette use is associated with smoking initiation in British youth and weak and inconclusive evidence that e-cigarette use is associated with progression to more frequent smoking.
Selya and colleagues (2017) examined the association between past 30-day e-cigarette and combustible tobacco cigarette use frequency in a young adult sample (age 19–23) originally recruited to be enriched with smokers attending high schools in Chicago, Illinois (81.1 percent had a history of smoking). Participants were surveyed annually four times from 2011 to 2015 and a cross-lagged structural equation model was used to examine the average estimate of association of e-cigarette use with combustible tobacco cigarette use at the subsequent wave for all prospective paths (i.e., Wave 1 → Wave 2, Wave 2 → Wave 3, Wave 3 → Wave 4). The effects of combustible tobacco cigarette dependence severity and use at the previous wave was adjusted for and the lowest possible score on the smoking dependence scale was imputed for never smokers. The results showed that e-cigarette use was not associated significantly with later increases in combustible tobacco cigarette smoking (β = 0.02, p = 0.08). This study was not well positioned to address whether e-cigarette use contributed to ever smoking and subsequent progression of smoking behavior because the sample was recruited to be enriched for smoking in 2006 (prior to the availability of e-cigarettes). Thus, it is likely that the majority of the sample began using combustible tobacco cigarettes prior to using e-cigarettes, including those who sought out e-cigarettes as a cessation aid. The authors acknowledge this limitation and that they did not adjust for any possible confounders of the association. In sum, this study provides negligible evidence that can be used to address the research questions posed in this review.
Loukas and colleagues (2018) administered four semiannual surveys to 2,558 never smoking 18- to 25-year-old (19.71 ± 1.61) students from 24 Texas colleges four times over a 1.5-year follow-up period beginning in 2014–2015 with retention rates ranging from 79 percent to 81 percent across waves. Transition probability from baseline never smoking to ever
smoking during the follow-up period was 20.1 percent versus 8.4 percent for baseline e-cigarette–never users (versus ever). Multivariable, multilevel discrete-time hazard models indicated that baseline e-cigarette–ever use was associated with subsequent transition to combustible tobacco cigarette ever smoking (adjusted OR = 1.36; 95% CI = 1.01–1.83) after adjusting for demographics, combustible tobacco cigarette use susceptibility, family-of-origin tobacco use, friend cigarette use, and other tobacco product use. Analyses stratified by other tobacco product use at baseline showed that e-cigarette–ever use was associated with greater odds of combustible tobacco cigarette–ever use during follow-up in baseline never users of cigars, hookah, or smokeless tobacco (OR = 2.26; 95% CI = 1.35–3.76) but not among ever users of other products at baseline (OR = 1.13; 95% CI = 0.81–1.58). The strengths of the study include a longer follow-up period than most prior research (i.e., 18 months here versus 12 months) and comprehensive adjustment of interpersonal risk factors for smoking. The statistical adjustment of use of other tobacco products and the estimation of associations among young adults who had never used any other tobacco products is another strength, which suggests that the associations were not explained entirely by a non-specific constellation of poly-tobacco product use behavior that generalizes to any form of nicotine or tobacco use. Limitations include the application of ever use in operationalizing the e-cigarette exposure, precluding investigation of dose–response associations between level of e-cigarette use and odds of becoming an ever smoker, and the omissions of some covariates that may provide a more comprehensive adjustment for endogenous liability to smoking (e.g., sensation seeking, depression). In sum, this study provides further evidence of an association of e-cigarette use with transition to ever smoking in young adult college students.3
___________________
3 Two studies were published after the end of the committee’s search dates for their systematic review that otherwise met the committee’s inclusion criteria. Hammond and colleagues (2017) surveyed Canadian high school students (n = 17,318) and found that past 30-day e-cigarette use among baseline never smokers was associated with increased likelihood of smoking a whole cigarette by follow-up and that past 30-day e-cigarette use among baseline never daily smokers was associated with increased odds of having smoked daily for at least 7 consecutive days by follow-up after adjusting for covariates. Bold and colleagues (2017) surveyed high school students (n = 808) across three waves (2013, 2014, and 2015) in three public schools in Connecticut. Past-month e-cigarette use was associated with subsequent past month combustible tobacco cigarette use (Wave 1–2, odds ratio [OR] = 7.08, 95% CI = 2.34–21.42; Wave 2–3, OR = 3.87, 95% CI = 1.86–8.06). These studies provide additional evidence consistent with those included in the committee’s review, showing an association between e-cigarette use with subsequent ever smoking and past 30-day smoking, and with progression to more frequent (i.e., daily) smoking.
Supporting Evidence
Studies Addressing Conceptually Plausible Mechanisms by Which E-Cigarette Use Affects Smoking
Studies of psychosocial variables expected to moderate or mediate the association of e-cigarette use with smoking based on conceptual models of the e-cigarette use–smoking link were interpreted by the committee as supportive evidence. For possible moderation effects, the catalyst and diversion models lead to distinct predictions regarding the subgroups of youth and young adults for whom the e-cigarette–smoking association may be most salient. The catalyst hypothesis proposes that e-cigarettes increase smoking among low-risk youth who would be unlikely to have started smoking in the absence of e-cigarettes (Schneider and Diehl, 2016). By contrast, the diversion hypothesis proposes that e-cigarettes reduce smoking among high-risk users who would be liable to initiate smoking in the absence of e-cigarettes by providing an alternative to satisfy a propensity to explore novel experiences (Etter, 2017; Warner, 2016). Finally, a common liability hypothesis would propose that e-cigarette use and smoking may be statistically associated due to a shared propensity to experiment with substances (Etter, 2017); thus, a positive association would be present only in youth who possess risk factors indicative of a liability to smoking.
Of longitudinal studies that provide evidence of an association between e-cigarette use and ever smoking among low-risk youth, all six studies have shown that the positive association of e-cigarette use with ever smoking in adolescents and young adults is present in low-risk youth or significantly stronger among lower- (versus higher-) risk youth in the form of a statistical interaction. The e-cigarette–smoking positive association has been shown to be present or significantly amplified for several indicators of lower-risk status, including among youth who report not being susceptible or willing to start smoking (Barrington-Trimis et al., 2016a; Best et al., 2017; Primack et al., 2015; Wills et al., 2016c), have no friends who smoke (Best et al., 2017; Conner et al., 2017), perceive that smoking poses great risk to health (Miech et al., 2017), have high parental support (Wills et al., 2016c), do not perceive themselves as rebellious (Wills et al., 2016c), and have never used other tobacco products (Loukas et al., 2018). Such findings are concordant with the catalyst hypothesis that e-cigarette use increases smoking by changing the likelihood of smoking in low-risk youth who otherwise would not have been liable to become ever smokers.
The catalyst hypothesis also proposes several mediating processes through which e-cigarette use increases smoking (Schneider and Diehl, 2016). Because e-cigarette use may produce positive sensations in the
airways and pleasant tastes and lack aversive effects like lung discomfort, the catalyst hypothesis proposes that e-cigarette use may cause adolescent or young adult never smokers to change their perceptions about combustible tobacco cigarettes to be more favorable and increase their willingness to try smoking. Consistent with this notion, longitudinal studies of baseline never smokers have found that e-cigarette use is associated with future increases in positive perceptions about smoking, willingness to smoke, and lower perceptions that smoking is harmful (Miech et al., 2017; Primack et al., 2015; Wills et al., 2016a), which, in turn, mediate increases in probability of becoming an ever smoker (Wills et al., 2016a). Another mediating mechanism proposed by the catalyst model is that youth who enjoy the pharmacological effects of nicotine or develop initial symptoms of nicotine dependence via e-cigarettes may be more inclined to use other tobacco products with nicotine-related pharmacological effects, such as combustible tobacco cigarettes (Schneider and Diehl, 2016). If this mechanism accounted for some of the association of e-cigarette use with increased future smoking, the amount of nicotine used in e-cigarettes should differentiate the future smoking behavior of youth who vape. Concordant with this notion, a dose–response association between the level of nicotine concentration used in e-cigarettes and increases in future smoking frequency and intensity has been reported among adolescent e-cigarette users (Goldenson et al., 2017).
One qualitative focus group study of perceptions about the effect of e-cigarette use on smoking among Swiss youth and young adult (age 16–26) users and non-users and of combustible tobacco cigarettes, e-cigarettes, or both products addresses the plausibility of the catalyst and diversion models (Akre and Suris, 2017). The majority of testimonials were consistent with the catalyst model, and included statements such as
- “It’s maybe a little smoother to start [vaping] … than by directly starting with a normal cigarette” (Akre and Suris, 2017, p. 450);
- “The [e-cigarette] can make the gesture a commonplace, one will lose track of the danger of smoking by starting with the [e-cigarette] just for the taste … and after why not pass on to [tobacco cigarettes] which is the following step” (Akre and Suris, 2017, p. 450);
- “I think [vaping is] fun for a little while but it lacks the whole aspect of the normal cigarette” (Akre and Suris, 2017, p. 450); and
- “I believe [e-cigarettes] could be a trampoline, like me, I started vaping, and finally I started smoking, while maybe I wouldn’t have started smoking if I hadn’t vaped” (Akre and Suris, 2017, p. 450).
While the majority of testimonials in this study were consistent with the catalyst model, the authors reported that a minority of dual-user
young adults had opinions that vaping could be a diversion for young adolescents with statements such as, “I think it’s not that bad that the tendency goes toward e-cigarettes for the young ones because if it wasn’t for the e-cigarettes they would all turn to tobacco” (Akre and Suris, 2017, p. 452), and “Youths now discover the e-cigarette, there are lots of flavors, and then they’ll say to themselves ‘why would I turn to tobacco?’ and they will get used to these flavors and will find tobacco cigarettes disgusting, which is good” (Akre and Suris, 2017, p. 452). Although the results of the study provided unique contextual information, the results should be interpreted with caution, because the sample was small (n = 42) and non-representative.
Trends in E-Cigarette and Combustible Tobacco Cigarette Use in the Adolescent and Young Adult Population
Trends in the prevalence of e-cigarette use relative to combustible tobacco cigarette smoking over time may also shed light on whether and how e-cigarette use affects smoking. Holding all other factors constant, if e-cigarette use increased risk of smoking, then changes in the prevalence of e-cigarette use should parallel changes in the prevalence of smoking. If e-cigarette use prevented combustible tobacco cigarette smoking, changes in the prevalence of e-cigarette use and smoking over time should oppose each other. Evidence from the National Youth Tobacco Survey (NYTS) show that from 2011 to 2016, the past 30-day use of e-cigarettes increased (non-linearly) and combustible tobacco cigarette use decreased linearly in middle and high school students (Jamal et al., 2017) (see Figure 16-3).
The population-level ecological data are more consistent with the diversion hypothesis than the catalyst hypothesis and run counter to individual-level results, which predominately show that youth and young adults who use e-cigarettes are more likely to become ever smokers and past 30-day smokers. A more granular examination of the population-level data indicates that the pattern of trends in use for the two products are not unequivocally concordant. There is a non-linear trend for past 30-day e-cigarette use in NYTS high school students, which increased from 1.5 percent in 2011 to 16.0 percent in 2015 and then decreased to 11.3 percent in 2016. For past 30-day combustible tobacco cigarette use in the NYTS, use decreased from 15.8 percent in 2011 to 9.3 percent in 2015 and was 8.0 percent in 2016. Due to the methodological limitations of interpreting causality from this descriptive population-level trend analysis and the multitude of influences on population-level e-cigarette use (e.g., policy changes, cultural changes, historical cohort effects), the ecological analysis does not provide strong evidence to rule out a possible risk-enhancing effect of e-cigarette use on youth smoking. Some population-based ecological studies report that downward trends in smoking rates in youth
SOURCE: Jamal et al., 2017.
across time stem back for decades. The rate of such reductions did not accelerate over the past several years after e-cigarette use was introduced into the U.S. market and became popular among youth (Barrington-Trimis et al., 2016a,b). In an analysis of NYTS data from 2004 to 2014, interrupted time series of ever (1 puff or more) and current (last 30 days) smoking comparing the rate of deceleration in combustible tobacco cigarette use before and after 2009 when e-cigarettes first became available showed no significant change in the overall linear trend in reduction in smoking by year before versus after 2009 (p = 0.57 and 0.23). Hence, the overall concordance in the ecological data is not clear. Overall, the population-based data broadly show opposing trends in e-cigarette and cigarette use prevalence across time among U.S. youth in recent years and thus do not provide confirmatory evidence of the epidemiological person-level positive associations of vaping and smoking.
Studies of the Association of E-Cigarette Use Policy Change and Youth Smoking
Three studies have examined whether changes in the prevalence of combustible tobacco cigarette use differ among youth who reside across
different states as a function of when laws prohibiting sales of e-cigarettes to minors were enacted. The diversion model would hypothesize that magnitude of downward trends in smoking would be slowed before and after enactment of sales restrictions because youth would not have access to e-cigarettes as an alternative to satisfy exploratory drives and would be more likely to resort to smoking (Etter, 2017; Warner, 2016). The catalyst model would hypothesize that e-cigarette sales bans would accelerate downward trends in smoking by reducing the likelihood that one would be exposed to a product that increases risk of smoking (i.e., e-cigarettes). The findings differed across the three studies. The most recent article on the topic by Abouk and Adams (2017) concluded that e-cigarette sales restrictions were associated with reduced adolescent smoking through 2014, while two earlier articles by Friedman (2015) and Pesko and colleagues (2016) came to the opposite conclusion, that e-cigarette sales restrictions were associated with higher levels of adolescent smoking through 2013. Abouk and Adams (2017) noted that the analysis in their study was more “granular” because it took into account the exact month that e-cigarette bans went into place, while the other articles take into account year of the ban. Overall, the small and inconsistent evidence base on this topic fails to provide confirmatory evidence for or against individual-level associations found in the principal epidemiological data. Incidentally, a 2015 Institute of Medicine report addressing the effect of sales restrictions on youth tobacco use concluded that if bans effectively reduce availability of tobacco from commercial sources, the effect of this on use by youth is uncertain because of the continued availability of tobacco from non-commercial sources (IOM, 2015). Hence, these policy change studies provide minimal weight in interpreting the direction and causality of the association of e-cigarette use with youth smoking.
Analogous Studies Involving Other Tobacco Products and Studies of Associations of E-Cigarette Use with Other Risk Behaviors
Given that the catalyst model hypothesizes that e-cigarette use may increase smoking in youth by sensitizing youth to nicotine in a form that is more palatable and lacks aversive qualities of cigarettes (Schneider and Diehl, 2016), other products that share properties with e-cigarettes would also be expected to be associated with increased risk of smoking. Hookah waterpipe is one such product that mirrors e-cigarettes in that it also delivers nicotine, is available in sweet flavorings, and is less irritating to the airways due to water-based filtration and cooling of hookah smoke. Consistent with this notion, Soneji and colleagues (2017) found in a nationally representative sample of U.S. adolescents and young adult never smokers, hookah ever use at baseline was associated with cigarette
smoking initiation (adjusted OR = 2.56; 95% CI = 1.46–4.47), past 30-day cigarette smoking (adjusted OR = 2.48; 95% CI = 1.01–6.06), and higher intensity of cigarette smoking (adjusted proportional OR = 2.55; 95% CI = 1.48–4.38) at a 1-year follow-up. In addition, e-cigarettes would be expected to increase risk of other combustible tobacco products per the catalyst hypothesis, which has been shown in several studies (Barrington-Trimis et al., 2016a; Leventhal et al., 2015). However, these studies can also be interpreted as providing evidence for the common liability model. Youth who have a liability to experiment with multiple tobacco products would show indiscriminate forms of poly-tobacco product use involving the use of multiple non-cigarette and cigarette products in various sequences. However, evidence showing that e-cigarette use is associated with later combustible tobacco cigarette use in samples excluding users of other tobacco products suggests that indiscriminate patterns of polytobacco product use are unlikely to explain the association (Leventhal et al., 2015; Loukas et al., 2018). Regardless, studies of associations involving analogous tobacco products can be interpreted as providing no evidence of inverse associations between use of analogous tobacco products with e-cigarettes and cigarettes, suggesting confirmatory evidence against the diversion hypothesis.
In addition to considering other tobacco products the committee considered associations of e-cigarette use with other risky behaviors. Some (but not all) of the putative mechanisms linking e-cigarette use with later smoking proposed by the catalyst hypothesis are expected to selectively increase risk of smoking and not impact likelihood of engaging in other risky behaviors (Schneider and Diehl, 2016). One longitudinal study of young adults in Los Angeles found that at 1-year follow-up, baseline past 30-day e-cigarette use was more strongly associated with past 30-day combustible tobacco cigarette (OR = 3.32; 95% CI = 1.55–7.10) than past 30-day cannabis use (OR = 1.97; 95% CI = 1.01–3.86) (Unger et al., 2016). The estimate appears larger for combustible tobacco cigarette than cannabis use; however, the confidence intervals for the estimates are overlapping with one another. Thus, this study provides suggestive evidence of specificity of the association from e-cigarette use to smoking aligned with the catalyst hypothesis and adds some further support to the conclusion from the primary review.
SYNTHESIS
Conclusion 16-1. There is substantial evidence that e-cigarette use increases risk of ever using combustible tobacco cigarettes among youth and young adults.
In the primary review of observational studies, there was consistent evidence from 10 of 10 studies4 that the association between e-cigarette use and transition from never to ever combustible tobacco cigarette smoking was positive in direction. Results were consistent across a number of different methodologies, age ranges, research groups, and locations, and the associations were of considerable strength. While there were moderate to high attrition rates in most of these studies, analyses of attrition effects showed that the impact of attrition on the observed associations was modest. Across the studies, a wide range of covariates were adjusted for that spanned a number of sociodemographic, interpersonal, environmental, and intrapersonal factors, including use of other substances (see Table 16-1), which the committee considered a comprehensive selection of confounding factors. Use of tobacco products other than e-cigarettes and combustible tobacco cigarettes was adjusted for in several of the studies, and two studies reported positive associations of e-cigarette use and subsequent transition to ever smoking among baseline never users of any tobacco product. Covariate adjustment had non-uniform effects on the magnitude of associations, with some reports showing no effect of covariate adjustment and other studies showing a reduction in the estimate from unadjusted to adjusted models. However, the committee found no published reports of non-significant associations after adjusting for covariates. To the extent to which the adjusted covariates addressed the influence of confounders, it is unlikely that confounding entirely accounts for the association because reductions in estimates of association from unadjusted to adjusted models were not consistently observed in the literature. Only a small number of studies provide evidence to assess for the presence of a dose–response association between e-cigarette use and smoking initiation, but the results of these few studies suggested that higher levels of e-cigarette use were associated with increased odds of smoking in baseline non-smokers.
The primary evidence consisted of longitudinal cohort studies that assessed e-cigarette use at baseline and smoking at a future follow-up assessment among baseline never smokers. The committee deliberated at length regarding the strengths and weaknesses of this design and how it impacted interpretation of the evidence. Removal of baseline ever smokers from the analyses is critical for eliminating the possibility of reverse causation. However, this method also systematically alters the population under study in several ways, which could inflate the likelihood of finding
___________________
4 As noted in the evidence review, one additional study published after the end of the committee’s search dates for their systematic review that otherwise met the committee’s inclusion criteria (Hammond et al., 2017) provides additional evidence of an association between e-cigarette use and subsequent ever smoking.
a positive association between e-cigarette use and ever smoking. Restriction to baseline never smokers removes youth and young adults who have highest liability for early-onset smoking. Because the diversion hypothesis would predict that protection against smoking initiation due to e-cigarette use would be most pronounced among those with highest liability for early-onset smoking, removing baseline smokers could restrict the capacity to detect diversion effects. The remaining sample of never smokers still likely retains some meaningful variation in liability for smoking onset that is not a consequence of e-cigarette use and due to other sources (e.g., social acceptance of smoking, residential proximity to tobacco product retailers). Such variance in smoking liability in the remaining sample of baseline never smokers could potentially influence e-cigarette use and thus confound associations between e-cigarette use and later transition to ever smoking. If, following the premises stated above, removal of baseline ever smokers were to significantly impact capacity to detect diversion effects, estimates of association between e-cigarette use and subsequent ever smoking would be expected to vacillate markedly across studies of populations that differ in liability for smoking. This was not the case. Robust positive associations between e-cigarette use in baseline never smokers and later ever smoking was observed in studies of young adolescents, older adolescents, and young adults, although these populations are known to differ in risk of smoking onset (Johnston et al., 2017). Different regions of the United States and other countries have unique tobacco product policies and sociocultural backdrops that may alter the availability and population-level risk of e-cigarette use or combustible tobacco cigarette use in each location. Associations between e-cigarette use and subsequent ever use of combustible tobacco products were observed in regional U.S. samples from California, Hawaii, Texas, and Virginia, and in nationally representative U.S. samples; and in Canadian, Scottish, and British samples. The overwhelming consistency of results across studies from different locations and across studies that differed in other ways (e.g., wording of survey questions; paper versus Internet survey; with versus without biochemical verification of self-reported smoking; length of follow-up) strengthened the committee’s confidence in the robustness, validity, and causality of the association of e-cigarette use with transition from never to ever smoker status.
In the supplemental review, some results confirmed the primary evidence, whereas others did not. Studies of whether the association varied across moderator variables consistently supported the plausibility of the catalyst hypothesis. Eight longitudinal cohort studies found that e-cigarette use increases risk of ever smoking among youth with fewer traditional risk factors for smoking (e.g., fewer friends who smoke)—a subgroup of youth who would be expected to have low likelihood of
becoming an ever smoker if e-cigarettes were not otherwise available. Other longitudinal evidence showed that e-cigarette use was associated with increased levels on mediator variables (e.g., more favorable perceptions of smoking) that are presumed by the catalyst hypothesis to channel the effect of e-cigarette use on to smoking. One study found that youth who vaped a higher concentration of nicotine were more likely to subsequently smoke at higher frequency and intensity levels, which further supports the catalyst hypothesis and suggests specificity of the association to nicotine and nicotine-containing products. Collectively, there was very strong evidence from longitudinal cohort studies of high plausibility that e-cigarette use may be a catalyst for smoking initiation, which strengthened the committee’s confidence in a possible causal link from e-cigarette use to combustible tobacco cigarette ever use. By contrast, ecological trends in e-cigarette use and smoking prevalence in youth across time failed to provide confirmatory support that e-cigarette use causes smoking initiation, and, if anything, are more consistent with the notion that e-cigarette use is associated with reduced smoking. However, ecological studies of trends going back a decade found that the rate of reduction of smoking in U.S. youth has remained consistent and has not accelerated in recent years when e-cigarettes have become popular. In addition, the changes in the prevalence of tobacco product use by U.S. high school students from 2015 to 2016 declined substantially for e-cigarettes and marginally for combustible tobacco cigarettes, raising questions about whether there is a systematic concordance between e-cigarette and combustible tobacco cigarette use over time in the population. Results from studies of e-cigarette sales restrictions and analogous associations involving cigarette and non-cigarette tobacco products were found to provide negligible weight toward the overall conclusion. Suggestive evidence of specificity of the association of e-cigarette use with combustible tobacco cigarette use relative to cannabis use from one study added marginal weight to the conclusion that e-cigarette use increases risk of becoming an ever smoker. In sum, because the supplemental review provided strong evidence of plausibility and specificity of a possible causal effect of e-cigarette use on smoking, and did not find conclusive evidence to refute the catalyst explanation, the committee considered the overall body of evidence of a causal effect of e-cigarette use on risk of transition from never to ever smoking to be substantial.
Conclusion 16-2. Among youth and young adult e-cigarette users who ever use combustible tobacco cigarettes, there is moderate evidence that e-cigarette use increases the frequency and intensity of subsequent combustible tobacco cigarette smoking.
Conclusion 16-3. Among youth and young adult e-cigarette users who ever use combustible tobacco cigarettes, there is limited evidence that e-cigarette use increases, in the near term, the duration of subsequent combustible tobacco cigarette smoking.
Primary review of the observational literature yielded several studies showing a positive association between e-cigarette–ever use and past 30-day smoking status, which is a weak proxy for patterns of smoking indicative of progression in frequency and intensity. Among studies better positioned to address progression in frequency and intensity, the evidence supported a positive association of more frequent e-cigarette use with progression in smoking frequency and intensity.5 Dose–response associations were evident or suggestive across most analyses among those four studies. Two studies included multiple follow-up time points and addressed whether e-cigarette use is associated with longer duration of smoking. One study found that e-cigarette use was associated with an acceleration of smoking intensity over time. Another study showed that changes in smoking status across two follow-up times did not significantly differ between baseline e-cigarette–ever users compared with e-cigarette–never users. The supplemental review found that youth nonsmokers who vape higher concentrations of nicotine were more likely to subsequently smoke at higher frequency and intensity rates, which added weight to the plausibility that e-cigarette use affects progression of smoking in some manner. There were no reports across any of the studies reviewed by the committee showing that e-cigarette users were associated with lower likelihood or speed of progression of smoking frequency, intensity, or duration. Hence, it is highly unlikely that e-cigarette users who become ever smokers are overrepresented by youth who may just be temporarily experimenting at low levels of smoking.
REFERENCES
Abouk, R., and S. Adams. 2017. Bans on electronic cigarette sales to minors and smoking among high school students. Journal of Health Economics 54:17–24.
Akre, C., and J. C. Suris. 2017. Adolescents and young adults’ perceptions of electronic cigarettes as a gateway to smoking: A qualitative study in Switzerland. Health Education Research 32(5):448–545.
Ambrose, B. K., H. R. Day, B. Rostron, K. P. Conway, N. Borek, A. Hyland, and A. C. Villanti. 2015. Flavored tobacco product use among U.S. youth aged 12–17 years, 2013–2014. Journal of the American Medical Association 314(17):1871–1873.
___________________
5 As noted in the evidence review, one additional study published after the end of the committee’s search dates for its systematic review that otherwise met the committee’s inclusion criteria (Hammond et al., 2017) provides additional evidence of an association between e-cigarette use and progression to more frequent smoking.
Barrington-Trimis, J. L., R. Urman, K. Berhane, J. B. Unger, T. B. Cruz, M. A. Pentz, J. M. Samet, A. M. Leventhal, and R. McConnell. 2016a. E-cigarettes and future cigarette use. Pediatrics 138(1). doi.org/10.1542/peds.2016-0379 (accessed December 29, 2017).
Barrington-Trimis, J. L., R. Urman, A. M. Leventhal, W. J. Gauderman, T. B. Cruz, T. D. Gilreath, S. Howland, J. B. Unger, K. Berhane, J. M. Samet, and R. McConnell. 2016b. E-cigarettes, cigarettes, and the prevalence of adolescent tobacco use. Pediatrics 138(2). 10.1542/peds.2015-3983 (accessed December 29, 2017).
Best, C., F. Haseen, D. Currie, G. Ozakinci, A. M. MacKintosh, M. Stead, D. Eadie, A. MacGregor, J. Pearce, A. Amos, J. Frank, and S. Haw. 2017. Relationship between trying an electronic cigarette and subsequent cigarette experimentation in Scottish adolescents: A cohort study. Tobacco Control 20(1):140–144. doi: 10.1136/tobaccocontrol-2017-053691 (accessed February 2, 2018).
Bold, K. W., G. Kong, D. A. Cavallo, D. R. Camenga, and S. Krishnan-Sarin. 2016. E-cigarette susceptibility as a predictor of youth initiation of e-cigarettes. Nicotine & Tobacco Research. https://academic.oup.com/ntr/article-abstract/20/1/140/2754532 (accessed February 2, 2018).
Bold, K. W., G. Kong, D. R. Camenga, P. Simon, D. A. Cavallo, M. E. Morean, and S. Krishnan-Sarin. 2017. Trajectories of e-cigarette and conventional cigarette use among youth. Pediatrics. doi: 10.1542/peds.2017-1832 (accessed February 2, 2018).
CDC (Centers for Disease Control and Prevention). 2016. Quickstats: Cigarette smoking status among current adult e-cigarette users, age group—National Health Interview Survey, United States, 2015. Morbidity and Mortality Weekly Report 65(42):1177.
Chassin, L., P. J. Curran, C. C. Presson, S. J. Sherman, and R. J. Wirth. 2009. Monograph 20: Phenotypes and endophenotypes: Foundations for genetic studies of nicotine use and dependence. https://cancercontrol.cancer.gov/BRP/tcrb/monographs/20/m20_5.pdf (accessed December 20, 2017).
Conner, M., S. Grogan, R. Simms-Ellis, K. Flett, B. Sykes-Muskett, L. Cowap, R. I. Lawton, L. J. Armitage, D. Meads, C. Torgerson, R. West, and K. Siddiqi. 2017. Do electronic cigarettes increase cigarette smoking in U.K. adolescents? Evidence from a 12-month prospective study. Tobacco Control. http://tobaccocontrol.bmj.com/content/early/2017/07/28/tobaccocontrol-2016-053539 (accessed February 2, 2018).
Doran, N., K. Brikmanis, A. Petersen, K. Delucchi, W. K. Al-Delaimy, S. Luczak, M. Myers, and D. Strong. 2017. Does e-cigarette use predict cigarette escalation? A longitudinal study of young adult non-daily smokers. Preventive Medicine 100:279–284.
Dutra, L. M., S. A. Glantz, N. E. Lisha, and A. V. Song. 2017. Beyond experimentation: Five trajectories of cigarette smoking in a longitudinal sample of youth. PLoS ONE 12(2):e0171808. https://doi.org/10.1371/journal.pone.0171808 (accessed February 2, 2018).
Etter, J. F. 2017. Gateway effects and electronic cigarettes. Addiction. doi: 10.1111/add.13924 (accessed February 2, 2018).
Friedman, A. S. 2015. How does electronic cigarette access affect adolescent smoking? Journal of Health Economics 44:300–308.
Goldenson, N. I., A. M. Leventhal, M. D. Stone, R. S. McConnell, and J. L. Barrington-Trimis. 2017. Associations of e-cigarette nicotine concentration and subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatrics 171(12):1192–1199.
Hammond, D., J. L. Reid, A. G. Cole, and S. T. Leatherdale. 2017. Electronic cigarette use and smoking initiation among youth: A longitudinal cohort study. Canadian Medical Association Journal 189(43):E1328.
HHS (U.S. Department of Health and Human Services). 2012. Preventing tobacco use among youth and young adults: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
HHS. 2014. The health consequences of smoking—50 years of progress: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Disease Prevention and Health Promotion, Office on Smoking and Health.
HHS. 2016. E-cigarette use among youth and young adults: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
Hornik, R. C., L. Gibson, and C. Lerman. 2016. Pos5-30: Prediction of cigarette use from six-month prior electronic and combustible cigarette use for a U.S. national sample of 13-25 year olds [abstract]. http://c.ymcdn.com/sites/www.srnt.org/resource/resmgr/Conferences/2016_Annual_Meeting/Program/SRNT_2016_Rapids_WEB2.pdf (accessed September 20, 2017).
IOM (Institute of Medicine). 2015. Public health implications of raising the minimum age of legal access to tobacco products. Washington, DC: The National Academies Press.
Jamal, A., B. A. King, L. J. Neff, J. Whitmill, S. D. Babb, and C. M. Graffunder. 2016. Current cigarette smoking among adults—United States, 2005–2015. Morbidity and Mortality Weekly Report 65(44):1205–1211.
Jamal, A., A. Gentzke, S. Hu, K. A. Cullen, B. J. Apelberg, D. M. Homa, and B. A. King. 2017. Tobacco use among middle and high school students—United States, 2011–2016. Morbidity and Mortality Weekly Report 66(23):597–603.
Johnston, L. D., P. M. O’Malley, R. Miech, J. G. Bachman, and J. E. Schulenberg. 2017. Monitoring the Future national survey results on drug use, 1975–2016: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf (accessed February 2, 2018).
Kasza, K. A., B. K. Ambrose, K. P. Conway, N. Borek, K. Taylor, M. L. Goniewicz, K. M. Cummings, E. Sharma, J. L. Pearson, V. R. Green, A. R. Kaufman, M. Bansal-Travers, M. J. Travers, J. Kwan, C. Tworek, Y. C. Cheng, L. Yang, N. Pharris-Ciurej, D. M. van Bemmel, C. L. Backinger, W. M. Compton, and A. J. Hyland. 2017. Tobacco-product use by adults and youths in the United States in 2013 and 2014. New England Journal of Medicine 376(4):342–353.
Kong, G., M. E. Morean, D. A. Cavallo, D. R. Camenga, and S. Krishnan-Sarin. 2015. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine & Tobacco Research 17(7):847–854.
Kozlowski, L. T., and K. E. Warner. 2017. Adolescents and e-cigarettes. Objects of concern may appear larger than they are. Drug and Alcohol Dependence 174:209–214.
Leventhal, A. M., D. R. Strong, M. G. Kirkpatrick, J. B. Unger, S. Sussman, N. R. Riggs, M. D. Stone, R. Khoddam, J. M. Samet, and J. Audrain-McGovern. 2015. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. Journal of the American Medical Association 314(7):700–707.
Leventhal, A. M., M. D. Stone, N. Andrabi, J. Barrington-Trimis, D. R. Strong, S. Sussman, and J. Audrain-McGovern. 2016. Association of e-cigarette vaping and progression to heavier patterns of cigarette smoking. Journal of the American Medical Association 316(18):1918–1920.
Loukas, A., C. N. Marti, M. Cooper, K. E. Pasch, and C. L. Perry. 2018. Exclusive e-cigarette use predicts cigarette initiation among college students. Addictive Behaviors 76(Supple-ment C):343–347.
Lydon, D. M., S. J. Wilson, A. Child, and C. F. Geier. 2014. Adolescent brain maturation and smoking: What we know and where we’re headed. Neuroscience & Biobehavioral Reviews 45:323–342.
Miech, R., M. E. Patrick, P. M. O’Malley, and L. D. Johnston. 2016. What are kids vaping? Results from a national survey of U.S. adolescents. Tobacco Control 26(4):386–391.
Miech, R., M. E. Patrick, P. M. O’Malley, and L. D. Johnston. 2017. E-cigarette use as a predictor of cigarette smoking: Results from a 1-year follow-up of a national sample of 12th grade students. Tobacco Control 26:e106–e111. http://tobaccocontrol.bmj.com/content/26/e2/e106.info (accessed February 2, 2018).
Neff, L. J., R. A. Arrazola, R. S. Caraballo, C. G. Corey, S. Cox, B. A. King, C. J. Choiniere, and C. G. Husten. 2015. Frequency of tobacco use among middle and high school students—United States, 2014. Morbidity and Mortality Weekly Report 64(38):1061–1065.
Patel, D., K. C. Davis, S. Cox, B. Bradfield, B. A. King, P. Shafer, R. Caraballo, and R. Bunnell. 2016. Reasons for current e-cigarette use among U.S. adults. Preventive Medicine 93:14–20.
Pesko, M. F., J. M. Hughes, and F. S. Faisal. 2016. The influence of electronic cigarette age purchasing restrictions on adolescent tobacco and marijuana use. Preventive Medicine 87:207–212.
Phillips, C. V. 2015. Gateway effects: Why the cited evidence does not support their existence for low-risk tobacco products (and what evidence would). International Journal of Environmental Research and Public Health 12(5):5439–5464.
Primack, B. A., S. Soneji, M. Stoolmiller, M. J. Fine, and J. D. Sargent. 2015. Progression to traditional cigarette smoking after electronic cigarette use among U.S. adolescents and young adults. JAMA Pediatrics 169(11):1018–1023.
Primack, B. A., A. Shensa, J. E. Sidani, B. L. Hoffman, S. Soneji, M. J. Fine, J. Everette, and J. Sargent. 2016. Initiation of cigarette smoking after e-cigarette use: A nationally representative study [abstract]. http://www.sbm.org/UserFiles/file/2016AbstractSupplement.pdf (accessed September 20, 2017).
Sargent, J. D., J. Gabrielli, A. Budney, S. Soneji, and T. A. Wills. 2017. Adolescent smoking experimentation as a predictor of daily cigarette smoking. Drug and Alcohol Dependence 175:55–59.
Schneider, S., and K. Diehl. 2016. Vaping as a catalyst for smoking? An initial model on the initiation of electronic cigarette use and the transition to tobacco smoking among adolescents. Nicotine & Tobacco Research 18(5):647–653.
Selya, A. S., J. S. Rose, L. Dierker, D. Hedeker, and R. J. Mermelstein. 2017. Evaluating the mutual pathways among electronic cigarette use, conventional smoking and nicotine dependence. Addiction 113(2):325–333.
Soneji, S., J. L. Barrington-Trimis, T. A. Wills, A. M. Leventhal, J. B. Unger, L. A. Gibson, J. Yang, B. A. Primack, J. A. Andrews, R. A. Miech, T. R. Spindle, D. M. Dick, T. Eissenberg, R. C. Hornik, R. Dang, and J. D. Sargent. 2017. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: A systematic review and meta-analysis. JAMA Pediatrics 171(8):788–797.
Spindle, T. R., M. M. Hiler, M. E. Cooke, T. Eissenberg, K. S. Kendler, and D. M. Dick. 2017. Electronic cigarette use and uptake of cigarette smoking: A longitudinal examination of U.S. college students. Addictive Behaviors 67:66–72.
Unger, J. B., D. W. Soto, and A. Leventhal. 2016. E-cigarette use and subsequent cigarette and marijuana use among Hispanic young adults. Drug and Alcohol Dependence 163:261–264.
Warner, K. E. 2016. Frequency of e-cigarette use and cigarette smoking by American students in 2014. American Journal of Preventive Medicine 51(2):179–184.
Whiting, P., J. Savovic, J. P. Higgins, D. M. Caldwell, B. C. Reeves, B. Shea, P. Davies, J. Kleijnen, R. Churchill, and ROBIS group. 2016. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. Journal of Clinical Epidemiology 69:225–234.
Wills, T. A., F. X. Gibbons, J. D. Sargent, and R. J. Schweitzer. 2016a. How is the effect of adolescent e-cigarette use on smoking onset mediated: A longitudinal analysis. Psychology of Addictive Behaviors 30(8):876–886.
Wills, T. A., R. Knight, J. D. Sargent, F. X. Gibbons, I. Pagano, and R. J. Williams. 2016b. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tobacco Control 26(1):34–39.
Wills, T. A., J. D. Sargent, F. X. Gibbons, I. Pagano, and R. Schweitzer. 2016c. E-cigarette use is differentially related to smoking onset among lower risk adolescents. Tobacco Control 26(5):534–536.
Young, A. F., J. R. Powers, and S. L. Bell. 2006. Attrition in longitudinal studies: Who do you lose? Australian and New Zealand Journal of Public Health 30(4):353–361.
Zhuang, Y. L., S. E. Cummins, Y. S. J, and S. H. Zhu. 2016. Long-term e-cigarette use and smoking cessation: A longitudinal study with U.S. population. Tobacco Control 25(Sup-plement 1):i90–i95.
















































