7
Modes of Action
Although the use of electronic cigarettes has increased steadily since their introduction into the market about a decade ago, much is unknown about their safety profile. As concluded in Chapter 5, the number of chemicals and their content in combustible tobacco cigarette smoke is much higher than emissions from most e-cigarette products, yet there are still concerns about the toxic properties of the variable combination of chemicals present in e-liquids and the additional chemicals generated during the aerosolization of e-liquids. The toxicology of e-cigarettes is a fertile area of investigation and one of current vigorous activity. Whereas previous chapters discussed toxicology of individual constituents, this section discusses toxicology of e-cigarette aerosols as a whole. The majority of the published work on the subject is in the form of acute in vivo studies, along with multiple in vitro studies using various human- and animal-derived cell lines, including endothelial, fibroblast, and cardiomyocytes, to name a few. Figure 7-1 shows the trend in publications per year on e-cigarettes and in vitro systems. The first publications (n = 5) appeared in 2013. Since then, there has been a steady increase in the number of publications, with a cumulative total of 124 as of September 2017. This section discusses two modes of action—endothelial cell dysfunction and oxidative stress—that are associated with the development of a range of health outcomes. Appendix D contains a summary of in vitro studies in which cytotoxicity is assessed.
ENDOTHELIAL CELL DYSFUNCTION
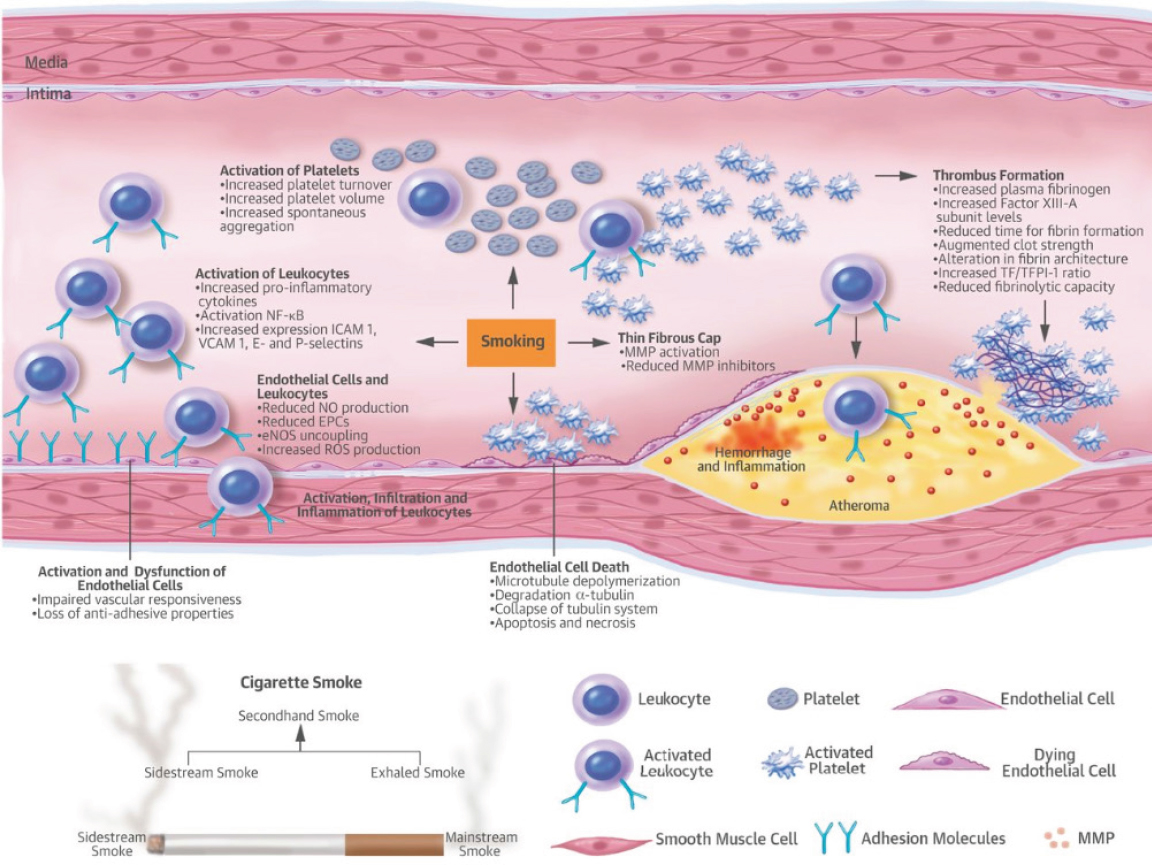
Smoking is among the most prominent preventable contributing risk factors for the development of various diseases, primarily cancers and cardiovascular diseases. The role of chemicals generated from traditional tobacco smoke on endothelial cell function has been well documented. With the introduction and recent use of electronic cigarettes, a key question is whether e-cigarette use and the chemicals present in e-liquids as well as those generated during use produce similar effects on endothelial cell function. The two emerging questions are whether such effects are seen with e-cigarette use and what the magnitude of these effects is compared with traditional combustible tobacco cigarette smoke or to neither e-cigarette nor combustible tobacco use. It is well accepted that endothelial cell dysfunction produced by traditional tobacco burning involves various key initiating events, including a reduction in nitric oxide (NO) net availability, increased reactive oxygen species (ROS) generation, and increased expression of adhesion molecules that facilitate trans-endothelial movement and deposition of activated complement components. More details on the effect of tobacco smoking on endothelial cell activation and dysfunction, and the molecular mechanisms involved can be found in the review by Morris and colleagues (2015). Figure 7-2,
from this review, highlights the key events associated with endothelial cell dysfunction by tobacco smoke.
Evidence Review
In a study by Antoniewicz and colleagues (2016), the effect of e-cigarette inhalation on vascular function was evaluated in healthy sporadic smokers (10 combustible tobacco cigarettes or fewer per month). Circulating endothelial progenitor cells (EPCs) and microvesicles (MVs) in blood were the two endpoints measured. Elevation in circulating levels of EPCs in blood is indicative of and a commonly used marker of vascular endothelial injury. EPCs are stem cells produced primarily in the bone marrow. Their increased presence in blood reflects regeneration of endothelial cells following vascular injury. MVs of endothelial origin can also be used as a biomarker of endothelial cell activation and/or death via apoptosis. Both biomarkers are used when assessing the risk of cardiovascular complications and are also implicated in pulmonary disease (chronic obstructive pulmonary disease [COPD] and emphysema). Their results show that levels of circulating EPCs increased significantly with short-term exposure to e-cigarette inhalation, while MV remained unchanged. The authors concluded that the effect of e-cigarette use on EPCs is similar in magnitude to that produced by combustible tobacco cigarette smoking, and is indicative of vascular injury. They attributed the unaffected MV values to insufficient exposure time because cotinine levels were much lower in their e-cigarette subjects compared with combustible tobacco cigarette users (Antoniewicz et al., 2016). The question of which of these two biomarkers (EPCs versus endothelial-derived MVs) is a more sensitive and earlier indicator of endothelial dysfunction well in advance of detection of ultrastructural changes in endothelial cells in response to e-cigarette use warrants further scrutiny.1
Another study, by Putzhammer and colleagues (2016), investigated the effect of e-cigarette aerosol extracts in human umbilical vein endothelial cells (HUVECs). Cells were exposed to hydrophilic fractions from aerosols obtained from various types of e-cigarette devices. Cell death was measured, as well as generation of ROS, cell proliferation rates, and cell morphology. ROS was assessed by measuring the oxidation of 2’,7’-dichlorodihydrofluorescein diacetate. Their results showed that 5 of the 11 e-cigarette aerosols analyzed produced acute cytotoxicity. Similarly, 5 of the 11 aerosols tested reduced cell proliferation rates, while only 1 of
___________________
1Chapter 9 also includes the Antoniewicz et al. (2016) study in its review and focuses on the effects of e-cigarette exposure on E-selectin MVs. The committee finds no conflict between the evidence presented in this chapter and the evidence presented in Chapter 9.
the aerosol extracts led to the generation of ROS. The aerosols generated from different liquids using the same e-cigarette show substantial differences, pointing to the liquids as an important source of toxicity. As previously demonstrated with combustible tobacco cigarette smoke extracts, exposure of HUVECs to e-cigarette aerosols produces prominent changes in cell morphology and alters the functional endothelial monolayer. The authors clearly demonstrated that the source of the e-liquid and type of device used are determining factors in the cytotoxic potency of e-cigarette aerosols and the capacity to change endothelial cell morphology. Equally important, some e-cigarette products showed toxic effects similar to those of combustible, high-nicotine, tobacco cigarettes. Of note, formation of ROS by the e-cigarette extracts tested in this study did not always correlate with their toxic potential, which is an intriguing observation due to the well-proven role of ROS in the cytotoxicity of combustible tobacco cigarette smoke. The authors suggest that the mechanisms of toxicity of certain e-cigarette vapors in endothelial cells may be distinct from those of combustible tobacco cigarettes, where ROS are central to disease etiology. Additionally, two of the three highly toxic e-liquids did not contain nicotine, but contained flavoring or herbal constituents (Putzhammer et al., 2016).
Another study on HUVECs exposed to extracts from e-cigarette aerosol or combustible tobacco cigarette smoke showed that both exposures produced apoptotic and necrotic cell death and that this cytotoxicity is associated with the generation of ROS and DNA damage (Anderson et al., 2016). Not surprisingly, the cytotoxicity was dose dependent for both treatments. The study also addressed the question of what level of oxidative stress is produced in the HUVECs exposed to the extract from e-cigarette aerosol in comparison to that produced by combustible tobacco cigarette smoke exposure. A concentration of 500 µM was used for both types of extracts. This treatment concentration was selected based on the results of their initial cytotoxicity assay with the e-cigarette aerosol extract. The results of the ROS generation fluorescence-based assay indicate that the e-cigarette aerosol extract produces a significant 4.5-fold increase in ROS levels over control values. However, this value is lower than the 7.8-fold increase produced by combustible tobacco cigarette smoke (relative to controls). The role of oxidative stress in the toxic response of these endothelial cells to e-cigarette aerosol exposure was further documented by the use of the antioxidants α-tocopherol and N-acetyl-l-cysteine. Although antioxidant treatment was capable of preventing necrotic cell death, it only afforded partial protection against e-cigarette aerosol-induced apoptotic cell death. This indicates that ROS indeed play a role, in part, in e-cigarette-induced cytotoxicity (Anderson et al., 2016). The lack of complete blockade of endothelial cell apoptosis by
antioxidants, in contrast to the prevention of necrotic cell death, indicates that components other than ROS in extracts from e-cigarette aerosol are also contributing to cytotoxicity of endothelial cells.
A study by Barber and colleagues (2016) goes further in identifying the specific components of e-cigarettes responsible for alterations in endothelial cell function and viability. Again, HUVECs were used as an in vitro model of endothelial cell function/dysfunction. HUVECs were exposed to extracts from combustible tobacco cigarettes and e-cigarettes. In some experiments, pure nicotine was used at a concentration that approximates the blood concentration of someone who smokes one combustible tobacco cigarette. The endpoints analyzed for all exposures included inflammatory response, cell viability and density, and metabolic activity, the latter determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
The results indicate that endothelial cells exposed to tobacco or e-cigarette products, but not nicotine, experienced a significant decrease in viability. By contrast, all forms of exposures (including pure nicotine at a final concentration of 50 nM) reduced cell density by approximately 25 percent. Interestingly, the responsiveness of endothelial cells to changes in cell density was not dependent on the concentration of nicotine present in the formulations tested, or apparently the composition of the “toxic” gases generated from these formulations, because no differences in magnitude of changes in cell density were detected between tobacco smoke extracts and e-cigarette aerosol extracts. The five e-cigarette products tested contained nicotine concentrations ranging from 0 to 18 mg/ml.
The results of the MTT assay revealed that for the majority of e-cigarette aerosol extracts and for all tobacco smoke extracts, metabolic activity is significantly reduced to comparable levels. Moreover, pure nicotine exposure did not change endothelial cell metabolic activity in comparison to control exposures. The authors emphasize observations indicating that endothelial cell metabolic activity was sensitive to the presence of the extracts, but not to the exact formulation of each extract. Worth also noting is that pure nicotine did not change endothelial cell metabolic activity (Barber et al., 2016).
Lastly, enhanced deposition of various components of complement onto endothelial cells was detected with exposure to both tobacco smoke and e-cigarette aerosol extracts. This was typically independent of the exact formulation. Specifically, deposition of the complement component C4d was of a lesser magnitude as a result of exposure to e-cigarette aerosol than to tobacco smoke. From these studies, the authors conclude that fine particulate matter from both e-cigarette aerosol and tobacco smoke extracts, and not nicotine or toxic combustion products, may be responsible for alterations in complement deposition that might be pivotal for
the inflammatory response in endothelial cells produced by tobacco and e-cigarette use. Of note, what is referred to as “toxic combustion product” in this article is rather broad and unclear. Nevertheless, the strength of the paper is that it addresses and/or attempts to define the specific components of combustible tobacco smoke and e-cigarette aerosols mediating detrimental effects on endothelial function. The evidence here is strongly suggestive of particulate matter as the main culprit responsible for endothelial cell dysfunction with e-cigarette aerosol (Barber et al., 2016).
The British American Tobacco Investments Ltd. recently developed a novel hybrid tobacco product consisting of a warm aerosol stream generated by electronic aerosolization and tobacco flavor produced by passing the aerosol through a bed of blended cut tobacco (Breheny et al., 2017; Poynton et al., 2017). In vitro studies addressed toxicological responses from this novel hybrid tobacco product in comparison with those from commercially available and prototype tobacco heating products, as well as a 3R4F reference combustible tobacco cigarette. Exposure matrices consisted of total particulate matter, whole aerosol, and aqueous aerosol extracts for all products tested. Endothelial dysfunction was among a battery of toxicological endpoints measured. The scratch wound assay using HUVECs was employed to measure rates of endothelial cell migration upon in vitro exposure to aqueous aerosol extracts from all test products. The results showed that wound repair after exposure to aqueous extract from the hybrid tobacco product and the commercial tobacco heating product was not significantly impaired, in contrast with the 3R4F reference combustible tobacco cigarette extract, which significantly impaired wound repair. This process was also inhibited by the aqueous extract from the prototype tobacco heating product tested, but at a lesser magnitude than that produced by the 3R4F reference combustible tobacco cigarette exposure. Similarly, outcomes of other toxicological endpoints such as mutagenicity, oxidative stress, and cytotoxicity were negative with the hybrid tobacco product. The study also measured carbonyls and nicotine levels in the aqueous aerosol extracts from these tobacco heating products. The carbonyls analyzed were acetaldehyde, acetone, acrolein, butyraldehyde, crotonaldehyde, formaldehyde, methyl ethyl ketone, and propionaldehyde. Overall, the most abundant carbonyls in the products tested (and in the 3R4F cigarette) were acetaldehyde and formaldehyde. Approximately 80 percent to 90 percent reductions in yield of total carbonyls were observed for extracts from tobacco heating and the novel hybrid tobacco products, by comparison with levels of carbonyls present in 3R4F and 3R4F-derived extract samples.
Interestingly, these reductions in yields of carbonyls with these devices correlated well with the significantly lower in vitro cytotoxicity responses obtained and the largely negative results from most of the end-
points measured, such as mutagenicity, genotoxicity, tumor promotion, oxidative stress, and endothelial cell dysfunction.
Flow-mediated dilation (FMD) is a non-invasive, ultrasound-based test to measure endothelial function (Raitakari and Celermajer, 2000). In this test, the arterial diameter is measured in response to an increase in sheer stress, causing endothelium-dependent dilatation. It is well documented that traditional tobacco smokers develop endothelial dysfunction, as evidenced by lower FMD values. Abnormal performance in this test is an early indicator of atherogenesis and closely associated with coronary artery disease (Carnevale et al., 2016).
In a crossover, single-blind study by Carnevale and colleagues (2016), 40 healthy subjects without cardiovascular disease (20 smokers and 20 non-smokers) smoked combustible tobacco cigarettes for a week. In the second phase of the study, all subjects were switched to smoking a tobacco-flavored e-cigarette product with a mean nicotine content of 16 mg per cartridge (equivalent to 250 puffs of tobacco cigarettes), which is approximately the same nominal nicotine content of combustible tobacco cigarettes. The overall goal of this study was to evaluate the differences between combustible tobacco cigarette and e-cigarette smoking in oxidative stress and endothelial dysfunction. For this purpose, ultrasound assessment of basal brachial diameter and endothelial-dependent FMD of the brachial artery were investigated according to established guidelines. Other parameters measured included blood levels of 8-iso-prostaglandin F2α, nitric oxide, soluble NOX2-derived peptide, and vitamin E (Carnevale et al., 2016).
Both combustible tobacco cigarette smoking and/or e-cigarette use resulted in a significant increase in soluble NOX2-derived peptide and 8-iso-prostaglandin F2α, while nitric oxide and vitamin E values were significantly decreased. Furthermore, the outcome of the FMD test showed that the brachial artery was significantly altered by both traditional and e-cigarette smoking, indicative that endothelial dysfunction occurs with both forms of smoking. E-cigarette use also appears to produce a less pronounced effect on levels of soluble NOX2-derived peptide, 8-iso-prostaglandin F2α, and nitric oxide content than combustible tobacco cigarette smoking. Future studies are warranted to clarify the chronic vascular effects of e-cigarette smoking. Although the authors are right to conclude that their results raise some degree of concern about the potential adverse vascular effect of e-cigarettes, they also acknowledged some of the limitations of the work, including the lack of assessment of chronic effects of e-cigarettes on endothelial function and no measurements of blood nicotine levels (Carnevale et al., 2016).
Reactive toxic aldehydes are commonly generated during combustion processes, including tobacco burning. In fact, a plethora of reactive
aldehydes can be found at high concentrations in tobacco smoke. Acrolein is a prominent tobacco-generated aldehyde and a well-known risk factor for cardiovascular diseases and pulmonary disease (COPD and emphysema) in smokers (Bein and Leikauf, 2011; Moghe et al., 2015). Although the impact of high levels of exposure to acrolein on cardiovascular health has been well studied, the systemic effects of exposure to lower levels of acrolein such as those found in combustible tobacco smoke and also in some electronic cigarettes are not known. In this context, Conklin and colleagues (2017) investigated the effect of exposing mice to 0.5 or 1 ppm acrolein for 12 weeks. This inhalation dosing regimen resulted in a significant increase in the primary urinary metabolite of acrolein 3-hydroxypro-pyl mercapturic acid. Concurrently, acrolein-protein adducts, expression of acrolein-metabolizing enzymes, and Nrf2-dependent gene products were all increased in the lungs of acrolein-treated mice.
Although exposure to acrolein reduced circulating levels of EPCs by 40 percent to 50 percent, there was no evidence of a lung inflammatory response or endoplasmic reticulum stress. Neither were elevations in circulating levels of endothelial-derived microparticles (MPs; referred to in other articles as endothelial-derived MVs) detected. From these findings, the authors suggest that circulating levels of specific EPCs could be used as sensitive biomarkers of inhaled acrolein-induced lung injury and that low-level acrolein exposure, such as that reported in e-cigarettes, poses a risk for cardiovascular diseases by hampering repair of endothelial cells (Conklin et al., 2017). Although proposing EPC changes as a sensitive and early biomarker of endothelial cell dysfunction for acrolein and other carbonyl compounds known to be generated in some e-cigarette aerosols is justifiable based on the evidence in this article, no remarkable changes in MPs were observed (as reported by others investigating e-cigarette exposures), and other key events associated with endothelial cell dysfunction, such as NO bioavailability, were not measured. Overall, what distinguishes this from other in vivo studies is the use of acrolein as a single toxicant in a chronic inhalation exposure regimen and at a concentration likely to be found in aerosols of combusted or heated tobacco products, including some e-cigarettes.
In another in vitro study, Teasdale and colleagues (2016) investigated the capacity of combustible tobacco cigarette smoke and electronic cigarette aerosol extracts to induce a stress response in endothelial cells. This study is distinguished from other in vitro studies because it used human coronary artery endothelial cells (HCAECs) instead of the more commonly used HUVECs. In this study, mainstream smoke from a single Marlboro Gold combustible tobacco cigarette was drawn through 10 ml of endothelial cell growth media MV2 to generate the combustible tobacco cigarette smoke extract. The e-cigarette aerosol extract was created using
the same apparatus, an iStick battery at 10.8-W (4.3-V) constant power output with an Aerotank Mini atomizer with Haven fluid USA Mix 18 mg/ml nicotine solution (80 percent glycerol/20 percent propylene glycol). A higher power output was selected with this e-cigarette unit to generate an e-cigarette aerosol extract expected to contain a greater proportion of potentially harmful chemicals. Any particulate matter from both combustible tobacco cigarette smoke and e-cigarette aerosol extracts was removed by filtration. The final samples applied to the cultured media contained the same nicotine concentration. Nicotine only (350 ng/ml) in media was used as an additional control.
Endpoints analyzed included Nrf2 nuclear localization by immunohistochemistry (indicative of an antioxidant stress response) and qPCR analysis of Nrf2-dependent and other genes. The genes selected were HMOX1, GCLM, OSGIN1, PAR4, CYP1A1, and CYP1B1. Their selection criteria were based on clear evidence that the expression of these genes changes in response to combustible tobacco cigarette smoke extract exposure to values greater than twofold (relative to controls). Not all these genes are Nrf2-dependent. For example, the CYP450 isoforms CYP1A1 and CYP1B1 were included in the analysis because of the previous evidence of their regulation by combustible tobacco cigarette smoke extract. The results demonstrated that exposure of HCAECs to combustible tobacco cigarette smoke extract resulted in Nrf2 activation, nuclear translocation, and transcriptional regulation of its target genes. In addition, the expression of the Nrf2-independent genes CYP1A1 and CYP1B1 was also upregulated by combustible tobacco cigarette smoke extract. In contrast, e-cigarette aerosol extracts did not affect expression of any of the genes analyzed, nor did it result in Nrf2 activation or changes in nuclear translocation levels. They also assessed interleukin 8 (IL8) and neuronal pentraxin-I (NTPX1) expression because both genes are known to be regulated by combustible tobacco cigarette smoke extract (observations from their unpublished data). Combustible tobacco cigarette smoke extract similarly upregulated the expression of both IL8 and NTPX1, but e-cigarette aerosol extract did not. Interestingly, IL8 expression was reduced and NTPX1 was increased by nicotine. Based on the responsiveness of human coronary artery endothelial cells, the authors concluded that the use of e-cigarettes as a substitute for combustible tobacco cigarettes is likely to reduce harm to the cardiovascular system (Teasdale et al., 2016).
Rubenstein and colleagues (2015) proposed a novel concept in which hepatic Kupffer cell function and activation can be a contributing factor to cardiovascular disease initiation and/or progression associated with tobacco use. In addition to the well-known role of Kupffer cells as resident macrophages in the liver, primarily responsible for the clearance of pathogens and other foreign particles from portal blood, Kupffer cells
recently have been shown to interact with platelets and leukocytes via an adhesion process—an interaction that results in inflammatory processes. Then, the reentry of platelets and leukocytes into the systemic circulation after interacting with Kupffer cells could lead to changes in the systemic circulation, producing adverse effects on the cardiovascular system, including vascular endothelial cell function. For these studies, the authors hypothesized that upon exposure to tobacco smoke or e-cigarette aerosol extracts, Kupffer cells would initiate inflammatory responses that would subsequently contribute to cardiovascular disease initiation and/or progression. In these in vitro studies, Kupffer cells were incubated with tobacco smoke extracts, e-cigarette aerosol extracts, or pure nicotine. An immortalized Kupffer cell line derived from Sprague-Dawley rats was employed. Exposure to both tobacco smoke and e-cigarette aerosol extracts were at a final concentration of 1 cigarette/5 L, while pure nicotine exposure was at a concentration of 50 nM, with all exposures lasting 48 hours. Endpoints measured included complement deposition, complement receptor expression, oxidative stress production, cytokine release, and cell viability and density. Their results conclusively showed that both tobacco and e-cigarette extracts induced a pronounced inflammatory response. Markers of oxidative stress, production and release of cytokines from Kupffer cells, were also significantly increased by both exposures with no notable differences between tobacco and e-cigarette extracts.
Complement C1q and C4d deposition onto Kupffer cells, which is indicative of classical complement pathway activation, was increased significantly by exposure to tobacco and e-cigarette extracts. Deposition of other components of the complement pathways (classical as well as alternative) was also enhanced significantly by both types of exposures. The effects on cell viability were less pronounced (about 80 percent) than on cell density (reductions by approximately 50 percent) for both tobacco and e-cigarette extracts compared with control exposures. Their overall conclusion is that by releasing cytokines and oxidative stress–mediator molecules, altered Kupffer cell function in the liver by e-cigarette aerosol and tobacco smoke exposure can affect the functionality of other cardiovascular cells, such as platelets, endothelial cells, and leukocytes (Rubenstein et al., 2015).
Schweitzer and colleagues (2011, 2015) conducted two studies examining effects of e-cigarettes on endothelial cells. The earlier study showed that in addition to damaging the pulmonary epithelium, soluble components of combustible tobacco cigarette smoke can directly damage lung endothelial cells by disrupting endothelial barrier function (Schweitzer et al., 2011). What is not entirely known is whether nicotine itself or exposure to aerosols released by electronic cigarettes have similar effects to those of combustible tobacco cigarettes on lung endothelia. In one of their more
recent studies (Schweitzer et al., 2015), the researchers investigated the effect of nicotine itself or an e-cigarette on lung endothelial cell injury and the mechanism(s). These studies employed primary rat lung endothelial cells (RLECs) and the human bronchial epithelial cell line BEAS-2B. Cell monolayers were exposed to nicotine, e-cigarette solution, or condensed e-cigarette aerosol (1–20 mM nicotine) or to nicotine-free combustible tobacco cigarette smoke extract or e-cigarette solutions.
Reductions in endothelial monolayer permeability as determined by transcellular electrical resistance (TER) measurements are indicative of endothelial cell monolayer disruption. As expected, exposure of primary RLECs to nicotine-containing combustible tobacco cigarette smoke extract (10 percent vol:vol) results in increased monolayer permeability in a time-dependent manner, a reduction of approximately 40 percent at 5 hours and 50 percent at 20 hours. A significantly diminished effect was observed with nicotine-free combustible tobacco cigarette smoke extract at the same time points. This indicates that nicotine itself is an important contributor to the damaging effects of soluble combustible tobacco cigarette smoke extract on the endothelial barrier. To confirm this, RLECs were then incubated with increasing concentrations of nicotine (up to 50 mM for up to 15 hours). They noted a significant time- and dose-dependent decrease in TER. The use of both mouse- and human-derived cells produced the same results, indicating that nicotine’s effects on endothelial monolayer disruption are not species specific (Schweitzer et al., 2015).
Two separate nicotine-containing solutions used in commercially available e-cigarettes also induced barrier dysfunction in RLECs, with the dysfuction proportional to the nicotine concentration in the e-liquid. Furthermore, barrier dysfunction was also observed with exposures to similar volumes of an e-cigarette solution without nicotine. However, the effect of an aerosol condensate from the non-nicotine–containing e-cigarette solution in altering the RLEC barrier was less potent.
Of note, the nicotine-free e-cigarette 2 was marketed as having the same flavor as nicotine-containing e-cigarette 2 and shared the same manufacturer. Because aerosolization of e-cigarette solutions may generate different metabolites than the original solution due to heating, the authors investigated whether the condensed aerosol isolated from an e-cigarette affected endothelial function. Overall, the in vitro studies showed that the endothelial barrier–disruptive effect of e-cigarette solutions is nicotine dose dependent and of a comparable magnitude to that produced by 3 percent combustible tobacco cigarette smoke extract exposure (a concentration known not to cause cell death). Furthermore, they concluded that the effects of e-cigarette solutions and aerosols on endothelial function are only in part dependent on nicotine (Schweitzer et al., 2015).
In addition, animal experiments were carried out in which C57BL/6
mice were nebulized using either one dose of nicotine (2 µg) and harvested immediately, or two doses of e-cigarette solution (1 µg each) and harvested 30 minutes or 24 hours later. The results showed a trend toward a rapid increase in polymorphonuclear cells in bronchoalveolar lavage fluid, indicative of higher extravasation of inflammatory cells by the endothelial barrier dysfunction. In addition, systemic oxidative and nitroxidative stress, as evidenced by increased levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG) and nitrotyrosine levels in plasma, was observed in response to inhalation of analytical-grade nicotine. A similar in vivo experiment was done with an e-cigarette liquid, but the data were not shown. The authors state that oxidative stress tended to increase by approximately 10–15 percent compared with a saline vehicle in mice exposed to e-cigarette solutions, as indicated by measured levels of 8-OHdG in plasma.
Lastly, mechanistic studies showed that the endothelial barrier–disruptive effects of cigarette smoke are associated with increased intracellular ceramides, p38 mitogen-activated protein kinase (MAPK) activation, and myosin light-chain phosphorylation. Furthermore, they showed that this signaling cascade is dependent on the function of Rho-associated kinase. The remaining question is whether the same is true for e-cigarettes. Figure 7-3, obtained from Schweitzer and colleagues (2015), depicts the proposed signaling cascade triggered by nicotine that partially overlaps with that used by combustible tobacco cigarette smoke extracts to disrupt the endothelial cell barriers and cell proliferation. A recent microRNA (miRNA) profiling study was conducted using normal human bronchial epithelial (NHBE) cells to determine the global effects of e-cigarette exposure on the miRNA transcriptome in lung epithelia (Solleti et al., 2017). The study first determined whether exposure to an e-cigarette induces oxidative stress in NHBE. They analyzed expression of various oxidative stress response genes by qPCR. NHBE were exposed to 2 percent non-aerosolized or aerosolized and condensed e-cigarette liquid that either contained or lacked nicotine. Their analysis showed that exposure of NHBE to any e-liquid resulted in induction of various oxidative stress–response genes, including GCLM, GCLC, GPX2, NQO1, and HO-1. Most of these are Nrf2-responsive genes. Of note, aerosolized e-liquid and the presence of nicotine in the exposure regimens resulted in a greater oxidative stress response. Their genome-wide transcriptional analysis of miRNAs identified 578 miRNAs with altered expression from e-cigarette exposure. Nicotine-containing e-cigarette aerosol produced the most profound changes in miRNA expression. They further validated the differential expression of eight miRNAs predicted to be affected significantly by treatment with any e-cigarette liquid by qPCR. While the expression of multiple miRNAs was increased, reduced expression of others was also noted. Overall, these results indicate that e-cigarette exposure has the
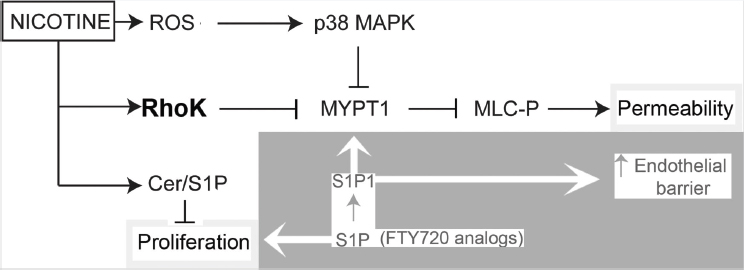
NOTES: Arrows indicate activation, and blocked lines indicate inhibition. Nicotine activates Rho-kinase, which in turn inhibits the myosin phosphatase target subunit 1, MYPT1, enhancing phosphorylation of myosin light chains (MLC-P) to increase endothelial permeability. Rho-kinase may have other targets in the cell to increase endothelial permeability because nicotine-induced oxidative stress-dependent p38 MAPK activation also contributed to MLC-P, but not sufficiently to alone increase permeability. Nicotine also increases the ceramide/sphingosine1-phosphate (S1P) ratios, which may inhibit lung endothelial cell proliferation. Enhancing S1P signaling opposes the decreased cell proliferation and the increase in permeability induced by nicotine in part by inhibiting MLC-P and restoring the lung endothelial barrier function. ROS = reactive oxygen species.
SOURCE: Schweitzer et al., 2015.
capacity to alter the miRNA transcriptome of an endothelial cell line. The importance of these changes and potential role in endothelial dysfunction is yet to be determined.
Previous studies by Fearon and colleagues (2012) reported the development and use of a “scratch wound” assay to measure the rate of HUVEC migration in vitro following artificial wound infliction. In those studies, wounding was inflicted to HUVEC with or without exposure to combustible tobacco cigarette smoke extracts. The results show that rates of HUVEC migration are reduced by combustible tobacco cigarette smoke extracts. In a more recent publication, Taylor and colleagues (2016) compared the effects of e-cigarette aqueous extracts generated from two commercial products to e-cigarette aqueous extracts from the 3R4F reference combustible tobacco cigarette on human bronchial epithelial cell (HBEC) migration. A “cig-a-like” cartomizer style (Vype eStick) and a closed modular (Vype ePen) device were used with blended tobacco–flavored variant containing 36 mg/ml nicotine (cartomizer style; operated at 3.7 V) and
18 mg/ml nicotine (closed modular; operated at 4.0 V). Aqueous aerosol extracts from the e-cigarette devices and the 3R4F reference cigarette were generated by procedures standard to this laboratory.
In the study by Fearon and colleagues (2012), 20 hours of exposure of HUVEC to the 3R4F extract produced a concentration-dependent inhibition of cell migration. This inhibition was complete with concentrations of 3R4F extract greater than 20 percent. As a point of reference, the aqueous extract from 3R4F at concentrations ranging from 0 to 30 percent provides nicotine exposure values of 0.09 to 1.98 µg/ml. By contrast, extracts from the e-cigarette devices at concentrations between 40 percent and 100 percent (equivalent to 1.56 and 1.90 to 4.76 µg/ml nicotine exposure) did not produce any significant reductions in cell migration rates, even when the concentration of nicotine was twice as high as that found in the 3R4F extract. Based on the conditions employed, the authors concluded that the commercial e-cigarette units used in this study do not inhibit endothelial cell migration in vitro. Clearly, analysis of other types of e-cigarette products and e-liquids are required to determine this in in vitro systems.
OXIDATIVE STRESS
Oxidative stress is the cornerstone of many chronic inflammatory diseases. Because there is a large body of evidence on the damage from oxidative stress due to cigarette smoking (both acutely and chronically), evaluation of oxidative stress with e-cigarette use is essential to identify and understand the potential biological and toxicological consequences of any pro-oxidant state produced by chemicals present and/or generated during e-cigarette use. The emerging in vitro literature investigating oxidative stress from e-cigarette use employs either immortalized cell lines, tumor cell lines, or primary cells in culture. Not surprisingly, the publication trend for in vitro oxidative stress by e-cigarettes parallels that presented previously for e-cigarettes and in vitro systems. This publication trend is captured in Figure 7-4, showing numbers of studies on e-cigarettes and oxidative stress.
The type of cell culture system used to study chemical-induced oxidative stress is a very important consideration because major differences in study outcomes can be explained by cell-type selection and the predictive value of the model. To better illustrate this, primary hepatocytes differ widely from commonly used hepatoma cell lines, such as HepG2, in that they are metabolically poor (e.g., biotransformation and disposition capacity) compared with hepatocytes. This is particularly important because chemicals may act directly as toxicants on cells and organs or following bioactivation into reactive intermediates by drug-metabolizing enzymes. However, it must be acknowledged that immortalized cell lines do offer
various advantages over primary cells, such as unlimited life span, lack of interindividual (or donor) variability, ease of use, and availability, making them popular and useful for in vitro toxicological screening. The selection of different in vitro systems with a wide range of metabolic competencies to study oxidative stress in response to exposure to e-cigarette constituents is one of multiple variables responsible for the wide spectrum of responses reported in the literature to date. Direct addition of e-liquids to cells in culture, as opposed to exposure to either the aerosolized form of or extracts from e-liquid aerosols, adds further variability to the oxidative stress responses measured in cell culture systems. This section will summarize the relevant literature on oxidative stress induced by e-cigarettes into in vitro systems. Establishing comparisons among different studies is somewhat challenging because of the multiple cell types used (primary versus immortalized cells), type and length of exposure (e-liquids versus aerosols and extracts), type of e-cigarette device, and e-liquid selection and composition. In addition, selected in vivo studies assessing the capacity of e-cigarettes to produce oxidative stress also will be discussed in support of and/or complimentary to in vitro studies.
Evidence Review
In a study by Anthérieu and colleagues (2017), human bronchial epithelial BEAS-2B cells, a commonly used bronchial epithelial cell line for respiratory toxicology studies, were exposed to e-cigarette aerosols and combustible tobacco cigarette smoke generated by a smoking machine. Specifically, samples were applied to air–liquid interface cultures of BEAS-2B cells. The puffing frequency/intervals used for cigarette smoke are recognized as the gold standard procedure for smoking machine use of cigarettes, while high puff volume and frequency were selected for e-cigarette aerosols because no standard regimens of exposure are defined and because it has been suggested that the e-cigarette use profile is more intense than the International Organization for Standardization combustible tobacco cigarette smoking profile. The time of exposure to both combustible tobacco cigarette smoke and e-cigarette aerosols ranged from 8 to 288 minutes. Effects on BEAS-2B cells analyzed were cytotoxicity, oxidative stress, and inflammatory response. Additionally, transcriptomic studies were carried out to identify changes in gene expression and potential mechanistic pathways linked to any adverse outcome from these exposures. As expected, exposure to cigarette smoke produced a strong time-dependent decrease in cell viability. However, no cytotoxicity was noted with e-aerosols up to 288 minutes of exposure. Oxidative stress was evaluated by measuring reduced glutathione (GSH) and oxidized glutathione (GSSG) levels. In agreement with the cytotoxicity studies, GSSG/GSH ratios were significantly elevated at both 8- and 48-minute exposure time points relative to controls, with no alterations detected for e-aerosols at any of the time points examined. This indicates, based on a single assay (or endpoint), that the e-aerosol exposure regimen employed in this study does not produce oxidative stress. The authors also analyzed the secretion of 10 inflammatory mediators from BEAS-2B cells in culture media, employing a Luminex-based assay. Exposure to e-cigarette aerosols induced secretion of only IL-6. Exposure to cigarette smoke (8 minutes) similarly induced IL-6 secretion, while also increasing IL-8 secretion. In contrast, secretion of GRO-α, MCP-1 in culture media was decreased by cigarette smoke. A longer exposure to cigarette smoke (48 minutes) reduced the expression of GRO-α, MCP-1, and IL-8. These effects were not seen with exposure to e-cigarette aerosols. Lastly, and as expected, the transcriptomic data showed dysregulation of a large number of genes by cigarette smoke, including genes mediating oxidative stress and/or responses to oxidative stress (e.g., heme oxygenase, superoxide dismutase), cell death (e.g., caspase 10, tumor necrosis factor), and cell signaling pathways associated with cytotoxic responses (e.g., FOS, JUN, STAT1). In contrast, no to very small numbers of genes were found to be differentially regulated by e-cigarette aerosols. Overall, the results
of this study strongly suggest lower oxidative stress and cytotoxicity of e-cigarette aerosols in comparison to cigarette smoke in the BEAS-2B cell line. The accompanying gene ontology analysis supports these findings.
Although the respiratory and cardiovascular systems are major targets in tobacco-related pathologies, cigarette smoking apparently accelerates the progression of chronic kidney disease, with nicotine being a proven exacerbator of ischemia-mediated renal injury via oxidative stress. Although the in vitro cytotoxicity of e-liquids has been investigated in various cardiovascular-derived cell lines, the effects of e-cigarettes in the renal system are unknown. A study by El Golli and colleagues (2016) determined whether or not e-cigarette liquid produces renal oxidative stress by generating free radicals or by altering antioxidant responses. In this in vivo study, four groups of rats were injected intraperitoneally with either vehicle saline, e-liquid without nicotine, e-liquid containing nicotine (0.5 mg of nicotine/kg of body weight/day), or nicotine alone (0.5 mg of nicotine/kg of body weight/day). Blood urea, creatinine, and uric acid were measured as endpoints of renal function. The results indicate that there are no significant differences in creatinine values between the e-liquid and vehicle control treated groups, whereas nicotine alone produced a significant increase in creatinine. However, urea and uric acid levels were significantly reduced in both the nicotine and the e-liquid without nicotine groups in comparison to vehicle controls. The e-liquid with nicotine produced a less pronounced decrease in these two endpoints. Total renal sulfhydryl content was increased in all three groups in comparison to vehicle controls, whereas superoxide dismutase and catalase activities were decreased. Renal glutathione-S-transferase activity was also decreased with e-liquid treatment. However, no evidence of lipid peroxidation (as evidenced by lack of changes in malondialdehyde levels) was found with e-liquid exposure. Tissue histology also showed the presence of ultrastructural changes, primarily in collecting ducts, that are consistent with an oxidative stress–mediated event by e-liquid treatment. Lastly, the strong correlation observed between superoxide dismutase activity and the renal biomarkers uric acid and urea is also indicative of the presence of oxidative stress/antioxidant response. Although the outcome of this study supports a renal oxidative stress/antioxidant response by e-liquid exposure, the overall relevance of this finding must be interpreted in the context of the route (intraperitoneal) and form of exposure (e-liquid as opposed to aerosol) selected for this study.
In a study that combines both in vivo and in vitro approaches, Husari and colleagues (2016) examined and compared the effect of cigarette smoke to electronic cigarette aerosol generated from a commercially available prefilled cartomizer that contained a propylene glycol/glycerol (80/20) solution of nicotine (18 mg/ml). The in vivo study consisted
of three groups of mice: control, e-cigarette, and cigarette smoke. The exposure regimen was 6 hours per day for 3 days, using smoke generator “nose-only” exposure chambers. A characterization of total particulate matter, nicotine, and aldehyde concentration produced by the smoke generation was also carried out in association with this in vivo study. Total particulate matter exposure for the e-cigarette was set at higher levels than for cigarette smoke. Lung injury was determined by measuring wet-to-dry tissue ratio, albumin concentration in bronchoalveolar lavage fluid, gene expression of selected inflammatory mediators, oxidative stress, and histopathological analysis. Oxidative stress was evaluated by confocal microscopy of dihydroethidium fluorescence. This is a commonly used indicator probe of reactive oxygen species production.
For in vitro studies, the human alveolar basal epithelial cell line A549 was selected. Cells were exposed to e-cigarette extract (0–64 mg/ml) or cigarette smoke extract (0–8 mg/ml) for 24 hours. Cytotoxicity and cell morphology were evaluated.
The in vivo studies showed that exposure to e-cigarette aerosol does not result in any increases in lung tissue oxidative stress in comparison to control mice, even when significantly higher concentrations of several chemicals (e.g., various volatile aldehydes, nicotine) and total particulate matter were detected in e-cigarette aerosol in comparison to cigarette smoke. Concurrently, a significant number of TUNEL-positive cells and apoptotic nuclei were detected in lung tissues of cigarette smoke–exposed mice. This was not observed in the lungs of e-cigarette–exposed animals. The other endpoints of lung injury analyzed were similarly unaffected by exposure to e-cigarette aerosol, with the exception of a significant increase in IL-1β. Alterations in these parameters and endpoints are commonly associated with the ability of xenobiotics and their metabolites to generate ROS and induction of oxidative stress.
The outcome of the in vitro studies was also similar. Cytotoxicity and cell morphology changes were evident in A549 cells exposed to combustible tobacco cigarette smoke, but not to the e-cigarette extract. Although oxidative stress in A549 cells was not assessed, it is safe to assume based on cytotoxicity and cell morphology that oxidative stress most likely occurred with cigarette smoke, but not e-cigarette exposure. Overall, the authors concluded that despite higher exposure conditions, exposure to e-cigarette aerosol produces less oxidative stress and less toxic effects both in vivo and in vitro in comparison to exposure to cigarette smoke (Husari et al., 2016).
Ji and colleagues (2016) evaluated the effect of e-cigarette aerosols on oral epithelial cells in vitro. Various e-liquids with different nicotine content and flavors were used to generate aerosols using a homemade puffing machine as described in the article. Particle number concentration
and size distribution in e-cigarette aerosols were measured. Furthermore, the physiochemical characteristics of e-cigarette aerosol particles in liquid were also analyzed, determining their size and distribution in water and cell culture media. Technologies such as transmission electron microscopy, X-ray spectroscopy, and dynamic light scattering analysis were employed for particle characterization.
In both water and culture media liquid phases, the size of nanoparticles was significantly larger than those found in the gas phase, which is most likely due to the aggregation of nanoparticles in the liquid phase. The cytotoxicity of e-cigarette aerosols was assessed using normal human oral keratinocytes (NHOKs) in culture. In vitro analysis of e-cigarette aerosol-treated NHOKs show that these aerosols are able to induce oxidative stress as reflected by the significantly lower levels of intracellular GSH. Exposure to fumed silica as a positive control produce similar reductions in GSH as e-cigarette aerosols in NHOKs. This reduction in GSH by e-cigarette aerosol was dose dependent. Oxidative stress represents a dynamic equilibrium between antioxidant defense that acts to restore redox equilibrium and oxidant injury responses that can result in toxicological outcomes. The combined reduction in GSH content, the significant cytotoxicity reflected by drastic reductions in NHOK ATP levels (to approximately 20 percent of control values), and the increased expression of the Nrf2-dependent gene heme oxygenase is suggestive of induction of oxidative stress. Overall, the results of this in vitro study suggest that e-cigarette aerosols could produce cytotoxicity to the oral cavity epithelium through a mechanism that potentially involves oxidative stress induced by nanoparticles and chemicals present in these aerosols. However, the magnitude of these effects by e-cigarette aerosols on NHOKs in relation to traditional tobacco smoke is uncertain because tobacco smoke exposures were not included in this study (Ji et al., 2016).
There is ample evidence indicating that cigarette smoke promotes cerebrovascular conditions that can lead to cerebral ischemia and stroke via generation of ROS, inflammation, and impairment of blood–brain barrier functions. Furthermore, the role of cigarette smoke in promoting vascular endothelial dysfunction and the role of oxidative stress are also well documented. More recent findings support an additive effect on the release and/or formation of angiogenic, oxidative, and inflammatory factors by endothelial cells in the blood–brain barrier in response to hyperglycemia and/or stroke conditions with simultaneous exposure to extracts from cigarette smoke (Prasad et al., 2015, 2017). From these studies, the authors suggested that the blood–brain barrier dysfunction and increased risk for stroke from combustible tobacco smoke resembles the cerebrovascular diseases seen in pathogenic stages of type 2 diabetes that also involve some of the same mediators, such as oxidative stress,
inflammation, and changes in antioxidant responses regulated by the transcription factor Nrf2.
In a recent study by the same group, brain ischemic injury was induced by transient middle artery occlusion in groups of mice previously exposed chronically (for 2 weeks total) to either combustible tobacco cigarette smoke (3R4F reference cigarette) or e-cigarette aerosol (blu brand e-cig) via direct inhalation using standard inhalation protocols (Kaisar et al., 2017). The combustible tobacco cigarette smoke and e-cigarette aerosol groups of mice were subdivided and treated with either metformin or saline. Metformin treatment was included because previous studies have shown that this antidiabetic drug has the capacity to reduce oxidative stress and inflammatory responses associated with stroke injury via an Nrf2- and AMP-activated protein kinase (AMPK)dependent mechanism(s) (Ashabi et al., 2015). In addition to the in vivo studies, C57BL/6 mouse primary brain microvascular endothelial cell (mBMEC) cultures were exposed to 5 percent soluble cigarette smoke or e-cigarette extracts for 24 hours. These extracts were prepared using a standard smoking protocol with a Single Cigarette Smoking Machine. In addition, transwell cultures of mBMEC cells were set up to measure changes in transendothelial electrical resistance (TEER) as an indicator of cell monolayer integrity to measure potential endothelial dysfunction by exposure to extracts.
The results showed that the levels of oxidative stress produced by e-cigarette aerosol and combustible tobacco cigarette smoke in mBMEC cells were not dissimilar and significantly greater than in control cultures. Oxidative stress was measured using the fluorogenic probe CellROX. Correspondingly, strong activation of the transcription factor Nrf2, master sensor and regulator of genes responsive to oxidative stress, was observed with both exposures. Induction of NAD(P)H:quinone oxidoreductase-1 protein, an Nrf2-dependent target, was of very similar magnitude with e-cigarette aerosol and combustible tobacco cigarette smoke exposure. Similarly, nicotine-only exposure of mBMEC cells produced a time- and concentration-dependent Nrf2 activation, as evidenced by its nuclear accumulation. Overall, the in vitro data led the authors to suggest that from a functional perspective, e-cigarette exposure is as damaging as that of combustible tobacco smoke, both eliciting similar levels of oxidative stress and thus oxidative stress–mediated inflammation. Similar correlative results were obtained in the in vivo studies. Interestingly, the in vivo studies show that metformin treatment confers partial support against the detrimental effects of both combustible tobacco cigarette smoke and e-cigarettes in stroke injury by reducing oxidative stress and inflammation (Kaisar et al., 2017).
A study by Moses and colleagues (2017) investigated the impact of
electronic cigarette aerosols on the global gene expression profile of primary HBECs, compared with the effect of combustible tobacco cigarette smoke. Cells were isolated from the lungs of a 23-year-old man with no history of smoking or lung disease. Gene expression analysis was carried out in cells grown at the air–liquid interface and exposed to one of four different e-cigarette aerosols, combustible tobacco cigarette smoke (3R4F reference cigarette), and clean air. The authors studied the blu brand of e-cigarettes. The blu e-cigarettes used were labeled as either menthol or tobacco flavored and with or without nicotine (24 mg/cartridge).
The authors first measured cytotoxicity via cell viability and TEER. Although HBECs exposed to combustible tobacco cigarette smoke (six 3R4F cigarettes) exhibited cytotoxicity, no significant effect was observed with up to 400 puffs of electronic cigarette exposure. Furthermore, no significant cytotoxicity was detected when HBECs were exposed to 400 puffs of electronic cigarette aerosols from the four different blu products tested. The gene expression profiling was then carried out under the same conditions (six 3R4F cigarettes or 400 blu electronic cigarette puffs).
Principal component analysis of gene expression data was carried out to examine the effect of all exposures. This is depicted in Figure 7-5. This type of analysis compares how gene expression patterns for combustible tobacco cigarette smoke, e-cigarette aerosols, and air are organized (and whether there is clustering) relative to each other. Of note, the gene expression pattern for the four groups of cells exposed to different blu products clustered together (ECIGs cluster in upper right quadrant). A total of 546 genes were determined to be differentially expressed among the three exposure groups. Gene ontology was also performed to identify gene expression patterns with roles in specific biological processes or signaling pathways highly enriched within the different clusters. Among the genes upregulated by e-cigarette aerosol and combustible tobacco cigarette smoke, they found enrichment for genes involved in oxidative stress, apoptosis, and DNA damage. However, relative to air control, the magnitude of changes in gene expression was greater for combustible tobacco cigarette smoke than for e-cigarettes. The studies also showed that these changes in gene expression are more pronounced in the nicotine-containing e-cigarette than the product without nicotine and that the induction of genes supportive of greater ROS production appears to be dose dependent. It is worth noting that even at the high exposure levels of e-cigarette aerosols (400 puffs) used, induction and/or activation of oxidative stress signaling and xenobiotic metabolism pathways is only a fraction of the magnitude of change produced by cigarette smoke exposures. The genomics data were validated for a selected number of genes by qPCR and also with an immunoassay that detects generation of reactive oxygen species.
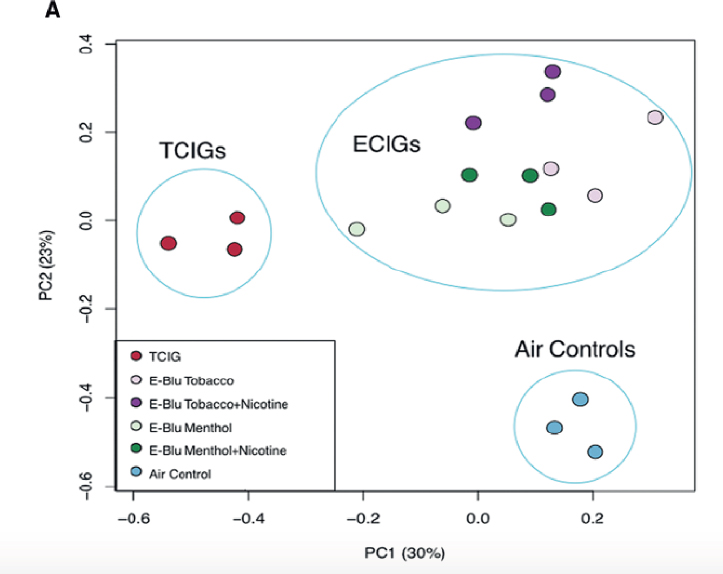
NOTE: ECIG = e-cigarette; TCIG = combustible tobacco cigarette.
SOURCE: Moses et al., 2017.
An intriguing component of this study is that the authors compared the results of the gene expression profiles between bronchial epithelial samples obtained from former cigarette smokers and former smokers who switched to e-cigarette use. The authors justify the importance of this new genomics analysis with e-cigarette exposure based on their past evidence documenting alterations in airway epithelial gene expression patterns by combustible tobacco cigarette smoke that can serve as a biomarker of pulmonary diseases in smokers, including the early detection of cancer (Beane et al., 2007; Silvestri et al., 2015). By gene enrichment analysis, the authors compared gene expression signatures from the in vitro e-cigarette exposures to those generated from bronchial epithelial brushings of current cigarette smokers and former smokers who switched to e-cigarettes. Interestingly, the gene expression differences observed in vitro were concordant with differences observed in airway epithelium collected from
e-cigarette users. Overall, e-cigarette aerosols can induce changes in gene expression in vitro, in the bronchial airway epithelium, with a subset of these changes in common with regular cigarette smoke, but of a much lesser magnitude. This study also demonstrates commonalities between in vitro responses and what is observed in individuals who are e-cigarette users (Moses et al., 2017).
A comparative study by Scheffler and colleagues (2015) analyzed the sensitivity of various cell lines, along with primary NHBE cells, to e-liquid aerosol and cigarette smoke. NHBE cells were obtained from healthy tissue from a 75-year-old patient with non-small cell lung cancer. The cells were named NHBE48. For immortalization, the cells underwent transfection with well-defined immortalization genes. The immortalized cells were named CL-1548. In addition, the widely used adenocarcinomic human alveolar basal epithelial cells A549 were selected for analysis. NHBE, NHBE48, and A549 cells were then exposed at the air–liquid interface to e-liquid aerosol, cigarette mainstream smoke, or clean air in a CULTEX RFS compact module.
For e-cigarette aerosol exposure, 200 puffs from a Reevo Mini-S e-cigarette were taken, while for mainstream cigarette smoke, 10 K3R4F cigarettes were used (each puffed six times). Details of puff volume, duration, and blow-out time are described in the article. The tested refill e-liquids were propylene glycol–based (75 percent propylene glycol, 25 percent glycerol) with nicotine concentrations of 0.0 percent and 2.4 percent (24 mg/ml).
Viability data showed that primary NHBE48 cells are the most sensitive cells, showing decreases in viability by 60 percent and 52 percent for nicotine- and non-nicotine–containing e-liquid aerosol exposure, respectively, compared with clean air controls. This is a much smaller response than that seen in combustible tobacco cigarette smoke–exposed cells, where 7 percent viability was measured. The A549 cells were least sensitive to both combustible tobacco cigarette smoke (21 percent viability compared with control air) and both e-liquid aerosols (88 percent viability relative to control air). The immortalized CL-1548 cells were less sensitive to e-liquid aerosols and combustible tobacco cigarette smoke exposure compared with the NHBE48 cells, but significantly more sensitive than A549 cells to both e-liquid aerosols (75 percent and 70 percent viability) and combustible tobacco cigarette smoke (10 percent viability). For all cell types, the presence or absence of nicotine did not significantly affect cell viability values for e-liquid aerosols.
To also analyze oxidative stress, samples from all the different cell types exposed to e-liquid aerosols, combustible tobacco cigarette smoke, and clear air were analyzed using the ROS-Glo™ H2O2 fluorescence-based assay. The results were in complete agreement with the cell viability data.
The levels of oxidative stress are highest in primary NHBE48 cells, followed by those in CL-1548 cells, and finally in A549 cells, with both e-liquid aerosols and combustible tobacco cigarette smoke. Also in agreement with the cell viability data, accumulation of oxidative stress with either e-liquid aerosol is only a fraction of that seen with combustible tobacco cigarette smoke. Not only does this study document the use of a new immortalized HBEC cell line for in vitro toxicity testing of e-cigarettes, but it also adds further evidence that e-cigarette aerosols are cytotoxic and produce oxidative stress at levels that are significantly lower than those produced by combustible tobacco cigarette smoke (Scheffler et al., 2015).
A study by Taylor and colleagues (2016) investigated whether a human bronchial epithelial cell line can be employed as a reliable in vitro model of airway epithelium to differentiate cellular stress responses to aqueous aerosol extracts obtained from traditional cigarette smoke and e-cigarette aerosols. The human bronchial epithelial cell line (NCI-H292) was selected as in vitro model. For endpoints, the authors analyzed cellular ratios of reduced GSH to the oxidized form (GSSG), generation of ROS, and transcriptional activation of gene antioxidant response element (ARE) as an indirect indicator of Nrf2 activation and nuclear translocation.
In addition, caspase 3/7 activity was analyzed as a marker of initiation of apoptotic responses to oxidative stress. To generate extracts from both combustible tobacco cigarettes and e-cigarettes, reference 3R4F cigarettes and two Vype e-cigarettes (cigarette-like, cartomizer style; and a closed modular product) were used, respectively. Nicotine and total carbonyl concentrations in the aqueous extracts were quantified to ensure batch-to-batch consistency among extracts used in this study.
As expected, a concentration-dependent induction of cytotoxicity was observed following exposure to cigarette smoke aqueous extract. By contrast, no cytotoxicity was detected with either type of e-cigarette aerosol extracts. Similarly, activation of caspase 3/7 was detected (up to a maximum of 40 percent compared with control exposure), with no changes detected with e-cigarette extracts, even when applied to cells undiluted. Intracellular generation of reactive oxygen species increased by up to 83 percent, while the GSH/GSSG ratios were lowered by more than 90 percent with cigarette smoke aqueous extract. Changes in GSH status and generation of reactive oxygen species are shown in Figure 7-6.
Also, activation of the ARE luciferase reporter gene increased by approximately 300 percent. None of these endpoints of oxidative stress and ROS generation were affected by any of the e-cigarette extracts. The methodology employed was of suitable sensitivity for comparative studies of traditional tobacco and e-cigarettes. Collectively, these results led the authors to conclude that aqueous extracts from the e-cigarettes
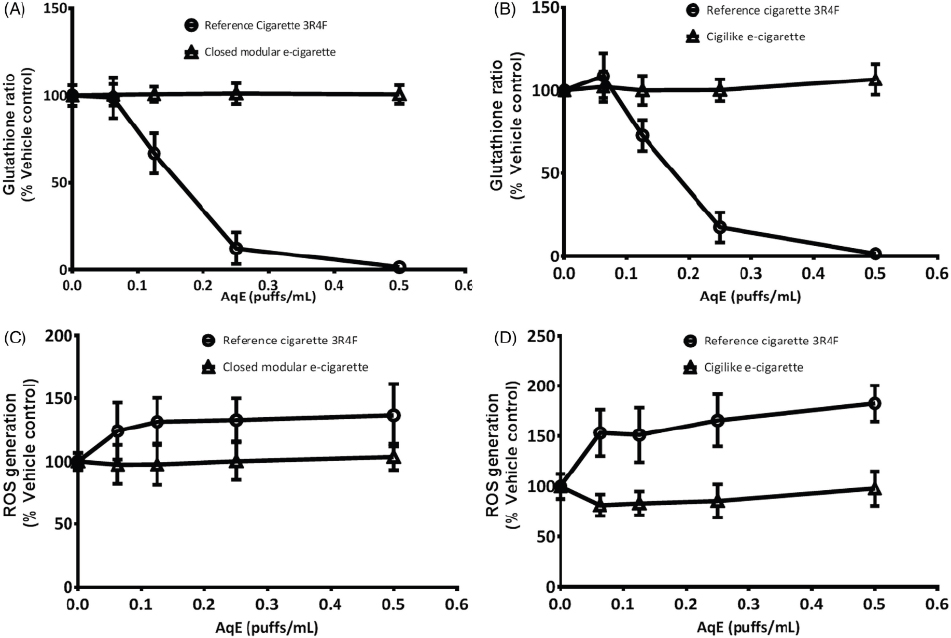
NOTE: AqE = aqueous extract; ROS = reactive oxygen species.
SOURCE: Taylor et al., 2016.
tested in the NCI-H292 human bronchial epithelial cells do not contain either the chemical drivers or the sufficient concentrations to produce oxidative stress or cytotoxicity.
Recent studies by Tran and colleagues (2015) documented that proteostasis and autophagy impairment is a novel mechanism for promoting aggresome formation and apoptosis in COPD–emphysema induced by cigarette smoke. Furthermore, it is well established that oxidative stress is an important mediator of autophagy impairment, which in turn is correlated with induction of proteostasis imbalance and accumulation of ubiquitinated proteins (Korovila et al., 2017). The role of oxidative stress in this response is further supported by the effects of promising new drug candidates for treating COPD, such as a cysteamine-based drug with antioxidant properties that are able to restore autophagy while inhibiting and/or neutralizing reactive oxygen species.
A more recent study by the same group (Shivalingappa et al., 2015) evaluated whether exposure to e-cigarette aerosols modulates proteostasis and autophagy as a potential mechanism for inflammatory and oxidative stress induced by nicotine and/or other chemical components in
e-cigarettes. This study also evaluated whether chemical modulation of autophagy can alleviate e-cigarette aerosol–mediated inflammatory and oxidative stress responses.
The study evaluated the effects of e-cigarette aerosol exposure (2.5 or 7.5 mg) on the accumulation of total polyubiquitinated proteins in BEAS-2B cells, an immortalized human bronchial epithelial cell line. Cell treatments with e-cigarette aerosol–exposed culture media lasted 1, 3, or 6 hours. The results showed a time-dependent increase in ubiquitin, primarily in the insoluble protein fractions. Then, autophagy activity in BEAS-2B cells was investigated by immunoblot analysis of the aberrant autophagy marker p62. Significant increases of p62 translocation and accumulation in the insoluble protein fractions at 6 hours were observed, suggesting an impairment of autophagy by exposure to e-cigarette aerosol. Aggresome formation, a cytoplasmic structure containing misfolded proteins, was similarly induced by e-cigarette aerosol.
In order to determine if e-cigarette aerosol induces inflammatory and oxidative stress via autophagy impairment, activation of the transcription factor NFκB and nitrotyrosine protein adduct formation were measured in the presence or absence of the autophagy inducers carbamazepine and cysteamine; the latter has also been an antioxidant. NFκB activation and nitrotyrosine protein adducts were used as markers of inflammation and oxidative stress, respectively. The results showed that BEAS-2B cells preincubated with carbamazepine for 6 hours and then exposed to e-cigarette aerosol for 1 hour showed an attenuation in the normal increases in total protein NFκB levels and nitrotyrosine protein adduct formation induced by e-cigarette aerosol–only exposure, confirming that this autophagy inducer ameliorates the oxidative and inflammatory stress produced by e-cigarette aerosols. The capacity of cysteamine to alter e-cigarette aerosol–induced oxidative stress in BEAS-2B cells was also assessed. E-cigarette aerosol by itself produced a significant increase in levels of intracellular ROS, which were significantly reduced by preincubation with cysteamine. Similarly, cysteamine treatment was capable of rescuing e-cigarette aerosol–induced aggresome formation and aberrant autophagy.
Collectively, this study demonstrated for the first time that exposure to e-cigarette aeosols impairs proteostasis and autophagy, which cascades into accumulation of ubiquitinated proteins, oxidative stress, apoptosis, and cell senescence (Shivalingappa et al., 2015). All these events can be reduced by autophagy inducers, such as carbamazepine and cysteamine. However, in the absence of comparative experiments that include exposure to cigarette smoke, the magnitude and physiological relevance of the effects observed with e-cigarette relative to combustible tobacco cigarette smoke remain uncertain.
CONCLUSIONS
Conclusion 7-1. There is substantial evidence that e-cigarette aerosols can induce acute endothelial cell dysfunction, although the long-term consequences and outcomes on these parameters with long-term exposure to e-cigarette aerosol are uncertain.
Conclusion 7-2. There is substantial evidence that components of e-cigarette aerosols can promote formation of reactive oxygen species/oxidative stress. Although this supports the biological plausibility of tissue injury and disease from long-term exposure to e-cigarette aerosols, generation of reactive oxygen species and oxidative stress induction are generally lower from e-cigarettes than from combustible tobacco cigarette smoke.
Adverse outcome pathway (AOP) is a relatively new, knowledge-driven concept that provides a framework that organizes in a sequential fashion molecular initiating events, intermediate key events, and an adverse outcome spanning layers of biological organization. Various AOPs have been developed for chemical-induced pathologies, such as cholestasis, liver fibrosis, and steatosis. The other use of AOPs is their potential for predicting disease risk. Pertinent to the health effects and risk analysis of e-cigarette use is a recently developed AOP for the onset of hypertension by oxidative stress–mediated perturbation of endothelial function. This AOP specifically describes how vascular endothelial peptide oxidation leads to hypertension through perturbation of endothelial nitric oxide bioavailability, resulting in impaired vasodilation (Lowe et al., 2017). The authors proposed that this and related AOPs can serve as a tool for the regulatory assessment of the harm reduction potential of e-cigarettes relative to combustible tobacco products. Another use of this or related AOPs is the assessment of harm associated with individual or combined components in e-cigarette products as well as device characteristic and mode of use. Lastly, as new biomarkers are identified and/or validated that can distinguish harm and magnitude of e-cigarette use from traditional cigarette smoking, they can be integrated with AOPs to better assess health risks associated with e-cigarettes and related nicotine products.
REFERENCES
Anderson, C., A. Majeste, J. Hanus, and S. Wang. 2016. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicological Sciences 154(2):332–340.
Anthérieu, S., A. Garat, N. Beauval, M. Soyez, D. Allorge, G. Garçon, and J. M. Lo-Guidice. 2017. Comparison of cellular and transcriptomic effects between electronic cigarette vapor and cigarette smoke in human bronchial epithelial cells. Toxicology in Vitro 45(Part 3):417–425.
Antoniewicz, L., J. A. Bosson, J. Kuhl, S. M. Abdel-Halim, A. Kiessling, F. Mobarrez, and M. Lundback. 2016. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis 255:179–185.
Ashabi, G., L. Khalaj, F. Khodagholi, M. Goudarzvand, and A. Sarkaki. 2015. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metabolic Brain Disease 30(3):747–754.
Barber, K. E., B. Ghebrehiwet, W. Yin, and D. A. Rubenstein. 2016. Endothelial cell inflammatory reactions are altered in the presence of e-cigarette extracts of variable nicotine. Cellular and Molecular Bioengineering 10(1):124–133.
Beane, J., P. Sebastiani, G. Liu, J. S. Brody, M. E. Lenburg, and A. Spira. 2007. Reversible and permanent effects of tobacco smoke exposure on airway epithelial gene expression. Genome Biology 8(9):R201.
Bein, K., and G. D. Leikauf. 2011. Acrolein—A pulmonary hazard. Molecular Nutrition & Food Research 55(9):1342–1360.
Breheny, D., J. Adamson, D. Azzopardi, A. Baxter, E. Bishop, T. Carr, I. Crooks, K. Hewitt, T. Jaunky, S. Larard, F. Lowe, O. Oke, M. Taylor, S. Santopietro, D. Thorne, B. Zainuddin, M. Gaça, C. Liu, J. Murphy, and C. Proctor. 2017. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (part 2): In vitro biological assessment and comparison with different tobacco-heating products. Food and Chemical Toxicology 106(Part A):533–546.
Carnevale, R., S. Sciarretta, F. Violi, C. Nocella, L. Loffredo, L. Perri, M. Peruzzi, A. G. M. Marullo, E. De Falco, I. Chimenti, V. Valenti, G. Biondi-Zoccai, and G. Frati. 2016. Acute impact of tobacco vs. electronic cigarette smoking on oxidative stress and vascular function. Chest 150(3):606–612.
Conklin, D. J., M. V. Malovichko, I. Zeller, T. P. Das, T. V. Krivokhizhina, B. H. Lynch, P. Lorkiewicz, A. Agarwal, N. Wickramasinghe, P. Haberzettl, S. D. Sithu, J. Shah, T. E. O’Toole, S. N. Rai, A. Bhatnagar, and S. Srivastava. 2017. Biomarkers of chronic acrolein inhalation exposure in mice: Implications for tobacco product-induced toxicity. Toxicological Sciences 158(2):263–274.
El Golli, N. E., A. Jrad-Lamine, H. Neffati, H. Dkhili, D. Rahali, Y. Dallagi, M. V. El May, and S. El Fazaa. 2016. Impact of e-cigarette refill liquid exposure on rat kidney. Regulatory Toxicology and Pharmacology 77:109–116.
Fearon, I. M., D. O. Acheampong, and E. Bishop. 2012. Modification of smoke toxicant yields alters the effects of cigarette smoke extracts on endothelial migration: An in vitro study using a cardiovascular disease model. International Journal of Toxicology 31(6):572–583.
Husari, A., A. Shihadeh, S. Talih, Y. Hashem, M. El Sabban, and G. Zaatari. 2016. Acute exposure to electronic and combustible cigarette aerosols: Effects in an animal model and in human alveolar cells. Nicotine & Tobacco Research 18(5):613–619.
Ji, E. H., B. B. Sun, T. K. Zhao, S. Shu, C. H. Chang, D. Messadi, T. Xia, Y. F. Zhu, and S. Hu. 2016. Characterization of electronic cigarette aerosol and its induction of oxidative stress response in oral keratinocytes. PLoS ONE 11(5):e0154447. https://doi.org/10.1371/journal.pone.0154447 (accessed February 6, 2018).
Kaisar, M. A., H. Villalba, S. Prasad, T. Liles, A. E. Sifat, R. K. Sajja, T. J. Abbruscato, and L. Cucullo. 2017. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is metformin a viable countermeasure? Redox Biology 13:353–362.
Korovila, I., M. Hugo, J. P. Castro, D. Weber, A. Hohn, T. Grune, and T. Jung. 2017. Proteostasis, oxidative stress and aging. Redox Biology 13:550–567.
Lowe, F. J., K. Luettich, M. Talikka, V. Hoang, L. E. Haswell, J. Hoeng, and M. D. Gaça. 2017. Development of an adverse outcome pathway for the onset of hypertension by oxidative stress-mediated perturbation of endothelial nitric oxide bioavailability. Applied In Vitro Toxicology 3(1):131–148.
Moghe, A., S. Ghare, B. Lamoreau, M. Mohammad, S. Barve, C. McClain, and S. Joshi-Barve. 2015. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicological Sciences 143(2):242–255.
Morris, P. B., B. A. Ference, E. Jahangir, D. N. Feldman, J. J. Ryan, H. Bahrami, M. F. El-Chami, S. Bhakta, D. E. Winchester, M. H. Al-Mallah, M. Sanchez Shields, P. Deedwania, L. S. Mehta, B. A. P. Phan, and N. L. Benowitz. 2015. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: Clinical perspectives from the Prevention of Cardiovascular Disease Section Leadership Council and Early Career Councils of the American College of Cardiology. Journal of the American College of Cardiology 66(12):1378–1391.
Moses, E., T. Wang, S. Corbett, G. R. Jackson, E. Drizik, C. Perdomo, C. Perdomo, E. Kleerup, D. Brooks, G. O’Connor, S. Dubinett, P. Hayden, M. E. Lenburg, and A. Spira. 2017. Molecular impact of electronic cigarette aerosol exposure in human bronchial epithelium. Toxicological Sciences 155(1):248–257.
Poynton, S., J. Sutton, S. Goodall, J. Margham, M. Forster, K. Scott, C. Liu, K. McAdam, J. Murphy, and C. Proctor. 2017. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (part 1): Product operation and preliminary aerosol chemistry assessment. Food & Chemical Toxicology 106(Part A):522–532.
Prasad, S., R. K. Sajja, J. H. Park, P. Naik, M. A. Kaisar, and L. Cucullo. 2015. Impact of cigarette smoke extract and hyperglycemic conditions on blood-brain barrier endothelial cells. Fluids Barriers CNS 12:18. https://doi.org/10.1186/s12987-015-0014-x (accessed February 6, 2018).
Prasad, S., R. K. Sajja, M. A. Kaisar, J. H. Park, H. Villalba, T. Liles, T. Abbruscato, and L. Cucullo. 2017. Role of Nrf2 and protective effects of metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biology 12:58–69.
Putzhammer, R., C. Doppler, T. Jakschitz, K. Heinz, J. Forste, K. Danzl, B. Messner, and D. Bernhard. 2016. Vapours of U.S. and E.U. market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS ONE 11(6):e0157337. https://doi.org/10.1371/journal.pone.0157337 (accessed February 6, 2018).
Raitakari, O. T., and D. S. Celermajer. 2000. Flow-mediated dilatation. British Journal of Clinical Pharmacology 50(5):397–404.
Rubenstein, D. A., S. Hom, B. Ghebrehiwet, and W. Yin. 2015. Tobacco and e-cigarette products initiate Kupffer cell inflammatory responses. Molecular Immunology 67(2):652–660.
Scheffler, S., H. Dieken, O. Krischenowski, and M. Aufderheide. 2015. Cytotoxic evaluation of e-liquid aerosol using different lung-derived cell models. International Journal of Environmental Research and Public Health 12(10):12466–12474.
Schweitzer, K. S., H. Hatoum, M. B. Brown, M. Gupta, M. J. Justice, B. Beteck, M. Van Demark, Y. Gu, R. G. Presson, Jr., W. C. Hubbard, and I. Petrache. 2011. Mechanisms of lung endothelial barrier disruption induced by cigarette smoke: Role of oxidative stress and ceramides. American Journal of Physiology—Lung Cellular and Molecular Physiology 301(6):L836–L846.
Schweitzer, K. S., S. X. Chen, S. Law, M. Van Demark, C. Poirier, M. J. Justice, W. C. Hubbard, E. S. Kim, X. Lai, M. Wang, W. D. Kranz, C. J. Carroll, B. D. Ray, R. Bittman, J. Goodpaster, and I. Petrache. 2015. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. American Journal of Physiology—Lung Cellular and Molecular Physiology 309(2):L175–L187.
Shivalingappa, P. C., R. Hole, C. V. Westphal, and N. Vij. 2015. Airway exposure to e-cigarette vapors impairs autophagy and induces aggresome formation. Antioxidants & Redox Signaling 24(4):186–204.
Silvestri, G. A., A. Vachani, D. Whitney, M. Elashoff, K. Porta Smith, J. S. Ferguson, E. Parsons, N. Mitra, J. Brody, M. E. Lenburg, A. Spira, and A. S. Team. 2015. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. New England Journal of Medicine 373(3):243–251.
Solleti, S. K., S. Bhattacharya, A. Ahmad, Q. Wang, J. Mereness, T. Rangasamy, and T. J. Mariani. 2017. MicroRNA expression profiling defines the impact of electronic cigarettes on human airway epithelial cells. Scientific Reports. https://doi.org/10.1038/ s41598-017-01167-8 (accessed February 6, 2018).
Taylor, M., T. Carr, O. Oke, T. Jaunky, D. Breheny, F. Lowe, and M. Gaça. 2016. E-cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicology Mechanisms and Methods 26(6):465–476.
Teasdale, J. E., A. C. Newby, N. J. Timpson, M. R. Munafo, and S. J. White. 2016. Cigarette smoke but not electronic cigarette aerosol activates a stress response in human coronary artery endothelial cells in culture. Drug and Alcohol Dependence 163:256–260.
Tran, I., C. Ji, I. Ni, T. Min, D. Tang, and N. Vij. 2015. Role of cigarette smoke-induced aggresome formation in chronic obstructive pulmonary disease-emphysema pathogenesis. American Journal of Respiratory Cell and Molecular Biology 53(2):159–173.