6
Assessing Safety and Toxicology
PATIENT SUSCEPTIBILITIES IN PRECLINICAL DRUG SAFETY ASSESSMENT
Brian Berridge, senior GlaxoSmithKline (GSK) fellow and director and head of WW Animal Research Strategy at GSK, talked about the successes and challenges of testing for patient susceptibilities in preclinical drug safety assessment. There are many modeling platforms used throughout the drug development process, but animal models are used strategically throughout
the process to help in critical decision making regarding target identification and validation, candidate selection, and preclinical safety. The dependence on animal models is in large part due to extensive similarities in anatomy, physiology, response to injury, pathobiology, and pharmacology between animals and humans. Berridge noted that the cardiovascular system, in particular, is well conserved in animal models. While there are differences that have to be accounted for, Berridge said there is a logical rationale for the dependence on animals as a surrogate for the human system.
Citing a study that investigated the concordance of drug toxicity between humans and animals, Berridge said that the use of animal models, and the high level of concordance, has helped protect patients from harm in the early stages of clinical trials (Olson et al., 2000). Despite this confidence, serious adverse events resulting in severe toxicity, irreversible harm, and even death have occurred in phase I clinical trials, said Berridge (e.g., Eddleston et al., 2016; Suntharalingam et al., 2006). In these events, there was no indication from preclinical studies that these toxicities could occur. While these acute toxicity events are extremely unfortunate, they are very rare, said Berridge. More troublesome, in his opinion, is the possibility of chronic effects in susceptible patients. For example, a clinical trial for the use of bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease was halted when an increase in cardiovascular events in the treatment group was discovered. By that time, patients had been exposed to the study drug for an average of 7 months. This is the type of adverse event that, unfortunately, is usually not detected until data from large numbers of patients over long periods of time are analyzed. The reasons for the disconnect between animal studies and clinical settings are not hard to find, said Berridge. Experimental animals tend to be young, healthy, genetically homogeneous, and live in a controlled environment, whereas patient populations tend to be all ages, usually sick, genetically heterogeneous, and live in uncontrolled and variable environments (Olson et al., 2000). Berridge noted that one reason why animal model studies operate under such controlled conditions is that such control leads to more discrete data, which makes it easier to make a decision. Unfortunately, the more controlled the platform, the less similar it is to human patients, he said.
Another complication, said Berridge, is that patient susceptibility to toxicity varies, perhaps due to synergy between the drug and the patient’s disease or genetic predisposition, or due to other reasons. Cancer treatments give us an opportunity to study toxicities and the concordance between animal and human studies, because higher levels of toxicity from cancer drugs are generally tolerated. One study on cardiovascular liabilities associated with cancer drugs found that, while there was an overall low incidence of most events, the intra-patient incidence varied widely, suggesting that individual patients are more susceptible, Berridge said.
Despite these challenges, the current approach to safety assessment protects patients quite effectively. Citing a study on first-in-human clinical trials, Berridge said that animal safety studies have a high negative predictive value (Monticello et al., 2017). That is, when a lack of toxicity is observed across multiple species of animals, and there is concordance across those species, there is a high degree of confidence that the compound will be safe in humans. Opportunities to improve this system, particularly how well models can predict liabilities in susceptible patients, include next-generation vivaria and alternative models.
Next-Generation Vivaria
Berridge noted that, when humans visit a doctor, certain parameters (e.g., blood pressure, weight, and body temperature) are always measured, regardless of the reason for the visit. Some of these parameters are difficult to routinely measure in animals. Technological developments in vivaria, said Berridge, will enable researchers to monitor animals in a more holistic way. The data from these physiological parameters will add to, and give context to, the data already routinely measured in animal studies.
Alternative Models
Rather than using animal models that are homogeneously young and healthy, the use of alternative models—animals with diseases or stresses—can help elucidate potential susceptibilities. For example, a study on doxorubicin found that hypertensive rats were more sensitive to doxorubicin cardiotoxicity (Herman et al., 1985).
Berridge concluded that, on the whole, the current approach to assessing preclinical drug safety largely protects patients from harm. However, the system is not designed to identify those rare clinical events associated with individual patient susceptibilities. As precision medicine advances, there may be an expectation that preclinical assessment will identify these individual susceptibilities, and as technological capabilities advance, it may be possible to do so.
CO-EXPRESSED GENE NETWORK ANALYSIS AS A BRIDGE FOR EXTRAPOLATION BETWEEN SPECIES
Biological systems are incredibly complex, said James Stevens, distinguished research fellow at the Lilly Research Laboratory. This complexity makes it challenging to model pathophysiology in a way that allows for meaningful mechanistic inferences. To address this challenge, a European Innovative Medicine Initiative II (IMI2) project called Translational and
Quantitative Systems Toxicology1 is developing a multi-level computational modeling approach to bridge cell, tissue, organ, and whole organism signaling and response to injury.
One foundational component of this effort is the application of weighted gene co-expression network analysis (WGCNA). WGCNA is not used to examine individual gene changes, but rather to assess highly connected networks of genes—called “modules”—that frequently respond together or are “co-expressed.” Though the approach is currently focused on transcriptomic data (i.e., mRNA), it could be applied more generally to other -omic data sets, said Stevens. By mapping networks to pathways and other biological information, the approach leverages the inter-dependence of biological functions and signaling represented by the modules and gene regulatory networks.
Stevens said that focusing on modules of genes can define biological or pathophysiological responses while reducing complexity compared to analysis at individual gene level, thus avoiding the “curse of dimensionality”—that is, the need for more and more data as the dimensions of the data increase. At the same time, modules retain biological content and help improve data interpretation through network visualization, rather than analysis of individual elements or genes.
There are several fundamental principles behind WGCNA, said Stevens. First, cellular biology is driven by coalescent properties. This means that individual genes and protein activities are not random, but act in integrated and coordinated ways. Second, these activities are co-regulated with many basic biological functions that are highly conserved across species. Third, statistical models are good at defining patterns in complex data.
Stevens gave an example of the use of WGCNA to study toxic liver injury (Sutherland et al., 2017). First, an open source WGCNA algorithm (Langfelder and Horvath, 2008; https://labs.genetics.ucla.edu/horvath/CoexpressionNetwork/Rpackages/WGCNA) was applied to gene expression data from the public database DrugMatrix. The data represented about 1,000 treatments that induced a wide spectrum of toxic responses. The algorithm defined self-assembling modules from these data; 415 modules were defined for rat liver. This approach, said Stevens, was validated by comparing the preservation of modules to a similar database built by the Japanese National Toxicogenomics Program (TG-GATES; Igarashi et al., 2015), in which 95% of the modules were preserved. Modules were hierarchically clustered to form a dendrogram, using their average eigengene scores for all modules across all experiments—modules cluster in regions with similar responses. An eigengene score is a single statistical measure of perturbation of all of the genes in the module for a given treatment. By
___________________
1 See http://transqst.org.
overlaying the dendrograms with pathway and GO terms enrichment clusters of modules with complementary functions are found in specific regions of the dendrograms. Important pathophysiological associations become recognizable as patterns of modular gene responses in the dendrogram. Importantly, said Stevens, mapping to pathways in the Molecular Signatures Database (MSigDB) to modules reveals blank regions in the WGCNA dendrogram with high association with the co-occurrence of pathology but for which no pathway has been defined for the modules, suggesting there is much to be learned regarding mechanisms of pathogenesis by better defining the biology represented in the gene networks.
Stevens told workshop participants that correlation of individual histological morphologies of injury with changes in WGCNA modules reveals identifiable and interpretable patterns. For example, modular gene changes in a rat model of bile duct ligation demonstrate that the WGCNA module activity parallels the changes in traditional measures of liver injury, such as histological morphology and serum biochemistry. In addition, assessing the activation of the c-Jun N-terminal kinase (JNK) pathway (common to many forms of pathological injury) by looking for the binding of c-Jun (the transcription factor that controls JNK activation) reveals enrichment in modules that are active with bile duct ligation.
WGCNA can also differentiate stereotypic or non-specific responses to injury from compound or mechanism-specific responses, said Stevens. He gave the example of liver responses in a rat model of partial ischemia. Using this model, and analyzing the module expression in ischemic and non-ischemic liver at 4 hours post-ischemia, researchers found distinct responses such as heat shock, cell cycle arrest, and cell–cell junction changes in the ischemic lobes, compared to ribosomal DNA processing in the non-ischemic lobes. However, similar activities (i.e., convergent pathobiology) are present in both ischemic and non-ischemic regions at 24 hours post-ischemia; both regions show DNA replication, ribosomal biogenesis, and tubulin formation. Stevens said that a similar phenomenon is observed by looking at other surgical liver injury models, such as partial hepatectomy.
Stevens said that additional applications of WGCNA include comparison of biological responses across models within a species (e.g., whole organs versus cell response in vitro), and across species for the same organ. For example, he cited work showing that mouse liver is more predictive of responses in rat liver than cultures of primary rat hepatocytes (Sutherland et al., 2016). Stevens told workshop participants that WGCNA offers an opportunity to get a better sense of the comparative relevance or predictivity of preclinical animal models for responses in human patients.
USING GENETICALLY DIVERSE MICE TO TEST SUSCEPTIBILITY TO TOXINS
Alison Harrill, a geneticist at the National Toxicology Program of the National Institute for Environmental Health Sciences, said assessing the susceptibility of humans to environmental chemicals is challenging for a number of reasons. First, although humans are routinely exposed to chemicals in their environments, few, if any, data about specific exposure levels and health effects are available. Second, exposure and susceptibility to these chemicals vary considerably between populations. For example, people exposed to chemicals as part of their job have longer-duration exposures compared to the general population. Infants may be more at risk of exposure to certain toxins because they put things in their mouth and have lower body weight. Third, genetic variations are linked to different susceptibility levels; for example, susceptibility to methyl mercury exposure can vary 50,000-fold between individuals due to genetic differences. Understanding these population dynamics can help inform the establishment of safe exposure thresholds.
The risk assessment process for environmental chemicals involves collecting data on a number of factors. These data are generally collected from whole animal studies and in vitro research. Data are needed to answer questions such as the following:
- What is the hazard to the body and what tissues are affected?
- What dose and potency exposure carries an agreeable risk?
- Is the risk different depending on whether exposure is acute or cumulative?
- Are certain populations more susceptible, based on, for example, age or life stage?
Safety assessment of chemicals has traditionally focused on determining the average “safe” exposure, rather than taking into consideration all of the variability in genetics and susceptibility. New genetically diverse mouse models have made it possible and desirable to study genetic diversity in chemical safety assessments. The Diversity Outbred (DO) mouse population is a rationally interbred population with highly randomized polymorphisms that mimics human genetic diversity, said Harrill. These mice were created through a genetic reshuffling of genes from eight founder strains, each with a different genetic profile and different propensities for diseases (see Figure 6-1). This genetic profile of the population allows researchers to look at chemical toxicity on a background of comorbidities that is very similar to that in the human population.

SOURCE: Harrill, slide 8.
These mice are used
- to identify specific polymorphisms associated with xenobiotic toxicity,
- to evaluate biomarker performance toward assessing human clinical adverse outcomes, and
- as a tool for population-based estimates of chemical potency for risk assessment.
To Identify Specific Polymorphisms Associated with Xenobiotic Toxicity
A diverse mouse population can be used to identify specific gene variants that influence susceptibility to drug and chemical toxicity. Harrill gave an example from her own research (Church et al., 2015), in which DO mice were given a short-term oral dose of a component of green tea extract (epigallocatechin gallate). Among the two-thirds of the susceptible population, a very small subpopulation showed extreme sensitivity analogous to the severe idiosyncratic liver injuries observed in humans. Harrill noted that these idiosyncratic toxicities had been difficult to study because the dose–response relationship was unclear, and the mechanism of action was not well understood. GWAS analysis identified a peak on chromosome four. Mice that inherited alleles from one specific founder strain (NOD) were less
susceptible to this toxicity. Subsequently, researchers sought to validate the candidate SNPs from the identified region using data from humans who had experienced hepatotoxicity associated with the green tea extract.
To Evaluate Biomarker Performance Toward Assessing Human Clinical Adverse Outcomes
Harill said that often the identification of biomarkers is conducted in a specific (limited) genetic background (e.g., Sprague Dawley rats), prior to clinical studies. Using the DO mice, Harrill compared the gold standard biomarkers of creatinine and blood urea nitrogen (BUN) to newly identified protein biomarkers, following exposure to cisplatin and the subsequent proximal renal tubule injury. The response to cisplatin was variable, with 13 of the 45 mice showing no signs of renal tubular necrosis. Twenty-six mice showed signs of necrosis, with 17 showing grade 1 necrosis and 9 showing grade 2 necrosis. BUN levels, as expected, did not increase until grade 2 necrosis was present. Unexpectedly the other urinary biomarkers were similarly not elevated until grade 2 necrosis was established. This finding suggests that these new biomarkers do not represent an improvement over BUN, said Harill.
As a Tool for Population-Based Estimates of Chemical Potency for Risk Assessment
A study to examine benzine toxicity used DO mice to calculate its benchmark concentration (the concentration of a substance that produces a defined response). Research on 44 human subjects had found a benchmark concentration at 7.2 parts per million. However, the calculated value from data collected from the DO mice was 0.205 parts per million—orders of magnitude lower. This type of study may help to inform risk assessments for humans that result in more protective levels of acceptable exposure, concluded Harill.
INTEGRATING EVIDENCE FROM ANIMAL AND HUMAN STUDIES
The Integrated Risk Information System (IRIS) was created in 1985 to enable consistent evaluations of chemical toxicity, said Kristina Thayer, who serves as director of the IRIS Division at the Environmental Protection Agency (EPA). Prior to the establishment of IRIS, multiple divisions at the EPA conducted independent assessments and often came up with conflicting results. The comprehensive IRIS risk assessments involve a rigorous, multi-step review process, with opportunities for public input, and provide toxicity values for both cancer and non-cancer effects. These technical as-
sessments, when combined with additional information, such as exposure levels, cost of cleanup, and regulatory options, are used to make regulatory decisions under the Clean Water Act, the Safe Water Drinking Act, the Toxic Substances Control Act, and other laws.
The IRIS assessments use data from both animal and human studies, conducting wide and deep analysis of the literature to find research that is relevant to the chemical in question. The assessments are performed using a systematic review method that screens and evaluates the quality of the evidence at each step, and synthesizes and integrates the evidence using a structured framework. The end result of the systematic review process is a conclusion about the toxicity of the chemical, accompanied by a confidence rating for the conclusion—that is, based on the quality of the evidence, how confident the assessor is in the conclusion about toxicity.
The assessment begins with determining the exact parameters of the assessment, performing a literature review to find relevant research, and extracting the data. Once studies have been identified—both human and animal—each study is individually evaluated on criteria incl uding internal validity, bias, sensitivity, applicability to the question, and reporting quality. For example, the bias analysis might look at whether or not the study used randomization; sensitivity analysis might look at the variation in exposure levels; applicability analysis might determine how relevant a specific animal model is to human health; and reporting quality analysis might look at whether the specific form of the chemical was reported. Each of these criteria receives an overall score of good, adequate, poor, or critically deficient.
Based on these ratings, each study is given a score of high, medium, low, or uninformative. Thayer compared this scoring to a heat map, in which a study with a lot of green (i.e., criteria scored as “good”) would fare better than a study with a lot of red (i.e., criteria scored as “poor” or “critically deficient”). Figure 6-2 demonstrates this visualization (taken from Taylor, 2017):
- High: No notable deficiencies or concerns identified; potential for bias unlikely or minimal and sensitive methodology.
- Medium: Possible deficiencies or concerns noted, but resulting bias or lack of sensitivity would be unlikely to be of a substantive degree.
- Low: Deficiencies or concerns were noted, and the potential for substantive bias or inadequate sensitivity could have a significant impact on the study results or their interpretation.
- Uninformative: Serious flaw(s) makes study results unusable for hazard identification. (Ibid)
After the individual evaluation of each study, the studies are examined together to synthesize the information within evidence streams. A similar heat map approach is used in this step, with, for example, all animal stud-
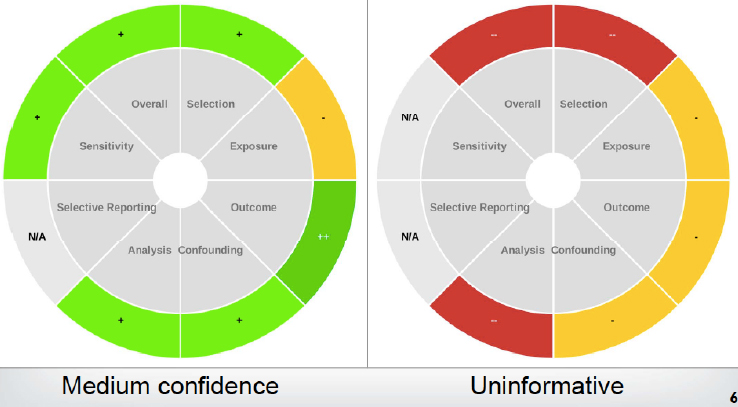
SOURCE: Thayer, slide 7.
ies displayed in one table (see Figure 6-3). The synthesis process considers questions such as the following:
- What outcomes are relevant to each health hazard domain and at what level (e.g., health effect or subgroupings) should synthesis occur?
- What populations were studied (e.g., general population, occupations, life stages, species, etc.)?
- Can study results be described across varying exposure patterns, levels, duration, or intensity?
- Are there differences in the confidence in study results for different outcomes, populations, or exposure?
- Does toxicokinetic information influence differences in responses across route of exposure, other aspects of exposure, or life stages?
Human and animal studies are synthesized separately, but their evidence streams are integrated together, a step that also includes mechanistic evidence that can inform the study results. Mechanistic evidence is particularly important when the studies are of lower quality and a determination cannot be made on the studies alone. In these cases, if mechanistic evidence supports the findings of the studies, this may increase the confidence in the overall conclusion. Evidence is organized into a table that describes the gen-
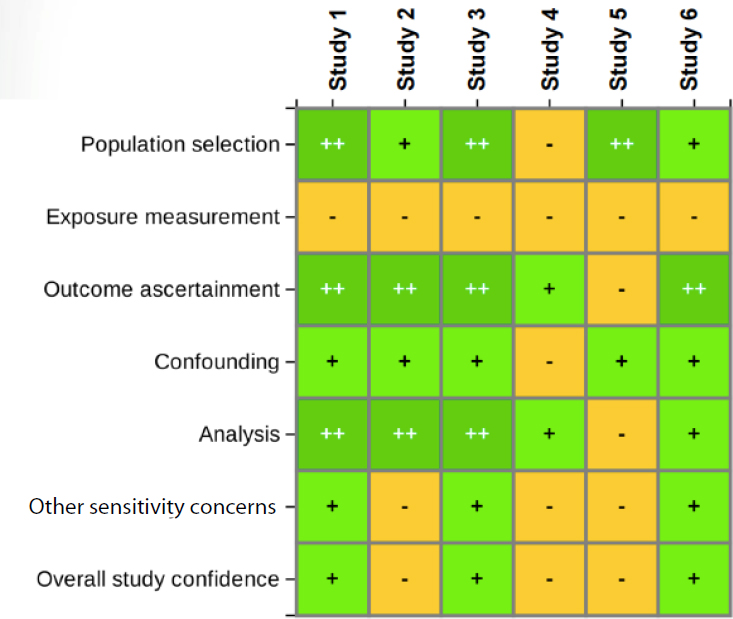
SOURCE: Thayer, slide 8.
eral findings, the factors that increase or decrease confidence in the findings, a judgment rating about the strength of the evidence, the inference across evidence streams, and a final conclusion about the evidence (see Figure 6-4).
Thayer noted that each step of these assessments relies heavily on the judgment of experts: generally two experts perform parts of the assessments independently and then reconcile their answers. If there is an abundance of studies, the assessment will focus on those that are of medium or high confidence levels, and those studies rated as “poor” or “critically deficient” will be dropped from the analysis. Inconsistencies between studies may be due to the stratification of the evidence—for example, different studies show different results because they focused on different populations or durations of exposure. However, if the inconsistencies are unexplained, the confidence level of the assessment is downgraded. Thayer noted that some studies cannot be used in their assessments because of a lack of complete information about features such as randomization, blinding, or exact chemical used, a
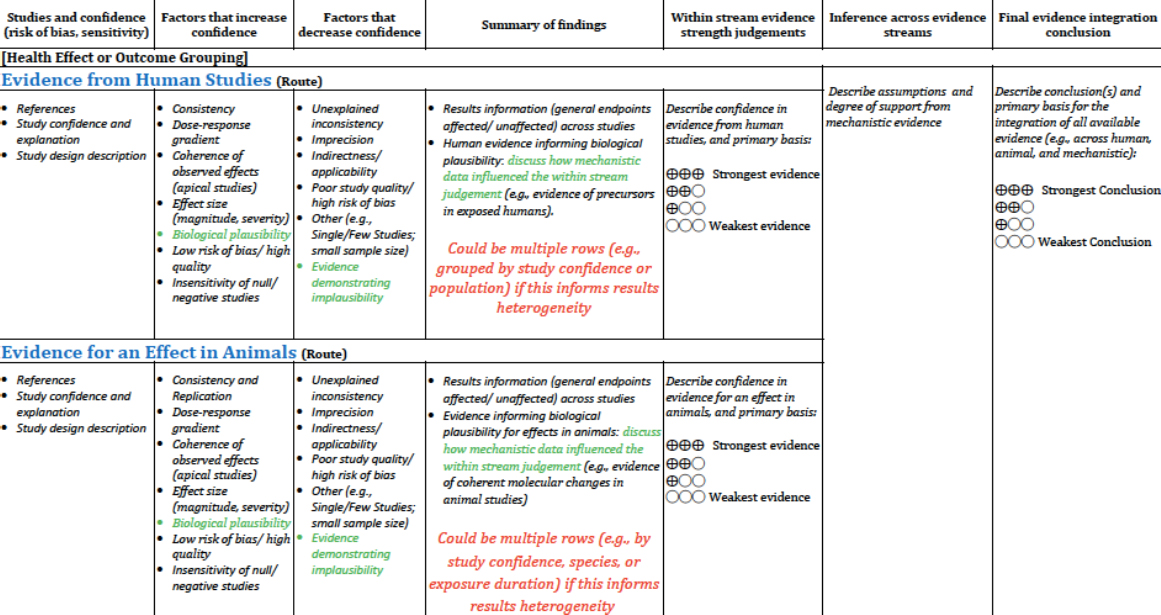
SOURCE: Thayer, slide 13.
statement that echoes comments made by Dirnagl and Hoitinga during their presentations at the end of the first day.
The current approach to integration of animal and human studies is largely qualitative, said Thayer, though structured frameworks like IRIS are becoming more commonplace. Animal models in environmental health are assumed to be relevant to human health unless there are data to suggest otherwise. “Exact findings in animals and humans aren’t necessarily required. . . . [T]he animal models can be canaries in the coal mine,” she said.













