4
Compatibility of BioWatch Improvements Within the Existing Biological Detection Architecture
This section draws on a paper commissioned by the Planning Committee on Strategies for Effective Biological Detection Systems, “BioWatch Improvements Within Existing Biological Detection Architecture,” by George Dizikes (see Appendix I). In his discussion of his invited paper, Dizikes, director of the Tennessee Department of Health’s Knoxville Regional Laboratory, provided an overview of the current BioWatch detection architecture, which has not changed substantially in 14 years, and then made some modest suggestions for improvements using existing methodologies. The rationale for an early warning system such as BioWatch, said Dizikes, is to provide response authorities with information concerning the presence of a biothreat agent before the public shows signs of infection. This window of opportunity between when an agent is released and when people become symptomatic and seek medical assistance, which could be days to weeks depending on the agent, is a time when prophylaxis with medical countermeasures may still be effective.
As with other types of environmental and clinical testing, there are three distinct phases to BioWatch, explained Dizikes: preanalytical, analytical, and postanalytical. Field operations serve as the preanalytical phase, while the laboratory operations represent the analytical phase. He said that, prior to their introduction to the program, the reagents used in polymerase change reaction (PCR) analysis and other laboratory operations have been extensively evaluated with regard to sensitivity, specificity, and other standard parameters of method validation.
A single primer probe is used to screen for each agent, and if a positive reaction occurs, the testing laboratory will run several real-time PCR reactions with additional primer probes to challenge and enhance overall specificity. “Only if the proper combination of primer probe sets is reactive for a given agent will a positive result be considered, and only then will activities commence for the possible declaration of a BioWatch Actionable Result, or BAR, along with its follow-on notifications and activities,” said Dizikes. In addition, a number of control reactions are set up to test for possible contamination and whether or not the DNA preparations contain inhibitors to PCR.
The postanalytical phase of BioWatch begins after completing all routine testing. For the vast majority of samples, the initial real-time PCR screening will produce a nonreactive signal and the laboratory will report a negative result to the Laboratory Response Network (LRN) results messenger. “Except for cleanup and paperwork, this will essentially end the day’s activities,” said Dizikes. A positive initial result followed by repeat testing results that do not fit the appropriate interpretive algorithm is also reported as a negative result through the LRN results messenger. However, if the follow-on results satisfy the algorithm and a sample is deemed presumptively positive, significant postanalytical activities commence and the process of BAR determination begins.
Historically, said Dizikes, presumptive positive results have been extremely rare and have warranted considerable scrutiny and review. The first step in the review process is to consult with the laboratory’s BioWatch director, who serves as the representative of the local public health jurisdiction for laboratory affairs, and the BioWatch staff. The laboratory will undertake various quality assurance (QA) measures, including a review of any recent work of the agent in question as part of a positive QA filter, a proficiency testing filter, or work by either the BioWatch staff or personnel of the host laboratory. Consultations with the Centers for Disease Control and Prevention (CDC), the BioWatch program office, and the local laboratory will occur to review the circumstances surrounding the results in question. “At this point, the laboratory may be advised to cut an additional quarter of the implicated filter and repeat the analysis using the full set of primers and probes for the agent in question,” said Dizikes.
Based on all available information, it will be the decision of the host laboratory authority—the resident BioWatch laboratory director or designee—to complete the BAR data form, declare a BAR, and commence notifications and conference calls over the next several hours. These conference calls serve to provide situational awareness at a local and national level and recruit additional resources to further evaluate the implications of the BAR to the larger national preparedness and response community. The ultimate question being investigated, said Dizikes, is whether the BAR represents a public health emergency warranting a local and national response or if the circumstances surrounding the presumptive positive result are explicable as a natural phenomenon and thus do not require triggering response activities, including syndromic surveillance by the local public health apparatus. To date, he added, all BARs have been interpreted as nonthreatening, natural events.
Since the BioWatch program’s inception, there have been a number of evolutionary, rather than revolutionary, changes to the analytical process. The first changes were made to increase throughput to jurisdictions with larger numbers of portable sampling units, such as changing from individual shaker tubes and filtration cartridges to a 96-well block shaker and 96-well filter manifold. The protocol was also changed to allow sample pooling, though the disadvantage of doing that is the need to disaggregate the pool and repeat the initial screen if the pool is reactive. Over time, there have been upgrades to the PCR instruments as well.
The most fundamental change to the BioWatch program, said Dizikes, was the introduction of the QA program that Molly Isbell previously discussed in her invited paper and its summary presentation. The QA program, he noted, helps prevent and detect errors that can often result from the lapses in attention and procedural drift that can occur with any highly routine, potentially monotonous process such as the daily BioWatch procedure. Still, he added, constant vigilance and efforts to vary staff jobs as much as possible also serve as essential components of a total QA process.
The BioWatch laboratory work is conducted by individuals who are essentially guests at a host laboratory, and, as such, the facilities allocated to BioWatch may not be ideal and their improvement or upgrade may not be a very high priority for local or state government budgets, said Dizikes. In addition, interactions between the computer information technology component of BioWatch and the host information technology structure may at times be at odds.
As Dizikes explained, for the BioWatch laboratory to function successfully, it is essential for there to be a lead worker with excellent managerial and interpersonal skills. It is also essential that a good working relationship be established between the BioWatch lead and the representatives of the host laboratory with whom he or she interacts. “Beyond this, it is important that these two individuals, while representing the laboratory, establish good working relationships with other members of the BioWatch Advisory Committee and that the anticipated events following a BAR be fully exercised before there is an actual event,” said Dizikes.
As currently designed, BioWatch can only find what it is looking for, he noted. “The holy grail of a biological detection system would be the virtually instantaneous detection and identification of any agent posing a biological threat,” said Dizikes. An even more ambitious system, he added, would be one that could detect and identify the presence of any threat: biological, chemical, or radiological. “Unfortunately, a universal detection system, while appealing, is likely beyond any foreseeable capabilities even going out 10 years,” he added. In his opinion, select agents and toxins pose the most immediate and greatest threat of clandestine release. “Although it seems that most bad actors still prefer to shoot, blow things up, or drive trucks over people, many chemical agents by their nature make their presence known almost immediately and radiological agents are more difficult to obtain than biological ones, which can be grown from the environment and transported with little risk of detection,” he explained.
With regard to possible future implementations of BioWatch, Dizikes said the automated biological detection system developed for and deployed by the U.S. Postal Service could serve as a prototype for an autonomous collection, detection, and reporting system. So, too, could some of the technologies developed for the BioWatch Generation 3 program that the Department of Homeland Security (DHS) canceled following a Government Accounting Office review1 (NASEM, 2016). One challenge of the current system is that if a release occurs early in the
___________________
1 See http://www.gao.gov/assets/680/673531.pdf (accessed December 4, 2017).
collection cycle, more than a day will pass by the time results are available. Collecting and processing samples more often would improve time resolution, but costs would increase dramatically. Autonomous detection would address this issue, too, but the necessary instrumentation, in Dizikes opinion, would require custom fabrication and be expensive to operate. “I think we are asking too much of autonomous detectors to say we are going to make one and it will be good enough to put everywhere,” he said. “Maybe we should just focus on indoor for the autonomous detectors and use off-the-shelf technology.” He added that indoor autonomous detection would enable the ability to limit the spread of material, something that could not be done effectively with an outdoor release.
For outdoor release, he suggested replacing filters with cyclone detectors, which collect samples in liquid form and enable automated extraction, to simplify some of the subsequent processing in the BioWatch laboratory. Robotic extraction and preparation of the PCR mixtures would reduce staffing needs, lessen the chances of error, and, in the long run, reduce costs. “The savings could be used to pay for additional collections,” Dizikes suggested. “That improvement would be the first time in BioWatch that the actual turnaround time would have a significant reduction and that reduction, which could be on the order of 8 to 10 hours, could be the difference in thousands of lives in an event.”
Having the samples in liquid form could also make it possible to use other analytical techniques, such as mass spectroscopy, affinity-based enrichment and identification using magnetic antibody beads, and culture-based drug susceptibility testing. “These are things you could not easily do in an autonomous system out in the field because adapting units to that environment would be too expensive,” he added.
Opportunities for and Challenges to Deploying BioWatch Indoors
This panel session included a presentation by David Silcott, chief executive officer and founder of S3I, on aspects of how bioaerosols behave experimentally in indoor spaces. The other four panelists—Maureen Sullivan, emergency preparedness and response laboratory supervisor at the Minnesota Public Health Laboratory; Mark Buttner; Toby Merlin; and George Dizikes—then provided their perspectives on indoor surveillance. An open discussion, moderated by David Cullin, followed the panelists’ reflections.
Key Points on Indoor Biological Surveillance
An attack on a facility or mass transit system is a low-probability but high-impact event, with a high loss of life, major disruption of operations and corresponding major loss of revenue, and costly and slow restoration of operations, said David Silcott. He noted that he has spoken with many property owners over the past 15 years and they are just as concerned about the latter two consequences as they are with protecting the occupants of their facilities. He added that any
venue where large numbers of people congregate, including airports, subways, commercial and financial centers, and sport and concert venues, are potential bioterrorism targets.
There are some subtle differences between an outdoor and indoor attack, said Silcott. In the outdoor environment, explosives, backpack sprayers, or crop dusters likely disperse the agent as a combination of single particles and aggregates as large as 10 microns in diameter. In contrast, indoor dispersal will largely be in the form of single particles below 2 microns in diameter generated by medical nebulizers and other dry-powder dispensers. “It is a subtle distinction, but an important one when it comes to real-time monitoring architectures,” said Silcott. The smaller size of particles released indoors translates into an ability to be transported over very long distances, he added.
Since 2003, he and his colleagues have conducted dispersion tests in a variety of facilities, producing large data sets on indoor dispersions and millions of hours of continuous run times of real-time optical biological threat detectors. While outdoor releases typically produce trace concentrations of particles, an important lesson from indoor release studies is that release from even small dispersal devices such as a handheld nebulizer produces a concentrated plume of particles that is easily distinguished from ambient particle levels by real-time optical detectors (see Figure 4-1).
To demonstrate the effectiveness of optical detection, Silcott discussed the results of an aerosol dispersion test in an airport with a heating, ventilation, and air conditioning (HVAC) system countermeasure consisting of a fire exhaust routine that overpressurizes neighboring HVAC zones and initiates an emergency exhaust system in the release zone. Twenty-four optical sensors continuously sampled the air in the airport ventilation system at various locations throughout the airport following a 3-gram release of tracer particles over 10 minutes—equivalent to the material in a single Advil tablet being released per minute—into a 600,000 square-foot facility. In the absence of the countermeasure, the tracer spreads throughout the entire airport within 2 hours, but with the sensor system triggering the HVAC countermeasure, the spread was confined to the initial release area.
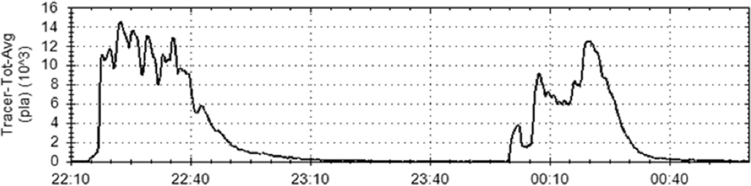
In a similar study in a 52-story building outfitted with 14 sensors placed between floors 1 and 44, Silcott and his colleagues released tracer particles using a handheld nebulizer from the building’s first floor lobby outside of a security checkpoint. The sensors detected tracer particles throughout the building within 30 minutes, with elevators transporting particles to the building’s upper floors. The question here is if the facility management would accept stopping elevator traffic until obtaining a confirmatory measurement given the disruption to 4,000 people who work in the building as a means of minimizing the possibility of contaminating a multibillion-dollar facility.
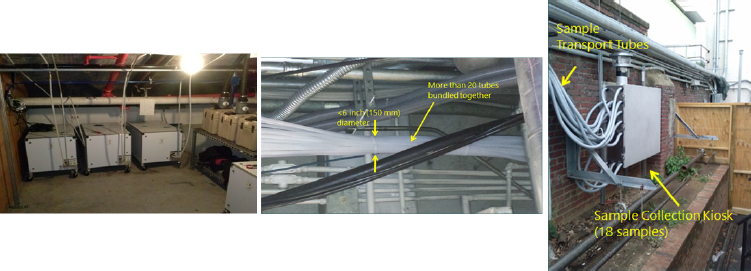
Indoor monitoring architectures have been deployed since 2006, mostly for infrastructure protection, said Silcott. In a typical system, triggers are configured to have either a 1-minute or a 5-minute trigger rate. Any trigger starts an automated filter-based sample collection system, and an accompanying automated delivery system that uses pneumatic tubes to send samples from the hot zone to a sample kiosk in a cold zone. One system Silcott discussed, called FAR-PASS™ (see Figure 4-2), contains a 30-day supply of filters and sends samples to a collection kiosk located several miles away. The kiosks serve as HEPA (High Efficiency Particulate Air) filters and tests have shown that the sample-containing tubes are free of particles that might contaminate the person retrieving the tubes.
Threat confirmation by PCR occurs within 2 to 3 hours after the release. For one of his company’s customers, Silcott worked with MRIGlobal to develop a multiplex PCR process that could conduct simultaneous analysis of up to 35 FAR-PASS™ samples.
Silcott said trigger devices have matured over the past 15 years, getting smaller, more rugged, and less expensive. Current indoor triggers based on optical techniques such as elastic scattering, autofluorescence, and polarized scattering now cost less than $10,000. These devices, some of which have millions of hours of operating time, sit in mechanical rooms or above ceiling tiles and look for particle plumes in real time.
One question Silcott has heard from many property owners has to do with public health response when the facility is not a subway environment or an airport. “What should the concept of operations be for something like that?” he asked. Most property owners, he said, want to have protection capability so they can have the information themselves to make decisions before waiting to get an official result from public health. In his opinion, detection by a property owner would not trigger a public health or BioWatch Actionable Result until the LRN confirmed the result, but a triggered biosurveillance architecture does give property owners low- to medium-regret actions they can perform to minimize spread while waiting for that confirmatory result.
In closing, Silcott said that mature and affordable indoor biosurveillance does exist for property owners. “These real-time warning systems do have a place in this type of architecture and it has been very useful,” he said. He ended his presentation with two key questions:
- What is an acceptable detection confidence level for an onsite confirmatory result to merit taking something to an LRN for further analysis?
- What should be used to determine the required sensitivity of the architecture given the variability in biological agent lethal dose or expected agent concentration from an attack?
Discussion
Toby Merlin began the discussion by asking Silcott if published data exist on how often the optical detectors trigger an alarm and whether the resulting samples have ever tested positive. Silcott replied that the alarm settings are programmable for both response time and sensitivity level to meet the end user’s needs. As an example, Silcott said that a sensor set to trigger an alarm after detecting 100 particles per liter of air over a 4-minute time period would trigger every couple of months. Changing the response time to 5 minutes would increase the trigger rate to once every couple of years. Deciding on sensitivity and time will depend on the ambient particle levels in a given facility, the expected threat concentration, the lethal dose of an expected threat, and other factors. He noted that sensors in some facilities have been set at both the individual sensor level and the network level, and that periodic test releases of harmless tracer materials serve as confidence checks that the sensor array is working at the desired sensitivity.
Merlin, commenting from the perspective of a public health professional, said the general approach he and his colleagues take is to base decisions on performance data. “It sounds as if this application is designed for the property owner and the comfort level of the property owner to shut down or alter the property operations, and to my mind, that is not a public health concern,” said Merlin. “A property owner who wishes to shut off the elevators in their buildings or change the HVAC settings is welcome to do that.” The interface with public health, he added, would occur when a sample tests positive and the facility owner then wants
to send the sample to a public health laboratory for confirmation. “Private property owners can acquire whatever they want, but I think it is useful for them to have a conversation with public health labs before they start sending things to public health labs for confirmation,” said Merlin, a statement Maureen Sullivan reiterated.
Sullivan then asked Silcott if there have been any instances in which secondary testing occurred, and Silcott said there have been in some critical infrastructure facilities. In those cases, a Department of Defense laboratory handled the analysis. He added that there is affordable, commercially available technology that private facilities could operate to make an informed decision before sending a sample to the LRN for final confirmation. Dizikes said he did not think there is a clear mechanism for a private entity to submit a specimen to the LRN, and that they may need to first go to the Federal Bureau of Investigation. “It is just too easy for private companies to come up with their own new system to sell to businesses, and now all of a sudden the market is flooded with people that want confirmatory tests,” said Dizikes. An unidentified participant said New York regulates this type of testing, which further complicates private entities operating their own testing systems.
As BioWatch moves indoors, said Sullivan, conversations between facility managers and public health must take place. In Minnesota, for example, management of an indoor facility expressed interest in having BioWatch collectors installed without understanding that a positive signal was going to mean the facility would have to close, even if that signal was for a naturally occurring organism. After several months, the facility management team came back to Sullivan with a consequence plan and was ready to have more productive talks, which eventually led to the conclusion that BioWatch was not right for that venue at that time.
Mark Buttner said Las Vegas has been proactive about reaching out to property owners and educating them on bioterrorism release scenarios. As a result, the city has generated interest among property owners to deploy BioWatch at special indoor events. Hotel casino vice presidents for security are very forward thinking and proactive about protecting their customers, he said, and they have come to understand the implications of monitoring events at their properties. One item hotel security executives have said they would like is technology that integrates biological, chemical, and radiological detection, and many of them have volunteered their facilities to serve as test beds for such systems.
Buttner then asked Silcott about background particle levels based on the millions of hours of run time for the optical sensors, and Silcott said background levels of fluorescent particles are most directly related to people traffic, with a much smaller contribution from the outdoors. Typically, he said, for fluorescent particles 2 microns and larger, levels would be in the range of 20 to 100 particles per liter of air. In a release scenario, levels at the sensors would rise to many thousands of particles per liter of air within approximately 6 minutes, depending on the sensor architecture. Silcott added that more quantitative data are available, but not for discussion in a public forum. He also noted that this type of system
would not detect the type of exposures that occurred with the anthrax-contaminated letters in a postal service facility because resuspension of particles occurs at too low of a level.
Buttner then asked Silcott how he could see the type of system he discussed being integrated into BioWatch. Silcott said the main role would be to compress the time from release to sampling. “The role of the trigger would be to provide the early warning,” he said. Buttner said he suspected that there would be several venues in Las Vegas that would volunteer to serve as a test bed for this type of technology, and Silcott replied that this system has been deployed in several test beds already, including one that the Navy Surface Warfare Center assessed independently. He agreed with Merlin that data from such test beds have to speak for themselves.
Merlin remarked that a question for BioWatch as its moves indoors is whether triggered collection, rather than the current continuous collection design, is a better approach. “Certainly, if there is a good signal-to-noise ratio and there are few alarms, you end up with fewer collections, and that decreases the burden on the lab and the cost,” said Merlin. Silcott noted that some locations, in fact, did change their collection practices after gaining confidence in the system once it had been running for some time.
One issue Merlin raised was where DHS and BioWatch’s responsibility ends and where a private property owner’s responsibility begins. Sullivan said she and her colleagues have discussed that very issue many times and decided if there is an incident, public health is going to be involved. As an example, restaurants are privately owned, but public health inspects them. “Eventually, we are going to be involved, which is where the up-front communication comes in,” said Sullivan. “There has to be some way that public health is aware of these different technologies that are out in the field.” Walter agreed, adding that facility owners need to take advantage of the local BioWatch Advisory Committees. He noted, too, that regardless of whether a facility is public or private, public health will dictate what will happen if a facility is attacked. As an example, he explained that if the Pentagon was attacked, Arlington County Public Health would decide when the military can go back into that facility.
An unidentified participant asked if sensors, even if not used to trigger sample collection, would be useful to deploy because they would provide a time stamp for when a release occurred. Walter agreed that would be useful information for public health because it would provide information about incubation period and symptom onset. He noted that conversations with local BioWatch Advisory Committees have repeatedly highlighted the need to have any trigger connected to a collection device. One way to satisfy that desire, said Walter, would be to have a handheld identification technology, one that fulfills the program’s quality assurance criteria, and use it to identify if a target agent was responsible for an alarm. No such device exists, he acknowledged, and the field needs to get moving on developing such a device.
Wayne Bryden remarked that when he was with the Defense Advanced Research Projects Agency (DARPA), the agency’s Immune Building program examined vulnerabilities and active protection measures for indoor releases of biological and chemical threats at military bases. This program ran a large number of tests of systems using HVAC controls to minimize the spread of an agent released both inside and outside of various test facilities, and while the data from those studies are classified, what he could say publicly was that the program demonstrated a protection factor of six orders of magnitude or more using that type of countermeasure. “You are protecting a lot of people by keeping an agent from spreading,” said Bryden. He added that at the time, the type of system Silcott described did not exist and the available system was too expensive to operate, so it was discontinued in 2007. He did note that parts of that system have been deployed in “special facilities” and have been operating for many years.
George Dizikes commented that the best time to deploy a sensor array would be when building a facility, rather than having to adapt a sensor array to the existing layout of a building. The good news, replied Bryden, is that fire suppression and fire-activated HVAC control for fire and smoke spread are already incorporated in many buildings, although it would be necessary to add one of several available low-cost technologies for removing the agent from the fire control system’s exhaust plume to prevent releasing the agent into the outdoor environment. He added that a problem DARPA encountered in its tests was that the HVAC countermeasures were so effective at sequestering a release that particle levels were never high enough in other areas of the test facility to set off other alarms.
Lisa Foddrill from the Tauri Group asked if triggers would go off in response to spikes of naturally occurring bacteria or in response to someone sneezing near a sensor. Silcott said that, even in the dirtiest buildings, the level of fungal spores, for example, would be orders of magnitude less than in a release plume. Dizikes asked if any of the sensor systems look at the size uniformity of the particles, and Silcott replied that two of the available technologies do measure particle size and distribution as well as concentration.
OPERATIONAL CONSIDERATIONS FOR NOVEL TECHNOLOGIES AND MODALITIES
The next panel session featured presentations by three panelists. Dee Pettit, assistant director for the North Carolina State Laboratory of Public Health, discussed what she sees as limitations of the current BioWatch approach to surveillance; M. Allen Northrup, chief executive officer of MIODx, provided an industry perspective on implementing new technologies in BioWatch; and Toby Merlin then addressed the issue of operationalizing a major shift in technology. An open discussion moderated by Grace Kubin, planning committee member, and including George Dizikes, followed the three presentations.
A Public Health Perspective on BioWatch Capabilities
Dee Pettit first reminded the workshop that the mission of the BioWatch program is to provide an early warning to prepare for and respond to biological incidents. To have a rapid and effective response, she continued, it is critical to both detect and characterize the agent that triggers a response. In a perfect world, BioWatch would have continuous, real-time aerosol monitoring that produces quality data at low cost, but quick, high-quality, and inexpensive rarely occur in the same technological package, she noted. The ultimate detection system would also determine if a detected organism was viable and infectious and characterize its antibiotic susceptibility, items that Pettit thought might be feasible using molecular markers. Even though such a technology might exist in 10 years, an important issue is whether it can do so with one environmental sample.
Once an organism is detected and characterized, public health needs to respond. That response, she noted, can be agent specific. If an agent is not endemic in the local environment, an initial response might be to deploy the Strategic National Stockpile, though there may be issues in the community about taking an antibiotic in the absence of symptoms. For an endemic organism, questions arise regarding when and where to conduct enhanced surveillance to determine if detection is related to natural occurrence of the organism or an act of terrorism.
In Virginia, for example, Francisella tularensis occurs naturally and the state sees as many as three cases a year, typically from April through September and largely through exposure to activities such as hunting, handling wild rabbits, being exposed to ticks, and landscaping, explained Pettit. In 2005, more than 15 sensors in the Washington, DC, area reported detection of F. tularensis at the time of an antiwar demonstration in the city. Public health responded by moving from a system of passive disease surveillance to active surveillance and notifying emergency departments and clinical diagnostic laboratories to be alert for signs of the disease.
The technologies used to detect F. tularensis include the direct fluorescence antibody test, which takes 3 hours, or real-time PCR, which takes 4 to 6 hours. F. tularensis grows slowly, culturing the organism takes 48 hours, and pulsed field gel electrophoresis from the cultured organism takes about 1 week to provide strain identification genetic typing. This last technology, said Pettit, provides a genetic signature that when compared with the genetic signatures from F. tularensis seen in the native environment provides a good clue as to whether the detected organism was intentionally released in an act of terrorism, she explained. In the case of the 2005 incident, the available data suggested this was a natural event, but if the detection of an agent that was not endemic in the community had occurred, Pettit said the response would have been much different.
With regard to enhanced surveillance, laboratories are asked to hold growth plates for 5 to 7 days because that is how long it can take for F. tularensis to grow enough to be detected. Pettit thought a better approach for enhanced surveillance would be to have a rapid diagnostic that could quickly analyze a blood sample to find bloodstream infections.
Going forward, Pettit thought it important to explore how to leverage environmental testing and clinical surveillance strategies. She also said it was important to question the return on investment regarding the money spent on BioWatch over the past 15 years. That money, she said, allowed jurisdictions that conduct BioWatch testing to establish biodefense advisory committees, and it has allowed enhanced relationships and critical partnerships to develop. It has also enabled the development of concepts of operation to address biodetection events. Nonetheless, she said, “we need added value to the program in the absence of an attack, because right now, when we have not had attacks, what is the value to the system?” she asked.
In her opinion, she would like to see the response to a BioWatch trigger include culture-independent testing using next-generation sequencing, for example. “I think development of that technology in a clinical diagnostic setting and then applying what we learn there to address our needs in environmental settings would be appropriate,” said Pettit. She questioned whether environmental monitoring adequately supports evidence-based decision making, and thought it too much to ask for today’s system and perhaps for any system developed over the next 10 years. She asked if it was possible to think about developing more than one test and more than one approach in addition to environmental surveillance. “I think it is important that we also enhance clinical diagnostic testing as a means of taking a more systematic approach to include environmental and clinical diagnostic testing,” said Pettit.
Other questions she had included whether environmental monitoring provides the data needed to identify populations most at risk. For example, individuals who are immunocompromised would, in her opinion, be the first to come forward with symptoms following a release, and the issue is how to identify those individuals. She suggested that diagnostic testing could be a better approach for identifying atypical and emerging threats, as the current system which looks for a handful of agents cannot. “A diagnostic approach would allow for the identification of those organisms if we are using next-generation sequencing,” said Pettit. She also hoped the BioWatch program would consider investing in enhanced clinical surveillance.
To conclude her remarks, Pettit listed some immediate considerations that came to her while listening to the 2 days of presentations. The first was that the current program only monitors a limited number of jurisdictions. “I think we are appropriately targeting high-risk areas,” she said, “but I do think that increasing event-based surveillance or a more targeted approach to surveillance could help partners really enhance the relationships that we know are very important and that BioWatch has done a great job of enhancing.”
In terms of the BioWatch process, Pettit said she believes PCR is a suitable technology and that the program is devoting appropriate attention to enhancing PCR testing, multiplexing, and next-generation sequencing to produce more data. What she did not hear much about was how to enhance sample collection to shorten the time between release and response and to support advanced molecular diagnostics to quickly identify index cases in individuals exposed to a biological
agent. She also suggested focusing on clinical surveillance that would look closely at individuals with bloodstream infections and acute respiratory illness, which in her opinion would produce a better return on investment than from the current structure of the BioWatch program. It is also important going forward to develop a better understanding of how the public will respond to and abide by public health advisories in the absence of morbidity and mortality in the community. It is unclear, said Pettit, that everyone would be receptive to taking an antibiotic in the absence of reports of people becoming ill.
An Industry Perspective on Implementing New Technologies
The U.S. Postal Service began using an autonomous biological detection system within a year of anthrax-containing letters being sent through the mail. The PCR technology used in the system was invented by Cepheid, where Allen Northrup was the chief technology officer at the time, and the technology has processed over several million samples since its installation. He was also the chief executive officer of Microfluidic Systems, which developed over 25 automated airborne detection systems for the Department of Homeland Security for testing as part of the m-BAND program. Microfluidic Systems was also a subcontractor developing technologies for BioWatch Generation 3.
With that as background, Northrup said his attitude is to stop working on detection. “It is done,” he said. “There are so many ways to detect nucleic acids and proteins that if I were deciding to go forward with technology development, I would just put detection on the back burner and focus on sample prep, sample processing, sample collection, and then, on the back end, the interpretation of the data and results.” He explained that with a quality DNA sample, there already exists at least five reliable ways to identify what it is, and the same is largely true as far as telling whether an organism is alive or not. In his opinion, if automated sample processing is what BioWatch decides to go forward with, he would consider using well-established existing detection technologies that are commercially available and modify them to fit into BioWatch. As an example, he cited the Roche COBAS system, a clinical system that analyzes 96 samples at a time with little human input other than loading samples into the system.
Northrup suggested developing automated preanalytical technologies, distributing them to users for evaluation and feedback, improving the technology, and then moving on to the next component of the system, such as detection. “It is extremely important to work with the users, the laboratorians, the decision makers, from day one,” he said. He advised taking an iterative approach that improves the system component-by-component rather than an all-inclusive “black box” with the expectation that the laboratories would just accept it as is without being involved in the development process. Part of this cooperative process would include identifying both technical and economic bottlenecks and setting priorities for short-term and long-term needs. The key, as Suzet McKinney noted in her presentation, is to build confidence in new technologies among the users and
decision makers and work with users to adopt technologies about which they are confident.
Operationalizing a Major Shift in Technology
In Toby Merlin’s opinion, BioWatch is a remarkably high-performing, well-established program, from sample collection to sample processing, getting the result to the BioWatch Advisory Committees, the quality assurance program, and the exercises it runs. It works, he said, and public health, public safety, transit departments, the CDC, and state laboratories have confidence in it. “It generates 230,000 to 240,000 results a year and the people who see those results know what those results mean, they know what causes errors, and they know how to troubleshoot errors,” said Merlin.
Given that, making a substantial change to the system will take time, and, as Northrup suggested, should be done in a stepwise, one-technology-at-a-time manner, with piloting at one or more sites, generating data, sharing those data, and developing a detailed rollout plan. Merlin said the old system and new system should be operated in parallel, and only when people are comfortable with and confident in the new system should the old system be shut down. Merlin noted that the LRN has experience with such a process that BioWatch can learn from and implement as it develops new technology.
Currently, for example, the LRN is in the process of introducing mass spectroscopy to replace a mouse assay for botulinum toxin detection using a full-sized mass spectrometer and an elegant assay developed at the CDC. The rollout of this new technology is taking 3 to 5 years, he added, and has been complicated by the unforeseen obstacles that arise in rolling out a program in the federal government, including the need to spend money when budgets are flat. He noted that the paper he and Duncan MacCannell wrote (see Appendix H) includes the description of a new system for detecting and characterizing food-borne pathogens that is being implemented in public health laboratories.
“The bottom line for me is there is the technology assessment process and selection process,” said Merlin in closing. “But do not underestimate the complexity of implementing a technology across a broad network.”
Discussion
Grace Kubin opened the discussion by asking the panelists to identify their most important consideration for BioWatch improvements. Pettit said the thing she grapples with most when a detection occurs is assessing whether there is an actual threat given that the result is based on looking at DNA, so she would like to see an effort to enhance the ability to assess viability of infectiousness of a detected organism. Northrup said his number one issue is ensuring that users are confident in any new technology before it gets implemented across the network
and to implement new technologies in a stepwise manner. Merlin’s primary concern is the availability of performance data in a real operational environment given that such data are often classified. Dizikes’s prime consideration is cost given that BioWatch depends on government funding.
Norman Doggett noted his concern that any new amplicon-based detection system will not have an option for user-defined primers, either because the primers are provided by BioWatch and their identity is classified or because the companies developing a technology provide their own proprietary primers. Northrup replied that nearly every commercial PCR instrument can take any user-defined primer and if it cannot, he would not use it. Merlin acknowledged Doggett’s point and noted that the Warrior Panel developed for the BioFire instrument uses primers and probes that are classified and whose distribution is limited to the Department of Defense and its designated facilities. He also pointed out that it is hard and dangerous to develop off-the-shelf primers for detecting potentially lethal organisms, which is why the federal government develops those primers.
Merlin concluded the discussion by comparing what BioWatch does and what he and his colleagues in public health do regarding infectious disease detection. “It is easier for us to roll out a new assay because we are detecting events that occur on a regular basis,” he said. “We are detecting influenza or Zika or West Nile virus or botulinum toxin, and we can compare the new detection method to the old detection method on actual occurring events. BioWatch does not have any real events to compare its detection methods with, and it is much harder to develop a system to detect something that you are hoping does not ever happen.”















