INTRODUCTION AND OVERVIEW
The U.S. Army conducted tests with human subjects to study the effects of a variety of agents, including chemical warfare and biological agents (Brown 2009 a,b). The tests were conducted between 1942 and 1975. The potential long-term health effects on the test subjects from their exposures have been evaluated periodically, most recently in a report titled Assessment of Potential Long-Term Health Effects on Army Human Test Subjects of Relevant Biological and Chemical Agents, Drugs, Medications and Substances: Literature Review and Analysis (the Report), which was prepared by a contractor (Ho-Chunk Technical Solutions 2016) to assist the Army with making determinations about providing medical care to former test subjects. In response to a request by the Army, the National Academies of Sciences, Engineering, and Medicine (the National Academies) formed the Committee to Review the Report on Long-Term Health Effects on Army Test Subjects. The committee’s task was to focus on whether the Report appropriately identified potential long-term health effects from exposure to the test agents and whether an adequate weight-of-evidence approach was used to characterize the strength of the associations between the agents and their potential health effects (see Attachment 1 for the statement of task). The committee was made aware at its first meeting on November 30, 2017, that the Army had already begun to receive applications for medical care and that some determinations may need to be made before the committee’s evaluation of the Report was completed. Because of this urgency, the Army developed a process by which applications for medical care will be reviewed, and as a result, the committee was given the additional task of reviewing the Army’s Memorandum that describes the approach that will be used by the Army to evaluate agent- and outcome-specific associations (see Attachment 2). This interim report was prepared to facilitate the Army’s deliberations. A review of the Report is presented first, followed by a review of the Memorandum. The committee is currently preparing a more detailed final report that will provide more technical details about how it arrived at its findings and recommendations. That report is expected to be released in May 2018.
REVIEW OF THE REPORT
The Report is a literature review of potential long-term health effects associated with exposures to more than 100 test agents. The review focuses on studies published after June 30, 2006, the last date when test subjects were provided information about their participation in the testing. Brief descriptions of the studies on each agent were provided and 18 agents were identified as having evidence of potential long-term health effects. The committee reviewed the Report to determine whether it applied best practices in conducting hazard identification.
The Army is required to provide medical care to former test subjects whose participation in the Army’s research is deemed to be the proximate cause1 of an injury or a disease. The committee considered “proximate cause” to be a legal term and not a medical or a scientific term. In scientific or medical evaluations, causation is evaluated by describing the strength of the association between an exposure and a health outcome. Typically, scientifically based determinations about potential associations require a comprehensive and rigorous analysis of the evidence, including consideration of the weight of evidence from epidemiological, toxicological, and mechanistic data on specific agents or exposures (IARC 2006; NRC 2009, 2014; NTP 2015). Examples of well-established hazard identification schemes include those conducted by the National Toxicology Program (NTP 2015), the U.S. Environmental Protection Agency (EPA 2005), and the International Agency for Research on Cancer (IARC 2006). Although the various schemes use different descriptors to characterize the strength of these associations, they all specify the types of scientific evidence to be reviewed, describe an explicit classification scheme, and define criteria on which the classifications are based. The committee considered a scheme most relevant to the Army’s
___________________
1 “Any health condition having a sufficiently strong causal link such that a reasonable person could find that the injury or disease was caused by testing exposure or participation in research” (see Attachment 3, Section 8a).
needs, which is one developed by the Institute of Medicine (IOM, which is now known as the Health and Medicine Division as part of the National Academies) for the U.S. Department of Veterans Affairs (VA) to characterize associations between exposure to Agent Orange during the Vietnam War and long-term health effects (IOM 1994, 2016). The scheme was also used to characterize associations between a variety of agents, including chemical warfare agents, environmental chemicals, vaccines, and infectious agents, and health effects in Gulf War Veterans (IOM 2000, 2004, 2007). The classifications based on the IOM scheme have been used by the VA to make decisions about treating and compensating veterans.
The committee finds that although the Report provides a survey of recent literature on the test agents, it does not constitute a hazard-identification assessment. The Report does not contain sufficient information about the methods used to conduct the literature review and analysis. Specifically, no information was provided regarding the literature search strategy, the databases that were searched, the search terms, and the results of the searches. The criteria and methods to screen literature for relevance were not described. Short summaries on the individual agents were provided in the Report, but no critical evaluation of the studies or explanation of how different lines of evidence were weighed in drawing conclusions about the hazard categorization of each agent was provided. The strength of association between each exposure and its potential long-term health effects was classified as high, medium, or low, but the methods used to categorize agents were inadequately explained. The committee finds that the Report should not be used as the sole scientific basis to assess the potential long-term health effects from exposure to biological and chemical agents, drugs, medications, and substances.
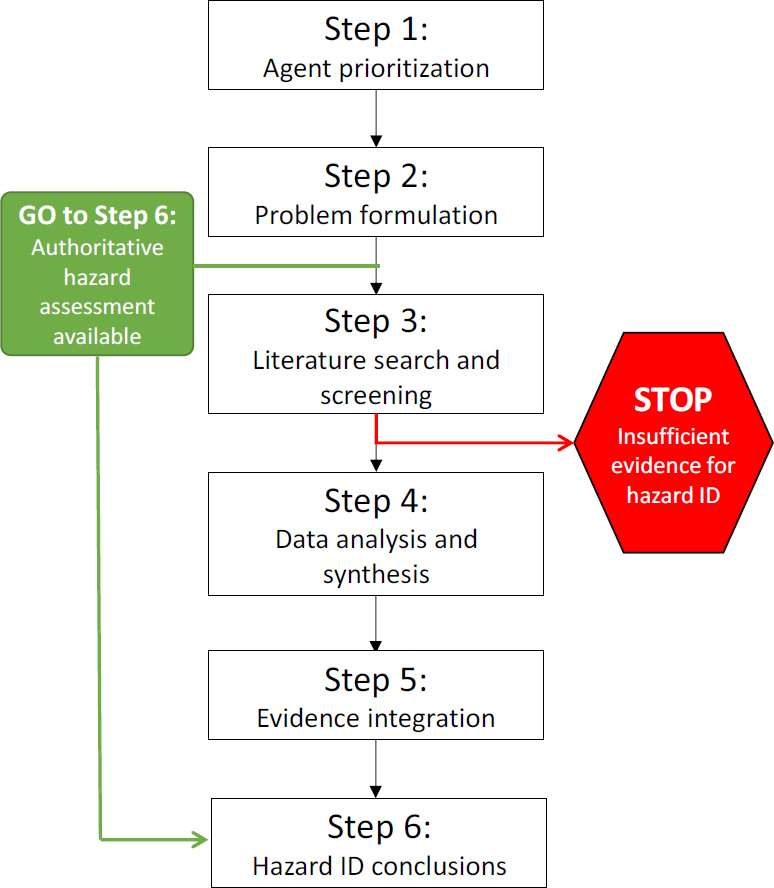
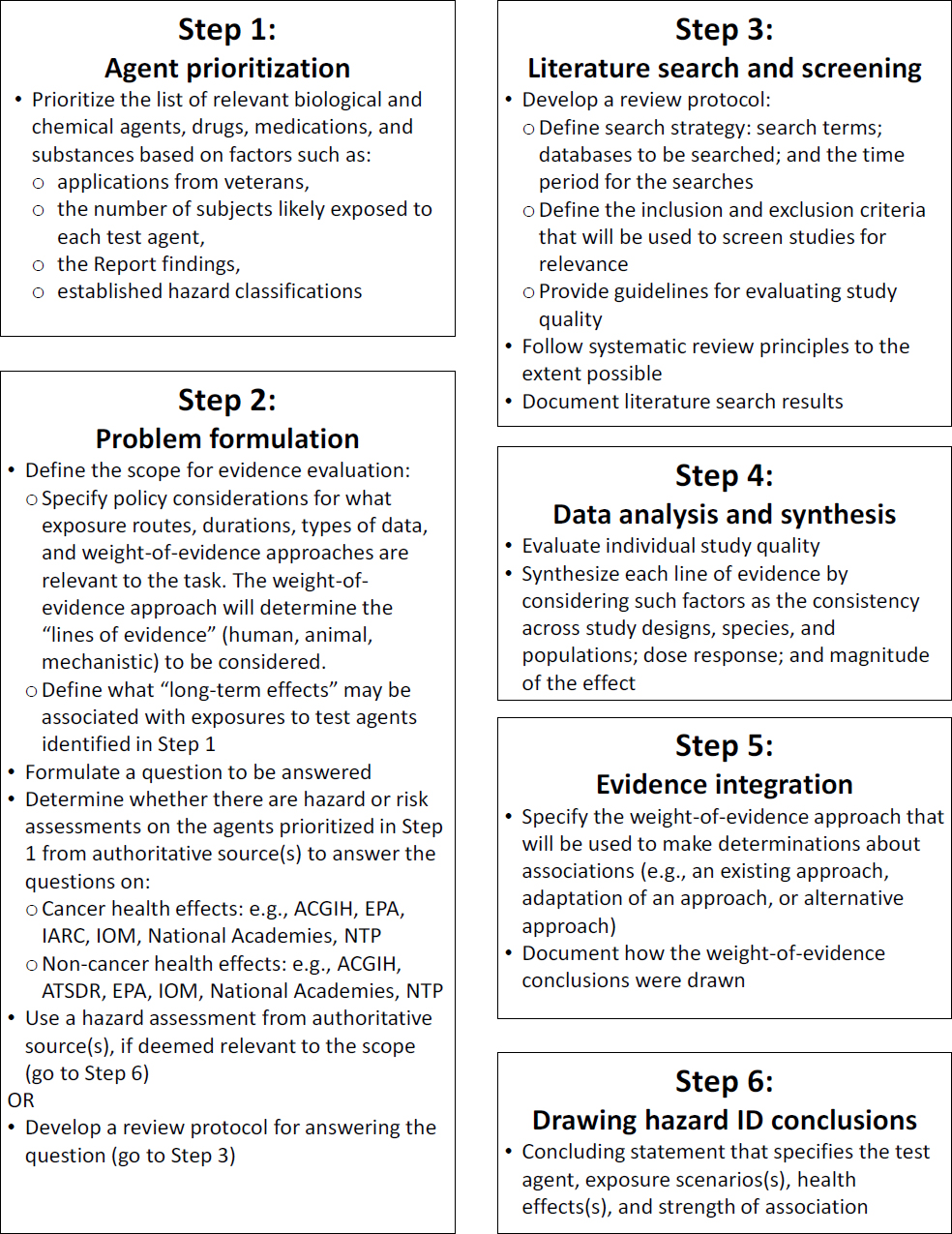
The committee recommends that the Army develop a streamlined, scientifically rigorous approach to categorize health hazards and, given the number of agents to be reviewed, a strategy to prioritize the evaluations. The committee proposes the strategy outlined in Figures 1 and 2. It is based on best practices in hazard identification and systematic review (e.g., IOM 1994, 2000; NTP 2015), which the Army can tailor to its needs. The strategy is designed to be a flexible approach that would allow the Army to rely on comprehensive reviews conducted by other public health agencies when available, as well as to conduct its own analyses. More information about the elements of the strategy will be provided in the final report. The Army is encouraged to define a weight-of-evidence approach to guide its analyses. The committee recognizes, however, that the Army is working under time constraints that may require it to adapt elements of existing approaches to meet its deadlines.
REVIEW OF THE ARMY’S CAUSATION METHODOLOGY
The Army developed an approach to assess proximate cause to assist a Benefits Application Panel (BAP) in making determinations about providing medical care. The Memorandum that describes the approach is presented in Attachment 2. The Memorandum describes a review and adjudication process, causation methodology, and documentation of the BAP’s review and decisions in sections 7–9.
With respect to the review and adjudication process (Section 7), the Memorandum states that previous reviews will be supplemented with specific reviews of the associations between the agent and health outcome of interest to each applicant. The committee agrees that previous literature reviews might be useful, but the Memorandum does not provide sufficient detail for the committee to understand the process by which the BAP will conduct its review of the pertinent information.
With respect to the causation methodology (Section 8), the Memorandum presents two major concepts. One pertains to the “general causation,” which is a process of establishing an association between an agent and a health outcome. The Memorandum specifies that the weight-of-evidence evaluations will follow the framework used in the IOM report Adverse Effects of Vaccines: Evidence and Causality (IOM 2012). The framework (the IOM Vaccine Approach) applies systematic review principles to identify and evaluate the evidence. Separate weight-of-evidence analyses of the epidemiological literature and mechanistic literature are performed and then the two lines of evidence are integrated to make determinations about a causal relationship. Under the framework, the epidemiological evidence is weighed more heavily
and “mechanistic information can only support causation” (IOM 2012, p. 49). The IOM Vaccine Approach classifies the evidence into one of four categories of causality conclusions: (1) evidence convincingly supports a causal relationship; (2) evidence favors acceptance of a causal relationship; (3) evidence is inadequate to accept or reject a causal relationship; or (4) evidence favors rejection of a causal relationship. The committee agrees that this classification terminology may be appropriate for the assessment of potential long-term effects on the Army test subjects. The committee recognizes that for human vaccines, a robust set of epidemiological studies would be available, but this is likely not the case for most agents and substances on the Army’s list. Therefore, the committee questions whether the IOM Vaccine Approach, beyond the categories of causality conclusions, is the most suitable for the Army’s purposes. It is likely that animal and mechanistic data will be important in hazard evaluations for many test agents and substances; therefore, other weight-of-evidence frameworks, such as those from IOM (1994, 2000, 2016), may also be used as guides to establish causality. The final report will review the similarities and differences of the IOM approaches that have been used for evaluating weight of evidence related to exposures to military personnel and veterans.
The second concept addressed in Section 8 of the Memorandum pertains to “specific causation,” which is a process of characterizing the risk based on the individual’s exposure to a specific test agent or a substance. The Memorandum states that consideration will be given to whether the test subject’s exposure was of sufficient magnitude to produce the alleged medical condition, whether the exposure was temporally related to the onset of the alleged medical condition, whether alternate causes of the medical condition can be ruled out, and whether there is coherence and consistency in the evidence. The Army has noted that information on the individual test subjects, such as the exposures they experienced and potential confounders, are unlikely to be available, which will limit the ability of the BAP to draw definitive conclusions with respect to specific causation. The committee agrees that it is unlikely that the exposures during the tests can be adequately characterized or that potential confounders can be ruled out for most outcomes; consequently, some presumptions about exposure and disease may have to be made.
The Memorandum indicates that the members of the BAP will be making both the general causation and the specific causation determinations. The committee recommends that the Army consider uncoupling the two processes, so that general causation is evaluated and determined by a separate group. The committee judges this to be a preferable approach to ensure that appropriate subject matter expertise is used to make determinations about general causation and to preclude bias in the individual adjudication decisions. This approach would be similar to how the VA makes determinations about compensation related to claims by veterans of the Vietnam War and Gulf War (e.g., IOM 1999, 2003).
With respect to documentation (Section 9), the Memorandum states that the decision rationale will be documented. The committee agrees that it is crucial to provide supporting documentation, but the Memorandum does not describe the elements to be provided in support of the decision.
REFERENCES
Brown, M. 2009a. Military chemical warfare agent human subjects testing: Part 1 – History of six-decades of military experiments with chemical warfare agents. Mil. Med. 174(10):1041-1048.
Brown, M. 2009b. Military chemical warfare agent human subjects testing: Part 2 – Long-term health effects among participants of U.S. military chemical warfare agent testing. Mil. Med. 174(10):1049-1054.
EPA (U.S. Environmental Protection Agency). 2005. Guidelines for Carcinogen Risk Assessment. EPA/630/P-03/001F. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC. March 2005 [online]. Available: https://www.epa.gov/sites/production/files/2013-09/documents/cancer_guidelines_final_3-25-05.pdf [accessed January 5, 2018].
Ho-Chunk Technical Solutions. 2016. Assessment of Potential Long-Term Health Effects on Army Human Test Subjects of Relevant Biological and Chemical Agents, Drugs, Medications and Substances: Literature Review and Analysis. Contract no. W81XWH-14-R-0102. February 4, 2016 [online]. Available: http://www.dtic.mil/dtic/tr/fulltext/u2/1009505.pdf [accessed July 24, 2017].
IARC (International Agency for Research on Cancer). 2006. Preamble to the IARC Monographs (amended January 2006). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: IARC [online]. Available: http://monographs.iarc.fr/ENG/Preamble/index.php [accessed January 8, 2018].
IOM (Institute of Medicine). 1994. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington, DC: National Academy Press.
IOM. 1999. Veterans and Agent Orange: Update 1998. Washington, DC: National Academies Press.
IOM. 2000. Gulf War and Health, Volume 1: Depleted Uranium, Pyridostigmine Bromide, Sarin, Vaccines. Washington, DC: National Academy Press.
IOM. 2003. Gulf War and Health, Volume 2: Insecticides and Solvents. Washington, DC: National Academies Press.
IOM. 2004. Gulf War and Health, Updated Literature Review of Sarin. Washington, DC: The National Academies Press.
IOM. 2007. Gulf War and Health, Volume 5: Infectious Diseases. Washington, DC: National Academies Press.
IOM. 2012. Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press.
IOM. 2016. Veterans and Agent Orange: Update 2014. Washington, DC: The National Academies Press.
NRC. 2009. Science and Decisions: Advancing Risk Assessment. Washington, DC: The National Academies Press.
NRC. 2014. Review of EPA’s Integrated Risk Information System (IRIS) Process. Washington, DC: The National Academies Press.
NTP (National Toxicology Program). 2015. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration, January 9, 2015. Office of Health Assessment and Translation, Division of the National Toxicology Program, National Institute of Environmental Health Sciences [online]. Available: https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf [accessed December 28, 2017].






