1
Introduction and Background1
Chronic pain is one of the most prevalent, costly, and disabling health conditions in the United States. Estimates show that more than 11 percent of the American population suffer from chronic pain (Nahin, 2015), yet the federal pain research investment has been minimal, said Christin Veasley, co-founder and director of the Chronic Pain Research Alliance.
In parallel with a gradual increased recognition of the problems of treating chronic pain, the opioid epidemic has emerged as a growing public health emergency. According to the Centers for Disease Control and Prevention (CDC), opioid overdoses account for 115 American deaths each day,2 contributing to the dramatic increase in overall drug overdose deaths since 1999 (see Figure 1-1). In 2016, at least 46 overdose deaths involved prescription opioids each day (Hedegaard et al., 2016). The intersection of these two crises lies in the fact that an unintended consequence of treating pain has been an increasing number of opioid prescriptions and diversion of drugs for illicit purposes, said Story Landis, vice chair of the National Academies of Sciences, Engineering, and Medicine’s Forum on Neuroscience and Nervous System Disorders and former director of the National Institute of Neurological Disorders and Stroke (NINDS). Sharon Walsh, professor of behavioral science, psychiatry, pharmacology, and pharmaceutical
___________________
1 The planning committee’s role was limited to planning the workshop, and the Proceedings of a Workshop was prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants, and have not been endorsed or verified by the National Academies of Sciences, Engineering, and Medicine. They should not be construed as reflecting any group consensus.
2 For more information, go to https://www.cdc.gov/drugoverdose/epidemic/index.html (accessed December 27, 2017).
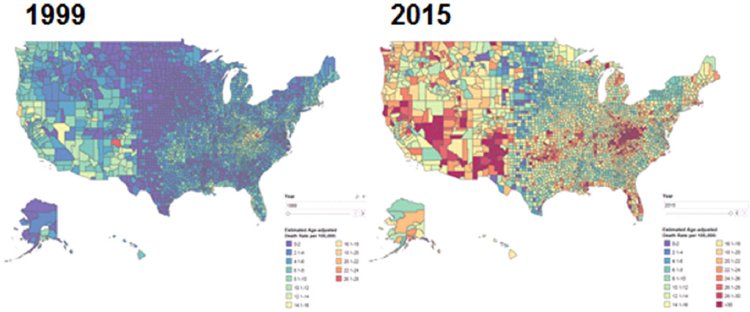
SOURCES: Presented by Nora Volkow, October 11, 2017. Center for Disease Control and Prevention/National Center for Health Statistics, National Vital Statistics System; designed by L. Rossen, B. Bastian, and Y. Chong.
sciences at the University of Kentucky, added that patients with chronic pain and patients with opioid use disorders have much in common. Both populations are stigmatized, and because their providers may also be stigmatized, even adequate treatment may be difficult to obtain, she said. Moreover, both disorders are misunderstood, and health professionals lack the tools needed for proper diagnosis and treatment, said Walsh.
One intervention or strategy by itself will not resolve the opioid crisis, said Nora Volkow, director of the National Institute on Drug Abuse. Developing non-addictive pain medications will, however, address the needs of patients with severe pain and reduce the likelihood that they will become addicted to opioids, she added. In parallel, treatments are needed for those who become addicted, and interventions are needed to prevent or reverse overdosing, said Volkow. She suggested that being in the midst of this crisis may motivate companies and academic institutions to move with greater urgency toward addressing the roadblocks to progress.
In May 2017, Francis Collins, director of the National Institutes of Health (NIH), and Volkow announced a public–private partnership to develop solutions to the opioid crisis and cut in half the time it takes to develop non-addictive analgesics (Volkow and Collins, 2017). Walter Koroshetz,
director of NINDS, noted that following this announcement, Collins convened government agencies and researchers from industry and academia for a series of three meetings, focusing on (1) medications development for opioid use disorders and for overdose prevention and reversal; (2) development of safe, effective, non-addictive pain treatments; and (3) understanding the neurobiological mechanisms of pain. To advance the planning of NIH’s anticipated public–private partnerships, the National Academies’ Forum on Neuroscience and Nervous Systems Disorders hosted this public workshop that brought together a diverse group of stakeholders from academia, federal agencies, advocacy organizations, and companies developing therapeutics for pain and opioid use disorders (see Box 1-1).
WORKSHOP OBJECTIVES
The purpose of the workshop, Koroshetz explained, was to discuss potential strategies to accelerate development of non-addictive pain medications and treatments for opioid use disorders. He reported that NIH and other federal and industry partners are working to create public–private partnerships to address the dual problems of treating pain and preventing and treating opioid addiction and overdose. The workshop was intended to explore the relative value of potential projects and think about how these projects could be operationalized to ensure that the science is advanced and that industry partners get what they need in order to move research and development forward. Topics discussed at the workshop aligned with the priorities identified by the Federal Pain Research Strategy (FPRS).
While recognizing the value of non-pharmacological approaches to pain management, the workshop focused on developing medications. Other important issues that are beyond the scope of the workshop and this report include pain education and workforce issues.
THE PUBLIC HEALTH IMPACT OF PAIN AND OPIOID USE DISORDERS
Opioid medications can be very effective for the treatment of acute pain, yet they are also highly rewarding and addictive, said Volkow. In addition, opioids are not very effective for chronic pain because tolerance develops, and as a result, people take higher doses, increasing the risk of addiction, she added. Overprescribing opioids contributed to the diversion of these drugs to the black market. After stricter clinical guidelines were introduced and education increased, the number of prescription opioids decreased, and some those who were already addicted to the prescription opioids transitioned to heroin, which was cheaper and, in many instances more accessible, said Volkow. This incentivized the black market, and imports of very pure heroin markedly increased in the United States, fueling the heroin epidemic and accelerating the number of deaths due to heroin overdoses, she added. More recently, synthetic opioids such as fentanyl, which is 50 times more potent than heroin, have flooded the market either laced with heroin or prescription opioids, and in some instances by itself. Volkow said that while it is not known whether the combination of fentanyl and heroin is more lethal than either drug alone, fentanyl overdoses are
much harder to reverse than those from heroin (CDC, 2013; Schumann et al., 2008).
The number of lives lost to drug overdoses has climbed dramatically in recent years, from 114 per day in 2013 to 144 per day in 2015 (Rudd et al., 2016), said Jessica Hulsey Nickel, president and chief executive officer of the Addiction Policy Forum. According to the CDC, overdose deaths involving opioids in 2016 were five times higher than in 1999 (2017). A recent study found that opioid overdoses have reduced life expectancy in the United States by 2 months (Dowell et al., 2017). Shame, guilt, and embarrassment cause this illness to become a dark family secret, said Nickel, and the lack of treatment options precipitates long-term effects on affected individuals and their families. The disparity between how opioid use disorder is treated compared with other illnesses perpetuates the crisis. Nickel recounted a statement made by a mother whose two sons died of drug overdoses, “Had they suffered from diabetes or skin cancer, they would have been provided the medical care and attention necessary to live a full life.”
SELECTED FEDERAL INITIATIVES TO ADDRESS THE CHALLENGE OF TREATING PAIN AND OPIOID USE DISORDERS AND RELATED NATIONAL ACADEMIES’ REPORTS
A provision in the Patient Protection and Affordable Care Act (ACA) required the Secretary of Health and Human Services to create the Interagency Pain Research Coordinating Committee (IPRCC),3 with representation from all federal agencies as well as the public, according to Linda Porter, director of the Office of Pain Policy at NINDS. A second requirement of the ACA was a report by the National Academies on pain in America, which was published in 2011 (IOM, 2011) (see Box 1-2). At the request of the Food and Drug Administration (FDA), the National Academies published a consensus study report in 2017 (see Box 1-3) with recommendations on actions that the FDA and other organizations should take to address the opioid use epidemic (NASEM, 2017). In conjunction with these efforts, CDC released guidelines for prescribing opioids for chronic pain in 2016.
A consequence of the 2011 IOM report was the strong suggestion for a federal pain strategy. IPRCC, in conjunction with the NIH Office of Pain
___________________
3 For more information, go to https://iprcc.nih.gov (accessed December 27, 2017).
Policy, released the Federal Pain Research Strategy (FPRS) in November 2017—the same month the President’s Commission on Combating Drug Addiction and the Opioid Crisis published its final report. This strategy encompasses both basic and clinical science efforts across the full continuum of pain, including prevention and management of acute pain, the transition from acute to chronic pain, and the management of chronic pain, said Porter.
The Federal Pain Research Strategy
The FPRS was set up under the ACA and includes recommendations made to the Secretary of Health and Human Services to ensure that NIH and other federal agencies are not duplicating efforts. IPRCC maintains a database of funding programs across all federal agencies, including NIH, the Departments of Veterans Affairs and Defense, the FDA, the CDC, and the Agency for Healthcare Research and Quality. Porter noted that through a series of crosscutting workgroup meetings, a list of priorities emerged
for the FPRS, namely to identify opportunities for therapeutic development of non-addictive pain medicines and to address clinical challenges and immediate needs for developing pain therapeutics (see Box 1-4 for a list of selected top priorities). Porter said that an aim of the FPRS is to complete the National Pain Strategy, a broad-ranging federal effort to change how pain is managed, educate professionals, and raise public awareness, which follows the recommendations of the 2011 IOM report on pain (IOM, 2011).
She also mentioned a Common Fund proposal in development for a project that will involve scientists from diverse areas of research and employ many advanced technologies to identify acute-to-chronic pain signatures. Common Fund projects are supported with set-aside funds that NIH uses for projects that are too big and too disorder-neutral for any single agency to support, said Porter.
Non-Pharmacological Approaches to Treating Pain
David Shurtleff, acting director of the National Center for Complementary and Integrative Health (NCCIH), added that while medications clearly play a role in treating opioid use disorders—with many tools already in the armamentarium, including methadone, buprenorphine, naltrexone, and NARCAN®—behavior therapies are needed to give people the tools they need to cope with their cravings, addictions, and relapses. Cognitive-behavioral therapies and mindfulness-based approaches teach people new strategies to manage their disorders. He noted that these non-pharmacological therapies, could also address pain and other co-morbidities such as depression, anxiety, and posttraumatic stress disorder, which are common in people with opioid use disorders. NIH has programs in place to move both non-pharmacological and natural product approaches forward, including the Stimulating Peripheral Activity to Relieve Conditions (SPARC)4 program, projects exploring brain circuitry and neuromodulation as part of the Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative,5 and the natural products portfolio at NCCIH, said Shurtleff.
Expanding research on non-pharmacological therapies for pain, including behavioral modification, cognitive therapy, and neuromodulation, would broaden the scope of a public–private partnership to address the chronic pain and opioid epidemic, said Porter and Volkow.
ORGANIZATION OF PROCEEDINGS
The following proceedings summarizes the workshop presentations and discussions. This chapter provides background for the motivation for the workshop and an overview of the opioid epidemic, including related federal initiatives to address it. Chapter 2 opens with the patient perspective on living with pain and includes a summary of challenges that have slowed development of adequate treatments for pain and opioid use disorders. Chapter 3 provides a brief overview of the state of the science on opioid and non-addictive pain medications, including the latest research on the molecular physiology and genetics of pain, as well as on preclinical
___________________
4 For more information, go to https://commonfund.nih.gov/sparc (accessed December 27, 2017).
5 For more information, go to https://www.braininitiative.nih.gov (accessed December 27, 2017).
models for pain therapy development. Chapter 4 focuses on clinical efforts to develop non-addictive pain medications, including biomarker-based drug discovery, research under way to prevent the acute-to-chronic pain transition, and a discussion of regulatory issues related to the approval of pain medications. Chapter 5 shifts focus to the development of treatments for opioid use disorders and reversing overdose. Chapter 6 concludes with discussions about existing and proposed partnerships and the role of payers in developing treatments for pain and opioid use disorders.










