9
Cognitive Aging, Dementia, and the Future of an Aging Population
INTRODUCTION
Dementia, a decline in memory and other cognitive functions leading to disability in daily function, is a common and feared geriatric condition. Over the past two decades, Alzheimer’s disease (AD) and Alzheimer’s disease–related dementias (ADRD) have been the focus of increasing attention from researchers, clinicians, and policy makers due to the projected significant increase in the number of dementia cases—and the associated burdens to patients, families, and government support programs—that is expected to result from the worldwide growth in the elderly population. However, a growing number of population-based studies have reported that the age-specific incidence and prevalence of dementia in a number of high-income countries may have declined over the past 25 years, suggesting that trends in some combination of demographic, behavioral, medical, and environmental factors have led to a decrease in dementia risk among older adults in these countries.
A better understanding of the potential causes for the decline in past decades in dementia risk, and whether there will be continued decline, leveling off, or an increase in lifetime dementia risk in future decades, has huge implications for individual and public health and for public policy. Dementia is unique in the extent to which its impact often ripples out across family members, affecting the lives of multiple generations due to the daily care-giving typically required. When families are unable or unwilling to
___________________
1 M.D., Ph.D., University of Michigan and Veterans Affairs Ann Arbor Healthcare System, Ann Arbor.
provide the care needed, institutional long-term care is often required—at high financial costs to both families and public programs.
In addition to the optimistic and welcome trend of declining risk for dementia in a number of countries over the past few decades, an increasing number of studies point to a troubling trend in the United States: namely, growing disparities across socioeconomic status (SES) and race/ethnicity in dementia risk, general health status, and life expectancy. The nature and causes of these disparities, how they have grown in past decades, and how they might be mitigated in the future are also extremely important issues for demographers, policy makers, and other researchers to address in the coming decades.
Finally, there have been important advances in understanding the biology and pathophysiology of cognitive decline and dementia in recent decades, including the importance of cardiovascular risk factors (CRFs) that increase the risk for AD and dementia, as well as new technologies for identifying biomarkers that may aid in the early diagnosis, prevention, and treatment of cognitive decline. These new technologies bring with them important questions for the future of an aging population regarding their costs and cost effectiveness and regarding equitable access to their use among those at risk for cognitive decline and dementia.
GROWING IMPORTANCE OF COGNITIVE DECLINE AND DEMENTIA IN AGING POPULATIONS
Cognitive function plays a central role in determining the well-being and quality of life of adults as they pass from midlife to older ages, including their decisions to work, retire, and spend or save their money. Dementia due to AD or ADRD is characterized by a decline in cognitive function severe enough to cause the loss of independence in daily function (McKhann et al., 2011). Dementia has wide-ranging, direct and indirect effects on the well-being of older adults, their families, and the costs imposed on public programs, such as Social Security, Medicare, and Medicaid.
There were approximately 4.2 million adults living with dementia in the United States in 2010 and more than 135 million worldwide (Hurd et al., 2013; Prince et al., 2013). The economic impact of dementia has been estimated at $200 billion per year in the United States (Hurd et al., 2013) and $600 billion worldwide (Wimo et al., 2013), including a significant burden of unpaid family care-giving. Because the incidence of dementia rises sharply at ages greater than 75, the expected growth in the worldwide elderly population in the decades ahead has been projected to lead to a tripling of dementia cases by 2050, absent new interventions to prevent or slow the trajectory of cognitive decline (Prince et al., 2013; Langa, 2015).
As noted above, tempering the projections of large increases in dementia cases as the older population grows are recent studies suggesting a decline in dementia incidence and prevalence in high-income countries over the past 25 years, perhaps attributable to higher levels of educational attainment and more intensive treatment of CRFs (e.g., hypertension, diabetes, smoking, and hyperlipidemia) that increase the risk for cognitive decline (Schrijvers et al., 2012; Matthews et al., 2013, 2016; Satizabal et al., 2016; Langa et al., 2017; Leggett et al., 2017; Wu et al., 2017). However, it is unclear whether this positive trend in high-income countries will continue, as the prevalence of obesity and diabetes have grown (Larson et al., 2013; Langa, 2015), and it is also unclear whether there has been a similar or opposite trend in low- and middle-income countries (Chan et al., 2013; Wu et al., 2013, 2014).
The relationship of education to lifetime risk for cognitive decline and dementia is especially important to consider for a number of reasons. First, more years of education has consistently been associated with a lower dementia risk in a wide range of studies across different countries over the past two decades (Valenzuela and Sachdev, 2005; Meng and D’arcy, 2012). Second, low educational attainment has been identified as likely the largest contributor to preventable dementia risk worldwide, with an estimated population-attributable risk of nearly 20 percent of dementia cases in 2010 (Norton et al., 2014). Third, SES and racial/ethnic disparities in educational attainment, as well as the quality of education received, have been identified as potential causes for the SES and racial/ethnic disparities in dementia incidence and prevalence in the United States and other countries around the world (Glymour and Manly, 2008). And finally, as noted above, increases in average levels of educational attainment in high-income countries over the past few decades have been associated with declines in dementia incidence and prevalence (Larson et al., 2013; Langa et al., 2017; Wu et al., 2017), suggesting that expanding access to education may help decrease population dementia risk.
How might more years of education decrease one’s risk for dementia? Education-related increases in “cognitive reserve” is a widely cited theory for how education may decrease dementia risk. The cognitive reserve hypothesis posits that the cognitive challenges and stimulation associated with education lead to changes in brain structure (e.g., more neurons and more connections among the neurons) that allow one to better compensate when pathologies accumulate in the aging brain (Stern, 2012; Meng and D’arcy, 2012). Individuals with high cognitive reserve can continue thinking normally with significantly greater levels of neuropathology than those with low cognitive reserve, so they are less likely to experience the significant cognitive decline leading to dementia. Although years of formal education is the variable most often used as an indicator of cognitive reserve (likely because it is routinely collected in many epidemiological studies), other
studies suggest that level of cognitive stimulation and cognitive challenge throughout life, including the characteristics of one’s occupation, how one spends her leisure time, whether one partakes in “cognitive training” exercises, and the extent of social interactions may all play a role in building or maintaining cognitive reserve and decreasing lifetime dementia risk (Vemuri et al., 2014). The optimistic and increasing evidence that aging brains retain their plasticity to grow new neurons and compensate for age-related pathologies through “life long learning” and cognitive stimulation will likely be an important focus of both future research and potential preventive interventions in the decades ahead (Lindenberger, 2014; Gutchess, 2014).
Of course, there are likely many additional pathways besides a direct biological impact of education on the brain by which one’s level of education is associated with better later-life health, cognition, and life expectancy. Hayward and colleagues (2015), in reviewing the literature on the relationship of education to life expectancy, provide a useful conceptual model that highlights the multiple positive health benefits of education, including information regarding the benefits of certain health behaviors and health care services, better jobs and lifetime income, larger and more supportive social networks, and cognitive skills that promote a greater sense of control and agency. Each of these pathways is likely important in promoting brain health, as well as a longer life.
While there has been a general trend toward increasing levels of education around the world in both high-income and low-income countries (Becker et al., 2010), there are significant international differences in the timing and magnitude of those increases, which will likely lead to differences in dementia trends across countries. For instance, while there has been a significant increase in the level of educational attainment among older adults in the United States over the past few decades, there will not be further significant increase in the next few decades, since education levels among those ages 65 to 69 now are similar to the education levels of those ages 25–29 (Lutz et al., 2014). However, educational attainment among China’s older population, for example, will continue to increase in the decades ahead because the education level of those ages 25–29 now is significantly higher than those ages 65–69 now (Lutz et al., 2014). Tracking dementia trends across countries with differing time trends in educational attainment may provide opportunities for a better understanding of the causal pathways leading from early-life education to late-life dementia risk (Langa et al., 2018).
Recent large-scale studies incorporating genetic data from genome-wide association studies (GWASs) have raised some additional complexities for sorting out the life course impact of education on brain health and late-life dementia risk. A number of studies have found genetic loci (single-nucleotide polymorphisms, or SNPs) that are associated with the level of
educational attainment, possibly through pathways that affect brain biology or structure, suggesting that there may be a component of genetically determined brain biology that might lead to both greater educational attainment and to lower risk for later-life dementia (Rietveld et al., 2013; Okbay et al., 2016). Other GWASs have shown a number of SNPs related to general cognitive function in mid- and late life (Davies et al., 2015), cognitive decline in later life (Deary et al., 2012), and the size of the hippocampus (the brain region associated with short-term memory typically affected by Alzheimer’s disease) (Hibar et al., 2017). Future genetic epidemiological research on cognitive decline may help us better understand the complexity of how genetics and one’s lifelong social and environmental exposures interact and combine to increase or decrease the risk for late-life dementia.
THE OVERLAP AND INTERACTION OF ALZHEIMER’S DISEASE, VASCULAR DISEASE, AND OTHER BRAIN PATHOLOGIES
Zlokovic (2011) has proposed a “two-hit vascular hypothesis” for AD, which highlights the likely overlap and interaction of vascular pathways and the classic AD amyloid-beta pathway in the onset of neurodegeneration and subsequent dementia (see Figure 9-1). In this model, vascular disease resulting from known risk factors, such as hypertension and diabetes, leads to decreased blood flow to brain cells as well as disruption of the “blood-brain barrier,” both of which may lead to increased production or decreased clearance of amyloid-beta, resulting in neurodegeneration, loss of synapses and neurons, and eventual cognitive decline and dementia (Snyder et al., 2015). A key implication of this two-hit hypothesis is that prevention or adequate treatment of known CRFs may decrease the risk for AD and other dementias, both by decreasing vascular-related injury to the brain (including strokes) and by decreasing amyloid-beta-related injury to the brain (Zlokovic, 2011). In other words, the prevention or control of CRFs may also act as a “disease-modifying” treatment for AD by preventing or slowing the build-up of cerebral amyloid-beta.
For instance, recent studies show that having more CRFs in midlife is not only associated with a greater risk of cognitive decline in later life (Gottesman et al., 2014) but also associated with higher levels of amyloid-beta in the brain, consistent with the two-hit vascular hypothesis that CRFs may directly influence the development of AD pathology (Gottesman et al., 2017). Yaffe and colleagues (2014) showed that a life course perspective regarding the impact of CRFs on cognitive decline is important, as the cumulative exposure to elevated levels of CRFs over 25 years of follow-up in individuals who were 18 to 30 years old at baseline was associated with significantly worse cognitive function in middle age. As discussed in more detail below, the importance of CRFs, especially in early and midlife, as
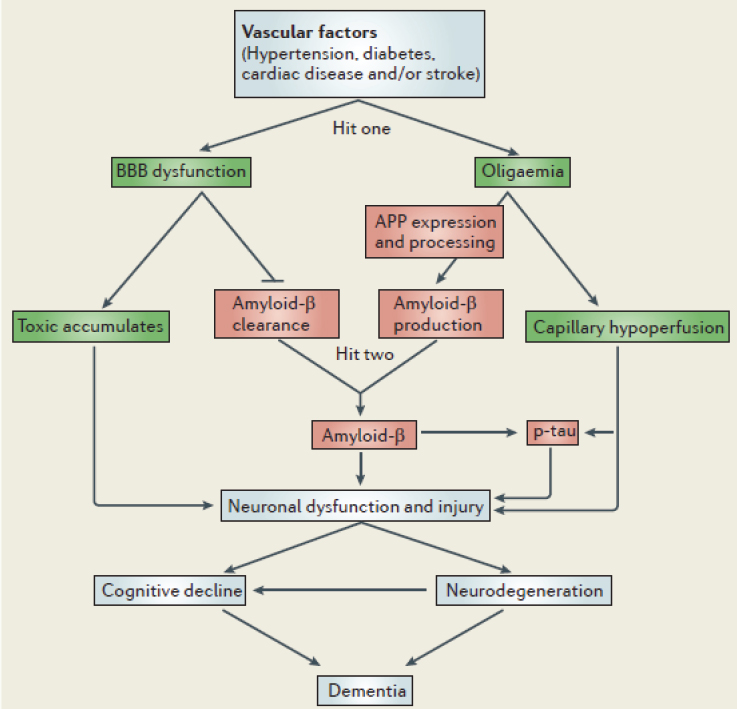
SOURCE: Reprinted with permission from Springer Nature: Springer. Zlokovic, B.V. (2011, Box 1, p. 733). Nature Reviews Neuroscience, ©2011.
potential causes for late-life dementia may be one reason for the clear SES disparities in brain health and dementia risk, since CRFs are more prevalent in individuals from low-SES groups and the SES disparities in the prevalence of those risk factors has been increasing in recent decades.
An important issue regarding the relationship of CRFs to late-life dementia risk is whether better prevention or treatment of CRFs will not only reduce age-specific dementia risk later in life but will also lead to an extension of life expectancy that will, in turn, lead to a greater number of dementia cases due to people living to much older ages, when dementia risk increases sharply. Norton and colleagues (2014) concluded that a significant reduction of mid-life CRFs would lead to a significant decline
in future dementia incidence and prevalence, but their analysis did not account for a possible increase in life expectancy due to the decline in CRFs. A more recent paper by Zissimopolous and colleagues (2018) used a microsimulation model that accounted for the expected increase in life expectancy associated with better prevention and treatment of hypertension and diabetes, along with the decrease in future age-specific dementia risk. They concluded that dementia cases would actually increase in future years, due to more individuals living to older ages when dementia risk is high. The different conclusions from these two analyses hinge on the relative magnitude of the decrease in dementia incidence associated with a decline in CRFs compared to the decrease in future mortality risk; this relative magnitude is currently uncertain. Future research to more clearly define how prevention and treatment of CRFs throughout the life course are related to both future dementia risk and mortality at older ages will be especially important to determine the public health implications of population-level trends in CRFs and their treatment and to determine whether additional years of life are more likely to be spent with good or poor cognitive function (Schoeni et al., 2018).
In addition to AD and vascular disease as causes for dementia, there are likely additional important disease processes and pathological pathways leading to cognitive decline and dementia that are not yet well understood. Boyle and colleagues (2013) showed that, surprisingly, only about 40 percent of late-life cognitive decline could be explained by the known common neuropathologies of AD, vascular disease, and Lewy body disease, a result that strongly suggests that other factors and causal pathways need to be identified and better understood to develop successful preventive interventions and treatments that address the multiple and mixed pathologies that are typically found in the brains of older adults (Neuropathology Group of the Medical Research Council Cognitive Function and Aging Study, 2001; Langa et al., 2004; Schneider et al., 2007; Sonnen et al., 2007). For instance, recent evidence suggests that hyperphosphorylated transactive response DNA-binding protein 43 (TDP-43), a pathological protein first identified in the brains of patients with frontotemporal dementia and amyotrophic lateral sclerosis, is also often present in those diagnosed with AD, and when present it significantly increases the risk for dementia (James et al., 2016).
Overall, the evidence gathered during the past 20 years from population-based studies, especially those that have included brain autopsies, has led to a much better understanding that cognitive decline and dementia are typically caused by a mix of neuropathologies that result from a complex interaction of risk factors (both social and biological) across the life course, likely including pathologies that are yet to be fully understood. As discussed in more detail below, an additional complexity identified with new neuro-
imaging techniques is that the neuropathologies leading to cognitive decline and dementia are often present decades prior to the onset of cognitive symptoms, further supporting the importance of a full life course perspective when identifying the causes and the potential preventive interventions for late-life dementia.
DISPARITIES IN LIFE EXPECTANCY, GENERAL HEALTH, AND BRAIN HEALTH IN THE UNITED STATES
An increasing number of studies show that health status has become significantly more unequal in the United States over the past few decades. Perhaps the clearest evidence of this growing disparity is the trends in life expectancy across rich and poor over the past 30 years. A recent National Academies report (National Academies of Sciences, Engineering, and Medicine, 2015), using data from the Health and Retirement Study, found that life expectancy at age 50 for women in the lowest income quintile and born in 1930 was 32.3 years, but life expectancy at age 50 has actually declined to 28.3 years for low-income women born in 1960. In contrast, life expectancy at age 50 for women in the highest income quintile born in 1930 was 36.2 years, but it had increased to 41.9 years for those in the same income quintile born in 1960. So the gap in life expectancy between rich and poor women in the United States has grown by nearly 10 years, from 3.9 to 13.6, in recent decades. The gap in life expectancy between rich and poor men also increased but by a slightly smaller amount (8 years) than for women.
A recent study by Chetty and colleagues (2016) used tax records to examine income and life expectancy across time and region in the United States between 2001 and 2014. Similar to the National Academies analysis, those with high income had significantly longer life expectancies, and the income gap in life expectancy increased significantly during the 13-year period. Life expectancy for those with low income also varied significantly across geographic regions and was correlated with smoking rates in those regions.
Disparities in general health status among older U.S. adults have also widened in recent years. For instance, the (age- and sex-adjusted) proportion of older U.S. adults who reported being in excellent or very good health (in the nationally representative Medical Expenditure Panel Survey) increased significantly between 2000 and 2014 among those who were white, well educated, or in the highest income quartile. But this proportion decreased significantly during this same time period for those who were Black, Hispanic, had a high school degree or less, or were in the lowest income quartile, thereby widening already significant differences across these groups (Davis et al., 2017).
As noted above, the presence and severity of CRFs in middle-aged and
older adults are closely linked to the risk for subsequent cognitive decline and dementia, so trends in the presence of CRFs and their treatment may be especially important for understanding trends and growing disparities in dementia risk in recent decades. Using data from the National Health and Nutrition Examination Survey, Odutayo and colleagues (2017) found that mean systolic blood pressure and current smoking declined significantly between 1999 and 2014 among those with high income but did not decline among those at or below the poverty level. As a result, the proportion of individuals with high cardiovascular disease risk (i.e., > 20% 10-year risk) widened significantly between those at low- and high-income levels in recent years.
SES and racial/ethnic disparities in dementia risk have been well documented in the United States. For instance, a recent study of older adults in California found significant differences in age-adjusted dementia incidence between 2000 and 2013, with African Americans having the highest risk (26.6 cases per 1,000 person-years), Latinos and Whites having intermediate risk (19.6 and 19.3 cases, respectively), and Asian Americans having the lowest risk (15.2 cases) (Mayeda et al., 2016). These differences were found for both men and women and across the entire age range. Differences across racial/ethnic groups in the presence of CRFs explained some of the difference in dementia incidence but not all of it. A similar pattern of racial/ethnic differences in overall cognitive function was found for a nationally representative sample of 19,000 U.S. adults ages 51 and older in 2010 (Díaz-Venegas et al., 2016).
Another community-based prospective cohort study of older adults in two U.S. locations (Pittsburgh and Memphis; the Health, Aging, and Body Composition Study) found a similar disparity in dementia incidence for African Americans compared to Whites over 12 years of follow-up, with African Americans having a greater risk (unadjusted hazard ratio 1.44) of dementia incidence (Yaffe et al., 2013). Most of the racial disparity was explained by socioeconomic factors (household income, education level, and literacy level), leading the authors to conclude that education’s impact on the building of cognitive reserve throughout the life course, and chronic stress that may result from low SES, are likely important pathways leading to SES and racial/ethnic disparities in late-life dementia risk. Many other studies support the value of using a life course perspective to better understand and identify the complex and overlapping factors that have led to the large and growing SES and racial/ethnic disparities in dementia risk in the United States (for a review, see Glymour and Manly, 2008).
The potential importance of geography as a contributor to SES and racial/ethnic disparities in cognitive function and dementia risk has been an increasing focus of recent research. A study by Gilsanz and colleagues (2017) found that being born in a state that has a high stroke mortality
rate (e.g., the “stroke belt” states in the southeastern United States, such as Alabama, Arkansas, Louisiana, and Mississippi) was associated with a significantly increased risk of dementia, even among individuals who moved away from those states during their life. The authors noted that “place of birth may reflect a host of social and environmental conditions in early life that could be some of the primary drivers of racial inequalities in rates of dementia,” including access to and quality of education, poverty, poor nutrition, chronic stress, and the establishment of health behaviors that may increase cardiovascular risk later in life (e.g., smoking and physical inactivity) (Gilsanz et al., 2017, p. 1061).
Place of residence throughout life may also have an impact on dementia risk and on SES disparities in dementia risk, due to exposures to environmental toxins that affect brain development and health. For instance, individuals living closer to major roadways in Ontario, Canada, were found to have a significantly increased risk for dementia over 12 years of follow-up, possibly related to greater exposure to traffic-related air pollution (Chen et al., 2017). A growing number of other studies have also found a relationship between air pollution levels in one’s region of residence and increased risk for dementia (Cacciottolo et al., 2017) or poor cognitive function (Ailshire et al., 2017).
Other characteristics of one’s neighborhood, separate from potential toxins in the physical environment, have also been shown to be related to cognitive function and dementia risk. For instance, rural residence in the United States was associated with an increased risk for cognitive impairment and dementia compared to urban residence, although that gap decreased somewhat in recent years, possibly related to greater gains in educational attainment among those in rural areas (Weden et al., 2017). Other studies have shown that living in a neighborhood with institutional resources that may help promote cognitive reserve (e.g., community centers, libraries, and recreational facilities) was associated with better cognitive function, independent of individual risk factors for cognitive decline (Clarke et al., 2012).
THE IMPLICATIONS OF DIAGNOSING AND TREATING “PRECLINICAL ALZHEIMER’S DISEASE”
Over the past 20 years, there have been important advances in understanding the natural history of the brain pathologies leading to AD and ADRD, as well as the development of new diagnostic tests and technologies to identify those pathologies. The identification of biomarkers in the blood and cerebrospinal fluid that accompany the development of AD-related brain pathologies, as well as new neuroimaging technologies that can identify changes in brain structure (e.g., AD-related shrinkage of the hippocampus) and the extent of amyloid-beta protein deposition in the
brains of individuals who do not yet show symptoms of cognitive decline, will have important and wide-ranging implications for prevention, treatment, and even the definition of who has AD and dementia. Karlawish and colleagues (2017, p. 379) called this the “Next Frontier” in understanding and management of AD and proposed that in the future, AD may no longer be “a disease leading to irrevocable cognitive and functional decline and death, but rather a chronic condition like cardiovascular disease, AIDS, or some cancers that can often be managed with early intervention.”
One especially important new insight is that the brain pathologies and changes leading to AD and dementia are often present for decades prior to the onset of any cognitive decline. Jack and colleagues (2013) have proposed a hypothetical model (see Figure 9-2) based on the increasing number of biomarker and neuroimaging studies in older adults with and without dementia that found that a significant proportion of individuals had amyloid-beta protein in their brains but were still cognitively “normal.” New diagnostic criteria proposed in 2011 by the National Institute on Aging and the Alzheimer’s Association identify these individuals as having “Preclinical Alzheimer’s Disease.” In other words, they have the typical biomarker and neuroimaging findings of AD, but they do not yet show the typical cognitive decline associated with AD (Sperling et al., 2011). In addition, a descriptive classification system—the “A/T/N” classification scheme—has been proposed to synthesize, and more clearly organize, the presence or absence of key currently known biomarkers of AD, so they can be combined systematically to classify all individuals in both clinical and population-based research (Jack et al., 2016). In this proposed scheme, the “A” denotes biomarkers of amyloid protein accumulation in the brain; the “T” denotes biomarkers of tau protein accumulation; and the “N” denotes biomarkers of neurodegeneration or neuronal injury.
The hope is that targeting future AD treatments at individuals with preclinical AD will successfully prevent or delay the onset of cognitive decline, in much the same way that statin treatment for those with atherosclerosis and CRFs can prevent or delay future myocardial infarctions. There are currently multiple clinical trials testing potential AD-preventive treatments, such as the Anti-Amyloid Treatment in Asymptomatic Alzheimer (A4) Study (Sperling et al., 2014). The A4 Study is testing whether 4 years of treatment with solanezumab, a drug designed to decrease brain amyloid-beta deposition, slows the rate of cognitive decline in older adults with preclinical AD.
While the potential to prevent AD and dementia through new treatments such as solanezumab is understandably creating significant interest and optimism, a number of key issues and uncertainties regarding preventive treatments for those with preclinical AD should be considered (Karlawish and Langa, 2016). Perhaps most important is the danger of “overdiagnosis”
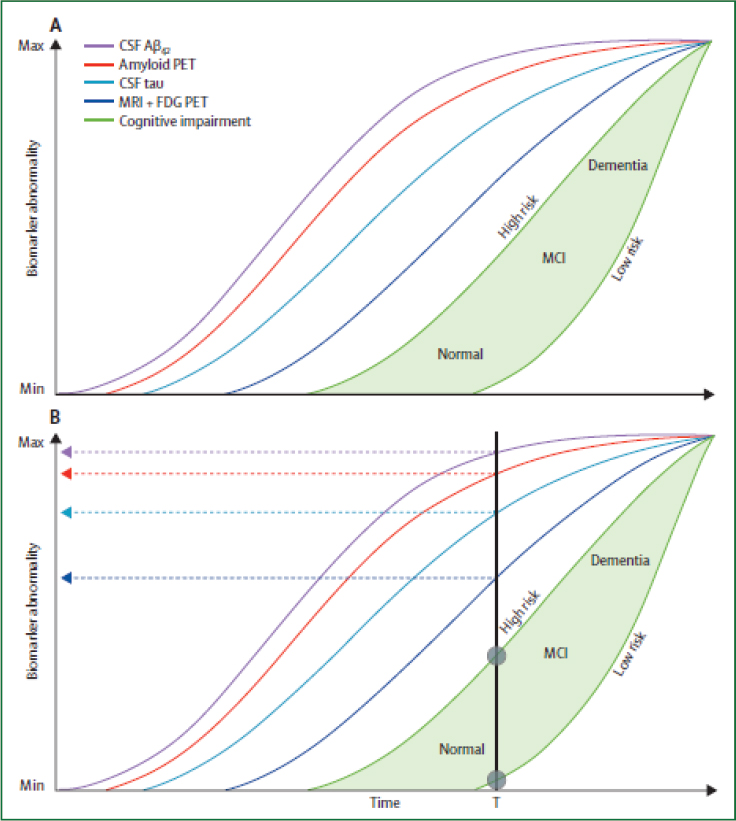
SOURCE: Reprinted with permission from Elsevier. Jack et al. (2013, Fig. 5, p. 211). The Lancet Neurology, ©2013.
(Welch et al., 2011)—in other words, identifying a pathological change in the brain based on a biomarker abnormality that will not ultimately lead to dementia, prior to an individual dying from other causes. Overdiagnosis exposes individuals to the potential negative side effects, complications, and costs of treatment, without providing benefits in terms of actual prevention of a future disease. This important possible downside of “early diagnosis” for chronic diseases of aging has gained wider attention related
to negative outcomes of screening for conditions such as prostate cancer, breast cancer, and osteoporosis (Welch et al., 2011). It could be especially prominent in screening for AD in middle-aged and older adults because the onset of AD or ADRD might not be expected for up to 25 years in the future and there will typically be significant competing risks for mortality from other causes during that time, especially cardiovascular disease (Langa and Levine, 2014).
Other important questions raised by screening for and treating preclinical AD include the costs and cost effectiveness of potential treatments, as well as the capacity of the health care financing and delivery systems to safely provide those treatments in an equitable way. For instance, solanezumab, the medication being tested in the A4 Study, is administered as an intravenous infusion requiring a monthly visit to an infusion site staffed by trained health care personnel. While the maker of solanezumab (Eli Lilly) has not announced what the target price of solanezumab would be for treatment of preclinical AD, similar monoclonal antibody drugs recently introduced for treatment of high cholesterol (evolocumab and alirocumab) currently cost about $14,000 per year (Kazi et al., 2016). A recent study estimated that at least 35 million adults in the United States likely have preclinical AD characterized by amyloid-beta deposition in the brain (Brookmeyer et al., 2017). Assuming a cost per person of $10,000 per year for solanezumab and assuming that only 30 percent of the estimated 35 million adults with preclinical AD obtain treatment, the total cost would be a staggering $105 billion per year (which was approximately one-third of the total expenditures for prescription medications in the United States in 2016) (Hartman et al., 2017). And since AD preventive treatments might be used for decades in someone who starts treatment at age 60 to 65 years, preclinical AD treatment would add a large and ongoing expenditure to the U.S. health care system. Given the likely high costs for the current preclinical AD treatments being studied, future analyses of the cost effectiveness of these interventions, as well as the distributional implications of the financing and delivery of these treatments, will be extremely important.
Finally, given the significant SES and racial/ethnic disparities in dementia risk noted above, one other key issue regarding the generalizability of current data is important to consider regarding potential treatments for preclinical AD. Since nearly all of the data on biomarker and neuroimaging testing to identify preclinical AD have thus far come from volunteer samples, with very limited representation of less-educated and minority individuals, there is still significant uncertainty regarding the generalizability of those studies to low-SES and minority populations. Future studies of biomarkers, neuroimaging, and treatments in more representative populations will be especially
important, given the higher risk for dementia in these populations (Falk et al., 2013; Karlawish et al., 2017).
CONCLUSIONS AND PRIORITIES FOR FUTURE RESEARCH
As the elderly population grows in the decades ahead, a better understanding of the life course factors that increase or decrease the risk for cognitive decline and dementia will be increasingly important, given the wide-ranging effects that cognitive decline has on older individuals, their families, and public expenditures. This review of recent developments in cognitive aging and dementia research points to a number of opportunities and challenges for future research and for potential interventions to prevent or delay the incidence of dementia.
As the brain is the most complex organ in the body, highly sensitive to any interruption in blood flow, it seems appropriate that the recent genetic, biological, and epidemiological evidence reviewed in this chapter highlight the complexity of identifying the causes for cognitive decline and dementia. One obvious theme is the growing evidence that dementia is best understood as a late-life condition that is influenced by a complex set of factors from across the entire life course: from genetics and early-life environment and education to mid- and late-life CRFs, to multiple factors in later life (e.g., cognitive stimulation and social networks) that will hopefully preserve and maintain cognitive reserve and the plasticity of the aging brain. Given the prominent role of education’s association with dementia risk, future research that helps unpack the “black box” of how early-life education decreases later-life dementia risk would be extremely important for helping to develop and target useful public health and public policy initiatives. What are the “active ingredients” of education, and how can they be delivered efficiently to children across the SES spectrum? Is online education a lower-cost option that will provide similar benefits for building cognitive reserve?
The growing evidence that Alzheimer’s disease and vascular disease are closely linked and may even be “two hits” of the same pathological process provides an important potential target for decreasing dementia risk, given the very high prevalence of CRFs among middle-aged and older adults and the known behavioral and pharmacologic interventions to prevent or treat them. However, the challenge of designing individual and population-level interventions for CRFs that address the large and growing SES and racial/ethnic disparities in those risks should be given especially high priority. Multifactorial interventions for life-style and CRF improvements, such as the FINGER study, have shown benefits for lowering dementia risk in clinical trials (Ngandu et al., 2015). Can those interventions be translated to successful implementation in low-SES populations that have the highest prevalence of CRFs and the greatest risk for dementia?
A recent National Academies report that comprehensively reviewed studies regarding risk factors for dementia recommended a number of areas that would be most useful to target for future research (National Academies of Sciences, Engineering, and Medicine, 2017). In line with the issues covered in this chapter, that report recommended future research on how treatments and behavioral interventions can better address CRFs (including hypertension, diabetes, high cholesterol, physical activity, and diet). It also recommended additional research on cognitive training interventions for older adults that have shown some benefit in randomized controlled trials for preventing cognitive decline and disability (Willis et al., 2006). Other promising avenues identified for research investment included interventions to improve sleep quality, treat depression, and facilitate social engagement at older ages. Additional pharmaceutical research on potential preventive medications, as discussed above, was also recommended.
It seems clear that given the complexity of the brain and the multiple factors that affect the brain’s health across the life course, it is unlikely that a single “magic bullet” will be found that prevents dementia in the growing population of older adults (Larson, 2017). Given the centrality of good cognitive function to living with independence and intention, making good decisions and planning for the future, and contributing productively to the labor force and one’s family and neighbors, the large and growing SES and racial/ethnic disparities for cognitive decline and dementia in the United States are especially pernicious and threatening to an aging society that hopes to provide some equity of access to the resources and environments that allow people to live long and well. Addressing these growing disparities in both dementia risk and life expectancy should be the main focus of public health and public policy in the decades ahead.
REFERENCES
Ailshire, J., Karraker, A., and Clarke, P. (2017). Neighborhood social stressors, fine particulate matter air pollution, and cognitive function among older U.S. adults. Social Science & Medicine, 172, 56–63.
Becker, G.S., Hubbard, W.H.J., and Murphy, K.M. (2010). Explaining the worldwide boom in higher education of women. Journal of Human Capital, 4(3), 203–241.
Boyle, P.A., Wilson, R.S., Yu, L., Barr, A.M., Honer, W.G., Schneider, J.A., and Bennett, D.A. (2013). Much of late-life cognitive decline is not due to common neurodegenerative pathologies. Annals of Neurology, 74(3), 478–489.
Brookmeyer, R., Abdalla, N., Kawas, C.H., and Corrada, M.M. (2017). Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimer’s & Dementia, 14(2), 121–129. doi: 10.1016/j.jalz.2017.10.009.
Cacciottolo, M., Wang, X., Driscoll, I., Woodward, N., Saffari, A., Reyes, J., Serre, M.L., Vizuete, W., Sioutas, C., Morgan, T.E., et al. (2017). Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Translational Psychiatry, 7(1), e1022.
Chan, K.Y., Wang, W., Wu, J.J., Liu, L., Theodoratou, E., Car, J., Middleton, L., Russ, T.C., Deary, I.J., Campbell, H., et al. (2013). Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet, 381, 2016–2023.
Chen, H., Kwong, J.C., Copes, R., Tu, K., Villeneuve, P.J., van Donkelaar, A., Hystad, P., Martin, R.V., Murray, B.J., Jessiman, B., et al. (2017). Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet, 389(10070), 718–726.
Chetty, R., Stepner, M., Abraham, S., Lin, S., Scuderi, B., Turner, N., Bergeron, A., and Cutler, D. (2016). The association between income and life expectancy in the United States, 2001-2014. Journal of the American Medical Association, 315(16), 1750–1766.
Clarke, P.J., Ailshire, J.A., House, J.S., Morenoff, J.D., King, K., Melendez, R., and Langa, K.M. (2012). Cognitive function in the community setting: The neighbourhood as a source of “cognitive reserve”? Journal of Epidemiologic Community Health, 66(8), 730–736.
Davies, G., Armstrong, N., Bis, J.C., Bressler, J., Chouraki, V., Giddaluru, S., Hofer, E., Ibrahim-Verbaas, C.A., Kirin, M., Lahti, J., et al. (2015). Genetic contributions to variation in general cognitive function: A meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53949). Molecular Psychiatry, 20(2), 183–192.
Davis, M.A., Guo, C., Sol, K., Langa, K.M., and Nallamothu, B.K. (2017). Trends and disparities in the number of self-reported health of older adults in the United States, 2000 to 2014. Journal of the American Medical Association Internal Medicine, 177(11), 1683–1684.
Deary, I.J., Yang, J., Davies, G., Harris, S.E., Tenesa, A., Liewald, D., Luciano, M., Lopez, L.M., Gow, A.J., Corley, J., et al. (2012). Genetic contributions to stability and change in intelligence from childhood to old age. Nature, 482(7384), 212–215.
Díaz-Venegas, C., Downer, B., Langa, K.M., and Wong, R. (2016). Racial and ethnic differences in cognitive function among older adults in the USA. International Journal of Geriatric Psychiatry, 31(9), 1004–1012.
Falk, E.B., Hyde, L.W., Mitchell, C., Faul, J., Gonzalez, R., Heitzeg, M.M., Keating, D.P., Langa, K.M., Martz, M.E., Maslowsky, J., et al. (2013). What is a representative brain? Neuroscience meets population science. Proceedings of the National Academy of Sciences of the United States of America, 110(44), 17615–17622.
Gilsanz, P., Mayeda, E.R., Glymour, M.M., Quesenberry, C.P., and Whitmer, R.A. (2017). Association between birth in a high stroke mortality state, race, and risk of dementia. Journal of the American Medical Association Neurology, 74(9), 1056–1062.
Glymour, M.M., and Manly, J.J. (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18, 223–254.
Gottesman, R.F., Schneider, A.L., Albert, M., Alonso, A., Bandeen-Roche, K., Coker, L., Coresh, J., Knopman, D., Power, M.C., Rawlings, A., et al. (2014). Midlife hypertension and 20-year cognitive change: The atherosclerosis risk in the Communities Neurocognitive Study. Journal of the American Medical Association Neurology, 71(10), 1218–1227.
Gottesman, R.F., Schneider, A.L., Zhou, Y., Coresh, J., Green, E., Gupta, N., Knopman, D.S., Mintz, A., Rahmim, A., Sharrett, A.R., et al. (2017). Association between midlife vascular risk factors and estimated brain amyloid deposition. Journal of the American Medical Association, 317(14), 1443–1450.
Gutchess, A. (2014). Plasticity of the aging brain: New directions in cognitive neuroscience. Science, 346(6209), 579–582.
Hartman, M., Martin, A.B., Espinosa, N., Catlin, A., and the National Health Expenditure Accounts Team. (2017). National health care spending in 2016: Spending and enrollment growth slow after initial coverage expansions. Health Affairs, 37(1), 150–160. doi: 10.1377/hlthaff.2017.1299. E-pub online Dec. 6.
Hayward, M.D., Hummer, R.A., and Sasson, I. (2015). Trends and group differences in the association of educational attainment and U.S. adult mortality: Implications for understanding education’s causal influence. Social Science & Medicine, 127, 8–18.
Hibar, D.P., Adams, H.H.H., Jahanshad, N., Chauhan, G., Stein, J.L., Hofer, E., Renteria, M.E., Bis, J.C., Arias-Vasquez, A., Ikram, M.K., et al. (2017). Novel genetic loci associated with hippocampal volume. Nature Communications, 8, 13624.
Hurd, M., Martorell, F., Delevande, A., Mullen, K., and Langa, K.M. (2013). The monetary costs of dementia in the United States. New England Journal of Medicine, 368, 1326–1334.
Jack, C.R., Jr., Knopman, D.S., Jagust, W.J., Petersen, R.C., Weiner, M.W., Aisen, P.S., Shaw, L.M., Vemuri, P., Wiste, H.J., Weigand, S.D., et al. (2013). Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurology, 12(2), 207–216.
Jack, C.R., Bennett, D.A., Blennow, K., Carrillo, M.C., Feldman, H.H., Frisoni, G.B., Hampel, H., Jagust, W.J., Johnson, K.A., Knopman, D.S., et al. (2016). An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology, 87(5), 539–547.
James, B.D., Wilson, R.S., Boyle, P.A., Trojanowski, J.Q., Bennett, D.A., and Schneider, J.A. (2016). TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain, 139(11), 2983–2993.
Karlawish, J., and Langa, K.M. (2016). Unfinished business in preventing Alzheimer disease. Journal of the American Medical Association Internal Medicine, 176(12), 1739–1740.
Karlawish, J., Jack, C.R., Jr., Rocca, W.A., Snyder, H.M., and Carrillo, M.C. (2017). Alzheimer’s disease: The next frontier. Alzheimer’s & Dementia, 13(4), 374–380.
Kazi, D.S., Moran, A.E., Coxson, P.G., Penko, J., Ollendorf, D.A., Pearson, S.D., Tice, J.A., Guzman, D., and Bibbins-Domingo, K. (2016). Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. Journal of the American Medical Association, 316(7), 743–753.
Langa, K.M. (2015). Is the risk of Alzheimer’s disease and dementia declining? Alzheimer’s Research & Therapy, 7(1), 34.
Langa, K.M., and Levine, D. (2014). The diagnosis and management of mild cognitive impairment: A clinical review. Journal of the American Medical Association, 312(23), 2551–2561.
Langa, K.M., Foster, N., and Larson, E.B. (2004). Mixed dementia: Emerging concepts and therapeutic implications. Journal of the American Medical Association, 292(23), 2901–2908.
Langa, K.M., Larson, E., Crimmins, E., Faul, J., Levine, D., Kabeto, M., and Weir, D. (2017). A comparison of the prevalence of dementia in the United States in 2000 and 2012. Journal of the American Medical Association Internal Medicine, 177(1), 51–58.
Langa, K.M., Crimmins, E., and Hayward, M.D. (2018). Examining trends in dementia incidence and prevalence using an Age-Period-Cohort framework. In Oxford Textbook of Neurological and Neuropsychiatric Epidemiology (in press). Oxford, UK: Oxford University Press.
Larson, E. (2017). Prevention of late-life dementia: No magic bullet. Annals of Internal Medicine, 68(1), 77–79. doi: 10.7326/M17-3026. Epub Dec 19.
Larson, E., Yaffe, K., and Langa, K.M. (2013). New insights into the dementia epidemic. New England Journal of Medicine, 369(24), 2275–2277.
Leggett, A., Clarke, P., Zivin, K., McCammon, R.J., Elliott, M.R., and Langa, K.M. (2017). Recent improvements in cognitive functioning among older U.S. adults: How much does increasing educational attainment explain? Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, published online, January 28.
Lindenberger, U. (2014). Human cognitive aging: Corriger la fortune? Science, 346(6209), 572–578.
Lutz, W., Butz, W., and Samir, K.C. (2014). World Population and Human Capital in the Twenty-First Century. Oxford, UK: Oxford University Press.
Matthews, F.E., Arthur, A., Barnes, L.E., Bond, J., Jagger, C., Robinson, L., Brayne, C., on behalf of the Medical Research Council Cognitive Function and Ageing Collaboration. (2013). A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: Results of the Cognitive Function and Ageing Study I and II. Lancet, 382(9902), 1405–1412.
Matthews, F.E., Stephan, B., Robinson, L., Jagger, C., Barnes, L., Arthur, A., and Brayne, C. (2016). Cognitive Function and Ageing Studies (CFAS) Collaboration. A two-decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nature Communications, 7, 11398.
Mayeda, E.R., Glymour, M.M., Quesenberry, C.P., and Whitmer, R.A. (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia, 12(3), 216–224.
McKhann, G.M., Knopman, D.S., Chertkow, H., Hyman, B.T., Jack, C.R., Kawas, C.H., Klunk, W.E., Koroshetz, W.J., Manly, J.J., Mayeux, R., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263–269.
Meng, X., and D’Arcy, C. (2012). Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One, 7(6), e38268.
National Academies of Sciences, Engineering, and Medicine. (2015). The Growing Gap in Life Expectancy by Income: Implications for Federal Programs and Policy Responses. Washington, DC: The National Academies Press.
National Academies of Sciences, Engineering, and Medicine. (2017). Preventing Dementia: A Way Forward. Washington, DC: The National Academies Press.
Neuropathology Group of the Medical Research Council Cognitive Function and Aging Study. (2001). Pathologic correlates of late onset dementia in a multicentre, community based population in England and Wales. Lancet, 357, 169–175.
Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., Bäckman, L., Hänninen, T., Jula, A., Laatikainen, T., et al. (2015). A 2-year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet, 385(9984), 2255–2263.
Norton, S., Matthews, F.E., Barnes, D.E., Yaffe, K., and Brayne, C. (2014). Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurology, 13, 788–794.
Odutayo, A., Gill, P., Shepherd, S., Akingbade, A., Hopewell, S., Tennankore, K., Hunn, B.H., and Emdin, C.A. (2017). Income disparities in absolute cardiovascular risk and cardiovascular risk factors in the United States, 1999–2014. Journal of the American Medical Association Cardiology, 2(7), 782–790.
Okbay, A., Beauchamp, J.P., Fontana, M.A., Lee, J.J., Pers, T.H., Rietveld, C.A., Turley, P., Chen, G.B., Emilsson, V., Meddens, S.F., et al. (2016). Genome-wide association study identifies 74 loci associated with educational attainment. Nature, 533(7604), 539–542.
Prince, M., Guerchet, M., and Prina, M. (2013). Alzheimer’s Disease International. Policy Brief for Heads of Government: The Global Impact of Dementia 2013–2050. London: Alzheimer’s Disease International.
Rietveld, C.A., Medland, S.E., Derringer, J., Yang, J., Esko, T., Martin, N.W., Westra H.-J., Shakhbazov, K., Abdellaoui, A., Agrawal, A., et al. (2013). GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science, 340(6139), 1467–1471.
Satizabal, C.L., Beiser, A.S., Chouraki, V., Chene, G., Dufouil, C., and Seshadri, S. (2016). Incidence of dementia over three decades in the Framingham Heart Study. New England Journal of Medicine, 374(6), 523–532.
Schneider, J.A., Arvanitakis, Z., Bang, W., and Bennett, D.A. (2007). Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology, 69, 2197–2204.
Schoeni, R., Freedman, V., and Langa, K.M. (2018). Introduction to a supplement on population level trends in dementia: Causes, disparities, and projections. Journals of Geronotology: Social Sciences, in press.
Schrijvers, E.M., Verhaaren, B.F., Koudstaal, P.J., Hofman, A., Ikram, M.A., and Breteler, M.M. (2012). Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology, 78(19), 1456–1463.
Snyder, H.M., Corriveau, R.A., Craft, S., Faber, J.E., Greenberg, S.M., Knopman, D., Lamb, B.T., Montine, T.J., Nedergaard, M., Schaffer, C.B., et al. (2015). Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimer’s & Dementia, 11(6), 710–717.
Sonnen, J.A., Larson, E.B., Crane, P.K., Haneuse, S., Li, G., Schellenberg, G.D., Craft, S., Leverenz, J.B., and Montine, T.J. (2007). Pathological correlates of dementia in a longitudinal, population-based sample of aging. Annals of Neurology, 62, 406–413.
Sperling, R.A., Aisen, P.S., Beckett, L.A., Bennett, D.A., Craft, S., Fagan, A.M., Iwatsubo, T., Jack, C.R., Jr., Kaye, J., Montine, T.J., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 280–292.
Sperling, R.A., Rentz, D.M., Johnson, K.A., Karlawish, J., Donohue, M., Salmon, D.P., and Aisen, P. (2014). The A4 study: Stopping AD before symptoms begin? Science Translational Medicine, 6(228), 228fs13.
Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology, 11(11), 1006–1012.
Valenzuela, M.J., and Sachdev, P. (2005). Brain reserve and dementia: A systematic review. Psychological Medicine, 35, 1–14.
Vemuri, P., Lesnick, T.G., Przybelski, S.A., Machulda, M., Knopman, D.S., Mielke, M.M., Roberts, R.O., Geda, Y.E., Rocca, W.A., Petersen, R.C., et al. (2014). Association of lifetime intellectual enrichment with cognitive decline in the older population. Journal of the American Medical Association Neurology, 71(8), 1017–1024.
Weden, M.M., Shih, R.A., Kabeto, M.U., and Langa, K.M. (2017). Secular trends in dementia and cognitive impairment of U.S. rural and urban older adults. American Journal of Preventive Medicine, published online, December 6.
Welch, G., Schwartz, L., and Woloshin, S. (2011). Overdiagnosed: Making People Sick in Pursuit of Health. Boston: Beacon Press.
Willis, S.L., Tennstedt, S.L., Marsiske, M., Ball, K., Elias, J., Koepke, K.M., Morris, J.N., Rebok, G.W., Unverzagt, F.W., Stoddard, A.M., et al. (2006). Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association, 296(23), 2805–2814.
Wimo, A., Jönsson, L., Bond, J., Prince, M., and Winblad, B. (2013). Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimer’s & Dementia, 9, 1–11.
Wu, Y.T., Lee, H.Y., Norton, S., Chen, C., Chen, H., He, C., Fleming, J., Matthews, F.E., and Brayne, C. (2013). Prevalence studies of dementia in mainland China, Hong Kong and Taiwan: A systematic review and meta-analysis. PLoS One, 8, e66252.
Wu, Y.T., Lee, H.Y., Norton, S., Prina, A.M., Fleming, J., Matthews, F.E., and Brayne, C. (2014). Period, birth cohort and prevalence of dementia in mainland China, Hong Kong and Taiwan: A meta-analysis. International Journal of Geriatric Psychiatry, 29(12), 1212–1220.
Wu, Y.T., Beiser, A.S., Breteler, M.M.B., Fratiglioni, L., Helmer, C., Hendrie, H.C., Honda, H., Ikram, M.A., Langa, K.M., Lobo, A., et al. (2017). The changing prevalence and incidence of dementia over time—current evidence. Nature Reviews Neurology, 13(6), 327–339.
Yaffe, K., Falvey, C., Harris, T.B., Newman, A., Satterfield, S., Koster, A., Ayonayon, H., Simonsick, E., and Health ABC Study. (2013). Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. BMJ, 19, 347.
Yaffe, K., Vittinghoff, E., Pletcher, M.J., Hoang, T.D., Launer, L.J., Whitmer, R., Coker, L.H., and Sidney, S. (2014). Early adult to midlife cardiovascular risk factors and cognitive function. Circulation, 129(15), 1560–1567.
Zissimopoulos, J., Crimmins, E., and St. Clair, P. (2018). The impact of changes in population health and mortality on future prevalence of Alzheimer’s disease and other dementias in the United States. Journal of Geronotology: Social Sciences, in press.
Zlokovic, B.V. (2011). Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neuroscience, 12(12), 723–738.




















