Summary1
Biospecimens from research participants are an essential resource for a broad range of studies, from exploratory, basic science inquiries to clinical trials using well-validated tests. These types of research have been enormously valuable in advancing knowledge about almost every aspect of human health and disease. The conduct of research with human volunteers is dependent on a collaborative, productive relationship between participants who give their time and samples and the investigators and research teams that conduct the research. This complex relationship has many elements, but in the past the communication of individual research results to participants has generally not been one of them.
In the last several decades, questions have been raised about the practice of not returning test results generated in a research study to the study’s participants; early on, much of the discussion was focused on returning results from imaging studies, while more recently the focus has moved more to the disciplines of genetics and environmental research. At the same time, the push for increased community and participant engagement across the research study life cycle and the rise of technology-enabled open science and data-sharing movements have added further momentum to the issue. Recent significant changes to federal regulations have promoted transparency and allowed individuals greater access to their clinical and research test results. These changes include the elimination of the laboratory exclusion from the Health Insurance Portability and Accountability Act (HIPAA) privacy rule and revisions to the Common Rule that require prospective participants to be told during the consent process whether clinically
___________________
1 This Summary does not include references. Citations for the discussion presented in this Summary appear in subsequent report chapters.
relevant individual research results will be returned. On the other hand, the Clinical Laboratory Improvement Amendments of 1988 (CLIA) bars laboratories that are not CLIA certified from reporting individual research results. This creates a dilemma when research results that are clinically relevant or otherwise valuable to participants, particularly those that might not otherwise be discovered, are generated in research laboratories that are not CLIA certified. See Box S-1 for a brief description of these federal regulations. (Box 6-1 in Chapter 6, from which Box S-1 is adapted, includes additional laws and regulations relevant to the return of individual research results.)
Over the last couple of decades expert groups have written position statements supporting the return of individual research results and secondary findings2 under certain conditions, such as when the results are clinically actionable, valid, and reliable. However, participant demand for individual research results is driven not just by the potential benefits that individuals could gain by learning about clinically actionable information, but also by their desire to learn about themselves from information that they would not otherwise obtain. More specific guidance is needed on how stakeholders should consider the benefits, risks, and costs associated with the return of individual research results, including the broad spectrum of results which may not be accurate, medically actionable, or have clear meaning.
Seeking guidance on these issues from a consensus body of experts representing diverse perspectives, the Centers for Medicare & Medicaid Services (CMS), the Food and Drug Administration (FDA), and the National Institutes of Health (NIH) asked the National Academies of Sciences, Engineering, and Medicine to conduct a study and generate a report that reviews and evaluates the ethical, societal, regulatory, and operational issues related to the return of individual-specific research results generated from research on human biospecimens. The full Statement of Task for the committee is presented in Box S-2.
SCOPE AND KEY TERMINOLOGY
The topic of the return of research results is exceptionally broad in scope and encompasses all fields of human research (e.g., biomedical, psychological, behavioral). During its first meeting on July 19, 2017, the committee had an opportunity to clarify the scope of the study with representatives of the three sponsoring federal agencies. In the course of that discussion, the study sponsors clarified that the committee was intended to focus on research results that are generated from the analysis of human biospecimens (samples of material collected from the human
___________________
2 Secondary findings are results that are not the primary objective of the research. Such findings are referred to in the literature by a variety of terms, such as “additional,” “secondary,” “incidental,” “ancillary,” “supplemental,” etc., and these terms can be combined with additional clarifiers such as “unanticipated” and “anticipated.”
body, such as urine, blood, tissue, and cells). The committee was not to consider the return of results from imaging, behavioral, or cognitive tests, for example. Of note, the committee’s charge was not limited to the return of genetic test results, as many other kinds of research are performed on human biospecimens. These include, for example, basic science studies using tumor biopsies to identify a new biomarker for colon cancer, clinical trials that evaluate blood samples for antibody levels induced by a new malaria vaccine, and epidemiological studies measuring the level of a suspected toxin in urine samples for an environmental exposure study—all of these involve laboratory tests on human biospecimens and are in the scope of this report.
In recent years, this topic has generated immense interest and debate among bioethicists and scientists, particularly in the fields of genetics and environmental exposure research. In the field of genetics, much of the debate has been focused on the return of clinically actionable secondary findings—results that were not the primary objective of the research. This is an important issue in the broader context of returning information generated in the course of research to individual
participants, but the sponsors clarified that it was not intended to be a central focus of this committee’s report. Instead, in this report the committee uses the term individual research results to refer to results that are generated in the course of a study to help answer the research question or otherwise support the study objectives (e.g., to determine clinical trial inclusion/exclusion) and that are specific to one participant. Distinctions can also be made between different types of individual research results according to the kind of information provided—i.e., uninterpreted data versus interpreted findings. In the genetics field there is also an ongoing discussion about the return of sequencing information, which is generally referred to as “raw data.” For the purposes of this report, all of these types of information are included in the term “individual research results.” Chapter 5 discusses ways to facilitate the understanding of different types of individual research results.
While not a primary focus of the committee’s deliberations, there was recognition that secondary findings remain an important part of the discussion about returning research results, given that many sequencing and other “omics” research studies have no primary target. Moreover, the issue of returning secondary findings has a long history (e.g., in the context of returning results from imaging tests), and the committee recognized that the lessons learned from those experiences might be relevant to the committee’s task. Furthermore, the committee acknowledged that the recommendations in this report may have impact beyond their application to results generated from biospecimens. In addressing its charge, the committee considered three general scenarios in which consideration of the return of individual research results is relevant:
- the planned offer of anticipated individual research results to participants,
- the return of individual research results upon the request of participants, and
- the offer of unanticipated research results to an individual participant.
For the purposes of this report, anticipated results are those results that are actively sought or are expected to arise when using a particular research test on human biospecimens. This includes results that are not the primary objective of the test or study. Unanticipated results are those that are unexpected either because they could not have been anticipated given the current state of scientific knowledge or because the research team did not consider the potential of generating them using a particular research test. In designing a study, investigators can anticipate several types of results and possible outcomes that may arise from the tests and analyses used over the course of investigation, and very few results should be unanticipated. However, despite investigators’ ability to predict the possible outcomes of their research, unanticipated results cannot be entirely avoided, as the state of the science may change over the course of a study or a participant
may have an unknown or undiagnosed condition that becomes apparent over the course of biospecimen analysis, thus leading to unforeseen results.
Frequently, the considerations that stakeholders will need to take into account for the return of individual-specific research results to research participants for any of the three scenarios described above will be identical. Therefore, throughout the report the committee uses the shorthand phrase “return of results” to refer to the practice of returning individual research results in any of the three scenarios described above when it is not important to make a distinction between them.
During the discussion of the charge at the committee’s first meeting, the following additional areas were identified by the study sponsors as falling outside the study scope, although it should be noted that only some of these are explicitly excluded in the Statement of Task:
- Specific assays or test results (i.e., the committee was not asked to generate a list, for example, of specific genes associated with disease susceptibility that, when tested for, should or should not be returned);
- The return of aggregate research data or study-level results;
- The return of results from anonymized or de-identified specimens that investigators cannot link back to the contributing participant, as well as the role or obligation of biobanks that retain identifiers that would enable the return of individual research results generated by investigators using de-identified biobank specimens (e.g., for secondary research);
- The infrastructure and policies needed for the implementation of a system to return results from secondary research; and
- Laboratory developed tests (LDTs) and the associated LDT regulations.
In discussions with the sponsors, the committee also clarified the scope as it applies to CLIA. The sponsors indicated to the committee that it would be appropriate to include in its description of the current regulatory environment for the return of individual research results CMS’s current interpretation of the scope and applicability of CLIA, which is that “only those facilities performing research testing on human biospecimens that do not report patient-specific results may qualify to be excepted from CLIA certification.” Although CMS’s current interpretation has been questioned by some legal scholars, the committee was advised that making any comments, analysis, or conclusions regarding the appropriateness of that interpretation would be beyond what was intended in the Statement of Task. Furthermore, the committee was not asked to make recommendations to Congress regarding changes to the CLIA law. However, recommendations on changes to the CLIA regulations were within the study scope if the committee felt that such changes were needed to better align the regulatory environment with the risks and benefits of the return of research results. Chapter 6 addresses the committee’s recommendations on clarifying and revising federal regulations.
BENEFITS AND RISKS OF RETURNING INDIVIDUAL RESEARCH RESULTS
We know from research and shared experience that participants often want and value their individual research results. Participants may benefit from the return of individual research results that inform clinical decision making, life or reproductive planning, and other decisions that may affect health and quality of life. Additionally, results may have personal value to participants by providing a newfound understanding about a health condition. Participants, patients, and their advocates want to be active contributors to the research process and are at the forefront of the movement to get participants involved in the research process from planning to completion. Many advocates consider the practice of withholding results based on concerns about participant welfare to be paternalistic, and they question the notion that participants cannot understand the distinction between clinical results and individual-specific results generated in a research context. Because of its potential to increase public engagement and trust in the research enterprise, the return of individual research results could have multiple positive effects, including possible improvements in the efficiency, generalizability, and participant-centeredness of research. These considerations suggest that the return of individual research results should be an important element of research in this transition toward more transparency and more robust participant engagement in the conduct of research.
On the other hand, important countervailing considerations have been raised concerning whether and how research results should be returned to participants. For instance, research participants do not have the same relationship with investigators as clinicians do with their patients. This means the ethical obligation to return results is less clear (see Appendix D). Furthermore, we know that research participants may conflate the research and clinical care relationships, having what has been termed a “therapeutic misconception.” The problem here is that some participants may misinterpret the goals of clinical care (individual benefit) with the goals of research (generalizable knowledge) and mistakenly assume that a research study will yield reliable results with clinical value. By its very nature research often produces results that are of uncertain value and, depending on the stage of research, may not be analytically or clinically valid.3 The return of uncertain, poorly validated, or poor-quality results poses a risk that participants will make important clinical or life decisions based on information that subsequently proves to be wrong or is misinterpreted.
The risks associated with returning individual research results may be minimized by improving result validity through the adoption of an externally accountable quality system by research laboratories. Furthermore, the use of effective communication strategies can minimize the risk of misinterpretation or
___________________
3Analytic validity indicates how well a test measures the property or characteristic it was intended to measure, whereas clinical validity is a measure of how consistently and accurately a test detects or predicts the intermediate or final outcomes of interest.
over-interpretation of research results. However, implementing such strategies may be a significant challenge for many research laboratories, which often operate with little in the way of formal quality assurance processes and often with constrained resources as well. In addition, few investigators have been specifically trained and have the resources needed to communicate results to participants in an effective manner. Clearly, this expanded activity will require additional resources, including resources devoted to planning, ensuring laboratory quality, and the time, effort, and expertise necessary for engaging participants. The cost and feasibility of any additional requirements or expectations on investigators, research laboratories, and institutions is a serious concern, especially when the level of funding for research from government bodies is uncertain. To the extent that additional resources cannot be found to address these costs, a central question is how to balance the value of return of individual research results with the costs, including the opportunity costs, and how to use existing resources. Careful consideration must be given to how the return of individual research results could more broadly affect the research enterprise, health care, and society.
The committee carefully considered the potential benefits, risks, and competing ethical justifications of investigators to return, or not to return, individual research results. Strong justifications can be made for returning results in many circumstances beyond traditional and current practices. The committee identified situations with compelling reasons for the return of individual results to participants, as well as situations with reasons to limit or constrain the returning of results. In determining whether to return results for any given study, ethical principles must be balanced, and the benefits and risks must be carefully considered based on the specific context of the study.
Investigators, with oversight from their IRBs and institutions, will ultimately be responsible for making decisions on a case-by-case basis regarding whether and how to return individual research results, as the decisions require the careful consideration of many factors, which are described below. However, research sponsors and funding agencies also have an important role in developing policies to support reasonable consistency across research studies and institutions. Although these oversight mechanisms are no guarantee against harm, the committee believes that at this time institutional review is the most practical and reasonable approach to support decision making regarding the return of individual research results. Chapter 4 presents the committee’s framework that can support
investigators and IRBs in their decision making. The committee recognizes that it will be challenging for IRBs to foster the return of results and to assess the risks and benefits of this practice in the near future before experience and an evidence base has fully developed. In the meantime, we encourage IRB professionals to approach the issue reflectively, regularly engage stakeholders, attend to accumulating data and institutional experiences, and share experiences, data, and protocols with colleagues through professional meetings and publications. Current practices and research into the return of results taking place in NIH-funded research like the All of Us Research Program, the Clinical Sequencing Evidence-Generating Research (CSER) consortium, and the Electronic Medical Records and Genomics (eMERGE) Network can be used to develop initial guidance for IRBs. NIH could also assist IRBs by convening a workshop or working group with other research funders to examine current practices regarding the return of results from biospecimens and explore lessons learned from biomonitoring programs and other domains such as radiology, imaging, and social and behavioral health research. As the evidence base expands, there may be a further role for government agencies to develop guidance to support investigators and their IRBs in their decision-making process.
GUIDING PRINCIPLES FOR RETURNING INDIVIDUAL RESEARCH RESULTS
The purpose of research is to create generalizable information for the benefit of society, and, unlike clinical care, research is not primarily focused on providing personal benefit for individual participants. Given this perspective, what is the nature of the relationship and expectations between investigators and participants and does our evolving conception of the relationship and expectations require more transparency with respect to individual research results? To what extent should the established ethical obligations to research participants, as codified in international and national guidelines, such as those laid out by the Council for International Organizations of Medical Sciences, the Declaration of Helsinki, the Belmont Report, and the Common Rule, be extended to entail obligations or responsibilities to promote the return of individual research results?
The complexity of these broad questions is substantial because of the sometimes competing, deeply held values involved. The research enterprise has been criticized for its lack of transparency and for the transactional nature of taking from participants without creating value when the results are too often not published or shared. This lack of transparency and true collaboration may factor into the contemporary concerns regarding current difficulties with research participant recruitment and retention, which in turn contribute to the escalating costs of conducting clinical studies. Amid growing consumer expectations for user-centeredness, engagement, and value, these criticisms have led to calls for a paradigm shift. At the same time, the productivity of research in an era of
uncertain resources is dependent on making prudent decisions in the allocation of those resources in the pursuit of valuable ends.
Efforts to transform the culture of the research enterprise involve actions and attitude changes along many fronts. One such change involves important and powerful modifications in terminology. Throughout this report—and consistent with use in the broader research community—the committee refers to human research volunteers as “participants” rather than research “subjects,” terminology we recommend for adoption by federal regulators in guidance and new regulations. The use of such language goes beyond semantics; it represents a conceptual move from the passive language of subjects to the active language of participants, it is in accordance with the ethical principles of autonomy and respect for persons, and it reflects the growing movement for participants to be engaged more robustly in the design and conduct of biomedical research.
Two general themes should be evident in this report. First, through its findings, conclusions, and recommendations, the committee is encouraging more frequent return of individual results than is currently the practice in research involving human biospecimens. While careful consideration on a study-by-study basis is important, the committee believes that if the return of individual research results becomes a more common practice, it will demonstrate respect for participants and support transparency and the development of trust with participants, in turn bringing benefits to participants, investigators, sponsors, funding agencies, research institutions, and society. Second, because this is a relatively unfamiliar practice to many investigators, sponsors, funding agencies, and institutions, and because it is a practice that requires the mobilization of resources, we do not expect our recommendations to change standards and practices immediately. We understand that accomplishing the goals articulated in this report will take time and resources and that best practices in terms of when and how to return results will emerge with experience and with new research focused on these very questions. Our hope is that this report will motivate stakeholders in ways that will ultimately transform research practices in parallel with other changes that promote transparency and trust, participant engagement, higher research quality, and improved reproducibility of research findings.
Taking into account the complex ethical and societal considerations underpinning the movement to increase the return of individual research results, the committee formulated the following six principles to help guide its deliberations and the development of the recommendations presented in this report:
- Principle 1: Participants bring essential and valuable information to the research enterprise without which research cannot be conducted. Because research results have value to many participants, as a matter of reciprocity, respect, transparency, and trust, the return of results should be routinely considered in the design of research protocols involving human participants.
- Principle 2: Research has significant societal value. The potential value of returning individual research results must be carefully considered along with the trade-offs for research participants, investigators, research institutions, and society.
- Principle 3: When individual research results are offered, participants have the right to decide whether to receive or to share their results.
- Principle 4: When individual research results are returned, the process of communication is important to promote understanding of the meaning, potential uses, and limitations of the information.
- Principle 5: The value of research results to investigators, participants, and society depends on the validity and reliability of the result. High standards of laboratory quality, from the acquisition of specimens to the communication of results, enhance the validity and reliability of the results generated in research laboratories.
- Principle 6: The conduct of high-quality, generalizable, and equitable research involves the inclusion of diverse populations and requires investigators to return individual research results in a manner that accommodates the full spectrum of community needs and preferences, regardless of participant social or economic status. The potential value of results, which is best assessed with input from the participant, community, or trusted proxy, should be considered.
QUALITY MANAGMEMENT SYSTEMS FOR LABORATORIES TESTING HUMAN BIOSPECIMENS
Establishing laboratory processes to give all stakeholders (investigators, institutions, regulators, and participants) confidence in the validity of the individual research results being returned is critical to ensuring the accuracy of information provided to research participants as well as the quality of the science. However, many research laboratories without CLIA certification currently do not have the systems in place to provide confidence in the validity of individual participants’ research results. Certainly, many research laboratories produce high-quality science, but without the documentation of practices under a quality management system (QMS),4 it is difficult to know which laboratories can generate accurate and reliable individual research results with proper assignment of the individual results to the correct research participants. Questions about validity and thus quality of individual research results pose a barrier to the responsible return of research results to participants. More broadly, the lack of established quality processes poses a problem for the rigor and reproducibility of the science.
___________________
4 Quality management systems (QMSs) are defined by the World Health Organization, the International Organization for Standardization, and the Clinical and Laboratory Standards Institute as “coordinated activities to direct and control a laboratory with regard to result validity and reliability.”
When individual research results are intended for use in clinical decision making in the study protocol, tests must be performed in laboratories that are CLIA certified. When the study protocol does not call for individual research results to be used in clinical decision making (see Box S-3), CLIA certification may not always be an appropriate or necessary mechanism to ensure that the quality of the research test results is sufficient to permit the return of results. While CLIA has significantly improved the quality of clinical laboratory results used in clinical decision making, its requirements are not always a good fit with the kinds of testing performed in the research context, such as tests relevant to biomonitoring for environmental contaminants. Moreover, current CLIA regulatory requirements have not kept up with the rapid pace of technological innovation (e.g., genetic sequencing technologies). For example, current CLIA requirements do not address the complexity of the required informatics analyses, interpretation, and reporting required with next-generation sequencing technologies or other omics testing. Furthermore, while the direct cost of CLIA certification may not be prohibitory, meeting the requirements to obtain the certification by compliance with all of the regulatory requirements would come with significant costs for most research laboratories, although the extent of the burden would depend on the infrastructure and processes already in place in the laboratory (see Chapter 3 for more detail).
However, if investigators plan to return individual research results to participants, it is essential that the quality of the laboratory analysis is sufficient to provide confidence in the result to be returned. Currently, there is no accepted QMS for research laboratories that could serve as an alternative to CLIA certification. For these reasons, the committee recommends that NIH lead an effort with the Centers for Disease Control and Prevention, FDA, CMS, and other relevant federal agencies and nongovernmental organizations, including patient and community groups, to develop a QMS with external accountability5 for research laboratories that perform tests on human biospecimens. Outside the United States, several governmental and nongovernmental organizations are already working in this arena, including ongoing initiatives in Europe aimed at producing guidance and recommendations to assist investigators in meeting quality essentials in laboratory practice. Prior to the development of the recommended QMS for research laboratories, or in the event that results are generated over the course of a study that may be valuable to a participant but were not anticipated by the investigator, IRB review should serve as an alternative pathway for determining if certain conditions have been met and if the return of results not intended for use in clinical decision making is permissible (see Recommendation 3 and Figure S-1).
___________________
5 External accountability means that a research laboratory’s compliance with defined QMS standards is assessed by an entity independent of the laboratory.
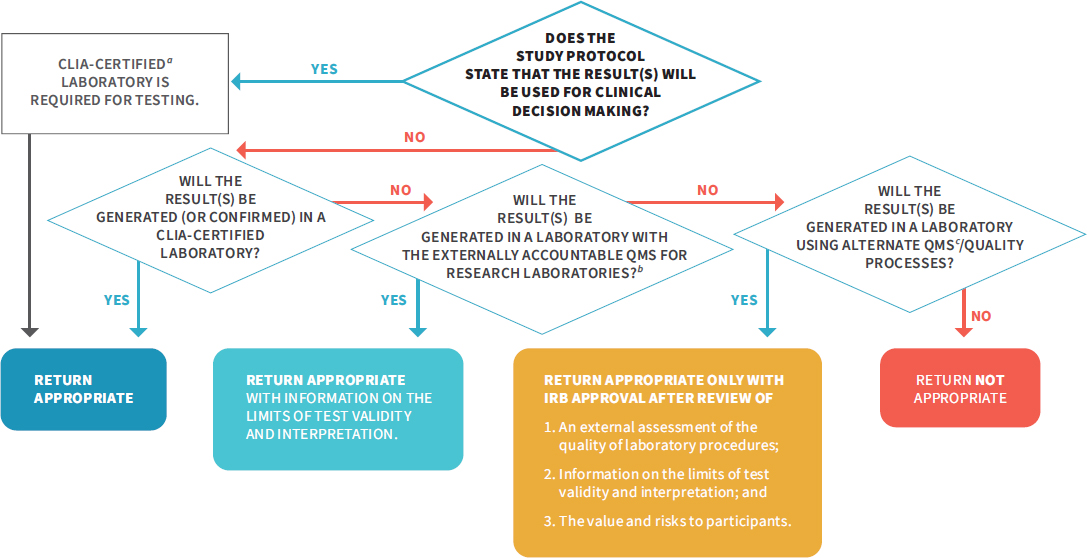
NOTE: CLIA = Clinical Laboratory Improvement Amendments of 1988; DRS = designated record set; HIPAA = Health Insurance Portability and Accountability Act of 1996; NIH = National Institutes of Health; QMS = quality management system.
Quality management systems have been shown to make work more efficient, facilitate the training of new staff, improve reproducibility, increase patient safety, and enhance data integrity. However, putting a QMS in place will have multiple impacts on research laboratories, in terms of both their research processes and resource requirements. Adoption of the quality system will likely require changes to the laboratory operations and the training environment. Additional resources may be needed for the analytical and clinical validation of testing procedures, equipment maintenance standards, and more stringent staffing and staff training and competency assessment requirements. To minimize the burden for research laboratories, sponsors, funding agencies, and research institutions need to facilitate access to resources and support quality management system training and the development of the necessary laboratory infrastructure to ensure that human biospecimen testing is performed under high-quality standards. The initial training, cost, and time commitment will likely be significant, but the value added will be considerable, both for participants and for science.
A DECISION-MAKING FRAMEWORK FOR THE RETURN OF INDIVIDUAL RESEARCH RESULTS
Decisions about whether and how to return individual research results are influenced by many factors. These include the potential value of the information to the participant; the nature of the relationship, if any, between the participant and investigator; the analytic and clinical validity of the research result; and the feasibility of return. Benefits to the participants and to the research enterprise have to be weighed against risks, including potential harms to individuals, the diversion of resources and investigator efforts from conducting research, liabilities, risks of privacy breach, and discrimination. Furthermore, investigators may be legally required to disclose a result if a participant makes a request under HIPAA, which ensures individuals a right to access any personal health information contained within the DRS of a HIPAA-covered entity.
A small number of well-defined cases present clear and broadly accepted rationales for when the return of results should be obligated or discouraged (see Box S-4). But, for the majority of scenarios, decisions have to be made on a case-by-case basis by weighing several factors. As the potential value of the result to participants and the feasibility of return increase, the justification for returning results becomes stronger (see Figure S-2 for a conceptual framework). Value in this context means the value of a result from the perspective of the participant and might entail clinical utility or personal utility as well as personal meaning (e.g., lineage information). This participant-centric approach recognizes that the value of a result is not necessarily tied to its use. To clarify, defining value in this way is not meant to imply that each participant needs to be queried regarding the results that would be meaningful to him or her, but it does require the investigator to consider value from the participant perspective rather than from the more traditional clinical perspective. Feasibility is also determined by multiple factors, including potential challenges, the costs and burdens of returning results, whether biospecimens can be linked to a specific participant, and the resources available to communicate the results effectively and appropriately.
Ascertaining Participant Needs, Preferences, and Values
Investigators, institutions, and research sponsors and funding agencies need to be cautious about making assumptions regarding the kinds of results that participants may find meaningful. Expert-identified criteria do not always reflect participant preferences and values, as the value of a research result to participants will be influenced by both their perspectives and the contexts in which they are participating in the research. Incorporating the needs, preferences, and values of community representatives and advocacy groups into decision making regarding the return of individual research results is important for helping investigators to better understand what participants value and to weigh the benefits and risks of disclosure.
Ascertaining and incorporating participant needs, preferences, and values into decision-making processes regarding whether or not to return individual research results can be undertaken at the study level but also in the development of policy or guidance. Both are critical to advancing a more participant-centric research paradigm. For some kinds of studies—particularly those that will involve significant interactions between researchers and participants—obtaining representative input from relevant and representative community members in
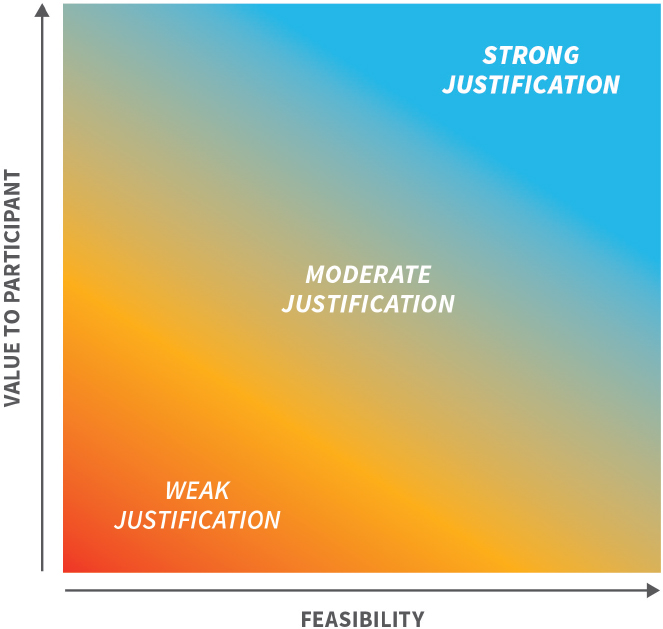
NOTES: This figure demonstrates that as the potential value of the result to participants and the feasibility of return increase, the justification for returning results becomes stronger. Value in this context means the value of a result from the perspective of the participant and might entail clinical utility or personal utility as well as personal meaning. Feasibility is determined by multiple factors, including potential challenges, the costs and burdens of returning results, and whether participants’ biospecimens are linked to the participant identity as well as the resources available to communicate the results effectively and appropriately.
the study design phase (e.g., through advocacy groups or community advisory boards) can help ensure that decisions on whether and how to return results are aligned with participant values and needs. For other types of studies (e.g., when biospecimens have been de-identified or if investigators can reasonably rely on existing documentation of participant needs, preferences, and values in the literature or from past experiences working with community groups), engagement may not be as important.
Many investigators will be new to participant and community engagement activities and will need to rely on existing models, guidance, and informational resources as they develop study protocols and consider return of individual research results. Investigators may need to be made aware of the existence of these resources or receive training in order to effectively engage participants in discussions of their preferences for the return of individual research results. To minimize the burdens on individual investigators, research sponsors and institutions can help investigators understand the preferences and needs of their prospective participants by leveraging their core resources (e.g., Clinical and Translational Science Awards Program cores, community advisory boards) and by engaging community and participant representatives to develop policies and guidance (see Chapter 4 for additional information on the range of engagement in the return of individual research results).
PLANNING FOR THE RETURN OF INDIVIDUAL RESEARCH RESULTS
The development of a plan at the design phase of a study that addresses whether, when, and how results will be offered to participants as part of the study protocol, or provided in response to a participant request or upon discovery of an unanticipated but potentially valuable result, can help maximize the benefits and prevent or mitigate the potential harms associated with the return of research results. Incorporating the plan into the research protocol ensures transparency and appropriate budgeting, while IRB review ensures that the risks and benefits to participants are carefully considered in a peer-review process.
The planning process should consider the types of results that might be shared (such as routine clinical results generated in the course of research, test results generated in a research laboratory, or urgent findings) and when in the study life cycle they might be shared without threatening the scientific integrity of the study. By requiring and reviewing plans and providing support for the return of individual research results, research institutions and sponsors can help foster a culture in which the return of individual research results is more routinely considered and practiced.
EFFECTIVELY COMMUNICATING INDIVIDUAL RESEARCH RESULTS TO PARTICIPANTS
The return of individual research results to participants is relatively uncommon in the research enterprise. As a result, few standardized practices or even guidance on how to accomplish this challenging communication task have been developed. Different communication approaches may be appropriate in different contexts and may be associated with different costs or burdens to investigators. Given the scientific community’s general lack of experience with returning individual research results to participants, as well as the complexity and uncertainty inherent in results generated through research, the development of guidance and best practices may help address inconsistency in practices and minimize the risk of harm from the return of research results.
To establish an empirical evidence base for the development of best practices, the research community will need to develop a learning system in which processes for returning research results are evaluated for benefits and harms and communication practices are refined. This will require the accumulation of experience over time. In the absence of such empirically derived best practices, applying existing principles for clear communication, such as considering audience characteristics and needs and having a clearly defined communication objective, represents a clear strategy for improving the quality of return-of-results practices now. Being clear and transparent during the consent process regarding whether, under what circumstances, and how investigators will offer and return research results
can help to set appropriate expectations and build trust. The use of established communication principles is also important in order to enhance the likelihood of participants understanding research results and the appropriate use of that information.
The ability of participants to understand and make use of research results depends on the provision of relevant contextual information that clarifies what is known or unknown about the meaning of a specific result. When relevant contextual information (such as reference standards) for a result is not known, studies should weigh the benefits and risks of return and consider whether the return of only aggregate results would be more appropriate than the return of individual results. Understanding is also facilitated by providing a clear takeaway message that includes a statement regarding actionability. For more complex studies, it can be challenging to effectively communicate to research participants the degree of uncertainty that the research results entail, especially in contrast to the more familiar context of clinical testing. As a result, the return of individual research results should often be accompanied by caveats and qualifiers that address potential inaccuracies and uncertainties.
The appropriate return of individual research results requires investment and careful forethought regarding the necessary contextualizing information, takeaway messages, and caveats. It also requires a consideration of the need to communicate in ways appropriate for participants with different needs, resources, and backgrounds. However, upfront investments to improve investigator access to resources, training, and expertise can be scalable, and the development of best practices over time will improve the consistency and quality of the process of returning individual research results.
When it comes to funding empirical research for the return of individual research results, NIH is the obvious, and likely primary, sponsor who would fund such an endeavor. However, this responsibility should not fall to NIH alone.
The return of research results will soon become an integral part of the research enterprise—it is a global endeavor and all sponsors of research using human biospecimens should direct resources to addressing the needs of investigators and participants through the funding of empirical research in the practice. The development of unified guidance on returning individual research results will help prevent dramatic variability in practice between institutions and will aid IRBs in making informed decisions. Funding agencies have a responsibility to ensure that processes for return are both feasible and implemented appropriately.
RESHAPING THE REGULATORY ENVIRONMENT
The legal and regulatory requirements and restrictions pertaining to the return of individual results are currently uncertain, thus causing variable interpretation and action across IRBs and research sites. As currently written and implemented, the laws and regulations governing access to laboratory results, both clinical and research, are not harmonized; they afford inconsistent and inequitable access for participants to their individual research results, and regulatory conflicts create dilemmas for laboratories. Specifically, CMS’s interpretation of CLIA blocks any laboratory from returning a test result if the laboratory is not CLIA certified, but HIPAA requires the return of results upon a request by the patient if the results are part of their DRS. In some cases regulations are too restrictive, while in others they are not restrictive enough, allowing for the return of results of poor or unsubstantiated quality without appropriate caveats or context. Moreover, the regulations governing the protection of human participants do not address the return of results, meaning that the guidance available to research participants and investigators in inadequate. Overall, the current regulatory environment for the return of individual research results is not well aligned with the benefits and risks associated with the practice.
The current absolute prohibition of the return of results from non-CLIA-certified laboratories fails to account for several factors, including the high quality maintained by some research laboratories, the value that many participants place on results despite uncertain validity, and the access rights afforded by HIPAA to individual results regardless of quality standards. Additionally, there is little evidence of harm from the return of research results, although the overall body of evidence is limited and may reflect a lack of evidence rather than conclusive evidence of a lack of effect. Accordingly, the committee believes that, in certain circumstances, results can be provided to participants when laboratories have not achieved CLIA certification. However, the committee is cognizant of the potential harms to participants and the research enterprise if laboratory quality systems are not in place in research laboratories or if loopholes are created that can be abused to perform clinical testing in a research laboratory without CLIA certification. Therefore, the Office for Civil Rights of the Department of Health and Human Services should limit access to individual research results under HIPAA to those
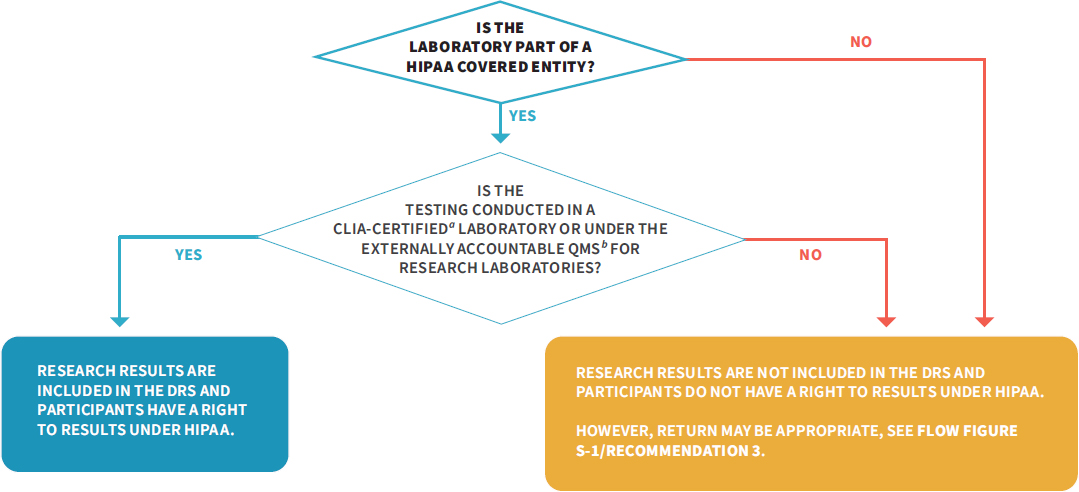
NOTE: CLIA = Clinical Laboratory Improvement Amendments of 1988; DRS = designated record set; HIPAA = Health Insurance Portability and Accountability Act of 1996; NIH = National Institutes of Health; QMS = quality management system.
generated in a CLIA-certified laboratory or in a laboratory that has adopted the QMS for research laboratories recommended by the committee (see Figure S-3). Through its recommendations, the committee promotes an approach that requires high-quality standards when investigators plan to return results, but also supports a thorough peer review and approval process for the potential return of valuable results generated in laboratories not meeting CLIA requirements.
FINAL THOUGHTS
The recommendations in this report, if followed, will result in substantial and potentially controversial changes to the research regulations and the research enterprise involving research with human biospecimens. The opportunity for change has arisen in response to the evolving relationship between investigators and participants and is supported by an assessment of the potential benefits and risks of returning individual research results. The need for higher standards of quality in many research laboratories is clearly illustrated in this report (see Chapter 3). Yet, despite the inherent limitations in the validity and interpretability of some research results, our assessment is that the risks associated with the communication of results have been overstated, particularly for the many research projects that are unlikely to yield highly sensitive or clinically meaningful results. Furthermore, the potential benefits of results disclosure to individual participants and to the research enterprise have been understated.
Therefore, we are recommending that the current absolute standard—that all disclosed results must be generated in a CLIA-certified laboratory—should be replaced with a process-oriented standard, meaning that a peer-review process can be used in some circumstances to weigh competing considerations regarding the return of individual results. We recommend that such a process take into account, on a case-by-case basis, the values of the participants, the risks and benefits of the return of particular results, the quality of the research laboratory and test
performance, and the feasibility for investigators to pursue this course. There are risks to moving away from an absolute standard, but we believe that the risks can be mitigated through improvements in laboratory quality, a case-by-case assessment of the risks and benefits, and the promotion and development of communication strategies to help place results in the proper context for participants. The committee believes that the benefits of this more nuanced approach will greatly exceed the adverse impacts and costs.
The committee is well aware that more frequent return of individual research results will create new demands on the research enterprise. Many institutions and researchers currently lack the experience and resources to return individual research results in a deliberate and effective manner. The committee does not expect that a more widespread return of results will happen immediately. However, the committee foresees an evolving set of responsibilities and offers recommendations that it believes will help stakeholders prepare for these added responsibilities and develop the necessary expertise over time.
At a broader level, the justification for fundamental changes in the research landscape can be found in our changing understanding of the ethics of human participant research as well as in our recognition that failures to support transparency and to earn respect and trust from individuals in the community are hampering the conduct of science. The vision is that a dedicated commitment to collaboration will better honor participants, benefit science, and promote the welfare of society. While the standards and practices related to the return of individual results are but one set of elements in this evolving landscape, the return of research results is a tangible, measurable piece that we know is valued by participants and is feasible in many more circumstances than are reflected in current practice. Our hope is that this report will promote the practice through selected changes in research regulations, the use of quality management systems that ensure the quality of research results, and the commitment of all stakeholders (see Table S-1) to innovative, collaborative processes in the planning and conduct of research.
TABLE S-1 Recommendations by Stakeholdera
| STAKEHOLDER | RECOMMENDED ACTION |
|---|---|
| HHS | RECOMMENDATION 12G – Chapter 6 Refer to research volunteers as participants, not subjects in all regulations relevant to human research |
| CMS | RECOMMENDATIONS 12C and D – Chapter 6 Revise CLIA regulations to allow for the return of individual research results from non-CLIA-certified laboratories when results are requested under the HIPAA access right and when the quality of results has been established and they are not intended for use in clinical decision making |
| RECOMMENDATION 12E – Chapter 6 Work with OCR to harmonize definitions of key terms relevant to the return of individual research results in the federal regulations |
|
| FDA | RECOMMENDATION 12H – Chapter 6 Clarify and provide additional guidance regarding how the return of individual research results affects IDE requirements for research studies |
| NIH | RECOMMENDATION 2 – Chapter 3 Lead an interagency effort with nongovernmental stakeholders to develop standards for a quality management system for research laboratories testing human biospecimens |
| RECOMMENDATION 12F – Chapter 6 Work with OCR and OHRP to harmonize the definitions of key terms relevant to the return of individual research results in the federal regulations |
|
| OCR | RECOMMENDATION 12A – Chapter 6 Revise the definition of the designated record set (DRS) |
| RECOMMENDATION 12B – Chapter 6 Require HIPAA-covered entities that conduct research on human biospecimens to develop a plan for the release of individual research results in the DRS when requested under HIPAA |
|
| RECOMMENDATIONS 12E and F – Chapter 6 Work with CMS, OHRP, and NIH to harmonize definitions of key terms relevant to the return of individual research results in the federal regulations |
|
| OHRP | RECOMMENDATION 12F – Chapter 6 Work with OCR and NIH to harmonize definitions of key terms relevant to the return of individual research results in the federal regulations |
| Research sponsors and funding agencies | RECOMMENDATION 4 – Chapter 3 Ensure adequate resources and infrastructure to generate high-quality individual research results |
| RECOMMENDATION 5 – Chapter 4 Engage community and participant representatives in the development of policy and guidance related to the return of individual research results |
| RECOMMENDATION 7 – Chapter 4 Ensure planning for the return of individual research results in applications for funding |
|
| RECOMMENDATION 11 – Chapter 5 Support research to expand the empirical evidence base relevant to the return of individual research results |
|
| Research institutions | RECOMMENDATION 1 – Chapter 2 Consider whether and how to return individual research results on a study-specific basis |
| RECOMMENDATION 3 – Chapter 3 Ensure the high quality of individual research results that are returned to participants |
|
| RECOMMENDATION 4 – Chapter 3 Ensure adequate resources and infrastructure to generate high-quality research results |
|
| RECOMMENDATION 5 – Chapter 4 Enable and facilitate investigator access to relevant community and participant networks, resources, and training |
|
| RECOMMENDATION 8 – Chapter 4 Develop policies and procedures that support the assessment of plans for the return of individual research results, and ensure that IRBs and research teams have or have access to the necessary expertise and resources to assess plans. |
| Research institutions | RECOMMENDATION 10 – Chapter 5 Enable the understanding of individual research results by research participants |
| IRBs | RECOMMENDATION 3 – Chapter 3 Ensure the high quality of individual research results that are returned to participants |
| RECOMMENDATION 7 – Chapter 4 Review the return-of-results plan and ensure the consent process aligns with it |
|
| Investigators | RECOMMENDATION 1 – Chapter 2 Consider whether and how to return individual research results on a study-specific basis |
| RECOMMENDATION 5 – Chapter 4 Seek information on participant needs, preferences, and values related to return of individual research results |
|
| RECOMMENDATION 6 – Chapter 4 Include plans for return of individual research results in research protocols |
|
| RECOMMENDATION 9 – Chapter 5 Ensure transparency regarding return of individual research results in the consent process |
| RECOMMENDATION 10 – Chapter 5 Enable understanding of individual research results by research participants |
|
| Participants | RECOMMENDATION 5 – Chapter 4 Engage researchers to ensure that participant needs, preferences, and values are incorporated in decision making about the return of individual research results |
a An interactive version of this table can be found at http://resources.nationalacademies.org/ReturnofResults/index.html (accessed August 13, 2018).
This page intentionally left blank.






































