2
High-Entropy Materials
AN ASSESSMENT OF THE HIGH-ENTROPY FIELD
Daniel Miracle, Senior Scientist, Air Force Research Laboratory
Daniel Miracle highlighted the two fundamental objectives that exist in the high-entropy alloy (HEA) community: (1) to examine the unknown central region of multicomponent alloy phase space (Cantor et al., 2004); and (2) to favor solid solutions over intermetallic phases by regulating the configurational entropy in complex alloys (Yeh et al., 2004). Although both ideas are compelling, Miracle asserted that the latter is not as well supported by experimental data as the former.
Two definitions currently exist within the HEA community, Miracle continued—one is composition-based (composed of five or more principal elements, each with a concentration between 5 and 35 atomic percent), while the other is entropy-based (retains an ideal, configurational entropy greater than or equal to 1.61R, where R is the gas constant). According to Miracle, both definitions support the motivation of favoring solid solution formation through configurational entropy while placing no restrictions on phase numbers or types. However, Miracle noted, there are three issues with these two definitions: (1) solid solutions are rarely ideal, even in the liquid state; (2) people incorrectly believe that in order to be classified as an HEA, the material must be both a single-phase and solid solution; and (3) the composition-based and entropy-based definitions have some contradictions.
Miracle introduced another important term, multi-principal-element alloy (MPEA), which should be used when the community is interested in looking only
at the central region of the complex composition space to avoid some of the confusion that results from the standard definition of HEA. The label HEA is reserved for times when configurational entropy or the production of single-phase solid solution microstructures is important. The literature suggests that studying single-phase solid solution alloys is necessary because intermetallic compounds in brittle alloys are difficult to process and have little practical use, whereas solid solution alloys are strong and ductile. Miracle admitted that this explanation is exaggerated because there are certain situations in which the benefits of using intermetallics outweigh those of solid solution alloys, which are not always strong and ductile. Miracle’s overall goal is to revive a study of the full range of microstructures and compositions.
Miracle shared four hypotheses that are currently unproven yet are still often taken as fact within the community:
- High-entropy effect. High configurational entropy in complex alloys favors simple (face-centered cubic [FCC], body-centered cubic [BCC], or hexagonal-close packed [HCP] based) solid solution phases relative to intermetallic phases.
- Severe lattice distortion. Distortions (from different atom sizes) are more severe in HEAs than in conventional alloys and influence strength and other properties.
- Sluggish diffusion. Diffusion will be more sluggish in HEAs than in conventional alloys.
- Cocktail effect. The end result of the synergistic mixture is unpredictable and greater than the sum of the parts.
Miracle noted that he would discuss the merits of only the first three hypotheses. He commented that the only way to determine whether a phase will have intermetallics or solid solutions is through competition of the Gibbs energy of all the potential phases that exist. In this case, the enthalpies and the entropies of both the solid solutions and the intermetallics must be considered. It is difficult to measure the enthalpy of solid solutions, Miracle remarked. This process is more accurate than estimating with the Miedema model; that model, however, generates more data. He noted that the enthalpy of solid solutions can be either positive or negative and that the enthalpy of multi-element solutions is complicated. Generally, according to Miracle, the enthalpy of the intermetallic phase is more negative than the enthalpy of mixing of the disordered solid solution (for the same pair of elements at the same composition). Miracle continued that many concepts are based on binary systems, and these concepts are not as useful when intermetallics have more atom species than sublattices.
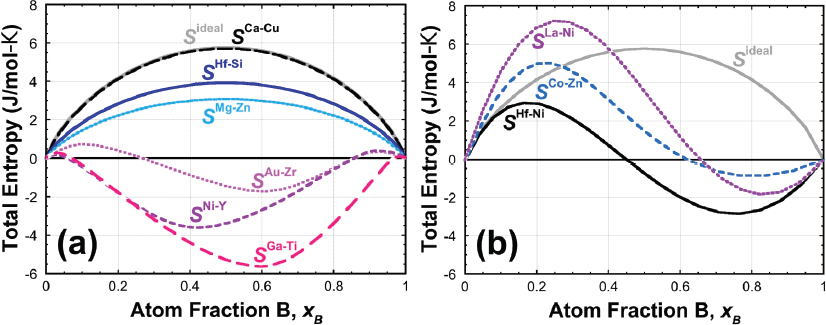
Miracle next discussed the entropy of solid solutions. He noted that configurational entropy can be estimated by the Boltzmann equation for ideal and regular solutions (see Figure 2.1).
In an analysis of 1,500 binary atom pairs, Miracle found that only approximately 4 percent were ideal solutions and 11 percent were regular solutions in the liquid state. He explained that there are additional entropy terms to consider, such as the excess configuration term from atoms of varying sizes and nonconfigurational terms from vibrations as well as electronic and magnetic effects. The size of the configurational term relative to the other terms is the important question that remains to be answered.1
Configurational terms cannot be ignored for the entropy of intermetallics either, Miracle asserted. The configurational entropy of an intermetallic in an MPEA can be as much as 60 percent of the configurational entropy of the disordered solid solution. He reiterated that the Gibbs energy of the competing phases needs to be considered, and he cautioned that the Gibbs phase rule2 is often misinterpreted in the available literature.
Miracle moved to a discussion of taxonomy. As of 2014, 385 unique alloys had been identified (only some of which are HEAs by definition), using 37 elements. There are only seven alloy families (groups of elements) currently in existence, and
___________________
1 For more information about this topic, see the summary of Michael Gao’s presentation to the workshop.
2 The Gibbs phase rule states that the number of degrees of freedom of a system is equal to the number of components minus the number of phases plus 2.
four of those were introduced in 2014. The family known as the first-row transition metal alloys is responsible for 348 of the 385 known alloys. This particular family can be characterized further into branches: there are 126 four-element branches from a collection of nine elements, and 5 of those branches are in more than half of the first-row transition metal alloys. One of the branches, CoCrFeNi, has an uncommon preference for combinations of elements that are ideal or regular solutions, he noted. In 2010, the Air Force Research Laboratory was the first to express interest in developing HEAs for high-temperature structural materials, which led to the development of refractory metal MPEAs. By the end of 2014, 26 alloys had been identified. Additional alloy families (encompassing the remaining 2 percent of the alloys) include low-density materials, lanthanides, brasses and bronzes, precious metals, and bulk ceramic MPEAs.
Miracle provided two definitions of microstructure phase classifications: (1) structures with one crystal lattice are disordered solid solutions; and (2) structures with two or more sublattices and chemical long-range order are ordered, intermetallic, or compound. Both types can have one or more phases. He explained that although there are 542 different microstructural reports in the literature, not all of these reports agree. Generally, two-phase microstructures are the most common (41 percent), while single-phase microstructures are a close second (36 percent), and microstructures with three or more phases develop only 25 percent of the time. Solid solution plus intermetallic microstructures are the most common (49 percent), solid solution microstructures are almost as common (45 percent), and intermetallic microstructures are uncommon (6 percent). Although these data support the view that HEAs provide single-phase solid solutions with simple structures more often than expected for complex alloys, Miracle explained that it is a far more complicated issue. He presented four issues with the observations from the literature:
- Use of as-cast versus annealed observations. 75 percent of studies use the as-cast condition instead of the annealed condition, which is misleading because annealing reduces solid solution alloys, increases the number of solid solution plus intermetallic alloys, decreases single-phase alloys, and increases alloys with two or more phases.
- Experimental bias from nonrandom alloy selection. Because elements studied are not chosen at random but are chosen to build on a known single-phase solid solution, the results of studies are insufficient for probability distributions.
- Contiguity of solid solution phase fields in hyperdimensional composition space. The large number of single-phase solid solution alloys in the first-row transitional metal complex concentrated alloy (CCA) family may
- represent different parts of the same solid solution phase in hyperdimensional composition phase.
- Difficulty in characterizing intermetallic phases in CCAs. X-ray diffraction is often not as useful as transmission electron microscopy in finding intermetallic phases. Consequently, intermetallic phases are often underreported.
A new area of exploration within the community includes high-throughput calculations of phase diagrams, Miracle explained. The Air Force Research Laboratory has calculated phase diagrams for more than 135,000 complex alloys; this data set of calculations can be used in comparison to the actual experiments (see Figure 2.2).
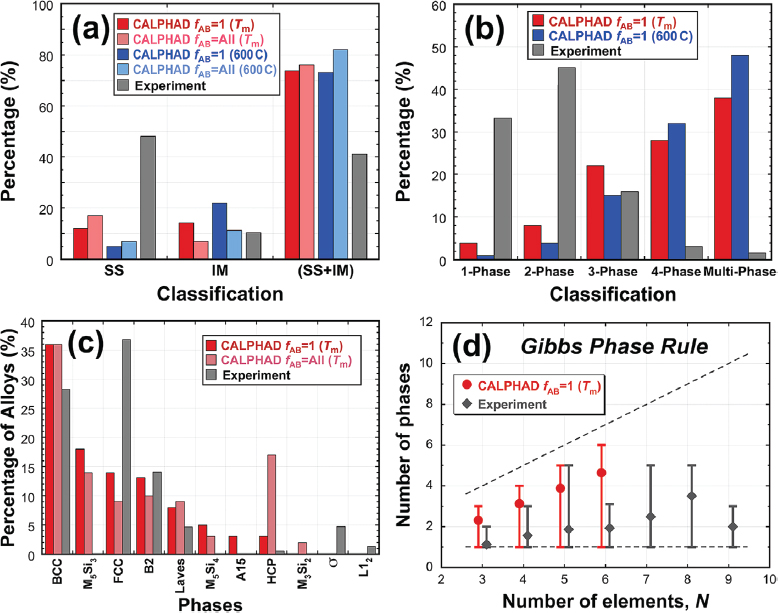
Miracle acknowledged Computer Coupling of Phase Diagrams and Thermochemistry’s (CALPHAD’s) benefits but also noted that there are some issues of reliability. To combat this, the Air Force Research Laboratory is developing a credibility factor fAB (fraction of assessed binaries). Other approaches can also be used, including ab initio or molecular dynamics. Miracle’s group developed a new analysis to try to combat some of the problems with CALPHAD, referred to as structure in–structure out, which analyzes the HEA effect. The weighting of elements is different in this analysis in that the crystal structure of the element at the melting temperature is weighted based on how often the element is used. This method has generated results that show the observed and predicted solutions to be the same. Miracle’s group also learned that the more elements present, the less likely single-phase solid solutions will form, which is the opposite of what the HEA effect would predict.
Regarding mechanical properties, Miracle noted that data do not support the notion that diffusion is sluggish in HEAs; it is likely similar to that in conventional alloys. An emerging field is refractory metal CCAs, so the data are not yet normalized, and there is great potential for continued activity in this field. Miracle concluded his talk by offering his recommendations for the future: (1) continue to explore and develop MPEAs for structural use; (2) develop strategy for high-throughput computational and experimental exploration and development of MPEAs; (3) expand the range of functional materials in the MPEA field; and (4) establish basic scientific concepts.
Peter Liaw asked Miracle about the future of multicomponent alloys. Miracle said that there needs to be a new strategy for alloy development for structural materials. Structural materials are difficult to measure and very few candidates are eliminated. High-throughput experiments and computations, as well as new strategies for material design and development, are needed, and HEAs could be used as a platform to advance this work and develop new tools, Miracle explained. Valerie Browning inquired about the potential to expand the range of functional materials. Miracle responded that although there are currently no specific approaches, any material can be approached from a high-entropy perspective.
MECHANICAL AND THERMODYNAMIC INSTABILITIES IN REFRACTORY HIGH-ENTROPY ALLOYS
Michael Widom, Professor, Carnegie Mellon University
Michael Widom’s presentation focused on the problem of predicting the stability of HEAs, particularly when enthalpy and entropy are considered. He noted that all of the systems he planned to discuss have nonideal entropy. Widom explained
that a parameter-free first-principle computational approach is needed because there are too many elements to use an empirical approach. Looking at just one element, he continued, requires tuning, while evaluating two elements requires modeling of cross interactions.
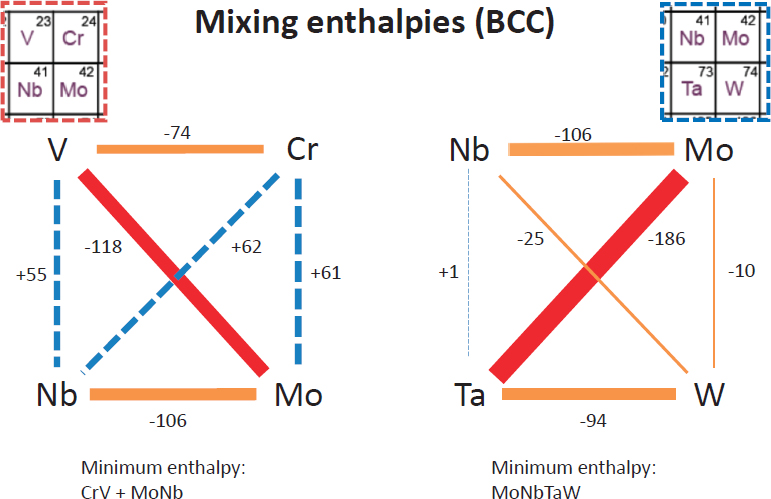
Widom suggested that researchers interested in identifying HEAs should look in certain regions of the periodic table and mix together elements from those particular regions. Within square groupings of elements, it is possible to arrange the elements in order relative to atomic size and electronegativity. For example, Widom demonstrated that BCC HEAs can be made out of elements that would typically prefer HCP alloys.
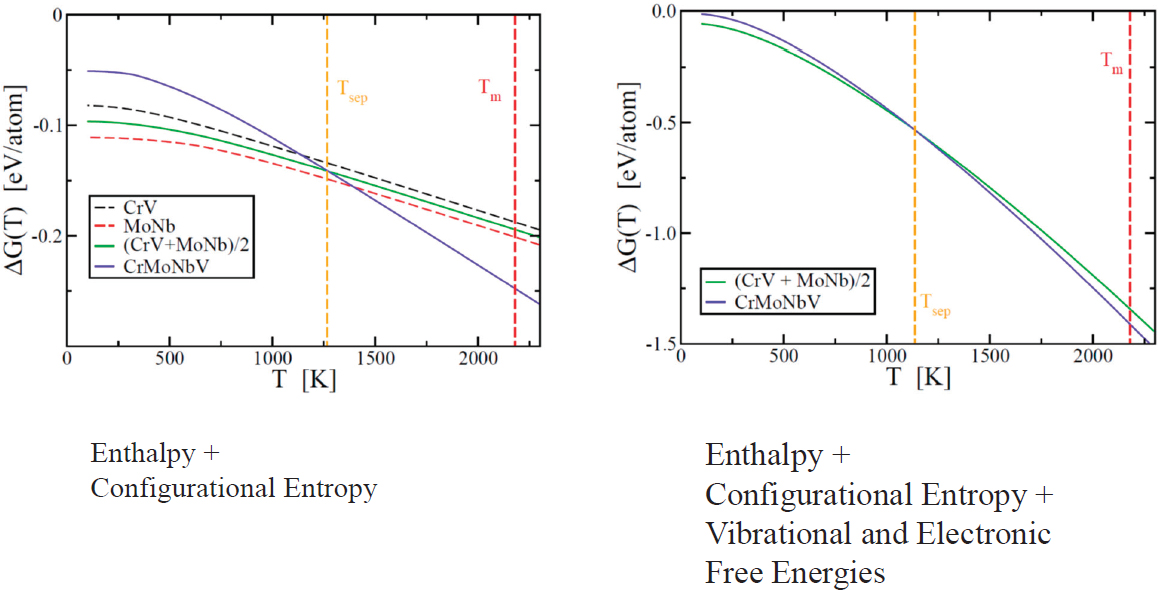
In Figure 2.3, MoNbTaW is stable, even though such strong interactions generate nonideal entropies of mixing. Widom relies on (1) a combination of molecular dynamics and Monte Carlo simulations and (2) ab initio calculations of thermodynamic potential (Gibbs free energy) to sample the configuration space and understand entropies of mixing. Widom then relies on the replica exchange method, which allows simultaneous runs at various temperatures, to increase efficiency. To exchange temperatures, a Boltzmann-like probability is used to trade replicas. In doing this, a single run has the potential to explore an entire range of
temperatures. Disentangling all of these results allows for the relative free energy across temperature ranges to be reconstructed. By looking at the pair correlation functions, Widom emphasized that the entropies of these systems are not ideal. Configurational entropy is evaluated using the cluster variation method, which was originally designed to work with ad hoc approximate interaction potentials. However, in this case, using a first-principles approach, the interaction potentials are not needed. Knowing that at 1,000 K, 15 percent of entropy has been lost, the configurational entropy can be quantified.
Similar methods were applied to AlCrFeCoNi, the most popular high-entropy family. Results included the onset of phase separation and long-range chemical order. According to Widom, the chromium-rich phase would like to separate, and the aluminum rarely partners with aluminum; aluminum atoms alternate with cobalt and nickel, forming a B2 phase. Thus, in order to see the true phase separation in the simulation cell, two cells must be used.
Beyond enthalpy and mixing entropy, vibrational and electronic free energy contributions also exist. Because the vibrational free energy is large, the vibrational free energy of separation is small and so does not drive phase separation. Combining all of these results, one can evaluate the relative free energies that are calculated from first principles (see Figure 2.4).
Widom presented another example, this one with two BCC formers and two HCP/BCC formers: NbTiVZr. A combination of molecular dynamics and Monte Carlo simulations is used again to calculate the pair correlation functions. The resulting solution is not nearly as stable as the previous solution. A distinction is lost between neighbors in correlation, he explained. Essentially, the BCC structure is preserved at long range but since the nearest and next-nearest neighbor split is missing, the BCC structure has been lost at short range. Widom hopes that someone will use diffuse X-ray scattering to identify the consequences of this. He has cut through diffraction patterns to show relative diffuse intensity of the HCP/BCC combinations, which indicates that a local disorder happens when HCP and BCC formers are mixed. Despite the local disorder, the BCC structure is still well ordered on long length scales.
Widom concluded his talk by reiterating the importance of applying first principles, total energies, and Monte Carlo and molecular dynamics methods to simulate HEAs; the benefit of using the cluster variation method to evaluate configurational entropy; and the presence of local structure collapse without long-range structure collapse in HCP/BCC combinations. Widom also concluded that the newly predicted CrMoNbV is likely unstable to separation below 1,100 K.
Peter Liaw asked about the possibility of applying high-throughput calculations to first-principle calculations. Widom noted that it is possible to calculate the mixing
enthalpies in high-throughput calculations. However, this process is expensive, Widom advised, and will require multiple days to calculate each vibrational free energy. Haydn Wadley asked about the precision of calculations when two systems are crossing in free energy. Widom agreed that locating the crossing point is a problem and that one must be extremely careful to avoid a mistake that would result in a large shift. He noted that the error bars impact this as do the slopes of the lines: when the slopes are small, there is a larger range. The goal is to target one millielectron volt accuracy in enthalpies. The errors for vibrational free energies will of course be larger, he noted. He suggested that the calculations be done in compatible cell sizes and shapes to avoid systematic errors. Kenny Lipkowitz, Office of Naval Research, asked how many configurations were done as well as how many should be done. He also asked how sensitive the results are to density functional theory, as well as why there was not a third effect measured in the cluster expansions. Widom explained that there are approximately 1,000 configurations; when the answers appear to stop changing (converging time can vary), they know they have done enough configurations. Four-point correlations are rare and can be error-prone, and three-point correlations often provide more unreliable results than the two-point correlations. Ward Plummer asked Widom why he is not studying thermoelectric materials. Widom noted that he has not done much in this area yet because evaluating thermoelectric properties is expensive, and elastic properties are easier to study. Daniel Miracle added that the stacking of energies is another important consideration in this discussion.
DEVIATION FROM HIGH-ENTROPY CONFIGURATIONS IN THE ATOMIC DISTRIBUTIONS OF A MULTI-PRINCIPAL-ELEMENT ALLOY
Peter Liaw, Ivan Racheff Chair and Professor, Department of Materials Science and Engineering, University of Tennessee
Peter Liaw’s current research objective is to understand and relate the advantages of the configurational entropy mixing in single-phase HEAs and MPEAs, with the hope of making new materials appropriate for applications where high strength, fatigue, toughness, and corrosion resistance at higher temperatures are essential (Santodonato et al., 2015; Zhang et al., 2014; Yeh et al., 2004; Senkov et al., 2010; Feuerbacher, Heidelmann, and Thomas, 2015; Youssef et al., 2015; Hemphill et al.,
2012; Tang et al., 2015; Gludovatz et al., 2014).3,4 Liaw defined HEAs as multiple elements mixed in near equimolar ratios and explained that if more elements are added into a material, the entropy will typically be higher. The phase with the lowest Gibbs free energy is stable at high temperatures, and a random solid solution phase of elements may be stabilized by the large configurational entropy of mixing, though other factors should be considered, Liaw explained.
Simple crystal structures in HEAs are formed in one of the following ways (or combination of the following): FCC, BCC, or HCP phases (Zhang et al., 2014; Feuerbacher, Heidelmann, and Thomas, 2015; Youssef et al., 2015). Liaw noted that the local lattice strain—which does not occur with conventional materials—can affect the mechanical behaviors of these HEA structures. In calculating the pair-distribution function, it is evident that the difference between the observed neutron and calculated pair-distribution function indicates local lattice strain in the multicomponent alloys.
Liaw also found that HEAs can likely be used for industrial applications because they have high strength at elevated temperatures. He summarized the work that his team has done on the fatigue performance of HEAs: As the tensile strength is increased in materials, the fatigue-endurance limit also generally increases with great fatigue resistance in HEAs (Hemphill et al., 2012; Tang et al., 2015). This experiment suggests that HEAs would perform well if used for structural components subjected to fatigue loading. HEAs, Liaw noted, also have higher fracture toughness than conventional materials (Gludovatz et al., 2014).
The next study that Liaw presented merged the work in microscopy, neutron scattering, synchrotron X-ray diffraction, and molecular-dynamic simulations (Santodonato et al., 2015). This study tested Al1.3CoCrCuFeNi, which has a more complicated microstructure than originally thought. Low-magnification scanning
___________________
3 This presentation was informed by the work of both Peter Liaw and Louis J. Santodonato (Department of Materials Science and Engineering, University of Tennessee; Instrument and Source Division, Oak Ridge National Laboratory).
4 Liaw acknowledged Drs. Y. Zhang, M. Feygenson, C.M. Parish, M.C. Gao, R.J.K. Weber, J.C. Neuefeind, and K.A. Dahmen for their research interactions and discussions on HEA research. He also acknowledged the Department of Energy (DOE), Office of Fossil Energy, National Energy Technology Laboratory (DE-FE-0008855 and DE-FE-0024054, and DE-FE-0011194), with Mr. V. Cedro and Mr. R. Dunst as program managers. He thanked the support from the project of DE-FE-0011194 with program manager Dr. J. Mullen. He acknowledged the support of the U.S. Army Research Office project (W911NF-13-1-0438) with program manager Dr. D.M. Stepp. He noted the support from the National Science Foundation (CMMI-1100080 and DMR-1611180) with program directors Dr. C. Cooper and D. Farkas. He thanked the Spallation Neutron Source, High Flux Isotope Reactor, and the Center for Nanophase Materials Sciences (DOE Office of Science User Facilities operated by the Oak Ridge National Laboratory) for use of their resources. He acknowledged the resources at the Advanced Photon Source, a DOE Office of Science User Facility operated by Argonne National Laboratory.
electron microscopy reveals segregation, which suggests that the alloy is not randomly mixed. Copper-rich FCC structures are found throughout the BCC/B2 crystal grains. In the B2 region, numerous elements are mixed in the major phases. The nearest-neighbor pair-correlation trends in the liquid state are similar to those in the solid state, Liaw explained, suggesting a type of precursor behavior. The disordered BCC structure is fully specified by the phase composition and lattice parameter. Thus, all sites must be populated equally since there are multiple-principal elements at each site. In order to fully specify the B2 ordered structure, additional information is needed, including the calculation of the configurational entropy.
Regarding diffraction studies, the crystal lattice parameters must be determined from the peak positions; the order parameters must be obtained from the relative peak intensities; and a twofold strategy must be used to cross-check the order parameters (e.g., conducting complementary neutron and X-ray scattering studies and introducing a single-parameter fitting technique). Looking at the results of the neutron-diffraction studies, there are always three phases present (FCC, disordered BCC, and B2 structures) in the range of 24°C to 800°C. Knowing this trend, Liaw explained and set up a boundary condition for calculating the entropy term. Thus, the multiple element structures are solved using complementary neutron and X-ray diffraction methods. Because the crystal models fit the two boundaries of neutron and synchrotron diffraction results, the ordering parameter can be determined confidently, and the entropy term can be calculated using both the higher-temperature and room-temperature models.
Liaw discussed an innovative experiment being performed with aerodynamic levitators to study the behavior of the phase transformation of multicomponent alloys from the melting point to room temperature. This process uses aerodynamic forces and CO2 laser-beam heating to achieve containerless measurements. This method prevents contamination and heterogeneous nucleation, while maintaining supercooled liquids and clean liquid surfaces. When the scattering intensity is plotted at different temperatures, the following changes are revealed: at room temperature, all three phases are apparent; at 800°C, all three phases remain; at 1,050°C, the FCC phase begins to dissolve; and at 1,200°C, the only phase visible is the BCC structure. By 1,400°C, the diffuse scattering indicates the transition to a liquid state. With this information, it is possible to calculate the pair-distribution function. Comparing this trend with the model prediction helps determine the local structure. Liaw found that for the liquids, the long-range periodicity is lost; however, the local structure of some liquid HEAs may still be similar to that of the solid structures. His team modeled this behavior with BCC and FCC unit cells to confirm the observed pair-distribution function.
From the experimental neutron-scattering study at room temperature, Liaw observed that HEAs may exhibit a significant deviation from one unit cell to the subsequent unit cell. Furthermore, an “average” crystal structure surfaces over
long distances. In the same study, but at the melting point it is evident that some crystal structure remains in the liquid. When the behavior of various atom pairs is compared at 1,400°C, the liquid strays from random mixing, and Liaw suggested that the local structure of the liquid state is a precursor to the solid state (i.e., knowledge about liquid state structures can be used to inform work on solid state microstructures), as shown by ab initio molecular dynamic (AIMD) simulations. Considering all of these results, and combining experimental and theoretical calculations, one can better understand the atomic-mixing behavior as a function of temperature and across numerous length scales.
To summarize his talk, Liaw reiterated the following: (1) local and long-range ordering behavior is apparent in Al1.3CoCrCuFeNi starting at room temperature up to the molten state; (2) configurational entropy of mixing during cooling is quantifiable; and (3) the local structure of the liquid state is a precursor to the long-range-ordered structure that forms in solid state. Liaw noted that his future research goals include predicting and controlling the mixing behavior of HEAs as well as expanding the scope of multicomponent alloy-design applications.
Luigi Colombo asked about the amount of copper and aluminum lost during the melting process and the resulting effect on phase separation. Liaw said that copper travels faster than other alloys, which results in the segregation, although additional elements may also be lost. A workshop participant asked Liaw to confirm that his data showed some immiscibility in the liquid state. He also asked if the corrosion effects will be different for HEAs than they will be for conventional alloys. Liaw acknowledged that it depends on the microstructure; some have high corrosion resistance. Ultimately, it is important to revisit the design stage and eliminate materials with the “undesirable” corrosive properties. In addition to increasing corrosion resistance, doing so may also enhance fatigue and fracture toughness, Liaw noted. Ward Plummer asked if the interfaces in the phase separation must have different structure and composition to affect the physical properties. Liaw responded that although this issue has not yet been studied in great depth, the mechanical and thermoelectric properties may be impacted.
HIGH-ENTROPY ALLOYS: THERMODYNAMICS
Michael Gao, Principal Materials Scientist, AECOM; Contractor, U.S. Department of Energy
Michael Gao’s presentation discussed how thermodynamics are used to design or identify new single-phase HEAs. He explained that single-phase HEAs are currently limited to four, five, or six elemental components, and he said that total thermodynamic properties and mixing properties are the two slightly different sets of parameters. He noted that the contributions to the total entropy comprise
the configurational entropy, vibrational entropy, magnetic entropy, and electronic entropy. However, the magnetic entropy will be small because the critical magnetic ordering temperatures are well below room temperature for reported single-phase HEAs. Research also demonstrates that the electronic entropy is negligible.
Gao defined excess entropy, which could be positive, zero, or negative, as the total entropy of mixing minus the ideal configurational entropy. Using seven empirical parameters, Gao highlighted the disordered HEA formation criteria, which is an important component in understanding the number of single-phase HEAs that exist (fewer than 20 are currently known in equimolar composition):
- Enthalpy versus atomic size difference
- Omega parameter
- Valence electron concentration
- Electronegativity difference
- Phi parameter
- Root mean square residual strain
- Gibbs free energy comparison
Gao acknowledged that further experimentation is needed to determine whether these empirical rules are universal, while some rules work better than the others. He noted that these rules are useful in fast screening for solid solution compositions, but they are inconclusive. Furthermore, they do not predict the crystal structures of the solid solution or the intermetallic compounds. Figure 2.5 illustrates three disordered HEA formation rules.
Phase diagrams assisted by CALPHAD are powerful strategies to identify single-phase HEAs, as are first-principles density functional theory (DFT) modeling and experimental validations with casting and characterization. Gao noted that the success of CALPHAD is critically dependent on the database that is used (e.g., TCNI8, TTNI8, and PanHEA), so work needs to be done to verify and understand the results of its predictions. Inspection of existing binary/ternary phase diagrams—namely, the combination of isomorphous solid solution, large terminal solubility, and intermediate phase with wide homogeneity—can provide useful hints on the proper elemental combination to form solid solution HEAs. Gao said that by combining phase diagram inspection and CALPHAD modeling, hundreds of quaternary and higher-order equimolar compositions in the Dy-Er-Gd-Ho-Lu-Sc-Sm-Tb-Tm-Y, Ba-Ca-Eu-Sr-Yb, Mo-Nb-Ta-Ti-V-W, and Mo-Nb-Re-Ta-Ti-V-W systems have been suggested. Gao explained that a team at Oak Ridge National Laboratory (ORNL) found that out of six alloys, a single FCC phase was observed in only one alloy, CoCrFeMnNi. Composite microstructures were observed in the other alloys, he noted. CALPHAD modeling was able to reasonably
reproduce ORNL’s experimental observation. Temperature effect, composition, and pressure can also be considered using CALPHAD. Gao explained that for solid solution HEAs, the entropy of mixing of the liquid phase can be close to the ideal configurational entropy. He noted that CALPHAD addresses more problems than those empirical rules (e.g., the Miedema model), such as calculating entropy of mixing and enthalpy of mixing as a function of composition and temperature for a given system.
Gao’s team observed that in a BCC system, when the total entropy is compared to the entropy of mixing behavior, total entropy increases as the temperature or the number of elements increases. However, entropy of mixing can also increase at lower temperatures due to magnetic contributions in certain phases, he continued. For the FCC Co-Cr-Fe-Mn-Ni HEA systems, he showed that the maximum entropy of mixing for the Co-Cr binary greatly exceeds the maximum configurational entropy of a binary system. As the number of principal components in the alloy increases, Gao explained, the contribution to the Gibbs free energy associated with the entropy also increases. While CALPHAD does not always indicate why the excess entropy is positive, DFT simulations reveal that positive excess entropy in the FCC Co-Cr-Fe-Mn-Ni system is a result of the positive vibrational entropy of mixing.
Gao showed examples of BCC alloys (i.e., Mo-Nb-Ta-Ti-V-W system) with negative excess entropy that were predicted using CALPHAD. DFT confirmed that the vibrational entropy of mixing is negative, which is partly responsible for the negative excess entropy. While there currently is no firm conclusion as to whether it is better to have a higher or a lower number of principal components in an alloy, Gao believes that it is best to balance the entropy and enthalpy so as to maximize the phase field of the target solid solution phase. He continued that higher entropy is also not always best because it can cause destabilization of the solid solution phase by promoting intermetallics formation. Gao commented that very little experimental data have been collected on enthalpy formation of solid solution HEAs.
Rosario Gerhardt asked Gao what his reference is for the atomic size difference of the single-phase HEAs. Gao responded that the atomic size difference parameter is calculated as the square root of the summation product of the composition-weighted average atomic radius. He continued that the enthalpy for a single-phase solid solution alloy needs to be neither too positive nor too negative, and the atomic size difference parameter should be small (generally less than 6.6 percent). While AIMD simulation is a useful tool to reveal atomic structure at different temperatures, it is not a conclusive tool for phase identification. DFT is used to calculate the lattice vibration, and the results show that the vibrational entropy of mixing
was positive for the FCC CoCrFeNi alloy, negative for the BCC MoNbTaW alloy, and close to zero for the HCP CoOsReRu alloy. The electronic entropy of mixing for these three HEAs is close to zero, Gao described. By using the combination of Monte Carlo and molecular dynamic simulations (based on DFT) suggested by Michael Widom, Gao said, it is possible to calculate configurational entropy as a function of temperature. For select FCC, BCC, and HEA alloys, Gao said that the DFT-predicted lattice parameters and bulk modulus are close to the averages of the pure elements and obey the rule of mixture. Gao continued that HEAs do not always exhibit solid solution strengthening. An example was shown in the HCP GdHoLaTbY HEA, which depends upon the constituent components. Solid solution strengthening is apparent, however, in many single-phase FCC and BCC HEAs, and the measured hardness, yield strength, and fracture strength exceed the averages of the pure elements tremendously; Gao attributed this to the differences in the lattice parameter, the shear modulus, and the so-called lattice distortion effect of HEAs.
Gao concluded by noting that although there are a series of empirical rules proposed in the literature to predict solid solution HEA formation, the total number of single-phase HEAs reported to date is very limited. More experimental and computational data are needed on the physical, thermodynamic, kinetic, and mechanical properties of single-phase HEAs. He reiterated that CALPHAD is the only tool that considers all phases in the system as a function of temperature and composition, although more effort is needed to develop robust and self-consistent databases that accurately represent HEA systems. He noted that maximum entropy of mixing may not necessarily occur at equimolar compositions; he also reiterated that entropy may not always dominate, enthalpy cannot be ignored, and enthalpy of the liquid does not necessarily correlate well with enthalpy of the solid solution.
Daniel Miracle asked if the magnitude of the negative excess vibrational entropy for BCC materials was sufficient to overcome the positive term associated with the configurational entropy. Gao said no because although it is small, it is not too small. Long-Qing Chen, Pennsylvania State University, asked Gao to share this range in terms of percentage, and Gao responded that it is less than 25 percent. Peter Liaw said that based on Gao’s elastic constant calculation, some of the modulus reported in the literature seemed very low. He asked if it is possible that there is an experimental error or if it is possible to achieve that low of a modulus. Gao said that this issue has been raised in the past, but he has not seen any results to the contrary. He said that this is a good potential area to focus on, and there have been few reports on elastic properties of HEAs. Plastic properties, on the other hand, can be sensitive to processing and the resulting microstructures (e.g., proper grain sizes, proper treatment, etc.).
PANEL DISCUSSION: HIGH-ENTROPY MATERIALS
| Participants: |
Long-Qing Chen, Donald W. Hamer Distinguished Professor of Materials Science and Engineering and Professor of Engineering Science and Mechanics, Pennsylvania State University Karin Dahmen, Professor, University of Illinois, Urbana-Champaign Zi-Kui Liu, Professor of Materials Science and Engineering, Pennsylvania State University |
| Moderator: |
Haydn Wadley, University Professor and Edgar Starke Professor of Materials Science and Engineering, University of Virginia |
Defining High-Entropy Alloys
Long-Qing Chen, the first panelist, works primarily on mesoscale microstructural modeling, although he also works on computational modeling of phase transformation and microstructural evolution in alloys, thin films, and energy materials. Chen added that some of his colleagues both perform DFT calculations and work with experimental groups. He noted the confusion in defining high-entropy materials. One way, according to Chen, is to compare the absolute value of maximum entropy of a material using the Boltzmann constant or gas constant since entropy is really an issue of thermodynamics. He also noted that the current unit to measure entropy (Joule/Kelvin) is too complex and should be its own individual unit, perhaps named after Boltzmann. Chemical potential should also be measured separately, according to Chen. He observed that high or low entropy is determined by the balance of enthalpic and entropic contributions. The amount of thermal energy is the entropy multiplied by the temperature, and the Gibbs free energy is really the amount of enthalpy; the system cannot be maintained without this free energy. Chen concluded that high entropy should be defined as the ratio of entropy and enthalpy.
Daniel Miracle agreed that it is impossible to label a material as high entropy without asking, relative to what? Miracle coined the phrase “high entrothalpy” to represent an alloy with both high entropy and enthalpy. Noam Bernstein added that this can be meaningful only if the characteristic temperature (e.g., melting temperature or half the temperature) is present. Chen responded that his calculation (dividing enthalpy by entropy) does include the system’s characteristic temperature. Entropy will dominate if the bonding is weak, and increasing a temperature increases the entropy of a system, Chen explained. Michael Gao
said that this discussion about high entropy is not new; the ratio of entropy and enthalpy has been labeled in various publications as the Omega parameter. If that is the case, Chen asked why are more researchers not using it?
Gao acknowledged that although Omega is used, HEAs may not always form. He pointed toward Peter Liaw’s research for further information on the issue. Miracle added that people may not use the Omega parameter because although the entropy and enthalpy of mixing terms are written as if they compete for a stable solution, they actually work together to achieve a stable solution. To avoid this complication, researchers should instead look at the entropy of the intermetallic versus the enthalpy of the solid solution.
Chen wondered if the single-phase, two-phase, or multiphase alloy is best, in terms of properties. He hypothesized that the two-phase alloy and the multiphase alloy are better because the single-phase alloy requires a search. Miracle noted that the answer to this question depends on what the researcher wants to achieve: single-phase alloys can be processed at low cost with corrosion resistance, while two-phase alloys have both high strength and a good balance of fracture properties. Because of this, Miracle believes that it is a mistake to focus only on the single-phase solid solutions. Liaw acknowledged Miracle’s point but noted that another benefit of starting with the single-phase HEA is to better understand the deformation mechanism.
Understanding Slips in Stress-Strain Curves
The second panelist, Karin Dahmen, models slip avalanches (i.e., the noise) in stress-strain curves. She presented a series of slides to demonstrate the nonequilibrium behavior of the HEAs. She explained that when a weak spot slips, it triggers other weak spots to slip and weaken in what is called a slip avalanche. This can aid in the prediction and extraction of useful material deformation mechanisms. Slip sizes should increase as temperatures increase, Dahmen noted, but with very high temperatures all of the slips disappear. She found that the experimental data on nanocrystals/microcrystals, bulk metallic glasses, granular materials, rocks, and earthquakes confirmed what her model had predicted. She also noted that these models could be useful in the future as a way to learn more about material microstructure, to improve nondestructive materials testing and failure prediction, and to design better materials less prone to failure. Peter Liaw asked Dahmen if there is a way to normalize her results. She noted that although it is possible with additional data, it has not yet been done. Liaw asked Dahmen if there is a physical meaning associated with the 1.5 prediction exponent. Dahmen said that this represents uncorrelated disorder, which leads to the development of weak spots, each of which has a failure threshold. She concluded that the prediction is independent of the microscopic details.
Daniel Miracle observed that Dahmen’s model is robust and applicable over many orders of magnitude and many materials. He asked how the information can be extracted on the microstructure of materials, for example, since the underlying model is consistent across scales and materials. Dahmen explained that there actually is a difference in the slip behavior of the crystals and the bulk metallic glass (i.e., crystals have small slips, while bulk metallic glass has larger slips). Dahmen noted that her model compared only the small slips; although small slips do not provide information about the microstructure, they can be scaled universally.
Modeling Instabilities
Zi-Kui Liu, the third and final panelist, does both CALPHAD modeling and first-principles calculations and combines the two to design materials and processes. He discussed the relationship between entropy and enthalpy and the strength of CALPHAD modeling and its ability to model phase stabilities of multicomponent systems. Liu explained that multicomponent databases need to be modified and updated with new data; DFT can be useful in the data-collection part of the process, but it remains to be seen how experimenters can use these databases more efficiently.
Liu’s first example relating to the relationship between entropy and enthalpy was about water vapor and liquid water mixing together with increased pressure and temperature to a critical point. At this critical point, entropy change with respect to temperature is infinite. To manipulate the entropy, temperature and pressure can be modified, Liu said. The entropy changes dramatically only when the system is unstable, and this produces a condition in which DFT cannot work. Liu noted that he is currently working on ways to predict the instability point. Considering the water vapor example, the mixture can be modeled as pressure and temperature are steadily increased, and DFT can be used to calculate a large number of states and mix them together. For some HEAs, entropy can actually be made higher than the ideal configurational entropy; this is the impetus for more modeling with multicomponent systems, Liu concluded.
Michael Widom referred to Liu’s discussion of the infinity of the change in entropy over the change in temperature but pointed out that the change in entropy is not necessarily infinite. When taking the derivative of entropy, it is possible that the result is a relatively normal size difference. Widom suggested calculating the fluctuations in energy (related to the derivative of entropy with respect to temperature) using DFT and then running models on increasing system sizes to observe how these fluctuations behave and diverge. However, because DFT is computationally intensive, it would be more efficient to fit empirical models to DFT using software such as the Alloy Theoretic Automated Toolkit.5 Widom noted
___________________
5 See the Alloy Theoretic Automated Toolkit website at https://www.brown.edu/Departments/Engineering/Labs/avdw/atat/, accessed July 13, 2016.
that magnetic and vibrational entropy will play a large role in increasing entropy. It is important to note, he continued, that vibrational entropies tend to cancel one another out when comparing systems, but they are very large on their own and can grow without bound. Liu highlighted the crux of this discussion: when materials are engineered to bring the system close to instability, entropy changes drastically.
Peter Liaw noted that it may be possible to apply this theory of instability to HEAs. He asked if it is possible to study temperature and strain rate, for example. Liu responded that instability, because of its complexity, is a concept not often considered by the simulation community; this is important work for engineering designers to take up. Miracle added that although the shear instability may be attractive from an entropic perspective, from a mechanical properties perspective, it is a nightmare because the material will fall apart. Noam Bernstein, on the other hand, maintained that one will not approach shear instability close enough for the material to fall apart. Liu added that in the single-phase region where the system can be controlled above the critical point and those colossal changes are reversible, the slip avalanche discussed by Dahmen represents one type of local instability where the material does not fall apart.
Advancing High-Entropy Alloy Research
Moderator Haydn Wadley posed a question to the panelists and the participants: Which properties of HEAs will drive applications to move the field forward? Liaw responded that the future of HEA research depends on the applications of HEAs; the science alone is not enough to advance the field. Liaw indicated that coating is an example of a potential HEA application that could increase the efficiency of the oil drill. Wadley added that the toughness exhibited by HEAs could also be useful in creating materials for ballistic protective armor. There is also preliminary research on fatigue, Liaw noted, but more needs to be understood in terms of creep deformation behavior, especially at higher temperatures. Bernstein asked why HEAs have good properties for mechanical applications. In other words, which aspects of the microstructure contribute to the “good” properties? Miracle responded that the mechanical properties of HEAs are not that different from those of conventional alloys. One exception to this, Miracle noted, is the remarkable mechanical fracture toughness of Cantor alloys due to nanotwinning at and below room temperature. Miracle acknowledged that much work remains to be done to optimize the microstructure and processing. Liaw added that nanotwinning is very important for HEAs, not just for fracture toughness but also for fatigue resistance. Studying twinning, Liaw explained, will aid in a better understanding of deformation mechanisms.
Miracle returned to Wadley’s original question about the properties of HEAs that will drive applications forward. He noted that what is most important is the
vastness of compositions, which is more of a characteristic than a property. Since there are millions of HEA compositions, researchers choose what to study at random and then study using the same methods they have always used. Studying just one property at a time is a difficult and time-consuming way to explore such a vast spectrum, Miracle asserted. He suggested that the initial selection process be thought about differently; a high-throughput approach is needed, as are new tools and new computations, to advance the field.
Wadley introduced a second question for the panel and participants to consider: What is the best approach for discovery and optimization of these materials, given their complexity? Miracle said that it is not yet clear which high-throughput experiments are needed to establish a foundation, but he is hopeful that more will be discovered soon; this is an excellent opportunity for university researchers. Liu added that high-throughput computations, DFT in particular, have a few centers in universities, so high-throughput database management is needed. Yellapu Murty, MC Technologies, asked if this is the appropriate time for industry to get involved. Liaw responded that Alcoa is already excited about HEAs; they have purchased high-temperature alloy companies, plan to switch their aluminum research to this area, and view HEAs as a great opportunity. Samsung and some of the automobile companies have also expressed interest in HEAs, Liaw noted. Murty asked when consumers will start to see HEAs used in commercial applications such as airplanes and automobiles. Liaw responded that industry and university communities first need to work together to identify potential applications, which will take some time. Miracle agreed that it is important for industry to work together with academia and government on such technological developments, as researchers in these arenas may not fully appreciate the constraints under which industry operates. Greater collaboration would help lessen the barriers that exist between these communities and focus the talent on the most crucial needs.
Wadley posed his third and final question to the group: Given the many issues with experimental and modeling techniques, is there low-hanging fruit related to modeling methods in which the community could invest to begin to make advancements? Miracle noted that the current models, especially those for modeling entropy, are cumbersome and could stand to be improved in terms of efficiency and effectiveness. Widom agreed with Miracle’s questioning of the accuracy and validity of basic quantum mechanical calculations because they contain uncontrolled errors. There are knowledge and principles that could improve accuracy, but easy-to-use codes are not yet available. Widom noted that developing codes that could convey this knowledge would be helpful, though this is a difficult process and would not be considered low-hanging fruit. Liu added an example of low-hanging fruit: the computational community should improve uncertainty quantification of models, model parameters, and model predictions.
__________________






















