4
Biologic Mechanisms
The committee reviewed all the relevant experimental studies of 2,4-dichlorophenoxyacetic acid (2,4-D), 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), 4-amino-3,5,6-trichloropicolinic acid (picloram), dimethylarsinic acid (DMA, also called cacodylic acid), and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) that have been published since Update 2014 (NASEM, 2016a). The findings have been incorporated into this chapter when it is appropriate and into the biologic-plausibility sections of Chapters 6–11 when they are of consequence for particular health outcomes. For each substance, this chapter includes a review of its toxicokinetic properties, a brief summary of the toxic outcomes investigated in animal experiments, and a discussion of underlying mechanisms of action as illuminated by in vitro studies. The final section of this chapter discusses factors that complicate the extrapolation of findings from laboratory experimentation to humans. Additionally, information about three emerging subjects in molecular and biologic science—epigenetics, developmental immunotoxicology, and oxidative stress—are discussed because they provide insights into the potential mechanisms that could explain biologic responses associated with exposure to the herbicides sprayed in Vietnam.
The establishment of biologic plausibility through laboratory studies strengthens the case for a cause–effect relationship between herbicide exposure and health effects that has been reported in epidemiologic studies. Such information supports the existence of the less stringent relationship of association, which is the target of this committee’s work. Experimental studies of laboratory animals or cultured cells make it possible to observe the effects of herbicide exposure under controlled conditions, which is difficult or impossible to do in epidemiologic studies. The conditions that can be controlled in a laboratory animal study
include genetic differences, the frequency and magnitude of exposure, exposure to other chemicals, dietary intake, preexisting and confounding health conditions, stressors, and the tissues or cells under examination. The limitations of extrapolating results of laboratory studies to human responses is discussed later in this chapter.
Once a chemical contacts the body, it becomes subject to the processes of absorption, distribution, metabolism, and excretion. The combination of those four biologic processes determines the concentration of the chemical in the various tissues and organs in the body and how long each organ or tissue is exposed to the chemical and thus influences its pharmacologic and possibly toxic activity (Lehman-McKeeman, 2013).
The absorption of a substance in an organism can occur through different routes. If ingested, it normally is taken up into the bloodstream from mucous surfaces, such as the intestinal walls of the digestive tract. If inhaled, the substance enters the bloodstream through the alveoli in the lungs. Some compounds can also be absorbed across the skin (dermal adsorption). Animal studies may involve additional routes of exposure that are not ordinarily encountered by humans, such as intravenous or intraperitoneal injection, when a chemical is injected into, respectively, the bloodstream or the abdominal cavity. The route of exposure and other factors influence how much of a chemical dose is absorbed by the organism. For example, the hydrophobicity of a chemical and its solubility in fat influence how much of that chemical is absorbed. Thus, absorption is a critical determinant of a chemical’s bioavailability—that is, the fraction of the chemical that reaches systemic circulation.
Once a chemical is absorbed, it is affected by distribution. This refers to the movement of a substance from the site of entry to the different tissues and organs in the organism. Distribution takes place most commonly via the bloodstream. As the chemical is moved through the body, it may enter a target tissue where it may have its ultimate toxic effect, or it may enter into tissues that sequester it. As a chemical is distributed in the organism, it will also begin to undergo metabolism.
Biotransformation or metabolism is the process by which a foreign substance is chemically modified when it enters an organism. For many environmental toxicants, this process takes place largely in the liver via the action of enzymes, including cytochromes P450, which catalyze the oxidative metabolism of many chemicals. As metabolism occurs, the parent chemical is converted into new chemicals called metabolites, which are often more water-soluble (polar) and thus more readily excreted. When the resulting metabolites are pharmacologically or toxicologically inert, metabolism has deactivated the administered dose of the parent chemical and thus reduced its effects on the body. Metabolism may, however, generate a chemical that is more potent or more toxic than the parent compound. This process is often referred to as bioactivation. A chemical’s structure influences the organism’s ability to metabolize (structurally transform) the
chemical, and this ability ultimately determines whether it persists in the body or is excreted.
Excretion is the removal of substances or their metabolites from the body, most commonly in urine or feces. This is different from elimination, which refers to the disappearance of the parent molecule from the bloodstream. The rate of excretion of a chemical from the body is often limited by the rate of metabolism of the parent chemical into more water-soluble, readily excreted metabolites. Excretion is often incomplete, especially in the case of chemicals that resist biotransformation. Incomplete excretion results in the accumulation of foreign substances that can adversely affect biologic functions.
Elimination is referred to as “first-order” when its rate is directly proportional to the amount of chemical in the body, in which case the chemical’s half-life is independent of dose. A half-life is defined as the time required for the plasma concentration or the amount of a chemical in the body to be reduced by half. The half-life of TCDD in humans is estimated to vary from 0.4 to more than 10 years, based on measurements of TCDD in serum samples; the half-life depends on a number of factors, including a person’s body mass index (BMI), age, sex, and exposure (Aylward et al., 2005a; Emond et al., 2005; Flesch-Janys et al., 1996; Geusau et al., 2002; Kumagai and Koda, 2005; Leung et al., 2006; Michalek et al., 2002; Milbrath et al., 2009; Needham et al., 1994; Pirkle et al., 1989; Sorg et al., 2009). Shorter half-lives were observed in humans during the first months after exposure or in severely contaminated persons, which is consistent with the nonlinear elimination predicted by physiologically based pharmacokinetic models. Several studies of the half-life of TCDD have been conducted in monkeys, mice, and rats, but the estimates are generally shorter than those found in humans, ranging from 8 to 74 days, and are generally proportional to body weight (DeVito and Birnbaum, 1995; Emond et al., 2006; Gasiewicz et al., 1983; Hurst et al., 1998; Koshakji et al., 1984; Minero et al., 2001; Neubert et al., 1990; Viluksela et al., 1996).
Collectively, the routes and rates of absorption, distribution, biotransformation or metabolism, and excretion of a toxic substance make up the toxicokinetics (or the pharmacokinetics for chemicals used as pharmaceutical agents) of the substance. Those processes determine the amount of a particular substance or metabolite that will reach specific organs or cells and the amount of a particular substance that persists in the body. Understanding the toxicokinetics of a chemical is useful for assembling a valid reconstruction of a human exposure. It is also important in assessing the concentration of the active chemical in target tissues, which influences the risk of disease. The basic principles involved in toxicokinetics are similar from chemical to chemical, but the precise way in which principles are applied will depend on the structure and other inherent properties of the particular chemical under consideration. For example, the lipophilicity or hydrophobicity of a chemical influences how long that chemical will persist in the body because those factors influence how much of the chemical is absorbed into
fat cells, metabolized, and excreted. The degree to which different toxicokinetic processes influence the toxic potential of a chemical depends on the metabolic pathways, which often differ among species. Animal and cell culture studies are often conducted at higher exposures and for shorter durations than are typical in human exposures, which can influence biotransformation. For that reason, attempts to extrapolate from experimental animal studies to human exposures must be done extremely carefully.
Many chemicals were used by the U.S. armed forces in Vietnam. The nature of the substances themselves was discussed in detail in Chapter 4 of the original Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam (VAO) report (IOM, 1994). Four herbicides documented in military records were of particular concern in that report and are examined here: 2,4-D; 2,4,5-T; picloram; and cacodylic acid. This chapter also examines TCDD, the most toxic congener of the tetrachlorodibenzo-p-dioxins, also commonly referred to as dioxin, which is a contaminant of 2,4,5-T. Considerably more information is available on TCDD than on the herbicides themselves. Other contaminants present in 2,4-D and 2,4,5-T are of less concern. Except as noted, the laboratory studies of the chemicals of concern used pure compounds or formulations; the epidemiologic studies discussed in later chapters often tracked exposures to mixtures.
PICLORAM
Chemistry
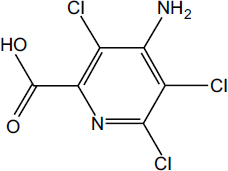
Picloram (Chemical Abstracts Service Number [CAS No.] 1918-02-1; see chemical structure in Figure 4-1) was used with 2,4-D in the herbicide formulation Agent White, which was sprayed in Vietnam. It is also used commonly in Australia in a formulation that has the trade name Tordon 75D®. Tordon 75D contains several chemicals, including 2,4-D; picloram; a surfactant, diethyleneglycolmonoethyl ether; and a silicone defoamer. A number of studies of picloram used such mixtures as Tordon formulations or other mixtures of 2,4-D and picloram that are similar to Agent White.
Toxicokinetics
The original VAO committee reviewed studies of the toxicokinetics of picloram (IOM, 1994). Studies of animals showed a rapid absorption through the gastrointestinal tract and a rapid elimination of picloram in unaltered form in urine. In humans, Nolan et al. (1984) examined the toxicokinetics of picloram in six healthy male volunteers who were given a single oral dose of either 0.5 or 5.0 mg/kg or a dermal dose of 2.0 mg/kg. In the oral study picloram was rapidly absorbed and rapidly excreted unchanged in urine. More than 75% of the dose was excreted within 6 hours, and the remainder with an average half-life of 27 hours. On the basis of the quantity of picloram excreted in urine in the dermal study, the authors concluded that only 0.2% of the picloram applied to the skin was absorbed. Because of its rapid excretion, picloram has low potential to accumulate in humans.
In general, the literature on picloram toxicity continues to be sparse. Studies of humans and animals indicate that picloram is rapidly eliminated as the parent chemical. Studies of animals indicate that picloram has low toxicity even at high doses (IARC, 1991).
Toxicity Profile
The original VAO committee reviewed studies of the carcinogenicity, genotoxicity, acute toxicity, chronic systemic toxicity, reproductive and developmental toxicity, and immunotoxicity of picloram (IOM, 1994). In general, there is some evidence of benign liver carcinogenicity in female Osborne-Mendel rats but picloram was not found to be carcinogenic for either male or female Osborne-Mendel rats or B6C3F1 mice (NCI, 1978). Because of some concern that contaminants in picloram (in particular, hexachlorobenzene) might be responsible for the carcinogenicity, picloram itself has not been established as a chemical carcinogen. Furthermore, studies conducted by the Environmental Protection Agency (EPA, 1988) yielded no evidence that picloram is a genotoxic agent.
Acute Toxicity
Picloram is considered a mild irritant. At high doses, it has produced erythema in rabbits. Some neurologic effects—including hyperactivity, ataxia, and tremors—were reported in pregnant rats exposed to picloram at 750 or 1,000 mg/kg (Thompson et al., 1972). However, the available information on the acute toxicity of picloram is very limited.
Chronic Systemic Toxicity
There is some evidence from chronic experimental animal studies that ingesting high doses of picloram affects the liver. Several studies have reported various effects of technical-grade picloram on the livers of rats. In the carcinogenicity
bioassay conducted by Stott et al. (1990), treatment-related hepatomegaly, hepatocellular swelling, and altered tinctorial properties were noted in the central regions of the liver lobules in groups of rats exposed at 60 and 200 mg/kg per day. Gorzinski et al. (1987) reported a dose-related increase in hepatocellular hypertrophy and changes in centrilobular tinctorial properties in male and female F344 rats exposed for 13 weeks to picloram at 150 mg/kg per day and higher in the diet. In a 90-day study, cloudy swelling in the liver cells and bile duct epithelium occurred in male and female F344 rats given 0.3% or 1.0% technical picloram in the diet (EPA, 1988). No other toxic effects of chronic exposure to picloram have been reported.
Reproductive and Developmental Toxicity
The reproductive toxicity of picloram was evaluated in a two-generation study. No toxicity was detected at the highest dose tested, which was 150 mg/kg per day; however, only a few animals were evaluated (EPA, 1988). Some developmental toxicity was produced in the offspring of pregnant rabbits exposed to picloram by gavage at 400 mg/kg per day on gestation days 6–18. Fetal abnormalities were forelimb flexure, fused ribs, hypoplastic tail, and omphalocele, each occurring in a single litter (John-Greene et al., 1985). Some maternal toxicity was observed at that dose, however, and EPA concluded on the basis of the sporadic nature of the findings that the malformations were not treatment related (EPA, 1988). No teratogenic effects were produced in the offspring of rats given picloram by gavage at up to 1,000 mg/kg per day on gestation days 6–15, but the occurrence of bilateral accessory ribs was significantly increased (Thompson et al., 1972).
Immunotoxicity
Studies of the potential immunotoxicity of picloram have included dermal sensitization in humans and rodent immunoassays. In one study, 53 volunteers received nine 24-hour applications of 0.5 mL of a 2% potassium picloram solution on the skin of both upper arms. Each volunteer received challenge doses 17–24 days later. The particular formulation of picloram (its potassium salt) was not a skin sensitizer or an irritant (EPA, 1988). In a similar study, a 5% solution of picloram (M-2439, Tordon 101 formulation) produced a slight dermal irritation and a sensitization response in 6 of the 69 volunteers exposed. When the individual components of M-2439—picloram, triisopropanolamine (TIPA) salt, and 2,4-D TIPA salt—were tested separately, no sensitization reaction occurred (EPA, 1988). Tordon K+, but not technical-grade picloram, was also found to be a skin sensitizer in guinea pigs (EPA, 1988). CD1 mice exposed to Tordon 202C (94% 2,4-D and 6% picloram) had no consistent adverse effects on antibody responses (Blakley, 1997), but the lack of a consistent response may be due to the fact that CD1 mice are outbred.
Mechanisms
No well-characterized mechanisms of toxicity for picloram are known.
CACODYLIC ACID
Chemistry
Arsenic (As) is a naturally occurring element. It exists in inorganic forms that can be trivalent (As+3 or AsIII) or pentavalent (As+5 or AsV). It can also exist in organic forms that are often methylated. See Figure 4-2 for the chemical structures of selected arsenic-containing compounds. Sodium arsenite, which contains AsIII, is generally considered to be the most toxic of these arsenic compounds. Inorganic arsenic is commonly present in drinking-water sources that are associated with volcanic soils and can reach high concentrations (more than 50 parts per billion). Numerous human health effects have been attributed to drinking water contaminated with inorganic arsenic, particularly bladder, skin, kidney, and lung cancers; developmental effects on learning and memory; reproductive endpoints; skin lesions; and diabetes and vascular diseases (NRC, 2001; WHO, 2018). Inorganic arsenic is readily metabolized in humans and other species into organic forms of arsenic. Although organic forms of arsenic can be converted into inorganic forms by microorganisms in the soil, there is no evidence that this can occur in humans or other vertebrate species (Cohen et al., 2006).
The arsenic in cacodylic acid (CAS No. 75-60-5) is an organic form of arsenic and has a valence of +5. Cacodylic acid (also known by its more standard chemical name, dimethylarsinic acid [DMAV]) was the form of arsenic used in Agent Blue, one of the mixtures used for defoliation in Vietnam. DMAV made up about 30% of Agent Blue. Agent Blue was chemically and toxicologically unrelated to Agent Orange, which consisted of phenoxy herbicides contaminated with dioxin-like compounds. Potential cacodylic acid exposure of Vietnam veterans would have involved direct exposure to exogenous DMAV, rather than exposure to inorganic arsenic, which would have led to the endogenous formation of MMAV and MMAIII and then DMAV. The old hypothesis that methylation of inorganic arsenic was a detoxifying mechanism has been dispelled by newer studies. The direct treatment of laboratory animals with these metabolic products has demonstrated the products to be linked to an increased incidence of cancers and non-cancer health outcomes. However, it is still the trivalent species (AsIII and MMAIII) that appear to be the most toxic. No studies of health effects in humans following direct exposure to DMA have been identified by the VAO committees. If there were, such studies could provide epidemiologic evidence of an association between DMA and health effects. However, most studies have focused on exposure to inorganic arsenic, which human metabolism turns into DMA. Consequently, VAO committees have excluded studies where the source
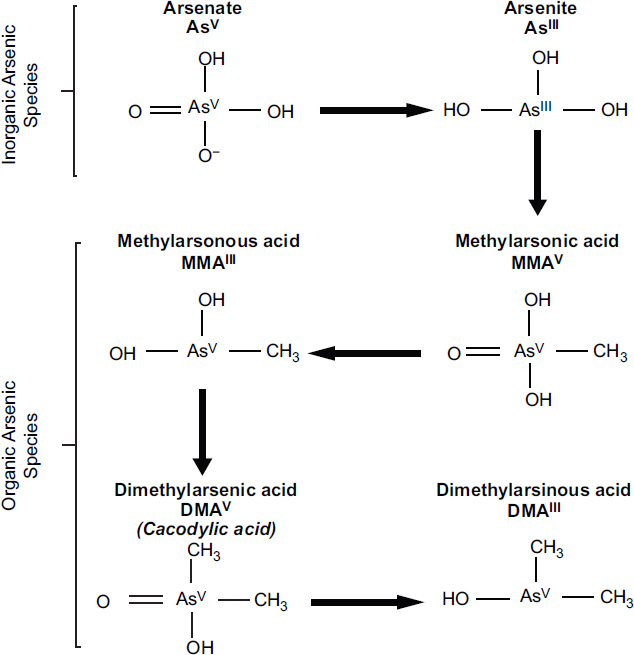
NOTES: Inorganic arsenic (AsV and AsIII) is commonly found in the environment and is metabolized by humans to form methylated organic arsenic species (MMA and DMA). The methylation process is incomplete in humans, and both inorganic and organic arsenic species are excreted in urine when approximately 10% is in inorganic forms (AsV and AsIII), 10–20% is MMA, and 70–80% is DMA. Cacodylic acid, which is the type of arsenic found in Agent Blue, is the same as DMAV and is excreted in urine as DMA.
of exposure was inorganic arsenic and have only considered and reviewed toxicologic studies in which the animals were directly exposed to DMA. Information about effects of inorganic arsenic exposure may be found in reviews by the International Agency for Research on Cancer (IARC, 2012a) and the National Research Council (NRC, 2013).
Toxicokinetics
In humans, metabolism studies have generally found DMAV to be rapidly excreted and mostly unchanged in the urine after an exposure has occurred (Cohen
et al., 2006; S. Suzuki et al., 2010). However, rats differ from most other mammals (including humans) in that a larger percentage of DMAV binds to hemoglobin in their red blood cells, which leads to a considerably longer half-life in blood (Cui et al., 2004; K. T. Suzuki et al., 2004). The binding of DMAV to hemoglobin is 10 times higher in rats than in humans (M. Lu et al., 2004). Chronic exposure of normal rat hepatocytes to DMAV results in decreased uptake and increased excretion, so that over time the rats developed resistance to the cytotoxic effects of DMAV (Kojima et al., 2006). This tolerance is mediated by the induction of glutathioneS-transferase activity and of multiple-drug-resistant protein expression. Recent work by Banerjee et al. (2014, 2016) found that multiple-drug-resistant protein 4 on renal proximal tubules transports DMAV, which facilitates urinary excretion, and that polymorphic variants of this protein can alter the transport activity and change local cellular responses. Rats also appear to biotransform DMAV to DMAIII. Adair et al. (2007) examined the tissue distribution of DMA in F344 rats after they were exposed to DMA for 14 days through drinking water. They found that DMAV was extensively metabolized to trimethylated forms that may play a role in toxicity. In a study of DMA treatment of Wistar rats for 10 weeks, the metabolism to trimethylated forms was far less apparent, and the tissue distribution of DMAV and trimethylated metabolites was strikingly different (S. Liu et al., 2015). Thus, DMA distribution and metabolism may differ according to exposure duration or the strain of rat, or both.
A physiologically based pharmacokinetic model of intravenous and ingested DMAV has been developed on the basis of mouse data (Evans et al., 2008). Similar models have been developed for humans on the basis of exposure to inorganic arsenic (El-Masri and Kenyon, 2008), but these models have limited relevance for assessing potential harm to Vietnam veterans, who are presumed to have been directly exposed to DMAV.
Although epidemiologic studies of direct exposure to DMAV are not available, investigations into the relationship between health outcomes and the metabolic profiles of humans exposed to inorganic arsenic provide some insight into the roles of the individual metabolites in producing adverse outcomes. An increased incidence of urothelial cancer (cancer of the bladder, kidney, renal pelvis, ureter, and urethra) among people exposed to high levels of inorganic arsenic in drinking water was found in those who generate more MMA and less DMA endogenously (S. K. Huang et al., 2008). Also, a lower risk of arsenical skin lesions was associated with evidence of higher arsenic methylation capacity in people in areas of high arsenic exposure via the drinking water (Q. Zhang et al., 2014a,b) as well as in smelter workers (Wen et al., 2012). These results could suggest that elevated cumulative levels of urinary MMA may be causally associated with an increased risk of inorganic arsenic-induced adverse health outcomes, but they could also imply that complete methylation of inorganic arsenic to DMA and resulting enhanced excretion are relatively protective.
Toxicity Profile
This section discusses the toxicity associated with organic forms of arsenic, most notably DMAV because it is the active ingredient in Agent Blue. The toxicity of inorganic arsenic is not considered to be relevant to veteran exposures to Agent Blue.
Neurotoxicity
Kruger et al. (2006) found that both DMAIII and DMAV significantly attenuated neuronal ion currents through N-methyl-D–aspartate receptor ion channels, whereas only DMAV inhibited ion currents through α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. The data suggest that DMA may have neurotoxic potential.
Immunotoxicity
Previous studies have shown that a low concentration of DMAV (10–7 M) could increase the proliferation of human peripheral blood monocytes after their stimulation with phytohemagglutinin, whereas it took a high concentration (10–4 M) to inhibit the release of interferon-γ. This suggested that different concentrations of DMAV may produce various immunomodulatory effects (Di Giampaolo et al., 2004).
Skin Toxicity
In an evaluation of the effects of topical exposure of pregnant mice to DMA (valence not stated) on the skin of the dams and offspring (E. Kim et al., 2012), no effects were observed in offspring, but the exposure did increase skin thickness in the area of application and alter the expression of multiple apoptosis-related genes (Bcl-2, Bad, Casp-12). The results suggested that transient DMA exposure can be a skin irritant and produce dermatitis.
Genotoxicity and Carcinogenicity
DMAIII and DMAV are genotoxic and increase oxidative stress and cause DNA damage, particularly aneuploidy, but they are poor mutagens (Rossman and Klein, 2011). Gómez et al. (2005) demonstrated that DMAIII induces a dose-related increase in DNA damage and oxidative stress in Jurkat cells. DMAIII was considerably more potent than DMAV in inducing DNA damage in Chinese hamster ovary cells (Dopp et al., 2004), which was associated with a greater uptake of DMAIII into the cells. Similarly, an analysis of arsenical dimethylated metabolites in human bladder cancer cells found dimethylmonothioarsinic acid
(DMMTAV) and DMAIII to be more toxic and DMAV to be less toxic in terms of DNA damage (Naranmandura et al., 2011). DNA damage from DMMTAV was shown to be related to the accumulation of reactive oxygen species and to the down-regulation of p53 and p21 (DNA repair proteins); these processes were mediated in part through the intracellular conversion of DMMTAV to DMAV and DMAIII (Naranmandura et al., 2011). Thus, although extracellular DMAV has little toxic effect in cells because of its low uptake, intracellular DMAV can be highly toxic. Gene-expression profiling of bladder urothelium after chronic exposure to DMAV in drinking water showed significant increases in genes that regulate oxidative stress (Sen et al., 2005). Additionally, hepatic gene-expression profiling showed that DMAV exposure induced changes consistent with oxidative stress (Xie et al., 2004). In vivo, DMAV-induced proliferation of the urinary bladder epithelium could be attenuated with the antioxidant N-acetylcysteine (M. Wei et al., 2005). Arsenicals, including DMA, also interfere with certain DNA repair mechanisms (both base- and nucleotide-excision repair) and may thereby act as co-carcinogens enhancing the effect of other genotoxic carcinogens (Rossman and Klein, 2011). In fact, MMAIII is a stronger inhibitor of nucleotide-excision repair than inorganic arsenic (Shen et al., 2008).
Toxicological data suggest DMAIII and DMAV are carcinogenic. Cancers have been induced in the urinary bladder, kidneys, liver, thyroid glands, and lungs of laboratory animals exposed to high concentrations of DMA. In a 2-year bioassay of F344/Crl rats fed a diet containing 40 or 100 parts per million (ppm) DMAV, the females consuming the highest dose (100 ppm) developed urothelial carcinomas and papillomas in the bladder, and males and females at both dose levels (40 and 100 ppm) developed hyperplastic non-neoplastic changes in the bladder (Arnold et al., 2006). M. Wei et al. (2002) exposed male F344/DuCrj rats to DMA (valence not stated) via their drinking water and found statistically significant incidences of bladder hyperplasia and transitional cell papillomas and carcinomas at doses of 50 and 100 ppm. Similarly, A. Wang et al. (2009) found that exposure of F344 rats to DMAV in drinking water at 1, 4, 40, or 100 ppm resulted in a change in the urinary bladder epithelium, but there were no changes in DNA repair capacity. In another study, Cohen et al. (2007b) exposed F344 rats to DMAV in the diet for 2 years and found an increase in bladder tumors in those receiving 100 ppm. As noted above, at least some strains of rats can reduce DMAV to DMAIII, and thus these researchers proposed that that trimethylated forms of arsenic (MMAIII and DMAIII) may be responsible for bladder cancer in rats. Direct intravesical administration of 90 mg/kg DMAV to female adult rats resulted in increased bromodeoxyuridine labeling in urothelial cells, indicating DNA damage; weak neutrophil infiltration; and the proliferation of urothelial epithelium mediated through modest increases in oxidative-stress indexes (Takahashi et al., 2011). Increased urothelial cell proliferation was also found following DMA (valence not stated) exposure via the drinking water (M. Wei et al., 2002). It is noteworthy that co-treatment with an antioxidant, N-acetylcysteine, worsened
the DMAV-induced bladder injury rather than ameliorating it as expected. This suggests that the carcinogenic mechanism of DMAV is more complicated than a simple production of oxidative stress. In the mouse lung, DMAV acts as a tumor initiator (Yamanaka et al., 2009) and as a tumor promoter (Mizoi et al., 2005). DMAV can also act as a complete carcinogen, inducing lung tumors in susceptible strains of mice, including those with deficient DNA-repair activity (Hayashi et al., 1998; Kinoshita et al., 2007). In F344/DuCrj rats treated with a mixture of carcinogens for 4 weeks, subsequent exposure to DMA (no indication of whether this was DMAIII or DMAV) via their drinking water for 24 weeks caused tumor promotion in the urinary bladder, kidney, liver, and thyroid gland but inhibited the induction of tumors of the nasal passages (S. Yamamoto et al., 1995). In a similarly designed experiment, DMA (valence not stated) was found to be a bladder tumor promotor after treatment with the bladder carcinogen N′-butyl-N′-(4-hydroxybutyl) nitrosamine (Wanibuchi et al., 1996). Yamanaka et al. (2009) suggested that DMAIII can act as a tumor promoter through the formation of a DMAIII radical after the reduction of DMAV. Studies have also found that an oral exposure of adult mice to 200 ppm DMAV in addition to fetal inorganic arsenic exposure can act as a promoter of renal and hepatocellular carcinoma, markedly increasing tumor incidence beyond that produced by the fetal arsenic exposure alone (Tokar et al., 2012). These findings emphasize how multiple life events can contribute to an adverse health outcome in which adult DMAV exposure triggered an otherwise dormant disease.
Mechanisms
It is believed that arsenic exerts its toxicity through several different mechanisms. Anwar-Mohamed et al. (2014) found that DMAV and other pentavalent arsenic metabolites induce the carcinogen-activating enzyme CYP1A1. There is also considerable evidence that arsenic induces oxidative stress, which can induce cancers in animals. Ebert et al. (2016) found that thio-DMAV exposure upregulated DNA repair and damage response proteins in human urothelial cells, and studies have shown that mice that are deficient in enzymes associated with the repair of oxidative DNA damage are highly susceptible to the induction of tumors, particularly lung tumors, by DMAV (Kinoshita et al., 2007). Some studies have also suggested that methylated arsenicals (MMAIII and DMAIII) can induce aneuploidy in mammalian cells at concentrations below those required to produce oxidative stress after in vitro exposure (Kligerman and Tennant, 2007; Rossman and Klein, 2011). The chemical reaction of arsenicals with thiol groups in sensitive target tissues, such as red blood cells and kidneys, may also be a mechanism by which organic arsenicals act (Naranmandura and Suzuki, 2008).
Cohen et al. (2007b) postulated that regenerative cell proliferation in response to cytotoxicity in the urothelium is a major factor in the carcinogenicity of DMA to the rat bladder, and, indeed, urothelial cell proliferation has been
shown to increase following DMA exposure (Takahashi et al., 2011; M. Wei et al., 2002). There may also be epigenetic effects involved in DMA carcinogenicity, as suggested by a study in humans in which there was a significant association between global DNA methylation and urinary DMA levels (Tellez-Plaza et al., 2014). Unterberg et al. (2014) observed DNA hypomethylation in urothelial cells that had been incubated for 21-days with inorganic AsIII or thio-DMAV.
The variation in the susceptibility of various animal species to tumor formation caused by inorganic and organic arsenic is thought to arise in large part from differences in metabolism and distribution. Thus, genetic differences may play an important role. Numerous investigators have examined potential human susceptibility factors and gene polymorphisms that may increase a person’s risk of cancer and other diseases induced by arsenicals (Aposhian and Aposhian, 2006; Hernandez et al., 2008; S. K. Huang et al., 2008; Y. K. Huang et al., 2008; McCarty et al., 2007; Meza et al., 2007; Steinmaus et al., 2007, 2010), but no polymorphisms that may contribute to a person’s susceptibility to DMA-induced cancers or tissue injury have yet been identified.
Summary
DMA is genotoxic and carcinogenic in certain animal models and in vitro assays, including studies in human cells. It is believed to exert a direct toxic effect by generating oxidative stress, inducing epigenetic dysregulation, binding to thiols, and interfering with DNA repair mechanisms. These mechanisms of action may be involved in gene damage and carcinogenesis. However, it is not clear whether these effects would also occur in humans directly exposed to DMAV.
PHENOXY HERBICIDES: 2,4-DICHLOROPHENOXY ACID AND 2,4,5,-TRICHLOROPHENOXYACETIC ACID
Chemistry
2,4-D (CAS No. 94-75-7) is an odorless crystalline powder that, when pure, is white in color (see Figure 4-3); it may appear yellow when phenolic impurities

are present. The melting point of 2,4-D is 138° C, and the free acid is corrosive to metals. It is soluble in water and in a variety of organic solvents (such as acetone, alcohols, ketones, ether, and toluene). 2,4,5-T (CAS No. 93-76-5) is an odorless, white to light-tan solid with a melting point of 158° C. 2,4,5-T is noncorrosive and is soluble in alcohol and water. It reacts with organic and inorganic bases to form salts and with alcohols to form esters.
Uses of 2,4-D and 2,4,5-T
2,4-D has been used commercially in the United States since World War II to control the growth of broadleaf plants and weeds on range lands, lawns, golf courses, forests, roadways, parks, and agricultural land; it remains a widely used herbicide that is approved for use by both the European Union and EPA. Formulations include 2,4-D amine and alkali salts and esters, which are mobile in soil and readily absorbed through the leaves and roots of many plants. Like 2,4-D, 2,4,5-T was developed and marketed as a herbicide during World War II. However, the registration for 2,4,5-T was canceled by EPA in 1978 when it became clear that it was contaminated with TCDD during the manufacturing process. It is recognized that the production of 2,4-D also involves the generation of some contaminants with dioxin-like activity, but the fraction of TCDD represented in the toxic equivalent (TEQ) is comparatively very small.
The herbicidal properties of 2,4-D and 2,4,5-T are related to the chemical’s ability to mimic the plant growth hormone indole acetic acid. They are selective herbicides in that they affect the growth of only broadleaf dicots (which include most weeds) and do not affect monocots, such as wheat, corn, and rice.
Toxicokinetics
Several studies have examined the absorption, distribution, metabolism, and excretion of 2,4-D and 2,4,5-T in animals and humans. Data on both compounds are consistent among species and support the conclusion that the absorption of oral or inhaled doses is rapid and complete. One study indicates that 2,4-D can bind to innate intestinal, intracellular lipid-binding proteins, which may be how these compounds move through columnar absorptive epithelial cells from the intestines to systemic distribution (Carbone and Velkov, 2013). Absorption through the skin is much lower but may be increased with the use of sunscreens or alcohol (Brand et al., 2002; Pont et al., 2004). After absorption, 2,4-D and 2,4,5-T are distributed widely in the body but are eliminated quickly, predominantly in an unmetabolized form in urine (Sauerhoff et al., 1977), but 2,4,5-trichlorophenol and 2,4-dichlorophenol have been identified as trace metabolites in urine. The half-life of single doses of 2,4-D or 2,4,5-T in humans has been estimated to be about 18–23 hours and is highly dependent on urinary pH (Gehring et al., 1973; Kohli et al., 1974; Sauerhoff et al., 1977;
WHO, 1984). Hines et al. (2003) found that concentrations of 2,4-D and its metabolites in the urine of herbicide applicators—that is, those who apply the herbicides—were consistent with 2,4-D urinary half-life estimates of 13–40 hours in humans.
Toxicity Profile
The toxicity database on 2,4-D is extensive, whereas the available data on the toxicity of purified 2,4,5-T, independent of its contamination by TCDD, are sparse. TCDD is much more toxic than 2,4,5-T, and much of the toxicity that had been attributed to 2,4,5-T in early studies was later shown to be caused by the TCDD contaminant. The following summary therefore focuses on 2,4-D toxicity, and information on pure 2,4,5-T is added when it is available.
A 2018 monograph from the IARC classified 2,4-D as possibly carcinogenic to humans. This statement originated from limited evidence available on the carcinogenic effects in animals and inadequate evidence in humans (IARC, 2018). When reviewing animal literature, studies show that after a single oral dose, 2,4-D is considered to produce moderate acute toxicity with a dose lethal to 50% of exposed animals (LD50) of 375 mg/kg in rats, 370 mg/kg in mice, and from less than 320 to 1,000 mg/kg in guinea pigs. Rats and rabbits have dermal LD50s of 1,500 mg/kg and 1,400 mg/kg, respectively. 2,4,5-T itself also produces moderate acute toxicity, with oral LD50s of 389 mg/kg in mice and 500 mg/kg in rats. Death from acute poisoning with 2,4-D or 2,4,5-T has been attributed to the ability of the chemicals to uncouple oxidative phosphorylation, a vital process used by almost all cells in the body as the primary means of generating energy. After an animal is exposed to a high dose, death due to multiple organ failure can occur rapidly. Studies in rats, cats, and dogs indicate that the central nervous system is the principal target organ for acute 2,4-D toxicity in mammals and suggest that the primary site of action is the cerebral cortex or the reticular formation (Arnold et al., 1991; Dési et al., 1962a,b). Based on case reports, in humans the predominant effect of acute inhalation and oral exposure to 2,4-D is neurotoxicity; symptoms include stiffness of the arms and legs, lack of coordination, lethargy, anorexia, stupor, and coma. 2,4-D is also an irritant of the gastrointestinal tract that causes nausea, vomiting, and diarrhea.
Chronic exposure to 2,4-D at relatively high concentrations has been shown to produce a variety of toxic effects, including hepatic and renal toxicity, neurotoxicity, and hematologic changes. A no-observed-effect level of 2,4-D of 1 mg/kg was identified for renal toxicity in rats (Hazleton Laboratories America, 1986). Exposure to 2,4-D was associated with reduced survival and decreased growth rates in offspring of mothers fed high doses during pregnancy; these doses were also associated with maternal toxicity (Munro et al., 1992). Charles et al. (2001) found that 2,4-D did not affect fertility or produce teratogenic effects in the offspring of rats or rabbits given doses lower than 90 mg/kg/day by gavage, which did, however, cause overt
maternal toxicity. A one-generation study in which rats were fed diets containing 2,4-D (females: up to 40 mg/kg/day; males: up to 45 mg/kg/day) from 4 weeks before breeding through 3 weeks of lactation confirmed these results and furthermore found that even at the highest exposure there is no evidence of interaction with the androgen, estrogen, or steroidogenesis pathways in the pups (Marty et al., 2013). Other studies, however, suggest that exposure to 2,4-D does have an impact on the male reproductive system (Alves et al., 2013; Joshi et al., 2012). Alves et al. (2013) found that the exposure of rat Serotoli cells in culture at 10 μM 2,4-D resulted in alterations in cellular metabolism linked to effective spermatogenesis, which could be a mechanism that would reduce sperm density. Mazhar et al. (2014) treated pregnant rats by gavage on gestation days 1–19 with 100 mg/kg/day of 2,4-D alone or administered with 100 mg/kg/day of the antioxidant vitamin E. Morphological and skeletal defects and low birth weight were observed in the fetuses of dams treated with only 2,4-D, but not in those whose mothers were also treated with vitamin E, thereby suggesting that 2,4-D exposure elicits fetotoxicity through inducing oxidative stress. In vitro exposure of human erythrocytes to 2,4-D caused changes in antioxidant enzyme activity as well as increased protein carbonyls, indicating induction of oxidative stress (Bukowska, 2003). Similar changes were observed in the liver of exposed rats (Tayeb et al., 2010, 2013). The immunotoxicity of 2,4-D has been reported in a small number of studies, including a few studies of 2,4-D applicators showing both immunosuppression (Faustini et al., 1996) and immunostimulation (Figgs et al., 2000; Holland et al., 2002). At high doses that produced clinical toxicity in experimental animals, a suppression of the antibody response was observed, whereas other measures of immune function were normal. The immunotoxicity of 2,4,5-T has not been evaluated in laboratory animals.
The carcinogenicity of 2,4-D and 2,4,5-T has been studied in rats, mice, and dogs after exposure in their food, direct placement in their stomachs, or exposure on their skin. Early studies in mice (NTIS, 1968) and rats (W. H. Hansen et al., 1971) found little to suggest tumor induction when animals were treated with 2,4-D by gavage or subcutaneously. Hazelton Laboratories of America (1986, 1987) conducted a series of studies in rats and mice, which all had negative results except one that found an increased incidence of brain tumors in male rats—but not female rats—that received the highest dose of 45 mg/kg/day 2,4-D in their feed. The occurrence of malignant lymphomas in dogs kept as pets was reported to be higher when owners reported that they used 2,4-D on their lawns than when they did not (Hayes et al., 1991, 1995), but a detailed reanalysis did not confirm this finding (Kaneene and Miller, 1999). A controlled study that used dogs exposed to 2,4-D in the laboratory had negative results. Timchalk (2004) suggested that dogs are not relevant for comparative evaluation of human health risk attributable to 2,4-D exposure because they excrete 2,4-D less efficiently than rats or humans. Given the degree of variability observed in humans (Hines et al., 2003), however, the canine information might be applicable for some people. 2,4-D is not metabolized to reactive intermediates capable of interacting with
DNA, and the evidence supports the conclusion that 2,4-D is not a genotoxic carcinogen. However, Sandal and Yilmaz (2011) found that lymphocytes from smokers show genotoxic damage after exposure to 2,4-D, whereas lymphocytes from non-smokers do not. In the Agricultural Health Study (AHS), Andreotti et al. (2015) found that a cumulative metric of 2,4-D exposure in pesticide applicators was associated with shorter relative telomere length in leukocytes. Although 2,4-D may not be a carcinogen, it may have some role influencing the activity of known carcinogens or potentially carcinogenic changes within a cell.
Genetic Studies
In recent years, a number of investigators have used telomere length as a sensitive marker of exposure to a variety of chemical pollutants. Telomere length generally decreases with age and the assumption is that the activation of many different biochemical pathways affects telomere maintenance and repair. The alterations in these pathways can in turn either reduce or increase telomere length, depending on which factors they affect. Since Update 2014, two large studies of telomere length have examined the effects of exposure to the chemicals of interest (COIs).
Andreotti et al. (2015) studied 568 cancer-free male subjects from the AHS who were between 31 and 94 years, taking blood samples from them between 2006 and 2008. Relative telomere length in DNA from blood was examined for associations with self-reported cumulative pesticide use of 57 types, with exposure data collected at enrollment (1993–1997) and in two follow-up telephone questionnaires (1998–2003, 2005–2008). The researchers used three metrics of pesticide use (ever use, lifetime days, and intensity-weighted lifetime days), divided into tertiles and applied multivariable linear regression analysis to examine associations, adjusting for age at blood-draw and the use of other pesticides. Further adjustments were made for potential confounding from the use of other pesticides. The analysis showed an association between blood telomere shortening, independent of age, and the cumulative use of 2,4-D in lifetime days (p < 0.01) for ever versus never used, but is borderline significant when using lifetime intensity-weighted days (p < 0.05). Similar effects had previously been observed for buccal relative telomere length in a subset of 40 AHS subjects, but their sample buccal and blood relative telomere length values were uncorrelated. A significant trend (p < 0.001) was shown for categorical 2,4-D lifetime use (low, medium, high) (Andreotti et al., 2015); however, the 2,4-D never used category had substantially longer telomeres than any other group in the study and some of the other chemicals were shown to lead to longer telomeres and this could confound analysis. Thus, relative telomere length might show an effect with cumulative 2,4-D exposure in blood leukocytes, but the mechanisms of action and consequences for disease are unknown.
2,3,7,8-TETRACHLORODIBENZO-P-DIOXIN
Chemistry
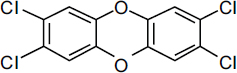
TCDDs are polychlorinated dibenzo-p-dioxins that have a triple-ring structure consisting of two benzene rings connected by an oxygenated ring with four attached chlorine atoms; in the case of the dioxin congener of greatest concern, 2,3,7,8-TCDD (commonly called simply TCDD), the chlorine atoms are attached at the 2, 3, 7, and 8 positions of the benzene rings (see Figure 4-4). The chemical properties of TCDD include a molecular weight of 322, a melting point of 305–306°C, a boiling point of 445.5°C, and a log octanol–water partition coefficient of 6.8 (National Toxicology Program substance profile). It is very lipophilic, or fat soluble; is virtually insoluble in water (19.3 ng/L); and is soluble in organic solvents, such as benzene and acetone. It has been suggested that the volatilization of dioxin from water may be an important mechanism of transfer from the aqueous to the atmospheric phase (EPA, 2004); however, because of its very low water solubility, most TCDD is bound to sediments and particulate matter.
Toxicokinetics
The disposition of TCDD (which includes its absorption, distribution, biotransformation, and excretion) has been extensively studied in humans and a number of other animal models over the past 30 years. Given the plethora of data, this section highlights and summarizes only key findings. TCDD is absorbed into the body rapidly but is eliminated slowly. Because it is very lipophilic, resistant to biotransformation, and slowly eliminated, the concentration of TCDD in the lipid fraction of blood serum is thought to be in dynamic equilibrium with that in the lipid fraction in other tissue compartments. Thus, the lipid-adjusted blood serum concentration of TCDD is used to estimate total body burdens; however, at high TCDD concentrations, the liver sequesters some of the dioxin, so a lipid adjustment that ignores the hepatic fraction would underestimate the total body burden. The exposure of humans to TCDD is thought to occur primarily via the mouth, skin, and lungs. In laboratory animals, oral administration of TCDD has been shown to result in absorption of 50–93% of the administered dose (Nolan et al., 1979; Rose et al., 1976). Similarly, a study performed in a 42-year-old man found that 87% of the oral dose was absorbed (Poiger and Schlatter, 1986). Dermal absorption appears to be dose-dependent: lower absorption occurs at
higher doses (Banks and Birnbaum, 1991). In vitro studies of tissues isolated from humans indicate that intact human skin may not be readily penetrable (Weber et al., 1991). The varied and complex environmental matrices make environmental exposures difficult to quantify. Animal studies have demonstrated that the presence of soil or lipophilic agents dramatically reduces dermal absorption of TCDD: application in an activated carbon–water paste essentially eliminates absorption in contrast with the absorption of the pure compound dissolved in solvents. The oral bioavailability of TCDD and related compounds also depends on the matrix: contaminated breast milk and food products have much higher bioavailability than soil-bound or sediment-bound TCDD, and activated carbon essentially blocks oral bioavailability (Olson, 2012).
After ingestion and gastrointestinal absorption, TCDD associates primarily with the lipoprotein fraction of the blood and later partitions into the cellular membranes and tissues (Henderson and Patterson, 1988). TCDD is distributed to all compartments of the body; the amounts differ from organ to organ, but most studies indicate that the primary distribution of TCDD is in the liver and adipose tissues. For example, in a human volunteer it was found that 135 days after ingestion, 90% of TCDD was in fat (Poiger and Schlatter, 1986), and TCDD persists in adipose tissue in the rhesus monkey (Bowman et al., 1989). The specific details of the distribution and elimination of TCDD depend on the tissue examined, the time that has elapsed since exposure, total exposure, and other factors. For example, the concentration of cytochrome P450 1A2 (CYP1A2) in the liver is increased by TCDD (Poland et al., 1989). Direct binding of TCDD to CYP1A2 is thought to result in the sequestration of TCDD in the liver and to inhibit its distribution to other tissues. The importance of CYP1A2 concentrations for the toxic actions of TCDD has also been demonstrated in several laboratory situations; for instance, CYP1A2-knockout mice were more susceptible than wild-type mice to TCDD immunotoxicity (Smialowicz et al., 2008), and maternal hepatic CYP1A2 was found to sequester TCDD and protect mouse fetuses against TCDD-induced teratogenesis (Dragin et al., 2006). In addition, the distribution of TCDD is age dependent, as shown by studies in which young animals displayed the highest concentration of TCDD in the liver and aged animals the highest concentrations in kidneys, skin, and muscle (Pegram et al., 1995). Finally, the rate of elimination of TCDD, particularly after low exposures, depends heavily on the amount of adipose tissue mass (Aylward et al., 2005a; Emond et al., 2005, 2006).
In laboratory animals, TCDD is metabolized slowly. It is eliminated primarily in feces as both the parent chemical and its more polar metabolites. However, elimination appears to be dose dependent; at low doses, about 35% of the administered dose of TCDD was detected in the feces, while at higher doses, about 46% was observed (Diliberto et al., 2001). The dose-dependent occurrence of TCDD metabolites in the feces is thought to be due to the increased expression of metabolizing enzymes at higher doses and to hepatic sequestration, which makes dioxins more available for metabolism. Milbrath et al. (2009) conducted
a comprehensive review of studies that reported the congener-specific elimination rates of TCDD and related compounds and analyzed the relationships between the apparent half-lives of the compounds as a function of age, body fat, smoking status, and breastfeeding. In infants (less than 2 years old), the compounds have a reported half-life of 0.4 year (Leung et al., 2006), and in adults a half-life of 7.2 years, based on the regression method used by Flesch-Janys et al. (1996) (Milbrath et al., 2009). Aging results in an increase in and redistribution of body fat and lipophilic chemicals that alters their rate of elimination (Van der Molen et al., 1996). Human studies of the Ranch Hand cohort have consistently found a similar relationship between an increasing half-life of TCDD and an increasing BMI (Michalek and Tripathi, 1999; Michalek et al., 1992, 1996). Smoking and breastfeeding are associated with promoting the elimination of TCDD and, in the case of breastfeeding, exposing infants through breast milk. Polycyclic aromatic hydrocarbons (PAHs) in cigarette smoke are capable of inducing CYP1A1, 1A2, and 1B1, which in turn may increase the rate of metabolism and subsequent elimination of TCDD. Smoking has been associated with a 30% decrease in TCDD plasma half-life (Flesch-Janys et al., 1996). Update 2014 (NASEM, 2016a) includes a table (4-1; pp. 95–96) summarizing the results of studies of TCDD half-life in humans and animals.
Special Case of the Poisoning of Victor Yushchenko
In 2004 Victor Yushchenko, a candidate for the presidency of the Ukraine, was poisoned with TCDD. It led to severe chloracne and a blood serum TCDD concentration of 108,000 ppt (pg/g lipid), which was about 50,000 times as great as that in the general population. The incident provided an unfortunate opportunity to assess the toxicokinetics of TCDD after a single large exposure. Serum and fat analysis of TCDD supports the first-order elimination half-life of 15.4 months in Yushchenko, and the similarity in the decay curves of serum lipids and subcutaneous fat confirmed that TCDD was in equilibrium between the two (Sorg et al., 2009). The elimination half-life of 15.4 months is much shorter than the 7.2-year reference half-life reported by Milbrath et al. (2009) and supports a dose-dependent elimination of TCDD, which is associated with the induction of potential TCDD-metabolizing enzymes (CYP1A1, 1A2, and 1B1) in very high TCDD exposures. Two metabolites of TCDD (2,3,7-trichloro-8-hydroxydibenzo-p-dioxin and 1,3,7,8-tetrachloro-2-hydroxydibenzo-p-dioxin) were detected in Yushchenko’s feces, serum, and urine but not in his fat or skin. Over a 12-month period, about 38% of the TCDD-derived material was eliminated as metabolites (95% in feces, 5% in urine) and 62% as parent chemical. The metabolite-to-TCDD ratio in the blood serum was about one-fiftieth of that in the feces; this supports the conclusion that the metabolites were not originally ingested with TCDD (Sorg et al., 2009). The very slow metabolism of TCDD has been previously reported in laboratory animal models (Gasiewicz et al., 1983; Olson, 1986;
Olson et al., 1980; Poiger and Schlatter, 1979) and in humans (Wendling et al., 1990). It is also noteworthy that the structures of the human metabolites are the same as previously reported in the rat and dog (Poiger et al., 1982; Sawahata et al., 1982). A continued analysis of Yushchenko’s condition has revealed putative metabolomic and transcriptomic biomarkers that may prove useful for predicting health effects in populations with significant TCDD exposures (Jeanneret et al., 2014; Saurat et al., 2012).
In light of the variables discussed above and the effect of differences in physiologic states and metabolic processes, which can affect the mobilization of lipids and possibly of the compounds stored in them, complex physiologically based pharmacokinetic models have been developed to integrate exposure dose with organ mass, blood flow, metabolism, and lipid content in order to predict the movement of toxicants into and out of each organ. A number of modeling studies have been performed in an effort to understand the relevance of animal experimental studies to the exposures that occur in human populations (Aylward et al., 2005a,b; Beaudouin et al., 2010; Emond et al., 2005).
Toxicity Profile
Effects on Tissues and Organs of Laboratory Animals
The effects of TCDD in laboratory animals have been observed in several species (rats, mice, guinea pigs, hamsters, monkeys, cows, and rabbits) after the administration of a variety of doses and after periods that represent acute exposures (less than 24 hours), sub-chronic exposures (1 day–3 months), and chronic exposure (more than 3 months). Some differences have been observed between species, particularly with respect to the degree of sensitivity, but in general the effects observed are qualitatively similar. Some differences in sensitivity have been observed within rodent species, and has been found to be related to species-specific aryl hydrocarbon receptor (Ahr)1 AHR polymorphisms (Ema et al., 1994; Pohjanvirta et al., 1998). Relatively high exposures of TCDD affect a variety of organs and result in organ dysfunction and death. The lethal toxicity of TCDD varies widely among animal species; the oral LD50 of the chemical varies from 1 μg/kg in guinea pigs to 5,000 μg/kg in hamsters. The developing fetus, however, is especially vulnerable to TCDD exposure, and there is only about a 10-fold variability in fetal lethal potency among these species (Kransler et al., 2007; Peterson et al., 1993; Poland and Knutson, 1982). One characteristic of TCDD exposure is a wasting syndrome that includes the loss of adipose and muscle tissue and severe weight loss, but the specific mechanisms of lethality remain unknown. Hwang et al. (2016) suggested that mitochondrial dysfunction caused by Ahrmediated, TCDD-induced toxicity contributes to wasting syndrome and metabolic
___________________
1 The aryl hydrocarbon receptor is denoted as AHR when in human cells and Ahr in rodent models.
dysfunction. In most rodents, exposure to TCDD leads to hepatic enlargement, the presence of hepatic lesions, and impaired hepatic function (Harrill et al., 2016); work by X. Cheng et al. (2014) suggest a role for TCDD-AHR acting on fibroblast growth factor 21 as a contributor to liver effects. The thymus is also sensitive. Recent work by Diani-Moore et al. (2017) in a chick-embryo model found poly(ADP-ribose) polymerase activation and resulting NAD+ loss offers a possible unifying mechanism for the effects of TCDD exposure such as wasting, hepatosteatosis, and thymus atrophy. Finally, in both humans and nonhuman primates, TCDD exposure results in chloracne and associated dermatologic changes. As will be discussed in more detail in Chapters 6–11, studies performed in animal models have indicated that exposure to TCDD adversely affects the heart, the skin, and the immune, endocrine, and reproductive systems and increases the incidence of cancers of the liver, skin, thyroid, adrenal cortex, hard palate, nasal turbinates, tongue, and respiratory and lymphatic systems (ATSDR, 1998; Barouki et al., 2012; Birnbaum, 1994; Huff et al., 1994; Knerr and Schrenk, 2006; Tian et al., 2015). When TCDD has been administered to pregnant animals, birth defects—such as cleft palate, malformations of the reproductive organs of male and female progeny, and abnormalities in the cardiovascular, pulmonary, endocrine, skeletal, and nervous systems—have been observed. Of course, effects arising from perinatal exposure are not in question for Vietnam veterans themselves, but this activity is of concern with respect to their offspring. The developmental origins of health and disease are discussed in more detail in Chapter 8.
Effects on Enzymes, Hormones, and Receptors in Laboratory Animals and Cultured Cells
In addition to adversely affecting the ability of specific organs to fulfill their normal physiologic roles, TCDD has been found to alter the function and expression of essential proteins, particularly a number of enzymes. The enzymes that are most affected by TCDD are ones that act on or metabolize xenobiotics and hormones, often by changing the chemicals’ polarity (water solubility) and thus promoting the elimination of the metabolites. Among the enzymes affected by TCDD, the best studied is CYP1A1, which metabolizes some xenobiotics. In laboratory animals, exposure to TCDD commonly results in an increase in CYP1A1 in most tissues; CYP1A1 therefore is often used as a marker of TCDD exposure. Related enzymes whose levels are also increased with TCDD exposure include CYP1B1 and CYP1A2, which together with CYP1A1 are capable of biotransforming some procarcinogens to potentially mutagenic and carcinogenic metabolites.
In addition to CYP1A1 and CYP1A2, TCDD can affect other enzymes that metabolize hormones, such as thyroid hormones, retinoic acid, testosterone, estrogens, and adrenal steroids (reviewed in Kung et al., 2009, and Safe et al., 2000). Those hormones transmit their signals by interacting with specific proteins
called receptors and in this manner initiate a chain of events in many tissues of the body. For example, the binding of the primary female sex hormone, estrogen, to the estrogen receptor promotes the formation of breasts and the thickening of the endometrium, regulates the menstrual cycle, and influences brain development. Exposure to TCDD can increase the metabolism of estrogen and thus lead to a decrease in the amount of estrogen available for binding and activating the estrogen receptor. The ultimate effect of TCDD is an interference with all the bodily functions that are regulated by estrogens. Similarly, the actions of TCDD on the adrenal steroids can adversely affect their ability to regulate glucose tolerance, insulin sensitivity, lipid metabolism, body weight, vascular function, and cardiac remodeling. In addition to changing the amount of hormone present, TCDD has been found to interfere with the ability of receptors to fulfill their role in transmitting hormone signals. Those actions of TCDD on enzymes and hormone receptors are thought to underlie, in part, the observed developmental and reproductive effects and cancers that are hormone responsive.
Effects on Paths of Cellular Differentiation
The broad spectrum of TCDD effects on hormone and growth factor systems, cytokines, and other signal-transducer pathways indicates that TCDD is an extremely powerful growth dysregulator (Birnbaum, 1994). Research performed primarily in cultured cells has shown that TCDD can affect the ability of cells to undergo such processes as proliferation, differentiation, and apoptosis (Duan et al., 2014; Marlowe and Puga, 2005). During the proliferation process, cells grow and divide. When cells are differentiating, they are undergoing a change from less specialized to more specialized. Cellular differentiation is essential for an organism to mature from a fetal to an adult state. In the adult, proper differentiation is required for the normal functioning of the body—for example, in maintaining a normally responsive immune system. The processes of controlled cell death, such as apoptosis, are similarly important during the development of the fetus and are necessary for normal physiologic functions in the adult. Apoptosis is a way for the body to eliminate damaged or unnecessary cells. The ability of a cell to undergo proliferation, differentiation, and apoptosis is tightly controlled by an intricate network of signaling molecules that allows the body to maintain the appropriate size and number of all the specialized cells that form the fabric of complex tissues and organs. Any disruption of the network that alters the delicate balance of cell fate can have severe consequences, including impairment of the function of the organ because of the absence of specialized cells. Alternatively, the presence of an excess of some kinds of cells can result in the formation and development of tumors. Thus, the ability of TCDD to disrupt the normal course of a specific cell to proliferate, differentiate, or undergo apoptosis is thought to underlie (at least in part) its adverse effects on the immune system and the
developing fetus and its ability to promote the formation of some cancers (Kolluri et al., 2017).
Mechanisms
TCDD binds and activates AHR in the cells of virtually every tissue in the body. The ability of TCDD to bind to AHR with high affinity is necessary—but not sufficient—to produce most of the adverse effects associated with TCDD exposure, including those from direct TCDD binding to and activation of the AHR and later alterations in the expression of TCDD-regulated genes as well as changes to those signaling pathways altered through interactions with the canonical AHR pathway (Poland and Knutson, 1982; Safe, 1990; Schmidt and Bradfield, 1996; Whitlock, 1990).
In the canonical pathway, AHR functions as a ligand-activated nuclear transcription factor. Upon binding of agonists (ligands), such as TCDD, AHR forms a heterodimer with a structurally related protein called AHR nuclear translocator (ARNT). The dimeric complex (structural model proposed by Corrada et al., 2017) binds to core DNA sequences called xenobiotic-responsive elements or dioxin-responsive elements in the promoter region of responsive genes and enhances the transcription of those genes. Many of the AHR-regulated genes encode drug-metabolizing enzymes, such as CYP1A1, CYP1A2, CYP1B1, and a variety of Phase II conjugating enzymes. Although the up-regulation of these enzymes is a sensitive biomarker of exposure to TCDD and may contribute mechanistically to some of the adverse effects of TCDD, the tissue-, species-, time-, and dose-specific modulation (increase or decrease) of many genes is thought to contribute to the wide array of toxic responses to TCDD exposure (Black et al., 2012; Boverhof et al., 2006; Y. Chen et al., 2017; Ovando et al., 2006, 2010; Perdew, 2008; Puga et al., 2009; Schneider et al., 2014). Many of these effects may be the result of activation of non-canonical cytoplasmic AHR pathways that lead to SRC activation, calcium release, and binding to other transcription factors, and their downstream effects as reviewed in Larigot et al. (2018).
AHR Signaling Pathways
The primary and most intensely studied pathway by which TCDD elicits biologic responses is depicted in Figure 4-5. In the absence of a bound ligand, the inactive AHR is retained in the cytoplasm of the cell in a complex consisting of two molecules of the heat-shock protein HSP90, one molecule of prostaglandin E synthase 3 (p23) (Kazlauskas et al., 1999), and one molecule of the immunophilin-like protein hepatitis B virus X-associated protein 2 (XAP2) (Petrulis et al., 2003), previously identified as either AHR-interacting protein (Ma and Whitlock, 1997) or AHR-associated protein 9 (Carver and Bradfield, 1997). The HSP90 dimer–p23 complex plays multiple roles in the protection of the AHR
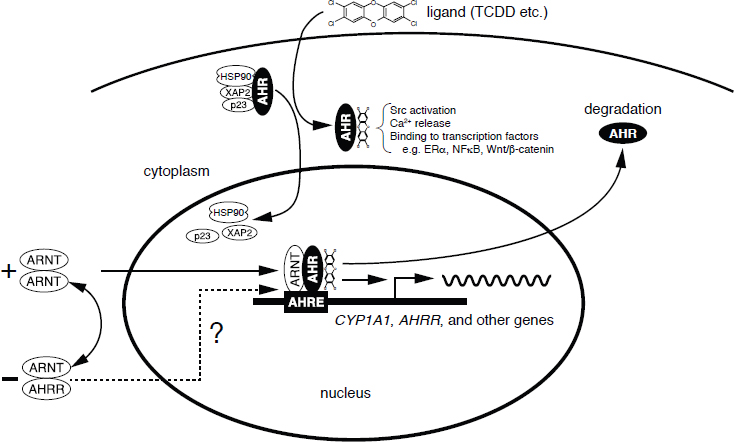
NOTES: In the canonical pathway, activation of the AHR though binding TCDD or other ligands, the AHR undergoes conformational changes that result in the dissociation of the AHR/HSP90 complex and its translocation to the nucleus. Once in the nucleus, the AHR establishes protein–protein interactions with the AHR nuclear translocator (ARNT) and additional coactivators and transcription factors. These complexes bind to AHRE (aryl hydrocarbon response elements) to control the transcriptional activity of target genes (e.g., CYP1A1, AHR repressor [AHRR]). In the non-canonical pathway, activation of the AHR through binding TCDD, or other ligands, results in cytoplasmic interactions leading to rapid changes in intracellular calcium, activation of signaling pathways, and direct binding to other transcription factors.
from proteolysis, maintaining it in a conformation that makes it accessible to ligand binding at the same time that it prevents the premature binding of ARNT (Carver et al., 1994; Pongratz et al., 1992; Whitelaw et al., 1993). XAP2 interacts with the carboxyl terminus of HSP90 and with the AHR nuclear-localization signal, a short amino acid domain that targets the receptor for interaction with nuclear-transport proteins. The binding of XAP2 blocks such an interaction, preventing the inappropriate trafficking of the receptor into the nucleus (Petrulis et al., 2003).
The binding of ligands (such as TCDD) induces the release of XAP2 and the exposure of the nuclear localization signal and leads to the binding of nuclear-import proteins and the translocation of the cytosolic complex into the nucleus (Davarinos and Pollenz, 1999; Song and Pollenz, 2002). Once the cytosolic complex is in the nucleus, HSP90, p23, and XAP2 dissociate from the AHR, which allows the binding of ARNT (Hoffman et al., 1991; Probst et al., 1993).
The activated AHR–ARNT heterodimeric complex is then capable of directly or indirectly interacting with DNA by binding to recognition sequences in the regulatory region of responsive genes (Dolwick et al., 1993; Probst et al., 1993). DeGroot and Denison (2014) found that the nucleotide specificity of DNA binding by the activated AHR-ARNT is not affected by structurally different ligands.
The canonical DNA recognition motif of the AHR–ARNT complex is referred to as the AHR-responsive element (AHRE, also referred to as the dioxin- or xenobiotic-response element, respectively). This element is found in the promoter region of AHR-responsive genes and contains the core sequence 5′-GCGTG-3′ (Shen and Whitlock, 1992), which is part of a more extensive consensus-binding sequence, 5′-T/GNGCGTGA/CG/CA-3′ (Lusska et al., 1993; Yao and Denison, 1992). The AHR–ARNT complex binds to the AHRE core sequence in such a manner that ARNT binds to 5′-GTG-3′ and AHR binds to 5′-TC/TGC-3′ (Bacsi et al., 1995; Swanson et al., 1995). A second type of element, termed AHRE-II, 5′-CATG(N6)C[T/A]TG-3′, has been shown to be capable of acting indirectly with the AHR–ARNT complex (Boutros et al., 2004; Sogawa et al., 2004). The end result of the process is the recruitment of the transcriptional machinery associated with RNA polymerase II and the initiation of differential changes in the expression of the genes bearing the AHR–ARNT recognition motif. Many of the genes code for proteins responsible for detoxification reactions directed at the elimination of the ligand. Research suggests that posttranslational modifications in histone proteins may modify the response (Hestermann and Brown, 2003; J. B. Kim et al., 2017; Schnekenburger et al., 2007).
In the canonical pathway, the actions of TCDD are mediated by the binding of the activated AHR–ARNT dimer to AHREs on DNA, which results in altered gene expression (see Figure 4-5). More recently, studies suggest that a non-canonical pathway within the cytoplasm also contributes to the toxic effects of TCDD, as first reviewed by Matsumura (2009). The TCDD-mediated activation of AHR within the cytoplasm does not involve binding to ARNT or DNA and appears to contribute to the rapid inflammatory responses associated with TCDD (Sciullo et al., 2008). In several cell lines, the activation of protein kinase C and the later activation of the serine phosphorylated form of cytosolic phospholipase A2 (cPLA2) takes place within 15 minutes of TCDD exposure (Dong and Matsumura, 2008; S. Park et al., 2007). It is proposed that within the cytoplasm, the TCDD-mediated activation of AHR leads to a rapid increase in intracellular Ca2+, plus activation of cPLA2, protein kinases, and pro-inflammatory proteins, such as cyclooxygenase (COX-2) (Matsumura, 2009). This pathway and other alternative mechanisms of TCDD-mediated AHR activation have also been reviewed by Denison et al. (2011), Larigot et al. (2018), Perdew (2008), and Wright et al. (2017).
AHR Physiology
The vertebrate AHR is presumed to have evolved from its counterpart in invertebrates, in which it serves a ligand-independent role in normal development processes. The ancestral function of the AHR appears to be the regulation of specific aspects of embryonic development, it having acquired the ability to bind xenobiotic compounds only during vertebrate evolution (Hahn, 2001; Hahn et al., 2017). The invertebrate AHR also functions as a transcription factor and binds to the same dimerization partner (ARNT) and DNA-response elements as the vertebrate protein, but it does not respond to any of the environmental ligands recognized by the vertebrate receptor. Instead, it regulates diverse developmental processes that are independent of exogenous ligand exposure, such as neuronal differentiation during worm development in Caenorhabditis elegans (X. Huang et al., 2004; Qin and Powell-Coffman, 2004) or the normal morphogenesis of legs, antennae, and bristles in Drosophila melanogaster (Adachi-Yamada et al., 2005; Céspedes et al., 2010). In developing vertebrates, AHR seems to play a role in cellular proliferation and differentiation and, in keeping with this role in invertebrates, also has a developmental role in craniofacial, muscle, pulmonary, renal, cardiovascular, and reproductive tract morphogenesis and blood cell differentiation (Birnbaum et al., 1989; C. Chiu et al., 2014; Fernandez-Salguero et al., 1997; Lahvis et al., 2005). Other potential functional roles of AHR include reproduction, innate immunity, tumor suppression, and blood-pressure regulation (Fujii-Kuriyama and Kawajiri, 2010). Dietrich (2016) reviewed antioxidant functions of the AHR.
The clearest adaptive physiologic response to AHR activation is the induction of xenobiotic-metabolizing enzymes involved in the detoxification of toxic ligands. Evidence of that response, which was described above, was first observed in conjunction with the induction of CYP1A1, which resulted from exposure to PAHs or TCDD and was directly related to the activation of the AHR signaling pathway (Israel and Whitlock, 1983, 1984). Because of the presence of the AHRE motif in their gene promoters, other metabolizing genes were tested and found to be induced by AHR ligands, which led to the identification of a so-called AHR gene battery of phase I and phase II detoxification genes that code for the drug-metabolizing enzymes CYP1A1, CYP1A2, CYP1B1, NQO1, ALHD3A1, UGT1A2, and GSTA1 (Nebert et al., 2000). Presumably, vertebrates have evolved those enzymes to detect a wide array of foreign, potentially toxic chemicals, represented in the wide variety of substrates that the AHR is able to bind to and whose biotransformation and elimination it is able to facilitate.
A potential complication of the adaptive responses elicited by AHR activation is the induction of a toxic response. Toxicity may result from the adaptive response itself if the induction of metabolizing enzymes results in the production of toxic metabolites. For example, the PAH benzo[a]pyrene, an AHR ligand, induces its own metabolism and detoxification by the AHR-dependent signaling
mechanism described earlier, but paradoxically it becomes bioactivated to a toxic metabolite in several tissues by a metabolism that depends on CYP1A1 and CYP1B1 activity (Harrigan et al., 2004). A second potential source of AHR-mediated toxicity may be aberrant changes in global gene expression beyond those observed in the AHR gene battery. The global changes in gene expression may lead to deleterious changes in cellular processes and physiology. Microarray and other transcriptomic analyses have proved invaluable in understanding and characterizing that response (Boverhof et al., 2006; Martinez et al., 2002; Ovando et al., 2006, 2010; Puga et al., 2000, 2004; Takeda et al., 2012; Vezina et al., 2004).
Endogenous AHR functions likely involve interaction with endogenous ligands that activate specific physiological processes. Several chemicals and chemical classes have been identified as putative endogenous ligands, including equilenin, indigoids, 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester, leukotrienes, heme metabolites, arachidonic acid metabolites, tryptophan metabolites, and ultraviolet (UV) photoproducts of tryptophan (Guyot et al., 2013; Nguyen and Bradfield, 2008). The tryptophan catabolite and AHR ligand kynurenine has been identified as a tumor promoter that suppresses anti-tumor immune responses and promotes tumor-cell survival and motility (Opitz et al., 2011). The tryptophan UV photoproduct 6-formylindolo[3,2-b] carbazole (FICZ) is a high-affinity AHR ligand, an inducer of CYP1A1 (Y.-D. Wei et al., 1998), and a substrate for CYP1A1 (Wincent et al., 2012). This autoregulatory loop maintains endogenous low levels of FICZ, which influence circadian rhythms, responses to UV light, homeostasis associated with pro- and anti-inflammatory processes, and genomic stability (Wincent et al., 2012). FICZ has been shown to reduce the inflammatory response in skin inflammation models (Di Meglio et al., 2014) and to enhance natural killer cell control of tumors (Shin et al., 2013).
It is clear that AHR is an essential component of the toxicity of dioxin and of dioxin-like chemicals. Studies of certain genetic polymorphisms in AHR in human cells suggest an association with differential susceptibility to dioxins (Kovalova et al., 2016; G. Liu et al., 2015), and experimental alteration of human AHR can suppress ARNT function (J. H. Xie et al., 2014). The homozygous deletion of Ahr in mice leads to a phenotype that is resistant to the toxic effects of TCDD and to the carcinogenic effects of benzo[a]pyrene (Fernandez-Salguero et al., 1996; Lahvis and Bradfield, 1998; Schmidt and Bradfield, 1996). The Ahr knockout mice, however, have other phenotypic effects, including reduced liver size, hepatic fibrosis, and cardiovascular abnormalities. Ahr knockout rats also demonstrate resistance to the toxic effects of TCDD and, in contrast to mice, display pathological alterations to the urinary tract in the absence of TCDD (Harrill et al., 2013). Hence, it is likely that dioxin has effects that are due to the disruption of endogenous AHR functions and that are unrelated to the intrinsic toxicity of some of its ligands.
Definition of Dioxin-Like Compounds, Toxic Equivalence Factor, and Toxic Equivalents
TCDD has the highest affinity for the AHR, but many other chemicals have dioxin-like properties: they have similar chemical structures, have similar physiochemical properties, and cause a common battery of toxic responses because of their relatively high affinity for the AHR. Because of their hydrophobic nature and resistance to metabolism, these chemicals persist and bioaccumulate in the fatty tissues of animals and humans. Although there are several hundred polychlorinated, polybrominated, and mixed polychlorinated–polybrominated dibenzo-p-dioxins, dibenzofurans, and biphenyls, only a relatively small number of congeners of these chemical classes display dioxin-like activity. Only 17 polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) with chlorine at the 2, 3, 7, and 8 positions and a few of the coplanar polychlorinated biphenyls (PCBs) that are often measured in environmental samples are recognized as being dioxin-like chemicals.
In the context of risk assessment, these polychlorinated–polybrominated dibenzo-p-dioxin, polychlorinated dibenzofuran, and biphenyl dioxin-like chemicals are commonly found as complex mixtures when detected in environmental media and biologic tissues or when measured as environmental releases from specific sources. That complicates the human health risk assessment that may be associated with exposures to varied mixtures of dioxin-like chemicals. To address the problem, the concept of toxic equivalence has been elaborated by the scientific community, and the toxic equivalence factor (TEF) has been developed and introduced to facilitate the risk assessment of exposures to those chemical mixtures (reviewed in Tavakoly Sany et al., 2015; van den Berg et al., 2006). On the most basic level, TEFs compare the potential toxicity of each dioxin-like chemical found in a mixture with the toxicity of TCDD, the most toxic member of the group. The procedure involves assigning individual TEFs to the dioxin-like chemicals on the basis of in vivo and in vitro potency relative to TCDD, which is assigned a TEF of 1.0. The dioxin-like chemicals have been assigned TEFs ranging from 0.00001 to 1.0 by the World Health Organization (WHO) (van den Berg et al., 2006, as summarized in Table 4-1). This approach has significant limitations due to the lack of in vivo human data and the difficulty of extrapolating cell line and rodent data to humans.
Larsson et al. (2015) used 17 in vitro assays from multiple species and in silico models to develop consensus toxicity factors that were then compared to internationally recognized WHO TEF values. The vast majority of the consensus toxicity factors for PCDDs and PCDFs were generally lower than the WHO TEFs but within an order of magnitude. The greatest differences between the human (but not rodent) consensus toxicity factor and TEQ values were in the effects of PCBs, which were inactive in the human cell line assays. The computational tools used by Larsson et al. (2015) could be quite useful in future discussion of TEF values, however, in vivo data must have more weight.
| Chemical | TEF |
|---|---|
| Chlorinated dibenzo-p-dioxins | |
| 2,3,7,8-TCDD | 1.0 |
| 1,2,3,7,8-PeCDD | 1.0 |
| 1,2,3,4,7,8-HxCDD | 0.1 |
| 1,2,3,6,7,8-HxCDD | 0.1 |
| 1,2,3,7,8,9-HxCDD | 0.1 |
| 1,2,3,4,6,7,8-HpCDD | 0.01 |
| OctoCDD | 0.0003 |
| Chlorinated dibenzofurans | |
| 2,3,7,8-TCDF | 0.1 |
| 1,2,3,7,8-PeCDF | 0.03 |
| 2,3,4,7,8-PeCDF | 0.3 |
| 1,2,3,4,7,8-HxCDF | 0.1 |
| 1,2,3,6,7,8-HxCDF | 0.1 |
| 1,2,3,7,8,9-HxCDF | 0.1 |
| 2,3,4,7,8,9-HxCDF | 0.1 |
| 1,2,3,4,6,7,8-HpCDF | 0.01 |
| 1,2,3,4,7,8,9-HpCDF | 0.01 |
| OctoCDF | 0.0003 |
| Non-ortho-substituted PCBs | |
| PCB 77—3,3′,4,4′-tetraCB | 0.0001 |
| PCB 81—3,4,4′,5-tetraCB | 0.0003 |
| PCB 126—3,3′,4,4′,5-pentaCB | 0.1 |
| PCB 169—3,3′,4,4′,5,5′-hexaCB | 0.03 |
| Mono-ortho-substituted PCBs | |
| PCB 105—2,3,3′,4,4′-pentaCB | 0.00003 |
| PCB 114—2,3,4,4′,5-pentaCB | 0.00003 |
| PCB 118—2,3′,4,4′,5-pentaCB | 0.00003 |
| PCB 123—2′,3,4,4′,5-pentaCB | 0.00003 |
| PCB 156—2,3,3′,4,4′,5-hexaCB | 0.00003 |
| PCB 157—2,3,3′,4,4′,5′-hexaCB | 0.00003 |
| PCB 167—2,3′,4,4′,5,5′-hexaCB | 0.00003 |
| PCB 189—2,3,3′,4,4′,5,5′-heptaCB | 0.00003 |
NOTE: CB, chlorinated biphenyl; CDD, chlorinated dibenzo-p-dioxin; CDF, chlorinated dibenzofuran; PCB, polychlorinated biphenyl; TEF, toxicity equivalence factor.
SOURCE: Adapted from van den Berg et al., 2006.
Interim TEF values have been established for brominated congeners by the 2011 joint WHO–UN Environment Programme meeting to evaluate the WHO TEF scheme. The recommendation is to use the TEF of the corresponding chlorinated congener as an interim TEF value for brominated congeners for human risk assessment (van den Berg et al., 2013).
When several chemicals are present in a mixture, the toxicity of the mixture is estimated by multiplying the TEF of each dioxin-like chemical in the mixture
by its mass concentration and summing the products to yield the TCDD TEQs of the mixture. In that approach to assessing the dioxin-like activity of a complex real-world mixture of dioxin-like chemicals, an environmental or biologic specimen with a 100-ppt (100-pg/g) TEQ is toxicologically equivalent to 100-ppt TCDD. There are two accepted specialized methods for assessing the dioxin-like chemicals in a complex biologic or environmental specimen: one involves analytic chemistry that quantifies specific dioxin-like chemicals (high-resolution gas chromatography–mass spectroscopy), and the other is a reporter-gene biologic screen that assesses dioxin-like activity due to binding to AHR in a transformed cell line (CALUX, EPA method 4435). Epidemiologic studies discussed in this and other updates assess exposure by reporting the specific concentration of TCDD in a specimen or by expressing dioxin-like activity in a complex mixture in units of TEQs.
Carcinogenic Classification
EPA and IARC have developed criteria to classify the potential carcinogenicity of chemicals on the basis of the weight of scientific evidence from animal, human, epidemiologic, mechanistic, and mode-of-action studies. EPA classified TCDD as a “probable human carcinogen” in 1985 and as “carcinogenic to humans” in a 2003 reassessment. In 1998 an IARC panel of experts concluded that the weight of scientific evidence supported the classification of dioxin as a class I carcinogen, that is, as “carcinogenic to humans.” Four years later the U.S. National Toxicology Program upgraded its classification to “known to be a human carcinogen.” In 2006 a panel of experts convened by the National Research Council to evaluate the EPA reassessment concluded that TCDD was “likely to be carcinogenic to humans”; this designation reflected the revised EPA Guidelines for Carcinogen Risk Assessment made public in 2005.
Genotoxicity
Genotoxicity refers to a deleterious action that affects the integrity of a cell’s DNA. Genotoxic substances are known to be potentially mutagenic or carcinogenic. Although TCDD is carcinogenic in humans and laboratory animals, it is generally classified as nongenotoxic and nonmutagenic (Wassom et al., 1977). There is no evidence of covalent binding of TCDD or its metabolites to DNA (Poland and Glover, 1979). TCDD does interact with DNA through a receptor-mediated pathway that involves the initial binding of TCDD to AHR, binding of the activated receptor complex to dioxin responsive elements on DNA and later alterations in the expression of TCDD-regulated genes, and altered signaling of the biologic pathways that interact with the AHR signal-transduction mechanism (Poland and Knutson, 1982; Safe, 1990; Schmidt and Bradfield, 1996; Whitlock,
1990). TCDD, 2,4,5-T, and 2,4-D were not mutagenic in Salmonella typhimurium with or without the addition of liver metabolic-activation enzymes (Blevins, 1991; Mortelmans et al., 1984). TCDD-induced cytogenetic damage in laboratory mice showed no increase in the frequencies of sister-chromatid exchanges, chromosomal aberrations, or micronuclei in the bone marrow cells of either C57Bl/6J or DBA/2J mice after the administration of a single high dose of TCDD—up to 150 μg/kg (Meyne et al., 1985). TCDD did not alter the frequency or the spectrum of mutations in male and female Big Blue transgenic rats (Thornton et al., 2001). There is one report of a positive result with TCDD in a test that measured the induction of chromosomal deletions resulting from intrachromosomal recombination in mouse embryos in vivo (Schiestl et al., 1997). More recently, Scinicariello and Buser (2015) found associations of dioxin-like PCBs and dioxins (other than TCDD) with longer leukocyte telomere length. In that study, leukocyte telomere length asays were used to assess the effects of exposure to dioxin-like PCBs (126, 169), PCDDs, and 1,2,3,4,6,7,8-heptachlorodibenzofuran. The study sample consisted of 2,413 adults from the 1999–2002 cycle of National Health and Nutrition Examination Survey (NHANES). Lipid-adjusted PCBs, PCDDs, and PCDFs were measured for a randomly selected one-third of all NHANES subjects. Both individual values and TEQs were estimated. Multivariate linear regression was used to study associations between natural log-transformed leukocyte telomere length and persistent organic pollutant quartiles. Individuals in the 3rd and 4th quartiles of the sum of PCBs were associated with, respectively, 8.33% (95% confidence interval [CI] 4.08–13.88) and 11.63% (95% CI 6.18–17.35) longer leukocyte telomere length, compared with the lowest quartile, with an evidence of a dose–response relationship (p-trend < 0.01). Most of these effects were stronger in the non-dioxin-like congeners than in the dioxin-like congeners, which brings into question the direct relevance to the committee’s charge. No significant associations were observed between leukocyte telomere length and the sum of PCDD; however, the congener 1,2,3,6,7,8-hexachlorodibenzo-p-dioxin was associated with a longer leukocyte telomere length. By TEQs, the highest quartiles of dioxin-like and the TEQ PCBs were associated with 11.63% (95% CI 4.08–18.53) and 6.18 (95% CI 1.01–11.63) longer leukocyte telomere length; both demonstrated a dose–response relationship (p < 0.01). This study suggests that telomere length may be influenced by exposure to dioxin and dioxin-like chemicals, however, the association between telomere length and disease is not well understood.
In summary, although TCDD does have some genotoxic activity, the vast majority of studies did not detect mutagenic activity of TCDD in a variety of in vitro and in vivo short-term tests.
Epigenetic Activity
Chromosomes contain the genetic material of an organism and are composed of both DNA and proteins called histones. Interactions between DNA
and histones regulate the accessibility of DNA to binding factors that activate or suppress gene transcription. This interaction is controlled by chemical modifications of the DNA (generally methylation of cytosine bases in cytosine–guanine dinucleotides or CpG sites) and histones (such as methylation, acetylation, sulfation, and ubiquitination), which are maintained by enzymatic processes. These modifications are considered “epigenetic” because they control the function of genes without changing the coding sequence. TCDD has been associated with epigenetic marks on the chromosomes, indicating that it could potentially be altering the function of numerous genes. Below is a brief summary of the epigenetic effects of TCDD. More detailed information about epigenetic mechanisms in general can be found later in this chapter, particularly concerning somatic modifications in an individual, and again in Chapter 8 with respect to effects that may affect offspring of an exposed organism.
The exposure of rodents to TCDD has been shown to result in the methylation of DNA in the adult rodents’ tissues—sperm, mammary tissue, liver, and a number of other solid tissues (Amenya et al., 2016; Papoutsis et al., 2013; Somm et al., 2013). The effects on DNA methylation were found to persist in rats for three generations following maternal exposure to 100 ng/kg TCDD and so could essentially be considered permanent and heritable (Manikkam et al., 2012a). As Ahr activation is generally considered necessary, but not sufficient, to induce adverse biological responses, and the dose–response relationship for Ahr activation is generally quite steep, the relevance of high-dose rodent studies such as these to human “low-dose exposures” is not always clear. When Olsvik et al. (2014) fed female zebrafish TCDD in their diet prior to breeding, they found no change in the level of DNA methylation throughout the genomes of fish or their embryos, but altered methylation was identified by probes for the promoter regions of a number of specific genes, with corresponding marked elevations in the expression of CYP1A1 and CYP1B1 in the mothers and embryos (Olsvik et al., 2014). To date, however, there have not been publications reporting on the persistence of methylation or other epigenetic modifications in the offspring of males exposed to TCDD as adults. In vitro studies in cell lines have identified altered DNA methylation and expression in genes related to adipocyte differentiation following TCDD exposure (van den Dungen et al., 2017) and in CYP1A1 following PCB 126 exposure (Vorrink et al., 2014).
Other Toxic Outcomes
Chloracne is a signature effect of high exposure to TCDD and dioxin-like chemicals in some species and in humans who are sensitive. There is an extensive body of evidence from experimental studies in animal model systems that TCDD, other dioxins, and several dioxin-like chemicals are immunotoxic (Kerkvliet, 2009, 2012; Kreitinger et al., 2016). Although the available evidence on dioxin immunotoxicity in humans is scant, mechanistic considerations support
the suggestion that chemical alterations of immune function would cause adverse health outcomes because of the critical role that the immune system plays in general protection—fighting off infection and eliminating cancer cells at early stages. Because of those considerations, the chemicals are potential immunotoxicants.
Similarly, reproduction and embryonic development clearly are targets of TCDD (Chen and Chan, 2016), other dioxins, and dioxin-like chemicals; it is a consistent finding that the adverse effects are more prevalent during fetal development than in the adult. Data on the developmental effects of dioxin-like chemicals in humans have begun to emerge over the past 10 years (Mocarelli et al., 2008). Human and animal studies have revealed other potential health outcomes, including cardiovascular disease, hepatic disease, thyroid dysfunction, lipid disorders, neurotoxicity, and metabolic disorders such as diabetes.
A number of effects of TCDD exposure in vitro appear to be independent of AHR-mediated transcription and, in at least one instance, perhaps independent of AHR itself. Guo et al. (2004) showed that TCDD induced expression of transforming growth factor α and other genes involved in extracellular matrix deposition in cells from mice that had homozygous ablation of the Ahr gene. Studies have shown that TCDD can mobilize calcium from intracellular sources and increase calcium imported from the culture medium (Puga et al., 1995). Mitochondrial oxidative stress has been shown to be induced when calcium is mobilized (Senft et al., 2002). Calcium mobilization by TCDD may have an important effect on signal-transduction mechanisms that control gene expression, inasmuch as several proto-oncogenes, such as c-fos, are activated by calcium changes.
Earlier VAO Update reports address the other toxic effects of TCDD in greater detail, including the relationship between human and animal-model sensitivity to exposure.
Summary of Biologic Plausibility That TCDD Induces Adverse Effects in Humans
Mechanistic studies in vitro and in laboratory animals have characterized the biochemical pathways and types of biologic events that contribute to the adverse effects of exposure to TCDD. For example, much evidence indicates that TCDD, acting via AHR in partnership with ARNT, alters gene expression. Receptor binding may result in the release of other cytoplasmic proteins that alter the expression or activity of other cell-regulatory proteins. Mechanistic studies also indicate that many other cellular-component proteins contribute to the gene-regulatory effect and that the response to TCDD exposure involves a complex interplay between genetic and environmental factors. Comparative data from animal and human cells in vitro and from tissues suggest a strong qualitative similarity among species in their response to TCDD, and this further supports the applicability to humans of the generalized model of initial events in response to dioxin exposure.
Biochemical and biologic responses to TCDD exposure are considered adaptive or simply reflective of exposure and not adverse in themselves if they take place within the normal homeostatic ranges of an organism; the exception to this is during development. However, they may exceed normal physiologic boundaries or constitute early events in a pathway that leads to damage in sensitive members of the population. In the latter case, the response is toxic and would be expected to cause an adverse health effect. Those qualitative generalizations about dose–response and individual variability are central to establishing biologic plausibility, which in the case of TCDD largely relies on extrapolation from animal studies to human risks. However, there are significant quantitative differences between responses in humans and rodents. Evidence from highly exposed human populations is an important means for corroborating biological plausibility.
OVERARCHING TOXICOLOGIC ISSUES RELATED TO THE CHEMICALS OF INTEREST
Limitations of Extrapolating Results of Laboratory Studies to Human Responses
In some instances, the toxic responses identified in laboratory-animal and cell-culture studies are not detected in epidemiologic studies with human exposure to the same chemicals. Although animal and cell-culture studies provide important links to understanding the biochemical and molecular mechanisms associated with toxicity induced by xenobiotics, many factors must be considered in extrapolating their results to human disease and disease progression. The following are key factors that might limit the ability of laboratory studies to predict human responses completely and accurately.
- Magnitude and duration of exposure In many instances, animal and cell-culture studies are conducted at higher exposures and for shorter durations than are typical in human exposures. For example, the concentrations of TCDD used in animal studies can be many times higher than was typical in the TCDD exposures of Vietnam veterans during their military service. In addition, TCDD is a persistent organic pollutant, and this results in human exposure that occurs over a lifetime, whereas animal studies seldom examine chronic low-level exposures that occur over a period of many months or years, except those that evaluate chronic toxicity or carcinogenicity. Animal studies that establish a measurement of body burden over a specific period provide the best potential for extrapolation to humans.
- Toxicokinetics The toxicokinetics—absorption, distribution, metabolism, and excretion—of xenobiotics can differ sharply between laboratory animals and humans. As described previously, the biologic half-life of TCDD
- varies from 8 to 29 days in rats and mice, while it is about 7 years in humans even though the drug-metabolizing enzymes—including cytochrome P450 1A1, 1A2, and 1B1—are up-regulated or induced via TCDD-mediated activation of the AHR in both rat and human liver (Black et al., 2012).
- Timing of exposure Many organ systems are more susceptible to xenobiotic exposure during critical stages of development, differentiation, or function—such as during gestation or in the face of another external challenge (for example, antigens, smoking, dietary salt, and fat)—than at other times. Therefore, the response of some systems (such as the immune or cardiovascular systems) may depend on the timing of exposure relative to the other challenges.
- Exposure composition Most animal and cell-culture studies involve exposure to single chemicals or to a well-defined mixture, but most human exposures are to complex mixtures from multiple sources, so it is difficult to definitively attribute any observed effects to a particular component of the environment.
- Difference in AHR affinity The binding affinity of AHR for TCDD differs between species (discussed in Okey et al., 2005). Many of the strains of mice used for toxicologic studies harbor a high-affinity Ahr allele (Ahrb), and these mice exhibit greater sensitivity to hepatic CYP1A induction, immunosuppression, birth defects, and other responses than do strains that carry the low-affinity allele (Ahrd). Such a simple allelic difference in AHR affinity has not been observed in humans, and the TCDD-binding affinity of the AHR found in most humans more closely resembles the low-affinity mouse Ahrd allele. Nonetheless, Nebert et al. (2004) reported that some people have TCDD-binding affinity that is 12 times higher than that found in others. Thus, although humans are generally considered to be less sensitive on the basis of an AHR that has low TCDD-binding affinity, this assumption may not apply to everyone.
- Complex disease etiology and environment The etiology of human diseases is highly influenced by genetics, environmental factors, and gene–environment interactions; these factors can be protective as well as deleterious. In addition to the COIs, the environmental factors that commonly influence human responses include diet, prescription and over-the-counter pharmaceuticals, cigarette smoking, alcohol consumption, physical activity, the microbiome, and stress. Stress (not to be confused with oxidative stress) produced via known or unknown sources is a well-known modifier of human disease responses (for example, immune and cardiovascular responses). Furthermore, stress is an ever-present factor that is difficult to assess or control for in epidemiologic studies because there is substantial individual variation in response to it (Cohen et al., 2007a). In contrast, laboratory studies are often conducted with inbred strains of animals and under tightly controlled experimental conditions, thus possibly underestimating or overestimating the potential
- contribution of a single chemical exposure to disease development. On the other hand, direct cause-and-effect relationships are more easily established in animal studies because of their standardization.
- Sex differences There are well-known differences in susceptibility to xenobiotic exposures between male and female animals, some of which are modified by sex steroids. For example, female Sprague Dawley rats are significantly more responsive to the hepatotoxic (neoplastic and non-neoplastic) effects of TCDD than are males of the same strain (Kociba et al., 1978).
Epigenetics
Epigenetics, as previously noted, is the term used to describe the mechanisms that regulate gene expression and genomic stability but that involve no changes in DNA sequence. The epigenetic marks on DNA and bound histones are mitotically stable because they are maintained every time a cell divides. The totality of epigenetics marks in each cell, termed the epigenome, creates and maintains the identity and function of the cell type (Christensen and Marsit, 2011; Cortessis et al., 2012; Skinner et al., 2010).
Conrad Waddington used the term “epigenetics” in the 1940s to describe environment–gene interactions that alter biologic traits (Waddington, 1940, 1953, 1956). It was not until the 1970s, however, that the first molecular epigenetic factor was described: DNA methylation, the chemical addition of a methyl group to DNA (Holliday and Pugh, 1975). In the 1980s, the role of DNA methylation in modifying gene expression—turning genes on and off—was established (Chen and Riggs, 2005). In the 1990s, the chemical modification of histone proteins associated with DNA was shown to also modify gene expression, thus establishing a second molecular epigenetic mechanism (Turner, 1998). In the early 2000s, various small noncoding RNA molecules were shown to regulate DNA activity (Sato et al., 2011). Around 2005, the first mapping of the yeast epigenome was conducted (Pokholok et al., 2005). Since that time, the mapping of cell-specific human epigenomes has accelerated under the National Institutes of Health’s Epigenomics Roadmap and ENCODE Project, and more than 100 human cell lines and tissues have been epigenetically characterized (Kundaje et al., 2015). The studies show that epigenetic marks act together in an exquisitely choreographed fashion to control cellular differentiation and the cellular ability to interact with, process, and initiate events and to respond to the signals and needs of the individual and local tissue environment. New National Institutes of Health efforts are under way within the Toxicant Exposures and Responses by Genomic and Epigenomic Regulators of Transcription Program to use mouse models of human relevant exposures to address the role of the environment in disease susceptibility as a function of changes to the epigenome, including TCDD and arsenic (T. Wang et al., 2018).
Today, the processes recognized as epigenetic mechanisms are DNA methylation (Chen and Riggs, 2005; Holliday and Pugh, 1975), histone modification (Turner, 1998), alterations in chromatin structure (Murr, 2010), and modulation of expression by some small and long non-coding RNA molecules (Valeri et al., 2009). DNA methylation is the addition of a methyl group onto specific nucleotides. In mammals it occurs mostly at cytosine nucleotides that are adjacent to guanine nucleotides (CpG sites), but it can also occur at cytosine nucleotides followed by other bases in embryonic cells and brain cells (Lister et al., 2013). Methylation of DNA in the promoter region of the gene can reduce the expression of the adjacent gene. Other modulations of DNA include hydroxymethylation (which is prominent in stem cells and brain cells), formylcytosine, and carboxylcytosine (X. Cheng et al., 2014; Song et al., 2013). Histones are the proteins that bind and form complex structures with DNA called nucleosomes in which DNA is wrapped around the histone core. The combination of DNA and histones is called chromatin. Chemical modifications of histones, such as methylation and acetylation, can alter the histone structure and modify gene expression by attracting protein complexes that can stimulate or repress transcription, in part by changing nucleosome spacing (Reid et al., 2009; Zhang and Pradhan, 2014). The influence of regulatory non-coding RNA including small interfering RNA and piwi-interacting RNA, as well as long non-coding RNA, on gene transcription is another field of epigenetic gene regulation that is now emerging. For example, recent findings suggest that micro-RNAs may be important regulators of cytokines involved in T-cell polarization and the allergic response (Tay et al., 2014).
Small RNAs can elicit both epigenetic and non-epigenetic responses depending on whether they are inhibiting transcription via the RNA-induced initiation of transcriptional silencing (RITS) pathway (epigenetic) or degrading mRNA through RNA-induced silencing complex (RISC) complex (not epigenetic). For example, short antisense RNA transcripts are produced within the nucleus by the action of the enzyme Dicer, which cleaves double-stranded RNA precursors into 21–26 nucleotide long RNA species (Matzke and Birchler, 2005; Verdel et al., 2005). These then associate with silencing–effector complexes, such as RISC, which directs cleavage of cognate mRNA or causes translational repression and RITS, which mediates heterochromatin formation at target loci and abrogates gene expression (Verdel et al., 2005). Thus, regulation mediated by small noncoding RNAs occurs both at the posttranscriptional and transcriptional levels. The latter transcriptional regulation is referred to as “epigenetic silencing,” and is mediated either by covalent modifications of chromatin (such as H3 methylation at Lys9) or by DNA methylation (Verdel and Moazed, 2005).
The interaction of all those epigenetic processes creates the epigenome, which has a critical role in regulating gene expression (Christensen and Marsit, 2011; Cortessis et al., 2012; Skinner et al., 2010). The variation that is possible in the epigenome is remarkable: The histone proteins that control chromatin configurations have dozens of possible modifications, and there are upwards of
50 million nucleotides in the DNA where methylation can occur and participate in regulating the cellular state. That implies that trillions of configurations of the epigenome are possible, although typically only about half of all genes are expressed in any given cell. Epigenetic marks are erased and re-established at two times during the life cycle—shortly after fertilization and during gametogenesis—to allow gamete-specific epigenomes to be converted to cell-specific epigenomes and vice versa (Dean, 2014).
Environmental epigenetics is the study of how environmental factors such as nutrition, toxicants, and stress alter epigenetic programming. In particular, epigenetics provides a molecular mechanism—other than mutations in the DNA itself—by which environmental factors can influence disease etiology (Jirtle and Skinner, 2007; Szyf, 2007). Epigenetics has been shown to have a role in the disease etiology of cancers and a number of other diseases (Christensen and Marsit, 2011; Cortessis et al., 2012; Skinner et al., 2010). In addition, exposure to environmental factors at critical times of development when epigenomes are shifting has the ability to alter epigenetic programming and cause changes in gene expression because these are times when epigenomes are evolving rapidly as stems cells differentiate into more mature cell types (Skinner et al., 2010). Hence, immune responses, fetal development, and gamete formation are important examples of physiological processes whose functioning can be affected by environmentally induced epigenetic changes.
New investigative tools and a more refined understanding of the epigenetic process have given rise to active research on the nature of the relationship between environmental exposure to epigenetically active agents and the occurrence of diverse disease states, including cancers, reproductive-developmental problems, immune dysregulation, diabetes, obesity, and psychiatric illnesses (Brookes and Shi, 2014). The committee sought to review data on the potential relationship of the exposures of interest with adverse epigenetic effects in directly exposed veterans in an attempt to find evidence linking the exposures to disease processes that might have been mediated epigenetically. The committee also sought to review relevant data on female veterans and male veterans separately inasmuch as the epigenetic consequences of exposures could be different, particularly in the case of adverse reproductive outcomes. Of note, possibly the greatest limitation to environmental epigenetic studies in human cohorts is access to the target tissue of interest. Researchers rely on more accessible proxy tissues such as blood leukocytes, saliva, buccal cells, or placenta. A second consideration is that DNA methylation levels change as a function of age in both humans and animal models and that these age-related changes in methylation are gene- and tissue-specific, can be either random or predictable, and are thought to play a role in chronic disease development.
A relevant example within the COIs is that the in vitro exposure of preimplantation embryos to TCDD alters the DNA methylation of imprinted genes (Q. Wu et al., 2004). Imprinted genes are a unique category of genes in which only
one copy is expressed in a parent-of-origin specific manner, and imprinted genes are important for growth and development. Similar results to those of Q. Wu et al. were obtained more recently when several solid tissues and sperm DNA were analyzed in adult male mice exposed to TCDD in utero (Somm et al., 2013). In utero exposure to TCDD was also associated with altered DNA methylation and reduced expression of the BRCA1 (breast cancer) gene in mammary tissue in adult female offspring (Papoutsis et al., 2013). More generally, studies of the developmental origins of health and disease have shown that early-life exposures or environmental influences can be associated with the onset of disease much later in life (Barker et al., 2010). These early developmental alterations in the epigenome provide a molecular mechanism by which environmental exposures of female veterans can have effects on their children into adulthood. In humans, using data from the AHS, Rusiecki et al. (2017) evaluated high pesticide exposure events and altered DNA methylation in the blood of male pesticide applicators (n = 695). A candidate gene approach was used to investigate three gene promoters as well as the repetitive element LINE-1. Ever having had a high pesticide exposure event was associated with increased DNA methylation at the GSTp1 promoter, an effect that was more pronounced among applicators older than 59 years. High pesticide exposure events were also associated with decreased DNA methylation at the MGMT promoter among applicators older than 59 and in applicators with less than 16.6 ng/mL plasma folate.
Of note, there are almost no toxicological studies with animal models that have evaluated more than a single, and at times very high dose, of the COIs. The potential for epigenetic changes induced via TCDD in Vietnam veterans and their descendants remains uncertain until more dose–response studies are completed to determine if epigenetic effects are secondary to AHR activation or separate from AHR activation. If epigenetic responses are the result of downstream events following extensive and prolonged AHR activation, they may be irrelevant to exposure scenarios that are inadequate to extensively induce AHR. On the other hand, if evidence demonstrates that TCDD can induce epigenetic changes at doses that cause no or minimal induction of AHR, it could well be the most important toxicological outcome to potentially occur in humans. There is precedent within the endocrine disrupting chemical literature for epigenetic alterations to have low-dose and non-monotonic effects, which are not necessarily linked to blocking or mimicking hormones, but may occur through other mechanisms such as oxidative stress or direct interactions with any of the many epigenetic enzymes and co-factors necessary for epigenetic gene regulation (Tapia-Orozco et al., 2017).
Most epigenetic modifications occur in somatic cells and are heritable only within the altered cell line, thereby having the potential to generate effects in the exposed individual but not in that individual’s offspring. Other studies have found direct effects of exposures on the germline (e.g., sperm or oocytes), which can be multi-generationally transmitted to the next generation. The possibility exists, however, of epigenetic transgenerational inheritance, which involves the
environment promoting a stable alteration in the germline that is transmitted to later unexposed generations (Schmidt, 2013; Skinner, 2014; Skinner et al., 2010; Y. Wei et al., 2015). Manikkam et al. (2012b) showed that the exposure of pregnant female rats to TCDD at critical times of development of the germline (when epigenetic programming is being established) can lead to abnormalities in the third-generation offspring, including kidney disease and changes in the ovaries and sperm of the offspring. There are few data on possible male-mediated heritable effects of TCDD or other environmental compounds. The sparse data include the results of studies of lead, a known developmental and neurologic toxicant. Lead exposure can alter semen quality in males (Alexander et al., 1996) and has been shown to induce paternally mediated developmental toxicity in rats (Anjum et al., 2011); this demonstrates that male-mediated effects on reproduction can be induced by reproductive toxicants. It has been suggested that environmental exposures can result in reduced fertility (Guerrero-Bosagna and Skinner, 2014; Paoloni-Giacobino, 2014). However, a serious need exists for additional study of the question, particularly of the effects of the COIs to this committee.
In summary, the ability of epigenetic mechanisms to regulate gene expression coupled with the interaction of the epigenome and the environment, including multi- and trans-generational effects, might underlie the ability of xenobiotic exposure to contribute to disease development and the potential for offspring to inherit the effects of the disrupted epigenetic processes.
Developmental Immunotoxicity
A second emerging field in the biologic sciences that may provide insight into the mechanism of xenobiotic-induced disease is developmental immunotoxicity, the study of the disruption of the developing immune system by xenobiotic exposure. The developing immune system is among the most sensitive physiologic targets of prenatal and childhood environmental insult. The sensitivity is due, in part, to the novel processes of gene rearrangement, somatic-cell selection, and immune-cell distribution that are required to produce a security system that can effectively protect not only the child but also the aging adult from disease. To produce that security system, the immune system, as it matures, must coordinate steps that result in highly specialized immune cells that are capable of self-versus-non-self recognition and that are tailored to the specialized functional environments of different tissues and organs (such as brain, lungs, skin, liver, gastrointestinal tract, and reproductive tract). A disruption of immune development can place the integrity of the organism at risk.
Among the known risk factors for developmental immunotoxicity are various chemicals including heavy metals, some pesticides, TCDD, industrial solvents such as trichloroethylene, and PCBs. The adverse outcomes of developmental immunotoxicity may become apparent soon after exposure or can emerge much later in life (Gascon et al., 2013). Often, childhood or adult infections can
trigger the appearance of developmental immunotoxicity-associated immune problems that were established earlier in life (Dietert, 2009). Developmental immunotoxicity-induced alterations can also contribute to myriad health problems related to dysfunction or pathologic conditions in virtually any tissue or organ. Chemicals, drugs, infectious agents, and physical and emotional stressors can act synergistically and increase the risk of developmental immunotoxicity. Not everyone is at identical risk for developmental immunotoxicity. People who have particular genotypes may be at increased risk for specific chemical-induced developmental immunotoxicity on the basis of heritable factors that affect metabolism or immune vulnerability.
The heightened sensitivity of the developing immune system is due to the existence of critical developmental windows of vulnerability during which environmental interference with key steps of immune maturation can change the entire course of immune development and result in later-life immune dysfunction and an increased risk of disease. The events programmed for these critical developmental windows have several basic features:
- They are necessary, usually one-time events of early development, with no equivalents in adults.
- They lock in building blocks on which additional maturational events rely.
- If they do not occur both on time and efficiently, the ramifications are usually profound, prolonged, and irreversible.
Examples of critical windows of immune vulnerability and the chemicals that can cause disruptions have been described in several reviews (R. Dietert and J. Dietert, 2008; R. Dietert and Piepenbrink, 2006; R. Dietert et al., 2000; Hessel et al., 2015; Holsapple et al., 2003; Landreth, 2002) and include
- The process of the seeding of immune cells in tissues where they grow into resident populations.
- The selection process of thymocytes in the thymus to distinguish nonself from self during the development of acquired immunity.
- The maturation of macrophage populations in the lung, in the brain, and elsewhere.
- The maturation of dendritic cells to provide balanced immune responses.
- The initial development, expansion, and seeding to the periphery of regulatory cell populations.
- Comparison with current tolerable daily intakes and their underlying overall no observed adverse effect levels showed that for some of the compounds, the tolerable daily intakes may need reconsideration based on developmental immune parameters.
The increased sensitivity of the fetal, neonatal, and juvenile immune systems compared with the immune system of an adult can manifest itself as a sensitivity to lower doses of chemical exposure than the doses that affect the adult, a greater persistence of the immune problems that follow exposure than are seen in adults, a broader array of immune problems than are experienced by adults, and a greater likelihood that a second later-life chemical exposure or environmental stressor will trigger an unexpected immune problem (Rooney et al., 2008). It is also important to note, similar to epigenetic mechanisms discussed above, the relevance of single or high-dose animal model studies remains uncertain until more dose–response studies are completed to determine if developmental immunotoxicity effects are secondary to AHR activation or separate from AHR activation.
Disruption of immune maturation is not the only route for developmental immunotoxicity. Early-life chemical exposure may affect the status of genes (the epigenome) in such a way that their pattern of expression in later life is affected and thereby alters immune functional capacity (J. Xu et al., 2016a). Such changes in gene status that affect immune status could occur in the exposed generation (for people exposed in utero or during childhood), or they could carry through one or more additional generations as a result of epigenetic alterations.
MEDIATORS OF HEALTH OUTCOMES
Identifying and assessing the mediators of health outcomes, which are effectors in the pathway of exposure to disease and may contribute to disease progression, is essential for characterizing the impact of toxicant exposures on health outcomes. Such mediators include oxidative stress, the cytogenetic status of cells, and factors that affect normal function and the development of the blood–brain barrier.
Recently, the study of oxidative stress has emerged as a mechanism for investigating toxicity. Oxidative stress is classically defined as an imbalance in oxidant and antioxidant species within a system in which oxidant species are predominant (Lushchak et al., 2014). Although oxidative stress and exposure to toxicants have been associated with many of the same health effects, they are rarely studied in parallel. For example, both oxidative stress and toxicant exposures have been associated with metabolic syndrome, insulin resistance, diabetes, obesity, and cardiovascular complications (Bonomini et al., 2015; Lang et al., 2008).
Using a longitudinal study of non-smoking Iowa corn farmers (n = 30) and non-farming controls (n = 10), Lerro et al. (2017) evaluated the effects of atrazine and 2,4-D exposures on three markers of oxidative stress in 225 samples collected during five agricultural periods (pre-planting, planting, growing, harvest, and off-season). Farmers exhibited higher urinary levels of atrazine and 2,4-D than controls. 2,4-D exposure (but not atrazine exposure) was associated with elevated levels of 8-OHdG (a marker of oxidative damage) and 8-isoPGF (a marker of lipoprotein peroxidation). No effects on the oxidative stress biomarker
malondialdehyde were observed in either atrazine or 2,4-D. Thus, 2,4-D may induce oxidative stress and contribute to the pathogenesis of cancer and other chronic diseases.
Using the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) cohort, Kumar et al. (2014a) evaluated serum persistent organic pollutants levels and several biomarkers of oxidative stress in 992 male and female subjects 70 years and older. Measurements included PCBs, organochlorine pesticides, octachlorinated dibenzo-p-dioxin, and polybrominated diphenyl ethers, and TEQ values were calculated for dioxin and dioxin-like mono-ortho PCBs via the van den Berg method. Oxidative stress markers consisted of protein stress markers (e.g., homocysteine, GSH, GSSG), lipid stress (e.g., conjugated dienes), and total antioxidant capacity. Several PCBs showed a significant positive association with oxidized low density lipoproteins (PCB 99, 138, 153, 156, 170, 180, 194, 206, and 209). In multivariable-adjusted analyses, the sum of PCBs was positively associated with oxidized low density lipoproteins and negatively associated with glutathione-related markers. Other summary measures did not show any significant associations with oxidative stress markers.
Sycheva et al. (2016) studied the cytogenetic status of cells isolated from the buccal and nasal epithelium from a sample of cases (n = 26) and controls (n = 35) from a rural population in Vietnam. The cases were individuals living in dioxin-contaminated areas (TCDD in soil 2.6 ng/kg), and the controls were villagers living in an area with negligible contamination (TCDD in soil 0.18 ng/kg). A variety of cellular damage parameters were examined, and a small increase in cell death was found for both types of epithelial cells in males from the contaminated area, indicating that dioxins had a more pronounced effect and that years of residence and aging affected the results inversely. Thus, the increase in apoptosis could suggest a dioxin effect and serve as a marker for cellular damage generally. However, the small sample size and the lack of a dioxin association with oral or nasopharyngeal cancers limits the confidence with which conclusions can be drawn from this study.
There is increasing investigation into the mechanics of the interactions between TCDD (and dioxin-like chemicals) and the normal function and development of the blood–brain barrier. Filbrandt et al. (2004) first demonstrated that TCDD was active in regulating gene expression in isolated murine cerebral vascular endothelial cells and astrocytes. X. Wang et al. (2011) demonstrated that TCDD exposure results in an increased expression and activity of several xenobiotic efflux transporters at the blood–brain barrier in rats and isolated capillaries in vitro. This has the potential to alter the delivery of drugs or endogenous chemicals to the brain. Miyazaki et al. (2016) reported that exposure to TCDD during blood–brain barrier formation disrupts and impairs blood–brain barrier function in part by the suppression of glial cell derived neurotrophic factor action, which may contribute to the adverse effects of TCDD on the fetal central nervous system. In a human cerebral microvascular endothelial cell line, Jacob et al. (2015)
did not observe a change in the expression or activity of two of the major efflux transporters in the human cerebral endothelium (ABCB1 and ABCG2) after exposure to TCDD. In a second study using the same cell line, Jacob et al. (2017) demonstrated that TCDD exposure increased the levels of the pro-inflammatory cytokines IL-1β and IL-6, which could induce blood–brain barrier breakdown and contribute to the pathogenesis of a variety of neurologic disorders.














































