8
Reproductive Health Effects and Effects on Descendants
Chapter Overview
Based on new evidence and a review of prior studies, the current committee did not find any new associations between outcomes related to the reproductive health of veterans or effects on their descendants and exposure to the chemicals of interest (COIs; 2,4-dichlorophenoxyacetic acid [2,4-D], 2,4,5-trichlorophenoxyacetic acid [2,4,5-T], picloram, dimethylarsinic acid [DMA or cacodylic acid], 2,3,7,8-tetrachlorodibenzo-p-dioxin [TCDD]).
Thus, the findings on these outcomes can be summarized as follows:
- None of the outcomes met the committee’s criteria for determining that there was sufficient evidence of an association with exposure to the COIs.
- None of the outcomes met the committee’s criteria for determining that there was limited or suggestive evidence of an association with exposure to the COIs.
- There is inadequate or insufficient evidence to determine whether there is an association between exposure to the COIs and endometriosis; decreased sperm counts or sperm quality, subfertility, or infertility; spontaneous abortion, stillbirth, neonatal death, or infant death; and low birth weight or preterm delivery, birth defects, childhood cancers, or other disease in their children as they mature or in later generations.
- There is limited or suggestive evidence of no association between paternal exposure to TCDD and spontaneous abortion.
This chapter summarizes the scientific literature published since Veterans and Agent Orange: Update 2014, hereafter referred to as Update 2014 (NASEM, 2016a), on the association between exposure to herbicides and adverse effects on the reproductive health of male and female Vietnam veterans and the health of their children and later generations. The literature considered in this chapter includes studies of a broad spectrum of reproduction-related effects in veterans and in other populations exposed occupationally or environmentally to the herbicides sprayed in Vietnam or to TCDD. Because some polychlorinated biphenyls (PCBs), some polychlorinated dibenzofurans (PCDFs or furans), and some polychlorinated dibenzodioxins (PCDDs) other than TCDD have dioxin-like biologic activity, studies of populations exposed to these chemicals were reviewed if their results were presented in terms of TCDD toxic equivalents (TEQs). As noted in Chapter 3, studies that report TEQs based only on mono-ortho PCBs (which are PCBs 105, 114, 118, 123, 156, 157, 167, and 189) are considered even though their TEQs are several orders of magnitude lower than those of the non-ortho PCBs (77, 81, 126, and 169), based on the revised World Health Organization (WHO) toxicity equivalency factor (TEF) scheme of 2005 (La Rocca et al., 2008; van den Berg et al., 2006). This is because the lower TEQs of the mono-ortho PCBs may be counterbalanced by their abundance, which is generally many orders of magnitude higher than the abundance of the non-ortho PCBs (H.-Y. Park et al., 2010).
The adverse outcomes evaluated in this chapter are male reproductive health effects such as alterations in sperm quality, semen, sex ratio, or hormonal levels; female reproductive health effects, including endometriosis and outcomes related to alterations in hormonal levels such as polycystic ovary syndrome and gestational diabetes; increased fetal loss (spontaneous abortion and stillbirth); neonatal and infant mortality; adverse gestational outcomes of low birth weight and preterm delivery; and the possibility of adverse health outcomes (birth defects, cancer; and changes in growth and physical parameters and in immune, allergic, motor development, cognitive, behavioral and socio-emotional outcomes) at any time during the lives of all progeny of Vietnam veterans. The committee responsible for Updates 2012 and 2014 separated those outcomes most directly related to reproductive health and to the health of progeny into separate chapters. This report combines them because the committee believes that reproduction-related effects are best understood as a continuum.
Because the vast majority of Vietnam veterans are men, the primary focus of the Veterans and Agent Orange (VAO) series has been on potential adverse effects of herbicide exposure on men, and the etiologic importance of the exposed party’s sex does not play the same dominant role in non-reproductive outcomes that it does in reproductive outcomes. However, an estimated 7,500 women are thought to have served in Vietnam (VA, 2017a), so findings relevant to female reproductive health, such as those concerning endometriosis, are also included in the chapter. Whenever the information was available, an attempt has
been made to evaluate the effects of exposure on males and females separately. It should be kept in mind, though, that the amount of research providing reliable information on the consequences of paternal exposure is extremely sparse for the COIs in the VAO report series and also for the full array of environmental agents that may pose threats to the health of future generations.
In addition, for published epidemiologic or experimental results to be fully relevant to the evaluation of the plausibility of reproductive effects in Vietnam veterans, whether female or male, the veteran’s exposure needs to have occurred before the conception of the child. With the exception of female veterans who became pregnant while serving in Vietnam, pregnancies that might have been affected occurred after deployment, when primary exposure had ceased but fetal exposure via dioxin stored in maternal tissue was possible. In the case of pregnancies of women who have previously been substantially exposed to the lipophilic dioxins, the direct exposure of the fetus throughout gestation is possible through the mobilization of toxicants from the mother’s adipose tissue. In contrast, adverse effects on offspring mediated by male veterans would be via alterations in the sperm genome and associated ribonucleic acids (RNAs) or semen that would have been transmitted after exposure and deployment.
The categories of association and the approach to categorizing the health outcomes are discussed in Chapter 3. To reduce repetition throughout the report, Chapter 5 characterized study populations and presents design information related to new publications that report findings or that revisit study populations considered in earlier updates.
BIOLOGIC PLAUSIBILITY OF REPRODUCTIVE HEALTH EFFECTS
There have been few studies of the effects on reproductive outcomes of exposure to the four herbicides in question, particularly picloram and cacodylic acid, and the available studies generally have shown toxicity only at very high doses. Much of the following discussion thus concerns TCDD, which, other than in controlled experimental circumstances, usually occurs in a mixture of dioxins (dioxin congeners in addition to TCDD).
TCDD is stored in fat tissue and has a long biologic half-life, so internal exposure at generally constant concentrations may continue after an episodic, high-level exposure to an external source is discontinued. If a person had a high exposure, then high amounts of dioxins may still be stored in fat tissue and be mobilized, particularly at times of weight loss. That would not be expected to be the case for nonlipophilic chemicals, such as cacodylic acid.
Dioxin exposure has the potential to disrupt male reproductive function by altering the expression of genes that are pertinent to spermatogenesis and by altering steroidogenesis (Wong and Cheng, 2011); it has the potential to disrupt female reproductive function by altering the expression of genes relevant to
ovarian follicle growth and maturation, uterine function, placental development, and fetal morphogenesis and growth (Bruner-Tran et al., 2017).
A father’s direct contribution to a pregnancy is limited to the contents of the sperm that fertilizes an egg; those contents had long been thought to consist of greatly condensed, transcriptionally inert deoxyribonucleic acid (DNA) constituting half the paternal genome (a haploid set of chromosomes). Consequently, it was once believed that paternally derived damage to the embryo or offspring could only result from changes in sperm DNA, and dioxins have not been shown to mutate the DNA sequence. However, as discussed in greater detail below, TCDD can have epigenetic effects that modify the expression of a cell’s genetic material, and those modifications persist in the daughter cells following cell division, whether the division involves an individual’s own somatic tissues or the production of his (or her) gametes. This provides an alternative pathway to creating permanent (heritable) changes in gene expression—a pathway that does not involve altering the DNA sequence. Epigenetic changes include chemical modifications made to DNA (usually involving methylation) or to other cellular components such as histones and RNAs (Jirtle and Skinner, 2007). As a sperm matures, most of its histones are replaced by protamines, which renders it transcriptionally quiescent and permits extensive DNA compaction. The core histones that are retained in human sperm carry epigenetic modifications to maintain open nucleosomes, which permits the transcription of genes that are important during embryo development (Casas and Vavouri, 2014). Sperm also carry a considerable collection of RNA fragments (Kramer and Krawetz, 1997; Krawetz et al., 2011), including ribosomal RNAs, messenger RNAs, and small noncoding RNAs (Casas and Vavouri, 2014; Lane et al., 2014). Small RNAs have been found to play critical roles in fertilization (Amanai et al., 2006), early embryonic development (Hamatani, 2012; Suh and Blelloch, 2011), and epigenetic modifications (Gapp et al., 2014; Kawano et al., 2012). Therefore, male infertility or fetal loss associated with exposure to the COIs might be mediated by epigenetic modifications to components of sperm other than the DNA (Krawetz, 2005; Vecoli et al., 2016).
A mother’s contribution to a pregnancy is obviously more extensive, and damage to an embryo or offspring can result from epigenetic changes in the egg DNA or from the direct effects of exposure on placenta formation and on the fetus during gestation. The mobilization of dioxin during pregnancy may be increased because the body is drawing on fat stores to supply nutrients to the developing fetus. TCDD has been measured in human circulating maternal blood, cord blood, and placenta. Thus, dioxin in the mother’s bloodstream could cross the placenta and expose the developing embryo and fetus. Data indicate that dioxin can accumulate in placental tissue and that dioxin can transfer from the placenta to the developing fetus (Mose et al., 2012).
On the basis of laboratory animal studies, it is known that TCDD can affect reproduction, so a connection between TCDD exposure and human reproductive and gestational effects is biologically plausible. However, making definitive
conclusions based on animal studies about the potential for TCDD to cause reproductive and gestational toxicity in humans is complicated by differences in sensitivity and susceptibility among different species, including strain-specific differences; by differences in the route, dose, duration, and timing of exposure between experimental protocols and real-world exposure; and by substantial differences between laboratory animals and humans in the toxicokinetics of TCDD. Experiments with 2,4-D and 2,4,5-T indicate that these chemicals have subcellular effects that could constitute a biologically plausible mechanism for reproductive and gestational effects. However, the preponderance of evidence from animal studies indicates that these chemicals do not have reproductive effects. There is insufficient information on picloram and cacodylic acid to assess the biologic plausibility of their potential reproductive or gestational effects.
The sections on the biologic plausibility of the specific outcomes considered in this chapter present more detailed toxicologic findings that are of particular relevance to the outcomes discussed.
MALE REPRODUCTIVE HEALTH
Male reproductive function is under the control of a variety of components whose proper coordination is important for normal fertility. Several of these components and some health outcomes related to male fertility, including reproductive hormones and sperm characteristics, can be studied as indicators of fertility. The reproductive neuroendocrine axis involves the hypothalamus, the anterior pituitary gland, and the testis. Gonadotropin-releasing hormone is secreted from the hypothalamus in a pulsatile fashion and acts on the anterior pituitary gland, leading to the release of both follicle-stimulating hormone and luteinizing hormone. Both are secreted into the circulatory system in episodic bursts by the anterior pituitary gland and are necessary for normal spermatogenesis. In the testis, luteinizing hormone interacts with receptors on Leydig cells, where it stimulates increased testosterone synthesis. Follicle-stimulating hormone and the testosterone from the Leydig cells interact with Sertoli cells in the seminiferous tubule epithelium to regulate spermatogenesis. A more detailed review of the male reproductive hormones can be found elsewhere (Strauss and Barbieri, 2013). Several agents, such as lead and dibromochloropropane, affect the neuroendocrine system and spermatogenesis (for reviews, see Schrader and Marlow, 2014; Sengupta, 2013). Reviews on the effects of various environmental toxicants, including TCDD, on testicular steroidogenesis and spermatogenesis provide insights into the potential underlying mechanisms, including reducing testosterone production in Leydig cells and inhibiting the formation of cyclic adenosine monophosphate (Mathur and D’Cruz, 2011; Svechnikov et al., 2010).
The committee responsible for the original VAO report (IOM, 1994) concluded that there was inadequate or insufficient evidence of an association between exposure to 2,4-D, 2,4,5-T, picloram, cacodylic acid, or dioxin and
alterations in sperm characteristics or other male reproductive health parameters, finding, generally, that existing studies reported inconsistent or non-significant results. Additional information available to the committees responsible for subsequent updates did not change these conclusions. Reviews of the relevant studies are presented in the earlier reports. Table 29, which can be found at www.nap.edu/catalog/25137, summarizes the results of studies related to male reproductive health outcomes.
Update of the Epidemiologic Literature
Environmental Studies
Mumford et al. (2015) examined the relationship between exposure to a number of persistent organic pollutants (POPs) and semen quality as part of the Longitudinal Investigation of Fertility and the Environment (LIFE) Study of environmental influences on human fecundity and fertility. Participants were 501 male partners of couples discontinuing contraception for the purposes of becoming pregnant, who were recruited in Michigan and Texas during 2005–2009. Upon enrollment, in-person interviews were conducted with each male partner to ascertain health, demographic, and reproductive histories. All data and biospecimens were collected in the home, and baseline interviews were followed by a standardized anthropometric assessment for the determination of BMI conducted by research nurses, and the research nurse also obtained non-fasting blood (10 mL) for quantification of serum chemicals and lipids. The quantification of POPs in serum included 1 polybrominated biphenyl (PBB 153); 9 organochlorine pesticides, and 10 polybrominated diphenyl ethers. PCBs with TEFs include 105, 114, 118, 156, 157, 167, and 189. PCDFs and the dioxin-like PCBs 77, 81, 123, 126, and 169 were not measured, leading to an underestimation of the TEQ. A baseline semen sample was obtained, followed by a second sample approximately 1 month afterwards, irrespective of the couples’ pregnancy status. A total of 35 semen parameters were measured, including five reflecting general characteristics (volume, straw distance [a motility marker], sperm concentration, total sperm count, and percent hypo-osmotic swollen [a marker of sperm quality]), 8 motility measures, 12 morphometry measures, 8 morphology measures, and 2 sperm chromatin stability assay measures. The models were adjusted for age, BMI, cotinine (a marker of tobacco smoke exposure), research site, total serum lipids, fish consumption, abstinence time, and sample age. A total of 468 men had measured chemical concentrations and semen quality and were included in the analysis. The levels of PBBs and some other chemicals were lower in this cohort than those observed in the U.S. National Health and Nutrition Examination Survey (NHANES) although the exposures were comparable. When males with chemical concentrations in the fourth quartile were compared with those in the first quartile, significant associations (at the 0.05 level) were found for several individual POPs and semen quality parameters. Although the majority of the comparisons were null, the researchers did observe associations between each chemical
class and each type of semen quality parameter, with results indicating both positive and negative associations with semen quality. At the 0.05 level of significance, among the PCBs with a TEF, only the 4th quartile of PCB 156 showed a positive association with a sperm morphology marker indicative of reduced semen quality. At the 0.01 level of statistical significance, PCBs 157 and 189 were associated with markers of improved semen quality. This report was based on a relatively large study cohort, and the results were adjusted for BMI and other potential confounders. Without a direct measurement of TCDD, though, the report is of modest utility.
Den Hond et al. (2015) measured biomarkers of exposure in 163 men recruited through academic fertility clinics in Belgium. All were under 50 years of age, with a body mass index (BMI) ≤ 35 and no known congenital, genetic, or acquired cause of infertility. Blood, urine, and two serially collected semen samples were obtained along with patient-provided information on smoking, food intake, physical activity, socioeconomic status, health status, and living conditions. Exposure characterization consisted of an evaluation of the serum for levels of endocrine-disrupting chemicals, including dioxins and dioxin-like PCBs. Men who had total motility counts of less than 20 million were classified as subfertile. The investigators found that elevated levels of PCDDs and PCDFs were associated with a non-significant increase in the risk of subfertility (1.59; 95% CI 0.96–2.65; p = 0.07), and elevated levels of dioxin-like PCBs were associated with a non-significant decrease in that risk (0.45; 95% CI 0.17–1.22; p = 0.12) after adjustment for confounders. This study was limited by its very small sample size and by a failure to use all of the semen quality markers available. It also had a confusing sampling frame with cases and controls sampled first based on an unsuccessful conception within 12 months status, and then further divided by total motile count. The generalizability of results from fertility clinic patients to Vietnam veterans is also uncertain. The report is thus of limited utility for the committee.
Galimova et al. (2015) measured PCDD/F levels in the semen of 168 infertile and 49 fertile men in Ufa, Russia, a city close to a manufacturing plant that produced, among other herbicides, 2,4,5-T during the 1960s through the 1980s. All subjects were patients of reproductive health clinics. Testing found PCDD/F TEQ levels to be 2.2–2.3 times higher in the ejaculate of infertile men than in that of fertile men. The highest concentration of the 2,3,7,8-TCDD congener was found in the ejaculate of men with abnormal sperm. 2,3,7,8-TCDD did not make a material contribution to the total level of dioxin load—its share was 12% of the equivalent dose, whereas the major part of toxicity as measured by the TEQ was determined by the presence of PCDFs. Other congeners also showed consistently higher levels among the men in the infertile groups. The paper lacked many details on the recruitment of the men, the number of men was small, and no analysis of the impact of adjustment for other factors was presented.
Mínguez-Alarcón et al. (2017) examined a group of young Russian men to determine whether peripubertal serum organochlorine concentrations affect
semen parameters. The analysis was based on the Russian Children’s Study, which is an ongoing prospective study of 516 males. Boys were enrolled at age 8–9 years and underwent a physical exam, blood sampling and, together with the mother or guardian, completed a questionnaire. Annual follow-ups were conducted. Because of loss to follow-up and other reasons, the analysis is based on 133 males (18–19 years) who had serum organochlorine concentration data collected and who provided one or two semen samples. One hundred twenty-three men provided two semen samples a week apart, and 10 provided one sample. Semen analysis included sperm motility, semen volume, and sperm concentration. Sera samples were used to measure 7 PCDDs, 10 PCDFs, 4 coplanar PCBs, 6 mono-ortho-substituted PCBs, and 31 other PCBs (non-dioxin-like PCBs). The measured TCDD concentrations (pg TEQ/g lipid) were: minimum, 0.35; 25th percentile, 1.77; 50th percentile, 2.9; 75th percentile, 4.2; and maximum, 12.1. A general pattern of decreased semen quality (concentration, count, motile sperm) with increasing TCDD was found. In adjusted models, men in the highest quartile of serum TCDD TEQs had, on average, a lower sperm concentration, lower sperm count, and lower total motile sperm count than those in the lowest quartile. There were no significant associations for the summed concentration of PCDD, PCDFs, coplanar PCBs or ∑CBs. This report is based on a well-designed study, including a prospective follow-up and adjustment for multiple potential confounders. The study was not able to isolate possible in utero exposure and postnatal exposure. Moreover, its utility is limited by the fact that subjects were exposed to dioxins in a different period of their life (infancy, childhood, and adolescence) than the Vietnam veterans, and the generalizability of the results is open to question.
Case-Control Studies
Paul and colleagues (2017) conducted a case-control study of the association between serum and semen levels of dioxin-like PCBs and post-testicular sperm maturation. The study group comprised 56 adult (aged 30–55 years) males from subfertile couples who were being evaluated for infertility at an in vitro fertilization clinic in Alicante, Spain, from May 2012 to June 2014. Cases (n = 24) were men whose semen quality was considered low based on having at least an alteration in at least one semen quality parameter as compared with baseline values. Controls (n = 26) were men with normal semen quality (all parameters above WHO 2010 cutpoints). Participants underwent a complete clinical examination; completed a questionnaire soliciting socioeconomic information, medical history, tobacco and alcohol consumption, and likely exposure to environmental chemicals; and gave blood and semen samples. The semen parameters that were measured included sperm concentration, volume, percentage of motile sperm, and percentage of sperm that were morphologically normal. The levels of 12 non-ortho (77, 81, 126, 169) and mono-ortho (105, 114, 118, 123, 156, 157, 167, 189) dioxin-like PCBs were measured in the serum samples. The authors also examined total TEQs as the sum of the TEQs obtained from the dioxin-like
PCBs. The mean levels (expressed at WHO-TEQ/g lipid) of the total dioxin-like PCBs and the non-ortho PCBs were higher in the low-semen-quality case group (22.32 ± 21.33 pg WHO-TEQ/g lipid and 22.52 ± 21.2 pg WHO-TEQ/g lipid, respectively) than in the control group (14.00 ± 10.82 pg WHO-TEQ/g lipid and 13.85 ± 10.69 pg WHO-TEQ/g lipid, respectively), although the differences were not statistically significant. The levels of mono-ortho PCBs were statistically significantly higher in low-semen-quality cases than in controls. When levels were expressed as pg/g lipid, all comparisons showed higher levels in the case group, which were statistically significant. Several specific PCBs had higher levels among cases than among controls, including PCB 126, but for only one (PCB 105) was the difference statistically significant (p = 0.031). Significant decreased correlations were found between PCB 126 and viability (r = −0.645; p = 0.013) and between PCB 77 (r = 0.671; p = 0.009) and PCB 81 (r = 0.552; p = 0.041) and sperm morphology among the cases. Other PCBs showed a positive correlation with specific sperm parameters, while parameters such as sperm count and concentration showed no correlation with total PCB levels. The study results suggest an association between exposure to dioxin-like PCBs and semen quality, but it is limited by the small number of participants, and its generalizability is uncertain.
Other Identified Studies
Cremonese and colleagues (2017) conducted a cross-sectional study of 99 rural and 36 urban men aged 18–23 years living in southern Brazil. Occupational exposure to herbicides (not otherwise specified) and other agricultural chemicals was assessed via a structured questionnaire. Information was also gathered on demographics, occupation, and other factors. Whole blood and semen samples were collected. The investigators found a statistically significant dose–response relationship between higher reported herbicide use and both poorer sperm morphology (p < 0.001) and reduced levels of luteinizing hormone (p = 0.002). The study’s strengths include the use of trained researchers to collect data on randomly sampled subjects who were selected to be representative of the underlying population, while its primary weakness is the small size of that cohort and the lack of specificity of the exposures.
Among the newly reviewed studies, Tan et al. (2016) found that the introduction of 2,4-D to in vitro samples of ejaculated human sperm did not affect their viability, capacitation, or spontaneous acrosome reactions, but it inhibited total motility, progressive motility, the ability to penetrate viscous media, and progesterone-induced capacitation and acrosome reaction rates in a dose-dependent manner. Sun and colleagues (2016) measured levels of prostate-specific antigen, dioxins, and steroid hormones in the serum of 97 men who had resided in a dioxin “hot spot” in Vietnam near a former U.S. airbase. Eighty-five men from a non-sprayed region in the north of the country served as controls. The investigators collected information on the subjects’ health status, residence history, smoking
habit, alcohol consumption, and occupation via a questionnaire. While dioxins, furans, and non-ortho PCBs levels were significantly higher in men in the hot spot region, prostate-specific antigen concentrations did not differ significantly between the groups. Levels of testosterone (p = 0.003) and estradiol (p = 0.024) were significantly higher in hot spot subjects and those of dehydroepiandrosterone (p = 0.047) significantly lower, but there were no significant differences for the other steroid hormones (androstenedione, cortisol, cortisone, dihydrotestosterone, estrone, and progesterone) measured. The study’s primary weaknesses are its cross-sectional design, and its lack of control for possible confounders, and the fact that prostate-specific antigen level is not a health outcome.
Biological Plausibility
Although a study reported that doses of 2,4-D greater than 50 mg/kg/day produce acute testicular toxicity in male rats (Joshi et al., 2012), there is little evidence that lower doses of either 2,4-D or 2,4,5-T (when free of TCDD contamination) given chronically have substantial effects on either the reproductive organs or fertility (Charles et al., 2001; Munro et al., 1992). The no-observed-adverse-effect level for 2,4-D is recognized as 15 mg/kg/day (Gervais et al., 2008). In contrast, many diverse laboratory studies have provided evidence that TCDD can affect reproductive-organ function and reduce fertility in both males and females.
The administration of TCDD to male animals elicits reproductive toxicity by affecting testicular, epididymal, prostate, and seminal vesicle weight and function and by decreasing the rate of sperm production (Foster et al., 2010; Rider et al., 2010; Schneider et al., 2014). The mechanisms underlying those effects are not known, but the primary hypotheses are that the changes are mediated through the dysregulation of testicular steroidogenesis, altered Sertoli cell function, and increased oxidative stress. The exposure of cultured testicular Leydig cells to 25 nM TCDD markedly alters gene expression (Naville et al., 2011), and the exposure of cultured Sertoli cells to 5 nM TCDD decreases viability and increases markers of oxidative stress (Aly and Khafagy, 2011). The exposure of adult rats or mice to TCDD (2–7 μg/kg/week for 45–60 days) reduces testicular and reproductive function, and these effects can be attenuated by co-treatment with various antioxidants (Beytur et al., 2012; Ciftci et al., 2012; Sönmez et al., 2011; H. P. Yin et al., 2012). The results of those studies are supported by the transgenic mouse model that harbors a constitutively active aryl hydrocarbon receptor (Ahr) in which testicular and ventral prostate weights and sperm number are reduced (Brunnberg et al., 2011).
The discussion of the influence of paternal exposures on outcomes in offspring later in the chapter contains additional information on the biologic plausibility of COI exposure affecting male reproductive health.
Synthesis
Reproduction is a sensitive toxic endpoint of TCDD and dioxin-like chemicals in rodents, and there are several species and strains of animals for which the fetus is more sensitive than the adult rodent to the adverse effects of TCDD. The sensitivity of these endpoints in humans, however, is less apparent. Although AHR plays an important role in normal sperm development (D. A. Hansen et al., 2014), there remains little evidence that exposure to dioxin is associated with a reduction in sperm quality or a reduction in fertility. Some of the studies reviewed in this update found an association between dioxin or dioxin-like PCB exposure and one or more parameters associated with male reproductive health, primarily poor sperm morphology (Mínguez-Alarcón et al., 2017; Paul et al., 2017). However, others reported only non-significant associations or none at all. The various weaknesses in these studies’ methodologies that were noted by the committee, including small sample sizes and difficulty in generalizing the results to the exposure experience of Vietnam veterans, greatly limits their usefulness.
Conclusion
On the basis of its evaluation of the evidence reviewed here and in previous VAO reports, the committee concludes that there is inadequate or insufficient evidence of an association between exposure to the COIs and alterations in semen quality or other male reproductive health markers.
FEMALE REPRODUCTIVE HEALTH
Studies of the relationship between chemicals and fertility are less common in women than in men. Some chemicals may disrupt the female hormonal balance necessary for proper functioning. Normal menstrual-cycle functioning is also important in the risk of hormonally related diseases, such as osteopenia, breast cancer, and cardiovascular disease. Generally speaking, chemicals can have multiple effects on the female system, including the modulation of hormone concentrations that results in uterine-cycle or ovarian-cycle irregularities, changes in menarche and menopause, and the impairment of fertility (Bretveld et al., 2006a,b). Past and current literature reviews have found studies relevant to the committee’s Statement of Task addressing endometriosis, hormonal levels, polycystic ovary syndrome, and gestational diabetes.
The committee responsible for the original VAO report (IOM, 1994) concluded that there was inadequate or insufficient evidence of an association between exposure to 2,4-D, 2,4,5-T, TCDD, picloram, or cacodylic acid and alterations in female reproductive health outcomes. Additional information available to the committees responsible for subsequent updates did not change the conclusion that exposure to the COIs had not been found to be associated with these outcomes.
Reviews of the relevant studies are presented in the earlier reports. Tables 30 and 31, which can be found at www.nap.edu/catalog/25137, summarize the results of studies related to endometriosis and other female reproductive health outcomes reviewed in the VAO series.
Endometriosis
Endometriosis (International Classification of Diseases, 9th revision [ICD-9] 617; ICD-10 N80.8) affects more than 5 million women in the United States and Canada at any given time (NICHD, 2017). The endometrium, the tissue that lines the inside of the uterus, is built up and shed each month during menstruation. In endometriosis, endometrial cells are found outside the uterus—usually in other parts of the reproductive system, in the abdomen or pelvis, or on surfaces near the reproductive organs. The ectopic tissue develops into growths or lesions that continue to respond to hormonal changes in the body and break down and bleed each month in concert with the menstrual cycle. Unlike blood released during normal shedding of the endometrium, blood released from degenerating ectopic endometrium has no way to leave the body. The blood sets up an inflammatory reaction causing pain, adhesions (scars), infertility, intestinal problems, or hematuria (blood in urine).
There are several theories of the etiology of endometriosis, including one that posits a genetic contribution, but the cause remains unknown. Estrogen dependence and immune modulation are established features of endometriosis, but they do not adequately explain its cause. It has been proposed that endometrium is distributed through the body via blood or the lymphatic system; that menstrual tissue backs up into the fallopian tubes, implants in the abdomen, and grows; and that all women experience some form of tissue backup during menstruation but only those who have immune-system or hormonal problems experience the tissue growth associated with endometriosis. Despite numerous symptoms that can indicate endometriosis, definitive diagnosis is possible only through laparoscopy or a more invasive surgical technique. Several treatments for endometriosis are available, but there is no cure.
Endometriosis was first reviewed in Update 2002, which identified two relevant environmental studies. Additional studies considered in later updates did not change the conclusion that the evidence is inadequate or insufficient to support an association with herbicide or dioxin exposure. Table 30, which can be found at www.nap.edu/catalog/25137, summarizes the results of studies related to endometriosis.
Update of the Epidemiologic Literature
No Vietnam-veteran, occupational, or environmental studies of exposure to the COIs and endometriosis have been published since Update 2014. The new
case-control studies regarding this outcome that met the committee’s criteria for review are summarized below.
Case-Control Studies Martínez-Zamora et al. (2015) used a case-control design to evaluate levels of dioxin-like chemicals in the adipose tissue in 30 women diagnosed with deep infiltrating endometriosis versus women in a control group who were undergoing laparoscopic surgery due to benign adnexal gynecological diseases other than endometriosis. All subjects were recruited from a university hospital located in Catalonia, Spain. Seventeen dioxins (7 PCDDs and 10 PCDFs) and 12 PCBs were analyzed. The investigators found that the TEQs and concentrations of both dioxins and PCBs were significantly higher in patients with deep infiltrating endometriosis than in the control group (p = 0.05), due primarily to significantly higher values of TCDD (odds ratio [OR] = 1.41, 95% confidence interval [CI] 1.12–2.10; p < 0.01) and 1,2,3,7,8-PeCDD (OR = 1.82, 95% CI 1.36–7.14, p < 0.01), which the authors observed was suggestive of a potential role for dioxin-like chemicals in the pathogenesis of deep infiltrating endometriosis. A strength of the study was the measurement of dioxins and PCBs in adipose tissue and the calculation of TEQs; its limitations included the small sample size (30 cases; 30 controls) and the selection bias induced by the recruitment of hospital surgery patients as subjects, which limits the generalizability of the results to other populations like female Vietnam veterans.
Ploteau et al. (2017) examined whether deep infiltrating endometriosis with or without concurrent ovarian endometrioma was associated with levels of POPs measured in the adipose tissue of cases and controls undergoing surgery in a clinic in the Pays de la Loire region of France during 2013–2015. Cases (n = 55) were 18 to 45 years old with a surgical diagnosis of deep infiltrating endometriosis; controls (n = 44) were women of similar age and BMI who had presented for other benign gynecological conditions. All subjects were interviewed for information on their health, physical state, and other factors thought to be associated with POP exposure. Serum was collected the day before the surgical procedure that led to their participation in the study; parietal and omental fat samples were obtained during the procedure. Biospecimens were tested for a number of POPs, including dioxins and dioxin-like PCBs. The investigators found statistically significant associations between deep infiltrating endometriosis and adipose tissue levels of four mono-ortho dioxin-like PCBs: 105 (adjusted odds ratio [aOR] = 2.09, 95% CI 1.24–3.77), 114 (aOR = 1.89, 95% CI 1.04–3.69]), 118 (aOR = 2.30, 95% CI 1.31–4.36), and 123 (aOR = 2.47, 95% CI 1.42–4.66) versus controls. The statistical significance held up when subjects with deep infiltrating endometriosis with ovarian endometrioma were examined. No such association was observed for the dioxin-like PCB 169. The authors noted that the associations were stronger for the subjects with deep infiltrating endometriosis with ovarian endometrioma but that the small number of such cases complicated the interpretation of the results. They did not calculate a TEQ, limiting the usefulness of their findings.
Other Identified Studies A 2017 review article by Parazzini et al. concluded that there were few studies on endometriosis and exposure to dioxin and that the available literature had inconsistent findings. Soave and colleague’s 2015 review contains information on several endometrial studies referenced in Update 2014, plus the Martínez-Zamora paper reviewed above.
Biological Plausibility
As observed in Update 2014, laboratory studies that used animal models and examined gene-expression changes associated with human endometriosis provide evidence of the biologic plausibility of a link between TCDD exposure and endometriosis. Genetic polymorphisms in the AHR signaling complex have been associated with a susceptibility to advanced endometriosis in humans (D. Li et al., 2013; C. H. Wu et al., 2012), although another study found no association in Japanese women (Matsuzaka et al., 2012). The first suggestion that TCDD exposure may be linked to endometriosis came as a secondary finding of a study that exposed female rhesus monkeys (Macaca mulatta) chronically to low concentrations of dietary TCDD for 4 years (Bowman et al., 1989). Ten and 13 years after the exposure ended, the investigators documented an increased incidence of endometriosis in the monkeys that correlated with the TCDD exposure concentration (Rier et al., 1993, 2001). The sample was too small to yield a definitive conclusion that TCDD was a causal agent of endometriosis, but this study led to additional studies of the ability of TCDD to promote the growth of pre-existing endometriotic lesions (Bruner-Tran et al., 1999; Johnson et al. 1997; J. Z. Yang et al., 2000).
There are a number of mechanisms by which TCDD may promote endometrial lesions, which provide additional support for the biologic plausibility of a link between TCDD and endometriosis. Human endometrial tissue and cultured human endometrial epithelial cells both express AHR; its dimerization partner, the aryl hydrocarbon nuclear translocator (Khorram et al., 2002); and three AHR target genes—CYP1A1, 1A2, and 1B1 (Bulun et al., 2000; Willing et al., 2011). These findings suggest that endometrial tissue is responsive to TCDD. M. N. Singh et al. (2008) showed that CYP1A1 expression is greater in ectopic endometrial tissue than in eutopic uterine tissue in the absence of TCDD exposure, which suggests that CYP1A1 may play a role in the etiology of the disease or that AHR and its signaling pathway have been activated by an endogenous ligand other than TCDD. Other mechanisms by which TCDD may promote endometriosis include altering the ratio of progesterone receptor A to progesterone receptor B and blocking the ability of progesterone to suppress matrix metalloproteinase expression—actions that promote endometrial-tissue invasion and that are observed in women who have endometriosis (Igarashi et al., 2005).
TCDD also induces changes in gene expression that mirror those observed in endometrial lesions. In addition to the induction of CYP1A1 noted above,
TCDD can induce expression of histamine-releasing factor, which is increased in endometrial lesions and accelerates their growth (Oikawa et al., 2002, 2003). TCDD disrupts cannabinoid signaling in endometrial stromal cells by inhibiting the progesterone-induced expression of cannabinoid receptor type 1, which is also observed in women with endometriosis (Resuehr et al., 2012). TCDD also stimulates the expression of RANTES (regulated on activation, normal T-cell–expressed, and secreted protein) in endometrial stromal cells, and RANTES concentration and bioactivity are increased in women who have endometriosis (Zhao et al., 2002). The two CC-motif chemokines (chemotactic cytokines), RANTES and macrophage-inflammatory protein-1α (MIP-1α), have been identified as potential contributors to the pathogenesis and progression of endometriosis. Previous studies have shown that the combination of 17β-estradiol and TCDD increases the secretion of RANTES and MIP-1α in endometrial stromal cells (Yu et al., 2008), and a more recent study showed that the same combination suppresses the expression of tetraspanin CD82, a tumor-metastasis suppressor, and thus promotes the invasion of endometrial stromal cells (M. Q. Li, 2011). Those results support the idea that TCDD in combination with estradiol may contribute to the development of endometriosis by increasing the invasiveness of endometrial cells. Despite that evidence, chronic exposure of rats to TCDD, non-dioxin-like PCB 153, dioxin-like PCB 118 or PCB 126, or 2,3,4,7,8-PeCDF (the furan congener with the highest TEF), either individually or in various combinations, fails to alter endometrial histology in a consistent manner (Yoshizawa et al., 2009). The differences between rodent and human endometrium could account for the lack of observed effects in rats.
In summary, experimental studies, particularly ones that used human eutopic and ectopic endometrial tissue, provide evidence of the biologic plausibility of a link between TCDD exposure and endometriosis.
Synthesis
The human studies linking dioxin exposure with endometriosis are few and inconsistent; information related to exposures to 2,4-D, 2,4,5-T, TCDD, picloram, or cacodylic acid is lacking. Although animal studies support the biologic plausibility of an association, contemporary human exposures may be too low to show an association should one exist.
Conclusion
On the basis of the evidence reviewed here, in VAO, and in the previous VAO updates, the committee concludes that there is inadequate or insufficient evidence to determine whether there is an association between exposure to the COIs and endometriosis.
Other Female Reproductive Health Outcomes
Update of the Epidemiologic Literature
Polycystic Ovary Syndrome (PCOS) PCOS is a hormonal disorder characterized by overproduction of the androgen testosterone. Three potentially relevant studies published since Update 2014 addressed this condition. Vagi et al. (2014) used a case-control pilot study design with Cedars Sinai Medical Center, Los Angeles, California, patients. Serum PCBs—including the dioxin-like PCBs 105, 118, and 156—organochlorine pesticides, and other compounds were measured in the urine of 52 cases and 50 controls. None of the dioxin-like PCBs was significantly elevated in cases compared to controls.
Q. Yang et al. (2015) used a case control design to evaluate serum PCBs, pesticides, and polycyclic aromatic hydrocarbons in 50 cases and 30 control ethnic Han females recruited from the Peking University Third Hospital in northern China. Exposure to dioxin-like PCBs was associated with an OR of 4.89 (95% CI 1.81–13.2), which remained significant after adjusting for confounders including education and occupation (aOR = 5.52; 95% CI 1.51–20.2). Study limitations include a small sample size and metabolic differences between the cases and control that could affect the absorption, distribution, metabolism, and excretion of COIs.
J. Zhang et al. (2014) used a cross sectional stratified case control design to examine PCOS in females aged 12–44 years in Chengdu, China; 169 cases and 338 controls were analyzed. Questionnaire responses were used to characterize exposure. No exposures to COIs were measured, although they can be inferred for two categories characterizing a history of contact with polybrominated biphenyls: “pesticide,” which yielded an odds ratio of 1.76 (95% CI 1.09–2.87) and “eating fruit with pericarp” 5.00 (95% CI 2.67–9.48). However, the considerable uncertainty associated with this inference greatly limits the usefulness of the study results to the committee.
Gestational Diabetes Mellitus Vafeiadi et al. (2017) used a cross-sectional/semi-prospective design to evaluate PCBs and other compounds in first-trimester maternal serum (dioxin-like PCBs 118 and 156 summed) with relation to gestational diabetes mellitus measured at 24–28 weeks in 939 mother–infant pairs from the Rhea pregnancy cohort in Crete, Greece. Sixty-eight (7%) mothers presented with gestational diabetes mellitus. The adjusted odds ratio of gestational diabetes mellitus in women in the “medium” (middle) tertile of dioxin-like PCBs was 5.63 (95% CI 1.81–17.51) and for the high tertile 4.71 (95% CI 1.38–16-01). The study controlled for maternal fish consumption, but the authors could not rule out undiagnosed type 1 or type 2 diabetes or other unmeasured lifestyle factors as confounders, limiting the usefulness of the results.
Alteration in Hormonal Levels Anh et al. (2017) examined the relationship between adrenal hormone disruption in lactating mothers and their children in two locations of Vietnam, one of which was known to be contaminated with dioxin. Thirty-seven mother–child pairs were recruited from the area near the former U.S. air base at Bien Hoa, which stored tactical herbicides during the Vietnam War and where at least four accidental releases of Agent Orange took place; 47 control pairs from a rural community in northern Vietnam—where no U.S. spraying took place—were also examined. Breast milk and blood samples were collected from all mothers 4–16 weeks after the birth of their first child. Data on subjects’ social characteristics, diseases (including hormonal therapy), and body measurements were obtained via questionnaire. The researchers found a statistically significant difference (p < 0.001) between serum androstenedione1 levels in mothers who resided in the dioxin contaminated region (n = 37; 1.91 ng/mol ± 1.00) versus the non-contaminated region (n = 47; 0.61 ng/mol ± 0.27). They also noted an association between maternal levels of several dioxin congeners and subsequent salivary dehydroepiandrosterone levels in children (total TEQ β = 0.42; p < 0.001). These results suggested to them that dioxin disrupts adrenal androgens in mothers and breastfeeding children through the same mechanism. However, since the children were exposed both before and after birth, this study is of limited relevance to the committee.
A cross-sectional study by Lignell and colleagues (2016) evaluated PBDE, PCB, and dioxin exposures in relation to thyroid hormone status early in pregnancy in randomly selected first-time mothers (1996–1999) from Uppsala County, Sweden. Maternal body burden of PCDD/Fs was inversely associated with first-trimester T3 thyroid hormone levels. In fully adjusted models, no PCBs were associated with first-trimester thyroid hormone levels; however, in crude analysis di-ortho and mon-ortho PCB TEQ concentrations were negatively associated with serum lipids in the third trimester and T3 in the first trimester. This result was deemed noteworthy because maternal thyroid hormone status influences fetal development in early pregnancy. However, reverse causality cannot be discounted in this study as maternal T3 could influence the absorption, distribution, metabolism, and excretion of toxicants.
Biological Plausibility
Many studies have examined the effects of TCDD on the female reproductive system. The two primary mechanisms that are believed to contribute to abnormal follicle development and decreased numbers of ova after TCDD exposure are the “cross-talk” of the AHR with the estrogen receptor and the dysregulation of the hypothalamic–pituitary–gonadal axis (Pocar et al., 2005; Safe and Wormke,
___________________
1 Androstenedione and dehydroepiandrosterone are so-called “pro-hormones” that are precursors in the production of testosterone and estrogen.
2003). Oocytes are directly responsive to TCDD, so TCDD’s effects on hormone concentrations, hormone-receptor signaling, and ovarian responsiveness to hormones all probably contribute to TCDD-induced female reproductive toxicity. The data of Jung et al. (2010) in rats show that a single high-dose gavage treatment of 32 μg/kg TCDD reduces the proliferation of granulosa cells and thus attenuates cell-cycle progression and potentially contributes to the reduction in ovulation rates observed in other studies. In contrast, Karman et al. (2012) found that 1 nM TCDD exposure in vitro did not reduce the rates of growth of murine antral follicles, but did reduce the secretion of progesterone and estradiol by the follicles. The concentrations of those hormones could be restored by the addition of the precursor pregnenolone, which suggests that TCDD acts upstream of pregnenolone formation. Baldridge et al. (2015) have also shown that rat granulosa cells are highly sensitive to low-level (femtomolar) TCDD, resulting in disrupted steroid hormone secretion. A similar result was demonstrated by Jablonska et al. (2014) in porcine granulosa cells. This would be consistent with previous observations in zebrafish that 10, 40, and 100 parts per billion TCDD in food depressed estradiol biosynthesis (Heiden et al., 2008).
Dioxin’s effects on early embryo development and on placenta formation are well documented (S. C. Chen et al., 2010; Ishimura et al., 2009; Tsang et al., 2012). Petroff et al. (2011) used a rat in vitro fertilization model to demonstrate that 100 nM TCDD perturbs chromatin and cytoskeletal remodeling at the earliest stages of embryo development, but these changes failed to result in any apparent morphologic changes at later stages of development. The long-term potential effects of these early changes on pregnancy outcome are unknown. It has previously been shown that TCDD may have direct effects on human trophoblast formation at 0.2–2.0 nM in vitro and thus may have the capacity to influence the developing fetus (S. C. Chen et al., 2010). That idea is supported by a study showing the ability of 5 nM TCDD to activate the AHR signaling pathway in both rat and human placental trophoblasts (Stejskalova et al., 2011). Finally, a study has demonstrated that TCDD at 0.1, 1.0, and 10.0 nM reduces in a dose-dependent fashion the ability of trophoblastic spheroids (which constitute an embryo surrogate) to attach to endometrial epithelial cells (Tsang et al., 2012). The more recent literature continues to support the biologic plausibility of TCDD having effects on male and female reproduction.
Synthesis
Eskenazi et al. (2010)—reviewed in Update 2010—published the only study to date that has examined dioxin exposure in women with respect to time-to-pregnancy (number of contraceptive-free months before pregnancy) and infertility (more than 12 contraceptive-free months to pregnancy). Dose–response relationships between TCDD serum levels in women who were less than 40 years of age at the time of the Seveso accident and both time-to-pregnancy and
infertility were observed, which is consistent with published observations in the rat model. Epidemiologic studies have not provided sufficient data to interpret the effects of dioxin specifically on menstrual-cycle function in humans.
The studies reviewed for this update yielded inconsistent results regarding any association between exposure to the COIs and the outcomes studied, and they exhibited weaknesses such as the failure to measure dioxin levels or calculate TEQs that limit their applicability to the evaluation of outcomes for Vietnam veterans.
Conclusion
On the basis of its evaluation of the evidence reviewed here and in previous VAO reports, the committee concludes that there is inadequate or insufficient evidence of an association between exposure to the COIs and disturbances in hormonal levels in females and diseases that may be associated with such disturbances.
GESTATION AND NEONATAL EFFECTS IN OFFSPRING
The gestation and neonatal periods are uniquely vulnerable times in life. Data on health outcomes either occurring or first identified during these periods have been collected on the offspring of those exposed to the COIs. Information concerning spontaneous abortion, stillbirth, neonatal death, and infant death; sex ratio; birth weight and preterm delivery; and birth defects are discussed below.
General Biologic Plausibility
Influence of Paternal Exposure
James (2006) has interpreted the perturbation of sex ratios by dioxins and other agents as being an indicator of parental endocrine disruption and, indeed, a population-level finding of a paternally mediated effect would be a strong indicator that dioxin exposure can interfere with the male reproductive process. If this observation were demonstrated to be true, then it would be concordant with a reduction in testosterone in exposed men (Egeland et al., 1994). Another pathway to an altered sex ratio might involve male embryos experiencing more lethality from the induction of mutations due to their unmatched X chromosome. TCDD has not been thought to have genotoxic effects, but sex-specific adverse consequences of the modified imprinting of gametes might be a possible mechanism leading to the observation of altered sex ratios at birth. To date, however, the proportion of sons among the children of fathers exposed to dioxin-like chemicals does not present a clear pattern of reduction.
The idea that the exposure of either parent to a toxicant before conception could result in an adverse outcome in offspring is not new and remains a topic of much interest (Schmidt, 2013). Epidemiologic studies have reported occasional findings of paternally transmitted adverse outcomes associated with paternal exposures to certain agents, but none has been replicated convincingly. Even in instances in which an agent is recognized as mutagenic or potentially carcinogenic for exposed men, adverse consequences have not been demonstrated in their children. For example, the hypothesis was extensively investigated in the early 1990s in relation to fathers’ exposure to ionizing radiation before conception and an increase in leukemias in their offspring. The initial study (Gardner et al., 1990) was conducted in men who worked at the Sellafield nuclear facility in West Cumbria, United Kingdom. It was presumed that the men were exposed to radiation as a result of working at Sellafield. An association was found between radiation exposures to fathers before their children’s conception and an increase in leukemias among those children. However, later studies failed to confirm that finding (Draper et al., 1997; Kinlen, 1993; Kinlen et al., 1993; Parker et al., 1993; Urquhart et al., 1991). Similarly, a rigorous follow-up of children of atomic-bomb survivors has not demonstrated increased risks of cancer or birth defects (Fujiwara et al., 2008; Izumi et al., 2003; Schull, 2003), and other studies of birth defects and cancer in the children of male cancer survivors after chemotherapy or radiation treatment have found little support for paternal transmission (Chow et al., 2009; Dohle, 2010; Howell and Shalet, 2005; Madanat-Harjuoja et al., 2010), although sperm and fertility clearly are adversely effected (Green et al., 2010).
An additional problem when trying to determine whether adult male exposure of any type (including to the COIs) can lead to pathological effects in descendants is that almost all experimental exposure studies designed to identify male transmission have been limited to developmental exposures in rodents (Guerrero-Bosagna and Skinner, 2014; Paoloni-Giacobino, 2014). An early experiment examining male mice treated with simulated Agent Orange mixtures prior to breeding with unexposed females failed to find an increase in a variety of different birth defects in progeny compared with the progeny of untreated males (Lamb et al., 1981).
Prior VAO committees have been unable to identify epidemiologic evidence that convincingly demonstrated paternal exposure to any particular chemical before conception that resulting in cancers or birth defects in offspring. However, few data exist to address the hypothesis of paternal exposure and adverse effects in human offspring in which the exposure occurred before conception only to the father, and what little information exists comes from the radiation effects literature. Thus, it is difficult to assert conclusively that the available epidemiologic evidence either supports or does not support paternal transmission; considerable uncertainty remains on many fronts and would presumably vary by agent and mode of exposure. Several systematic reviews of the topic have been conducted
(Chia and Shi, 2002; Weselak et al., 2007, 2008; Wigle et al., 2007, 2008), and none have established firm relationships between specific agents and particular effects in offspring. Paternal occupation (characterized by exposure to chemicals via job title or job–exposure matrices) has been linked to an increased risk of selected birth defects (Desrosiers et al., 2012; Fear et al., 2007; Shaw et al., 2002) and neuroblastoma (De Roos et al., 2001a,b). Moreover, increased risks of childhood brain cancer have been reported in relation to paternal exposure to selected pesticides, particularly herbicides and fungicides (van Wijngaarden et al., 2003), although the authors noted considerable uncertainty in the robustness of the findings. Therefore, the hypothesis that paternal preconception exposure to toxic agents may result in harm to a man’s children remains unresolved in significant part because of the sparseness of epidemiologic research on the subject.
Paternal Preconceptual Exposure There is no evidence that dioxins can mutate DNA sequences (see Chapter 4). Genetic changes in sperm genes due to preconception exposures to TCDD—as has been shown, for example, in connection with irradiation or the anticancer drug cyclophosphamide (Codrington et al., 2004)—are thus unlikely. The potential does exist, however, for TCDD to alter the sperm cells of adults before fertilization through epigenetic pathways. The sperm epigenome is distinctive from that of the egg (oocyte) or somatic cells (all other non-gamete cells in the body). The mature sperm cell has less global methylation than somatic cells, particularly at gene promoters, and has unique DNA methylation marks (particularly on paternally imprinted genes) that put the sperm genomes in a pluripotent-like state before fertilization (Hales et al., 2011). However, rapid demethylation of most of the remainder of the paternal genome occurs shortly after fertilization (Dean, 2014), suggesting that additional changes are required for the nascent embryo to become truly pluripotent. Chemical alterations of DNA methylation foci of adult sperm have the potential to contribute to permanent effects in offspring, as has been suggested for male transmittance in fetal alcohol syndrome (Jenkins and Carrell, 2012a). During spermatogenesis in the adult, most sperm histones are replaced by protamines, which render the sperm transcriptionally quiescent and permit extensive DNA compaction. However, some core histones are retained in human sperm with appropriate epigenetic modifications in order to maintain open nucleosomes at sites that are important during embryo development (Casas and Vavouri, 2014), so their perturbation by exogenous chemicals remains a possibility. This is particularly important because although genome-wide DNA demethylation occurs in paternal DNA after fertilization (Dean, 2014) and should erase most sites that have been reprogrammed by chemicals, histone modification patterns are retained and thus may transmit chemical-induced alterations across generations (Puri et al., 2010).
Despite the exclusion of almost all cytoplasm, mature sperm, as noted above, have been found to carry a diverse spectrum of RNAs, including messenger RNAs, ribosomal RNAs, and small noncoding RNAs, which may affect the developing
embryo (Casas and Vavouri, 2014; Hamatani, 2012; Kawano et al., 2012; Krawetz, 2005; Krawetz et al., 2011; Lane et al., 2014; Suh and Blelloch, 2011). For example, small RNAs of paternal origin may direct epigenetic modifications during embryo development and lead to changes in phenotype later in life (Hales et al., 2011). When newborn male mice were stressed by unpredictable separation from their mothers, messenger RNAs in their sperm have been shown to transmit the effects of this early trauma for two generations (Gapp et al., 2014). Heavy metals interact with sperm’s nuclear proteins, and this mechanism is suspected to be a basis of the paternally mediated effects of lead (Quintanilla-Vega et al., 2000). Disturbances in the establishment of the epigenetic marks in mature sperm may change cell fate in the early embryo and have effects throughout development and postnatal life (Jenkins and Carrell, 2012b).
Direct evidence of dioxin-mediated changes in the epigenome of mature sperm is not available. However, dioxins have been shown to modify DNA methylation in somatic cells (Hou et al., 2012), so an epigenetic pathway is plausible.
Paternal Postconceptual Exposure Contaminants such as TCDD that are present in the tissues and blood of exposed males can be transported as parent compounds or as metabolites into seminal fluid, the noncellular component of the ejaculate. Typically, the concentrations of contaminants in seminal fluid are lower than those in serum, but no direct assessments of the ratios of serum to seminal fluid in TCDD have yet been reported. Seminal-fluid contaminants can be transmitted to a female during sexual intercourse and be absorbed through the vaginal wall; if the concentrations are high, then they could potentially affect a current pregnancy (Chapin et al., 2004; Klemmt and Scialli, 2005). TCDD and other POPs have been identified and quantified in the seminal plasma of exposed men, including Vietnam veterans (Schecter et al., 1996; Schlebusch et al., 1989; Stachel et al., 1989); thus, this transmission route is theoretically possible. In the Schecter et al. (1996) study, serum TCDD was measured in 50 Vietnam veterans from Michigan who had a confirmed or self-reported potential for herbicide exposure and had blood drawn an average of 26 years after the possible exposure. Of those, six had TCDD levels greater than 20 parts per trillion (ppt) on a lipid-adjusted basis, which supports the idea that some veterans had high initial exposures. A subgroup of 17 men contributed semen at the time of blood draw, and dioxin congeners were analyzed in three randomly pooled samples—a process necessary to provide sufficient volume for chemical analysis. Although the measured concentrations were very low, the results documented the existence of dioxins and dibenzofurans in the seminal plasma of the veterans long after the possible herbicide exposure to TCDD-contaminated herbicides. Because the results on serum and semen concentrations could not be linked to individual veterans and because it is unknown whether any of the individuals who had high serum dioxin concentrations after 26 years contributed semen for the seminal-fluid measurements, the value of this information is
minimal. Seminal-fluid concentrations of TCDD and related chemicals closer to the period of exposure in Vietnam have not been determined, so it is not possible to assess the clinical consequences of this exposure route for female partners and gestating offspring. Banked Ranch Hand specimens, however, might provide a valuable resource for comparing TCDD concentrations in serum and seminal fluid. A 2015 Institute of Medicine report describes the available data and biospecimens from the Air Force Health Study (AFHS) and the potential for future analyses (IOM, 2015).
Despite the potential for a seminal fluid route of exposure, the critical question of dose sufficiency remains unanswered. That is, could absorbed TCDD concentrations be high enough to transmit adverse effects to the fetus? To answer that question, one must take into account several factors. First, the volume of seminal plasma is relatively low (1–5 mL) and, because of leakage, only a fraction of seminal constituents is absorbed across the vaginal wall. Moreover, the dilution of absorbed chemicals in the female bloodstream before transmission across the placenta is estimated at 3 orders of magnitude or more (Klemmt and Scialli, 2005), which reduces a serum concentration of 20 ppt to a scale of parts per quadrillion (10–15). Although no studies have been undertaken to address the issue directly, the dilution factor makes it extremely unlikely that adverse fetal and offspring outcomes would occur as a consequence of seminal plasma exposures to TCDD during pregnancy. One caveat to this conclusion, however, is that seminal fluid is now known to play an important role in the metabolic phenotype of offspring because it stimulates embryotrophic factors (Bromfield, 2014; Bromfield et al., 2014). Whether TCDD contamination of the seminal fluid can affect this function is not known and should be tested.
Influence of Maternal Exposure
Maternal exposures can affect a pregnancy and the resulting offspring far more extensively than can paternal exposures. Because of the long half-life of TCDD and its bioaccumulation in adipose tissues, women exposed to herbicides in Vietnam would have the potential to expose their offspring to TCDD directly during later pregnancies. Thus, damage to the resulting offspring or future generations could result from epigenetic changes in an egg before conception or from the direct effects of exposure on the fetus during gestation and on the neonate during lactation. Dioxin in the mother’s bloodstream can cross the placenta and expose the developing embryo and fetus.
Furthermore, the mobilization of dioxin during pregnancy or lactation may be increased because the body is drawing on fat stores to supply nutrients to the developing fetus or nursing infant. TCDD has been measured in circulating human maternal blood, cord blood, placenta, and breast milk (G. Suzuki et al., 2005), and it is estimated that an infant breastfed for 1 year accumulates a dose of TCDD that is six times as high as an infant who is not breastfed (Lorber and Phillips, 2002).
The offspring effects of maternal exposures may not be manifested immediately and could be a result of a dioxin-mediated reprogramming of developing organs and lead to a disease onset later in life. As noted elsewhere, placental structure and function are believed to play a major role in fetal growth, and TCDD has been shown to alter placental vascular remodeling (Y. Wu et al., 2013, 2014).
As mentioned in conjunction with the role of the placenta in fetal development, the developmental basis of adult disease (Barker et al., 2012) is being actively researched through the investigation of maternal nutritional exposures, stress, and alcohol exposure, and more recent studies have examined exposures to TCDD and other environmental toxicants. The molecular basis of the later-life effects is believed to be primarily epigenetic. Maladies that may be manifested later in life include neurologic and reproductive disorders, thyroid changes, diabetes, obesity, and adult-onset cancers. Furthermore, germ cells (eggs and spermatogonia) in offspring pass through critical developmental stages during fetal life (D. A. Hansen et al., 2014), and emerging evidence demonstrates that fetal exposures are capable of altering the germ cells epigenetically, resulting in a transmission of adverse effects to future generations (intergenerational and transgenerational inheritance) (D. A. Hansen et al., 2014).
Spontaneous Abortion, Stillbirth, Neonatal Death, and Infant Death
Spontaneous abortion is the expulsion of a nonviable fetus—generally before 20 weeks of gestation—that is not induced by physical or pharmacologic means. The background risk of recognized spontaneous abortion is generally 11–22% (Avalos et al., 2012), but it is established that many more pregnancies terminate before women become aware of them (Wilcox, 2010). Such terminations are known as subclinical pregnancy losses and generally are not included in studies of spontaneous abortion. The estimates of the risk of recognized spontaneous abortion vary with the design and method of analysis. Studies have included cohorts of women asked retrospectively about pregnancy history, cohorts of pregnant women (usually those receiving prenatal care), and cohorts of women who are monitored for future pregnancies. The value of retrospective reports can be limited by the differential recall of details (exposure history, for example) specific to pregnancies that occurred long before the interview. Studies that enroll women who present for prenatal care require the use of life tables and specialized statistical techniques to account for differences in the times at which women seek medical care during pregnancy. The enrollment of women before pregnancy provides the theoretically most valid estimate of risk, but it can attract non-representative study groups because the study protocols are demanding for the women.
Countries and U.S. states have different legal definitions of the age of fetal viability and apply these terms differently. The American College of Obstetricians and Gynecologists defines “stillbirth” as the delivery of a fetus that shows no signs of life (that is, an absence of breathing and heartbeat; pulsations in umbilical cord
are absent; no voluntary movement of muscle) at 20 weeks or greater of gestation (if the gestational age is known) or at a weight greater than or equal to 350 g if the gestational age is not known (Da Silva et al., 2016). “Neonatal death” refers to the death of a live-born infant within 28 days of birth (WHO, 2006) and “postnatal death” refers to a death that occurs before the first birthday (Andrews et al., 2008).
The causes of stillbirth and early neonatal death overlap considerably, so they are commonly analyzed together in a category referred to as “perinatal mortality” (Andrews et al., 2008). Stillbirths make up less than 1% of all births (CDC, 2000). The most common causes of mortality during the neonatal period are low birth weight (< 2.5 kg at birth), preterm delivery, congenital malformations, pregnancy or delivery complications, and placenta or cord conditions. The most common causes of postnatal death in infants is SIDS (sudden infant death syndrome) (Andrews et al., 2008).
The committee responsible for the original VAO report concluded that there was inadequate or insufficient evidence of an association between exposure to 2,4-D, 2,4,5-T, TCDD, picloram, or cacodylic acid and spontaneous abortion or perinatal death. Additional information available to the committees responsible for Update 1996, Update 1998, and Update 2000 did not change that conclusion.
The committee responsible for Update 2002, however, found that there was enough evidence available to conclude that there was “limited or suggestive evidence of no association” between paternal exposure to TCDD and the risk of spontaneous abortion. That conclusion was based primarily on a National Institute for Occupational Safety and Health study (Schnorr et al., 2001) that investigated a large number of pregnancies fathered by workers whose serum TCDD concentrations were extrapolated back to the time of conception; no association was observed up to the highest-exposure group (1,120 ppt or higher). Indications of a positive association were seen in studies of Vietnam veterans (CDC, 1989c; Field and Kerr, 1988; S. D. Stellman et al., 1988b), but the committee responsible for Update 2002 asserted that they might be due to an exposure to phenoxy herbicides rather than to TCDD and concluded that there was insufficient information to determine whether there is an association between maternal exposure to TCDD and the risk of spontaneous abortion or between maternal or paternal exposure to 2,4-D, 2,4,5-T, picloram, or cacodylic acid and the risk of spontaneous abortion.
The additional information (none of which concerned paternal exposure) reviewed by the committees responsible for the Update 2004 through Update 2014 reports did not change these conclusions.
The relevant studies concerning perinatal death are reviewed in the earlier reports. Table 32, which can be found at www.nap.edu/catalog/25137, summarizes the results of studies related to spontaneous abortion.
Update of the Epidemiologic Literature
No Vietnam-veteran, occupational, environmental or case-control studies of exposure to the COIs and spontaneous abortion or perinatal death have been published since Update 2014.
Biological Plausibility
Laboratory animal studies have demonstrated that TCDD exposure during pregnancy can alter the concentrations of circulating steroid hormones and disrupt placental development and function and thus contribute to a reduction in the survival of implanted embryos and to fetal death (L. Huang et al., 2011; Ishimura et al., 2009; J. Wang et al., 2011; Y. Wu et al., 2013, 2014). There is no evidence of a relationship between paternal or maternal exposure to TCDD and spontaneous abortion. Exposure to 2,4-D or 2,4,5-T causes fetal toxicity and death after maternal exposure in experimental animals. However, that effect occurs only at high doses and in the presence of maternal toxicity. No fetal toxicity or death has been reported to occur after paternal exposure to 2,4-D.
Animal studies of maternal TCDD exposure during pregnancy have demonstrated the induction of fetal death; neonatal death, however, is only rarely observed and is usually the result of TCDD-induced cleft palate, which leads to an inability to nurse. A single study by Y. Wu et al. (2016) suggests that AHR may be involved in unexplained recurrent spontaneous abortion without consideration of TCDD or other ligands. Further study in this area is warranted. Studies addressing the potential for perinatal death as a result of paternal exposure to TCDD or herbicides are at this point inadequate to support drawing any conclusions.
Synthesis
No studies concerning the COIs and spontaneous abortion, stillbirth, neonatal death, or infant death have been published since Update 2014, and available toxicologic studies do not provide clear evidence for the biologic plausibility of an association with paternal exposures.
Conclusion
On the basis of the evidence reviewed to date, the committee concludes that there is limited or suggestive evidence that paternal exposure to TCDD is not associated with risk of spontaneous abortion and that insufficient information is available to determine whether there is an association between maternal exposure to TCDD or either maternal or paternal exposure to 2,4-D, 2,4,5-T, picloram, or cacodylic acid and the risk of spontaneous abortion. The committee concludes that there is inadequate or insufficient evidence to determine whether there is an
association between exposure to the COIs and stillbirth, neonatal death, or infant death.
Sex Ratio
Although it would not constitute an adverse health outcome in an individual veteran, perturbations in the sex ratio of children born to an exposed population would suggest that the exposure had an impact on the reproductive process. Previous reports in the VAO series have reviewed the literature on this topic. Update 2014 contains a summary of this work, which is also cataloged in Table 33, which may be found in the supplementary tables available at www.nap.edu/catalog/25137. In brief, studies have found an alteration—most often, a reduction—in the expected proportion of male infants at birth, but results are inconsistent and only some reach statistical significance.
Update of the Epidemiologic Literature
One occupational cohort study on TCDD exposure and sex ratio has been published since Update 2014 was released. 't Mannetje et al. (2017) conducted a retrospective study of workers employed for at least 1 month between 1969 and 1984 at a phenoxy herbicide production plant in New Zealand. In 2007–2008, a sample of 430 workers out of 631 known to be alive under the age of 80 years and living in New Zealand was randomly selected. Of these, 244 responded, and 212 had a biological child. Blood samples were collected and a survey was conducted regarding biological children, their birthdates, sex, and medical conditions. Logistic regression was conducted with the outcome of male versus female sex, with adjustment for the parent’s age at the time of birth, BMI at the time of blood draw, and smoking status. Both female and male employees were included, and analyses were conducted separately; however, the number of female employees was small. Exposures were estimated for the time of the child’s birth by back-calculation using first-order toxicokinetics and a half-life of 7.6 years. Results showed reduced odds of a male birth when comparing high- versus low-TCDD exposure. For a father’s exposure in 2007–2008, reduced odds of a male birth (OR = 0.46, 95% CI 0.29–0.73) were observed for TCDD serum concentration ≥ 4 versus < 4 pg/g lipid (OR = 0.49, 95% CI 0.30–0.79). Similarly, for a father’s exposure at time of the child’s birth, reduced odds of a male birth were observed, comparing TCDD concentration ≥ 20 versus < 20 pg/g lipid (OR = 0.49, 95% CI 0.30–0.79). Additionally, a significant dose–response was observed, both for the TCDD as measured in 2007–2008 (p trend = 0.01) and for exposure estimated at time of the child’s birth (p trend = 0.007). In both of these analyses, the upper two exposure categories showed significant ORs of approximately half the probability of a male child compared with fathers whose exposure to TCDD was in the low-dose group. The study found that paternal serum TCDD concentrations in
excess of an estimated 20 pg/g lipid at time of conception were associated with a reduced sex ratio. No significant association was observed for the mother’s exposure to TCDD, although the ORs were elevated, not reduced. These findings are consistent with a male-mediated reduction in sex ratio, which had been observed in some prior studies (del Rio Gomez et al., 2002; Hertz-Picciotto et al., 2008; Mocarelli et al., 1996, 2000). The lack of an association for maternal exposure is consistent with an endocrine-mediated outcome.
Biological Plausibility
In a previously unreviewed paper, Ishihara et al. (2007) found that male ICR mice administered TCDD orally with an initial loading dose of 2,000 ng TCDD/kg followed by a weekly maintenance dose of 400 ng/kg (T2,000/400) prior to mating produced a significantly lower proportion of male offspring than controls (controls: 53.1% ± 1.7; T2000/400: 46.2% ± 2.1) while there was no alteration in litter size. The authors speculated that TCDD might selectively reduce the fertility potential of Y-bearing gametes before conception but stipulated that no mechanism could be identified.
An investigation by You et al. (2018) treated mouse spermatozoa with different concentrations of TCDD in vitro (0.25, 25, 2,500 ng/mol) and then performed in vitro fertilization. The sex ratio of two-cell embryos was significantly decreased at all TCDD treatment groups compared with the control (p < 0.05). In addition, the sex ratio of blastocysts decreased significantly for the 25 and 2,500 ng/ml of TCDD treatment groups (p < 0.01), and embryo sex ratios were negatively correlated with live X sperm proportion (two-cell embryos, r = –0.697; blastocyst, –0.856; p < 0.05). The researchers concluded that TCDD may affect the fertility of Y spermatozoa more than X spermatozoa but asserted that further studies are needed to evaluate the source of the difference of fertilizing capability between X and Y spermatozoa exposed to TCDD.
Synthesis
Prior to this update, committees had not identified any experimental animal studies that specifically examined the effects of the COI on the sex ratios of offspring, nor had any alterations in sex ratio been reported in animal studies that examined the developmental effects of the COIs on offspring. However, the recent publication of You and colleagues’ (2018) study and the previously unreviewed Ishihara et al. (2007) paper yielded information for their consideration. The committee finds that the papers’ results are consistent with those observed in human studies but that the lack of an identified mechanism underlying the observations limits their usefulness. These data, along with the new 't Mannetje et al. (2017) retrospective study add incrementally to the body of knowledge on the topic but do not change any of the conclusions.
Conclusion
On the basis of its evaluation of the evidence reviewed here and in previous VAO reports, the committee concludes that there is inadequate or insufficient evidence of an association between exposure to the COIs and altered sex ratio.
Birth Weight and Preterm Delivery
Birth weight and the length of the gestation period can have important effects on neonatal morbidity and mortality and on health over the life span. Typically, low birth weight (LBW) is defined as a birth weight under 2,500 g (~5.5 lbs.) (UNICEF, 2004). In the absence of congenital malformations or chromosomal anomalies, LBW is the consequence of either preterm delivery or an intrauterine growth restriction. Preterm delivery is delivery at less than 259 days or 37 weeks of gestation from the date of the first day of the last menstrual period (Jones and Lopez, 2013). Intrauterine growth restriction is functionally defined in terms of LBW relative to gestational age when compared to local or national fetal-growth graphs (Romo et al., 2009). LBW occurs in about 7% of live births. When no distinction is made between intrauterine growth restriction and preterm delivery, the factors most strongly associated with LBW are maternal tobacco use during pregnancy, multiple births, and race or ethnicity. Other potential risk factors are low socioeconomic status, malnutrition, maternal weight, birth order, maternal complications during pregnancy (such as severe pre-eclampsia or intrauterine infection), obstetric history, job stress, and cocaine or caffeine use during pregnancy (Alexander and Slay, 2002; Alexander et al., 2003; Ergaz et al., 2005; Jones and Lopez, 2013; Peltier, 2003). Established risk factors for preterm delivery include race (black), extremes of maternal age, low socioeconomic status, previous LBW or preterm delivery, multiple gestations, tobacco use, and low maternal pre-pregnancy weight or poor pregnancy weight gain (Rubens et al., 2014).
The importance and interpretation of risk factors for associations with birth weight are often unclear and a subject of controversy among researchers (Barker et al., 2012; Wilcox, 2010). Across populations, the frequency distribution of birth weight is Gaussian, with an extended lower tail, or “residual distribution,” that includes preterm and LBW infants. The predominant, normal distribution corresponds largely to term births. In general, shifts in the predominant distribution do not tend to correspond to notable shifts in infant mortality (Wilcox, 2001). A number of factors may result in shifts in the predominant distribution; altitude, race or ethnicity, and maternal smoking are among the better studied, and these factors can produce either a larger or, sometimes, smaller percentage of LBW babies. However, populations that have a larger percentage of LBW infants do not always have higher infant mortality (Wilcox, 2001, 2010). While birth weight is tracked internationally as a public health indicator to identify opportunities for
intervention and to understand country-specific infant mortality (UNICEF, 2004), strategies to increase birth weight have not been effective in reducing mortality.
The committee responsible for VAO concluded that there was inadequate or insufficient evidence to determine whether there is an association between exposure to the COIs and LBW or preterm delivery, and additional information available to the committees responsible for the subsequent updates did not change that conclusion. Reviews of the relevant studies are presented in the earlier reports. Tables 34 and 35, which can be found at www.nap.edu/catalog/25137, summarize the results of studies related to birth weight after paternal and maternal exposure to the COIs.
Update of the Epidemiologic Literature
No Vietnam-veteran, occupational, or case-control studies of exposure to the COIs and LBW or preterm delivery have been published since Update 2014. Four new environmental studies are summarized below.
A study of 234 couples by Robledo et al. (2015) examined how birth size varied with maternal and paternal exposures to 63 POPs from five major classes, including 36 PCBs, seven of which were dioxin-like (PCBs 105, 114, 118, 156, 157, 167, and 189). Exposure was measured in parental serum collected before conception. Differences in birth weight and other growth-related parameters were estimated using multiple linear regression per 1 standard deviation increase in natural log-transformed (ln-transformed) chemicals. The subjects were participants in the LIFE prospective cohort study,2 which was conducted in Michigan and Texas between 2005 and 2009. Models were estimated separately for each parent and adjusted for maternal age, maternal prepregnancy BMI, and other confounders, and all models included an interaction term between infant sex and each chemical. The authors found that for every 1 standard deviation increase in ln-transformed concentration of paternal serum levels of PCB 167, the mean birth weight among female newborns (n = 117) was 97.49 g lower (95% CI [–187.45, –7.54]), and mean length (β = −0.57 cm; 95% CI [–1.12, −0.02]) and head circumference (β = −0.45 cm; 95% CI [−0.86, −0.03]) were smaller. No statistically significant outcomes were observed for paternal exposures and male newborns (n = 113). For maternal serum PCB 167 levels, male newborn birth weight was significantly lower (−129.24 g, 95% CI [−228.16, −30.31]) and head circumference smaller (−0.47 cm, 95% CI [–0.95, 0.00]), and there were no statistically significant outcomes for female newborns.
A birth cohort study of 484 children in Hokkaido, Japan, by Kobayashi et al. (2017)—the Hokkaido Study on Environment and Children’s Health—measured total dioxin levels as the sum of 29 congeners in maternal blood samples taken
___________________
2 Cited earlier in this chapter in the review of a paper by Mumford et al. (2015) in the section on male reproductive health outcomes.
either during the third trimester or within 1 week after delivery. (In Japan, the largest source of dioxin exposure is dietary, and of the total dioxin intake in this population, the main source is seafood.) This new study expanded results from a previous study conducted in the same birth cohort (Konishi et al., 2009; reviewed in Update 2010) that reported a significant adverse effect on birth weight for total PCDDs TEQ levels (adjusted β = −231.5 g, 95% CI [−417.4, −45.6]) and total PCDFs TEQ levels (adjusted β = −258.8 g, 95% CI [−445.7, −71.8]). Sex-stratified analyses suggested that these reductions in birth weights were stronger in boys. Here, the investigators assessed polymorphisms in the women’s genes encoding the aryl hydrocarbon receptor (AHR [G > A, Arg554Lys]), cytochrome P450 (CYP1A1) (T6235C), and glutathione S-transferase mu 1 (GSTM1; non-null/null), genes that code for three metabolizing enzymes that had previously been related to dioxin or birth weight.3 Linear regression analyses that were adjusted for confounding factors found that pregnant women with a GSTM1 null genotype—a genotype previously established to be associated with a high inducibility of cytochrome P450 1A1 gene transcription—gave birth to infants with a 345 g (95% CI [−584, −105]) reduction in birth weight for each 10-fold increase in total dioxin TEQ. They also noted birth weight reductions for the children of GSTM1-null mothers for some of the individual congeners. These results suggest that PCDDs and PCDFs may accumulate in the placenta and, through interference with placental function, affect birth weight.
Miyashita and colleagues (2015b) investigated 70 PCB congeners, including dioxin-like PCBs, in the blood of a smaller subgroup of 367 woman–child pairs from the Hokkaido Study cohort cited above. Mothers completed self-administered questionnaires on demographic characteristics, socioeconomic status, personal habits, and food consumption; medical records were used to extract details for the delivery and characteristics of the infants. The authors found no associations between the concentrations of dioxin-like PCBs (or non-dioxin-like PCBs) and newborn anthropometric measurements of birth weight (small for gestational age), length, chest circumference, and head circumference in analyses with multiple linear regression models with or without adjustment for confounding factors. A reduction in birth weight and other measures of size was observed for dioxin-like PCBs, but these results were not statistically significant.
A study by Van Tung et al. (2016) of residents of a dioxin hot spot area in Vietnam4 reported on 58 mother–infant pairs who were compared with 62 pairs from a region thought to be uncontaminated. Breast milk from lactating mothers with infants ages 7 and 16 weeks at the time of sampling was analyzed for
___________________
3 Research indicates that dioxins bind AHR and induce CYP1A1 expression (Harper et al., 2002) and absence of human glutathione S-transferase mu 1 (GSTM1; null genotype) is associated with increased induction of CYP1A1 expression (Vaury et al., 1995).
4 Males in this study cohort were reported on by Sun et al. (2016) in a paper addressed in the male reproductive outcomes discussion earlier in this chapter.
dioxin congener levels. Samples from mothers living in the hot spot had fivefold higher levels of dioxin than breast milk from controls. Birth weight was inversely correlated with both 2,3,7,8-TCDD and 2,3,4,7,8-PeCDF congener levels. The rate of newborns with a birth weight less than 2,500 g was threefold higher in the hot spot (12%) than in the control region (4%). However, at 8–9 or 12–14 weeks of age, no significant associations were observed between infant size and dioxin isomer levels.
Biological Plausibility
The available evidence from experimental animal studies indicates that TCDD exposure during pregnancy can reduce body weight at birth, but only at high doses. A study in human placental explants suggests that TCDD exposure may enhance placental inflammation and may increase the risk of preterm births associated with infection (Peltier et al., 2013). Laboratory studies of the potential male-mediated developmental toxicity of TCDD and herbicides resulting from the prior exposure of the fathers are inadequate to support conclusions. TCDD and herbicides are known to cross the placenta, which leads to the direct exposure of the fetus. Data from studies of experimental animals also suggest that the pre-implantation embryo and developing fetus are sensitive to the toxic effects of 2,4-D and TCDD after maternal exposure.
Synthesis
Studies reviewed in this update continue the pattern observed in earlier research of identifying either no or small decrements in birth weight and size parameters for children born to parents with the highest levels of dioxin exposure. As has been noted by previous committees, there are a number of challenges in conducting these types of epidemiologic studies in a rigorous way. First, the prenatal and immediate postpartum period is not a stable pharmacokinetic state; there are substantial changes in body volume and fat mobilization during that time. Biomarker measures during pregnancy may be substantially affected by weight change during pregnancy. Moreover, the extrapolation of a more recent biomarker measure back many years to a more relevant period is complicated by intervening pregnancy and breastfeeding events, which result in a substantial uncertainty in the index exposure level. Overall, although the committee notes that the animal literature does support an effect of TCDD exposure at high doses on birth weight, the epidemiologic literature is insufficiently robust to allow a final determination.
Conclusion
On the basis of the evidence reviewed here and in previous VAO reports, the committee concludes that there is inadequate or insufficient evidence to determine whether there is an association between exposure to the COIs and LBW or preterm delivery.
Birth Defects
A birth defect is an abnormality of structure, function, or metabolism, whether genetically determined or resulting from an environmental influence during embryonic or fetal life (Christianson et al., 2006). Other terms for birth defect, often used interchangeably, are “congenital anomaly” and “congenital malformation.” Major birth defects, which occur in 2–3% of live births, are abnormalities present at birth that are severe enough to interfere with viability or physical well-being. Birth defects are detected in another 5% of babies through the first year of life. Genetic factors, exposure to some medications, exposure to environmental contaminants, occupational exposures, maternal infections, and lifestyle factors have been implicated in the etiology of birth defects (Christianson et al., 2006), although the causes of the vast majority of birth defects are unknown. Most etiologic research has focused on the effects of maternal and fetal exposures, but, as discussed in the beginning of this chapter, it is theoretically possible that epigenetic alterations in the paternal gamete caused by preconception exposures could result in paternally mediated effects. It should be noted that a substantial amount of epidemiologic research on suspect toxic agents has been conducted, but none of it has definitively established paternal preconception exposures as a contributing factor to the occurrence of birth defects (Chow et al., 2009; Desrosiers et al., 2012; Dohle, 2010; Schull, 2003).
The committee responsible for VAO concluded that there was inadequate or insufficient evidence to determine whether there is an association between exposure to 2,4-D, 2,4,5-T or its contaminant TCDD, picloram, or cacodylic acid and birth defects in offspring. Additional information from the AFHS available to the committee responsible for Update 1996 led it to conclude that there was limited or suggestive evidence of an association between at least one of the COIs and spina bifida in the children of veterans; there was no change in the conclusions regarding other birth defects.
The Update 2002 committee, which reviewed a study of female Vietnam veterans that reported significant increases in birth defects in their offspring (Kang et al., 2000a), did not find those results sufficient to modify prior conclusions. Nonetheless, Congress mandated that a number of birth defects in the children of female Vietnam veterans be assigned service-related status. Later committees have not encountered data that would merit changing the conclusion that the evidence
is inadequate to support an association between exposure to the COIs and birth defects (aside from spina bifida) in the offspring of either male or female veterans.
The Update 2014 committee concluded that the new evidence it identified concerning the occurrence of birth defects in association with exposure to the COIs, in combination with existing evidence, was inadequate or insufficient to support an association for birth defects overall in the children of Vietnam veterans. In light of the fact that evidence anticipated by the committee responsible for Update 1996 that would support an association between paternal exposure to the COIs and spina bifida had not materialized, that committee concluded that spina bifida should be moved from the category of limited or suggestive evidence of an association to the default category of inadequate or insufficient evidence of an association. An increased scrutiny of mechanisms by which paternal exposure might contribute to adverse effects in offspring has not definitively established the biologic plausibility of this phenomenon, whereas the understanding of how maternal exposures may disrupt fetal development has grown substantially. There were, however, no epidemiologic results supporting an association between maternal exposure to the COIs and spina bifida specifically, so spina bifida in association with exposure of either parent was moved to the inadequate or insufficient category of association. Chapter 10 of Update 2014 contains a detailed discussion of the evidence and reasoning underlying this determination.
Tables 36 and 37, which can be found at www.nap.edu/catalog/25137, summarize the results of studies related to birth defects and specifically of neural-tube defects such as spina bifida.
Update of the Epidemiologic Literature
No Vietnam-veteran studies of exposure to the COIs and birth defects have been published since Update 2014. New occupational, environmental, and case-control studies addressing neural-tube defects, cryptorchidism or hypospadias, gastrointestinal tract defects, cardiovascular defects, and general or multiple congenital anomalies are addressed below. All are classified as “Other Identified Studies” (as delineated in Chapter 3) because the exposures they addressed were not described in sufficient detail to allow the committee to determine their relevance to the experience of Vietnam veterans.
Other Identified Studies–Occupational Studies Makelarski et al. (2014) report results from a case-control analysis of maternal periconceptional occupational exposure to pesticides and association with neural-tube defects in offspring. Cases and controls were derived from the U.S. National Birth Defects Prevention Study (NBDPS), a large national case-control study of risk factors for birth defects. For any insecticide and herbicide use the odds ratio for spina bifida was 2.1 (95% CI 1.0–4.1), based on 14 exposed. There was no dose–response gradient
for cumulative exposure to insecticide and herbicide and spina bifida. The study’s strengths include the national context of the study with a relatively large, well-defined case group and extensive covariate information. The limitations include a lack of specific herbicide exposure information, the use of the general job-exposure matrix for exposure assessment, and the fact that small sample sizes precluded analyses of exposure to herbicides only.
Using cases and controls from the NBDPS, Pettigrew and colleagues (2016) examined the joint effect of parental occupational exposure to pesticides on the risk of spina bifida in offspring (291 cases). The analysis included joint parental exposure to insecticides and herbicides and fungicides, which yielded an odds ratio of 0.8 (95% CI 0.4–1.5). There was only one case with joint parent herbicide-only exposure (the odds ratio was not estimated). Herbicide exposure adjusted for other pesticides had decreased odds of spina bifida (OR = 0.6, 95% CI 0.3–125.0). The study’s strengths include the national context of the study with a well-defined case group and extensive covariate information. Its limitations include the lack of specific herbicide exposure information, the lack of paternal self-reported occupational history (it was reported by the mother), the use of the general job-exposure matrix for exposure assessment, and the very small sample sizes for herbicide exposure.
Rocheleau et al. (2015) examined the association between maternal occupational exposure to fungicides, insecticides, and herbicides and the risk of congenital heart defects among offspring. Cases and controls were drawn from the NBDPS, a multisite case-control study. Maternal occupational exposure to pesticides was analyzed for 3,318 cases and 2,979 controls using data for births in 1997–2002. Only 3 case mothers and 3 control mothers were determined to have had exposure to only herbicides. Analyses of estimated high exposure to herbicides and insecticides found associations with any simple, isolated congenital heart defects (OR = 1.90, 95% CI 1.05–3.44), pulmonary valve stenosis (OR = 3.64, 95% CI 1.31–10.15), right ventricular outflow tract obstruction (OR = 3.40, 95% CI = 1.41–8.16), and hypoplastic left heart syndrome without ventricular septal defect or anomalous pulmonary venous return (OR = 5.11; 95% CI 1.70–15.35). The study’s strengths include its use of a large population-based case-control design with careful ascertainment and classification of congenital heart defects, adjustment for multiple potential confounders, and an expert-based assessment of potential pesticide exposure. The major study limitation was the lack of information on exposure to specific pesticides.
Jorgensen and colleagues (2014) used the national Danish registries to determine parental employment in farming or horticulture and to identify their male offspring born between 1980 and 2007. Boys were followed for a hospital diagnosis of cryptorchidism recorded in the Danish National Patient Registry. Hazard ratios (HRs) and 95% CIs were calculated using Cox regression to compare the occurrence of cryptorchidism among the male children of mothers and fathers working as horticultural workers and farmers with the male children of
parents in other occupations. Adjustment was made for birth year, parental age, parity, and geographic region. A total of 618,081 boys born to actively employed mothers during 1980–2007 were identified, including 2,105 and 4,520 born to maternal horticultural workers and farmers, respectively. The adjusted HR for cryptorchidism showed an elevated risk among boys of maternal horticultural workers (HR = 1.20, 95% CI 0.95–1.52) and an increased risk among boys of maternal farmers (HR = 1.31, 95% CI 1.12–1.53) compared with boys of mothers in all other occupations. For fathers, a total of 708,283 boys were identified, and 18,648 were diagnosed with cryptorchidism during follow-up. Fathers working in horticulture or farming had 2,157 and 24,348 sons, respectively, of whom 72 (3.3 %) and 742 (3.0%), respectively, were diagnosed with cryptorchidism. The HRs for cryptorchidism were 1.20 (95% CI 0.96–1.51) for boys of paternal horticultural workers and 1.04 (95% CI 0.96–1.12) for boys of paternal farmers. The study’s strengths included a large national registry-based cohort of parental occupational information and outcome information for offspring. However, data on specific chemical exposures for the parents were not available, limiting the usefulness of the study for the evaluation of risks from the COIs.
Other Identified Studies–Environmental Studies Markel and colleagues (2015) conducted an ecologic study of hypertrophic pyloric stenosis identified by the Indiana Birth Defects Registry from 2005–2009. Birth defect cases were linked with their birth certificate record to obtain demographic and other information. County-level pesticide use data from available from the U.S. Geological Survey annual agricultural pesticide use database. The county-level pesticide data were categorized into high, moderate, and low level of use. Data were also subdivided into fungicides, fumigants, insecticides, and herbicides. Herbicides comprised 91% of all pesticides used. Total pesticide levels in the county of residence correlated significantly with the incidence of hypertrophic pyloric stenosis (r = 0.029, p = 0.004), as did herbicide use (r = 0.029, p = 0.005). This analysis is limited by the ecologic nature of the exposure data and outcome data. Although statistically significant, the correlation values were rather small and while logistic regression was used, the report only presents p-values and no effect estimates. There was no information on specific herbicides, other than to note that glyphosate and atrazine, which are not COIs, were the most heavily used.
A study on the island of Guam by Noel and colleagues (2015) examined the relationship between village-level estimates of alleged Agent Orange exposure and infant mortality due to congenital anomalies. Total births by village (n = 19) and infant mortality due to congenital anomalies for each year from 1970 to 1989 were obtained from the Office of Vital Statistics in the Guam Department of Public Health and Public Services. Each birth and death was assigned to a village based on the usual residence of the mother. The 1980 U.S. Census was used to obtain data on the median age of the village females, village fertility
ratio, population density, persons per household, single-mother households, and the number of married females. Agent Orange exposure data were obtained from a U.S. Air Force veteran who conducted ground-level Agent Orange spraying for vegetation control. The veteran provided village-level spray estimates based on his recollection; however, there was no reported independent verification of this information. Eleven villages were classified as high risk (cause-specific death rate was 2.96 per 1,000 live births) and eight as low risk (1.31 per 1,000 live births). Twelve villages were considered to have Agent Orange spraying (10 high risk and 2 low risk, p = 0.006). In the multivariable linear regression model, which included an adjustment for the median age of village females, the association between Agent Orange spraying area and infant mortality due to congenital anomalies was statistically significant (standardized regression coefficient β = 2.02; 95% CI 0.08–3.96; p = 0.042) with the model explaining approximately 51% of the variance. Models adjusting for other Census-based covariates yielded similar results. The study had several limitations, prominently the highly subjective nature of the exposure characterization. The assignment of potential village exposure to Agent Orange spraying was obtained from a single source and was not independently confirmed by records or biomarker data. Lacking congenital anomaly prevalence data, the study relied instead on mortality data, which may be affected by village-specific differences in infant survival due to differences in medical care and other factors.
Other Identified Studies–Case-Control Studies Shaw et al. (2014) examined cases and controls ascertained in the San Joaquin Valley in California. Cases were born from 1997 to 2006 with gastroschisis confirmed by clinical geneticists; those with single-gene conditions or chromosomal abnormalities or with identifiable syndromes were ineligible. Controls were non-malformed live-born infants randomly selected from birth hospitals in the study area. Data were collected from maternal telephone interviews using a standardized, computer-based questionnaire. Interviews were conducted with mothers of 72% of eligible cases (n = 193) and 69% of controls (n = 974). Mothers with pregestational diabetes were excluded from the analyses. Exposure assessment was performed for 461 individual chemicals and 62 physicochemical groupings that were applied at > 100 lb. in any of eight San Joaquin Valley counties in any year during the study period. An exposure-time window of 1 month before to 2 months after the maternal reported date of conception was assigned for each case or control mother. Exposure assignments were made for 156 cases and 785 controls whose mothers lived in the geocoded addresses more than 68 days during the window. To estimate pesticide applications, statewide pesticide use reporting records from the California Department of Pesticide Regulation describing agricultural pesticide applications occurring in the study period were obtained. Pesticide exposure was based on pounds of pesticides used during the relevant time window within a 500-meter radius of a case or control’s geocoded address. Logistic regression was
used to estimate odds ratios for pesticide exposure (yes/no), with adjustments for race/ethnicity, prepregnancy BMI, any use of folic acid–containing supplements, and smoking during the month before and the first 2 months of pregnancy. 2,4-D dimethylamine salt exposure had an adjusted odds ratio of 1.7 (95% CI 0.7–4.1) based on 8 exposed cases. The study’s strengths include the population-based design, comprehensive case ascertainment and classification, and extensive covariate data. The exposure assessment was performed using pesticide application data, although misclassification is expected because of the lack of data on individual factors that may influence exposure. Random error cannot be excluded when considering the result for 2,4-D dimethylamine salt.
Using the same study design as Shaw et al. (2014) above, Carmichael and colleagues (2016) examined the association between pesticide exposure and the risk of five different types of birth defects ascertained in the San Joaquin Valley of California. The analysis included 367 cases with one of five types of birth defects and 785 controls without any identified malformations born in 1997–2006. The case groups (with at least 50 cases) included anotia/microtia, anorectal atresia/stenosis, transverse limb deficiency, craniosynostosis, and diaphragmatic hernia. Among the published results (the criteria for presentation were an odds ratio > 2.0 or < 0.5 or confidence intervals that excluded 1.0), the authors reported associations between dichlorophenoxy acid or ester exposure and transverse limb deficiency (7 exposed cases; OR = 2.5; CI 1.1–6.0) and anotia/microtia (12 exposed cases; OR = 3.4; CI 1.6–7.6). The study’s strengths and limitations are similar to those of the Shaw et al. (2014) study, although the sample sizes for the defects in this study were relatively small and the estimate imprecise.
Rappazzo et al. (2016) conducted a case-control study using subjects drawn from a cohort of geocoded singleton live births (n = 335,729) in North Carolina between 2003 and 2005. Birth records were linked to the North Carolina Birth Defects Monitoring Program, an active birth defects surveillance system. Crop maps and pesticide application data were combined to estimate the quantities and types of pesticides applied during the time window of interest (1 month before pregnancy through the third trimester). Women who either had no crops within the buffer or who had no exposure during the relevant window of pregnancy were considered unexposed. The analysis included 6,358 cases and 298,548 controls. A total of 42 birth defects were ascertained, and 4,634 cases had a single (isolated) congenital anomaly. Logistic regression was used to estimate the odds ratio for exposure (yes/no) and each birth defect with adjustments made for race, education, marital status, maternal age, and maternal smoking. Elevated adjusted odds ratios were observed for atrial septal defects (OR = 1.70, 95% CI 1.34–2.14) and for patent ductus arteriosus (OR = 1.50, 95% CI 1.22–1.85) for the highest (≥ 90th percentile) exposure subjects. The study used a population-based design with birth defects surveillance and linked crop and pesticide records. Its limitations include the lack of individual-level pesticide exposure data for specific
pesticides, potential residual confounding due to the use of birth certificate data and unmeasured factors, and the small number of cases for some comparisons.
Koskenniemi and colleagues (2015) based their case-control study of 44 cases of cryptorchidism and 38 controls on subjects who were operated on for inguinal or umbilical hernia or hydrocele at two hospitals: one in Turku, Finland (2002–2006), and the other in Copenhagen, Denmark (2004–2005). Twelve of the Finnish cases and two of the Danish were referred for surgical procedures from a prospective cohort study. A subcutaneous adipose tissue biopsy was taken during the operation, and samples were analyzed for 37 PCBs, 17 PCDD/Fs and 14 PBDEs. Covariate data were obtained through parental interview and from medical records. Associations between adjusted and unadjusted chemical concentrations and the risk of cryptorchidism were calculated using logistic regression. The analysis included an adjustment of the chemical concentrations by factors influencing postnatal exposure (age at operation and duration of breastfeeding) by linear regression, but no adjustment was made for factors related to chemical concentrations in the mother (e.g., BMI, maternal age, parity). In a sensitivity analysis, cases and controls were excluded if the mother had gestational diabetes. After adjustment for the country of origin, age at operation, and the duration of breastfeeding, total TEQ (OR = 3.2, 95% CI 1.3–9.1) and sum of PCDD/Fs (OR = 3.7, 95% CI 1.5–10.9) were associated with an increased risk of cryptorchidism. The sum of PCBs had an elevated, but non-significant, odds ratio (OR = 1.9, 95% CI 0.9–4.0). The sum of PBDEs was not associated with an elevated odds ratio of cryptorchidism (OR = 0.86, 95% CI 0.47–1.54). The effect estimates were not materially different after restricting to boys who were born full term, biopsied at less than 5 years of age, and whose mothers did not have gestational diabetes. The study provides some evidence of an association with dioxin-like chemicals (TCDD-specific results were not reported). However, the study was very small and some potential confounders were not adjusted. The study is of limited value to the committee’s assessment.
Kalfa et al.’s (2015) case-control study examined 408 boys with isolated hypospadias and 302 controls identified at multiple institutions in the south of France between 2009 and 2014. Control boys were matched by ethnic origin and had no congenital malformation; no urological, genital, or nephrological condition; and no inguinal hernia or endocrine disease. The reasons for hospitalization for the controls included acute appendicitis, idiopathic intussusception, minor abdominal trauma, and pyloric stenosis. Information about maternal and paternal occupational and professional exposure to endocrine-disrupting chemicals was obtained using a standardized questionnaire and a job-exposure matrix. Environmental exposure was estimated by geocoding the residence postal code at the time of pregnancy and factoring in the types of surrounding hazards and their distance from that residence. The odds ratio for maternal exposure to herbicides (otherwise unspecified) was 1.00 (95% CI 0.007–13.97); the paper did not specify whether this imprecise estimate was adjusted. Environmental
exposure to intensive agriculture (residence within a 3-km radius) was statistically significantly different between cases and controls, as was residence in an industrial area or proximity to an incinerator or a waste area. The committee did not consider this study to be informative because results for specific COIs were not presented and there are concerns that the analysis and exposure information is based on questionnaire and a determination by simple distance to source for the environmental exposures.
A paper by Ueker and colleagues (2016) describes the results of a hospital-based case-control study conducted in Cuiabá, Mato Grosso, Brazil in 2011. The cases were children aged less than 5 years with a diagnosis of a congenital malformation in medical records who were identified at four referral institutions. The controls were children of those ages diagnosed with other conditions (respiratory diseases, infectious diseases, disorders of the perinatal period, and endocrine and metabolic diseases) at the same institutions. Controls were pair-matched with cases on sex. Information on parental exposure to pesticides (unspecified, except to note that glyphosate is the most commonly used) and other factors was collected by a maternal interview. The analysis group consisted of 137 cases and 274 controls. In the unadjusted analysis, several maternal pesticide exposure variables had an elevated odds ratio for birth defects, including living close to crop spraying with pesticides (OR = 1.61, 95% CI 0.88–3.03) and pesticide use at work (OR = 1.52, 95% CI 0.65–3.53). Paternal application of pesticides was also associated with an elevated odds ratio for birth defects (OR = 2.75, 95% CI 1.05–7.19). This study has several important limitations, including the lumping of all different birth defects into a single case group, a small sample, self-reported pesticide exposure (including maternal report of paternal exposures), no information on specific pesticides, and no information on case and control response rates.
Biological Plausibility
As was noted in Update 2014, 2,4-D has been previously shown to be a teratogen, although at exposure levels that exceed maternal renal clearance and thus are not relevant to herbicide exposure in Vietnam. A 2011 study showed that late in utero and early postnatal 2,4-D exposure can result in nephrotoxicity in offspring at one-sixth of the LD505 (Troudi et al., 2011). Other herbicides of interest can induce fetal malformations but typically only at high doses that are toxic to pregnant women. TCDD is a potent teratogen in all laboratory species that have been studied, although the patterns of birth defects that are produced are often species-specific. However, specific mechanisms that link TCDD exposure to specific birth defects have not been fully elucidated.
___________________
5 Lethal dose 50%: the dose required to kill half of the test population, usually expressed as a function of body weight.
A variety of animal model studies, including in utero exposures, work with cultured cells, and studies with zebrafish embryos, have investigated the mechanisms underlying various TCDD-induced birth defects, including hydronephrosis, cleft palate, reproductive organ anomalies, neurogenesis, and perturbed heart, kidney, and lung development (Dong et al., 2010; Falahatpisheh et al., 2011; Jacobs et al., 2011; Lanham et al., 2012; Latchney et al., 2011; Neri et al., 2011; Tait et al., 2011; Yamada et al., 2014; Yoshioka et al., 2012; Yuan et al., 2012). Interestingly, AHR is required for TCDD-induced birth defects. In contrast, the induction of cytochrome P450 1A1 is not required (Dragin et al., 2006; Jang et al., 2007; Mimura et al., 1997). When pregnant Ahr-null mice are exposed to TCDD, the fetuses do not exhibit any of the typical developmental malformations associated with TCDD exposure, but fetuses of TCDD-exposed pregnant CYP1A1-null mice do. In addition, an Ahr antagonist can attenuate TCDD-induced birth defects in mice. Thus, the activation of Ahr by TCDD during development appears to be a key first step in mediating TCDD’s developmental toxicity, but this step does not depend on CYP1A1 activity. Although structural differences in AHR have been identified among species, it functions similarly in animals and humans. Therefore, a common mechanism mediated by AHR in which tissue growth and differentiation processes are affected probably underlies the developmental toxicity of TCDD in humans and animals.
Antioxidant treatment provides protection against some TCDD-induced teratogenicity, which suggests that reactive oxygen species might be involved in the pathways that lead to these structural changes (Jang et al., 2008). A few studies indicate that the stem cells and organ-specific progenitor cells may be direct targets and that maternal TCDD exposures interfere with proliferation and cell differentiation through AHR and result in defects in organ morphogenesis (Latchney et al., 2011; Neri et al., 2011). Few laboratory studies of potential male-mediated developmental toxicity (and specifically birth defects) attributable to exposure to TCDD and herbicides have been conducted. As noted, the feeding of simulated Agent Orange mixtures to male mice produced no adverse effects in offspring (Lamb et al., 1981).
In sum, studies with maternal exposure in animal models suggest that a role for TCDD and related chemicals in causing birth defects is plausible and also that AHR plays a causal role. However, translating these results to human populations has been difficult.
Synthesis
Given the long-standing concern of Vietnam veterans about the potential of the COIs to adversely affect the health of their children, birth defects have been among the outcomes considered by VAO committees since the first comprehensive review was published in 1994. Embryonic and fetal development in some species including rodents are sensitive to the toxic effects of exposure to TCDD and dioxin-like chemicals, and there are several species and strains of animals for
which the fetus is more sensitive than the adult to the adverse effects of TCDD. Human data are generally lacking, however, and the sensitivity to developmental disruption in humans is less apparent, in part because contemporary studies of environmental dioxin exposure and birth defects have involved extremely low exposures. As noted, recent human population-based studies attempting to link TCDD or the other COI exposures to birth defects have provided mixed results.
Moreover, the study of birth defects in any population is complicated by the relatively rarity of specific birth defects. Attributing any one defect to a specific exposure is often difficult, and it is extremely difficult to conduct rigorous epidemiology studies assessing risks to children arising from their parents’ exposure, particularly when a distinction between maternal and paternal contributions is sought. These challenges are highlighted in the studies considered by the committee, which exhibit sometimes significant weaknesses that limit their usefulness—particularly in assessing the effects resulting from the exposures experienced by Vietnam veterans. The new epidemiologic studies considered by the committee were mostly not informative because of a small sample or the absence of exposure data on COIs, or both. There were two relatively rigorous epidemiologic studies that suggested an association between two different COIs (2,4-D dimethylamine salt; dichlorophenoxy acid) and an increased risk of two unrelated birth defects. However, the recent studies did not change the previous conclusion of inadequate or insufficient evidence to support an association for birth defects overall in the children of Vietnam veterans.
Conclusion
The committee’s review of newly published studies in combination with existing information leads it conclude that evidence remains inadequate or insufficient to determine whether there is an association between exposure to the COIs and birth defects in the children of Vietnam veterans.
EFFECTS OCCURRING LATER IN OFFSPRING’S LIVES
Information is available concerning parental exposure to the COIs and several outcomes occurring later in the lives of their offspring. Studies investigating cancers; growth and physical parameters; motor development, cognitive, behavioral, and socio-emotional outcomes; immune and allergic outcomes; and reproductive health are reviewed below.
Cancer in Offspring
The American Cancer Society estimates that 10,590 children under 15 years old will receive a new diagnosis of cancer in the United States in 2018 (ACS, 2018f). The treatment and supportive care of children who have cancer continue to improve. The 5-year survival rate for children who receive a cancer diagnosis
has increased from less than 60% in the 1970s to more than 80% in 2013, the most recent year for which data are available. Despite advances, cancers remain the second leading cause of death in children under 15 years old (after accidents); 1,180 deaths are projected for 2018 (ACS, 2018f).
Leukemias are the most common cancer in children, accounting for about 29% of all childhood cancer cases. The second-most common group of cancers in children is cancer of the brain and tumors of other parts of the central nervous system (CNS) (26%), followed by neuroblastomas (6%) and Wilms tumor (also called nephroblastoma; 5%) (ACS, 2018f). Other cancers in children include lymphomas, bone cancers, soft-tissue sarcomas, renal cancers, eye cancers, and adrenal cancers. In contrast with adult cancers, relatively little is known about the etiology of most childhood cancers, especially about potential environmental risk factors and the effects of parental exposures.
The committee responsible for VAO concluded that there was inadequate or insufficient evidence to determine whether there is an association between exposure to 2,4-D, 2,4,5-T, TCDD, picloram, or cacodylic acid and childhood cancers. The additional information available to the committees responsible for Update 1996 and Update 1998 did not change that conclusion. The committee responsible for Update 2000 reviewed the material in earlier VAO reports and in newly available published literature and concluded that there was limited or suggestive evidence of an association between exposure to at least one of the COIs and acute myeloid leukemia (AML).6 However, after the release of Update 2000, investigators involved in one study discovered an error in their published data. The Update 2000 committee reconvened to evaluate the previously reviewed and new literature regarding AML, and it produced Acute Myelogenous Leukemia (IOM, 2002). It reclassified AML from “limited/suggestive evidence of an association” to “inadequate evidence to determine whether an association exists.”
The committees responsible for Update 2002, Update 2004, Update 2006, Update 2008, Update 2010, Update 2012, and Update 2014 reviewed the material in earlier VAO reports and in newly available published literature and agreed that there remained inadequate or insufficient evidence to determine whether there is an association between exposure to the COIs and childhood cancers.
Table 38, which can be found at www.nap.edu/catalog/25137, summarizes the results of studies related to cancers in children.
___________________
6 AML (ICD-9 205; ICD-10 C92.A0) is also referred to as acute myelogenous leukemia, acute myeloblastic leukemia, and acute nonlymphocytic leukemia. For consistency, this report uses “acute myeloid leukemia,” or AML, regardless of the usage in the source materials.
Update of Epidemiologic Literature
New Vietnam-veteran, occupational, environmental, and case-control studies addressing childhood leukemias, central nervous system tumors, rhabdomyosarcoma, and retinoblastoma are addressed below.
Vietnam-Veteran Studies Grufferman and colleagues (2014) evaluated the role of parental military service in Vietnam and service-related exposures in the risk of rhabdomyosarcoma7 occurring in offspring. Cases of rhabdomyosarcoma diagnosed under the age of 20 years were identified from the Intergroup Rhabdomyosarcoma Study Group clinical trial, which included hospitals in 46 U.S. states and the District of Columbia from 1982 to 1988. Controls were identified by telephone random-digit dialing and were matched to cases on race, sex, and age. Case and control families were interviewed by telephone. The interview included questions about childhood environmental exposures, parental occupational exposures, family demographic characteristics, parental lifestyle and behavioral characteristics, and medical history. Questions related to military service focused on the Vietnam War period. Each parent was asked if he or she had ever served in the armed forces before the date of the index child’s diagnosis and, if so, in which years the service occurred. The parent was also asked if he or she was in contact with nuclear, chemical, and biological weaponry, radiation, radar or microwaves, or Agent Orange. Among the 440 cases that were eligible, 351 completed interviews, and, of those, 319 eligible cases had available information on parental occupation. Subjects were matched on age, sex, and race, and analyses were adjusted for family income, maternal education and recreational drug use, length of pregnancy, and maternal spotting/bleeding/cramping during pregnancy. The study reported that 3.4% of case mothers and 1.4% of control mothers reported a history of military service. Maternal history of military service was associated with an elevated adjusted odds for rhabdomyosarcoma (OR = 2.75, 95% CI 0.71–10.62). There were too few cases to refine this analysis by time period of service. A history of paternal military service was not associated with rhabdomyosarcoma (OR = 0.85, 95% CI 0.58–1.25); restricting the time of paternal military service to 1962–1970 yielded an adjusted odds ratio of 0.78 (95% CI 0.50–1.21), based on 53 cases (18.5%) and 64 controls (22.3%). Only 9 cases (1.7%) and 5 controls (3.1%) reported potential Agent Orange exposure during Vietnam War service. The adjusted odds ratio was elevated for this exposure, but the estimate was not statistically significant (OR = 1.72, 95% CI 0.55–5.41). This report was based on a nationally ascertained case groups and included a parental interview asking questions about a wide array of potential risk factors and confounders, with specific questions on military
___________________
7 Rhabdomyosarcoma is a cancer of the muscle tissue. It is the most common form of soft-tissue sarcoma in children. See https://www.cancer.gov/types/soft-tissue-sarcoma/patient/rhabdomyosarcoma-treatment-pdq (accessed October 28, 2018).
service and Agent Orange exposure. Concerns include the use of a control group based on telephone random-digit dialing and the recall of potential Agent Orange exposure. The analysis of paternal Agent Orange exposure was based on a very small number of exposed cases, and the confidence intervals associated with the odds ratios were correspondingly broad. This report found no association between paternal military service in Vietnam or Agent Orange exposure and an increased risk of rhabdomyosarcoma in offspring.
Other Identified Studies–Occupational Study Febvey et al. (2016) pooled three population-based case-control studies of childhood CNS tumors conducted from France, Germany, and the United Kingdom. Cases were children less than 15 years of age with CNS tumors; controls were matched by gender and age. Interview data (telephone or in-person) on parental occupational histories were collected. Parental occupational data were harmonized, and an existing general population job-exposure matrix was used to assign pesticide exposure into no-, low-, or high-exposure categories for insecticides, herbicides, fungicides, and all pesticides combined. The analysis included 1,361 cases and 5,498 controls. Only 0.9% of mothers and 4.2% of fathers were found to have occupational exposure to pesticides during the studied pregnancy. No association was observed between CNS tumors and maternal pesticide exposure (pooled OR = 0.76, 95% CI 0.41–1.41) and statistically significantly decreased odds were seen with paternal occupational pesticide exposure (OR = 0.71, 95% CI 0.53–0.95). The study included a relatively large number of subjects and used a common job-exposure matrix to assign potential pesticide exposure across studies. However, the analysis was limited by the low prevalence of parental exposure, precluding examination of dose categories and a breakdown of pesticide categories. No information was available on specific pesticides.
Other Identified Studies–Environmental Study Bailey and colleagues (2015) used pooled individual-level data from 12 studies that participated in the Childhood Leukemia International Consortium to examine the association between home pesticide exposure and the risk of childhood leukemia. The analysis included data from 12 consortium studies conducted in North America, Europe, and Australasia over a 30-year period. Self-reported home pesticide exposure data were harmonized across studies. The time periods of potential exposure included the period prior to conception, during pregnancy, and after the child’s birth. Exposure could be to the mother, father, or child. A variety of factors were examined as potential confounders. The analyses were stratified by leukemia subgroups, including acute lymphoblastic leukemia (ALL) and AML, and cytogenetic data. Weakly elevated odds ratios for ALL were found for home herbicide use (otherwise unspecified) during the three time periods of interest: for example, the odds ratio for reported use within 1–3 months before conception was 1.23 (95% CI 1.04–1.45), based on 5 studies. Home herbicide use during pregnancy
was associated with decreased odds of AML (OR = 0.84; 95% CI 0.56–1.26). These were the only two analyses with reported results for the herbicide exposure group. The study’s strengths include a large pooled resource from multiple international studies. Limitations include self-reported home pesticide exposure and the inability to isolate effects of specific herbicides.
Other Identified Studies–Case-Control Studies Gunier et al. (2017) analyzed data from the California Childhood Leukemia Study, a population-based case control study conducted in 35 counties from 1995 to 2008. Cases were ascertained within 72 hours after diagnosis at Northern and Central California hospitals. Controls were randomly selected from California birth certificate files and individually matched to cases on the child’s age, sex, race, and Latino ethnicity, and on maternal race. In-person interviews collected information on occupational history for each parent. The interview also collected information on whether the parent worked regularly with pesticides, insecticides, fungicides, or herbicides, otherwise unspecified, and whether the parent worked in agricultural occupations. Detailed occupational information was collected using specific job module interview questions for occupations with potential pesticide exposure, including farm or ranch worker; gardener, landscaper, nursery worker, or groundskeeper; agricultural packer; and pesticide applicator. The analysis was based on 669 children diagnosed with ALL and 1,021 controls. After adjustment for child’s sex, age, ethnicity, mother’s race, and household income an increased odds ratio of ALL was found for paternal occupational exposure to any pesticides (OR = 1.7, 95% CI 1.2–2.5). A statistically significant odds ratio was found for paternal pesticide exposure and ALL in children diagnosed before 5 years of age (OR = 2.3, 95% CI 1.3–4.1). Maternal pesticide exposure had a weakly elevated odds ratio for ALL (OR = 1.3, 95% CI 0.8–2.4). The study strengths include a population-based design and a comprehensive in-person interview-based collection and assessment of parental occupational exposures, including occupations associated with pesticide use. However, the study was not large enough to assess the effect of specific pesticides including the COIs.
Omidakhsh and colleagues (2017) conducted research based on data from a multi-center case-control study of retinoblastoma.8 Cases included patients with sporadic retinoblastoma who were diagnosed or treated at the Children’s Oncology Group institution or at the Wills Eye Institute in Philadelphia, Pennsylvania, between 2006 and 2011. A total of 282 cases (186 unilateral and 96 bilateral) were recruited. Controls were selected based on friends or non-biological relatives nominated by case families and with a child in the same age range as the index case child. Phone interviews were used to collect information on residential
___________________
8 “Retinoblastoma is a rare childhood cancer in which malignant (cancer) cells form in the tissues of the retina. The retina is a thin layer of nerve tissue that lines the inside of the back of the eye and is sensitive to light.” See https://www.cancer.gov/types/retinoblastoma (accessed October 28, 2018).
pesticide use before conception and during pregnancy, including the use of professional lawn or landscape services; the use of pest control professionals or exterminators for their home; in-home use of insect or rodent killers; use of indoor foggers; home or garden use of herbicides, mold removal products, antifungals, or weed killers; insect repellant; head lice treatment on the children; and, for pets, the use of flea collars or flea or tick shampoos. When parents indicated that they had applied a product themselves in the home or garden, they were asked to identify the name of the product. The investigators’ analysis was based parents of 99 unilateral and 56 bilateral case-control pairs. Odds ratios were adjusted for mother’s race, mother’s age, mother’s work status, and whether the father was living at home at the start of the pregnancy. Parental home use of a weed killer (OR = 2.3; 95% CI 0.9–5.4) was associated with an elevated odds ratio for unilateral retinoblastoma. The use of weed control products on the garden or lawn had an adjusted odds ratio of 2.3 (95% CI 0.9–5.4). Associations between maternal residential pesticide exposure in the month before or during pregnancy and bilateral retinoblastoma were very imprecise. For example, the use of professional lawn or landscape services had an odds ratio of 3.4 (95% CI 0.6–18.0), while the use of weed control products on a garden or lawn had an adjusted odds ratio of 1.0 (95% CI 0.3–3.3). The patterns of association were similar for different exposure factors, including the types of products, locations, timing, and frequency of pesticide use. When asked about the specific pesticide product used, about half of participants responded with a product name for which ingredients could be identified. Reported products used for weed control included 2,4-D, dicamba, glyphosate, and 2-methyl-4-chlorophenoxyacetic acid (MCPA). The study was based on national case and control groups and a detailed interview regarding residential pesticide use and other factors. In addition, cases could be divided into unilateral and bilateral subgroups. Limitations included the use of friend controls that may introduce selection bias based on shared geography and other factors, the relatively small number of cases, and the inability to analyze by specific herbicides that may have been used, including those of particular interest (2,4-D and dicamba).
Biologic Plausibility
Laboratory animal studies have established that TCDD can affect development, so a connection between TCDD exposure and effects on offspring, including developmental disruption and disease onset in later life, is biologically plausible. It has been established in several animal studies that TCDD at high doses is a potent teratogen. Studies with rodent models have demonstrated male, female, and sex-independent effects in the immediate offspring of females exposed during pregnancy. These include epigenetic modification of imprinted genes (Somm et al., 2013), increased DNA methylation of the BRCA1
tumor suppressor gene in mammary tissue (Papoutsis et al., 2013), altered uterine response to estradiol (K. A. Burns et al., 2013), the dysregulation of lipid metabolism in the presence of a high-caloric diet (Sugai et al., 2014), aberrant emotional behaviors (A. T. Nguyen et al., 2013), a reduced capacity for lymphocyte differentiation (Ahrenhoerster et al., 2014), testicular inflammation (Bruner-Tran et al., 2014), and a variety of adult diseases, including kidney, prostate, ovarian primordial follicle loss, and polycystic ovarian disease (Manikkam et al., 2012a). Transgenerational inheritance to the F3 generation was shown for the last two studies. However, definitive conclusions based on animal studies about the potential for TCDD to cause later-life effects in human offspring are complicated by differences in sensitivity and susceptibility among individual animals, strains, and species; by differences in the route, dose, duration, and timing of exposure in experimental protocols and real-world exposure; and by differences in the toxicokinetics of TCDD between laboratory animals and humans. Experiments with 2,4-D and 2,4,5-T indicate that they have subcellular effects that could constitute a biologically plausible mechanism for developmental effects, but only at very high doses. There is insufficient information on picloram and cacodylic acid to assess the biologic plausibility of their developmental or delayed effects in offspring.
Paternal or maternal exposure to xenobiotics potentially could increase the susceptibility of offspring to cancer through multiple mechanisms. Susceptibility could be increased by causing a tumor-promoting mutation in germ cells that would be present in all of the somatic cells of the child. This de novo mutation could then be passed on to subsequent generations via Mendelian inheritance, assuming that the child survived to reproduce. However, as discussed earlier in this chapter and in earlier chapters, TCDD and other COIs are not genotoxic (i.e., they do not cause mutations), which makes the mutation-induction and inheritance scenario unlikely. Alternatively, a maternally mediated increase in susceptibility to childhood cancers could result from the direct exposure of a fetus in utero or of the newborn via lactation to a xenobiotic that induces epigenetic alterations that increase cancer susceptibility.
As noted elsewhere in this chapter, there are several pre- and post-conception scenarios for how toxicant exposures could cause disease in first-generation offspring and perhaps in later generations based on epigenetic mechanisms (Vaiserman, 2014). Perhaps the most straightforward scenario is in utero exposure affecting the developing epigenome, predisposing the child to cancer. The best example of this happening is when otherwise very rare vaginal cancers arose in the daughters of women who took the estrogenic agent diethylstilbestrol to prevent miscarriage (Herbst et al., 1971). Thus, this scenario is plausible for humans. Although TCDD can have antiestrogenic effects, its toxicity via AHR likely involves transcriptional changes that could induce epigenetic mechanisms. With regard to cancers, if the affected gene or genes are involved in cancer pathways
and epigenetic modifications stabilize the gene-expression changes, then the susceptibility to cancer could increase.
Prenatal TCDD exposure of rats is associated with altered mammary gland differentiation and an increase in the number of mammary adenocarcinomas (Brown et al., 1998). Perhaps related, prenatal TCDD exposure led to increased DNA methylation at the BRCA1 (breast cancer) gene promoter in the female offspring of exposed pregnant rats (Papoutsis et al., 2013). The demonstration that early postnatal TCDD exposure does not increase mammary-cancer risk (Desaulniers et al., 2004) does not contradict the finding that TCDD-induced changes in utero mediate the increase in cancer susceptibility (Fenton et al., 2000, 2002), and it is consistent with the ultimate carcinogenic effect being greatest when epigenomic changes are the most dynamic. Thus, developmental epigenetic alterations may be involved in the prenatal effects. TCDD has been shown to suppress the expression of two tumor-suppressor genes, p16Ink4a and p53, via an epigenetic mechanism that appears to involve DNA methylation (Ray and Swanson, 2004). Similarly, it was reported that prenatal TCDD exposure increases the DNA methylation of two growth-related imprinted genes, H19 and Igf2, in the developing fetus (Q. Wu et al., 2004).
No direct evidence from animal models shows that TCDD increases the risk of childhood cancers, such as acute leukemia and germ-cell tumors; although a 2014 study showed a reduced capacity of hematopoietic stem cells to undergo differentiation in offspring (Ahrenhoerster et al., 2014). Emerging research suggests that prenatal TCDD exposure can disrupt epigenetic imprinting patterns and alter organ differentiation and thus could contribute to an increased susceptibility to cancer later in life. Smith et al. (2005) showed that chromosomal rearrangements associated with childhood ALL are evident in the neonatal blood spots, which suggests that childhood leukemias begin before birth, perhaps due to maternal exposures to carcinogenic xenobiotics.
Synthesis
Newly identified studies of cancer in children of parents potentially exposed to the COIs included a case-control study in which parents were asked about military service and potential Agent Orange exposure. The study was small, and while an elevated odds ratio was found, the estimate was very imprecise and random error could not be ruled out. Other identified occupational and environmental studies had methodologic challenges and did not report results for specific COIs. In sum, the evidence is sparse and inconclusive.
Conclusion
On the basis of the evidence reviewed here and in previous VAO reports, the committee concludes that there is inadequate or insufficient evidence to determine whether there is an association between exposure to the COIs and childhood cancers.
Growth and Physical Parameters
Since Update 2014, seven studies identified as relevant by the committee have examined exposure to the COIs and the subsequent growth and development of children.
A case control study by Hsieh et al. (2017) explored whether polymorphisms and haplotypes of arsenic methyltransferase (AS3MT), glutathione-S-transferase omegas (GSTOs), and purine nucleoside phosphorylase (PNP) affect arsenic methylation capacity and developmental delay. From 2010 to 2014, 179 children with developmental delay and 88 children without delay were recruited from the Shin Kong Wu Ho-Su Memorial Teaching Hospital in Taiwan. Urinary arsenic species, including arsenite (AsIII), arsenate (AsV), monomethylarsonic acid (MMAV), and DMAV were measured using a high-performance liquid chromatography-linked hydride generator and atomic absorption spectrometry. The polymorphisms of AS3MT, GSTO, and PNP were analyzed using the Sequenom MassARRAY platform with iPLEX Gold chemistry. Polymorphisms of AS3MT genes were found to affect susceptibility to developmental delay in children, but GSTO and PNP polymorphisms were not. Participants with the AS3MT rs3740392 A/G+G/G genotype, when compared with those with the AS3MT rs3740392 A/A genotype, had a significantly lower secondary methylation index. This may result in an increased odds ratio for developmental delay. Participants with the AS3MT high-risk haplotype had a significantly higher OR than those with AS3MT low-risk haplotypes (OR = 1.59; 95% CI 1.08–2.34). This study shows a joint dose–response effect of an AS3MT high-risk haplotype with inefficient arsenic methylation capacity on developmental delay, but it does not constitute persuasive evidence that the AS3MT genotype causes developmental delay by affecting arsenic methylation.
Three European birth cohorts (Belgian, Norwegian, Slovak) that assessed dioxin exposures in cord blood or breast milk were pooled by Iszatt et al. (2016) to investigate the influence of perinatal exposure to dioxins and dioxin-like chemicals on infant growth and BMI in childhood. The exposure was highest in the Belgian and lowest in the Norwegian cohort (the median and interquartile range of the pooled sample were 13 and 12.3 pg CALUX9 TEQ/g lipid
___________________
9 CALUX (Chemical-Activated LUciferase gene eXpression bioassay) is a test that may used to determine dioxin-like activity.
respectively). Infant growth was defined as the cohort- and sex-specific change in weight-for-age z-score between birth and 24 months (n = 367). The investigators also calculated BMI at ~7 years of age in 251 children. Overweight was defined according to international standards for children equivalent to an adult BMI > 25 kg/m2. In multivariate models based on generalized estimating equations, perinatal exposure to dioxins and dioxin-like chemicals appeared to be associated with increased growth between birth and 24 months (adjusted estimate for change in z-score: β = 0.07, 95% CI −0.01–0.14) and at age 7 years, and dioxin exposure was associated with a statistically significant increase in BMI in girls (adjusted estimate for BMI units β = 0.49, 95% CI 0.07–0.91) but not in boys (β = −0.03, 95% CI −0.55–0.49) (p-interaction = 0.044). Furthermore, only girls had an increased risk of being overweight at age 7 (54%, 95% CI −6% –151%). While these studies suggest that perinatal exposure to dioxin and dioxin-like chemicals may increase early infant growth and contribute to increased BMI in school age girls, the studies were still quite small in size, and additional studies of larger sizes are needed to re-examine these associations.
Mayhoub and colleagues (2014) used data from the MecoExpo study to investigate the relationship between parental exposures to pesticides (as reported by the mother) and neonatal parameters. The study included 993 mother–newborn pairs from the Picardy region of northern France. In this cohort, each mother completed a questionnaire that probed potential occupational, domestic, environmental, and dietary sources of parental exposure to pesticides during her pregnancy. Multivariate regression analyses found that maternal occupational exposure was associated with an elevated risk of low birth weight (OR = 4.2, 95% CI 1.2–15.4). Paternal occupational exposure to pesticides was associated with a lower-than-average gestational age at birth (−0.7 weeks, p = 0.0002) and an elevated risk of prematurity (OR = 3.7, 95% CI 1.4–9.7).
A study of 234 couples by Robledo et al. (2015)—also reviewed above in the section regarding low birth weight and preterm delivery—examined how birth size varied with maternal and paternal exposures to 63 POPs from five major classes, including 36 PCBs, seven of which were dioxin-like (PCBs 105, 114, 118, 156, 157, 167, and 189). Exposure was measured in parental serum collected before conception. Differences in length, head circumference, and ponderal index were estimated using multiple linear regression per 1 standard deviation increase in natural log-transformed (ln-transformed) chemicals. The subjects were participants in the LIFE prospective cohort study, which was conducted in Michigan and Texas between 2005 and 2009. Models were estimated separately for each parent and adjusted for maternal age, maternal pre-pregnancy BMI, and other confounders, and all models included an interaction term between infant sex and each chemical. Among girls (n = 117), mean length (β = −0.57 cm; 95% CI [−1.12, −0.02]) and head circumference (β = −0.45 cm; 95% CI [−0.86, −0.03]) were smaller in association, with a 1
standard deviation increase in ln-transformed maternal serum concentrations of the dioxin-like PCB 167. No statistically significant differences in length, head circumference, and ponderal index were noted among boys (n = 113) relative to maternal or paternal exposure to the COIs.
Tai et al. (2016) enrolled 217 mother–infant pairs living in a dioxin-contaminated area in Vietnam and collected data on the birth cohort’s physical growth during the first 3 years of life. Perinatal dioxin exposure of infants was estimated by the measurement of dioxin levels in the breast milk of the nursing mothers. Growth parameters—including weight, height, and head and abdominal circumferences—were measured at birth, 1 and 4 months, and 1 and 3 years of age. Multivariate mixed models were applied for analyzing repeated measures. All body size measures in boys were decreased in the high-exposure groups of TCDD (for example, head circumference age-adjusted z-score high-exposure: −0.87, 95% CI [−1.28, −0.46], versus low-exposure: −0.18, 95% CI [−0.29, −0.06]; p < 0.01). In girls, high TCDD exposure was associated with increased head circumference (age-adjusted z-score high-exposure: 0.18, 95% CI [−0.08, 0.44], versus low-exposure: −0.28, 95% CI [−0.42, −0.15]; p < 0.01). This study suggests that perinatal dioxin exposure affects the physical growth of infants and children in the first 3 years of life in a sex-specific manner.
S. Thomas and colleagues (2015) measured arsenic and chemical levels in blood or urine samples from the first and third trimesters in 1,835 pregnant women from across Canada to study the association with fetal growth. The investigators adjusted for maternal age, parity, pre-pregnancy BMI, and smoking and examined potential effect modification by single nucleotide polymorphisms (SNPs) in GSTP1 and GSTO1 genes. No association was found for arsenic and small-for-gestational-age as an outcome, but the researchers found an increased risk for small-for-gestational-age for the highest compared to the lowest tertile of exposure for the organic arsenic species arsenobetaine (> 2.25 μg/L, RR = 1.65, 95% CI 1.10–2.47) after adjusting for parity and smoking. A statistically significant interaction was observed in the relationship between DMA levels in urinary arsenic and small-for-gestational-age between strata of GSTO1 A104A (p for interaction = 0.02). This study was deemed to be of limited usefulness to the committee because the relevance of the exposures to those experienced by Vietnam veterans is debatable.
Motor Development, Cognitive, Behavioral, and Socio-Emotional Outcomes
Since Update 2014, a number of studies identified as relevant by the committee have examined exposure to the COIs and cognitive or motor development outcomes in children.
Berghuis and colleagues (2014) reported on the results of a birth cohort study conducted in the Netherlands, with recruitment from 1998–2000. Maternal blood
samples were collected in the second or third trimester and analyzed for 10 PCB congeners and 6 hydroxylated metabolites. Three mono-ortho PCBs (105, 118, and 156), but no coplanar PCBs were measured; the hydroxylated metabolite for PCBs 105 and 118 was measured. These prenatal exposures were analyzed in association with scores on the Touwen neurological examination administered at 3 months of age. Many empirical analyses were conducted, with results showing, in general, that prenatal PCB exposures were associated with more optimal development, both before and after adjustment for confounders of birth weight and child’s age at assessment. None of the results assessing the association between prenatal exposure to mono-ortho PCBs and non-optimal development were statistically significant: PCB 105 (0.833 per 1 ng/g lipid weight, CI 0.550–1.262), 118 (0.932, CI 0.728–1.191), or 156 (0.910, CI 0.831–1.019) (both genders; after adjustment).
Braun and colleagues (2014) conducted an analysis of the effects of gestational exposure to endocrine-disrupting chemicals on 4- to 5-year-old children enrolled in the Health Outcomes and Measures of the Environment Study conducted in the Cincinnati, Ohio, metropolitan area. The study examined a total of 25 PCBs—including the dioxin-like PCBs 105, 118, 156, 157, 167, and 170—along with many phthalates, PBDEs, pesticides, and polyfluorinated compounds in relation to scores on the Social Responsiveness Scale (SRS). No dioxin-like PCB was found to be associated with SRS scores (data were presented in graphs with no accompanying numbers). The authors cautioned, though, that their modest sample size (n = 175) precluded them from dismissing possible effects from chemicals with null associations.
Caspersen et al. (2016) used data from the Norwegian Mother and Child Cohort Study, also known as MoBa, to estimate maternal dietary exposure to dioxins and PCBs using a validated food frequency questionnaire administered mid-pregnancy and a database of dioxin and PCB concentrations in foods. Exposure to dioxins and dioxin-like PCBs was characterized in terms of TEQs. Children’s language skills at age 3 were assessed by parental report, including a grammar rating scale and questions about communication skills from the Ages and Stages Questionnaire (ASQ). The total sample analyzed was 44,092 children, and a thorough control for confounders included maternal age, education, parity, pre-pregnancy BMI, household income, bilingualism, maternal smoking, alcohol, and folate supplement use, dietary fatty acids intake, and dietary exposure to methylmercury during pregnancy. Statistically significant results included associations of either high maternal TEQ exposure from the diet or high intake (above the 97.5th percentile) of the non-dioxin-like PCB 153, with higher odds of incomplete grammar in boys and girls (aORs 1.1 to 1.3) and severe language delay (aOR = 1.6, 95% CI 1.1–2.4 for dioxin-like chemicals, and aOR = 1.9, 95% CI 1.1–3.4 for PCB 153). Higher exposure to dioxin-like chemicals was also associated with lower ASQ communication scores in girls (aOR = 1.4, 95% CI 1.1–1.9).
Hui et al. (2016) recruited 161 mothers and their 11-year-old children living in Hong Kong to participate in a study of prenatal dioxin exposure and neurocognitive development that was a follow-up to earlier work. Neuropsychological assessments were performed by clinical psychology trainees or senior research assistants with graduate training in psychology who were blinded to the participating children’s dioxin exposure. A registered clinical psychologist was responsible for ensuring assessment quality and interpreting the assessment results. All children first completed the cognitive assessments, which took approximately 3 hours, before other health assessments were performed. Most assessments were done in the research unit, but 15 were performed at home. Testing venues were required to be quiet and distraction free. No associations were found between any of the neurocognitive or behavioral tests administered with the TEQ levels from maternal breast milk obtained from their mothers 2 to 6 weeks postpartum, with p values for interaction ranging from 0.34 to 0.99 for children who were breastfed exclusively during infancy.
Kono and colleagues (2015) used a general population sample from an ongoing breast milk survey that has been conducted in several prefectures and cities in Japan since 1997, from which TEQs are calculated based on PCDD/Fs and coplanar PCBs. When the children were 6 to 13 years of age, parents were asked to complete the extended Japanese version of the Strengths and Difficulties Questionnaire (SDQ), which consists of 25 items on specific strengths and difficulties, with an overall rating of whether the child had behavioral or psychological problems. Four SDQ subscales were obtained—emotional symptoms, conduct problems, hyperactive/inattention, and peer problems—as well as a total difficulties score (TDS) based on the four subscale scores. Analyses were conducted stratified by age and sex of the child. Few covariates were considered in the multivariate analysis—maternal age, birth weight, any history of maternal cigarette smoking, and age at SDQ assessment—raising concerns about lack of control for the socioeconomic status of the family. None of several different metrics estimating the dioxin exposure showed any association with the TDS score in any of the analyses of boys, girls, or different age groups: in linear regression analyses, breast milk TEQ level was not significantly related to the TDS in boys (partial regression coefficient = 2.29; 95% CI [−7.60, 12.18]), or in girls, (partial regression coefficient = −1.04; 95% CI [−9.24, 7.15]) after adjustment for covariates. According to the authors, levels of dioxins in this population were comparable to other studies from the same time period that estimated dioxin exposures from breast milk in Japan, the United Kingdom, and Eastern Europe.
Nakajima et al. (2017) examined sex-specific differences in the effect of prenatal exposure to dioxin-like chemicals on neurodevelopment in children who were participants in the Sapporo cohort of the Hokkaido Study on Environment and Children’s Health. Data included a blood draw from the mother in the second trimester of the pregnancy; a self-administered questionnaire that collected
information on the mother’s dietary habits, exposure to chemicals during daily life and at work site, home environment, smoking habit, and medical histories as well as the medical histories of the mother’s partners; maternal and child medical records information; and a neurodevelopmental test (Bayley Scales of Infant Development, 2nd Edition) administered to the child at either 6 or 18 months. The exposure assessment included levels of a number of dioxin-like chemicals and TEQ values. One hundred ninety mother–infant pairs in the 6-month-old group and 122 mother-child pairs in the 18-month-old group were studied. The investigators reported that levels of 10 dioxin-like chemicals in male children were significantly negatively associated with the Psychomotor Developmental Index (PDI) scores at 6 months of age after adjustment for potential confounding variables (−0.21 point change in developmental score per dioxin level [common logarithm], adjusted; p < 0.05) but that the associations disappeared at 18 months of age. For female children, the level of only one mono-ortho PCB isomer (PCB 123) was significantly negatively associated with PDI at 6 months of age. However, in contrast to the males, levels of four dioxin-like chemicals (PCBs 114, 156, 157, and 189) in 18-month-old female children were significantly positively associated (p < 0.05) with Mental Developmental Index scores.
An English-language abstract of a paper published in Chinese by Ni et al. (2016) reports the results of a study involving 3,771 children whose mothers were recruited during pregnancy. Maternal occupational and life exposures were assessed in the period 6 months prior to pregnancy, and the BRIEF (Behavior Rating Inventory of Executive Function–Preschool version) was used to assess the preschool children’s executive function. The investigators’ findings included that maternal exposure to pesticides in the 6 months before the pregnancy were associated with Inhibitory Self-Control Index dysplasia (OR = 3.60, 95% CI 1.45–8.95), Flexibility Index dysplasia (OR = 6.72, 95% CI 2.50–18.07), and Global Executive Composite dysplasia (OR = 2.39, 95% CI 1.02–5.58) in the preschool children. No statistically significant associations between paternal pesticide exposures and adverse outcomes were reported. It is unclear from the abstract, however, whether any COI exposures were involved.
In a companion study to Pham et al. (2015), reviewed later in this section, Nishijo et al. (2014) examined 153 mother–infant pairs residing in a dioxin-contaminated area near a former U.S. base in Vietnam to evaluate potential associations between perinatal dioxin exposure and autism spectrum disorders in the children. Parents completed a full-length Autism Spectrum Rating Scale (ASRS) survey—which has not been validated in a Vietnam population but for which no comparable test was available—and researchers calculated several outcome and treatment scale scores from the results. These data were analyzed in conjunction with the Bayley-III neurodevelopmental battery test results described by Pham et al. The investigators found that high-TCDD-exposed (≥ 3.5 pg per g fat in mother’s breast milk sampled 1 month after birth) male and female children showed significantly higher ASRS scores than less-TCDD-exposed children,
without concomitant differences in their mental and psychomotor scores. In contrast, high TEQ level boys (≥ 17.9 pg-TEQ per g fat), had significantly lower neurodevelopmental scores than lesser-exposed boys without a comparable difference in their ASRS scores. These results suggest that perinatal TCDD exposure has an effect on autistic traits in childhood that is separate from neurotoxic effects of dioxin exposure in general.
A second follow-up to Pham et al. (2015) conducted by Nishijo et al. (2015) focused on urinary metabolite levels in 26 children who were part of their study cohort. The investigators found that urinary histidine is associated with dioxin exposure–induced neurodevelopmental deficits, suggesting that it might be a useful marker of dioxin-induced neurodevelopmental deficits. However, given the very small number of subjects and the very wide natural variability of urinary histidine (along with that of other urinary metabolites such as glycine and tryptophan), the results are not likely to be reliable and do not present data bearing on any direct evidence of a link between TCDD and clinical outcomes in descendants.
The Duisburg (Germany) birth cohort study by Nowack and colleagues (2015) recruited pregnant women from September 2000 to October 2002. Maternal blood samples were collected primarily in the third trimester (a few in the immediate postnatal period) and used for the quantitation of PCDD/Fs and dioxin-like PCBs in order to obtain a final TEQ for PCDD/Fs, PCBs, and PCDD/Fs+PCBs. These TEQ values were then examined in relation to child scores on the SRS, a standardized instrument suitable for administration to the general population that collects data from the parent on symptoms related to autism spectrum disorders. Parents rated the children on 65 items that were grouped into five subscales. In this study, the children were 9–12 years of age when the SRS was completed. Results showed that SRS Social Communication scores were significantly lower in male and female children exposed to higher TEQs from PCDD/Fs or from PCBs (p < 0.05). Autistic Mannerisms scores also correlated significantly with TEQs from PCDD/Fs or from PCDD/Fs+PCBs (p < 0.05). In sex-stratified analysis, the only significant association in boys was between TEQ from PCDD/Fs and the Social Motivation subscale (p < 0.05). In girls, however, significant associations were found for PCDD/Fs alone or combined with PCBs in relation to the total SRS score (p < 0.05) and the Social Communication subscale score (p < 0.05); and for all three TEQs (PCDD/Fs, PCBs, and PCDD/Fs+PCBs) in relation to the Social Cognition (p < 0.05) and Autistic Mannerisms (p < 0.01) subscales. The study’s limitations were that postnatal exposures were not accounted for in the analysis of prenatal exposures and that the sample size for this follow-up was small (n = 100 for boys and girls combined). Also, other risk factors for autism spectrum disorder that have emerged were not measured: air pollution, maternal nutrition, maternal diabetes, and inter-pregnancy interval. However, the study’s major strengths were the measurement of TEQs from the prenatal period and its control for potential
confounders, such as maternal age, education, the presence of older siblings, the age of the child, alcohol and smoking during pregnancy, and nationality. Some factors were also associated with attrition, thereby reducing selection bias.
In a paper related to the Nowack et al. (2015) research described above, Neugebauer et al. (2015) examined attention performance and attention-related behavior among 117 school-aged children who were part of the Duisburg cohort. Increased prenatal PCDD/F and PCB concentrations were significantly (p < 0.05) associated with a higher number of omission errors in the subtest Divided Attention (47% and 42%; 95% CI 1.08–2.00 and 1.07–1.89, respectively). Orenstein and colleagues (2014) evaluated verbal memory, visual memory, and learning in 393 children born to mothers residing in New Bedford, Massachusetts, near a Superfund Site. They calculated TEQs based on measurements of the dioxin-like mono-ortho substituted PCBs 105, 118, 156, 167, and 189 in cord serum. Verbal memory, visual memory, and a learning index from the Wide-Range Assessment of Memory and Learning at 8 years of age were evaluated, with adjustments made for a broad array of confounders, including examiner, child’s age at examination, sex, birth year, school grade, parental education, maternal age at birth, maternal birth place, household income, prenatal tobacco smoke exposure, prenatal alcohol exposure, prenatal omega-3 exposure, and maternal IQ. No significant associations were found.
A birth cohort studied by Pham et al. (2015) was established in Da Nang, Vietnam, in 2008–2009, at the site of a former U.S. air base, which is an area of documented high exposures to TCDD and other PCDD/Fs (Hatfield Consultants, 2009a,b). The study includes two districts in a surrounding area of 10 kilometers from the former air base. Exposure measurements were made in breast milk collected at approximately 1 month after delivery, and the chemical measured included 17 PCDD/Fs, including 2,3,7,8-TCDD. The Bayley Scales of Infant Development, 3rd edition (Bayley-III), was administered at age 12 months by an examiner who was supervised by a pediatric psychologist experienced with the Bayley-III. Adjustments were made for the gender, parity, gestational week, age (in days), and birth weight of the infants; the age, education, and drinking habit during pregnancy and residential location of the mothers; and the family income and smoking status of family members. Analyses found that the TEQs for three different metrics of exposure (all PCDD/Fs, TCDD, and the daily dioxin intake) showed virtually no associations with the overall cognitive score; the language composite, receptive language and expressive language subscales; and the motor composite, fine motor and gross motor subscales. The one exception was the daily dioxin intake (in pg TEQ/kg/day), which was significantly associated with improved overall cognitive scores in each of the three upper levels of exposure—mild (102.8; p = 0.033), moderate (102.1; p = 0.042), and high (102.4; p = 0.041), as compared with low exposures (97.7). No adaptive behavior skills showed any association with the exposures examined. However, based on parent report (not observation), scores on a social–emotional scale that assesses
functional emotional skills and self-regulation were lower in the children with the highest category of prenatal overall TEQ and the highest category of the TCDD TEQ, as compared with the corresponding lowest-exposure categories (p = 0.09).
Tai et al. (2016)—whose results regarding physical growth in offspring are reviewed earlier in this chapter—enrolled 217 mother–infant pairs living in a dioxin-contaminated area in Vietnam and followed the neurodevelopment of the birth cohort longitudinally during the first 3 years of life. The perinatal dioxin exposure of infants was estimated by the measurement of dioxin levels in breast milk of the nursing mothers. The neurodevelopment of infants and children, including cognitive, language, and motor development, was determined at 4 months, 1 year, and 3 years of age. Multivariate mixed models were applied for analyzing repeated measures. In boys, composite motor (94.9, 95% CI 89.9–100, p = 0.49) and gross motor scores (9.1, 95% CI 7.9–10.2, p = 0.36) were significantly decreased with increasing exposure of 2,3,7,8-TCDD. The high PCDDs/PCDFs-TEQ group showed a significant decrease in expressive communication score compared with the low-exposure group, as measured by Bayley-III (8.4, 95% CI 7.8–8.8, p = 0.30). In girls, there was no decreased score in any neurodevelopment aspects in high-exposure groups. This study suggests that perinatal dioxin exposure affects the neurodevelopment of infants and children in the first 3 years of life in a sex-specific manner.
Tran et al. (2016) investigated the effects of early life exposure to dioxins in children living around Da Nang City, Vietnam, a location known to be contaminated with dioxin. Exposure was assessed via a breast milk sample from the nursing mother taken 1 month after birth. Demographic and confounding factors data were collected from the mother; physical measurements of the subjects—176 children—were taken at birth and 5 years of age. In boys, the total test (7.7 versus 9.7; p = 0.018) and balance (7.3 versus 9.3; p = 0.013) scores of the Movement Assessment Battery for Children-2 (Movement ABC-2) were significantly lower in the high-TEQ-PCDDs/Fs group compared with the moderate- and low-exposure groups. Nonverbal Index scores (77.0 versus 98.1; p = 0.034) and the pattern reasoning subscale of the Kaufman Assessment Battery for Children, 2nd Edition (KABC-II) (5.3 versus 7.1; p = 0.041), which measures planning ability, were also significantly lower in the high-TCDD-exposure group compared with the low-exposure group of boys. However, in girls no significant differences in Movement ABC-2 or KABC-II scores were found among the different TEQ-PCDDs/Fs and TCDD exposure groups. The five boys and one girl who were highly exposed to TEQ-PCDDs/Fs and TCDD were found to be at increased risk for difficulties in both motor coordination and cognitive function. Overall, these results suggest differing impacts of TEQ-PCDDs/Fs and TCDD exposure on motor coordination and higher cognitive ability, respectively. Moreover, high-TEQ--
PCDDs/Fs exposure combined with high-TCDD exposure may increase autistic traits combined with developmental coordination disorder.
Immune and Allergic Outcomes
Since Update 2014, five studies identified as relevant by the committee have examined exposure to the COIs and immune or allergic outcomes in children.
P. H. Su et al. (2015) conducted a study of 56 8-year-old children from Taiwan. The subjects were stratified into high- and low-exposure groups based on maternal PCB and PCDD/F serum levels. No changes were found, although there were differences (t-test) in thyroid hormones, thyroxine binding globulin, IGFBP-3, and growth hormone.
El Majidi and colleagues (2014) used a procedure previously developed to standardize PCB biological concentration data between published studies to perform a systematic analysis of associations between PCB exposure and thyroid hormones (THs) (total and free T3 and T4) or thyroid-stimulating hormone (TSH) in pregnant women and newborns. The weight of evidence of a significant impact of PCB exposure on TSH and TH levels at the described biological levels in pregnant women and newborns (mean < 1,000 μg PCBΣEQ kg−1 lipids) appeared low, according to this analysis.
S. Hansen et al. (2014) examined the association between maternal serum concentrations of the dioxin-like PCB 118 and the risk of asthma in their offspring. Compared with subjects in the first tertile of maternal PCB 118 concentration, those in the third tertile had an adjusted HR of 1.90 (95% CI 1.12–3.23). Offspring were exposed in utero, and this is thus evidence of a developmental exposure leading to a later-life health impact.
A second study by S. Hansen et al. (2016) examined a cohort of 965 (421 in the follow-up) pregnant women and measured POPs, including PCBs. Exposures were separated into tertiles for the analysis, based on week-30 POP levels. A clinical evaluation of offspring took place at age 20. No association with asthma was found, but dioxin-like PCB exposure in the mother was associated with offspring airway obstruction (FEV1/FVC < 75%; OR = 2.96, 95% CI 1.14–7.70), with 9 cases versus 20 cases. This is an interesting finding in that this condition may be associated with chronic obstructive pulmonary disease (COPD) later in life.
Nicolle-Mir (2014)—in a paper published in French—examined the association between prenatal exposure to PCBs, hexachlorobenzene, and p-p′-DDE and the risk of asthma in children born to women included in a cohort of Danish births in 1988–1989 (the Danish Fetal Origins Cohort). The concentrations of POPs (congeners 118, 138, 153, 156, 170, and 180 for PCBs) were determined in a maternal serum sample taken at 30 weeks of pregnancy. Nine hundred sixty-five children in the cohort were contacted in 2008 to complete an online questionnaire covering their health status, lifestyle, and diet; 872 (90%) returned data that could
be included in the analysis. Their government personal identification numbers were cross-referenced with the national prescriptions file, and subjects who had been treated for asthma between the ages of 6 and 20 years were classified as asthmatics. Those who had received a single prescription of beta-2 mimetics or inhaled corticosteroids were excluded. Three secondary criteria were also used: the diagnosis of asthma made by a doctor, the use of asthma treatments during the past 12 months (questions asked in the follow-up questionnaire), and a hospital diagnoses of asthma (outpatient or emergency, based on a central admissions registry). The cross-tabulation of these different sources showed a good match between the data from the national prescription file and the responses to the questionnaire. The prevalence of asthma was equal to 12.7% according to the file and to 13.6% according the questionnaire; 76% of subjects classified as asthmatics on the basis of the file report had an asthma diagnosis, and 72% of those who reported having been diagnosed with asthma were identified in the file. Serum concentrations of POPs were divided into tertiles, and the HR of asthma was calculated for the second and third tertiles with reference to the first. Analyses included those carried out with each individual congener and the sum of the dioxin-like congeners (PCBs 118 and 156). Models were adjusted to the child’s sex and birth weight as well as to several maternal parameters: age at birth, pre-pregnancy BMI, parity, smoking, alcohol consumption, education level, plasma concentrations of triglycerides, and total cholesterol. The main analysis (asthma defined according to the national prescriptions file) showed an increase in the risk of asthma in the third tertile concentration of dioxin-like PCBs with reference to the first tertile (HR = 1.75, 95% CI 1.02–2.98), with significant dose–response trends. The association between dioxin-like PCBs and asthma is mainly due to the 118 congener (HR = 1.9, 95% CI 1.12–3.23). For PCB 156, PCBs with no dioxin activity, and the sum of the PCBs, the increases were not significant. The results of the secondary analyses were consistent: the strongest associations were observed with PCB 118 when the criterion “treatment of the asthma during the last 12 months” was used (HR in the last tertile respectively equal to 2.49 [95% CI 1.03–5.99] and 4.18 [95% CI 1.57–11.15]). The authors indicate, however, that the plasma levels of the dioxin-like PCBs and hexachlorobenzene were highly correlated (Spearman’s R-value 0.53–0.99), with the mutual adjustments weakening the associations observed in the main analysis to HR = 1.47 (95% CI 0.75–2.86) for PCB 118. In addition, in the absence of information about breastfeeding, it is unclear whether these associations are attributable to prenatal or postnatal exposures or a combination of both.
Other Outcomes
No studies identified as relevant by the committee have examined exposure to the COIs and reproductive function in offspring since the publication of Update
2014. However, since this category was included in the Update 2014 report, it is noted here for completeness.
Li and colleagues (2015) conducted a study of the so-called “Yucheng children,” descendants of mothers exposed to PCBs/PCDFS via a contaminated cooking oil incident in 1978–1979 in Taiwan, to evaluate the effect of this insult on their auditory function later in life. Exposed children were born June 1978–December 1998; control children were matched by age, sex, neighborhood, mother’s age, and parents’ educational level and occupation. Demographic data were collected via questionnaire, and pure-tone audiograms were obtained. Eighty-six Yucheng children and 97 controls participated in the study. Serum taken from the mothers during other studies had previously been screened for dioxins and PCBs. The study found that gestational exposure to 2,3,4,7,8-PeCDF was associated with an increase in hearing loss (increase in the pure tonal frequency threshold), although the frequency deficits were not the same in the right and left ears. Exposures to 1,2,3,4,7,8-HxCDF were associated with hearing loss in the left ear at 4,000 Hz. There was no effect of PCB exposure on hearing, although the PCB levels as measured by TEQ were rather low.
Biologic Plausibility
As noted in Update 2014, the results of studies in rodent models provide support for the idea that prenatal exposure to TCDD can result in adverse effects in offspring later in life, including immune disorders, behavioral disturbances, reproductive impairment, kidney disease, and cancers (Foster et al., 2010; Prescott, 2011; Puga, 2011; Takeda et al., 2012). Using two mouse models, investigators showed that prenatal TCDD (2.5–5.0 mg/kg) modified multiple immune signatures in the adult offspring that were indicative of adult-onset autoimmunity (Holladay et al., 2011). Adult-onset inflammatory disease and lupus-like autoimmunity were also observed in mice at 36 weeks of age after high-dose prenatal TCDD exposures (Mustafa et al., 2011). A single prenatal exposure of rats to TCDD (0.7 μg/kg of body weight) reduced brain developmental myelination and compromised remyelination potential in adults (Fernández et al., 2010), and in utero TCDD in mice altered neural progenitor differentiation (Mitsuhashi et al., 2010). However, another study suggested that, in contrast to wild-type murine neural progenitor cells (mNPCs), “human NPCs and AhR-deficient mNPCs were insensitive to AhR agonism or antagonism” (Gassmann et al., 2010, p. 1571). Maternal exposure to TCDD (0.2–0.4 μg/kg of body weight) in pregnant rats perturbed neuroendocrine function as measured by thyrotropin and growth hormone concentrations in exposed offspring through peripubertal postnatal day 30, which supports the idea of continued later-life thyroid hormone disturbances (Ahmed, 2011, 2014). These disturbances include the epigenetic modification of imprinted genes (Somm et al., 2013), increased DNA methylation of the BRCA1
tumor suppressor gene in mammary tissue (Papoutsis et al., 2013), altered uterine response to estradiol (Burns et al., 2013), dysregulation of lipid metabolism in the presence of a high-caloric diet (Sugai et al., 2014), aberrant emotional behaviors (A. T. Nguyen et al., 2013), a reduced capacity for lymphocyte differentiation (Ahrenhoerster et al., 2014), testicular inflammation (Bruner-Tran et al., 2014), and a variety of adult diseases including kidney disease, prostate disease, ovarian primordial follicle loss, and polycystic ovarian disease (Manikkam et al., 2012a).
Other studies have focused on neurobehavioral outcomes following perinatal exposure, which is of relevance in understanding the possible consequences in the offspring of Vietnam veterans, particularly the offspring of female veterans. Haijima et al. (2010) found that gavage treatment of pregnant mice with 3 μg/kg TCDD on gestation day 12.5 (resulting in in-utero and lactational exposure of the offspring) impaired memory in the male offspring. Mitsui et al. (2006) reported that hippocampus-dependent learning could be impaired in male rats exposed to TCDD in utero and that the impairment could affect fear conditioning. Curran et al. (2011) assessed the effect of CYP1A2 and Ahr genotypes on altered learning and memory in mice exposed to an environmentally relevant mixture of dioxin-like (coplanar) and non-dioxin-like PCBs in utero and during lactation. They observed the most significant deficits in response to PCB treatment in Ahrb1 CYP1A2(–/–) mice, including impaired novelobject recognition and increased failure rate in the Morris water maze test. Studies in week-old rodents have also detected molecular effects of TCDD in cerebellar granule cells and neuroblasts, which may be relevant to motor function and cognitive processes (Kim and Yang, 2005; Williamson et al., 2005). Stürtz et al. (2008) found alterations in how female rats that had been fed on postpartum days 1–7 with diets containing 15, 25, or 50 mg/kg 2,4-D interacted with their pups. The specific relevance of these findings to neurobehavioral effects in humans exposed as adults is unclear.
Another mode of epigenetic change is the modification of the spatial arrangement of chromosomes, which can influence gene expression and cell differentiation. Oikawa et al. (2008), for example, found that TCDD—through the AHR—modifies the positions of chromosomes in the interphase nuclei of human preadipocytes.
The studies discussed above suggest that TCDD has the potential to influence the epigenome and therefore could promote changes in offspring that lead to disease later in life, although the research addressing this issue in model systems is still at an early stage.
Synthesis
Epidemiologic studies designed to examine the effects of the COIs in more mature offspring have evaluated a variety of growth and development, neurologic, immunologic, reproductive, and endocrine system health outcomes. More studies are required before conclusions can be reached as to whether such outcomes in
the offspring of exposed parents are replicable. In particular, it would be of interest to obtain information on neuropsychiatric conditions, such as attention-deficit hyperactivity disorder and other clinically defined neurodevelopmental outcomes in children who were exposed in utero.
Conclusion
There is inadequate or insufficient evidence to determine whether there is an association between the exposure of men and women to 2,4-D, 2,4,5-T, TCDD, picloram, or cacodylic acid before conception or during pregnancy and the adverse health outcomes in their children addressed in this section. Although the results of laboratory research support the plausibility of such clinical conditions, there are not yet sufficient human data to support an association between the COIs and such adverse outcomes in human offspring.
BIOLOGIC PLAUSIBILITY OF POSSIBLE EFFECTS IN SUBSEQUENT GENERATIONS
In response to a special request from the Department of Veterans Affairs, continuing inquiries from veterans and their families, and increasing attention in research efforts, the committee responsible for Update 2010 explored the possibility of intergenerational or transgenerational effects resulting from exposure-related epigenetic changes in the parents or exposed fetuses that would lead to adverse health effects in later generations, such as grandchildren. Effects in persons exposed in utero are not considered transgenerational because the fetus was likely exposed directly. This exception includes the children of women exposed in Vietnam even if they are conceived after their tour of duty was over because TCDD remains in the body for a long time and is mobilized during pregnancy. Likewise, the children of men exposed to TCDD in Vietnam and born after the soldiers’ tour of duty was over could possibly have health outcomes due in part to TCDD’s effect on the sperm epigenome. In contrast, any adverse health effects in grandchildren associated with exposure would be considered to be transgenerational. Figure 8-1 illustrates the partitioning between intergenerational and transgenerational effects due to the exposure of a parent, delineating between those exposures that biologically persistent (like dioxin) and therefore dwell in the body long after exposure versus those that do not.
The Update 2010 committee did not identify any relevant scientific studies to review and concluded that there was inadequate or insufficient evidence to determine whether there is an association between exposure to the COI and diseases in grandchildren or later descendants of Vietnam veterans. Committees responsible for Updates 2012 and 2014 likewise failed to find any relevant human studies and affirmed the conclusion of inadequate or insufficient evidence.
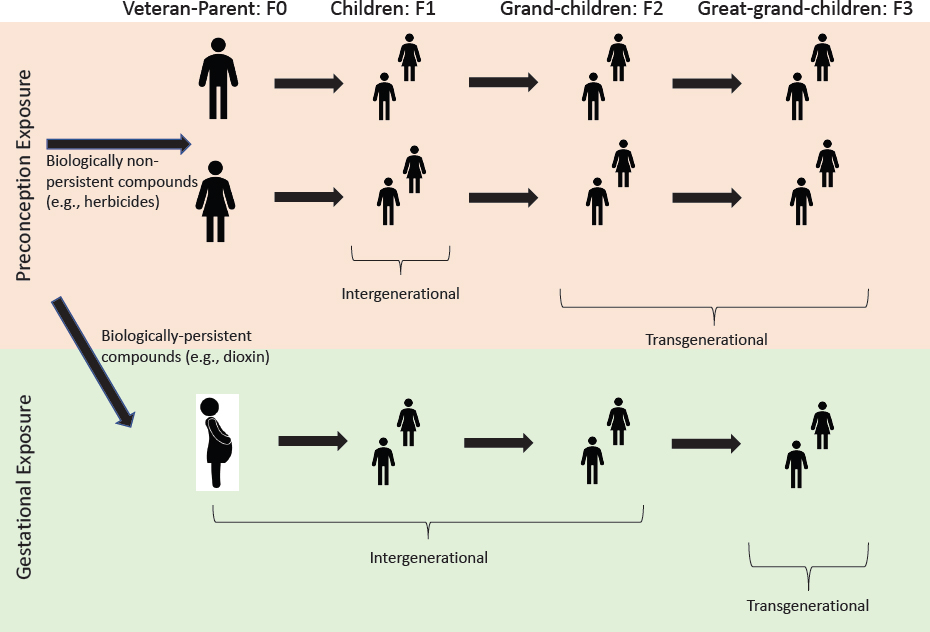
SOURCE: Adapted from NASEM, 2018, Figure 3-2.
Congress put forward and passed the Jeff Miller and Richard Blumenthal Veterans Health Care and Benefits Improvement Act in 2016, and it became Public Law 114-315. Among the law’s provisions is Section 632, which called on the Secretary of Veterans Affairs to enter into a contract with the National Academies of Sciences, Engineering, and Medicine to conduct an assessment on scientific research relating to the descendants of individuals with toxic exposure. As part of its response to this directive, VA tasked this committee to “assess the current research available on possible generational health effects that may be the result of exposures to [the COIs]—including the biologic plausibility or potential for an exposure to lead to an increased risk of birth defects or other adverse conditions in the descendants of male Veterans.”10
Epidemiological Studies
No relevant studies of potential transgenerational effects of exposure to the COIs in humans have been reported to date. The only transgenerational effect shown in humans has been from a comparison of food supplies in Sweden during the 1800s and health outcomes in the children and grandchildren of men who were prepubescent when food supplies were relatively high or low. These studies found an association between high food supply levels in grandfathers and decreased longevity and increased risk of cardiovascular disease and diabetes in grandsons that was paternally transmitted, although no mechanistic information was obtained (Kaati et al., 2002, 2007). Whether transgenerational effects can occur in humans from chemical exposures is unknown at this time.
Biologic Plausibility
Research on the biologic mechanisms that might underlie the potential effects of exposure to the COIs experienced by grandchildren and later generations is still sparse, although it has greatly expanded in the past few years. Epigenetic effects have been shown for male gametes in adult mice exposed to an endocrine-disrupting pesticide (methoxychlor) and fungicide (vinclozolin) (Paoloni-Giacobino, 2014). However, the chemically induced DNA methylation changes in sperm DNA were not transmitted from one mouse generation to the next for imprinted genes; they were, presumably, lost during the period of active demethylation that occurs shortly after fertilization. This observation suggests that transgenerational effects on imprinted genes in mice that might be paternally transmitted may not necessarily involve DNA methylation (Iqbal et al., 2015). Nonetheless, a 2013 study showed that odor fear-conditioning in the father could be paternally transmitted to the F2 generation (as well as the F1) and implicated
___________________
10Gulf War and Health, Volume 11 (NASEM, 2018) addresses this mandate as it applies to exposures experienced during the conflicts in the Southwest Asia theater of military operations.
reduced DNA methylation in the responsible odor receptor gene (Dias and Ressler, 2013). Thus, more research is required to understand better how transgenerational effects can be transmitted paternally once they have been demonstrated (Dias and Ressler, 2014).
A few animal studies have provided evidence of transmission of adverse effects to later generations. The mechanisms that could underlie later-life effects in offspring and effects in later generations (transgenerational inheritance) could involve epigenetic processes, as described earlier in this chapter. Research into dioxin’s potential as an epigenetic agent is in its early stages, but a few studies have suggested that dioxin has such properties that are, in significant part, linked to the AHR. Direct evidence, however, is limited to maternal exposures of the developing embryo or fetus during in utero growth, and no reports exist showing paternal TCDD exposure and later-life effects in offspring or paternally mediated transgenerational effects in humans. Q. Wu et al. (2004) demonstrated that TCDD exposure of mouse embryos before implantation in unexposed females resulted in epigenetic changes, including increased DNA methylation and a reduced expression of imprinted genes, which implied that early embryonic exposure alone was sufficient to alter gene expression in the resulting offspring. The transmission of effects to later generations would involve epigenetic alterations in the developing germ cells of a fetus that was directly exposed to maternal TCDD in utero.
Results of a few studies support a transgenerational inheritance due to in utero exposure to TCDD. Exposing pregnant mice to TCDD (at 10 μg/kg) reduced fertility and increased premature birth in three later generations (Bruner-Tran and Osteen, 2011); effects were transmitted through both male and female offspring (Ding et al., 2011; McConaha et al., 2011). Exposing gestating female rats (F0) to dioxin (TCDD) at 100 ng/kg was shown to result in an earlier puberty in the offspring (F1) and in two later generations (F2 and F3) and to reduce ovarian follicle numbers in the females of the F3 generation; this implies transgenerational inheritance (Manikkam et al., 2012a). The F3 effects appear to be transmitted through the sperm that were initially exposed to maternal dioxin in utero. In a second paper by the same research team, additional diseases appeared later in life in the first generation (directly exposed offspring), including prostate disease in males and ovarian follicle loss and polycystic ovarian disease in females (Manikkam et al., 2012b). Further third-generation effects were noted, including kidney disease in males and polycystic ovarian disease in females, which imply transgenerational inheritance. The latter appear to be transmitted through the sperm originally exposed to maternal dioxin in utero inasmuch as sperm DNA methylation changes were observed at 50 chromosomal sites in generations F1–F3. Testicular inflammation from TCDD exposure has also been reported to manifest in multiple generations (Bruner-Tran et al., 2014).
The zebrafish has been used as a model to examine transgenerational effects from dioxin exposure, although different groups have reported different aspects of these effects since Update 2012. One group reported that exposing zebrafish at
3 and 7 weeks old (during sexual development) to TCDD in water for 1 hour at 50 pg/ml increased female-to-male ratios and skeletal abnormalities and reduced fertility in the F1 and F2 descendants (equivalent to F2 and F3 in mammals) (Baker et al., 2014a,b). Another group studying DNA methylation changes in the offspring of mothers fed 20 μg/kg TCDD in their food reported no changes in global methylation in offspring when looking at the total levels of DNA methylation in the genome. However, gene-specific increases or decreases in promoter DNA methylation were observed with a tiling array assay for a limited number of genes in the F1 generation. CYP1A1 transcription, a marker of TCDD exposure, was elevated in F1 offspring. Unfortunately, no F2 fish were generated from the TCDD exposure because the F1 fish died 1 to 2 weeks post hatching (Olsvik et al., 2014). Further work with this model will be helpful for providing targets for mammalian biologists as they continue to probe for transgenerational effects from TCDD and the other COIs.
Synthesis
No epidemiological information exists to evaluate whether paternal or maternal exposure to the COIs results in health effects in grandchildren or subsequent generations of descendants of Vietnam veterans. The animal literature contains evidence that environmental agents mediated by maternal exposure affect later generations through fetal and germline modifications, but in the case of adult male exposures before the conception of the next generation, there is insufficient evidence on which to draw a conclusion regarding transgenerational effects.
Conclusion
On the basis of the evidence reviewed here and in previous VAO reports, the committee concludes that there is inadequate or insufficient evidence to determine whether there is an association between paternal or maternal exposure to the COIs and health effects in grandchildren or later generations of descendants of Vietnam veterans.
This page intentionally left blank.




































































