5.10 Searching for Life Across Space and Time: Proceedings of a Workshop
A Proceedings of the Space Studies Board, Joseph R. Schmitt, Rapporteur
Setting the Stage
Michael Moloney, the Director of Space and Aeronautics at the National Academies of Sciences, Engineering, and Medicine, opened the December 5-6, 2016, workshop1 entitled “Searching for Life across Space and Time” by welcoming all those attending the workshop (see Appendix B),2 both in person at the Arnold and Mabel Beckman Center (Irvine, California) and online watching the webcast.3,4 The Space Studies Board carries out a major workshop every 2 years for NASA’s Science Mission Directorate, who sponsors the workshop. The National Academies’ workshops are designed to promote dialogue, not to form a consensus opinion or to make official recommendations. Opinions and recommendations contained within this workshop proceedings are those of the speakers themselves and are not intended to represent the opinions or recommendations of the workshop or the National Academies as a whole. In addition to fostering discussion, Moloney and the Space Studies Board also see this workshop as an early step in preparation for two upcoming decadal surveys—one in astronomy and astrophysics starting in 2018 and one in planetary science starting in 2020.
INTRODUCTION TO THE WORKSHOP
The search for life is one of the most active fields in space science and involves a wide variety of scientific disciplines, including planetary science, astronomy and astrophysics, chemistry, biology, chemistry, geoscience, and so forth. These workshop proceedings cover the very stimulating discussions that were held by experts in the various fields about the possibility of habitable environments in the solar system and in exoplanets (i.e., those outside the solar system) and techniques for detecting life and the instrumentation used. The cross-disciplinary discussions were designed to be a highlight of the workshop format.
James Kasting, Pennsylvania State University, and chair of the workshop’s organizing committee (see Appendix E), began the workshop by describing the approach the committee took to organizing the workshop, breaking it down into four regions of parameter space (also see Table 1.1):
- In situ detection in the solar system of life as we know it (e.g., Mars),
- In situ detection in the solar system of life as we don’t know it (e.g., Titan),
- Remote detection on exoplanets of life as we know it (e.g., “exo-Earths”), and
- Remote detection on exoplanets of life as we don’t know it (target unknown).
These four categories can be used to help guide the approach to detecting life beyond the Earth. Each region has its own characteristic set of biosignatures and will require a different set of technologies, instruments, knowledge, and expertise to determine whether life can or does exist in each environment. This workshop is intended to foster dialogue on the best way to accomplish the goal of detecting life beyond Earth.
___________________
NOTE: “Setting the Stage” reprinted from Searching for Life Across Space and Time: Proceedings of a Workshop, The National Academies Press, Washington, D.C., 2017, pp. 1-14.
1 The workshop agenda is included as Appendix A.
2 There were 120 in-person and 92 webcast attendees on day one, and 80 in-person and 72 webcast attendees on day two.
3 The statement of task is included as Appendix C.
4 A summary of the material presented during poster sessions is included as Appendix D.
TABLE 1.1 Four Regions of Parameter Space with an Example of the Type of World in which We Might Find It
| In situ detection (solar system bodies) | Remote detection (exoplanets) | |
|---|---|---|
| Life as We Know It | Mars SOURCE: NASA/JPL/USGS. |
Artist’s concept depicting Kepler 22b SOURCE: NASA/JPL and NASA/Ames Research Center. |
| Life as We Don’t Know It | Titan SOURCE: NASA/JPL/University of Arizona/University of Idaho. |
S Exoplanet, UCF-1.01 SOURCE: NASA/JPL. |
HOW LIKELY IS IT THAT LIFE EXISTS BEYOND EARTH?
John Baross of the University of Washington presented the first talk of the workshop on the likelihood that life exists beyond Earth. He started his talk by stating that he would focus on “life as we know it” and leave the “weird life” discussions for later talks. The most important aspect of this workshop, in Baross’s opinion, is how life is initially acquired. Panspermia, which includes the notion that life can be transported to Earth by meteorites, comets, or even spacecraft, is one possibility, but the limits of such an occurrence in terms of distance from the source, time of origin, survival, characteristics, and so on are unknown. The biggest challenge in Baross’s mind, however, is a de novo origin of life (or abiogenesis). Open questions on abiogenesis include what, if any, are the essential planetary conditions for life and how they might be detected or inferred on other planetary bodies in the solar system and beyond.
Life on Earth
Baross then listed the basic requirements for life as we know it. Life uses either light or chemical energy. For example, H2 was probably the first energy source of microbial systems. Life also requires oxidants. The most abundant elements in the universe (C, H, N, O, P, S, and Fe) are required for life, but also many trace elements like boron, vanadium, tungsten, and nickel. In total, more than 30 elements are required. Baross mentioned that, according to new research, the two earliest catalytic systems on Earth likely required tungsten and molybdenum. Pyrococcus furiosus, a high-temperature microorganism from hydrothermal vents, has been found with never-before-seen metalloproteins, including proteins containing lead and uranium. Life also builds catalytic and energy-transfer organic macromolecules around metals and metal-sulfur clusters. Baross said that it is now thought by many that mineral catalysis preceded protein catalysis, providing the backbone of reaction networks that led to metabolism.
Life on Earth has a common ancestor, Baross said. This idea is based on what he called the “unity of biochemistry,” which is the fact that all life has the same biochemical and molecular characteristics: the same nucleotide bases, the same 20 amino acids (along with selenocysteine), the same genetic code, lipids with straight, methyl-branched chains, and metabolic energetics that use phosphate anhydrides and thioesters. This unity of biochemistry is reflected in the global phylogenetic tree. Baross then asked whether life on other planetary bodies would exhibit a similar sort of unity of biochemistry and if Darwinian selection would allow the most fit genes to survive there too.
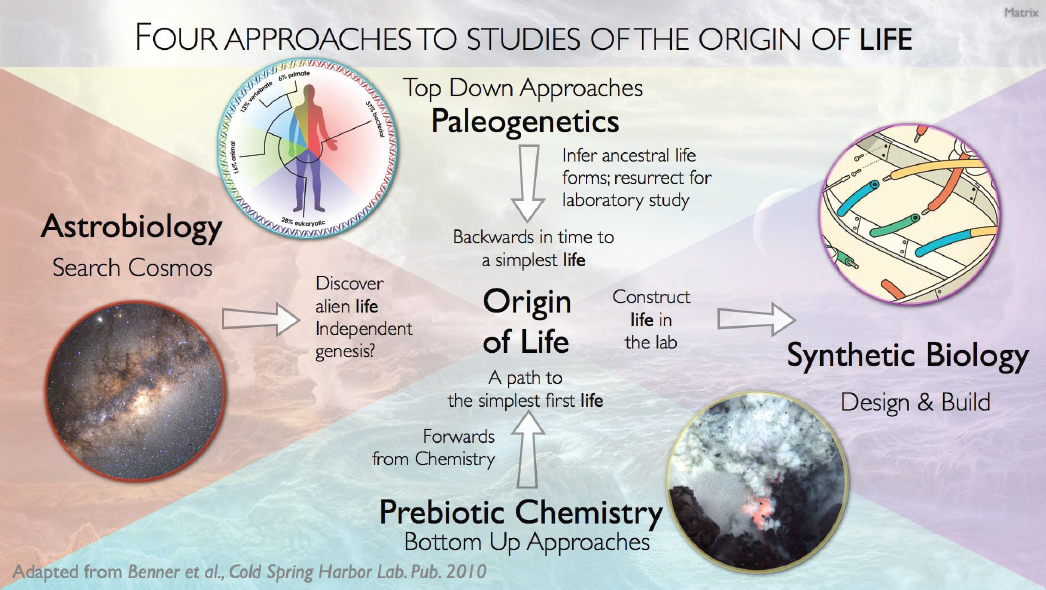
Switching topics, Baross said that Earth’s geophysical and geochemical characteristics are important because they are the sources of the essential elements and minerals used. He then said he believed that plate tectonics and hydrothermal vent systems are two such essential processes; life as we know it cannot form without them. Plate tectonics can be dated back as far as the end of the Hadean era (~4 billion years [Gyr] ago), according to Baross, although there is wider agreement on a date of 3 Gyr ago. Combining geology with biology is a field Baross calls paleogenetics, which is one of the four ways to approach the issue of abiogenesis. The other three are astrobiology (specifically exobiology and finding alien life), studying prebiotic chemistry to map out the simplest form of life, or creating new life with synthetic biology (see Figure 1.1).
Life is at least 3.7 Gyr old, according to all of the research that has come out, according to Baross.5 One such paper indicated the existence of stromatolites 3.7 Gyr ago from the Isua sub-crustal belt in SW Greenland, which likely came from a shallow, seawater environment. Three months after the workshop, there was a report of potential 3.77 Gyr old microfossils from a super-crustal belt in Quebec, Canada. The putative fossils were found in iron-containing rocks indicating a seafloor hydrothermal vent setting. If this report is substantiated, Baross said, it would indicate that hydrothermal systems were the earliest habitats for life and perhaps were also instrumental in the origin of life.
Baross said that all evidence points to hydrogen as being the earliest source of chemical energy for both photosynthetic and non-photosynthetic organisms. Methanogens (methane-producing organisms) appear to be ancient and possibly even the root of the Archaea tree. Presumably, hydrothermal vents would have provided the ingredients (hydrogen, sulfur, nitrogen, and other compounds) and the needed physical, chemical, and spatial gradients necessary for life.
New discoveries, Baross continued, may lead to changes in the way we think about life on Earth. One such major change is that, according to Baross, the evidence is starting to become overwhelming that the domain of Eukarya might instead reside within the lineage of the Archaea domain (see Figure 4.2), the root of which was likely methanogens. Some of this evidence comes from the Loki hydrothermal vent site in the Arctic Mid-Ocean Ridge, which contained Archaean organisms called Lokiarchaea. Lokiarchaea possess many characteristics originally thought only to be present in eukaryotes, such as a cytoskeleton, membrane remodeling, ubiquitin modification of proteins, and endosymbiosis and/or phagocytosis. The divergence of Lokiarchaeota and Eukaryota may have coincided with a merger with a bacterial endosymbiont (i.e., mitochondria). This paleogenetic approach points to hydrothermal systems as the providers of carbon, energy sources (mainly H2), and other essential elements for life, and also possibly as the location of the ancestor of modern-day eukaryotes.
___________________
5 There are competing viewpoints. See, for example, B. Grierson, “The Big Debate Over the Oldest Life on Earth,” Discover Magazine, December 2011.
Life Beyond Earth
Baross would envisage a search for geophysical processes and water-rock reactions on exoplanets and solar system planets and moons. Enceladus, a moon of Saturn, is an icy body that may have (or have had) active water-rock interactions like the serpentinization systems on Earth that could support life. Serpentinization occurs when olivine [(Mg,Fe2+)2SiO4] becomes hydrated and, in the process, produces H2, CH4, and other hydrocarbons out to at least C5, formate, acetate, and pyruvate. This process results in two things: (1) it raises the temperature to as high as 268°C, and (2) it expands the rock by a factor of about one-third. Serpentinization allows up to 300 kg of water to be taken up by one cubic meter of rock. An oceanic plate undergoing subduction releases this water deep in the lithosphere, which helps give rise to volcanoes and hydrothermal vent systems.
Enceladus has many of the same properties as the so-called Lost City system of hydrothermal vents in the mid-Atlantic, such as detections of CO2, H2, CH4, higher-order hydrocarbons, and a high pH value. However, there are many unknowns in the Enceladus system, like the abundances of important metals, which makes it difficult to say whether there exist the conditions for life. In the Lost City, there is a single species of archaea in the highest-temperature regions involved in methane cycling that can both consume (oxidize) methane and produce it anaerobically. It demonstrates what Baross thinks is a living vestige of mineral catalysis (as opposed to protein catalysis), which he said could be the most ancient catalytic pathway on Earth. Several metals are required though, such as iron, tungsten, selenium, cobalt, zinc, and nickel. Returning to the subject of Enceladus, Baross wondered if tidal heating could mimic subduction in hydrating and dehydrating rocks.
Baross finished by saying that we do not know how life on Earth came to be—whether through abiogenesis or panspermia. Other planets or the moons in the solar system may be habitable if they could acquire the right kind of life. Abiogenesis, he said, would have required a tectonically active, rocky planet with plentiful resources. Finally, he concluded by saying that paleogenetics has inferred that the earliest group of microbes, potentially including the ancestors of modern eukaryotes, were associated with hydrothermal vents.
Audience Participation
A member of the audience said that, for models of abiogenesis at hydrothermal vents, the H2 coming from serpentinization reduced CO2 dissolved in the ocean because there was a large, thick, CO2 atmosphere at the time. He then asked what the CO2 source would be on Enceladus and whether this could be the limiting ingredient for life there. Baross said that CO2 is present there, but was not sure of the concentration. On a similar line of questioning, another audience member then said that the relative abundance of CO and N2 on Enceladus, both probably primordial, is being debated, considering they both have the same number of atomic mass units. Some CO would convert to CO2 through OH from water. He then asked what happens when N2 is put into the system and what kind of biosignature there might be. Baross brought up a proposal from a group who wanted to capture particles in the plume of Enceladus to analyze any organic polymers associated with them. He went on to say that many groups in the international community think that serpentinizing environments were the source of the origin of life. If that were the case, he said, Enceladus would be a good test to see if the correct organic compounds and polymers were there.
A workshop participant then asked whether the trace elements were really necessary for life or if just sulfur and iron were needed. Baross said that there are two approaches to creating life. The “metabolism first” approach is the one most interested in serpentinization because of the gradients present there (like pH). The organisms associated with serpentinization, Baross said, are thought by many to be the most ancient CO2-fixing pathway in life. The other approach is making RNA first, but Baross has no idea how to make RNA in a serpentinizing environment. Baross then said that he thinks a global Earth is necessary for the origin of life, not just individual serpentinizing systems that could be habitable for the first living organism.
Moving to the importance of geology for life, another participant at the workshop asked whether having a tectonically active planet was necessary for the origin of life or whether it was only necessary for sustained habitability. Baross answered that, in his opinion, while planets and moons may be able to sustain life, a planet having had a de novo origin of life must have had plate tectonics or other, similar geophysical processes.
A member of the audience then focused on false positives of biosignatures. He said that organics could be produced in serpentinizing systems, but life in these systems could also produce organics. He then asked how one would go about teasing apart these signatures. Baross emphasized that serpentinization is just one of several systems that can produce organics. Greigite (Fe3S4), for example, can also produce acetate and pyruvate. Even cosmic dust shows abiotic synthesis of organic compounds, such as ether-linked lipids. Baross wished more money would go into understanding the origin of life, such as mineral catalysis and how the synthesis of organics depends on temperature, pH, and other conditions, to better understand how abiotic processes could produce organic materials. The audience member then commented that the very systems that are needed for abiogenesis could also mimic biosignatures. Baross then wondered what the most compelling biosignatures would be to avoid that scenario, thinking maybe that organic biochemistry might have to be combined with other processes, like isotopic analysis or disequilibrium. Another audience member agreed, saying multiple detection techniques are needed to be convincing. She then said that the detection of certain molecules may not be a potent biosignature, but that their relative ratios to other molecules might make them a biosignature. One example she gave was the lipid fatty acid pattern with either a C2 or C5 addition, which could suggest life, since abiotic synthesis can only add one carbon at a time.
An example to support Baross’s earlier claim that a global Earth is necessary for the origins of life rather than just localized serpentinization systems was then provided by one audience member. He said that trace metals are important for abiogenesis, but at high pH, most of them are very insoluble. Abiogenesis then requires a way to decrease the pH or to produce redox gradients. Atmospheric escape would work for both, and it operates at a global scale. The audience member then said that sugars in prebiotic chemistry are hard to figure out because they’re so unstable. He said that reduced carbon, which could have come out of the atmospheric envelopes of red giants, could get irradiated, which would add OH groups to molecules. This cosmic organic matter could provide the unstable carbohydrate-like compounds that would be hard to produce in a hydrothermal system. Baross again said that he supported looking at a global Earth for abiogenesis. He then said that, if the proper minerals are found under certain conditions, sugars will be found. For example, ribose has been found to be catalyzed by borate minerals. He also said that a four-carbon organoboron compound in a ring structure, believed to be very ancient, is used as a signaling compound in bacteria and looks just like ribose. He then wondered whether there could be a compound that is non-ribose that could have formed and served as the early backbone in RNA.
Going back to geology, another workshop participant said that Mars does not have tectonics, but it does contain almost all of the trace elements required for life. He then asked if there was any reason to exclude places like Mars from having abiogenesis just because they don’t have plate tectonics, even if they have atmospheric photochemistry and ways to aqueously alter minerals to enhance trace metals. Baross answered by saying that Norm Sleep of Stanford University thinks that they could have been “mushy plate tectonics” in the first tens or even maybe hundreds of millions of years on Mars that could have been a source of subterranean metals. Baross then admitted that any place like Mars that has the key metals and other elements necessary for life could support life, but he still did not think that it could be the origin of life without plate tectonics. He then again emphasized the need to keep these two ideas apart: abiogenesis versus just being able to sustain Earth-like life if it were moved there.
The last person to comment on the talk then posed what he called the “paradox of a biosignature,” which he said is the fact that life needs organic compounds that only life can make. Darwinism, however, is the solution to the paradox. For example, he said that homochirality is essential for Darwinism to act on proteins. Isotope effects do not do a good job of this because they only indicate how the molecules, particularly carbon, were fixed. An unusual fixing process could produce a large isotopic effect abiotically. Finishing, he then addressed a previous audience member and said that carbohydrates are indeed unstable in alkaline conditions of pH 12. However, this brings up the question of whether the planet is always flooded. If not, then carbohydrates could be quite stable in areas where there are lots of evaporites, such as boron. However, on Europa, there may never have been dry land to concentrate these elements and molecules. Additionally, these substances would suffer from dilution in the oceans as well.
THE LIMITS OF LIFE AND ITS INTERACTION WITH THE ENVIRONMENT
Tori Hoehler of the NASA Ames Research Center began his presentation by recalling the previous report by the National Research Council called The Limits of Organic Life in Planetary Systems6 (2007), which has become known as the “Weird Life Report.” Although that report explored the limits of all potential life, Hoehler planned to focus his presentation on the limits of life as we know it and trying to create a link between them.
Requirements for Life
The so-called “Weird Life Report” identified four fundamental requirements for life (in order of decreasing certainty): thermodynamic disequilibrium (Gibbs free energy), an environment capable of maintaining covalent bonds (especially between C, H, and other atoms), a liquid environment, and a molecular system that can support Darwinian evolution. The report went on to say that thermodynamic disequilibrium “is not disputable as a requirement for life. Other criteria are not absolute.”7
Earth life, Hoehler said, only uses a small subset of available energy forms, light and chemical energy, and even then only a small subset of those two forms. For example, life is only known to use light in visible to near-infrared wavelengths and is only known to capture the energy released by oxidation-reduction reactions. Both of these processes create electron flow, which appears to be fundamental to the processes by which Earth life captures and stores energy. It is not clear if this constraint holds for all life. Hoehler then compared life to a laptop computer, saying that both process information at the expense of energy, and both have two types of energy requirements. The laptop requires a minimum voltage (energy per quantity of electrons) and a minimum power (energy per unit time). Life as we know it has analogous requirements: Gibbs energy change (energy per quantity of substrate consumed) and power. Both must be met at minimum levels for life to function properly. There is also a corresponding maximum amount of voltage and power life can handle.
Hoehler then addressed the other three requirements for life from the Weird Life Report. Noting that Darwinism is rarely considered when talking about habitability or biosignatures, Hoehler said that it is nevertheless a fundamental aspect of life as we understand it. The requirement for a molecular system capable of such evolution significantly constrains life’s requirements. For example, if Darwinian evolution is fundamental to biology, then information processing is a core attribute of life, and this would require molecular recognition with a very high level of fidelity—a requirement that may limit the range of chemistries, solvents, and environments that are suit-
___________________
6 National Research Council, The Limits of Organic Life in Planetary Systems, The National Academies Press, Washington, D.C., 2007.
7 Ibid, p. 8.
able for life. Hoehler described molecular recognition as one example of reversible (i.e., non-covalent) molecular interactions which, although fundamental to the way our life works, are not always considered when defining life’s requirements. Hoehler used the example of a ribosome to distinguish covalent and non-covalent interactions. A ribosome is composed of covalent bonds (electrons shared between atoms). Its job, protein synthesis, is ultimately to create covalent bonds. It does this job only by virtue of non-covalent interactions. For example, the folded 3D shape that gives the ribosome catalytic function and supports the recognition of specific amino acids is made possible by a myriad of interactions caused by non-covalent forces within the molecule. The strength and nature of those forces depend as much on the solvent (water) as on the molecule itself. Other liquids that are considered as potential solvents for life must therefore be evaluated on their potential not only to support the synthesis of covalent bonds, but also to support the wide range of non-covalent interactions that confer “life-like” function on a system.
Hoehler then went on to discuss the basic elements that life on Earth needs, the chemical symbols of which spell “CHNOPS” (carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur) or “SPONCH” (sulfur, phosphorus, oxygen, nitrogen, carbon, and hydrogen). Carbon is used as the scaffolding element that allows for a large diversity of molecular structures. That diversity is greatest when carbon is in the intermediate oxidation state (between CO2 and CH4).8 Heteroatoms (nitrogen, oxygen, phosphorus, and sulfur) support a diverse range of covalent chemistry and also have polar bonds that allow for a variety of non-covalent interactions. Hydrogen provides hydrogen bonding (obviously), which is part of what allows for high-fidelity molecular recognition in our biochemistry. Watson-Crick base-pairing in DNA, for example, is based on hydrogen bonding between complementary nucleobases. Potential alternatives to these elements, Hoehler said, would have to fill these same roles and do so as part of molecules that are stable over meaningful time scales.
Hoehler then described the known environmental limits of life on Earth. Life, he said, is found between –25°C and 122°C, at pH between 0 and 13, at pressures up to at least 200 MPa, and at water activity as low as 0.6. (Water activity is defined as the vapor pressure of water over the solution in question divided by the vapor pressure of pure water at the same temperature; it is a measure of how chemically “available” water is in a given solution and decreases as the concentration of solutes, like salts, increases.) These limits represent the known record holders and are often established in laboratory settings where factors other than the extreme being considered are optimized. Hoehler said that life in natural environments, where other factors are not always optimal, may not be able to reach these same extremes. It also may not be possible for life to originate at these extremes. Whereas existing life has had the benefit of extensive evolution by which to develop tolerance to extremes, the conditions that foster prebiotic chemistry may be narrower. Life at the limits may sacrifice diversity, abundance, and productivity just to survive. As with solvent and elemental requirements, Hoehler said, the environmental conditions suitable for life must support both covalent bonding and non-covalent interactions. The latter may define a much narrower range of possible environments than the former.
How Life Can Alter Its Environment
Hoehler then moved onto the subject of what life can do to alter its environment and the planet as a whole. The potential to do so depends on how abundantly life’s requirements are met. For example, biomass densities on land range from practically nothing in the most arid regions of the planet to hundreds of kilograms per square meter in the rain forests, a variation that reflects the availability of water. The oceans have 3 to 4 orders of magnitude variation in the density of photosynthetic biomass, and this variation reflects the availability of nutrients and key elements. Such differences could influence our ability to detect life on another world. Moreover, Hoehler noted, we must be mindful that our intuition about what an inhabited world looks like is based on a world, Earth, in which life’s requirements are abundantly met. The same may not be true of some of the worlds currently considered as potential abodes of life. Hoehler pointed to the availability of light energy to Earth’s biosphere, but not to worlds like Europa or Enceladus, to quantify this point. The amount of energy available (such as from light) can limit the ability of life to create recognizable biosignatures. On Earth, photosynthetic organisms capture about 1 percent of the Sun’s 173,000 TW of power incident on the top-of-atmosphere and create chemical energy in biomass at the rate of 63 to 105 TW. Comparatively, non-photosynthetic chemical energy fluxes on Earth, such as the flux of hydrothermal vent fluids into the oxidizing ocean, amount to only about 0.006 TW in forms that can be utilized
___________________
8 Life wants carbon in an intermediate oxidation state, and on Earth, the carbon available is fully oxidized in CO2. To perform this chemical reduction, life needs a source of electrons, which it finds abundantly in water (in the bonds between O and H).
by life. The way in which we search for life must take these differences and their likely impact on the abundance and quality of biosignatures into account.
Energy flux, Hoehler continued, places upper limits on several aspects of life. Energy constrains the abundance of biomass sustained. Energy flux also constrains metabolic and biosynthetic rates and thus the rate at which biosignature molecules can accumulate in the environment. This rate is important because low accumulation rates can make it difficult to maintain a pool of biosignatures against physical, chemical, or biological attrition. An example is the racemization of amino acids, which can exist in either of two non-superimposable (i.e., chiral) mirror images called “enantiomers.” Earth life typically produces/utilizes one enantiomer exclusively, while abiotic mechanisms generally produce a mixture of both enantiomers. Finding a large excess of one enantiomer over the other would thus serve as good evidence for life. However, chemical processes spontaneously interconvert (racemize) the two enantiomer forms, thereby continuously erasing the signature of life. When energy fluxes are very low, the rate at which the biological signal is replenished by biosynthesis may be overwhelmed by racemization.
When living organisms do have access to abundant energy, Hoehler explained, a commonly mentioned biosignature is the presence of a disequilibrium. He said that, for such cases, the signature of life lies not just in the presence of a disequilibrium, but in its type and magnitude, considered within its environmental context. Oxygen-producing photosynthesis, for example, is not an inevitable outcome of a photosynthetic environment. Its occurrence on Earth is the result of a specific biochemical need expressed in a specific environmental context. Extracting electrons from water yields O2 as a by-product. Life that either has access to carbon in an intermediate oxidation state, uses a different biochemistry, or has a different solvent might not yield this same by-product of photosynthesis. Moreover, it is important to consider that thermodynamic disequilibrium can result not just from biological processes, but also abiotic ones. For example, O2 can form abiotically when sufficiently energetic photons fragment oxygen-containing molecules like CO2 or H2O. In an Earth-like setting, abiotic processes can produce about 1011 O2 molecules/cm2/s. Life on Earth, on the other hand, can produce 1019 O2 molecules/cm2/s. Thus, the fact that Earth displays evidence of its biosphere in the form of an oxygen-rich atmosphere derives not from the uniqueness of O2 as a biological product, but from the much greater efficiency (8 orders of magnitude) with which life uses sunlight to create O2. More generally, this illustrates that for “disequilibrium biosignatures,” it is the abundances of molecules, not just their presence, that constitutes the sign of life.
Hoehler concluded that, when searching for evidence of life as it appears on Earth, one must consider how and why life does what it does physically and chemically—for example, thinking of the potential for alternative solvents or biochemistries to support non-covalent interactions—which significantly constrains what life can be and do and what evidence it may leave of itself. Lastly, he emphasized the importance of changing our concept of habitability from a binary construct (1 or 0, life either being present or not) to one in which the abundance and productivity of life are seen as a continuum of possibility that depends on a range of environmental factors. Thinking in this way would allow us to distinguish among environments that may have greater or lesser capacity to express evidence of a biosphere.
Audience Participation
One audience member challenged Hoehler’s claim that the limits of life have been established primarily in laboratory settings. He said that there are many organisms in nature that are unknown, saying, “The truth is out there.” Hoehler clarified that the currently known limits of life generally correspond to observations made in a laboratory setting, rather than to organisms or biological activity in natural settings. He also said that natural environments provide the feedstock to explore the biochemical breaking points, but that the most extreme limits are often expressed in the laboratory under optimal conditions. The audience member thought that this was still a naïve point of view. He said that he does not think that the conditions these organisms require can be produced in laboratories effectively. For example, microbes are often smothered by what we give them. Hoehler agreed with this point. He said that it is uncertain whether the ultimate limits on life are going to be found in nature or in a laboratory.
A workshop participant then referred to Hoehler’s discussion of the upper limits imposed on biosignature formation by energy flux and asked why he placed metabolic rates and biosynthetic rates together on the same line. Part of the reason, Hoehler said, was to save time and space. However, both have also been considered as potential biosignatures. Biosynthesis creates clearer biosignatures, because it manufactures molecules that are abiotically improbable to produce. However, metabolic intermediates and end products can also serve to indicate
biological activity if they have not achieved equilibrium with the environment or bear the hallmarks of biological catalysis, such as isotopic discrimination. More properly though, Hoehler agreed that they should be considered separately, as they are not directly coupled.
Another audience member then said that Hoehler had convinced her that using disequilibrium as a biosignature would be impossible because of the difficulty with disentangling the abiotic sources from the biotic sources. Hoehler agreed that it would be hard to say definitively that disequilibrium is a biosignature without better understanding the environmental context, which would be hard to see on a planet that is simply a point of light. However, he thinks that examples like creating O2, at which life is 100 million times more efficient than abiotic sources, could be indisputable if placed in the proper context. The audience member countered, saying that if we know the environmental context, we would also already know whether or not the planet had life. The challenge, she said, is for less visible signatures. Hoehler said that that could be true. He gave photosynthesis as an example that has yielded two biosignatures visible from space: O2 and the “red edge” (the fact that photosynthetic plants are highly reflective in the near-infrared).
A question from an evolutionary biologist asked whether Darwinian evolution is a fundamental feature of life and whether a diversity of life would be possible without it, considering that the process is often included in the definition of life. Hoehler answered that he thinks that it is an essential part of life. He noted Steven Benner’s description of evolution as the answer to the “paradox of a biosignature”—the observation that life depends on complex, improbable molecules that only life can create in the first place. Hoehler noted that evolution, despite its central place in biology, is not usually considered when discussing the inherently biological concept of habitability because it is not, in general, directly observable. Lastly, he said that evolution is especially interesting in places where the energy flux is very low, because that would affect the rate at which evolution can explore the possible parameter space of genetics. A simple linear extrapolation, he said, might be a naïve idea. He concluded by speculating whether there may be a critical level of energy flux below which evolution is not possible.
IS LIFE A COSMIC IMPERATIVE: HOW WOULD THERMODYNAMICS FORCE LIFE INTO EXISTENCE?
Eric Smith of the Santa Fe Institute discussed life as a cosmic imperative and whether the existence of a biosphere might be viewed as the ineluctable result of thermodynamics. He said that there is an idea that the emergence of life is a necessary stage in planetary evolution. Evidence of this may be hidden in biochemistry and higher-level architecture of cellular organization. However, work needs to be done to change this from just hand-waving storytelling into a solid theory. Lastly, he wanted to address how to extend this idea and subsequent analysis to exoplanets.
The Ancient Geochemical Landscape for Life
Smith noted the importance of the concept of system-level order to the question of whether life could be a necessary step in a planet’s evolution. Addressing this question, he argued, begins with a familiar mathematical concept: the phenomena of breakdown processes, which can be recognized as robust states of dynamical order on short time scales. Familiar physical examples include plasma channels (e.g., lightning), convective storms, and fracture propagation through materials. (In contrast, while diffuse energy stresses of the familiar sort such as heat do not typically create order, breakdown phenomena differ in that they generally result from feedback processes that can focus this energy to create order.) Of the many disequilibria found on Earth, the one that might best explain the emergence of a biochemistry is redox disequilibrium, which is deeply connected to life. On the early Earth, this disequilibrium was driven primarily by hydrogen escape. Photolysis of water vapor can result in the Jeans escape of hydrogen leading to an excess of oxygen in the upper atmosphere. This process has likely occurred throughout Earth’s history and maintains a state of persistent redox disequilibrium between the atmosphere/oceans and the bulk Earth. (Such a disequilibrium would not persist in a system dominated by diffusion.)
Smith continued, explaining the consequences of this disequilibrium and how it behaves at large scales. Tracing the emergence of this disequilibrium, he noted the bulk Earth’s oxidation state is between Fe and Fe+2, which forms a redox gradient between the atmosphere, which shows relatively higher oxidation states, usually between Fe+2 and Fe+3. This is seen in the Earth’s two major reservoirs of water: seawater and sub-surface water are each brought to their respective equilibriums, which as noted are at two very different oxidation states. Mixing
zones between the two can produce redox potentials of several tenths of an electron volt at distances of just a few atomic diameters. Today’s living systems use these potentials to produce chemical order. The question this raises, of relevance to the origin of life, is whether we can infer a link from the earlier abiotic geochemical processes to today’s redox potentials of the sort observed in these mixing zones.
Smith then returned to his initial question of whether life is a necessary step in a planet’s evolution. If we are correct that the gradient in redox potential is the relevant geochemical disequilibrium that allows for life, where, Smith asked, might we find evidence of this in the biochemistry? He said that one thing to look for is continuity of ancient, universal biochemical signatures with selective organic geochemistry observed today. The ancient signatures include those that either became “locked in” and are unchanging, or are paths of least resistance, or small-molecule chemistry unchanged by modern enzymes. A second place to look for verification of the role of redox potentials is in what Smith calls “upward causation,” or metabolic patterns imprinted on higher levels of cellular architecture where you would otherwise not expect such imprints to be, since information usually devolves, in the opposite direction, from higher to lower levels. (This is further discussed below in the subsection, “Upward Causation”.)
The Core of Metabolic Biochemistry
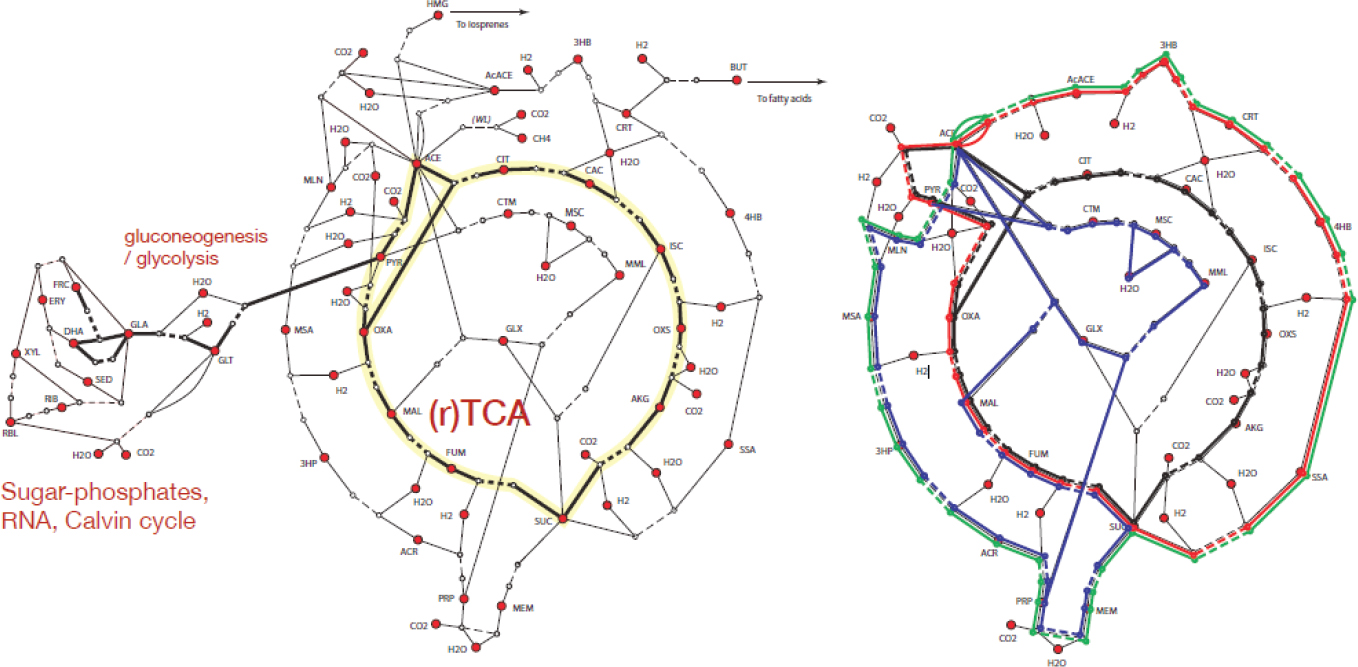
Smith then went on to discuss metabolism and the degree to which it has or has not experienced innovation during the 4 billion years of life on earth. He showed a slide illustrating a “universal core” of metabolic biochemistry that is visible always at the ecosystem level, sometimes within the level of particular organisms, and that is organized around the citric acid cycle (see Figure 1.2). (The original version of this cycle is now known to be its reductive version, not the oxidative direction of cycling that was first discovered by Krebs.) Of the six autotrophic carbon fixation pathways that are currently known, five are self-amplifying loops, and four of these remain close to the template of the citric acid cycle. (The only loop that is not closed is the Calvin-Benson cycle.) The evolutionary innovation that appears to have given rise to these other loops usually takes place at the pathway segment level, and the cycles as a whole stay very close to the citric acid cycle intermediates. If these intermediates are not all in a particular cycle that fixes carbon, they are added by auxiliary pathways known as anaplerotic reactions. Two pinch points in the map of cycle diversification are acetic acid in the form of acetyl-CoA and succinic acid, which may or may not be succinyl-CoA.
Smith then opined that there has been almost no innovation in carbon fixation over the last four billion years. This set of autotrophic reactions essentially consists of easy pair transfer chemistry in a water environment (two-electron reductions) or hydrations and dehydrations. The distinguishing points of departure for different pathways tend to be their opening steps, which are typically a carbon attachment followed immediately by a reduction. Those reactions are also usually associated with strictly conserved proteins with metal centers at the active site. Many researchers have noted the similarity between the metal center proteins and mineral metal centers. Certain transition metals allow for coordination geometries that can be adjusted by evolution. Smith then agreed with Baross’s talk that said that mineral chemistry appears to have been placed into a control system.
Upward Causation
Smith then moved on to upward causation—that is, the imprint of patterns that are native in metabolism being found at higher levels of cellular architecture. The critical question is the compatibility of this evidence of upward hierarchical movement of information with the dogma of the genetic code, which argues for information moving in the opposite direction. In particular, the process of translation from RNA to peptides should acts as a firewall that insulates the fundamental structural non-symmetries of biosynthetic chemistry from the protein sequence-space in order to allow Darwinian selection to pick the best sequence without having to overcome arbitrary biases. The expectation that translation should be a firewall also suggests that the codon assignments of amino acids to nucleobase triplets should be arbitrary, in the sense that they could have been different than they actually are.9 The actual genetic code, however, does not really have arbitrary assignments; in information-theoretic terms, the genetic code is enormously compressible and could, for example, be represented as a decision tree for the selection
___________________
9 One can back off slightly from the assumption of arbitrariness by recognizing that coding happens with errors, and error buffering can be provided by grouping similar amino acids at related codons.
of amino acids.10 Thus, it turns out that the first base in the genetic code tells you which citric acid cycle precursor the amino acid that the codon specifies is built from. Thus, for example, if the first base is C, this precursor is glutamate; for A it is aspartate, and for U it is pyruvate. And what the second base is telling you is how these precursors have been modified; for example, if U is the second base, a second copy of pyruvate is added to make hydrophobic amino acids. The evolutionary steps that have produced this type of regularity are a mystery that needs to be explained.
Thermodynamics, Kinetics, and Trajectories
Smith’s next point was that the maintenance and error correction of systems in disequilibrium are revealed not only by making lists of the structures they form, but more so by considering kinetics and trajectories (i.e., time evolution of a system in phase space). Smith said that thermodynamics is not fundamentally about energy or equilibrium, but rather is about the emergence of a stable macro-world, and that this idea needs to be applied to disequilibrium environments (i.e., those in which the system has a tendency to evolve to a new macrostate) to explain the robustness of the error-correcting processes that operate in the biosphere. A mathematical system to do this, he said, already exists.11 He went on to explain what is meant when saying the fundamental concept behind thermodynamics is the emergence of macro-phases. The general idea is that, under aggregation of their constituent particles and components, systems and the probability distributions that describe their fluctuations can converge on exponential families.12 This convergence is why systems of large and definite size are possible. The scale of the system separates from the structures the system is capable of taking on—the macrostructures. This is true for equilibrium systems, but, dynamically, the same convergence to exponential families is possible. In the mathematical system noted above, the role played by equilibrium free energy is subsumed by an effective action associated with the trajectory, and dynamical phase transitions are the shifts of the central tendencies of macro-states. Making an analogy with modern electronics and its incorporation of error correction, Smith offered that thermodynamics is in effect an error correction process. The mathematics of fluctuations leads us to expect that error correction in evolution will tend to have a three-way tradeoff between robustness (the probability of error), the complexity of the number of states capable of being maintained, and any associated costs (such as the time or system size needed to take a system back to its central tendency).
In a view of life based on dynamical phase transitions, Smith explained, the abiotic Earth becomes a dynamically metastable condition. The most probable path (also known as “the maximum path entropy condition” or “path of least resistance”) will be the path for which there are the most ways to scatter into and the fewest ways to scatter out of. This explains part of the importance of “easy” chemistry, such as water-base pair and group transfer. Catalysis of these reactions is extensively duplicated; enzyme families are either divergent or recurrently evolved. “Hard” chemistry is the electron transfer chemistry, which isn’t possible due to radical production in water being disfavored. The need for single-electron transfer processes in biochemistry suggests a mineral or metal-ligand complex origin for these processes. The whole network needs a positive, self-amplifying feedback to concentrate matter and energy flows. Short feedback loops and feedbacks with few alternatives are best suited for this because they have less of a problem with diffusion while also allowing for the evolution of control mechanisms.
Problems and Future Work
Smith then went through some outstanding problems with this explanation. A major problem is that the pathways that seem specific and necessary biologically do not seem inevitable or special geochemically. Another problem is that reactivity is self-defeating. For example, a one-carbon reduction sequence from CO2 to CH4 goes through CH2O (formaldehyde), which is extremely reactive. For the origin of life, the process needs a kinetic way to focus reactivity into a thermodynamically disfavored domain because that is the only place where reactivity is available. Recognizing this allows us to see that the formose reaction (i.e., the formation of sugars from formaldehyde) is in a different disequilibrium class than processes of reductive carboxylation, such as Fischer-Tropsch
___________________
10 S.D. Copley, E. Smith, and H.J. Morowitz, 2005, A mechanism for the association of amino acids with their codons and the origin of the genetic code, Proceedings of the National Academy of Sciences U.S.A. 102(12): 4442-4447.
11 See, for example: Touchette, H. 2009. The large deviation approach to statistical mechanics. Physics Reports 478: 1-69.
12 Koopman, B.O. 1936. “On distributions admitting a sufficient statistic”. Trans Amer. Math Soc. 39(3):399-409.
reactions. In the formose reaction, electrons cannot enter or leave the system, so they are trapped on a surface of redox constraint, ensuring that reactivity is preserved. In reductive carboxylations, the flow of electrons into or through the system is the driving force behind creating organic complexity, but there is no natural constraint to preserve reactivity. The contrast between reductive carboxylations and constrained systems such as the formose reaction underscores the problem of understanding what the right concept of disequilibrium should be. Smith gave another example of this problem: the dominant motifs in biochemistry are cascades of group transfers, which can be understood partly in equilibrium-thermodynamic terms because they take place in a context that Yoshitsugu Oono has termed “compartmented quasi-equilibrium.” In contrast, most origin-of-life research emphasizes cycling of physical conditions such as temperature or water activity so that the system “chases equilibrium,” which differs from the group transfer just mentioned.
Referring to the understanding of thermodynamics showing that little extra structure is needed to make exergonic reactions feasible, Smith said that a similar knowledge is needed for kinetics. He gave a list of things that need to be better known: mineral-metal center catalytic selectivity and activity, including edge, vertex, and face effects; and roles of impurities and mineral-mineral interfaces. He said that we need to combine our knowledge of ligand field theory in a mineral context with the same for soluble metal-ligand complexes. Smith then said that a connection needs to be made between the Hadean atmospheric and oceanic conditions, such as the redox state of the atmosphere (CO vs. CO2).
In his closing thoughts, Smith said that we need to stop just trying to get to an organic material and instead think about how the organics were created out of equilibrium. He then emphasized that big and random molecules are not necessarily complex. Complexity implies selectivity. Finally, he said that disequilibria (e.g., redox, radioactivity, thermal activation, and dehydration) are not all the same in the context of the origin of life.
Audience Participation
A member of the audience asked Smith whether there is enough of a free energy gradient on Enceladus and Europa for abiogenesis, which is what is needed in a metabolism-first method. Smith said that the Archean era on Earth had a longstanding disequilibrium due to having a reduced interior with a steady process of Jeans escape of hydrogen from the upper atmosphere. He further responded that the important question to answer is where the terminal electron acceptors are produced on Enceladus. (Smith later learned in off-line conversation that the production of peroxides on surface ice through particle bombardment is believed to be the major process generating such acceptors.) There needs to be a flow of electrons. He asked whether there is a way to get a disequilibrium of multiple tenths of an electron volt at distances of just a few atomic diameters, as the key consideration.
A potential problem in reconstructing history using modern day biochemistry, another workshop participant said, could have been flexibility in the earliest stages of Earth and life. Gibbs free energy has been suggested as a way to do this historical reconstruction. She then asked if there was any alternative way to try to reconstruct the past. Smith said that biology is overwhelmingly preoccupied by the role of innovation. However, the discovery of chemoautotrophy in the 1970s led to the realization that there is a chemoautotrophic core in everything with oxygenic shells wrapped around it. There is also the question of the extent to which evolution just wrapped controlling systems around pre-existing processes versus how much it actually built new, innovative pathways. The facts that the deep core of metabolism is so conservative and that the innovations built with it are pretty serial suggest to Smith that error correction is a difficult thing to invent. Therefore, the reconstruction of deep history may not be as difficult as it is usually expected to be by biologists because biology has been dominated by an emphasis on historical contingency. The examples of the architecture of biochemistry were adduced to suggest that in the very deep past, the role of direct historical reconstruction may give way to a science of prediction based on first principles, which are inferred from the structural regularities in this architecture. In agreement with the questioner, Smith actually finds the possibility of flexibility in early stages of Earth and life an important and interesting question.
Moving the discussion away from Earth, an audience member said that, on ocean worlds, icy and solid materials formed together under certain conditions, but once together again at lower temperatures, they were in disequilibrium. He then asked Smith why this long time period where the icy and solid materials are trying to approach equilibrium is not the same as the reductive Earth interior with Jeans escape of hydrogen. Smith said that he is open to the idea that it need not be fundamentally different.
A workshop participant agreed with Smith’s talk that there appears to be a serial set of innovations to get to the necessary biomolecules for life. She then said that there is an idea to focus on intermediate molecules as
a biosignature that would not necessarily be favored in an abiotic system and asked Smith to comment on that. Smith broadly agreed with the position presented by the questioner. Smith said that there are two views of life, one that is all about innovation and one that is all about conservation with a bit of innovation. One position is that Darwinism will be the smoking gun that there is life. However, Smith thinks that this is too strong of a position and that the statistical mechanics of the biochemical systems can also be informative.
The last comment was about cofactors, which are very geochemically reactive and not well preserved. Smith responded that, in his mind, this is one of the most important questions in the origin of life. He said that a key distinction involves whether cofactors were a stepping stone towards a more structured polymer world or if they are an artifact of a polymer world that was already in place.














