3
Personalized Nutrition in the Real World
Continuing the session 1 discussion, four presenters discussed applications of nutrigenomics “in the real world.” This chapter summarizes their presentations and the discussion that followed, with highlights provided in Box 3-1.
EXPLORING PERSONAL, DENSE, DYNAMIC DATA CLOUDS AND THE FUTURE OF PERSONALIZED MEDICINE
Nathan Price, Institute for Systems Biology, addressed personalized nutrition in the clinical setting. He began by emphasizing the complexity of nutrition’s health effects, as reflected in the fact that different foods have been shown to both prevent and cause cancer (Schoenfeld and Ioannidis, 2013). Given this complexity, he argued, “there is a need for personalization, a need for understanding.” He referred to Patsy Brannon’s opening presentation in session 1 and her discussion of the complex interrelationships between diet and health, particularly when one considers the molecular details of how the body processes food and how these molecular details are related to disease (see Chapter 1 for a summary of Brannon’s presentation). Additionally, he emphasized how the many different biological systems add to this complexity and the many different ways to think about it, citing Douglas Wallace’s perspective on mitochondria and their impacts as an excellent example (see Chapter 2 for a summary of Wallace’s presentation).
Scientific Wellness
The health care industry costs in the United States are approaching a staggering $4 trillion per year, according to Price. But it has been estimated that about 30 percent of a person’s lifetime health is attributable to genetics; about 60 percent to behavior and the environment, a large part of which is nutrition; and only about 10 percent to the health care system (Schroeder, 2007). “So there is a huge need, obviously, to focus on the 90 percent,” Price said. Yet, physicians receive only about 2 hours of training in nutrition, he observed, and the “wellness industry” has a mixed reputation, being characterized by many not very scientifically based approaches. Thus, he and his colleagues have been advocating what he called “scientific wellness.” He explained that the goals of scientific wellness are to provide a data-rich basis for rigorously quantifying wellness, to try to predict and prevent disease before it happens, and to focus on optimization of health in the individual.
To help launch this new scientific wellness industry, Price and his colleague, Leroy Hood, announced in 2014 a project called the 100K Wellness Project (Hood and Price, 2014). The project has one major goal: to collect a dataset enabling observation of enough people over time to detect all of the early warning signs for all of the major human diseases, and predict and prevent those diseases to the extent possible. The initial vision was to collect dense information on 100,000 individuals, including data on genomics, proteomics, metabolomics, the microbiome, and clinical chemistry, plus data from wearable devices.
Price explained that, to test whether he and his team could collect all the types of data they wanted to collect, they first conducted a prototype, or feasibility, study called the Pioneer 100 Wellness Project. The results of that 9-month longitudinal study of 108 individuals were published in Nature Biotechnology in 2017 (Price et al., 2017). As Price explained, multiple types of data were collected on the participants at three different times. Each time, the investigators measured about 150 clinical chemistries, about 700 metabolites, and about 400 proteins from blood; they also measured stress hormones from saliva over the course of a day. The initial data collection included whole-genome sequence data as well. In addition, individuals’ microbiomes were analyzed at three different times, and participants used wearable devices for continual self-tracking and lifestyle monitoring. With these data, Price and his team created what they call “personal, dense, dynamic data (PD3) clouds” for each participant—personal in the sense that they are individualized, dense because they include a large amount of information, and dynamic because they change over time.
In addition to the data collection, Price continued, participants received wellness coaching. He concurred with previous speakers on the critical role
of behavior change and the difficult challenge it presents, noting that his team’s study engaged a behavioral coach as well as a study physician.
Price stressed that a key to retaining participants in a program like this is making the data relevant. “How do you take these data,” he asked, and make them “actionable for the person, in the moment?” In the long run, he said, the researchers want to be able to mine PD3 clouds to enable new health discoveries that can then be returned back to the participants.
Meanwhile, Price reported, just over the course of the 9 months of the Pioneer 100 Wellness Project, the investigators saw improvements in a number of clinical markers, including a 21 percent improvement in markers for nutrition, a 33 percent improvement in markers for diabetes, a 12 percent improvement in markers for inflammation, and a 6 percent improvement in markers for cardiovascular disease. He noted that participants who have stayed with the program, through Arivale,1 have shown continued improvements; for example, improvement in markers for cardiovascular disease rose to 20 percent.
In addition to clinical markers, Price continued, his team monitored a number of dietary factors. Through this monitoring, they discovered, for example, that one individual who had high mercury levels also ate tuna sushi three times a week. When this person switched to salmon sushi, the amount of mercury in his blood had decreased by half within 3 months. After he had remained with the program for 1 year, his mercury blood level had normalized completely. According to Price, there were a number of such cases.
Scientific Wellness and Discovery: The Manifestation of Genetic Risk in the Body
In addition to mining the data and returning new health discoveries back to the Pioneer 100 Wellness Project participants, Price and his collaborators have been studying the nearly 4,000 correlations detected among the different types of data collected, including associations between the microbiome and metabolites, metabolites and proteins, proteins and lifestyle factors, and metabolites and genetic risk scores. “All these data types had never before been measured simultaneously on a population of people,” Price said. Given the complexity of the associations between these factors, he argued, “this is just the tip of the iceberg in terms of understanding how these things are interrelated.”
Price went on to acknowledge that some in the field of medicine believe his team is collecting too many measures, a view with which he strongly
___________________
1 Price disclosed that he was co-founder of and on the board of directors at Arivale, a scientific wellness company that partially funded and may license discoveries from the Pioneer 100 Wellness Project.
disagrees. He explained, however, that it is important to distinguish what he and his collaborators do with respect to discovery—trying to understand how the human body is interconnected—from efforts focused on implementation in populations as the field moves forward. The basis for the initial study, he said, was the former. He clarified further that the relationships among different data types can be modularized into subsets, each having greater interconnectivity within than without, and those subsets then used for further study.
As an example, Price used the relationship between cystine, the dimer form of cysteine that is in the blood, and risk for inflammatory bowel disease (IBD). He and his collaborators found that it is not the disease itself but genetic risk for IBD that is related to differences in blood cystine levels. In this particular case, the correlation detected by his team was a negative one: on average, the higher the genetic risk score for IBD, the lower was the amount of cystine in the blood. Price noted that case control studies have shown that blood cystine level is also one of the best candidate biomarkers for the disease itself. But what Price found interesting was that even without the disease present, he and his collaborators found that this difference in blood cystine level preexists across the life span, in individuals ranging in age from their 20s through 89-plus, so the entire life span. He interprets this finding to mean that this deficiency in cystine is genetic.
Another interesting aspect of this cystine–IBD relationship for Price is that cystine lies upstream of the synthesis of glutathione, which is what combats oxidative stress in the body, and that depressed cystine can lead to an inability to process oxidative stress efficiently (Sido et al., 1998). Because oxidative stress is a trigger for IBD, he explained, this depressed ability to combat oxidative stress could be a potential mechanism for the association between cystine genetics and increased risk for IBD. He suspects that over a lifetime, individuals with a cystine deficiency are more likely to surpass what he called their “reservoirs of resiliency,” thereby transitioning to some sort of disease state. What is really fascinating to Price if this is true is that an individual at high genetic risk for IBD could potentially change to one at low risk for the disease through a shift in nutrition. Price termed this finding “the manifestation of genetic risk in the body.” “Gene variants do not mean anything in a test tube,” he remarked. “They mean something in the body.” In his opinion, using a resource such as the PD3 clouds created by his research team increases the chances of being able to see potential interventions not suggested directly by genetics.
Personalized Nutrition in the Real World
Price reported that Arivale, which, as noted above, was launched after the successful completion of the Pioneer 100 Wellness Project, and which
collects the same types of data and provides the same type of wellness coaching, now has 175 employees and has raised about $54 million, and thousands of people are participating in the program. He added that the Institute for Systems Biology has access to deidentified data from individuals who participate in the program and who agree to donate their data anonymously for research.
With respect to the value for participants, he said, “It’s really about empowering them with data.” The program provides coaching to help link the data collected with individuals’ behaviors so they can take action. Technologies such as mobile apps and dashboards are used to amplify the coaching relationship, Price noted, so that participants are receiving nearly daily texts from their coaches. His hope is that people will remain with the program over the course of their lifetime and that it will have an enormous impact on their health.
Already, Price continued, there have been many cases in which participants “have done the usual things,” such as lose weight and get healthier, but there have also been people who have been directed to their physicians because of early warning signs. As an example, he mentioned a woman who had an early warning sign that led to the discovery of a stage III colon cancer that was surgically removed just before it was likely to metastasize.
To provide workshop participants with a sense of other activities in the scientific wellness space, Price briefly shared what is being done by another personalized nutrition company, Habit, a spin-off of Campbell’s and a company for which Price sits on the scientific advisory board. For each individual, he reported, the company measures about 40–50 DNA variants and blood biomarkers in participants, collects metabolic data after they consume a “challenge shake” (a meal replacement drink), collects information on their habits and goals, and then analyzes all these data. Then, in a step Price characterized as very important, the company delivers personalized food back to participants. This is important, he explained, because integrating all of the information being provided and changing one’s behavior is difficult. Thus in addition to telling customers that they need to eat more x, y, or z, the company actually provides them with more x, y, or z.
In closing, Price observed, “We are starting to be able to gather immensely more kinds of data.” Additionally, the concept of the PD3 cloud is already being put into practice. Price believes that “this kind of data can be the foundation for the future of personalized medicine.”
SICKLE CELL DISEASE: AN ARGININE DEFICIENCY SYNDROME WITH DISTINCTIVE NUTRITIONAL REQUIREMENTS
Claudia Morris, Emory University School of Medicine, began by stating, “I am an emergency medicine physician. Most people scratch their
head wondering what I am doing talking about nutritional interventions.” She explained that essential amino acids are dietary dependent, whereas nonessential amino acids are not. But, she added, there are also conditionally essential amino acids, which are nonessential but indispensable under stress when the capacity of endogenous synthesis is surpassed. Arginine is one of these amino acids. Morris explained that although she would be talking mainly about arginine deficiency and its role in sickle cell disease (SCD), she would also be discussing the role of arginine deficiency in trauma. She noted that a number of other conditions are linked to acquired arginine deficiencies, including critical illness, burns, surgery, pregnancy (where Morris observed that the deficiency is probably protective), sepsis, pulmonary hypertension, asthma, and other hemolysis conditions in addition to SCD (e.g., thalassemia, malaria).
What Is Arginine?
Morris explained that arginine is found naturally in the diet, with high concentrations in meat, dairy products, seafood, nuts, and watermelon, but it is challenging to obtain enough arginine from the diet to reverse an acquired arginine deficiency. She noted that cardiovascular trials and her studies in SCD utilize 7–10 grams two to three times per day, whereas normal adult ingestion is about 2–7 grams per day. Arginine is available as a nutritional supplement with a low toxicity and, according to Morris, is now being marketed in the supplement world as a “natural alternative to Viagra” in addition to its touted role in cardiovascular health.
Ultimately, Morris explained, arginine is the obligate substrate for nitric oxide (NO) production via the NO synthase enzyme. NO, in turn, is a potent vasodilator with multiple functions: it plays a role in blood pressure modulation, but it also inhibits platelet aggregation, has immune response and anti-inflammatory properties, and can be a signaling molecule.
Arginine is a substrate for arginase as well, Morris continued, which means that arginase, an important intracellular enzyme in the urea cycle, competes with the NO synthase enzyme (see Figure 3-1 for a schematic of the metabolism of these different arginine processes). There are two mammalian isoforms of arginase: arginase I, which is cytosolic, and arginase II, which is mitochondrial. They are present in most cell types, including the red blood cells, which, for Morris, makes arginase a very intriguing enzyme to study in hemolytic disorders because when a red blood cell ruptures, the arginase “gets dumped” into circulation in a physiologically active form. It is also induced from inflammation by cytokines, she noted.
Morris went on to explain that in the presence of arginase, arginine is converted to ornithine and urea. Interestingly, she remarked, arginine and ornithine use the same amino acid transporters, CAT-1 and CAT-2, which
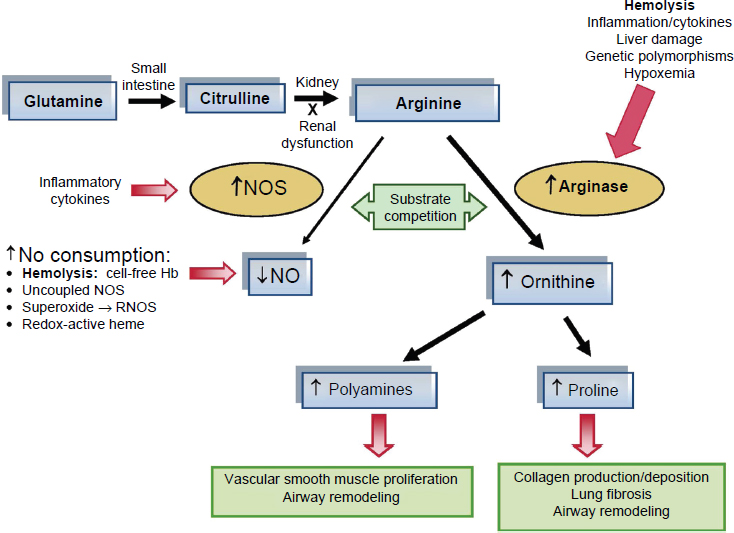
NOTE: Hb = hemoglobin; NOS = nitric oxide synthases; RNOS = reactive nitric oxide species.
SOURCES: Presented by Claudia Morris on December 5, 2017, from Bakshi and Morris, 2016. Reprinted with permission from Dove Medical Press Limited.
means that when ornithine concentration is increased, it competitively inhibits cellular uptake of arginine, thus limiting arginine bioavailability and so decreasing NO bioavailability as well. As shown in Figure 3-1, the downstream by-products of arginase activity are the polyamines and prolines. The polyamines are involved in part in vascular smooth muscle proliferation and airway modeling. The prolines play a role in collagen production and deposition, lung fibrosis, and airway remodeling, which Morris identified as the kinds of structural changes seen in pulmonary hypertension and asthma and as common complications in SCD.
Arginine is semiessential, Morris continued, because the body has the ability to synthesize it through what is called “the intestinal renal axis.” The amino acid L-glutamine is taken up in the diet, then converted into citrulline in the enterocytes of the small intestine, and the citrulline is converted into arginine in the kidney. Morris mentioned that glutamine was recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of SCD, which makes it the first FDA-approved drug for pediatric SCD and only the
second FDA-approved drug for adults with the disorder. Morris clarified that although glutamine is now considered a “drug” for treatment of SCD, it is still an amino acid and a nutritional supplement. It is also what she called “an arginine prodrug” through the intestinal–renal axis.
Turning to the bioavailability of arginine, Morris characterized it as much more complex than the amount of arginine in the plasma. She added that the term “global arginine bioavailability ratio”—arginine/(ornithine + citrulline)—was coined to take into account a number of different mechanisms that impact arginine bioavailability, such as the effects of arginase activity and renal dysfunction.
Amino Acid Deficiencies: Sickle Cell Disease as a Model
Morris defined SCD as an autosomal recessive inherited disease of the red blood cells. The genetic mutation responsible for SCD, she explained, is a single-point mutation, a substitution of valine for glutamic acid at the six position of the beta unit of the hemoglobin molecule, which causes the hemoglobin molecule to polymerize under stress or deoxygenation. This “sickling,” in turn, causes a cascade of effects that ultimately decrease blood flow. The tissue hypoxia that results causes both acute and chronic damage to nearly every organ system, according to Morris. She added that SCD affects about 100,000 individuals in the United States but millions of people worldwide, and comes at significant economic cost. Interestingly, she said, the clinical phenotype of SCD extends across a broad spectrum, from mild to severe, far exceeding what would be expected from a single-point mutation. So there are many other factors that come into play and that contribute to disease severity.
In Morris’s opinion, SCD is an excellent model for distinctive nutritional requirements that develop from a metabolic process, the metabolic process in the case of SCD being hemolysis. She noted that a decline in an amino acid does not necessarily translate to a clinically significant deficiency. Rather, a nutritional deficiency requires a biological process that is dependent on the nutrient that is being compromised. This compromise, in turn, must lead to an abnormal physiological response that causes a poor outcome, which needs to be reversible by replacement of the amino acid. In the case of SCD, Morris elaborated, the low arginine bioavailability that results from hemolysis is the nutritional deficiency; the endothelial dysfunction is the biological process that develops in the presence of low arginine bioavailability; pulmonary hypertension is one of the abnormal physiological responses that occurs as a result, and the one Morris said she would be using as a model for the remainder of her presentation; and in SCD patients, pulmonary hypertension is associated with increased mortality, which can be reversed by amino acid replacement.
According to Morris, in addition to being a model for distinctive nutritional requirements developing from a metabolic process, SCD is a model for vasculopathy and endothelial dysfunction, as it involves not only hemolysis but also inflammation, NO depletion, and arginine depletion. With SCD-related vasculopathy and endothelial dysfunction depletion, NO depletion takes “center stage,” she said. This is the case because with hemolysis, all of the contents that are normally packaged within a red blood cell dump into circulation when the cell breaks apart. These contents include hemoglobin, which, now cell-free, rapidly consumes NO, as well as arginase, which when dumped into circulation rapidly consumes the obligate substrate for NO production. As arginine level depletes, Morris continued, the NO synthase enzyme also begins to malfunction, uncoupling and producing superoxide in lieu of NO and adding to what she described as “this milieu of oxidative stress.” In sum, she said, there is a “global dysfunction of the arginine-NO pathway,” with many of these breakdowns occurring simultaneously.
Morris went on to explain that decreased NO bioavailability has several biological consequences, including endothelial activation, increased endothelin-1 (a potent vasoconstrictor), increased vasoconstriction, and increased platelet activation. There are several clinical consequences as well, she observed, including pulmonary hypertension, asthma, stroke, renal insufficiency, priapism, and leg ulcers. Although she said she would go on to focus on pulmonary hypertension in particular, she noted that all of these clinical manifestations are hemolytic subphenotypes of both SCD and low arginine bioavailability.
Also within the red blood cells, Morris noted, is lactase dehydrogenase (LDH). She explained that LDH is commonly thought not to be of clinical significance when dumped into circulation, but makes for a convenient biomarker of the hemolytic subphenotype of SCD first reported by Dr. Greg Kato and colleagues (Kato et al., 2006).
Arginine Deficiency and Sickle Cell Disease
Morris went on to discuss her work with SCD and arginine insufficiency, beginning with a 2005 study through which she and her research team discovered that patients with SCD have a plasma arginine insufficiency and elevated plasma arginase activity (Morris et al., 2005a). She clarified that because arginase is an intracellular enzyme, it is released only upon cell damage or cell death. “It just should not be in your plasma if you are a healthy individual,” she said. As part of the same study, Morris and colleagues (2005a) also examined the arginine-to-ornithine ratio, again because in the presence of arginase, arginine converts into ornithine, and they found a low arginine-to-ornithine ratio in patients with SCD compared
with normal controls. But “where it got interesting,” Morris said, was that the lowest levels were found in the patients at highest risk for pulmonary hypertension. She noted that not all patients with SCD develop pulmonary hypertension—only about 10 percent—but that these results led her to pay more attention to pulmonary hypertension.
Morris and her collaborators hypothesized, “if it is low, give it back.” So they conducted a small study of arginine replacement and observed a greater than 15 percent decrease in pulmonary systolic pressures, as estimated by Doppler echocardiography (Morris et al., 2003). Morris characterized this finding as “pretty impressive” (see Figure 3-2), noting that this decline is similar to that observed with on-the-market pulmonary hyperten-
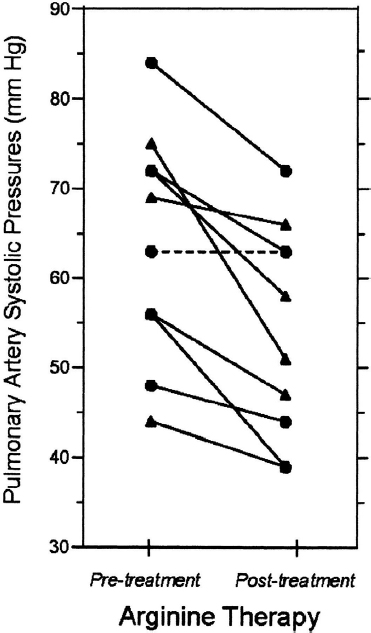
SOURCES: Presented by Claudia Morris on December 5, 2017, from Morris et al., 2003. Reprinted with permission of the American Thoracic Society. Copyright © 2018 American Thoracic Society. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
sion medicine. She interprets this finding to mean that there is a condition of endothelial dysfunction, manifesting as pulmonary hypertension, that appears to be reversed with arginine therapy. In addition to being excited about the decline shown in Figure 3-2, Morris and her team observed, anecdotally, that leg ulcers started to heal on two of these patients, both of whom had been experiencing chronic leg ulcers for years.
The next step, Morris said, was to consider another hemolytic anemia, thalassemia, that was also known to be associated with a high prevalence of pulmonary hypertension. So her team looked at some of their patients in the thalassemia clinic and found a similar pattern of arginine dysregulation (Morris et al., 2005b). More specifically, compared with controls, patients with thalassemia had, on average, higher-than-normal arginase activity, lower arginine-to-ornithine ratios, and elevated levels of the downstream by-products proline and citrulline. The higher level of citrulline suggested to Morris that there might be problems with converting citrulline to arginine.
Morris reported, however, that it was not until her team returned to the thalassemia clinic 10 years later and looked specifically at patients who were at risk for pulmonary hypertension that they found that some of the thalassemia patients had completely normal arginine levels, and that it was mainly patients who were at risk for pulmonary hypertension who had dysregulated arginine metabolism (Morris et al., 2015). That is, compared with thalassemia patients who were not at risk for pulmonary hypertension, those who were at risk had low arginine levels, high arginase activity, low arginine-to-ornithine ratios, and low global arginine bioavailability ratios.
In her studies of both SCD and thalassemia, Morris observed that that the severity of pulmonary hypertension and cardiopulmonary dysfunction correlated strongly with biomarkers of hemolytic rate. But what she characterized as “really fascinating” was that low global arginine bioavailability was also associated with increased risk of death in adults with SCD: there had been no deaths among patients with the highest bioavailability and the greatest number of deaths among those with the lowest bioavailability. She noted that Cox and colleagues (2018) obtained the same finding in children with SCD in Tanzania. Additionally, she said, low arginine bioavailability predicts early mortality in adults with malaria.
Morris reported that in separate work, Dr. Stan Hazen, a cardiologist, and his colleagues followed a group of nearly 1,000 patients who were at risk for cardiovascular disease and were undergoing right heart cardiac catheterization. After 3 years, they found that a reduced global arginine bioavailability ratio was prospectively associated with an increased incidence of major adverse cardiovascular events (Tang et al., 2009). The researchers looked at death, myocardial infarction, and stroke. Additionally, Morris said, they found that the global arginine bioavailability ratio was more predictive than cholesterol of cardiovascular disease, suggesting
to her, first, that this ratio is important for survival and, second, that it is a biomarker of vasculopathy that goes beyond SCD.
Morris pointed out that, while there are a number of different mechanisms of arginine dysregulation, many acting simultaneously, a common theme in arginine deficiency syndromes is excess arginase activity. So, she stated, whether hepatic, immune, or from hemolyzed red blood cells during hemolysis or transfusion reactions—that is, regardless of cellular origin—the physiological effects and clinical consequences of excess extracellular arginase are similar.
Arginine Deficiency and Trauma
In addition to conditions involving endothelial dysfunctions such as SCD, Morris continued, are arginine deficiency–related conditions related to T cell dysfunction, trauma being one example. Arginine is essential for naïve T cell activation, she explained, and T cells are “exquisitely sensitive” to arginine depletion. T cell proliferation is blunted and T lymphocyte–mediated cytotoxicity and memory responses are almost entirely abolished when arginine is depleted. But again, Morris said, “give it back, and you fix the problem.” Indeed, providing arginine to culture media restores T cell function.
As Morris had suggested earlier, T cell dysfunction may be protective in pregnancy, when a women needs to suppress her immune system to protect both herself and the fetus. But with trauma, she said, T cell dysfunction increases susceptibility to infection. Plasma arginine levels decrease within minutes to hours of a traumatic event and can remain low for up to a week or longer. Morris pointed out that it was one of the pioneers in the field, Dr. Juan Ochoa, who helped her and her team understand plasma arginase activity increases in trauma patients as a result of myeloid-derived suppressor cells that express arginase I after trauma. So in contrast to SCD, she continued, where it is the red blood cells that are dumping arginase from hemolysis, the cell source in trauma is different, but it similarly increases arginase. The increased arginase leads in turn to the arginine deficiency, ultimately translating into T cell dysfunction and increased susceptibility to infection.
Morris went on to observe that more than 15 million injuries occur each year in the United States, and about 10 percent of trauma patients develop wound infections. The infection rate increases to 30 percent for patients who have been in an intensive care unit (ICU) for more than 48 hours. Infections are the leading cause of late organ failure, Morris noted, and they contribute to about 10 percent of trauma deaths. Thus, she suggested, strategies aimed at infection prevention after trauma should result in significant decreased mortality, morbidity, and cost.
The Therapeutic Potential of L-Arginine
According to Morris, the therapeutic potential of arginine for SCD has been studied for 20 years. She reported that in a sickle cell mouse model, arginine supplementation has been shown to improve perfusion, increase glutathione levels (with an effect on oxidative stress), decrease inflammation, help heal lung injury, reverse micro-vascular vaso-occlusion, and decrease mortality. However, what Morris finds interesting about a sickle cell mouse model is that mice do not have arginase in their red blood cells as do humans, yet they have increased arginase activity. So the arginase is coming from a source other than the erythrocyte, she explained, and not necessarily through the process of hemolysis.
Morris noted further that several human phase II studies have shown that arginine therapy improves leg ulcers. Also in humans, she and her team conducted a vaso-occlusive pain trial in which children with SCD admitted to the hospital with acute SCD-related pain were treated with arginine versus placebo. She reported that those treated with arginine showed a 55 percent decrease in total opioid use (mg/kg) and lower pain scores at discharge. She mentioned that she was currently working with FDA to complete a second phase II trial, with plans for a phase III multicenter study. She also referred to data she had presented earlier showing decreased pulmonary hypertension with arginine therapy (see Figure 3-2). Anecdotally, she added, arginine has improved priapism in the emergency room the few times she has used it for that purpose.
With respect to trauma, Morris reported that arginine therapy has been shown to enhance wound healing after trauma and hemorrhagic shock; immunonutrition has been found to improve immune responses and T cell function; and high arginine formulas have been shown to decrease infection complications in critically ill patients, with the greatest benefit for surgical patients. However, she added, there is also evidence of harm in sepsis and following acute myocardial infarction. “So we don’t have all the answers yet,” she said. Moreover, most of these studies have had a number of methodological weaknesses, and, as discussed below, there is a paucity of data in children.
Immunonutrition in Critically Ill Children
Regarding immunonutrition in critically ill children, Morris said she was shocked by results of a 2009 Cochrane review that found insufficient evidence either for or against nutritional support in children during the first week of a critical illness, mainly because the appropriate studies had not been performed. She noted that some pediatric ICU doctors have interpreted this finding to mean that some of the sickest children in the hospital
should not be fed—even trauma patients, some of whom, she said, are basically being starved for days.
In addition to the Cochrane review, Morris mentioned a randomized controlled trial of arginine/glutamine-fortified formula among 40 children with traumatic brain injury (Briassoulis et al., 2006). The primary outcome measure was mortality. The authors found no difference in mortality compared with standard formula. According to Morris, however, fewer than 10 percent of pediatric trauma patients die, so the study was severely underpowered for its primary outcome. What she did find enlightening was that 69 percent of patients who received fortified formula had a positive nitrogen balance by day 5, compared with 31 percent of patients who received standard formula. Morris cited another, multicenter prospective cohort study of more than 1,000 mechanically ventilated children, in which Mehta and colleagues (2015) found decreased 60-day mortality among patients who had adequate protein intake. She reported that the same group recently published data showing similar findings in surgical trauma patients (Velazco et al., 2017). “This certainly gives me pause,” she said, “and it also suggests that we have gotten it wrong all these years. It is not overall calories, but it is enteral protein delivery [impacting important clinical outcomes].” She described enteral protein delivery as “a modifiable risk factor for mortality that is in dire need of a shift in our current practice, given the potential for improved outcomes.”
Therapeutic Strategies
Morris suggested several other therapeutic strategies to consider in addition to arginine supplementation: arginine precursors such as citrulline and glutamine; combination therapies that target multiple mechanisms; and immunonutrition, particularly targeted enteral formulas, although the ideal formulas for trauma, critical illness, hemoglobinopathies, and pediatrics do not yet exist. She urged more research in this area.
Final Remarks
Morris concluded by listing several final points:
- Arginine is a conditionally essential amino acid that becomes essential under conditions of stress and catabolic states, when the capacity of endogenous amino acid synthesis is exceeded. This occurs in such cases as critical illness, trauma, and hemolysis.
- SCD and trauma represent arginine deficiency syndromes, and they pose a distinct nutritional requirement that develops because of their metabolic abnormalities. Thus, they may benefit from arginine replacement therapy.
- There are at least two broad categories of arginine deficiency syndromes: (1) T cell dysfunction (e.g., trauma), and (2) endothelial dysfunction (e.g., SCD).
- The global arginine bioavailability ratio may be a novel biomarker of arginine deficiency, and as such warrants further study.
- Arginine-fortified immunonutrition may be a treatment for acquired arginine deficiencies. Morris called for future studies to identify subpopulations that would benefit most, with potential adverse events being minimized.
Morris closed by stating, “Nutrition is medicine!”
PERSONALIZED NUTRITION IN THE REAL WORLD: WHERE DO WE STAND?
David Alpers, Washington University School of Medicine, began his presentation by saying, “We are in this room to try to figure out where nutrigenomics is.” “We have heard a lot of evidence that has tremendous promise,” he added, including evidence indicating that behavior has an enormous influence on what people do in terms of nutrition and that this behavior may be modifiable (Meisel et al., 2015). Yet, he asserted, despite this evidence and despite the great number of genetic tests already available, most based on single nucleotide polymorphisms (SNPs), few studies are informative about how to influence clinical behavior in nutrition. He discussed why useful studies will be difficult, but not impossible, and why “we are just at the early stages of where we can utilize this information.”
According to Alpers, nutrigenomics studies are difficult, first, because they require isolation of the effects of different individual genes and different individual foods, initially by themselves and then in combination, using adequate control arms. Thus far, he said, upon examination of either isolated genes or isolated nutrients, such as antioxidants and vitamin A, the results “have not been very impressive.” A second reason for the difficulty of nutrigenomics studies is the challenge of proving causation from associations, a challenge he discussed in more detail, as summarized below.
The Challenge of Nutrigenomics Studies: Proving Causation
Alpers explained how the Bradford-Hill criteria for causal association are particularly difficult to achieve in nutrition-related studies (Bruemmer et al., 2009). He noted that while the Bradford-Hill criteria are not “absolute criteria,” they do provide a rough “scaling” of how to interpret associations.
For example, one of the Bradford-Hill criteria is that there be a strong association. According to Alpers, however, this is not the case for many of
the associations that have been reported between nutrition and either genetic or metabolic change. A second Bradford-Hill criterion is that the association be constant. But at present, Alpers said, most nutrigenomics associations are based on only a few studies, which makes it difficult to assess constancy. A third Bradford-Hill criterion, Alpers continued, is that there be one cause and one effect. He pointed out, however, that in nutrition, particularly in nutrigenomics, where the goal is to prevent disease in relatively healthy people, it is very difficult to narrow an association down to one cause and one effect because there are so many components to the diet and because those components interact, causing multiple metabolic changes to the diet. He added that this complexity extends even to inherited diseases caused by single genetic changes, such as what Morris had discussed. A fourth Bradford-Hill criterion is that there be a dose-response relationship. Alpers noted that although these relationships exist in genetic studies when one allele is variably knocked out, they are not usually available for the spontaneous SNPs found in association studies. A fifth Bradford-Hill criterion is that there be scientific justification for an association. According to Alpers, although there is always scientific justification for nutrition-related gene associations, some of it quite convincing in his opinion, few of these justifications are based on clinical data. Most are based on in vitro and animal data. A sixth Bradford-Hill criterion is that the association be coherent with other data. In nutrition, however, other data are often quite limited, Alpers explained. A final Bradford-Hill criterion is that interventions have been tested in randomized controlled trials. Again, however, such studies are very difficult to perform, Alpers said.
The Challenge of Nutrigenomics Studies: Isolating Nutrition-Related Phenotypic Effects
Alpers continued by pointing out that, in addition to the challenge of proving causation, another challenge to linking nutrition to genomics is the fact that, except for diseases caused by single gene defects, it is very difficult to isolate which components of the phenotype are related to nutrition and which to other factors. He elaborated on this challenge in the context of malnutrition, but remarked that his conclusions were just as applicable to the prevention of disease.
Alpers observed that, although a number of organizations have developed consensus criteria for malnutrition—that is, criteria that indicate whether a person is malnourished (White et al., 2012)—in clinical practice there are in fact two major types of malnutrition (Jensen et al., 2010). The first is pure starvation with no or limited inflammation, whereby if nutrients are given back, the phenotype is reversed. The second is malnutrition either in chronic disease, where mild or moderate inflammation and/or other fac-
tors are frequently superimposed, or in acute disease or injury with marked inflammation.
Alpers explained that chronic diseases with malnutrition include, for example, obesity, rheumatoid arthritis, inflammatory bowel disease, and cancer. But in the case of chronic disease, he noted, clinicians can have no idea what effect replacing nutrition will have on the phenotype. Although there are certainly differences among individuals, he said, “usually by the time we see a well-developed chronic disease, the effects of the disease itself are more potent than that of nutritional deficiencies.” Acute diseases or injuries with marked inflammation include trauma, as discussed by Morris, and major infections and burns.
According to Alpers, many of these same kinds of chronic disease/malnutrition conditions are the same disorders for which genomic links have been sought in past studies (e.g., obesity, cancer, type 2 diabetes, aging, even pregnancy). But again, he noted, most of these conditions have a component of inflammation, and only conditions with no or limited inflammation respond in a clear manner to nutrient supplementation. Thus, he observed, any change in phenotype may be related to nutritional supplementation only by chance or in part.
According to Alpers, the question then arises of what approaches have been used to try to link genetic or genomic effects to nutritional phenotypes. He referenced the large body of in vitro and in vivo animal studies carried out to support the rationale for such a link. He cited the example of cancer research, with many studies showing that curcumin, turmeric, garlic extract, and other nutrient components have potent roles in preventing some of the changes that occur during cancer in cells or in animals. But these findings have yet to be translated into human data, he cautioned.
Alpers went on to point out that the few human data that do exist are often less suggestive, citing studies on caffeine metabolism, omega-3 supplementation, and antioxidant supplementation. For example, he observed, even though the antioxidant pathway has been well explicated in a variety of diseases, including cancer and a number of degenerative diseases, the antioxidant theory of human disease, which was quite prevalent many years ago, has not yet been proven by clinical studies. He finds the mitochondrial story potentially a very powerful one (see Chapter 2 for a summary of Douglas Wallace’s presentation on mitochondria), but one without the necessary clinical data at present.
Alpers observed further that with human data, some genetic factors that can be detected—such as human leukocyte antigen (HLA) subtypes for celiac disease—are not sensitive enough. It is known, he pointed out, that the two subtypes found in almost all cases of celiac disease are also highly prevalent among individuals who do not have celiac disease. Sometimes, he noted, more information is needed than just the genetic change.
Alpers also pointed to other genetic factors that add little to clinical information. To illustrate this point, he explained that while it has been known for decades which genetic changes are determinative of lactose intolerance in humans, this same determination can be made clinically by removing milk products from an individual’s diet and seeing whether the person responds. “So we have not needed that information yet to get personalized in that particular condition,” he said.
Alpers cited as a final challenge to the scientific approaches linking genomics to nutrition that again, many chronic diseases—obesity in particular—are also related to the phenotype of chronic inflammation/malnutrition. He identified as the challenge with obesity and other nutritional disorders, including to some extent even diabetes, that management with the standard-of-care nutritional advice is difficult by itself and often is not fully implemented. While the use of individual coaches is, he said, “a wonderful thing” (see the summary of Price’s presentation earlier in this chapter), with such coaching being what trained dietitians provide, he believes the field will need to move much further along in terms of its use of cell phones and other technologies before individualized coaching becomes a widespread phenomenon.
Alpers expects a long lag time before strong nutrigenomics data become available, and he predicted that such data would become available one disease at a time. “There is not going to be any great breakthrough,” he said. “I think we need definite effects on a clinical basis before we can really implement these things fully commercially.”
Exploring Current and Future Directions of Personalized Nutrition
Alpers identified three current and future trends in personalized nutrition. First, many currently available personalized Internet services provide people with information based on an analysis of their dietary patterns, although, according to Alpers, none of these services have anything to do with genomics (Gibney and Walsh, 2013). Moreover, he asserted, the dietary patterns reported by individuals are often biased, although he believes this perhaps could be changed with education. He suggested that the difference between meal-based and food component–based information also will need to be addressed. He added that many of these services are mobile phone–based. Thus at present, the uptake of these systems has been much greater among adolescents, who have grown up with cell phones, than among adults, Alpers expects this situation will change with time and as the population becomes more accustomed to the technology. And despite these challenges, he predicts that in the future, personalized Internet services will become a potent method for modifying behavior, potentially leading to changes in clinical outcomes (once those changes in clinical outcomes have
been identified). He cautioned, however, that recidivism may be a problem, as it currently is with weight loss diets, noting that “it is very hard to keep up a change in behavior that hasn’t been lifelong up until now.” He added that while these programs have as yet nothing to do with genomics, if it can be shown that behavior is changed in a meaningful way, those lessons can be incorporated into what is learned in the future about the role of genetics in modifying disease phenotypes.
A second personalized nutrition trend discussed by Alpers is the use of phenotypic data. He remarked that although the phenotype is more difficult to study than the genome because of the difficulty of conducting a large phenotypic study with clear clinical relevance, there are a few examples of such studies. He cited a study in which monitoring urine sodium and decreasing sodium-containing foods was found to have an effect on blood pressure (Yamasue et al., 2006). This intervention appeared to work, he observed, although in the face of relatively high sodium intake, indicating that the effect of altering sodium intake is modest. He cited another example involving the use of a wristwatch accelerometer to monitor physical activity and deliver relevant information (Hurling et al., 2007). He described these studies as being among a number of wellness programs, suggesting that such programs do work. But again, he stressed, what is missing is knowledge of whether this type of program will actually change a disease phenotype, as well as how long people will stay with the program. As a final example, Alpers cited a number of studies of blood tests that are used to analyze metabolites and develop metabolic profiles. He clarified that these are metabolic analyses, not metabolomics analyses, which he said “are coming.” One of these studies looked at the effect of vitamin D supplementation, finding no effect on metabolites (O’Sullivan et al., 2011). In sum, Alpers stated that these types of studies have many problems that he did not have time to discuss during his presentation, but that such studies will be necessary to examine personalized nutrition with respect to phenotypic changes.
The third and final trend Alpers discussed is personalized nutrition based on genomic data. He observed that at present, most of the information in this area comes from observational studies linking SNPs to dietary patterns. “That’s not really enough in itself,” he argued. While it has been shown, he elaborated, that certain SNPs can cause metabolic changes—such as in the methylenetetrahydrofolate reductase gene, MTHFR—that have been shown to change homocysteine levels in TT individuals who receive riboflavin supplementation (McNulty et al., 2006), again that association has yet to be translated into a change in disease phenotype.
Alpers emphasized that, with all three of these approaches, it is difficult to predict the extent to which a user will want just a “one-off” result or will maintain the service in the long term. In his opinion, some of the
programs currently available are providing good incentives for people to stay with them, but what is needed is a service that can be delivered over the long term and will eventually show that its delivery leads to a change in disease progression or occurrence. While just making people feel good is worthwhile, he said, “that is not really what we are talking about [in this workshop].”
In closing, Alpers mentioned that some Food4Me studies under way in the European Union are developing data on consumer responses. These include a study just published in the American Journal of Clinical Nutrition in which individuals who were told that they had the FTO variant associated with risk of obesity lost more weight over 6 months relative to individuals who were not told whether they had the variant (Celis-Morales et al., 2017). The difference was small, Alpers said, but he suspected that it would have been greater if the study had lasted longer than 6 months. Additionally, he noted that members of the control group were told nothing about their genetic risk and that to serve as a real control group, they should have been told something definite but unrelated.
In closing, Alpers stated, “The concept of genomics for personalized nutrition is a sound one, and many of the strategies are in place. What is missing is the data that translate those strategies or the preclinical work to actual clinical outcomes. It will occur. It will be difficult but it will occur slowly. We just need to be patient.”
IS GENETIC TESTING FOR PERSONALIZED NUTRITION READY FOR PRIME TIME?
Ahmed El-Sohemy, University of Toronto, began by saying that he would be discussing some of the translational activities under way at a company, Nutrigenomix, he had founded and for which he serves as chief science officer. He also holds shares in the company. He clarified that Nutrigenomix is not a direct-to-consumer genetic testing company, but provides a service to health care practitioners, mainly registered dietitians but also some physicians who practice functional and integrative medicine. At the time of the workshop, the company was serving more than 6,000 practitioners in 35 countries and providing reports in 8 languages.
El-Sohemy asserted that genetic testing for personalized nutrition is indeed ready for prime time. While he agreed with many of Alpers’s comments, he said he would also be presenting evidence to support his opinion that many of the skeptics’ criticisms “are actually not true.” However, he acknowledged that the field is not without controversy, as there are many different kinds of operators, and the evidence base behind much of what is being offered is quite varied. He characterized some of what is being offered as “on the fringe or not really rooted in robust scientific evidence.”
He pointed to the many articles in the media questioning the validity of personalized dietary tests, and while he agrees that it is important to keep these companies honest, he asserted, “It is also not right to lump all of them in one basket and say that the whole field is all just snake oil.”
Part of the controversy, in El-Sohemy’s opinion, is due to the different kinds of tests on the market. He emphasized the importance of distinguishing between disease risk genes, which are often identified through genetic association studies, and what he refers to as modifier, or metabolic, genes. The latter are genes that are not by themselves related to any phenotype or health outcome, but modify the effect of an environmental factor on a phenotype or health outcome. El-Sohemy cited as an example that a genetic variant for a drug-metabolizing enzyme or a drug transporter does not necessarily cause any adverse effect by itself, but if someone with one of these particular genetic variants is prescribed a certain drug, he or she may experience an adverse reaction. He noted that although this analogy is from pharmacogenetics, it applies to nutrition as well.
Why Are Genetic Differences Important for Nutrition?
With respect to why it is important to look at genetics in the field of nutrition, El-Sohemy remarked that if one looks at the link between virtually any nutritional factor and any health outcome for which there have been enough observational studies, one will see a heterogeneity of responses, with some studies showing increased effects, some no effect, and others completely opposite effects. There are, he observed, many reasons for the inconsistencies among studies, but an important consideration is the genetics of the groups or population being studied. For example, he noted, some people who go on a low-sodium diet actually experience an increase in blood pressure. These people used to be thought of as outliers, he said, but these so-called outliers are increasingly being recognized as very real. “So what if you are an outlier,” he asked, “and the advice that we are giving you is actually causing harm?” If there is a way to identify these individuals and find alternative strategies for them, he suggested, that is something to consider. “One size does not fit all,” he pointed out.
As proof of concept, El-Sohemy described some of his early work on caffeine and cardiovascular disease. He and his collaborators found that in fast metabolizers (CYP1A2 AA), moderate coffee consumption was associated with a lower risk of myocardial infarction, whereas in slow metabolizers (CYP1A2 AC + CC), consumption of even two to three cups per day was linked with a higher risk of that outcome (Cornelis et al., 2006; see Figure 3-3). A couple of years later, he reported, these findings were replicated by a research group in Italy, looking not at myocardial infarction but at the risk of developing hypertension (Palatini et al., 2009). Theirs was
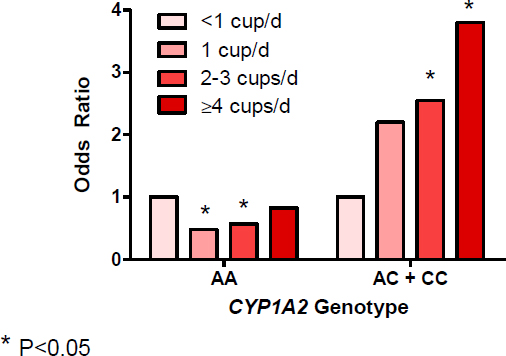
SOURCES: Presented by Ahmed El-Sohemy on December 5, 2017, from Cornelius et al., 2006.
a prospective study, he explained, for which the researchers recruited people who were prehypertensive, genotyped them, assessed their coffee consumption, then monitored them over a few years. They found a similar pattern, with the fast metabolizers being protected against the risk of hypertension, while the slow metabolizers experienced the opposite effect. The pattern was replicated yet again a few years later with respect to risk of prediabetes, with coffee consumption being found to increase the risk of impaired fasting glucose among slow metabolizers but not among fast metabolizers (Palatini et al., 2015). More recently, El-Sohemy’s group collaborated with the Italian research group to look at kidney function and again found a similar pattern, with no increased risk of impaired kidney function (as measured by glomerular filtration rate) among fast metabolizers but a strong increased risk among slow metabolizers.
In addition to these observational studies, El-Sohemy and his team conducted a randomized controlled intervention trial of endurance performance in athletes. They recruited individuals and, based on genotype, randomized them to receive either placebo or one of two different doses of caffeine. They found that fast metabolizers benefited more from the caffeine, whereas slow metabolizers showed no improvement in performance (Guest et al., 2018).
In summary, El-Sohemy said, myocardial infarction, hypertension, prediabetes, and kidney function all show the same pattern. “If you are a slow
metabolizer,” he observed, “you should probably limit your intake to no more than two cups a day. If you are a fast metabolizer, you are lucky. You can follow the general recommendations, which suggest that you can drink up to four cups a day.”
Media Messages
El-Sohemy then showed the image of a headline for a 2015 The Washington Post article: “Government panel said drinking coffee is harmless. Why that might be wrong.” The subheadline read: “A U.S. panel said coffee can be part of a healthy diet. That might be true for only half of us.” According to El-Sohemy, the journalist cited some of the work on fast versus slow metabolizers and wondered why these one-size-fits-all recommendations are still be being issued when the science suggests otherwise (Whoriskey, 2015).
El-Sohemy added that one of the quotes in the article was from Sander Greenland, an epidemiology professor emeritus at the University of California, Los Angeles, who said, “There are spectacular metabolic differences in people, and to expect that coffee will have the same health effects on everyone is absurd.” El-Sohemy agreed with this assessment. He found it interesting that the journalist also interviewed a member of the government panel, who said, “Unfortunately, because genetic testing is expensive and rarely done, most people have little idea which gene variant they carry.” El-Sohemy agreed that this, too, was a fair observation, saying, “There is no point in making recommendations based on a bit of information that people do not have access to.”
For El-Sohemy, this quote by the panel member is significant because it implies that, if genetic testing were inexpensive and everyone knew what gene variant he or she had, the recommendation about drinking coffee would to some extent have taken the science into account. Such issues that relate to the economic and social aspects of genetic testing, such as how to make the information accessible to everyone, are very legitimate topics of discussion, in his view. He believes “there are some really good examples of proof of concept at how . . . a single SNP can modify the association between a dietary component and a variety of different health outcomes.”
El-Sohemy then cited a paper that appeared in The BMJ just prior to the workshop. Poole and colleagues (2017) conducted a review of about 200 meta-analyses of coffee consumption and multiple health outcomes and concluded that the totality of the evidence suggests a protective effect for a number of these outcomes. A BBC News article responding to this was headlined, “Three cups of coffee a day may have health benefits” (Roxby, 2017). Unfortunately, El-Sohemy said, “at the end of the day, people want to know what they can do for themselves, and when they see headlines like
this, they falsely assume that coffee is safe to consume.” But he emphasized that if slow metabolizers were to follow those recommendations, they would actually increase their risk for multiple health conditions. He asserted that the conclusion of the review suggests that the majority of participants in the studies included in the review were probably fast metabolizers. He pointed out that if a study population comprised only 60 percent fast metabolizers and 40 percent slow metabolizers, the fast metabolizer effect would still predominate. “We need to move away from these kinds of studies,” he argued. “Bigger is not necessarily better. You have to look at the quality of the scientific evidence.”
To this end, El-Sohemy and Raffaele De Caterina wrote what he said was the first consensus article of the International Society for Nutrigenetics and Nutrigenomics reviewing the scientific evidence that would enable DNA-based dietary advice on the consumption of caffeine (De Caterina and El-Sohemy, 2016). He mentioned another article, published recently in Genes and Nutrition, in which he and his co-authors propose certain guidelines for evaluating the scientific evidence for formulating DNA-based dietary advice (Grimaldi et al., 2017). He also cited an article that appeared in The New York Times headlined “For Coffee Drinkers, the Buzz May Be in Your Genes.” Although the research had been completed several years earlier, he added, the journalist reminded readers that the association between coffee and health appears to be dependent on individual genetic variation (O’Connor, 2016).
Still, El-Sohemy continued, there is no shortage of articles that question the validity of the entire field, referring in particular to an article that had appeared in the week just prior to the workshop titled “DNA-Based Diet Advice Is Big Business with Little Scientific Support” (Entis, 2017). He pointed out that one of the individuals interviewed in this article was a pediatrician and author of The Bad Food Bible (Carroll, 2017). The journalist wrote:
There’s no evidence that some people respond better to high-fat diets while others are more receptive to diets packed with protein or complex carbs. “It doesn’t exist,” [Carroll] says. Even if it did, “there’s no evidence we could detect it” through DNA sequencing. Metabolic illnesses and disorders such as celiac disease or lactose intolerance aside, humans’ genes are very similar. We have evolved to be able to eat the same foods.
El-Sohemy reminded the workshop participants, however, that José Ordovás had already presented evidence showing an association between APOA2 and saturated fat (see Chapter 2 for a summary of Ordovás’s presentation). El-Sohemy cited other studies as well, such as the POUNDS LOST study, a 2-year randomized controlled trial comparing the effects of
a high-protein versus a low-protein diet, that have found similar associations. He reported that, as part of the POUNDS LOST study, Zhang and colleagues (2012) had found a significant loss of fat mass after 2 years of a high-protein diet, but only among individuals with an FTO AA genotype. Individuals with an AA genotype showed no change in fat mass on a low-protein diet, and individuals with either a TT or TA genotype showed no change in fat mass on either a high- or low-protein diet. “When it comes to the gold standard of scientific evidence,” El-Sohemy asserted, “I think this is pretty robust.” He added that this evidence has since been replicated in a distinct population in Spain. In that study, de Luis and colleagues (2015) showed that a high-protein diet is effective for weight loss only in individuals with the AA genotype. El-Sohemy and his team have replicated these findings yet again, in a distinct, East Asian population, in which individuals with the AA genotype had a waist circumference roughly 10 cm greater when not on a high-protein diet (Merritt et al., 2018). What these findings show, El-Sohemy argued, is that while FTO has been used in the past as a predictor for obesity, it is also a modifier gene that indicates whether someone is likely to benefit from a high-protein diet.
Personalized Dietary Advice Versus Public Health Recommendations
El-Sohemy agreed with Patsy Brannon’s prediction that the future would likely see the integration of personalized dietary advice with public health recommendations (see Chapter 1 for a summary of Brannon’s presentation). In the meantime, he said, there is some disagreement about whether providing people with genetic information is actually helpful. Some argue that if a person knows that something is “in my genes,” he or she will not be motivated to do anything in response. Others argue the opposite: that someone aware of having a particular gene will be motivated to watch what he or she eats. For example, people who knew they were slow metabolizers of coffee would cut back on coffee. Likewise, people told that they have the risk variant for salt-sensitive hypertension would lower their sodium intake.
El-Sohemy and his collaborators decided to put this notion to the test and conduct a randomized controlled trial comparing DNA-based dietary advice with standard recommendations (Nielsen and El-Sohemy, 2012). They found that those who received the DNA-based dietary advice actually understood the recommendations to a greater extent relative to those who received the standard recommendations, and were motivated to change their eating habits. In fact, El-Sohemy reported, they did change their eating habits and had maintained those changes 1 year later (Nielsen and El-Sohemy, 2014). The biggest effect, he noted, was with salt-sensitive hypertension in individuals told that they had the risk variant for that phenotype.
That DNA-based dietary advice can motivate behavior change has since been replicated by a group in Finland, El-Sohemy continued, with a different kind of genetic information and different outcomes (Hietaranta-Luoma et al., 2014), as well as in the Food4Me trial (Celis-Morales et al., 2017; Livingstone et al., 2016). He cited yet another study of behavior change in response to receiving genetic information, in which Green and Farahany (2014) showed that 42 percent of people surveyed reported positive changes in their health behavior. Many people reported changing their exercise habits (61 percent), and the vast majority reported changing their dietary patterns (72 percent). Finally, El-Sohemy cited a recent study by Nielsen and colleagues (2017), showing again that while not everyone changes behavior in response to receiving genetic information, providing people with the right kind of information can be a very useful way to get at least some people to change their eating habits.
What Do the Skeptics Say?
Again, El-Sohemy posed the question, What do the skeptics say? He mentioned the Academy of Nutrition and Dietetics’ position paper on nutritional genomics, which states: “Applying nutritional genomics in clinical practice through the use of genetic testing requires that registered dietitian nutritionists understand, interpret, and communicate complex test results in which the actual risk of developing a disease may not be known” (Camp and Trujillo, 2014). El-Sohemy questioned the use of the word “complex” and remarked that risk in nutrition is always relative, never actual, viewing this as a message to “spook” dietitians away from this type of testing. He noted that the position paper had been pulled a couple of months prior to the workshop so that the scientific evidence could be re-reviewed.
More generally, El-Sohemy continued, a frequent comment is that single SNPs are useless. In his opinion, there are a number of good examples of single SNPs that modify the effects of specific dietary factors. Another skeptics’ argument, he noted, is that people are not going to change their behaviors, and he reiterated that research has shown the contrary to be true. He also asked the question of when this has ever been an issue, pointing out that some people still smoke, and it is unlikely that they have not heard that smoking causes cancer. Finally, he observed, some skeptics argue that “it’s all about the microbiome.” Yet, he argued, there is good evidence that host genetics determines to a large extent the kinds of bacteria that colonize the gut (Blekhman et al., 2015; Goodrich et al., 2016; Turpin et al., 2016). Although he did not go into detail, he remarked that there are other criticisms that also are not necessarily valid.
Where Are We Today?
El-Sohemy closed by stating that, while the current dietary recommendations are based on science, he believes they are based on what he considers “old science.” The question this raises for him is, How much more science do we need before we can actually start using DNA-based information? “It is not meant to be revolutionary,” he said. “It is just meant to be evolutionary.” In his opinion, yes, DNA-based dietary advice is ready for prime time.
DISCUSSION
Following El-Sohemy’s presentation, he and the other session 1 speakers (including those whose presentations are summarized in Chapter 2) participated in an open discussion with the audience, summarized here.
Unintended Consequences of Information Provided to Consumers
Session moderator Naomi Fukagawa asked the speakers to reflect on the potential unintended consequences of providing consumers with genetic information and on ways to achieve a balance such that dietary recommendations do not end up being punitive—that is, with people feeling guilty about their dietary choices when they know they have an SNP that increases their risk for a particular health outcome.
Douglas Wallace replied that providing genetic information without genetic counseling can be highly problematic. He commented on recreational genetics2 companies that sequence various parts of an individual’s DNA, including mitochondrial DNA, and then provide that sequence to the consumer. It is quite common, he suggested, for people to search the Internet and scan the literature to see whether their nucleotides have been correlated with any kind of clinical problem. He receives phone calls from people who have done just that, asking him what they should do to “save themselves from this terrible disease.” People can find information on almost any nucleotide, he observed, with either positive or negative associations. “Without appropriate genetic counseling,” he said, “we are not doing people a real service to give them this kind of information.”
Fasting and the Mitochondria
Panelist Tim Morck commented on what he called a “new genre” of weight loss—fasting, not just fasting intended to mimic diets but also fast-
___________________
2 Recreational genetics refers to direct-to-consumer genetic testing for genealogy and health diagnostic services.
ing itself, that is, going without any nutrients. He wondered whether fasting affects the mitochondria.
There is no question, Wallace replied, that periodic fasting changes one’s metabolic state, and it has been shown repeatedly in model organisms and in some primate studies to affect longevity and other risk factors. There is also clear evidence, he added, that fasting affects mitochondrial metabolism by increasing antioxidant defenses and respiration rate. Additionally, he explained, people make more mitochondria during fasting because they are trying to cope, first, with low carbohydrates, and then with what is basically a high-fat diet (from stored fats). According to Wallace, these aspects of fasting have generated a great deal of interest, as has the question of whether fasting increases mitophagy—the way the body pulls out the accumulation of defective mitochondrial DNA—and how it might be related to longevity. “But I think there is still a huge amount of unknown information that we need to really understand this in any causal way,” he said.
When Morck asked about potential stem cell stimulation in particular, Wallace replied that in fact, some of the most interesting studies have involved introducing mutations into the nuclear-encoded mitochondrial DNA polymerase that increased mitochondrial DNA mutation rates and caused premature aging. But the main effect, he said, was on stem cell biology. “So, yes,” he said, “there is a lot of interaction there.”
Behavior, Behavioral Feedback, and the Brain
In response to the emphasis placed by many speakers on the importance of behavior, Morck asked about the need for better markers of improvement that could help reinforce behaviors. He asked whether “true” metabolomics panels that show benefit might be sufficient to help motivate people.
Nathan Price agreed that behavioral feedback is important and pointed out that it is a focus of Arivale and was emphasized in the Pioneer 100 Wellness Project. The risk of a disease in the far future is not very motivating to individuals, he observed, and at present, the company does not explicitly provide people information on disease risk. But with the markers provided, individuals can see changes in their body that are happening now. “In our experience,” he said, “we have seen that to be quite motivating for people.”
Wallace identified as a problem in clinical medicine that “if we don’t have a test, it is invisible to us.” In the field of what he called bioenergetic medicine, there are no good outcome variables, making it difficult to know whether an individual has a low- or high-energy state and whether a nutraceutical or other kind of intervention is actually having an effect. He mentioned that a great deal of time is currently being spent on developing microelectronic and nanoelectronic systems with which to monitor bioenergetics in tissues so there can be some kind of quantifiable outcome variable.
While the discussion was on the topic of behavior, David Alpers observed, “The organ that has been left out of this whole discussion is the brain.” He stressed that many behavior changes are dependent on an individual’s makeup. What drives or motivates a person to stay in a program is not always a test, he asserted; sometimes it is a feeling the person has or something about the way he or she looks. “So that is an enormously important part of the whole equation,” he said, “which usually isn’t looked into very often in this particular setting.”
Behavior and Coaching
An audience member asked about the longitudinal follow-up of the coaching that Price had described, wondering whether he and his team had followed participants beyond 6 months to see whether they continued to show improvement either with or without continued coaching. Price replied that many of the Pioneer 100 Wellness Project participants had entered Arivale’s commercial program. So yes, he and his collaborators do have a couple of years of follow-up data on those participants. But, he acknowledged, there was also a gap between the end of the Pioneer 100 Wellness Project and participants’ entry into the program, during which they received no coaching. During that period, many participants had reverted back, he said, so they had not done so well when they were not receiving coaching. When they reentered the program, however, they improved again.
Questions About the Pioneer 100 Wellness Project
In response to the previous question about whether the microbiome sequencing conducted during the Pioneer 100 Wellness Project was metagenomic sequencing, Price replied that, while metagenomic sequencing is better and he is advocating for a switch, 16S sequencing was used in the project because of cost considerations. Another audience member asked about the demographics of the project’s population, including diversity of socioeconomic status, and what Price hopes the demographics will be for the 100K Wellness Project. Price replied that the population in the Pioneer 100 Wellness Project was representative of Seattle, so in terms of ethnicity, it was heavily Caucasian, with some Asians and a few representatives of other lineages. With respect to the Arivale program, he explained that, because it is a commercial program, it is “definitely heavily biased socioeconomically.” He said the company would love to launch the program among groups of lower socioeconomic status and had attempted to do so in West Virginia, for example, but the financing had fallen through. He added that the company is exploring different
models of payment in cases where individuals, including those of low socioeconomic status, are at very high risk for disease, and there are other parties with a financial interest in their health.
Caffeine Fast Metabolizers Versus Slow Metabolizers
An audience member commented that most cardiologic health events are multifactorial, not single-factor events. She asked El-Sohemy whether studies on fast metabolizers versus slow metabolizers of caffeine had collected nutritional information other than caffeine intake, such as tea intake and overall diet, and whether they had considered the concentration of the coffee people were drinking. El-Sohem 3y acknowledged that many factors determine the “chemical soup” of a cup of coffee, such as type of bean, the extent of roasting, and other factors. In his opinion, however, those other factors are just “noise,” because whatever the fast metabolizers are drinking, or misreporting (e.g., their cup sizes), the slow metabolizers are drinking or misreporting as well. He explained that the gene he had discussed, CYP1A2, does not affect preference—for example, whether someone prefers Arabica, which has less caffeine than Robusta. Regarding other sources of caffeine, in one of his group’s study populations, 90 percent of caffeine intake came from coffee, even with the consumption of cola beverages and tea. With regard to consumption of other nutrients, he commented that in the myocardial infarction study he had referenced (Cornelis et al., 2006), the reported odds ratios were multivariate adjusted odds ratios that accounted for several confounding factors (e.g., sugar added to coffee, physical activity, smoking). Session moderator Fukagawa added that there are other bioactive compounds in coffee and coffee products that could have had salient interactions as well.
Treating Specific Nutritional Deficiencies
In response to a question about when the field will begin treating the type of very specific nutritional deficiencies discussed by Morris—that is, those with clear causality constructs—Morris reframed the question as, Are we really replacing a nutritional deficiency, or is arginine, or glutamine, working and functioning as a drug that works for everyone? Her personal experience in patients with SCD was that glutamine supplementation had the greatest effect on those who were the most glutamine deficient and that glutamine deficiency in those patients’ red blood cells was correlated strongly with their tricuspid regurgitant jet velocity on Doppler echocardiography (a measure of pulmonary hypertension risk). So her bias, she said, is that the patients who should be targeted first are those with the most severe deficiencies.
Additionally, Morris has learned from her studies that the global arginine bioavailability ratio is predictive of clinical outcome years ahead. Thus while children, for instance, have normal arginine levels at baseline, their levels drop acutely during times of pain crisis. Morris was involved with a phase II study that showed that administering arginine to these children during a pain crisis had an impact on pain outcomes.
Finally, when Morris and her collaborators gave arginine to SCD patients at baseline, they saw no effect on NO bioavailability. In fact, they observed a paradoxical decrease in NO that was not overcome by dose. But when they gave arginine to the same patients during a vaso-occlusive pain episode, they saw a rise in NO. “So again,” Morris said, “the patients who have a deficiency are probably the ones who are most likely to respond.”
Ordovás added that, regarding the genetic component, criticism is often raised that there is no clinical proof of benefit. He mentioned a long-term nutrition intervention study of people at high risk of cardiovascular disease, in which participants were administered either a Mediterranean or a low-fat diet. Using the gene TCF7L2, which is related to diabetes, Ordovás and colleagues found that even in people who were aged 60 and older, there was a benefit to using what he described as “the right diet for the right genotype” in terms of reducing the number of cardiovascular events that occurred over the 5 years of the study (Corella et al., 2016).
































